Abstract
High‐pressure processing (HPP) is a non‐thermal treatment in which, for microbial inactivation, foods are subjected to isostatic pressures (P) of 400–600 MPa with common holding times (t) from 1.5 to 6 min. The main factors that influence the efficacy (log10 reduction of vegetative microorganisms) of HPP when applied to foodstuffs are intrinsic (e.g. water activity and pH), extrinsic (P and t) and microorganism‐related (type, taxonomic unit, strain and physiological state). It was concluded that HPP of food will not present any additional microbial or chemical food safety concerns when compared to other routinely applied treatments (e.g. pasteurisation). Pathogen reductions in milk/colostrum caused by the current HPP conditions applied by the industry are lower than those achieved by the legal requirements for thermal pasteurisation. However, HPP minimum requirements (P/t combinations) could be identified to achieve specific log10 reductions of relevant hazards based on performance criteria (PC) proposed by international standard agencies (5–8 log10 reductions). The most stringent HPP conditions used industrially (600 MPa, 6 min) would achieve the above‐mentioned PC, except for Staphylococcus aureus. Alkaline phosphatase (ALP), the endogenous milk enzyme that is widely used to verify adequate thermal pasteurisation of cows’ milk, is relatively pressure resistant and its use would be limited to that of an overprocessing indicator. Current data are not robust enough to support the proposal of an appropriate indicator to verify the efficacy of HPP under the current HPP conditions applied by the industry. Minimum HPP requirements to reduce Listeria monocytogenes levels by specific log10 reductions could be identified when HPP is applied to ready‐to‐eat (RTE) cooked meat products, but not for other types of RTE foods. These identified minimum requirements would result in the inactivation of other relevant pathogens (Salmonella and Escherichia coli) in these RTE foods to a similar or higher extent.
Keywords: High‐pressure processing, microbial inactivation, food, milk, ready‐to‐eat products, safety concern
Summary
Following a request from the European Commission, the Scientific Panel on Biological Hazards (BIOHAZ) was asked to provide a scientific opinion on the efficacy and safety of high‐pressure processing (HPP) of food. It was clarified that high‐pressure homogenisation and multipulsed HPP are out of scope of the assessment. HPP was considered a non‐thermal treatment (with product temperature < 45°C during the treatment) to be applied at different points in the food processing and preservation chain aiming to achieve microbial inactivation, particularly of pathogenic vegetative bacteria, to improve food safety.
In Term of Reference 1 (ToR1), EFSA was requested to assess the efficacy and microbiological and chemical safety of the use of HPP when applied to relevant foodstuffs. This ToR consisted of three subquestions.
ToR1a provides an overview of the foods to which HPP is or could be applied along with the processing conditions (e.g. pressure, time, temperature). The food categories/foods to increase microbiological food safety were considered, placing the focus on those foods that are being commercially subjected to HPP in the EU. A literature search and questionnaire were used. The latter consisted of questions related to the products being treated using HPP along with their processing conditions (for establishments) as well as the relative importance and recommendations for HPP (for equipment providers). Competent authorities (CA) were asked to provide information about evaluations of the impact of HPP on food safety. It was concluded that almost all types of food can be treated with HPP, although low moisture food is not usually treated with this technology due to low microbial inactivation when the water content is below 40%. The relative importance of the food type being treated with HPP in comparison to other food types for which HPP is used was ranked based on the information obtained from the questionnaire. Within the industrial context, pressures of between 400 and 600 MPa are most often applied for microbial inactivation, with common holding times ranging from 1.5 to 6 min.
ToR1b focused on listing the food intrinsic and extrinsic factors that may influence the efficacy of HPP. Only the impact in terms of reduction (log10 units) of vegetative microorganisms has been covered, as non‐thermal HPP treatment does not inactivate spores. A literature search was conducted, and it was concluded that the main intrinsic (i.e. food related) factors that influence the efficacy of HPP of foodstuffs in terms of microbial reduction of vegetative microorganisms are the water activity (aw) and pH of the food. The main extrinsic (i.e. processing related) factors are the target pressure and the holding time. The type of microorganisms, taxonomic unit and strain and the physiological state of the microorganisms to be inactivated also affect the efficacy of HPP. The efficacy of HPP in different food matrices is variable due to the interactions between the intrinsic factors which makes it difficult to predict the efficacy of HPP in a complex food matrix thereby necessitating validation in real foods.
ToR1c required an evaluation of the potential chemical and microbiological food safety risks in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods with the purpose to increase microbiological food safety, if any. The microbiological food safety risks are referring to a physiological, biochemical or genetic effect on a pathogen that could result in an increased risk (e.g. potential activation of spores or prion infectivity), compared to other treatments routinely applied to these foods (e.g. thermal pasteurisation). The potential chemical food safety concerns addressed include the formation of process contaminants and food contact materials (FCM). The whole duration of the shelf‐life of the foods was considered. Information provided through the questionnaire, related to the awareness of any food safety problems originating from food subjected to HPP, was considered together with information retrieved using specific literature searches. It is judged 99–100% certain (almost certain), that HPP of food will not present any additional microbial food safety concerns to consumers when compared to other treatments routinely applied to these foods. It is judged, with more than 95% certainty, that mycotoxins and process contaminants evaluated in the scientific opinion will not present an increased concern due to HPP‐treated food intake compared to conventional food. It was also concluded that the use of HPP does not give rise to additional chemical food safety concerns from FCM in HPP‐treated food compared to food treated under similar temperature and time (T/t) conditions without HPP.
In ToR2, EFSA was requested to assess the efficacy of HPP when applied to raw milk and raw colostrum from ruminants. It was clarified that raw milk and colostrum from ruminants refer to cow, sheep, goat, camel and/or buffalo. This ToR consisted of three subquestions.
ToR2a focused on recommending minimum requirements as regards time and pressure of the HPP, and other factors if relevant, for the control of Mycobacterium spp., Brucella spp., Listeria monocytogenes, Salmonella spp. and Shiga toxin‐producing Escherichia coli (STEC), to achieve an equivalent efficacy to that of pasteurisation. The requestor clarified that other relevant pathogens may be added. Based on a previous scientific opinion (EFSA BIOHAZ Panel, 2015) and using food‐borne outbreak (FBO) data, it was concluded that the relevant additional hazards to be reduced by thermal pasteurisation of raw milk/colostrum from ruminants are Campylobacter spp., tick‐borne encephalitis virus (TBEV) and Staphylococcus aureus. The end point in the assessment is the raw milk/colostrum for direct human consumption and the minimum T/t requirements for thermal pasteurisation of milk according to EU legislation (i.e. at least 72°C for 15 s, at least 63°C for 30 min or equivalent) were applicable as reference condition. Thermal pasteurisation of milk according to these legal requirements is expected to result in more than 10 log10 reductions of most of the pathogens (i.e. STEC, L. monocytogenes, Salmonella spp., S. aureus and Campylobacter spp.), while lower reductions are expected for Brucella spp. and M. bovis (using Mycobacterium avium subsp. paratuberculosis (MAP) as surrogate) and even lower for TBEV for which there is a significant lack of data. HPP cannot achieve equivalent log10 reductions to those achieved by thermal pasteurisation of milk according to these legal requirements, but target pressure–holding time (P/t) combinations can be identified that achieve lower log10 reductions (i.e. 5, 6, 7 and 8 log10 reductions) as pasteurisation performance criteria (PC) recommended by international agencies. For STEC, L. monocytogenes, Salmonella spp., S. aureus and Campylobacter spp., it is judged 99–100% certain (almost certain) that the PC of 8 log10 reduction is achieved using thermal pasteurisation of raw milk and by HPP treatment of raw milk/colostrum by using defined P/t combinations. For example, by using 600 MPa – 8 min, 550 MPa – 10 min and 500 MPa – 15 min for S. aureus, the most HPP resistant of these biological hazards. For M. bovis, it is judged 95–99% certain (extremely likely) that the PC of 5 log10 reduction is met using thermal pasteurisation of milk. This 5 log10 reduction can be achieved with 99–100% certainty (almost certain) by HPP treatment of raw milk/colostrum using, e.g. 600 MPa – 2.5 min, 550 MPa – 4.5 min and 500 MPa – 7.5 min. For B. melitensis and TBEV, minimum HPP requirements could not be set due to the lack of data. The most stringent HPP condition currently used industrially (600 MPa for 6 min), based on the information collected, would achieve the PC (i.e. 5 logs for M. bovis and 8 logs for S. aureus, STEC, L. monocytogenes, Salmonella spp. and Campylobacter spp.), except for S. aureus as this HPP condition would achieve 6 log10 reductions.
ToR2b gives an overview of appropriate indicators to verify the efficacy of HPP, either as part of the validation and verification in the HPP facility and/or in the end‐product on the market. A literature search was conducted, and the screening considered whether the record contained information about the effect of HPP on an inherent milk/colostrum component that could serve as an indicator for pasteurisation. Raw milk/colostrum of all animal and human milk was considered, although the focus was on ruminant milk/colostrum. It was concluded that alkaline phosphatase, the endogenous milk enzyme that is widely used to verify adequate thermal pasteurisation of cows’ milk, is relative pressure resistant and its use would be limited to that of an overprocessing indicator, while inactivation of other milk enzymes (γ‐glutamyltransferase or xanthine oxidase) or denaturation of some whey proteins (β‐lactoglobulin, lactoferrin) would occur at HPP processing conditions closer to those necessary for the 5 log10 inactivation of S. aureus. Nevertheless, based on available evidence, it is judged 90–95% certain (very likely) that none of the evaluated indicators can currently be proposed as an appropriate indicator to be used under the technologically and commercially feasible HPP conditions applied by the industry (400 and 600 MPa for 1.5–6 min).
ToR2c provides a comparative assessment of the risk to human health that could derive from the consumption of HPP‐treated vs. raw vs. pasteurised vs. ultra‐high temperature (UHT)‐treated milk or colostrum. A comparative assessment was undertaken of the relative levels of exposure to selected pathogens from the consumption of the four types of milk, for those pathogens for which it was possible to estimate the log10 reductions resulting from both HPP and thermal treatments. A model was used to estimate the level of contamination in a serving of 250 mL of milk and the probability of a serving being contaminated (carrying at least one CFU), immediately after treatment. The batch to be treated was considered to be contaminated, although, for each pathogen, scenarios of fixed theoretical levels of initial contamination ranging from 1 to 8 log10 CFU/mL were assumed. It was concluded that even the most stringent HPP condition used industrially (600 MPa for 6 min) would lead to less log10 reductions compared to UHT milk (considered to achieve 12 log10 reductions), except for Campylobacter spp. The impact of these reductions on the relative levels of exposure depend on the initial contamination levels. Even the least stringent HPP condition used industrially (450 MPa for 5 min) leads to a lower probability of exposure to contaminated servings to all pathogens than raw milk. Comparative assessments could not be made for pathogens in colostrum and for Brucella spp. or TBEV in milk due to lack of HPP and/or thermal inactivation data. When comparing to thermally pasteurised milk, considering the minimum and maximum PC recommended by international agencies (i.e. 5 and 8 log10 reductions), the HPP conditions assessed (500 MPa – 5 min, 600 MPa – 3 min or 600 MPa – 6 min) as well as the initial contamination levels of milk have an impact on the outcome of the comparative exposure assessment. For all relevant pathogens, except for S. aureus, present in milk at initial contamination levels below 5 log10 CFU/mL, the most stringent HPP condition used industrially would lead to such high log reduction that more than 99 out of 100 servings would not be contaminated. The public health significance of a small number of surviving bacteria after HPP could not be estimated due to lack of data.
In ToR3, EFSA was requested to assess the efficacy of HPP when applied to foods known to cause human listeriosis. First, the ready‐to‐eat (RTE) foods were identified considering those known to be associated with human listeriosis in the EU based on previous scientific opinions (EFSA BIOHAZ Panel, 2018, 2020b) and FBO data, and relevant to be treated by HPP. The RTE foods identified were within the categories of cooked meat products, soft and semi‐soft cheese, fresh cheese and smoked or gravad fish. Frozen vegetables are currently not treated with HPP because of its detrimental effects on the structure of the product.
Furthermore, this ToR consisted of two subquestions.
ToR3a focused on recommending minimum requirements as regards of time and pressure of the HPP, and other factors if relevant, to reduce significantly L. monocytogenes levels and assuming that the parameters influencing the growth of L. monocytogenes remain unchanged. The minimum HPP requirements to reduce L. monocytogenes in the selected RTE food categories were estimated through the calculation of the equivalent P/t combinations required for achieving a target log10 reduction, in the form of isoreduction plots. The model developed by Santillana Farakos and Zwietering (2011) including pressure and time was used. This model was built through a meta‐analysis of literature data for Listeria spp. in various food treated at 200–700 MPa. Minimum requirements to reduce L. monocytogenes levels by specific log10 reductions could be identified when HPP is applied to RTE cooked meat products. For example, a reduction of more than 2 log10 could be achieved on RTE cooked meat products applying 600 MPa‐2.3 min, 550 MPa‐3.4 min and 500 MPa‐5.0 min while more than 5 log10 reductions could be achieved by extending the holding times and by applying 600 MPa‐4.7 min, 550 MPa‐6.9 min and 500 MPa‐10.1 min. For the other types of RTE foods, generic minimum HPP requirements could not be set and specific validation studies following international guidelines are needed for each specific food.
ToR3b required an assessment of the efficacy on other relevant pathogens when applying the identified minimum requirements. From FBO data, Salmonella spp. and pathogenic E. coli were identified as the most important relevant hazards (i.e. apart from L. monocytogenes), in the above listed foods known to be associated with human listeriosis. The microbial reduction for these other two pathogens was assessed from data gathered through a literature search. It was concluded that, in the RTE foods considered, these pathogens are generally more sensitive to HPP than L. monocytogenes and it is judged 66–90% certain (likely) they will be inactivated to a similar or higher extent than L. monocytogenes.
It is recommended that future research on the impact of intrinsic factors on the efficacy of HPP treatments should consider interactions of different components and these studies should be undertaken in real food matrices. Further, it is recommended to perform an in‐depth analysis on the effect of HPP treatments on inherent compounds in milk or colostrum for which a significant effect is found at P/t combinations intended to be used for pasteurisation. Studies on pathogen behaviour in milk and colostrum during storage after HPP should be performed. Further research is required on HPP inactivation of L. monocytogenes and other relevant pathogenic bacteria for RTE foods (as smoked and gravad fish and soft/semi‐soft cheese) from a quantitative perspective which could facilitate the construction of a suitable predictive model to set the generic minimum requirements for HPP to assure the food safety of these food products.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
High pressure processing (HPP), also referred as high‐hydrostatic pressure processing (HHP) or ultra‐high‐pressure processing (UHP) is a non‐thermal technique of food preservation that inactivates pathogens and vegetative spoilage organisms. HPP subjects liquid and solid foods usually to pressures of about 400 to 600 MPa at refrigeration or mild process temperatures (< 45°C). It has minimal effects on taste, texture, appearance or nutritional value (Muntean et al., 2016).
HPP is applied mainly to pre‐packed juices, sauces, dips, fishery products, meat products and ready‐to‐eat meals (RTE). There is also an increasing interest for the use of HPP of milk as an alternative for pasteurisation.
HPP is not specifically regulated at EU level. Overall food safety requirements such as good hygiene practices (GHP), procedures based on the Hazard Analysis and Critical Control Point (HACCP) principles, traceability and labelling requirements apply. HPP is considered as processing within the meaning of Regulation (EC) No 852/20041, and standalone establishments carrying out HPP on products of animal origin are subject to approval. Clarification on the implementation of hygiene requirements in case of HPP have been recently introduced under section 9.6 of the Guidance document on the implementation of certain provisions of that Regulation.2
The efficacy of HPP treatments will be dependent on the pressure applied, the holding time and temperature, the characteristics of the food and the target microorganism. Some possible food safety concerns have been described in scientific literature.
The European Commission funded research on HPP for example under the FP6 a project called “Hipster” and under FP7 a project called “High Tech Europe”, from which the internet portal is available at respectively https://hipster‐project.eu/ and https://www.foodtech‐portal.eu/index.php?title=Categorv:Technology_Sheet.
Apart from an overall assessment on the efficacy and safety of HPP of the food categories described above, it is appropriate to provide a more detailed assessment on the use of HPP for two specific purposes: as an alternative for pasteurisation and ultra‐high temperature (UHT) treatment of raw milk and raw colostrum, and for the control of Listeria monocytogenes in RTE foods.
When raw milk, colostrum, dairy or colostrum‐based products undergo heat treatment, such treatment must comply with the requirements in point II. 1 of Chapter II to Section IX of Annex III to Regulation (EC) No 853/20043. Specifically, these products need to be pasteurised achieving certain requirements (i.e. at least at 72°C for 15 s or at least at 63°C for 30 min or any other combination of temperature‐time (T/t) conditions to obtain an equivalent effect) or be subjected to UHT treatment. There is an increasing demand to allow HPP as an alternative treatment because it is expected to keep the properties closer to those of raw milk and colostrum.
L. monocytogenes contamination of RTE foods continues to be of public health concern in the EU. This was illustrated by EFSA in its recent scientific opinion on the risks to public health from consumption of RTE foods contaminated with L. monocytogenes.4 An increasing trend was found of the notified incidence rate of confirmed human invasive listeriosis cases in the EU/EEA over 2008‐2015 for the elderly (> 75 years old) and females between 25 and 44 years old (probably related to pregnancies). This increase likely resulted from an increased proportion of persons with underlying health conditions but may also have been caused by the rise in consumption of RTE foods and an improved surveillance in some Member States. The RTE foods typically associated with human listeriosis, such as smoked fish, heat‐treated meat and soft and semi‐soft cheese, continue to be of public health significance. However, yet unconsidered RTE food categories of plant‐derived origin, under certain conditions, can also support growth and have the potential to contribute to the burden of disease. The EU summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 20185 and a recent L. monocytogenes outbreak due to frozen vegetables6 seem to confirm these conclusions. An ongoing EFSA opinion on L. monocytogenes in frozen fruit, vegetables and herbs, blanched during processing,7 primarily addresses control options during processing and consumption.
EFSA is asked to deliver a scientific opinion on the efficacy (reduction of the levels of food‐borne pathogens) and safety of HPP of food. Quality issues and organoleptic properties are not part of this mandate.
More specifically, EFSA is asked:
ToR1. To assess the efficacy and microbiological and chemical safety of the use of HPP when applied to relevant foodstuffs, and in particular:
To provide an overview of the foods to which HPP is or could be applied along with the processing conditions (e.g. pressure, time, temperature).
To list the intrinsic and extrinsic factors that may influence the efficacy of HPP.
To evaluate the potential chemical and microbiological food safety risks in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods with the purpose to increase microbiological food safety, if any (e.g. pasteurisation of juices).
ToR2. To assess the efficacy of HPP when applied to raw milk and raw colostrum from ruminants, and in particular:
To recommend minimum requirements as regards time and pressure of the HPP, and other factors if relevant, for the control of Mycobacterium spp., Brucella spp., L. monocytogenes, Salmonella spp. and Shiga toxin‐producing Escherichia coli (STEC), to achieve an equivalent efficacy to that of pasteurisation;
To propose appropriate indicators to verify the efficacy of HPP, either as part of the validation and verification in the HPP facility and/or in the end‐product on the market;
If data allow, to provide a comparative assessment of the risk to human health that could derive from the consumption of HPP‐treated vs. raw vs. pasteurised vs. UHT‐treated milk or colostrum.
ToR3. To assess the efficacy of HPP when applied to foods known to cause human listeriosis (e.g. RTE smoked or gravid fish, soft and semi‐soft cheese and cooked meat products and (blanched) frozen vegetables such as peas or corn that are consumed without prior cooking) and in particular:
To recommend minimum requirements as regards time and pressure of the HPP, and other factors if relevant, to reduce significantly L. monocytogenes levels (e.g. by a certain log reduction or reduction of the probability of illness per serving), and assuming that the parameters influencing the growth of L. monocytogenes remain unchanged (e.g. shelf‐life and storage conditions);
To assess the efficacy on other relevant pathogens when applying the minimum requirements identified in a.
1.2. Interpretation of the Terms of Reference
HPP is a non‐thermal treatment in which foods are subjected to isostatic pressures. Traditionally, this is an in‐batch process that can be applied to both solid and liquid prepacked foods. Pressure is transmitted rapidly and uniformly in an isostatic manner so that all parts of the food are subjected to the same pressure simultaneously. It was clarified with the requestor that high‐pressure homogenisation (HPH; also called dynamic high‐pressure homogenisation, dynamic‐HPH) is out of the scope of the assessment. HPH is based on the same principles as the homogenisation process used in the dairy industry for reducing the size of fat globules, but it works at higher pressures (100–400 MPa). Not only pressure, but the combined action of other physical effects (such as cavitation, shear stress, turbulence, impingement and temperature, that may increase due to friction) causes inactivation of vegetative microorganisms (Patrignani and Lanciotti, 2016).
Multipulsed HPP (mpHPP) treatment, with few exceptions, is more effective than the classical or single‐pulsed HPP (spHPP) treatment for inactivation of microorganisms in fruit juice, dairy products, liquid whole egg, meat products and sea foods (Buzrul, 2015). However, it is not likely to be applied commercially because it is a longer application and may significantly increase the wear and tear of the equipment and reduce the quality of the food (Buzrul, 2015; Colussi et al., 2018; Balakrishna et al., 2020), and for this reason, it is outside the scope of this assessment.
This scientific opinion will consider the HPP as a non‐thermal treatment and will not consider treatments causing an increase in product temperature above 45°C. Depending on the food composition, the temperature increase of the product might range from 2–3°C/100 MPa (in aqueous phase) to 8.7–9.7°C/100 MPa (in oil phase) (Rasanayagam et al., 2003; Patazca et al., 2007). The compression heating, which causes a (quasi)adiabatic increase of temperature, is completely reversible upon pressure release, but the temperature of the product does not return to the initial value, due to the heat transfer within the high‐pressure chamber and temperature equilibration during holding time (particularly for longer pressure holding times) (Ghafoor et al., 2020). As such, applications are excluded using either heat‐assisted HPP (referred to in this document as high‐pressure thermal processing or HPTP) (Sevenich and Mathys, 2018) or HPP where the maximum temperature of the product during the treatment exceeds 45°C, in which microbial inactivation may result partly or entirely from thermal energy.
Apart from its common application as a preservation technology, HPP has been applied in many other food technology processes and in the development of new products. These include but are not limited to freezing, enzyme control, cold gelatinisation of starch, protein unfolding, shucking of shellfish, enhancing mass transfer phenomena and even for promoting microbial growth under mild processing conditions, thus enhancing fermentation processes and reducing fermenting time (Barba et al., 2015; Ghafoor et al., 2020). The processing parameters (i.e. level of pressure, temperature and holding time) differ according to the targeted applications (Ghafoor et al., 2020). Within the food industry context, the main reason to apply HPP to a food matrix is the non‐thermal inactivation of pathogenic and spoilage vegetative microorganisms (Sevenich and Mathys, 2018) to increase microbiological safety and microbial shelf‐life of the processed food with, in general, minimal impact on thermally sensitive compounds (e.g. nutrients and vitamins) and sensory attributes. This scientific opinion will focus on the use of HPP for microbial inactivation, particularly of pathogenic vegetative bacteria, with the aim of improving food safety. Quality issues and sensory properties, as well as nutritional aspects are out of scope.
HPP can be applied at different points in the food processing and preservation chain. It can be applied to intact or minimally processed raw materials or products (e.g. milk, fruit juices, smoothies, dips, sauces) and as a final lethal in‐package treatment to reduce the potential recontamination of the food after the primary lethal treatment (e.g. thermal pasteurisation, cooking), due to post‐process handling, e.g. slicing of cooked meat products, preparation of RTE meals, etc. This is usually referred to as post‐lethality treatment. When HPP is applied to achieve pathogen inactivation as a control measure, it has to be integrated within the HACCP plan, as such, the efficacy of HPP has to be validated by demonstrating that the HPP conditions applied achieve the target inactivation of the relevant microorganism in the specific food product.
For most of the conventional HPP applications, foods are packaged before treatment (in‐package HPP), which is a discontinuous batch‐based process. For liquid food, semicontinuous production processing can be applied using equipment with small to medium vessels working in parallel followed by aseptic packaging (bottling). The liquid food is then in direct contact with the equipment parts (piston, vessel, plugs and seals). More recently, the so‐called ‘in‐bulk HPP’ has been developed using a large vessel (similar to in‐pack HPP). In this case, liquid food is processed in a flexible plastic bag with a volume and size similar to the vessel, allowing no direct contact with the steel parts of the equipment, followed by ultra clean/aseptic packaging (Tonello‐Samson et al., 2020).
Specifically, for ToR1a, the food categories/foods that are treated with HPP worldwide with the purpose to increase microbiological food safety will be considered, placing the focus on those foods that are being commercially processed by HPP in the EU.
For ToR1b, as the non‐thermal HPP treatment will be considered, for evaluating the factors that may influence the efficacy of HPP, only the impact on vegetative microorganisms will be covered, as this treatment does not inactivate spores.
For ToR1c, the microbiological food safety risks are not those for which the efficacy (pathogen reduction) is being evaluated. Instead, it is referring to a physiological, biochemical or genetic effect on a pathogen that could result in an increased risk e.g. potential activation of spores (germination) or prion infectivity (due to conformational change of prion proteins), compared to the food that was not HPP treated, as opposed to the risk reduction that is normally the goal of HPP treatment. The potential concerns will be contextualised to account for the potential food safety risks that are not exclusive of HPP. The whole duration of the shelf‐life of the foods is to be considered in the assessment of the potential chemical and microbiological food safety risks.
For ToR2, raw milk and colostrum from ruminants refer to cow, sheep, goat, camel and/or buffalo. For ToR2a, other relevant pathogens may be added, e.g. Campylobacter. The end point in the assessment is the raw milk or colostrum for direct human consumption. Its further use for other dairy products (e.g. for cheese or yoghurt production) is out of the scope of this scientific opinion. The efficacy of the processing conditions is of relevance (not the post‐processing contamination) considering the minimum time and temperature requirements for thermal pasteurisation of milk from legislation are applicable (as reference condition) to recommend minimum requirements as regards time and pressure of the HPP for the control of the relevant pathogens. UHT treatments will be considered only in ToR2c, where a comparative assessment will be undertaken of the relative levels of exposure to selected pathogens [or probability of illness, if data allow] from the consumption of pasteurised, UHT or HPP‐treated milk or colostrum.
For ToR3, the requestor clarified that the assessment should focus on ‘foods known to cause human listeriosis in the EU’, not on foods that could potentially cause listeriosis (based on the risk factors associated with the processing conditions, exposure to contamination, growth supporting characteristics, etc.) but have no recorded cases/outbreaks in the EU to date.
The ToRs have been translated into assessment questions (AQs). These are for ToR1 as follows:
AQ1/What are the (broad) food categories for which there is evidence that HPP could in principle be applied with the purpose of increasing microbiological food safety, focusing on those foods that are being commercially processed by HPP? What are the processing conditions (e.g. pressure, time, temperature) and packaging applied by the industry?
AQ2/What are the intrinsic (i.e. food‐related) and extrinsic (i.e. processing‐related) factors that may influence the efficacy of HPP in terms of reduction (log10 units) of vegetative microorganisms when applied to foodstuffs?
AQ3/What are the potential microbiological food safety concerns in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods, with the purpose of increasing microbiological food safety (e.g. thermal pasteurisation of milk)?
AQ4/What are the potential chemical food safety concerns through formation of process contaminants in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods with the purpose of increasing microbiological food safety?
AQ5/What are the potential chemical food safety concerns through food contact materials (FCM) in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods with the purpose of increasing microbiological food safety?
For ToR2, the AQs have been formulated as:
AQ6/What log10 reduction of Mycobacterium spp., Brucella spp., L. monocytogenes, Salmonella spp. and STEC (or other relevant vegetative pathogens) is achieved by thermal pasteurisation of raw milk and raw colostrum from ruminants according to the legal requirements?
AQ7/What are the minimum requirements of HPP (i.e. time and pressure and any other relevant factor) of raw milk and raw colostrum from ruminants to achieve an equivalent efficacy (in terms of log10 reduction) to that of thermal pasteurisation for the control of the pathogens deemed relevant according to AQ6?
AQ8/Which inherent components of the milk or colostrum could be used as appropriate indicators to verify the efficacy of HPP of raw milk and raw colostrum from ruminants, either as part of the validation and verification immediately after such treatment (e.g. in the processing plant) and/or in the end‐product on the market, considering the minimum requirements as defined in AQ7?
AQ9/What are the relative levels of exposure [or probability of illness, if data allow] for the pathogen(s) to be defined per serving through the consumption of industrially HPP‐treated milk or colostrum in comparison to raw vs. thermally pasteurised vs. UHT‐treated milk or colostrum [according to the legal requirements] [considering that the batch to be treated is contaminated]?
For ToR3, the AQs have been formulated as:
AQ10/What are the minimum requirements of HPP (i.e. time and pressure and any other relevant factor) when applied to the food categories known to be associated with human listeriosis to reduce significantly L. monocytogenes levels by specific log10 reductions, assuming that the parameters influencing the subsequent growth of L. monocytogenes remain unchanged (e.g. product characteristics, shelf‐life and storage conditions)?
AQ11/What is the efficacy (log10 reduction) on other relevant pathogens when applying the minimum requirements of HPP identified in AQ10?
1.3. Additional information
1.3.1. Approach to answer the ToRs
The approach to answer the ToRs was defined in advance and is described in the protocol (Annex A). It covers both the problem formulation (i.e. what the assessment aims to address) and which methods will be used for addressing the problem. The problem formulation (‘what’) includes the clarification of the mandate (see further refined in Section 1.2) and consists of the steps (1) translation of the mandate into scientifically answerable AQs, (2) definition of the subquestions (SQs) of each AQ and their relationship (conceptual model) and (3) the selection of the approach for the assessment. The planning of the methods for conducting the assessment (‘how’) consists of (1) specifying the evidence needs and the methods for answering each SQ, including uncertainty analysis and (2) the methods for integrating evidence across SQ and addressing of the remaining and overall uncertainty. Protocol development followed the draft framework for protocol development for EFSA’s scientific assessments (EFSA, 2020).
The SQs can be found below; their relationship can be found in the protocol.
SQ1 (for AQ1)/What are the (broad) food categories for which there is evidence that HPP could in principle be applied with the purpose of increasing microbiological food safety, focusing on those foods that are being commercially processed by HPP?
SQ2 (for AQ1)/What are the processing conditions (e.g. pressure, time, temperature) and packaging applied by industry?
SQ3 (for AQ2)/What are the intrinsic (e.g. food‐related) and extrinsic (e.g. processing‐related) factors that may influence the efficacy of HPP in terms of reduction (log10 units) of vegetative microorganisms when applied to foodstuffs?
SQ4 (for AQ3)/What are the potential microbiological food safety concerns in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods with the purpose to increase microbiological food safety?
SQ5 (for AQ4)/What is the effect of HPP‐treated foods, if any, on the levels of specific contaminants compared to untreated or conventionally treated foods?
SQ6 (for AQ4)/What would be the contributions of these levels to the total exposure to the specific contaminants?
SQ7 (for AQ5)/What is the effect of HPP, if any, on the migration potential (including diffusivity and partitioning effects) of FCM substances during the treatment, compared to migration from the FCM with the same food/simulant, t/T/surface area (SA), etc. but without HPP?
SQ8 (for AQ5)/What is the chemical effect of HPP, if any, during the treatment on the number and nature of reaction/degradation products (non‐intentionally added substances, NIAS) in/from the FCM?
SQ9 (for AQ5)/What is the effect, if any, on the morphology, physical and chemical properties of the treated FCM (‘permanent’ change) that may influence migration to the food after the HPP treatment?
SQ10 (for AQ5)/Does HPP affect the characteristics of the food and so impact its potential to elicit migration?
SQ11 (for AQ6)/What are the relevant pathogens to be reduced by thermal pasteurisation of raw milk and raw colostrum from ruminants?
SQ12 (for AQ6)/What are the thermal inactivation parameters for the control of the pathogens deemed relevant according to SQ11 in raw milk and raw colostrum from ruminants?
SQ13 (for AQ6)/What log10 reduction of the pathogens deemed relevant according to SQ11 is achieved by thermal pasteurisation of raw milk and raw colostrum from ruminants using the minimum legal requirements?
SQ14 (for AQ7)/What are the relevant factors that affect the efficacy of HPP (in terms of log10 reduction of the pathogens deemed relevant according to SQ11) of raw milk and raw colostrum from ruminants?
SQ15 (for AQ7)/What is the most resistant pathogen considering the pathogens deemed relevant according to SQ11 when treating raw milk and raw colostrum from ruminants using HPP?
SQ16 (for AQ7)/What are the minimum requirements of HPP (relevant factors from SQ14) of raw milk and raw colostrum from ruminants to achieve an equivalent efficacy to that of thermal pasteurisation for the control of the pathogens deemed relevant according to SQ11?
SQ17 (for AQ8)/Which inherent components of the milk or colostrum could be used as appropriate indicators to verify the efficacy of HPP of raw milk and raw colostrum from ruminants, either as part of the validation and verification immediately after such treatment (e.g. in the processing plant) and/or in the end‐product on the market, considering the minimum requirements as defined in SQ16?
SQ18 (for AQ9)/What are the relative levels of exposure [or probability of illness, if data allow] for the pathogen(s) to be defined per serving through the consumption of industrially HPP‐treated milk or colostrum [conditions as being used by industry from SQ2] in comparison to raw vs. thermally pasteurised vs. UHT‐treated milk or colostrum [according to the minimum legal requirements] [considering that the batch to be treated is contaminated]?
SQ19 (for AQ10)/What are the most relevant foods known to be associated with human listeriosis in the EU and that are relevant to be treated with HPP (i.e. there is evidence of use)?
SQ20 (for AQ10)/What are the relevant factors that affect the efficacy of HPP (in terms of log10 reduction of L. monocytogenes) in the RTE foods identified in SQ19?
SQ21 (for AQ10)/What are the minimum requirements of HPP (e.g. time, pressure) according to the relevant factors related to food (from SQ20) when applied to the foods identified in SQ19 to reduce significantly L. monocytogenes levels by specific log10 reductions, assuming that the parameters influencing the subsequent growth of L. monocytogenes remain unchanged (e.g. product characteristics, shelf‐life and storage conditions)?
SQ22 (for AQ11)/ What are the other relevant pathogens (apart from L. monocytogenes) in the foods identified in SQ19?
SQ23 (for AQ11)/ What is the efficacy (log10 reduction) on other relevant pathogens identified in SQ22 when applying the minimum requirements of HPP identified in SQ21 according to the relevant factors related to food (from SQ20)?
2. Data and methodologies
2.1. Questionnaire on the commercial use of HPP of foods
A questionnaire on the commercial use of HPP in foods with the purpose to increase microbiological food safety was sent on 24 November 2020 through the European Commission WG on food hygiene to the competent authorities (CA) in each Member State (MS). Part of the questionnaire was to be filled by the CA and they were requested to forward the other part of the questionnaire to the establishments using HPP in their country. The questionnaire addressed to establishments has also been shared for information with the members of the Advisory Group on the Food Chain and Animal and Plant Health (a body gathering representatives of stakeholders) and the EFSA stakeholders. The questionnaire for the equipment providers has been sent to Hiperbaric, JBT‐Avure Technologies, Multivac/Thyssenkrupp – Uhde High Pressure Technologies, and Stansted Fluid Power on 11 December 2020.
The questionnaires can be found in Appendix A, together with a summary of the replies. It consists of general questions related to the products being treated using HPP along with their processing conditions (for establishments) as well as relative importance and general recommendations for HPP (for equipment providers), and the validation process of the HPP treatment (for establishments and equipment providers). CA were also asked to provide information about past and current evaluation of the impact of HPP on food safety. A question was also included on expected important technological changes in the near future (for CA, establishments and equipment providers) and in‐bulk treatment using a batch system (for equipment providers).
A specific question was included on the food microbiology/hygiene and asked about the awareness of any microbiological food safety problems originating from food subjected to HPP (for CA, establishments and equipment providers).
Also, specific questions related to food contaminants and FCM were included (for CA, establishments and equipment providers), further detailed in Sections 2.2.4 and 2.2.5, respectively.
2.2. Efficacy and microbiological and chemical safety of the use of HPP when applied to relevant foodstuffs
2.2.1. Overview of the food categories to which HPP is being applied for food safety reasons
The strategy for conducting the literature searches and screening is provided in Appendix B. A general search was conducted in Web of ScienceTM Core Collection (2010–present) as described in Appendix B.1 to retrieve review papers, book sections and books summarising information about the food products being treated with HPP worldwide with the purpose of increasing microbiological food safety. As clarified in the ToR, this scientific opinion only considers the HPP as a non‐thermal treatment. Therefore, the search excluded all the studies focused on heat‐assisted HPP or using HPP with an initial fluid temperature such that, due to pressure‐associated temperature increase, the maximum temperature of the product during the treatment exceeds 45°C.
The records were screened for information about the use of HPP of food products only with the aim of increasing food safety in three steps: screening of (1) titles, (2) abstracts and (3) full‐text documents to further identify records to be excluded based on criteria related to report and study characteristics considering whether the record contains info about the use of HPP of food products only to improve food safety. Selected full‐text documents were screened to extract the relevant information needed to answer AQ1.
Replies to the general questions provided through the questionnaire were also considered and summarised (see Section 2.1) to provide an overview of the food categories to which HPP is being applied for food safety reasons along with the processing conditions used by industry.
2.2.2. Food intrinsic and extrinsic factors that may influence the efficacy of HPP
The records retrieved as described in Section 2.2.1 were screened in two steps for relevant information on HPP inactivation of microorganisms for bacteria considering the vegetative form only (as non‐thermal HPP does not inactivate spores): screening of title and abstract (Ti/Ab) and further at full text level. The reference lists of these documents were further screened for additional relevant information. Studies were considered eligible when they fulfilled the following criteria: (i) report inactivation of above‐mentioned microorganisms by HPP at temperatures < 45°C, (ii) deal with bacterial pathogens or non‐pathogens, since their inactivation may be influenced by the same factors, (iii) be conducted in food or in laboratory media.
2.2.3. Potential microbiological food safety concerns in HPP‐treated food
Information provided through the questionnaire related to the awareness of any microbiological food safety problems originated from food subjected to HPP was considered (see Section 2.1). The following examples were provided: spore activation leading to more rapid and more complete germination and outgrowth, prion activation by conversion of normal form to amyloid form and the induction of virulence or of toxin gene expression.
With respect to the literature search of relevant information, eligible studies should report on microbiological concerns which are generated or increased specifically by HPP treatment. They should fulfil the following criteria: (i) deal with the effect of HPP on food‐borne pathogens including vegetative and/or spore‐forming bacteria, viruses, mycotoxin‐producing moulds, protozoa or prions, (ii) report an increased or new risk, or a physiological, biochemical or genetic effect on a pathogen that could result in an increased risk, compared to the food that was not HPP treated, as opposed to the risk reduction that is normally the goal of HPP treatment. A two‐step strategy was followed to retrieve relevant studies: (i) the records from Section 2.2.1 were screened first using the Ti/Ab, and then at full‐text level to identify specific HPP‐associated concerns.
Subsequently, a targeted search was conducted with specific search strings for each specific concern identified as specified in Appendix B.2: (i) focusing on spore activation/germination with HPP (97 records), (ii) related to prions (38 records) and (iii) induction of virulence and toxin gene expression (25 records). With respect to records about these specific searches, the results were narrowed down in several steps: (i) records meeting the eligibility criteria based on Ti/Ab were first selected, and then (ii) full papers were further screened.
2.2.4. Potential chemical food safety concerns in HPP‐treated food through formation of process contaminants
An extensive literature search has been performed with the aim to retrieve information reporting or reviewing chemical changes related to contaminants during the HPP compared to non‐treated or conventional processes. To search for different types of publications providing information on chemical contaminants during HPP, two databases were used; search queries used in individual databases and the subsequent screening to retrieve potentially relevant studies can be found in Appendix B.3. The records were screened for relevance in two levels: (i) The retrieved articles were screened first at Ti/Ab level for information on chemical substances that are modified or produced due to HPP and (ii) the selected records were screened at the full‐text level for information that is related to contaminants.
In addition, in the questionnaires (see Section 2.1), a question on the awareness of studies on the effect of HPP on the potential formation or degradation/modification of contaminants in foods during and after HPP treatment was included.
2.2.5. Potential chemical food safety concerns in HPP‐treated food through food contact materials
In order to retrieve information on the effect of HPP on the packaging and ultimately on the migration potential of substances from packaging to food or food simulants, (i) questionnaires were developed and sent to the CA, the establishments using HPP and the equipment providers (see Section 2.1) as well as to the EFSA FIP FCM Network, (ii) expert knowledge from a representative of the US Food and Drug Administration (US‐FDA) was considered through a technical hearing held on 14 October 2020 and (iii) a general literature search was conducted.
In the questionnaires, questions specific to FCM were included such as special considerations or technical requirements on the properties of FCM, awareness of studies on the effect of HPP on FCM including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment, and whether there are types of foods for which HPP has an adverse effect or for which HPP is not suitable or recommended.
The literature was searched in two databases, covering the period from 1990 to 9 October 2020 as described in Appendix B.4. It considered English primary articles. Reviews, books and conference proceedings were also included, but no conclusions were drawn from them, without recourse to an evaluation of the original papers (without applying the 1990 cut‐off date).
The selection for inclusion/exclusion of studies was performed based on a three‐step strategy. First, the identified records were screened by title and abstract and classified according to a conceptual framework of the factors that determine the identity and quantity of chemicals migrating from FCM. Second, the selected records were screened as full text to further identify records to be included/excluded based on criteria related to report characteristics and study characteristics. Third, the included records were evaluated to address the main question: ‘What is the effect of HPP treatment on the food packaging and ultimately on chemical migration to food?’.
2.3. Efficacy of HPP when applied to raw milk and raw colostrum from ruminants
2.3.1. Minimum HPP requirements for the control of pathogens in raw milk and raw colostrum to achieve an equivalent efficacy to thermal pasteurisation
2.3.1.1. Additional hazards to be reduced by thermal pasteurisation of raw milk and raw colostrum from ruminants
The previous scientific opinion by the BIOHAZ panel on the public health risks related to the consumption of raw drinking milk (EFSA BIOHAZ Panel, 2015) was consulted for the selection of the hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants. Data on ‘strong and weak’ evidence food‐borne outbreaks (FBOs) at EU/EEA level from 2008 to 2019 implicating milk as the food vehicle (i.e. the food (or foodstuff) that is suspected of causing human cases) were extracted from the EFSA zoonoses database and the available epidemiological evidence summarised, including the causative agent and number of outbreaks. Further information in other data elements, such as ‘more food vehicle information (fboVehicleInfo)’ and ‘contributory factors (fboFactor)’, was consulted, when available (reported as free text). More information about the reporting on FBOs can be found in the technical report titled ‘Zoonoses, antimicrobial resistance and food‐borne outbreaks guidance for reporting 2020 data’ (EFSA, 2021b).
2.3.1.2. Relevant factors to describe the requirements of HPP of raw milk and raw colostrum from ruminants
A literature search was performed to identify the relevant factors determining the effect of HPP in raw milk and raw colostrum from ruminants. The search is described in Appendix B.5 and the screening process was undertaken in two steps: screening of (1) Ti/Ab and (2) full text to further identify records to be excluded based on criteria related to report characteristics (e.g. not in English) and study characteristics. The search also included literature reviews using the same string as described in Appendix B.1 as well as relevant references from WG member’s knowledge. A record was excluded when the study dealt with laboratory media experiments, unless it could help in identifying (qualitatively) a relevant factor. A study was considered eligible when:
included a challenge test, e.g. the pathogen was inoculated to a target concentration and under controlled conditions;
done in milk or colostrum, for which, experimental information (preferably from quantitative perspective) such as the following is provided: strain and its physiological state, the origin (species) of raw milk/colostrum and related factors (e.g. fat and antimicrobial compounds) and HPP technological conditions; and
provided inactivation data such as log10 reduction, kinetic parameter (e.g. inactivation rate) and/or mathematical model(s) about log10 reduction or inactivation rate.
Data were extracted using predefined tables. Besides raw milk/colostrum‐related factors and HPP technological conditions, other relevant issues were collected when available: microbial strain (and conditions for preparing the inoculation culture), and analysis (method and media, time and conditions after HPP that may overcome sublethal damage with a consequent overestimation of the efficacy of the treatment).
2.3.1.3. Most resistant pathogen when treating raw milk and raw colostrum from ruminants using HPP
The most resistant pathogens to be considered when treating raw milk/colostrum from ruminants using HPP were identified by means of a literature review aimed at capturing the pathogen‐specific log10 reductions in comparable conditions (i.e. time/pressure, media), scientific evidence in terms of log10 reductions (ideally the decimal reduction time at a given target pressure, Dp‐value) of pathogens when raw milk or previously heat‐treated milk is treated by using HPP.
The search is described in Section 2.3.1.2. It included records of milk from other species should limited data be available when considering only cows’ milk. After preliminary screening of Ti/Ab, studies were retained if the pathogen‐specific parameters were reported and were obtained from HPP treatment of milk/colostrum. An MS Excel spreadsheet containing the data used for modelling is made available through the Knowledge Junction under 10.5281/zenodo.5998538.
A global modelling approach, with a log‐linear (single inactivation phase) or a biphasic primary inactivation model (Cerf, 1977), both encompassing a Bigelow secondary model term for the impact of pressure on microbial inactivation, was fitted to the log10 reduction (logR), data extracted from the relevant literature studies.
The following inactivation models were used:
- Log‐linear model
(1) -
Biphasic model
(2) where: is the decimal reduction time or D‐value (min) at the reference pressure (Pref) of 600 MPa, zp is the change in pressure (MPa) required for 10‐fold change of the Dp‐value, f is the fraction (0–1) of the sensitive subpopulation and k1 and k2 the inactivation rates (i.e. ln(10)/D) of the sensitive and resistant to pressure subpopulations. Fitting of the equations was performed by means of nls regression in R version 4.0.5. (R Core Team, 2021).
The Root Mean Square Error (RMSE) and the % of predicted log10 reductions within and outside the Acceptable Prediction Zone (APZ) were used as the goodness‐of‐fit criteria. The APZ was defined as a range ± 1 log10 from the 100% accurately predicted value, i.e. the value that makes the difference between prediction and observation equal to 0.
The global modelling aimed to: (i) describe the combined effect of pressure (MPa) and holding time (min) on the inactivation (expressed as log10 reduction) of relevant hazards in raw milk and colostrum, (ii) assist in ranking the relevant hazards based on their resistance to pressure via plotting of the dependency of their log Dp‐values on pressure (MPa) and (iii) to calculate the equivalent pressure–holding time (P/t) combinations for achieving specific (target) log reduction of the (most resistant) hazard to pressure, in the form of isoreduction plots.
2.3.1.4. Minimum requirements of HPP of raw milk and raw colostrum from ruminants to achieve an equivalent efficacy to that of thermal pasteurisation
Data gathered from the studies retrieved from the search described in Section 2.3.1.2 were used to evaluate the HPP treatment conditions needed to achieve the equivalent log10 reduction of thermal pasteurisation. The aim was to identify the log10 reductions reached in raw milk/colostrum for a specific pathogen when different HPP processing conditions (P/t combinations) were applied.
In order to identify the HPP conditions resulting in an equivalent efficacy to that of thermal pasteurisation, performance criteria (PC) normally recommended by international agencies have been considered. This was done by first carrying out a literature review aimed at identifying the pathogen‐specific thermal inactivation parameters (DT and/or zT‐values and/or time to specific log10 reductions) or log10 reductions achieved by thermal pasteurisation (65°C for 30 min and 72°C for 15 s).
The strategy for conducting the literature searches and screening to retrieve records reporting those pathogen‐specific thermal inactivation parameters or log10 reductions in raw milk or colostrum is provided in Appendix B.6. A study was considered eligible for the evaluation of the impact of temperature on the relevant hazards (as outcome of Section 2.3.1.1) when it reported on the thermal treatment of milk or colostrum (from any species), considering that: (i) priority was given to cows’ milk but milk from other animal species was also considered if the evidence for some of the pathogens was scarce (population); (ii) a heat treatment needs to be conducted (intervention) and the thermal inactivation parameters and/or log10 reductions of the hazards reported (outcome); (iv): industrial, pilot or laboratory conditions were used (setting). When data for the estimation of the log10 reductions of the relevant pathogens in milk and colostrum were not available, evidence for surrogate microorganisms was used. This is the case for Mycobacterium avium subsp. paratuberculosis (MAP), which was considered adequate to serve as surrogate for M. bovis for the scope of this scientific opinion. Knowing that the resistance of MAP to thermal and HPP treatments is higher than M. bovis, the assessment based on MAP data could be considered as a worst‐case scenario. For STEC, the evidence was used considering the entire body of evidence for any E. coli strain subjected to HPP treatment.
When the log10 reductions were not reported, or reported for T/t combinations different from the minimum legal requirements of 63°C for 30 min or 72°C for 15 s, the log10 reduction achieved by thermal pasteurisation under the conditions of interest was estimated from the DT‐ and zT‐values as follows:
| (3) |
where t is the time (in s), the milk is held at Tref (i.e. 72°C or 63°C) and DTref is the DT‐value for a specific pathogen at Tref (i.e. 72°C or 63°C). The pathogen‐specific DTref is derived from:
| (4) |
Where is the DT‐value for a specific pathogen recorded at temperature TX (selected out of those, for which the corresponding values are also available in the literature) and zT is the pathogen‐specific thermal resistance constant.
The evidence gathered by the literature review was then used to evaluate, for each relevant hazard, whether the specific log10 reductions proposed by international agencies (i.e. 5, 6, 7 and 8 log10 reductions, here in called PC) are achieved using the minimum legal requirements for thermal pasteurisation. If the target log10 reductions were achieved (or exceeded), pathogen‐specific HPP equivalent conditions were derived, when data allowed for, for each PC. If none or only some of the target log10 reductions were achieved, HPP equivalence was assessed based on the log10 reductions that are estimated to be achieved by the minimum legal pasteurisation requirements (T/t conditions), according to the thermal inactivation parameters of the hazard.
2.3.2. Appropriate indicators to verify the efficacy of HPP treatments on raw milk or raw colostrum
A search was conducted to retrieve records dealing with HPP of milk or colostrum (see Appendix B.5). If another reference was potentially relevant from the reference list of the reviewed papers, it was retrieved and screened. The screening considered whether the record contained information about the effect of HPP on an inherent milk/colostrum component that could serve as an indicator for pasteurisation. Raw milk/colostrum of all animal and human milk was considered, although the focus was on ruminant milk/colostrum. Records were discarded when the effect of HPP was evaluated on previously heat‐treated milk, in which the natural properties of the components could be altered, or on other dairy products (e.g. cheese, yogurt or milk mixed with other ingredients).
The screening of the relevance of each study (appropriateness to answer AQ8) was performed at full‐text level. Data were extracted from the selected records using predefined tables compiled with information at the record level including the following questions:
Was the study performed using raw ruminant milk/colostrum?
Was the inherent milk compound evaluated as an indicator to assess HPP pasteurisation‐like treatments?
Did the study consider HPP conditions within the range for industrial applications of pasteurisation‐like HPP treatments (i.e. temperature < 25°C, pressure < 600 MPa and time < 15 min)?
Did the study contain quantitative data, in the range proposed for the pasteurisation‐like HPP treatments, that would allow the estimation of the effect of HPP on the compound?
Did the study include kinetic data expressed as Dp, zp or similar, easily comparable with lethality parameters determined for pathogen microorganisms?
Did the study compare the effect of HPP on the compound itself with the effect on a target microorganism?
Did the study include data about the stability of the effect on the compound caused over time after the treatment?
The information extracted from the selected records was appraised in a narrative way to determine which of the compounds could be suitable to determine if HPP is applied correctly as a pasteurisation method. The following criteria were applied:
Quality of the literature support considering the number of records appraised and the quality of the retrieved studies (based on the number of affirmative answers to questions for the record‐appraisal).
Is the effect of HPP on the compound significant in the range of HPP conditions (P/t) according to SQ16?
Is the effect similar for milk/colostrum from all ruminant species?
Does the effect remain stable after the treatment and shelf‐life?
Has the effect been compared/validated with any of the target pathogens from SQ11?
Is the method of analysis used feasible for a Critical Control Point (CCP) monitoring/HACCP validation purpose?
For a better evaluation of the potential indicators, when the data provided by the records were adequate (e.g. at least three or more pressure and holding times for the same matrix) the P/t conditions necessary to reduce 90% of the initial concentration/activity of the compound were estimated and compared with the conditions necessary to achieve 5 log10 reduction of the most resistant pathogen as described in Section 2.3.1.3. Decimal reduction time at a given target pressure (Dp) was recorded, either as reported by the authors or, when not available, estimated by fitting the reduction of the concentration (e.g. whey proteins) or activity (enzymes) expressed as log10 (Cf/C0), where C0 is the initial concentration/activity of the compound and Cf is the residual concentration/activity after applying the HPP treatment. The linear regression for each case was estimated in MS‐Excel. The Dp (in min) was calculated as the inverse of the estimated inactivation rate (k) defined by the slope of the curve. A secondary linear model was fit to the log10 transformed Dp values vs. pressure (P) to derive the pressure resistant constant (zp) as the inverse of the slope of the linear regression.
2.3.3. Comparative assessment of the risk to human health from the consumption of HPP‐treated milk or colostrum
The comparative exposure assessment of the risk to human health from the consumption of HPP‐treated milk or colostrum was done by estimating the probability of contaminated servings immediately after treatment to the relevant pathogens through the consumption of four types of milk, i.e. raw milk, thermally pasteurised milk, UHT milk and industrially HPP‐treated milk. The batch to be treated was considered to be contaminated, although different initial contamination levels were established through different scenarios.
The comparison was carried out for the pathogens for which it was possible to estimate the log10 reductions resulting from both HPP and thermal treatments. A model was used to estimate the colony forming units (CFUs) in milk after thermal or HPP treatment. For purpose of comparison, two outputs were generated:
the variability in the level of contamination in a serving (with serving size of 1 cup of ~ 250 mL) after processing as described using the location parameters of the distribution (5th, 50th (median) and 95th percentiles); and
the probability of at least one CFU being present in that serving.
The model assumed for each pathogen, scenarios of fixed theoretical levels of initial contamination (N0) ranging from 1 to 8 log10 CFU/mL. The rationale for evaluating these scenarios was to ease comparisons across the outputs and to evaluate to what extent the outcome is dependent on the initial contamination levels.
From the initial level of contamination and assuming the bacteria are homogeneously distributed in milk, the probability of at least 1 CFU being present after processing, P(N1 > 0), was estimated as:
| (5) |
where P(N1 = 0) the theoretical probability of observing 0 CFU derived from a Poisson distribution of rate = λ × V, where V is the consumption unit of interest (250 mL). The microbial contamination was assumed to be randomly and homogeneously distributed in the milk system without aggregation of cells, because of the liquid (homogenous) character of the milk. Thus, the distribution of cells in milk was assumed to follow a Poisson distribution. When the distribution of cells would be heterogeneous, e.g. due to clumping of cells, another distribution such as the negative binomial distribution, may be more appropriate (Jongenburger et al., 2012).
The residual level of contamination in a serving of milk after heat treatment was estimated as:
| (6) |
where logR is the log10 reduction assumed to be achieved by thermal pasteurisation for a minimum (TT5, log R = 5) and maximum scenario (TT8, log R = 8) of log10 reductions. For UHT treatment, log R = 12 was assumed.
Raw milk was assumed to receive any processing or heat treatment (logR = 0) and therefore:
For HPP‐treated milk, the pathogen‐specific log10 reductions achieved when considering the minimum, maximum and an intermediate HPP processing conditions for milk reported to be applied by the industry were informed through the questionnaire (minimum = 450 MPa for 5 min, maximum = 600 MPa for 6 min, intermediate = 600 MPa for 3 min). More stringent conditions were not considered because these were outside the range of commonly applied conditions by the industry. The parameter log R (the predicted mean log10 reductions) in Equation 6 was informed through the global modelling described in Section 2.3.1.3.
For the four types of milk and all pathogens, the theoretical location parameters of the distributions describing the level of contamination in log10 CFU per serving (N1) after the thermal or HPP treatment (or no treatment for raw milk) were extracted to prepare a graph with the cumulative distributions, describing, for all scenarios (in terms of initial level of contamination N0), the variability in the number of bacteria per serving under the assumption that bacteria are homogeneously distributed in milk:
| (7) |
2.4. Efficacy of HPP when applied to foods known to be associated with human listeriosis
2.4.1. Most relevant foods known to be associated with human listeriosis in the EU
The aim was to retrieve information on the RTE foods known to be associated with human listeriosis in the EU and that are relevant to HPP either because they are already commercialised after HPP, or the literature provided some evidence that they are technologically appropriate to be treated by HPP. The previous scientific opinions by the BIOHAZ panel on (i) the L. monocytogenes contamination of RTE foods and the risk for human health in the EU (EFSA BIOHAZ Panel, 2018) and (ii) the public health risk posed by L. monocytogenes in frozen fruit and vegetables including herbs, blanched during processing (EFSA BIOHAZ Panel, 2020b), were reviewed for relevant information.
Data on ‘strong and weak evidence’ FBO from 2008 to 2019 at EU/EEA level were extracted from the EFSA zoonoses database. The data related to L. monocytogenes (as causative agent) were used to summarise the number of outbreaks and number of human cases for the reported food vehicles. When available, further information about the food vehicle was taken from the data element ‘more food vehicle information (fboVehicleInfo)’ (reported as free text). Also, the multicountry outbreaks (2012–2021 period) were screened for evidence that the causative agent was L. monocytogenes.
To complement this information, relevant documents on FBOs associated with L. monocytogenes in different RTE foods occurring in EU countries were identified and reviewed. These documents included scientific papers, book chapters, non‐peer review papers, regulations, guidance documents from (inter)national authorities and scientific opinions. The reference list of these documents was further screened to identify additional relevant publications until reaching a coverage of the subject considered sufficient.
2.4.2. Relevant factors to describe the requirements of HPP of the most relevant foods known to be associated with human listeriosis in the EU
A literature search was performed to identify the most relevant factors determining the effect of HPP in the selected most relevant foods known to be associated with human listeriosis in the EU and are relevant to be treated with HPP (Section 2.4.1). The search is described in Appendix B.7 and the screening process was undertaken in two steps: screening of (1) Ti/Ab and (2) full text to further identify records to be excluded based on criteria related to report characteristics (e.g. not in English) and study characteristics. A record was excluded when the study dealt only with laboratory media experiments, unless they could help in identifying (qualitatively) a relevant factor. A study was considered eligible when it:
included a challenge test, e.g. L. monocytogenes was inoculated at known level and controlled conditions;
was performed in a real food matrix (most relevant RTE foods from Section 2.4.1), for which, experimental information (preferably from a quantitative perspective) such as the following is provided: strain and its physiological state, food type and related factors (pH, aw, fat content, preservatives) and HPP technological conditions; and
provided inactivation data such as log10 reduction, kinetic parameters (e.g. inactivation rates or Dp‐values) and/or mathematical model(s).
Data were extracted using predefined tables. Besides food‐related factors and HPP technological conditions, other relevant information was collected when available: microbial strain (history and conditions for preparing the inoculation culture) and experimental approaches (method and media, time and conditions after HPP that may overcome sublethal damage with a consequent overestimation of the efficacy of the treatment).
2.4.3. Minimum HPP requirements to reduce L. monocytogenes levels in foods known to be associated with human listeriosis
The minimum HPP requirements to reduce L. monocytogenes in the selected RTE food categories were estimated through the calculation of the equivalent P/t combinations required for achieving a given log10 reduction (PC), in the form of isoreduction plots.
Data gathered from the studies retrieved from the search described in Section 2.4.2 were used to evaluate the HPP treatment conditions associated with a given log10 reduction. An MS Excel spreadsheet containing the data used in the model is made available through the Knowledge Junction under 10.5281/zenodo.5998538.
When directly available, the reported log10 reduction result was retrieved or digitalised from figures. In some cases, the log10 reduction (in log10 units) was calculated from the reported concentrations as Equation 8:
| (8) |
where N0 and N are the concentrations (CFU/g) of the pathogen before and after HPP, respectively. In most cases, N0 refers to the inoculation level used (Ni) in the challenge test. In studies dealing with inactivation kinetics, N0 usually refers to the concentration (CFU/g) of the pathogen after a HPP treatment with only a pressure come‐up time (CUT) and no holding time (i.e. a cycle of pressure increase until the target pressure is reached, immediately followed by pressure release).
Two different approaches were followed:
-
a
Modelling the collected data in order to obtain a mathematical model describing the combined effect of pressure (MPa) and holding time (min) on the inactivation (log10 reduction) of L. monocytogenes in the selected RTE foods.
First, attempts were made to fit a polynomial model including linear, quadratic and interaction terms of HPP parameters (pressure, holding time and initial fluid temperature) to the overall log10 reduction data for each type of RTE food included in the assessment. Secondly, the classical two‐step (primary and secondary) modelling approach was applied using the retrieved studies providing inactivation kinetic data. The decimal reduction time at a given target pressure (Dp) was recorded, either as reported by the authors or, when not available, it was estimated by fitting the primary inactivation model to the log10 reduction data. The log‐linear model without (Equation 9) or with tail (Equation 10) was used to fit the inactivation curve at each target pressure (Hereu et al., 2012b). The statistical significance of the tail was tested by comparing the fit of Equations 9 and 10 through an F‐test (van Boeijen et al., 2008; Santillana Farakos and Zwietering, 2011) and when a more complex model (i.e. with tail) was statistically significant (p < 0.05), the inactivation parameters estimated with Equation 10 were used.
| (9) |
| (10) |
where log10(N/N0)i is the log10 reduction at time zero of the HPP (no holding time, when the data for a cycle of pressure come‐up immediately followed by the pressure release were not available, the inoculum level before HPP treatment was used); log10(N/N0)res is the maximum log10 reduction achieved associated with the occurrence of a resistant tail; kmax is the inactivation rate expressed in natural logarithm per unit of time and t is the holding time at the target pressure (P).
The Dp (in min) was calculated as the inverse of the estimated inactivation rate (Equation 11).
| (11) |
A secondary linear model fit to the log10 transformed Dp values vs. pressure (P) was applied to derive the pressure resistant constant (zp), i.e. the parameter expressing the dependence of pressure resistance on pressure changes, as the inverse of the slope of the linear regression. The 95% confidence interval (CI) and prediction interval of the linear regression were estimated in MS‐Excel and the goodness‐of‐fit parameters with the JMP® statistical software (JMP®, Version 14, SAS Institute Inc., Cary, NC, 1989–2019).
The global regression approach nesting the primary and secondary models as in Section 2.3.1 was used to fit the entire set of log10 reduction data.
-
b
Use of predictive models available in the literature.
The model to predict the Dp as a function of pressure developed by Santillana Farakos and Zwietering (2011) (Equation 12) was evaluated. This model was built through a meta‐analysis of literature data for Listeria spp. in various food treated at pressures ranging from 200 to 700 MPa. Since in the present assessment no thermal effects are expected, the model including pressure (P) (but not temperature or the interaction between temperature and pressure) was selected.
| (12) |
Additionally, the predictive model developed by Hereu et al. (2012b) for mortadella (Equation 13) to predict log10 reduction as a function of pressure and holding time was also evaluated. This model was based on the log‐linear with tail primary model combined with a linear relationship between pressure and the log10 transformed inactivation rate. The model coefficients were obtained through one‐step global fitting the log10 reduction data generated with a series of challenge test of L. monocytogenes (strain CTC1034) in mortadella pressurised between 300 and 600 MPa for up to 15 min.
| (13) |
The predictive performance of these models was assessed by comparing the log10 reduction data retrieved in the literature search for different P/t combinations with the log10 reduction predicted with the predictive models. For the Hereu et al. (2012b) model, the log10 reduction data used to fit the model for mortadella were excluded. The proportion of simulated log10 reduction within an Acceptable Simulation Zone (ASZ), i.e. less than ± 1 log10 from the observation was calculated.
2.4.4. Other relevant pathogens (apart from L. monocytogenes) in those foods known to be associated with human listeriosis in the EU
The extracted data on ‘strong and weak evidence’ FBO from 2008 to 2019 were also used for the identification of pathogens, in addition to L. monocytogenes, in foods known to be associated with human listeriosis in the EU (Section 2.4.1). The following vehicle categories were considered, based on the foods that were identified: meat and meat products, pig meat and products thereof, bovine meat and products thereof, other or mixed red meat and products thereof, broiler meat (Gallus gallus) and products thereof, fish and fish products, and cheese.
Manual screening of the data elements ‘more food vehicle information (fboVehicleInfo)’ and ‘comment (resComm)’ (reported as free text) was performed to gather information about the specific food product identified as a vehicle of the outbreak for each pathogen other than L. monocytogenes. The pathogen was deemed relevant when the incriminated food type was identified within the most relevant foods from Section 3.3.1.1, i.e. causing listeriosis and technologically and commercially suitable for HPP: cold‐smoked fish, hot‐smoked fish, gravad fish, cooked meat products (including cooked sausages and pâté) and cheese (soft and semi‐soft cheese). Also, the multicountry outbreaks (2012–2021 period) were screened for evidence of other hazards in the foods known to be associated with human listeriosis. It was agreed that only pathogens relevant for at least two categories of the selected food known to be associated with human listeriosis would be included.
To complement this information, relevant documents from FBOs occurring in EU countries covering the relevant RTE food were identified and reviewed, using the same approach as described in Section 2.4.1.
2.4.5. Efficacy of HPP on other relevant pathogens when applying the identified minimum requirements
The microbial reduction for the other relevant pathogens identified in Section 2.4.4 was assessed from data gathered from the studies retrieved from the literature search. The search string is described in Appendix B.7 and the screening process was undertaken in two steps: screening of (1) Ti/Ab and (2) full text to further identify records to be excluded based on criteria related to report characteristics (e.g. not in English) and study characteristics. The eligibility of a study was assessed as described in Section 2.4.2 but considering Salmonella spp. and/or E. coli instead of L. monocytogenes. Data were extracted using predefined tables as described in Section 2.4.2.
2.5. Uncertainty
Based on the EFSA guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a) and scientific opinion on the principles and methods behind EFSA’s Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018b), special attention was given to: (i) the interpretation of the ToRs, i.e. framing of the mandate and the AQs, (ii) identifying sources of uncertainty and (iii) their impact on the outcome of the assessment. The identified assumptions and other sources of uncertainty were listed, and, in some cases, the impact was quantified. The experts elicited the overall uncertainty associated with the final outcome of the AQs through expert group judgement taking into account the quantified and non‐quantified sources of uncertainty. For quantifying overall uncertainty by expert judgement, the subjective probability scale recommended for harmonised use in EFSA was applied. Considering the formulation of ToR1a (‘to provide an overview’) and ToR2b (‘to list’), the overall uncertainty was not expressed when answering these.
When answering the ToRs, uncertainties may derive from the incomplete identification or misclassification of the following items: microbiological food safety concerns, additional hazards, RTE food categories and appropriate indicators in milk. The uncertainty analysis was limited to the quantification by expert judgement of the probability of incompleteness or misclassification of these items. For their incompleteness, expert knowledge was elicited on the probability that at least one factor was missed in the outcome table. For the misclassification, the experts assessed the probability of the correct inclusions of the factors in the final outcome.
Uncertainty associated with the identification of the minimum requirements of HPP needed for the inactivation of the relevant pathogens in selected commodities was evaluated taking into account the amount and representativeness of the data, the modelling approach, model structure and its predictive performance as well as the factors influencing the efficacy of HPP. Expert judgement was used to quantify the overall uncertainty on the identification of the HPP conditions needed to inactivate selected hazards on the general conclusions.
Uncertainty associated with the results of the comparative exposure assessment was evaluated considering the following: the uncertainty in the parameters used to quantify the log10 reductions achieved following HPP, the distribution (i.e. Poisson) used to describe the variability in the number of bacteria per serving after treatment and the lack of data that would enable the estimation of the exposure arising from consumption of HPP‐treated milk or colostrum, following storage until the consumption.
For the assessment of the potential chemical food safety concerns from FCM in HPP‐treated food, the impact of the uncertainty on the outcome of the assessment was not expressed in the conclusions as there was no chemical food safety concern and this cross‐links with the regulatory products area.8
3. Assessment
3.1. Efficacy and microbiological and chemical safety of the use of HPP when applied to relevant foodstuffs
3.1.1. Overview of the food categories to which HPP is being applied for food safety reasons along with the processing conditions used by industry
Conventional heat‐based processing methods are sometimes associated with certain deleterious changes in the nutritional, physico‐chemical and sensorial attributes of food products. This has resulted in the development of novel techniques that may be regarded as mild technologies (Ghafoor et al., 2020). HPP is a non‐thermal preservation technique presently used in the food and beverages industry in which products are subjected to isostatic pressures of up to 600 MPa for a relatively short pressure holding time.
Nineteen CAs from 13 MS and Switzerland, 23 establishments using HPP and two equipment providers, provided answers to the questionnaires. Three reports from research institutions were also included. Based on the answers provided by the CAs, there is a large variability on the level of implementation of the HPP technology by the food industry in Europe. On the other hand, based on the replies obtained from establishments, many establishments are already using this technology in some countries while in other countries, its use is marginal or non‐existent. However, this assessment was considered uncertain as some MS stated that they would not know and compiled data on the approved/registered food establishments or service providers using the HPP technology is not available in most countries. The answers from the CAs indicated that national authorities have not been active in regulating the HPP of foods. Around the year 2000, some countries considered HPP‐treated foods as novel foods and undertook first steps in preparing safety assessments, but this was discontinued.
Based on the answers given by the establishments as well as the equipment providers, the food business operator (FBOp) is usually responsible for the validation. Replies from the CA indicated that FBOp should assess the impact of HPP on food safety as a part of their Food Safety Management System (FSMS), which is regularly audited by the CA. There are different strategies used by the establishments to validate the HPP treatments. Most respondents indicated that mostly, available literature is used as a starting point to determine the impact of specific HPP treatments on microbial food safety. However, if information for a specific food formulation is not available, challenge tests are needed to obtain product‐specific information. Based on the answers, it could be concluded that when possible, the equipment providers support FBOp with shelf‐life tests but in certain cases, a systematic initial check on a new product by shelf‐life tests is needed.
3.1.1.1. Commercial high‐pressure equipment
High pressure can be generated by an external pump, by a moving piston or by heating of the pressure‐transmitting fluid in a closed chamber. In batch systems, pressure is transmitted by a low compressibility medium (usually water) and the process consists of three stages with associated duration: (i) the pressure CUT during the compression stage (also referred to as pressurisation stage) during which the liquid medium is pumped into the pressure chamber using pumps and pressure intensifiers, (ii) the holding time during which the product is maintained at target pressure and (iii) the come‐down time or CDT during the pressure release stage (also referred to as depressurisation or de‐compression stage), in which the pressure drops rapidly. A representation of the temperature and pressure profile of a food when exposed to a HPP treatment is shown in Figure 1.
Figure 1.
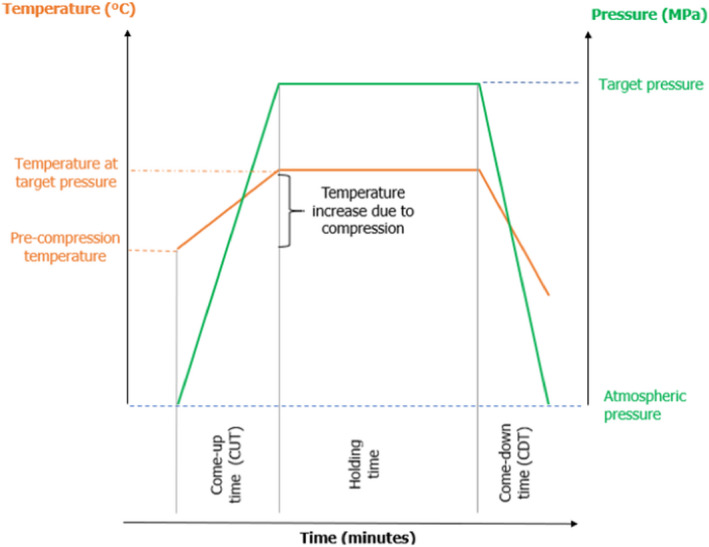
Representation of temperature and pressure profile of a food when exposed to a HPP treatment
The HPP target pressure and holding time (and also the initial fluid temperature) are process parameters that can be selected according to the food matrix and the expected microbial inactivation outcomes. The CUT and CDT depend on the equipment capabilities. The system could be chilled or heated by a complementary equipment or by using pressure‐transmitting fluid at certain temperature to achieve the target temperature during the process. Commercial applications of HPP in the food industry use pressures up to 600 MPa, whereas laboratory equipment can achieve pressures up to 1,400 MPa, sometimes connected with cooling or heating systems (Ghafoor et al., 2020).
As extracted from the questionnaire, the HPP processing conditions recommended and used vary depending on a number of factors. According to one equipment provider, the features of the industrial equipment available determine that 600 MPa is the upper limit for commercial scale HPP, while holding times applied for the vast majority of products are 1–6 min. The pressure‐transmitting fluid on present commercial HPP systems is water. Recirculating water can be cooled to 10–15°C or even lower to 4–8°C, depending on refrigeration capacity, water volume, vessel filling capacity, cycle time and room temperature. Another equipment provider added that target pressures depend on the application: shucking of seafood ~ 250–300 MPa, pasteurisation 400–600 MPa. Maximum holding time, once the target pressure is reached, is around 3 min with a maximum of 15 min. The CUT and CDT are 3 min and 10–45 s, respectively. The initial water temperature is usually 5–15°C. The expected rise in temperature depends on the product with values per 100 MPa being provided. According to Hiperbaric9, HPP is a non‐thermal preservation technique for food and beverages in which (already packaged) products are introduced into a vessel and subjected to 300–600 MPa during a holding time ranging from some seconds up to 10 min.
Usually, products are packed before HPP treatment (‘In‐pack’). The in‐pack process can be used for liquid, semi‐solid and solid foods. Generally, the products are prepacked under vacuum conditions in flexible and compressible material packages to eliminate air bubbles (Ghafoor et al., 2020). All responses from the questionnaire indicated that food products are processed in‐pack. The In‐pack systems prevent recontamination of the product, as it is processed in its final packaging (Georget et al., 2015). Figure 2 shows a diagram of an industrial scale HPP equipment with a horizontal vessel system for batch processing of packed food products.
Figure 2.
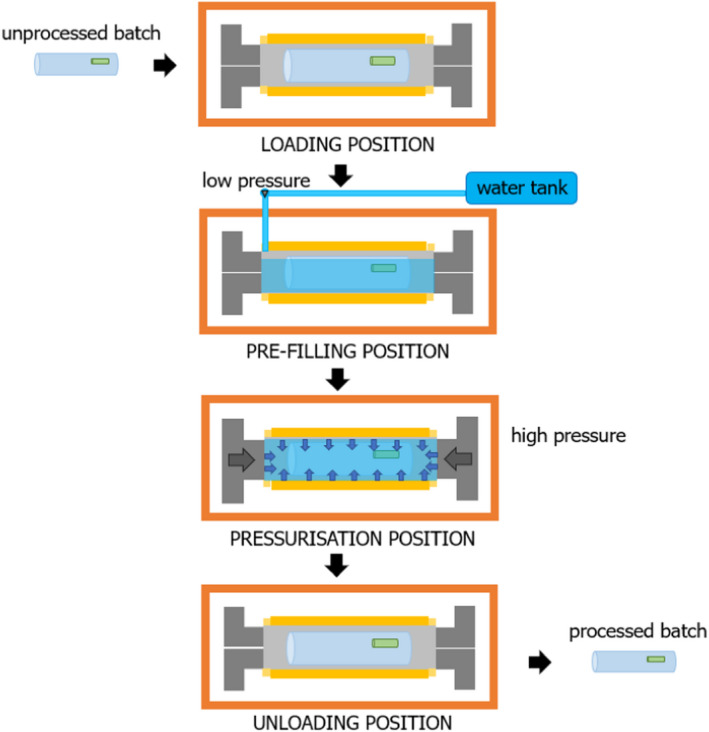
Diagram representing a commercial HPP treatment equipment
Hiperbaric recently introduced a new equipment for the processing of bulk liquids before packaging (‘In‐Bulk’) (Tonello‐Samson et al., 2020). The process begins when the liquid food fills the inlet tank. The pressurised beverage fills the outlet tank through sterilised tubes and is bottled in an ultra‐clean filling line10. One establishment reported that this is the latest advance in commercial equipment. This allows packaging after the treatment and therefore removes packaging material limitations; thus, the use of rigid packages such as glass and aluminium becomes possible. A commercial bulk machine was installed in a French company, a co‐packer of HPP‐processed beverages.11
3.1.1.2. Treated food categories and processing conditions
In principle, almost all types of food can be subjected to HPP. However, aerated foods that have air bubbles entrapped (e.g. bread, cakes, whole or fresh‐cut fruit and vegetables) are not suitable for HPP because their porous structure will be negatively impacted or dissipated (Otero and Prestamo, 2009; Jung, 2016). Low moisture foods such as powdered products and dried fruit are not usually subjected to HPP due to the low microbial inactivation in food with moisture content below 40% (Abera, 2019). The impact of the food composition on microbial inactivation will be described in Section 3.1.2.
Fruit puree was the first food product that was subjected to the industrial application of HPP, in Japan in the early 1990s (Yamamoto, 2017). HPP shows several advantages against other conventional technologies such as thermal treatments, which lead to the extension of this technology to a wide variety of food and beverages including juices, meat products, as well as RTE products such as RTE meals, vegetable dips and baby food. More recently, HPP has been applied to dairy and seafood products, although to a lesser extent (Ghafoor et al., 2020).
Manufacturers in Europe, North America and Japan have been actively developing commercial applications, and the production of HPP products has increased yearly. Approximately 500,000 tons of HPP products are produced around the globe yearly, with RTE meat products in a convenient format (e.g. sliced prepacked meat products) representing the largest application among all HPP products. In addition, the clean label trend in developed countries boosted the development of HPP juices, which has become a popular topic in the field of HPP technology with a focus on coconut water. Juice products hold a high market share, similar than that of meat products, in the USA food market (Huang et al., 2017).
According to the information provided through the questionnaire (Appendix A as summarised in Tables A.3 and A.5), some foods in the categories meat and meat products, i.e. RTE sliced meat products, deli meat, hot dogs and other cooked, comminuted meat products and dry‐cured meat products, have a high relative importance being HPP in comparison to other food types on which HPP is used. In comparison, ground meats have a low relative importance. In the group fruit, vegetables, juices and other products thereof, high acid fruit/vegetable juices and guacamole have high relative importance, while fruit puree, wet salads (pH < 5) (also known as deli salads) and other dips (e.g. hummus, salsa) have medium relative importance. Fish and fishery products and crustaceans, shellfish, molluscs and products thereof have low and medium relative importance, respectively. In the last category, HPP is used also for increasing the meat yield and aiding shell removal. All foods in the dairy food categories have a relatively low importance: milk, cheeses derived from raw milk and pasteurised milk, processed cheese dips/spreads and dairy products (other than cheeses). Mixed food/composite products have a medium/high relative importance. Most frequently, pressures between 400 and 600 MPa (the upper limit for commercial scale HPP equipment) were applied, with durations from 1.5 to 8 min (in one instance in a double cycle). The temperature of the water used as pressure transmitting medium before HPP treatment (i.e. not including the compression heating) ranges from 0 to 20°C, often chilled at 4–8°C. In‐pack food temperature during HPP is not captured. Milder conditions were reported within the category ‘crustaceans, shellfish, molluscs and products thereof’ using 250–300 MPa, but with a different reason for using HPP (i.e. labour saving/shucking of lobster, texture enhancement). Storage of perishable products after HPP treatment is under refrigeration mostly for several days up to several months. For self‐stable dry‐cured meat products, storage was declared to be up to several months at ambient temperature. Most of the commonly used food packaging materials were reported to be used for HPP products: specifically, various types of polyolefins, polyethylene terephthalate (PET), ethylene‐vinyl alcohol copolymer (EVOH), polyamides and polystyrene as monolayers or multilayers.
Table A.3.
Summary of the replies to the questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety by the establishments
| Food category | Food type | No( b ) | Reasons for using HPP( b ) | HPP conditions( a ) | ||
|---|---|---|---|---|---|---|
| To increase product safety | To extend product shelf‐life | Other | ||||
| Meat and meat products | Heat treated/cooked meat product | 5 | 5 | 5 | 0 | 580–600 MPa/2–6 min/6–17°C |
| Dry‐cured (uncooked) meat product | 4 | 4 | 3 | 1: to be preserved without refrigeration | 500 MPa/8 min/unknown to 600 MPa/5–8 min/3–10°C+NR | |
| Not specified | 6 | 6 | 6 | 0 | 450 MPa/5 min/NR to 600 MPa/3–8 min/4°C+NR | |
| Fruit, vegetables, juices and other products thereof | Acid (pH < 4.5) or unspecified fruit and vegetable juices | 3 | 1 | 3 | 2: preservation of food ingredients, sensory/organoleptic properties, freshness | 600 MPa/2–3 min/15–20°C |
| Fruit puree, hummus, dips, pesto | 2 | 1 | 2 | 1: preservation of food ingredients, sensory/organoleptic properties | 500–600 MPa/2–3 min/10–20°C | |
| Not specified or various | 9 | 9 | 9 | 0 | 350–600 MPa/1–5 min/4–15°C( c ) | |
| Fish and fishery products | Fish roe | 1 | 1 | 0 | 1: side effect, faster maturation of fish roes | 600 MPa/5 min/7–10°C/double cycle |
| Not specified | 3 | 3 | 3 | 0 | 400 MPa/5 min/0°C with ice to 600 MPa/3–5 min/4°C | |
| Crustaceans, shellfish, molluscs and products thereof | Octopus and prawns | 1 | 1 | 1 | 1: to maintain organoleptic/nutritional characteristics vs. other technologies | 600 MPa/3–5 min/3–10°C( c ) |
| Not specified | 1 | 1 | 1 | 0 | 400 MPa/5 min/0°C with ice | |
| Not specified | 2 | 0 | 0 |
2: labour saving/shucking of lobster 1: texture enhancement |
250–300 MPa/2–3 min/7–10°C to 300–350 MPa/1–2 min | |
| Milk | Not specified | 1 | 1 | 1 | 0 | 450 MPa/5 min to 600 MPa/6 min |
| Cheese | Lactic fermentation; hard cheese made from cooked paste | 1 | 1 | 0 | 0 | 600 MPa/3–5 min/4°C |
| Soft cheese | 1 | 1 | 1 | 0 | pH > 4.5: 600 MPa/2–3 min/4°C | |
| Not specified | 1 | 1 | 1 | 0 | 450 MPa/5 min to 600 MPa/6 min( d ) | |
| Mixed food/composite product and other food category | RTE meals | 10 | 9 | 6 | 1: organoleptic change | 500 MPa/5 min/6°C to 600 MPa/3–8 min/3–15°C |
Target pressure/treatment time once target pressure reached/water temperature before HPP treatment.
Number of establishments reporting.
Depending on the product treated.
Pressure depends of the sensory properties in this case increment of texture.
Table A.5.
Summary of the replies to the questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety by the equipment providers
| Food category | Equipment provider | Food type and relative importance of this food type being HPP in comparison to other food types on which HPP is used | Reasons for using HPP | HPP conditions( a ) | What are the packaging conditions? | Which is the packaging material used? |
|---|---|---|---|---|---|---|
| Meat and meat products | 1 |
RTE sliced meats, deli meat, hot dogs and other cooked, comminuted meat products: high Ground meats: low |
Pathogen reduction; shelf‐life extension | 400–600 MPa/2–4 min/4–8°C | Vacuum, skin or modified atmosphere |
PET, PE, PP, EVOH, OPA, others as necessary Films, trays and pouches Monolayer and polymer according to needs |
| 2 |
Heat treated meat product: high Hot smoked and cured meat product: high |
To increase product safety, to extend product shelf‐life | 600 MPa/3 min/5°C( a ) | Modified atmosphere packed (heat treated meat product) and vacuum packed (smoked meat product) | PET/PE EVOH (heat treated meat product) and PE (hot smoked and cured meat product) | |
| Fruit, vegetables, juices and other products thereof | 1 |
High acid fruit/veg juices: high Guacamole: high Wet salads (pH < 5): medium Other dips (e.g. hummus, salsa): medium |
Pathogen reduction; shelf‐life extension | 400–600 MPa/1.5–3 min/4–8°C | Bottles, film lidded cups, pouches in vacuum or modified atmosphere flush |
PET, PE, PP, EVOH, OPA, others as necessary Bottles, pouches, film, cups, trays Monolayer and polymer according to needs |
| 2 |
Fresh pressed juices (pH < 4.5): high Fruit puree: medium Guacamole: high Hummus and other dips: medium Vegetarian products based on beans: low |
To extend product shelf‐life, freshness | 600 MPa/3 min/5°C | Bottle (juices), cups (the rest) | PET (juices), PET, PP, PE (the rest) | |
| Milk | 1 | Low | N/A | N/A | N/A | N/A |
| 2 | High( b ) | To extend product shelf‐life, taste | 600 MPa/3 min/25°C | 750 mL | PET | |
| Cheese | 1 |
Raw milk and pasteurised cheese: low Processed cheese dips/spreads: low |
Shelf‐life extension; pathogen reduction | 400–600 MPa/2–6 min/4–8°C | Vacuum or modified atmosphere |
PET, PE, PP, EVOH, OPA, others as necessary Monolayer and polymer according to needs |
| 1 | Shelf‐life extension; pathogen reduction | N/A | N/A | |||
| 2 | Low | |||||
| Dairy products (other than cheeses) | 1 | Low | N/A | N/A | N/A | N/A |
| 2 | Low | |||||
| Fish and fishery products | 1 | Low | N/A | N/A | ||
| Crustaceans, shellfish, molluscs and products thereof | 1 | Medium | Meat yield, aid shell removal; pathogen reduction (e.g. Vibrio spp.) | 250–600 MPa/1.5–3 min/4–20°C | Lidded trays and skin trays (vacuum or modified atmosphere flush) |
PET, PE, PP, OPA, EVOH, others as necessary Monolayer and polymer according to needs |
| 2 | Crab: medium | To increase product safety, to extend product shelf‐life | 600 MPa/3 min/5°C | Vacuum packed | PE | |
| Mixed food/composite product | 1 | Medium | 400–600 MPa/2–5 min/4–8°C | |||
| 2 | Ready to eat meals: high | To extend product shelf‐life, freshness | 600 MPa/3 min/5°C | Various | PE/PET | |
| Other food category | 2 | Baby food: medium | To extend product shelf‐life, freshness | 600 MPa/3 min/5°C | Various | PE, PP |
PE: polyethylene, PP: polypropylene, PET: polyethylene terephthalate, OPA: oriented polyamide, EVOH: ethylene‐vinyl alcohol copolymer, N/A: not applicable.
The specification of 600 MPa for 3 min is a standard and could be adjusted for different products; for example a fresh juice with low pH for 2 min or a meat product could be also processed for more than 5 min. Also, the water treatment temperature could be adjusted, it depends on the product. The only pressure deviation is in case of shucking of seafood; in that case 300 MPa is sufficient to separate the meat from the shell, but no long shelf‐life increase will be added. The decompression rate of 50 MPa/s can be also adjusted to 0.2 MPa/s from a defined point lower than 50 MPa. This will take place to protect MAP packages during pressure release. Beside of tolling service we also manufacture machines for our global clients in that case also dairy products are used, for example raw milk in Australia.
Whole milk from cow (~ 4% and ~ 5% fat) in being processed in Australia, but we are not aware of HPP milk in the EU. There is huge interest and upcoming requests of the industry for HPP of raw cows’ milk in the last year especially.
Meat and meat products. The use of HPP by the meat industry is well established. HHP has primarily been used in the RTE meat products industry as a new post‐packaging, non‐thermal decontamination method (Bajovic et al., 2012; Meloni, 2019). Many studies have demonstrated the suitability of HPP to improve the safety of raw meat and meat products. Jofré and Serra (2016) summarised the successful applications of HPP in meat and meat products and the inactivation levels of L. monocytogenes in different meat products submitted to HPP. The USDA Food Safety and Inspection Service recognised HPP technology as a useful post‐lethality treatment (PLT) to prevent or eliminate post‐processing contamination by L. monocytogenes in RTE meat products such as whole or formed ham, whole and split roast beef, turkey ham, chicken breast fillets and strips, and sliced ham, sliced turkey, and sliced roast beef. Within the US ‘Listeria rule’, PLTs have to be included in the HACCP plan of the FBOp; therefore, the efficacy of HPP has to be previously validated according to the procedures described in the FSIS guideline (FSIS, 2014). Health Canada also encourages FBOp to use validated PLTs (such as HPP) to RTE foods that are exposed to contamination after a lethality treatment (such as in cooked meat products) (Health Canada, 2011). Among pressure‐treated products, meat and meat products represent approximately 30% of market share in US (Huang et al., 2017; Li et al., 2020).
Fruit, vegetables, juices and other products thereof. The HPP technology has been approved by governmental authorities in the US, Europe and Canada for reducing pathogens in fruit juices and vegetable products and extending their shelf‐life (Huang et al., 2017). Pressurised fruit juices and vegetable products are widely available, showing a similar market share as RTE meat products (Daher et al., 2017). Fruit and vegetable beverages are usually treated with pressures ranging between 400 and 600 MPa, which are typically applied from a few seconds to 5 min, at refrigerated or room temperature. Of particular interest are fruit and vegetable beverages that require refrigerated storage to inhibit spore germination (Juliano, 2016). These refrigerated pressurised products include many fruit‐based and, to a lesser extent, vegetable‐based food products currently on the international market, including a range of fruit smoothies, jams, juices, apple sauce, fruit preparations as ingredients for yogurts, fruit blends, guacamole and other avocado products, tomato‐based salsa and meal kits containing acidified sliced bell peppers and onions (Doona et al., 2015). The FDA Juice regulations require the FBOp to implement a validated control measure (among which HPP is cited), to achieve a 5 log10 reduction in the number of the ‘pertinent microorganisms’, which is the most resistant microorganism of public health significance of those that are likely to occur in juice, e.g. E. coli O157:H7, L. monocytogenes, Salmonella spp. (FDA, 2004).
Fish and fishery products, crustaceans, shellfish, molluscs and products thereof. Fish and shellfish are highly perishable food products, due to their high aw, neutral pH and presence of autolytic enzymes (Tabilo‐Munizaga et al., 2016). The development of HPP technology is very significant for the quality and microbial safety of aquatic products as it inhibits the growth of pathogens, reduces the accumulation of biogenic amine compounds, maintains the fresh mouthfeel and extends the shelf‐life of aquatic products, all of which are impossible using traditional thermal processing technology (Mujica‐Paz et al., 2011; Huang et al., 2017). There are patents related to the use of HPP in seafood that focuses on food safety, such as the patent of a process for the elimination of bacteria in shellfish and of shucking shellfish. The purpose of this patent is to use HPP (138 MPa and 1034 MPa for 1–15 min) for eliminating pathogenic organisms and other bacteria from raw food products, such as raw shellfish. Another example is the method for shelf‐life extension of oysters by HPP.
Milk and dairy products (other than cheeses). The use of HPP in milk was one of the first applications. However, up to now, the industrial relevance of this technology in the dairy sector is low and there are still very few applications for dairy products, although patents for milk and dairy products exist. One reason for this lack of application for dairy products might be that HHP affects several constituents of milk (Trujillo et al., 2016). Comprehensive inquires have disclosed the potential profits of HPP as an alternative to heat treatments by affecting the structure of milk components, particularly proteins and fats (Ravash et al., 2020). On the other hand, HPP is successfully applied to high quality dairy products such as cheese to inactivate pathogenic microorganisms. HPP application of cheese can reduce total number of microorganisms or inactivate pathogenic microorganisms, thus increasing the shelf‐life and safety of the product. There are many examples of the effectiveness of HPP inactivating pathogenic microorganisms including L. monocytogenes in cheese using 300–500 MPa for 5–10 min without significantly affecting the sensory characteristics (Trujillo et al., 2016).
Other food categories. HPP has been suggested as an alternative to thermal pasteurisation to improve the microbiological safety of egg and egg‐derived products (Naderi et al., 2017). In 2015, Health Canada approved applications for HPP‐treated egg products (such as egg salad, egg dips and egg spreads). The treatment was designed to apply 600 MPa for a 2‐min cycle on boiled eggs to extend their shelf‐life during refrigeration (Health Canada, 2015; Naderi et al., 2017). Concurrently, the possibility of using HPP to develop a novel alternative to liquid whole egg heat pasteurisation was evaluated (Monfort et al., 2012). Precooked egg products (such as egg patties, omelettes, scrambled eggs) are generally preserved and sold as frozen food, with the main priority being ensuring food safety.
3.1.1.3. Concluding remarks
In principle, almost all types of food can be subjected to HPP. However, aerated foods that have air bubbles entrapped (e.g. bread, cakes, whole and fresh‐cut fruits and vegetables) are not suitable for HPP because their porous structure will be negatively impacted or dissipated. Low moisture foods such as powdered products and dried fruits are not subjected to HPP due to the low microbial inactivation by HPP in foods with moisture below 40%.
Currently, a wide variety of food and beverages are subjected to HPP including meat and meat products, fruit and vegetable juices and other products thereof, fish and fishery products, seafood products, milk and dairy products, RTE meals, wet salads, vegetable dips and baby food. They have been classified in different levels of importance of the food type being HPP in comparison to other food types on which HPP is used, according to the information extracted from the questionnaires.
The replies from the questionnaire indicate that there is a large variability in the level of implementation of the HPP technology by the food industry in the EU.
HPP consists of three stages with associated time duration: (i) the pressure CUT during the compression stage, (ii) the holding time during which the product is maintained at target pressure and (iii) the CDT during the pressure release stage.
The pressure level and holding time (and initial fluid temperature) are process parameters that can be selected according to the food matrix and desired effect. The CUT and CDT depend on the equipment capabilities.
Most frequently, pressures between 400 and 600 MPa (i.e. the upper limit for commercial scale equipment) are applied, with common holding times ranging from 1.5 to 6 min and a marginal use of longer holding times between 8 and 15 min.
The temperature of the water used as pressure transmitting fluid for HPP treatment (i.e. not including the compression heating) ranges from 0 to 20°C, and is often previously chilled at 4–8°C.
Usually, products (liquid, semi‐solid and solid food) are packed before HPP treatment (‘In‐pack’) to avoid recontamination of the product after HPP. Most of the commonly used food packaging materials were reported to be used for HPP products: specifically, various types of polyolefins, PET, EVOH, polyamides and polystyrene as monolayers or multilayers. Equipment for the processing of bulk liquids before packaging (‘In‐bulk’) is, based on gathered information, only commercially available from one establishment.
Storage of perishable products after HPP treatment is under refrigeration, mostly for several days up to several weeks. For shelf‐stable dry‐cured meat products, storage was declared to be up to several months at ambient temperature.
3.1.2. Food extrinsic and intrinsic factors that may influence the efficacy of HPP
Different factors should be considered during the application of HPP in food processing as these may influence the efficacy of the treatments in terms of microbial inactivation, including factors associated with the microorganisms, food extrinsic factors (related to HPP processing conditions) and the food intrinsic factors (e.g. composition and physico‐chemical characteristics) as summarised in Table 1 (Rendueles et al., 2011; Evelyn and Silva, 2017).
Table 1.
Factors affecting the efficacy of HPP in terms of microbial inactivation
| Type of factor | Factors |
|---|---|
| Factors related to microorganisms |
Type of microorganisms Taxonomic unit (e.g. species, genus) Strain Physiological state |
| Food extrinsic factors |
Target pressure Holding time Processing temperature CUT and CDT Packaging atmosphere |
| Food intrinsic factors |
Macronutrient and micronutrient contents pH (acidity) Water activity Antimicrobial compounds |
CDT: come‐down time; CUT: come‐up time.
3.1.2.1. Factors related to microorganisms
In this section, factors related to microorganisms potentially contaminating the foods will be discussed. Only the impact of vegetative forms of pathogenic microorganisms will be covered as the scope of the scientific opinion is the non‐thermal HPP treatment, which does not inactivate spores when used alone.
The microbial resistance to HPP depends on the type of microorganism, with variations also among the taxonomic unit, including genera and species, and the strains. Usually, the higher the structural complexity the more sensitive is the organism. Thus, eukaryotic vegetative microorganisms (moulds and yeasts) are more susceptible to HPP than prokaryotic microorganisms (e.g. bacteria). The HPP resistance of viruses is widely variable depending on the structural diversity, while parasites are usually inactivated at relatively low pressures (100–400 MPa) (Rendueles et al., 2011; Roos, 2020). Within vegetative bacteria, cocci are usually more resistant than bacilli, and often Gram‐positive are more resistant than Gram‐negative bacteria (Datta and Deeth, 2011), with a few exceptions. Reviews have shown that S. aureus appears to have the highest resistance among the vegetative pathogens, followed by E. coli and lastly L. monocytogenes and Salmonella spp. (Borda et al., 2013; Cebrian et al., 2016; Evelyn and Silva, 2017; MPI, 2019). Within the same species, the strain plays a major role in the microbial resistance to HPP as illustrated by two examples. A study with 39 STEC strains inoculated individually into 80% lean ground beef and treated at 350 MPa (4°C) reported Dp‐values ranging from 0.89 to 25.7 min (Sheen et al., 2015). Another study compared the inactivation of 13 L. monocytogenes strains on cooked chicken by 600 MPa for 2 min (20°C) and concluded that although eight strains were reduced by 5 log10, only about 1 log10 reduction was detected with two other strains (Patterson et al., 2011). In validation studies, the most pressure resistant strains (or a cocktail of several strains) should be used to help account for strain variability (MPI, 2019).
The conditions to which microbial cells are exposed to before contaminating the food and once they colonise the food before it is subjected to HPP, is a relevant issue which can affect the efficacy of HPP in pathogen reduction, due to cross protection or sensitisation of cells to HPP. The physiological state associated with the growing phase of cells has also an influence, as it has been demonstrated that cells in the stationary phase of the growth cycle can be more resistant than cells in the exponential growth phase (Rendueles et al., 2011).
On the other hand, the survival of microorganisms is also impacted by the conditions during storage. HPP treated foods are generally stored and distributed under refrigerated conditions (T < 7°C), which might favour the survival and subsequent growth of psychrotrophic microorganisms (Silva and Evelyn, 2020).
3.1.2.2. Food extrinsic factors
Factors extrinsic to foods are mainly those related to HPP processing conditions. The target pressure and holding time at this target pressure (in the second stage of the HPP cycle) are the major extrinsic factors determining the efficacy of a HPP process (Evelyn and Silva, 2017; MPI, 2019). In general, the higher the pressure or holding time, the higher will be the microbial inactivation, although the increase in pressure is reported to be more effective than increase in holding time. The same level of microbial inactivation can be achieved using several HPP conditions (P/t combinations), in which higher pressures require less time for the same microbial inactivation as quantitatively assessed in Sections 3.2.3 (for milk and colostrum) and 3.3.2 (for RTE foods). For a specific microorganism/food combination, there is a threshold pressure below which there is no relevant microbial inactivation. As HPP is an isostatic process, the target pressure is transmitted instantaneously and uniformly to all points of the food contained inside the HPP vessel, irrespectively of the shape and size (Smelt, 1998), and no difference in process efficiency is expected for common batch ‘in pack’ and more recently developed ‘in bulk’ HPP processes.
Reviews reported in other papers that demonstrated findings that although inactivation can occur due to the CUT to target pressure and release (without holding time), most studies used the target pressure and holding time to characterise the HPP process, as this is usually the longest stage of the cycle where most microbial inactivation occurs (Evelyn and Silva, 2017, 2019). The duration of the compression (CUT) may have an effect on microbial inactivation, particularly when pressures of 500–600 MPa are applied (Hereu et al., 2012b). The CDT is much shorter than the CUT. Rademacher et al. (2002) concluded that rates of compression and decompression in the range of 1.7–8.3 MPa/s did not affect inactivation of Listeria innocua. The extra microbial inactivation that might occur during the CUT and CDT will also depend on the food matrix.
Processing temperature is another important factor, particularly when it reaches values above 45°C and thermal inactivation effects may occur (scenario not considered in the present scientific opinion). However, temperatures above or below ambient temperature have been reported to increase the inactivation rate, which has been attributed to changes in the cell membrane structure and fluidity (Rendueles et al., 2011). Processing temperatures mainly depend on the initial temperature of the food and the pressure transmission fluid in the equipment, as well as the compression heating during the CUT. Some small‐scale equipment has the ability to regulate (cooling) the fluid temperature during the HPP cycle counteracting the heating caused by compression. The compression heating is dependent on the pressure increase and on the product composition. The higher the target pressure, the higher will be the temperature increase. The temperature increase of the product might range from 2–3°C/100 MPa (in aqueous phase) to 8.7–9.7°C/100 MPa (in oil phase) (Rasanayagam et al., 2003; Patazca et al., 2007), which may create processing temperature differences within heterogenous food matrixes (Grauwet et al., 2016). In order to operate under non‐thermal conditions to preserve the original quality of the food (e.g. flavour and thermolabile food constituents), the initial temperature of the food (i.e. before HPP, also referred as pre‐compression temperature) and the temperature of the pressure transmitting fluid (usually water) can be lowered to stay within a temperature limit during processing (< 45°C).
The type of packaging system is another factor that can influence HPP microbial inactivation. Usually, foods are vacuum packed in a flexible film or bottle (e.g. PET), as gas inside the food package can cause package bursting during the pressure release stage. However, HPP can also be applied to foods with modified atmosphere packaging, and in this case, the dissolved CO2 has been shown to enhance microbial inactivation compared to vacuum packaging (Amanatidou et al., 2000b).
3.1.2.3. Food intrinsic factors
Intrinsic factors are related to the food formulation/components such as moisture, fat, protein, carbohydrates. The composition of the food has a great effect on HPP microbial inactivation, as carbohydrates, protein and fat of food matrixes (e.g. milk, meat) confer a protective effect against microbial inactivation compared with the simpler matrix of laboratory culture media (Patterson et al., 1995; Rendueles et al., 2011; Georget et al., 2015).
Studies conducted with model foods or laboratory media provide important information regarding the effect of individual food components on microbial inactivation during HPP. However, HPP achieves higher inactivation rates in model food systems as compared to real food systems at comparable treatment conditions, most likely due to the presence of various food constitutes, which provide a protective effect (Georget et al., 2015). In addition, the kinetics of microbial inactivation may change from linear to non‐linear depending on the food composition (Tassou et al., 2007). Therefore, HPP microbial inactivation validation tests in real foods are recommended (Claeys et al., 2003a; MPI, 2019).
The higher the water activity (aw) the more effective the HPP on microbial inactivation, contrary to foods with low aw values where microorganisms may be protected from the lethal effects of HPP due to protein stabilisation, which prevents its denaturation and limits transfer of pressure in the food matrix via water (Rendueles et al., 2011). The type of solutes determining the aw have different piezo‐protective effects, with sodium chloride being reported to be less protective compared to sorbitol, glycerol and fructose (Setikaite et al., 2009). A dose‐dependent protective effect of solutes (Balamurugan et al., 2016) or the aw values (Bover‐Cid et al., 2015, 2017) has also been reported.
With respect to pH (acidity), studies demonstrated that microbial inactivation by HPP increased when pH decreased (Syed et al., 2016). Microbial inactivation can be affected by the type of acid, the pH range and microorganism tested. The acid‐base equilibria of a system submitted to HPP is affected by pressure and a temporary reduction of pH and an increase in the dissociated form of the organic acids could be present (Hayert et al., 1999). In carbonated drinks, the dissolved CO2 acidifies the beverage and can also enhance microbial inactivation (similar to lowering pH).
Antimicrobial compounds (e.g. preservatives) have been highlighted as important food intrinsic factors that may affect the HPP microbial inactivation. The effect of preservatives (additives, such as organic acids) on HPP microbial inactivation can vary: some studies reported enhanced inactivation of Salmonella in a raw meat matrix when lactic acid was added (Serra‐Castello et al., 2019), while when used as a salt (i.e. lactate) they show a dose‐dependent piezo‐protective effect on L. monocytogenes in laboratory media (Serra‐Castello et al., 2021a) and in different types of meat products (Marcos et al., 2008b; Lerasle et al., 2014; Stollewerk et al., 2014; Serra‐Castello et al., 2021b).
Fat content. In an oily food, microorganisms entrapped in the fat are more resistant to HPP. The increased fat content of milk provided a protective effect of E. coli, with inactivation greater in skimmed milk 0.05% fat than in whole milk 3.6% fat (Garcia‐Graells, 1999). In contrast, Bover‐Cid et al. (2015) reported similar inactivation of 6.6 and 6.3 log10 reductions of L. monocytogenes in dry cured ham with 10 and 50% fat content after HPP at 600 MPa for 5 min.
In certain foods (e.g. meat and fish), proteins also have a protective effect for microbes against HPP inactivation (Rosario et al., 2021). Any heterogeneous distribution within the food matrix of the food components described above may result in a variable impact of the same HPP treatment.
Based on the available literature it is difficult to draw clear conclusions regarding the impact of HPP in different food matrices due to the occurrence of several factors that may interact and lead to different microbial inactivation levels. Thus, it is important to conduct validation tests in real food samples.
3.1.2.4. Concluding remarks
-
Intrinsic (i.e. food related) and extrinsic (i.e. processing related) factors, and also factors related to the contaminant microorganisms influence the efficacy of HPP in terms of reduction (log10 units) of vegetative microorganisms when applied to foodstuffs.
-
○
The main intrinsic factors are the aw and pH of the food and its components. Microbial inactivation by HPP is enhanced at higher aw‐ and lower pH‐values. Carbohydrates, proteins and lipids and/or presenting a low moisture content and water activity, exert a protective effect on microorganisms, which decreases microbial reduction by HPP in foods.
-
○
The main extrinsic factors are the target pressure and the holding time. In general, the higher the pressure or holding time, the higher will be the microbial inactivation, although the increase in pressure is reported to be more effective than increase in holding time.
-
○
The type of microorganisms, taxonomic unit and strain, as well as the physiological state (e.g. growth phase and injuries) of the microorganisms to be inactivated are also relevant. Cells in the stationary growth phase are generally more resistant than cells in the exponential growth phase.
-
○
The efficacy of HPP in different food matrices is variable due to the interactions between the intrinsic factors impacting on the microbial inactivation. This makes it difficult to predict the efficacy of HPP in a complex food matrix and makes validation in real foods necessary.
3.1.3. Potential microbiological food safety concerns in HPP‐treated food
HPP has been recognised as an effective technology able to provide safe foods with high quality. However, there are still a number of remaining questions regarding the safe use of HPP, including some potential risks associated with the application of the technology (Aganovic et al., 2021). In this section, the major microbiological food safety concerns in HPP‐treated foods are assessed, including:
the control of surviving microbial spores (e.g. with refrigerated storage or additives) and HPP‐induced spore activation (with subsequent germination and conversion to vegetative cells);
the induction of sublethal injury in cells, including in the viable but not culturable (VBNC) state, that might lead to an overestimation of the HPP efficacy by routine detection methods;
the conversion of the normal form of prions to amyloid forms; and
the induction of virulence, of toxin gene expression, and of cross‐resistance to other stresses.
As clarified in Section 1.2, the whole duration of shelf‐life of the foods is to be considered within the potential microbiological concerns, as it might affect the potential growth of microorganisms during storage.
The following sections summarise the answers provided through the questionnaire (see Appendix A) followed by an assessment of the evidence provided in the scientific literature.
3.1.3.1. Information from the questionnaire
All respondents of the 19 CA and of the equipment providers stated that they were not aware of any microbiological food safety problems originating from food subjected to HPP. Seven out of the 23 respondents of the establishments/companies using HPP reported on the spore concern and one on the HPP effect on prions.
Control of spores. In general, establishments are aware of the difficulties related to spore inactivation when using HPP treatments. One establishment reported that HPP inactivation of bacterial spores remains a challenge due to spore resistance to the HPP treatment limits of currently available industrial HPP units (i.e. ~650 MPa and 50°C). For that reason, another establishment mentioned that they use other hurdles to inactivate spore formers.
Spore activation and germination have also been referred to. One equipment provider replied that, depending on the intrinsic factors of certain foods and the physiological conditions of spores, excessively long cycle time has the potential for bacterial spore activation and possible outgrowth when intrinsic and extrinsic factors are not controlled. This is considered by manufacturers when selecting the commercial production parameters. Another establishment added that HPP can induce the first stage of spore germination of Bacillus spp. (but not of Clostridium spp.). However, the subsequent stages of the germination process are not accelerated, and this germination is not homogeneous due to the great variability within a spore population. The physico‐chemical properties of the food are used to reduce the risk associated with spore presence and germination. One establishment subjects all finished products to a final microbiological analysis and another one evaluates the presence of anaerobic spore‐forming bacteria before and after the HPP treatment. The change from spore‐forming to the vegetative form is avoided by the low pH value of the food and/or by maintaining the cold chain. One establishment reported a contamination problem of ginger and turmeric with Bacillus cereus that was solved using a prewash step of the vegetables before processing. Another establishment observed activation of mesophilic aerobic spores resulting in an increase of the total plate count (TPC) level.
Prions. One establishment reported, without providing the supporting evidence, that the effect of HPP on the structure of proteins depends on their composition. The conversion of the normal (host) form of the prion protein to the disease‐associated amyloid form requires the formation of new molecular interactions within the prion protein structure. The establishment reported that it was observed that high pressure can open the structure of some prions to make them more accessible for digestion by proteinases. However, the effect of HPP on different prions is unknown.
3.1.3.2. Information from the scientific literature
Surviving spores. Similar to other pasteurisation processes (e.g. thermal), HPP at room temperature does not inactivate pathogenic bacterial spores (such as the spores of Clostridium botulinum, Clostridium perfringens, B. cereus) (Evelyn and Silva, 2017, 2019; Silva and Evelyn, 2018).
Spore activation and germination can be triggered by HPP, leading to a more rapid and higher germination rate, and possible outgrowth during storage. The spore germination behaviour of different spore species/strains in response to pressure can vary. For example, C. botulinum spores inoculated (105 CFU/mL) in coconut water (pH 5.2) and HPP treated (550 MPa, 3 min, 10°C) were not able to germinate after 61 days of storage regardless of O2 concentration (< 0.5–11 mg/l) or storage temperature (4 and 20°C) (Gonzalez‐Angulo et al., 2020). On the other hand, a five‐strain cocktail of non‐proteolytic C. botulinum spores inoculated in chicken mince and subjected to a heat process of 80°C – 1 min followed by HPP 600 MPa for 2 min at 20°C were able to germinate and grow up to 106 CFU/g after 6‐week storage at 8°C (Linton et al., 2014). In the case of Clostridioides difficile spores, Doona et al. (2016) did not observe germination upon 150 MPa HPP treatment, but Clostridium perfringens spores released dipicolinic acid (DPA) and became heat sensitive, although most spores did not complete germination. Differences were also observed for B. subtilis strains. For example, Doona et al. (2014) observed 100% germination of three B. subtilis strains after a HPP process of 150 MPa room temperature (37°C) for 2–4 min, while Reineke et al. (2012) concluded that 100% germination of B. subtilis spores required a minimum pressure of 300 MPa (germination occurred up to 700 MPa). In another study, one strain of B. subtilis germinated (> 60%) after 1 min HPP at 150 MPa and 37°C, while 10% germination was reported for two other strains, and one strain presented no germination under the same processing conditions (Luu et al., 2015). The germination of Bacillus amyloliquefaciens with the same HPP process for 5 min was < 10% (Luu et al., 2015). Bacillus licheniformis did not germinate at 550 MPa – 37°C (Borch‐Pedersen et al., 2017).
In addition, the germination response of spores within the same population (species/strain) to HPP is very heterogeneous. For example, Zhang et al. (2020) detected four subpopulations of B. subtilis spores after 150 MPa HPP treatment at 37°C: heat‐resistant super‐dormant spores (unaffected by HPP), heat‐sensitive and culturable germinated spores, heat‐sensitive and partially culturable germinated spores and membrane‐compromised cells with barely detectable culturability.
Taking into account that bacterial spores may germinate during storage, proper control of the cold chain during distribution of HPP treated food is important, especially if the product is held for an extended period under their optimum growth conditions. To reduce the risks linked to the spore germination during storage, challenge tests can be performed to assess if spores of concern for a particular food/beverage are able to germinate and grow during the intended shelf‐life (Demazeau et al., 2018).
Induction of sublethal injury in cells. Severely injured, yet metabolically active, cells of food‐borne pathogens such as Salmonella, E. coli, Shigella and Campylobacter that cannot be resuscitated under routine laboratory conditions can enter a VBNC state and maintain their pathogenicity (Wesche et al., 2009). Depending on the pressure level and holding time, as well as the intrinsic microbial factors, HPP treatment can cause the death of some, but not necessarily all, bacterial cells. Several studies demonstrated the recovery of microorganisms subsequent to a HHP inactivation treatment, indicating sublethal damage caused by the process and the associated repair of the damage (Schottroff et al., 2018).
Ferreira et al. (2016) observed loss of viability followed by growth of L. monocytogenes in HPP treated cheese after one‐week storage at low temperature (4°C). In general, specific product properties can exert pronounced effects due to osmoregulation (Schottroff et al., 2018). The food composition affects the recovery and growth of possible VBNC and surviving cells (MPI, 2019). For example, the acidity of foods prevents or retards the repair and growth of HPP sublethally injured cells. Similarly, high sugar or salt concentrations in the matrix can decrease the susceptibility of the cells to high pressure, associated with the accumulation of compatible solutes inside the cell (Molina‐Hoppner et al., 2004; Gayan et al., 2013; Schottroff et al., 2018).
A direct consequence of the sublethal damage is the overestimation of the efficacy of the HPP, as previously reported for many other inactivation treatments, such as chemical disinfection (Truchado et al., 2021).
The performance of challenge tests to evaluate the potential induction of injured cells during storage of HPP treated food represents a good strategy to minimise this safety concern. In these HPP validation studies, the method used to enumerate surviving cells after HPP should be capable of resuscitating and detecting stressed/injured cells.
Prions or other transmissible spongiform encephalopathy (TSE) agents can be present in specified risk materials (in particular bone or brain tissue) causing infection in humans (Simonin et al., 2012). Prions are misfolded proteins with the ability to act as a template for further misfolding of the normal cellular isoform of the same protein. High pressure has the power to change the protein conformation and can dissociate both native and non‐native oligomers (Simonin et al., 2012). The protein primary structure formed by peptide chains containing covalent bonds between amino acids is not affected by HPP. Similarly, the protein secondary structures, consisting of peptide chains α‐helices and β‐pleated sheets formed by intra‐molecular or intermolecular hydrogen bonds are not ruptured by commercial HPP operations, as pressures above 700 MPa (combined with temperatures above 120°C) are required for irreversible denaturation of prions, causing only 2 log10 reductions in infectivity (Brown et al., 2016). However, the 3D subunits of protein tertiary structure, formed due to non‐covalent bonds between the amino acids of the peptide chains can be changed by pressures above 200 MPa (Rastogi et al., 2007). Likewise, the protein quaternary structure, consisting of a few subunits also aggregated by non‐covalent bonds, is also susceptible to pressure (Rastogi et al., 2007; Silva and Sulaiman, 2019). Prions are very resistant to thermal pasteurisation, and also HPP pasteurisation. Although thermal pasteurisation is not efficient for prion denaturation, a thermal sterilisation of 5 min exposure to 132°C at 0.3 MPa in a steam autoclave destroyed the prion (Brown et al., 2016). Torrent et al. (2004) confirmed that heat and pressure do not lead to identical unfolded protein conformations, and completely unfolded proteins were not achieved by HPP. This study also demonstrated that pressures above 400 MPa could induce protein aggregation, potentially causing a conformational change of prion proteins into potentially pathogenic isoforms. However, the relationship between the changes in protein aggregation and infectivity of the protein has not been proven. Brown et al. (2016) did not find an effect on prion infectivity after a 2‐h treatment at 400 MPa – 60°C or 800 MPa – 60°C. El Moustaine et al. (2008) reported no protein aggregation for neutral and slightly acidic solutions, although irreversible formation of protein aggregates at pH 8.5 for pressures between 350 and 600 MPa was registered. Furthermore, the protein aggregation was reversible, as shown by complete dissolution of aggregates after pressure release (El Moustaine et al., 2008). It is important to highlight that although there are studies demonstrating that HPP treatments can cause folding and unfolding of proteins, the activation of prions after these treatments has not been demonstrated.
Induction of genes related to virulence factors, toxin gene expression and cross‐resistance to other stresses. Some studies evaluated the induction or upregulation of the expression of microbial virulence factors genes by HPP. In general, the application of commercial HPP conditions did not induce virulence, toxin gene expression and cross‐resistance to other stresses in the tested microorganisms. For example, Baptista et al. (2015) demonstrated that HPP up to 600 MPa for 15 and 30 min had no effect on the virulence factors of three strains of S. aureus (enterotoxic and non‐enterotoxic). HPP at 200 MPa did not induce toxin gene expression in STEC (Fang et al., 2017). Sanz‐Puig et al. (2017) registered lower virulence of S. Typhimurium subjected to 250 MPa HPP for 5 min. Martinez‐Gomariz et al. (2009) suggested a reduction of virulence of B. cereus based on different expression of some proteins after 700 MPa. However, Song et al. (2019) used HPP at a low pressure of 52 MPa, as a standard method to induce the expression of genes responsible for encoding a putative toxin by a piezophilic hyperthermophile microorganism. One study conducted with Enterococcus spp. isolates showed HPP for 5 min at 400 MPa or 600 MPa could increase the expression of some virulence genes, although no phenotypic features were observed (Zarzecka et al., 2022).
3.1.3.3. Uncertainty analysis
Sources of uncertainty identified for the microbial food safety concerns considered for HPP treated foods and potentially affecting the conclusions are listed in Table C.1. One source of uncertainty relates to the incompleteness in the identification of those potential concerns. It is expected that all concerns have been identified from the current available evidence. The other source of uncertainty relates to the exclusivity of the identified concerns to HPP and the lack of evidence in the assessment of (an) identified concern(s). Some concerns have been reported, but there is a lack of evidence in the literature. There is enough information to confirm that all the identified safety concerns are not exclusive of HPP. The impact of these two uncertainties on the conclusions is expected to be low.
Table C.1.
Potential sources of uncertainty identified in the assessment of potential microbiological food safety concerns in HPP‐treated food
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Incompleteness in the identification of potential microbiological food safety concerns | It is possible that the final list of potential food safety concerns is not complete. The list was populated relying on the available scientific literature as well as the expert knowledge. | The impact of this uncertainty is expected to be low as it is believed that all potential microbiological food safety concerns have been identified from the current available evidence. In case a potential concern would have been omitted, the concern for human health may have been underestimated. |
| Exclusivity of the identified microbial food safety concerns to HPP and the lack of evidence in the assessment of (an) identified potential microbiological food safety concern(s) | It is possible that the identified food safety concerns, which have been considered common to other thermal and novel inactivation technologies, is exclusive to HPP. Additionally, if could be possible that a potential safety concern has been identified but there is a lack of evidence in the literature for the identified specific microbiological safety concerns. | The impact of this uncertainty is expected to be low because none of the identified safety concerns have been exclusive for the HPP treatment, so the lack of evidence will not impact the final assessment. |
HPP: high‐pressure processing.
3.1.3.4. Concluding remarks
All the identified microbial food safety concerns are shared with conventional treatments such as thermal treatments and with other novel non‐thermal methods. Therefore, any safety concern relating to the application of HPP should be contextualised to account for the potential food safety concerns that are not exclusive to HPP.
Similar to thermal pasteurisation, spores can survive HPP non‐thermal treatments. Spore activation and germination can be observed after HPP treatments, depending on storage conditions. Proper control of the cold chain (temperature < 7°C) during distribution and storage prevents outgrowth of most germinated spores.
Sublethally injured cells, such as those in the VBNC state, have been recovered after HPP, indicating sublethal damage caused by the process and the associated repair of the damage.
The induction of sublethally injured cells in HPP‐treated foods can be minimised by the application of optimised conditions identified based on appropriate validation studies (e.g. challenge tests) carried out with the commercial HPP food/beverage product inoculated with specific resistant strains of microorganisms.
Prions are highly resistant to heat and HPP, but it has been shown that HPP can promote changes in the proteins’ normal conformation. However, an increase of prion infectivity due to HPP has not been demonstrated.
No study has demonstrated the induction of the expression of genes related to virulence factors, toxin gene expression and cross‐resistance to other stresses by HPP operating at commercial pressures. However, more studies are needed to draw definitive conclusions, as literature is scarce.
The uncertainty related to the microbiological food safety concerns was considered low and it is judged 99–100% certain (almost certain) that all the relevant potential microbiological food safety concerns associated with HPP were identified and that none were exclusive for HPP.
3.1.4. Potential chemical food safety concerns in HPP‐treated food through formation of process contaminants
3.1.4.1. Information from the questionnaire related to food contaminants
All 19 respondents of the CAs, 19 of 23 respondents of the establishments and the two respondents of the equipment providers replied that they are not aware of studies on the effect of HPP on the potential formation or degradation/modification of contaminants in foods during and after HPP treatment. One establishment stated that, by definition, HPP do not induce the formation or dissociation of strong covalent bonds. Therefore, according to that establishment, the HPP process will not ‘induce the formation or dissociation of strong molecular interactions (hydrogen bonds, covalent bonds, etc.). Therefore, the HPP process will not form food contaminants. Likewise, it cannot destroy low molecular weight compounds such as food toxins.’ Another establishment stated that ‘different studies obtained showed that high pressure affected in different ways the different stages of the Maillard reaction and that such effects were strongly influenced by pressure‐induced changes in the pH of the systems. This aspect is important overall when HPP is combined with temperature’. The answers to the questionnaires included suggestions for reports and review articles associated with the formation/degradation/modification of contaminants in HPP‐treated foods. These were considered and used as a source to retrieve primary studies, if relevant.
3.1.4.2. Safety concerns through the formation of process contaminants in HPP‐treated food compared to untreated food or food submitted to treatments routinely applied to these foods, with the purpose to increase microbiological food safety
The chemical changes that would lead to the formation or transformation of contaminants in HPP‐treated food compared to untreated food, or food conventionally treated, are evaluated. Consequently, chemical changes that are normally expected to happen in foods during processing such as lipid oxidation not related to contaminants (e.g. in Wiggers et al., 2004; Gou et al., 2010; Escobedo‐Avellaneda et al., 2011; Borda et al., 2013; Medina‐Meza et al., 2014; Yi et al., 2017; Carrera et al., 2020) are not considered.
The effects of HPP on the food system follow the Chatelier’s principle because the increase in pressure affects the equilibrium of the system that will lead to a reduction in the volume by e.g. changes in molecular configuration and reactions. Increase in pressure can therefore alter the interatomic distances and high pressure may affect weak interactions in the molecules such as hydrophobic interactions of proteins, electrostatic forces and van der Waals forces, and, therefore, could disrupt cell membranes and protein structures (Medina‐Meza et al., 2014; Hao et al., 2016; Woldemariam and Emire, 2019) but should not normally affect the formation or disruption of covalent bonds. Therefore, in principle, it is expected that organic contaminants will not be formed or transformed during HPP (Medina‐Meza et al., 2014; Hao et al., 2016; Woldemariam and Emire, 2019).
Information on process contaminants like furans, 2‐monochloropropane‐1,2‐diol and 3‐monochloropropane‐1,2‐diol (2‐ and 3‐MCPD), hydroxymethylfurfural and acrylamide is available only for high‐pressure thermal sterilisation (HPTS) which is beyond the scope of this evaluation. However, due to the low temperature used in the process, processing contaminants are not expected to be formed.
As described above, HPP can disrupt or damage the cell membrane leading to cell death also for some bacteria and fungi. Therefore, the growth of microorganisms that can form toxins in the treated food product will be decreased and the amount of toxins is expected to be lower due to the HPP treatment compared to non‐treated products. The decrease in the mycotoxin formation has been shown for several fungi‐producing citrinin, deoxynivalenol (DON) and zearalenone (ZEA) (e.g. Tokusoglu et al., 2010; Huang et al., 2014; Kalagatur et al., 2018). Studies that reported on the mycotoxin levels are summarised in Table 2.
Table 2.
Studies reporting on contaminants levels in food following HPP under specified conditions
| Contaminant | Food | Pressure | Temperature | Time | Levels of contaminant | Reference |
|---|---|---|---|---|---|---|
| Patulin | Apple juice | 300, 400, 500 MPa | 20, 30, 40, 50°C | 5 min | ↓ | Avsaroglu et al. (2015) |
| Juices | 400, 500, 600 MPa | 11°C | 1, 3, 5 min | ↓ | Hao et al. (2016) | |
| Tomato juice | 600 MPa | 28°C | 5, 10 min | Unchanged | Scaccabarozzi et al. (2020) | |
| Citrinin | Olives | 100–400 MPa | 4°C or 25°C | 4–5 days | ↓ | Tokuşoğlu et al. (2010) |
| DON, ZEA | Maize | 100–550 MPa | 30–60°C | 10–30 min | ↓ | Kalagatur et al. (2018) |
| PSP toxins | Oysters | 200, 400, 600 MPa | 5°C or 35°C | 1 + 4 min or 5 min | Unchanged | Turner et al. (2014) |
| Cadmium | Oysters | 300 MPa | N/A | 90 s | Unchanged | Rasmussen and Morrissey (2007) |
DON: deoxynivalenol; N/A: not applicable; PSP: Paralytic Shellfish Poison, ZEA: zearalenone; ↓: decreased levels.
Studies on the stability on mycotoxins as such are limited to patulin (also reported in Table 2). In the papers by Avsaroglu et al. (2015) and Hao et al. (2016), changes in juices spiked with patulin before HPP treatment were studied. Patulin concentrations decreased when HPP was applied to the samples. According to the authors, the results could be explained by formation of adducts with compounds in the juice that possess sulfhydryl groups. Only cysteine adducts of patulin have been studied and according to Lindroth and von Wright (1990) are less toxic than patulin itself. These findings could not be supported by Scaccabarozzi et al. (2020) who studied the impact of HPP in tomato juice that was spiked with patulin. According to the authors, no change in patulin concentration was obtained after the HPP treatment.
For contaminants other than mycotoxins, also very limited information has been identified.
In 2007, AFSSA published a dossier evaluation of HPP treatment of duck meat that was dried and/or smoked. The conditions of the HPP treatment were briefly described as 600 MPa for 5 min with a temperature in the meat of about 25–27°C. The French Agency concluded that the contents of nitrosamines and polycyclic aromatic hydrocarbons (PAHs) were comparable in the reference product and the treated products (AFSSA, 2007). At the follow up evaluation of this dossier in 2010, the agency concluded that the mentioned HPP treatment does not significantly modify the content of undesirable substances in foods (ANSES, 2010).
In a study by Turner et al. (2014), the storage stability of Paralytic Shellfish Poison (PSP) toxins was studied as part of the production of reference material. Oyster tissues were prepared with different levels of toxins and exposed to HPP. The biological activity was reduced substantially after HPP treatment compared to control samples. The toxins analysed were: Gonyautoxins (GTX) 1&4, 2&3; neosaxitoxin (neoGTX) 5; saxitoxin (STX) dihydrochloride, decarbamoylsaxitoxin (dcSTX), N‐sulfocarbamoyl toxins (C1&2), decarbamoylneosaxitoxin (dcneoSXT), decarbamoylgonyautoxins (dcGTX) 2&3. Overall, no differences were obtained in the concentrations between the control samples and the HPP treated samples when the standard deviation was taken into account. Only for 200 MPa, 5 min, 5°C, the results were in general lower compared to the concentrations in the control sample.
Rasmussen and Morrissey (2007) studied the concentration of cadmium (Cd) in pacific oysters after processing and storage. The study was performed because HPP treatment causes the release of the oyster adductor muscle, and therefore, no shucking is needed after HPP treatment. Effects of HPP at 300 MPa for 90 s (temperature not stated) treatment followed by washing, draining and jar‐packing were examined. The treated and packed samples were analysed at days 0, 5 and 10 and compared to samples that had been subjected to shucking by a conventional method, draining, washing and jar‐packing. The concentration of Cd after HPP treatment was significantly lower compared to the conventional groups (1.1 mg/kg compared to 1.4 mg/kg) but on day 10 the concentrations were similar (0.8 mg/kg and 0.9 mg/kg, resp.).
3.1.4.3. Uncertainty analysis
It is a major uncertainty that there are only few studies available on the impact of HPP on the formation or transformation of a limited number of contaminants in treated foods. However, the literature is consistent so far in not reporting any significant HPP‐related increase in the levels of these contaminants in foods compared to foods treated with conventional processes. Also due to the low temperature used in the process, processing contaminants are not expected to be formed.
Based on expert judgement, the certainty that mycotoxins and process contaminants evaluated in this scientific opinion will not present an increased safety concern in HPP treated food compared to conventional food would correspond to ‘extremely likely’ or higher, i.e. above 95% (EFSA CONTAM Panel, 2017).
3.1.4.4. Concluding remarks
The responses to the questionnaires did not indicate concerns on the formation or transformation of contaminants when HPP under the conditions examined in this mandate are applied.
There are few studies available on the impact of HPP on the formation or transformation of a limited number of contaminants in treated foods.
The limited literature is overall consistent so far in not reporting any significant HPP‐related increase in the levels of these contaminants in treated foods compared to foods treated with conventional processes.
Due to the low temperature used in the process, processing contaminants that have been evaluated in this scientific opinion are not expected to occur (see above).
Based on expert judgement, it is concluded with more than 95% certainty (extremely likely or higher) that mycotoxins and process contaminants evaluated in this scientific opinion will not present an increased concern due to HPP treated food intake compared to conventional food.
3.1.5. Potential chemical food safety concerns in HPP‐treated food through food contact materials
3.1.5.1. Introduction
The question to be answered is whether HPP has an effect on the migration from FCM to foods, thus, on the consumer exposure and food safety, with an emphasis on whether it may increase migration and/or form new migrating substances. For the legal framework, an answer is needed as to whether the current migration testing for plastics, i.e. the regulated simulants, the testing conditions and the applied correcting factors for simulant D2 (simulating fatty food), covers FCM used with HPP. To this end, the evaluation is focussed on the mechanisms described below that are involved in HPP of packed food and that could, in principle, have an effect on migration.
Migration from an FCM is controlled by diffusion and partitioning coefficients that are influenced notably by material characteristics like crystallinity, the ratio of ordered to amorphous regions of polymers and the glass transition temperature (Tg), which may be affected by HPP.
HPP and the compression heating may affect the food, such as melting fat (although the melting points of fats are increased under pressure) and increase the area of direct contact between the food and the FCM. Both may have an effect on the migration from the FCM.
Upon release of the pressure, there is immediate decompression cooling. The properties of the food and the FCM may or may not restore fully. The duration of storage after HPP is much longer (weeks to months) compared to the duration of the HPP step (few minutes). Therefore, a significant effect of HPP on migration over the whole life‐time of a packaged food could only be expected if there is either a strong change in migration during the short HPP duration or if a possibly weaker effect persists as a long‐lasting change in the properties of the food or the FCM.
High pressure can affect chemical reactions, accelerating or slowing these. It may, in principle, form new compounds in the food as well as in the FCM or increase/decrease the rate of such reactions.
3.1.5.2. Information from the questionnaire as relating to food contact materials
From the questionnaire (see Section 3.1.1), it seemed that each food producer using HPP has a preferred food packaging material. Most of the commonly used plastics were reported: specifically, various types of polyolefins, PET, EVOH, polyamides and polystyrene, as monolayers or multilayers (Table A.5). No problematic plastics were mentioned, such as multilayers with a metal layer, known from literature to sometimes cause integrity problems. Packaging materials appeared to be considered as a minor issue or a non‐issue. Where migration was addressed, it was reported that overall migration is not affected by HPP.
Since in the current applications the temperature before pressure increase does not exceed 20°C (Table A.5), the maximum temperature of the FCM resulting from compression heating during HPP can be estimated. Pressure increase takes 1–2 min and the temperature will be largely determined by heat exchange with the pressurising fluid outside and the food inside the pack. For water, compression to 600 MPa implies a temperature increase of about 6 × 3°C/100 MPa, resulting in a maximum of 38°C (when the initial water temperature is 20°C). If the food in contact with the plastic consists of fat, e.g. for a food immersed in oil, and a temperature increase of 9°C/100 MPa is assumed for fat, the maximum could, in principle, be 74°C. Hence, the midpoint FCM temperatures would be up to around 55°C.
Food storage after HPP treatment ranges from refrigeration for several days to storage at ambient temperature for several months (corresponding to ‘all storage times of up to 6 months at room temperature’12 simulated for compliance testing for 10 days at 50°C).
Looking to the future, two developments were noted. First, the next generation of HPP equipment might be capable of sterilisation. This would require higher temperatures (90–120°C; also called HPTP) and/or higher pressures. The resistance of the FCM to these conditions seems to be an important challenge. HPTP is, however, out of the scope of this mandate. Second, equipment providers have commercial machinery for in‐bulk processing. In‐bulk processing involves the use of a holding bag (also called a bladder) for the liquid food/beverage that is to be processed. Repeated pressurising‐depressurising may cause material fatigue and may change the migration properties of the bag.
3.1.5.3. Effect of HPP on the migration potential of substances
In some of the studies used for the assessment, a higher pressure and/or temperature were applied compared to those used commercially, as reported in the questionnaires and also in the background of the mandate. Such conditions are more severe for FCM and are, therefore, expected to better reveal potential effects. Moreover, if no concerns arise from using these conditions, it is also not expected under milder commercial conditions.
Permeation studies
Of the over 2,100 records screened by title and abstract, 60 were taken for full‐text evaluation on the topic of permeation and 11 were considered relevant and reliable for inclusion in this assessment.
Food packaging plastics are frequently specified for their gas barrier properties, since this can influence the keeping properties of the food. So, studies are available on the influence of HPP, if any, on gas permeation rates. Since permeation is a measure of the diffusion of molecules through the polymer network, these studies give indirect information on the likely effects of HPP on chemical migration to foods.
Nine studies on gas permeation were considered as relevant and reliable for this assessment (Caner et al., 2000; Lambert et al., 2000a; López‐Rubio et al., 2005; Le‐Bail et al., 2006; Yoo et al., 2009; Bull et al., 2010; Koutchma et al., 2010; Largeteau et al., 2010; Sterr et al., 2015). The gas most frequently studied was oxygen (8/9) followed by water vapour (4/9) and then carbon dioxide (1/9). In a typical experimental set‐up, described here for illustrative purposes only, the packaging film was made into pouches which were filled with water and then subjected to HPP. The pressure applied in these nine studies covered the range of 200–800 Mpa with the most commonly applied pressure being 500–600 MPa. The temperatures used in these studies covered the range from −20°C to 121°C with the temperature most commonly used being around 20°C. The pouches were then emptied, and the films tested for permeation of the gas. Thus, the test was performed after a period of time had elapsed after HPP. No tests were reported on gas permeation during HPP. For our purposes, a common shortcoming of the studies is that the control sample giving the reference permeation rate was typically a film taken ‘straight off the reel’ and not subjected to liquid contact, etc., as done with the HPP pouches.
In the selected studies, there was no significant effect on the gas transmission rates through the films following HPP. In contrast, increased permeation was reported in several papers that were amongst those 60 taken for full text evaluation but were not considered as relevant and reliable for this assessment. They are mentioned here to illustrate the potential pitfalls if the gas barrier is provided by a thin metalised or inorganic layer (or similar) in a multilayer film structure. It was observed that mechanical stress and deformation during HPP can damage (e.g. crack) the layer structure. Also, eruption of gas bubbles during the pressure release stage can give rise to pinholes, blisters and delamination that compromises the barrier properties. Such damage causes permeation rates to increase and presents pack‐integrity problems. From the answers provided in the questionnaire, it seems that these problems have been mastered by the industry by a proper selection of the food packaging materials and do not occur in practice.
Two publications were identified where the permeation of larger molecules was studied, both during and after HPP. The permeants were raspberry ketone (4‐(4‐hydroxyphenyl)butan‐2‐one, MW = 164 Da) (Schmerder et al., 2005) and raspberry ketone along with benzoic acid (MW = 122 Da), carvacrol (5‐isopropyl‐2‐methylphenol, MW = 150 Da) and β‐ionone ((3E)‐4‐(2,6,6‐trimethylcyclohex‐1‐en‐1‐yl)but‐3‐en‐2‐one, MW = 192 Da) (Richter et al., 2010). The films were monolayers of polyamide (PA) (Schmerder et al., 2005) and PA along with low‐density polyethylene (LDPE) (Richter et al., 2010). During HPP, permeation rates were reduced several fold, but after HPP, they returned to their original values. Analysis of the permeation kinetics indicated that this effect was due to reduced diffusion coefficients brought about by compression of the polymer which then relaxed back (within minutes or less) when the pressure was released.
Overall, these data support that HPP compresses the FCM and slows permeation. Upon depressurising, data indicate that FCM relax rapidly back to their original state and their rate of permeation during subsequent storage return to pre‐HPP values.
Migration studies
Of the over 2,100 records screened by title and abstract, 17 were taken for full‐text evaluation on the topic of migration and six were considered suitable for this assessment. Additionally, a paper published in early 2021 that was discussed as a prepublication during the technical hearing held with the author (a representative of the US‐FDA) was included.
For 11 of the 18 records evaluated, migration tests were ancillary to studies on structural and physical changes of packaging submitted to HPP and could not be used. For instance, there were no adequate controls, the descriptions of the analytical results were insufficient, the packaging materials did not withstand the HPP conditions or the packaging materials were developed for research purposes.
Six studies were scored as suitable for this assessment. Either pouches were made with the studied polymers, filled with food simulant, submitted to HPP and then stored under defined T/t conditions (Lambert et al., 2000b; Caner and Harte, 2005; Yoo et al., 2014), or polymer strips were immersed in food simulant (Mauricio‐Iglesias et al., 2010), or polymers were in contact with food (Rivas‐Canedo et al., 2009a,b) and subjected to HPP followed by storage. The migration levels, measured in the simulants or derived from the amount lost from the film, were compared with the migration results from control samples that were similarly treated, but without undergoing HPP.
The studies used monolayer polyolefin films (Caner and Harte, 2005; Mauricio‐Iglesias et al., 2010; Yoo et al., 2014) or multilayers (Lambert et al., 2000b; Rivas‐Canedo et al., 2009a,b). In all cases, the food contact layer was a polyolefin. The simulants used were according to the Commission Regulation (EU) No 10/2011.12 Specific migration of two additives was followed for the monolayers: (i) the antioxidant octadecyl‐3‐(3,5‐di‐tert‐butyl‐4‐hydroxyphenyl)‐propionate, MW = 531 Da (Caner and Harte, 2005; Mauricio‐Iglesias et al., 2010; Yoo et al., 2014) and (ii) the light stabiliser 2,5‐thiophenediylbis(5‐tert‐butyl‐1,3‐benzoxazole, MW = 431 Da (Mauricio‐Iglesias et al., 2010). Overall migration was monitored for the multilayers.
In some studies (Lambert et al., 2000b; Rivas‐Canedo et al., 2009a,b; Yoo et al., 2014), the applied HPP conditions (up to 600 MPa and up to 25°C) were in the range currently used commercially, while in others (Caner and Harte, 2005; Mauricio‐Iglesias et al., 2010; Yoo et al., 2014), they were higher (up to 800 MPa and up to 115°C). The HPP treatment lasted 5–10 min (Caner and Harte, 2005; Rivas‐Canedo et al., 2009a,b; Mauricio‐Iglesias et al., 2010; Yoo et al., 2014), except 30 min in one report (Lambert et al., 2000b). In one study (Mauricio‐Iglesias et al., 2010), the concentration of the two migrated additives was measured immediately after HPP, while in the others (Lambert et al., 2000b; Caner and Harte, 2005; Rivas‐Canedo et al., 2009a,b; Yoo et al., 2014), the migrating substances were measured only after subsequent storage at different time points up to 40 days at 23–25 or 40°C.
In the four studies on the migration into food simulants (Lambert et al., 2000b; Caner and Harte, 2005; Mauricio‐Iglesias et al., 2010; Yoo et al., 2014), specific and overall migration after HPP and storage was not significantly changed with respect to the untreated control samples. Except for one experiment, the migration was not tested immediately after HPP and, therefore, any impact of HPP may not have become apparent, being masked by the migration during the far longer storage period. In the only study (Mauricio‐Iglesias et al., 2010) that determined the migration immediately after HPP (800 MPa), there was no significant change in the specific migration of the tested additives compared to the controls.
When studying migration into foods, in two studies (Rivas‐Canedo et al., 2009a,b) on the effect of HPP on pre‐packed sliced meat products (Spanish dry‐fermented salchichón sausage and dry‐cured Serrano ham), conflicting results were obtained. The studies focused on the release from the meat samples and scalping (sorption) in the plastic of aroma and flavour substances, but substances attributed to the LDPE/ethylene vinyl acetate (EVA)/vinylidene chloride (VDC) packaging material were also detected in the foods. The application of HPP at 400 MPa for 10 min at 12°C followed by storage for 3 days at 4°C caused a decrease in the migration of packaging‐derived hydrocarbons into ham, but an increase into sausage, compared to no‐HPP controls. Compression heating may have caused some melting of the sausage fat and a more intimate contact with the plastic packaging by compressing air pockets, thereby increasing migration.
The Song et al. (2021) paper was considered as the most extensive and robust, hence is more thoroughly described here. Ethylene‐propylene copolymer films were spiked with low molecular mass organic compounds (chloroform, toluene, methyl salicylate and phenylcyclohexane) selected to model the migration of molecules representative of general classes of volatile polar, volatile non‐polar, non‐volatile polar and non‐volatile non‐polar substances. The films were placed into pouches made of fluorinated ethylene propylene copolymer and then Miglyol 812 (a medium‐chain triglyceride mixture) or 10% ethanol were poured into the pouches as food simulants. The samples were subjected to HPP (700 MPa, 71°C peak temperature, 5 min) using the following cycle run: preheating for the pouches, come‐up (compression), holding and come‐down (decompression) followed by cooling. To distinguish the effect of pressure on the migration, a comparable thermal processing (TP) at atmospheric pressure (0.1 MPa) was developed. The migration level in the simulant was analysed for samples withdrawn at the initial point, after preheating (451 s), after come‐up (498 s) and after holding at 0.1 MPa or 700 MPa for 5 min duration (798 s). The migration was also analysed with further storage at 25°C up to 5 days (with five sampling time points) for Miglyol or up to 10 days for 10% ethanol. The content of the four model substances that remained in the films at each time point was determined too, to check for the mass balance.
The results indicated that during HPP the migration of the model substances from polypropylene (PP) into Miglyol and 10% ethanol significantly decreased in comparison with the TP controls. In the Miglyol test, the migration of phenylcyclohexane decreased the most during the compression cycle of HPP when compared to the TP, followed by toluene, chloroform and methyl salicylate. These differences were attributed to the influence of molecular size and the different affinities to the non‐polar plastic. In 10% ethanol, a significant reduction of migration in comparison to the TP was observed in the compression cycle for chloroform, toluene and methyl salicylate, while the migration of phenylcyclohexane showed only a small decrease. This was attributed by the authors to partitioning effects due to different affinities for the polar simulant.
Diffusion coefficients of the model substances estimated by modelling were at least two orders of magnitude lower under high pressure than at atmospheric pressure. The glass transition temperature of the PP increased under HPP, which is in line with the observed decrease of diffusivity and consequently, with a decrease in migration.
With Miglyol, after HPP and during the storage period (up to 5 days) at 25°C, the migration was initially slower compared to the untreated samples, but after 8–24 h, the migration rates showed comparable profiles for both the HPP treated and untreated samples.
With 10% ethanol, after HPP and during the extended storage period (up to 10 days) at 25°C, the migration was also slower at the early stages compared to the untreated films, but as with Miglyol this difference became insignificant after 8–24 h of storage.
In conclusion, the studies support that the compression of the plastics used as packaging material tends to reduce the migration during the HPP period compared to the same conditions without HPP. Upon decompression, the materials return to their original diffusion properties as the polymer chains relax to their original free volume state and Tg. This reduction in migration may, however, be negligible after storage, since the duration of storage of the packed foods far exceeds that of HPP and the temperatures involved in HPP are in the same range, i.e. migration during the storage dominates and renders the effect of HPP insignificant.
3.1.5.4. Effect of HPP on the formation of reaction/degradation products in FCM
As mentioned in Section 3.1.4.2 on the formation of process contaminants in HPP‐treated food, increasing pressure may, in principle, induce the formation of new reaction products or enhance their concentration. It accelerates chemical reactions if they are accompanied by a reduction in the molecular volume and, conversely, slows them if the volume increases (Le Chatelier Principle). However, no observations have been reported that such reactions are relevant in FCM. Pressure can have a pronounced effect on the rate of gas‐phase reactions, since compression of the gas(es) gives rise to a greater frequency of intramolecular collisions, but this effect is far less important for liquids and solids, such as foods and FCM, that have a lower compressibility.
At the initial fluid temperatures presently applied in HPP (up to 20°C, Table A.5) and/or within the scope of the terms of reference (≤ 45°C including compression heating), pressures of around 600 MPa have significant effects only when transition activation energies are low. As HPP is short and temperatures are mild, the chemical stress HPP exerts is insignificant compared to that during the total lifetime of the packaging, particularly when taking into account the usually high temperature employed in manufacturing the plastic FCM.
3.1.5.5. Effect of HPP on food that may influence migration
HPP may increase chemical migration into food by causing a more intimate contact. To this end, the types of contact should be distinguished.
Contact may be direct as for liquid foods, which means that the molecules of the food and the FCM touch each other and the transfer does not require a high transition energy.
Contact may be indirect as for foods only partially touching the FCM. If there is a gap between the food and the FCM of at least a few nanometres, transfer presupposes that the energy of a molecule exceeds that of its solvation in the FCM, i.e. it must evaporate and diffuse through the gas phase before it can reach the food and recondense there. Migration is almost entirely through the gas phase, which restricts migration to substances of at least slight volatility over the FCM surface (boiling points up to ca. 300–400°C for storage at ambient temperature).
Contact may be a mixture of direct and indirect contact for many solid foods. For instance, sliced meat in a vacuum pack will have predominantly direct contact. Fat and lean meat establishes contact, but there remain indentations into which the plastic film does not enter. HPP compresses air pockets – more than ambient pressure acting on vacuum packs – and increases the area of direct contact, which is likely to increase migration of some substances.
A question relevant for the mandate is whether conventional migration testing covers HPP. It was considered that it would do so as long as the testing is by a liquid simulant, since liquids make complete contact. The situation is different for dry foods for which the simulant specified is poly(2,6‐diphenyl‐p‐phenylene oxide) (MPPO, simulant E). HPP would press the MMPO powder particles against the dry food as well as against each other, promoting migration. However, this effect was considered as weak, since gas pockets are likely to be restored after HPP and the duration of HPP is short compared to the storage duration. Also, according to the answers to the questionnaires, no dry food is submitted to HPP.
For the migration testing for some fatty foods with simulant D2, legislation provides for the application of a ‘correcting (dividing) factor’ to account for the exaggeration of using oil or its substitutes as fatty food simulants D2 (Commission Regulation (EU) No 10/2011).13 This ‘correcting factor’ takes several factors into account, the relevant one here being that food contact is only partial (e.g. chocolate, fatty confectionery products, meat products). Since HPP can establish additional direct contact zones, in principle the corresponding correcting factors could be reduced accordingly. However, the Panel also considers this effect as weak for the same reasons as given above for MMPO.
HPP may melt fat in a food (e.g. meat), increasing fatty contact. However, the fatty food simulant provided for FCM is liquid (i.e. Table 2 of Annex II in Commission Regulation (EU) No 10/2011), which covers this situation.
HPP affects solubility in liquids, i.e. may change the solubility of sparingly soluble components in foods or simulants, such as substances of low polarity in aqueous media for which migration is determined by solubility. However, solubility is likely to decrease (which would give rise to lower migration), and also the effect would only be relevant over the short period of HPP.
Overall, HPP may intensify the food contact and (slightly) increase migration into food, but this is covered when testing FCM with liquid simulants. Effects on the ‘correcting (dividing) factor’ are too small to require any change. Moreover, since the migration during the storage dominates, the possible effect of HPP due to the increased area of contact over the short period of treatment is considered negligible.
3.1.5.6. Uncertainties and limitations
The experimental studies include a range of plastic FCM that are representative of the materials used in current commercial HPP processes along with conditions of time, temperature and pressure that are the same or more severe than those in current commercial processes. The experimental findings on migration are backed‐up by permeation studies, by investigations of related material properties and by theoretical considerations, that all arrive at the same general conclusions. Considering the relevance and reliability of these lines of evidence and the consistency between them, there was a high level of certainty in these conclusions.
With regard to the possible application of in‐bulk HPP, the migration behaviour of polymers under repeated HPP uses has not been reported in the literature. It is noted that normally, without HPP, migration levels generally decrease when a polymeric material is used for repeated contact.
3.1.5.7. Concluding remarks
According to the answers from the questionnaires, all packaging materials used for foods that are submitted to HPP are plastics. The commercially used plastics have been adequately covered in the literature studying the effect of HPP.
The studies with liquid food simulants are consistent in not reporting significantly increased HPP‐related migration compared to the same conditions without HPP. Indeed, in the most diagnostic studies, there is a decrease in migration/permeation during HPP compared to contact without HPP. In principle there may be an increase when the food contact is intensified and if MPPO is used for simulation, but since the duration of storage of the packed foods far exceeds that of HPP, whereas the temperatures involved are in the same range, migration during the storage dominates and renders any effects during HPP insignificant.
As regards the possible formation of reaction products, the HPP step is short and at mild temperatures, so that the chemical stress it exerts is expected to be insignificant compared to that during the total lifetime of the packaging, particularly when taking into account the usually high temperature employed in manufacturing the FCM.
Since there is no significant contribution to migration during HPP (migration during the storage dominates), the migration testing performed with food simulants does not need to include the HPP step.
It is concluded that the use of HPP does not give rise to additional chemical food safety concerns from FCM in HPP‐treated food compared to food treated under similar T/t conditions without HPP.
3.2. Efficacy of HPP when applied to raw milk and raw colostrum from ruminants
3.2.1. Additional hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants
The hazards referred to in ToR2 as Mycobacterium spp. and Brucella spp. were further specified as Mycobacterium bovis and Brucella melitensis according to the scientific opinion by the BIOHAZ panel related to raw drinking milk (EFSA BIOHAZ Panel, 2015) and the single outbreak of Brucella melitensis in raw drinking milk.
In addition to those hazards, mandated by ToR2 (Mycobacterium spp., Brucella spp., L. monocytogenes, Salmonella spp. and STEC), some hazards have been included in the present assessment as shown in Table 3. Campylobacter spp. and tick‐borne encephalitis virus (TBEV) were added as these were also identified as main microbiological hazards in the above‐mentioned scientific opinion (EFSA BIOHAZ Panel, 2015). These two hazards were also involved in FBO associated with consumption of contaminated milk (TBEV with genus name Flavivirus).
Table 3.
Selection of the hazards to be reduced by thermal pasteurisation of raw milk and raw colostrum from ruminants based on the Terms of Reference of the mandate, on their identification in the scientific opinion by the BIOHAZ panel related to raw drinking milk (EFSA BIOHAZ Panel, 2015) and whether they were reported as a FBO agent in the ‘strong and weak evidence’ food‐borne outbreaks (FBO) with milk as a vehicle EU/EEA in EFSA’s zoonoses database (2008–2019)
| Hazard | Mandated in ToR2 | Identified in RDM scientific opinion | Involved in strong and weak evidence FBO | Included or excluded from the assessment with justification |
|---|---|---|---|---|
| Mycobacterium bovis | Y (as Mycobacterium) | Y | N | Included as listed in ToR2 |
| Brucella melitensis | Y (as Brucella) | Y | Y (1 B. melitensis in raw milk) | Included as listed in ToR2 |
| Listeria monocytogenes | Y | N | N | Included as listed in ToR2 |
| Salmonella spp. | Y | Y | Y (22 Salmonella) | Included as listed in ToR2 |
| Shiga toxin‐producing Escherichia coli | Y | Y | Y (11 E. coli) | Included as listed in ToR2 |
| Campylobacter spp. | N | Y | Y (84 Campylobacter) | Included based on RDM scientific opinion and FBO |
| Tick‐borne encephalitis virus | N | Y | Y (17 Flavivirus) | Included based on RDM scientific opinion and FBO |
| S. aureus | N | N | Y (26 Staphylococcal enterotoxins and Staphylococcus) | Included based on FBO |
| Bacillus cereus | N | N | Y (6 B. cereus in pasteurised cows’ milk, vanilla milk, milk, milk) | Excluded because spore‐forming bacteria |
| Yersinia enterocolitica and Yersinia pseudotuberculosis | N | N | Y (1 Y. pseudotuberculosis in unpasteurised milk) | Excluded because caused relatively few FBO and limited data support the relevance to the safety of raw drinking milk |
| Cryptosporidium | N | N | Y (1 Cryptosporidium in raw milk) | Excluded because caused relatively few FBO and limited data support the relevance to the safety of raw drinking milk |
FBO: food‐borne outbreak; N: no; RDM: raw drinking milk; ToR: Terms of Reference; Y: yes.
Staphylococcus aureus, B. cereus, Yersinia pseudotuberculosis and Cryptosporidium were also involved in FBO associated with consumption of contaminated milk. Of these, only S. aureus was included in the current assessment, as it is a toxigenic bacterium. S. aureus is highly prevalent and at high concentrations in raw milk and, as a coccus, it is expected to be more HPP resistant than bacilli. Therefore, if the background biota is reduced by HPP, it may overcome the competitive disadvantage and grow to toxin producing levels during post‐treatment storage. HPP, similarly to pasteurisation, might be a potential treatment aiming to eliminate or reduce the population of this organism. This is expected to contribute to the prevention of enterotoxin production until consumption of treated milk. Bacillus cereus was excluded because it is a spore‐forming bacterium. Yersinia and Cryptosporidium were also excluded because they caused relatively few FBO and there is limited data supporting their relevance to the safety of raw drinking milk (EFSA BIOHAZ Panel, 2015).
3.2.1.1. Uncertainty analysis
The main source of uncertainty identified for the selection of the hazards to be reduced by thermal pasteurisation of raw milk/colostrum from ruminants is presented in Table C.2 and refers to potential microbiological hazards of public health concern not being identified. The selection for the additional most important hazards was based on the scientific opinion by the BIOHAZ panel related to raw drinking milk (EFSA BIOHAZ Panel, 2015) and the best available information on FBO in the EU/EEA during a 10‐year period implicating milk as the food vehicle. The impact of this uncertainty on the conclusions is therefore expected to be low.
Table C.2.
Potential sources of uncertainty identified in the assessment of the additional hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Incomplete identification of the additional hazards of concern to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants | It is possible that the final list of eight microbiological hazards of major concern to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants is not complete. The list was populated relying on their identification in the scientific opinion by the BIOHAZ panel related to RDM (EFSA BIOHAZ Panel, 2015) and their reporting as a FBO agent in the ‘strong and weak evidence’ FBOs with milk as a vehicle in the 2008–2019 period. | The impact of this uncertainty is expected to be low as it is believed that the most important microbiological hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants have been identified using the currently available evidence from the specified sources. |
FBO: food‐borne outbreak; HPP: high‐pressure processing; RDM: raw drinking milk.
3.2.1.2. Concluding remarks
In addition to M. bovis, B. melitensis, L. monocytogenes, Salmonella spp. and STEC described in ToR2, the other relevant hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants are Campylobacter spp., TBEV and S. aureus.
The uncertainty on the identification of microbiological food safety hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants was considered low and it is judged 99–100% certain (almost certain) that the currently most important microbiological hazards have been identified using the specified data sources.
3.2.2. Pathogen reduction by thermal pasteurisation in raw milk/raw colostrum from ruminants
The log10 reduction of the relevant hazards defined in Section 3.2.1 using thermal pasteurisation of raw milk and raw colostrum from ruminants using the minimum legal requirements were derived in order to define the minimum HPP requirements to achieve an equivalent efficacy to that of thermal pasteurisation.
Part II of Chapter II of Section IX of Annex III to Regulation (EC) No 853/20041 laying down specific hygiene rules for food of animal origin describes the specific requirements for heat treatment of raw milk, colostrum and dairy or colostrum‐based products. FBOp must ensure that the treatment satisfies the requirements laid down in Chapter XI of Annex II to Regulation (EC) No 852/200414. In particular, they shall ensure that thermal pasteurisation is achieved by a treatment involving at least 72°C for 15 s (i.e. high temperature short time, HTST); at least 63°C for 30 min (i.e. low temperature long time, LTLT); or any other combination of T/t conditions to obtain an equivalent effect, such that the products show, where applicable, a negative reaction to an ALP test immediately after such treatment. The pasteurisation process of milk has been described in EFSA (2021a).
3.2.2.1. Thermal inactivation parameters of pathogens by thermal pasteurisation in raw milk/raw colostrum from ruminants
The assessment related to the thermal inactivation parameters of pathogens in raw milk/raw colostrum from ruminants can be found in Appendix D.
The scientific literature presenting thermal inactivation experiments of pathogens in raw milk and colostrum is very heterogeneous with a variety of equipment used to measure heat resistance of milk‐borne pathogens. Quantitative inactivation data for some pathogens could be retrieved only (i.e. Brucella spp.) or mostly (i.e. C. jejuni) from old studies where the methods used in these studies could not be considered as representative of commercial heat treatments. In addition, the body of literature meeting the eligibility criteria for extraction of thermal inactivation parameters (e.g. reporting on the DT‐ and/or zT‐values or time to specific log10 reductions) was scarce for some pathogens (i.e. Brucella spp. and TBEV) when considering raw milk as the matrix of interest and absent for all pathogens but MAP (surrogate for M. bovis) when considering colostrum. Heterogeneity is also observed in the equipment and methods that were used to conduct the thermal inactivation experiments, particularly for Brucella spp., Campylobacter spp. and MAP. For batch pasteurisation experiments, these ranged, for example, from sealed tubes totally or partially submerged in water bath (with or without agitation) to elaborated ad‐hoc laboratory apparatus. Similarly, methods for HTST pasteurisation included laboratory‐scale batch pasteurising unit immersed in water bath at 72°C, laboratory‐scale HTST pasteuriser (with or without turbulent flow and with or without agitation), commercial‐scale HTST pasteuriser or strips of tubes and a thermocycler used to reproduce both LTLT and HTST conditions. Variability was also observed in relation to the initial physiological state of the suspension of bacteria used to contaminate the milk (e.g. frozen and thawed stock cultures vs. freshly grown cultures). The laboratory equipment, methods and state of initial inoculum may affect the estimation of the thermal inactivation parameters and/or log10 reductions. These range from an overestimation of heat resistance due to inactivation occurring during a prolonged temperature come up time to the non‐uniform dispersion of bacteria in the medium that might alter both the uniform exposure to the target temperature for the required time and the number of bacteria in the sample extracted for enumeration. Again, not ensuring turbulent flow of the milk during the temperature holding phase affects the residence time of the fastest moving particles (Pearce et al., 2001) and use of a suspension of bacteria that have been frozen might result in a reduced heat resistance with subsequent overestimation of heat inactivation parameters or log10 reductions. For these reasons, identification of the pathogen‐specific thermal inactivation parameters or log10 reductions was done considering the experimental conditions evaluated to be the most representative of commercial pasteurisation.
Thermal inactivation parameters (DT‐ and zT‐values) for L. monocytogenes, S. aureus, Salmonella spp. and STEC in raw milk were extracted from a study by Pearce et al. (2012) where the most resistant bacterial strains were systematically identified and conditions of commercial pasteurisation were faithfully reproduced. Considering the DT‐values (in seconds) reported for the higher temperature tested, D64 for E. coli O157:H42 was 16 s (sd = 2 s), D64 for L. monocytogenes was 14 s (sd = 3 s), D60 for Salmonella spp. was 18 s (sd = 2 s) and the D64 for S. aureus was 14 s (sd = 2 s). Estimated zT‐values were 3.00, 2.90, 2.09 and 3.53°C for E. coli O157:H42, L. monocytogenes, Salmonella spp. and S. aureus, respectively.
None of the identified studies could be considered as representative of the commercial LTLT or HTST pasteurisation conditions for Brucella spp. and Campylobacter spp. However, the available evidence converges to the conclusion that C. jejuni is not heat resistant. Considering the most resistant strain amongst those tested by Waterman (1982), a D55 of 1.1 min associated with a zT‐value of 5.43°C strongly suggests that both LTLT and HTST are effective in inactivating C. jejuni, even if present at high concentrations. This is also supported by the pooled mean values (range) of thermal inactivation parameters estimated for Campylobacter spp. in liquid media (i.e. not only milk) by Sörqvist (2003): D55 = 50 s (44–57 s) and D72 = 0.1 s (0.1–0.2 s), associated with a zT‐value of 5.5°C (sd = 1.1°C), all markedly lower than the corresponding values of the other bacterial pathogens. Specific evidence for Brucella melitensis could not be retrieved from the literature review; from reduction data of other Brucella species (i.e. B. suis and B. abortus), LTLT and HTST T/t conditions resulted in at least 8.6 log10 reductions (Park et al., 1932) and 6.6 log10 reductions (Davies and Casey, 1973), respectively.
For MAP (used as surrogate for M. bovis), the DT‐ and zT‐value reported in the study by Pearce et al. (2001) involving experiments on five strains of MAP performed under similar conditions of commercial pasteurisation were a zT‐value of 8.6°C, a D63 ranging from 10.75 to 26.62 s and a D66 ranging from 4.38 to 7.59 s depending on the strain. Only one strain had survivors at 69°C for 15 s and for this one, the D69 was 4.13 s. According to the authors, the absence of data points at 69 and 72°C indicates that the kill exceeded the 4 log10 to 5 log10 detection limit. The mean extrapolated D72 for the five strains of MAP examined was 2.03 s, representing 7 log10 kill at the 95% CI.
Scarce and contrasting evidence was found for thermal inactivation data of TBEV in milk. Indeed, when considering the experiment done with the not‐attenuated strain used in Saier et al. (2015), it seems that less than 3 log10 reductions can be expected after HTST, while evidence for milk contaminated with 106 focus forming units (FFU)/mL) of an attenuated strain showed complete inactivation of the virus after HTST pasteurisation conditions (i.e. not detected in the sample unit after the treatment) (Offerdahl et al., 2016).
3.2.2.2. Thermal pasteurisation in raw milk and raw colostrum from ruminants using the minimum legal requirements
The PC normally recommended by international agencies as reference values for thermal pasteurisation of milk are summarised in Table 4. The minimum target of 5 log10 reductions to be achieved by thermal pasteurisation is considered as the minimum reference value for all the target pathogens in this Scientific Opinion.
Table 4.
Overview of the performance criteria (PC) proposed by international agencies as reference values for thermal pasteurisation of milk
| Agency | Reference | Performance criteria for thermal pasteurisation | Log10 reduction considered in the assessment |
|---|---|---|---|
| Codex Alimentarius Commission | CAC (2004) | As C. burnettii is the most heat‐resistant non‐sporulating pathogen likely to be present in milk, pasteurisation is designed to achieve at least a 5 log10 reduction of C. burnettii in whole milk (4% milk fat) | 5, 6, 7, 8 |
| Food Safety Authority of Ireland | FSAI (2020) | Minimum 6 log10 reduction in the number of vegetative cells of L. monocytogenes because it is currently regarded as the most heat‐resistant food‐borne pathogen that does not form spores | |
| New Zealand Government | MPI (2021) |
5 log10 reduction of Campylobacter spp., L. monocytogenes, STEC, Salmonella spp. and S. aureus; and 6 log10 reduction for MAP (used as surrogate for M. bovis) (domestic market) > 7 log10 reduction for these hazards to achieve an equivalent outcome to thermal pasteurisation (export product should meet at least this standard) |
|
| European Food Safety Authority | EFSA BIOHAZ Panel (2020a) | Usually 6 log10 reduction (according to a reported range from 4 to 8 log10 reduction( a )) of relevant vegetative pathogen depending on the type of commodity/raw materials used |
MAP = Mycobacterium avium subsp. paratuberculosis; STEC = Shiga toxin‐producing E. coli.
Not necessarily related to milk.
The evidence gathered by the literature review described in Section 3.2.2.1 was used to evaluate, for each relevant pathogen, whether the specific log10 reductions proposed by international agencies (i.e. 5, 6, 7 and 8 log10 reductions) are achieved using the minimum legal requirements for thermal pasteurisation. It was found that the target log10 reductions were exceeded for most of the pathogens (i.e. STEC, L. monocytogenes, Salmonella spp. (using S. Typhimurium), S. aureus and Campylobacter spp.), as it was estimated that a > 10 log10 reduction would be achieved. For Brucella spp. and M. bovis (using MAP as surrogate), the reductions achieved by thermal pasteurisation were lower and were compared against the PC target of 6 and 5 log10 reductions. For TBEV, the reductions were even lower and were thus compared against the PC target of 5 log10 reductions.
The pathogen‐specific HPP equivalent conditions have been evaluated, when data allowed for it, for all the PC and further identified in relation with the highest PC achieved in Section 3.2.3.
3.2.2.3. Uncertainty analysis
The sources of uncertainty associated with the assessment of the thermal inactivation parameters are presented in Table C.3. Uncertainties refer to the possibility that information for some pathogens/product had not been intercepted by the literature review and the lack of accuracy of the data (DT‐ and zT‐values, log10 reductions) reported in scientific literature. The literature search was adequately comprehensive and therefore the impact of this uncertainty on the conclusions is judged to be low. For data on S. aureus, L. monocytogenes, Salmonella spp. and STEC the impact of the sources of uncertainty related to the strain being used (most resistant or not) and experimental conditions were judged to be low. In fact, for these pathogens, it was possible to identify the thermal inactivation parameters or log10 reductions associated with purposely selected resistant strains tested under commercial pasteurisation conditions. For this reason, the uncertainty in the achievement of the PC for these pathogens was considered to be low.
Table C.3.
Potential sources of uncertainty identified in the assessment of the pathogen reduction by thermal pasteurisation in raw milk/raw colostrum from ruminants using the minimum legal requirement
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Impact of information not retrieved from the literature | It is possible that not all the information (DT and zT‐values or log10 reductions) for the pathogens and/or products of interest may be retrieved using the search strategy. | The impact of this uncertainty is expected to be low as it is believed that the relevant evidence has been identified by the literature review. |
| Inaccurate approximation of targeted T conditions and/or maintenance at the target T, to which DT‐values refer | The estimates of inactivation kinetics are uncertain, or do not accurately correlate to the specified target T‐values, i.e. in most studies, the organisms were exposed to non‐isothermal conditions, and receive more intense heat treatment (process lethality) than that specified in the papers, due to the experimental set up. | For the pathogens for which proceeding with the comparative exposure assessment was possible (i.e. thermal and HPP inactivation data were available), the thermal inactivation data used to evaluate whether the PC for thermal pasteurisation are met, were extracted from experiments where commercial pasteurisation conditions were more closely reproduced. The impact of this uncertainty is therefore judged to be low. |
| Pathogen‐specific representativeness of the DT‐values or log10 reductions being found | The thermal inactivation parameters (DT‐value or log10 reductions) reported in the studies intercepted by the literature review are strain‐specific | For the pathogens for which proceeding with the comparative assessment was possible (i.e. thermal and HPP inactivation data were available), the thermal inactivation data of the most resistant strains were preferably used as reference values. As this translates in a likely underestimation of log10 reductions for less resistant strains, the impact of this uncertainty is low. |
| Validity of reported DT‐value estimate | The DT‐value has been calculated assuming log‐linear inactivation kinetics models | The impact of this uncertainty is expected to be moderate and presents an underestimation or overestimation of the log10 reductions depending on the raw inactivation data being fitted assuming log‐linear inactivation kinetic model. |
DT: decimal reduction time at a certain temperature; HPP: high‐pressure processing; PC: performance criteria; T: temperature; zT: thermal resistance constant.
Evidence for Campylobacter spp. and particularly Brucella spp. was old and equipment/methods used to carry out the experiments represent an intrinsic source of uncertainty possibly leading to an over‐ or underestimation in the reported results. As previously mentioned, scarce and contrasting evidence was found for thermal inactivation data for TBEV in milk, which represent a source of uncertainty. For MAP (used as surrogate of M. bovis), heterogeneous findings are reported in literature also when considering studies where commercial pasteurisation conditions were closely reproduced. In fact, from a study reporting extrapolated inactivation parameters, it seems that the PC of 5 log10 reductions could be considered as met; however, other experiments under similar conditions reported the presence of few survivors after HTST treatment, and these were identified mostly from milk samples where the initial level of contamination was 5 log10 CFU/mL.
3.2.2.4. Concluding remarks
Based on the thermal inactivation parameters (DT‐ and zT‐values reported in Section 3.2.2.1) using milk pasteurised under commercial conditions, it is judged 99–100% certain (almost certain) that the higher PC of 8 log10 reduction for thermal pasteurisation using the T/t conditions defined in the legal requirements for milk pasteurisation is met for STEC, L. monocytogenes, Salmonella spp. (using S. Typhimurium), S. aureus and Campylobacter spp.
Considering direct reduction data of B. suis and B. abortus, milk subjected to LTLT and HTST pasteurisation conditions is likely to result in at least 8.6 and 6.6 log10 reductions, respectively. For Brucella spp., it is therefore judged 95–99% certain (extremely likely) that the PC for thermal pasteurisation of 6 log10 reduction is met and 33–66% certain (as likely as not) that the other criteria are met (i.e. 7–8 log10 reductions).
Variability was observed in the thermal inactivation parameters for MAP (used as surrogate for M. bovis) based on the laboratory set‐up. Using a pilot‐scale pasteuriser operating under validated turbulent flow in one study, thermal pasteurisation resulted in > 4 log10 reductions of MAP. Based on the thermal inactivation parameters, 7 log10 reductions is expected considering the 95% CI. For M. bovis (using MAP as surrogate), it is judged 95–99% certain (extremely likely) that the PC for thermal pasteurisation of 5 log10 reduction is met and 33–66% certain (as likely as not) that the other criteria are met (i.e. 6–8 log10 reductions).
Based on a single study, in which pasteurisation conditions (HTST) are not sufficient to guarantee a 3 log10 reductions of TBEV, it is judged > 50% certain (more likely than not) that the PC for thermal pasteurisation of 5 log10 reduction is not met.
Studies meeting the eligibility criteria for extraction of thermal inactivation data for the pathogens of interest in colostrum were not found from the literature review.
3.2.3. Minimum requirements of HPP of raw milk/raw colostrum from ruminants to achieve an equivalent efficacy to that of thermal pasteurisation
3.2.3.1. Relevant factors to describe the requirements of HPP of raw milk and raw colostrum from ruminants
The intrinsic and extrinsic factors that may influence the efficacy of HPP have already been summarised in Section 3.1.2. This section aims to give information on the specific issues related to the HPP of milk and colostrum.
The relevant factors that need to be taken into account when defining the requirements of the use of HPP on milk or colostrum from ruminants include aspects associated with the pathogens that could be present, the processing conditions and other extrinsic factors, as well as the intrinsic factors (physico‐chemical characteristics) of the milk/colostrum to be treated.
Factors related to microorganisms
The piezo resistance of the microorganisms, the growth phase at the moment of HPP and sublethal injury of cells after HPP will be discussed in this section.
Different microorganisms can show different degrees of resistance to HPP treatment. Indeed, although inactivation data for HPP‐treated milk are still scarce or absent for some relevant pathogens such as Brucella spp., M. bovis or TBEV, significant differences among other bacterial species of major concern are widely reported in the literature (Considine et al., 2008). It has been reported that Gram‐negative bacteria are more susceptible to pressure than Gram‐positive bacteria mostly due to the complexity of the Gram‐negative cell membrane, and cocci are more resistant than rod‐shaped bacteria (Smelt, 1998; Considine et al., 2008). Strain variation in pressure resistance has also been observed; although the extent to which this variability poses a risk in terms of incomplete inactivation following HPP is likely to be species‐specific and not entirely clear for all microorganisms. For example, Martinez‐Rodriguez and Mackey (2005) concluded that pressure resistance between different Campylobacter species and amongst different strains varies considerably. When milk was treated at 300 MPa for 10 min, 2.5 log10 and > 7.2 log10 reductions were observed for two different C. jejuni strains; however, raising the pressure to 400 MPa was capable of overcoming the piezo‐resistance with complete elimination (> 7 log10) of all the strains tested. This pressure value was also suggested by Solomon and Hoover (2004) after observing 2–3 log10 reductions of C. jejuni in milk, which let the authors conclude that 400 MPa is sufficient to inactivate this pathogen in this food. While the probability of Campylobacter surviving commercial HPP processing can be considered to be low, the strain variability observed for other major pathogens is of more concern. For example, a difference up to 3 log10 units of inactivation between the most sensitive and the most resistant strains of E. coli and L. innocua (used as surrogate for L. monocytogenes) was observed in skimmed milk treated at 550 and 400 MPa for 10 min (Garcia‐Graells et al., 2000). The definition of the P/t combinations for HPP treatment of milk or colostrum should be established not only based on the most pressure‐resistant pathogen but, ideally, also the most pressure‐resistant strain.
Bacterial cells in the exponential growth phase are typically more sensitive to HPP than cells in the stationary phase probably due to the synthesis of proteins that protect against a range of adverse conditions (Voight et al., 2015; Hill et al., 2002). For instance, in E. coli, the higher resistance of stationary phase cells to HPP is partly due to the presence of the RpoS protein while in L. monocytogenes, is the SigB protein (Considine et al., 2008). However, except for experiments under controlled conditions, the physiological state of pathogens in food prior to HPP is unknown. Definition of requirements for use of HPP on milk should be based on experiments using cells in stationary phase. In addition, following HPP, the bacterial population can be considered as a mixture of completely inactivated cells, stressed or injured survivors and unaffected survivors, with the ratio between these three subpopulations being modified by the bacterial composition, the combined effects of the HPP processing conditions and physico‐chemical characteristics of the product (Alpas et al., 2000). Therefore, in addition to the unaffected survivors, stressed or sublethally injured cells may recover and subsequently grow if conditions are favourable (Koseki et al., 2008; Ferreira et al., 2016; Syed et al., 2016). Indeed, the rate of recovery of injured cells and also the rate of growth itself (for both the culturable and recovered cells) is dependent upon both the post‐processing conditions (such as storage T/t or packaging under modified atmosphere) and the intrinsic characteristics of the product (such as the pH or presence of additives). The definition of requirements for use of HPP on milk aims to achieve a target log10 reduction (PC) internationally recognised. When the probability of occurrence of survivors is relevant (e.g. because the target log10 reduction applied to a reasonably foreseeable initial contamination level is not enough for the complete inactivation), the growth potential of these survivors (either unaffected or recovered from injury) need to be taken into account when setting the shelf‐life of pressurised milk (EFSA BIOHAZ Panel, 2020a).
Food extrinsic factors
As already mentioned in Section 3.1.2, factors extrinsic to foods are those related to HPP processing conditions. The target pressure and the holding time at this target pressure are the major extrinsic factors determining the efficacy of a HPP process as quantitatively addressed in Section 3.2.3.2.
Food intrinsic factors
The fat content is considered the most relevant intrinsic factor in milk. Whether or not, and the extent to which the fat content may alter the efficacy of HPP treatments for inactivation of pathogens in milk is still unclear. In fact, few studies have compared the inactivation kinetics of the same pathogen inoculated in milk samples of different % fat content.
In Garcia‐Graells et al. (1999), an increased fat content resulted in a protective effect for four E. coli strains, with inactivation (HPP treatment of 600 MPa/20°C/15 min) progressively higher in skimmed milk (0.05% fat) than in semi‐skimmed milk (1.55% fat) and whole milk (3.5% fat). However, the same trend was not observed by Gervilla et al. (2000) in a study in which reductions of single strains of E. coli, L. innocua and S. aureus were recorded for pasteurised ewe’s milk/cream of different fat content (i.e. 0, 6% and 50%) at temperatures of 4 and 25°C, and HPP processing conditions of 200–400 MPa for 15 min. In that case, a protective effect was observed only for L. innocua in milk treated at 4°C, the log10 reductions being ~ 3, 2.5 and 1.5 for the 0, 6 and 50% fat content milk, respectively. Similarly, no apparent protective effect was reported by Solomon and Hoover (2004) when different Campylobacter strains were inoculated into UHT whole and skimmed milk subsequently treated at pressure values up to 350 MPa for 10 min. In Martinez‐Rodriguez and Mackey (2005), milk of unspecified %fat content was found to result in substantially lower inactivation of Campylobacter spp. compared to water or broth when treated at 200, 250 or 300 MPa for 10 min, but not at 400 MPa which resulted in complete inactivation in all media.
The above data suggest that a protective effect of the fat content on the inactivation of pathogens during HPP of milk cannot be excluded. Overall, the normal fat content of whole milk (3.3–5.4%, Claeys et al., 2014) did not seem to be relevant for pathogen inactivation compared to skimmed milk. However, more evidence is needed to confirm the hypothesis and particularly to understand how and to what extent this effect is dependent upon other processing conditions (i.e. pressure, holding time, temperature).
Other factors also appear to have an impact in the efficacy of HPP, such as milk homogenisation, animal species, preheating of milk, etc. The impact of these intrinsic factors in the HPP efficacy is further described in Section 3.2.3.2.
3.2.3.2. Pathogen reduction by HPP in raw milk and raw colostrum from ruminants
A total of 35 studies were considered relevant for the evaluation of the impact of HPP on the selected pathogens in different human or cows’ milk types, including raw, pasteurised or UHT milk, whole or reduced fat milk and colostrum. No records were found for the HPP impact on B. melitensis and TBEV (Flavivirus) and thus, no relevant conclusions could be drawn for these hazards. Whenever reported, the CUTs increased with pressure level, also depending on the equipment performance (Xu et al., 2009; Ramos et al., 2015; Misiou et al., 2018). The reduction of the pathogens during the CUT was dependent on the microorganism and target pressure, with higher pressures tending to cause a higher magnitude of reductions in the compression stage. An overall (expected) trend, similarly to thermal pasteurisation, is that high pressures (500–600 MPa) require short treatment durations (up to 5 min) to achieve equivalent pathogen inactivation as lower pressures (< 500 MPa) with extended treatment times (i.e. > 10 min up to 45 min, especially at ≤ 400 MPa) (Dogan and Erkmen, 2004; Erkmen, 2009, 2011; Ramos et al., 2015; Allison et al., 2018; Stratakos et al., 2019).
For modelling purposes, the log10 reductions referring only to the holding time were chosen for describing the reduction of pathogens as a function of pressure and time, as the reductions in the compression stage could not (reliably) be attributed to a specific pressure level. Given that certain records did not specify during which pressure stage the reported pathogen inactivation refers to, the data set of log10 reductions vs. time and pressure for each hazard were fitted twice, i.e. including and excluding the non‐specifying records. The model with the best performance was selected, based on RMSE and the magnitude of parameter standard errors relatively to the parameter estimates, i.e. the lower the better. Furthermore, it was found (based both on the literature and preliminary model fitting) that UHT milk leads to a distinct inactivation pattern compared to the other milk types, with on average lower (i.e. ~ 1 to > 3) magnitude of log10 reductions at similar pressure and time combinations, or with longer treatment durations required to achieve comparable pathogen reduction to those in other milk types (Patterson et al., 1995; Garcia‐Graells et al., 2000; McClements et al., 2001; Pandey et al., 2003; Buzrul et al., 2008; Misiou et al., 2018; Stratakos et al., 2019; Komora et al., 2020). A possible reason may be that the antimicrobial compounds naturally present in raw milk and possibly enhancing the bactericidal effect of HPP are denatured in UHT (Viazis et al., 2008). As such, considering the scope of the present mandate, data for UHT milk were excluded from the final fitting used to identify the most pressure‐resistant pathogen and estimate the HPP conditions that deliver equivalent reductions to those achieved by the two regulated (reference) thermal pasteurisation conditions for milk. For Campylobacter, as the only relevant studies were using UHT milk, modelling was carried out including UHT milk.
Finally, the data used to fit the models refer to stationary phase cells, which, in general, are known to be more resistant than exponential phase cells (unless certain adaptation treatments increase their barotolerance) and thus, the worst‐case scenario of microbial resistance to HPP is considered (Pagan and Mackey, 2000). Thus, if the current assessment relied only on the P/t combinations needed for the inactivation of exponential cells, this would lead to a significant overestimation of HPP efficacy compared to the inactivation of stationary phase cells. According to Rocha‐Pimienta et al. (2020) if S. aureus cells are acclimatised for 2 h at 25°C in thermally ‐treated milk (118°C for 10 min) prior to HPP treatment, they enter the exponential phase and the reductions achieved by HPP using 379–593 MPa are 2–3 log10 units higher compared to results of studies assessing the inactivation of stationary phase cultures at the same pressure range. This study was excluded to fit the models because the milk was intensively heat treated (resembling UHT) and exponential phase cells were used.
Next, the impact of HPP applied in milk on each of the five documented relevant hazards (out of the seven originally identified and two excluded per above), as well as the modelling results are detailed.
Listeria monocytogenes. Fifteen studies were found to assess the effect of HPP on the bacterium in various types of milk, including whole (pasteurised) milk, raw and UHT milk, from humans and cows, as well as in milk buffer, i.e. a lab‐made solution composed of the same minerals and lactose as whey from rennet casein (Gao et al., 2006) (see Figures 3 and 4 which do not include UHT milk). Overall, inactivation of L. monocytogenes by HPP depended on the pressure intensity and time. Lower pressures required longer treatment durations. Limited reductions (< 0.5 log10) of L. monocytogenes were evident in raw milk treated at 200 MPa (20°C) or 250 MPa (25°C) for 5, 10 or 15 min (Huang et al., 2015; Ramos et al., 2015). Likewise, 200 MPa for 10 min at 25°C did not affect the survival of L. monocytogenes in UHT milk. Conversely, reductions of L. monocytogenes by HPP clearly increased with higher pressures during the holding time. Treatment of raw milk with 300, 350 and 400 MPa for 5 min caused 1, 4.4 and 6.3 log10 reductions, respectively, and by doubling the holding time to 10 min, the respective drops in L. monocytogenes levels increased to 2.6, 5.8 and 7.5 log10 units (Huang et al., 2015).
Figure 3.
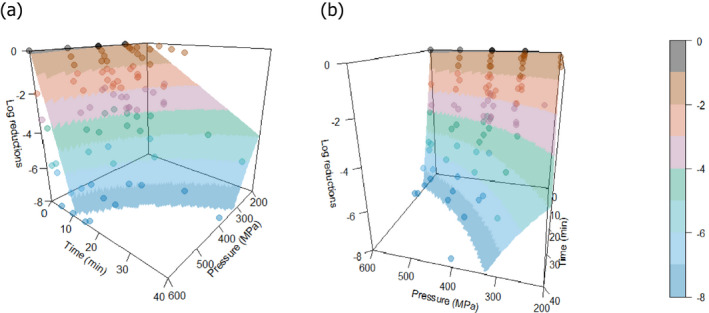
Observed (points) and predicted (response surface) log10 reductions of Listeria monocytogenes in response to pressure (P, MPa) and holding time (min), in various milk types (excluding UHT milk)
- Heat map bars represent magnitudes of log10 reductions. The two figures (a and b) represent two different angles of the same 3D graph. Source of data: Patterson et al. (1995); Dogan and Erkmen (2004); Gao et al. (2006); Hayman et al. (2008); Viazis et al. (2008); Xu et al. (2009); Amina et al. (2010); Mishra et al. (2013); Huang et al. (2015); Ramos et al. (2015); Allison et al. (2018); Misiou et al. (2018); Stratakos et al. (2019); Komora et al. (2020).
Figure 4.
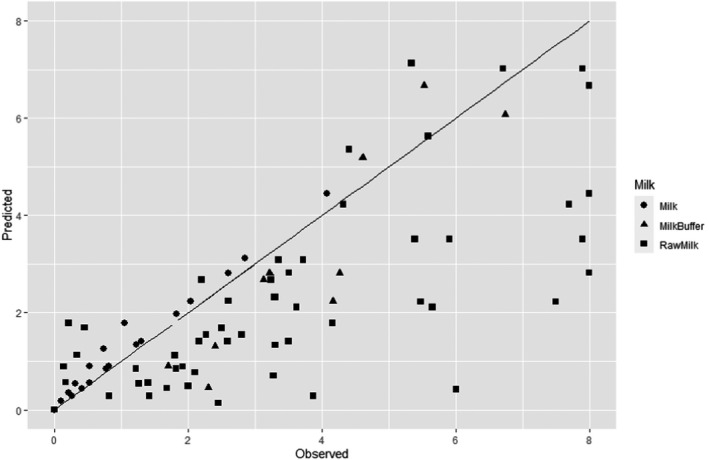
Predicted vs. observed log10 reductions of Listeria monocytogenes by HPP in different milk types, as predicted by the global linear model fitting
During HPP treatment of raw milk at 400 and 500 MPa (18–20°C), increasing the holding time from 5 to 15 min caused a concomitant rise in L. monocytogenes inactivation from 2.16 (at 400 MPa) and 5.48–5.91 (at 500 MPa) to 7.68–7.90 log10 at both pressures (Ramos et al., 2015; Stratakos et al., 2019). At 300 and 310 MPa, the reductions of L. monocytogenes were similar after 9 and 15 min of holding, equal to 2.2–2.6 log10 (Ramos et al., 2015; Allison et al., 2018), whereas 9–12 min of 380 MPa (4 or 25°C) delivered at least one additional log10 reduction (Allison et al., 2018). Equivalent P/t combinations found to kill 7.5–7.7 log10 of L. monocytogenes in raw milk were 75 min at 300 MPa, 40 min at 400 MPa and 17 min at 600 MPa, with Dp‐values of 10.99, 6.00 and 2.43 min, respectively (Dogan and Erkmen, 2004).
Xu et al. (2009) assessed the impact of both the CUT and holding time at a controlled temperature (30°C) on the HPP inactivation of L. monocytogenes in 3.2% fat milk, treated at 300, 400 and 500 MPa. The CUT of 1.2, 2.5 and 3.5 min needed to reach the aforementioned pressures caused limited reductions of L. monocytogenes, from 0.16 to 0.99 log10 units. Thus, the major inactivation occurred during the subsequent 10 min of holding time, leading to 1.12, 2.79 and 4.88 log10 reductions at 300, 400 and 500 MPa, respectively. The average corresponding DP‐values, based on log‐linear inactivation were 9.56, 3.84 and 2.45 min. In another study, with human milk (21°C) treated at 400 MPa, the CUT was 60 s and together with the subsequent holding time of 0.5 min yielded 6 log10 reductions of L. monocytogenes (Viazis et al., 2008).
Application of 400 or 500 MPa to UHT milk delivered L. monocytogenes reductions of 2.2–4.8 and 6.2 log10 units, respectively (Misiou et al., 2018).
Staphylococcus aureus. Seven studies were deemed relevant for the inactivation of S. aureus by HPP in raw, thermally pasteurised and UHT milk from humans, ewes or cows (see Figures 5 and 6 which do not include UHT milk). The reported reductions were the lowest observed among all pathogens assessed in the range 400–600 MPa, for 5–15 min of total treatment duration.
Figure 5.
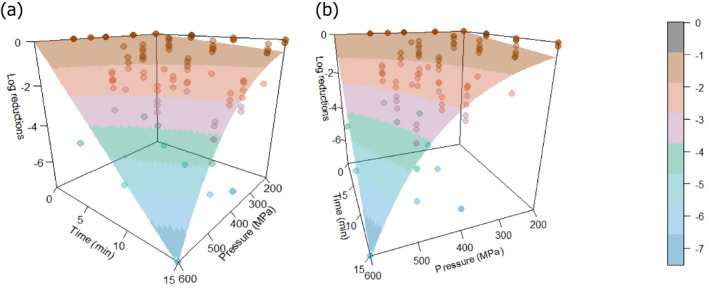
Observed (points) and predicted (response surface) log10 reductions of Staphylococcus aureus in response to pressure (P, MPa) and holding time (min), in various milk types (excluding UHT milk)
- Heat‐map bars represent magnitudes of log10 reductions. The two figures (a and b) represent two different angles of the same 3D graph. Source of data: Records included in text and used for data extraction: Patterson et al. (1995); Patterson and Kilpatrick (1998); Gervilla et al. (1999); Viazis et al. (2008); Tabla et al. (2012); Ramos et al. (2015); Windyga et al. (2015).
Figure 6.
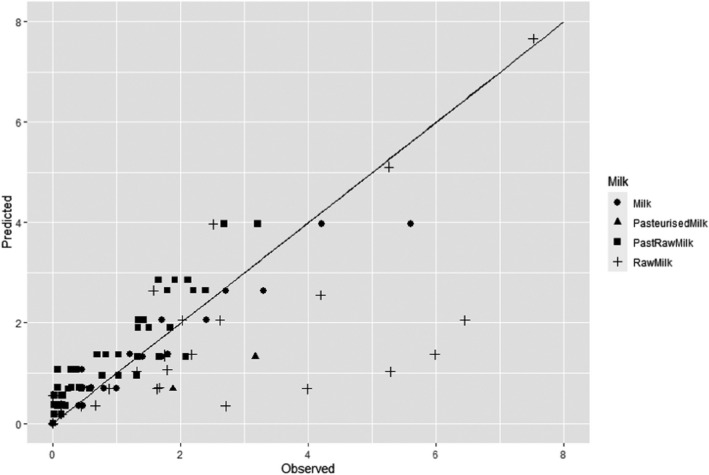
Predicted vs. observed log10 reductions of Staphylococcus aureus by HPP in different milk types, as predicted by the global linear model fitting
Limited (< 0.5 log10 units) or no reductions were observed at 200 and 300 MPa in ovine and human milk (Gervilla et al., 1999; Windyga et al., 2015). At 400 MPa, the observed decrease of S. aureus in raw human milk treated for 15 min ranged from 1.70 to 2.61 log10 (Gervilla et al., 1999; Viazis et al., 2008; Windyga et al., 2015). The inactivation was not enhanced by the slightly higher pressure of 450 MPa (Gervilla et al., 1999). In pasteurised milk, the reported reductions of S. aureus by treatment with 400 MPa at 10°C for 5 min were less than 0.5 log10 (Tabla et al., 2012). Nonetheless, extending the duration of the 400 MPa treatment up to 30 min increased the S. aureus inactivation by almost 3 log10 units compared to 15 min (Viazis et al., 2008). Contrary to what was expected, is that for one of the strains tested in this study, the survivors counted on non‐selective media, i.e. tryptic soy agar, suggested much faster inactivation than that deduced from enumeration on selective media, e.g. Baird Parker agar. Notably, although negligible reductions of S. aureus were observed in pasteurised milk after 5 min treatment with 400 and 500 MPa at 10°C, the population of the pathogen in the treated milk decreased by 1.5 and 3 log10, respectively, after 2 h of storage at 4°C (Tab la et al., 2012). According to the available evidence, HPP inactivation may also differ with milk type. The reductions of S. aureus in ewes’ milk treated with 500 MPa for 15 min were ~ 3.2 log10 units, which is almost 10‐fold higher than in cows’ milk (Gervilla et al., 1999; Ramos et al., 2015).
In UHT milk, the magnitude of S. aureus inactivation was markedly lower than in raw or thermally pasteurised milk. Fifteen min treatment of UHT milk at 600 MPa, caused a 2.1 log10 reduction (Patterson et al., 1995) in comparison to > 7 log10 reduction (no survivors) reported for the same conditions in raw skimmed milk (Ramos et al., 2015). This magnitude of S. aureus inactivation in raw milk was not reached in UHT milk using a 30‐min treatment at 600 MPa (resulting in a 5.2 log10 reduction). However, strain variation in pressure resistance may impact the aforementioned differences between milk types and lead to comparative decreases of S. aureus in UHT and non‐UHT milk. In particular, within 10 min of treatment at 400, 500 and 600 MPa, the observed reductions in UHT milk were 1.3, 2.5 and 5.2 log10 units, which were close to those found in raw cows’ milk (Patterson and Kilpatrick, 1998; Ramos et al., 2015).
STEC. Thirteen studies were reviewed for the impact of HPP on E. coli and STEC (E. coli O157:H7) in UHT, raw human and cows’ milk, reconstituted powdered milk and colostrum, at pressures ranging from 100 to 600 MPa and variable treatment durations (5–80 min) (see Figures 7 and 8 which do not include UHT milk). In this assessment, the evidence was used considering the entire body of evidence for any E. coli strain. The reported reductions clearly suggest that the milk type (human, ewe and cow) and strain variability have a major impact on sensitivity to pressure, and hence on the efficacy of HPP at > 400 MPa. More specifically, 10 and 15 min of raw human milk treatment with 400 MPa, achieved 1.89 and 3.23 log10 reductions of E. coli, respectively, and the same pressure applied in raw cows’ milk yielded reductions of 1.31–3.02 log10 units after 10 min, 1.4–4.1 log10 units after 15 min and notably, 2.5 and 4.5–5 log10 units within only 5 and 7 min in raw and pasteurised cows’ milk, respectively (Gervilla et al., 2000; Dogan and Erkmen, 2003; Pandey et al., 2003; Viazis et al., 2008; Ramaswamy et al., 2009; Stratakos et al., 2019). However, after 30 min of holding raw human milk at 400 MPa, the obtained reductions of E. coli exceeded 6 log10 units, leading almost to complete inactivation of the initial inoculated levels (Dogan and Erkmen, 2003; Viazis et al., 2008). The 2.7 log10 reductions observed in pasteurised cows’ milk after 8 min of exposure to 300 MPa, were achieved either after 15 min of holding the same milk at 300–400 MPa (Gervilla et al., 2000), or after 15–20 and 30–45 min of holding raw cows’ milk at 400 and 300–350 MPa, respectively (Pandey et al., 2003; Ramaswamy et al., 2009). The decrease of E. coli by 4.05 log10 units in pasteurised cows’ milk within 16 min at 300 MPa was not reached in raw cow’s milk even after 60 min of treatment where a max. of 3.33 log10 reductions was observed but required at least 5 min of treatment at 500 MPa to ensure equivalent efficacy (Stratakos et al., 2019).
Figure 7.
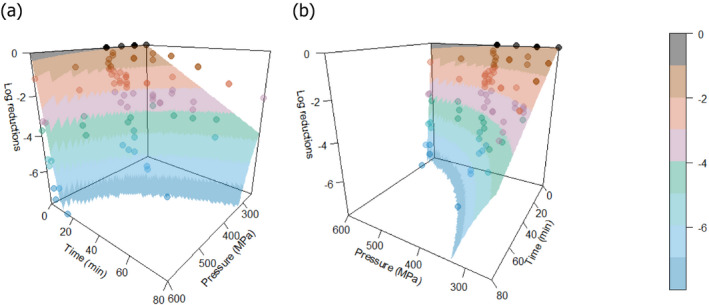
Observed (points) and predicted (response surface) log10 reductions of Escherichia coli (including E. coli O157:H7 and non‐pathogenic E. coli) in response to pressure (P, MPa) and holding time (min), in various milk types (excluding UHT milk)
- Heat‐map bars represent magnitudes of log10 reductions. The two figures (a and b) represent two different angles of the same 3D graph. Source of data: Patterson et al. (1995); Patterson and Kilpatrick (1998); Garcia‐Graells et al. (2000); Gervilla et al. (2000); Dogan and Erkmen (2003); Pandey et al. (2003); Buzrul et al. (2008); Buzrul et al. (2009); Foster et al. (2016); Bernedo‐Navarro et al. (2016); Machado et al. (2019); Stratakos et al. (2019); Viazis et al. (2008).
Figure 8.
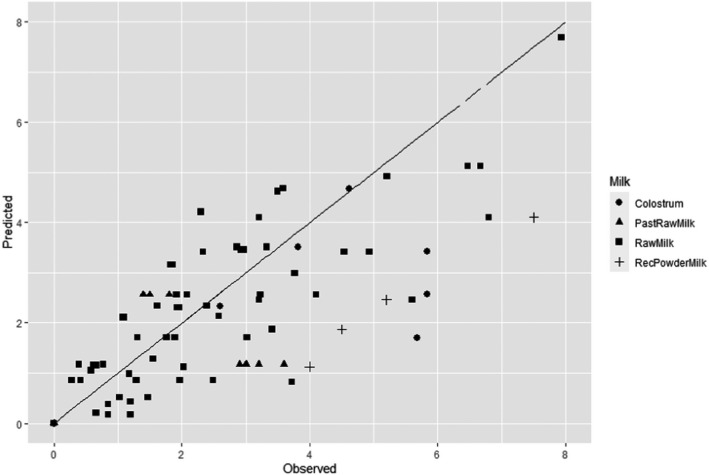
Predicted vs. observed log10 reductions of Escherichia coli by HPP in different milk types, as predicted by the global linear model fitting
- ‘PastRawMilk’ in the legend, refers to raw milk that was centrifuged and standardised to different fat contents (6%) and pasteurised without prior homogenisation (Gervilla et al., 2000).
Regardless of strain variability (in pressure resistance) in the different studies, within a single study, inactivation increased with holding time at a given pressure, e.g. from 3.02 to 4.1 (at 400 MPa) and 6.6–7.93 log10 units (at 600 MPa) after 10–15 min, respectively, or with target pressure at a given time, e.g. from 3 to 6.6 and 4.1 to > 7.9 log10 units after 10 min at 400 and 600 MPa, respectively (Dogan and Erkmen, 2003). According to a single study, E. coli reductions in colostrum, were on average higher than in other milk types, i.e. 5.6–5.8 log10 units after 10 min at 400 MPa, but rather limited, i.e. 2.5–4.62 log10 units, at 300 MPa for 30–60 min (Foster et al., 2016).
The greatest differences in E. coli inactivation among studies were evident in UHT milk. The magnitude of 2.4–2.6 log10 reductions of STEC was common among the following combinations: 45–60 min at 250 MPa (Bernedo‐Navarro et al., 2016), 15 min at 550 MPa (Garcia‐Graells et al., 2000) and 15–30 min at 600 MPa (Patterson et al., 1995; Patterson and Kilpatrick, 1998), also suggesting a potentially higher STEC resistance to pressure than other E. coli strains. In addition, as with the other pathogens assessed, the reductions of E. coli in UHT milk, albeit variable, were on average lower than in other milks, and particularly 0.45–1.4, 2.06, 0.43–3.1 and 1.8–5.7 log10 units, during 15 min of holding at 200–400, 450, 500 and 550 MPa, respectively (Buzrul et al., 2008, 2009). Likewise, 80 min at 400 MPa caused only a 5.25 log10 reduction, although the combinations of 40 min at 500 MPa, 30 min at 550 MPa and 20 min at 600 MPa delivered 7.51–8.20 log10 reductions (Buzrul et al., 2008). Patterson and Kilpatrick (1998) also suggested that temperature rise from 10 to 40°C enhances HPP inactivation at pressures greater than 400 MPa possibly because of the thermal effect during treatment at initial temperatures of 40°C (these data have been excluded for modelling). The temperature enhanced HPP inactivation showed a concomitant increase in injury, as determined by comparative enumeration on Tryptone Soya Yeast Extract Agar (TSAYE) with 0.5% NaCl (allowing colony formation by all viable cells) or 2% NaCl (allowing colony formation only by non‐injured cells). Contrary to UHT milk, holding reconstituted powder formula for 5 min at 500 and 600 MPa, caused 4.5 and 7.5 log10 unit reductions in E. coli, approximating the efficacy recorded in other milk types (Machado et al., 2019).
Salmonella spp. Five records were included for the impact of HPP on various Salmonella enterica serovars, including Senftenberg, Anatum, Agona, Enteritidis, Typhimurium and Dublin, in raw cows’ milk and colostrum. In general, Salmonella seemed to be more sensitive in the pressure range of 400–600 MPa than L. monocytogenes, S. aureus and E. coli (Figures 9 and 10). Three minutes of holding at 300 MPa and 2–4.5 min at 400 MPa caused 3.19 and 2.5–5.7 log10 reductions of S. Enteritidis, respectively (Xu et al., 2009). Again, serovar and strain variations introduced variability in the efficacy of HPP at 400 MPa between studies. For instance, even though reductions of S. Typhimurium in raw milk treated with 300 MPa for 5 min were similar among studies, ca 2.36–2.89 (Erkmen, 2009, 2011; Stratakos et al., 2019), after 10 min of holding at the same pressure, reductions differed among studies, ranging from 3.8 to 5.7 log10 units, possibly due to milk batch and strain differences (Erkmen, 2009, 2011). Furthermore, as a result of the biphasic inactivation of Salmonella, high reduction rates were obtained shortly, e.g. 5–10 min, after exposure to target pressure, leading to an estimated 2 log10 unit reduction, followed by a slower inactivation phase that may accumulate > 6 log10 reductions within 15–30 min of treatment at 300 and 400 MPa (Figure 9).
Figure 9.
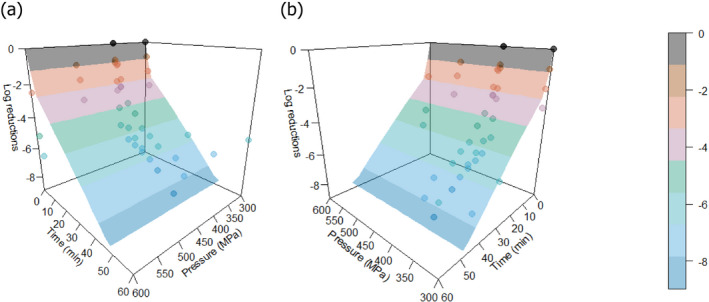
Observed (points) and predicted (response surface) log10 reductions of Salmonella spp. in response to pressure (P, MPa) and holding time (min), in various milk types
Figure 10.
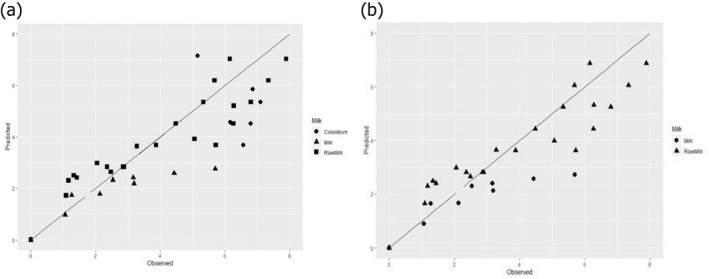
Predicted vs. observed log10 reductions of Salmonella spp. by HPP in different milk types, as predicted by the global biphasic model fitting, including (a) or excluding (b) the records relevant to colostrum
- ‘Milk’ in the legend, refers to data from Xu et al. (2009), where the milk type was not specified.
One study assessing S. Dublin HPP inactivation in colostrum, reported 6.2–6.6 log10 reductions attributable to total treatment duration of 30 and 10 min at 300 and 400 MPa, respectively (Foster et al., 2016). Finally, at a given holding time of 5 min, log10 reductions of Salmonella in raw cows’ milk increased with target pressure from 2.3 at 400 MPa, to 3.27 at 500 MPa and 6.27 log10 units at 600 MPa (Stratakos et al., 2019).
Campylobacter spp. Only two studies evaluated the impact of HPP in the range 100–375 MPa on five strains of C. jejuni and only in UHT milk from cows (skimmed or full‐fat) (see Figures 11 and 12).
Figure 11.
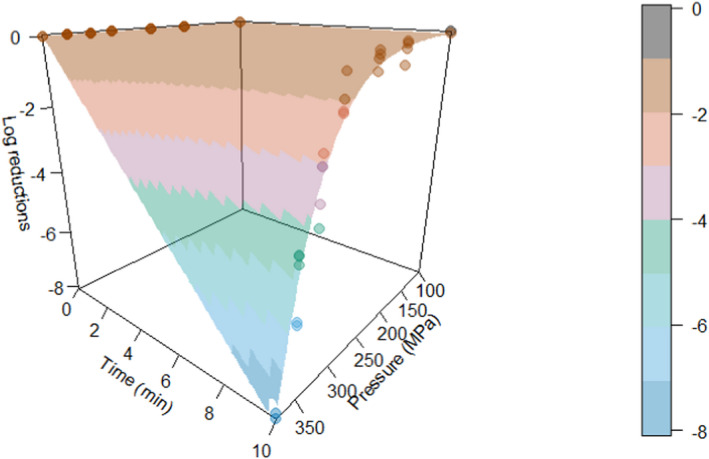
Observed (points) and predicted (response surface) log10 reductions of Campylobacter jejuni in response to pressure (P, MPa) and holding time (min), in various milk types
- Heat‐map bars represent magnitudes of log10 reductions.Source of data: Martinez‐Rodriguez and Mackey (2005), Solomon and Hoover (2004).
Figure 12.
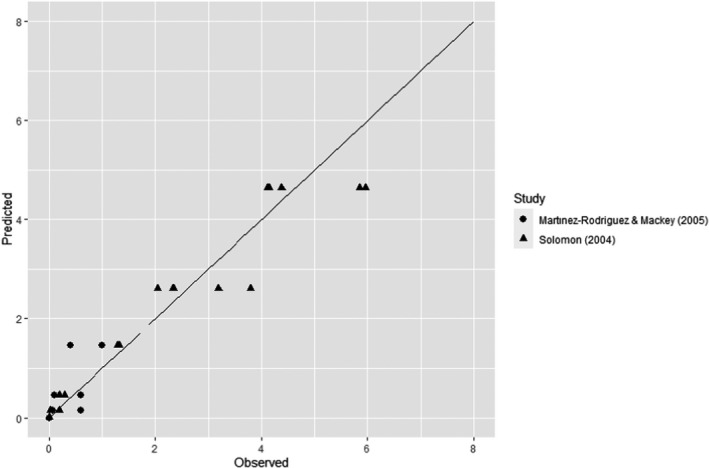
Predicted vs. observed log10 reductions of Campylobacter jejuni by HPP in UHT milk, as predicted by the global linear model fitting
Nonetheless, despite the limited evidence, the findings suggest that C. jejuni was the most sensitive pathogen to high pressure as compared to the other bacteria assessed. This is further corroborated by the high magnitude of reported log10 reductions at low pressures, i.e. up to 375 MPa, particularly leading to 1.31, 2.33, 4.15–5.85 and 8.92–8.09 log10 units within 10 min of total HPP treatment duration (not specified whether CUT is included or not) at 300, 325, 350 and 375 MPa (Solomon and Hoover, 2004; Martinez‐Rodriguez and Mackey, 2005). In agreement with data presented in Section 3.2.3.1, comparison of C. jejuni inactivation in skimmed and full‐fat milk did not reveal any significant impact of fat on the efficacy of HPP probably due to the low percentage of fat in whole milk (3.3–5.4%).
Mycobacterium bovis . Two studies were assessed on the impact of HPP (300–600 MPa) on the surrogate MAP in UHT milk and one study in colostrum; all three for a total treatment duration of 10 min (see Figures 13 and 14). These studies did not specify whether the CUT was included in the inactivation. Overall, the data suggest limited inactivation of MAP at 300 MPa and a sharp increase in log10 reductions at higher pressures, a fact that is further supported by the lower zp‐value of MAP compared to other hazards and explains the slope describing the dependence of logDP on pressure, in Figure 15. In particular, the maximum reported MAP reductions for 10 min at 300 MPa were less than 0.75 log10 units (López‐Pedemonte et al., 2006), whereas significant strain variability in sensitivity to pressure was evident at 400 MPa, but this was less at 500 MPa (López‐Pedemonte et al., 2006; Donaghy et al., 2007). The reported reductions during 10 min of UHT treatment at 400 MPa ranged from 0.73 to 2.9 log10 and from 4.07 to 6.29 log10 at 500 MPa, for the same treatment duration (López‐Pedemonte et al., 2006; Donaghy et al., 2007). The latter variability was eliminated at 600 MPa, as the intensity of this pressure likely masked strain differences, resulting in 6.13–7.43 log10 reductions within a short treatment duration of 5 min (Donaghy et al., 2007). In colostrum, HPP inactivation of MAP was less than 0.7 log10 units during 60 and 20 min of HPP treatment at 300 and 400 MPa, respectively, whereas reported reductions unexpectedly exceeded 6 log10 units within 5 or 10 min at 600 MPa (Foster et al., 2016).
Figure 13.
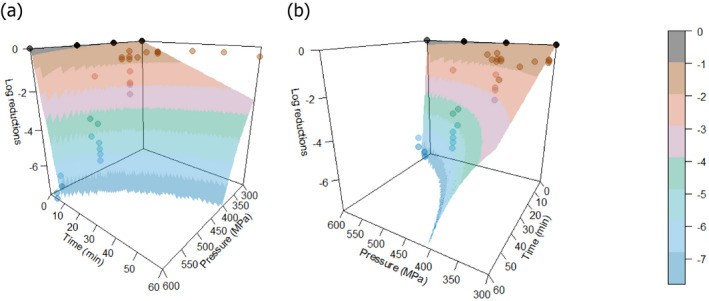
Observed (a) and predicted (b) log10 reductions of Mycobacterium bovis (using Mycobacterium avium subsp. paratuberculosis as surrogate) in response to pressure (P, MPa) and holding time (min), in UHT milk and colostrum
Figure 14.
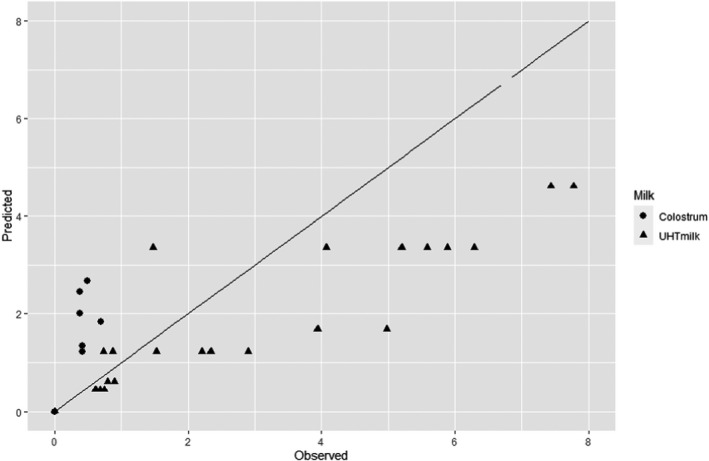
Predicted vs. observed log10 reductions of Mycobacterium bovis (using Mycobacterium avium subsp. paratuberculosis as surrogate) by HPP in UHT milk and colostrum, as predicted by the global linear model fitting
Figure 15.
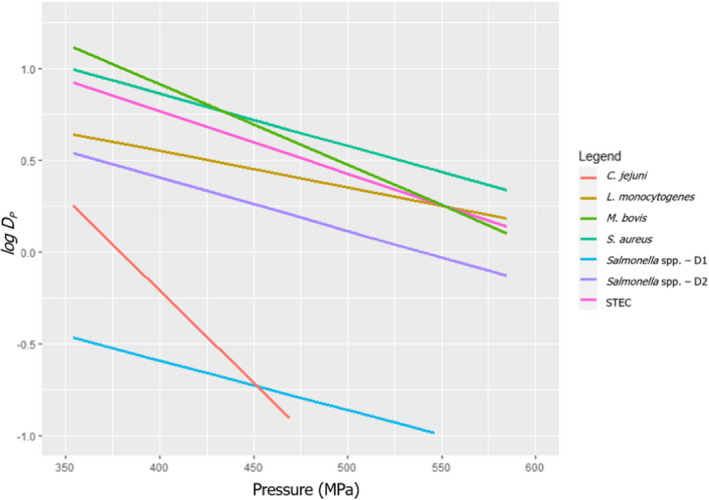
Dependence of D p ‐values on pressure, based on D ref and z p ‐values estimated by global fitting of log‐linear (single‐phase inactivation) or biphasic primary models shown in Table 5
- For Listeria monocytogenes not including UHT milk and colostrum; for Staphylococcus aureus not including UHT milk and colostrum; for STEC not including UHT milk and colostrum with the studies used to fit the model used only O157:H7 and non‐pathogenic Escherichia coli; for Salmonella spp. (including colostrum)– D1 considering the first rapid inactivation phase; for Salmonella spp. – D2 considering the second slower death phase; for Campylobacter jejuni only including UHT milk; for Mycobacterium bovis not including UHT milk and colostrum and using Mycobacterium avium subsp. paratuberculosis (MAP) as surrogate.
Table 5 summarises the parameter estimates, and goodness‐of‐fit statistics of the global models fitted to the data of log10 reduction per pathogen, extracted from the literature studies, in response to pressure and time. The Bigelow model was nested in (primary) linear models for describing the log10 reductions as a function of pressure and time for all pathogens except for Salmonella, for which, a biphasic primary model was used instead.
Table 5.
Overview of model parameter estimates ( in min, z p in MPa) and performance indices of the global model fitted to log10 reduction vs. pressure and time data for different hazards in milk, with P ref fixed at 600 MPa
| Hazard | Primary model type | Parameter estimates ± standard error | RMSE | % predictions within APZ( a ) | % fail‐safe prediction | % fail‐dangerous predictions |
|---|---|---|---|---|---|---|
| L. monocytogenes ( b ) | Log‐Linear |
: 1.423 ± 0.115 zp: 504.1 ± 54.7 |
1.797 | 59.8 | 31.5 | 8.7 |
| S. aureus ( b ) | Log‐Linear |
: 1.958 ± 0.172 zp: 345.0 ± 35.7 |
0.983 | 88.7 | 7.8 | 3.5 |
| STEC( (b) , (c) ) | Log‐Linear |
: 1.219 ± 0.059 zp: 293.5 ± 14.4 |
1.347 | 62.2 | 24.5 | 13.3 |
| Salmonella spp. (+C)( d ) | Biphasic |
: 0.074 ± 0.037 : 371.8 ± 128.4 : 0.666 ± 0.044 : 343.2 ± 16.0 f: fixed at 0.99 |
1.256 | 61.3 | 27.3 | 11.4 |
| Salmonella spp.( e ) | Biphasic |
: 0.067 ± 0.031 : 354.8 ± 104.6 : 0.655 ± 0.043 : 323.9 ± 15.9 f: fixed at 0.99 |
1.111 | 63.9 | 25.0 | 11.1 |
| Campylobacter jejuni ( f ) | Log‐Linear |
: 0.006 ± 0.001 zp: 99.5 ± 4.9 |
0.401 | 92.3 | 5.8 | 1.9 |
| Mycobacterium bovis ( (b) , (e) ) | Log‐Linear |
: 1.084 ± 0.101 zp: 227.9 ± 22.5 |
1.423 | 66.0 | 20.0 | 14.0 |
APZ = Acceptable Prediction Zone defined as a range ± 1 log10 from the 100% accurately predicted value, i.e. the value that makes the difference between prediction and observation equal to 0; RMSE = Root Mean Square Error.
Not including UHT milk and colostrum.
The studies used to fit the model used only O157:H7 and non‐pathogenic E. coli.
Including colostrum in which those records associated with colostrum did not specify whether the CUT was included or not, but colostrum is a highly relevant product for the current mandate.
Excluding colostrum.
Only including UHT milk.
Mycobacterium avium subsp. paratuberculosis was used as surrogate for M. bovis.
3.2.3.3. Most resistant pathogen when treating raw milk/raw colostrum from ruminants using HPP
The parameter estimates of Table 5 were used to generate Figure 15, which illustrates the dependence of log Dp on pressure, for the five documented pathogens (out of the seven identified as relevant in Section 3.2.1 and for which there was evidence available as described in Section 3.2.3.2).
Pressures above 450 MPa are considered as these are applicable for the treatment of milk according to the responses provided in the questionnaires. S. aureus was the pathogen most resistant to these pressures, followed closely (considering also the 95% CI; not shown for clarity) by M. bovis (using MAP as surrogate), STEC (using E. coli as surrogate) and L. monocytogenes. Salmonella spp. was clearly less resistant than these four pathogens, whereas C. jejuni was identified as the most pressure sensitive pathogen.
Due to the use of biphasic inactivation model fitted to Salmonella data, there are two trends of log Dp vs. pressure for this pathogen, which corresponds to the Dp‐values of the two inactivation phases, i.e. the first rapid phase characterised by a low and a high zp‐value indicating high sensitivity over the whole pressure range assessed, followed by a second, slower death phase, expressed by a higher and comparable ‐value (i.e. higher overall pressure resistance) representing a (hypothetical) resistant subpopulation.
MAP appears to be the most resistant organism at pressures < 450 MPa. This should be viewed with caution as this may be caused by a fitting artefact, due to the low number of available records for fitting.
3.2.3.4. Minimum requirements of HPP of raw milk/raw colostrum from ruminants to achieve an equivalent efficacy to that of thermal pasteurisation
Evaluation of the HPP treatment conditions that achieve the equivalent log10 reductions of thermal pasteurisation was assessed considering the PC normally recommended by international agencies as explained in Section 3.2.2.2 and summarised in Table 4. The minimum target of 5 log10 reductions to be achieved by thermal pasteurisation is considered as the minimum reference value for all the pathogens under current investigation. Furthermore, a comparison between the thermal and HPP treatment conditions capable of achieving higher reductions (i.e. 6, 7 and 8 log10 reductions) was performed to evaluate the maximum level of inactivation that can be practically achieved by HPP. The pathogen specific HPP equivalent conditions have been evaluated, when data allowed, for all the PC and further identified in relation with the highest PC achieved by thermal pasteurisation as assessed in Section 3.2.2.2.
The parameter estimates of global models for each relevant pathogen (Table 5) were used to generate the corresponding isoreduction plots for different target PC shown in Figure 16. The higher the targeted log10 reductions, the longer the required holding time (min) using 500–600 MPa, to deliver the desired inactivation. Contour plots of Figure 16 illustrate the equivalent HPP conditions (P/t combinations) that the global model predicted to achieve 5, 6, 7, 8 and 9 log10 reductions for each of the six relevant pathogens.
Figure 16.
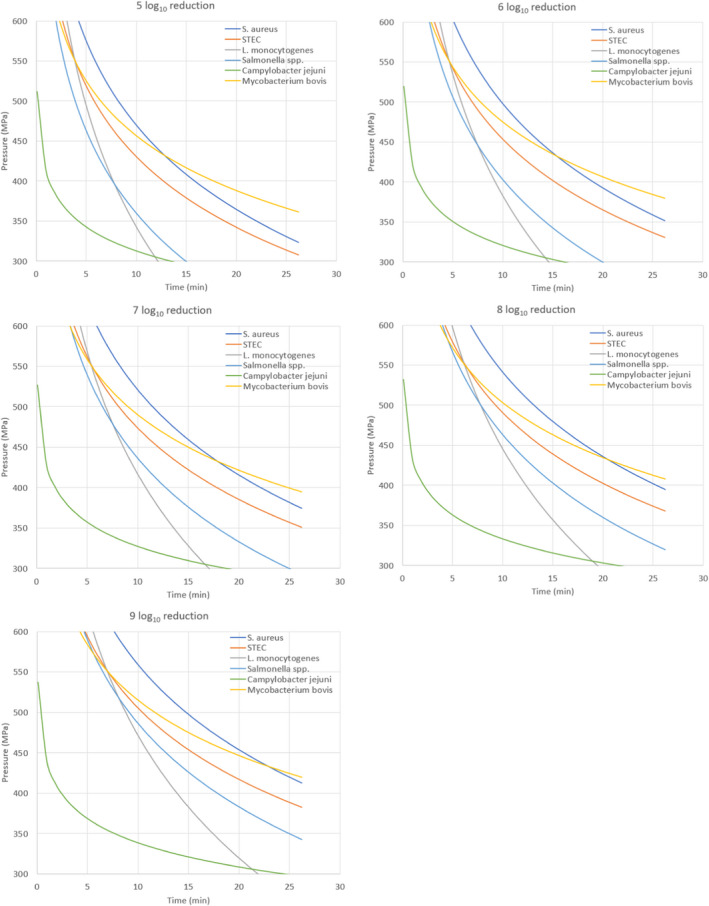
Isoreduction curves of HPP conditions (pressure/holding time combinations) needed to achieve the four targeted performance criteria (log10 reductions), according to the global model parameters shown in Table 5 for all six relevant pathogens in milk
Given that the performance of the global models was assessed with a ± 1 log margin of error, a conservative approach was applied. Specifically, the mathematical model was used to estimate the target pressure and holding time combinations needed to achieve 1 log10 reduction higher than each target PC (log10 reduction). For S. aureus, i.e. the most pressure‐resistant pathogen according to Section 3.2.3.3, the following target P/t combinations are needed as minimum requirements of HPP of raw milk/raw colostrum from ruminants:
at least 4 log10 reduction would be achieved with 600 MPa – 4 min, 550 MPa – 6 min and 500 MPa – 8 min (combinations for which the mathematical model predicted 5 log10 reductions);
at least 5 log10 reduction would be achieved with 600 MPa – 5 min, 550 MPa – 7 min and 500 MPa – 10 min (combinations for which the mathematical model predicted 6 log10 reductions),
at least 6 log10 reduction would be achieved with 600 MPa – 6 min, 550 MPa – 8 min and 500 MPa – 12 min (combinations for which the mathematical model predicted 7 log10 reductions);
at least 7 log10 reduction would be achieved with 600 MPa – 7 min, 550 MPa – 9 min and 500 MPa – 13 min (combinations for which the mathematical model predicted 8 log10 reductions); and
at least 8 log10 reduction would be achieved with 600 MPa – 8 min, 550 MPa – 10 min and 500 MPa – 15 min (combinations for which the mathematical model predicted 9 log10 reductions).
As mentioned above, S. aureus was identified as the most pressure‐resistant microorganism above 450 MPa. However, the risk of this pathogen is related to it growing up to 5 log10 CFU/mL and toxin production, which would be difficult to achieve under the reasonably foreseeable refrigeration conditions (even with mild abuse) in which milk is usually stored. STEC is also relatively pressure resistant above 450 MPa and may be more relevant than S. aureus in terms of risk, given the low infective dose (ID50) of STEC, even if it cannot grow under refrigeration storage. Notably, C. jejuni is inactivated in less than 1 min at 500 MPa.
For the other five pressure‐resistant pathogens (STEC, Salmonella spp., L. monocytogenes and M. bovis), the range of times needed to achieve equivalent reductions at different pressures, increases at pressures less than 550 MPa. At 500–600 MPa, the predicted range of holding time duration required for equivalent reductions of the five above pathogens by 7–9 log10 is generally narrower than that for 5 and 6 log10. In particular, equivalent times for 5 log10 reductions, range from ca. 2 to 3.2 min at 600 MPa, 3–4.2 min at 550 MPa and 4–7 min at 500 MPa. The equivalent ranges for 8 log10 reductions are 4–5 min at 600 MPa, 5.5–6 min at 550 MPa and 8–10.1 min at 500 MPa and for 9 log10 reductions, the equivalent ranges are 4.8–5.7 min at 600 MPa, 6.4–7 min at 550 MPa and 9–11.5 min at 500 MPa.
3.2.3.5. Uncertainty analysis
The uncertainty associated with the assessment of the minimum requirements of HPP of raw milk/raw colostrum from ruminants to achieve an equivalent efficacy to that of thermal pasteurisation is associated with the data used to fit the model and the structure of the model used to describe the data (see Table C.4). In particular, for each pathogen, there is uncertainty about the representativeness and sufficiency of the data (i.e. number of records, impact of strain variability and physiological state of cells) for describing the variability in the effectiveness of HPP treatments in different milk types, especially colostrum. The aforementioned data‐ and model‐related uncertainties explain the descrepancies between predicted and reported log10 reductions. Furthermore, the underestimation of observed log10 reductions by the linear models is possibly driven by the majority of data putting more weight to the lower magnitude of reductions. Given these uncertainties, a conservative approach was applied considering the APZ, the P/t combinations needed to achieve a given target log10 reduction were estimated for 1 log10 reduction higher than the target value. In this way, a conservative approach was provided which is judged 99–100% certain (almost certain) to provide the minimum HPP conditions required to reduce S. aureus in raw milk.
Table C.4.
Potential sources of uncertainty identified in the assessment of the minimum HPP requirements for the control of pathogens in raw milk and raw colostrum to achieve an equivalent efficacy to thermal pasteurisation
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Data used to fit the models (number of studies available per pathogen, number of strains used in eligible studies) |
Representativeness of the variability in the efficacy of HPP against the relevant pathogens in different types of milk. Log10 reduction data were all based on experimental data subjected to error, variability and uncertainty. In many studies mean log10 reduction of few replicates are reported, missing the variability (lower and higher values than that collected). |
The impact of this uncertainty is estimated to be low‐moderate as a high number of studies were included in the assessment covering a relatively wide range of experimental conditions, which resulted in a wide variability of the log10 reduction values. This uncertainty may result in an under‐ or over‐estimation of the HPP efficacy. However, based on model prediction, this source may have contributed more to the underestimation of the HPP efficacy. |
| Model structure | Log‐linear or biphasic inactivation with two linear parts is assumed to describe the inactivation trends in response to P and t. Non‐linear trends may also exist that could not be adequately captured by fitting all data by a global model | This uncertainty source may lead to over‐ or under‐estimation of HPP efficacy |
| Relevant factors determining the efficacy of HPP processing: microbiological factors |
The physiological state of cells, which is highly dependent on culture preparation (e.g. exponential vs. stationary phase cells, or stress adapted cells) may influence the efficacy of HPP. The resistance of the strain and its physiological state are very hard to elucidate under real industrial conditions due to the variability of the strains that can contaminate the food processing environment and the food products, as well as the variety of stresses that these strains can be exposed to. This factor is particularly relevant when the pathogenic bacteria contaminate raw materials (e.g. raw milk) and are exposed to heat during manufacturing, which may confer resistance to the cell and reduce the efficacy of HPP compared to what could be observed if the pathogen would contaminate the final product during post‐processing conditions. The effect of the level of contamination before HPP is experimentally assessed through the inoculum concentration, which may be a limiting factor to quantify the number of log10 reductions caused by the HPP treatment if the contamination level is not high enough or the HPP causes a reduction of the microorganisms below the quantification limit. This adds uncertainty about the actual magnitude of the inactivation or the occurrence of pseudo‐tails within a range of concentrations of 2.4 log10 units, between the detection limit 1 cell/25 g (i.e. −1.4 log10 CFU/g) up to just below the quantification limit (i.e. 1 log10 CFU/g). Analytical methods (culture media) to count L. monocytogenes after HPP, can make the recovery of the sublethally injured cells difficult, leading to an overestimation of the HPP efficacy. Many data used in the present assessment used selective agar (e.g. PALCAM, Oxford and more recently ALOA). The overestimation is less relevant since ALOA became of extensive use, as pressure injured L. monocytogenes are able to grow when directly plated on ALOA without previous repair incubation period (Jantzen et al., 2006). |
This uncertainty source may lead to over‐ or under‐estimation of HPP efficacy. However, the impact of this uncertainty is estimated to be low‐moderate as a high number of studies were included in the assessment covering a relatively wide number of different strains at stationary phase, which represents the worst‐case scenario of HPP resistance. The majority of log10 reduction data has been obtained from high inoculum level, allowing a proper quantitative determination of the survivors. Though some studies have been used selective media for enumeration (that may fail to recover sublethally injured cells), in the case of Listeria monocytogenes the use by many studies of ALOA and ALOA‐like media is known to recover HPP injured cells (Jantzen et al., 2006; Morales et al., 2006). This uncertainty may result in an over‐estimation of the HPP efficacy. |
| Relevant factors determining the efficacy of HPP: Impact of fat content from different animal species (few inconclusive studies were available) | Fat could play a protective role on microorganisms against HPP | This source would lead to under‐estimation of HPP efficacy. |
| Relevant factors determining the efficacy of HPP: processing parameters (P, t, T) |
Some extrinsic factors, mainly temperature during processing (once at the target pressure) is not reported during HPP cycle in many studies. Besides the uncertain compression heating, the impact of temperatures below 45°C has not been studied. Most records did not specify whether the CUT was included or not in the period during which, HPP inactivation was assessed. The compression stage may cause some inactivation, depending on the microorganism, the product and the pressure come‐up rate. |
This uncertainty source may lead to over‐ or under‐estimation of HPP efficacy. When the considered duration of the HPP treatment considers also CUT, the assessed log10 reductions practically refer to shorted holding time and thus, the killing effect is underestimated. |
ALOA: Agar Listeria Ottavani & Agosti; CUT: come‐up time; HPP: high‐pressure processing; P: pressure; t: time; T: temperature.
3.2.3.6. Concluding remarks
The relevant factors that affect the efficacy of HPP for the inactivation of pathogens in raw milk/colostrum from ruminants are the microorganism related factors and extrinsic factors, but available evidence suggests that the protective effect of the fat content on the inactivation of pathogens during HPP of milk cannot be excluded, although this does not seem to be a relevant factor when considering the common fat content of whole milk (3.3–5.4%) in comparison to skimmed milk.
A total of 35 relevant studies were available for the evaluation of the HPP impact on the selected pathogens (M. bovis, L. monocytogenes, Salmonella spp., STEC, Campylobacter spp. and S. aureus), in different human or ruminant milk types, including raw, pasteurised or UHT milk, whole or reduced fat milk and colostrum. No records were found for the HPP impact on B. melitensis and TBEV and thus, no relevant conclusions could be drawn for these hazards.
A global modelling approach, with a log‐linear or a biphasic primary inactivation model, both encompassing a Bigelow secondary model term for the impact of pressure on microbial inactivation, was successful in describing the combined effect of pressure (MPa) and holding time (min) on the inactivation of six hazards in various types of milk and colostrum, excluding UHT milk.
S. aureus was the most resistant pathogen, followed closely by M. bovis (using MAP as surrogate), STEC (using E. coli as surrogate) and L. monocytogenes, at pressures commonly applied by the industry (> 450 MPa). Salmonella was more sensitive to these pressures, while C. jejuni was the most pressure sensitive pathogen.
HPP cannot achieve equivalent log10 reductions to those achieved by thermal pasteurisation of milk according to these legal requirements. HPP equivalent conditions (at processing temperatures < 45°C) can be identified for lower log10 reductions (PC) as recommended by international agencies (i.e. 5, 6, 7 and 8 log10 reductions).
-
The equivalent HPP conditions (P/t combinations) required to achieve 5, 6, 7, 8 and 9 log10 reductions of the various pathogens were identified. As an example, for S. aureus (as the most pressure resistant pathogen), according to recommended PC for thermal pasteurisation, these were defined based on the parameter estimates of the global model. Given the uncertainties associated with data and the model, a conservative approach was applied considering the APZ. Thus, the combinations needed to achieve a given target log10 reduction were estimated for 1 log10 reduction higher than the target value as follows:
at 500 MPa, 8–15 min holding time would be needed to comply with 4–8 log10 reduction, respectively;
at 550 MPa, 6–10 min holding time would be needed to comply with 4–8 log10 reduction, respectively; and
at 600 MPa, 4–8 min holding time would be needed to comply with 4–8 log10 reduction, respectively.
It is judged 99–100% certain (almost certain) that the predictions performed using the selected model targeting 1 log10 reduction more than the given PC, provide a conservative approach regarding the minimum HPP conditions required to reduce S. aureus and the other pathogens in raw milk and raw colostrum.
3.2.4. Appropriate indicators to verify the efficacy of HPP treatments on raw milk or raw colostrum
Most of the reference and official methods used to assess heat treatment in milk are based on the evaluation of the modifications of some milk components following the thermal process, such as the determination of enzyme activities (ALP and lactoperoxidase), whey proteins, Maillard reaction compounds (generally furosine) and lactulose (Ritota et al., 2017). However, there is no direct correlation between the effect of high pressure and the effect of temperature on the same compounds, mostly because some pressure sensitive enzymes are very heat resistant and vice versa. Consequently, it is not clear that the compounds used or proposed as time temperature integrators (TTIs) for thermally processed milk are applicable as pressure/temperature time integrators (PTTIs) for equivalent HP processes (Claeys et al., 2003).
The suitability of ALP as an indicator to verify the effectiveness of HPP is first described in Section 3.2.2.1 as it is used to verify adequate pasteurisation of cows’ milk. Other endogeneous enzymes are then discussed in Section 3.2.2.2 followed by other inherent components, such as β‐lactoglobulin (β‐Lg), in Section 3.2.2.3. Some of the enzymes and the other inherent compounds have not been sufficiently studied as potential indicators (neither as TTI nor PTTI) in the relevant studies, but in many cases were evaluated to assess the consequences of HPP of milk or colostrum in the production of dairy products. However, those components on which HPP cause a significant effect within those conditions applied to guarantee the safety of milk or colostrum, have been highlighted as potential indicators to be used as a tool in validation and verification of the process.
Tables E.1–E.3 summarise the information extracted from the records retrieved. The information is organised by major compound categories (e.g. enzymes, whey proteins, caseins and other compounds), with subgroups within each category (e.g. ALP).
Table E.1.
Information extracted from the records retrieved concerning enzymes to verify the efficacy of HPP on raw milk or raw colostrum according to the criteria described in Section 2.3.2
| Compound | Significant effect of HPP | Type of milk/colostrum | Stability after the HPP treatment | Effect compared/validated with target pathogens | Methods available to use | References |
|---|---|---|---|---|---|---|
| Enzymes | ||||||
| Alkaline phosphatase (ALP) |
Significant inactivation occurs at pressures above 600 MPa. Complete inactivation requires 800 MPa. Dp‐ and zp‐values: D400 = 301 min; zp = 368 MPa (2); D625 = 256.7 min; zp = 113.4 MPa (5); D650 = 146.6 min; D700 = 59.5 MPa. Inactivation may indicate HPP overprocessing (6). |
Most surveys included data on bovine milk (1, 2, 4–9). No effect of HPP at 500 MPa for 10 min was reported in goat milk (3). | Slight reactivation is described after incubation at 37°C for 2.5 h. During cold storage reactivation of ALP was not observed (6) | The kinetics of ALP inactivation have not been directly contrasted with that of any pathogen. One survey (6) suggests that ALP inactivation to < 0.1% can indicate the absence of pressure resistant pathogens E. coli and L. monocytogenes (according to resistance data from literature: Patterson (2005). |
Easily measurable both qualitatively and quantitatively and does not require any instrument for a qualitative determination. A fluorometric standardised method is available (ISO standard 11816‐1:1997). |
(1) López‐Fandiño et al. (1996); (2) Mussa and Ramaswamy (1997); (3) Felipe et al. (1997); (4) Rademacher et al. (1998); (5) Ludikhuyze et al. (2000); (6) Rademacher and Hinrichs (2006); (7) Kouassi et al. (2007); (8) Koncza et al. (2007); (9) Martínez‐Monteagudo et al. (2014) |
| y‐Glutamyltransferase (GGT) |
Less pressure‐stable than ALP: inactivation starts at 500 MP and complete inactivation needs 600 MPa at 20°C for 30 min. Dp‐ and zp‐values: D400 = 555.5 min; zp = 543.5 MPa (2) |
Only data on bovine milk are available (1–3). | No data available | The kinetics of GGT at 20°C and > 500 MPa are close to inactivation of L. monocytogenes and E. coli (3) (according to resistance data from literature: Patterson (2005). | Colorimetric method based on determining spectrophotometrical formation of p‐nitroaniline (yellow) at 410 nm. | (1) Rademacher et al. (1998); (2) Pandey and Ramaswamy (2004); (3) Rademacher and Hinrichs (2006) |
| Phosphohexoseisomerase (PHI) | Inactivation starts at 400 MPa. Nearly complete inactivation after 15 min at 500 MPa (2). | Only data on bovine milk are available (1, 2) | No data available | No data available | Colorimetric method. based on the formation of fructose‐6‐phosphate from glucose‐6‐phosphate and a red dye that is determined spectrophotometrically at 490 nm. | (1) Rademacher et al. (1998); (2) Rademacher and Hinrichs (2006) |
| Xanthine oxidase (XOx) | At 500 MPa 46% of XOx is inactivated in 60 min and at 600 MPa 83% of the activity was destroyed within 12 min. | Only data on bovine milk are available | No data available | No data available | Activity determined from the rate of formation of urate from xanthine measured by the increasing absorbance of urate at 290 nm using a spectrophotometer | Olsen et al. (2004) |
| Acid Phosphatase (ACP) | Less resistant to HPP than ALP. 600 MPa for 10 min causes 75% of inactivation. | Only data on bovine milk are available | No data available | No data available | Activity determined by a fluorometric method that evaluates nitrophenol release from fluorophos reagent. | Balci et al. (2002) |
| Plasmin | Reductions up to 18% have been described for 450 and 650 MPa treatment at room temperature. | Six surveys included data on bovine milk (1–5, 7), and one on ovine milk (6) | Cold storage of milk treated at 600 MPa for 30 min did not cause plasmin reduction (4). | No data available | Fluorometric method using N‐succinyl‐L‐alanyl‐L‐phenylalanyl‐L‐lysyl‐7‐amido‐4‐methyl coumarin and measured with a fluorescence spectrophotometer (excitation WL 350 nm; emission WL 460 nm). | (1) Garcia‐Risco et al. (2000); (2) Scollard et al. (2000a); (3) Scollard et al. (2000b); (4) Huppertz et al. (2004a); (5) Borda et al. (2004); (6) Moatsou et al. (2008a); (7) Moatsou et al. (2008b) |
| Lipoprotein lipase (LPL) | Lipoprotein lipase resisted inactivation at 300–400 MPa. No data using higher pressures were reported. | Only data on bovine milk are available | No data available | No data available | Titrimetric determination of hydrolysis of triglycerides in olive oil | Pandey and Ramaswamy (2004) |
| Lysozyme | Lysozyme retains most of its activity after HPP treatments up to 650 MPa for 30 min | No data available for ruminant milk. Only human milk (1, 2) | No data available | No data available | Micrococcus lysodeikticus turbidimetric assay | (1) Viazis et al. (2007); (2) Mayayo et al. (2016) |
| Lactoperoxidase (LPO) | LPO retains 100% of its activity at 600 MPa for 15 min (2). Pressures up to 700 MPa exerts a protective effect on LPO activity (3). | Only data on bovine milk are available (1–5). | No data available | No data available | Spectrophotometric method at 412 nm using 2,2’‐azino‐bis‐3‐ethylbenzothiazoline‐6‐sulfonic acid (ABTS) as reagent and H2O2 as substrate | (1) López‐Fandiño et al. (1996); (2) García‐Graells et al. (2000); (3) Ludikhuyze et al. (2001); (4) Mazri et al. (2012b); (5) Ramos et al. (2015) |
| Lactate dehydrogenase (LDH) | Loss of activity of LDH starts at 206 MPa and complete inactivation is achieved at 482 MPa. | Only data on bovine milk are available | No data available | No data available | Spectrophotometrically (beta‐NADH at 340 nm). | Kouassi et al. (2007) |
Dp: decimal reduction time at a certain pressure; HPP: high‐pressure processing; TVC: Total Viable Counts; zp: pressure resistance constant; WL: wavelength.
Table E.3.
Information extracted from the records retrieved concerning casein micelles, other chemical compounds and changes in physical properties of milk proteins to verify the efficacy of HPP on raw milk or raw colostrum according to the criteria described in Section 2.3.2
| Compound | Effect of HPP | Type of milk/colostrum | Stability after the HPP treatment | Effect compared/validated with target pathogens | Methods available to use | References |
|---|---|---|---|---|---|---|
| Casein micelle (effect on size and composition) | ||||||
| Casein micelle size | Treatments between 400 and 600 MPa resulted in complete disintegration of casein micelles. | Most surveys done with bovine milk, one with buffalo milk and (6) and one with camel (9) milk. Some differences in the primary structure of micelles may explain some differences reported | Changes were generally irreversible on subsequent storage at 5°C, but reassociation is described at 20°C (3, 4) | No data available |
EM (1, 2) PCS (4, 5, 6, 7) MADLS (7, 8, 9, 10) |
(1) Garcia‐Risco et al. (2000); (2) Needs et al. (2000); (3) Huppertz et al. (2004a); (4) Huppertz et al. (2004b); (5) Huppertz et al. (2004c); (6) Huppertz et al. (2005); (7) Leu et al. (2017); (8) Bogahawaththa et al. (2018); (9) Omar et al. (2018); (10) Liu et al. (2020) |
| Solubilisation of casein fractions | Micelle disintegration also implies the solubilisation of casein fractions that follows the course: β → χ → αs1 → αs2‐casein. | Two surveys done with bovine milk. No specific differences reported in goat (1) and buffalo milk (4) | No data available | No data available | Urea‐PAGE | (1) Law et al. (1998); (2) Kiełczewska et al. (2004); (3) Huppertz et al. (2004a); (4) Huppertz et al. (2005) |
| Mineral balance | HPP had no apparent effect on levels of colloidal Ca and P concentrations. | Goats’ milk (1, 2, 3). | Soluble Ca and P concentrations increased during the first day of cooling after HPP (3) | No data available |
MS (1,3) AAS (2) |
(1) Law et al. (1998); (2) De la Fuente et al. (1999); (3) Nassar et al. (2019) |
| Other chemical compounds | ||||||
| Fat globule size | 400–800 MPa treatments increased mean diameter and broadened the size distribution. | Ovine milk (2). | No data available | No data available | Coulter Counter system (1) | (1) Kanno et al. (1998); (2) Gervilla et al. (2001) |
| Fatty acid composition | 250–900 MPa treatments at room temperature did not result in alteration of the composition of neutral and polar lipids, and fatty acids. | Most surveys done with bovine milk, one with ovine (1) and one with caprine (6) milk. | During refrigerated storage the impact of HPP on the attributes of caprine milk fat became apparent only after 14 days (6) | No data available |
GC‐FID (3) HPLC‐LSD (4, 5) |
(1) Gervilla et al. (2001); (2) Delgado et al. (2014); (3) Martinez‐Monteagudo et al. (2014); (4) Rodríguez‐Alcalá et al. (2014); (5) Rodriguez‐Alcala et al. (2015); (6) Kiełczewska et al. (2020) |
| Ribonucleosides | No significant changes reported. | All surveys done with bovine milk. | No data available | No data available | HPLC | (1) Martin et al. (2001); (2) Martin and Meisel (2006) |
| Maillard and volatile compounds | No relevant Maillard reaction (furosine formation), lactulose formation or lactosylation of β‐Lg occur after 100–400 MPa for 10–60 min at room temperature. | Two surveys done with bovine milk and two with human milk (3, 4). | No data available | No data available |
IP RP HPLC (1) GC–MS (3,4) |
(1) López‐Fandiño et al. (1996); (2) Nabhan et al. (2004); (3) Contador et al. (2015); (4); Garrido et al. (2015) |
| Changes in physical properties of milk | ||||||
| Colour | HPP reduces the lightness values (L*) and increases the total colour differences (∆E) and whiteness (WI). Changes were attributable mainly to the disruption of casein micelles. |
Two surveys were done with bovine milk, one with ovine (1), one with Caprine (6) and one with camel (5) milk. |
L* of untreated milk decreased slightly while in milk treated at 300–600 MPa increased slightly during the first 24 h of storage (2) | No data available | Colorimeter | (1) Gervilla et al. (2001); (2) Huppertz et al. (2004c); (3) Huppertz et al. (2005); (4) Omar et al. (2018); (5) Nassar et al. (2019); (6) Kiełczewska et al. (2020) |
| Viscosity | Viscosity increases after HPP. | Caprine | Viscosity continued increasing during storage at 4°C (Nassar et al., 2019). | No data available | Rheometer (2) | Nassar et al. (2019) |
| Turbidity | Turbidity (A320) decreases markedly after treatments up to 300 MPa, but little further decrease was observed at pressures up to 600 MPa. | Bovine | No data available | No data available | Spectrophotometer | Needs et al. (2000) |
AAS: atomic absorption spectrophotometry; ∆E: total colour differences; EM: electron microscopy; FID: Flame‐Ionisation Detection; GC: gas chromatography; HPLC: high‐pressure liquid chromatography; IP RF: ion‐pair reversed‐phase; L*: lightness value; LSD: light scattering detector; MADLS: Multi‐Angle Dynamic Light Scattering; MS: mass spectrometry; PAGE: polyacrylamide gel electrophoresis; PCS: photon correlation spectroscopy; WI: whiteness.
3.2.4.1. Alkaline phosphatase
ALP is an endogenous milk enzyme and is widely used to verify adequate pasteurisation of cows’ milk; inactivation of ALP takes place at temperatures slightly higher than those necessary to kill M. tuberculosis, S. Senftenberg or L. monocytogenes (Claeys et al., 2002). As described in Section 3.2.1.2, FBOp must ensure that the thermal pasteurisation is achieved by a treatment involving T/t conditions such that the products show, where applicable, a negative reaction to an ALP test immediately after such treatment. According to Chapter II in Annex III to Commission Implementing Regulation (EU) No 2019/627,15 such a test is considered to give a negative result if the measured activity in cows’ milk is not higher than 350 milliunits of enzyme activity per litre (mU/L) using the ISO reference method 11816‐1.16
López‐Fandiño et al. (1996) and Mussa and Ramaswamy (1997) indicated a higher resistance of ALP to pressure inactivation (up to 400 MPa) than the natural microbiota present in milk, reporting higher Dp‐values compared to those obtained for total viable counts (TVC), and a zp‐value of about twice for ALP than that for the microorganisms. This implies that when a 4D reduction of the TVC was achieved the inactivation of ALP was only ~ 10%. As a result, the authors suggested that the criteria for the application of ALP test as an indicator for HPP pasteurisation of milk should be different from that established for thermal pasteurisation, considering a percentage destruction of this enzyme more indicative than complete inactivation. Rademacher et al. (1998) and Rademacher and Hinrichs (2006) found that loss of ALP activity starts around 600 MPa, but that complete inactivation requires 800 MPa. Rademacher and Hinrichs (2006) considered ALP more resistant to HPP treatment than L. monocytogenes and E. coli (according to their HPP resistance described by Patterson (2005)). These authors suggested that inactivation of the activity (< 0.1%) of ALP could indicate the absence of these pathogens in pressure‐treated milk but concluded that its use as indicator for pressure pasteurisation of milk at room temperatures or below may lead to overprocessing.
Kouassi et al. (2007) suggested that ALP owes its pressure resistance to the stability of its secondary structure, demonstrated by the preservation of its R‐helix content and the limited formation of aggregates following various pressure treatments (up to 620 MPa). Differences in raw milk sources and the existence of two or more isoenzymes of ALP with different pressure stability have been highlighted as the main reasons for the observed variability in the inactivation behaviour (Rademacher and Hinrichs, 2006). Inactivation of ALP is accelerated by increasing the temperature, indicating a synergistic effect of pressure and temperature on the inactivation reaction (Rademacher and Hinrichs, 2006).
Concerning the stability of the inactivation caused by HPP over time, Rademacher and Hinrichs (2006) reported that reactivation of ALP was not observed during cold storage of pressure‐treated milk samples and concluded that its reactivation should not be a problem in industrial practice since pasteurised milk has to be kept cold during storage.
Only one report described the effect of HPP on milk from species other than cows (goats’ milk), where the activity of ALP was unchanged after 10 min at 500 MPa (Felipe et al., 1997). None of the available records included data about colostrum.
3.2.4.2. Other endogeneous enzymes
γ‐glutamyltransferase (GGT) is a membrane‐bound enzyme and nearly all its activity is located in the milk fat globule membrane (Pandey and Ramaswamy, 2004). Rademacher et al. (1998) evaluated the suitability of GGT as a pressure process marker alternative to ALP in raw bovine milk and found that a pressure treatment above 500 MPa is needed to achieve inactivation. Rademacher and Hinrichs (2006) when applying pressures in the range of 400–800 MPa, concluded that GGT is less pressure stable than ALP and complete inactivation was achieved at 600 MPa for 30 min at 20°C. The influence of process temperature on the pressure‐induced inactivation of GGT is limited within the range 5–40°C. When comparing GGT inactivation kinetics with the inactivation of pathogens such as L. monocytogenes and E. coli, Rademacher and Hinrichs (2006) suggested that the kinetics of GGT at 20°C and pressures above 500 MPa were sufficiently close to these microorganisms (according to Patterson, 2005) that GGT may therefore provide a useful process marker for their destruction. No data were available about the stability of GGT during milk storage after HPP treatments.
Phosphohexoseisomerase (PHI) is more sensitive to HPP than ALP and GGT, starting its inactivation at about 400 MPa (Rademacher et al., 1998) and is completely inactivated after treatments at 500 MPa for less than 15 min, showing a strong influence of pressure on the inactivation reaction (Rademacher and Hinrichs, 2006). Nevertheless, more studies would be needed to confirm this.
Xanthine oxidase (XOx) and acid phosphatase (ACP) were also studied as HPP indicators of bovine milk. XOx showed resistance to 400 MPa at 25°C, but at higher pressures the enzyme was inactivated. Treatment of milk at 400 MPa for 120 min resulted in a loss of 17% in enzyme activity while at 500 MPa 46% of XOx was inactivated in 60 min and 83% of the activity was destroyed within 12 min at 600 MPa (Olsen et al., 2004). On the other hand, according to Balci et al. (2002), ACP was shown to be much less resistant to pressure than ALP and the majority of ACP activity was lost within 10 min of exposure to 500 and 550 MPa, thereafter the decline in activity was relatively slow. Results showed that, after 600 MPa for 10 min, ACP still showed 25% of activity. A marginally higher inactivation was observed with skimmed milk compared to whole milk for the same time and pressure combination, although activity in skimmed milk was 80% that of whole milk, suggesting ACP as a potential indicator to discriminate between thermal and high‐pressure treatments.
Lactoperoxidase (LPO) is well known as a process indicator of high‐temperature pasteurised milk, but it presents a high stability to pressure. LPO activity was not reduced after treatments at 400 MPa and 25°C during 60 min (López‐Fandiño et al., 1996) and 600 MPa at 20°C for 15 min (García‐Graells et al., 2000). Ludikhuyze et al. (2001) also reported that LPO was only slightly affected by treatments at pressures up to 700 MPa combined with temperatures between 20°C and 65°C. The pressure stability of LPO may be due to its monomeric structure, which is stabilised by eight disulfide bonds (Mazri et al., 2012b), and consequently, according to Ramos et al. (2015) it cannot be considered as a potential marker for the inactivation of bacteria in pressurised milk.
A contrasting situation applies to lactate dehydrogenase (LDH). Kouassi et al. (2007) reported that even the lowest HPP treatment tested (206 MPa for 6 min at room temperature) induced a reduction in LDH activity, and total inactivation occurred at 482 MPa and above after 6 min treatment, caused by a combined effect of denaturation and aggregation of the enzyme.
The effect of HPP on lipoprotein lipase (LPL) has been described by Pandey and Ramaswamy (2004). After HPP treatment combinations using pressures up to 400 MPa and times up to 120 min (at 3°C), LPL was not inactivated but rather enhanced. No data using higher pressures or temperatures were reported on the activity of this enzyme.
Available literature indicates that HPP treatments at 300 and 400 MPa applied between 10 and 30 min only slightly reduced the plasmin activity in the milk by 25–30% (Garcia‐Risco et al., 2000; Scollard et al., 2000a; Moatsou et al., 2008a). However, similar treatments combined with higher temperatures (up to 60°C) considerably increased plasmin inactivation. Treatments at 600 MPa for 10 and 30 min in bovine milk showed a plasmin activity reduction between 16 and 50% (Scollard et al., 2000a; Huppertz et al., 2004a; Moatsou et al., 2008a). Moatsou et al. (2008a) also reported reductions in activities of plasminogen activators between 38 and 62% at 450 and 650 MPa, at room temperature. In ovine milk, similar results were reported for both plasmin (24%) and plasminogen activators (34%) activities after pressure treatments of 450 and 650 MPa (Moatsou et al., 2008b).
The effect of HPP on lysozyme was evaluated in human milk showing it to be very resistant to HPP and resisting treatments of 400 MPa for up to 120 min (Viazis et al., 2007) or increasing its levels of activity when pressure increased to up of 650 MPa for 30 min (Mayayo et al., 2016).
3.2.4.3. Effect of HPP on other inherent compounds
The effect of HPP has been studied in other compounds inherent in milk/colostrum of different species, especially for whey proteins, caseins (both in micelle size and composition) or fat. The effect that HPP has on the possible formation of new substances from reactions between compounds due to the action of high pressure has also been evaluated, and which have been used as indicators of the intensity of other applied treatments, such as thermal ones. The consequences that the changes caused by HPP treatments on these compounds have resulted in some physical properties of milk/ colostrum, such as colour, degree of turbidity or viscosity, have also been studied.
Major whey proteins. The major whey proteins of milk are β‐Lg, α‐lactalbumin (α‐La), serum albumin (SA), lactoferrin (LF) and immunoglobulins (Ig). The determination of the level of denaturation of whey proteins has been proposed as an indicator of the effectiveness of heat treatments, and relatively fast methods have been proposed to quantify this effect, such as the measurement of acid‐soluble tryptophan by fluorescence or the Aschaffenburg turbidity test (EFSA, 2021a). However, in the case of HPP treatments, most surveys have evaluated the effect on the different whey proteins individually, but very few have quantitatively studied the effect on the whey proteins as a whole. Pressurisation of milk cause an increase in the amount of denatured whey proteins that significantly increases with the pressure level (Kiełczewska et al. 2004; Bravo et al., 2013). Kiełczewska et al. (2004) reported that for cows’ milk the level of whey protein denaturation achieved after 15 min HPP treatment at 400 MPa was 35.7%, and this increased to just 51.1% at 600 MPa. HPP leads to unfolding and aggregation of whey proteins, which is accompanied by an increase in water‐binding capacity. Hence, similar to heating, HP denaturation of whey proteins changes the functional milk properties (Claeys et al., 2003). However, HPP influences differently each of the whey protein fractions; α‐La, SA, LF and Ig are more stable under HPP than β‐Lg (Bogahawaththa et al., 2018; Liu et al., 2020).
The denaturation of the different whey proteins by HPP is commonly evaluated in the acid whey fraction based on its loss of solubility at pH 4.6 and it is determined by different methods such as sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE), reversed‐phase high‐pressure liquid chromatography (RP‐HPLC) and radial immunodiffusion, among others.
β‐lactoglobulin (β‐Lg) . Table 6 summarises the effect reported of HPP treatments from 400 to 600 MPa on the denaturation level of β‐Lg. β‐Lg showed to be the most sensitive whey protein to HPP. Although pressures up to 100 MPa did not induce denaturation at room temperature (López‐Fandino et al., 1996; Huppertz et al., 2004a; Ye et al., 2004), denaturation increased with pressure and longer holding times (Kleber et al., 2007; Bravo et al., 2012). Treatments up to 30 min at 400–500 MPa in bovine milk caused 30%‐60% β‐Lg denaturation (Scollard et al., 2000a) and may increase to above 90% at pressures above 600 MPa (Ye et al., 2004; Leu et al., 2017). In an experiment where 600 MPa for 15 min were applied to achieve a reduction of at least 7 log10 cycles of three food‐borne bacteria (L. monocytogenes, S. aureus and Pseudomonas aeruginosa) about 80% of the β‐Lg was denatured (Ramos et al., 2015). Temperature increases the level of denaturation, achieving 100% in milk treated at 400 MPa and above and temperatures up to 60°C (Garcia‐Risco et al., 2000; Kiełczewska et al., 2004; Moatsou et al., 2008a).
Table 6.
Level of β‐lactoglobulin denaturation in milk of different species after HPP treatments at 400–600 MPa at different times and initial temperatures up to 20°C
| Reference | Product | Species | Type of milk | Pressure (MPa) | Temp (°C) | Time (min) | % Denaturation |
|---|---|---|---|---|---|---|---|
| Scollard et al. (2000a) | Milk | Cow | Whole | 400 | 20 | 10 | 47.56 |
| 400 | 20 | 30 | 70.24 | ||||
| 500 | 20 | 10 | 72.13 | ||||
| 500 | 20 | 30 | 82.52 | ||||
| 600 | 20 | 10 | 77.17 | ||||
| 600 | 20 | 30 | 82.84 | ||||
| Huppertz et al. (2004b) | Milk | Cow | Skimmed | 400 | 5 | 30 | 60.60 |
| 400 | 20 | 30 | 92.60 | ||||
| 600 | 5 | 30 | 88.50 | ||||
| 600 | 20 | 30 | 98.00 | ||||
| Nabhan et al. (2004) | Milk | Cow | Whole | 400 | 20 | 5 | 35.00 |
| 400 | 20 | 10 | 49.50 | ||||
| 400 | 20 | 15 | 57.70 | ||||
| 400 | 20 | 30 | 72.00 | ||||
| 400 | 20 | 60 | 79.70 | ||||
| Nabhan et al. (2004) | Milk | Cow | Whole | 400 | 20 | 30 | 67.30 |
| 600 | 20 | 30 | 79.00 | ||||
| Zobrist et al. (2005) | Milk | Cow | Skimmed | 400 | 20 | 30 | 90.70 |
| 600 | 20 | 30 | 99.60 | ||||
| Mazri et al. (2012a) | Milk | Cow | Skimmed | 450 | 20 | 10 | 22.90 |
| 500 | 20 | 10 | 53.24 | ||||
| 550 | 20 | 10 | 60.69 | ||||
| 600 | 20 | 10 | 74.21 | ||||
| 500 | 20 | 15 | 58.76 | ||||
| 550 | 20 | 15 | 71.72 | ||||
| 600 | 20 | 15 | 79.72 | ||||
| Bravo et al. (2013) | Milk | Cow | Skimmed | 450 | 15 | 5 | 57.62 |
| 550 | 15 | 5 | 75.69 | ||||
| Ramos et al. (2015) | Milk | Cow | Skimmed | 600 | 20 | 15 | 79.31 |
| Leu et al. (2017) | Milk | Cow | Skimmed | 600 | 20 | 5 | 60.07 |
| Omar et al. (2018) | Milk | Cow | Whole | 400 | 20 | 30 | 64.58 |
| 600 | 20 | 30 | 79.61 | ||||
| Law et al. (1998) | Milk | Goat | Whole | 400 | 20 | 10 | 77.70 |
| 500 | 20 | 10 | 81.60 | ||||
| Huppertz et al. (2005) | Milk | Buffalo | Whole | 400 | 20 | 30 | 99.10 |
| 600 | 20 | 30 | 100.00 |
Concerning the effect on milk from other species, Felipe et al. (1997) observed that the denaturation curve of β‐Lg in raw goats’ milk showed a slow increase up to 150–200 MPa, followed by a rapid increase up to 350 MPa. Above this pressure, most of the β‐Lg can be found in aggregates, and consequently, the denaturation rate was slower. In ovine milk, Moatsou et al. (2008b) also observed a low level of denaturation at 200 MPa, but substantially increased at 450 and 650 MPa, remaining very low levels of native β‐Lg after treatment at 650 MPa. In buffalo milk, Huppertz et al. (2005) reported that a treatment of 250 MPa denatured > 85% and a 400–800 MPa treatment achieved 100% denaturation, higher than that reported for bovine, ovine or caprine milk. It must be taken into consideration that the initial concentration of β‐Lg is different depending on the species; sheep’s milk has the highest concentration of β‐Lg and it is about 2.5 times higher than in cows’ and goats’ milk (EFSA, 2021a).
Comparable to thermal denaturation, pressure‐induced unfolding of β‐Lg is extensive and irreversible at neutral pH (Claeys et al., 2003). The majority of denatured β‐Lg is associated with casein micelles as β‐Lg unfolds results in exposure of its free thiol group, which in turn can react with proteins containing disulfide bonds including other β‐Lg, αs2‐casein, κ‐casein and α‐La through thiol‐disulfide interchange reactions (Huppertz et al., 2004b, 2005; Bogahawaththa et al., 2018). This explains why β‐Lg is more pressure sensitive in milk than in an aqueous system as they could associate with caseins and precipitate with them at their isoelectric point (Bravo et al., 2013; Ramos et al., 2015).
α‐lactalbumin (α‐La) . Quantitative data on the effect of HPP on α‐La at pressures between 400 and 600 MPa are included in Table 7. Unlike the β‐Lg, α‐La in bovine milk is resistant to pressurisation up to 400 MPa at room temperature (López‐Fandino and Olano, 1998; Garcia‐Risco et al., 2000; Huppertz et al., 2004b; Mazri et al., 2012a; Leu et al., 2017; Liu et al., 2020). Ye et al. (2004) described that only about 10% of the α‐La was denatured at 600 MPa and about 50% at 800 MPa. Increased α‐La denaturation may be obtained by increasing temperatures to 50–60°C reaching almost 60% of denaturation after treatments at 400 MPa (Lopez‐Fandiño et al., 1996; Garcia‐Risco et al., 2000). Moatsou et al. (2008a), reported that residual native α‐La ranged from 73% after 650 MPa treatment at 20°C to 27% at 55°C.
Table 7.
Level of α‐lactalbumin denaturation in milk of different species after HPP treatments at 400–600 MPa at different times and initial temperatures up to 20°C
| Reference | Product | Species | Type of milk | Pressure (MPa) | Temp (°C) | Time (min) | % Denaturation |
|---|---|---|---|---|---|---|---|
| Moatsou et al. (2008b) | Milk | Buffalo | Skimmed | 400 | 20 | 30 | 6.00 |
| 600 | 20 | 30 | 52.50 | ||||
| Milk | Camel | Whole | 400 | 20 | 30 | 29.08 | |
| 600 | 20 | 30 | 30.61 | ||||
| Law et al. (1998) | Milk | Goat | Whole | 400 | 20 | 10 | 0.00 |
| 500 | 20 | 10 | −3.20 | ||||
| Ye et al. (2004) | Milk | Cow | Whole | 400 | 20 | 5‐30 | 0.0 |
| 400 | 20 | 60 | 4.00 | ||||
| 600 | 20 | 30 | 9.15 | ||||
| Huppertz et al. (2004b) | Milk | Cow | Skimmed | 400 | 5 | 30 | −2.00 |
| 400 | 20 | 30 | 1.70 | ||||
| 600 | 5 | 30 | 21.60 | ||||
| 600 | 20 | 30 | 42.00 | ||||
| Mazri et al. (2012a) | Milk | Cow | Skimmed | 500 | 20 | 10 | 5.22 |
| 500 | 20 | 15 | 7.38 | ||||
| 550 | 20 | 10 | 11.22 | ||||
| 550 | 20 | 15 | 15.29 | ||||
| 600 | 20 | 10 | 1.13 | ||||
| 600 | 20 | 15 | 10.65 | ||||
| Bravo et al. (2013) | Milk | Cow | Skimmed | 450 | 5 | 15 | 5.33 |
| 550 | 5 | 15 | 16.12 | ||||
| Ramos et al. (2015) | Milk | Cow | Skimmed | 600 | 20 | 15 | 5.96 |
| Leu et al. (2017) | Milk | Cow | Skimmed | 600 | 20 | 5 | −1.37 |
A similar trend has been found in milk from other species. Felipe et al. (1997) and Law et al. (1998) reported a small amount of aggregation of α‐La in goats’ milk at pressures up to 500 MPa, which has been attributed to the absence of free SH groups and the initial difficulty in forming covalent links to other proteins. Moatsou et al. (2008b) observed that denaturation of α‐La in ovine milk occurred only at 650 MPa and increased with temperature. In pasteurised skimmed buffalo milk the level of α‐La that was denatured after a HP treatment at 800 MPa was higher (> 90%) than that observed in bovine milk (70%) (Huppertz et al., 2005). In contrast, Omar et al. (2018) reported that the level of denatured α‐La of camel milk after 800 MPa treatment was considerably lower than that of the previously reported species.
This lower pressure sensitivity of α‐La with respect of β‐Lg can be explained by the number of bonds stabilising the conformational structure (two and four disulfide bonds, respectively), and the relative numbers of non‐covalent interactive forces (Bogahawaththa et al., 2018).
Immunoglobulins (Ig) . Table 8 shows the effect of HPP on different types of Ig. At pressures up to 600 MPa, a denaturation around 44% was observed in cows’ milk (Kiełczewska et al., 2004). Nevertheless, denaturation increased to 96% when treatments are performed at an initial temperature of 30°C (Bogahawaththa et al., 2018). In goats’ milk only partial denaturation of the Ig fraction occurred between 300 and 500 MPa (Felipe et al., 1997; Law et al., 1998). In human milk, IgM and IgA maintained their initial levels after treatments at 400 MPa for 6 min and retained 75% of their immunoactivity after 120 min, but at higher pressure (600 MPa) their immunoactivity was reduced by 40–64% after treatments for up to 30 min (Viazis et al., 2007; Contador et al., 2013; Mayayo et al., 2016).
Table 8.
Level of immunoglobulins denaturation in milk of different species after HPP treatments at 400–600 MPa at different times and initial temperatures up to 20°C
| Reference | Indicator | Product | Species | Type of milk | Pressure (MPa) | Temp (°C) | Time (min) | % Denaturation |
|---|---|---|---|---|---|---|---|---|
| Viazis et al. (2007) | IgA | Milk | Human | Whole | 400 | 21 | 30 | 17.4 |
| 400 | 21 | 60 | 12.9 | |||||
| 400 | 21 | 90 | 19.4 | |||||
| 400 | 21 | 120 | 24.6 | |||||
| Mayayo et al. (2016) | IgA | Milk | Human | Whole | 400 | 20 | 30 | 42.00 |
| 500 | 20 | 30 | 53.00 | |||||
| 600 | 20 | 30 | 64.00 | |||||
| Law et al. (1998) | Ig‐ | Milk | Goat | Whole | 400 | 20 | 10 | 0.54 |
| 500 | 20 | 10 | 20.50 | |||||
| Ramos et al. (2015) | IgG | Milk | Cow | Skimmed | 600 | 20 | 15 | 42.95 |
| Sousa et al. (2014) | IgA | Colostrum | Human | Whole | 400 | 8 | 15 | 0 |
| 400 | 8 | 30 | 0.4 | |||||
| 600 | 8 | 15 | 19.7 | |||||
| 600 | 8 | 30 | 25.73 | |||||
| IgM | Colostrum | Human | Whole | 400 | 8 | 15 | 0 | |
| 400 | 8 | 30 | 6.29 | |||||
| 600 | 8 | 15 | 59.1 | |||||
| 600 | 8 | 30 | 59.8 | |||||
| IgG | Colostrum | Human | Whole | 400 | 8 | 15 | 8.57 | |
| 400 | 8 | 30 | 8.78 | |||||
| 600 | 8 | 15 | 35.3 | |||||
| 600 | 8 | 30 | 39.8 | |||||
| Foster et al. (2016) | IgG | Colostrum | Cow | Whole | 400 | 20 | 10 | 0.92 |
| 400 | 20 | 15 | 8.64 | |||||
| 400 | 20 | 20 | 12.58 | |||||
| Trujillo et al. (2007) | IgG | Colostrum | Goat | Whole | 400 | 20 | 10 | 19.76 |
| 500 | 20 | 10 | 38.03 |
The effect of HPP on Ig was also evaluated in colostrum of different species. Foster et al. (2016) reported that HPP treated bovine colostrum maintained an acceptable IgG level while Trujillo et al. (2007) reported that after 500 MPa the IgG concentration in caprine colostrum was reduced by 38%. In human colostrum, treatment at 600 for 2.5 min resulted in the maintenance of IgA and losses of 21% for both IgG and IgM, which was demonstrated to be the most sensitive, obtaining Dp‐values of 441 min at 400 MPa and 40 min at 600 MPa, followed by IgA, with a Dp‐value of 1,887 min at 400 MPa and 158 min at 600 MPa, with IgG being the most resistant, with Dp‐values of 4,941 min at 400 MPa and 235 min at 600 MPa (Sousa et al., 2014).
Lactoferrin (LF) . Mazri et al. (2012b) reported a Dp‐value of 201 min for LF denaturation in bovine milk at 450 MPa and 20°C, but this reduced to a Dp‐value of 34.47 min at 600 MPa and 11.37 min at 700 MPa. This substantial denaturation of LF at 600 MPa (around 70%) was also reported by Ramos et al. (2015). In camel milk, Omar et al. (2018) reported a reduction of less than 3% of LF after 600 MPa for 30 min. This greater stability of camel LF was similar to that the observed for other whey proteins when compared with their counterparts in bovine milk. In human milk, Mayayo et al. (2014) reported that the loss of concentration of immunoreactive LF was approximately 43% after 7 min of treatment at 600 MPa. Data on the effect of HPP on LF denaturation in milk of different species is summarised in Table 9.
Table 9.
Level of lactoferrins denaturation in milk of different species after HPP treatments from 400 to 600 MPa at different times and initial temperatures up to 20°C
| Reference | Product | Species | Type of milk | Pressure (MPa) | Temp (°C) | Time (min) | % Denaturation |
|---|---|---|---|---|---|---|---|
| Ramos et al. (2015) | Milk | Cow | Skimmed | 600 | 20 | 15 | 70 |
| Omar et al. (2018) | Milk | Camel | Whole | 400 | 20 | 30 | < 3 |
| 600 | 20 | 30 | < 3 | ||||
| Mayayo et al. (2014) | Milk | Human | Whole | 400 | 20 | 7 | 19 |
| 500 | 20 | 7 | 30 | ||||
| 600 | 20 | 7 | 43 |
Serum albumin (SA) . Very few investigations on the effect of pressure on pure SA have been reported, but it has been found to be quite resistant to pressure treatment up to 600 MPa, probably due to its extremely stable structure associated with the high number of intramolecular disulfide bonds and the presence of several separate domains (Considine et al., 2007; Patel and Huppertz, 2014). Omar et al. (2018) reported 16.3% SA denaturation in bovine milk and 2.5% in camel milk after a 30 min HPP treatment at 600 MPa at room temperature.
Casein. Pressurisation of milk may cause fragmentation of casein micelles accompanied by a release of calcium and phosphorus into the milk serum resulting from the breaking of linkages between caseins and inorganic constituents and weakening or rupture of electrostatic and hydrophobic interactions between casein constituents (Claeys et al., 2003). A variety of methods have been used to determine casein micelle size in HP‐treated milk, such as electron microscopy or photon correlation spectroscopy (PCS) among others. Solubilisation of micelle fractions were analysed mainly using SDS‐PAGE and concentrations of soluble calcium contents were determined by atomic absorption spectrophotometry.
In raw bovine milk, pressure treatments performed at low pressure (200 MPa) and room temperature caused only a partial disintegration of casein micelles, changing the sizes between 250 and 300 MPa with an apparent increase in density while treatments at 400 and 600 MPa resulted in its complete disintegration (Garcia‐Risco et al., 2000; Needs et al., 2000; Huppertz et al., 2004b,c; Leu et al., 2017; Bogahawaththa et al., 2018; Omar et al., 2018).
The reduction in casein micelle size in bovine milk is likely to be due to the disruption of the intra‐micellar van der Waals, hydrophobic and electrostatic interactions and changes in the solubilisation of micellar calcium phosphate (Needs et al., 2000; Huppertz et al., 2004a; Omar et al., 2018; Liu et al., 2020). These changes were generally irreversible on subsequent storage at 5°C, although considerable reassociation of caseins is described at 20°C (Huppertz et al., 2004a,b). Micelle disintegration also implies the solubilisation of casein fractions that follows the course: β → χ → αs1 → αs2‐casein and is closely related to the level of phosphate residues in casein and the transformation of colloidal calcium phosphate into a dissolved form (Huppertz et al., 2004a; Kiełczewska et al., 2004). Similar results were observed in goats’ milk (Law et al., 1998), but Huppertz et al. (2005) reported that in skimmed buffalo milk the effect of HPP treatments at 400 or 800 MPa increased by 10 and 35% the micelle size, respectively, differing considerably from those observed in milk of other species, such as bovine milk. In contrast, the decrease in the size of casein micelles in camel milk was considerably smaller than that in bovine milk, which might be due to the differences in the primary structure of micelles between species (Hailu et al., 2016).
Law et al. (1998) observed in raw caprine milk that HPP had no significant effect on levels of colloidal Ca and P concentrations, and the ratio of Ca to P in the colloidal phase was maintained at the ratio is about two after each treatment. Nassar et al. (2019) also reported that in raw caprine milk, the soluble Ca and P concentrations increased during the first day of cooling after HPP treatment which could be a consequence of the solubilisation of Ca and P during cold storage.
Fat globule structure and composition. Kanno et al. (1998) reported that HPP pressures below 400 MPa did not affect the mean diameter of milk fat globules, but at higher pressures (400–800 MPa), the diameter increased and broadened the size distribution. Nevertheless, no damage to the milk fat globule membrane was thought to occur since no increase in products of lipolysis was detected. HPP up to 900 MPa at room temperature did not significantly affect the lipid profile of cows’ milk (Rodriguez‐Alcalá et al., 2014, 2015) and similar findings are reported for caprine milk (Kiełczewska et al., 2020). According to Gervilla et al. (2001), temperature during HPP is the main factor explaining 95% of the variability caused in the lipidic fraction in ovine milk.
Other chemical compounds. Some compounds that have been proposed as TTIs can be formed in milk as a consequence of the Maillard and other degrative reactions due to thermal treatments (e.g. 5‐hydroxymethylfurfural, lactulose, furosine, lysinoalanine (EFSA, 2021a), but little information is available about their formation during HPP treatments.
López‐Fandiño et al. (1996) observed no Maillard reaction (furosine formation or lactulose formation) after 100–400 MPa HPP treatments at 25°C for 10–60 min. Nabhan et al. (2004) reported that lactosylation of β‐Lg, another reaction that usually occurs in heat‐treated milk samples, seemed to be very limited after HPP treatments as only trace amounts of glycosylated aggregates were found. Contador et al. (2015) reported that treatments at 400 and 600 MPa for 3 min maintained the volatile compounds at similar levels to those found in control milk samples, although the application of 600 MPa for 6 min changed the original volatile compounds of human milk, even more than thermal pasteurisation. Milk treatments may also influence on the content of ribonucleosides and some changes in these may be used for the characterisation of heat treatments (e.g. formation of N6‐methyladenosine) (Martin and Meisel, 2006). However, these changes are largely affected by the enzyme adenosine deaminase, inactivated by high pressure, thus resulting in lower inosine contents in the high‐pressure treated samples (Martin et al., 2001).
Changes in physical properties of milk. Changes in the physical properties of milk, especially in the optical properties, have been reported as a consequence of disruption of casein micelles and other macromolecules caused by HPP treatments.
Needs et al. (2000) reported that turbidity (A320) of skimmed raw cows’ milk held at 4°C after pressure treatment decreased markedly as a result of treatments up to 300 MPa, but little further decrease was observed at pressures up to 600 MPa. Concerning the HPP effect on milk colour parameters, different authors reported that HPP at 200–800 MPa reduced the Lightness values (L*) and an increase in total colour differences (∆E) and whiteness (WI) of bovine milk although L*‐value may increase during further storage at 5°C (Huppertz et al., 2004c; Omar et al., 2018). Mussa and Ramaswamy (1997) estimated that L* decrease follows a first‐order reaction, with a D400 of 456 min and a zp of 532 MPa. In ovine milk, an increase of greenness (a*) and yellowness (b*) is also reported (Gervilla et al., 2001). Similar changes were also reported for caprine milk (Nassar et al., 2019; Kiełczewska et al., 2020), buffalo milk (Huppertz et al., 2005) and camel milk (Omar et al., 2018), but in that last case at a considerably lower level than bovine milk. In all cases, changes were more evident when the pressure increased. Nevertheless, most authors concluded that even the colour changes caused by the most extreme HPP treatment would not induce consumers to reject the milk. In caprine milk, Kiełczewska et al. (2020) reported that the modification of colloidal and emulsion components led to changes in the colour parameters of HP‐treated milk excluding greenness, causing an increase in ∆E with pressure, and that storage exerted a significant effect on all, minimising the total colour difference. Nassar et al. (2019), reported that the L*‐values of the HP‐treated caprine milk samples decreased with increasing pressure compared with those of the control milk sample, whereas the a*‐values increased and the b*‐values showed the same general trend as the a*‐values. All reported surveys attribute these colour changes mainly to the disruption of casein micelles, which form small fragments that increase the translucence of milk and to reformation of micellar particles through hydrophobic interactions during cold storage.
Mussa and Ramaswamy (1997) reported in HPP processed cows’ milk that viscosity increased with pressure and time, with a D400 of 222 min and a zp of 404 MPa. According to De La Fuente et al. (1999), the viscosity of raw caprine milk increased from 1.93 to 2.80 MPa and from 3.51 to 4.25 MPa after the application of HP at 500 MPa (for 25 min at 25°C). Viscosity continued increasing during storage at 4°C (Nassar et al., 2019) probably as a. consequence of the solubilisation of Ca and P during cold storage and the distribution of casein between the micellar and soluble phases of milk.
3.2.4.4. Comparison among potential indicators to verify the efficacy of HPP treatments on raw milk or raw colostrum
Based on the results obtained in Section 3.2.3, the minimum requirements of HPP of raw milk to achieve a target 5 log10 reductions of S. aureus can be achieved, e.g. with 600 MPa for 10 min, 550 MPa for 13 min or 500 MPa for 19 min, as it is shown in Figure 17. According to that, the P/t combinations needed to obtain a 1 log10 reduction of the activity of some milk inherent enzymes (ALP, GGT and Xox) or denaturation of whey proteins (β‐Lg and LF) of some compounds are higher than those needed to cause 5 log10 reductions of S. aureus. In some cases (GGT and β‐Lg) the necessary P/t combinations are quite close, according to some surveys, but data are scarce, especially for the range of pressures intended to be used for milk processing, and therefore, they cannot be reliably proposed as a suitable indicator to verify the efficacy of HPP treatments on raw milk or colostrum. More studies would be needed to confirm the observed trends before any inherent compound can be recommended as a potential indicator as in some cases, the available information is contradictory.
Figure 17.
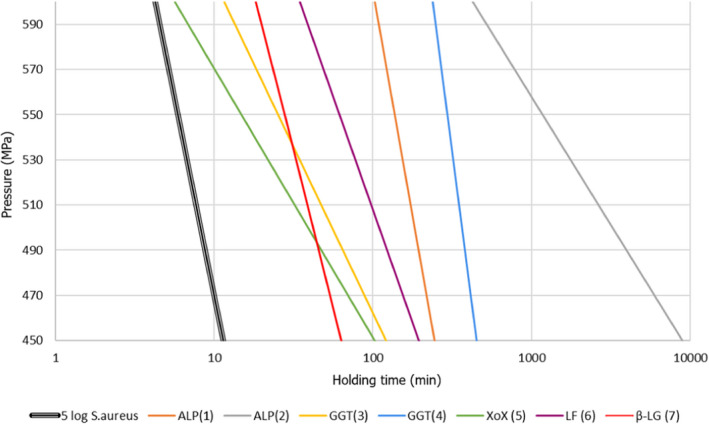
Isoreduction plot of HPP conditions (pressure/holding time combinations) that cause 90% reduction of the activity (enzymes) or denaturation (whey proteins) of some milk inherent compounds compared with the same HPP conditions that cause 5 log10 reductions of Staphylococcus aureus in raw milk (see Section 3.2.3.4)
- ALP: Alkaline phosphatase; GGT: γ‐Glutamil Transferase; Xox: Xanthine oxidase; LF: Lactoferrin; β‐Lg: β‐Lactoglobuline. Sources: (1) Mussa and Ramaswamy (1997), estimated from zp/Dp‐values; (2) Ludikhuyze et al. (2000), estimated from zp/Dp‐values; (3) Rademacher and Hinrichs (2006), estimated from Cf/Co‐values; (4) Pandey and Ramaswamy (2004), estimated from z/D values; (5) Olsen et al. (2004), estimated from Cf/Co‐values; (6) Mazri et al. (2012b), estimated from Cf/Co values; (6) Mazri et al. (2012a), estimated from Cf/Co‐values (see Section 2.3.2).
3.2.4.5. Uncertainty analysis
The possible sources of uncertainty identified in attempting to propose appropriate indicators to verify the effectiveness of HPP, either as part of the validation and verification in the HPP installation and/or in the final product on the market, are included in Table C.5. Uncertainties refer to the possibility that information on some potential indicators had not been obtained, although the impact is considered low as the search has been extensive and a wide range of compounds has been covered. Other possible sources of uncertainty would be the scarcity of data on the P/t combinations intended to be used for milk pasteurisation, the limited data about milk of other origin than bovine and the lack of data about the stability of the HPP effect overtime for most compounds. These uncertainties have a large influence on the conclusions. Finally, the lack of data on colostrum is also a major uncertainty regarding this product.
Table C.5.
Potential sources of uncertainty identified in the assessment of appropriate indicators to verify the efficacy of HPP of raw milk and raw colostrum of ruminants
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Incomplete identification of potential intrinsic compounds of raw milk/raw colostrum from ruminants to be used as HHP indicators. | A potential intrinsic compound from ruminants milk or colostrum, able to be used as HHP indicator, may not have been identified in the selected literature or the expert knowledge. | The impact of this uncertainty is expected to be low as it is believed that the most relevant intrinsic compounds of raw milk/raw colostrum from ruminants able to be used as HHP indicator of raw milk/raw colostrum from ruminants have been identified using the specified sources. |
| Lack of evidence in the assessment of an identified potential intrinsic HHP indicator | The potential indicator has been identified, but there is a lack of evidence in the literature for the specific raw milk/raw colostrum compound identified, since in most cases the surveys evaluating the effect of HPP on them consider pressures and times different to those intended to be used to eliminate pathogens. Different types of milk (e.g. different species of origin and fat content) have not always been considered and these variables may influence the results. | The impact of this uncertainty is expected to be high because scarce data are available concerning the effect of HPP on most of the identified compounds which will impact the final assessment. |
| Lack of information about colostrum and colostrum‐based products | Information about the effect of HPP on colostrum from ruminants is null for most of the compounds, except for Immunoglobulins | The impact of this uncertainty is expected to be high because of the almost complete lack of evidence about the effect of HPP on raw colostrum from ruminants |
HPP: high‐pressure processing.
3.2.4.6. Concluding remarks
Comparing the scarce data obtained from the literature about some milk components (i.e. ALP, GGT, XOx, LF and β‐Lg) with P/t combinations necessary to inactivate at least 5 log of S. aureus (considered the most resistant pathogen to HPP), it can be concluded that much greater times and/or pressures are necessary to inactivate/denature most of them by at least 90% of their initial level. However, there are major differences reported, depending on the source, therefore, more information is required to find an appropriate indicator to be used under the conditions currently applied by the HPP industry.
Other components of milk and colostrum are not sensitive enough (e.g. LPO or α‐La) to be inactivated/destroyed/modified to an extent that can be quantified robustly to set a threshold that is useful to indicate a minimum HPP intensity correlated with the microbial destruction or, conversely, are highly sensitive and become completely inactivated/destroyed at lower pressures and shorter times than necessary to inactivate pathogens (e.g. PHI, LDH).
ALP, the enzyme used as TTI to verify adequate pasteurisation of cows’ milk, is quite pressure resistant and has been suggested as an HPP over‐processing indicator. Alternative enzymes to ALP have been proposed, such as GGT, but the HPP effect on them differs greatly depending on the survey and more data are required to verify their suitability. Other enzymes such as XOx and ACP could also be considered, but only a limited number of studies are available.
HPP shows a relevant effect on the denaturation of β‐Lg, since denaturation levels are close to or greater than 90% when applying 600 MPa. Similar findings have been reported for lactoferrin, although much less data are available. The analytical procedures are diverse and require sophisticated equipment and expertise for the subsequent quantitative analysis, as well as a high degree of processing of the sample prior to analysis (e.g. precipitation of the proteins).
The effect of HPP on casein was not as clear as in the case of enzymes or β‐Lg, and the determination also requires the use of complex procedures and/or equipment.
HPP at moderate temperatures (< 45°C) seem to have little impact on the fat fraction of milk, as well as on the formation of compounds derived from reactions between the components of milk, such as those derived from Maillard reactions (e.g. furosine or lactulose, used as TTIs).
HPP modifies milk's optical properties, especially turbidity and colour. Measurement of these parameters is simple and does not require sophisticated equipment, but the retrieved data do not allow assessment of the level of changes that would be useful to assess HPP efficacy on microorganisms, and do not always remain stable after processing.
More in‐depth analysis including milk from different species, considering the great variability existing on these compounds between them, are necessary. This need is especially important in the case of colostrum, which has been the subject of very few studies.
The scarce data available on the P/t combinations intended to be used for HPP of milk or colostrum from ruminants (and sometimes its inconsistency) has been identified as the main source of uncertainty in attempting to propose appropriate indicators to verify the effectiveness of HPP.
Considering the available evidence, it is judged 90–95% certain (very likely) that none of the evaluated indicators in milk can currently be proposed as an appropriate indicator to be used under the conditions currently applied by the HPP industry.
3.2.5. Comparative assessment of the risk to human health from the consumption of HPP‐treated milk or colostrum
The exposure per serving through the consumption of four types of milk and colostrum from ruminants (HPP‐treated, raw, thermally pasteurised and UHT‐treated) was assessed for S. aureus, L. monocytogenes, STEC, Campylobacter spp., M. bovis (using MAP as surrogate) and Salmonella spp. For these pathogens, the decimal reductions could be estimated for both thermal treatments and HPP. In order to compare results for a comprehensive range of possible scenarios in terms of initial level of contamination before treatment, the comparative assessment was made assuming a different theoretical initial level of contamination (N0) ranging from 1 to 8 log10 CFU/mL, for each pathogen.
3.2.5.1. Pathogen‐specific decimal reductions in milk achieved by HPP applied by industry
This comparative assessment considered the log10 reductions achieved by HPP as estimated through the global model presented in Section 3.2.3 using the three P/t combinations (Minimum or HPPMin using 450 MPa for 5 min, Intermediate or HPPInt using 600 MPa for 3 min, Maximum or HPPMax using 600 MPa for 6 min) applied by industry as informed through the questionnaire. More stringent conditions resulting in higher log10 reductions and lower exposure were not tested as judged as not representative of the P/t conditions currently applied for HPP treatment of milk. As comparison, the minimum and maximum reference values (PC) to be achieved by thermal pasteurisation normally recommended by international agencies (i.e. 5 and 8 log10 reductions) were used as well as a 12 log10 reduction for UHT treatment as shown in Figure 18.
Figure 18.
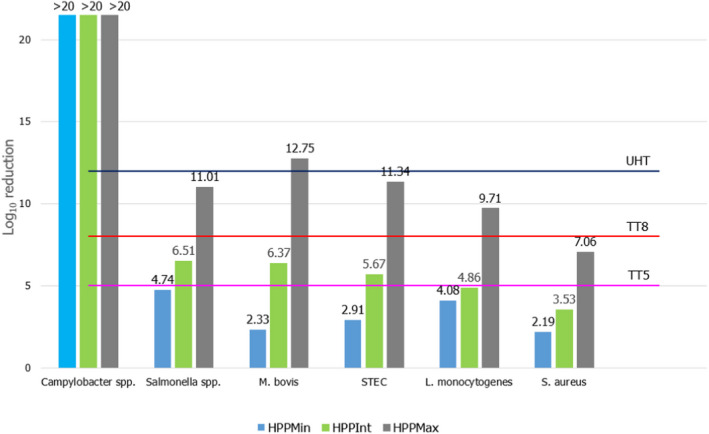
Estimates of the pathogen‐specific log10 reductions in milk achieved by HPP at three different pressure‐time combinations (Minimum or HPPMin using 450 MPa for 5 min, Intermediate or HPPInt using 600 MPa for 3 min, Maximum or HPPMax using 600 MPa for 6 min) as reported to be applied by the industry
- The minimum and maximum reference values to be achieved by thermal pasteurisation (i.e. 5 log10 reductions or TT5 and 8 log10 reductions or TT8) are shown as well as the assumed 12 log10 reductions for UHT milk. Mycobacterium avium subsp. paratuberculosis (MAP) was used as surrogate for Mycobacterium bovis while Shiga toxin‐producing E. coli (STEC) considered E. coli O157:H7 and non‐pathogenic E. coli.
3.2.5.2. Estimated probabilities of at least one cell being present in a typical serving and variability in the number of bacteria present in a typical serving
The variability in the level of contamination in a serving of 250 mL immediately following processing (N1) and the estimated probabilities of at least one CFU being present in that serving, P(N1 > 0), of each of the four types of milk (raw milk, heat treated milk (using target values of 5 and 8 log10 reductions), UHT milk (considering 12 log10 reductions) and HPP at the three established processing conditions) are summarised for each pathogen in the following paragraphs. Results are graphically presented considering fixed N0‐levels ranging from 1 to 8 log10 CFU/mL.
Staphylococcus aureus. The comparative assessment for S. aureus shows substantial differences in both P(N1 > 0) and N1 after each treatment (see Figure 19).
Figure 19.
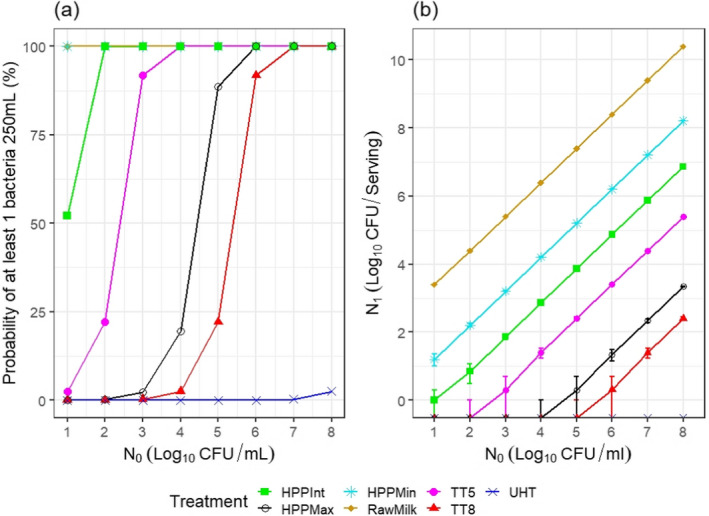
Probability of at least 1 CFU being present per serving of 250 mL (a) and location parameters (i.e. 5th, 50th and 95th percentiles) of the Poisson distributions describing the variability in the level of S. aureus in a 250 mL serving of milk (N1, in log10 CFU in 250 mL) (b)
- Results are presented disaggregated by initial contamination level (N o ).
Thermal pasteurisation treatments achieving 8 log10 reductions (TT8) were more effective in reducing the S. aureus levels compared to all the considered HPP conditions from N0 = 5 log10 CFU/mL. At this initial contamination level, P(N1 > 0) was 22.1% and 88.6% for TT8 and milk treated at 600 MPa for 6 min (HPPMax) with values at 50th and 95th percentiles of 0 and 1 vs. 0.3 and 0.7 log10 CFU/250 mL for TT8 and HPPMax, respectively. However, a treatment of 600 MPa for 6 min (HPPMax) was more effective as compared to thermal pasteurisation treatments achieving 5 log10 reductions (TT5) from N0 = 3 log10 CFU/mL where the P(N1 > 0) was substantially higher for TT5 as compared to HPPMax (91.7% vs. 21.5%) and only 1 CFU/250 mL is predicted at 99th percentile for HPPMax as compared to 0.30 and 0.69 log10 CFU/250 mL at 50th and 95th percentile for TT5.
Pressure treatment at 600 MPa for 3 min (HPPInt) was however less effective than TT5. Indeed, 1 CFU and 0.30 log10 CFU/250 mL were predicted at 50th and 95th percentile for HPPInt when N0 = 1 log10 CFU/mL and up to 0.84 and 1.07 log10 CFU/250 mL at the same percentiles for N0 = 2 log10 CFU/mL; for these initial contamination levels, P(N1 > 0) was 52.2% and 100%, respectively. These values compare with P(N1 > 0) = 22.1% and only 1 CFU/250 mL at 95th percentile for TT5 when N0 = 2 log10 CFU/mL.
Listeria monocytogenes . Both P(N1 > 0) N1 for L. monocytogenes after each treatment were substantially different, exception when considering TT5 and milk treated at 600 MPa for 3 min (see Figure 20).
Figure 20.
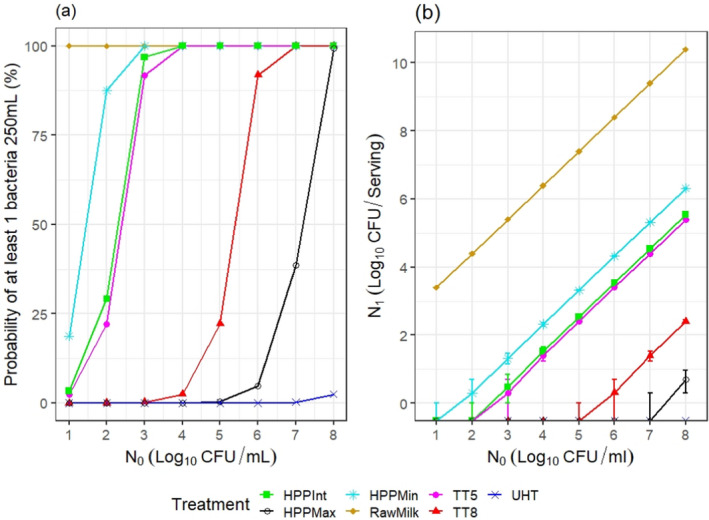
Probability of at least 1 CFU being present per serving of 250 mL (a) and location parameters (i.e. 5th, 50th and 95th percentiles) of the Poisson distributions describing the variability in the level of L. monocytogenes in a 250 mL serving of milk (N1, in log10 CFU in 250 mL) (b)
- Results are presented disaggregated by initial contamination level (N 0 ).
Results show that a HPP treatment at 600 MPa for 6 min (HPPMax), achieves a higher reduction level of L. monocytogenes than thermal pasteurisation treatments achieving both 5 (TT5) or 8 (TT8) log10 reductions. Although when considering TT5, P(N1 > 0) was equal to 22.1% for N0 = 2 log10 CFU/mL, location parameters (0 and 1 CFU/250 mL at 50th and 95th percentile) suggest it is unlikely for more than 1 CFU per serving to be present if the initial concentration is ≤ 2 log10 CFU/mL. From N0 = 3 log10, differences between TT5, TT8 and HPPMax are, however, substantial with P(N1 > 0) = 91.7% and predicted values at 50th and 95th percentiles of 0.3 and 0.7 log10 CFU/250 mL for TT5 as compared to P(N1 > 0) = 0.24% and 0.005% for TT8 and HPPMax, respectively (for both the treatments, 0 CFU/250 mL are predicted up to 99th percentiles). For TT8, 1 CFU/250 mL is estimated only at the 99th percentile when N0 = 4 log10 CFU/mL. This differs from HPPMax, where 1 CFU/250 mL is estimated at the 99th percentile for N0 = 6 log10 CFU/mL. For milk treated for a shorter time at 600 MPa (3 min, HPPInt), 50th and 95th percentiles were 0 and 1 CFU/250 mL for N0 = 2 log10 CFU/mL and 0.47 and 0.84 log10 CFU/250 mL when N0 = 3 log10 CFU/mL. The same estimates were obtained for TT5 and N0 = 2 log10 CFU/mL and slightly lower values when N0 = 3 log10 CFU/mL; HPPInt and TT5 lead to similar log10 reductions across all the initial contamination levels tested.
Salmonella spp. Both P(N1 > 0) and N1 considering Salmonella spp. after each treatment are presented in Figure 21.
Figure 21.
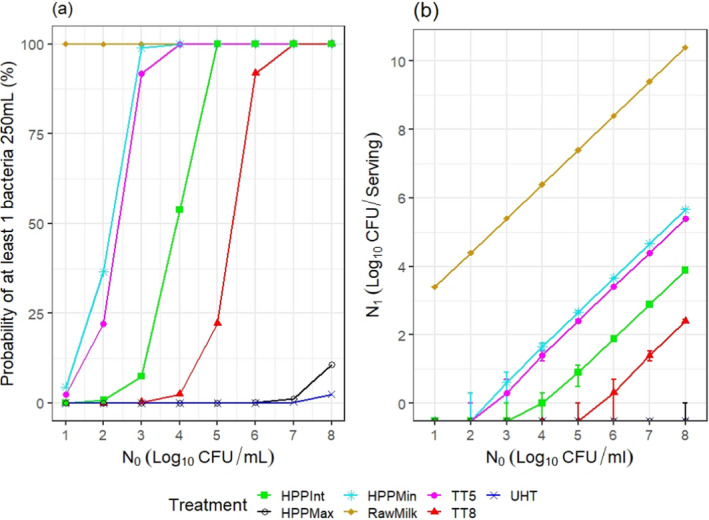
Probability of at least 1 CFU being present per serving of 250 mL (a) and location parameters (i.e. 5th, 50th and 95th percentiles) of the Poisson distributions describing the variability in the level of Salmonella spp. in a 250 mL serving of milk (N1, in log10 CFU in 250 mL) (b)
- Results are presented disaggregated by initial contamination level (N 0 ).
Both P(N1 > 0) and N1 were of similar magnitude when considering TT5 and milk treated at 450 MPa for 5 min (HPPMin) across the different scenarios of N0; UHT treatment and milk treated at 600 MPa for 6 min (HPPMax) also resulted of similar effectiveness until N0 = 7 log10 CFU/mL.
Indeed, 0 and 1 CFU/250 mL at 95th percentiles were estimated for UHT and HPPMax, respectively, for N0 = 7 log10 CFU/mL; for the same initial contamination level, P(N1 > 0) was 1.1% and 0.2% for HPPMax and UHT, respectively. Thermal treatments achieving 8 log10 reductions (TT8) lead to N1 values comparable to both treatments at 600 MPa for 6 min (HPPMax) and at 600 MPa for 3 min (HPPInt) until N0 = 3 log10 CFU/mL. Here, 0 CFU/250 mL were predicted at 95th percentile for both TT8 and HPPMax as compared to only 1 CFU/250 mL for HPPInt. Values of P(N1 > 0) for the same treatments and N0 = 3 log10 CFU/mL, were 0.25%, 0.0004% and 7.4% for TT8, HPPMax and HPPInt, respectively. Although this indicates that treatments at 600 MPa for 3 min can lead to a higher proportion of contaminated servings as compared to TT8 or stronger HPP treatments, these are very unlikely to contain more than 1 CFU/serving if the initial contamination level is low (1–3 log10 CFU/mL).
STEC. Estimates for both P(N1 > 0) and N1 after treatment are presented in Figure 22.
Figure 22.
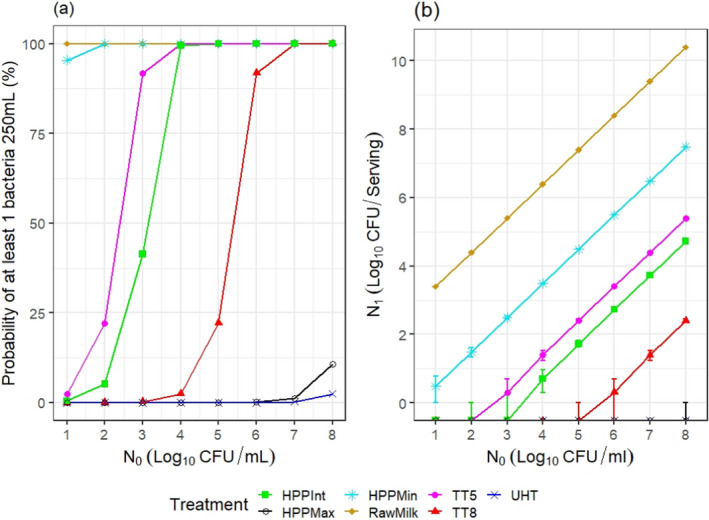
Probability of at least 1 CFU being present per serving of 250 mL (a) and location parameters (i.e. 5th, 50th and 95th percentiles) of the Poisson distributions describing the variability in the level of E. coli in a 250 mL serving of milk ((N1, in log10 CFU in 250 mL) (b)
- Results are presented disaggregated by initial contamination level (N0).
Thermal treatment achieving 8 log10 reductions (TT8) resulted in a similar inactivation as milk treated at 600 MPa for 6 min (HPPMax) and UHT milk until N0 = 5 log10 CFU/mL. For this initial level of contamination, the predicted 95th percentile for TT8, HPPMax and UHT milk were 1, 0 and 0 CFU/250 mL, respectively.
Milk treated under HPPMax conditions also resulted in similar reductions achieved for UHT milk until N0 = 8 log10 CFU/mL. At this initial contamination level, P(N1 > 0) was 10.7% for HPPMax and 2.5% for UHT. However, although the expected proportion of servings containing at least 1 CFU is considerably higher for pressure‐treated milk, these are very unlikely to contain more than 1 CFU/250 mL. In fact, only 1 CFU/250 mL was predicted at 95th percentile of N1 for milk treated at 600 MPa for 6 min (0 at same percentile for UHT milk).
Milk treated at 450 MPa for 5 min (HPPMin) resulted in a very high probability of bacteria being present per serving with a = P(N1 > 0) 95.3% already at N0 = 1 log10 CFU/mL; at this initial contamination level, 0.47 and 0.78 CFU/250 mL at 50th and 95th percentile, respectively, were estimated for N1. The difference in the performance between TT5 and milk treated at 600 MPa for 3 min (HPPInt) became evident from N0 = 3 log10 CFU/mL where P(N1 > 0) was 91.7% for TT5 and 41.4% for HPPInt. At this initial contamination level, location parameters shown 0.3 and 0.69 log10 CFU/250 mL for TT5 milk at 95th and 99th percentile and 0 and 0.3 log10 CFU/250 mL at the same percentiles for HPPInt.
M. bovis. Results for M. bovis using MAP as its surrogate in terms of P(N1 > 0) and N1 are presented in Figure 23.
Figure 23.
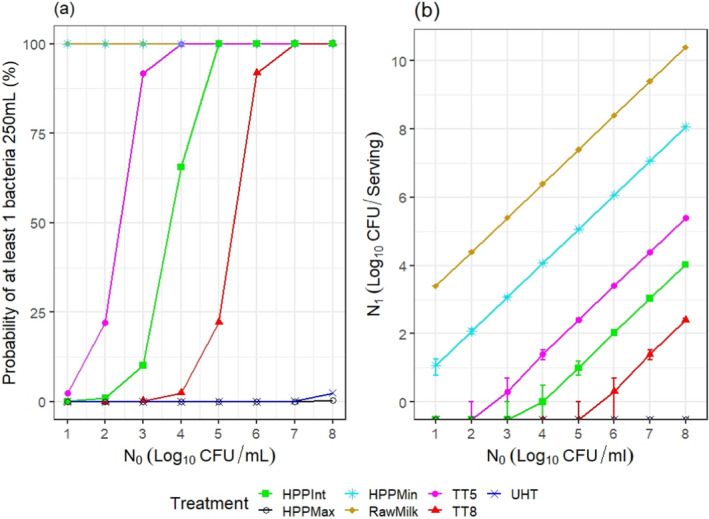
Probability of at least 1 CFU being present per serving of 250 mL (a) and location parameters (i.e. 5th, 50th and 95th percentiles) of the Poisson distributions describing the variability in the level of M. bovis in a 250 mL serving of milk (N 1 , in log10 CFU in 250 mL) (b)
- Results are presented disaggregated by initial contamination level (N 0 ).
From N0 = 5 log10 CFU/mL, substantial differences are observed for P(N1 > 0) between TT8 milk where P(N1 > 0) = 22.1% and HPPMax milk where P(N1 > 0) = 0.0004%. However, the location parameters of the distributions describing the variability in the number of bacteria per serving for TT8 and HPPMax (1 and 0 CFU/250 mL at 95th percentile, respectively) suggest it is very unlikely for more than 1 CFU to be present in contaminated servings if the initial level of contamination is ≤ 5 log10 CFU/mL.
Similarly, substantial differences are observed between TT5 and milk treated at 600 MPa for 3 min (HPPInt), in fact, P(N1 > 0) were 22.1% and 1.06% for TT5 and HPPInt, respectively, when N0 = 2 log10 CFU/mL. However, as before, location parameters for the number of bacteria per serving suggest that it is extremely unlikely for more than 1 CFU to be present in contaminated servings. Substantial differences in N1 between TT5 and HPPInt are evident from N0 ≥ 3 log10 CFU/mL where values at 50th and 95th percentiles were 0.3 and 0.69 log10 CFU/250 mL for TT5 and 1 CFU/250 mL only at 99th percentile for HPPInt. On the other hand, milk treated by HPP at 450 MPa for 6 min (HPPMin) led to differences greater than 2 log10 as compared to both TT5 or TT8 already from an initial contamination level of 1 log10 CFU/mL.
Campylobacter spp. From the comparative assessment, Campylobacter spp. appeared to be very sensitive to pressure treatments, also HPP at minimum processing settings (in terms of pressure and time) performed better than the thermal treatments. This is particularly evident for the scenarios evaluating high initial levels of contamination. Indeed, at N0 = 7 log10 CFU/mL for example, the location parameters of the distributions describing the variability in the number of bacteria per serving show 0 CFU/250 mL at 99th percentile, also for milk treated at 450 MPa for 5 min (HPPMin).
3.2.5.3. Uncertainty analysis
Potential sources of uncertainty identified for the assessment of the relative levels of exposure to selected pathogen(s) per serving through the consumption of industrially HPP‐treated in comparison to raw vs. thermally pasteurised vs. UHT‐treated milk or colostrum are listed in Table C.6. The first source of uncertainty refers to the accuracy of the pathogen‐specific log10 reductions achieved in HPP treated milk as a function of pressure and time. The log10 reductions are estimated from the pathogen‐specific models described in Table 5 of Section 3.2.3.2, and therefore, the uncertainties and impact (moderate underestimation of the log10 reductions) listed in that section are also valid for the comparative assessment. Another source of uncertainty lies in the probability distribution used to describe the variability in the number of bacteria being present per serving before and after HPP and thermal treatments. The number of bacteria per serving was modelled assuming a homogeneous dispersion of the cells in the milk, which was considered to be appropriate given the motility of certain bacteria and the fact that milk is generally an emulsion with fat particles randomly dispersed in an aqueous environment. In such a case, the use of alternative distributions (e.g. mixture Gamma‐Poisson) to describe the heterogeneity of count data in milk would have similar impact on all scenarios (N0) tested. As such, the overall conclusions in terms of efficacy would not be affected, unless HPP has a distinct impact on the distribution of the cells in the treated milk (this cannot be assessed with available data).
Table C.6.
Potential sources of uncertainty identified in the assessment of the relative levels of exposure to selected pathogen(s) per serving through the consumption of industrially HPP‐treated in comparison to raw vs. thermally pasteurised vs. UHT‐treated milk or colostrum
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Log10 reductions achieved by HPP | Model to describe the pathogen‐specific log reductions achieved in HPP treated milk as a function of pressure and time | Over/under‐estimation of HPP efficacy |
| Variability in the number of bacteria per serving before HPP and thermal treatment | Probability distribution (Poisson) assumed to adequately describe the number of bacteria dispersed in milk before HPP or thermal treatment | Impact of this uncertainty (over/under‐estimation of the number of bacteria per serving) is low. Use of the Poisson distribution is justified by the homogeneous nature of the matrix in which bacteria are dispersed. |
| Variability in the number of bacteria per serving after HPP and thermal treatment | Probability distribution (Poisson) assumed to adequately describe the number of survived bacteria homogeneously dispersed in milk after HPP or thermal treatment. The assumption would not be valid if for any reason bacteria are present in form of clusters or anyway not homogeneously dispersed and alternative distributions would be more suitable. | Impact of this uncertainty (over/under‐estimation of the number of survived bacteria per serving) is low. The use of alternative distributions would translate in wider (or skewed) distributions of the final number of bacteria per serving, but the effect would be the same across all the scenarios of initial levels of contamination (N0) tested. Therefore, the overall conclusions in terms of efficacy would not be affected unless HPP has a distinct impact on the distribution of the cells in the treated milk (this cannot be assessed with available data). |
| Variability in the number of bacteria per serving after HPP and thermal treatment | Non‐uniform piezo‐protective effect of the matrix | Impact of this uncertainty (over/under‐estimation of the number of survived bacteria per serving) is low. Use of the Poisson distribution is justified by the homogeneous nature of the matrix in which bacteria are dispersed. |
| Number of bacteria ingested at consumption | Could not be modelled due to lack of pathogen‐specific data needed to model growth during storage after HPP | Inconclusive |
HPP: high‐pressure processing.
The level of exposure was estimated through a model immediately after treatment and thus did not include storage. This is because pathogen‐specific data to model the growth of bacteria surviving HPP treatment during storage at refrigeration temperature are lacking. For M. bovis (using MAP as surrogate), it is judged 95–99% certain (extremely likely) that lack of these data would not have major impact on the number of CFU/serving at consumption. In fact, as an intracellular and slow growing (> 20 h generation time) bacterium, storage conditions are unlikely to affect the residual level of contamination after treatment, either thermal or HPP. Conversely, lack of these data would impact the number of CFU/serving at consumption for bacteria that are known to grow at refrigeration temperature, e.g. L. monocytogenes with 95–99% certainty (extremely likely).
3.2.5.4. Concluding remarks
The number of bacteria per serving (250 mL), immediately after treatment, of four types of milk from ruminants (HPP‐treated, raw, thermally pasteurised and UHT‐treated) was assessed for S. aureus, L. monocytogenes, STEC, Campylobacter spp., M. bovis (using MAP as surrogate) and Salmonella spp. The three HPP conditions applied by industry, as informed by the questionnaire responses, were used: 450 MPa for 5 min, 600 MPa for 3 min and 600 MPa for 6 min.
-
For S. aureus:
-
○
Treatments of 450 MPa for 5 min or 600 MPa for 3 min would result in a substantially higher probability of exposure to contaminated servings/number of bacteria per serving as compared to thermal pasteurisation treatments resulting in 5 log10 reductions (TT5); this difference is evident even if the initial level of contamination is as low as 1 log10 CFU/mL.
-
○
If the initial level of contamination is lower than 4 log10 CFU/mL and milk is treated at 600 MPa for 6 min, the probability of exposure to contaminated servings is higher than for thermal pasteurisation treatments resulting in 8 log10 reductions (TT8) but with a more than 99% probability that 1 CFU survives per contaminated serving.
-
○
For L. monocytogenes, Salmonella spp., STEC and M. bovis, a treatment of 600 MPa for 6 min leads to a lower probability of exposure and number of bacteria per serving as compared to TT8; however, a higher probability of exposure to more than 1 CFU per serving on average is observed for TT8 only from an initial level of contamination of 6 log10 CFU/mL.
For Salmonella spp., STEC and M. bovis, a treatment of 600 MPa for 3 min leads to a lower probability of exposure and number of bacteria per serving as compared to TT5; however, a higher probability of exposure to more than 1 CFU per serving on average is observed for TT5 only from an initial level of contamination of 3 log10 CFU/mL.
-
For L. monocytogenes:
-
○
A treatment of 450 MPa for 5 min always resulted in a higher probability of contaminated servings as compared to TT5, the average number of surviving bacteria per serving is unlikely to be greater than 1 CFU only if the initial contamination level is < 2 log10 CFU/mL.
-
○
A treatment of 600 MPa for 3 min results in a probability of exposure and number of bacteria per serving that is slightly higher but comparable overall to TT5, regardless of the initial contamination level.
-
○
For Salmonella spp., a treatment of 450 MPa for 5 min results in a probability of exposure and number of bacteria per serving that is slightly higher but comparable overall to TT5, regardless of the initial contamination level.
-
For STEC:
-
○
A pressure treatment of 450 MPa for 5 min always resulted in a higher probability of contaminated servings as compared to TT5; but if the initial level of contamination is lower than 1 log10 CFU/mL, there is a 99% probability that only 1 CFU survives in a contaminated serving.
-
○
Pressure treatment of 600 MPa for 3 min led to a higher probability of exposure and number of bacteria per serving as compared to TT8 but a higher probability of exposure to more than 1 CFU per serving for pressure‐treated milk is estimated only at initial levels of contamination greater than 4 log10 CFU/mL.
-
○
-
For M. bovis:
-
○
A treatment of 450 MPa for 5 min always resulted in a higher probability of contaminated servings as compared to TT5; if the initial level of contamination is ≤ 1 log10 CFU/mL, there is a 99% probability that only 1 CFU survives in a contaminated serving.
-
○
A treatment of 600 MPa for 3 min leads to a higher probability of exposure/number of bacteria per serving as compared to TT8 but a higher probability of exposure to more than 1 CFU per serving for pressure‐treated milk is observed only from an initial level of contamination of 5 log10 CFU/mL.
-
○
For Campylobacter spp., even the least stringent HPP condition tested (450 MPa for 5 min) leads to a lower probability of exposure and number of bacteria per serving as compared to UHT treatment resulting in 12 log10 reductions.
The public health significance of a small number of surviving bacteria after HPP cannot be realistically estimated due to lack of pathogen‐specific data concerning the physiological state of the surviving cells, the impact of HPP on the indigenous microbiota of milk and both of the above on the growth capacity of the pathogens in treated milk, using realistic T/t‐data during retail and domestic storage. Collectively, these uncertainties hamper the exposure assessment of the pathogens via the consumption of HPP‐treated milk.
Comparative assessment of levels of exposure for the pathogens in colostrum could not be carried out due to the lack of colostrum‐specific thermal and/or HPP inactivation data.
Comparative assessment could not be made for pathogens in colostrum and for Brucella spp. or TBEV in milk due to lack of HPP and/or thermal inactivation data.
3.3. Efficacy of HPP when applied to foods known to be associated with human listeriosis
3.3.1. Most relevant foods known to be associated with human listeriosis in the EU and that are relevant to be treated with HPP and other relevant pathogens (apart from L. monocytogenes) in those foods
3.3.1.1. Most relevant foods known to be associated with human listeriosis in the EU and that are relevant to be treated with HPP
The EU‐wide baseline survey examined the presence of L. monocytogenes in heat‐treated meat, RTE smoked and gravad fish and soft and semi‐soft cheese (EFSA, 2013, 2014). In EFSA BIOHAZ Panel (2018), it was concluded, based on strong‐evidence FBO (period 2008–2015), that the RTE food categories typically associated with human listeriosis, i.e. ‘meat and meat products’, ‘fish and fish products’ and ‘milk and milk products’ continued to be of significance from a food safety perspective. However, as most invasive listeriosis cases appear as sporadic infections and the detected outbreaks are usually small, the panel acknowledged the difficulty to establish links between human cases and causative foods. Yet, it was recognised that some plant derived RTE food categories can, under certain conditions, support the growth of L. monocytogenes and potentially contribute to the burden of disease. Indeed, other foods have been reported to be associated with listeriosis, including food from non‐animal origin, such as blanched frozen vegetables (EFSA BIOHAZ Panel, 2018). Over the last decade, an increasing number of events (i.e. outbreaks, sporadic cases or precautionary food product recalls) have been associated with foods not traditionally recognised as vehicles for Listeria transmission, and a rise in international events was noted. Changes in food production and distribution, and improved diagnostics may have contributed to this (Desai et al., 2019).
During the 2008–2019 period, the ‘meat and meat products’ food category was responsible for 26 of the strong and weak‐evidence FBO, causing 460 cases. ‘Fish and seafood’ and ‘dairy’ food categories were responsible for, respectively, 13 and seven outbreaks, causing 99 and 51 cases. These three categories caused 46 (61%) of the 76 FBOs with known vehicle and 610 (56%) of the 1,081 human cases. Food of non‐animal origin caused 13 outbreaks and 110 cases. In 56% (76/135) outbreaks, the vehicle was reported (Table F.1).
Table F.1.
Summary of the food vehicles in the ‘strong and weak evidence’ food‐borne outbreaks (FBO) associated with L. monocytogenes in the EU/EEA as reported in EFSA’s zoonoses database (2008–2019)
| FBO vehicle | No of FBO (cases) | Detailed info about the FBO vehicle (No of FBO) |
|---|---|---|
| Meat and meat products | 26 (469) | |
| Meat and meat products | 7 (303) | Cold cuts (1), cured pork belly with juniper or similar products (1), meat pâté (1), N/A (4) |
| Pig meat and products thereof | 7 (85) | Blood sausage (1), sliced jellied pork (1), N/A (5) |
| Bovine meat and products thereof | 6 (24) | Beef stew (sous vide) (1), potted beef (1), pressed beef also called potted beef and beef stew (1), N/A (3) |
| Other or mixed red meat and products thereof | 4 (41) | Different cold cuts (1), meat jelly (1), sausage (1), tongue, beef, pork, ham, chicken, turkey (1) |
| Broiler meat (Gallus gallus) and products thereof | 2 (16) | Chicken mayo sandwiches (1), RTE meat products (1) |
| Fish and seafood | 13 (99) | |
| Crustaceans, shellfish, molluscs and products thereof | 3 (10) | Crab meat (2), N/A (1) |
| Fish and fish products | 10 (89) | Cold smoked salmon (1), fermented fish (1), gravad salmon (1), half‐fermented trout (1), herring casserole in vegetable oil (1), smoked salmon (2), smoked trout and smoked halibut (1), N/A (2) |
| Dairy products | 7 (51) | |
| Cheese | 7 (51) | Acid curd cheese (1), cheese (acid curd) made from pasteurised milk (1), washed rind cheeses (1), N/A (4) |
| Food of non‐animal origin | 13 (110) | |
| Vegetables and juices and other products thereof | 10 (92) | Black olives and other delicatessen products (1), frozen corn (1), frozen sweetcorn (1), frozen corn and other frozen vegetables (1), leaf lettuce (1), mixed salad (1), pre‐cut salad (1), N/A (3) |
| Bakery products | 2 (16) | Pork pies (1), sponge cake (1) |
| Cereal products including rice and seeds/pulses (nuts, almonds) | 1 (2) | N/A (1) |
| Mixed and other food | 17 (352) | |
| Mixed food | 9 (212) | Composite meal (1), hummus and salads prepared in a small establishment (1), iceberg lettuce with yoghurt dressing, gouda cheese (1), likely dill which then contaminated crustaceans and cheese (1), rice pudding (1), sandwiches (1), sandwiches various and prepared salad dishes (1), N/A (2) |
| Other foods | 5 (93) | Cold cuts (1), salmon and cress sandwiches, egg mayonnaise sandwiches (1), N/A (3) |
| Buffet meals | 3 (47) | Food dishes: fresh chicken meat, pressed ham, meat chicken products ready to eat, cheeses made from cows' milk (1), sandwiches (1), N/A (1) |
| Unknown | 59 (209) | |
| Total | 135 (1,290) |
FBO: food‐borne outbreaks; N/A: not applicable as unknown.
Considering the detailed information about the FBO vehicle, the RTE food that caused most outbreaks and that are relevant for HPP from the technological perspective, based on the information extracted from the questionnaires (Section 3.1.1), are mostly those listed in the categories of meat and meat products, fish and seafood and cheese, which are also those more commonly associated with listeriosis in the EU/EEA and evaluated earlier (EFSA BIOHAZ Panel, 2018): cooked meat products including sausage and pâte, cold and hot smoked fish, gravad fish and soft and semi‐soft cheese including fresh cheese. Also, a multicountry outbreak of L. monocytogenes clonal complex 8 infections was linked to consumption of cold‐smoked fish products (2014–2019 period)17 and (marinated and smoked) salmon products (in 2017).18
This result is consistent with the outcome of the systematic literature review and meta‐analysis based on odds ratio (OR) estimated from case‐control studies of sporadic listeriosis performed by Leclercq et al. (2021) in order to determine the association between listeriosis and its main risk factors. The consumption of RTE seafood and dairy products were the main risk factors in the general and non‐perinatal population subset. The meta‐analysis on the seafood data did not reveal significant associations for crustaceans, molluscs and processed fish. Within dairy, the consumption of cheese (mainly soft cheese) and dairy products were significantly associated with listeriosis in the susceptible population. Within meat, a significant association was found for the consumption of processed meat. Within produce, the association was significant for the consumption of fruit by the susceptible population. The authors highlighted the difficulty to identify specific food at risk as the food categories have a different ability to support L. monocytogenes growth and the methodology used in the case‐control studies is a recognised source of bias.
Frozen vegetables have also been associated with human listeriosis in the EU/EEA. A multicountry outbreak of L. monocytogenes serogroup IVb, multilocus sequence type 6, infections was linked to frozen corn and possibly to other frozen vegetables (2014–2018 period)19. However, this food category is not currently being treated with HPP with the purpose to increase microbiological food safety because of the detrimental effects that HPP has on the quality of the product, particularly on the structure. It has been reported that application of HPP to whole fruit or vegetables causes a significant change in the appearance and texture of this type of products mostly due to liquid infiltration, air displacement, collapse of air pockets and shape distortion (resulting in shrinkage as air is more compressible than liquid and solid present in the matrix) (Otero and Prestamo, 2009; Jung, 2016; Janowicz and Lenart, 2018). For this reason, frozen vegetables have not been identified as relevant for HPP and they were excluded for the assessment.
Other food descriptions such as composite meal, hummus and salad, black olives and other delicatessen products, crab meat and rice pudding were observed associated with few outbreaks in that period. Additionally, based on the data obtained from the questionnaire (Table A.5), these specific foods have not been mentioned as food currently subjected to HPP by the establishments or if subjected to HPP, the primary reason is to extend the product shelf‐life (while the secondary reason is to increase the product safety) (e.g. hummus). For this reason, these food categories have not been included in the assessment.
3.3.1.2. Other relevant pathogens (apart from L. monocytogenes) in those foods known to be associated with human listeriosis in the EU
Other pathogens in the foods known to be associated with human listeriosis as identified in Section 3.3.1.1 in the EU (i.e. cold‐smoked fish, hot‐smoked fish, gravad fish, cooked meat and soft and semi‐soft cheese) may also cause illnesses apart from listeriosis.
The type of RTE cooked meat products (heat‐treated meat products) associated with human listeriosis were also implicated in outbreaks caused by E. coli and viruses (not specified), as reported in the EU from 2008 to 2019 (see Table F.2). In the systematic review performed by Omer et al. (2018) covering a period also before 2008, diseases caused by E. coli O157:H7 and Salmonella spp. were reported in several EU countries associated with meat products. Several multicountry outbreaks linked to meat and meat products have been associated with Salmonella spp.: monophasic S. Typhimurium in meats as suspected vehicle (in 2014)20 and S. Stanley in turkey meat (2011–2013 period).21
Table F.2.
Summary of the other pathogens in foods known to be associated with human listeriosis and are relevant for HPP based on the ‘strong and weak evidence’ food‐borne outbreaks (FBO) in the EU/EEA as reported in EFSA’s zoonoses database (2008–2019) and literature
| FBO vehicle | Identified pathogens causing FBO and details of the FBO vehicles (No of FBO) | Other info from literature |
|---|---|---|
| Meat and meat products | ||
| Meat and meat products; pig meat and products thereof; bovine meat and products thereof; other or mixed red meat and products thereof; broiler meat (Gallus gallus) and products thereof |
E. coli: cold meats (1) Salmonella spp.: pork, beef and chicken in jelly with vegetables (1); sliced cold cuts (pork) (1); chicken prepared (RTE) (1); ham and gammon (1); ham in the bread (1) |
Omer et al. (2018) E. coli O157:H7: turkey roll/tinned frankfurter sausage; cooked meat, beef cooked Salmonella: cold roasted pork, cooked ham, processed meat |
| Fish and seafood | ||
| Fish and fish products |
Salmonella spp.: smoked salmon (1), little salted salmon (1), cooked and smoked fish (1) Clostridium: cold smoked white fish (1); smoked fish (1); smoked whitefish, ‘Sik’ in Swedish (1) |
|
| Dairy products | ||
| Cheese |
Salmonella spp.: unpasteurised/raw goats’ milk soft cheese (2); soft and semi‐soft sheep’s milk cheese made from pasteurised or raw/low heat‐treated milk (2); from cows’ milk (1) E. coli: unpasteurised soft blue cheese (1); cheeses made from cows’ milk – unspecified (1); unpasteurised cheese (1); different types of cheese, predominantly raw milk cheeses, including semi‐hard cheese (1) S. aureus: soft cheeses and cheeses made from pasteurised or raw/low heat‐treated milk (15); from cows (7); sheep (9); goat (3) Brucella: soft cheese made of raw non‐pasteurised sheep and goats’ milk (1) Campylobacter: farm sheep’s cheese (1) Flavivirus: sheep cheese (3), goat cheese (1) |
Martínez‐Rodríguez et al. (2012) Salmonella: cantal cheese, raw milk cheese |
FBO: food‐borne outbreaks.
In the case of fish products, human salmonellosis cases have been attributed to smoked salmon. Clostridium has been reported for fish products, and a multicountry FBO of botulism neurotoxin type E was linked to dried and salted roach (Rutilus rutilus) (in 2016),22 but as a spore forming bacterium it is out of the remit of this assessment (see Section 1.2).
Different types of soft and semi‐soft cheeses, made of pasteurised or raw/low heat‐treated milk, have also been implicated in human salmonellosis cases in the EU from 2008 to 2019 and covered in the review of Martinez‐Rodríguez et al. (2012). FBOs associated with Brucella, Campylobacter, S. aureus and Flavivirus in cheese were also reported in the EU and a multicountry outbreak of STEC O26 was linked to Romanian cheese (in 2016).23 The occurrence of Brucella has been mostly linked to artisan cheeses made with raw milk (Griffin et al., 2020). Similarly, originating in the raw milk, Campylobacter can be present in cheese, although only remains viable in fresh cheese for a short period of time (Butzler and Oosterom, 1991). The TBEV belongs to the genus Flavivirus, which are transmitted from their natural hosts, mostly rodents, by ticks to humans. Cows, sheep and goats can also be infected by TBEV, in this case, the viruses can be shed via milk and consumption of contaminated raw milk can lead to infection in humans. Flavivirus are readily inactivated by heat treatment, detergents and organic solvents (EFSA BIOHAZ Panel, 2011). Cheeses associated with staphylococcal intoxication have been reported in the EU, though from the available information it could not be clarified whether the growth and toxin formation by S. aureus occurred during the manufacture, i.e. before the final product (cheese) is suitable for HPP. In this case, HPP does not constitute a control measure for S. aureus, unless HPP is applied to raw milk used to manufacture the cheese.
To conclude, the other relevant pathogens, apart from L. monocytogenes, which can be present in the different identified foods (i.e. cooked meat products including sausage and pâté; cold and hot smoked fish, gravad fish and soft and semi‐soft cheese including fresh cheese) are Salmonella spp., pathogenic E. coli, Brucella melitensis, Campylobacter spp. and Flavivirus. Only pathogens relevant for at least two categories of the selected foods known to be associated with human listerioris would be included. While Salmonella spp. have been associated with 13 outbreaks linked to food from the three main categories (cooked meat products, smoked fish and cheese) and E. coli has been associated with five outbreaks linked to food from two categories (cooked meat products and cheese), Brucella melitensis, Campylobacter spp. and Flavivirus have only been associated with fewer outbreaks involving cheese (i.e. one FBO linked to Brucella spp. and Campylobacter spp. and four Flavivirus have been reported in the EU/EEA in the 2008–2019 period). Therefore, Salmonella spp. and pathogenic E. coli have been identified as the main additional relevant hazards (apart from L. monocytogenes) in those food known to be associated with human listeriosis.
3.3.1.3. Uncertainty analysis
The potential sources of uncertainty identified for most relevant foods known to be associated with listeriosis in EU are included in Table C.7. Uncertainties refer to the incomplete information about the FBOs in the EFSA zoonoses database and literature. The impact of this uncertainty on the conclusions is expected to be low as the information from both sources of data are in agreement. Moreover, the identified food categories show the typical risk factors for L. monocytogenes (i.e. exposed to contamination, relatively long shelf‐life, physico‐chemical characteristics supporting the growth of the pathogen, etc.).
Table C.7.
Potential sources of uncertainty identified in the assessment of the most relevant foods known to be associated with human listeriosis in the EU and that are relevant to be treated with HPP and other relevant pathogens (apart from L. monocytogenes) in those foods
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Incomplete information in the FBO database and literature |
The link between listeriosis cases/outbreaks and the incriminated food is not always established (reported) and sometimes the description of the food is too general to accurately understand the type of food incriminated. Similarly, the description of food incriminated in a FBO caused by pathogens other than L. monocytogenes does not facilitate to understand whether it is a specific RTE food associated with listeriosis and relevant for HPP |
The impact of this uncertainty is expected to be low as it is believed that the most relevant foods known to be associated with human listeriosis in the EU have been identified. This is supported by the fact that there is good agreement between the FBO as retrieved from the EFSA zoonoses database and the retrieved scientific literature. Moreover, the identified food categories show the typical risk factors (exposed to contamination, relatively long shelf‐life, physico‐chemical characteristics supporting the growth of the pathogen, etc.). The impact of this uncertainty is expected to be low as it is believed that the other relevant hazards have been identified in the most relevant foods known to be associated with human listeriosis in the EU. |
FBO: food‐borne outbreak.
The potential sources of uncertainty identified for the other relevant pathogens (apart from L. monocytogenes) in those foods known to be associated with human listeriosis in the EU are also included in Table C.7. The impact of this uncertainty is also expected to be low as it is believed that the most important additional microbiological hazards have been identified in the most relevant foods known to be associated with human listeriosis in the EU.
3.3.1.4. Concluding remarks
According to the detailed information about the FBO vehicle, the most relevant foods known to be associated with human listeriosis in the EU that are also relevant to be treated with HPP include: RTE cooked meat products, soft and semi‐soft cheese, fresh cheese, smoked or gravad fish.
Although frozen vegetables have also been associated with human listeriosis in the EU, this food category has been excluded as these are currently not treated with HPP because of its detrimental effects on the quality of the product, particularly on the structure.
Other food categories such as composite meal, hummus and salad, black olives and other delicatessen products, crab meat and rice pudding have been associated with very few FBOs in the 2008–2019 period. However, these specific foods have not been mentioned as food currently subjected to HPP by the establishments or if subjected to HPP, the primary reason is to extend the product shelf‐life. For this reason, these food categories have not been included in the assessment.
Salmonella spp. and pathogenic E. coli have been identified as the main additionally relevant hazards (i.e. apart from L. monocytogenes) in the most relevant foods known to be associated with human listeriosis. Salmonella spp. have been associated with 13 outbreaks in the EU during 2008–2019 linked to food from the three categories (i.e. cooked meat products, smoked fish and cheese) and pathogenic E. coli with five outbreaks linked to food from two categories (i.e. cooked meat products and cheese).
Brucella, Campylobacter and Flavivirus have been associated with relatively fewer outbreaks involving only cheese and have therefore not been considered in the list of main additional hazards.
Cheeses associated with staphylococcal intoxication have been reported in the EU, though from the available information it could not be clarified whether the growth and toxin formation by S. aureus occurred during the manufacture. HPP does not constitute a control measure for staphylococcal toxin and S. aureus, unless HPP is applied to raw milk used to manufacture the cheese.
The uncertainty on the identification of the main food categories relevant for HPP that are associated with listeriosis are cooked meat products, smoked and gravad fish and soft/semi‐soft cheese. Other relevant pathogens in these food (besides L. monocytogenes) are pathogenic E. coli and Salmonella. It is judged 90–95% certain (very likely) that these were the most important RTE foods and most relevant additional microbiological hazards.
3.3.2. HPP requirements in foods known to be associated with human listeriosis
3.3.2.1. Relevant factors to describe the requirements of HPP of the most relevant foods known to be associated with human listeriosis in the EU
The intrinsic and extrinsic factors that may influence the efficacy of HPP have already been summarised in Section 3.1.2. This section aims to give further specific information regarding HPP of the most relevant RTE foods listed in Section 3.3.1 (i.e. cooked meat products including sausage and pâte, cold and hot smoked fish, gravad fish and soft and semi‐soft cheese including fresh cheese).
The relevant factors include aspects associated with the pathogen, the processing conditions and other extrinsic factors, as well as physico‐chemical characteristics of the RTE food to be subjected to HPP. It is worth mentioning that not all the factors described below are known and/or characterised under industrial conditions, and thus, they are sources of uncertainty.
Factors related to microorganisms. Differences in the intrinsic piezo resistance among L. monocytogenes strains (including within a given serovar) have been reported, although the genetic or cellular determinants and mechanisms are not yet well understood (Bucur et al., 2018). Experiments performed in specific RTE food have shown that different strains of L. monocytogenes follow different inactivation kinetics (Figure 24), either in terms of magnitude of the inactivation rate and/or the shape of inactivation curve, i.e. convex (with a shoulder), linear or concave (with a tail) (Simpson and Gilmour, 1997; Serra‐Castello et al., 2021b). Also, intra‐strain variability is shown by a wider scattering of the observed data (Serra‐Castelló et al., 2021b), which can be attributed to the heterogeneity of the population consisting of sensitive and resistant fractions because of physiological or genetic changes during HPP (Karatzas et al., 2005; van Boeijen et al., 2008). The occurrence of different inactivation patterns makes the comparison of the strain resistance difficult when only one or few holding times are tested. Consequently, when selecting the most resistant strain for assessing different HPP treatments, the kinetic approach is more relevant than the comparison of the log10 reduction values at given HPP conditions. In order to apply a conservative approach covering a worst‐case scenario of a potential contamination, the use of a versatile L. monocytogenes pool consisting of a mixture of three strains with different inactivation patterns depending on the HPP conditions and product characteristics was proposed in HPP validation studies for RTE cooked meat products (Serra‐Castello et al., 2021b). It is worth mentioning that the strain Scott A is a clinical isolate (serotype 4b), frequently used in assessing the effect of control measures (including HPP), that becomes the most resistant strain, only when relatively long HPP holding times are applied as shown in Figure 24. Strains more resistant to HPP than Scott A have been reported when being comparatively assessed in cooked meat products (Serra‐Castello et al., 2021b). In cheese, the Scott A strain was found to be more resistant than a strain of the same serotype from a culture collection (NCTC 11994, López‐Pedemonte et al., 2007) and the type strain (serovar 1/2, known as NCTC 10357; ATCC 15313 or CECT4031; Evert‐Arriagada et al., 2018).
Figure 24.
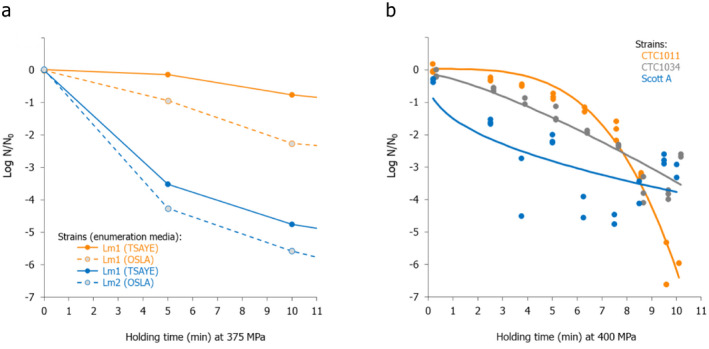
High‐pressure inactivation of different strains of L. monocytogenes inoculated on cooked chicken (a; enumeration done on non‐selective Tryptone Soya Yeast Extract (TSAYE) agar and selective Oxford Listeria Selective Agar (OLSA), Simpson and Gilmour, 1997) and sliced cooked ham (b; enumeration done in chromogenic agar, Serra‐Castelló et al., 2021b)
- Strains: Lm1 (NCTC 11994, isolate from cheese); Lm2 (isolate from poultry meat of a local processor); CTC1011 and CTC1034 (isolates from RTE meat products); Scott A (clinical isolate).
Besides the strain, the physiological state of L. monocytogenes prior to the HPP treatment is a key factor explaining in part the variability of the response to HPP treatments. As cells in the stationary phase are known to be more resistant compared to exponentially growing phase, the challenge tests in RTE food products reported in the reviewed scientific literature usually use L. monocytogenes cultures that have reached the (early) stationary phase. Other factors determine the physiological state of L. monocytogenes mainly associated with the conditions to which cells are grown or exposed to before the HPP treatment. Teixeira et al. (2016) found that L. monocytogenes inoculated in cooked ham was significantly more sensitive to 500 MPa when, prior to inoculation, it was adapted at 8°C compared to those pre‐grown at 32°C; however, this finding was contrary to what was observed in L. monocytogenes pressurised in laboratory media (tryptone soya broth) included in the same study. This study included 10 min of temperature equilibration of cooked ham prior to HPP that, according to the authors, could cause a rapid adjustment of the membrane fluidity partially hiding the effect of growth temperature on pressure resistance. In agreement with Teixeira et al. (2016), Hereu et al. (2014) found cold‐adapted cells (grown at 8°C prior inoculation and HPP) to be reduced on average 2.8 log10 units more than those kept frozen as observed in previous experiments (Bover‐Cid et al., 2011b). As frozen cells are exposed to concentrated solutes, they may respond similarly to osmotic stress, which is known to generate protection against HPP (Molina‐Hoppner et al., 2004). Though it is hard to know in advance the physiological state of L. monocytogenes when it contaminates RTE food in real conditions, the occurrence of osmotic stress is reasonably plausible in the food industry (e.g. on dry food contact surfaces). Additionally, some industrial processes to prepare pre‐packaged RTE products include a pre‐freezing step to facilitate the slicing process (Hereu et al., 2014).
With respect to the physiological state factors, it should be noted that HPP can also cause an impact on the physiological state of the surviving cells in the form of sublethal injury. The enumeration method used to quantify the surviving cells after HPP should ensure the recovery of injured cells enabling them to form colonies, otherwise the magnitude of HPP inactivation will be overestimated. For instance, Teixeira et al. (2016) reported survival curves with tailing when plating onto Tryptic Soy Agar (TSA), whereas when using PALCALM, the concentration fell below 2 log10 CFU/g. This is in agreement with the findings of Simpson and Gilmour (1997) when using Oxford Listeria Selective Agar (OLSA) compared with TSAYE (as illustrated in Figure 24). Though selective media are usually detrimental for sublethally injured cells, the Agar Listeria Ottavani & Agosti (ALOA) (chromogenic) selective and differential media has been shown to allow the recovering of high pressure‐injured L. monocytogenes cells from food matrixes (Jantzen et al., 2006; Morales et al., 2006).
The effect of the contamination level before HPP is experimentally assessed in challenge tests through the inoculum concentration. However, few studies were found dealing with the inactivation of L. monocytogenes in RTE foods. According to the experiments made by Hereu et al. (2014), the effect of HPP did not depend on the initial inoculum level (107 vs. 104 CFU/g). However, the inoculum level may be a limiting factor to quantify the number of log10 reductions caused by the HPP treatment if it is not high enough or the intensity of the treatments is strong and causes a pathogen reduction below the quantification limit, adding uncertainty about the actual magnitude of the inactivation or the occurrence of pseudo‐tails.
Regarding the potential impact of background microbiota of a RTE food in the pressure inactivation of L. monocytogenes, Teixeira et al. (2018) did not observe any influence when a cocktail consisting of Brochothrix thermosphacta, Carnobacterium maltaromaticum, Leuconostoc gelidum and Lactilobacillus sakei was inoculated in cooked ham together with L. monocytogenes. The effect of background competing microbiota was relevant during the subsequent refrigerated storage, as it prevented growth of the pathogen.
Food extrinsic factors. Extrinsic factors are mainly covered with the HPP conditions, namely target pressure, holding time and processing temperature, the effect of which is extensively addressed in Section 3.1.2 from the analysis of the data collected from the scientific literature.
In addition, packaging can also be a relevant factor. For instance, the use of modified atmosphere packaging with 50% CO2 and 50% O2 enhanced the L. monocytogenes inactivation by more than 1.5 log10 after a treatment of 150 MPa for 15 min compared with vacuum packaging of fresh salmon (Amanatidou et al., 2000a). However, no studies have been found assessing the effect of packaging for the relevant RTE foods.
Food intrinsic factors. Studies comparing the HPP inactivation of given strains of L. monocytogenes in various RTE foods processed under a similar experimental set up (i.e. using the same equipment and conditions such as 400 MPa, 10 min, 25°C) reported relevant differences depending on the product type, e.g. about 5 log10, 3 log10 and < 0.5 log10 reduction in milk, mozzarella cheese and smoked salmon, respectively (Misiou et al., 2018). The reduction of L. monocytogenes caused by HPP (600 MPa, 3 min) was not statistically different for cooked meat products (including pork, ham or turkey) formulated with low (1.8%) or high (2.4%) salt contents (Myers et al., 2013). However, Teixeira et al. (2016) found that L. monocytogenes was more resistant on cooked ham containing 3% sodium chloride as compared to 1% salt. Salt mainly causes a reduction of the aw, which is known to protect L. monocytogenes from the lethal effects of HPP (Morales et al., 2006; Evrendilek et al., 2008; Hereu et al., 2012a; Bover‐Cid et al., 2015; Teixeira et al., 2016). Therefore, as expected, HPP is much more effective in cooked meat products than in dry‐cured meat products (Bover‐Cid et al., 2011a; Hereu et al., 2012a,b), and it is also more effective in fresh cheese than in ripened cheese (Morales et al., 2006). In some RTE foods such as dry‐cured meat products or cheese, the aw decreases during ripening. The effect of aw was modelled in a dry‐cured ham matrix (0.86–0.96) and a clear linear piezo‐protection trend was found when lowering the aw of the matrix within the tested pressure range (347–852 MPa), with more than 4.5 log10 units difference in the inactivation observed in the lowest and highest aw matrixes pressurised at 600 MPa for 5 min (Bover‐Cid et al., 2015). For a fresh cheese matrix, log10 reduction of L. monocytogenes against aw‐values followed a more sigmoidal curve with about 3 log10 units difference in the inactivation between the lowest (0.904) and the highest (0.984) aw‐values when treated at 400 MPa for 10 min (Morales et al., 2006). The piezo‐protection given by low aw can be related to the stabilisation of proteins (particularly enzymes), reducing its pressure‐induced denaturation in biological systems (Georget et al., 2015). The fat content has also been reported as a relevant factor for HPP inactivation, although its effect has received less attention and the results are controversial. According to Hereu et al. (2012b, 2014), L. monocytogenes was significantly more resistant between 373 and 550 MPa in a fatty product (mortadella, with 17% fat) than in the lean product (cooked ham, with 4.6% fat). In dry‐cured meat, the influence of fat on L. monocytogenes inactivation was dependent on the pressure level; a high fat content was protective above ca. 700 MPa, but the opposite was true at lower pressures when increasing the fat content above 30% (Bover‐Cid et al., 2015).
Though no data was found on the effect of pH on L. monocytogenes inactivation in RTE food under HPP, the pH may be relevant in some specific RTE food such as cheese. For instance, the lower pH of Sainte Maure de Touraine cheese (pH of 4.7) compared to Gorgonzola cheese rind (pH of 7.0) was suggested to be one of the factors explaining the higher inactivation of L. monocytogenes in the more acidic cheese (Martinez‐Rodriguez et al., 2012).
Within the product formulation, some antimicrobial preservatives, used as food additives, may have a significant impact on the inactivation under HPP. In one study, the reduction of L. monocytogenes caused by HPP (600 MPa, 3 min) was not statistically different for cooked meat products (including pork ham or turkey) formulated with 200 ppm of nitrite (E‐250, cured product) or without (uncured) (Myers et al., 2013). However, lactate is a relatively common preservative (E‐325, E‐326) in cooked meat products, as it helps to extend the shelf‐life through the reduction of the growth of spoilage lactic acid bacteria as well as L. monocytogenes. Some studies have proved the piezo‐protection effect of potassium lactate on L. monocytogenes strains inoculated on cooked ham, with a dose‐dependent effect within the reasonably used amount, i.e. 2.8% (Serra‐Castello et al., 2021b). Though the effect might be partially related to the aw lowering effects of lactate, other mechanisms might be involved as the protection has been found even without changing the physico‐chemical characteristics of the product (Marcos et al., 2008a; Lerasle et al., 2014; Serra‐Castello et al., 2021a,b). Other organic acids in the form of salt, that can be used in RTE foods include acetate/diacetate (alone or in combination with lactate). These compounds have been found to have the opposite effect compared with lactate as they sensitised L. monocytogenes to HPP. Compared with the control cooked ham, the addition of 0.1% diacetate (E‐262) decreased the time required to achieve a 1 log10 reduction by 13–20% depending on the strain (Serra‐Castello et al., 2021b). Smoked fish may also be formulated with organic acids, however their effect, alone or in combination with other relevant hurdles for this RTE food (e.g. phenol from the smoke) on the HPP inactivation of L. monocytogenes has not been addressed.
The results emphasise the relevant influence of intrinsic factors on the L. monocytogenes inactivation by HPP. Therefore, it is necessary to assess and validate the efficacy of HPP on specific food products and consequently set process criteria adjusted to the characteristics of the given product, taking into consideration the uncertainties associated with the biological variability of the pathogen.
3.3.2.2. Minimum HPP requirements to reduce L. monocytogenes in foods known to be associated with human listeriosis
Potential target PC (i.e. target log10 reduction for L. monocytogenes)
The minimum HPP requirements in terms of holding time and pressure level depend on the target log10 reductions of L. monocytogenes in a RTE food (i.e. the target PC). Since such criteria have not been established in the current EU regulation, the assessment relied on references from guidelines and recommendations released by food safety organisations.
When HPP is applied as the main lethality treatment, potentially alternative to thermal pasteurisation (for example for fruit juices), the target PC is usually 6 log10 reduction (ranging from 4 to 8 log10 reduction) of the vegetative pathogen relevant for the food being pressurised (Table 4).
HPP can also be applied as the final in‐package lethality treatment (called a PLT) to eliminate potential L. monocytogenes re‐contamination of the RTE product after a thermal (cooking) treatment, e.g. during slicing, dicing, assembling, packing (for example in sliced cooked meat products). The PC for PLT are usually lower than for pasteurisation since pathogen concentrations due to re‐contamination are usually lower than those in raw materials. For some RTE food products, the processing during the manufacture may not be so effective in reducing or eliminating L. monocytogenes from raw materials (e.g. cheese made from raw milk, cold‐smoked salmon). In this case, the prevalence and concentration of the pathogen in packaged food not previously submitted to a lethal treatment can be higher than in cooked products (Uyttendaele et al., 2009; Jofré et al., 2016; EFSA BIOHAZ Panel, 2018).
HPP is explicitly recognised as a PLT for RTE food (meat) in several food safety regulations and guidelines, with different requirements regarding the target PC to be demonstrated through specific validation studies include:
1 log10 reduction as the minimum target for a PLT under the L. monocytogenes control strategies (alternative 1 and alternative 2a) of the US Listeria rule (FSIS, 2014). If properly validated, the FBOp may declare this on the label to inform the consumers, especially the vulnerable groups, about the safety of the product;
2 log10 reduction is recognised by the US Listeria rule dealing with RTE food as an increased level of control. When the FBOp demonstrates this level of control by the PLT, the FBOp will be relatively less sampled by FSIS compared with the above scenario (FSIS, 2014);
3 log10 reduction is recommended in RTE foods by the Health Canada Listeria Policy (Health Canada, 2011), which permits the frequency of recommended sampling to be due to a lower relative risk level;
4 log10 reduction is recommended for cooked meat products by the Spanish Food Safety Agency (ASEAN, 2004); and
5 log10 reduction is the requirement for reprocessing of contaminated RTE meat products, i.e. in case the RTE meat product is tested positive for L. monocytogenes or has passed over a food contact surface that was tested positive for the pathogen or is suspected to be positive due to issues in the sanitation or processing at the establishment (FSIS, 2012, 2014).
Carminati et al. (2004) indicated that at least a 5 log10 reduction in Gorgonzola cheese would be needed, considering the maximum levels of L. monocytogenes found on the rinds of Gorgonzola cheeses (i.e. up to 4 log10 CFU/g).
The impact on the risk to public health of some of these PC as means to control L. monocytogenes in RTE cooked meat products in Australia was evaluated by Ross et al. (2009) using a quantitative microbial risk assessment (QMRA). It was estimated that a treatment achieving a 1–2 and 3–4 log10 reduction would result in a risk reduction of the number of listeriosis cases/year of 99.33% (from 43.5 to 0.34 estimated listeriosis cases/year) and 99.84%, respectively (from 43.5 to 0.07 estimated listeriosis cases/year) with respect to the baseline scenario, i.e. without a PLT.
L. monocytogenes inactivation in RTE food treated with HPP reported in the literature
In total, 19 (category cooked meat products), nine (category smoked and gravad fish) and 13 (category soft or semi‐soft and fresh cheese) relevant records used for data extraction were retrieved. A high number of log10 reduction data could be retrieved for cooked meat products (n = 983) compared to smoked fish (n = 37; none related to gravad fish) and soft cheese (n = 145). Experiments covered HPP treatments involving pressures from 100 to 727 MPa, holding times from 0 to 40 min and initial fluid temperature (though not always reported) from −5°C to 32°C. Figure 25 shows the dispersion of the data gathered for each RTE food category.
Figure 25.
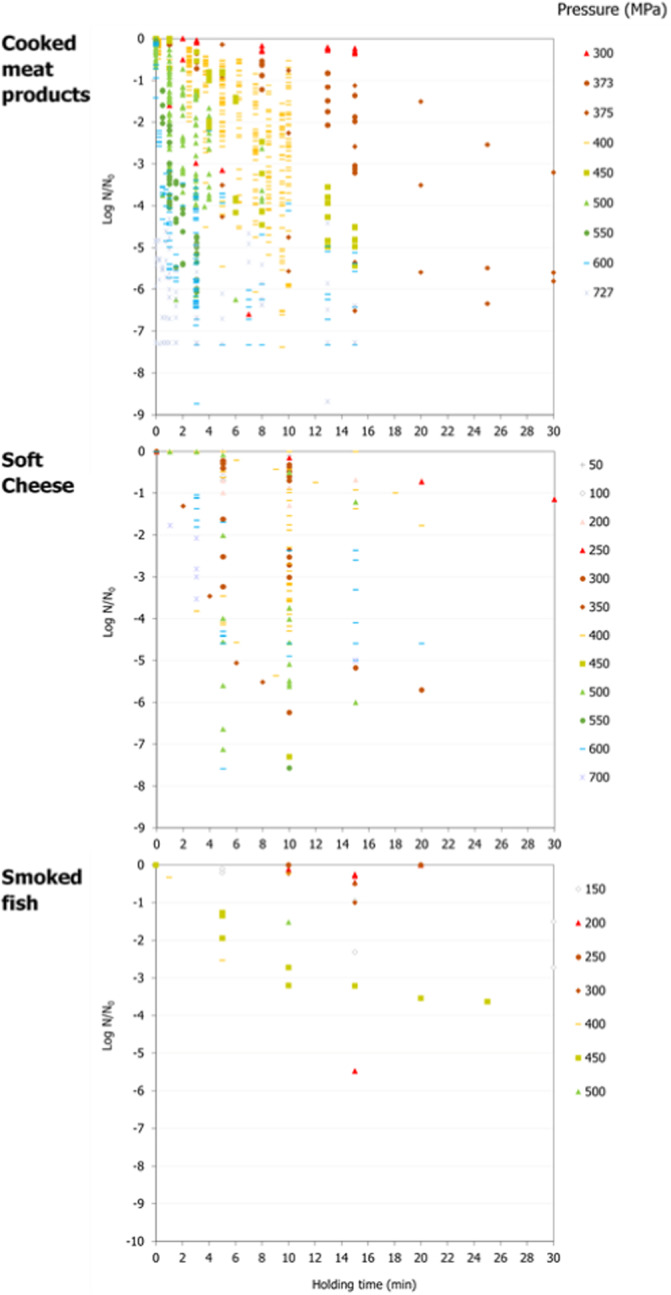
Listeria monocytogenes inactivation (log (N/N 0 )) when treating three RTE food categories (cooked meat products, soft cheese and smoked fish) using various pressure (in MPa) and holding time (in min) combinations
- Source of data for cooked meat products: Bover‐Cid et al. (2019); Chen (2007); Hayman et al. (2004); Hereu et al. (2012a); Hereu et al. (2014); Jofré et al. (2007); Jofré et al. (2008); Lavieri et al. (2014); Lucore et al. (2000); Marcos et al. (2008b); Montiel et al. (2015); Myers et al. (2013); Pavli et al. (2019); Serra‐Castelló et al. (2021b); Simpson and Gilmour (1997); Stratakos et al. (2015); Teixeira et al. (2016); Teixeira et al. (2018). Source of data for smoked and gravid fish: Amanatidou et al. (2000a); Ekonomou et al. (2020); Lakshmanan and Dalgaard (2004); Medina et al. (2009); Mengden et al. (2015); Misiou et al. (2018); Montero et al. (2007); Montiel et al. (2012); Montiel et al. (2014). Source of data for soft or semi‐soft and fresh cheese: Argues et al. (2005); Batty et al. (2019); Carminati et al. (2004); Evert‐Arriagada et al. (2018); Evrendilek et al. (2008); Goncalves et al. (2021); López‐Pedemonte et al. (2007); Martinez‐Rodriguez et al. (2012); Misiou et al. (2018); Morales et al. (2006); Opkala et al. (2010); Shao et al. (2006); Tomasula et al. (2014).
For each of the three RTE food categories, several attempts were made to fit a second‐order polynomial model including linear, quadratic and interaction terms of HPP parameters (pressure, holding time and initial fluid temperature) to the overall log10 reduction dataset). As the initial fluid temperature was not always a statistically significant parameter, a polynomial model without initial fluid temperature was also tested. However, the goodness‐of fit criteria (i.e. determination coefficient, residual sum of squares and root mean of squared error) indicated poor fitting, in agreement with the statistical significance of the lack of fit test (data not shown). Therefore, this statistical approach was withdrawn. Moreover, other factors related to the microorganisms and the food product known to influence the efficacy of HPP (Section 3.3.2.1) could not be statistically assessed or their impact considered by the model, as in most of the studies the information was not properly recorded and/or the results were highly variable, as also highlighted in the published meta‐analyses (Santillana Farakos and Zwietering, 2011; Sermen‐Moreno et al., 2015; Guillou and Membre, 2019) .
Inactivation kinetic data to derive Dp‐values were retrieved from 61 experiments dealing with cooked meat products (for pressures 100–600 MPa), three experiments with cold‐smoked salmon (at 150 and 450 MPa) and 11 experiments with cheese (for pressures 250–600 MPa). The collected log Dp‐values plotted against pressure for cooked meat products and cheese are shown in Figure 26, together with the linear regression fit enabling to estimate the mean zp‐value of 210 MPa for cooked meat products, which is between zp = 159 MPa for cooked meat products (Hereu et al., 2012b) and zp = 299 MPa for various foods (Santillana Farakos and Zwietering, 2011). The calculated zp‐value for cheese was 525 MPa. However, the slope of the regression line for cheese was not statistically significant (p > 0.05) and the R2 adj was very low (0.20), mostly due to scarce and scattered data available to fit the model. This higher zp‐value for cheese would indicate a higher piezo‐resistance of L. monocytogenes in cheese compared to cooked meat products, but this finding is not statistically supported. The model fitting to the entire data set for the three RTE foods (Figure 27) also provided a poor goodness of fit and the slope of the linear regression used for the calculation of the zp = 267 MPa was not statistically significant either. Therefore, these mathematical models were withdrawn (i.e. not considered to determine the HPP requirements to reduce L. monocytogenes in RTE food).
Figure 26.
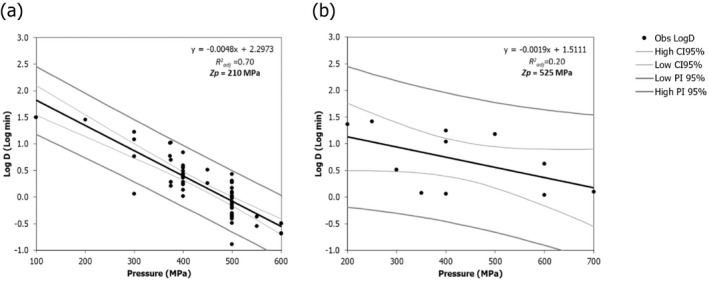
Log Dp‐values(dots) collected from HPP inactivation kinetic reported in the scientific literature as a function of pressure (MPa) for cooked meat products (a) and cheese (b)
- Black line represents the linear fit, light grey line shows the 95% confidence interval and dark grey line show the 95% prediction interval. Source of data for cooked meat products: Fonberg-Broczek et al. (2005); Hereu et al. (2012a); Lucore et al. (2000); Serra-Castelló et al. (2021b); Simpson and Gilmour (1997); Teixeira et al. (2016). Source of data for soft or semi-soft and fresh cheese: Carminati et al. (2004); Morales et al. (2006); Shao et al. (2006); Tomasula et al. (2014).
Figure 27.
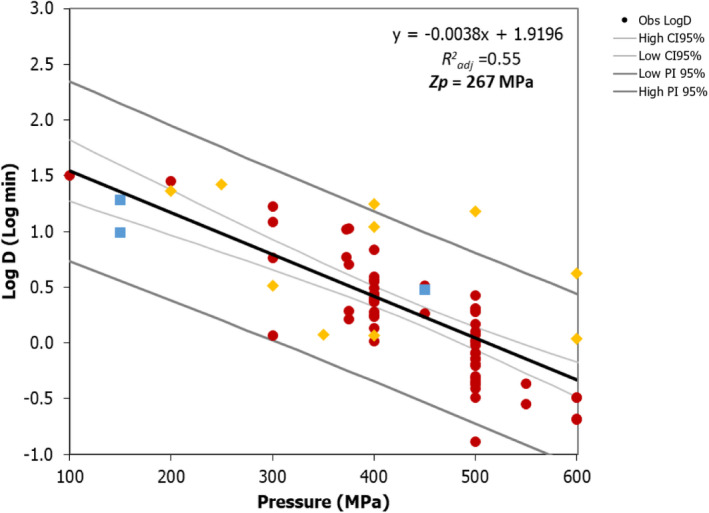
Log Dp‐values (dots) collected from HPP inactivation kinetic reported in the scientific literature as a function of pressure (MPa) for cooked meat products (red circles), cheese (orange diamonds) and smoked fish (blue squares)
- Black line represents the linear fit, light grey line shows the 95% confidence interval and dark grey line shows the 95% prediction interval. Source of data for cooked meat products: Fonberg-Broczek et al. (2005); Hereu et al. (2012a); Lucore et al. (2000); Serra-Castelló et al. (2021b); Simpson and Gilmour (1997); Teixeira et al. (2016). Source of data for soft or semi-soft and fresh cheese: Carminati et al. (2004); Morales et al. (2006); Shao et al. (2006); Tomasula et al. (2014). Source of data for smoked and gravid fish: Amanatidou et al. (2000a); Medina et al. (2009).
The model performance in predicting the log10 reductions for a given HPP treatment was assessed for two available predictive models about HPP inactivation of L. monocytogenes: (i) for various foods developed through the meta‐analysis of data collected from literature (Santillana Farakos and Zwietering, 2011) and (ii) for cooked meat products performing ad hoc experiments (Hereu et al., 2012b). The aim was to select a model that would be useful for setting the minimum HPP requirements to reduce L. monocytogenes in the relevant RTE foods. The predictive models were used to simulate HPP inactivation in terms of log10 reduction as a function of P/t combinations and the model output was compared with the inactivation collected from the experiments found in the scientific literature, including pressures from 200 to 727 MPa. The proportion of outputs within the ASZ for each RTE food category are summarised in Tables 10 and 11. The calculations were also performed for data within the range 400–600 MPa as the most representative pressures used in commercial HPP applications (Section 3.1.1).
Table 10.
Results of the comparison of the L. monocytogenes HPP inactivation (log10 reduction) outputs provided by predictive model developed by Santillana Farakos and Zwietering (2011) and the observations collected from experiments available in the scientific literature for different RTE food categories
| RTE food category | n( a ) | Fail dangerous( b ) | ASZ (± 1 log10)( c ) | Fail safe( d ) | ASZ and fail safe (conservative) |
|---|---|---|---|---|---|
| Pressure range 200–727 MPa and holding time up to 30 min | |||||
| Cooked meat products | 983 | 13% | 51% | 36% | 87% |
| Soft cheese | 141 | 43% | 37% | 21% | 57% |
| Smoked fish | 28 | 36% | 50% | 14% | 64% |
| Total | 1,152 | 17% | 49% | 33% | 83% |
| Pressure range 400–600 MPa and holding time up to 15 min | |||||
| Cooked meat products | 794 | 8% | 56% | 36% | 92% |
| Soft cheese | 88 | 50% | 30% | 20% | 50% |
| Smoked fish | 13 | 31% | 62% | 15% | 77% |
| Total | 895 | 13% | 53% | 34% | 87% |
RTE = ready‐to‐eat.
Number of observations (log10 reduction data) retrieved from literature compared with the log10 reduction provided by the model.
log10 reduction provided by the model (simulation) being at least 1 log10 higher than the observed value.
ASZ = Acceptable Simulation Zone; log10 reduction provided by the model (simulation) being less than 1 log10 unit higher or lower than the observed value.
log10 reduction provided by the model (simulation) being at least 1 log10 lower than the observed value.
Table 11.
Results of the comparison of the L. monocytogenes HPP inactivation (log10 reduction) outputs provided by predictive model developed by Hereu et al. (2012b) and the observations collected from experiments available in the scientific literature for different RTE food categories
| RTE food category | n( a ) | Fail dangerous( b ) |
ASZ (± 1 log)( c ) |
Fail safe( d ) |
ASZ and fail safe (conservative) |
|---|---|---|---|---|---|
| Pressure range 200–727 MPa and holding time up to 30 min | |||||
| Cooked meat products | 805 | 11% | 68% | 21% | 89% |
| Soft cheese | 141 | 35% | 36% | 28% | 65% |
| Smoked fish | 28 | 32% | 57% | 11% | 68% |
| Total | 974 | 15% | 63% | 22% | 85% |
| Pressure range 400–600 MPa and holding time up to 15 min | |||||
| Cooked meat products | 304 | 19% | 67% | 14% | 81% |
| Soft cheese | 88 | 42% | 30% | 28% | 58% |
| Smoked fish | 13 | 54% | 46% | 8% | 54% |
| Total | 405 | 25% | 58% | 17% | 75% |
RTE = ready‐to‐eat.
Number of observations (log10 reduction data) retrieved from literature compared with the log10 reduction provided by the model.
log10 reduction provided by the model (simulation) being at least 1 log10 higher than the observed value.
ASZ = Acceptable Simulation Zone; log10 reduction provided by the model (simulation) being less than 1 log10 unit higher or lower than the observed value.
log10 reduction provided by the model (simulation) being at least 1 log10 lower than the observed value.
Overall, the predictive performance of these two models can be considered similar. Though the model of Hereu et al. (2012b) showed a higher proportion of predictions within the ASZ, the model of Santillana Farakos and Zwietering (2011) showed slightly higher proportions of simulations within the ASZ and fail safe (i.e. underestimating the actual log10 reductions of L. monocytogenes) when considering the conditions most representative of the HPP commercial applications (i.e. pressure range 400–600 MPa and holding time up to 15 min).
When model predictions were compared for the 1,152 log10 reduction data covering the three RTE food categories, 49% were within the ASZ (±1.0 log) and 33% were fail‐safe, which means that 17% of the simulation outputs overestimated the HPP inactivation by more than 1 log10 unit (fail‐dangerous). The predictive model performed better for cooked meat products than for soft cheese and smoked fish, for which a proportion, generally lower than 70%, of the model predictions could be considered acceptable or fail safe.
The observed log10 reduction data (dots) for cooked meat products and the simulation of HPP inactivation (lines) according to the model by Santillana Farakos and Zwietering (2011) and by Hereu et al. (2012b) are shown in Figures 28 and 29, respectively.
Figure 28.
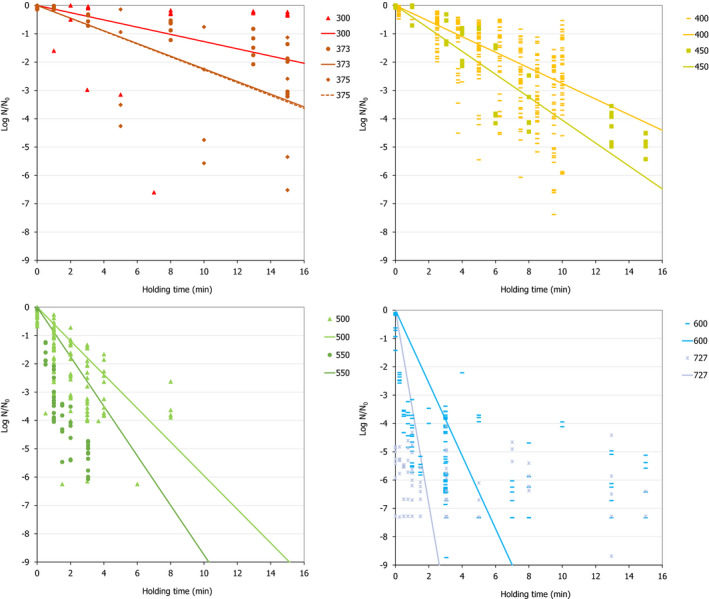
Observed log10 reduction data (dots) for cooked meat products and the simulation of HPP inactivation (lines) according to the model by Santillana Farakos and Zwietering (2011)
Figure 29.
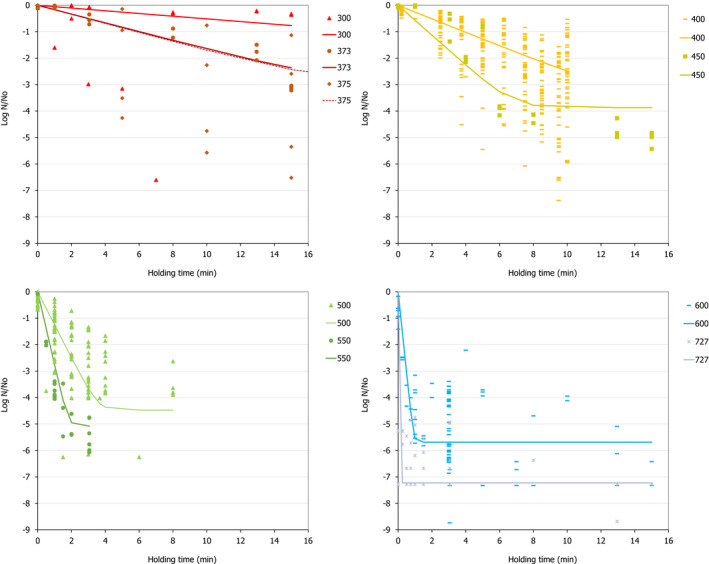
Observed log10 reduction data (dots) for cooked meat products and the simulation of HPP inactivation (lines) according to the model developed by Hereu et al. (2012b)
Based on the obtained results, the model of Santillana Farakos and Zwietering (2011) was used to determine the HPP conditions (P/t combinations) needed to achieve a given target PC for cooked meat products.
HPP processing parameters to comply with target PC
The isoreduction lines showing the P/t combinations for different target log10 reduction values according to the predictions provided by the model are plotted in Figure 30. Given that the performance of the model by Santillana Farakos and Zwietering (2011) was assessed with a ± 1 log margin of error, a conservative approach was applied. The model was used to estimate the target pressure and holding time combinations needed to achieve 1 log10 reduction higher than each target PC (log10 reduction) for L. monocytogenes in RTE cooked meat products. In this respect, Table 12 gathers the minimum HPP holding time (in min) at different pressures as conservative requirements for achieving each target PC (log10 reduction values) for L. monocytogenes in cooked meat products.
Figure 30.
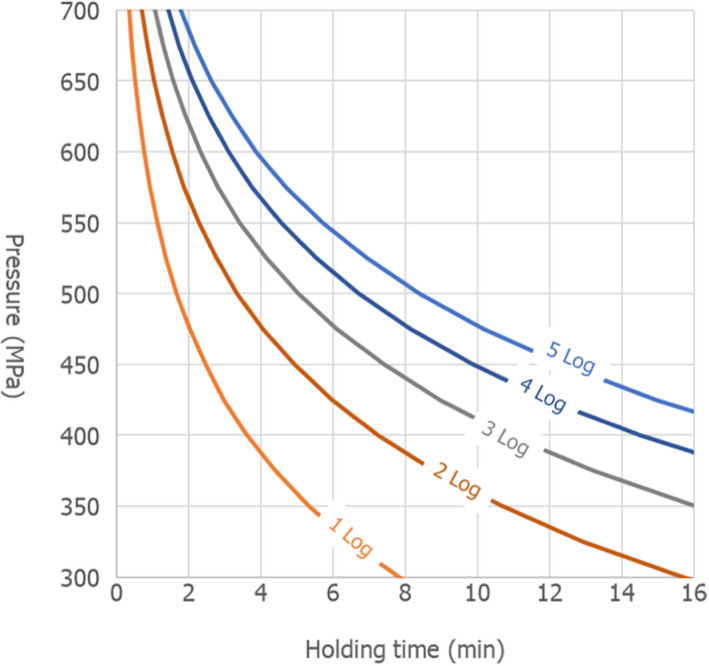
Isoreduction (log10 units) diagrams showing the combination of pressure and holding time for HPP causing a given number of log10 reductions for L. monocytogenes as predicted by the predictive model developed in Santillana Farakos and Zwietering (2011)
Table 12.
Minimum HPP holding time (in min) at different pressures as conservative requirements for achieving( a ) each target PC (log10 reduction values) for L. monocytogenes in cooked meat products (grey shadow: time < 10 min)
| Pressure (MPa) | 1 log10 reduction | 2 log10 reduction | 3 log10 reduction | 4 log10 reduction | 5 log10 reduction |
|---|---|---|---|---|---|
| 300 | 15.7 | 23.5 | 31.4 | 39.2 | 47.1 |
| 400 | 7.3 | 10.9 | 14.5 | 18.2 | 21.8 |
| 450 | 4.9 | 7.4 | 9.9 | 12.4 | 14.8 |
| 500 | 3.4 | 5.0 | 6.7 | 8.4 | 10.1 |
| 550 | 2.3 | 3.4 | 4.6 | 5.7 | 6.9 |
| 600 | 1.6 | 2.3 | 3.1 | 3.9 | 4.7 |
To achieve the target log10 reduction with a high certainty, the simulation with the predictive model was targeted one additional log10 reduction (i.e. to achieve the indicated 1 log10 reduction, the P/t combinations predicted by the model of Santillana Farakos and Zwietering (2011) to cause 2 log10 reductions are indicated in the table).
Considering the specific pressure levels most frequently used in the commercial application of HPP, for RTE cooked meat products for L. monocytogenes in RTE cooked meat products:
at least 1 log10 reduction would be achieved with 600 MPa – 1.6 min, 550 MPa – 2.3 min or 500 MPa – 3.4 min (combinations for which the mathematical model predicted 2 log10 reductions);
at least 2 log10 reductions would be achieved with 600 MPa – 2.3 min, 550 MPa – 3.4 min or 500 MPa – 5.0 min (combinations for which the mathematical model predicted 3 log10 reductions);
at least 3 log10 reductions would be achieved with 600 MPa – 3.1 min, 550 MPa – 4.6 min or 500 MPa – 6.7 min (combinations for which the mathematical model predicted 4 log10 reductions);
at least 4 log10 reductions would be achieved with 600 MPa – 3.9 min, 550 MPa – 5.7 min and 500 MPa – 8.4 min (combinations for which the mathematical model predicted 5 log10 reductions); and
at least 5 log10 reductions would be achieved with 600 MPa – 4.7 min, 550 MPa – 6.9 min and 500 MPa – 10.1 min (combinations for which the mathematical model predicted 6 log10 reductions).
Given the considerable impact of intrinsic characteristics of the food to be treated, the HPP conditions (P/t combinations) should be tailored to the intended foods, based on validation studies (e.g. challenge tests) carried out ad‐hoc, with the aim of providing food‐specific evidence for the HPP efficacy. Validation studies should be designed and carried out following international guidelines, taking into account the variability associated with the pathogen traits and product characteristics (e.g. CAC, 2008; NACMCF, 2010; Ceylan et al., 2021; ISO, under development).
The model performance for the other relevant RTE foods (soft cheese and smoked fish) was not acceptable to be used for setting HPP processing parameters mostly due to the lack of data. Therefore, for these types of RTE food, specific validation studies (e.g. challenge tests, designed and carried out following international guidelines) would be needed to set the HPP conditions required to achieve a given target log10 reduction.
3.3.2.3. Efficacy of HPP on other relevant pathogens when applying the identified minimum requirements
A literature search and screening resulted in 14 relevant papers, from which up to 194 of log10 reduction were retrieved, of which 169 were for E. coli and 25 for Salmonella. From the studies reporting on E. coli, a large amount of log10 reduction data could be retrieved for cooked meat products (n = 46) compared to smoked fish (n = 4) and cheese (n = 116). From the studies reporting on Salmonella, a substantial amount of log10 reduction data could be retrieved for cooked meat products (n = 8) compared to smoked and gravad fish (n = 1) and cheese (n = 16). Experiments covered HPP treatments involving pressures from 100 to 800 MPa and holding times from 0 (i.e. a pulse consisting of a CUT and no holding time) to 100 min. The initial fluid temperature was not reported in most of the cases.
Figure 31 shows the dispersion of the log10 reduction data with overlaid predictions of log10 reduction of L. monocytogenes provided by the mathematical models of Santillana Farakos and Zwietering (2011) and Hereu et al. (2012b).
Figure 31.
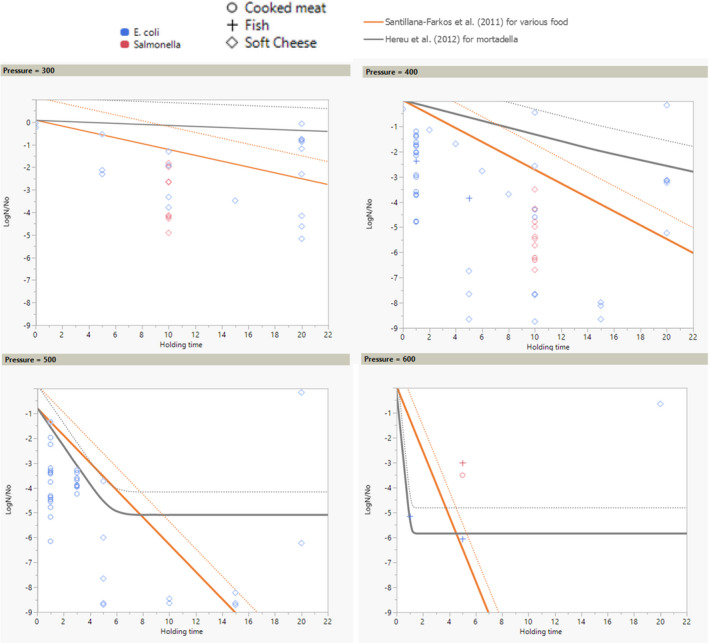
Dispersion of the log10 reduction data (symbols) of Escherichia coli and Salmonella vs. times grouped by pressure intensity, and simulated L. monocytogenes inactivation curves (solid lines) by the models of Santillana Farakos and Zwietering (2011) and Hereu et al. (2012b), including + 1 log10 (dotted lines)
- Source of data for E. coli in cooked meat products: Chung and Yousef (2010); Garriga et al. (2002); Waite‐Cusic and Yousef (2011); Salmonella in cooked meat products: Al‐Nehlawi et al. (2014); Garriga et al. (2002); Jofré et al. (2008); Kruk et al. (2011); Porto‐Fett et al. (2010); Stiebing et al. (2000); E. coli in smoked and gravid fish: Mengden et al. (2015), Salmonella in smoked and gravid fish: Stollewerk et al. (2014), E. coli in soft or semi‐soft and fresh cheese: Capellas et al. (1996); De Lamo‐Castellvı et al. (2006); Goncalves et al. (2021); O'Reilly et al. (2000); Rodriguez et al. (2005); Shao et al. (2007), Salmonella in soft or semi‐soft and fresh cheese: De Lamo‐Castellvı et al. (2007).
The scattered data did not allow fitting of a mathematical model for estimating the inactivation as a function of HPP processing conditions for E. coli and Salmonella. However, the log10 reduction data collected were benchmarked with the simulation of the predictive models used to estimate L. monocytogenes inactivation in RTE foods (Section 3.3.2.2). In general, for a given combination of pressure and time, the recorded inactivation of Salmonella and E. coli is higher than that predicted by the model for L. monocytogenes. Therefore, in general the P/t combinations required to treat RTE food for reducing E. coli and Salmonella would be at least as efficacious as for L. monocytogenes.
3.3.2.4. Uncertainty analysis
The potential sources of uncertainty identified in the assessment of the efficacy of HPP when applied to selected RTE foods known to be associated with human listeriosis are included in Table C.8 relate mainly to the data (quality and quantity, representativeness) and mathematical models used (to simulate HPP‐inactivation) as well as to the several factors influencing the HPP lethality (which are not always properly characterised and/or considered in the studies published in the literature). For many sources of uncertainties, both under‐ or overestimation of the HPP efficacy could occur. For setting the HPP requirements for cooked meat products, the mathematical model for L. monocytogenes showing the higher predictive performance with the lowest proportion of fail dangerous simulations (i.e. Santillana Farakos and Zwietering, 2011) was used to set the P/t combinations required to cause a given log10 reduction. Given the uncertainties, the P/t combinations needed to achieve a given target log10 reduction were estimated for 1 log10 reduction higher than the target value. In this way, a conservative approach was provided regarding the minimum HPP conditions required to reduce L. monocytogenes in RTE cooked meat products known to be associated with human listeriosis. In this respect, the outputs were judged to be 90–99% certain (very likely).
Table C.8.
Potential sources of uncertainty identified in the assessment of the efficacy of HPP when applied to selected foods known to be associated with human listeriosis (i.e. over/underestimation of the efficacy and the extent of the over/underestimation)
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Incomplete information retrieved from the literature | Not all the studies providing the data on inactivation of the hazards in the selected RTE foods may be retrieved using the search strategy. | The impact of this uncertainty is estimated to be low as the search string was complete and the reference lists in recent review papers were additionally checked and information added, if relevant. This uncertainty may result in an under‐ or over‐estimation of the log10 reduction due to HPP |
| Relevant factors determining the efficacy of HPP processing: microbiological factors |
The information about the microbial (e.g. strain, physiological state) factors determining the HPP efficacy were missing/not reported in most of the studies and thus they could not be considered in the assessment. The specific resistance of the strain affects the kinetic behaviour, both in terms of the shape of the inactivation curve at a given pressure (e.g. showing shoulders and/or tails) as well as the sensitivity to pressure increases (e.g. zp‐values). The resistance of the strain and its physiological state are very hard to elucidate under real industrial conditions due to the variability of the strains that can contaminate the food processing environment and the food products, as well as the variety of stresses that these strains can be exposed to. This factor is particularly relevant when the pathogenic bacteria contaminate raw materials (e.g. fresh meat, fish or raw milk) and are exposed to a series of hurdles during manufacturing (e.g. acidification, smoking, drying) which may confer resistance to the cell and reduce the efficacy of HPP compared to what could be observed if the pathogen would contaminate the final product during post‐processing conditions. However, not all available scientific studies take this factor into consideration (e.g. the inoculation of the pathogen for the challenge test is done in the final product and not in the raw material). The effect of the level of contamination before HPP is experimentally assessed through the inoculum concentration, which may be a limiting factor to quantify the number of log10 reductions caused by the HPP treatment if the contamination level is not high enough or the HPP causes a reduction of the microorganisms below the quantification limit. This adds uncertainty about the actual magnitude of the inactivation or the occurrence of pseudo‐tails within a range of concentrations of 2.4 log10 units, between the detection limit 1 cell/25 g (i.e. −1.4 log10 CFU/g) up to just below the quantification limit (i.e. 1 log10 CFU/g). Analytical methods (culture media) to count L. monocytogenes after HPP, can make the recovery of the sublethally injured cells difficult, leading to an overestimation of the HPP efficacy. Many data used in the present assessment used selective agar (e.g. PALCAM, Oxford and more recently ALOA). The overestimation is less relevant since ALOA became of extensive use, as pressure injured L. monocytogenes are able to growth when directly plated on ALOA without previous repair incubation period (Jantzen et al., 2006). |
The impact of this uncertainty is estimated to be low‐moderate as a high number of studies were included in the assessment covering a relatively wide number of different strains. Different procedures to prepare the inoculum has been carried out, covering different physiological state. The majority of log10 reduction data has been obtained from high inoculum level, allowing a proper quantitative determination of the survivors. Though some studies have been used selective media for enumeration (that may fail to recover sublethally injured cells), in the case of Listeria monocytogenes the use by many studies of ALOA (and ALOA like) media is known to recover HPP injured cells (Jantzen et al., 2006; Morales et al., 2006). This uncertainty may result in an under‐ or over‐estimation of the HPP efficacy. |
| Relevant factors determining the efficacy of HPP: food factors (mainly intrinsic, and packaging) |
The information about food intrinsic characteristics identified as relevant factors determining the HPP efficacy were not reported in most of the studies and thus they could not be considered in the assessment. In the particular case of cheese, often it is hard to understand whether the cheese reported in a scientific article is within the soft‐/semi‐soft cheese category. Almost all data came from experiments with vacuum packaged food, as it is more effective for HPP (the equipment can be loaded with higher amounts of product compared with MAP packaged products). Modified atmosphere packaging with CO2 could enhance the inactivation (only once study showing this effect). |
The impact of this uncertainty is estimated to be low. The type of RTE food covered a wider range of formulations, compositions, etc. including factors providing piezo‐protection (presence of lactate, high salt, etc.) or enhancing the inactivation (e.g. acetate). The predictive model provided in general fail‐safe estimations when compared with the log10 reduction observed in the experiments from the literature. In addition, the P/t‐combinations needed to achieve a given target log10 reduction were estimated for 1 log10 reduction higher than the target value. Overall, a conservative approach (under‐estimation of HPP efficacy) was provided regarding the minimum HPP conditions required to reduce L. monocytogenes in RTE cooked meat products known to be associated with human listeriosis |
| Relevant factors determining the efficacy of HPP: processing parameters (P, t, T) |
Some extrinsic factors, mainly temperature during processing (once at the target pressure) is not reported during HPP cycle in many studies. Besides the uncertain compression heating, the impact of the temperature at a range below 45°C has not been studied. The compression stage may cause some inactivation, depending on the microorganism, the product and the pressure come‐up rate. However, the predictive model does not consider this extra inactivation. |
The impact of this uncertainty is estimated to be low. The temperature during HPP can be estimated from the reported initial temperature and the compression heating usually reported for different matrixes (e.g. 3–4°C in most food matrixes, up to 8°C in oil). The impact of temperature (below 45°C) is not expected to be relevant compared with the impact of pressure and time. In case CUT causes some inactivation, the model predictions will underestimate the actual efficacy of HPP, as predictions only cover log10 reductions during holding time. As indicated above, the P/t combinations needed to achieve a given target log10 reduction were estimated for 1 log10 reduction higher than the target value. Overall, a conservative approach (underestimation of HPP efficacy) was provided regarding the minimum HPP conditions required to reduce L. monocytogenes in RTE cooked meat products known to be associated with human listeriosis. |
| Log10 reduction data |
Log10 reduction data were all based on experimental data subjected to error, variability and uncertainty. In many studies mean log10 reduction of few replicates are reported, missing the variability (lower and higher values than that collected). |
The impact of this uncertainty is estimated to be low‐moderate as a high number of studies were included in the assessment covering a relatively wide range of experimental conditions, which resulted in a wide variability of the log10 reduction values. This uncertainty may result in an under‐ or over‐estimation of the HPP efficacy. |
ALOA: Agar Listeria Ottavani & Agosti; CUT: come‐up time; HPP: high‐pressure processing; P: pressure; P/t: pressure‐holding time; t: time; T: temperature; RTE: ready‐to‐eat; zp: pressure resistance constant.
Regarding the other type of RTE food (smoked fish and soft cheese), much lower amount of data was available compared to RTE cooked meat products, leading to a high uncertainty as well as a poor predictive performance of the model when compared with cooked meat products. Therefore, minimum HPP requirements could not be reliably defined.
3.3.2.5. Concluding remarks
Besides the extrinsic factors (P/t) and the microorganism related factors, the food intrinsic factors that affect the efficacy of HPP (in terms of log10 reduction of L. monocytogenes) in the most relevant RTE foods known to be associated with human listeriosis are the type and the physico‐chemical characteristics of the RTE food including: aw, pH, fat as well as specific preservatives (such as lactate and diacetate).
The minimum HPP requirements to treat the RTE foods were defined using pressure and holding time as HPP processing conditions.
For RTE cooked meat products, the predictive performance of the model of Santillana Farakos and Zwietering (2011) was acceptable (with 92% of simulations within the ±1 log10 ASZ or being fail safe) and used to determine the HPP conditions required to achieve a specific log10 reduction for L. monocytogenes. Given the uncertainties, a conservative approach was applied by estimating the combinations needed to achieve a given target log10 reduction for 1 log10 reduction higher than the target value. At 500 MPa, 3.4–10.1 min holding time would be needed to comply with 1–5 log10 reduction. At 600 MPa, 1.6–4.7 min holding time would be needed to comply with 1–5 log10 reduction.
For smoked fish and soft cheese, the predictive performance of the models was not acceptable for setting HPP processing conditions. Product‐specific validation studies (e.g. challenge tests) would be needed to set the HPP conditions required to achieve a target log10 reduction. In these studies, the variability associated with the pathogen traits and product characteristics need to be taken into account following the international guidelines outlining the validation of lethal treatments.
The limited amount of quantitative data available for the HPP inactivation of Salmonella and pathogenic E. coli seem to indicate they are more sensitive than L. monocytogenes and therefore HPP would be generally at least as efficacious.
It is judged 90–95% certain (very likely) that the simulations performed using the selected model targeting 1 log10 reduction more than the given PC, provides a conservative approach regarding the minimum HPP conditions required to reduce L. monocytogenes in RTE cooked meat products.
4. Conclusions
ToR1: To assess the efficacy and microbiological and chemical safety of the use of HPP when applied to relevant foodstuffs, and in particular
ToR 1a/To provide an overview of the foods to which HPP is or could be applied along with the processing conditions (e.g. pressure, time, temperature)
-
Almost all types of food can be treated with HPP, although low moisture food is not usually treated with this technology due to low microbial inactivation when the water content is below 40%. The relative importance of the food type being treated with HPP in comparison to other food types for which HPP is used, as informed by relevant stakeholders, is as follows:
-
○
high: cooked meat products and RTE sliced cooked meats, deli meats, hot dogs and other cooked, comminuted meat products and dry‐cured (uncooked) meat products; acidic fruit and vegetable juices and guacamole, and RTE meals;
-
○
medium: fruit puree, wet salads (pH < 5) and other dips (e.g. hummus, pesto, salsa); crustaceans, shellfish, molluscs and products thereof; baby food; and
-
○
low: fish and fishery products; milk, raw milk and pasteurised cheese, processed cheese dips/spreads and dairy products (other than cheeses).
-
○
Within the industrial context, pressures of between 400 and 600 MPa are most often applied for microbial inactivation, with common holding times ranging from 1.5 to 6 min. The water used as pressure‐transmitting fluid for HPP treatment is often previously chilled at 4–8°C.
Usually products (liquid, semi‐solid and solid food) are packed in flexible plastic materials before HPP treatment (‘In‐pack’) to avoid re‐contamination of the product after HPP. Equipment for the processing of bulk liquids before packaging (‘In‐bulk’) is also available but is currently very rarely used.
ToR 1b/To list the food intrinsic and extrinsic factors that may influence the efficacy of HPP
The main food intrinsic factors that influence the efficacy of HPP of foodstuffs in terms of microbial reduction of vegetative microorganisms are aw and pH. Microbial inactivation is enhanced at higher aw and lower pH‐values. Carbohydrates, proteins and lipids exert a protective effect on microorganisms, which decreases microbial reduction by HPP in foods. The main extrinsic factors are the target pressure and the holding time. The type of microorganisms, taxonomic unit and strain and the physiological state of the microorganisms to be inactivated also affect the efficacy of HPP.
The efficacy of HPP in different food matrices is variable due to the interactions between the intrinsic food factors, which makes it difficult to predict the efficacy of specific HPP treatments in a complex food matrix thereby necessitating validation in real foods.
ToR 1c/To evaluate the potential chemical and microbiological food safety risks in HPP‐treated food compared to untreated food or food submitted to treatments, routinely applied to these foods with the purpose to increase microbiological food safety, if any (e.g. pasteurisation of juices).
It is judged 99–100% certain (almost certain) that HPP of food will not present any additional microbial food safety concerns to consumers (e.g. spore activation, induction of sublethal injury in cells, conversion of the normal form of prions to amyloid forms; and induction of virulence, toxin gene expression and cross‐resistance to other stresses) when compared to other treatments routinely applied to these foods.
It is judged with more than 95% certainty (extremely likely or higher) that mycotoxins and process contaminants evaluated in this scientific opinion will not present an increased concern due to HPP treated food intake compared to conventional food.
The use of HPP does not give rise to additional chemical food safety concerns from FCM in HPP‐treated food compared to food treated under similar T/t conditions without HPP.
ToR2: To assess the efficacy of HPP when applied to raw milk and raw colostrum from ruminants, and in particular
ToR 2a/To recommend minimum requirements as regards time and pressure of the HPP, and other factors if relevant, for the control of Mycobacterium spp., Brucella spp., L. monocytogenes, Salmonella spp. and STEC, to achieve an equivalent efficacy to that of pasteurisation.
In addition to Mycobacterium bovis, Brucella melitensis, L. monocytogenes, Salmonella spp. and STEC, the other relevant hazards to be reduced by thermal pasteurisation of raw milk/raw colostrum from ruminants are Campylobacter spp., TBEV and S. aureus.
Thermal pasteurisation of milk according to the legal requirements (i.e. at least 72°C for 15 s or at least 63°C for 30 min or equivalent) is expected to result in more than 10 log10 reduction of most of the pathogens (i.e. STEC, L. monocytogenes, Salmonella spp., S. aureus and Campylobacter spp.), while lower reductions are expected for Brucella spp. and M. bovis (using MAP as surrogate) and even lower for TBEV, for which there is a significant lack of data.
HPP cannot achieve equivalent log10 reductions to those achieved by thermal pasteurisation of milk according to these legal requirements. HPP equivalent conditions (at processing temperatures < 45°C) can be identified for lower log10 reductions (PC) as recommended by international agencies (i.e. 5, 6, 7 and 8 log10 reductions).
-
For STEC, L. monocytogenes, Salmonella spp., S. aureus and Campylobacter spp., it is judged 99–100% certain (almost certain) that the PC of 8 log10 reduction is achieved using thermal pasteurisation of milk and by HPP treatment of raw milk and raw colostrum using the following examples of minimum requirements (target pressure/holding time (P/t) combinations):
-
○
600 MPa – 8 min, 550 MPa – 10 min and at 500 MPa – 15 min for S. aureus;
-
○
600 MPa – 5 min, 550 MPa – 7 min and at 500 MPa – 11 min for STEC;
-
○
600 MPa – 6 min, 550 MPa – 7 min and at 500 MPa – 8 min for L. monocytogenes;
-
○
600 MPa – 5 min, 550 MPa – 6.5 min and at 500 MPa – 9 min for Salmonella spp.; and
-
○
600 MPa – 1 min, 550 MPa – 1 min and at 500 MPa – 1 min for Campylobacter spp.
-
○
For M. bovis (using MAP as surrogate), it is judged 95–99% certain (extremely likely) that the PC for thermal pasteurisation of 5 log10 reduction is met using thermal pasteurisation of milk. This 5 log10 reduction can be achieved with 99–100% certainty (almost certain) by HPP treatment of raw milk and raw colostrum using for example 600 MPa – 2.5 min, 550 MPa – 4.5 min and at 500 MPa – 7.5 min.
For B. melitensis and TBEV, minimum HPP requirements could not be set due to the lack of data.
The most stringent HPP condition used industrially, based on the information collected (600 MPa for 6 min), would achieve the PC (i.e. 5 logs for M. bovis and 8 logs for S. aureus, STEC, L. monocytogenes, Salmonella spp. and Campylobacter spp.), except for S. aureus as this HPP condition would achieve 6 log10 reduction.
ToR 2b/To propose appropriate indicators to verify the efficacy of HPP, either as part of the validation and verification in the HPP facility and/or in the end‐product on the market.
ALP, the endogenous milk enzyme that is widely used to verify adequate thermal pasteurisation of cows’ milk, is relatively pressure resistant and its use would be limited to that of an over‐processing indicator, while inactivation of other milk enzymes (GGT or XoX) or denaturation of some whey proteins (β‐Lg, LF), would occur at HPP processing conditions closer to those necessary for the 5 log10 inactivation of S. aureus.
Nevertheless, based on the available evidence, it is judged 90–95% certain (very likely) that none of the evaluated indicators can currently be proposed as an appropriate indicator to be used under the technologically and commercially feasible HPP conditions applied by the industry (400 and 600 MPa for 1.5–6 min).
ToR 2c/if data allow, to provide a comparative assessment of the risk to human health that could derive from the consumption of HPP‐treated vs. raw vs. pasteurised vs. UHT‐treated milk or colostrum.
Even the most stringent HPP condition used industrially (600 MPa for 6 min) would lead to less log10 reductions compared to UHT milk (considered to achieve 12 log10 reductions), except for Campylobacter spp. The impact of this on the exposure would depend on the initial contamination levels.
Even the least stringent HPP condition used industrially (450 MPa for 5 min) would lead to a lower probability of exposure to contaminated servings to all pathogens than raw milk.
Comparative assessment could not be made for pathogens in colostrum and for Brucella spp. or TBEV in milk due to lack of HPP and/or thermal inactivation data.
When comparing to thermally pasteurised milk, considering the minimum and maximum PC recommended by international agencies (i.e. 5 and 8 log10 reductions), the HPP conditions assessed (500 MPa for 5 min, 600 MPa for 3 min or 600 MPa for 6 min) as well as the initial contamination levels of milk have an impact on the outcome of the comparative exposure assessment.
For all relevant pathogens, except for S. aureus, present in milk at initial contamination levels below 5 log10 CFU/mL, the most stringent HPP condition used industrially would lead to such high log reduction that more than 99 out of 100 servings would not be contaminated.
The public health significance of a small number of surviving bacteria after HPP cannot be estimated, due to lack of pathogen‐specific data and information on realistic T/t conditions that are needed to quantify the impact of treatments and subsequent storage on pathogen growth and levels to be ingested via treated milk.
ToR3: To assess the efficacy of HPP when applied to foods known to cause human listeriosis (e.g. RTE smoked or gravad fish, soft and semi‐soft cheese and cooked meat products and (blanched) frozen vegetables such as peas or corn that are consumed without prior cooking) and in particular:
ToR 3a/To recommend minimum requirements as regards time and pressure of the HPP, and other factors if relevant, to reduce significantly L. monocytogenes levels (e.g. by a certain log reduction or reduction of the probability of illness per serving), and assuming that the parameters influencing the growth of L. monocytogenes remain unchanged (e.g. shelf‐life and storage conditions).
The most relevant foods known to be associated with human listeriosis and that are currently subjected to HPP in order to increase microbiological food safety in the EU are RTE cooked meat products, soft and semi‐soft cheese, fresh cheese and smoked or gravad fish. Frozen vegetables are currently not treated with HPP because of its detrimental effects on the structure of the product.
The food intrinsic factors that may influence the efficacy of HPP in these foods are their physico‐chemical characteristics, mainly pH and aw, and the presence of specific compounds such as proteins, fat and preservatives (e.g. lactate).
-
For RTE cooked meat products, the minimum requirements (target P/t combinations) at processing temperatures < 45°C that would achieve certain (1–5) log10 reductions for L. monocytogenes with 90–95% certainty (very likely) were derived; examples are:
-
○
≥ 2 log10 reduction: 600 MPa – 2.3 min, 550 MPa – 3.4 min and 500 MPa – 5.0 min;
-
○
≥ 4 log10 reduction: 600 MPa – 3.9 min, 550 MPa – 5.7 min and 500 MPa – 8.4 min; and
-
○
≥ 5 log10 reduction: 600 MPa – 4.7 min, 550 MPa – 6.9 min and 500 MPa – 10.1 min.
-
○
For the other type of RTE foods listed as most relevant for listeriosis, the high uncertainty due to the scarcity of data and poor predictive performance of the model did not allow generic minimum HPP requirements to be set. Specific validation studies following international guidelines are needed for each specific food.
ToR 3b/To assess the efficacy on other relevant pathogens when applying the minimum requirements identified in a
Salmonella spp. and pathogenic E. coli have been identified as the most important additional relevant hazards (i.e. apart from L. monocytogenes) in the above listed relevant foods known to be associated with human listeriosis.
In the RTE foods considered, these pathogens (Salmonella and E. coli) are generally more sensitive to HPP than L. monocytogenes and it is judged 66–90% certain (likely) they will be inactivated to a similar or higher extent than L. monocytogenes.
5. Recommendations
To consider interactions of different components in planning research on the impact of intrinsic factors in the efficacy of HPP treatments and to undertake such studies in real food matrices.
To perform an in‐depth analysis on the effect of HPP treatments on inherent compounds in milk or colostrum (from different species) for which a significant effect is found at P/t‐combinations intended to be used for pasteurisation, in order to verify their suitability as indicators of HPP efficacy.
To perform studies on pathogen behaviour in milk and colostrum during storage after HPP.
To perform further research on HPP inactivation of L. monocytogenes and other relevant pathogenic bacteria for RTE foods (as smoked and gravad fish and soft/semi‐soft cheese) from a quantitative perspective. This would facilitate the construction of a suitable predictive model to set the generic minimum requirements for HPP to assure the safety of these food products.
Abbreviations
- ∆E
total colour differences
- α‐La
α‐lactalbumin
- a*
Greenness
- AAS
atomic absorption spectrophotometry
- ACP
acid phosphatase
- ALOA
Agar Listeria Ottavani & Agosti
- ALP
alkaline phosphatase
- APZ
Acceptable Prediction Zone
- AQ
assessment question
- ASZ
Acceptable Simulation Zone
- aw
water activity
- β‐Lg
β‐lactoglobulin
- b*
yellowness
- CA
competent authority
- C1&2
N‐sulfocarbamoyl toxins
- C0
initial concentration/activity of a compound before the HPP treatment
- Cf
residual concentration/activity after the HPP treatment
- CCP
Critical Control Point
- Cd
cadmium
- CDT
come‐down time
- CE
capillary electrophoresis
- CFU
colony formation unit
- CI
confidence interval
- CUT
come‐up time
- dcGTX
dicarbamoylgonyautoxins
- dcNEO
dicarbamoylneosaxitoxin
- dcSTX
decarbamoylsaxitoxin
- DON
deoxynivalenol
- DPA
dipicolinic acid
- Dp
decimal reduction time at a certain pressure
- DT
decimal reduction time at a certain temperature
- ELISA
enzyme‐linked immunosorbent assay
- EM
electron microscopy
- EVA
ethylene vinyl acetate
- EVOH
ethylene‐vinyl alcohol copolymer
- f
fraction (0–1) of the sensitive subpopulation
- FBO
food‐borne outbreak
- FBOp
food business operator
- FCM
food contact material
- FFU
focus forming units
- FID
Flame‐Ionisation Detection
- FPLC
fast protein liquid chromatography
- FSMS
Food Safety Management System
- GC
gas chromatography
- GGT
γ‐glutamyltransferase
- GHP
good hygiene practices
- GTX
gonyautoxins
- HACCP
Hazard Analysis and Critical Control Point
- HHP
high‐hydrostatic pressure processing
- HPH
high‐pressure homogenisation
- HP
high pressure
- HPLC
High Pressure Liquid Chromatography
- HPP
high pressure processing
- HPTP
high pressure thermal processing
- HPTS
high pressure thermal sterilisation
- HTST
high temperature short time
- Ig
immunoglobulin
- IP RF
ion‐pair reversed‐phase
- k
inactivation rate
- k1
inactivation rate of the pressure sensitive subpopulations
- k2
inactivation rate of the pressure sensitive subpopulations
- L*
Lightness value
- LC‐MS
liquid chromatography–mass spectrometry
- LDH
lactate dehydrogenase
- LDPE
low‐density polyethylene
- LF
lactoferrin
- LPL
lipoprotein lipase
- LPO
lactoperoxidase
- LSD
light scattering detector
- LTLT
low temperature long time
- logR
log10 reduction
- MADLS
Multi‐Angle Dynamic Light Scattering
- MAP
Mycobacterium avium subsp. paratuberculosis
- mpHPP
multipulsed HPP
- 2‐MCPD
2‐monochloropropane‐1,2‐diol
- 3‐MCPD
3‐monochloropropane‐1,2‐diol
- MPPO
poly(2,6‐diphenyl‐p‐phenylene oxide
- MS
Member State
- MS
mass spectroscopy
- MW
molecular weight
- N0
initial contamination level of the pathogen
- N1
contamination level of the pathogen per serving
- N/A
not applicable
- NIAS
non‐intentionally added substances
- OLSA
Oxford Listeria Selective Agar
- OPA
oriented polyamide
- OR
odds ratio
- P
target pressure
- PA
Polyamide
- PAGE
polyacrylamide gel electrophoresis
- PAH
polycyclic aromatic hydrocarbons
- PATS
pressure assisted thermal sterilisation
- PC
performance criteria
- PCR
polymerase chain reaction
- PCS
photon correlation spectroscopy
- PE
Polyethylene
- PET
polyethylene terephthalate
- PHI
Phosphohexoseisomerase
- PLT
post‐lethality treatment
- PP
Polypropylene
- PSP
Paralytic Shellfish Poison
- P/t
pressure‐holding time
- PTTI
pressure/temperature time integrator
- QMRA
quantitative microbial risk assessment
- RDM
raw drinking milk
- RID
radial immunodiffusion
- RMSE
Root Mean Square Error
- RTE
ready‐to‐eat
- RP‐HPLC
Reversed‐Phase high Pressure Liquid Chromatography
- SA
surface area
- SA
serum albumin
- Sd
standard deviation
- SDS‐PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEC
size‐exclusion chromatography
- spHPP
single‐pulsed HPP
- SQ
Subquestions
- STEC
Shiga toxin‐producing E. coli
- STX
Saxitoxin
- t
Time
- T
Temperature
- TBEV
tick‐borne encephalitis virus
- TP
thermal processing
- Tg
glass transition temperature
- Ti/Ab
title/abstract
- TP
thermal processing
- TPC
total plate count
- TSA
Tryptic Soy Agar
- TSAYE
Tryptone Soya Yeast Extract Agar
- TSE
transmissible spongiform encephalopathy
- T/t
temperature–time
- TT5
a thermal pasteurisation achieving a 5 log10 reduction
- TT8
a thermal pasteurisation achieving a 8 log10 reduction
- TTI
time temperature integrator
- TVC
Total Viable Counts
- UHP
ultra‐high‐pressure processing
- UHT
ultra‐high temperature
- US‐FDA
US Food and Drug Administration
- V
consumption unit of interest
- VBNC
viable but not culturable
- VDC
vinylidene chloride
- WI
Whiteness
- XOx
xanthine oxidase
- ZEA
Zearalenone
- zp
pressure resistance constant
- zT
thermal resistance constant
Appendix A – Questionnaire on the commercial use of HPP of foods with the purpose to increase microbiological food safety
A.1 Questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety addressed to the competent authorities
The European Food Safety Authority (EFSA) received a mandate from the European Commission with a request for a scientific opinion on the efficacy and safety of high‐pressure processing of food. You can find further information about the mandate at EFSA‐Q‐2020‐00380.24
An ‘ad hoc’ working group (WG) including external experts and EFSA staff has been established to address the Terms of Reference of the mandate. The WG prepared a questionnaire addressed to the competent authorities and a questionnaire addressed to the establishments using HPP, in order to collect data that will be used to inform the assessment and could be summarised in the final scientific opinion. These data will complement and update the information collected in 2014 by the questionnaire from DG‐SANTE.
We would appreciate if you could fill in the questionnaire addressed to the competent authorities and send your answers until 4 January 2021 to biohaz@efsa.europa.eu. Additionally, we kindly request you to forward the questionnaire addressed to the establishments using HPP and the attached excel file to the concerned establishments of your country, if any (note that these establishments have the same deadline to answer their questionnaire).
Thank you very much for your co‐operation.
Please specify your contact point details
| Name of the competent authority | |
| Reporting country | |
| e‐mail | |
| Name of contact person |
GENERAL QUESTIONS
-
–
To your knowledge, how many establishments are using HPP technology in your country?
…… establishments, among which …… are service providers25
I do not know
1.
If you have establishments using HPP in your country, please send them the questionnaire addressed to the establishments using HPP and excel file (see attachments)
-
–
Has the impact of HPP on food safety been or is it currently being evaluated by the competent authority or other bodies?
Yes (please provide the details of the food safety assessments, providing references and/or your original documents if possible)
No
I do not know
-
–
Are you aware of any expected important technological changes in the near future (e.g. type of foods to be treated and their processing conditions)?
Yes (please provide this information)
No
SPECIFIC QUESTIONS RELATED TO FOOD MICROBIOLOGY/HYGIENE
-
–
Are you aware of any microbiological food safety problems (such as spore activation leading to more rapid and more complete germination and outgrowth, prion activation by conversion of normal form to amyloid form, induction of virulence or toxin gene expression) originated from food subject to HPP?
Yes (please provide the details of such incidents)
No
SPECIFIC QUESTIONS RELATED TO FOOD CONTACT MATERIALS (FCM)
-
–
Are there special considerations or technical requirements on the properties of FCM for HPP apart from flexibility and appearance, such as polymer morphology, molecular weight distribution, density, melt‐flow index, delamination, etc.?
Yes (please provide this information)
No
I do not know
-
–
Are you aware of studies on the effect of HPP on FCM, including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment? (Note that the WG will review the published literature and so this question seeks information only on unpublished data)
Yes (please provide this information)
No
-
–
Concerning possible strong and undesirable food‐packaging interactions and other changes that may give rise to migration, are there types of foods for which HPP has an adverse effect or for which HPP is not suitable/recommended? What would be the reason for this? Are there polymer types not recommended for HPP apart from aspects such as flexibility, tightness and appearance? Are there other pitfalls to be aware of, including the choice of treatment conditions?
Yes (please provide this information)
No
I do not know
SPECIFIC QUESTIONS RELATED TO FOOD CONTAMINANTS
-
–
Are you aware of studies on the effect of HPP on the potential formation or degradation/modification of contaminants in foods during and after HPP treatment? (Note that the WG will review the published literature and so this question seeks information only on unpublished data)
Yes (please provide this information)
No
The replies to the questionnaires replied by the CAs are summarised in Table A.1.
Table A.1.
Replies to the questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety by the Competent Authorities
| Question | Yes | No | I don’t know | |
|---|---|---|---|---|
|
GENERAL QUESTIONS |
1. To your knowledge, how many establishments are using HPP technology in your country? | N/A | N/A | N/A |
| 2. Has the impact of HPP on food safety been or is it currently being evaluated by the competent authority or other bodies? | 6( a ) | 9 | 4 | |
| 3. Are you aware of any expected important technological changes in the near future (e.g. type of foods to be treated and their processing conditions)? | 2( b ) | 17 | N/A | |
| SPECIFIC QUESTIONS RELATED TO FOOD MICROBIOLOGY/HYGIENE | 4. Are you aware of any microbiological food safety problems originated from food subject to HPP? | 0 | 19( c ) | N/A |
| SPECIFIC QUESTIONS RELATED TO FCM | 5. Are there special considerations or technical requirements on the properties of FCM for HPP apart from flexibility and appearance? | 3( d ) | 3 | 13( e ) |
| 6. Are you aware of studies on the effect of HPP on FCM, including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment? | 0 | 19 | N/A | |
| 7. Concerning possible strong and undesirable food‐packaging interactions and other changes that may give rise to migration, are there types of foods for which HPP has an adverse effect or for which HPP is not suitable/recommended? What would be the reason for this? Are there polymer types not recommended for HPP apart from aspects such as flexibility, tightness and appearance? Are there other pitfalls to be aware of, including the choice of treatment conditions? | 1( f ) | 2 | 16 | |
| SPECIFIC QUESTIONS RELATED TO FOOD CONTAMINANTS | 8. Are you aware of studies on the effect of HPP on the potential formation or degradation/ modification of contaminants in foods during and after HPP treatment? | 0 | 19 | N/A |
FCM: food contact materials; HPP: high‐pressure processing; N/A: not applicable.
one competent authority (CA) added that food business operators (FBOps) should assess the consequences of using of HPP in the Hazard Analysis and Critical Control Point (HACCP) system of their establishment. The HACCP system of establishments is checked by the CA; another CA clarified that one meat processing company had their products high‐pressure treated for export to the USA by an establishment in the EU. The company, together the University, carried out a challenge test regarding the growth of Listeria in order to be able to comply with the zero tolerance of US legal requirements for export. The influence of high‐pressure treatment was also taken into account. This project was accompanied by the local CA. One company is treating fruit juices by high pressure. To our knowledge, up to now it is not clear, whether high‐pressure treated food was used for human consumption to a significant degree within the EU before 15 May 1997 (see definition of ‘novel food’ in Regulation (EU) 2015/2283); another CA added that it is being evaluated. FBOp have incorporated the HPP technology in the HACCP system of their establishments which is checked by the CA; another CA added that, during the sanitary control, the results of storage tests presented by the FBOp (producer of fruit and vegetable juices) were analysed: the number of yeasts and moulds in 1 mL, the number of Escherichia coli bacteria in 1 mL, the number of mesophilic aerobic bacteria in 1 mL, the presence of Salmonella and Listeria monocytogenes in 25 mL; test results for the presence of spore‐forming anaerobic bacteria before and after the HPP treatment. Moreover, the results of organoleptic tests of juices before and after HPP treatment as well as tests of extract content, pH, lead and cadmium were presented; another CA informed that the impact of HPP on food safety is evaluated yearly during the audits performed by the Veterinary Central Competent Authority (CCA) and regional (County) veterinary authority. The frequency of the audits performed by the inspectors of County veterinary authority depends on the risk category of establishment using HPP equipment; for example, because the single HPP establishment had been classified into low risk category, the frequency of audits that should be performed by County veterinary authority is 1 audit/trimester (or 4 audits/year); another CA added that FBOp assess the impact of HPP on food safety as a part of their HACCP, which is checked by the CA.
one CA clarified that one service provider works or has worked on the sanitation of raw milk cheeses with the presence of L. monocytogenes and enteropathogenic E. coli; he also works on the treatment of raw milk for consumption (which poses a product classification problem); another CA added that the HPP technology is mainly used in meat products. Besides this, is currently used or planned to be used in RTE meals, some kind of salads or poultry meat products.
two CAs clarified that so far no public health problem has been clearly identified in connection with this HPP technology in their country; one CA clarified that any microbiological food safety problems have been identified in connection with this HPP technology.
one CA answered that the professional has in his specifications with his customers an obligation for them to provide proof of the foodstuffs of the packaging for the HPP treatment; another CA answered that the only problem that FBOp noticed concerning food contact material (FCM) was delamination, so they had to ask relevant guarantees from the producer of FCM. In any case FCM which are used in HPP technology have to be suitable for this use in accordance with the technical specifications of these products; another CA added that packages used are made of special materials that can withstand high pressure.
one CA clarified that FCM which are used within the framework of HPP technology have to be suitable for this use in accordance with the technical specifications of these products; another CA answered clarified that FCM which are used within the framework of HPP technology have to be suitable for this use in accordance with the technical specifications of these products.
One CA answered that FCM used in HPP applications are subject to the same legislative requirements as other FCMs in terms of their safety i.e. migration studies, applicability, etc. In general, FCM for HPP are required to be flexible enough to withstand the mechanical stress caused by hydrostatic pressure while maintaining physical integrity during the process. Due to their good flexibility, elasticity and water‐barrier characteristics, plastics are commonly used as packaging material subject to HPP treatment. Not all foods are suitable for HPP, liquids and sauces are best. It has effects on the quality of some food such as meat e.g. colour.
A.2 Questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety addressed to the establishments using HPP
The European Food Safety Authority (EFSA) received a mandate from the European Commission with a request for a scientific opinion on the efficacy and safety of high‐pressure processing of food. You can find further information about the mandate at EFSA‐Q‐2020‐00380.24
An ‘ad hoc’ working group (WG) including external experts and EFSA staff has been established to address the Terms of Reference of the mandate. The WG prepared a questionnaire for establishments using HPP to collect data that will be used to inform the assessment and could be used, after their anonymisation (by removing any reference to the establishment name and country), in the scientific opinion. By providing the data, you give the consent for its use for this assessment. These data will complement and update those already collected in 2014 by the European Commission.
We would appreciate if you could fill in the questionnaire and send it to the following email address until 4 January 2021 to biohaz@efsa.europa.eu.
It would be appreciated if you provide also any information not covered by the questionnaire and considered to be of relevance.
Thank you very much for your cooperation.
Please specify your contact point details
| Establishment name | |
| Establishment category (food producer and/or tolling centre) | |
| Country | |
| e‐mail | |
| Name of contact person |
GENERAL QUESTIONS
-
1
On which type of food do you use HPP, and how are they processed? Please provide detailed information in the table below or in the attached excel file (insert one row for each food subtype you process and consider the footnotes that provide clarifications).
-
2
Shortly describe how you carried out the validation of the HPP treatment, e.g. target microorganism, target log reduction, approach to document the safety/target log reduction (e.g. literature data, experimental trials such as challenge test, use of predictive models)26.
-
3
Are you aware of any expected important technological changes in the near future (e.g. type of foods to be treated and their processing conditions)?
Yes (please provide this information)
No
SPECIFIC QUESTIONS RELATED TO FOOD MICROBIOLOGY/HYGIENE
-
4
Are you aware of any microbiological food safety problems (such as spore activation leading to more rapid and more complete germination and outgrowth, prion activation by conversion of normal form to amyloid form, induction of virulence or toxin gene expression) originated from food subject to HPP?
Yes (please provide the details of such incidents)
No
SPECIFIC QUESTIONS RELATED TO FOOD CONTACT MATERIALS (FCM)
-
5
Are there special considerations or technical requirements on the properties of FCM for HPP apart from flexibility and appearance, such as polymer morphology, molecular weight distribution, density, melt‐flow index, delamination, etc.?
Yes (please provide this information)
No
I do not know
-
6
Are you aware of studies on the effect of HPP on FCM, including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment? (Note that the WG will review the published literature and so information only on any unpublished data is sought)
Yes (please provide this information)
No
-
7
Concerning possible strong and undesirable food‐packaging interactions and other changes that may give rise to migration, are there types of foods for which HPP has an adverse effect or for which HPP is not suitable/recommended? What would be the reason for this? Are there polymer types not recommended for HPP apart from aspects such as flexibility, tightness and appearance? Are there other pitfalls to be aware of, including the choice of treatment conditions?
Yes (please provide this information)
No
I do not know
SPECIFIC QUESTIONS RELATED TO FOOD CONTAMINANTS
-
8
Are you aware of studies on the effect of HPP on the potential formation or degradation/modification of contaminants in foods during and after HPP treatment? (Note that the WG will review the published literature and so information only on any unpublished data is sought)
Yes (please provide this information)
No
Table – Question 1
| FOOD CATEGORY | REASONS FOR USING HPP | HPP CONDITIONS | FOR PROCESSING IN‐PACK (final retail package) | FOR PROCESSING IN‐BULK( d ) using batch system | FOR PACKAGING AFTER TREATMENT | FOOD STORAGE INSTRUCTIONS TO CONSUMERS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Food category (sector) | Brief description of the food type on which HPP is used. Please insert one row for each food type | Treatment of the food prior to HPP and relevant to the preservation achieved | Treatment of the food post‐HPP and relevant to the preservation achieved | Primary reason for using the HPP technology for the commodity listed | Secondary reason for using the HPP technology for the commodity listed | Target pressure (in MPa)/treatment time once target pressure reached (in min)/water temperature before HPP treatment (in °C)( a ) | Rate of pressurisation/depressurisation (MPa/s) | Temperature of the food before, during and after HPP( b ) | What are the packaging conditions and what is the pack size? | Which is the packaging material used( c )? | Please provide information about the bag( e ) | Are there any measures taken to avoid recontamination between treatment and packaging (e.g. is aseptic packaging applied and verified)? | Temperature (°C) | Shelf‐life |
|
Options: ‐please specify ‐unknown ‐none |
Options: ‐please specify ‐unknown ‐none |
Options: ‐to increase product safety (i.e. inactivate pathogenic microorganisms) ‐to extend product shelf‐life (i.e. inactivate spoilage microorganisms) ‐other (please specify) |
Options: ‐increase product safety ‐extend product shelf‐life ‐other (please specify) |
Example: 400 MPa/5 min/21°C OR 400 MPa/5 min/unknown |
Example: T before = 10°C/T during = 25°C or unknown/T after = 10°C |
Example: vacuum‐packaged and 500 g | ||||||||
| Meat and meat products | E.g. Fresh meat, minced meat, heat treated meat product, fermented meat product, cured or not‐cured meat product, mechanically separated meat (MSM), OTHER (please specify) | |||||||||||||
| Fruit, vegetables, juices and other products thereof | E.g. acid (pH < 4.5) or non‐acid (pH > 4.5) fruit/vegetable juices, pre‐cut fruit, fruit salad, guacamole, sweets, OTHER (please specify) | |||||||||||||
| Milk | E.g. raw skimmed or whole fat milk from cow or other species, OTHER (please specify) | |||||||||||||
| Cheese | E.g. raw milk cheese, pasteurised milk cheese (type), OTHER (please specify) | |||||||||||||
| Dairy products (other than cheeses) | E.g. yoghurt, OTHER (please specify) | |||||||||||||
| Fish and fishery products | E.g. fresh fish, fish salads, surimi, fish proteins, OTHER (please specify) | |||||||||||||
| Crustaceans, shellfish, molluscs and products thereof | E.g. oysters, lobster, crab, OTHER (please specify) | |||||||||||||
| Mixed food/composite product | ||||||||||||||
| Other food category | ||||||||||||||
If for each food category a range of different parameters are used, please provide the range (e.g. min, typical, max). If there are inter‐relationships between, e.g. the time, pressure and temperature applied, please describe these inter‐relationships and how the different parameters are combined to bring about the desired effect on the food.
To take account of the compression heating phenomena during HPP, please provide information on the compression heating experienced by the pressure transmitting fluid and by the food itself. Actual measurements of the heat‐up during compression and the cool‐down during decompression would be most valuable. In the absence of data, a description based on modelling of the heat‐up and cool‐down using literature compression heating values, would be useful.
If a plastic is used, please provide the polymer type, whether the food contact material (FCM) is a monolayer or a multilayer and the layer thickness(es). If other materials than plastics are used, please describe them.
For instance, where milk or a beverage is filled into a large holding bag in the HPP chamber.
What is this bag made of (polymer and characteristics)? On which criteria is the bag changed following its repeated use? How is it cleaned between uses? Has the repeated pressurisation an effect on the integrity of the bag and the migration from the bag into food? Is the bag degraded, aged, etc., following the repeated uses?
The replies to the questionnaires by the establishments are provided in Table A.2 and summarised in Table A.3.
Table A.2.
Replies to the questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety by the establishments
| Question | Yes | No | I don’t know | |
|---|---|---|---|---|
|
GENERAL QUESTIONS |
1. On which type of food do you use HPP, and how are they processed? Please provide detailed information in the table below or in the attached excel file( a ) | N/A | N/A | N/A |
| 2. Shortly describe how you carried out the validation of the HPP treatment, e.g. target microorganism, target log reduction, approach to document the safety/target log reduction?( b ) | N/A | N/A | N/A | |
| 3. Are you aware of any expected important technological changes in the near future (e.g. type of foods to be treated and their processing conditions)? | 6( c ) | 17 | N/A | |
| SPECIFIC QUESTIONS RELATED TO FOOD MICROBIOLOGY/HYGIENE | 4. Are you aware of any microbiological food safety problems originated from food subject to HPP? | 7( d ) | 16 | N/A |
| SPECIFIC QUESTIONS RELATED TO FCM | 5. Are there special considerations or technical requirements on the properties of FCM for HPP apart from flexibility and appearance? | 6( e ) | 10( f ) | 7 |
| 6. Are you aware of studies on the effect of HPP on FCM, including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment? | 5( g ) | 18( h ) | N/A | |
| 7. Concerning possible strong and undesirable food‐packaging interactions and other changes that may give rise to migration, are there types of foods for which HPP has an adverse effect or for which HPP is not suitable/recommended? What would be the reason for this? Are there polymer types not recommended for HPP apart from aspects such as flexibility, tightness and appearance? Are there other pitfalls to be aware of, including the choice of treatment conditions? | 5( i ) | 9( j ) | 9 | |
| SPECIFIC QUESTIONS RELATED TO FOOD CONTAMINANTS | 8. Are you aware of studies on the effect of HPP on the potential formation or degradation/ modification of contaminants in foods during and after HPP treatment? | 4( k ) | 19 | N/A |
FCM: food contact materials; HPP: high‐pressure processing; N/A:not applicable.
In connection to replies using a table, some establishments gave following information: (i) use of HP treatment for the treatment of sealed products (treatment in the final packaging); (ii) fruit, vegetables, juices and other products thereof, 600 MPa 180 s; (iii) refrigerated fruit and vegetable juices; (iv) dry‐cured ham and sausage; (v) meat product ready for consumption, cured ham, for export to the US, mainly; (vi) we make cold pasta to fill sandwiches in the group's establishments. The pasta has a wide variety of ingredients such as mayonnaise, canned vegetables and fish, cheeses, smoked salmon, nuts, cooked meat, etc.; (vii) mainly cured meat products on a sliced presentation; (viii) the use of HPP has wide spectrum use at the moment, traditionally was used in meat specially cured meat but nowadays HPP is use for fresh fruit, fresh vegetables, fresh cheese, processed ready‐to‐eat (RTE) foods, soups, creams and fish and shellfish mainly. Each product needs specific treatment to ensure the safe use because it is important to obtain an equilibrium between safety and sensory properties mainly in the case of fresh products. Meanwhile in cured meat it is possible to applied maximum pressure (600 MPa) and times close to 6–7 min in other products is necessary to reduce pressure (450 MPa) and longer times (10 min) or double treatment since the loss of pressure has also an important effect. We have treated an important type of samples although most of them are associated with cured meats, juices and fresh cut fruit. The conditions depend on the type of product and all the treatments are applied at room temperature since the storage must be associated with refrigeration. However, it is important to have in mind that each product required specific validation.
- Target microorganisms in the validation studies were: Listeria monocytogenes, Salmonella spp., Clostridia.
- Microanalysis (Total Plate Counts, Enterobacteriaceae) done before and after treatment. Log reduction between 1 and 3 logs.
- Criteria applicable to finished products: European regulation + Fédération du Commerce et de la Distribution (FCD) criteria.
- Literature data and challenge tests provided by the HPP Machine supplier + systematic initial check on a new product by shelf‐life tests.
- To validate the system, non‐heat‐treated fruit purees were filled, and the initial contamination of the products was determined. The first part of the samples was directly HPP‐treated, and then, the contamination in the finished product was determined (GKZ, H/S, Enterobacteriaceae). Furthermore, samples were subjected to a stress test. These were pre‐incubated for up to 72 h at 30°C, and then, the contamination before and after the HPP treatment was determined. These tests showed a log reduction of up to 107. These experiments were tested with different parameters related to pasteurisation.
- With the help of a master student and of an external laboratory. Also, in combination with a sensory test (taste, smell, texture)
- Guidelines from manufacturer of HPP machine according to the aw of the product
- Validations of the HPP treatment were carried out using a combination of shelf‐life studies, challenge tests and literature data
- Validation of the treatment of microorganism tests in an accredited laboratory, and literature data
-
Storage research was performed and target values were established (with following parameters, content and unit):
-
○Number of yeasts: max. 10 units/mL
-
○Number of moulds: max. 10 units/mL
-
○Number of Escherichia coli: max. 100 units/mL
-
○Number of mesophilic aerobic bacteria: max. 1000 units/mL
-
○The presence of Salmonella: absent in 25 mL
-
○The presence of L. monocytogenes: absent in 25 mL
-
○
- Literature data, experimental trial and safety/target log
- Validation study with inoculation of food‐borne pathogens has been used to set HPP parameters. Validation shows a minimum of 5 log reduction in worst case scenario at a lower pressure than operational setting. These lower settings are now Critical Control Points (CCP’s) in the process, every batch is verified to meet the CCP
- Customer of tolling facility must show validation data and tell us what to do
- Validation: Seeding of 7 log of pathogenic microorganisms with growth capacity at pH of the product, application of the treatment and count of viable, proving a logarithmic reduction of 6; Verification: The effectiveness of the process is verified by verifying the absence of target microorganisms, quality indicators (moulds, yeasts and acidophilic bacteria)
- Experimental trials: shelf‐life studies
- It is performed on L. monocytogenes, verifying a reduction of three logarithmic units
- Since the implementation of the pasteurising equipment, we have worked hand in hand with its supplier (Hiperbaric). From Hiperbaric they helped us to carry out the initial validation of the pasteurisation recipes and we are in continuous contact as they recommend and advise us with everything that is needed. In our company, one of the main pillars is quality. We have an internal laboratory in which we analyse the vast majority of raw materials that go into manufacturing, process controls (manipulators, surfaces and environments), analysis of the finished product before being pasteurised and this same product once it has been subjected to the treatment of pasteurisation. In this way we control the microbiological load that enters the room and that which we can incorporate into the process. The high‐pressure pasteuriser (with the recipe that we currently apply 5,900 bars for 180 s) is capable of eliminating the pathogenic flora and the hygiene indicator microorganisms are reduced by 3 logarithmic units. That is why we try to work in the most hygienic way possible, counting on good raw materials so that, once the product has been pasteurised, it stores at 0 CFU / g of banal flora.
- Being a HPP treatment company for third parties, it is the client who must carry out the validation of the HPP treatment for their product. From our company, based on previously studied literature, acquired experience or our own challenge studies. The client is advised, depending on the conditions of the product (physico‐chemical characteristics, packaging, production or previous treatments, etc.), the treatment(s) to be tested and the validation that we consider necessary for them. These are usually useful life studies with the target microorganisms (according to legislation and also according to the type of product) and / or challenge tests towards certain pathogens.
- With the HPP treatment it is intended to destroy and / or prevent the proliferation of the microorganisms present such as mesophilic aerobes, E. coli, Staphylococcus aureus, Enterobacteria, lactic acid bacteria, Salmonella spp. and L. monocytogenes. The objective is achieved by means of a pressure‐time combination established according to the experience of various manufacturers, existing bibliography, trial–error tests and validation through analytics and shelf‐life studies in external laboratories.
- Validation of the process has been done on several pork meat products (cured products). Validation analysis was carried out at internal laboratory. Target microorganism: Salmonella, Listeria spp. and L. monocytogenes, total aerobic mesophilic count, Enterobacteriaceae, sulfate reducers, S. aureus, total coliforms, E. coli. A part of previous mention, our HPP outsourced supplier has validated L. monocytogenes and Salmonella spp. with results of log reduction of: Meat products with aw > 0.85 have to be treated at least 6 min under 6,000 bars pressure. To get reductions of 3–5 log of Listeria spp. To get reductions of from 5 log of Salmonella spp. Our products are aw < 0.92. The target is to reduce possible contamination of products although the products are safe even before treatment. (comply with EU regulations even before treatment).
- The typical parameters of an HPP treatment are more commonly 600 MPa for 1–5 min; however, you will need to validate your treatment parameters when food safety is implicated. Basically, is necessary: (a) Reference to scientific or technical literature, previous HPP treatment validation studies or historical knowledge of the performance of the control measure; (b) scientifically valid experimental data that demonstrate the adequacy of the control measure. Laboratory challenge testing designed to mimic process conditions and industrial or pilot plant trials of particular aspects of a food processing system are validation techniques that are used commonly, particularly in food processing unit operations. Quantitative demonstration and documentation of appropriate log reduction of a specified pathogen by a specific HPP process is an example of validation of a control measure by experimental trials; (c) Mathematical modelling. Mathematical modelling is a means of mathematically integrating scientific data on how factors affecting the performance of a control measure or combination of control measures affect their ability to achieve the intended food safety outcome.
One establishment informed that new raw materials and product categories are always presented in the course of the regular HACCP team meetings and the resulting risks are discussed and classified in the specialist group. In addition to the existing product range of fruit and vegetable juices in PET bottles and fruit and vegetable purees in pouches or cups (partly with grain and/or dairy products), no further products are currently planned that are pasteurised on the basis of pressure treatment. Another establishment informed that all data are sent by the AVURE manufacturer. Another establishment informed that there will be more and more foods fixed with methods that do not affect the organoleptic features, e.g. pascalisation, membranes). Another establishment informed that these are technological changes related to the digitalisation of processes, we are aware of, and fully active in their implementation. Regarding new foods to process in our sector, practically all the possibilities are implemented, and more specifically in our company). Another establishment added infant feeding, animal feeding. Another one informed that the treatment of liquid food in bulk equipment, developed by Hiperbaric company, is the latest advance in commercial equipment. This allows aseptic packaging after the treatment in any possible package, and therefore removes packaging material limitations, which are rigid packages (such as glass). On the other hand, and as far as we are aware, it is still far from occurring what would be the next generation of high‐pressure processing equipment, those capable at a commercial scale of processes that can result in a sterilisation. This implies the use of high temperature (90–120°C), and the resistance of the materials to these conditions under pressure cycles seems to be an important challenge.
One establishment informed that they observed an activation of mesophilic aerobic spore activation resulting in an increase TPC level; another establishment informed that all finished products are subject to a final microbiological inspection by the in‐house laboratory and additional microbiological analyses by an external service provider. The change from spore‐forming to the vegetative form is ensured by the pH value and by maintaining the cold chain. All the food produced is refrigerated. As it is a matter of ‘just in time’ productions, in which finished product is always examined microbiologically, contamination by toxin‐ producing microorganisms or their toxins is not to be expected or would be noticed due to microbial abnormalities in the products; another establishment added that once they had a problem with Bacillus cereus in one of their products. There was the problem, that our vegetables (ginger and turmeric) weren´t washed in the producing process and had a very high contamination. The product wasn´t at the consumer. We changed our producing process and there wasn’t any problem anymore); another establishment informed that the presence of anaerobic spore‐forming bacteria was checked before and after the HPP treatment – absent; another establishment added that they are aware that HPP does not inactivate sporeformers and therefore they use other hurdles against the risk of spore formers; another establishment added that high pressures can induce the first stage of spore germination of Bacillus spp. (not so of Clostridium sp.). However, the following stages of the germination process are not accelerated and continue the normal rhythm depending on characteristics of the food matrix. Furthermore, this germination is not homogeneous due to the great variability within a spore population (Response of Spores to High‐Pressure Processing). The physico‐chemical properties of the food are used as a control to reduce the associated risk to its presence. The effect of high pressures on the structure of proteins depends on their composition. Therefore, the effect on different prions is unknown. However, the conversion of normal form to amyloid form requires the formation of new interactions molecular structures within the prion (usually hydrogen bonds). By definition, high pressures do not create or destroy hydrogen bonds as they are intera strong molecular actions (high pressures act on weak molecular interactions such as electrostatic interactions, van der Waals forces, hydrophobic interactions…). Therefore, it can be expected that the conversion from normal to amyloid is dependent on other factors that are not related to high pressure. It has been seen that high pressure can open the structure of some prions to make them more accessible for digestion by proteinases. High pressure‐induced gene expression is a recent field in research.); another establishment informed that HHP‐induced inactivation of bacterial spores remains a challenge due to spore resistance to the treatment limits of currently available industrial HHP units (i.e. ~650 MPa and 50°C). And important number of equipment in the industry do not combined temperature and pressure. Several reports have demonstrated that high pressure can modulate the germination machinery of bacterial spores, rendering them susceptible to subsequent inactivation treatments. Unfortunately, high pressure‐induced germination is a unique phenomenon for spores of the genus Bacillus but not of Clostridium. Alternative strategies to inactivate bacterial spores at commercially available pressure and temperature levels include promoting the germination step by inclusion of known germinants into the food formulation to increase the lethality of HHP treatments on bacterial spores (Sarker MR, Akhtar S, Torres A and Paredes‐Sabja D, 2015. High hydrostatic pressure‐induced inactivation of bacterial spores. Critical Reviews in Microbiology, 41, 18–26).
One establishment informed that migration tests were carried out prior to the launch of the range of products treated with HP; another establishment mentioned EVOH component; another establishment informed that packages are made of special materials that withstand high pressure (pet bottle made of special polymers); another establishment clarified that, apart from flexibility, the requirements are general for MECAS in this technology, and in conventional pasteurisers; one establishment informed that some metallised multilayer materials can delaminate; one establishment clarified that there is a trend to use more plastic‐based, stronger, packaging materials that provide higher durability and meet the demand for higher line speeds and packaging waste reduction. Other newer barrier technologies which have been appearing are providing innovations that may be suitable for HPP. A few materials utilising PET, PE, PP, as well as PS‐EVOH, PE‐EVOH and other metallic films are currently being utilised for commercial production. In the case of HPP of foods mainly utilises flexible packaging materials. Many materials have been evaluated for their adequacy in the process although mostly PP, HDPE, PET, C‐PET, EVOH. There are a number of integrity requirements for these packaging materials that must be complied with for acceptance and use in different product applications. These include visual integrity, gas permeability, seal and physical strength properties and global migration of packaging components into the food, some of which are specific to either refrigerated or shelf‐stable products. HPP can alter integrity of multilayered materials including adhesion between layers. Adhesion between layers can be compromised if compressibility and resilience of individual layers differ significantly. The resulting gaps between the layers may even affect the microbiological safety of the package. Moreover, gas permeability and mechanical properties, the integrity of packaging materials can be characterised by sorption and migration parameters. Substances migrating from the packaging can affect the sensory quality and level of toxicity of the packaged product. Specifically, the food and the packaging interact through mass transport mechanisms such as permeation, sorption and migration. Different authors have tested the migration under different conditions and found that the levels of migration complied with the EU directive (less than 10 mg/dm2). For example, Lambert et al. (2000) tested the global migration of five PA/PE type packages and one PET/PVDC/PE laminate keeping packages sealed with food simulants (water, 3% acetic acid, and 15% ethanol or isooctane) for 10 days at 40°C. HP–LT treatment (500 MPa for 30 min at 20°C) did not increase the global migration, and all packages met the referred standard (10 mg/dm2). However, migration of liquids to the plastic after HPP has been reported under different conditions. A study Schauwecker et al. (2002) on the migration of components in the pressurising liquids into the package confirmed the impermeability of the aluminium layer in a retort package (PE/PA/Al/PP). Aluminium layer was impermeable to the migration of 1,2‐propanediol contained in the pressurising fluid into the package containing 95% ethanol at both HP–LT (400–827 MPa and 30–50°C) and HP–HT (600–827 MPa and 75°C) for 10 min. However, an EVOH‐based package (PA/EVOH/PE) showed significant migration of 1,2‐propanediol. In this case, pressures higher than 200 MPa significantly reduced migration levels compared to non‐pressure‐treated packages. Once all requirements for HP–LT and HP–HT packages have been thoroughly defined by the industry for specific product applications, more complete studies on materials will provide the whole picture that can assess their suitability for HP process. References: Lambert Y, Demazeau G, Largeteau A, Bouvier JM, Laborde‐Croubit S and Cabannes M, 2000. Packaging for high‐pressure treatments in the food industry. Packaging Technology and Science, 13, 63–71; (b) Schauwecker A, Balasubramaniam VM, Sadler G, Pascall MA and Adhikari C, 2002. Influence of high‐pressure processing on selected polymeric materials and on the migration of a pressure transmitting fluid. Packaging Technology and Science 15, 255–262.
One establishment informed that there is no additional requirement beyond adhering to materials approved to be in contact with food, which can be single layer, multilayer or made of different polymers. High pressure does not cause general or specific migration within the limits established by the Regulation (EU) No 10/2011.
One establishment informed that migration tests were satisfactory based on the conclusions of the laboratory that conducted the tests; another establishment informed that Hiperbaric has webinars to different topic for their customers; another establishment mentioned internal tests of PET bottle migration after HPP; another one informed that there are many published studies that the task force will find. We do not have any additional studies. Simply to insist on the idea that by definition high pressures do not form and destroy strong molecular interactions, including covalent bonds here. wait for the formation of new reaction products. HPP equipment for food processing in the food industry cannot work at pressures higher than 600 MPa or at temperatures higher than 25°C. Pressure holding times higher than 10 min are not common as they greatly reduce productivity. It is important to take this into account because there are studies where pressures higher than 600 MPa and temperatures higher than 25°C are analysed.
One establishment added that they have studies showing no changes in polymers.
One establishment added that with regard to the packaging, sufficient flexibility is important to be able to withstand the volume reduction of approx. 16% without damage. Good resistance to water is also important (glass containers, cans and laminated cardboard are not suitable). Flexible or partially flexible packaging such as skin films, trays with flexible sealing film and plastic bags, on the other hand, are very suitable. The barrier properties such as gas permeability must of course be adapted to the desired minimum shelf‐life); another establishment added that there are special packaging intended for pascalisation; another establishment replied that, if you use HPP for shelf‐life extension, the packaging material must be suitable to keep the product in good condition for a longer period, thus needs a good barrier against oxygen, moisture, light; another establishment recommended to take into account the amount of air/gas mixture that is introduced into the container in cases where the product does not go under vacuum. Gases can precipitate in the product and also a high amount of air/mixture gases can cause breakage problems during treatment; another establishment added that these aspects have been covered in Section 5 also, but summarising the package must be flexible and resistant, able to withstand pressure and maintain after the treatment such as the Polyethylene (PE), polyethyleneterephthalate (PET), polypropylene (PP), ethylene‐vinyl alcohol (EVOH), polyamide (PA) and nylon films are some of the packaging materials currently used in industrial food processing by HHP treatments since covered all the properties described. Also, it is important to minimise the headspace up to 30% to maximise the utilisation of the vessel capacity and minimise the time needed for preheating, if the treatment requires temperature. Usually, an HPP vessel will utilise its 50–70% volume capacity depending on the shape of the package and the vessel design. The main drawback is the combination of temperature with pressure overall if the systems rich high temperatures.
One establishment clarified that, in the literature review carried out on this topic, it can be seen that many studies have been carried out with a wide variety of polymers and food simulants. The general conclusion of these studies is that there is no migration (general or specific). The most extreme HPP conditions that can be applied industrially: 600 MPa for 10 min at 25°C.
One replier from an establishment, when working for another company, read different books and papers in the topic of HPP and visited a masterclass of Hiperbaric to learn about microorganisms and the effect of HPP in the growing and inactivation; one establishment informed that there are very incipient studies related to the degradation of agri‐food waste molecules in some foods (Reference provided: Pallarés N, Tolosa J, Gavahian M, Barba FJ, Khaneghah AM and Ferrer E, 2020. Chapter 7 ‐ The potential of HPP for minimising pesticides and toxins in food products. In: Barba FJ, Tonello‐Samson C, Puértolas E, Lavilla M. (eds.). Present and Future of High Pressure Processing. A Tool for Developing Innovative, Sustainable, Safe and Healthy Foods, Elsevier, ISBN 978‐0‐12‐816405‐1, pp. 173‐184); one establishment informed that, as mentioned before, by definition, high pressures do not induce the formation or dissociation of strong molecular interactions (hydrogen bonds, covalent bonds...). Therefore, the HPP process will not form food contaminants. Likewise, it cannot destroy low molecular weight compounds such as food toxins. There are published studies in this respect, such as: ‐ HPP does not inactivate S. aureus toxins of seafood products (saxitoxin, okadaic or domoic acid, brevetoxin...) High‐pressure processing of shellfish: A review of microbiological and other quality aspects ‐ HP does not inactivate the toxins produced by B. cereus and E. coli. Effect of High Pressure and Heat on Bacterial Toxins); another establishment informed that different studies obtained showed that high pressure affected in different ways the different stages of the Maillard reaction and that such effects were strongly influenced by pressure‐induced changes in the pH of the systems. This aspect is important overall when HPP is combined with temperature. Moreno FJ, Molina E, Olano A, and López‐Fandiño R, 2003. High‐pressure effects on Maillard reaction between glucose and lysine. Journal of Agricultural and Food Chemistry, 51, 394‐400).
A.3 Questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety addressed to the equipment providers
The European Food Safety Authority (EFSA) received a mandate from the European Commission with a request for a scientific opinion on the efficacy and safety of high‐pressure processing of food. You can find further information about the mandate at EFSA‐Q‐2020‐00380.24
An ‘ad hoc’ working group (WG) including external experts and EFSA staff has been established to address the Terms of Reference of the mandate. The WG prepared a questionnaire for equipment providers to collect data that will be used to inform the assessment and could be used, after their anonymisation (by removing any reference to the equipment provider if that is preferred), in the scientific opinion. By providing the data, you give the consent for its use for this assessment.
We would appreciate if you could fill in the questionnaire and send it to the following email address until 22 January 2021: winy.messens@efsa.europa.eu. The competent authorities have also been sent a questionnaire and asked to forward a more detailed questionnaire to the establishments using HPP.
It would be appreciated if you provide also any information not covered by the questionnaire and considered to be of relevance.
Thank you very much for your cooperation.
Please specify your contact point details
| Equipment provider | |
| Country | |
| e‐mail | |
| Name of contact person |
GENERAL QUESTIONS
-
9
Are you a high‐pressure equipment provider for the food industry?
Yes (please fill in the below questionnaire)
No (there is no need to complete the questionnaire but please inform EFSA)
-
10
On which type of food is HPP currently being used along with the processing conditions and relative importance? Please provide an overview in the table below or in the attached excel file (please insert one row for each food subtype and consider the footnotes that provide clarifications).
-
11
What are the general recommendations for HPP? Please consider the range of recommended maximum target pressures to be used taking into account the long‐term performance of the currently used equipment, the maximum treatment time once target pressure reached, the rate of pressurisation/depressurisation and the usual water temperature before HPP treatment and expected rise in temperature. To take account of the compression heating phenomena during HPP, please provide information on the compression heating experienced by the pressure transmitting fluid and by the food itself. Actual measurements of the heat‐up during compression and the cool‐down during decompression would be most valuable. In the absence of data, a description based on modelling of the heat‐up and cool‐down using literature compression heating values, would be useful.
-
12
Are you aware of any food product being treated in‐bulk using a batch system (for instance, where milk or a beverage is filled into a large holding bag in the HPP chamber)?
Yes (can you please provide information related to the food business operator and the food product being treated this way along with the processing conditions being applied. Please also inform about the bag that is recommended for being used in this situation: what is this bag made of (polymer and characteristics), on which criteria is the bag changed following its repeated use, how is it cleaned between uses and has the repeated pressurisation an effect on the integrity of the bag and the migration from the bag into food, is the bag degraded, aged, etc., following the repeated uses?)
No
-
13
Shortly describe how you recommend to carry out the validation of the HPP treatment for safety purposes, e.g. target microorganism, target log reduction, approach to document the safety/target log reduction (e.g. literature data, experimental trials such as challenge test, use of predictive models). 26
-
14
Are you aware of any expected important technological changes in the near future (e.g. type of foods to be treated and their processing conditions)?
Yes (please provide this information)
No
SPECIFIC QUESTIONS RELATED TO FOOD MICROBIOLOGY/HYGIENE
-
15
Are you aware of any microbiological food safety problems (such as spore activation leading to more rapid and more complete germination and outgrowth, prion activation by conversion of normal form to amyloid form, induction of virulence or toxin gene expression) originated from food subject to HPP?
Yes (please provide the details of such incidents)
No
SPECIFIC QUESTIONS RELATED TO FOOD CONTACT MATERIALS (FCM)
-
16
Are there special considerations or technical requirements on the properties of FCM for HPP apart from flexibility and appearance, such as polymer morphology, molecular weight distribution, density, melt‐flow index, delamination, etc.?
Yes (please provide this information)
No
I do not know
-
17
Are you aware of studies on the effect of HPP on FCM, including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment? (Note that the WG will review the published literature and so information only on any unpublished data is sought)
Yes (please provide this information)
No
-
18
Concerning possible strong and undesirable food‐packaging interactions and other changes that may give rise to migration, are there types of foods for which HPP has an adverse effect or for which HPP is not suitable/recommended? What would be the reason for this? Are there polymer types not recommended for HPP apart from aspects such as flexibility, tightness and appearance? Are there other pitfalls to be aware of, including the choice of treatment conditions?
Yes (please provide this information)
No
I do not know
SPECIFIC QUESTIONS RELATED TO FOOD CONTAMINANTS
-
19
Are you aware of studies on the effect of HPP on the potential formation or degradation/modification of contaminants in foods during and after HPP treatment? (Note that the WG will review the published literature and so information only on any unpublished data is sought)
Yes (please provide this information)
No
Table – Question 1
| FOOD CATEGORY | REASONS FOR USING HPP | HPP CONDITIONS | FOR PROCESSING IN‐PACK (final retail package) | FOOD STORAGE INSTRUCTIONS TO FOOD BUSINESS OPERATOR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Food category (sector) | Brief description of the food type on which HPP is used. Please insert one row for each food type | Relative importance of this food type being HPP in comparison to other food types on which HPP is used | Primary reason for using the HPP technology for the commodity listed | Secondary reason for using the HPP technology for the commodity listed | Target pressure range (in MPa)/treatment time once target pressure reached (in min)/water temperature before HPP treatment (in °C)( a ) | What are the packaging conditions? | Which is the packaging material used( b )? | Temperature (°C) | Shelf‐life |
|
Options: ‐high ‐medium ‐low |
Options: ‐to increase product safety (i.e. inactivate pathogenic microorganisms) ‐to extend product shelf‐life (i.e. inactivate spoilage microorganisms) ‐other (please specify) |
Options: ‐to increase product safety ‐to extend product shelf‐life ‐other (please specify) |
Example: 400 MPa/5 min/21°C OR 400 MPa/5 min/unknown |
Example: vacuum‐packaged | |||||
| Meat and meat products | E.g. Fresh meat, minced meat, heat treated meat product, fermented meat product, cured or not‐cured meat product, mechanically separated meat (MSM), OTHER (please specify) | ||||||||
| Fruit, vegetables, juices and other products thereof | E.g. acid (pH < 4.5) or non‐acid (pH > 4.5) fruit/vegetable juices, pre‐cut fruit, fruit salad, guacamole, sweets, OTHER (please specify) | ||||||||
| Milk | E.g. raw skimmed or whole fat milk from cow or other species, OTHER (please specify) | ||||||||
| Cheese | E.g. raw milk cheese, pasteurised milk cheese (type), OTHER (please specify) | ||||||||
| Dairy products (other than cheeses) | E.g. yoghurt, OTHER (please specify) | ||||||||
| Fish and fishery products | E.g. fresh fish, fish salads, surimi, fish proteins, OTHER (please specify) | ||||||||
| Crustaceans, shellfish, molluscs and products thereof | E.g. oysters, lobster, crab, OTHER (please specify) | ||||||||
| Mixed food/composite product | |||||||||
| Other food category | |||||||||
If for each food category a range of different parameters are used, please provide the range (e.g. min, typical, max). If there are inter‐relationships between, e.g. the time, pressure and temperature applied, please describe these inter‐relationships and how the different parameters are combined to bring about the desired effect on the food.
If a plastic is used, please provide the polymer type, whether the food contact material (FCM) is a monolayer or a multilayer and the layer thickness(es). If other materials than plastics are used, please describe them.
The replies to the questionnaires by the equipment providers are provided in Table A.4 and summarised in Table A.5.
Table A.4.
Replies to the questionnaire on the commercial use of high‐pressure processing (HPP) of foods with the purpose to increase microbiological food safety by the equipment providers
| Question | EP1 | EP2 | |||
|---|---|---|---|---|---|
| GENERAL QUESTIONS | 1. Are you a high‐pressure equipment provider for the food industry? | Yes | Yes, we manufacture high‐pressure equipment and provides turnkey solutions for the food industry: HPP plants and supercritical fluid extraction units | ||
| 2. On which type of food is HPP currently being used along with the processing conditions and relative importance? Please provide an overview in the table below or in the attached excel file | see table | We consider to process food products as indicated in the attached excel sheet. | |||
| 3. What are the general recommendations for HPP? Please consider the range of recommended maximum target pressures to be used taking into account the long‐term performance of the currently used equipment, the maximum treatment time once target pressure reached, the rate of pressurisation/depressurisation, and the usual water temperature before HPP treatment and expected rise in temperature. To take account of the compression heating phenomena during HPP, please provide information on the compression heating experienced by the pressure transmitting fluid and by the food itself. Actual measurements of the heat‐up during compression and the cool‐down during decompression would be most valuable. In the absence of data, a description based on modelling of the heat‐up and cool‐down using literature compression heating values, would be useful. |
The HPP processing conditions recommended and used vary depending on a number of factors. Equipment limitations ensure that 600 MPa is the upper limit for commercial scale HPP. Holding times for the vast majority of products are 1–6 min. Recirculating water temperature is often chilled to 4–8°C depending on refrigeration capacity, water volume, vessel filling capacity, cycle time, room temperature. The pressure transmitting medium on modern HPP systems is water. According to literature, the adiabatic temperature increase of water is 2.6–2.9°C/100 MPa (Rasanayagam et al., 2003). The adiabatic heat rise for various other substances/foods is also reported in Rasanayagam et al. (2003) but our machines do not capture data for discrete samples added to the pressure chamber. We do not, as a matter of course, calculate or account for adiabatic heat rise for the vast majority of refrigerated foods processed through HPP as the practical effects are limited. |
Maximum target pressures depending on the application: shucking of seafood ~ 250–300 MPa, pasteurisation 400–600 MPa. Maximum treatment time once target pressure reached ~ 3 min, max 15 min. Rate of pressurisation /depressurisation ~ 3 min/10–45 s. The initial water temperature is usually 5–15°C. The expected rise in temperature pro 100 MPa: | |||
| Substance | Initial temperature | Temperature change (°C/100) | |||
| Water | 20 | 2.8 | |||
| 25 | 3.0 | ||||
| 60 | 3.8 | ||||
| 80 | 4.4 | ||||
| Water/glycol (1:1) | 25 | 4.8–3.7a | |||
| Propylene glycol | 25 | 5.3 | |||
| Ethanol | 25 | 10.6–6.8a | |||
| Steel | 20 | ~0 | |||
| Orange juice, tomato salsa, 2% fat milk, water‐like substances | 25 | 2.6–3.0 | |||
| Chicken | 20 | 2.9 | |||
| Chicken fat | 25 | 4.5 | |||
| Chicken breast | 25 | 3.1 | |||
| Salmon | 25 | 3.0–3.2 | |||
| Beef fat (23%) | 25 | 4.4 | |||
| Extracted beef fat (85%) | 25 | 8.3–6.3a | |||
| Ground beef | 25 | 3.2 | |||
| Gravy beef | 25 | 3.0 | |||
| Milk fat | 20 | 8.5 | |||
| Whole milk (3.5% fat) | 25 | 3.2 | |||
| Yogurt | 25 | 3.1 | |||
| Cream cheese 24% fat | 25 | 4.8 | |||
| Cheese Gauda | 20 | 3.4 | |||
| Soy oil 100% | 25 | 9.1 to < 6.2a | |||
| Olive oil 100% | 25 | 8.7 to < 6.3a | |||
| Egg albumin | 25 | 3.0 | |||
| Egg yolk | 25 | 4.4 | |||
| Egg whole | 25 | 3.3 | |||
| Mayonnaise 75% fat | 25 | 7.2–5.3a | |||
| Tofu | 25 | 3.1 | |||
| Mashed potato | 25 | 3.0 | |||
| Hass avocado | 25 | 4.1–3.7a | |||
| Honey | 25 | 3.2 | |||
| a: Substances exhibited decreasing adiabatic heating as pressure increased | |||||
| 4. Are you aware of any food product being treated in‐bulk using a batch system (for instance, where milk or a beverage is filled into a large holding bag in the HPP chamber)? | Yes (There is a system offered by a competing HPP equipment provider for bulk liquid processing as described here. As any current users are not our customers, we can’t offer details on the products or materials in use) | No, only in PET bottles | |||
| 5. Shortly describe how you recommend to carry out the validation of the HPP treatment for safety purposes, e.g. target microorganism, target log reduction, approach to document the safety/target log reduction (e.g. literature data, experimental trials such as challenge test, use of predictive models) | We recommend challenge and shelf‐life testing be conducted on the food matrix intended to be HPP treated. Our recommendations for study protocols generally fall within the Parameters for Determining Inoculated Pack/Challenge Study Protocols set forth by the National Advisory Committee on Microbiological Criteria for Foods which have been adopted by major regulatory agencies in North America and Europe. For pathogen challenge studies we recommend a robust cocktail of strains appropriate for the food matrix in question and do not recommend use of surrogate organisms. The target log reduction is dependent on regulatory requirements of the country/food being treated. The most common target for log reduction of inoculated pathogens is 5 but some regulators have required less (e.g. 1–3). We recommend the target pressure for production be lowered by 1,000 psi for a challenge study to account for slight downward pressure drift during the holding time. | Our client is responsible for the validation. We support the client with shelf‐life test. In that case, the HPP processed product and a reference will be tested after several weeks and pathogens as well microbiological count will be analysed. The amount of microorganism shall not exceed the guided value given by standards. | |||
| 6. Are you aware of any expected important technological changes in the near future (e.g. type of foods to be treated and their processing conditions)? | No | Yes (Protein folding and unfolding would be interested for new properties of products tests take place; PATS is still in evaluation and tests takes place) | |||
| SPECIFIC QUESTIONS RELATED TO FOOD MICROBIOLOGY/HYGIENE | 7. Are you aware of any microbiological food safety problems originated from food subject to HPP? | Yes (Depending on intrinsic factors of certain foods and physiological conditions of spores excessively long cycle time has the potential of bacterial spore activation and possible outgrowth when intrinsic and extrinsic factors are not controlled. Current commercial production parameters by our clients take this into consideration) | No | ||
| SPECIFIC QUESTIONS RELATED TO FCM | 8. Are there special considerations or technical requirements on the properties of FCM for HPP apart from flexibility and appearance? | No (Extensive research both published and conducted by our company in collaboration packaging companies have shown that materials used for commercial HPP applications do not present these issues) | No (based on recently studies the HPP treatment has no impact on the permeation of polymers on food under pressure27 | ||
| 9. Are you aware of studies on the effect of HPP on FCM, including the potential formation of new reaction/degradation products and the migration of substances into food during and after HPP treatment? | Yes (our company has conducted tests and submitted results to regulatory agencies in the US, Canada and European Commission to show this does not occur) | No (Please see above Point 8) | |||
| 10. Concerning possible strong and undesirable food‐packaging interactions and other changes that may give rise to migration, are there types of foods for which HPP has an adverse effect or for which HPP is not suitable/recommended? What would be the reason for this? Are there polymer types not recommended for HPP apart from aspects such as flexibility, tightness and appearance? Are there other pitfalls to be aware of, including the choice of treatment conditions? | Yes (Only to the degree of oxygen transmission and moisture vapour transfer rates on product organoleptic and shelf‐life) | Yes (One consideration point is the relation of HPP and MAP packages. During pressure treatment the gas is solved and is able to move in between the layers of the foils. During pressure release the gas expands immediately and is able to damage the layers, called ‘white spots’. In that case it is necessary to regulate the pressure release and allow the gas the time to leave the space in between the layers. This effect differs between packaging, gas and product; Polymers based on renewable sources (sugarcane) are still in investigation for bottles for example) | |||
| SPECIFIC QUESTIONS RELATED TO FOOD CONTAMINANTS | 11. Are you aware of studies on the effect of HPP on the potential formation or degradation/ modification of contaminants in foods during and after HPP treatment? | No | No | ||
FCM: food contact materials; HPP: high‐pressure processing; PATS: pressure assisted thermal sterilisation; PET: polyethylene terephthalate.
Appendix B – Literature searches and screening
Several literature searches were conducted for this assessment.
B.1 Food categories to which HPP is being applied for food safety reasons
The search was performed on 9 October 2020 in the Web of ScienceTM Core Collection (1975–present) to retrieve review papers, book sections and books summarising information about the food products being treated with HPP. The search strategy is reported in Table B.1.
Table B.1.
Details of search strings used for literature searches on HPP of food products using Web of ScienceTM Core Collection (1975–present)
| Set number | Search query | No of records |
|---|---|---|
| #1 | TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*) ) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) | 21,262 ( a ) |
| #2 | TS = (food OR foods OR meal OR meals OR beverage OR beverages OR drink OR drinks OR snack OR snaks OR juice* OR dip OR dips OR salsa OR salsas OR sandwich* OR filling OR mayonnaise OR ready‐to‐eat OR “ready to eat” OR "ready meal*" OR "ready prepared" OR dressing* OR sauce* OR soup* OR cream* OR chowder* OR stew* OR pasta OR rice OR puree* OR coulis OR chunk* OR slice* OR pudding* OR jam OR ((“commercially prepared” OR “convenience” OR “pickled” OR “fermented” OR “preserved” OR “smoked” OR “cured” OR “gravid” or marinate* or marinad*) NEAR/2 (product*)) OR fruit* OR vegetable* OR smoothie* OR avocado* OR salad* OR tomato* OR guacamole* OR hummus OR taboulé OR pea OR peas OR bean OR beans OR chickpea* OR sprout* OR tofu* OR pomegranate* OR apple* OR carrot OR carrots OR broccoli* OR beetroot* OR meat OR sausage* OR pate OR pates OR ham OR hams OR “cold cut” OR “cold cuts” OR deli OR delis OR delicatessen* OR charcuterie OR salami* OR pepperoni OR hotdog* OR “hot dog*” OR frankfurter* OR burger* OR hamburger* OR chorizo OR meatloaf OR pastrami OR salchichon OR mortadella OR bologna OR baloney OR boloney OR polony OR terrine* OR rillette* OR tongue OR pork OR bovine OR beef OR chicken* OR turkey* OR lamb OR lambs OR poultry OR seafood OR seafoods OR “sea food” OR “sea foods” OR crustacean* OR shellfish OR mollusc* OR gravad OR fish* OR salmon OR cod OR hake OR mussel* OR crab* OR oyster* OR prawn* OR shrimp* OR lobster* OR “clam” OR “clams” OR “crayfish” OR scallop* OR trout OR trouts OR mackerel OR mackerels OR "gravad lax" OR gravlax OR sushi OR sashimi OR ceviche OR dairy OR milk OR colostrum OR cheese* OR curd OR yogurt* OR yoghurt* OR yoghourt* OR cream OR egg OR eggs OR feta OR brie OR camembert OR “queso blanco” OR chevre OR “blue‐vein*” OR “mold ripe*” OR “mould ripe*” OR “danish blue” OR gorgonzola OR roquefort OR ricotta) | 4,606,906 ( a ) |
| #3 | #1 AND #2 | 6,566 ( a ) |
| #4 | (#1 AND #2) AND DocType=(BOOK OR REVIEW OR BOOK CHAPTER) | 643 |
| #5 | #4 and Years=2010‐ | 499 |
DocType: All document types; Language: All languages; Timespan: All years.
B.2 Potential microbiological food safety concerns in HPP‐treated food
The search was performed on 28 June 2021 in the Web of ScienceTM Core Collection (1975–present) to retrieve records dealing with prions (conversion of normal form to amyloid forms) as possible HPP food safety concern. The search strategy is reported in Table B.2.
Table B.2.
Details of search strings used for literature searches on HPP and prions using Web of ScienceTM Core Collection (1975–present)
| Set number | Search query | No of records |
|---|---|---|
| #1 | TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) | 22,382 ( a ) |
| #2 | TS = (prion OR prions OR "Bovine Spongiform Encephalopathy” OR “Transmissible Spongiform Encephalopathy” OR TSE OR “TSE agent*” OR “BSE” OR (“Bovine Spongiform” W/3 encephalopath*) OR ( ( “mad cow” OR “chronic wasting”) W/3 disease*) OR scrapie) | 40,089 ( a ) |
| #3 | #1 AND #2 | 38 ( a ) |
DocType = All document types; Language = All languages; Timespan = All years.
The search was performed on 27 July 2021 in the Web of ScienceTM Core Collection (1975–present) to retrieve records dealing with spore activation as possible HPP food safety concern. The search strategy is reported in Table B.3.
Table B.3.
Details of search strings used for literature searches on HPP and spore activation using Web of ScienceTM Core Collection (1975–present)
| Set number | Search query | No of records |
|---|---|---|
| #1 | TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*) ) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) | 22, 531 ( a ) |
| #2 | TS =(spore* or endospore*) | 65,042 ( a ) |
| #3 | #1 AND #2 | 517 ( a ) |
| #4 | #3 AND DocType=(BOOK OR REVIEW OR BOOK CHAPTER) | 92 |
| #5 | TS = ((spore* OR endospore*) NEAR (activat* OR germinat*)) | 9,016 ( a ) |
| #6 | #1 AND #5 | 111 ( a ) |
| #7 | #6 NOT #4 | 97 ( a ) |
DocType = All document types; Language = All languages; Timespan = All years.
The search was performed on 12 August 2021 in the Web of ScienceTM Core Collection (1975–present) to retrieve records dealing with induction of virulence and toxin gene expression as possible HPP food safety concern. The search strategy is reported in Table B.4.
Table B.4.
Details of search strings used for literature searches on HPP and induction of virulence and toxin gene expression using Web of ScienceTM Core Collection (1975–present)
| Set number | Search query | No of records |
|---|---|---|
| #1 | TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*) ) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) | 22,597 ( a ) |
| #2 | TS = (((virulence* OR virulent* OR virul*) NEAR (induct* OR impact* OR gene* OR toxin OR express*))) | 48,299 ( a ) |
| #3 | #1 AND #2 | 25 ( a ) |
DocType = All document types; Language = All languages; Timespan = All years.
B.3 Potential chemical food safety concerns in HPP‐treated food through formation of process contaminants
To search for different types of publications providing information on chemical contaminants during HPP, the following databases were used: Web of Science Core Collection https://webofknowledge.com/WOS and PubMed https://www.ncbi.nlm.nih.gov/pubmed
The search was performed on 1 October 2020. For full transparency and reproducibility, all searches performed are recorded in search protocols, including search queries used in individual databases to retrieve potentially relevant studies for subsequent screening of titles and abstracts.
The search strategies used in PubMed and Web of ScienceTM Core Collection are reported in Tables B.5 and B.6.
Table B.5.
Details of search strings used for literature searches in PubMed on potential chemical food safety risks in HPP‐treated food through formation of process contaminants
| Set number | Search query | No of records |
|---|---|---|
| #7 | Search #1 AND #6 | 450 |
| #6 | Search #2 OR #3 | 8,542,857 |
| #5 | Search #1 AND #3 | 439 |
| #4 | Search #1 AND #2 | 41 |
| #3 | "reaction product"[All Fields] OR "metabolite*"[All Fields] OR ("metabolite*"[All Fields] AND "contaminant*"[All Fields]) OR "contaminant*"[All Fields] OR (("chemical"[All Fields] OR "chemical s"[All Fields] OR "chemically"[All Fields] OR "chemicals"[All Fields]) AND ("food safety"[MeSH Terms] OR ("food"[All Fields] AND "safety"[All Fields]) OR "food safety"[All Fields]) AND "risk*"[All Fields]) OR "impurit*"[All Fields] OR ("food safety"[MeSH Terms] OR ("food"[All Fields] AND "safety"[All Fields]) OR "food safety"[All Fields]) OR ("risk assessment"[MeSH Terms] OR ("risk"[All Fields] AND "assessment"[All Fields]) OR "risk assessment"[All Fields]) OR ("risk"[MeSH Terms] OR "risk"[All Fields]) OR (("toxic"[All Fields] OR "toxical"[All Fields] OR "toxically"[All Fields] OR "toxicant"[All Fields] OR "toxicant s"[All Fields] OR "toxicants"[All Fields] OR "toxicated"[All Fields] OR "toxication"[All Fields] OR "toxicities"[All Fields] OR "toxicity"[MeSH Subheading] OR "toxicity"[All Fields] OR "toxicity s"[All Fields] OR "toxics"[All Fields]) AND ("compound"[All Fields] OR "compound s"[All Fields] OR "compounds"[All Fields])) OR "toxic*"[All Fields] OR ("adverse"[All Fields] OR "adversely"[All Fields] OR "adverses"[All Fields]) OR ("undesirable"[All Fields] OR "undesirables"[All Fields] OR "undesirably"[All Fields] OR "undesired"[All Fields]) OR "harm*"[All Fields] OR "safe"[All Fields] OR ("safety"[MeSH Terms] OR "safety"[All Fields] OR "safeties"[All Fields]) OR ("hazard"[All Fields] OR "hazard s"[All Fields] OR "hazardous"[All Fields] OR "hazardously"[All Fields] OR "hazardousness"[All Fields] OR "hazards"[All Fields]) OR ("chemical"[All Fields] OR "chemical s"[All Fields] OR "chemically"[All Fields] OR "chemicals"[All Fields]) | 7,598,283 |
| #2 | ("nitrosamines"[MeSH Terms] OR "nitrosamines"[All Fields] OR "nitrosamine"[All Fields]) OR "polychlorinated naphthalene"[All Fields] OR "PCN"[All Fields] OR (("heterocycle"[All Fields] OR "heterocycles"[All Fields] OR "heterocyclic"[All Fields] OR "heterocyclics"[All Fields] OR "heterocyclization"[All Fields] OR "heterocyclizations"[All Fields] OR "heterocyclized"[All Fields]) AND ("aminal"[All Fields] OR "aminalization"[All Fields] OR "aminals"[All Fields] OR "aminate"[All Fields] OR "aminated"[All Fields] OR "aminating"[All Fields] OR "amination"[MeSH Terms] OR "amination"[All Fields] OR "aminations"[All Fields] OR "aminative"[All Fields] OR "amine s"[All Fields] OR "amines"[MeSH Terms] OR "amines"[All Fields] OR "amine"[All Fields] OR "aminic"[All Fields])) OR (("polycycle"[All Fields] OR "polycycles"[All Fields] OR "polycyclic"[All Fields] OR "polycyclics"[All Fields] OR "polycyclization"[All Fields] OR "polycyclizations"[All Fields]) AND ("aminal"[All Fields] OR "aminalization"[All Fields] OR "aminals"[All Fields] OR "aminate"[All Fields] OR "aminated"[All Fields] OR "aminating"[All Fields] OR "amination"[MeSH Terms] OR "amination"[All Fields] OR "aminations"[All Fields] OR "aminative"[All Fields] OR "amine s"[All Fields] OR "amines"[MeSH Terms] OR "amines"[All Fields] OR "amine"[All Fields] OR "aminic"[All Fields])) OR ("acrylamide"[MeSH Terms] OR "acrylamide"[All Fields] OR "acrylamides"[MeSH Terms] OR "acrylamides"[All Fields]) OR "MCPD"[All Fields] OR ("monochloropropanediol"[All Fields] OR "monochloropropanediols"[All Fields]) OR "chloropropanediol"[All Fields] OR ("mineral oil"[MeSH Terms] OR ("mineral"[All Fields] AND "oil"[All Fields]) OR "mineral oil"[All Fields]) OR "aflatoxin*"[All Fields] OR "glycoalkaloid*"[All Fields] OR ("healthc manage forum"[Journal] OR "hmf"[All Fields]) OR "grayanotoxin*"[All Fields] OR (("perfluoroalkyl"[All Fields] OR "perfluoroalkylated"[All Fields] OR "perfluoroalkylation"[All Fields] OR "perfluoroalkylations"[All Fields] OR "perfluoroalkyls"[All Fields]) AND "substance*"[All Fields]) OR "PFAs"[All Fields] OR ("ochratoxin a"[Supplementary Concept] OR "ochratoxin a"[All Fields] OR "ochratoxin a"[All Fields]) OR "OTA"[All Fields] OR (("chlorin"[Supplementary Concept] OR "chlorin"[All Fields] OR "chlorinate"[All Fields] OR "chlorinated"[All Fields] OR "chlorinates"[All Fields] OR "chlorinating"[All Fields] OR "chlorinations"[All Fields] OR "chlorinator"[All Fields] OR "chlorinators"[All Fields] OR "chlorine"[MeSH Terms] OR "chlorine"[All Fields] OR "chlorine s"[All Fields] OR "chlorines"[All Fields] OR "chlorins"[All Fields] OR "halogenation"[MeSH Terms] OR "halogenation"[All Fields] OR "chlorination"[All Fields]) AND "paraffin*"[All Fields]) OR (("quinolizidine"[All Fields] OR "quinolizidines"[MeSH Terms] OR "quinolizidines"[All Fields]) AND "alkaloid*"[All Fields]) OR (("cyanogen"[Supplementary Concept] OR "cyanogen"[All Fields] OR "cyanogenic"[All Fields] OR "cyanogens"[All Fields]) AND "glycoside*"[All Fields]) OR "perfluorooctane sulfonic acid"[All Fields] OR "perfluorooctanoic acid"[All Fields] OR "dioxin*"[All Fields] OR "dioxin‐like PCB"[All Fields] OR ("diacetoxyscirpenol"[Supplementary Concept] OR "diacetoxyscirpenol"[All Fields]) OR ("fumonisins"[MeSH Terms] OR "fumonisins"[All Fields] OR "fumonisin"[All Fields]) OR "opium alkaloid"[All Fields] OR ("moniliformin"[Supplementary Concept] OR "moniliformin"[All Fields]) OR "monochloropropane diol"[All Fields] OR ("furan"[Supplementary Concept] OR "furan"[All Fields] OR "furane"[All Fields] OR "furanes"[All Fields] OR "furanic"[All Fields] OR "furanics"[All Fields] OR "furans"[MeSH Terms] OR "furans"[All Fields]) OR ("methylfuran"[All Fields] OR "methylfurans"[All Fields]) OR "hydrocyanic acid"[All Fields] OR ("deoxynivalenol"[Supplementary Concept] OR "deoxynivalenol"[All Fields] OR "deoxynivalenols"[All Fields]) OR ("zearalenon"[All Fields] OR "zearalenone"[MeSH Terms] OR "zearalenone"[All Fields] OR "zearalenones"[All Fields]) OR ("tetrodotoxin"[MeSH Terms] OR "tetrodotoxin"[All Fields] OR "tetrodotoxins"[All Fields]) OR "TTX"[All Fields] OR ("TTX"[All Fields] AND ("analog"[All Fields] OR "analoge"[All Fields] OR "analoges"[All Fields] OR "analogic"[All Fields] OR "analogical"[All Fields] OR "analogizing"[All Fields] OR "analogous"[All Fields] OR "analogously"[All Fields] OR "analogs"[All Fields] OR "analogue"[All Fields] OR "analogues"[All Fields])) OR "T2 toxin"[All Fields] OR "HT2 toxin"[All Fields] OR ("erucic acid"[Supplementary Concept] OR "erucic acid"[All Fields]) OR ("malachite green"[Supplementary Concept] OR "malachite green"[All Fields]) OR ("monochloropropanediol"[All Fields] OR "monochloropropanediols"[All Fields]) OR "MCPD"[All Fields] OR ("phorbol esters"[MeSH Terms] OR ("phorbol"[All Fields] AND "esters"[All Fields]) OR "phorbol esters"[All Fields] OR ("phorbol"[All Fields] AND "ester"[All Fields]) OR "phorbol ester"[All Fields]) OR ("dronabinol"[MeSH Terms] OR "dronabinol"[All Fields] OR "tetrahydrocannabinol"[All Fields] OR "tetrahydrocannabinolic"[All Fields] OR "tetrahydrocannabinols"[All Fields]) OR ("dronabinol"[MeSH Terms] OR "dronabinol"[All Fields] OR "thc"[All Fields]) OR ("nitrofurane"[All Fields] OR "nitrofuranes"[All Fields] OR "nitrofurans"[MeSH Terms] OR "nitrofurans"[All Fields] OR "nitrofuran"[All Fields]) OR ("chlorates"[MeSH Terms] OR "chlorates"[All Fields] OR "chlorate"[All Fields]) OR ("acrylamide"[MeSH Terms] OR "acrylamide"[All Fields] OR "acrylamides"[MeSH Terms] OR "acrylamides"[All Fields]) OR ("nickel"[MeSH Terms] OR "nickel"[All Fields] OR "nickelous"[All Fields] OR "nickels"[All Fields]) OR ("mycotoxines"[All Fields] OR "mycotoxins"[MeSH Terms] OR "mycotoxins"[All Fields] OR "mycotoxin"[All Fields]) OR ("chloramphenicol"[MeSH Terms] OR "chloramphenicol"[All Fields] OR "chloramphenicols"[All Fields]) OR ("perchlorate"[Supplementary Concept] OR "perchlorate"[All Fields] OR "perchlorates"[MeSH Terms] OR "perchlorates"[All Fields] OR "perchloric"[All Fields]) OR ("beauvericin"[Supplementary Concept] OR "beauvericin"[All Fields] OR "beauvericins"[All Fields]) OR ("enniatin"[All Fields] OR "enniatins"[Supplementary Concept] OR "enniatins"[All Fields]) OR ("methylmercury compounds"[MeSH Terms] OR ("methylmercury"[All Fields] AND "compounds"[All Fields]) OR "methylmercury compounds"[All Fields] OR "methylmercury"[All Fields] OR "methylmercury s"[All Fields]) OR ("chromium"[MeSH Terms] OR "chromium"[All Fields]) OR "Tropane alkaloid"[All Fields] OR ("sterigmatocystin"[MeSH Terms] OR "sterigmatocystin"[All Fields] OR "sterigmatocystins"[All Fields]) OR ("endocrine disruptors"[Pharmacological Action] OR "endocrine disruptors"[MeSH Terms] OR ("endocrine"[All Fields] AND "disruptors"[All Fields]) OR "endocrine disruptors"[All Fields] OR ("endocrine"[All Fields] AND "disruptor"[All Fields]) OR "endocrine disruptor"[All Fields]) OR "mercury poisoning"[MeSH Terms] OR ("mercury"[All Fields] AND "poisoning"[All Fields]) OR "mercury poisoning"[All Fields] OR "mercury"[MeSH Terms] OR "mercury"[All Fields] OR "mercury s"[All Fields]) OR "pcdd f"[All Fields] OR "DL‐PCB"[All Fields] OR "Brominated Flame Retardant"[All Fields] OR "BFR"[All Fields] OR ("ergot alkaloids"[MeSH Terms] OR ("ergot"[All Fields] AND "alkaloids"[All Fields]) OR "ergot alkaloids"[All Fields] OR ("ergot"[All Fields] AND "alkaloid"[All Fields]) OR "ergot alkaloid"[All Fields]) OR "Mineral Oil Hydrocarbon"[All Fields] OR "Brominated Phenol"[All Fields] OR ("citrinin"[MeSH Terms] OR "citrinin"[All Fields]) OR ("phomopsin"[Supplementary Concept] OR "phomopsin"[All Fields] OR "phomopsins"[All Fields]) OR ("tetrabromobisphenol a"[Supplementary Concept] OR "tetrabromobisphenol a"[All Fields] OR "tetrabromobisphenol a"[All Fields]) OR "TBBPA"[All Fields] OR "Pyrrolizidine alkaloid"[All Fields] OR "Alternaria toxin"[All Fields] OR ("hexabromocyclododecane"[Supplementary Concept] OR "hexabromocyclododecane"[All Fields] OR "hexabromocyclododecanes"[All Fields]) OR ("hbcdd"[All Fields] OR "hbcdds"[All Fields]) OR "Polybrominated Diphenyl Ether"[All Fields] OR ("pentabromodiphenyl ether"[Supplementary Concept] OR "pentabromodiphenyl ether"[All Fields] OR "pbde"[All Fields] OR "halogenated diphenyl ethers"[MeSH Terms] OR ("halogenated"[All Fields] AND "diphenyl"[All Fields] AND "ethers"[All Fields]) OR "halogenated diphenyl ethers"[All Fields]) OR ("glycerol"[MeSH Terms] OR "glycerol"[All Fields] OR "glycerin"[All Fields] OR "glycerine"[All Fields]) OR "PBB"[All Fields] OR "marine"[All Fields] OR "marines"[All Fields]) AND ("biotoxin"[All Fields] OR "biotoxins"[All Fields])) OR ("brevetoxin"[Supplementary Concept] OR "brevetoxin"[All Fields] OR "brevetoxins"[All Fields]) OR "mercury poisoning"[MeSH Terms] OR ("mercury"[All Fields] AND "poisoning"[All Fields]) OR "mercury poisoning"[All Fields] OR "mercury"[MeSH Terms] OR "mercury"[All Fields] OR "mercury s"[All Fields]) OR "pcdd f"[All Fields] OR "DL‐PCB"[All Fields] OR ("ciguatoxins"[MeSH Terms] OR "ciguatoxins"[All Fields] OR "ciguatoxin"[All Fields]) OR (("cyclic"[All Fields] OR "cyclics"[All Fields]) AND ("imination"[All Fields] OR "imines"[MeSH Terms] OR "imines"[All Fields] OR "imine"[All Fields] OR "iminic"[All Fields])) OR ("spirolide"[All Fields] OR "spirolides"[All Fields]) OR ("gymnodimine"[Supplementary Concept] OR "gymnodimine"[All Fields] OR "gymnodimines"[All Fields]) OR ("pinnatoxin"[All Fields] OR "pinnatoxins"[All Fields]) OR "pteriatoxin"[All Fields] OR ("lead"[MeSH Terms] OR "lead"[All Fields]) OR ("melamine"[Supplementary Concept] OR "melamine"[All Fields] OR "melamines"[All Fields]) OR ("palytoxin"[Supplementary Concept] OR "palytoxin"[All Fields] OR "palytoxins"[All Fields]) OR ("high"[All Fields] AND ("viscosity"[MeSH Terms] OR "viscosity"[All Fields] OR "viscosities"[All Fields]) AND ("mineral oil"[MeSH Terms] OR ("mineral"[All Fields] AND "oil"[All Fields]) OR "mineral oil"[All Fields] OR ("white"[All Fields] AND "mineral"[All Fields] AND "oil"[All Fields]) OR "white mineral oil"[All Fields])) OR ("arsenates"[MeSH Terms] OR "arsenates"[All Fields] OR "arsenic acid"[Supplementary Concept] OR "arsenic acid"[All Fields] OR "arsenate"[All Fields] OR "arsenic"[MeSH Terms] OR "arsenic"[All Fields] OR "arsenics"[All Fields] OR "arsenic s"[All Fields] OR "arsenicals"[MeSH Terms] OR "arsenicals"[All Fields] OR "arsenical"[All Fields] OR "arsenism"[All Fields] OR "arsenous"[All Fields]) OR "Domoic acid"[All Fields] OR ("pectenotoxin"[All Fields] OR "pectenotoxins"[All Fields]) OR ("uranium"[MeSH Terms] OR "uranium"[All Fields] OR "uranium s"[All Fields]) OR ("saxitoxin"[MeSH Terms] OR "saxitoxin"[All Fields] OR "saxitoxins"[All Fields]) OR ("nitritation"[All Fields] OR "nitrites"[MeSH Terms] OR "nitrites"[All Fields] OR "nitrite"[All Fields]) OR ("cadmium"[MeSH Terms] OR "cadmium"[All Fields]) OR ("yessotoxin"[Supplementary Concept] OR "yessotoxin"[All Fields] OR "yessotoxins"[All Fields]) OR ("gossypol"[MeSH Terms] OR "gossypol"[All Fields] OR "gossypols"[All Fields]) OR ("azaspiracid"[Supplementary Concept] OR "azaspiracid"[All Fields] OR "azaspiracids"[All Fields]) OR "Polycyclic Aromatic Hydrocarbon"[All Fields] OR ("perfluorooctane sulfonic acid"[Supplementary Concept] OR "perfluorooctane sulfonic acid"[All Fields] OR "perfluorooctane sulfonate"[All Fields]) OR "PFOS"[All Fields] OR ("perfluorooctanoic acid"[Supplementary Concept] OR "perfluorooctanoic acid"[All Fields]) OR "PFOA"[All Fields] OR ("nitratation"[All Fields] OR "nitrates"[MeSH Terms] OR "nitrates"[All Fields] OR "nitrate"[All Fields]) OR ("diclazuril"[Supplementary Concept] OR "diclazuril"[All Fields]) OR ("nicarbazin"[MeSH Terms] OR "nicarbazin"[All Fields] OR "nicarbazine"[All Fields]) OR ("robenidine"[MeSH Terms] OR "robenidine"[All Fields]) OR ("decoquinate"[MeSH Terms] OR "decoquinate"[All Fields]) OR (("halofuginone"[Supplementary Concept] OR "halofuginone"[All Fields]) AND "hydrobromide"[All Fields]) OR ("okadaic acid"[MeSH Terms] OR ("okadaic"[All Fields] AND "acid"[All Fields]) OR "okadaic acid"[All Fields]) OR "Ethyl carbamate"[All Fields] OR ("hydrogen cyanide"[MeSH Terms] OR ("hydrogen"[All Fields] AND "cyanide"[All Fields]) OR "hydrogen cyanide"[All Fields] OR ("hydrocyanic"[All Fields] AND "acid"[All Fields]) OR "hydrocyanic acid"[All Fields]) OR (("hormon"[All Fields] OR "hormonal"[All Fields] OR "hormonally"[All Fields] OR "hormonals"[All Fields] OR "hormone s"[All Fields] OR "hormones"[Pharmacological Action] OR "hormones"[MeSH Terms] OR "hormones"[All Fields] OR "hormone"[All Fields] OR "hormons"[All Fields]) AND ("residue"[All Fields] OR "residue s"[All Fields] OR "residues"[All Fields])) | 1,618,572 |
| #1 | "high pressure process*"[All Fields] OR "high hydrostatic pressure process*"[All Fields] OR "ultra high pressure process*"[All Fields] | 450 |
Table B.6.
Details of search strings used for literature searches in Web of ScienceTM Core Collection on potential chemical food safety risks in HPP‐treated food through formation of process contaminants
| Set number | Search query | No of records |
|---|---|---|
| #7 |
#6 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
985 |
| #6 |
#3 OR #2 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
11,791,681 |
| #5 |
#3 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
809 |
| #4 |
#2 AND #1 Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
283 |
| #3 |
TS=(“reaction product" OR "potential reaction product" OR metabolite* OR "metabolite* of contaminant*" OR contaminant* OR "chemical food safety risk*" OR impurit* OR food safety OR risk assessment OR risk OR toxic compound OR toxic* OR adverse OR undesirable OR harm* OR safe OR safety OR hazard OR chemical) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
8,076,879 |
| #2 |
TS=(nanoparticle or nitrosamine or “polychlorinated naphthalene” OR PCN OR heterocyclic amines OR polycyclic amines OR acrylamide OR mcpd OR monochloropropanodiol OR chloropropanodiol OR monochloropropanediol OR chloropropanediol OR mineral oil OR aflatoxin* OR glycoalkaloid* OR HMF OR grayanotoxin* OR perfluoroalkyl substance* PFAs OR Ochratoxin A OR OTA OR chlorinated paraffin* OR quinolizidine alkaloid* OR cyanogenic glycoside* OR "perfluorooctane sulfonic acid" OR “perfluorooctanoic acid” OR dioxin* OR "dioxin‐like PCB" OR diacetoxyscirpenol OR fumonisin OR “opium alkaloid” OR moniliformin OR "monochloropropane diol" OR furan OR methylfuran OR "hydrocyanic acid" OR deoxynivalenol OR zearalenone OR tetrodotoxin OR TTX OR TTX analogue OR "T2 toxin" OR "HT2 toxin" OR Erucic acid OR Malachite green OR monochloropropanediol OR MCPD OR phorbol ester OR tetrahydrocannabinol OR THC OR nitrofuran OR chlorate OR acrylamide OR nickel OR mycotoxin OR Chloramphenicol OR perchlorate OR beauvericin OR enniatin OR methylmercury OR chromium OR "Tropane alkaloid" OR sterigmatocystin OR endocrine disruptor OR mercury OR PCDD/F OR DL‐PCB OR "Brominated Flame Retardant" OR BFR OR Ergot alkaloid OR "Mineral Oil Hydrocarbon" OR "Brominated Phenol" OR citrinin OR phomopsin OR Tetrabromobisphenol A OR TBBPA OR "Pyrrolizidine alkaloid" OR "Alternaria toxin" OR Hexabromocyclododecane OR HBCDD OR "Polybrominated Diphenyl Ether" OR PBDE OR glycerine OR PBB OR marine biotoxin OR Brevetoxin OR mercury OR PCDD/F OR DL‐PCB OR Ciguatoxin OR Cyclic imine OR spirolide OR gymnodimine OR pinnatoxin OR pteriatoxin OR Lead OR Melamine OR Palytoxin OR "high viscosity white mineral oil" OR Arsenic OR “Domoic acid” OR Pectenotoxin OR Uranium OR Saxitoxin OR Nitrite OR Cadmium OR Yessotoxin OR Gossypol OR Azaspiracid OR "Polycyclic Aromatic Hydrocarbon" OR Perfluorooctane sulfonate OR PFOS OR perfluorooctanoic acid OR PFOA OR Nitrate OR diclazuril OR nicarbazin OR robenidine OR decoquinate OR halofuginone hydrobromide OR okadaic acid OR “Ethyl carbamate” OR hydrocyanic acid OR hormone residue) Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
4,770,006 |
| #1 |
TS=("High pressure process*" OR "high‐hydrostatic pressure process*" OR "ultra‐high‐pressure process*") Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC Timespan=All years |
2,374 |
Table B.7 shows that duplicates were removed after merging retrieved references in individual searches. The duplicates were removed through both Endnote and Distiller software.
Table B.7.
Overview of retrieval of records concerning chemical contaminants during HPP
| Database | Search results |
|---|---|
| Pubmed | 450 |
| Web of Science | 985 |
| Pubmed and Web of Science, after de‐duplication | 1,297 |
The references were subsequently screened for relevance in Distiller in two levels: (i) the retrieved articles were screened first at Ti/Ab level for information on chemical substances that are modified or produced due to HPP (one reviewer) and (ii) the selected records were screened at the full text level for information that is related to contaminants (two reviewers).
The studies were excluded according to the following criteria:
-
–
Studies on nutritional aspects
-
–
Studies on microbiological aspects
-
–
HPP Engineering
In total 1,297 references were screened for relevance based on their titles and abstracts. After the resolution of conflicts, 151 articles were considered as potentially relevant and further screened. The relevant articles were used in the risk assessment and appraised narratively.
B.4 Potential chemical food safety concerns in HPP‐treated food through food contact materials
The search was performed on 9 October 2020 in the Web of ScienceTM Core Collection (1990–present) and Scopus to retrieve primary articles, reviews, books and proceedings about the effect of HPP on the packaging and ultimately on the migration potential to food or food simulants. The search concept is given in Table B.8 and the strategies used are reported in Tables B.9 and B.10.
Table B.8.
Search concept for literature searches on potential chemical food safety risks in HPP‐treated food through food contact materials
| High pressure | Migration | Food/Packaging |
|---|---|---|
|
High isostatic pressur* High hydrostatic pressur* HHP/HP/HPP/High/UHP/Ultrahigh pressure applicat* HHP/HP/HPP/High/UHP/Ultrahigh pressure pasteuriz/s* HHP/HP/HPP/High/UHP/Ultrahigh pressure preservat* HHP/HP/HPP/High/UHP/Ultrahigh pressure process* HHP/HP/HPP/High/UHP/Ultrahigh pressure steriliz/s* HHP/HP/HPP/High/UHP/Ultrahigh pressure technique* HHP/HP/HPP/High/UHP/Ultrahigh pressure technolog* HHP/HP/HPP/High/UHP/Ultrahigh pressure treat* HPP pressure* HHP pressure* Pascalisation Pascalization |
Barrier* Contamina* Diffusion* Leech* Mechanical propert* Physical propert* Migrat* Morpholog* Scalp* Sorpt* Transfer* |
Beverage* Drink/s Film/s Food/s Packag* Plastic/s Poly* Simulant* |
Table B.9.
Details of search strings used for literature searches in Scopus on potential chemical food safety risks in HPP‐treated food through food contact materials
| Set number | Search query | No of records |
|---|---|---|
| 7 | ( ( TITLE‐ABS‐KEY ( bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR ( ( "high pressure*" OR hp OR "ultrahigh pressure*" OR uhp OR hpp OR hhp ) W/3 ( applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* ) ) OR ( ( hpp OR hhp ) AND pressure* ) OR pascalisation* OR pascalization* ) ) AND ( TITLE‐ABS‐KEY ( barrier* OR contamin* OR diffusion* OR leech* OR ( ( mechanical OR physical ) W/3 propert* ) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer* ) ) AND ( TITLE‐ABS‐KEY (beverage* OR drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant* ) ) ) AND ( PUBYEAR > 1989 ) AND ( LIMIT‐TO ( LANGUAGE, "English" ) ) | 2,050 |
| 6 | ( ( TITLE‐ABS‐KEY ( bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR ( ( "high pressure*" OR hp OR "ultrahigh pressure*" OR uhp OR hpp OR hhp ) W/3 ( applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* ) ) OR ( ( hpp OR hhp ) AND pressure* ) OR pascalisation* OR pascalization* ) ) AND ( TITLE‐ABS‐KEY ( barrier* OR contamin* OR diffusion* OR leech* OR ( ( mechanical OR physical ) W/3 propert* ) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer* ) ) AND ( TITLE‐ABS‐KEY ( beverage* OR drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant* ) ) ) AND( PUBYEAR > 1989 ) | 2,178 |
| 5 | PUBYEAR > 1989 | 60,722,959 |
| 4 | ( TITLE‐ABS‐KEY ( bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR ( ( "high pressure*" OR hp OR "ultrahigh pressure*" OR uhp OR hpp OR hhp ) W/3 ( applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* ) ) OR ( ( hpp OR hhp ) AND pressure* ) OR pascalisation* OR pascalization* ) ) AND ( TITLE‐ABS‐KEY ( barrier* OR contamin* OR diffusion* OR leech* OR ( ( mechanical OR physical ) W/3 propert* ) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer* ) ) AND ( TITLE‐ABS‐KEY ( beverage* drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant* ) ) | 2,297 |
| 3 | TITLE‐ABS‐KEY ( beverage* OR drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant* ) | 9,586,813 |
| 2 | TITLE‐ABS‐KEY ( barrier* OR contamin* OR diffusion* OR leech* OR ( ( mechanical OR physical ) W/3 propert* ) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer*) | 7,742,858 |
| 1 | TITLE‐ABS‐KEY ( bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR ( ( "high pressure*" OR hp OR "ultrahigh pressure*" OR uhp OR hpp OR hhp ) W/3 ( applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* ) ) OR ( ( hpp OR hhp ) AND pressure* ) OR pascalisation* OR pascalization* ) | 31,132 |
Table B.10.
Details of search strings used for literature searches in Web of ScienceTM Core Collection on potential chemical food safety risks in HPP‐treated food through food contact materials
| Set number | Search query | No of records |
|---|---|---|
| # 5 |
(#4) AND LANGUAGE: (English) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI, CCR‐EXPANDED, IC Timespan=1990–2020 |
1,336 |
| # 4 |
#3 AND #2 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI, CCR‐EXPANDED, IC Timespan=All years |
1,361 |
| # 3 |
TI=(beverage* OR drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant*) OR AB=(beverage* OR drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant*) OR AK=(beverage* OR drink OR drinks OR film OR films OR food OR foods OR packag* OR plastic OR plastics OR poly* OR simulant*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,923,348 |
| # 2 |
TI=(barrier* OR contamin* OR diffusion* OR leech* OR ((mechanical OR physical) NEAR/3 propert*) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer*) OR AB=(barrier* OR contamin* OR diffusion* OR leech* OR ((mechanical OR physical) NEAR/3 propert*) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer*) OR AK=(barrier* OR contamin* OR diffusion* OR leech* OR ((mechanical OR physical) NEAR/3 propert*) OR migrat* OR morpholog* OR scalp* OR sorpt* OR transfer*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI, CCR‐EXPANDED, IC Timespan=All years |
5,098,198 |
| # 1 |
TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat*) ) OR ((HPP OR HHP) AND pressure*) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat*) ) OR ((HPP OR HHP) AND pressure*) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat*) ) OR ((HPP OR HHP) AND pressure*) OR Pascalisation* OR Pascalization*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI, CCR‐EXPANDED, IC Timespan=All years |
20,805 |
After removing the duplicates, 2,127 records remained that were screened for title and abstract blind to eliminate the possibility of bias, i.e. without knowledge of the identity of the authors, the country in which the study was conducted, nor the journal and date in which the findings were published. They were classified according to study/information available (migration, NIAS, integrity, material, barrier, scalping, food properties, multiple).
One hundred and eighteen records were selected for full‐text evaluation to which one book and six opinions from AFSSA were added. They were assessed against criteria related to characteristics (relevance, material and methods, reliability) and study characteristics (HPP treatments of food contact materials (with or without packaged food (simulants)); migration; FCM physico‐chemical characteristics; reaction products; food and packaging). Nineteen additional records were added from that step. Additionally, a paper published in early 2021 that was discussed as a pre‐publication during the technical hearing held with the author (a representative of the FDA), was included.
Finally, 18 papers have been selected and reported in this scientific opinion to address the main question: ‘What is the effect of HPP treatment on the food packaging and ultimately on chemical migration to food’.
B.5 High pressure processing of milk or colostrum
A search was conducted on 9 October 2020 in the Web of ScienceTM Core Collection (1975–present) to retrieve information about the HPP of milk or colostrum. The search strategy is reported in Table B.11.
Table B.11.
Details of search strings used for literature searches on HPP of milk or colostrum using Web of ScienceTM Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*) ) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) | 21,262 ( a ) |
| #2 | TS = (milk OR colostrum) | 229,681 ( a ) |
| #3 | #1 AND #2 | 1,093 ( a ) |
DocType = All document types; Language = All languages; Timespan = All years.
B.6 Pathogen‐specific thermal inactivation parameters in milk/colostrum
Another search was conducted on 17 December 2020 in the Web of ScienceTM Core Collection (1975–present) to retrieve information from experimental studies reporting pathogen‐specific thermal inactivation parameters (DT‐ and zT‐values) or log10‐reductions in raw milk/colostrum. The search strategy is reported in Table B.12.
Table B.12.
Details of search strings used for literature searches on pathogen‐specific thermal inactivation parameters in raw milk or colostrum using Web of ScienceTM Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TS=("thermal inactivation" OR inactiv* OR reduction OR D‐value OR z‐value OR F‐value OR "heat inactivation" OR "thermal resistance" OR "heat resistance" OR "thermal death time" OR "decimal red*" OR "heat‐sensitivity" OR inactiv* OR survival OR pasteurisation OR pasteurization) | 3,998,502(a) |
| #2 | TS=("Campylobacter" OR "Salmonella" OR "Listeria monocytogenes" OR "St. aureus" OR "S. aureus" OR STEC OR EHEC OR “Escherichia coli” OR “E. coli”) |
664,218 ( a ) |
| #3 | TS=(Milk OR "Raw milk" OR colostrum) | 252,485 ( a ) |
| #4 | TS=(colostrum) | 10,499 ( a ) |
| #5 | TS=(Brucella) | 11,434 ( a ) |
| #6 | TS=("Tick‐borne encephalitis virus" OR encephalitis OR flavivirus) | 53,912 ( a ) |
| #7 | TS=(Mycobacterium) | 102,610 ( a ) |
| #8 | #1 AND #2 AND #4 | 144 ( a ) |
| #9 | #1 AND #3 AND #5 | 70 ( a ) |
| #10 | #1 AND #3 AND #6 | 44 ( a ) |
| #11 | #1 AND #3 AND #7 | 426 ( a ) |
DocType = All document types; Language = All languages; Timespan = All years.
B.7 Impact of HPP on L. monocytogenes and other relevant pathogens when applied to foods known to be associated with human listeriosis
Another search was conducted on 14 January 2021–2017 May 2021 to retrieve information on the impact of HPP on L. monocytogenes when applied to foods known to be associated with human listeriosis. The search strategy is reported in Table B.13.
Table B.13.
Details of search strings used for literature searches on HPP when applied to foods known to be associated with human listeriosis using Web of ScienceTM Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TI=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AB=(bridgmanis* OR bridgmaniz* OR "high isostatic pressure*" OR "high hydrostatic pressure*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*) ) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) OR AK=(bridgmanis* OR bridgmaniz* OR "high isostatic pressur*" OR "high hydrostatic pressur*" OR (("high pressure*" OR HP OR "ultrahigh pressure*" OR UHP OR HPP OR HHP) NEAR/3 (applicat* OR pasteur* OR preserv* OR process* OR sterilis* OR steriliz* OR technolog* OR technique* OR treat* OR inactivat*)) OR ((HPP OR HHP) AND pressure) OR Pascalisation* OR Pascalization*) | 22,181(a) |
| #2 | TS = ((ready‐to‐eat OR “ready to eat” OR "ready meal*" OR RTE OR cook* OR roast* OR chopp* OR roll* OR lunch OR luncheon) NEAR/2 (meat OR pork OR bovine OR beef OR chicken* OR turkey* OR lamb OR lambs OR poultry) OR sausage* OR pate OR pates OR “cold cut” OR “cold cuts” OR deli OR delis OR delicatessen* OR charcuterie OR salami* OR hotdog* OR “hot dog*” OR frankfurter* OR burger* OR hamburger* OR meatloaf OR mortadella OR bologna OR baloney OR boloney OR polony OR terrine* OR rillette* OR tongue OR jelly OR “meat roll” OR Rullepølse OR seafood OR seafoods OR “sea food” OR “sea foods” OR crustacean* OR shellfish OR mollusc* OR gravad OR fish* OR salmon OR cod OR hake OR seabream OR flatfish OR flounder OR herring OR sardine OR sturgeon OR mussel* OR crab* OR oyster* OR prawn* OR shrimp* OR lobster* OR “clam” OR “clams” OR “crayfish” OR scallop* OR trout OR trouts OR mackerel OR mackerels OR "gravad lax" OR gravlax OR sushi OR sashimi OR ceviche OR cheese* OR curd OR feta OR brie OR camembert OR “queso blanco” OR chevre OR “blue‐vein*” OR “mold ripe*” OR “mould ripe*” OR vacherin OR “danish blue” OR gorgonzola OR roquefort OR ricotta OR mozzarella OR “pasta filata” OR halloumi OR Neufchâtel OR havarti OR munster and “port salut” OR cottage OR panela OR white OR “queso fresco” OR “queso burgos” OR “queso de burgos” OR “fromage blanc” OR “fromage frais” OR chhena OR cas OR paneer OR chèvre OR “breingen‐tortoille” OR ricotta OR “Irish Mellieriem Rochers” OR “Belgian Mellieriem Rochers” OR “hispanico‐type” OR “hispanico type” OR “hispanico‐style” OR “hispanico style” OR “latin‐type” OR “latin type” OR “spanico style” OR “spanico‐style” OR “spanish style” OR “spanish‐style” OR “Mexican style” OR “Mexican‐style” OR “Mexican type” OR “Mexican‐type”) | 1,690,412(a) |
| #3 | TS = (Listeria OR L.monocytogenes OR Lmonocytogenes OR monocytogenes OR salmonella OR “S Enterica” OR “S Enteritidis” OR “S Typhimurium” OR “S Hadar” OR “S Virchow” OR “S Infantis” OR “S Derby” OR “S Stanley” OR “S Newport” OR “S Bovismorbificans” OR “S Agona” OR “S Kentucky” OR “S Java” OR “S Muenchen” OR “S Brandenburg” OR “S Saintpaul” OR “S Oranienberg” OR “S Thompson” OR “S Braenderup” OR “S Montevideo” OR “S Goldcoast” OR “S Napoli” OR “S Mbandaka” OR “S Livingstone” OR “S Senftenberg” OR “S Weltevreden” OR “S Heidelberg” OR “S Havana” OR “S Anatum” OR “S London” OR “S Blockley” OR “S Reading” OR “S Eastbourne” OR “S Anatum” OR S Bredeney” OR “S Panama” OR S Monophasic” OR “S Corvalis” OR “S Meleagridis” OR “S Rissen” OR “S Worthington” OR “S Johannesburg” OR “S Muenster” OR “Escherichia coli” OR “E. coli” OR “E coli” OR STEC OR VTEC OR EHEC OR EPEC OR ETEC OR EIEC OR EAEC OR EAggEC OR eagg OR DAEC OR AIEC) | 597,464 |
| #4 | #1 AND #2 AND #3 | 325(a) |
Appendix C – Tables of the uncertainty analysis
Appendix D – Additional information on inactivation of pathogens by thermal pasteurisation of raw milk and raw colostrum from ruminants
L. monocytogenes, Salmonella spp., S. aureus and STEC. The thermal inactivation parameters reported in Pearce et al. (2012) under commercial‐type thermal pasteurisation conditions were used for L. monocytogenes, Salmonella spp., S. aureus and STEC (using E. coli O157:H7). The study followed the recommendations related to the choice of strains, culture maintenance, inoculum preparation, heating apparatus and treatment, recovery methods and statistical analysis as made by the International Dairy Federation International Workshop on Heat Resistance. In addition, for each pathogen, the most heat‐resistant strains from 30 representative strains were identified using a turbulent‐flow, pilot‐plant scale pasteuriser. The results are shown in Table D.1.
Table D.1.
DT‐ and zT values for the most resistant pathogens of each species as estimated in Pearce et al. (2012) together with the temperatures of inactivation during the 15‐s treatment under commercial‐type pasteurisation conditions
| Pathogen | DT‐value (s)( a ) | zT‐value (°C) | Log10 reduction | Strain, equipment and experimental conditions |
|---|---|---|---|---|
| STEC( b ) |
D61; mean = 435, sd = 157 D62; mean = 132, sd = 37 D63; mean = 39, sd = 7 D64; mean = 16, sd = 2 |
3.00 | > 6.8 (65°C) |
Attenuated strain, Commercial‐type pasteurisation conditions |
| L. monocytogenes |
D61; mean = 146, sd = 34 D62; mean = 61, sd = 8 D63; mean = 28, sd = 5 D64; mean = 14, sd = 3 |
2.90 | > 6.9 (65.5°C) |
Strain NZRM 4237, Commercial‐type pasteurisation conditions |
| Salmonella spp.( c ) |
D57; mean = 292 sd = 83 D58; mean = 111, sd = 31 D59; mean = 34, sd = 2 D60; mean = 18, sd = 2 |
2.09 | > 6.9 (61.5°C) |
Strain NZRM 4220 Commercial‐type pasteurisation conditions |
| S. aureus |
D60; mean = 312, sd = 226 D61; mean = 86, sd = 16 D62; mean = 36, sd = 3 D63; mean = 22, sd = 3 D64; mean = 14, sd = 2 |
3.53 | > 6.7 (66.5°C) |
Strain S12, Commercial‐type pasteurisation conditions |
DT: decimal reduction time at a certain temperature; sd: standard deviation; Shiga toxin‐producing E. coli; zT: thermal resistance constant.
DT‐values reported are the effective decimal reduction time for the entire heat treatment.
Using E. coli O157:H42.
Using Salmonella enterica ser. Typhimurium.
For the remaining hazards in Table 3 (i.e. Brucella melitensis, Campylobacter spp., M. bovis and TBEV, ad‐hoc literature reviews were conducted and results are presented in the following paragraphs.
Brucella melitensis . Of the 70 papers intercepted by the literature review, only three (old) studies contained quantitative heat inactivation data, one additional study, Foster et al. (1953), was further intercepted by cross‐referencing (see Table D.2).
Table D.2.
Heat resistance parameters for Brucella species extracted from the literature review DT
| Reference | DT‐value (min) | zT‐value (°C) | Log10 reduction | Strain, equipment and experimental conditions |
|---|---|---|---|---|
| Park et al. (1932) | n.a. | n.a. | ≥ 3.7 log10 (inoculum) |
B. suis strain 1922 and 2872; cotton‐stopped tube in water bath at 60°C for 30 min |
| Park et al. (1932) | n.a. | n.a. | ≥ 3.7 log10 (inoculum) |
B. suis strain 1922 and 2872; cotton‐stopped tube in water bath at and 62°C for 30 min |
| Park et al. (1932) | n.a. | n.a. | Survivors detected but not quantified (inoculum = 7 log10 CFU/mL) |
B. suis strain 1922 and 2872; cotton‐stopped tube in water bath at and 62°C for 30 min |
| Park et al. (1932) | n.a. | n.a. | Survivors detected but not quantified (inoculum = 8.7 log10 CFU/mL) |
B. suis strain 1922 and 2872; cotton‐stopped tube in water bath at and 62°C for 30 min |
| Park et al. (1932) | n.a. | n.a. | ≥ 8.7 log10 (inoculum) |
B. suis strain 1922 and 2872; Hermetically sealed tube in water bath at 60, 61 and 62°C for 30 min |
| Foster et al. (1953) | n.a. | 5.4( a ) | ≥ 5.5 log10 (inoculum) |
B. abortus strain 2308; Fit‐for‐purpose apparatus; Complete inactivation at: 61.5°C for 23 min; 65.5°C for 4 min; 71.1°C for 21 s and 72°C for 4 s; |
| Kronenwett et al. (1954) | n.a. | 4.3( a ) | ≥ 8.3 log10 (inoculum) |
B. abortus strain 19; Fit‐for‐purpose apparatus; Complete inactivation at: 64°C for 32–34 s; 64.4°C for 32–34 s; 64.6°C for 26–29 s; 64.9°C for 24 s; 65.4°C for 18 s and 65.9°C for 12 s; |
| Kronenwett et al. (1954) | n.a. | 4.3( a ) | ≥ 8.3 log10 (inoculum) |
B. abortus strain 659; Fit‐for‐purpose apparatus; Complete inactivation at: 65.1°C for 35–38 s; 65.3°C for 26–30 s; 66°C for 18–31 s; 66.7°C for 14 s and 67°C for 13 s; |
| Kronenwett et al. (1954) | n.a. | 4.3( a ) | ≥ 4.5 log10 (inoculum) |
B. abortus strain 2016; Fit‐for‐purpose apparatus; Complete inactivation at: 64.3°C for 70–73 s; 65.5°C for 38–40 s and 66.6°C for 20–25 s. |
| Kronenwett et al. (1954) | n.a. | 4.7( a ) | ≥ 4.5 log10 (inoculum) |
B. abortus strain 2308; Fit‐for‐purpose apparatus; Complete inactivation at: 65.1°C for 95–100 s; 63.3°C for 33 s and 66.5°C for 3 s. |
| Kronenwett et al. (1954) | n.a. | ≥ 8 log10 (inoculum) |
B. abortus strain 2308; Fit‐for‐purpose apparatus; Complete inactivation at: 61.6°C for 150–180 s; 65°C for 28–30 s; 66.7°C for 16–18 s and 66.8°C for 10–12 s. |
|
| Kronenwett et al. (1954) | n.a. | 4.7( a ) | ≥ 8 log10 (inoculum) |
B. abortus strain 3237; Fit‐for‐purpose apparatus; Complete inactivation at: 63.4°C for 36–38 s; 64.5°C for 22–25 s; 66.5°C for 9 s and 64.8°C for 16–18 s. |
| Kronenwett et al. (1954) | n.a. | 4.3( a ) | ≥ 8 log10 (inoculum) |
B. abortus strain McComb; Fit‐for‐purpose apparatus; Complete inactivation at: 61.5°C for 240–270 s; 65°C for 32–34 s; 65.6°C for 24–26 s; 66°C for 22–24 s and 66.5°C for 12–14 s. |
| Kronenwett et al. (1954) | n.a. | 4.8( a ) | ≥ 8 log10 (inoculum) |
B. abortus strain Thompson; Fit‐for‐purpose apparatus; Complete inactivation at: 61.5°C for 150–180 s; 63.5°C for 74–79 s; 64.3°C for 53 s; 65.1°C for 35–37 s; 65.4°C for 26–29 s and 66.3°C for 20–22 s |
| Kronenwett et al. (1954) | n.a. | 4.8( a ) | ≥ 8 log10 (inoculum) |
B. abortus strain Woodward; Fit‐for‐purpose apparatus; Complete inactivation at: 66.6°C for 24–26 s; 67.2°C for 16–20 s and 67.8°C for 14–16 s |
| Davies and Casey (1973) | n.a. | n.a. | 3.3 log10 |
B. abortus strain 544; Water bath system; 72°C for 60 s |
| Davies and Casey (1973) | n.a. | n.a. | 5 log10 |
B. abortus strain 544; Water bath system; 65.5°C for 1 min |
| Davies and Casey (1973) | n.a. | n.a. | 0.7 log10 |
B. abortus strain 544; Water bath system; 60°C for 1 min |
| Davies and Casey (1973) | n.a. | n.a. | 0.7 log10 |
B. abortus strain 544; Water bath system; 54.5°C for 10 min |
| Davies and Casey (1973) | n.a. | n.a. | ≥ 6.6 log10 (inoculum) |
B. abortus strain 544; HTST simulator; 72°C for 5 s |
DT: decimal reduction time at a certain temperature; n.a.: not available, HTST: high temperature short time; zT: thermal resistance constant.
DT‐ and zT‐values together with the strain and equipment used to carry out the experiment are reported.
zT‐value estimated by the original authors from the thermal death time.
Park et al. (1932) used sterile whole and skimmed milk samples spiked with two B. suis strains heated in cotton‐stopped tubes and hermetically sealed glass tubes at temperatures ranging from 56.6°C to 62.2°C for 30 min. In skimmed milk samples contaminated with 3.7 log10 CFU/mL and heated in cotton‐stopped tubes at 60 and 62°C, no survivors were found. Survivors (unquantified) were detected in whole milk samples contaminated at 6 and 7 but not at 4.7 log10 CFU/mL. Whole milk samples containing 8 log10 CFU/mL and sealed in glass tubes yielded viable Brucella after being heated 2 and 5 min at 62°C but were negative after being heated 7 min. Viable organisms were detected from whole milk samples contaminated at 8.6 log10 CFU/mL and heated at 56.6°C and 60°C for 30 and 15 min, respectively, but not after 20 min at 60°C. Brucella remained viable for 10 and 5 min at 61°C and 62°C, but was non‐viable in 15 and 7 min at the same temperature.
Higher temperatures ranging from 61.1°C to 72°C were tested in Foster et al. (1953) using milk samples at a concentration of 5.5 log10 CFU/mL. At 61.5, 65.5, 71.1 and 72°C B. abortus was completely inactivated after 23 min, 4 min, 21 s and 14 s, respectively, when pre‐heating and cooling time were considered instantaneous. A zT‐value of 5.4°C was estimated for the tested temperature range.
Kronenwett et al. (1954) established thermal death time curves for eight B. abortus strains in milk at concentrations ranging from 4.5 to 8.3 log10 CFU/mL and at temperature range of 61.5–67.8°C using the same equipment as Foster et al. (1953). The estimated zT‐values for the tested strains showed minimal differences and ranged from 4.3°C to 4.8°C.
In Van den Heever et al. (1982), 5 liters of B. abortus naturally contaminated milk were treated using LTLT (63°C for 30 min in batch) and HTST (72°C for 15 s) pasteurisation. No evidence of Brucella infection (serological testing and culture of spleen and other organs) was observed in guinea pigs that were intramuscularly injected with 1 mL of mixed sediment and cream obtained from centrifugation of pasteurised milk. However, no information is given about the initial contamination level of the milk (supposed to be low) or methods for pasteurisation.
Davies and Casey (1973) explored survival of B. abortus in milk and skimmed milk after thermal treatment using conventional thin‐walled glass tubes suspended in hot‐water bath and batch heating system simulating the commercial HTST pasteuriser (Franklin, 1965). Milk contaminated with about 7 log10 CFU/mL showed 3.3 log10‐reductions after 60 s at 72°C in the water bath system and 5, 0.7 and 0.7 log10‐reductions when held for 1, 1 and 10 min at 65.5, 60 and 54.5°C, respectively. Notably, 10 cells/mL were detected in milk heated at 48.8°C for 1 h. When fresh milk containing 6.6 log10 CFU/mL was pasteurised using the HTST simulator, complete inactivation was observed after either 5, 10 and 15 s at 72°C.
Campylobacter spp. Of the 161 records extracted by systematic literature review, five explored thermal resistance of Campylobacter in milk. Literature data on DT‐ and zT‐values of Campylobacter are summarised in Table D.3.
Table D.3.
Heat resistance parameters for Campylobacter species extracted from the literature review. DT‐ and zT‐values together with the strain and equipment used to carry out the experiment are reported
| Reference | DT‐value (min) | zT‐value (°C) | Experimental conditions |
|---|---|---|---|
| Doyle and Roman (1981) |
D48 = 12.3 D50 = 5.4 D53 = 1.57 D55 = 0.74 |
5.7 |
C. jejuni, strain FRI‐CF3 Erlenmeyer flask placed in shaking water bath |
|
D48 = 7.2 D50 = 4.7 D53 = 1.83 D55 = 1 |
8.02 |
C. jejuni, strain FRI‐CF6 Erlenmeyer flask placed in shaking water bath |
|
|
D48 = 7.7 D50 = 3.5 D53 = 1.85 D55 = 0.93 |
7.99 |
C. jejuni, strain FRI‐CF8 Erlenmeyer flask placed in shaking water bath |
|
|
D48 = 12.8 D50 = 4.4 D53 = 1.56 D55 = 1 |
6.37 |
C. jejuni, strain FRI‐CF12 Erlenmeyer flask placed in shaking water bath |
|
|
D48 = 11.7 D50 = 5.1 D53 = 1.95 D55 = 0.86 |
6.31 |
C. jejuni, strain FRI‐CF16 Erlenmeyer flask placed in shaking water bath |
|
| Waterman (1982) |
D50 = 5.7 D51.5 = 3.9 D53.5 = 1 |
4.54 |
C. jejuni, strain 24791 Test‐tubes fitted with cotton wool plugs placed in water bath |
|
D49.5 = 15.8 D51.5 = 4.7 D53.5 = 2.2 |
4.67 |
C. jejuni, strain 16000 Test‐tubes fitted with cotton wool plugs placed in water bath |
|
|
D50 = 7.2 D51.5 = 1.8 D56 = 0.3 |
4.61 |
C. jejuni, strain 21033 Test‐tubes fitted with cotton wool plugs placed in water bath |
|
|
D52 = 5.2 D54.5 = 0.8 D56 = 0.9 |
4.90 |
Campylobacter, strain: n.a. 1725 Test‐tubes fitted with cotton wool plugs placed in water bath |
|
|
D50 = 7.3 D51.75 = 5.6 D53 = 1.3 D55 = 1.1 |
5.43 |
C. jejuni, strain 16509 Test‐tubes fitted with cotton wool plugs placed in water bath |
|
|
D50 = 36 D52 = 12.5 D54 = 0.7 D55 = 0.6 |
2.78 |
C. jejuni, strain 5388 Test‐tubes fitted with cotton wool plugs placed in water bath |
|
| D63 = 0.6 | n.a. | Estimated from joint regression except data for strain 5388. | |
| Christopher et al. (1982) | D50 = 3.7 | n.a. | C. intestinalis, strain A6622, sealed glass ampules immersed in shaking water bath |
| D50 = 1.7 | n.a. |
C. intestinalis, strain Par3, sealed glass ampules immersed in shaking water bath |
|
| D50 = 2 | n.a. |
C. intestinalis, strain 13524, sealed glass ampules immersed in shaking water bath |
|
| D50 = 1.1 | n.a. |
C. intestinalis, strain B8833, sealed glass ampules immersed in shaking water bath |
|
| D50 = 1 | n.a. |
C. intestinalis, strain ‘Pedro’, sealed glass ampules immersed in shaking water bath |
|
| D50 = 4.5 | n.a. |
C. jejuni, strain 18177, sealed glass ampules immersed in shaking water bath |
|
| D50 = 1.3 | n.a. |
C. jejuni, strain 29428, sealed glass ampules immersed in shaking water bath |
|
| D50 = 2.5( a ) | n.a. |
C. jejuni, strain SK557, sealed glass ampules immersed in shaking water bath |
|
| D50 = 1.8( a ) | n.a. |
C. jejuni, strain S7197, sealed glass ampules immersed in shaking water bath |
|
| D50 = 1.5( a ) | n.a. |
C. jejuni, strain Par6, sealed glass ampules immersed in shaking water bath |
|
| Sörqvist (2003) |
D55 = 50 (13–190) s D60 = 8.2 (2.1–32) s D65 = 1.3 (0.3–5.4) s D72 = 0.1 (0.1–0.5) s |
6.4 (5.8–7.0) |
DT‐values and 95% CI for a predicted individual value reported. zT‐values obtained using the slopes of thermal inactivation lines constructed in the study and their 95% confidence limits |
CI: confidence interval; DT: decimal reduction time at a certain temperature; zT: thermal resistance constant.
Calculated from digitalised graphs.
Gill et al. (1981) tested heat resistance of five Campylobacter strains (three C. jejuni and two C. fetus) under HTST pasteurisation (at laboratory‐scale). Raw milk samples were spiked with up to 6.3 log10 CFU/mL and no survivors were observed at 72°C for 15 s. Different T/t combinations (i.e. 72°C from 5 to 70 s, 60°C from 20 to 180 s and 50°C from 60 to 240 s) were used to test the heat resistance of the human enteropathogenic strain C. jejuni V212. Evidence of survival was reported qualitatively (growth/no growth by plating on blood agar and Skirrow's medium) for milk treated at 60°C and 50°C for 1 and 4 min, respectively.
Doyle and Roman (1981) examined the heat resistance of five C. jejuni strains by means of flask method at 48, 50, 53 and 55°C. The higher observed D55 was 1 min, estimated zT‐values ranged between 5.7 and 8.02°C (Table D.3). Similar results are reported in Waterman (1982) who explored heat resistance of six C. jejuni strains over 49.5–56°C. The DT‐values calculated at the upper temperature range were D53.3 = 1 min (strain 24,791), D53.3 = 2.2 min (strain 16,000), D56 = 0.3 min (strain 21,033), D56 = 0.9 min (strain 17,259), D55 = 1.1 min (strain 16,509) and D55.5 = 1.1 min (strain 5388). The D63 was estimated based on extrapolation from pooled data as 0.6 s.
Christopher et al. (1982) examined the thermal resistance of 10 Campylobacter strains (five C. jejuni and five C. intestinalis) in skimmed milk and reported D50 ranging from 1.3 to 4.5 and from 1.0 to 3.7 for C. jejuni and C. intestinalis, respectively. No bacteria were recovered from skimmed milk samples contaminated with 105–106 and 105–107 CFU/mL and heated at 55 and 60°C for 1 min, respectively.
D’Aoust et al. (1988) tested the heat resistance of Campylobacter (cocktail of 4 C. fetus, 3 C. coli and 8 C. jejuni of various source and animal species) in whole milk by means of HTST pasteurisation at 60–72°C. Starting with an inoculum of 5.08, 5.36 and 5.40 log10 CFU/mL per trial, survivors (i.e. 1.04, 1.38 and 1.38 log10 CFU/mL resp.) were observed only in milk treated for 16.2 s (minimum holding time) at 60°C. No survivors were detected at 63, 66 and 72°C suggesting that at least 5 log10‐reductions are achieved at these temperatures.
A literature review on heat resistance of different food‐borne pathogens including Campylobacter in liquids was published by Sörqvist (2003). Although the authors grouped several experiments on different matrices (e.g. scalding water in pig slaughterhouse) the estimated mean DT and zT‐values are consistent with those described for milk so far.
From the available evidence, LTLT and HTST thermal pasteurisation should be well effective for complete elimination of Campylobacter.
Mycobacterium bovis
With no data being available for the estimation of the log10 reductions of M. bovis in milk by means of HPP, scientific evidence for MAP was considered adequate to serve as surrogate for M. bovis for the scope of this scientific opinion. Early experiments have identified MAP as more thermal‐resistant than M. bovis, and therefore, results provided for MAP should be considered as conservative estimates (worst‐case scenario) if extended to M. bovis. Availability of reduction data for MAP in HPP treated milk allowed comparative assessment of the risk of human exposure for Mycobacteria; this would not have been possible if relying on evidence for M. bovis. Literature on heat inactivation of MAP until 2002 has been critically reviewed in Lund et al. (2002) and the studies mentioned in that review were summarised (Table D.4) and considered as the baseline reference.
Table D.4.
Heat inactivation of MAP extracted from Lund et al. (2002)
| Reference | Heat treatment | |||||
|---|---|---|---|---|---|---|
| 63°C for 30 min | 75°C for 15 s | |||||
| Inoculum (log10) |
Reductions (log10) |
Experimental conditions | Inoculum (log10) |
Reductions (log10) |
Experimental conditions | |
| Chiodini and Hermon‐Taylor (1993) | 4 | < 2 | Water bath | 4 | < 2 | Double boiler |
| Grant et al. (1996) |
6–7 3–4 |
5–6 2–3.7 |
Tubes in water bath |
6–7 3–4 |
4.3–6 2–3.7 |
Batch pasteuriser unit |
| Grant et al. (1999) | 5–6 | 5.6–6 | Batch pasteuriser unit | |||
| Hope et al. (1996) | < 5 | ~5 | Small‐scale continuous flow pasteuriser | |||
| Stabel et al. (1997) | 6–7 | 0.5–3 | Small‐scale flow‐through pasteuriser, laminar flow | |||
| Sung and Collins (1998) | 5–6 | > 6 | Vials in water bath | 5–6 | 1–2 | Vials in water bath |
| Keswani and Frank (1998) | 6–7 | > 10 | Tubes in water bath | 5 | ~ 4 | Tubes in water bath |
Literature post‐2001 (included) was systematically reviewed to gather thermal inactivation data on Mycobacterium spp. in milk resulted in 11 studies considered as eligible and summarised in the following paragraphs. Literature data on DT‐ and ZT‐values for MAP (used as surrogate for M. bovis) are summarised in Table D.5.
Table D.5.
Heat resistance parameters for Mycobacterium avium subsp. paratuberculosis (MAP) species extracted from the literature review DT
| Reference | DT‐value (s) | ZT‐value (°C) | Experimental conditions |
|---|---|---|---|
| Pearce et al. (2001) |
D63 = 15 (mean) D66= 5.9 (mean) D72 < 2.03 (extrapolated) |
8.6 |
MAP strain: ATCC 19698, ATCC 43015, 3 bovine isolates. Pilot‐scale pasteuriser operating under validated turbulent flow |
| Stabel and Lambertz (2004) | D63 = 230 (mean) | n.a. |
MAP strains 167, 5007 and 6112. Slug‐flow pasteuriser |
| D72 = 1.83 (mean) | n.a. |
MAP strains 167, 5007 and 6112. Laboratory‐scale HTST pasteuriser |
|
| Rademaker et al. (2007) | D72 = 1.2 (mean) | 7.7 |
MAP strain unknown. Pilot‐scale turbulent‐flow pasteuriser plant with homogenisation |
| Lynch et al. (2007) | D65 = 20 | n.a. |
MAP strain 19698. Pilot‐scale pasteuriser plant with homogeniser unit; turbulent flow |
| Foddai et al. (2010) |
D63 = 78.8 (mean) D68 = 10.2 (mean) D72 = 4.4 (mean) |
7.1 |
MAP strains NCTC 8578, ATCC 19698, 806R, 796PSS. CFU method; milk dispersed into strips PCR tubes and treated using thermocycler |
|
D63 = 81.8 (mean) D68 = 9.8 (mean) D72 = 4.2 (mean) |
6.9 |
MAP strains NCTC 8578, ATCC 19698, 806R, 796PSS. Phage method; milk dispersed into strips PCR tubes and treated using thermocycler |
|
| Van Brandt et al. (2011) | D60 = 205.1 | 5.7 | MAP strain ‘Niebüll’, capillary glass tubes submerged in a water |
|
D60 = 195 (mean) D72 = 2 (extrapolated) |
6.1 | MAP strain NIZO B 2962, capillary glass tubes submerged in a water |
DT: decimal reduction time at a certain temperature; HTST: high temperature short time; MAP: Mycobacterium avium subsp. paratuberculosis; zT: thermal resistance constant.
DT‐ and zT‐values together with the strain and equipment used to carry out the experiment are reported.
Pearce et al. (2001) evaluated the thermal resistance in raw milk by means of a pilot‐scale pasteuriser operating under validated turbulent flow. Prior to the pasteurisation experiments, the level of MAP contamination in raw milk samples ranged from 2.8 to 4.2 log10 CFU/mL. Experiments were conducted using five different strains (one reference strain, one human strain and three bovine strains). No strains survived at 72°C for 15 s and only one (human isolate) strain survived at 69°C. Means of pooled D63 and D66 were 15.0 and 5.9 s, respectively. According to the authors, the absence of data points at 69 and 72°C indicates that the kill exceeded the 4 log10 to 5 log10 detection limit. The mean extrapolated D72 for the five strains of MAP examined was 2.03 s, representing 7 log10 kill at the 95% CI.
Grant et al. (2002) treated naturally contaminated raw milk with commercial‐scale HTST pasteuriser and isolated MAP from milk processed at 73°C for both 15 and 25 s, either with or without pre‐homogenisation. Even if the results were expressed as positive/negative only, the majority of the positive pasteurised samples were obtained during a particular period of the study where MAP in the source raw milk was detected frequently and with high abundance. In addition, although not statistically significant, lower number of survivors were consistently observed in treatments including homogenisation. This made the authors to hypothesise that (i) the actual number of MAP cells present in milk before pasteurisation plays an important role in terms of potential survival and (ii) homogenisation of the milk might result in declumping of MAP cells that would be more easily killed during heating.
Using artificially contaminated raw milk samples in tubes submerged in water, Gao et al. (2002) mimicked LTLT (63°C for 30 min, batch) and HTST (73°C for 15 s) pasteurisation with 11 replicated experiments. Survivors were observed only in one replicate of the set of samples contaminated with 5 log10 units and in one replicate of the set of samples contaminated with 7 log10 units survivors were enumerated resulting in 11 CFU on average of five slants. These results seem to support the hypothesis that MAP could survive HTST if present > 5 log10 units.
No MAP survivors after HTST pasteurisation at 71.7°C for 15 s were detected in the experiment described in Stabel et al. (2004) using commercial on‐farm pasteuriser and raw milk samples artificially contaminated up to 5.8 and 6.8 log10 units.
Stabel and Lambertz (2004) investigated the effectiveness of LTLT and HTST pasteurisation using a slug‐flow pasteuriser and a laboratory scale HTST equipment. Experiments were run inoculating three different MAP strains in UHT milk at 5 and 8 log10 units and processing the milk at five T/t combinations (62.7°C for 30 min, 65.5°C for 16 s, 71.7°C for 15 s, 71.7°C for 20 s and 74.4°C for 15 s) in triplicate, for a total of one hundred eighty experiments. Fewer survivors were observed in HTST as compared to LTLT treated milk and fewer survivors were detected in milk samples spiked with low MAP concentrations. On the other hand, significant number of survivors was found regardless strain, means of pasteurisation and inoculum level for subpasteurisation treatments at 65.5°C for 16 s. From the experiments using milk inoculated at high concentrations and across strains, the D72 was estimated as 1.83 s (HTST) and the D63‐value as 240 s (LTST).
Using a pasteuriser with comparable characteristics to full‐scale commercial pasteuriser, Grant et al. (2005) evaluated various T/t conditions in combination with homogenisation for inactivation of MAP. Raw milk samples were spiked with up to 5 log10 units of bacteria and survivors were detected in 27 out of 816 samples; five on Herrold’s egg yolk medium and 22 by BACTEC culture. Incorporation of homogenisation resulted in a significantly lower number of positive samples as compared to samples treated with pasteurisation when the holding time at temperatures of 72.5–78.5°C was 25 s. At the same temperatures but holding time of 15 s, no differences were observed; similarly, no differences were observed between holding times of 15 and 25 s when homogenisation was not applied.
McDonald et al. (2005) estimated a > 6 log10 reduction for 17 out of 20 artificially contaminated batches of milk subjected to homogenisation and pasteurisation using a commercial‐scale pasteuriser. Three trials (72°C for 15 s, 75°C for 25 s and 78°C for 15 s) showed evidence of survivors at concentrations from 0.004 to 0.002 CFU/mL in different trials corresponding to from 4 to 6 log10 reductions.
Rademaker et al. (2007) used a pilot plant scale pasteuriser to simulate HTST pasteurisation. Twenty‐two T/t combinations were evaluated, including 60–90°C at holding times of 6–15 s. Following treatments at 72°C for 6 s, 70°C for 10 and 15 s or more stringent conditions, no viable MAP cells were detected, resulting in 2–5 log10 reductions, depending on the original inoculum. Homogenisation of raw milk was found not to be significant in relation to MAP inactivation and kinetic modelling of quantitative data resulted in a corresponding D72‐value of 1.2 s and a zT‐value of 7.7°C.
Lynch et al. (2007) evaluated heat sensitivity of three MAP strains inoculated in raw milk at concentrations ranging from 102 to 105 CFU/mL and treated at 72.5°C for 27 s. None of the 16 trials using a pilot‐scale pasteurisation showed evidence of survivors regardless homogenisation. Partial kinetic inactivation data were obtained at 65°C where measurable heat inactivation data could be established. This resulted in a D65‐value of 20 s.
Foddai et al. (2010) performed thermal inactivation experiments using UHT milk contaminated with four MAP strains at levels of 106 to 107 CFU/mL. Spiked milk was dispersed into strips of PCR tubes and treated using thermal cycler. Temperature–time combinations were: 63°C for 3, 6 and 9 min; 68°C for 20, 40 and 60 s; and 72°C for 5, 10 and 15 s. The authors emphasised that the experiments run on the laboratory‐scale HTST pasteuriser were not specifically designed to evaluate heat resistance of MAP under commercial continuous‐flow HTST pasteurisation but rather to validate the use of optimised phage amplification bioassay for rapid enumeration of MAP after heat treatment. Mean D63, D68, and D72‐values based on the results produced using the optimised phage assay were 81.8, 9.8, and 4.2 s, respectively, resulting in a mean zT‐value of 6.9°C. When using culture method corresponding DT (D63 = 78.8 s, D68 = 10.2 s and D72 = 4.4 s) and ZT (7.1°C) values did not show significant differences. However, incomplete inactivation was observed under all the T/t combinations and MAP strains with thermal inactivation curves shown significant tailing. Therefore, DT‐ and zT‐values calculated from the slopes of linear regression lines should be considered carefully.
Van Brandt et al. (2011) investigated intrinsic heat characteristics of MAP expressed as kinetic parameters by inoculating milk samples with two MAP strains at levels ranging from 5 to 6 log10 CFU/mL for strain NIZO B 2962 and from 4 to 6 log10 CFU/mL for strain ‘Niebüll’. Milk samples were treated at different T/t combinations (1–35 min at 55–65°C) by means of capillary glass tubes submerged in a water bath. Data from three replicates resulted in D60‐values ranging from 114.3 to 244.5 s and zT‐values ranging from 4.2 to 6.8°C. A D72 of 2.0 ± 0.3 s was also estimated by extrapolation.
Tick‐borne encephalitis virus
Heat resistance of TBEV was explored in two studies summarised in Table D.6.
Table D.6.
Heat resistance parameters for Tick‐borne encephalitis virus extracted from the literature review DT
| Reference | DT‐value (s) | zT‐value (°C) | Log10 reduction | Experimental conditions |
|---|---|---|---|---|
| Saier et al. (2015) | D70 = 13 | 9.8 |
~2 (65°C x 55 s) ~2 (75°C x 9 s) |
PCR tubes in thermal cycler |
| Offerdahl et al. (2016) | n.a. | n.a. | 6 (72°C x 15 s) |
LGTV virus strain TP21, PCR tubes in thermal cycler |
DT: decimal reduction time at a certain temperature; n.a.: not applicable; PCR: polymerase chain reaction; zT: thermal resistance constant.
DT‐ and zT‐values together with the strain and equipment used to carry out the experiment are reported.
Saier et al. (2015) explored the thermal inactivation of TBEV in raw milk spiked with 7.6 TCID50/mL using a thermocycler at 65, 70, 75 and 80°C with holding times up to 350 s. The machine required 20 and 30 s, respectively, to stabilise at 65–70 and 75–80°C; but for the calculation of the kinetic data, the virus concentrations at time 0 were considered as the initial concentration. During the heating up to the target temperature, a loss of infectivity was found of about 2–3 log10 units. A holding time of 55 s at 65°C led to a further 2 log10 units decrease. Similarly, a 2 log10 reduction was observed for 9 s of holding time at 75°C; 3 log10 reductions were achieved after 350 s at 65°C. The authors estimated that a 3 log10 reduction can be achieved by either 4 s at 80°C or 12 s at 72°C.
Offerdahl et al. (2016) evaluated the thermal resistance of the naturally attenuated Langat virus (LGTV, a flavivirus that can used as a model of the more virulent tick‐borne encephalitis virus) in milk (6 log10 FFU/mL) when subjected to HTST pasteurisation using a thermocycler. No virus could be detected following this treatment.
Informed by the thermal inactivation parameters, pathogen reduction by thermal pasteurisation of raw milk/colostrum from ruminants has been estimated considering the minimum legal requirements of a HTST or LTLT thermal pasteurisation.
L. monocytogenes, Salmonella spp., S. aureus and STEC. These pathogens tested in Pearce et al. (2012) reached the maximum measurable level of inactivation of ~7 log10 reductions at temperatures ranging from 61.5 to 66.5°C. From the derived DT‐and zT‐values, it is evident that extrapolation using longer exposure times at the same temperature or using higher temperatures for the same exposure time would lead to substantially higher estimates of the log reductions. The best‐fit calculation for 2°C above the last measured temperature led to up to 12, 13.3 and 18 log10 reductions of S. aureus, pathogenic E. coli O157:H42 and L. monocytogenes, respectively, at 66°C and up to 29.4 log10 reductions for S. Typhimurium. The inactivation achieved for these pathogens by HTST pasteurisation seems to be too high to be estimated with any degree of certainty.
Brucella melitensis . The literature dealing with thermal resistance of Brucella in milk is old and the laboratory equipment used to run the experiments and evaluate the thermal resistance of Brucella in milk mainly consisted in tubes in water bath (Park et al., 1932, Davies and Casey, 1973), elaborated laboratory implants (Foster et al., 1953; Kronenwett et al., 1954), unspecified apparatus (Van den Heever et al., 1982) or Franklin HTST pasteuriser (Davies and Casey, 1973). None of them deemed adequate to reproduce the thermal inactivation achieved by commercial pasteurisation. However, from consideration of empirical reduction data of milk subjected to LTLT and HTST pasteurisation conditions, at least 8.6 (Park et al., 1932) and 6.6 (Davies and Casey, 1973) log10 reductions resp. can be expected.
Campylobacter spp. As for Brucella spp., none of the studies exploring thermal inactivation of Campylobacter spp. in milk could be considered as adequately representative of commercial pasteurisation conditions. However, in spite of the methodological limitations related to the equipment used in the different studies, Campylobacter spp. appears to be extremely vulnerable even to mild heat treatments. From reported DT‐ and zT‐values both LTLT and HTST pasteurisation should be effective for the elimination of even high Campylobacter levels in milk.
Mycobacterium bovis. Data for the estimation of the log10 reductions of M. bovis in milk by means of HPP were not available. Early experiments have identified MAP as more thermal‐resistant than M. bovis, and therefore, results provided for MAP should be considered as conservative estimates (worst scenario) if extended to M. bovis. In the studies identified by the literature review, attempts were made to reproduce the features of the commercial pasteurisation process. However, the methods and equipment available varied between studies, these ranged from test tubes or capillary tubes completely or partially submerged in water baths to laboratory‐scale and pilot‐scale pasteurisers with or without turbulent flow. In addition, MAP has the tendency to form clumps, which undoubtedly adds to the challenge of obtaining accurate enumeration of viable organisms and the heterogeneity observed in available studies with or without turbulent flow. Hence, not surprisingly, results showed marked variations.
When considering studies reporting kinetic data and performed under similar conditions of commercial pasteurisation including turbulent flow, Pearce et al. (2001) extrapolated a D72 of 2.03 s and a zT‐value of 8.6°C; Stabel and Lambertz (2004) estimated a D72 of 1.83 s while a D72 and zT‐value of 1.2 s and 7.7°C, respectively, were reported in Rademaker et al. (2007). Even considering the most conservative estimate of Pearce et al. (2001), pasteurisation at 72°C for 15 s under commercial conditions should result in 7.4 log10 reductions.
Tick‐borne encephalitis virus (TBEV) . The literature presenting inactivation parameters or log10 reduction of TBEV was limited to two studies. From reported data, it appears that heat resistance largely depends on the strain being tested. If considering only the evidence related to the not‐attenuated strain used in Saier et al. (2015), it seems that HTST pasteurisation conditions are not sufficient to guarantee even a 3 log10 reduction of the virus in milk.
Appendix E – Additional information on appropriate indicators to verify the efficacy of HPP of raw milk or raw colostrum
Table E.2. Information extracted from the records retrieved concerning whey proteins to verify the efficacy of HPP on raw milk or raw colostrum according to the criteria described in Section 2.3.2
| Compounds | Effect of HPP | Type of milk/colostrum | Stability after the HPP treatment | Effect compared/validated with target pathogens | Methods available to use | References |
|---|---|---|---|---|---|---|
| Whey proteins | ||||||
| β‐lactoglobulin (β‐Lg) |
β‐Lg Is the most sensitive whey protein to HPP. Denaturation increased with pressure and longer holding times. Some authors reported denaturation levels above 90% at 600 MPa (Table 6) |
Most surveys done with bovine milk, two with goats’ milk (2, 3), one with ovine milk (15) and one with buffalo milk (12). No great differences were reported, although buffalo milk β‐Lg (12) seems more sensitive to HPP than others | No data available | 600 MPa for 15 min treatment denatures about 80% of the β‐Lg and reduces at least 7 log10 cycles of L. monocytogenes, S. aureus and Pseudomonas aeruginosa |
FPLC (2, 3, 10) RP‐HPLC (1, 4, 5, 6, 7, 8, 9, 10, 12, 13, 14, 15,16, 21) SDS‐PAGE (2, 10, 15, 22), SEC (16) CE (17, 19, 23) RID (18, 20) LC‐MS Q‐ToF (24) |
(1) López‐Fandiño et al. (1996); (2) Felipe et al. (1997); (3) Law et al. (1998); (4) Lopez‐Fandiño and Olano (1998); (5) Garcia‐Risco et al. (2000); (6) Scollard et al. (2000a); (7) Huppertz et al. (2004a); (8) Huppertz et al. (2004b); (9) Kiełczewska et al. (2004); (10) Nabhan et al. (2004); (11) Ye et al. (2004); (12) Huppertz et al. (2005); (13) Zobrist et al. (2005); (14) Kleber et al. (2007); (15) Moatsou et al. (2008a); (16) Moatsou et al. (2008b); (17) Bravo et al. (2012); (18) Mazri et al. (2012a); (19) Bravo et al. (2013); (20) Ramos et al. (2015); (21) Leu et al. (2017); (22) Bogahawaththa et al. (2018); (23) Omar et al. (2018); (24) Liu et al. (2020) |
| Α‐lactalbumin (α‐La) | α‐La is resistant to up to 400 MPa at room temperature. Maximum denaturation level described were 42% (5) in bovine milk and 52.6% in buffalo milk (11) after 30 min treatments at 600 MPa at 20°C (Table 7) |
Most surveys done with bovine milk, two with goats’ milk (1,2), one with ovine milk (10), one with buffalo milk (9) and one with camel milk (18). Little interspecies differences were reported. |
No data available | No data available |
SDS‐PAGE (1, 7, 17) SEC (17) RP‐HPLC (3, 4, 5, 6, 9, 10, 11) CE (12, 14, 18) FPLC (1, 2, 7) RID (13, 15) LC‐MS Q‐ToF (19) |
(1) Felipe et al. (1997); (2) Law et al. (1998); (3) Lopez‐Fandiño and Olano (1998); (4) Garcia‐Risco et al. (2000); (5) Huppertz et al. (2004b); (6) Kiełczewska et al. (2004); (7) Nabhan et al. (2004); (8) Ye et al. (2004); (9) Huppertz et al. (2005); (10) Moatsou et al. (2008a); (11) Moatsou et al. (2008b); (12) Bravo et al. (2012); (13) Mazri et al. (2012a); (14) Bravo et al. (2013); (15) Ramos et al. (2015); (16) Leu et al. (2017); (17) Bogahawaththa et al. (2018); (18) Omar et al. (2018); (19) Liu et al. (2020) |
| Immunoglobulins (Ig) |
IgA seems more resistant to denaturation than IgG and IgM Maximum denaturation described after 30 min HPP at 600 MPa and 20°C was 60% for IgA in human colostrum (Table 8) |
Two surveys included data from goats’ milk (1,2) and four of human’s milk (5, 6, 7, 11). No differences were described. One survey included data from bovine colostrum (10), one from caprine colostrum (4) and one from human colostrum (8) |
No data available | No data available |
ELISA (4,6,8, 11,12) FPLC (1, 2) SDS‐PAGE (1) RP‐HPLC (3) RID (4, 9, 10) |
(1) Felipe et al. (1997); (2) Law et al. (1998); (3) Kiełczewska et al. (2004); (4) Trujillo et al. (2007); (5) Viazis et al. (2007); (6) Contador et al. (2013); (7) Delgado et al. (2013); (8) Sousa et al. (2014); (9) Ramos et al. (2015); (10) Foster et al. (2016); (11) Mayayo et al. (2016); (12) Bogahawaththa et al. (2018) |
| Lactoferrin |
Dp‐values described in literature: 201 min at 450 MPa; 34.47 min at 600 MPa and 11.37 min at 700 MPa Maximum reported denaturation of 70% after 600 MPa for 15 min (3) (Table 9) |
Most surveys done with bovine milk, one survey reported data on human milk (2) and another on camel milk (5). | No data available | No data available |
ELISA (1, 2, 3, 4) CE (5) |
(1) Mazri et al. (2012b); (2) Mayayo et al. (2014); (3) Ramos et al. (2015); (4) Bogahawaththa et al. (2018); (5) Omar et al. (2018) |
CE: capillary electrophoresis; ELISA: enzyme‐linked immunosorbent assay; FPLC: Fast protein liquid chromatography; LC‐MS: liquid chromatography–mass spectrometry; RID: radial immunodiffusion; RP‐HPLC: Reversed‐phase high‐pressure liquid chromatography; SDS‐PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis; SEC: size‐exclusion chromatography.
Appendix F – Tables supporting the food vehicles associated with L. monocytogenes and the other pathogens in foods known to be associated with human listeriosis and are relevant for HPP
Annex A – Protocol for the assessment of the efficacy and safety of high‐pressure processing of food
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7128
Annex B – Detailed tables of type of foods HPP treated and the processing conditions as informed by the establishments and equipment providers through the questionnaire
Annex B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7128
Supporting information
Protocol for the assessment of the efficacy and safety of high‐pressure processing of food
Detailed tables of type of foods HPP treated and the processing conditions as informed by the establishments and equipment providers through the questionnaire
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Castle L, Crotta M, Grob K, Milana MR, Petersen A, Roig Sagués AX, Vinagre Silva F, Barthélémy E, Christodoulidou A, Messens W and Allende A, 2022. Scientific Opinion on the efficacy and safety of high‐pressure processing of food. EFSA Journal 2022;20(3):7128, 195 pp. 10.2903/j.efsa.2022.7128
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00380
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to thank the following for the support provided to this scientific output: Chris Michiels (WG member until 22 January 2021) and Nikolaos Giannoulis and Carina Wenger (EFSA trainees). The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output. In addition, the BIOHAZ Panel wishes to thank the members of the Panel on Contaminants in the Food Chain (CONTAM Panel): Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace and the members of the Panel on Food Contact Materials, Enzymes and Processing Aids (CEP Panel): José Manuel Barat Baviera, Claudia Bolognesi, Andrew Chesson, Pier Sandro Cocconcelli, Riccardo Crebelli, David Michael Gott, Konrad Grob, Claude Lambré, Evgenia Lampi, Marcel Mengelers, Alicja Mortensen, Gilles Rivière, Vittorio Silano (until 21 December 2020†), Inger‐Lise Steffensen, Christina Tlustos, Henk Van Loveren, Laurence Vernis and Holger Zorn for the preparatory work on this scientific opinion.
†Deceased
Adopted: 26 January 2022
Note: Data used for modelling are available on the EFSA Knowledge Junction at: https://doi.org/10.5281/zenodo.5998538
Notes
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJ L 139, 30.4.2004, p. 1–54.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, p. 55–205.
EFSA Journal 2018;16(1):5134.
EFSA Journal 2018;16(12):5500.
The scientific opinion has been adopted by the BIOHAZ Panel on 19 March 2021 and is available at https://www.efsa.europa.eu/en/efsajournal/pub/6092
According to the implementation plan from 2018 of the uncertainty guidance document uncertainty analysis shall first be applied in the general scientific areas non‐regulated areas before being rolled out into the regulated products area (https://www.efsa.europa.eu/en/topics/topic/uncertainty-scientific-assessments).
Commission Regulation (EU) 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 12, 15.1.2011, p. 51–89.
“For food categories where in sub‐column D2 … the cross is followed by an oblique stroke and a figure, the migration test result shall be corrected by dividing the result by this figure. The corrected test result shall then be compared to the migration limit to establish compliance.”
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJL 139, 30.4.2004, p. 1–54.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) 2074/2005 as regards official controls. OJ L 131, 17.5.2019, p. 51.
ISO 11816‐1 [IDF 155‐1:2013]. Milk and milk products – Determination of alkaline phosphatase activity – Part 1: Fluorimetric method for milk and milk‐based drinks. International Organization for Standardization, Geneva, Switzerland.
https://efsa.onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1448 and https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2018.EN-1402.
https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2014.EN-592 and https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2012.2893.
https://registerofquestions.efsa.europa.eu/roqFrontend/questionLoader?question=EFSA-Q-2020-00380. The current link is https://open.efsa.europa.eu/questions/EFSA-Q-2020-00380.0
Service providers refers to establishments which do not produce food but have the HPP equipment and carry out the HPP (on pre‐packaged products) for food establishments (i.e. “tolling”)
For more information, the Codex Alimentarius Guideline for the Validation of Food Safety Control Measures (CXG 69‐2008) can be accessed at https://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/en/
Source: Journal of Applied Packaging Research, Flexible Packaging for High Pressure Treatments: Delamination Onset and Design Criteria of Multilayer Structures Page 56,58.; Source: J. Götz, H. Weisser Food Science & Emerging Technologies 3 (2002) 25_31; Source: Innovative Food Science & Emerging Technologies 3 Ž2002. 25_31 Permeation of aroma compounds through plastic films under high pressure: in‐situ measuring method.; Source Polymeric‐Based Food Packaging for High‐Pressure Processing Article in Food Engineering Reviews · December 2010 Received: 20 June 2010/Accepted: 16 July 2010 / Published online: 10 August 2010 Springer Science + Business Media, LLC 2010 Page 292.; Source: Elsevier journal homepage: www.elsevier.com/locate/foodres Food Research; International Effect of high‐pressure processing on characteristics of flexible packaging for foods and beverages Page 925.
References
- Abera G, 2019. Review on high‐pressure processing of foods. Cogent Food & Agriculture, 5, 1568725. 10.1080/23311932.2019.1568725 [DOI] [Google Scholar]
- AESAN (Agencia Española de Seguridad Alimentaria y Nutrición) , 2004. Opinión del Comité científico de la AESA sobre una cuestión presentada por la Dirección Ejecutiva, en relación con la aplicación de altas presiones en carne y productos cárnicos (Ref. AESA‐2003‐007). 36 pp. Revista Del Comité Científico, 1. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_comite/ALTAS_PRESIONES_CARNE.pdf pp. 36–76.
- AFSSA (Agence Française de sécurité des produits alimentaires) , 2007. Avis de l’Agence française de sécurité sanitaire des aliments (Afssa) relatif à l’autorisation de mise sur le marché de magrets de canards séchés, ou séchés et fumés, stabilisés par hautes pressions hydrostatiques comme nouvel aliment dans le cadre du règlement (CE) n°258/97. Saisine n° 2007‐SA‐0164. Available online: https://www.anses.fr/en/system/files/AAAT2007sa0164.pdf
- Aganovic K, Hertel C, Vogel RF, Johne R, Schluter O, Schwarzenbolz U, Jager H, Holzhauser T, Bergmair J, Roth A, Sevenich R, Bandick N, Kulling SE, Knorr D, Engel KH and Heinz V, 2021. Aspects of high hydrostatic pressure food processing: perspectives on technology and food safety. Comprehensive Reviews in Food Science and Food Safety, 20, 3225–3266. 10.1111/1541-4337.12763 [DOI] [PubMed] [Google Scholar]
- Allison A, Chowdhury S and Fouladkhah A, 2018. Synergism of mild heat and high‐pressure pasteurization against Listeria monocytogenes and natural microflora in phosphate‐buffered saline and raw milk. Microorganisms, 6. 10.3390/microorganisms6040102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Nehlawi A, Guri S, Guamis B and Saldo J, 2014. Synergistic effect of carbon dioxide atmospheres and high hydrostatic pressure to reduce spoilage bacteria on poultry sausages. Lwt‐Food Science and Technology, 58, 404–411. 10.1016/j.lwt.2014.03.041 [DOI] [Google Scholar]
- Alpas H, Kalchayanand N, Bozoglu F and Ray B, 2000. Interactions of high hydrostatic pressure, pressurization temperature and pH on death and injury of pressure‐resistant and pressure‐sensitive strains of foodborne pathogens. International Journal of Food Microbiology, 60, 33–42. 10.1016/s0168-1605(00)00324-x [DOI] [PubMed] [Google Scholar]
- Amanatidou A, Schlüter O, Lemkau K, Smid E and Knorr D, 2000a. Effect of combined application of high pressure treatment and modified atmospheres on the shelf‐life of fresh Atlantic salmon. Innovative Food Science & Emerging Technologies, 1, 87–98. 10.1016/S1466-8564(00)00007-2 [DOI] [Google Scholar]
- Amanatidou A, Slump RA, Gorris LGM and Smid EJ, 2000b. High oxygen and high carbon dioxide modified atmospheres for shelf‐life extension of minimally processed carrots. Journal of Food Science, 65, 61–66. 10.1111/j.1365-2621.2000.tb15956.x [DOI] [Google Scholar]
- Amina M, Panagou EZ, Kodogiannis VS and Nychas GJE, 2010. Wavelet neural networks for modelling high pressure inactivation kinetics of Listeria monocytogenes in UHT whole milk. Chemometrics and Intelligent Laboratory Systems, 103, 170–183. 10.1016/j.chemolab.2010.07.004 [DOI] [Google Scholar]
- ANSES , 2010. Avis relatif à l’évaluation de l’impact d’un traitement par hautes pressions sur de la viande de volaille marinée et de la viande de volaille farcie. Saisine no 2009‐SA‐0315 et 2009‐SA‐0316. [Google Scholar]
- Argues JL, Rodriguez E, Gaya P, Medina M and Nunez M, 2005. Effect of combinations of high‐pressure treatment and bacteriocin‐producing lactic acid bacteria on the survival of Listeria monocytogenes in raw milk cheese. International Dairy Journal, 15, 893–900. 10.1016/j.idairyj.2004.07.020 [DOI] [Google Scholar]
- Avsaroglu MD, Bozoglu F, Alpas H, Largeteau A and Demazeau G, 2015. Use of pulsed‐high hydrostatic pressure treatment to decrease patulin in apple juice. High Pressure Research, 35, 214–222. 10.1080/08957959.2015.1027700 [DOI] [Google Scholar]
- Bajovic B, Bolumar T and Heinz V, 2012. Quality considerations with high pressure processing of fresh and value added meat products. Meat Science, 92, 280–289. 10.1016/j.meatsci.2012.04.024 [DOI] [PubMed] [Google Scholar]
- Balakrishna AK, Wazed MA and Farid M, 2020. A review on the effect of high pressure processing (HPP) on gelatinization and infusion of nutrients. Molecules, 25. 10.3390/molecules25102369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan S, Ahmed R, Chibeu A, Gao AL, Koutchma T and Strange P, 2016. Effect of salt types and concentrations on the high‐pressure inactivation of Listeria monocytogenes in ground chicken. International Journal of Food Microbiology, 218, 51–56. 10.1016/j.ijfoodmicro.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Balci AT, Ledward DA and Wilbey RA, 2002. Effect of high pressure on acid phosphatase in milk. High Pressure Research, 22, 639–642. 10.1080/08957950212435 [DOI] [Google Scholar]
- Baptista I, Queiros RP, Cunha A, Saraiva JA, Rocha SM and Almeida A, 2015. Inactivation of enterotoxic and non‐enterotoxic Staphylococcus aureus strains by high pressure treatments and evaluation of its impact on virulence factors. Food Control, 57, 252–257. 10.1016/j.foodcont.2015.04.022 [DOI] [Google Scholar]
- Barba FJ, Terefe NS, Buckow R, Knorr D and Orlien V, 2015. New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A Review. Food Research International, 77, 725–742. 10.1016/j.foodres.2015.05.015 [DOI] [Google Scholar]
- Batty D, Meunier‐Goddik L and Waite‐Cusic JG, 2019. Camembert‐type cheese quality and safety implications in relation to the timing of high‐pressure processing during aging. Journal of Dairy Science, 102, 8721–8733. 10.3168/jds.2018-16236 [DOI] [PubMed] [Google Scholar]
- Bernedo‐Navarro RA, Duraes‐Carvalho R, de Souza AR, Miyachiro MM, Bonafe CFS and Yano T, 2016. Inactivation of Shiga‐toxin producing Escherichia coli (STEC) O157:H7 in milk by combined treatment with high hydrostatic pressure and aqueous pomegranate extract. Journal of Microbiology Biotechnology and Food Sciences, 6, 636–640. 10.15414/jmbfs.2016.6.1.636-640 [DOI] [Google Scholar]
- Bogahawaththa D, Buckow R, Chandrapala J and Vasiljevic T, 2018. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innovative Food Science & Emerging Technologies, 47, 301–308. 10.1016/j.ifset.2018.03.016 [DOI] [Google Scholar]
- Borch‐Pedersen K, Mellegard H, Reineke K, Boysen P, Sevenich R, Lindback T and Aspholm M, 2017. Effects of high pressure on Bacillus licheniformis spore germination and inactivation. Applied and Environmental Microbiology, 83, 13. 10.1128/aem.00503-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda D, Bleoanca I and Turtoi M, 2013. Advancements in high pressure processing & applications in vegetal origin foods and food safety indicators Annals of the University Dunarea De Jos of Galati. Fascicle Vi‐Food Technology, 37, 18–34. [Google Scholar]
- Borda D, Indrawati X, Smout C, Van Loey A and Hendrickx M, 2004. High pressure thermal inactivation kinetics of a plasmin system. Journal of Dairy Science, 87, 2351–2358. 10.3168/jds.S0022-0302(04)73357-3 [DOI] [PubMed] [Google Scholar]
- Bover‐Cid S, Belletti N, Garriga M and Aymerich T, 2011a. Model for Listeria monocytogenes inactivation on dry‐cured ham by high hydrostatic pressure processing. Food Microbiology, 28, 804–809. 10.1016/j.fm.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Bover‐Cid S, Belletti N, Aymerich T and Garriga M, 2015. Modeling the protective effect of aw and fat content on the high pressure resistance of Listeria monocytogenes in dry‐cured ham. Food Research International, 75, 194–199. 10.1016/j.foodres.2015.05.052 [DOI] [PubMed] [Google Scholar]
- Bover‐Cid S, Belletti N, Aymerich T and Garriga M, 2017. Modelling the impact of water activity and fat content of dry‐cured ham on the reduction of Salmonella enterica by high pressure processing. Meat Science, 123, 120–125. 10.1016/j.meatsci.2016.09.014 [DOI] [PubMed] [Google Scholar]
- Bover‐Cid S, Hereu A, Garriga M and Aymerich MT, 2011b. Pre‐culture conditions as a key factor of the behaviour of Listeria monocytogenes on high pressure processed RTE cooked meat products. TUBITAK‐MAM 4th International Congress on Food and Nutrition and SAFE Consortium 3rd International Congress on Food SafetyIstanbul, Turkey, p. 65. [Google Scholar]
- Bover‐Cid S, Serra‐Castello C, Dalgaard P, Garriga M and Jofré A, 2019. New insights on Listeria monocytogenes growth in pressurised cooked ham: a piezo‐stimulation effect enhanced by organic acids during storage. International Journal of Food Microbiology, 290, 150–158. 10.1016/j.ijfoodmicro.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Bravo FI, Felipe X, Lopez‐Fandino R and Molina E, 2013. High‐pressure treatment of milk in industrial and pilot‐scale equipments: effect of the treatment conditions on the protein distribution in different milk fractions. European Food Research and Technology, 236, 499–506. 10.1007/s00217-012-1902-9 [DOI] [Google Scholar]
- Bravo FI, Molina E and Lopez‐Fandino R, 2012. Effect of the high‐pressure‐release phase on the protein composition of the soluble milk fraction. Journal of Dairy Science, 95, 6293–6299. 10.3168/jds.2012-5490 [DOI] [PubMed] [Google Scholar]
- Brown P, Cardone F, Meyer R and Pocchiari M, 2016. High‐pressure inactivation of Transmissible Spongiform Encephalopathy Agents (Prions) in processed meats. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds). High Pressure Processing of Food: Principles, Technology and Applications. Springer, New York. pp. 317–330. 10.1007/978-1-4939-3234-4_16 [DOI] [Google Scholar]
- Bucur FI, Grigore‐Gurgu L, Crauwels P, Riedel CU and Nicolau AI, 2018. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Frontiers in Microbiology, 9, 18. 10.3389/fmicb.2018.02700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull MK, Steele RJ, Kelly M, Olivier SA and Chapman B, 2010. Packaging under pressure: Effects of high pressure, high temperature processing on the barrier properties of commonly available packaging materials. Innovative Food Science & Emerging Technologies, 11, 533–537. 10.1016/j.ifset.2010.05.002 [DOI] [Google Scholar]
- Butzler JP and Oosterom J, 1991. Campylobacter – Pathogenicity and significance in foods. International Journal of Food Microbiology, 12, 1–8. 10.1016/0168-1605(91)90043-o [DOI] [PubMed] [Google Scholar]
- Buzrul S, 2015. Multi‐pulsed high hydrostatic pressure treatment of foods. Foods, 4, 173–183. 10.3390/foods4020173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzrul S, Alpas H, Largeteau A and Demazeau G, 2008. Modeling high pressure inactivation of Escherichia coli and Listeria innocua in whole milk. European Food Research and Technology, 227, 443–448. 10.1007/s00217-007-0740-7 [DOI] [Google Scholar]
- Buzrul S, Alpas H, Largeteau A and Demazeau G, 2009. Efficiency of pulse pressure treatment for inactivation of Escherichia coli and Listeria innocua in whole milk. European Food Research and Technology, 229, 127–131. 10.1007/s00217-009-1033-0 [DOI] [Google Scholar]
- CAC (Codex Alimentarius Commission) , 2004. Code of hygienic practice for milk and milk products. CAC/RCP 57‐2004. Food and Agriculture Organization and World Health Organization (FAO and WHO). Available online: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/CXP_057e.pdf
- CAC (Codex Alimentarius Commission) , 2008. Guidelines for the Validation of Food Safety Control Measures. CXG 69‐2008. 10 pp. Available online: https://www.fao.org/input/download/standards/11022/CXG_069e.pdf
- Caner C and Harte B, 2005. Effect of high‐pressure processing on the migration of antioxidant Irganox 1076 from polypropylene film into a food simulant. Journal of the Science of Food and Agriculture, 85, 39–46. 10.1002/jsfa.1935 [DOI] [Google Scholar]
- Caner C, Hernandez RJ and Pascall MA, 2000. Effect of high‐pressure processing on the permeance of selected high‐barrier laminated films. Packaging Technology and Science, 13, 183–195. [DOI] [Google Scholar]
- Capellas M, MorMur M, Sendra E, Pla R and Guamis B, 1996. Populations of aerobic mesophils and inoculated E. coli during storage of fresh goat's milk cheese treated with high pressure. Journal of Food Protection, 59, 582–587. 10.4315/0362-028x-59.6.582 [DOI] [PubMed] [Google Scholar]
- Carminati D, Gatti M, Bonvini B, Neviani E and Mucchetti G, 2004. High‐pressure processing of Gorgonzola cheese: Influence on Listeria monocytogenes inactivation and on sensory characteristics. Journal of Food Protection, 67, 1671–1675. 10.4315/0362-028x-67.8.1671 [DOI] [PubMed] [Google Scholar]
- Carrera M, Fidalgo LG, Vazquez M, Saraiva JA and Aubourg SP, 2020. Comparative effect of a previous 150‐MPa treatment on the quality loss of frozen hake stored at different temperatures. Journal of the Science of Food and Agriculture, 100, 4245–4251. 10.1002/jsfa.10465 [DOI] [PubMed] [Google Scholar]
- Cebrian G, Manas P and Condon S, 2016. Comparative resistance of bacterial foodborne pathogens to non‐thermal technologies for food preservation. Frontiers in Microbiology, 7, 17. 10.3389/fmicb.2016.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf O, 1977. Tailing of survival curves of bacteria spores. Journal of Applied Bacteriology, 42, 1–19. 10.1111/j.1365-2672.1977.tb00665.x [DOI] [PubMed] [Google Scholar]
- Ceylan E, Amezquita A, Anderson N, Betts R, Blayo L, Garces‐Vega F, Gkogka E, Harris LJ, McClure P, Winkler A and den Besten HMW, 2021. Guidance on validation of lethal control measures for foodborne pathogens in foods. Comprehensive Reviews in Food Science and Food Safety, 20, 2825–2881. 10.1111/1541-4337.12746 [DOI] [PubMed] [Google Scholar]
- Chen HQ, 2007. Temperature‐assisted pressure inactivation of Listeria monocytogenes in turkey breast meat. International Journal of Food Microbiology, 117, 55–60. 10.1016/j.ijfoodmicro.2007.02.025 [DOI] [PubMed] [Google Scholar]
- Chiodini RJ and Hermon‐Taylor J, 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. Journal of Veterinary Diagnostic Investigation, 5, 629–631. 10.1177/104063879300500424 [DOI] [PubMed] [Google Scholar]
- Christopher FM, Smith GC and Vanderzant C, 1982. Effect of temperature and pH on the survival of Campylobacter fetus. Journal of Food Protection, 45, 253–259. 10.4315/0362-028x-45.3.253 [DOI] [PubMed] [Google Scholar]
- Chung H‐J and Yousef AE, 2010. Inactivation of Escherichia coli in broth and sausage by combined high pressure and Lactobacillus casei cell extract. Food Science and Technology International, 16, 381–388. 10.1177/1082013210366883 [DOI] [PubMed] [Google Scholar]
- Claeys W, Indrawati VL and Hendrickx M, 2003a. Review: are intrinsic TTIs for thermally processed milk applicable for high‐pressure processing assessment? Innovative Food Science & Emerging Technologies, 4, 1–14. 10.1016/S1466-8564(02)00066-8 [DOI] [Google Scholar]
- Claeys WL, Van Loey AM and Hendrickx ME, 2002. Intrinsic time temperature integrators for heat treatment of milk. Trends in Food Science & Technology, 13, 293–311. 10.1016/s0924-2244(02)00164-4 [DOI] [Google Scholar]
- Claeys WL, Van Loey AM and Hendrickx ME, 2003b. Influence of seasonal variation on kinetics of time temperature integrators for thermally processed milk. Journal of Dairy Research, 70, 217–225. 10.1017/s0022029903006137 [DOI] [PubMed] [Google Scholar]
- Claeys WL, Verraes C, Cardoen S, De Block J, Huyghebaert A, Raes K, Dewettinck K and Herman L, 2014. Consumption of raw or heated milk from different species: an evaluation of the nutritional and potential health benefits. Food Control, 42, 188–201. 10.1016/j.foodcont.2014.01.045 [DOI] [Google Scholar]
- Colussi R, Kaur L, Zavareze EdR, Guerra Dias AR, Stewart RB and Singh J, 2018. High pressure processing and retrogradation of potato starch: influence on functional properties and gastro‐small intestinal digestion in vitro . Food Hydrocolloids, 75, 131–137. 10.1016/j.foodhyd.2017.09.004 [DOI] [Google Scholar]
- Considine KM, Kelly AL, Fitzgerald GF, Hill C and Sleator RD, 2008. High‐pressure processing ‐ effects on microbial food safety and food quality. Fems Microbiology Letters, 281, 1–9. 10.1111/j.1574-6968.2008.01084.x [DOI] [PubMed] [Google Scholar]
- Considine T, Patel HA, Anema SG, Singh H and Creamer LK, 2007. Interactions of milk proteins during heat and high hydrostatic pressure treatments ‐ a review. Innovative Food Science & Emerging Technologies, 8, 1–23. 10.1016/j.ifset.2006.08.003 [DOI] [Google Scholar]
- Contador R, Delgado FJ, Garcia‐Parra J, Garrido M and Ramirez R, 2015. Volatile profile of breast milk subjected to high‐pressure processing or thermal treatment. Food Chemistry, 180, 17–24. 10.1016/j.foodchem.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Contador R, Delgado‐Adamez J, Delgado FJ, Cava R and Ramirez R, 2013. Effect of thermal pasteurisation or high pressure processing on immunoglobulin and leukocyte contents of human milk. International Dairy Journal, 32, 1–5. 10.1016/j.idairyj.2013.03.006 [DOI] [Google Scholar]
- Daher D, Le Gourrierec S and Perez‐Lamela C, 2017. Effect of high pressure processing on the microbial inactivation in fruit preparations and other vegetable based beverages. Agriculture‐Basel, 7. 10.3390/agriculture7090072 [DOI] [Google Scholar]
- D'Aoust JY, Park CE, Szabo RA, Todd EC, Emmons DB and McKellar RC, 1988. Thermal inactivation of Campylobacter species, Yersinia enterocolitica, and hemorrhagic Escherichia coli O157:H7 in fluid milk. Journal of Dairy Science, 71, 3230–3236. 10.3168/jds.s0022-0302(88)79928-2 [DOI] [PubMed] [Google Scholar]
- Datta N and Deeth HC, 2011. Heat treatment of milk|non‐thermal technologies: high pressure processing. In Encyclopedia of Dairy Sciences (Second Edition), 732–737. 10.1016/B978-0-12-374407-4.00219-3 [DOI] [Google Scholar]
- Davies G and Casey A, 1973. The survival of Brucella abortus in milk and milk products. British Veterinary Journal, 129, 345–353. 10.1016/S0007-1935(17)36436-9 [DOI] [PubMed] [Google Scholar]
- De la Fuente MA, Olano A, Casal V and Juarez M, 1999. Effects of high pressure and heat treatment on the mineral balance of goats' milk. Journal of Dairy Research, 66, 65–72. 10.1017/s0022029998003264 [DOI] [PubMed] [Google Scholar]
- De Lamo‐Castellvi S, Capellas M, Roig‐Sagues AX, Lopez‐Pedemonte T, Hernandez‐Herrero MM and Guamis B, 2006. Fate of Escherichia coli strains inoculated in model cheese elaborated with or without starter and treated by high hydrostatic pressure. Journal of Food Protection, 69, 2856–2864. 10.4315/0362-028x-69.12.2856 [DOI] [PubMed] [Google Scholar]
- De Lamo‐Castellvi S, Roig‐Sagues AX, Lopez‐Pedemonte T, Hernandez‐Herrero MM, Guamis B and Capellas M, 2007. Response of two Salmonella enterica strains inoculated in model cheese treated with high hydrostatic pressure. Journal of Dairy Science, 90, 99–109. 10.4315/0362-028x-69.12.2856 [DOI] [PubMed] [Google Scholar]
- Delgado FJ, Cava R, Delgado J and Ramirez R, 2014. Tocopherols, fatty acids and cytokines content of holder pasteurised and high‐pressure processed human milk. Dairy Science & Technology, 94, 145–156. 10.1007/s13594-013-0149-y [DOI] [Google Scholar]
- Delgado FJ, Contador R, Alvarez‐Barrientos A, Cava R, Delgado‐Adamez J and Ramirez R, 2013. Effect of high pressure thermal processing on some essential nutrients and immunological components present in breast milk. Innovative Food Science & Emerging Technologies, 19, 50–56. 10.1016/j.ifset.2013.05.006 [DOI] [Google Scholar]
- Demazeau G, Plumecocq A, Lehours P, Martin P, Couedelo L and Billeaud C, 2018. A new high hydrostatic pressure process to assure the microbial safety of human milk while preserving the biological activity of its main components. Frontiers in Public Health, 6, 8. 10.3389/fpubh.2018.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai AN, Anyoha A, Madoff LC and Lassmann B, 2019. Changing epidemiology of Listeria monocytogenes outbreaks, sporadic cases, and recalls globally: a review of ProMED reports from 1996 to 2018. International Journal of Infectious Diseases, 84, 48–53. 10.1016/j.ijid.2019.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan C and Erkmen O, 2003. Note: ultra high hydrostatic pressure inactivation of Escherichia coli in milk, and orange and peach juices. Food Science and Technology International, 9, 403–407. 10.1177/1082013203040264 [DOI] [Google Scholar]
- Dogan C and Erkmen O, 2004. High pressure inactivation kinetics of Listeria monocytogenes inactivation in broth, milk, and peach and orange juices. Journal of Food Engineering, 62, 47–52. 10.1016/s0260-8774(03)00170-5 [DOI] [Google Scholar]
- Donaghy JA, Linton M, Patterson MF and Rowe MT, 2007. Effect of high pressure and pasteurization on Mycobacterium avium ssp. paratuberculosis in milk. Letters in Applied Microbiology, 45, 154–159. 10.1111/j.1472-765X.2007.02163.x [DOI] [PubMed] [Google Scholar]
- Doona CJ, Feeherry FE, Feng H, Grove S, Krishnamurthy K, Lee A and Kustin K, 2015. Combining sanitizers and nonthermal processing technologies to improve fresh‐cut produce safety. In: Pillai SD and Shayanfar S (eds.). Electron Beam Pasteurization and Complementary Food Processing Technologies. Woodhead Publishing Series in Food Science, Technology and Nutrition. pp. 95–125. 10.1533/9781782421085.2.95 [DOI] [Google Scholar]
- Doona CJ, Feeherry FE, Setlow B, Wang SW, Li W, Nichols FC, Talukdar PK, Sarker MR, Li YQ, Shen A and Setlow P, 2016. Effects of high‐pressure treatment on spores of Clostridium species. Applied and Environmental Microbiology, 82, 5287–5297. 10.1128/aem.01363-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doona CJ, Ghosh S, Feeherry FF, Ramirez‐Peralta A, Huang Y, Chen H and Setlow P, 2014. High pressure germination of Bacillus subtilis spores with alterations in levels and types of germination proteins. Journal of Applied Microbiology, 117, 711–720. 10.1111/jam.12557 [DOI] [PubMed] [Google Scholar]
- Doyle MP and Roman DJ, 1981. Growth and survival of Campylobacter fetus subsp. jejuni as a function of temperature and pH. Journal of Food Protection, 44, 596–601. 10.4315/0362-028x-44.8.596 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready‐to‐eat foods in the EU, 2010–2011 part A: Listeria monocytogenes prevalence estimates. EFSA Journal 2013;11(6):3241, 75 pp. 10.2903/j.efsa.2013.3241 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Analysis of the baseline survey on the prevalence of Listeria monocytogenes in certain ready‐to‐eat foods in the EU, 2010–2011 Part B: analysis of factors related to prevalence and exploring compliance. EFSA Journal 2014;12(8):3810, 73 pp. 10.2903/j.efsa.2014.3810 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2020. Draft framework for protocol development for EFSA’s scientific assessments. EFSA supporting publication 2020;EN‐1843, 46 pp. 10.2903/sp.efsa.2020.EN-1843 [DOI]
- EFSA (European Food Safety Authority) , 2021a. The use of alkaline phosphatase and possible alternative testing to verify pasteurisation of raw milk, colostrum, dairy and colostrum‐based products. EFSA Journal 2021;19(4):6576, 73 pp. 10.2903/j.efsa.2021.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2021b. Zoonoses, antimicrobial resistance and food‐borne outbreaks guidance for reporting 2020 data. EFSA supporting publication 2021;EN‐6438, 112 pp. 10.2903/sp.efsa.2021.EN-6438 [DOI]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2011. Scientific Opinion on an update on the present knowledge on the occurrence and control of foodborne viruses. EFSA Journal 2011;9(7):2190, 96 pp. 10.2903/j.efsa.2011.2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2015. Scientific Opinion on the public health risks related to the consumption of raw drinking milk. EFSA Journal 2015;13(1):3940, 95 pp. 10.2903/j.efsa.2015.3940 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2018. Scientific Opinion on the Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Journal 2018;16(1):5134, 173 pp. 10.2903/j.efsa.2018.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Biohaz Panel (EFSA Panel on Biological Hazards) , 2020a. Scientific Opinion on guidance on date marking and related food information: part 1 (date marking). EFSA Journal 2020;18(12):6306, 74 pp. 10.2903/j.efsa.2020.6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , 2020b. Scientific Opinion on the public health risk posed by Listeria monocytogenes in frozen fruit and vegetables, including herbs, blanched during processing. EFSA Journal 2020;18(4):6092, 102 pp. 10.2903/j.efsa.2020.6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2017. Scientific Opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal 2017;15(9):4718, 345 pp. 10.2903/j.efsa.2017.4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Scientific Opinion on the principles and methods behind EFSA’s Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekonomou SI, Bulut S, Karatzas KAG and Boziaris IS, 2020. Inactivation of Listeria monocytogenes in raw and hot smoked trout fillets by high hydrostatic pressure processing combined with liquid smoke and freezing. Innovative Food Science & Emerging Technologies, 64, 11. 10.1016/j.ifset.2020.102427 [DOI] [Google Scholar]
- El Moustaine D, Perrier V, Smeller L, Lange R and Torrent J, 2008. Full‐length prion protein aggregates to amyloid fibrils and spherical particles by distinct pathways. Febs Journal, 275, 2021–2031. 10.1111/j.1742-4658.2008.06356.x [DOI] [PubMed] [Google Scholar]
- Erkmen O, 2009. High hydrostatic pressure inactivation kinetics of Salmonella typhimurium . High Pressure Research, 29, 129–140. 10.1080/08957950802338949 [DOI] [Google Scholar]
- Erkmen O, 2011. Effects of high hydrostatic pressure on Salmonella typhimurium and aerobic bacteria in milk and fruit juices. Romanian Biotechnological Letters, 16, 6540–6547. [Google Scholar]
- Escobedo‐Avellaneda Z, Pateiro‐Moure M, Chotyakul N, Torres JA, Welti‐Chanes J and Perez‐Lamela C, 2011. Benefits and limitations of food processing by high‐pressure technologies: effects on functional compounds and abiotic contaminants. Cyta‐Journal of Food, 9, 351–364. 10.1080/19476337.2011.616959 [DOI] [Google Scholar]
- Evelyn X and Silva FVM, 2017. Resistance of Byssochlamys nivea and Neosartorya fischeri mould spores of different age to high pressure thermal processing and thermosonication. Journal of Food Engineering, 201, 9–16. 10.1016/j.jfoodeng.2017.01.007 [DOI] [Google Scholar]
- Evelyn X and Silva FVM, 2019. Heat assisted HPP for the inactivation of bacteria, moulds and yeasts spores in foods: log reductions and mathematical models. Trends in Food Science & Technology, 88, 143–156. 10.1016/j.tifs.2019.03.016 [DOI] [Google Scholar]
- Evert‐Arriagada K, Trujillo AJ, Amador‐Espejo GG and Hernandez‐Herrero MM, 2018. High pressure processing effect on different Listeria spp. in a commercial starter‐free fresh cheese. Food Microbiology, 76, 481–486. 10.1016/j.fm.2018.07.012 [DOI] [PubMed] [Google Scholar]
- Evrendilek GA, Koca N, Harper JW and Balasubramaniam VM, 2008. High‐pressure processing of Turkish white cheese for microbial inactivation. Journal of Food Protection, 71, 102–108. 10.4315/0362-028x-71.1.102 [DOI] [PubMed] [Google Scholar]
- Fang Y, Mercer RG, McMullen LM and Ganzle MG, 2017. Induction of Shiga toxin‐encoding prophage by abiotic environmental stress in food. Applied and Environmental Microbiology, 83, 13. 10.1128/aem.01378-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farakos SMS and Zwietering MH, 2011. Data analysis of the inactivation of foodborne microorganisms under high hydrostatic pressure to establish global kinetic parameters and influencing factors. Journal of Food Protection, 74, 2097–2106. 10.4315/0362-028x.jfp-11-162 [DOI] [PubMed] [Google Scholar]
- FDA (USDA Food and Drug Administration) , 2004. Guidance for Industry: Juice Hazard Analysis Critical Control Point Hazards and Controls Guidance, First Edition. FDA‐2002‐D‐0298. Available online: https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/guidance‐industry‐juice‐hazard‐analysis‐critical‐control‐point‐hazards‐and‐controls‐guidance‐first
- Felipe X, Capellas M and Law AJR, 1997. Comparison of the effects of high‐pressure treatments and heat pasteurization on the whey proteins in goat's milk. Journal of Agricultural and Food Chemistry, 45, 627–631. 10.1021/jf960406o [DOI] [Google Scholar]
- Ferreira M, Almeida A, Delgadillo I, Saraiva J and Cunha A, 2016. Susceptibility of Listeria monocytogenes to high pressure processing: a review. Food Reviews International, 32, 377–399. 10.1080/87559129.2015.1094816 [DOI] [Google Scholar]
- Foddai A, Elliott CT and Grant IR, 2010. Maximizing capture efficiency and specificity of magnetic separation for Mycobacterium avium subsp. paratuberculosis cells. Applied and Environmental Microbiology, 76, 7550–7558. 10.1128/aem.01432-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonberg‐Broczek M, Windyga B, Szczawinski J, Szczawinska M, Pietrzak D and Prestamo G, 2005. High pressure processing for food safety. Acta Biochimica Polonica, 52, 721–724. [PubMed] [Google Scholar]
- Foster DM, Poulsen KP, Sylvester HJ, Jacob ME, Casulli KE and Farkas BE, 2016. Effect of high‐pressure processing of bovine colostrum on immunoglobulin G concentration, pathogens, viscosity, and transfer of passive immunity to calves. Journal of Dairy Science, 99, 8575–8588. 10.3168/jds.2016-11204 [DOI] [PubMed] [Google Scholar]
- Foster HG Jr, Lear SA and Metzger HJ, 1953. Time‐temperature studies on the inactivation of Brucella abortus strain 2308 in milk. Journal of Milk and Food Technology, 16, 116–120. 10.4315/0022-2747-16.3.116 [DOI] [Google Scholar]
- Franklin JG, 1965. A simple laboratory‐scale HTST milk pasteurizer. Journal of Dairy Research, 32, 281–289. 10.1017/S0022029900018653 [DOI] [Google Scholar]
- FSAI (Food Safety Authority of Ireland) , 2020. Appraisal of new and emerging food processing technologies and their potential risks to food safety. ISBN: 978‐1‐910348‐38‐3. 45 pp. Food Safety Authority of Ireland. Available online: https://www.fsai.ie/Appraisalofnewemerging_foodprocessingtechnologies_foodsafety/
- FSIS (Food Safety and Inspection Service of the US Department of Agriculture) , 2012. FSIS. Directive: High Pressure Processing (HPP) and Inspection Program Personnel (IPP) Verification Responsibilities. FSIS. Directive 6120.2, 5 pp. Available online: https://www.FSIS..usda.gov/sites/default/files/media_file/2020‐07/6120.2.pdf
- FSIS (Food Safety and Inspection Service of the US Department of Agriculture) , 2014. FSIS. Compliance Guideline: Controlling Listeria monocytogenes in Post‐lethality Exposed Ready‐to‐Eat Meat and Poultry Products. FSIS.‐GD‐2014‐0001, 143 pp. Available online: https://www.fsis.usda.gov/guidelines/2014‐0001
- Gao A, Mutharia L, Chen S, Rahn K and Odumeru J, 2002. Effect of pasteurization on survival of Mycobacterium paratuberculosis in milk. Journal of Dairy Science, 85, 3198–3205. 10.3168/jds.S0022-0302(02)74408-1 [DOI] [PubMed] [Google Scholar]
- Gao YL, Ju XR and Jiang HH, 2006. Statistical analysis of inactivation of Listeria monocytogenes subjected to high hydrostatic pressure and heat in milk buffer. Applied Microbiology and Biotechnology, 70, 670–678. 10.1007/s00253-005-0143-2 [DOI] [PubMed] [Google Scholar]
- Garcia‐Graells C, Masschalck B and Michiels CW, 1999. Inactivation of Escherichia coli in milk by high‐hydrostatic‐pressure treatment in combination with antimicrobial peptides. Journal of Food Protection, 62, 1248–1254. 10.4315/0362-028x-62.11.1248 [DOI] [PubMed] [Google Scholar]
- Garcia‐Graells C, Valckx C and Michiels CW, 2000. Inactivation of Escherichia coli and Listeria innocua in milk by combined treatment with high hydrostatic pressure and the lactoperoxidase system. Applied and Environmental Microbiology, 66, 4173–4179. 10.1128/aem.66.10.4173-4179.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Risco MR, Olano A, Ramos M and Lopez‐Fandino R, 2000. Micellar changes induced by high pressure. Influence in the proteolytic activity and organoleptic properties of milk. Journal of Dairy Science, 83, 2184–2189. [DOI] [PubMed] [Google Scholar]
- Garrido M, Contador R, Garcia‐Parra J, Delgado FJ, Delgado‐Adamez J and Ramirez R, 2015. Volatile profile of human milk subjected to high‐pressure thermal processing. Food Research International, 78, 186–194. 10.1016/j.foodres.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Garriga M, Aymerich MT, Costa S, Monfort JM and Hugas M, 2002. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiology, 19, 509–518. 10.1006/fmic.2002.0498 [DOI] [Google Scholar]
- Gayan E, Condon S, Alvarez I, Nabakabaya M and Mackey B, 2013. Effect of pressure‐induced changes in the ionization equilibria of buffers on inactivation of Escherichia coli and Staphylococcus aureus by high hydrostatic pressure. Applied and Environmental Microbiology, 79, 4041–4047. 10.1128/aem.00469-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georget E, Sevenich R, Reineke K, Mathys A, Heinz V, Callanan M, Rauh C and Knorr D, 2015. Inactivation of microorganisms by high isostatic pressure processing in complex matrices: a review. Innovative Food Science & Emerging Technologies, 27, 1–14. 10.1016/j.ifset.2014.10.015 [DOI] [Google Scholar]
- Gervilla R, Ferragut V and Guamis B, 2000. High pressure inactivation of microorganisms inoculated into ovine milk of different fat contents. Journal of Dairy Science, 83, 674–682. 10.3168/jds.S0022-0302(00)74928-9 [DOI] [PubMed] [Google Scholar]
- Gervilla R, Ferragut V and Guamis B, 2001. High hydrostatic pressure effects on color and milk‐fat globule of ewe's milk. Journal of Food Science, 66, 880–885. 10.1111/j.1365-2621.2001.tb15190.x [DOI] [Google Scholar]
- Gervilla R, Sendra E, Ferragut V and Guamis B, 1999. Sensitivity of Staphylococcus aureus and Lactobacillus helveticus in ovine milk subjected to high hydrostatic pressure. Journal of Dairy Science, 82, 1099–1107. 10.3168/jds.S0022-0302(99)75332-4 [DOI] [PubMed] [Google Scholar]
- Ghafoor K, Gavahian M, Marszałek K, Barba FJ, Xia Q and Denoya GI, 2020. Chapter 1 ‐ An overview of the potential applications based on HPP mechanism. In: Barba FJ, Tonello‐Samson C, Puértolas E and Lavilla M (eds.). Present and Future of High Pressure Processing. Elsevier, 3–11. 10.1016/B978-0-12-816405-1.00001-7 [DOI]
- Gill KPW, Bates PG and Lander KP, 1981. The effect of pasteurization on the survival of Campylobacter species in milk. British Veterinary Journal, 137, 578–584. 10.1016/S0007-1935(17)31534-8 [DOI] [PubMed] [Google Scholar]
- Goncalves SM, Gouveia FS, Chavez DWH, Rosenthal A, da Silva JPL and de Melo NR, 2021. Antimicrobial packaging and high hydrostatic pressure: combined effect in improving the safety of coalho cheese. Food Science and Technology International, 27, 301–312. 10.1177/1082013220953238 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Angulo M, Clauwers C, Harastani R, Tonello C, Jaime I, Rovira J and Michiels CW, 2020. Evaluation of factors influencing the growth of non‐toxigenic Clostridium botulinum type E and Clostridium sp. in high‐pressure processed and conditioned tender coconut water from Thailand. Food Research International, 134, 10. 10.1016/j.foodres.2020.109278 [DOI] [PubMed] [Google Scholar]
- Gou J, Choi KP, He X and Ahn J, 2010. Dimethylamine, Trimethylamine, and Biogenic Amine Formation in High‐Pressure Processed Semidried Squid (Todarodes pacificius) during Refrigerated Storage. Journal of Food Science, 75, M489–M495. 10.1111/j.1750-3841.2010.01731.x [DOI] [PubMed] [Google Scholar]
- Grant I, Ball H, Neill S and Rowe M, 1996. Inactivation of Mycobacterium paratuberculosis in cows’ milk at pasteurization temperature. Applied and Environmental Microbiology, 62, 631–636. 10.1128/AEM.62.2.631-636.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant IR, Ball HJ and Rowe MT, 1999. Effect of higher pasteurization temperatures, and longer holding times at 72°C, on the inactivation of Mycobacterium paratuberculosis in milk. Letters in Applied Microbiology, 28, 461–465. 10.1046/j.1365-2672.1999.00557.x [DOI] [PubMed] [Google Scholar]
- Grant IR, Hitchings EI, McCartney A, Ferguson F and Rowe MT, 2002. Effect of commercial‐scale high‐temperature, short‐time pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Applied and Environmental Microbiology, 68, 602–607. 10.1128/AEM.68.2.602-607.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant IR, Williams AG, Rowe MT and Muir DD, 2005. Efficacy of various pasteurization time‐temperature conditions in combination with homogenization on inactivation of Mycobacterium avium subsp. paratuberculosis in milk. Applied and Environmental Microbiology, 71, 2853–2861. 10.1128/AEM.71.6.2853-2861.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauwet T, Van der Plancken I, Vervoort L, Hendrickx M and Van Loey A, 2016. High‐pressure processing uniformity. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds.). High Pressure Processing of Food: Principles, Technology and Applications. Springer, New York, pp. 253–268. 10.1007/978-1-4939-3234-4_13 [DOI]
- Griffin S, Falzon O, Camilleri K and Valdramidis VP, 2020. Bacterial and fungal contaminants in caprine and ovine cheese: a meta‐analysis assessment. Food Research International, 137. 10.1016/j.foodres.2020.109445 [DOI] [PubMed] [Google Scholar]
- Guillou S and Membre JM, 2019. Inactivation of Listeria monocytogenes, Staphylococcus aureus, and Salmonella enterica under high hydrostatic pressure: a quantitative analysis of existing literature data. Journal of Food Protection, 82, 1802–1814. 10.4315/0362-028x.Jfp-19-132 [DOI] [PubMed] [Google Scholar]
- Hailu Y, Hansen EB, Seifu E, Eshetu M, Ipsen R and Kappeler S, 2016. Functional and technological properties of camel milk proteins: a review. Journal of Dairy Research, 83, 422–429. 10.1017/s0022029916000686 [DOI] [PubMed] [Google Scholar]
- Hao HY, Zhou T, Koutchma T, Wu F and Warriner K, 2016. High hydrostatic pressure assisted degradation of patulin in fruit and vegetable juice blends. Food Control, 62, 237–242. 10.1016/j.foodcont.2015.10.042 [DOI] [Google Scholar]
- Hayert M, Perrier‐Cornet JM and Gervais P, 1999. A simple method for measuring the pH of acid solutions under high pressure. Journal of Physical Chemistry A, 103, 1785–1789. 10.1021/jp983204z [DOI] [Google Scholar]
- Hayman MM, Anantheswaran RC and Knabel SJ, 2008. Heat shock induces barotolerance in Listeria monocytogenes . Journal of Food Protection, 71, 426–430. 10.4315/0362-028x-71.2.426 [DOI] [PubMed] [Google Scholar]
- Hayman MM, Baxter I, O'Riordan PJ and Stewart CM, 2004. Effects of high‐pressure processing on the safety, quality, and shelf life of ready‐to‐eat meats. Journal of Food Protection, 67, 1709–1718. 10.4315/0362-028x-67.8.1709 [DOI] [PubMed] [Google Scholar]
- Health Canada , 2011. Policy on Listeria monocytogenes in Ready‐to‐Eat Foods. Bureau of Microbial Hazards, Food Directorate, Health Products and Food Branch. FD‐FSNP 0071. 49 pp. Available online: https://www.canada.ca/en/health‐canada/services/food‐nutrition/legislation‐guidelines/policies/policy‐listeria‐monocytogenes‐ready‐eat‐foods‐2011.html
- Health Canada , 2015. Novel Food Information ‐ High Pressure Processing (HPP)‐Treated Egg Salad, Egg Dips, and Egg Spreads. Available online: https://www.canada.ca/en/health‐canada/services/food‐nutrition/genetically‐modified‐foods‐other‐novel‐foods/approved‐products/novel‐food‐information‐high‐pressure‐processing‐treated‐egg‐salad‐egg‐dips‐egg‐spreads.html
- Hereu A, Bover‐Cid S, Garriga M and Aymerich T, 2012a. High hydrostatic pressure and biopreservation of dry‐cured ham to meet the Food Safety Objectives for Listeria monocytogenes . International Journal of Food Microbiology, 154, 107–112. 10.1016/j.ijfoodmicro.2011.02.027 [DOI] [PubMed] [Google Scholar]
- Hereu A, Dalgaard P, Garriga M, Aymerich T and Bover‐Cid S, 2012b. Modeling the high pressure inactivation kinetics of Listeria monocytogenes on RTE cooked meat products. Innovative Food Science & Emerging Technologies, 16, 305–315. 10.1016/j.ifset.2012.07.005 [DOI] [Google Scholar]
- Hereu A, Dalgaard P, Garriga M, Aymerich T and Bover‐Cid S, 2014. Analysing and modelling the growth behaviour of Listeria monocytogenes on RTE cooked meat products after a high pressure treatment at 400 MPa. International Journal of Food Microbiology, 186, 84–94. 10.1016/j.ijfoodmicro.2014.06.020 [DOI] [PubMed] [Google Scholar]
- Hill C, Cotter PD, Sleator RD and Gahan CGM, 2002. Bacterial stress response in Listeria monocytogenes: jumping the hurdles imposed by minimal processing. International Dairy Journal, 12, 273–283. 10.1016/S0958-6946(01)00125-X [DOI] [Google Scholar]
- Hope AF, Tulk PA and Condron RJ, 1996. Pasteurization of Mycobacterium paratuberculosis in whole milk. Proceedings of the International Colloquium on Paratuberculosis. (5th, 1996. Madison, Wisconsin) International Association for Paratuberculosis, 1997, Rehoboth, MA. 377–382. [Google Scholar]
- Huang H‐W, Lung H‐M, Chang Y‐H, Yang BB and Wang C‐Y, 2015. Inactivation of pathogenic Listeria monocytogenes in raw milk by high hydrostatic pressure. Foodborne Pathogens and Disease, 12, 139–144. 10.1089/fpd.2014.1871 [DOI] [PubMed] [Google Scholar]
- Huang H‐W, Wu S‐J, Lu J‐K, Shyu Y‐T and Wang C‐Y, 2017. Current status and future trends of high‐pressure processing in food industry. Food Control, 72, 1–8. 10.1016/j.foodcont.2016.07.019 [DOI] [Google Scholar]
- Huang HW, Yang BB and Wang CY, 2014. Effects of high pressure processing on immunoreactivity and microbiological safety of crushed peanuts. Food Control, 42, 290–295. 10.1016/j.foodcont.2014.02.030 [DOI] [Google Scholar]
- Huppertz T, Fox PF and Kelly AL, 2004a. High pressure treatment of bovine milk: effects on casein micelles and whey proteins. Journal of Dairy Research, 71, 97–106. [DOI] [PubMed] [Google Scholar]
- Huppertz T, Fox PF and Kelly AL, 2004b. Properties of casein micelles in high pressure‐treated bovine milk. Food Chemistry, 87, 103–110. 10.1017/s002202990300640x [DOI] [Google Scholar]
- Huppertz T, Fox PF and Kelly AL, 2004c. Plasmin activity and proteolysis in high pressure‐treated bovine milk. Lait, 84, 297–304. 10.1051/lait:2004003 [DOI] [Google Scholar]
- Huppertz T, Zobrist MR, Uniacke T, Upadhyat V, Fox PF and Kelly AL, 2005. Effects of high pressure on some constituents and properties of buffalo milk. Journal of Dairy Research, 72, 226–233. 10.1017/s0022029905000701 [DOI] [PubMed] [Google Scholar]
- ISO (the International Organization for Standardization) , under development. ISO/DIS 20976‐2 Microbiology of the food chain — Requirements and guidelines for conducting challenge tests of food and feed products — Part 2: Challenge tests to study inactivation potential and kinetic parameters. 24 pp. International Organization for Standardization. Geneva.
- Janowicz M and Lenart A, 2018. The impact of high pressure and drying processing on internal structure and quality of fruit. European Food Research and Technology, 244, 1329–1340. 10.1007/s00217-018-3047-y [DOI] [Google Scholar]
- Jantzen MM, Navas J, de Paz M, Rodriguez B, da Silva WP, Nunez M and Martinez‐Suarez JV, 2006. Evaluation of ALOA plating medium for its suitability to recover high pressure‐injured Listeria monocytogenes from ground chicken meat. Letters in Applied Microbiology, 43, 313–317. 10.1111/j.1472-765X.2006.01950.x [DOI] [PubMed] [Google Scholar]
- Jofré A, Garriga M and Aymerich T, 2007. Inhibition of Listeria monocytogenes in cooked ham through active packaging with natural antimicrobials and high‐pressure processing. Journal of Food Protection, 70, 2498–2502. 10.4315/0362-028x-70.11.2498 [DOI] [PubMed] [Google Scholar]
- Jofré A, Garriga M and Aymerich T, 2008. Inhibition of Salmonella sp Listeria monocytogenes and Staphylococcus aureus in cooked ham by combining antimicrobials, high hydrostatic pressure and refrigeration. Meat Science, 78, 53–59. 10.1016/j.meatsci.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Jofré A, Garriga M, Aymerich T, Pérez‐Rodríguez F, Valero A, Carrasco E and Bover‐Cid S, 2016. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food. EFSA Supporting Publication 2016;13(12):EN‐1141, 184 pp. 10.2903/sp.efsa.2016.EN-1141 [DOI] [Google Scholar]
- Jofré A and Serra X, 2016. Processing of Meat Products Utilizing High Pressure. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds.). High Pressure Processing of Food: Principles, Technology and Applications. pp. 591–623. 10.1007/978-1-4939-3234-4_26 [DOI]
- Jongenburger I, Bassett J, Jackson T, Zwietering MH and Jewell K, 2012. Impact of microbial distributions on food safety I. Factors influencing microbial distributions and modelling aspects. Food Control, 26, 601–609. 10.1016/j.foodcont.2012.02.004 [DOI] [Google Scholar]
- Juliano P, 2016. Egg‐Based Product Development for High‐Pressure Processes at Low and High Temperature. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds.). High Pressure Processing of Food: Principles, Technology and Applications. pp. 671–684. 10.1007/978-1-4939-3234-4_28 [DOI]
- Jung S, 2016. Applications and Opportunities for Pressure‐Assisted Extraction. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds.). High Pressure Processing of Food: Principles, Technology and Applications. pp. 173–191. 10.1007/978-1-4939-3234-4_10 [DOI]
- Kalagatur NK, Kamasani JR, Mudili V, Krishna K, Chauhan OP and Sreepathi MH, 2018. Effect of high pressure processing on growth and mycotoxin production of Fusarium graminearum in maize. Food Bioscience, 21, 53–59. 10.1016/j.fbio.2017.11.005 [DOI] [Google Scholar]
- Kanno C, Mu TH, Hagiwara T, Ametani M and Azuma N, 1998. Gel formation from industrial milk whey proteins under hydrostatic pressure: effect of hydrostatic pressure and protein concentration. Journal of Agricultural and Food Chemistry, 46, 417–424. 10.1021/jf970652f [DOI] [PubMed] [Google Scholar]
- Karatzas KAG, Valdramidis VP and Wells‐Bennik MHJ, 2005. Contingency locus in ctsR of Listeria monocytogenes Scott A: a strategy for occurrence of abundant piezotolerant isolates within clonal populations. Applied and Environmental Microbiology, 71, 8390–8396. 10.1128/aem.71.12.8390-8396.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani J and Frank JF, 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. Journal of Food Protection, 61, 974–978. 10.4315/0362-028x-61.8.974 [DOI] [PubMed] [Google Scholar]
- Kielczewska K, Czerniewicz M, Michalak J and Brandt W, 2004. The effect of high pressure on nitrogen compounds of milk. Journal of Physics‐Condensed Matter, 16, S1067–S1070. 10.1088/0953-8984/16/14/017 [DOI] [Google Scholar]
- Kielczewska K, Jankowska A, Dabrowska A, Wachowska M and Ziajka J, 2020. The effect of high pressure treatment on the dispersion of fat globules and the fatty acid profile of caprine milk. International Dairy Journal, 102. 10.1016/j.idairyj.2019.104607 [DOI] [Google Scholar]
- Kleber N, Maier S and Hinrichs J, 2007. Antigenic response of bovine beta‐lactoglobulin influenced by ultra‐high pressure treatment and temperature. Innovative Food Science & Emerging Technologies, 8, 39–45. 10.1016/j.ifset.2006.05.001 [DOI] [Google Scholar]
- Komora N, Maciel C, Pinto CA, Ferreira V, Brandao TRS, Saraiva JMA, Castro SM and Teixeira P, 2020. Non‐thermal approach to Listeria monocytogenes inactivation in milk: the combined effect of high pressure, pediocin PA‐1 and bacteriophage P100. Food Microbiology, 86. 10.1556/AAlim.2007.0011 [DOI] [PubMed] [Google Scholar]
- Koncza A, Meszaros L, Farkas J, Pasztor‐Huszar K, Helt R and Lechner N, 2007. Pasteurisation of raw milk by high hydrostatic pressure. Acta Alimentaria, 36, 471–481. 10.1556/AAlim.2007.0011 [DOI] [Google Scholar]
- Koseki S, Mizuno Y and Yamamoto K, 2008. Use of mild‐heat treatment following high‐pressure processing to prevent recovery of pressure‐injured Listeria monocytogenes in milk. Food Microbiology, 25, 288–293. 10.1016/j.fm.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Kouassi GK, Anantheswaran RC, Knabel SJ and Floros JD, 2007. Effect of high‐pressure processing on activity and structure of alkaline phosphatase and lactate dehydrogenase in buffer and milk. Journal of Agricultural and Food Chemistry, 55, 9520–9529. 10.1021/jf071518q [DOI] [PubMed] [Google Scholar]
- Koutchma T, Song Y, Setikaite I, Juliano P, Barbosa‐Canovas GV, Dunne CP and Patazca E, 2010. Packaging evaluation for high‐pressure high‐temperature sterilization of shelf‐stable foods. Journal of Food Process Engineering, 33, 1097–1114. 10.1111/j.1745-4530.2008.00328.x [DOI] [Google Scholar]
- Kronenwett FR, Lear SA and Metzger HJ, 1954. Thermal death time studies of Brucella abortus in milk. Journal of Dairy Science, 37, 1291–1302. 10.3168/jds.S0022-0302(54)91406-X [DOI] [Google Scholar]
- Lakshmanan R and Dalgaard P, 2004. Effects of high‐pressure processing on Listeria monocytogenes, spoilage microflora and multiple compound quality indices in chilled cold‐smoked salmon. Journal of Applied Microbiology, 96, 398–408. 10.1046/j.1365-2672.2004.02164.x [DOI] [PubMed] [Google Scholar]
- Lambert Y, Demazeau G, Largeteau A, Bouvier JM, Laborde‐Croubit S and Cabannes M, 2000a. New packaging solutions for high pressure treatments of food. High Pressure Research, 19, 597–602. 10.1080/08957950008202556 [DOI] [Google Scholar]
- Lambert Y, Demazeau G, Largeteau A, Bouvier JM, Laborde‐Croubit S and Cabannes M, 2000b. Packaging for high‐pressure treatments in the food industry. Packaging Technology and Science, 13, 63–71. [DOI] [Google Scholar]
- Largeteau A, Angulo I, Coulet JP and Demazeau G, 2010. Evaluation of Films for Packaging applications in High Pressure Processing. Proceedings of the Joint AIRAPT‐22 and HPCJ‐50 Conference/International Conference on High Pressure Science and TechnologyTokyo, JAPANJul 26–31. 10.1088/1742-6596/215/1/012172 [DOI]
- Lavieri NA, Sebranek JG, Brehm‐Stecher BF, Cordray JC, Dickson JS, Horsch AM, Jung S, Larson EM, Manu DK and Mendonca AF, 2014. Investigating the Control of Listeria monocytogenes on a Ready‐to‐Eat Ham Product Using Natural Antimicrobial Ingredients and Postlethality Interventions. Foodborne Pathogens and Disease, 11, 462–467. 10.1089/fpd.2013.1702 [DOI] [PubMed] [Google Scholar]
- Law AJR, Leaver J, Felipe X, Ferragut V, Pla R and Guamis B, 1998. Comparison of the effects of high pressure and thermal treatments on the casein micelles in goat's milk. Journal of Agricultural and Food Chemistry, 46, 2523–2530. 10.1021/jf970904c [DOI] [Google Scholar]
- Le‐Bail A, Hamadami N and Bahuaud S, 2006. Effect of high‐pressure processing on the mechanical and barrier properties of selected packagings. Packaging Technology and Science, 19, 237–243. 10.1002/pts.727 [DOI] [Google Scholar]
- Leclercq A, Kooh P, Augustin J‐C, Guillier L, Thebault A, Cadavez V, Gonzales‐Barron U and Sanaa M, 2021. Risk factors for sporadic listeriosis: a systematic review and meta‐analysis. Microbial Risk Analysis, 17. 10.1016/j.mran.2020.100128 [DOI] [Google Scholar]
- Lerasle M, Guillou S, Simonin H, Anthoine V, Cheret R, Federighi M and Membre JM, 2014. Assessment of Salmonella and Listeria monocytogenes level in ready‐to‐cook poultry meat: effect of various high pressure treatments and potassium lactate concentrations. International Journal of Food Microbiology, 186, 74–83. 10.1016/j.ijfoodmicro.2014.06.019 [DOI] [PubMed] [Google Scholar]
- Leu M, Marciniak A, Chamberland J, Pouliot Y, Bazinet L and Doyen A, 2017. Effect of skim milk treated with high hydrostatic pressure on permeate flux and fouling during ultrafiltration. Journal of Dairy Science, 100, 7071–7082. 10.3168/jds.2017-12774 [DOI] [PubMed] [Google Scholar]
- Li H, Sun X, Liao X and Ganzle M, 2020. Control of pathogenic and spoilage bacteria in meat and meat products by high pressure: challenges and future perspectives. Comprehensive Reviews in Food Science and Food Safety, 19, 3476–3500. 10.1111/1541-4337.12617 [DOI] [PubMed] [Google Scholar]
- Lindroth S and von Wright A, 1990. Detoxification of patulin by adduct formation with cysteine. Journal of Environmental Pathology, Toxicology and Oncology, 10, 254–259. [PubMed] [Google Scholar]
- Linton M, Connolly M, Houston L and Patterson MF, 2014. The control of Clostridium botulinum during extended storage of pressure‐treated, cooked chicken. Food Control, 37, 104–108. 10.1016/j.foodcont.2013.09.042 [DOI] [Google Scholar]
- Liu G, Caroe C, Qin Z, Munk DME, Crafack M, Petersen MA and Ahrne L, 2020. Comparative study on quality of whole milk processed by high hydrostatic pressure or thermal pasteurization treatment. Lwt‐Food Science and Technology, 127. 10.1016/j.lwt.2020.109370 [DOI] [Google Scholar]
- López‐Fandino R, Carrascosa AV and Olano A, 1996. The effects of high pressure on whey protein denaturation and cheese‐making properties of raw milk. Journal of Dairy Science, 79, 929–936. 10.3168/jds.S0022-0302(96)76443-3 [DOI] [Google Scholar]
- López‐Fandino R and Olano A, 1998. Effects of high pressures combined with moderate temperatures on the rennet coagulation properties of milk. International Dairy Journal, 8, 623–627. 10.1016/s0958-6946(98)00093-4 [DOI] [Google Scholar]
- López‐Pedemonte T, Roig‐Sagues A, De Lamo S, Hernandez‐Herrero M and Guamis B, 2007. Reduction of counts of Listeria monocytogenes in cheese by means of high hydrostatic pressure. Food Microbiology, 24, 59–66. 10.1016/j.fm.2006.03.008 [DOI] [PubMed] [Google Scholar]
- López‐Pedemonte T, Sevilla I, Garrido JM, Aduriz G, Guamis B, Juste RA and Roig‐Sagues AX, 2006. Inactivation of Mycobacterium avium subsp paratuberculosis in cow's milk by means of high hydrostatic pressure at mild temperatures. Applied and Environmental Microbiology, 72, 4446–4449. 10.1128/aem.01924-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Rubio A, Lagaron JM, Hernandez‐Munoz P, Almenar E, Catala R, Gavara R and Pascall MA, 2005. Effect of high pressure treatments on the properties of EVOH‐based food packaging materials. Innovative Food Science & Emerging Technologies, 6, 51–58. 10.1016/j.ifset.2004.09.002 [DOI] [Google Scholar]
- Lucore LA, Shellhammer TH and Yousef AE, 2000. Inactivation of Listeria monocytogenes Scott A on artificially contaminated frankfurters by high‐pressure processing. Journal of Food Protection, 63, 662–664. 10.4315/0362-028x-63.5.662 [DOI] [PubMed] [Google Scholar]
- Ludikhuyze I, Claeys W and Hendrickx M, 2000. Combined pressure‐temperature inactivation of alkaline phosphatase in bovine milk: a kinetic study. Journal of Food Science, 65, 155–160. 10.1111/j.1365-2621.2000.tb15972.x [DOI] [Google Scholar]
- Ludikhuyze LR, Claeys WL and Hendrickx ME, 2001. Effect of temperature and/or pressure on lactoperoxidase activity in bovine milk and acid whey. Journal of Dairy Research, 68, 625–637. 10.1017/s0022029901005118 [DOI] [PubMed] [Google Scholar]
- Lund BM, Gould GW and Rampling AM, 2002. Pasteurization of milk and the heat resistance of Mycobacterium avium subsp. paratuberculosis: a critical review of the data. International Journal of Food Microbiology, 77, 135–145. 10.1016/s0168-1605(02)00057-0 [DOI] [PubMed] [Google Scholar]
- Luu S, Cruz‐Mora J, Setlow B, Feeherry FE, Doona CJ and Setlow P, 2015. The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Applied and Environmental Microbiology, 81, 2927–2938. 10.1128/aem.00193-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D, Jordan KN, Kelly PM, Freyne T and Murphy PM, 2007. Heat sensitivity of Mycobacterium avium ssp. paratuberculosis in milk under pilot plant pasteurization conditions. International Journal of Dairy Technology, 60, 98–104. 10.1111/j.1471-0307.2007.00317.x [DOI] [Google Scholar]
- Machado KIA, Roquetto AR, Moura CS, Lopes AD, Cristianini M and Amaya‐Farfan J, 2019. Comparative impact of thermal and high isostatic pressure inactivation of gram‐negative microorganisms on the endotoxic potential of reconstituted powder milk. LWT‐Food Science and Technology, 106, 78–82. 10.1016/j.lwt.2019.02.064 [DOI] [Google Scholar]
- Marcos B, Aymerich T, Monfort JM and Garriga M, 2008a. High‐pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiology, 25, 177–182. 10.1016/j.fm.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Marcos B, Jofré A, Aymerich T, Monfort JM and Garriga M, 2008b. Combined effect of natural antimicrobials and high pressure processing to prevent Listeria monocytogenes growth after a cold chain break during storage of cooked ham. Food Control, 19, 76–81. 10.1016/j.foodcont.2007.02.005 [DOI] [Google Scholar]
- Martin D, Lorenzen PC, Schwertfeger M, Buchheim M and Schlimme W, 2001. Ribonucleoside contents in high‐pressure treated milk samples. Kieler Milchwirtschaftliche Forschungsberichte, 53, 283–293. [Google Scholar]
- Martin D and Meisel H, 2006. Ribonucleosides: interesting nucleo compounds and chemical parameters for characterization of technological treatment of milk and milk products. Deutsche Lebensmittel‐Rundschau, 102, 501–508. [Google Scholar]
- Martinez‐Gomariz M, Hernaez ML, Gutierrez D, Ximenez‐Embun P and Prestamo G, 2009. Proteomic analysis by two‐dimensional differential gel electrophoresis (2D DIGE) of a high‐pressure effect in Bacillus cereus . Journal of Agricultural and Food Chemistry, 57, 3543–3549. 10.1021/jf803272a [DOI] [PubMed] [Google Scholar]
- Martinez‐Monteagudo SI, Ganzle MG and Saldana MDA, 2014. High‐pressure and temperature effects on the inactivation of Bacillus amyloliquefaciens, alkaline phosphatase and storage stability of conjugated linoleic acid in milk. Innovative Food Science & Emerging Technologies, 26, 59–66. 10.1016/j.ifset.2014.05.003 [DOI] [Google Scholar]
- Martinez‐Rodriguez A and Mackey BM, 2005. Factors affecting the pressure resistance of some Campylobacter species. Letters in Applied Microbiology, 41, 321–326. 10.1111/j.1472-765X.2005.01768.x [DOI] [PubMed] [Google Scholar]
- Martinez‐Rodriguez Y, Acosta‐Muniz C, Olivas GI, Guerrero‐Beltran J, Rodrigo‐Aliaga D and Sepulveda DR, 2012. High hydrostatic pressure processing of cheese. Comprehensive Reviews in Food Science and Food Safety, 11, 399–416. 10.1111/j.1541-4337.2012.00192.x [DOI] [Google Scholar]
- Mauricio‐Iglesias M, Jansana S, Peyron S, Gontard N and Guillard V, 2010. Effect of high‐pressure/temperature (HP/T) treatments of in‐package food on additive migration from conventional and bio‐sourced materials. Food Additives and Contaminants Part a‐Chemistry Analysis Control Exposure & Risk Assessment, 27, 118–127. 10.1080/19440040903268054 [DOI] [PubMed] [Google Scholar]
- Mayayo C, Montserrat M, Ramos SJ, Martinez‐Lorenzo MJ, Calvo M, Sanchez L and Perez MD, 2014. Kinetic parameters for high‐pressure‐induced denaturation of lactoferrin in human milk. International Dairy Journal, 39, 246–252. 10.1016/j.idairyj.2014.07.001 [DOI] [Google Scholar]
- Mayayo C, Montserrat M, Ramos SJ, Martinez‐Lorenzo MJ, Calvo M, Sanchez L and Perez MD, 2016. Effect of high pressure and heat treatments on IgA immunoreactivity and lysozyme activity in human milk. European Food Research and Technology, 242, 891–898. 10.1007/s00217-015-2595-7 [DOI] [Google Scholar]
- Mazri C, Sanchez L, Ramos SJ, Calvo M and Perez MD, 2012a. Effect of high‐pressure treatment on denaturation of bovine beta‐lactoglobulin and alpha‐lactalbumin. European Food Research and Technology, 234, 813–819. 10.1007/s00217-012-1695-x [DOI] [Google Scholar]
- Mazri C, Sanchez L, Ramos SJ, Calvo M and Perez MD, 2012b. Effect of high‐pressure treatment on denaturation of bovine lactoferrin and lactoperoxidase. Journal of Dairy Science, 95, 549–557. 10.3168/jds.2011-4665 [DOI] [PubMed] [Google Scholar]
- McClements JMJ, Patterson MF and Linton M, 2001. The effect of growth stage and growth temperature on high hydrostatic pressure inactivation of some psychrotrophic bacteria in milk. Journal of Food Protection, 64, 514–522. 10.4315/0362-028x-64.4.514 [DOI] [PubMed] [Google Scholar]
- McDonald WL, O'Riley KJ, Schroen CJ and Condron RJ, 2005. Heat inactivation of Mycobacterium avium subsp. paratuberculosis in milk. Applied and Environmental Microbiology, 71, 1785–1789. 10.1128/aem.71.4.1785-1789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M, Cabeza MC, Bravo D, Cambero I, Montiel R, Ordonez JA, Nunez M and Hoz L, 2009. A comparison between E‐beam irradiation and high pressure treatment for cold‐smoked salmon sanitation: microbiological aspects. Food Microbiology, 26, 224–227. 10.1016/j.fm.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Medina‐Meza LG, Barnaba C and Barbosa‐Canovas GV, 2014. Effects of high pressure processing on lipid oxidation: a review. Innovative Food Science & Emerging Technologies, 22, 1–10. 10.1016/j.ifset.2013.10.012 [DOI] [Google Scholar]
- Meloni D, 2019. High‐Hydrostatic‐Pressure (HHP) processing technology as a novel control method for listeria monocytogenes occurrence in mediterranean‐style dry‐fermented sausages. Foods, 8. 10.3390/foods8120672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengden R, Rohner A, Sudhaus N and Klein G, 2015. High‐pressure processing of mild smoked rainbow trout fillets (Oncorhynchus mykiss) and fresh European catfish fillets (Silurus glanis). Innovative Food Science & Emerging Technologies, 32, 9–15. 10.1016/j.ifset.2015.10.002 [DOI] [Google Scholar]
- Mishra N, Puri VM and Demirci A, 2013. Inactivation and injury of Listeria monocytogenes under combined effect of pressure and temperature in UHT whole milk. Journal of Food Process Engineering, 36, 374–384. 10.1111/jfpe.12004 [DOI] [Google Scholar]
- Misiou O, van Nassau TJ, Lenz CA and Vogel RF, 2018. The preservation of Listeria‐critical foods by a combination of endolysin and high hydrostatic pressure. International Journal of Food Microbiology, 266, 355–362. 10.1016/j.ijfoodmicro.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Moatsou G, Bakopanos C, Katharios D, Katsaros G, Kandarakis I, Taoukis P and Politis I, 2008a. Effect of high‐pressure treatment at various temperatures on indigenous proteolytic enzymes and whey protein denaturation in bovine milk. Journal of Dairy Research, 75, 262–269. 10.1017/s002202990800321x [DOI] [PubMed] [Google Scholar]
- Moatsou G, Katsaros G, Bakopanos C, Kandarakis I, Taoukis P and Politis I, 2008b. Effect of high‐pressure treatment at various temperatures on activity of indigenous proteolytic enzymes and denaturation of whey proteins in ovine milk. International Dairy Journal, 18, 1119–1125. 10.1016/j.idairyj.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Molina‐Hoppner A, Doster W, Vogel RF and Ganzle MG, 2004. Protective effect of sucrose and sodium chloride for Lactococcus lactis during sublethal and lethal high‐pressure treatments. Applied and Environmental Microbiology, 70, 2013–2020. 10.1128/aem.70.4.2013-2020.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort S, Ramos S, Meneses N, Knorr D, Raso J and Alvarez I, 2012. Design and evaluation of a high hydrostatic pressure combined process for pasteurization of liquid whole egg. Innovative Food Science & Emerging Technologies, 14, 1–10. 10.1016/j.ifset.2012.01.004 [DOI] [Google Scholar]
- Montero P, Gomez‐Estaca J and Gomez‐Guillen MC, 2007. Influence of salt, smoke, and high pressure on growth of Listeria monocytogenes and spoilage microflora in cold‐smoked dolphinfish (Coryphaena hippurus). Journal of Food Protection, 70, 399–404. 10.4315/0362-028x-70.2.399 [DOI] [PubMed] [Google Scholar]
- Montiel R, Bravo D, de Alba M, Gaya P and Medina M, 2012. Combined effect of high pressure treatments and the lactoperoxidase system on the inactivation of Listeria monocytogenes in cold‐smoked salmon. Innovative Food Science & Emerging Technologies, 16, 26–32. 10.1016/j.ifset.2012.03.005 [DOI] [Google Scholar]
- Montiel R, Martin‐Cabrejas I, Gaya P and Medina M, 2014. Reuterin and High Hydrostatic Pressure Treatments on the Inactivation of Listeria monocytogenes and Effect on the Characteristics of Cold‐Smoked Salmon. Food and Bioprocess Technology, 7, 2319–2329. 10.1007/s11947-014-1287-9 [DOI] [Google Scholar]
- Montiel R, Martin‐Cabrejas I and Medina M, 2015. Reuterin, lactoperoxidase, lactoferrin and high hydrostatic pressure on the inactivation of food‐borne pathogens in cooked ham. Food Control, 51, 122–128. 10.1016/j.foodcont.2014.11.010 [DOI] [Google Scholar]
- Morales P, Calzada J, Rodriguez B, de Paz M, Gaya P and Nunez M, 2006. Effect of cheese water activity and carbohydrate content on the barotolerance of Listeria monocytogenes Scott A. Journal of Food Protection, 69, 1328–1333. 10.4315/0362-028x-69.6.1328 [DOI] [PubMed] [Google Scholar]
- MPI (Ministry for Primary Industries) , 2019. Review of High Pressure Processes (HPP) applied as an alternative to thermal pasteurisation. Ministry for Primary Industries (MPI). New Zealand Food Safety, 120. Available online: https://www.mpi.govt.nz/dmsdocument/34659‐review‐of‐high‐pressure‐processes‐hpp‐applied‐as‐an‐alternative‐to‐thermal‐pasteurisation
- MPI (Ministry for Primary Industries) , 2021. Further Processing. Ministry for Primary Industries (MPI). New Zealand Food Safety, 87. Available online: https://www.mpi.govt.nz/dmsdocument/32007‐Guidance‐document‐Further‐processing
- Mujica‐Paz H, Valdez‐Fragoso A, Tonello Samson C, Welti‐Chanes J and Antonio Torres J, 2011. High‐pressure processing technologies for the pasteurization and sterilization of foods. Food and Bioprocess Technology, 4, 969–985. 10.1007/s11947-011-0543-5 [DOI] [Google Scholar]
- Muntean M‐V, Marian O, Barbieru V, Cătunescu GM, Ranta O, Drocas I and Terhes S, 2016. High pressure processing in food industry – characteristics and applications. Agriculture and Agricultural Science Procedia, 10, 377–383. 10.1016/j.aaspro.2016.09.077 [DOI] [Google Scholar]
- Mussa DM and Ramaswamy HS, 1997. Ultra high pressure pasteurization of milk: kinetics of microbial destruction and changes in physico‐chemical characteristics. Food Science and Technology‐Lebensmittel‐Wissenschaft & Technologie, 30, 551–557. 10.1006/fstl.1996.0223 [DOI] [Google Scholar]
- Myers K, Montoya D, Cannon J, Dickson J and Sebranek J, 2013. The effect of high hydrostatic pressure, sodium nitrite and salt concentration on the growth of Listeria monocytogenes on RTE ham and turkey. Meat Science, 93, 263–268. 10.1016/j.meatsci.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Nabhan MA, Girardet JM, Campagna S, Gaillard JL and Le Roux Y, 2004. Isolation and characterization of copolymers of beta‐lactoglobulin, alpha‐lactalbumin, kappa‐casein, and alpha(s1)‐casein generated by pressurization and thermal treatment of raw milk. Journal of Dairy Science, 87, 3614–3622. 10.3168/jds.S0022-0302(04)73499-2 [DOI] [PubMed] [Google Scholar]
- NACMCF (National Advisory Committee On Microbiological Criteria For Foods) , 2010. Parameters for Determining Inoculated Pack/Challenge Study Protocols. Journal of Food Protection, 73, 0362‐028X, 140‐202. 10.4315/0362-028X-73.1.140 [DOI]
- Naderi N, House JD, Pouliot Y and Doyen A, 2017. Effects of high hydrostatic pressure processing on hen egg compounds and egg products. Comprehensive Reviews in Food Science and Food Safety, 16, 707–720. 10.1111/1541-4337.12273 [DOI] [PubMed] [Google Scholar]
- Nassar KS, Zhang S, Lu J, Pang X, Ragab ES, Yue Y and Lv Jm 2019. Combined effects of high‐pressure treatment and storage temperature on the physicochemical properties of caprine milk. International Dairy Journal, 96, 66–72. 10.1016/j.idairyj.2019.03.003 [DOI] [Google Scholar]
- Needs EC, Stenning RA, Gill AL, Ferragut V and Rich GT, 2000. High‐pressure treatment of milk: effects on casein micelle structure and on enzymic coagulation. Journal of Dairy Research, 67, 31–42. 10.1017/s0022029999004021 [DOI] [PubMed] [Google Scholar]
- Offerdahl DK, Clancy NG and Bloom ME, 2016. Stability of a Tick‐Borne Flavivirus in Milk. Frontiers in Bioengineering and Biotechnology, 4. 10.3389/fbioe.2016.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen K, Kristensen D, Rasmussen JT and Skibsted LH, 2004. Comparison of the effect of high pressure and heat on the activity of bovine xanthine oxidase. Milchwissenschaft‐Milk Science International, 59, 411–413. [Google Scholar]
- Omar A, Harbourne N and Oruna‐Concha MJ, 2018. Effects of industrial processing methods on camel skimmed milk properties. International Dairy Journal, 84, 15–22. 10.1016/j.idairyj.2018.03.011 [DOI] [Google Scholar]
- Omer MK, Alvarez‐Ordonez A, Prieto M, Skjerve E, Asehun T and Alvseike OA, 2018. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathogens and Disease, 15, 598–611. 10.1089/fpd.2017.2393 [DOI] [PubMed] [Google Scholar]
- O'Reilly CE, O'Connor PM, Kelly AL, Beresford TP and Murphy PM, 2000. Use of hydrostatic pressure for inactivation of microbial contaminants in cheese. Applied and Environmental Microbiology, 66, 4890–4896. 10.1128/aem.66.11.4890-4896.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero L and Prestamo G, 2009. Effects of pressure processing on strawberry studied by nuclear magnetic resonance. Innovative Food Science & Emerging Technologies, 10, 434–440. 10.1016/j.ifset.2009.04.004 [DOI] [Google Scholar]
- Pagan R and Mackey B, 2000. Relationship between membrane damage and cell death in pressure‐treated Escherichia coli cells: differences between exponential‐ and stationary‐phase cells and variation among strains. Applied and Environmental Microbiology, 66, 2829–2834. 10.1128/aem.66.7.2829-2834.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey PK, Ramaswamy HS and Idziak E, 2003. High pressure destruction kinetics of indigenous microflora and Escherichia coli in raw milk at two temperatures. Journal of Food Process Engineering, 26, 265–283. 10.1111/j.1745-4530.2003.tb00601.x [DOI] [Google Scholar]
- Pandey PK and Ramaswamy HS, 2004. Effect of high‐pressure treatment of milk on lipase and gamma‐glutamyl transferase activity. Journal of Food Biochemistry, 28, 449–462. 10.1111/j.1745-4514.2004.02603.x [DOI] [Google Scholar]
- Park SE, Graham R, Prucha MJ and Brannon JM, 1932. Pasteurization of milk artificially infected with two strains of Brucella suis . Journal of Bacteriology, 24, 461–471. 10.1128/jb.24.6.461-471.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patazca E, Koutchma T and Balasubramaniam VM, 2007. Quasi‐adiabatic temperature increase during high pressure processing of selected foods. Journal of Food Engineering, 80, 199–205. 10.1016/j.jfoodeng.2006.05.014 [DOI] [Google Scholar]
- Patel HA and Huppertz T, 2014. Effects of High‐pressure Processing on Structure and Interactions of Milk Proteins. In: Singh H, Boland M and Thompson A (eds.). Milk Proteins: from Expression to Food, 2nd Edition. pp. 243–267. 10.1016/b978-0-12-405171-3.00008-8 [DOI]
- Patrignani F and Lanciotti R, 2016. Applications of high and ultra high pressure homogenization for food safety. Frontiers in Microbiology, 7. 10.3389/fmicb.2016.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MF, 2005. Microbiology of pressure‐treated foods. Journal of Applied Microbiology, 98, 1400–1409. 10.1111/j.1365-2672.2005.02564.x [DOI] [PubMed] [Google Scholar]
- Patterson MF and Kilpatrick DJ, 1998. The combined effect of high hydrostatic pressure and mild heat on inactivation of pathogens in milk and poultry. Journal of Food Protection, 61, 432–436. 10.4315/0362-028x-61.4.432 [DOI] [PubMed] [Google Scholar]
- Patterson MP, Mackle A and Linton M, 2011. Effect of high pressure, in combination with antilisterial agents, on the growth of Listeria monocytogenes during extended storage of cooked chicken. Food Microbiology, 28, 1505–1508. 10.1016/j.fm.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Patterson MF, Quinn M, Simpson R and Gilmour A, 1995. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate‐buffered saline and foods. Journal of Food Protection, 58, 524–529. 10.4315/0362-028x-58.5.524 [DOI] [PubMed] [Google Scholar]
- Pavli F, Argyri AA, Skandamis P, Nychas GJ, Tassou C and Chorianopoulos N, 2019. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials, 12, 26. 10.3390/ma12223726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LE, Smythe BW, Crawford RA, Oakley E, Hathaway SC and Shepherd JM, 2012. Pasteurization of milk: the heat inactivation kinetics of milk‐borne dairy pathogens under commercial‐type conditions of turbulent flow. Journal of Dairy Science, 95, 20–35. 10.3168/jds.2011-4556. [DOI] [PubMed] [Google Scholar]
- Pearce LE, Truong HT, Crawford RA, Yates GF, Cavaignac S and de Lisle GW, 2001. Effect of turbulent‐flow pasteurization on survival of Mycobacterium avium subsp. paratuberculosis added to raw milk. Applied and Environmental Microbiology, 67, 3964–3969. 10.1128/aem.67.9.3964-3969.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2021. R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R‐project.org/ [Google Scholar]
- Rademacher B, Hinrichs J and Kessler HG, 1998. Process optimization of high pressure treatment of milk by means of reaction kinetics. Proceedings of the Symposium on Fresh Novel Foods by High PressureHelsinkiFinland, 1998 Sep 21–22, 189–189. [Google Scholar]
- Rademacher B and Hinrichs J, 2006. Effects of high pressure treatment on indigenous enzymes in bovine milk: reaction kinetics, inactivation and potential application. International Dairy Journal, 16, 655–661. 10.1016/j.idairyj.2005.10.021 [DOI] [Google Scholar]
- Rademacher B, Werner F and Pehl M, 2002. Effect of the pressurizing ramp on the inactivation of Listeria innocua considering thermofluiddynamical processes. Innovative Food Science & Emerging Technologies, 3, 19–24. 10.1016/S1466-8564(02)00002-4 [DOI] [Google Scholar]
- Rademaker JL, Vissers MM and Te Giffel MC, 2007. Effective heat inactivation of Mycobacterium avium subsp. paratuberculosis in raw milk contaminated with naturally infected feces. Applied and Environmental Microbiology, 73, 4185–4190. 10.1128/aem.00326-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy HS, Jin H and Zhu SM, 2009. Effects of fat, casein and lactose on high‐pressure destruction of Escherichia coli K12 (ATCC‐29055) in milk. Food and Bioproducts Processing, 87, 1–6. 10.1016/j.fbp.2008.02.005 [DOI] [Google Scholar]
- Ramos SJ, Chiquirrin M, Garcia S, Condon S and Perez MD, 2015. Effect of high pressure treatment on inactivation of vegetative pathogens and on denaturation of whey proteins in different media. Lwt‐Food Science and Technology, 63, 732–738. 10.1016/j.lwt.2015.03.085 [DOI] [Google Scholar]
- Rasanayagam V, Balasubramaniam VM, Ting E, Sizer CE, Bush C and Anderson C, 2003. Compression heating of selected fatty food materials during high‐pressure processing. Journal of Food Science, 68, 254–259. 10.1111/j.1365-2621.2003.tb14148.x [DOI] [Google Scholar]
- Rasmussen RS and Morrissey MT, 2007. The effects of processing methods and storage on cadmium levels in Pacific oysters (Crassostrea gigas). Journal of Aquatic Food Product Technology, 16, 3–17. 10.1300/J030v16n03_02 [DOI] [Google Scholar]
- Rastogi NK, Raghavarao K, Balasubramaniam VM, Niranjan K and Knorr D, 2007. Opportunities and challenges in high pressure processing of foods. Critical Reviews in Food Science and Nutrition, 47, 69–112. 10.1080/10408390600626420 [DOI] [PubMed] [Google Scholar]
- Ravash N, Peighambardoust SH, Soltanzadeh M, Pateiro M and Lorenzo JM, 2020. Impact of high‐pressure treatment on casein micelles, whey proteins, fat globules and enzymes activity in dairy products: a review. Critical Reviews in Food Science and Nutrition, 10.1080/10408398.2020.1860899 [DOI] [PubMed] [Google Scholar]
- Reineke K, Doehner I, Schlumbach K, Baier D, Mathys A and Knorr D, 2012. The different pathways of spore germination and inactivation in dependence of pressure and temperature. Innovative Food Science & Emerging Technologies, 13, 31–41. 10.1016/j.ifset.2011.09.006 [DOI] [Google Scholar]
- Rendueles E, Omer MK, Alvseike O, Alonso‐Calleja C, Capita R and Prieto M, 2011. Microbiological food safety assessment of high hydrostatic pressure processing: a review. Lwt‐Food Science and Technology, 44, 1251–1260. 10.1016/j.lwt.2010.11.001 [DOI] [Google Scholar]
- Richter T, Sterr J, Jost V and Langowski HC, 2010. High pressure‐induced structural effects in plastic packaging. High Pressure Research, 30, 555–566. 10.1080/08957959.2010.531722 [DOI] [Google Scholar]
- Ritota M, Di Costanzo MG, Mattera M and Manzi P, 2017. New trends for the evaluation of heat treatments of milk. Journal of Analytical Methods in Chemistry, 2017. 10.1155/2017/1864832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐Canedo A, Fernandez‐Garcia E and Nunez M, 2009a. Volatile compounds in dry‐cured Serrano ham subjected to high pressure processing. Effect of the packaging material. Meat Science, 82, 162–169. 10.1016/j.meatsci.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Rivas‐Canedo A, Nunez M and Fernandez‐Garcia E, 2009b. Volatile compounds in Spanish dry‐fermented sausage ‘salchichon' subjected to high pressure processing. Effect of the packaging material. Meat Science, 83, 620–626. 10.1016/j.meatsci.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Rocha‐Pimienta J, Martillanes S, Ramirez R, Garcia‐Parra J and Delgado‐Adamez J, 2020. Bacillus cereus spores and Staphylococcus aureus sub. aureus vegetative cells inactivation in human milk by high‐pressure processing. Food Control, 113, 8. 10.1016/j.foodcont.2020.107212 [DOI] [Google Scholar]
- Rodriguez E, Arques JL, Nunez M, Gaya P and Medina M, 2005. Combined effect of high‐pressure treatments and bacteriocin‐producing lactic acid bacteria on inactivation of Escherichia coli O157: H7 in raw‐milk cheese. Applied and Environmental Microbiology, 71, 3399–3404. 10.1128/aem.71.7.3399-3404.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Alcala LM, Alonso L and Fontecha J, 2014. Stability of fatty acid composition after thermal, high pressure, and microwave processing of cow milk as affected by polyunsaturated fatty acid concentration. Journal of Dairy Science, 97, 7307–7315. 10.3168/jds.2013-7849 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Alcala LM, Castro‐Gomez P, Felipe X, Noriega L and Fontecha J, 2015. Effect of processing of cow milk by high pressures under conditions up to 900 MPa on the composition of neutral, polar lipids and fatty acids. Lwt‐Food Science and Technology, 62, 265–270. 10.1016/j.lwt.2014.12.052 [DOI] [Google Scholar]
- Roos YH, 2020. Water and pathogenic viruses inactivation‐food engineering perspectives. Food Engineering Reviews, 12, 251–267. 10.1007/s12393-020-09234-z [DOI] [Google Scholar]
- Rosario DKA, Rodrigues BL, Bernardes PC and Conte‐Junior CA, 2021. Principles and applications of non‐thermal technologies and alternative chemical compounds in meat and fish. Critical Reviews in Food Science and Nutrition, 61, 1163–1183. 10.1080/10408398.2020.1754755 [DOI] [PubMed] [Google Scholar]
- Ross T, Rasmussen S and Sumner J, 2009. Using a quantitative risk assessment to mitigate risk of Listeria monocytogenes in ready‐to‐eat meats in Australia. Food Control, 20, 1058–1062. 10.1016/j.foodcont.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Saier R, Maier G, Atamer Z and Hinrichs J, 2015. Thermal inactivation of tickborne encephalitis virus in milk. International Journal of Dairy Technology, 68. 10.1111/1471-0307.12230 [DOI] [Google Scholar]
- Santillana Farakos SM and Zwietering MH, 2011. Data analysis of the inactivation of foodborne microorganisms under high hydrostatic pressure to establish global kinetic parameters and influencing factors. Journal of Food Protection, 74, 2097–2106. 10.4315/0362-028x.Jfp-11-162 [DOI] [PubMed] [Google Scholar]
- Sanz‐Puig M, Lazaro E, Armero C, Alvares D, Martinez A and Rodrigo D, 2017. S. Typhimurium virulence changes caused by exposure to different non‐thermal preservation treatments using C. elegans . International Journal of Food Microbiology, 262, 49–54. 10.1016/j.ijfoodmicro.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Scaccabarozzi B, Brutti A and Berni E, 2020. Effect of long‐term storage, heat and high pressure processing on patulin reduction in tomato products. Journal of Food Processing & Technology, 11, 829. 10.35248/2157-7110.2020.11.829 [DOI] [Google Scholar]
- Schmerder A, Richter T, Langowski HC and Ludwig H, 2005. Effect of high hydrostatic pressure on the barrier properties of polyamide‐6 films. Brazilian Journal of Medical and Biological Research, 38, 1279–1283. 10.1590/s0100-879x2005000800018 [DOI] [PubMed] [Google Scholar]
- Schottroff F, Frohling A, Zunabovic‐Pichler M, Krottenthaler A, Schluter O and Jager H, 2018. Sublethal Injury and Viable but Non‐culturable (VBNC) state in microorganisms during preservation of food and biological materials by non‐thermal processes. Frontiers in Microbiology, 9, 19. 10.3389/fmicb.2018.02773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollard PG, Beresford TP, Murphy PM and Kelly AL, 2000a. Barostability of milk plasmin activity. Lait, 80, 609–619. 10.1051/lait:2000148 [DOI] [Google Scholar]
- Scollard PG, Beresford TP, Needs EC, Murphy PM and Kelly AL, 2000b. Plasmin activity, beta‐lactoglobulin denaturation and proteolysis in high pressure treated milk. International Dairy Journal, 10, 835–841. 10.1016/s0958-6946(01)00028-0 [DOI] [Google Scholar]
- Serment‐Moreno V, Fuentes C, Barbosa‐Canovas G, Torres JA and Welti‐Chanes J, 2015. Evaluation of high pressure processing kinetic models for microbial inactivation using standard statistical tools and information theory criteria, and the development of generic time‐pressure functions for process design. Food and Bioprocess Technology, 8, 1244–1257. 10.1007/s11947-015-1488-x [DOI] [Google Scholar]
- Serra‐Castello C, Ferrocino I, Jofré A, Cocolin L, Bover‐Cid S and Rantsiou K, 2021a. Unravelling the molecular mechanisms underlying the protective effect of lactate on the high‐pressure resistance of Listeria monocytogenes . Biomolecules, 11, 16. 10.3390/biom11050677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra‐Castello C, Jofré A, Garriga M and Bover‐Cid S, 2019. High pressure processing inactivation of Salmonella in raw pet food formulated with and without lactic acid. Proceedings of the Proceedings of the IAFP's European Symposium on Food Safety (NantesFrance). 106 pp.
- Serra‐Castello C, Jofré A, Belletti N, Garriga M and Bover‐Cid S, 2021b. Modelling the piezo‐protection effect exerted by lactate on the high pressure resistance of Listeria monocytogenes in cooked ham. Food Research International, 140, 8. 10.1016/j.foodres.2020.110003 [DOI] [PubMed] [Google Scholar]
- Setikaite I, Koutchma T, Patazca E and Parisi B, 2009. Effects of water activity in model systems on high‐pressure inactivation of Escherichia coli . Food and Bioprocess Technology, 2, 213–221. 10.1007/s11947-008-0069-7 [DOI] [Google Scholar]
- Sevenich R and Mathys A, 2018. Continuous versus discontinuous ultra‐high‐pressure systems for food sterilization with focus on ultra‐high‐pressure homogenization and high‐pressure thermal sterilization: a review. Comprehensive Reviews in Food Science and Food Safety, 17, 646–662. 10.1111/1541-4337.12348 [DOI] [PubMed] [Google Scholar]
- Shao Y, Ramaswamy HS and Zhu S, 2007. High‐pressure destruction kinetics of spoilage and pathogenic bacteria in raw milk cheese. Journal of Food Process Engineering, 30, 357–374. 10.1111/j.1745-4530.2007.00114.x [DOI] [Google Scholar]
- Sheen S, Cassidy J, Scullen B and Sommers C, 2015. Inactivation of a diverse set of shiga toxin‐producing Escherichia coli in ground beef by high pressure processing. Food Microbiology, 52, 84–87. 10.1016/j.fm.2015.07.001 [DOI] [PubMed] [Google Scholar]
- Silva FVM and Evelyn X, 2018. High‐Pressure Processing Effect on Microorganisms in Fruit and Vegetable Products. In: Houska M and Silva FVM (eds). Chapter 2 in: High Pressure Processing of Fruit and Vegetable Products, High pressure processing of fruit and vegetable products, CRC Press, pp. 3–38.
- Silva FVM and Evelyn X, 2020. Resistant moulds as pasteurization target for cold distributed high pressure and heat assisted high pressure processed fruit products. Journal of Food Engineering, 282, 8. 10.1016/j.jfoodeng.2020.109998 [DOI] [Google Scholar]
- Silva FVM and Sulaiman A, 2019. Polyphenoloxidase in Fruit and Vegetables: Inactivation by Thermal and Non‐Thermal Processes. In: Varelis P, Melton L and Shahidi F (eds.). Encyclopedia of Food Chemistry. Elsevier, Vol 2, pp. 287–301.
- Simonin H, Duranton F and de Lamballerie M, 2012. New insights into the high‐pressure processing of meat and meat products. Comprehensive Reviews in Food Science and Food Safety, 11, 285–306. 10.1111/j.1541-4337.2012.00184.x [DOI] [Google Scholar]
- Simpson RK and Gilmour A, 1997. The resistance of Listeria monocytogenes to high hydrostatic pressure in foods. Food Microbiology, 14, 567–573. 10.1006/fmic.1997.0117 [DOI] [Google Scholar]
- Smelt J, 1998. Recent advances in the microbiology of high pressure processing. Trends in Food Science & Technology, 9, 152–158. 10.1016/s0924-2244(98)00030-2 [DOI] [Google Scholar]
- Solomon EB and Hoover DG, 2004. Inactivation of Campylobacter jejuni by high hydrostatic pressure. Letters in Applied Microbiology, 38, 505–509. 10.1111/j.1472-765X.2004.01527.x [DOI] [PubMed] [Google Scholar]
- Song QH, Li Z, Chen RK, Ma XP, Xiao X and Xu J, 2019. Induction of a toxin‐antitoxin gene cassette under high hydrostatic pressure enables markerless gene disruption in the hyperthermophilic archaeon Pyrococcus yayanosii . Applied and Environmental Microbiology, 85, 12. 10.1128/aem.02662-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YS, Koontz JL, Juskelis RO, Patazca E, Limm W and Zhao K, 2021. Effect of high pressure processing on migration characteristics of polypropylene used in food contact materials. Food Additives and Contaminants Part a‐Chemistry Analysis Control Exposure & Risk Assessment, 38, 513–531. 10.1080/19440049.2020.1861341 [DOI] [PubMed] [Google Scholar]
- Sörqvist S, 2003. Heat resistance in liquids of Enterococcus spp., Listeria spp., Escherichia coli, Yersinia enterocolitica, Salmonella spp. and Campylobacter spp. Acta Veterinaria Scandinavica, 44, 1–19. 10.1186/1751-0147-44-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa SG, Delgadillo I and Saraiva JA, 2014. Effect of thermal pasteurisation and high‐pressure processing on immunoglobulin content and lysozyme and lactoperoxidase activity in human colostrum. Food Chemistry, 151, 79–85. 10.1016/j.foodchem.2013.11.024 [DOI] [PubMed] [Google Scholar]
- Stabel JR, Hurd S, Calvente L and Rosenbusch RF, 2004. Destruction of Mycobacterium paratuberculosis, Salmonella spp., and Mycoplasma spp. in raw milk by a commercial on‐farm high‐temperature, short‐time pasteurizer. Journal of Dairy Science, 87, 2177–2183. 10.3168/jds.S0022-0302(04)70038-7 [DOI] [PubMed] [Google Scholar]
- Stabel JR and Lambertz A, 2004. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp. paratuberculosis in milk. Journal of Food Protection, 67, 2719–2726. 10.4315/0362-028X-67.12.2719 [DOI] [PubMed] [Google Scholar]
- Stabel JR, Steadham EM and Bolin CA, 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Applied and Environmental Microbiology, 63, 4975–4977. 10.1128/aem.63.12.4975-4977.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr J, Fleckenstein BS and Langowski HC, 2015. The effect of high‐pressure processing on tray packages with modified atmosphere. Food Engineering Reviews, 7, 209–221. 10.1007/s12393-014-9081-z [DOI] [Google Scholar]
- Stollewerk K, Jofré A, Comaposada J, Arnau J and Garriga M 2014. NaCl‐free processing, acidification, smoking and high pressure: effects on growth of Listeria monocytogenes and Salmonella enterica in QDS processed (R) dry‐cured ham. Food Control, 35, 56–64. 10.1016/j.foodcont.2013.06.032 [DOI] [Google Scholar]
- Stratakos AC, Delgado‐Pando G, Linton M, Patterson MF and Koidis A, 2015. Synergism between high‐pressure processing and active packaging against Listeria monocytogenes in ready‐to‐eat chicken breast. Innovative Food Science & Emerging Technologies, 27, 41–47. 10.1016/j.ifset.2014.11.005 [DOI] [Google Scholar]
- Stratakos AC, Inguglia ES, Linton M, Tollerton J, Murphy L, Corcionivoschi N, Koidis A and Tiwari BK, 2019. Effect of high pressure processing on the safety, shelf life and quality of raw milk. Innovative Food Science and Emerging Technologies, 52, 325–333. 10.1016/j.ifset.2019.01.009 [DOI] [Google Scholar]
- Sung N and Collins MT, 1998. Thermal tolerance of Mycobacterium paratuberculosis . Applied and Environmental Microbiology, 64, 999–1005. 10.1128/aem.64.3.999-1005.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed QA, Buffa M, Guamis B and Saldo J, 2016. Factors affecting bacterial inactivation during high hydrostatic pressure processing of foods: a review. Critical Reviews in Food Science and Nutrition, 56, 474–483. 10.1080/10408398.2013.779570 [DOI] [PubMed] [Google Scholar]
- Tabilo‐Munizaga G, Aubourg S and Perez‐Won M, 2016. Pressure Effects on Seafoods. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds.). High Pressure Processing of Food: Principles, Technology and Applications. pp. 625–669. 10.1007/978-1-4939-3234-4_27 [DOI]
- Tabla R, Martinez B, Rebollo JE, Gonzalez J, Ramirez MR, Roa I, Rodriguez A and Garcia P, 2012. Bacteriophage performance against Staphylococcus aureus in milk is improved by high hydrostatic pressure treatments. International Journal of Food Microbiology, 156, 209–213. 10.1016/j.ijfoodmicro.2012.03.023 [DOI] [PubMed] [Google Scholar]
- Tassou CC, Galiatsatou P, Samaras FJ and Mallidis CG, 2007. Inactivation kinetics of a piezotolerant Staphylococcus aureus isolated from high‐pressure‐treated sliced ham by high pressure in buffer and in a ham model system: Evaluation in selective and non‐selective medium. Innovative Food Science & Emerging Technologies, 8, 478–484. 10.1016/j.ifset.2007.04.002 [DOI] [Google Scholar]
- Teixeira JS, Maier MB, Miller P, Ganzle MG and McMullen LM, 2016. The effect of growth temperature, process temperature, and sodium chloride on the high‐pressure inactivation of Listeria monocytogenes on ham. European Food Research and Technology, 242, 2021–2029. 10.1007/s00217-016-2700-6 [DOI] [Google Scholar]
- Teixeira JS, Repkova L, Ganzle MG and McMullen LM, 2018. Effect of pressure, reconstituted RTE meat microbiota, and antimicrobials on survival and post‐pressure growth of Listeria monocytogenes on Ham. Frontiers in Microbiology, 9, 11. 10.3389/fmicb.2018.01979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusoglu O, Alpas H and Bozoglu F, 2010. High hydrostatic pressure effects on mold flora, citrinin mycotoxin, hydroxytyrosol, oleuropein phenolics and antioxidant activity of black table olives. Innovative Food Science & Emerging Technologies, 11, 250–258. 10.1016/j.ifset.2009.11.005 [DOI] [Google Scholar]
- Tomasula PM, Renye JA, Van Hekken DL, Tunick MH, Kwoczak R, Toht M, Leggett LN, Luchansky JB, Porto‐Fett ACS and Phillips JG, 2014. Effect of high‐pressure processing on reduction of Listeria monocytogenes in packaged Queso Fresco. Journal of Dairy Science, 97, 1281–1295. 10.3168/jds.2013-7538 [DOI] [PubMed] [Google Scholar]
- Tonello‐Samson C, Queirós RP and González‐Angulo M, 2020. Advances in high‐pressure processing in‐pack and in‐bulk commercial equipment. In: Present and Future of High Pressure Processing. Elsevier, pp. 297–316. 10.1016/B978-0-12-816405-1.00013-3 [DOI]
- Torrent J, Alvarez‐Martinez MT, Heitz F, Liautard JP, Balny C and Lange R, 2004. Insights into alternative prion protein topologies induced under high hydrostatic pressure. Journal of Physics‐Condensed Matter, 16, S1059–S1065. 10.1088/0953-8984/16/14/016 [DOI] [Google Scholar]
- Truchado P, Gil MI and Allende A, 2021. Peroxyacetic acid and chlorine dioxide unlike chlorine induce viable but non‐culturable (VBNC) stage of Listeria monocytogenes and Escherichia coli O157:H7 in wash water. Food Microbiology, 100, 8. 10.1016/j.fm.2021.103866 [DOI] [PubMed] [Google Scholar]
- Trujillo AJ, Castro N, Quevedo JM, Arguello A, Capote J and Guamis B, 2007. Effect of heat and high‐pressure treatments on microbiological quality and immunoglobulin G stability of caprine colostrum. Journal of Dairy Science, 90, 833–839. 10.3168/jds.S0022-0302(07)71567-9 [DOI] [PubMed] [Google Scholar]
- Trujillo AJ, Ferragut V, Juan B, Roig‐Sagues AX and Guamis B, 2016. Processing of Dairy Products Utilizing High Pressure. In: Balasubramaniam VM, BarbosaCanovas GV and Lelieveld HLM (eds.). High Pressure Processing of Food: Principles, Technology and Applications. pp. 553–590. 10.1007/978-1-4939-3234-4_25 [DOI]
- Turner AD, Powell AL and Burrell S, 2014. Novel application of high pressure processing for the production of shellfish toxin matrix reference materials. Toxicon, 90, 1–14. 10.1016/j.toxicon.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Uyttendaele M, Busschaert P, Valero A, Geeraerd AH, Vermeulen A, Jacxsens L, Goh KK, De Loy A, Van Impe JF and Devlieghere F, 2009. Prevalence and challenge tests of Listeria monocytogenes in Belgian produced and retailed mayonnaise‐based deli‐salads, cooked meat products and smoked fish between 2005 and 2007. International Journal of Food Microbiology, 133, 94–104. 10.1016/j.ijfoodmicro.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Van Boeijen IKH, Moezelaar R, Abee T and Zwietering MH, 2008. Inactivation kinetics of three Listeria monocytogenes strains under high hydrostatic pressure. Journal of Food Protection, 71, 2007–2013. 10.4315/0362-028x-71.10.2007 [DOI] [PubMed] [Google Scholar]
- Van Brandt L, Coudijzer K, Herman L, Michiels C, Hendrickx M and Vlaemynck G, 2011. Survival of Mycobacterium avium ssp. paratuberculosis in yoghurt and in commercial fermented milk products containing probiotic cultures. Journal of Applied Microbiology, 110, 1252–1261. 10.1111/j.1365-2672.2011.04979.x [DOI] [PubMed] [Google Scholar]
- Van den Heever LW, Katz KW and Te Brugge LA, 1982. On the inactivation of Brucella abortus in naturally contaminated milk by commercial pasteurisation procedures. Journal of the South African Veterinary Association, 53, 233–234. [PubMed] [Google Scholar]
- Viazis S, Farkas BE and Allen JC, 2007. Effects of high‐pressure processing on immunoglobulin A and lysozyme activity in human milk. Journal of Human Lactation, 23, 253–261. 10.1177/0890334407303945 [DOI] [Google Scholar]
- Viazis S, Farkas BE and Jaykus LA, 2008. Inactivation of bacterial pathogens in human milk by high‐pressure processing. Journal of Food Protection, 71, 109–118. 10.4315/0362-028x-71.1.109 [DOI] [PubMed] [Google Scholar]
- Voigt DD, Kelly AL and Huppertz T, 2015. High‐pressure processing of milk and dairy products. In: Emerging Dairy Processing Technologies, 71–92. 10.1002/9781118560471.ch3 [DOI] [Google Scholar]
- Waite‐Cusic JG and Yousef AE, 2011. Inactivation of pathogenic bacteria by FD&RED No. 3 and high‐pressure processing combination treatment in food systems. Journal of Food Safety, 31, 472–479. 10.1111/j.1745-4565.2011.00323.x [DOI] [Google Scholar]
- Waterman SC, 1982. The heat‐sensitivity of Campylobacter jejuni in milk. Journal of Hyg (Lond), 88, 529–533. 10.1017/s0022172400070388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche AM, Gurtler JB, Marks BP and Ryser ET, 2009. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. Journal of Food Protection, 72, 1121–1138. 10.4315/0362-028x-72.5.1121 [DOI] [PubMed] [Google Scholar]
- Wiggers SB, Kroger‐Ohlsen MV and Skibsted LH, 2004. Lipid oxidation in high‐pressure processed chicken breast during chill storage and subsequent heat treatment: effect of working pressure, packaging atmosphere and storage time. European Food Research and Technology, 219, 167–170. 10.1007/s00217-004-0931-4 [DOI] [Google Scholar]
- Windyga B, Rutkowska M, Sokolowska B, Skapska S, Wesolowska A, Wilinska M, Fonberg‐Broczek M and Rzoska SJ, 2015. Inactivation of Staphylococcus aureus and native microflora in human milk by high pressure processing. High Pressure Research, 35, 181–188. 10.1080/08957959.2015.1007972 [DOI] [Google Scholar]
- Woldemariam HW and Emire SA, 2019. High pressure processing of foods for microbial and mycotoxins control: current trends and future prospects. Cogent Food & Agriculture, 5. 10.1080/23311932.2019.1622184 [DOI] [Google Scholar]
- Xu H, Lee H‐Y and Ahn J, 2009. High pressure inactivation kinetics of Salmonella enterica and Listeria monocytogenes in milk, orange juice, and tomato juice. Food Science and Biotechnology, 18, 861–866. [Google Scholar]
- Yamamoto K, 2017. Food processing by high hydrostatic pressure. Bioscience, Biotechnology, and Biochemistry, 81, 672–679. 10.1080/09168451.2017.1281723 [DOI] [PubMed] [Google Scholar]
- Ye A, Anema SG and Singh H, 2004. High‐pressure‐induced interactions between milk fat globule membrane proteins and skim milk proteins in whole milk. Journal of Dairy Science, 87, 4013–4022. 10.3168/jds.S0022-0302(04)73542-0 [DOI] [PubMed] [Google Scholar]
- Yi JJ, Kebede BT, Dang DNH, Buve C, Grauwet T, Van Loey A, Hu XS and Hendrickx M, 2017. Quality change during high pressure processing and thermal processing of cloudy apple juice. Lwt‐Food Science and Technology, 75, 85–92. 10.1016/j.lwt.2016.08.041 [DOI] [Google Scholar]
- Yoo S, Lee J, Holloman C and Pascall MA, 2009. The effect of high pressure processing on the morphology of polyethylene films tested by differential scanning calorimetry and X‐ray diffraction and its influence on the permeability of the polymer. Journal of Applied Polymer Science, 112, 107–113. 10.1002/app.29401 [DOI] [Google Scholar]
- Yoo S, Sigua G, Min D and Pascall MA, 2014. The influence of high‐pressure processing on the migration of irganox 1076 from polyethylene films. Packaging Technology and Science, 27, 255–263. 10.1002/pts.2030 [DOI] [Google Scholar]
- Zarzecka U, Zadernowska A and Chajecka‐Wierzchowska W, 2022. Effects of osmotic and high pressure stress on expression of virulence factors among Enterococcus spp. isolated from food of animal origin. Food Microbiology, 102, 9. 10.1016/j.fm.2021.103900 [DOI] [PubMed] [Google Scholar]
- Zhang YF, Delbruck AI, Off CL, Benke S and Mathys A, 2020. Flow cytometry combined with single cell sorting to study heterogeneous germination of Bacillus spores under high pressure. Frontiers in Microbiology, 10, 13. 10.3389/fmicb.2019.03118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobrist MR, Huppertz T, Uniacke T, Fox PF and Kelly AL, 2005. High‐pressure‐induced changes in the rennet coagulation properties of bovine milk. International Dairy Journal, 15, 655–662. 10.1016/j.idairyj.2004.07.025 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the assessment of the efficacy and safety of high‐pressure processing of food
Detailed tables of type of foods HPP treated and the processing conditions as informed by the establishments and equipment providers through the questionnaire


