Key Points
Question
Is higher-than-normal body mass index (BMI) associated with better clinical outcomes in patients with cystic fibrosis?
Findings
In this systematic review and meta-analysis of studies including 9114 patients with cystic fibrosis, BMI indicating overweight and obesity were associated with better pulmonary function and lower chance for exocrine pancreatic insufficiency and cystic fibrosis–related diabetes compared with normal BMI.
Meaning
The findings of this study suggest that guidelines should be updated to recommend a higher target BMI in patients with cystic fibrosis.
Abstract
Importance
The prevalence of overweight (body mass index [BMI] = 25-29.9 [calculated as weight in kilograms divided by height in meters squared]) and obesity (BMI ≥30) is increasing among patients with cystic fibrosis (CF). However, it is unclear whether there is a benefit associated with increasing weight compared with the reference range (ie, normal) in CF.
Objective
To evaluate the association of altered BMI or body composition and clinical outcomes in patients with CF.
Data Sources
For this systematic review and meta-analysis, the literature search was conducted November 2, 2020, of 3 databases: MEDLINE (via PubMed), Embase, and Cochrane Central Register of Controlled Trials.
Study Selection
Patients older than 2 years diagnosed with CF with altered body composition or BMI were compared with patients having the measured parameters within the reference ranges. Records were selected by title, abstract, and full text; disagreements were resolved by consensus. Cohort studies and conference abstracts were eligible; articles with no original data and case reports were excluded.
Data Extraction and Synthesis
Two authors independently extracted data, which were validated by a third author. Studies containing insufficient poolable numerical data were included in the qualitative analysis. A random-effects model was applied in all analyses.
Main Outcomes and Measures
Pulmonary function, exocrine pancreatic insufficiency (PI), and CF-related diabetes (CFRD) were investigated as primary outcomes. Odds ratios (ORs) or weighted mean differences (WMDs) with 95% CIs were calculated. The hypothesis was formulated before data collection.
Results
Of 10 524 records identified, 61 met the selection criteria and were included in the qualitative analysis. Of these, 17 studies were included in the quantitative synthesis. Altogether, 9114 patients were included in the systematic review and meta-analysis. Overweight (WMD, –8.36%; 95% CI, –12.74% to –3.97%) and obesity (WMD, –12.06%; 95% CI, –23.91% to –0.22%) were associated with higher forced expiratory volume in the first second of expiration compared with normal weight. The odds for CFRD and PI were more likely in patients of normal weight (OR, 1.49; 95% CI, 1.10 to 2.00) than in those who were overweight (OR, 4.40; 95% CI, 3.00 to 6.45). High heterogeneity was shown in the analysis of pulmonary function (I2 = 46.7%-85.9%).
Conclusions and Relevance
The findings of this systematic review and meta-analysis suggest that the currently recommended target BMI in patients with CF should be reconsidered. Studies with long-term follow-up are necessary to assess the possible adverse effects of higher BMI or higher fat mass in patients with CF.
This systematic review and meta-analysis compares the clinical outcomes of patients with cystic fibrosis by body mass index.
Introduction
Cystic fibrosis (CF) is a common, often lethal inherited disorder caused by recessive genetic variants in the CF transmembrane conductance regulator (CFTR) gene affecting 1 in 3300 neonates of White race.1 The variants result in diminished function of chloride, sodium, and bicarbonate ion transport, leading to thick mucus production that causes functional derangement of multiple organs (eg, lungs, gastrointestinal system, and reproductive system).2 Consequential frequent airway infections, chronic inflammation, exocrine pancreatic insufficiency (PI), and complications, such as CF-related diabetes (CFRD) and CF-related liver disease, result in general unease and poor quality of life.3
Among patients with CF, malnutrition is commonly seen, mainly caused by the combination of the following mechanisms: (1) nutrient malabsorption and fecal energy loss due to PI, (2) increased energy expenditure predominantly related to chronic inflammation and breathing efforts,4,5,6 and (3) loss of appetite caused by inflammation-related anorexia.7 Malnourishment may accelerate the progression of the disease; nevertheless, underweight (body mass index [BMI]<15th percentile, with BMI calculated as weight in kilograms divided by height in meters squared) is known to be associated with worse pulmonary function and increased morbidity and mortality in patients with CF.8
Monitoring the nutritional status and growth of patients, as well as the prevention and treatment of malnutrition, are demanding components of CF care. Currently, BMI is the generally accepted indicator for monitoring the nutritional status of patients with CF. In children older than 2 years, the target BMI is at least the 50th percentile; in adults, the target BMI is greater than or equal to 22 for women and greater than or equal to 23 for men.9 However, BMI does not distinguish between the major components of the body, namely, fat mass (FM), fat-free mass (FFM), total body water, bone mineral density, and bone mineral content. There is a growing body of evidence highlighting the importance of nutritional status in the diagnostic and therapeutic management of patients with CF,10,11,12 but there is a lack of comprehensive review on patients with a BMI above the target.
The European Society for Clinical Nutrition and Metabolism; the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition; and the European Cystic Fibrosis Society adult and pediatric dietary guideline focuses on nutritional failure with no recommendation on the management of individuals who are overweight or obese.9 However, an analysis of BMI changes found that the prevalence of overweight and obesity in adults with CF is 31.4% and has more than doubled over the past 2 decades in patients with CF.13 However, long-term adherence to the currently recommended high-fat and high-carbohydrate diet in CF also might have controversial effects on body composition and even on some clinical outcomes. It is unclear whether there is an advantage of increasing weight over the normal range in CF. For instance, mortality in pneumonia has been reported to be lower in individuals without CF who are obese, known as the obesity survival paradox.14 To fulfill the knowledge gap, we aimed to evaluate the differences in clinically significant outcomes, such as lung function, PI, and CFRD, in patients with CF having altered BMI and/or body composition by conducting a systematic review and meta-analysis of the literature.
Methods
The review protocol for this systematic review and meta-analysis was prospectively registered with PROSPERO. The only deviation from our protocol was the addition of Pseudomonas aeruginosa colonization incidence. Findings are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.15
Study Selection
The literature search was conducted November 2, 2020, in MEDLINE (via PubMed), Embase, and Cochrane Central Register of Controlled Trials. Key search terms included cystic fibrosis, body fat, body mass, and body weight without any restrictions. Further details regarding the strategy of the literature search and selection can be found in the eMethods in the Supplement.
Two of us (R.N. and P.P.) independently conducted the selection in duplicate using reference management software (Endnote X9 software; Clarivate Analytics; 2019). Removal of duplicates was performed automatically and after that manually. The records were selected by title, abstract, and full text based on a previously determined set of rules. Any disagreements were resolved by consensus between the 2 reviewers. After each step of the selection process, the rate of agreement was determined and documented by calculating the Cohen κ coefficient. Values may indicate slight (0-0.20), fair (0.21-0.40), moderate (0.41-0.60), substantial (0.61-0.80), and almost perfect agreement (0.81-1.00).16 References of each included study were checked, and records that were considered to be eligible were added to the pool.
Eligibility Criteria
Cohort studies, case series, and clinical trial or conference abstracts were eligible; case reports and articles with no original data were excluded from our systematic review. The research question was formulated using the Population, Exposure, Comparator, and Outcomes framework.17
Patients older than 2 years diagnosed with CF regardless of sex, transplant status, CFTR modulator therapy, or comorbidities with altered body composition (BMI, FFM, and FM values out of the reference ranges, eg, underweight, overweight, and obese) were compared with patients with the measured parameters within the reference ranges. Articles reporting coefficients regarding the association between BMI or body composition and clinical outcomes were also eligible.
The nutritional categories were accepted based on study definition; however, we intended to strictly follow the thresholds recommended by the World Health Organization18: underweight (BMI <18.5), normal weight (BMI = 18.5-24.9), overweight (BMI ≥25), and obese (BMI ≥30) when it was possible to analyze separately. We also compared the underweight group (BMI <20) with the nonunderweight group (≥20) and performed subgroup analyses based on the age of the participants (adults, children, and mixed population).
Primary outcomes included pulmonary function (expressed by forced expiratory volume in the first second of expiration [FEV1%]), PI, and CFRD. Diagnosis of PI and CFRD was determined according to the definitions used in the included studies. As secondary outcomes, we investigated parameters associated with metabolic status, including fasting glucose, fasting insulin, hemoglobin A1c (HbA1c), cholesterol, and triglyceride levels, and P aeruginosa colonization as an additional outcome.
Data Extraction
Two of us (R.N. and D.K) independently extracted data into a standardized data collection sheet (Excel 2019; Microsoft Corp), and data extraction was validated by another one of us (B-M.D.). Data collected included sex distribution, age distribution, genotype, patient numbers, and mean or median values of outcomes of interest. Correlation coefficients were also extracted regarding the association of BMI or body composition and clinical outcomes. Further details regarding data extraction can be found in the eMethods in the Supplement. Most of the eligible studies were cross-sectional. For longitudinal studies, we collected only baseline data. For overlapping populations, the study working with the most patients was chosen for each outcome.
Risk of Bias Assessment
Based on the recommendations of the Cochrane Prognosis Methods Group, the Quality in Prognostic Studies (QUIPS) tool was applied by 2 of us (R.N. and P.P.) to assess the risk of bias in the included studies for each outcome separately.19 Any disagreement was resolved based on consensus.
Statistical Analysis
A random-effects model was applied in all analyses using the DerSimonian-Laird estimation.20 Pooled odds ratios (ORs) with corresponding 95% CIs were calculated for dichotomous outcomes. Pooled mean difference was calculated for continuous outcomes (weighted mean difference [WMD]). Results of the meta-analyses are displayed in forest plots. Statistical heterogeneity was analyzed using the I2 and χ2 tests to gain probability values; P < .10 was defined to indicate significant heterogeneity. I2 values representing moderate (30%-60%), substantial (50%-90%), and considerable (75%-100%) heterogeneity were based on the Cochrane Collaboration recommendations.21 Sensitivity analyses were also carried out omitting 1 study and calculating the summary OR or WMD with the 95% CI to investigate whether there was an association between a single study and the final estimation. To check for publication bias, a visual inspection of funnel plots was performed with Egger tests.22 Statistical analyses were carried out using Stata, version 16 SE (StataCorp LLC). For continuous variables, P values were calculated using 2-tailed unpaired analysis. Results were considered significant at P < .05.
Results
Study Selection
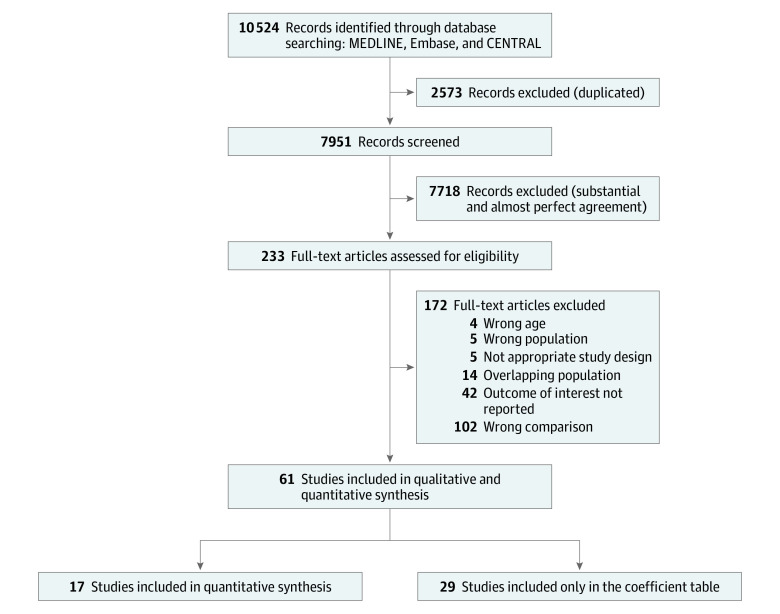
The systematic literature search yielded 10 524 records. After removal of duplicates, 7951 records were screened; of these, 61 records were included in the qualitative analysis and 16 full-text articles and 1 conference abstract were included in the quantitative analysis. Of the 61 studies, 33 contained correlational coefficients from which 29 did not report outcomes of interest according to BMI categories. These records and coefficients are included in the eTable in the Supplement. The selection process is shown in Figure 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses Flowchart.
CENTRAL indicates Cochrane Central Register of Controlled Trials.
Study Characteristics
Altogether, 9114 patients were included in the systematic review and meta-analysis. Of 9114 patients, 5301 were included based on BMI categories, and studies that reported coefficients resulted in 3813 involved patients. Five studies investigated only children (<18 years), 13 studies included only adults, and 14 studies examined a mixed patient population. The estimated proportion of children (mixed population studies did not give the number of children) is 30%. The mean (SD) values of BMI in the analyzed groups ranged from 18.5 (1.7) to 34.8 (5.7). The major characteristics of the included studies are reported in the Table.
Table. Characteristics of Included Studies.
| Source | Characteristic | Setting | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients, No. | Age group | Age, mean (SD), y | BMI | Pancreatic insufficiency, No. (%) | Genotype (DF508) | Growth parameter | Outcomes | |
| Altman et al,23 2017 (abstract) | 224 | Adults | 32.4 (10.6) | NA | NA | NA | BMI | CFRD, P aeruginosa colonization |
| Alvarez et al,24 2016 | 32 | Mixed | 26.1 (8.9) | 22.1 (2.9)a | 31 (96.9) | 56.3% Homozygote; 28.1% heterozygote | BMI, percent body fat, FFM | FEV1%, CFRD, FG |
| Barni et al,25 2017b | 73 | Mixed | 25.6 (7.3) | 21.0 (3.0)a | 66 (90) | 17.8% Homozygote; 45.2% heterozygote | BMI | FEV1%, PI, CFRD, P aeruginosa colonization |
| Bodnar et al,26 2014b | 59 | Mixed | 14.0 (4.8) | 20.4 (2.4)a | NA | 50.8% Homozygote | BMI, BMI percentile | FEV1%, P aeruginosa colonization |
| Bonhoure et al,27 2020b | 290 | Adults | 25.5 (7.9) | 21.7 (2.9)a | 232 (79.9) | 49.0% Homozygote; 41.3% heterozygote | BMI | FEV1%, PI, CFRD, FG, HbA1c%, TC, TG, HDL-C, LDL-C, FI |
| Bouma et al,28 2020 | 201 | Mixed | 13.3 (4.6) | 19.9 (3.7)a | NA | 51.2% Homozygote; 36.3% heterozygote | BMI, BMI z score | FEV1% |
| Brennan et al,29 2010 (abstract) | 348 | Adults | No data | No data | NA | NA | BMI | FEV1% |
| Cano Megias et al,30 2015b | 61 | Mixed | 26.8 (9.5) | 20.3 (3.3)a | NA | 24.6% Homozygote; 78.6% heterozygote | BMI, z score | FEV1% |
| Charatsi et al,31 2016 | 71 | Mixed | 12 (2.7) | 18.2 (3.4)c | 36 (90) | 49.3% Homozygote; 39.4% Heterozygote | FFM z score | FEV1%, PI, P aeruginosa colonization |
| Da Silva Garrote et al,32 2016 (abstract) | 34 | Children | 10.2 (5.3) | No data | NA | NA | BMI percentile | P aeruginosa colonization |
| Dray et al,33 2005b | 163 | Adults | 28.8 (8.4) | 19.1 (2.8)a | 137 (84) | 42.3% Homozygote; 38.6% heterozygote | BMI | PI, CFRD, P aeruginosa colonization |
| Dudina et al,34 2017 (abstract) | 435 | Adults | No data | No data | NA | NA | BMI | FEV1% |
| Engelen et al,35 2012b | 77 | Mixed | 14.8 (2.9) | 40.77 (26.4)d | 75 (97) | 63.6% Homozygote; 25.9% heterozygote | BMI, BMI percentile, FFM, z score | FEV1% |
| Gozdzik et al,36 2008b | 39 | Adults | 23.9 (3.7) | 19.5 (2.9)a | NA | NA | BMI | FEV1% |
| Hanna and Weiner,37 2015b | 226 | Children | 10.6 (4.9) | 18.5 (4.2)a | 181 (80) | NA | BMI percentile | FEV1%, PI |
| Harindhanavudhi et al,38 2020b | 484 | Adults | 35.2 (11.6) | 23.9 (4.4)a | 417 (85) | 46.9% Homozygote | BMI | FEV1%, PI, CFRD, HbA1c%, HDL-C, LDL-C |
| Hollander et al,39 2018 (abstract) | 224 | Adults | No data | No data | NA | NA | BMI | FEV1% |
| Ionescu et al,40 2003 | 56 | Adults | 23 (5.2) | 20.9 (1.6)a | 29 (100) | NA | FFM | FEV1% |
| González Jiménez et al,41 2012b | 109 | Children | 12.3 (8.8) | 21.6 (3.9)a | 371 (82) | 41.3% Homozygote; 46.7% heterozygote | BMI percentile | FG, HbA1c%, TC, TG, FI |
| González Jiménez et al,42 2017b | 451 | Mixed | 12.7 (3.2) | −0.3 (0.8)e | 93 (85) | 33.0% Homozygote; 49.8% heterozygote | BMI, z score | FEV1% |
| Kines et al,43 2012 (abstract) | 114 | Mixed | No data | No data | NA | NA | BMI | FEV1% |
| Kotsifas et al,44 2016b (abstract) | 44 | Adults | No data | No data | NA | NA | BMI | FEV1% |
| Maksimycheva et al,45 2018 (abstract) | 51 | Children | No data | No data | NA | NA | BMI | FEV1%, P aeruginosa colonization |
| Ochota et al,46 2019 (abstract) | 226 | Adults | No data | 23.5 (6.0)a | NA | NA | BMI | FEV1% |
| Panagopoulou et al,47 2008b | 43 | Mixed | 20.1 (8.5) | 19.4 (2.6)a | No data | NA | BMI, FFM | FI |
| Panagopoulou et al,48 2014 | 68 | Mixed | 19.81(9.0) | 19.8 (2.7)a | 56 (82) | 20.6% Homozygote; 48.5% heterozygote | BMI, BMI percentile | FEV1%, PI, CFRD, FG, TC, TG, P aeruginosa colonization |
| Papalexopoulou et al,49 2018 | 29 | Mixed | 15 (1.8) | −0.1 (−2.7–1.2)e | 28 (96.5) | 58.6% Homozygote; 31.0% heterozygote | BMI z scores, BMI percentile, FFM index z score | FEV1% |
| Proud et al,50 2012 (abstract) | 117 | Adults | 28 (9) | No data | NA | NA | BMI, FFM index | FEV1%, PI |
| Ramírez et al,51 2015b | 173 | Mixed | 11.43 (2.3) | No data | 145 (83.77) | 50.3% Homozygote; 36.9% heterozygote | BMI percentile | FEV1%, PI, P aeruginosa colonization |
| Stephenson et al,52 2013b | 651 | Adults | 33.8 (11.4) | 22.3 (4.1)a | 488 (75) | 39.3% Homozygote; 38.2% heterozygote | BMI | FEV1%, PI, CFRD, TC, TG, P aeruginosa colonization |
| Umławska et al,53 2014b | 89 | Children | 12.3 (3.5) | –0.8 (0.8)e | 80 (90) | 51.7% Homozygote; 33.7% heterozygote | BMI percentile | FEV1%, PI |
| Ziegler et al,54 2008 | 39 | Mixed | 23.7 (6.4) | 20.3 (2.2)a | NA | NA | BMI, BMI percentile | FEV1% |
Abbreviations: BMI, body mass index; CFRD, cystic fibrosis–related diabetes; FEV1%, forced expiratory volume in the first second of expiration; FFM, fat-free mass; FG, fasting glucose; FI, fasting insulin; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NA, not assessed; P aeruginosa, Pseudomonas aeruginosa; PI, exocrine pancreatic insufficiency; TC, total cholesterol; TG, triglycerides.
Value expressed as mean (SD).
Included in the quantitative synthesis.
Value expressed as median (IQR).
Value expressed as mean (SD) percentile.
Value expressed as z score.
Primary Outcomes
Forced Expiratory Volume in the First 1 Second of Expiration
Most studies (54 of 61) reported FEV1% as an indicator of pulmonary function. A total of 13 studies were included in the quantitative synthesis. Based on our results, patients whose weight was considered normal had significantly higher FEV1% values compared with those who were underweight (WMD, 14.61%; 95% CI, 10.39%-18.83%). Compared with patients whose BMI was considered normal, better pulmonary function was noted in patients who were overweight (82.96% vs 74.60%; WMD, –8.36%; 95% CI, –12.74% to –3.97%) or obese (84.63% vs 72.57%; WMD, –12.06%; 95% CI, –23.91% to –0.22%) (Figure 2). High heterogeneity was shown in the analysis of pulmonary function (I2 = 46.7%-85.9%). In the comparison of patients who were underweight vs not underweight, we found significantly lower FEV1% in children, adults, and mixed patient populations who were underweight (overall WMD, –19.12%; 95% CI, –23.53% to –14.71%) (eFigure 1 in the Supplement).
Figure 2. Pulmonary Function in Different Body Mass Index (BMI) Categories of Patients With Cystic Fibrosis.
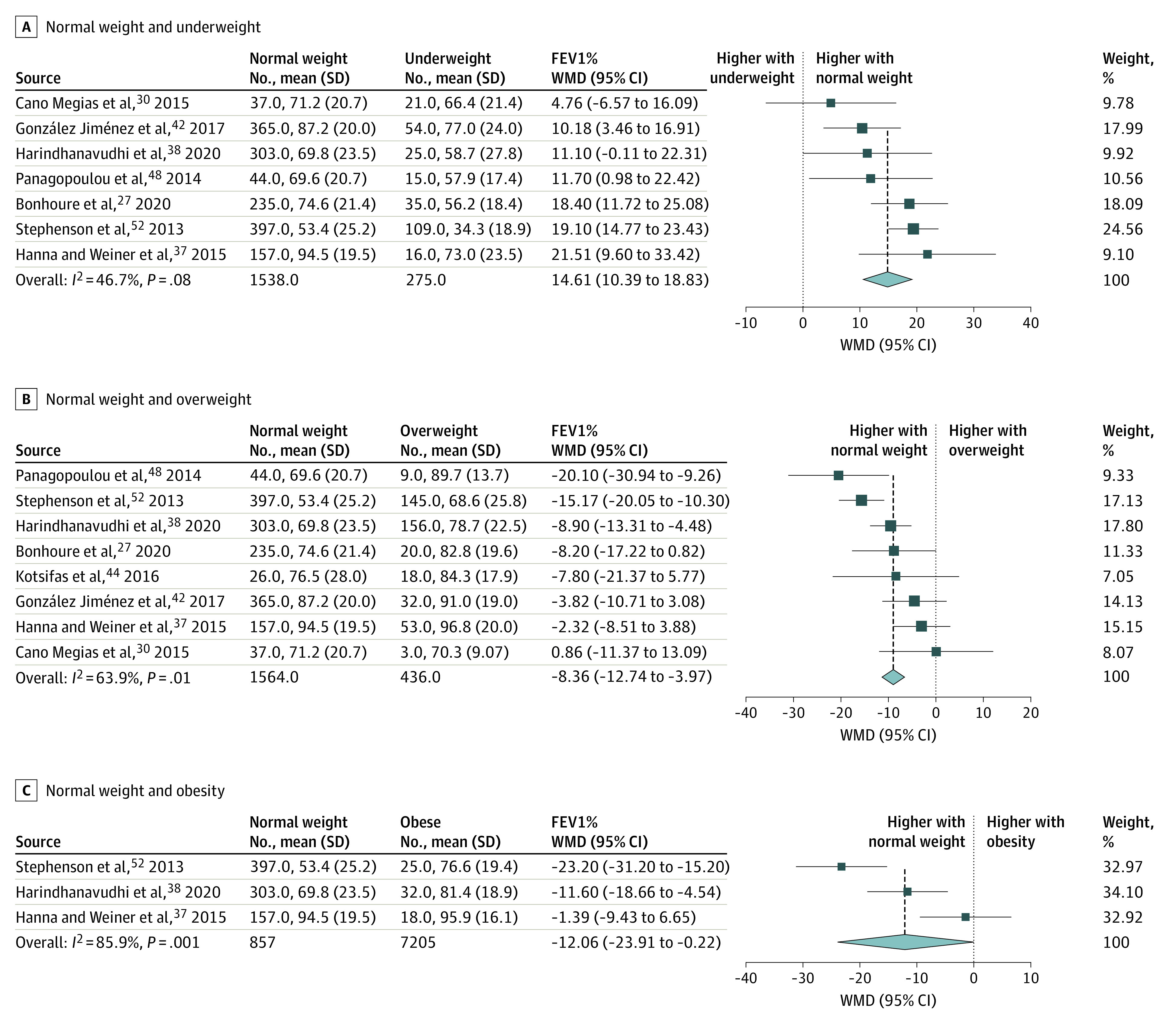
Comparison of patients with normal weight vs underweight (moderate heterogeneity detected) (A), normal weight vs overweight (substantial heterogeneity detected) (B), and normal weight vs obesity (considerable heterogeneity detected) (C). FEV1% indicates forced expiratory volume in the first second of expiration; WMD, weighted mean difference. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95% CI.
In addition to the 13 records in the quantitative synthesis, 15 studies, including conference abstracts, were added to the qualitative synthesis. The reasons for exclusion of these 15 studies from the quantitative synthesis were either different BMI categorization from the World Health Organization recommendation or insufficient data reporting. Among these 15 studies, 9 studies showed increased FEV1% values to be associated with higher BMI values in patients with normal BMI or overweight compared with those who were underweight. Two studies did not report significant differences in pulmonary function when comparing different BMI categories.49,54 Five studies investigated the connection between pulmonary function and FFM, and all of the studies reported an association between FFM and pulmonary function. Most (39 of 42 [92.9%]) of the extracted correlation coefficients indicated significant correlation between BMI or body composition parameters and FEV1%. Considered one of the main possible confounders, use of modulator therapy was rarely reported (2 of 54 [3.7%]); therefore, we were not able to perform further analysis of these participants.
Exocrine Pancreatic Insufficiency
Our results showed that normal BMI is associated with a lower odds for PI compared with underweight (OR, 0.45; 95% CI, 0.27-0.77) and a higher likelihood of PI compared with overweight (OR, 4.40; 95% CI, 3.00-6.45) and obesity (OR, 10.88; 95% Cl, 4.58-25.85) (Figure 3). Adults who were underweight had significantly higher odds for PI (OR, 3.16; 95% CI, 1.97-5.06) compared with those of normal weight (overall OR, 2.54; 95% CI, 1.53-4.23) (eFigure 2 in the Supplement). The studies of Ramírez et al51 reported a nonsignificant result and were not included in our quantitative synthesis owing to inadequate data reporting.
Figure 3. Odds of Exocrine Pancreatic Insufficiency in Different Body Mass Index Categories.
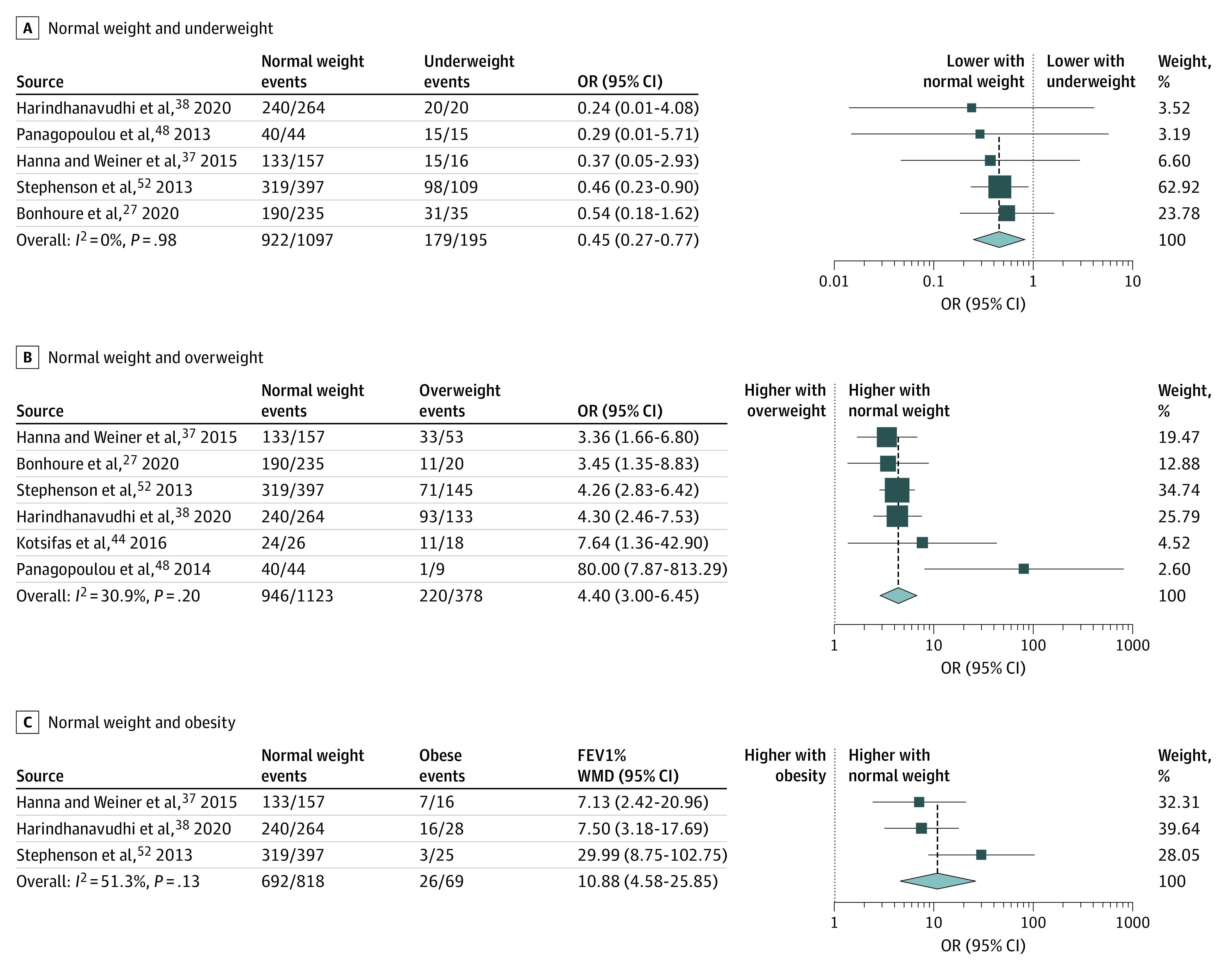
Comparison of patients with normal weight vs underweight (A), normal weight vs overweight (B), and normal weight vs obesity (C). OR indicates odds ratio. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95% CI.
CF-Related Diabetes
Our results suggest that CFRD is more common in patients who are underweight compared with those who are of normal weight (31% vs 29.4%; OR, 0.76; 95% CI, 0.53-1.09). In addition, normal BMI is associated with higher odds for CFRD compared with overweight (OR, 1.49; 95% CI, 1.10-2.00) (Figure 4). Based on the subgroup analysis, the overall comparison showed significantly higher odds of CFRD in patients with lower BMI vs those with normal BMI (OR, 1.43; 95% CI, 1.04-1.9) (eFigure 3 in the Supplement). The studies of Altman et al23 and Alvarez and Stecenko24 were not included in our quantitative synthesis owing to inadequate comparison categories; however, none of the studies reported significant differences between BMI groups regarding the CFRD outcome.24
Figure 4. Odds for Cystic Fibrosis–Related Diabetes in Different Body Mass Index Categories.
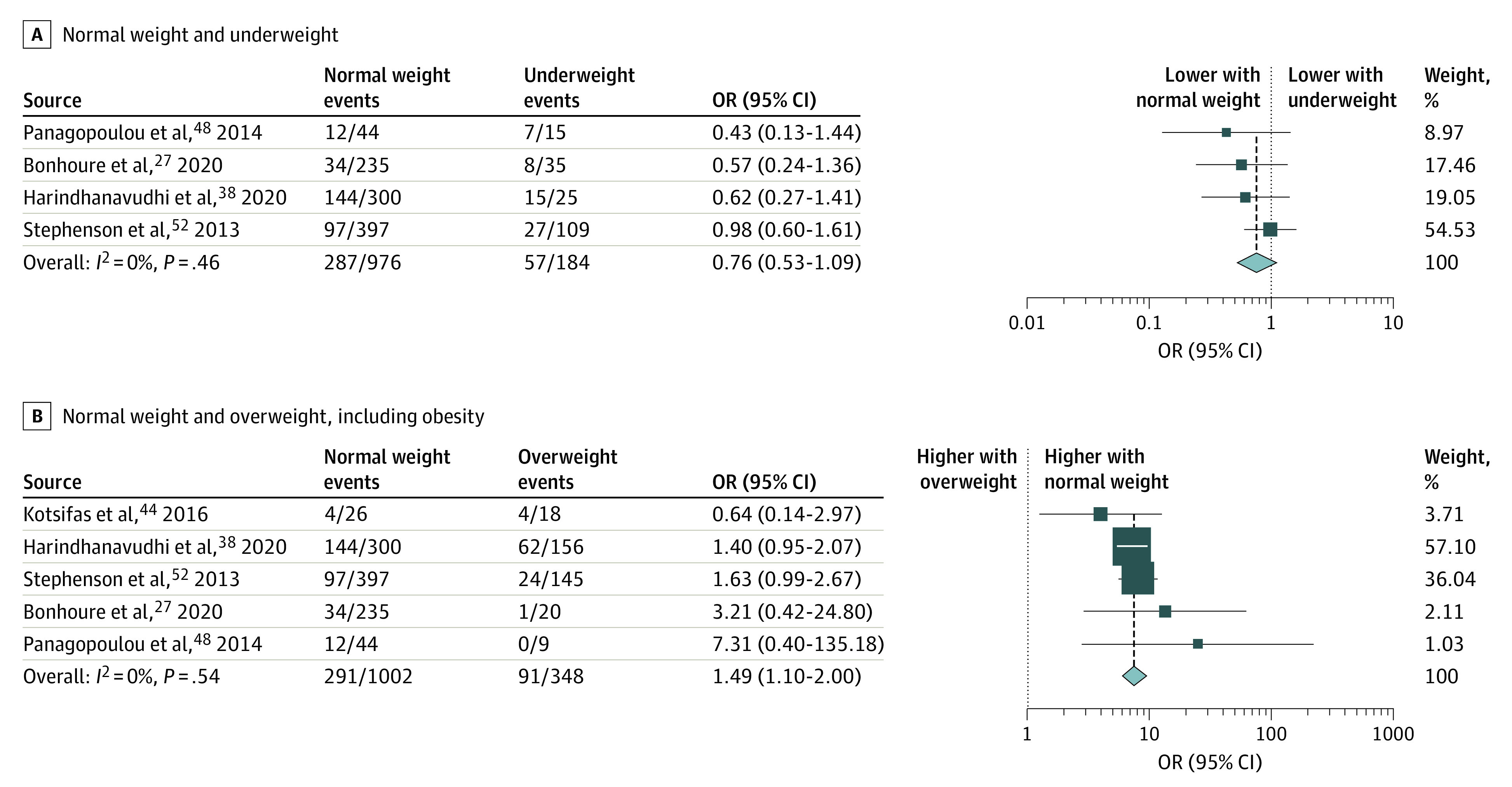
Comparison of patients with normal weight vs underweight (A) and normal weight vs overweight and obesity. OR indicates odds ratio. The size of squares is proportional to the weight of each study. Horizontal lines indicate the 95% CI of each study; diamond, the pooled estimate with 95% CI.
Secondary Outcomes
Glucose metabolic status indicators, such as fasting glucose, fasting insulin, and HbA1c levels did not significantly differ between BMI categories (eFigures 4-6 in the Supplement). However, in accordance with our hypothesis, compared with patients having normal weight, those who were overweight or obese had significantly higher total cholesterol levels (0.11 vs 0.09 mg/dL; WMD, –0.02 0.41 mg/dL; 95% CI, –0.03 to 0.01) and triglyceride levels (0.03 vs 0.02 mg/dL WMD, –0.005; 95% CI, –0.009 to 0.0005 [to convert to millimoles per liter, multiply by 0.0113]) (eFigure 7 and eFigure 8 in the Supplement). In the comparison of patients with normal weight vs underweight, both cholesterol levels (WMD, 0.008 mg/dL; 95% CI, 0.004 to 0.013) and triglyceride levels (WMD, 0.003 mg/dL; 95% CI, 0.001 to 0.006) were significantly higher in the normal weight group (eFigures 7-8 in the Supplement). Bonhoure et al,27 Harindhanavudhi et al,38 and Panagopoulou et al48 reported high-density and low-density lipoprotein cholesterol values; all of the studies showed significantly higher low-density lipoprotein cholesterol levels and not significantly lower high-density lipoprotein cholesterol levels in patients who were overweight. However, these results could not be included in the quantitative synthesis owing to insufficient data reporting by Harindhanavudhi et al.38
Additional Outcomes and Analysis
The ratio of patients with P aeruginosa colonization at the time of assessment was reported in 11 studies; of these, 6 studies were included in our quantitative synthesis. The results showed that patients who were underweight were significantly more likely to have P aeruginosa colonization compared with those who were not underweight (OR, 1.86; 95% CI, 1.34-2.59) (eFigure 9 in the Supplement). Of the studies included only in the qualitative synthesis, Maksimycheva et al45 and Da Silva Garrote et al32 found that underweight is associated with higher odds for P aeruginosa colonization, whereas 3 studies did not find significant differences between BMI and FFM categories.23,31,51
The studies by Stephenson et al,52 Bonhoure et al,27 and Harindhanavudhi et al38 were identified as showing significance regarding PI and CFRD by the leave-1-out sensitivity analysis (eFigures 10-13 in the Supplement). Funnel plots were created, and the Egger test was performed to detect publication bias. There was no small-study effect found in our analyses (eFigure 14 and eFigure 15 in the Supplement).
Quality Assessment of Studies
Regarding FEV1%, 23% of the eligible studies (3 of 13) were assessed to be high risk and 46% (6 of 13) as moderate risk. High risk was shown for PI (56% [5 of 9]) and CFRD (57% [4 of 7]). Detailed results of risk of bias assessments are shown in eFigures 16 to 24 in the Supplement.
Discussion
Our results suggest that higher BMI is associated with favorable clinical outcomes in patients with CF. Both overweight and obesity are associated with clinically significantly better pulmonary function compared with normal weight. A possible explanation could be the higher proportion of FFM in individuals who are overweight, which is associated with higher FEV1% and physical well-being.35,40,49,55,56 It has also been reported that patients with CF who are overweight have markedly fewer exacerbations that could contribute to loss of appetite.38 Moudiou et al57 described a negative correlation between resting energy expenditure (REE) and BMI z score. Resting energy expenditure is the amount of energy that is necessary to maintain basic body functions, such as digestion and breathing, and requires approximately 60% of the total calorie need.58 The worse the condition of the lungs, the higher the level of REE. Moreover, patients with PI were reported to have a higher REE compared with those with sufficient pancreatic function.5,6,58 Based on these data, we hypothesized that excess weight could cover the increased energy requirement during chronic inflammation.56 The favorable effect of weight gain has also been visible in the past few years as modulator therapy has become available as a novel treatment in CF.59 The continuous spread of modulators that aim to diminish the influence of CFTR dysfunction has been shown to have beneficial effect on body composition.60 In addition to weight gain, the proportion of FFM is increasing, which is associated with the reduction of REE.
Although we intended to include studies that compared patients with CF based not only on BMI but including FFM and FM, there were not enough eligible studies examining FFM and FM for a quantitative synthesis to be performed. All studies included in the qualitative synthesis highlighted the importance of the FFM proportion that leads to better pulmonary function. However, the influence of higher FM on FEV1% is not obvious. Alvarez et al61 reported that FEV1% is inversely associated with FM, whereas Panagopoulou et al48 described a correlation between body fat percentage and pulmonary function.
Our results regarding the association between higher BMI and the lower odds for PI are consistent with previous research.27,48,52 In patients with sufficient exocrine pancreatic function, adequate digestion and absorption are more likely to be present and can lead to higher BMI.
We found underweight to be associated with a higher prevalence of CFRD, which is in accordance with general clinical observations. In patients with CF who are underweight, there are several associated risk factors that contribute to the development of CFRD, such as PI or insulinopenia.62 We assumed that insulin resistance, as the minor component of CFRD, becomes more dominant in individuals who are overweight or obese and may lead to higher odds for diabetes compared with those of normal BMI. However, our results did not confirm this hypothesis, showing significantly lower odds for CFRD in patients with a BMI greater than or equal to 25.
Although our results showed that BMI greater than or equal to 25 does not have a statistically relevant association with glucose homeostasis (fasting glucose, fasting insulin, and HbA1c levels), overweight and obesity are associated with higher total cholesterol and triglyceride levels. However, none of these elevated values exceeded the upper limit of normal.
Based on our results, the higher the BMI, the better the investigated clinical indices; we found no obvious evidence to be associated with harmful effects. However, the assessment of FFM and FM could provide more precise information. Several studies emphasized the potential usefulness of FFM as a more detailed assessment of body composition compared with BMI. The prevalence of hidden FFM depletion (FFM<5th percentile and normal BMI) is unexpectedly high (10%-20%) among patients with CF and is associated with increased disease severity, including reduced lung function, frequent pulmonary exacerbations, and increased inflammation.31,35,40,55 Therefore, measuring body composition in patients with CF may be more informative than the single use of BMI as an indicator of optimal health and nutritional status.
Strengths and Limitations
To our knowledge, this is the first systematic review and meta-analysis assessing body composition in patients with CF in detail, including 3100 patients, with special focus on those who are overweight and obese. Moreover, we performed a meta-analysis regarding 9 outcomes, and subgroup analyses were performed for 3 outcomes.
Our study has limitations. There was substantial heterogeneity in the comparison of patients with normal weight and those who are obese regarding pulmonary function, and the source of substantial heterogeneity could not be identified by subgroup analysis. Furthermore, most of the studies did not report transplant status; thus, we could not perform subgroup analysis, and none of the pediatric studies reported the measurement method of respiratory function in children younger than 6 years.
Conclusions
Our findings suggest that nutritional status plays an important role in maintaining organ function in patients with CF. Because we noted that a higher BMI is associated with better clinical parameters, we advise clinicians to reconsider increasing the currently recommended target BMI (22 for women and 23 for men). The use of a nutritional strategy that increases BMI, at least until the upper limit of normal BMI is reached, should be included in the daily protocol. Our results suggest that careful evaluation of body composition (FFM and FM) should be incorporated into everyday clinical practice. Studies with long-term follow-up are required to investigate the possible harmful effects of higher BMI, higher FM, and high-fat diet. Further observational studies are necessary focusing on major components of body composition (FFM and FM) with BMI.
eMethods. Search Strategy and Selection
eFigure 1. Forest Plot Showing Pulmonary Function in Adults, Children, and Mixed Patient Population in the Comparison of Underweight and Non-Underweight Patients
eFigure 2. Forest Plot Displaying the Risk for Exocrine Insufficiency in Subgroup Analysis in the Comparison of Underweight and Non-Underweight Patients
eFigure 3. Forest Plot Displaying the Risk for CF-Related Diabetes in the Comparison of Underweight and Non-Underweight Patients
eFigure 4. Forest Plots Showing Fasting Glucose Level in the Comparison of Different BMI Categories
eFigure 5. Forest Plots Showing HbA1c% Level in the Comparison of Different BMI Categories
eFigure 6. Forest Plot Displaying Fasting Insulin Level in the Comparison of Underweight and Non-Underweight Patients
eFigure 7. Forest Plots Showing Total Cholesterol Level in the Comparison of Different BMI Categories
eFigure 8. Forest Plots Showing Triglyceride Level in the Comparison of Different BMI Categories
eFigure 9. Forest Plots Showing Pseudomonas aeruginosa Colonization in the Comparison of Different BMI Categories
eTable. Table of Correlation Coefficients
eFigure 10. Leave-1-Out Sensitivity Analysis Regarding Pulmonary Function in the Comparison of Different BMI Categories
eFigure 11. Leave-1-Out Sensitivity Analysis Regarding Exocrine Insufficiency in the Comparison of Normal Weight and Underweight Patients
eFigure 12. Leave-1-Out Sensitivity Analysis Regarding CF-Related Diabetes in the Comparison of Normal Weight and Underweight Plus Obese Groups
eFigure 13. Leave-1-Out Sensitivity Analysis Regarding CF-Related Diabetes in Subgroup Analysis in the Comparison of Underweight and Non-Underweight Patients
eFigure 14. Funnel Plot of the Included 8 Studies Regarding Pulmonary Function in the Comparison of Normal Weight and Overweight Patients
eFigure 15. Funnel Plot of the Included 12 Studies Regarding Pulmonary Function in Subgroup Analysis in the Comparison of Underweight and Non-Underweight Patients
eFigure 16. Risk of Bias Assessment at Study and Domain Level Regarding Pulmonary Function (FEV1%)
eFigure 17. Risk of Bias Assessment at Study and Domain Level Regarding Exocrine Insufficiency
eFigure 18. Risk of Bias Assessment at Study and Domain Level Regarding CFRD
eFigure 19. Risk of Bias Assessment at Study and Domain Level Regarding Fasting Glucose
eFigure 20. Risk of Bias Assessment at Study and Domain Level Regarding HbA1c%
eFigure 21. Risk of Bias Assessment at Study and Domain Level Regarding Total Cholesterol
eFigure 22. Risk of Bias Assessment at Study and Domain Level Regarding Triglycerides
eFigure 23. Risk of Bias Assessment at Study and Domain Level Regarding Pseudomonas aeruginosa Colonization
eFigure 24. Risk of Bias Assessment at Study and Domain Level Regarding Fasting Insulin
eReferences
References
- 1.Slieker MG, Uiterwaal CS, Sinaasappel M, Heijerman HG, van der Laag J, van der Ent CK. Birth prevalence and survival in cystic fibrosis: a national cohort study in the Netherlands. Chest. 2005;128(4):2309-2315. doi: 10.1378/chest.128.4.2309 [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183(11):1463-1471. doi: 10.1164/rccm.201009-1478CI [DOI] [PubMed] [Google Scholar]
- 3.Moran A, Doherty L, Wang X, Thomas W. Abnormal glucose metabolism in cystic fibrosis. J Pediatr. 1998;133(1):10-17. doi: 10.1016/S0022-3476(98)70171-4 [DOI] [PubMed] [Google Scholar]
- 4.Bell SC, Saunders MJ, Elborn JS, Shale DJ. Resting energy expenditure and oxygen cost of breathing in patients with cystic fibrosis. Thorax. 1996;51(2):126-131. doi: 10.1136/thx.51.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd RW, Greer RM, McNaughton SA, Wotton M, Cleghorn GJ. Energy expenditure and the body cell mass in cystic fibrosis. Nutrition. 2001;17(1):22-25. doi: 10.1016/S0899-9007(00)00470-6 [DOI] [PubMed] [Google Scholar]
- 6.Pencharz PB, Durie PR. Pathogenesis of malnutrition in cystic fibrosis, and its treatment. Clin Nutr. 2000;19(6):387-394. doi: 10.1054/clnu.1999.0079 [DOI] [PubMed] [Google Scholar]
- 7.Culhane S, George C, Pearo B, Spoede E. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract. 2013;28(6):676-683. doi: 10.1177/0884533613507086 [DOI] [PubMed] [Google Scholar]
- 8.Milla CE. Nutrition and lung disease in cystic fibrosis. Clin Chest Med. 2007;28(2):319-330. doi: 10.1016/j.ccm.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Turck D, Braegger CP, Colombo C, et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr. 2016;35(3):557-577. doi: 10.1016/j.clnu.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Sinaasappel M, Stern M, Littlewood J, et al. Nutrition in patients with cystic fibrosis: a European consensus. J Cyst Fibros. 2002;1(2):51-75. doi: 10.1016/S1569-1993(02)00032-2 [DOI] [PubMed] [Google Scholar]
- 11.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530-535.e1. doi: 10.1016/j.jpeds.2012.08.040 [DOI] [PubMed] [Google Scholar]
- 12.Bhudhikanok GS, Wang MC, Marcus R, Harkins A, Moss RB, Bachrach LK. Bone acquisition and loss in children and adults with cystic fibrosis: a longitudinal study. J Pediatr. 1998;133(1):18-27. doi: 10.1016/S0022-3476(98)70172-6 [DOI] [PubMed] [Google Scholar]
- 13.Cystic Fibrosis Foundation . Patient registry 2019 annual data report. 2020. Accessed March 2, 2021. https://www.cff.org/medical-professionals/patient-registry
- 14.Singanayagam A, Singanayagam A, Chalmers JD. Obesity is associated with improved survival in community-acquired pneumonia. Eur Respir J. 2013;42(1):180-187. doi: 10.1183/09031936.00115312 [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276-282. doi: 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121(pt 1):1027-1031. doi: 10.1016/j.envint.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . Body mass index—BMI. Accessed March 2, 2021. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 19.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-286. doi: 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane; 2021. [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altman K, DiMango E, Kramer A, Keating C. Identifying clinical characteristics associated with poor nutritional status in CF [abstract]. Pediatr Pulmonol. 2017;52:459-460. [Google Scholar]
- 24.Alvarez JA, Ziegler TR, Millson EC, Stecenko AA. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition. 2016;32(4):447-452. doi: 10.1016/j.nut.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barni GC, Forte GC, Forgiarini LF, Abrahão CLO, Dalcin PTR. Factors associated with malnutrition in adolescent and adult patients with cystic fibrosis. J Bras Pneumol. 2017;43(5):337-343. doi: 10.1590/s1806-37562016000000319 [DOI] [PubMed] [Google Scholar]
- 26.Bodnar R, Kadar L, Holics K, et al. Factors influencing quality of life and disease severity in Hungarian children and young adults with cystic fibrosis. Ital J Pediatr. 2014;40(1):50. doi: 10.1186/1824-7288-40-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonhoure A, Boudreau V, Litvin M, et al. Overweight, obesity and significant weight gain in adult patients with cystic fibrosis association with lung function and cardiometabolic risk factors. Clin Nutr. 2020;39(9):2910-2916. doi: 10.1016/j.clnu.2019.12.029 [DOI] [PubMed] [Google Scholar]
- 28.Bouma SF, Iwanicki C, McCaffery H, Nasr SZ. The association of grip strength, body mass index, and lung function in youth with cystic fibrosis. Nutr Clin Pract. 2020;35(6):1110-1118. doi: 10.1002/ncp.10583 [DOI] [PubMed] [Google Scholar]
- 29.Brennan AL, McKenna D, Roberts J, et al. Raised BMI in patients with cystic fibrosis related-diabetes [abstract]. Pediatr Pulmonol. 2010;45:432. doi: 10.1002/(ISSN)1099-0496 [DOI] [Google Scholar]
- 30.Cano Megías M, Guisado Vasco P, González Albarrán O, Lamas Ferreiro A, Máiz Carro L. Association of the relative change in weight and body mass index with lung function in teenagers and adults with cystic fibrosis: influence of gender and diabetes. Endocrinol Nutr. 2015;62(9):422-429. doi: 10.1016/j.endoen.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 31.Charatsi AM, Dusser P, Freund R, et al. Bioelectrical impedance in young patients with cystic fibrosis: validation of a specific equation and clinical relevance. J Cyst Fibros. 2016;15(6):825-833. doi: 10.1016/j.jcf.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 32.Da Silva Garrote M, Costa LDC, Da Silva Garrote Filho M, Duarte WDF, Baragatti RF, Fernandes RV. Respiratory colonization and nutritional status of children and adolescents in the cystic fibrosis clinic from clinical hospital-UFG [abstract]. Pediatr Pulmonol. 2016;51:S42. doi:10.1002/ppul.23409 [Google Scholar]
- 33.Dray X, Kanaan R, Bienvenu T, et al. Malnutrition in adults with cystic fibrosis. Eur J Clin Nutr. 2005;59(1):152-154. doi: 10.1038/sj.ejcn.1602039 [DOI] [PubMed] [Google Scholar]
- 34.Dudina AL, Fletcher G, Gallagher CG, McKone EF. The prevalence of obesity in Irish adults with cystic fibrosis: a registry study [abstract]. J Cyst Fibros. 2017;16:S49. doi: 10.1016/S1569-1993(17)30320-X [DOI] [Google Scholar]
- 35.Engelen MP, Schroder R, Van der Hoorn K, Deutz NE, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clin Nutr. 2012;31(6):927-933. doi: 10.1016/j.clnu.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 36.Gozdzik J, Cofta S, Piorunek T, Batura-Gabryel H, Kosicki J. Relationship between nutritional status and pulmonary function in adult cystic fibrosis patients. J Physiol Pharmacol. 2008;59(suppl 6):253-260. [PubMed] [Google Scholar]
- 37.Hanna RM, Weiner DJ. Overweight and obesity in patients with cystic fibrosis: a center-based analysis. Pediatr Pulmonol. 2015;50(1):35-41. doi: 10.1002/ppul.23033 [DOI] [PubMed] [Google Scholar]
- 38.Harindhanavudhi T, Wang Q, Dunitz J, Moran A, Moheet A. Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: a single-center analysis. J Cyst Fibros. 2020;19(1):139-145. doi: 10.1016/j.jcf.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollander FM, Roelofs J, De Roos NM, Heijerman HGM. Relationship between nutritional status and pulmonary function in adults with cystic fibrosis: cross-sectional and longitudinal analyses [abstract]. J Cystic Fibrosis. 2018;17:S114. doi: 10.1016/S1569-1993(18)30490-9 [DOI] [Google Scholar]
- 40.Ionescu AA, Evans WD, Pettit RJ, Nixon LS, Stone MD, Shale DJ. Hidden depletion of fat-free mass and bone mineral density in adults with cystic fibrosis. Chest. 2003;124(6):2220-2228. doi: 10.1378/chest.124.6.2220 [DOI] [PubMed] [Google Scholar]
- 41.González Jiménez D, Bousoño García C, Rivas Crespo MF, et al. Insulin resistance in overweight cystic fibrosis paediatric patients [in Spanish]. An Pediatr (Barc). 2012;76(5):279-284. doi: 10.1016/j.anpedi.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 42.González Jiménez D, Muñoz-Codoceo R, Garriga-García M, et al. Excess weight in patients with cystic fibrosis: is it always beneficial? [in Spanish]. Nutr Hosp. 2017;34(3):578-583. doi: 10.20960/nh.620 [DOI] [PubMed] [Google Scholar]
- 43.Kines K, Wooldridge N, Griffin R, Britton LJ, Hoover W, Gutierrez H. Nutritional status and disease severity in pre-adolescent and adolescent patients with cystic fibrosis [abstract]. Pediatr Pulmonol. 2012;47:419-420. [Google Scholar]
- 44.Kotsifas K, Antonogiannaki EM, Diamantea F, Inglezos I. Overweight and obesity in Greek adult cystic fibrosis patients: description and comparison to patients with optimal BMI [abstract]. J Cystic Fibrosis. 2016;15:S103. doi: 10.1016/S1569-1993(16)30442-8 [DOI] [Google Scholar]
- 45.Maksimycheva T, Kondratyeva E, Budzinskiy R, Zhekayte E, Voronkova A, Sherman V. Cost analysis of the treatment of exacerbations in children with CF depending on nutritional status [abstract]. J Cystic Fibrosis. 2018;17:S113-S114. doi: 10.1016/S1569-1993(18)30488-0 [DOI] [Google Scholar]
- 46.Ochota A, Frost F, Pandya S, Foster K, Walshaw M.. BMI: a predictor of bone mineral density in adult people with cystic fibrosis [abstract]. J Cystic Fibrosis. 2019;18:S20. [Google Scholar]
- 47.Panagopoulou P, Fotoulaki M, Manolitsas A, Pavlitou-Tsiontsi E, Tsitouridis I, Nousia-Arvanitakis S. Adiponectin and body composition in cystic fibrosis. J Cystic Fibrosis. 2008;7(3):244-251. doi: 10.1016/j.jcf.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 48.Panagopoulou P, Fotoulaki M, Nikolaou A, Nousia-Arvanitakis S. Prevalence of malnutrition and obesity among cystic fibrosis patients. Pediatr Int. 2014;56(1):89-94. doi: 10.1111/ped.12214 [DOI] [PubMed] [Google Scholar]
- 49.Papalexopoulou N, Dassios TG, Lunt A, et al. Nutritional status and pulmonary outcome in children and young people with cystic fibrosis. Respir Med. 2018;142:60-65. doi: 10.1016/j.rmed.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 50.Proud D, Rezaie M, Stoakes A, Ketchell I, Duckers J.. Body composition; expect the unexpected [abstract]! J Cystic Fibrosis. 2012;11:S121. doi: 10.1016/S1569-1993(12)60423-8 [DOI] [Google Scholar]
- 51.Ramírez I, Filbrun A, Hasan A, Kidwell KM, Nasr SZ. Improving nutritional status in a pediatric cystic fibrosis center. Pediatr Pulmonol. 2015;50(6):544-551. doi: 10.1002/ppul.23128 [DOI] [PubMed] [Google Scholar]
- 52.Stephenson AL, Mannik LA, Walsh S, et al. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: a population-based cohort study. Am J Clin Nutr. 2013;97(4):872-877. doi: 10.3945/ajcn.112.051409 [DOI] [PubMed] [Google Scholar]
- 53.Umławska W, Krzyżanowska M, Zielińska A, Sands D. Effect of selected factors associated with the clinical course of the disease on nutritional status in children with cystic fibrosis. Adv Clin Exp Med. 2014;23(5):775-783. doi: 10.17219/acem/37251 [DOI] [PubMed] [Google Scholar]
- 54.Ziegler B, Lukrafka JL, de Oliveira Abraão CL, Rovedder PM, Dalcin PdeT. Relationship between nutritional status and maximum inspiratory and expiratory pressures in cystic fibrosis. Respir Care. 2008;53(4):442-449. [PubMed] [Google Scholar]
- 55.King SJ, Nyulasi IB, Strauss BJ, Kotsimbos T, Bailey M, Wilson JW. Fat-free mass depletion in cystic fibrosis: associated with lung disease severity but poorly detected by body mass index. Nutrition. 2010;26(7-8):753-759. doi: 10.1016/j.nut.2009.06.026 [DOI] [PubMed] [Google Scholar]
- 56.Cancho Candela R, Alonso-Franch M, Calvo Romero C. Resting energy expenditure in cystic fibrosis [in Spanish]. Nutr Hosp. 1999;14(4):153-158. [PubMed] [Google Scholar]
- 57.Moudiou T, Galli-Tsinopoulou A, Vamvakoudis E, Nousia-Arvanitakis S. Resting energy expenditure in cystic fibrosis as an indicator of disease severity. J Cyst Fibros. 2007;6(2):131-136. doi: 10.1016/j.jcf.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 58.Dorlöchter L, Røksund O, Helgheim V, Rosendahl K, Fluge G. Resting energy expenditure and lung disease in cystic fibrosis. J Cyst Fibros. 2002;1(3):131-136. doi: 10.1016/S1569-1993(02)00076-0 [DOI] [PubMed] [Google Scholar]
- 59.Pettit RS, Fellner C. CFTR Modulators for the treatment of cystic fibrosis. P T. 2014;39(7):500-511. [PMC free article] [PubMed] [Google Scholar]
- 60.Bass R, Brownell JN, Stallings VA. The impact of highly effective CFTR modulators on growth and nutrition status. Nutrients. 2021;13(9):2907. doi: 10.3390/nu13092907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez JA, Stecenko A. Body composition and normal weight obesity in cystic fibrosis [abstract]. Pediatr Pulmonol. 2013;48:402. [Google Scholar]
- 62.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis–related diabetes. J Pediatr. 2005;146(5):681-687. doi: 10.1016/j.jpeds.2004.12.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy and Selection
eFigure 1. Forest Plot Showing Pulmonary Function in Adults, Children, and Mixed Patient Population in the Comparison of Underweight and Non-Underweight Patients
eFigure 2. Forest Plot Displaying the Risk for Exocrine Insufficiency in Subgroup Analysis in the Comparison of Underweight and Non-Underweight Patients
eFigure 3. Forest Plot Displaying the Risk for CF-Related Diabetes in the Comparison of Underweight and Non-Underweight Patients
eFigure 4. Forest Plots Showing Fasting Glucose Level in the Comparison of Different BMI Categories
eFigure 5. Forest Plots Showing HbA1c% Level in the Comparison of Different BMI Categories
eFigure 6. Forest Plot Displaying Fasting Insulin Level in the Comparison of Underweight and Non-Underweight Patients
eFigure 7. Forest Plots Showing Total Cholesterol Level in the Comparison of Different BMI Categories
eFigure 8. Forest Plots Showing Triglyceride Level in the Comparison of Different BMI Categories
eFigure 9. Forest Plots Showing Pseudomonas aeruginosa Colonization in the Comparison of Different BMI Categories
eTable. Table of Correlation Coefficients
eFigure 10. Leave-1-Out Sensitivity Analysis Regarding Pulmonary Function in the Comparison of Different BMI Categories
eFigure 11. Leave-1-Out Sensitivity Analysis Regarding Exocrine Insufficiency in the Comparison of Normal Weight and Underweight Patients
eFigure 12. Leave-1-Out Sensitivity Analysis Regarding CF-Related Diabetes in the Comparison of Normal Weight and Underweight Plus Obese Groups
eFigure 13. Leave-1-Out Sensitivity Analysis Regarding CF-Related Diabetes in Subgroup Analysis in the Comparison of Underweight and Non-Underweight Patients
eFigure 14. Funnel Plot of the Included 8 Studies Regarding Pulmonary Function in the Comparison of Normal Weight and Overweight Patients
eFigure 15. Funnel Plot of the Included 12 Studies Regarding Pulmonary Function in Subgroup Analysis in the Comparison of Underweight and Non-Underweight Patients
eFigure 16. Risk of Bias Assessment at Study and Domain Level Regarding Pulmonary Function (FEV1%)
eFigure 17. Risk of Bias Assessment at Study and Domain Level Regarding Exocrine Insufficiency
eFigure 18. Risk of Bias Assessment at Study and Domain Level Regarding CFRD
eFigure 19. Risk of Bias Assessment at Study and Domain Level Regarding Fasting Glucose
eFigure 20. Risk of Bias Assessment at Study and Domain Level Regarding HbA1c%
eFigure 21. Risk of Bias Assessment at Study and Domain Level Regarding Total Cholesterol
eFigure 22. Risk of Bias Assessment at Study and Domain Level Regarding Triglycerides
eFigure 23. Risk of Bias Assessment at Study and Domain Level Regarding Pseudomonas aeruginosa Colonization
eFigure 24. Risk of Bias Assessment at Study and Domain Level Regarding Fasting Insulin
eReferences