Key Points
Question
Does a clinical decision support system aimed at primary care clinicians improve cardiovascular health for people with serious mental illness?
Findings
In this cluster randomized clinical trial of 8937 patients with serious mental illness, intervention patients’ rate of change in total modifiable cardiovascular risk over 12 months was 4% lower than control patients’ rate of change. There were no significant differences in individual modifiable risk factors.
Meaning
Although treatment effects favored the intervention, the results were driven by cumulative effects of incremental and mostly nonsignificant changes in individual modifiable cardiovascular risk factors.
Abstract
Importance
Adults with schizophrenia, schizoaffective disorder, or bipolar disorder, collectively termed serious mental illness (SMI), have shortened life spans compared with people without SMI. The leading cause of death is cardiovascular (CV) disease.
Objective
To assess whether a clinical decision support (CDS) system aimed at primary care clinicians improves CV health for adult primary care patients with SMI.
Design, Setting, and Participants
In this cluster randomized clinical trial conducted from March 2, 2016, to September 19, 2018, restricted randomization assigned 76 primary care clinics in 3 Midwestern health care systems to receive or not receive a CDS system aimed at improving CV health among patients with SMI. Eligible clinics had at least 20 patients with SMI; clinicians and their adult patients with SMI with at least 1 modifiable CV risk factor not at the goal set by the American College of Cardiology/American Heart Association guidelines were included. Statistical analysis was conducted on an intention-to-treat basis from January 10, 2019, to December 29, 2021.
Intervention
The CDS system assessed modifiable CV risk factors and provided personalized treatment recommendations to clinicians and patients.
Main Outcomes and Measures
Patient-level change in total modifiable CV risk over 12 months, summed from individual modifiable risk factors (smoking, body mass index, low-density lipoprotein cholesterol level, systolic blood pressure, and hemoglobin A1c level).
Results
A total of 80 clinics were randomized; 4 clinics were excluded for having fewer than 20 eligible patients, leaving 42 intervention clinics and 34 control clinics. A total of 8937 patients with SMI (4922 women [55.1%]; mean [SD] age, 48.4 [13.5] years) were enrolled. There was a 4% lower rate of increase in total modifiable CV risk among intervention patients relative to control patients (relative rate ratio [RR], 0.96; 95% CI, 0.94-0.98). The intervention favored patients who were 18 to 29 years of age (RR, 0.89; 95% CI, 0.81-0.98) or 50 to 59 years of age (RR, 0.93; 95% CI, 0.90-0.96), Black (RR, 0.93; 95% CI, 0.88-0.98), or White (RR, 0.96; 95% CI, 0.94-0.98). Men (RR, 0.96; 95% CI, 0.94-0.99) and women (RR, 0.95; 95% CI, 0.92-0.97), as well as patients with any SMI subtype (bipolar disorder: RR, 0.96; 95% CI, 0.94-0.99; schizoaffective disorder: RR, 0.94; 95% CI, 0.90-0.98; schizophrenia: RR, 0.92; 95% CI, 0.85-0.99) also benefited from the intervention. Despite treatment effects favoring the intervention, there were no significant differences in individual modifiable risk factors.
Conclusions and Relevance
This CDS intervention resulted in a rate of change in total modifiable CV risk that was 4% lower among intervention patients compared with control patients. Results were driven by the cumulative effects of incremental and mostly nonsignificant changes in individual modifiable risk factors. These findings emphasize the value of using CDS to prompt early primary care intervention for adults with SMI.
Trial Registration
ClinicalTrials.gov Identifier: NCT02451670
This randomized clinical trial assesses whether a clinical decision support system aimed at primary care clinicians improves cardiovascular health for adult primary care patients with serious mental illness.
Introduction
People with bipolar disorder, schizophrenia, or schizoaffective disorder, collectively termed serious mental illness (SMI), die at 2.3 times the rate of people without SMI, shortening their life spans by 10 to 15 years.1,2 Cardiovascular (CV) disease is the leading cause of death for people with SMI,3 associated in part with higher relative risks of dyslipidemia (5-fold), smoking (2-fold to 3-fold), diabetes (2-fold), and obesity (1.5-fold to 2-fold).4,5 Some SMI medications may increase cardiometabolic risk by adversely affecting weight, insulin resistance, and lipid metabolism.6,7,8 In theory, excess CV mortality among people with SMI could be reduced by early recognition and management of modifiable CV risk factors, among other strategies.3,9,10,11
Clinical decision support (CDS) tools are typically technology-based interventions that provide patient-specific information, often at the point of care, to improve health and health care.12 Clinical decision support in primary care settings could help prompt clinicians to address gaps in evidence-based care, but many previous CDS studies have had null results, in part owing to poor CDS design and low use of the CDS tools.13,14,15,16,17,18 With improved design and implementation, more recent studies of electronic health record (EHR)–linked CDS tools have achieved high use rates and decreased CV risk in populations without SMI.19,20,21 This pragmatic cluster randomized clinical trial, designed to test the effectiveness of the intervention in real-world clinical practice, assessed whether an EHR-linked CDS system slowed increases in modifiable CV risk among adults with SMI.
Methods
Study Design and Study Setting
The design and methods of this cluster randomized clinical trial have been previously described.22 The trial protocol and statistical analysis plan can be found in Supplement 1. This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for cluster randomized studies. The study was conducted from March 2, 2016, to September 19, 2018, at 76 primary care clinics in 3 health care systems providing integrated health care for patients in Minnesota, North Dakota, and Wisconsin: Essentia Health, HealthPartners, and Park Nicollet. Study procedures were reviewed and approved by each site’s institutional review board. Waivers of consent for clinicians and patients were granted by each site’s institutional review board because CDS guidance was limited to evidence-based recommendations from national guidelines.
Clinic Randomization
Primary care clinics that treated at least 20 patients with SMI in the prior year were randomized by the study statistician (A.L.C.) using site-stratified restricted randomization to balance site-specific factors that could affect the intervention or its implementation.22,23 Site A clinics were balanced on the proportion of patients insured by Medicaid, the presence of onsite behavioral health services, and the number of patients with SMI. Site B clinics were balanced on urbanicity, the proportion of patients who smoked, and the proportion of patients younger than 30 years. Site C clinics were balanced on the proportion of patients insured by Medicaid, the proportion of patients receiving optimal vascular care, and the number of patients with SMI. Cluster randomization was chosen to minimize contamination. Given the nature of the intervention, clinicians were not blinded to clinic assignments.
Study Participants
Participant enrollment began on March 2, 2016, at site A; October 18, 2016, at site B; and March 15, 2017, at site C. Enrollment ended on September 19, 2017, and patients were followed up through September 19, 2018. Adults aged 18 to 75 years who were not pregnant, had SMI, had at least 1 modifiable CV risk factor not at the goal set by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines, and had an index visit and at least 1 postindex visit at any randomized clinic were eligible. Patients in nursing homes or hospice, requesting exclusion from research, or with active cancer diagnoses were excluded from analyses. An index visit was the first visit made by an eligible patient during the intervention. Patients were assigned to their index visit clinic’s treatment group. Patients were considered to have SMI if they had at least 1 inpatient or 2 outpatient EHR-documented diagnoses of bipolar disorder, schizophrenia, or schizoaffective disorder in the 2 years prior to the index visit (eTable 1 in Supplement 2). Patients with International Classification of Diseases, Ninth Revision and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes crossing SMI subtypes were considered to have schizoaffective disorder.
Intervention
For study-eligible patients, intervention clinic rooming staff members received EHR best practice advisories prompting them to print and distribute 1-page handouts for clinicians and patients. The clinician handout summarized and prioritized each patient’s modifiable CV risk factors: blood pressure, lipid levels, glucose and hemoglobin A1c (HbA1c) levels, smoking status, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared). The clinician handout also estimated the patient’s 10-year (for those aged 40-75 years) and/or 30-year (for those aged 18-59 years) ACC/AHA CV risk and gave patient-specific treatment recommendations based on national guidelines (eg, ACC/AHA,24,25 Framingham Heart Study,26 and United Kingdom Prospective Diabetes Study27,28). The clinician handouts included patient-specific suggestions regarding medications, diet, exercise, and smoking cessation. The patient handouts contained similar information designed for individuals with lower literacy levels, including suggestions regarding diet, exercise, lifestyle programs, and smoking cessation. Handouts were designed to be used as shared decision-making tools, and rooming staff members suggested that patients review and talk to their clinicians about the handouts. There were no alerts or printouts in control clinics. For study-eligible patients who presented for a subsequent visit with a psychiatric prescriber, the CDS alerted the prescriber when patients had active prescriptions of potentially obesogenic SMI medications (eTable 2 in Supplement 2) and had elevated BMIs or recent weight gain.
Data Collection
The CDS system ran in the background to collect EHR data, including demographic characteristics, vital signs, medications, comorbidities, allergies, and laboratory data, for study-eligible patients in intervention and control clinics for 2 years preceding the visit. Race and ethnicity were self-reported to health care systems and included Asian, Black, Hispanic, Native American, White, and other or unknown race and ethnicity (the “other or unknown race and ethnicity” group included people who were Pacific Islander and people for whom there were no race or ethnicity data recorded in the electronic health record). Electronic health record data were harvested to obtain safety event information, including suicidal ideation (as recorded on the Patient Health Questionnaire29), hospitalizations, and emergency department visits.
Outcomes
The primary outcome was patient-level rate of change in total modifiable CV risk during the 12 months after the index visit. Secondary outcomes were rates of change in individual modifiable CV risk factors (blood pressure, lipid levels, HbA1c level, smoking, BMI) and use of potentially obesogenic SMI medications in the 12 months after the index visit. Total modifiable CV risk and individual CV risk factors were calculated by the CDS at each clinic visit. Total modifiable CV risk was defined as the sum of modifiable CV risk factors, calculated via the following. (1) Ten-year risk equations estimated CV risk (modifiable plus nonmodifiable [age, sex, race and ethnicity]) using ACC/AHA24,25 and Framingham risk equations.26 For patients younger than 40 years, 10-year risk equations were calculated as if the patient were 40 years of age. (2) A risk component for each modifiable CV risk factor was calculated as the difference between the calculated risk using the patient’s values and the goal using ACC/AHA,24,25 Framingham Heart Study,26 and United Kingdom Prospective Diabetes Study equations.27,28 For BMI, the goal was a decrease of 3 BMI units for those with a BMI of 28 or more or a decrease to a BMI of 25 for those with BMIs of 25 to 27.9. Risk components for modifiable CV risk factors at the goal set by the ACC/AHA guidelines were calculated as zero. (3) Modifiable CV risk components were summed to calculate total modifiable CV risk.
Statistical Analysis
Statistical analysis was conducted on an intention-to-treat basis from January 10, 2019, to December 29, 2021. Total modifiable CV risk and individual modifiable CV risk factors were analyzed using general or generalized linear mixed models with distribution-appropriate link functions (eg, log-binomial function and log-negative binomial function) and a random patient × clinic intercept. Outcomes measured repeatedly per patient (modifiable CV risk, blood pressure, lipid levels, HbA1c level, BMI) were predicted from fixed effects of treatment, linear time in years from the index visit to each outcome, and the treatment × time interaction. The treatment effect was quantified as the rate of change between the index visit and 12 months after the index visit among patients in intervention clinics (rate ratio [RR]) relative to the RR among patients in control clinics (ie, RR for treatment × time). The 2-sided 95% CI around RR for treatment × time assessed its statistical significance. Covariates included sex, age, outcome value at the index visit, site, and balancing covariates.
The smoking status model estimated the likelihood that patients who were documented as current smokers at the index visit were documented as having quit at their last visit in the follow-up period. This model did not include a random patient intercept or baseline outcome value and added a covariate for the log-years elapsed between the index visit and the last visit. The obesogenic medication model also used this approach and was applied to patients with an active prescription for an obesogenic medication at the index visit and with either 7% or more weight gain in the previous 12 months or a most recent BMI increase greater than 2. Subgroup analyses by age and sex (a priori) and SMI subtype and race and ethnicity (post hoc) were conducted by adding subgroup main effect and interaction terms to these models. Confidence intervals around the subgroup treatment effects were not adjusted for multiple comparisons and should be considered exploratory. Study analyses were conducted using SAS, version 9.4 software (SAS Institute Inc).
Sample Size
An a priori power analysis estimated the minimum detectable standardized effect for the time × treatment parameter in a mixed-effects regression model (power = 0.80; 2-sided α = .05) estimating a normally distributed outcome. Assumptions were 52 clinics randomized on a 1:1 basis and 2250 patients with SMI, equally distributed across clinics with 4 outcome measures each (interclass correlation coefficient = 0.01, 0.02 for clinics; interclass correlation coefficient = 0.35, 0.90 for patients). The resulting minimum detectable standardized effect (Cohen d < 0.10) suggested power to detect very small differences in rates of change among patients in intervention clinics relative to those in control clinics.22 Between CDS implementation and September 19, 2017, 8937 patients had an index visit and a mean (SD) of 5.8 (5.5) postindex visits (median, 5 postindex visits) in 1 of 76 randomized clinics retained for analysis (median, 88 patients per clinic; range, 20-423 patients per clinic).
Results
A total of 45 clinics were randomized to the intervention group and 35 to the control group; 4 clinics were excluded for having fewer than 20 eligible patients, leaving 42 intervention clinics and 34 control clinics. In all, 8937 individuals with SMI (4922 women [55.1%]; mean [SD] age, 48.4 [13.5] years) made an index visit and at least 1 follow-up visit (Table 1; Figure). A total of 5901 participants (66.0%) had bipolar disorder, 1747 had schizoaffective disorder (19.5%), and 1289 had schizophrenia (14.4%). A total of 7483 individuals (83.7%) were White, 905 were Black (10.1%), 182 were Native American (2.0%), and 125 were Hispanic (1.4%). The mean (SD) 10-year total CV risk was estimated at 8.3% (8.8%), while the mean (SD) total modifiable CV risk was estimated at 3.7% (5.6%). Most patients’ 30-year CV risk estimates indicated 1 (4138 [46.3%]) or 2 or more (3602 [40.3%]) major CV risk factors that did not change for the better.
Table 1. Patient Characteristics and CV Risk at Index Visit by System and Treatment Group.
| Characteristic | All | Site A | Site B | Site C | ||||
|---|---|---|---|---|---|---|---|---|
| Control | CDS | Control | CDS | Control | CDS | Control | CDS | |
| No. | 4387 | 4550 | 2120 | 1807 | 1486 | 1818 | 781 | 925 |
| Women, No. (%) | 2392 (54.5) | 2530 (55.6) | 1157 (54.6) | 962 (53.2) | 797 (53.6) | 1040 (57.2) | 438 (56.1) | 528 (57.1) |
| Age, mean (SD), y | 48.1 (13.4) | 48.6 (13.3) | 47.8 (13.6) | 47.3 (13.2) | 47.8 (13.1) | 48.3 (13.3) | 49.6 (13.6) | 52.0 (13.0) |
| Age group, No. (%) | ||||||||
| 18-39 y | 1265 (28.8) | 1270 (27.9) | 632 (29.8) | 553 (30.6) | 434 (29.2) | 527 (29.0) | 199 (25.5) | 190 (20.5) |
| 40-49 y | 961 (21.9) | 962 (21.1) | 472 (22.3) | 400 (22.1) | 325 (21.9) | 397 (21.8) | 164 (21.0) | 165 (17.8) |
| 50-59 y | 1194 (27.3) | 1263 (27.8) | 550 (25.9) | 515 (28.5) | 418 (28.1) | 483 (26.6) | 226 (28.9) | 265 (28.6) |
| 60-64 y | 462 (10.5) | 493 (10.8) | 220 (10.4) | 163 (9.0) | 161 (10.8) | 194 (10.7) | 81 (10.4) | 136 (14.7) |
| 65-75 y | 505 (11.5) | 562 (12.4) | 246 (11.6) | 176 (9.7) | 148 (10.0) | 217 (11.9) | 111 (14.2) | 169 (18.3) |
| Race and ethnicity, No. (%) | ||||||||
| Asian | 55 (1.3) | 76 (1.7) | 35 (1.7) | 52 (2.9) | 8 (0.5) | 10 (0.6) | 12 (1.5) | 14 (1.5) |
| Black | 390 (8.9) | 515 (11.3) | 287 (13.5) | 329 (18.2) | 39 (2.6) | 73 (4.0) | 64 (8.2) | 113 (12.2) |
| Hispanic | 72 (1.6) | 53 (1.2) | 53 (2.5) | 31 (1.7) | 6 (0.4) | 11 (0.6) | 13 (1.7) | 11 (1.2) |
| Native American | 69 (1.6) | 113 (2.5) | 19 (0.9) | 15 (0.8) | 41 (2.6) | 98 (5.4) | 9 (1.2) | 0 |
| White | 3740 (85.3) | 3743 (82.3) | 1691 (79.8) | 1361 (75.3) | 1386 (93.3) | 1614 (88.8) | 663 (84.9) | 768 (83.0) |
| Other or unknowna | 133 (3.0) | 103 (2.3) | 88 (4.2) | 50 (2.8) | 12 (2.8) | 23 (1.3) | 33 (4.2) | 30 (3.2) |
| SMI subgroup, No. (%) | ||||||||
| Schizoaffective | 864 (19.7) | 883 (19.4) | 413 (19.5) | 382 (21.1) | 296 (19.9) | 295 (16.2) | 155 (19.8) | 206 (22.3) |
| Schizophrenia | 657 (15.0) | 632 (13.9) | 234 (11.0) | 262 (14.5) | 297 (20.0) | 245 (13.5) | 126 (16.1) | 125 (13.5) |
| Bipolar | 2866 (65.3) | 3035 (66.7) | 1473 (69.5) | 1163 (64.4) | 893 (60.1) | 1278 (70.3) | 500 (64.0) | 594 (64.2) |
| ACC/AHA 10-y CV risk | ||||||||
| No. | 3586 | 3708 | 1744 | 1500 | 1168 | 1377 | 674 | 831 |
| Mean (SD) | 8.1 (8.7) | 8.5 (8.9) | 7.8 (8.6) | 8.1 (8.7) | 8.6 (8.6) | 8.5 (8.8) | 8.2 (8.9) | 9.1 (9.6) |
| 25th Percentile | 2.3 | 2.4 | 2 | 2.3 | 2.6 | 2.5 | 2.4 | 2.4 |
| Median | 5.2 | 5.4 | 4.8 | 5.2 | 5.9 | 5.5 | 5.1 | 5.8 |
| 75th Percentile | 11.1 | 11.5 | 10.2 | 10.7 | 11.7 | 11.4 | 11.4 | 12.7 |
| Aged >39 y with no CHD | ||||||||
| No. | 2569 | 2704 | 1221 | 1059 | 845 | 994 | 503 | 651 |
| Mean (SD) | 9.1 (8.7) | 9.5 (9.1) | 8.8 (8.7) | 9.3 (9.1) | 9.5 (8.7) | 9.3 (8.7) | 8.9 (8.7) | 10.1 (9.9) |
| 25th Percentile | 3.0 | 3.2 | 2.6 | 3.1 | 3.4 | 3.2 | 2.8 | 3.3 |
| Median | 6.3 | 6.5 | 6.1 | 6.5 | 7.1 | 6.4 | 5.9 | 6.8 |
| 75th Percentile | 12.5 | 12.7 | 12.1 | 12.4 | 13 | 12.3 | 12.6 | 13.5 |
| Framingham 10-y CV risk | ||||||||
| No. | 4387 | 4550 | 2120 | 1807 | 1486 | 1818 | 781 | 925 |
| Mean (SD) | 7.1 (8.1) | 7.3 (8.2) | 6.7 (7.8) | 6.9 (8.0) | 7.7 (8.5) | 7.4 (8.2) | 7.3 (8.2) | 8 (8.7) |
| 25th Percentile | 1.8 | 1.9 | 1.7 | 1.6 | 2.1 | 2.0 | 1.9 | 2.0 |
| Median | 4.0 | 4.1 | 3.7 | 3.8 | 4.5 | 4.1 | 4.1 | 4.7 |
| 75th Percentile | 9.1 | 9.9 | 8.3 | 9.0 | 10.1 | 9.9 | 9.7 | 11.1 |
| Aged >39 y with no CHD | ||||||||
| No. | 2925 | 3077 | 1395 | 1184 | 984 | 1194 | 546 | 699 |
| Mean (SD) | 8.6 (8.6) | 8.8 (8.6) | 8.1 (8.3) | 8.5 (8.6) | 9.3 (9.0) | 8.8 (8.4) | 8.4 (8.4) | 9.2 (8.9) |
| 25th Percentile | 2.6 | 2.7 | 2.4 | 2.5 | 3.0 | 2.8 | 2.5 | 2.8 |
| Median | 5.6 | 5.7 | 5.1 | 5.6 | 6.4 | 5.7 | 5.4 | 6.1 |
| 75th Percentile | 11.6 | 12.1 | 10.9 | 11.3 | 12.6 | 12.4 | 11.3 | 12.9 |
| Modifiable CV risk | ||||||||
| Mean (SD) | 3.6 (5.5) | 3.7 (5.7) | 3.4 (5.6) | 3.6 (5.5) | 4.1 (5.6) | 3.9 (5.8) | 3.3 (5) | 3.4 (5.5) |
| 25th Percentile | 0.3 | 0.3 | 0.3 | 0.3 | 0.5 | 0.5 | 0.3 | 0.3 |
| Median | 1.6 | 1.6 | 1.4 | 1.5 | 2.1 | 2.0 | 1.4 | 1.3 |
| 75th Percentile | 4.3 | 4.3 | 3.9 | 4.3 | 5.1 | 4.4 | 3.9 | 4.1 |
| 30-y (Lifetime) CV risk | ||||||||
| Aged <60 y, No. | 2685 | 2733 | 1310 | 1181 | 884 | 1011 | 491 | 541 |
| Risk factors, % | ||||||||
| All optimal | 2.5 | 2.5 | 2.6 | 2.7 | 2.1 | 2.4 | 2.9 | 2.4 |
| ≥1 Not optimal | 7.5 | 7.4 | 9.2 | 7.5 | 4.9 | 6.8 | 7.7 | 8.5 |
| ≥1 Elevated | 3.9 | 3.8 | 4.5 | 3.6 | 3.5 | 3.5 | 3.3 | 5.2 |
| 1 Major | 46.3 | 46.3 | 48.9 | 46.7 | 41.9 | 44.3 | 47.3 | 45.3 |
| ≥2 Major | 39.8 | 40.7 | 34.8 | 39.5 | 47.6 | 43.0 | 38.9 | 38.6 |
| Aged <60 y with no CHD, No. | 2585 | 2627 | 1262 | 1140 | 847 | 963 | 476 | 524 |
| Risk factors, % | ||||||||
| All optimal | 2.6 | 2.6 | 2.7 | 2.8 | 2.2 | 2.5 | 2.9 | 2.5 |
| ≥1 Not optimal | 7.7 | 7.6 | 9.4 | 7.7 | 5 | 7 | 8 | 8.6 |
| ≥1 Elevated | 4.1 | 4.0 | 4.7 | 3.7 | 3.7 | 3.6 | 3.4 | 5.3 |
| 1 Major | 46.9 | 46.2 | 49.5 | 47.6 | 42.5 | 44.9 | 47.9 | 45.6 |
| ≥2 Major | 38.7 | 39.6 | 33.7 | 38.2 | 46.6 | 42.1 | 37.8 | 38 |
| Current smoking, No. (%) | 2027 (46.2) | 2131 (46.8) | 930 (43.9) | 840 (46.5) | 782 (52.6) | 982 (54.0) | 315 (40.3) | 309 (33.4) |
| Body mass indexb | ||||||||
| Mean (SD) | 32.8 (8.0) | 32.6 (7.8) | 32.6 (7.9) | 32.2 (7.6) | 32.8 (7.9) | 32.7 (8.1) | 32.9 (8.3) | 32.8 (7.6) |
| 25th Percentile | 27.3 | 27.2 | 27.4 | 27 | 27.2 | 27.2 | 27.4 | 27.3 |
| Median | 31.6 | 31.5 | 31.4 | 31.4 | 31.9 | 31.6 | 31.4 | 31.5 |
| 75th Percentile | 36.9 | 36.8 | 36.6 | 36.4 | 37.1 | 36.9 | 37.3 | 37.2 |
| Systolic blood pressure, mm Hg | ||||||||
| Mean (SD) | 124.2 (16.5) | 124.4 (16.6) | 123.9 (16.7) | 124.6 (17.2) | 125.1 (16.6) | 124.4 (16.1) | 123.2 (15.9) | 124.3 (16.5) |
| 25th Percentile | 112 | 113 | 112 | 113 | 114 | 114 | 111 | 112 |
| Median | 123 | 124 | 123 | 124 | 123 | 124 | 122 | 123 |
| 75th Percentile | 134 | 134 | 133 | 135 | 135 | 134 | 132 | 134 |
| Diastolic blood pressure, mm Hg | ||||||||
| Mean (SD) | 78.3 (11.4) | 78.3 (11.4) | 79.0 (11.7) | 79.9 (11.9) | 77.9 (10.8) | 77.7 (10.5) | 77.0 (11.5) | 76.5 (11.8) |
| 25th Percentile | 70 | 70 | 71 | 72 | 70 | 71 | 70 | 69 |
| Median | 78 | 78 | 79 | 80 | 78 | 78 | 76 | 76 |
| 75th Percentile | 85 | 86 | 86 | 87 | 84 | 84 | 84 | 84 |
| Blood pressure <140/90 mm Hg, No. (%) | 3712 (84.6) | 3816 (83.9) | 1744 (82.3) | 1417 (78.4) | 1296 (87.2) | 1590 (87.5) | 672 (86.0) | 809 (87.5) |
| LDL-C, mg/dL | ||||||||
| Mean (SD) | 104.6 (35.6) | 104.9 (35.4) | 104.3 (34.8) | 102.1 (34.4) | 105.3 (36.8) | 106.9 (36.3) | 104.1 (35.7) | 106.7 (35.4) |
| 25th Percentile | 80 | 80 | 80 | 78 | 79 | 81 | 79 | 81 |
| Median | 102 | 103 | 102 | 100 | 102 | 104 | 101 | 104 |
| 75th Percentile | 126 | 126 | 126 | 122 | 128 | 130 | 126 | 127 |
| HbA1c among those with diabetes | ||||||||
| No. | 866 | 947 | 375 | 376 | 309 | 337 | 182 | 234 |
| Mean (SD) | 7.3 (1.8) | 7.1 (1.7) | 7.4 (1.9) | 7.2 (1.6) | 7.2 (1.7) | 7.1 (1.6) | 7.4 (1.9) | 7.1 (1.7) |
| 25th Percentile | 6.1 | 6.0 | 6.1 | 6.1 | 6.1 | 6.0 | 6.2 | 6.0 |
| Median | 6.9 | 6.7 | 6.9 | 6.8 | 6.8 | 6.7 | 6.9 | 6.6 |
| 75th Percentile | 7.9 | 7.7 | 7.9 | 7.9 | 7.8 | 7.6 | 8.3 | 7.6 |
| HbA1c, No. (%) | ||||||||
| <7% | 464 (53.6) | 546 (57.7) | 191 (50.9) | 206 (54.8) | 177 (57.3) | 201 (59.6) | 96 (52.7) | 139 (59.4) |
| 7% to <8% | 186 (21.5) | 205 (21.6) | 91 (24.3) | 81 (21.5) | 59 (19.1) | 71 (21.1) | 36 (19.8) | 53 (22.6) |
| 8% to <9% | 83 (9.6) | 63 (6.7) | 37 (9.9) | 28 (7.4) | 28 (9.1) | 20 (5.9) | 18 (9.9) | 15 (6.4) |
| ≥9% | 133 (15.4) | 133 (14.0) | 56 (14.9) | 61 (16.2) | 45 (14.6) | 45 (13.4) | 32 (17.6) | 27 (11.5) |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CDS, clinical decision support; CHD, coronary heart disease; CV, cardiovascular; HbA1c, hemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; SMI, serious mental illness.
SI conversion factors: To convert LDL-C to millimoles per liter, multiply by 0.0259; and HbA1c to proportion of total hemoglobin, multiply by 0.01.
Included patients who were Pacific Islanders and patients for whom no race or ethnicity data were recorded in the electronic health record.
Calculated as weight in kilograms divided by height in meters squared.
Figure. Study Consolidated Standards of Reporting Trials Diagram.
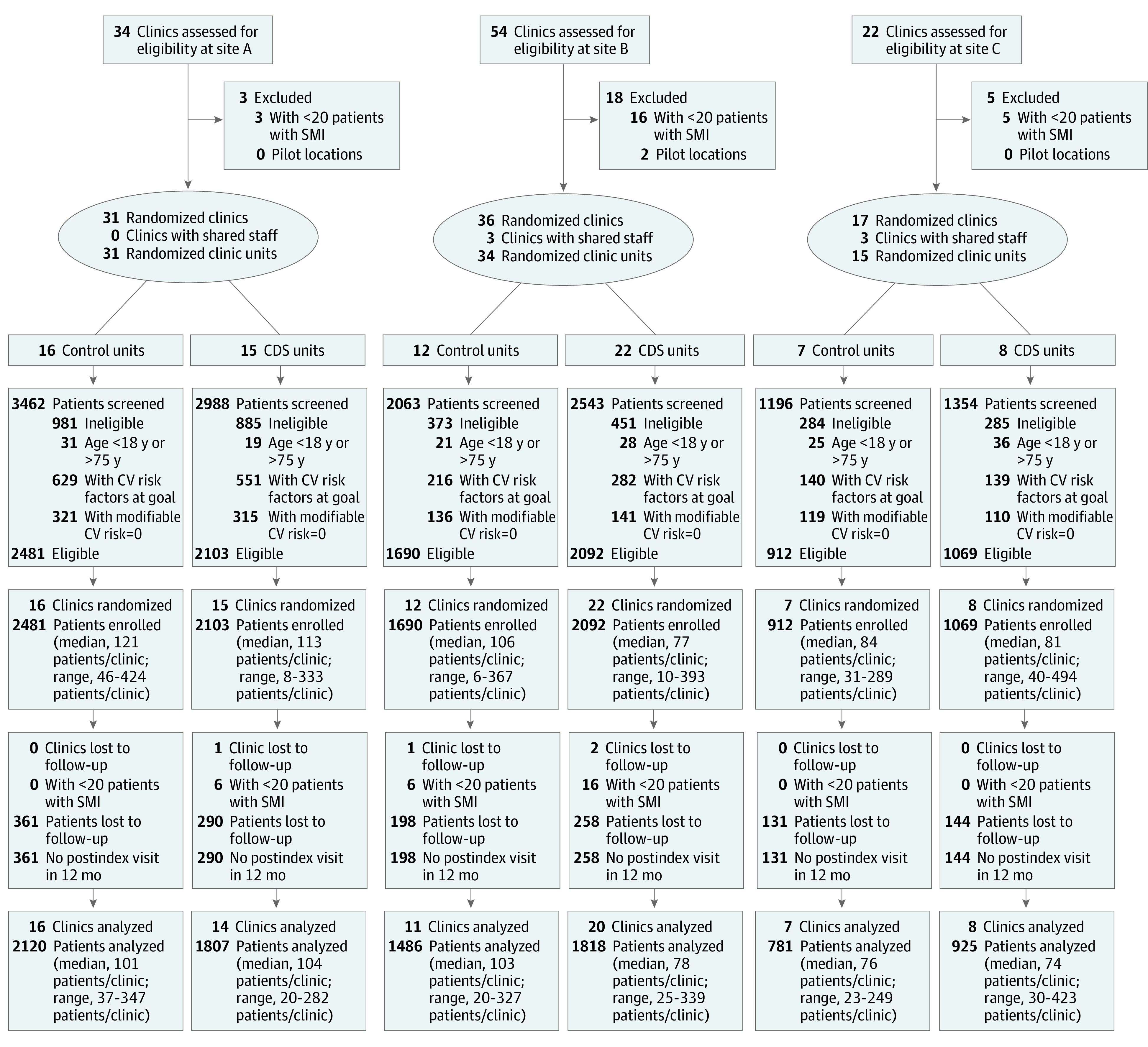
CDS indicates clinical decision support; CV, cardiovascular; and SMI, serious mental illness.
Intervention patients had an estimated 1% decrease in total modifiable CV risk (rate ratio [RR], 0.99; 95% CI, 0.98-1.01) over 12 months, while control patients had an estimated 4% increase (RR, 1.04; 95% CI, 1.02-1.05), for a net 4% lower increase in total modifiable CV risk among patients in intervention clinics relative to those in control clinics (relative RR, 0.96; 95% CI, 0.94-0.98) (Table 2).
Table 2. Total American College of Cardiology/American Heart Association 10-Year CV Risk at Index Visit, Model-Estimated Modifiable CV Risk at Index Visit and 12 Months, and Rate of Change by Time and Treatment × Time for Patients Overall and by Subgroupa.
| Characteristic | No. | Total CV risk at index visit, mean | Total modifiable CV risk | |||
|---|---|---|---|---|---|---|
| LS mean | Rate of change | |||||
| Index visit | 12 mo | RR for time | RR (95% CI) for treatment × time | |||
| All | ||||||
| Control | 4387 | 8.12 | 1.86 | 1.93 | 1.04 | 0.96 (0.94-0.98) |
| CDS | 4550 | 8.49 | 1.90 | 1.89 | 0.99 | |
| SMI subtype | ||||||
| Bipolar disorder | ||||||
| Control | 2866 | 7.39 | 1.64 | 1.73 | 1.05 | 0.96 (0.94-0.99) |
| CDS | 3035 | 7.96 | 1.69 | 1.72 | 1.02 | |
| Schizoaffective disorder | ||||||
| Control | 855 | 8.68 | 2.23 | 2.29 | 1.03 | 0.94 (0.90-0.98) |
| CDS | 877 | 8.73 | 2.20 | 2.13 | 0.97 | |
| Schizophrenia | ||||||
| Control | 657 | 10.33 | 2.84 | 3.18 | 1.12 | 0.92 (0.85-0.99) |
| CDS | 632 | 10.32 | 3.14 | 3.24 | 1.03 | |
| Total modifiable CV risk at index visit | ||||||
| 0%-2% | ||||||
| Control | 2389 | 4.25 | 0.65 | 0.88 | 1.36 | 0.96 (0.92-0.99) |
| CDS | 2467 | 4.37 | 0.66 | 0.86 | 1.30 | |
| 2%-5% | ||||||
| Control | 1048 | 7.77 | 2.99 | 3.26 | 1.09 | 0.96 (0.93-0.99) |
| CDS | 1084 | 8.10 | 3.04 | 3.19 | 1.05 | |
| 5%-10% | ||||||
| Control | 495 | 12.56 | 6.06 | 5.61 | 0.93 | 0.97 (0.93-1.01) |
| CDS | 544 | 12.90 | 6.14 | 5.52 | 0.90 | |
| >10% | ||||||
| Control | 455 | 20.27 | 13.02 | 10.67 | 0.82 | 0.96 (0.92-0.99) |
| CDS | 455 | 21.91 | 13.58 | 10.69 | 0.79 | |
| Sex | ||||||
| Female | ||||||
| Control | 2392 | 6.10 | 1.69 | 1.84 | 1.09 | 0.95 (0.92-0.97) |
| CDS | 2530 | 6.28 | 1.73 | 1.78 | 1.03 | |
| Male | ||||||
| Control | 1994 | 10.54 | 2.12 | 2.10 | 0.99 | 0.96 (0.94-0.99) |
| CDS | 2020 | 11.24 | 2.18 | 2.08 | 0.95 | |
| Age group, y | ||||||
| 18-29 | ||||||
| Control | 466 | 3.02 | 1.57 | 1.72 | 1.09 | 0.89 (0.81-0.98) |
| CDS | 439 | 3.07 | 1.48 | 1.44 | 0.97 | |
| 30-39 | ||||||
| Control | 799 | 3.71 | 1.60 | 1.73 | 1.08 | 0.97 (0.92-1.03) |
| CDS | 831 | 3.67 | 1.65 | 1.74 | 1.05 | |
| 40-49 | ||||||
| Control | 961 | 5.01 | 1.65 | 1.72 | 1.04 | 0.98 (0.94-1.03) |
| CDS | 962 | 4.94 | 1.74 | 1.78 | 1.02 | |
| 50-59 | ||||||
| Control | 1194 | 7.91 | 1.90 | 1.98 | 1.05 | 0.93 (0.90-0.96) |
| CDS | 1263 | 8.47 | 2.04 | 1.99 | 0.97 | |
| 60-75 | ||||||
| Control | 967 | 15.37 | 2.34 | 2.35 | 1.00 | 0.97 (0.94-1.01) |
| CDS | 1055 | 15.58 | 2.26 | 2.20 | 0.98 | |
| Race and ethnicity | ||||||
| Asian | ||||||
| Control | 55 | 7.59 | 1.95 | 1.69 | 0.86 | 1.11 (0.93-1.33) |
| CDS | 76 | 7.10 | 1.79 | 1.71 | 0.96 | |
| Black | ||||||
| Control | 390 | 9.43 | 2.15 | 2.33 | 1.09 | 0.93 (0.88-0.98) |
| CDS | 515 | 10.55 | 2.17 | 2.19 | 1.01 | |
| Hispanic | ||||||
| Control | 72 | 6.87 | 1.94 | 2.00 | 1.03 | 0.87 (0.75-1.01) |
| CDS | 53 | 9.06 | 2.36 | 2.12 | 0.90 | |
| Native American | ||||||
| Control | 68 | 7.07 | 2.13 | 1.97 | 0.93 | 0.98 (0.85-1.13) |
| CDS | 110 | 7.82 | 2.39 | 2.17 | 0.91 | |
| White | ||||||
| Control | 3736 | 8.03 | 1.83 | 1.90 | 1.04 | 0.96 (0.94-0.98) |
| CDS | 3740 | 8.27 | 1.86 | 1.85 | 1.00 | |
| Other and unknownb | ||||||
| Control | 66 | 8.73 | 1.76 | 1.62 | 0.92 | 1.16 (0.94-1.43) |
| CDS | 56 | 6.31 | 2.01 | 2.14 | 1.07 | |
Abbreviations: CDS, clinical decision support; CV, cardiovascular; LS, least-squares; RR, rate ratio; SMI, serious mental illness.
Analyses adjusted for age, sex, and index visit outcome value.
Includes Pacific Islanders and all patients for whom race or ethnicity was not indicated in the electronic health record.
Despite the overall positive treatment effect, there were no significant treatment effects for individual modifiable CV risk factors, except for BMI, for which small differences favored the control group (Table 3). Small nonsignificant changes in low-density lipoprotein cholesterol level, HbA1c level, and smoking favored the intervention group.
Table 3. Model-Estimated Rate of Change From Index Visit to 12 Months After Index Visit in Individual Modifiable CV Risk Factors and Obesogenic Medication Use Among Patients in CDS Clinics Relative to Control Clinics.
| Characteristic | Risk ratio or difference in difference (95% CI)a,b | |||
|---|---|---|---|---|
| All | Site A | Site B | Site C | |
| Quit smokinga | 1.11 (0.92 to 1.33) | 1.19 (0.91 to 1.55) | 1.04 (0.77 to 1.41) | 1.09 (0.48 to 2.44) |
| Body mass indexb | 0.07 (0.01 to 0.13) | 0.09 (0.01 to 0.17) | 0.04 (−0.08 to 0.15) | 0.19 (−0.06 to 0.44) |
| Low-density lipoprotein cholesterolb | −0.92 (−4.65 to 2.80) | −2.96 (−7.90 to 1.99) | 4.47 (−2.70 to 11.63) | −4.86 (−22.94 to 13.22) |
| Systolic blood pressureb | 1.03 (−1.04 to 3.09) | −0.26 (−3.29 to 2.77) | 3.64 (−0.30 to 7.58) | −1.65 (−8.25 to 4.96) |
| Hemoglobin A1cb | −0.04 (−0.13 to 0.05) | −0.03 (−0.15 to 0.09) | −0.06 (−0.23 to 0.11) | −0.11 (−0.46 to 0.24) |
| Obesogenic SMI medication discontinuationa | 1.02 (0.81 to 1.29) | 1.03 (0.76 to 1.39) | 0.92 (0.58 to 1.47) | 1.15 (0.56 to 2.38) |
Abbreviations: CDS, clinical decision support; CV, cardiovascular; SMI, serious mental illness.
Risk ratio (95% CI), adjusted for sex, age at index visit, log (year of index visit to last visit), site, and balancing factors.
Difference in difference (95% CI), adjusted for sex, age at index visit, index visit value, site, and balancing factors.
Change in modifiable CV risk varied by patient subgroup (Table 2). The effectiveness of the intervention varied by SMI subtype, with rate of change in intervention vs control patients 4% slower (RR, 0.96; 95% CI, 0.94-0.99) for patients with bipolar disorder, 6% slower (RR, 0.94; 95% CI, 0.90-0.98) for patients with schizoaffective disorder, and 8% slower (RR, 0.92; 95% CI, 0.85-0.99) for patients with schizophrenia. The intervention was similarly effective for women (RR, 0.95; 95% CI, 0.92-0.97) and men (RR, 0.96; 95% CI, 0.94-0.99). Change in total modifiable CV risk favored intervention patients aged 18 to 29 years (RR, 0.89; 95% CI, 0.81-0.98) and 50 to 59 years (RR, 0.93; 95% CI, 0.90-0.96) but not other ages. The intervention benefited patients who self-identified as Black (RR, 0.93; 95% CI, 0.88-0.98) or White (RR, 0.96; 95% CI, 0.94-0.98) patients but not Asian (RR, 1.11; 95% CI, 0.93-1.33), Native American (RR, 0.98; 95% CI, 0.85-1.13), or Hispanic (RR, 0.87; 95% CI, 0.75-1.01) patients or those of other or unknown race (RR, 1.16; 95% CI, 0.94-1.43). Patients of racial and ethnic minority groups generally had higher model-estimated total modifiable CV risk than White patients at the index visit and 12 months after the index visit. Patient and clinician handouts were printed at 61.1% of eligible encounters (37 079 of 60 685) in intervention clinics, and the rate of change in CV risk was not associated with print rates (eTables 3 and 4 in Supplement 2).
There were no clinician reports of adverse events. Surveillance of safety data aggregated from EHRs found no significant treatment × time effects for any monitored safety outcome (eTables 5-7 in Supplement 2).
Discussion
This cluster randomized primary care CDS intervention produced mostly nonsignificant improvements in individual modifiable CV risk factors that, when summed together, resulted in 4% lower total modifiable CV risk among adults with SMI at 12 months. The CDS intervention favored patients who were 18 to 29 years of age or 50 to 59 years of age, Black, or White.
To our knowledge, this is the first randomized clinical trial shown to improve CV health in a large population of US outpatients with SMI. Few previous studies have addressed CV risk among those with SMI, and those that did had smaller sample sizes (a few hundred at most) or focused on a single CV risk factor.30 Furthermore, effects on overall CV risk have been rarely reported.30 One US study randomized 269 outpatients with SMI (including depression) and 1 CV risk factor not at the goal set by the ACC/AHA guidelines to counseling and care coordination.31 Intervention patients achieved a 12.7% relative reduction in the 10-year Framingham Risk Score at 19 months compared with control patients. In a US study of collaborative care for 118 veterans with bipolar disorder, intervention patients had decreased systolic and diastolic blood pressure but not cholesterol level at 24 months.32 A British cluster randomized primary care trial involving personalized goal setting for 327 patients with SMI showed no difference in the primary outcome of total cholesterol level or a secondary outcome of total CV risk at 12 months.33 Finally, a Danish randomized trial of lifestyle coaching plus care coordination for 428 patients with schizophrenia and central obesity had no effect on 10-year CV risk, BMI, systolic blood pressure, HbA1c level, or smoking status at 12 months.34
Our finding of a 4% relative decrease in modifiable CV risk in intervention compared with control patients was modest yet clinically significant, particularly given the relatively low intensity of the intervention and the intention-to-treat analysis. For this population of patients with a baseline 10-year CV risk of 8%, approximately 80 of 1000 patients would be predicted to have a myocardial infarction or stroke in the next 10 years. The relative 4% decrease that we observed with the intervention would result in an estimated 7.7% 10-year CV risk, or approximately 77 of 1000 events, potentially preventing 3 events per 1000 patients. Thus, although a 4% lower CV risk appears modest, on a population level it is clinically meaningful, especially in the context of an observed upward trend in 10-year risk in the control group and a stable trend in the intervention group.
Similar to studies of total CV risk, studies focused on single CV risk factors among patients with SMI have had mixed results. In a UK study of 526 outpatients, smoking cessation rates were higher for intervention patients than for control patients at 6 months (14% vs 6%; risk difference, 7.7%; 95% CI, 2.1%-13.3%), but this effect was nonsignificant at 12 months, the primary end point.35 In a US trial of a behavioral weight-loss intervention for 291 outpatients with SMI (including depression), there was a mean between-group difference in weight of 3.2 kg (P = .002) favoring the intervention at 18 months.36 Although the intensity of our intervention was lower than in these studies, our CDS intervention reached thousands of patients with SMI and addressed multiple modifiable CV risk factors, resulting in lower total modifiable CV risk.
There were significant differences in intervention effectiveness by race and ethnicity. Black and White intervention patients had lower total modifiable CV risk relative to control patients at 12 months, while patients in other racial or ethnic groups did not. Patients in racial and ethnic minority groups started and ended the intervention with higher estimated modifiable CV risk than did White patients, consistent with the cumulative effects of structural racism on cardiometabolic risk.37,38 Existing research on CV risk of patients in racial and ethnic minority groups with SMI have been scarce. A 2014 literature review was limited by a small number of studies with small sample sizes and varied designs and methods.39 Despite these limitations, the authors found that Black patients with SMI, especially Black women with SMI, were at greater risk for obesity and, along with Hispanic patients, for diabetes, especially when exposed to antipsychotic medications. We encourage future studies to report outcomes by race and ethnicity and further address CV health disparities for patients in racial and ethnic minority groups with SMI.
Clinicians were asked to address CV risk starting at 18 years of age rather than waiting until the more standard age of 40 years. This decision was supported by findings that patients with SMI have higher CV burden at younger ages, resulting in shortened life spans.2 The intervention had significant effects for patients who were 18 to 29 years of age, resulting in a relative 11% decrease in change in total modifiable CV risk at 12 months. This finding is important as evidence emerges of improved lifelong outcomes when CV risk for patients with SMI is addressed earlier in the life span.40 Our findings support the value in addressing elevated 30-year risk with evidence-based interventions.
Some may question the usability and sustainability of a low-intensity CDS intervention. Clinicians have previously reported ignoring CDS owing to inconsequential alerts, distrust, alert fatigue, or workflow disruption.41,42,43 Our CDS intervention was designed to fit with rooming staff workflows to avoid interference with clinician workflows, leading to print rates of the CDS output at 61.1% of targeted visits. Furthermore, at the request of health care system leaders, the CDS was turned on for all primary care clinics at 2 participating health care systems the day after the study ended. The CDS has since been printed at 73.7% of eligible encounters, supported by monthly feedback of print rates to clinic leadership. These findings highlight the usability and sustainability of CDS interventions.
Limitations
This study has some limitations. It was conducted in 3 Midwestern integrated health care systems, and the results may not be generalizable to other settings. This pragmatic intervention was implemented on top of usual care, with patients returning to the clinic at intervals deemed appropriate by their care team, and 12-month outcomes were derived from available data. We relied on EHR diagnoses to identify patients with SMI, and some patients may have been misclassified.
Conclusions
In this large cluster randomized clinical trial of an EHR-integrated CDS tool, intervention patients had 4% lower change in total modifiable CV risk at 12 months compared with control patients. Intervention patients with SMI who were 18 to 29 years of age or 50 to 59 years of age, Black, or White had significantly lower change in modifiable CV risk at 12 months compared with control patients. The effects of our intervention on young adults with SMI highlights the value of using 30-year CV risk estimates to prompt primary care intervention for this population. Our intervention helped slow the otherwise increasing CV health disparities for Black patients with SMI, and we encourage future interventions to focus on further improving CV health in disadvantaged populations.
Trial Protocol
eTable 1. Serious Mental Illness (SMI) Diagnostic Codes
eTable 2. Potentially Obesogenic Medications for Serious Mental Illness (SMI)
eTable 3. Intervention Dose, ie, the Number and Percent of Visits (Index and Post-Index) at Which the CDS Was Printed by System and Treatment Group
eTable 4. Rate Ratios Estimating Rates of Change in Total Modifiable CV Risk From Index to 12 Months Post-Index Among Patients in Intervention Clinics by Print Rate Subgroups
eTable 5. Emergency Department Visit Rates by Health System and Treatment Group in the Year Prior to the First Visit (Pre) and Time Between First Visit and 9/19/2018 (Post)
eTable 6. Inpatient Stay Rates by Health System and Treatment Group in the Year Prior to the First Visit (Pre) and Time Between First Visit and 9/19/2018 (Post)
eTable 7. Suicide Attempt Rates by Health System and Treatment Group in the Year Prior to the First Visit (Pre) and Time Between First Visit and 9/19/2018 (Post)
Data Sharing Statement
References
- 1.Osby U, Correia N, Brandt L, Ekbom A, Sparén P. Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res. 2000;45(1-2):21-28. doi: 10.1016/S0920-9964(99)00191-7 [DOI] [PubMed] [Google Scholar]
- 2.Parks J, Svendsen D, Singer P, Foti ME, Mauer B. Morbidity and mortality in people with serious mental illness. National Association of State Mental Health Program Directors. Published October 2006. Accessed March 29, 2021. https://www.nasmhpd.org/sites/default/files/Mortality%20and%20Morbidity%20Final%20Report%208.18.08_0.pdf
- 3.Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database. Arch Gen Psychiatry. 2007;64(2):242-249. doi: 10.1001/archpsyc.64.2.242 [DOI] [PubMed] [Google Scholar]
- 4.Dixon L, Postrado L, Delahanty J, Fischer PJ, Lehman A. The association of medical comorbidity in schizophrenia with poor physical and mental health. J Nerv Ment Dis. 1999;187(8):496-502. doi: 10.1097/00005053-199908000-00006 [DOI] [PubMed] [Google Scholar]
- 5.Davidson S, Judd F, Jolley D, Hocking B, Thompson S, Hyland B. Cardiovascular risk factors for people with mental illness. Aust N Z J Psychiatry. 2001;35(2):196-202. doi: 10.1046/j.1440-1614.2001.00877.x [DOI] [PubMed] [Google Scholar]
- 6.Newcomer JW. Medical risk in patients with bipolar disorder and schizophrenia. J Clin Psychiatry. 2006;67(suppl 9):25-30. doi: 10.4088/JCP.1106e16 [DOI] [PubMed] [Google Scholar]
- 7.Pylvänen V, Knip M, Pakarinen A, Kotila M, Turkka J, Isojärvi JI. Serum insulin and leptin levels in valproate-associated obesity. Epilepsia. 2002;43(5):514-517. doi: 10.1046/j.1528-1157.2002.31501.x [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JA, Stroup TS, McEvoy JP, et al. ; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators . Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. doi: 10.1056/NEJMoa051688 [DOI] [PubMed] [Google Scholar]
- 9.Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder. Ann Clin Psychiatry. 2011;23(1):40-47. [PMC free article] [PubMed] [Google Scholar]
- 10.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121. doi: 10.1016/j.ahj.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572. doi: 10.1001/archpsyc.58.6.565 [DOI] [PubMed] [Google Scholar]
- 12.The Office of the National Coordinator for Health Information Technology (ONC) . Clinical decision support. Accessed December 21, 2021. https://www.healthit.gov/topic/safety/clinical-decision-support
- 13.Meigs JB, Cagliero E, Dubey A, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003;26(3):750-757. doi: 10.2337/diacare.26.3.750 [DOI] [PubMed] [Google Scholar]
- 14.Montori VM, Dinneen SF, Gorman CA, et al. ; Translation Project Investigator Group . The impact of planned care and a diabetes electronic management system on community-based diabetes care: the Mayo Health System Diabetes Translation Project. Diabetes Care. 2002;25(11):1952-1957. doi: 10.2337/diacare.25.11.1952 [DOI] [PubMed] [Google Scholar]
- 15.Crosson JC, Stroebel C, Scott JG, Stello B, Crabtree BF. Implementing an electronic medical record in a family medicine practice: communication, decision making, and conflict. Ann Fam Med. 2005;3(4):307-311. doi: 10.1370/afm.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orzano AJ, Strickland PO, Tallia AF, et al. Improving outcomes for high-risk diabetics using information systems. J Am Board Fam Med. 2007;20(3):245-251. doi: 10.3122/jabfm.2007.03.060185 [DOI] [PubMed] [Google Scholar]
- 17.Welch WP, Bazarko D, Ritten K, Burgess Y, Harmon R, Sandy LG. Electronic health records in four community physician practices: impact on quality and cost of care. J Am Med Inform Assoc. 2007;14(3):320-328. doi: 10.1197/jamia.M2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor PJ, Crain AL, Rush WA, Sperl-Hillen JM, Gutenkauf JJ, Duncan JE. Impact of an electronic medical record on diabetes quality of care. Ann Fam Med. 2005;3(4):300-306. doi: 10.1370/afm.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor PJ, Sperl-Hillen JM, Rush WA, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Ann Fam Med. 2011;9(1):12-21. doi: 10.1370/afm.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperl-Hillen JM, Crain AL, Margolis KL, et al. Clinical decision support directed to primary care patients and providers reduces cardiovascular risk: a randomized trial. J Am Med Inform Assoc. 2018;25(9):1137-1146. doi: 10.1093/jamia/ocy085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharbanda EO, Asche SE, Sinaiko AR, et al. Clinical decision support for recognition and management of hypertension: a randomized trial. Pediatrics. 2018;141(2):e20172954. doi: 10.1542/peds.2017-2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossom RC, O’Connor PJ, Crain AL, et al. Pragmatic trial design of an intervention to reduce cardiovascular risk in people with serious mental illness. Contemp Clin Trials. 2020;91:105964. doi: 10.1016/j.cct.2020.105964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials. 2004;1(3):297-305. doi: 10.1191/1740774504cn024oa [DOI] [PubMed] [Google Scholar]
- 24.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25, pt B):2935-2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S49-S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791-798. doi: 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 27.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56(9):1925-1933. doi: 10.1007/s00125-013-2940-y [DOI] [PubMed] [Google Scholar]
- 28.Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group . The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101(6):671-679. doi: 10.1042/CS20000335 [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gierisch JM, Nieuwsma JA, Bradford DW, et al. Interventions to Improve Cardiovascular Risk Factors in People With Serious Mental Illness: AHRQ Comparative Effectiveness Review No. 105. AHRQ publication 13-EHC063-EF. Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 31.Daumit GL, Dalcin AT, Dickerson FB, et al. Effect of a comprehensive cardiovascular risk reduction intervention in persons with serious mental illness: a randomized clinical trial. JAMA Netw Open. 2020;3(6):e207247. doi: 10.1001/jamanetworkopen.2020.7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilbourne AM, Goodrich DE, Lai Z, et al. Randomized controlled trial to assess reduction of cardiovascular disease risk in patients with bipolar disorder: the Self-Management Addressing Heart Risk Trial (SMAHRT). J Clin Psychiatry. 2013;74(7):e655-e662. doi: 10.4088/JCP.12m08082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborn D, Burton A, Hunter R, et al. Clinical and cost-effectiveness of an intervention for reducing cholesterol and cardiovascular risk for people with severe mental illness in English primary care: a cluster randomised controlled trial. Lancet Psychiatry. 2018;5(2):145-154. doi: 10.1016/S2215-0366(18)30007-5 [DOI] [PubMed] [Google Scholar]
- 34.Speyer H, Christian Brix Nørgaard H, Birk M, et al. The CHANGE trial: no superiority of lifestyle coaching plus care coordination plus treatment as usual compared to treatment as usual alone in reducing risk of cardiovascular disease in adults with schizophrenia spectrum disorders and abdominal obesity. World Psychiatry. 2016;15(2):155-165. doi: 10.1002/wps.20318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbody S, Peckham E, Bailey D, et al. Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial. Lancet Psychiatry. 2019;6(5):379-390. doi: 10.1016/S2215-0366(19)30047-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daumit GL, Dickerson FB, Wang NY, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013;368(17):1594-1602. doi: 10.1056/NEJMoa1214530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer LC, Cooper LA. Race, discrimination, and cardiovascular disease. Virtual Mentor. 2014;16(6):455-460. [PMC free article] [PubMed] [Google Scholar]
- 38.Churchwell K, Elkind MSV, Benjamin RM, et al. ; American Heart Association . Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142(24):e454-e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 39.Carliner H, Collins PY, Cabassa LJ, McNallen A, Joestl SS, Lewis-Fernández R. Prevalence of cardiovascular risk factors among racial and ethnic minorities with schizophrenia spectrum and bipolar disorders: a critical literature review. Compr Psychiatry. 2014;55(2):233-247. doi: 10.1016/j.comppsych.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corlin L, Short MI, Vasan RS, Xanthakis V. Association of the duration of ideal cardiovascular health through adulthood with cardiometabolic outcomes and mortality in the Framingham Offspring Study. JAMA Cardiol. 2020;5(5):549-556. doi: 10.1001/jamacardio.2020.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ash JS, Sittig DF, Campbell EM, Guappone KP, Dykstra RH. Some unintended consequences of clinical decision support systems. AMIA Annu Symp Proc. 2007;2007:26-30. [PMC free article] [PubMed] [Google Scholar]
- 42.Khalifa M, Zabani I. Improving utilization of clinical decision support systems by reducing alert fatigue: strategies and recommendations. Stud Health Technol Inform. 2016;226:51-54. [PubMed] [Google Scholar]
- 43.Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3(1):17. doi: 10.1038/s41746-020-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Serious Mental Illness (SMI) Diagnostic Codes
eTable 2. Potentially Obesogenic Medications for Serious Mental Illness (SMI)
eTable 3. Intervention Dose, ie, the Number and Percent of Visits (Index and Post-Index) at Which the CDS Was Printed by System and Treatment Group
eTable 4. Rate Ratios Estimating Rates of Change in Total Modifiable CV Risk From Index to 12 Months Post-Index Among Patients in Intervention Clinics by Print Rate Subgroups
eTable 5. Emergency Department Visit Rates by Health System and Treatment Group in the Year Prior to the First Visit (Pre) and Time Between First Visit and 9/19/2018 (Post)
eTable 6. Inpatient Stay Rates by Health System and Treatment Group in the Year Prior to the First Visit (Pre) and Time Between First Visit and 9/19/2018 (Post)
eTable 7. Suicide Attempt Rates by Health System and Treatment Group in the Year Prior to the First Visit (Pre) and Time Between First Visit and 9/19/2018 (Post)
Data Sharing Statement


