Abstract
Background:
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT) is a naturally occurring, short-acting psychedelic tryptamine, produced by a variety of plant and animal species. Plants containing 5-MeO-DMT have been used throughout history for ritual and spiritual purposes. The aim of this article is to review the available literature about 5-MeO-DMT and inform subsequent clinical development.
Methods:
We searched PubMed database for articles about 5-MeO-DMT. Search results were cross-checked against earlier reviews and reference lists were hand searched. Findings were synthesised using a narrative synthesis approach. This review covers the pharmacology, chemistry and metabolism of 5-MeO-DMT, as well epidemiological studies, and reported adverse and beneficial effects.
Results:
5-MeO-DMT is serotonergic agonist, with highest affinity for 5-HT1A receptors. It was studied in a variety of animal models, but clinical studies with humans are lacking. Epidemiological studies indicate that, like other psychedelics, 5-MeO-DMT induces profound alterations in consciousness (including mystical experiences), with potential beneficial long-term effects on mental health and well-being.
Conclusion:
5-MeO-DMT is a potentially useful addition to the psychedelic pharmacopoeia because of its short duration of action, relative lack of visual effects and putatively higher rates of ego-dissolution and mystical experiences. We conclude that further clinical exploration is warranted, using similar precautions as with other classic psychedelics.
Keywords: 5-methoxy-N, N-dimethyltryptamine, 5-MeO-DMT, classic psychedelic, hallucinogen, tryptamine
Introduction
Plant- and fungi-based psychedelics have been used for centuries for healing or ritual purposes (Schultes and Hofmann, 1980), and there is an active culture of self-medication with psychedelics for mental health (Carhart-Harris and Nutt, 2010). The classical psychedelic drugs were investigated extensively in psychiatry before they were placed in Schedule I of the UN Convention on Psychotropic Substances 1971 (United Nations Convention on Psychotropic Substances, 1971) and resulted in significant barriers to research and drug development with them (Johnson et al., 2008; Rucker et al., 2018; Weston et al., 2020).
Over the past two decades, research has resumed and encouraging early phase clinical trials assessing psilocybin-assisted psychotherapy have been reported in unipolar mood disorders and anxiety (Carhart-Harris et al., 2016, 2018; Griffiths et al., 2016; Grob et al., 2011; Ross et al., 2016) and substance use disorders (Bogenschutz et al., 2015, 2018; Johnson et al., 2014; Noorani et al., 2018).
The duration of action of classical psychedelics varies considerably. After oral ingestion, the subjective effect of lysergic acid diethylamide (LSD) lasts approximately 12 h (Holze et al., 2019), while psilocybin lasts approximately 6 h (Hasler et al., 2004). Short-acting psychedelics may have therapeutic benefit (Nutt et al., 2020). Several survey studies have examined reports of addiction recovery prompted by the use of dimethyltryptamine (DMT) (Garcia-Romeu et al., 2019; Johnson et al., 2017). If efficacious, an advantage of short-acting psychedelics may be lower treatment costs. This may allow wider delivery of treatment, if clinical trial data supports licensing.
History of discovery
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT) is a short-acting serotonergic psychedelic that was first synthesised in 1936 (Hoshino and Shimodaira, 1936) and later isolated from Dictyoloma incanescens in 1959 (Pachter et al., 1959). Subsequently, 5-MeO-DMT has been found in a large number of plants (reviewed in Trout, 2007), notably Anadenanthera, Phalaris and Virola spp. (Rätsch, 2005; Schultes et al., 2001; Schultes and Hofmann, 1980). 5-MeO-DMT is found in fungi Amanita citrina and Amanita porphyria (Tyler and Gröger, 1964), as well as the gland secretions of the Sonoran Desert toad Incilius (formerly Bufo) alvarius (Erspamer et al., 1967; Uthaug et al., 2019; Weil and Davis, 1994) and in mammals (Barker et al., 2012; Beaton and Morris, 1984).
Occurrence in nature
5-MeO-DMT is likely to be endogenously produced in humans, as it has been detected in blood, urine and cerebrospinal fluid (Christian et al., 1975; Corbett et al., 1978; Guchhait, 1976; Heller et al., 1970; Narasimhachari et al., 1971a, 1971b; Riceberg and Vunakis, 1978; Smythies et al., 1979; Tanimukai, 1967, Tanimukai et al., 1970), although several studies contradict this finding (Forsström et al., 2001; Himwich et al., 1972; Huszka et al., 1976; Narasimhachari et al., 1972, 1974). Pooling these studies together, 5-MeO-DMT was detected in urine of 2 out of 113 individuals, in blood of 20 out of 39 individuals and in cerebrospinal fluid of 40 out of 136 individuals. However, it is important to note that only the two later studies (Corbett et al., 1978; Smythies et al., 1979) used mass spectrometry, while the older studies used less reliable methods. The physiological role of 5-MeO-DMT is unknown and more research is needed to definitively answer if, when and where 5-MeO-DMT is endogenously produced.
Traditional use
Indigenous peoples of South America have used 5-MeO-DMT containing plants for thousands of years (Pochettino et al., 1999; Torres and Repke, 2006). Snuffs from the beans of Anadenanthera peregrina (called cohoba, yopo) are prepared in northern South America, although the use of this plant in pre-Colombian times has been documented as far as the West Indies (Schultes and Hofmann, 1980). In central and southern parts of South America, snuffs called vilca, huilca and cibil produced from A. colubrina are used. Many species of Virola trees (e.g. V. theiodora, V. calophylla, V. elongata) are utilised by the indigenous peoples in the Amazon region (Schultes et al., 2001). 5-MeO-DMT is present in plants that are sometimes used as constituents in ayahuasca (Holmstedt et al., 1980).
The popularity of toad secretions is a fairly recent phenomenon traceable to the publication of a booklet by Albert Most (1984). There is no conclusive historical evidence for the indigenous use of Incilius alvarius toads for their psychoactive properties prior to this (Ott, 1996).
Legal status
5-MeO-DMT was included in the Schedule 1 Controlled Substance Act in the United States of America in 2009 (Drug Enforcement Administration (DEA), Department of Justice, 2010) and is a controlled substance in the United Kingdom (Home Office, 2019), Australia (Federal Register of Legislation, 2016), New Zealand (New Zealand Legislation, 2021) and several other countries. It is not listed by the United Nations Convention on Psychotropic Substances, and in many countries, including Canada, this substance is not controlled (Canada Justice Laws, 1996).
Epidemiological surveys suggest increasing non-medical use of 5-MeO-DMT, with users often reporting improvements in outcomes relating to mental health (Davis et al., 2018; Uthaug et al., 2019, 2020b).
This narrative review of published 5-MeO-DMT research aimed to synthesise the available literature and provide a comprehensive overview of the pre-clinical and safety data. It is considered timely and important because interventional clinical trials with this compound are being initiated (Clinicaltrials.gov, 2019, 2021a, 2021b).
Methods
References for this article were identified via a search of PubMed from January 1965 to October 2020 using the terms ‘5-methoxy-N,N-dimethyltryptamine’ or ‘5-MeO-DMT’ and other variations on the chemical name (for full search terms, see Supplementary Material). Papers in English, Russian or Spanish were included, representing the fluent language proficiencies of the authors. The PubMed search was supplemented by additional articles, which were identified during the review of the bibliographies from the papers sourced through PubMed. References were then selected on the basis of relevance to the content of review.
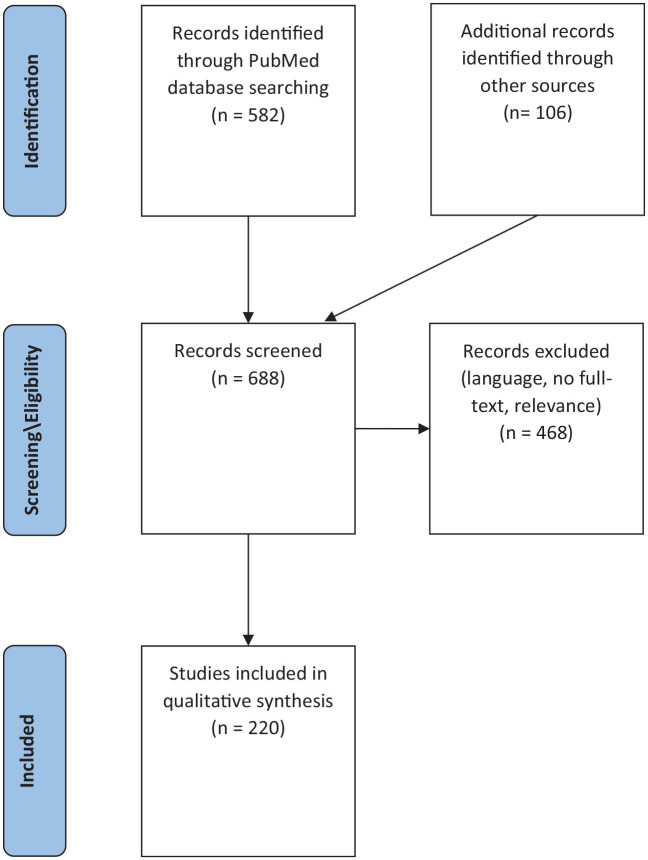
As this review aims to inform future clinical research, we excluded studies on the chemical synthesis or forensic detection of 5-MeO-DMT, articles identifying 5-MeO-DMT in plants and others that were not providing novel information about 5-MeO-DMT aside from its use at 5-HT agonist. The number of sources we identified, screened and included/excluded can be found in Figure 1.
Figure 1.
PRISMA flow diagram.
Description of studies/topics
Chemical properties
2-(5-methoxy-1H-indol-3-yl)-N,N-dimethylethanamine (5-methoxy-N,N-dimethyltryptamine, abbreviated to 5-MeO-DMT) is a tryptamine alkaloid, an aromatic ether and a tertiary amine with a molecular weight of 218.298 g/mol and the chemical formula C13H18N2O. As the freebase, 5-MeO-DMT is a white solid with a melting point of 69.5°C. Water solubility is >32.7 μg/mL (PubChem, 2021).
In vitro pharmacology
5-MeO-DMT is a non-selective serotonin (5-HT) receptor agonist, with affinity to other receptors, as well as to serotonin and norepinephrine transporters (Halberstadt et al., 2012; PDSP Database, 2021; Ray, 2010; see Table 1). 5-MeO-DMT is a weak 5-HT reuptake inhibitor but has no appreciable effects on monoamine release nor on noradrenaline or dopamine reuptake (Berge et al., 1983; Blough et al., 2014; Nagai et al., 2007). 5-MeO-DMT has high affinity for a range of 5-HT receptors, particularly (inhibition constant [Ki] < 100 nM) at cloned human 5-HT1A, 5-HT1B, 5-HT1D, 5-HT6 and 5-HT7 receptor subtypes. High affinity is seen for the 7-transmembrane G-protein-coupled 5-HT receptors, with no affinity for the ion-channel 5-HT3 receptor. 5-MeO-DMT’s binding affinity to sigma receptors is >10,000 nM, although one study indicated that 5-MeO-DMT can impact immune responses in human monocyte-derived dendritic cells via σ-1 (Szabo et al., 2014).
Table 1.
Receptor binding profiles for 5-MeO-DMT.
| Binding sites | Binding affinity, Ki (nM) Ray (2010) |
Binding affinity, Ki (nM) Halberstadt et al. (2012) |
Binding affinity, Ki (nM)PDSP Ki database |
|---|---|---|---|
| Serotonin (5-HT) receptors | |||
| 5-HT1A | 1.9 | 3.0 | |
| 5-HT1B | 74 | 14 | 351 |
| 5-HT1D | 6.3 | 2.3 | |
| 5-HT1E | 360.2 | 376 | |
| 5-HT2A | 2011 | 907 | 14 |
| 390 | |||
| 207 | |||
| 600 | |||
| 616 | |||
| 617 | |||
| 620 | |||
| 5-HT2B | 3884 | 36 | 1300 |
| 5-HT2C | 538 | 418 | 87.1 |
| 100 | |||
| 5-HT5A | 276.6 | 505 | |
| 5-HT6 | 35.2 | 6.5 | |
| 5-HT7 | 3.9 | 4.5 | |
| Dopamine receptors | |||
| D1 | 79.5 | >10,000 | |
| D2 | 3562 | >10,000 | |
| D3 | 497.6 | >10,000 | |
| D4 | 3120 | >10,000 | |
| D5 | >10,000 | >10,000 | |
| Norepinephrine receptors | |||
| α1A | >10,000 | 4373 | |
| α1B | >10,000 | 2188 | |
| α2A | 1890 | 938 | |
| α2B | 2640 | 430 | |
| α2C | 508.1 | 206 | |
| β2 | >10,000 | 2679 | |
| Other receptors and transporters | |||
| σ-1 | >10,000 | >10,000 | |
| σ-2 | >10,000 | 3689 | |
| H1 | ND | 7580 | |
| Serotonin transporter protein (SERT) | 2032 | 3603 | |
| Dopamine active transporter (DAT) | >10,000 | >10,000 | |
| Norepinephrine transporter (NET) | 2859 | >10,000 | |
The raw Ki data is from Supplementary Table S2, Ray (2010) or Table 1, Halberstadt et al. (2012), both based on cloned human receptors in cell lines. 5-MeO-DMT also binds to the trace amine-associated receptor 1 (TAAR1), but the Ki is not provided (Wallach, 2009). 5-MeO-DMT bound to the following sites with Ki values >10,000 nM: 5-HT3, Ca2+ channels, β1, β3, DOR, MOR, KOR, EP3, EP4, GABAA, H2, H3, H4, M1, M2, M3, M4, M5 (Halberstadt, 2012). Additionally, we present older data, based on rat or pig brain homogenates, retrieved from PDSP Ki database (Roth et al., 2000) in January 2021 (https://pdsp.unc.edu/databases/kidb.php).
Receptor binding profiles based on human cloned receptors in cell lines presented by Halberstadt et al. (2012) and Ray (2010) are shown in Table 1. Although their findings are similar for the 5-HT receptors, they differ for others; further research is required to resolve these discrepancies.
Radioligand binding studies show that 5-MeO-DMT has about 300-fold selectivity for the 5-HT1A (3 ± 0.2 nM) versus 5-HT2A (907 ± 170 nM) receptor subtypes (Halberstadt et al., 2012). Other receptor types have not been studied as extensively (Halberstadt and Geyer, 2011).
More research is needed to resolve the full receptor binding profile of 5-MeO-DMT and understand the functionally selective pharmacology at 5-HT2A and other receptors. Precise changes in receptor conformation result in different signalling cascades with different effects (e.g. behavioural or gene expression) (Urban et al., 2007). Put another way, every ligand has its own signalling signature, which may (or may not) be similar to the endogenous ligand.
For example, hallucinogenic and non-hallucinogenic 5-HT2A agonists differentially activate second messenger pathways (González-Maeso et al., 2007). Kurrasch-Orbaugh et al. demonstrated that 5-MeO-DMT activated phospholipase A2 (PLA2) signalling 13-fold more than phospholipase C (PLC) signalling (Kurrasch-Orbaugh et al., 2003). Added to this, β-arrestins are scaffolding proteins that can attenuate or facilitate G-protein-coupled receptor activity by, for example, receptor internalisation or the formation of heteroreceptors (Gurevich and Gurevich, 2004). Schmid and Bohn demonstrated that the actions of 5-HT require the β-arrestin-2 signalling pathway and activation of protein kinase B, while 5-MeO-DMT activates signalling cascades independent of β-arrestin-2 (Schmid and Bohn, 2010). Blough et al. (2014) confirmed this observation, demonstrating a 100-fold difference in potency for the G-protein-coupled compared to the β-arrestin signalling pathway for 5-MeO-DMT. Overall, the functional selectivity of exogenous versus endogenous ligands for receptors is highly complex, but likely important in understanding their observable effects.
Pharmacokinetics
The pharmacokinetics of 5-MeO-DMT has been studied in mice (Jiang et al., 2013, 2015, 2016a; Shen et al., 2010a, 2011b, 2011) and rats (Halberstadt, 2016; Sitaram et al., 1987a, 1987b, 1987c, 1987d).
Absorption
The maximum concentration (Cmax) in plasma is reached after 5–6 min following an intraperitoneal (IP) injection, and the terminal half-life (t1/2) is 12–19 min in mice (Shen et al., 2009). A similar profile is seen in rats, with Cmax = 5–10 min and t1/2 = 6–16 min (Sitaram et al., 1987a, 1987d).
Tissue distribution and protein binding
5-MeO-DMT is lipid-soluble (3.30 oil/water partition coefficient) and readily crosses the blood–brain barrier (BBB) (Gessner et al., 1968). 5-MeO-DMT distributes to the liver, kidneys and brain similarly in different animal models: rabbit, rat and mouse (Berger et al., 1978; Sitaram et al., 1987c, 1987d; Sitaram and McLeod, 1990). Brain concentrations of 5-MeO-DMT in the rat are 1.7-fold higher compared to plasma 45 min after IP injection, with highest concentrations in the cortex, thalamus, hippocampus, basal ganglia, medulla, pons and cerebellum (Barker et al., 2001; Sitaram et al., 1987c). In the mouse brain, 5-MeO-DMT distributes to the cortex, hippocampus, hypothalamus and striatum after IP administration (Shen et al., 2010b).
Metabolism and excretion
Shen et al. (2011) demonstrated that the pharmacokinetics of 5-MeO-DMT is non-linear for both IP and intravenous (IV) administration of high doses in mice. The estimated parameters for both IP and IV routes are as follows: maximum rate of reaction (Vmax), Michaelis constant (Km), clearance (CL) and additional clearance (CLCYP2D6) values are 2.76 mmol/min per kg, 13.2 mM, 0.21 min−1 kg−1 and 0.0256 L/min per kg, respectively. The CLCYP2D6 value represents the additional linear clearance of 5-MeO-DMT from the central compartment that is dependent on CYP2D6 protein.
5-MeO-DMT is extensively metabolised through oxidative deamination catalysed by monoamine oxidase A (MAOA). O-demethylation, N-demethylation and N-oxygenation are involved to a much smaller extent (Sitaram and McLeod, 1990). Metabolic studies performed in rats showed that 5-methoxyindoleacetic acid (5-MIAA) is the main urinary metabolite of 5-MeO-DMT (54%), followed by 5-hydroxy-N,N dimethyltryptamine glucuronide (23%), 5-hydroxyindoleacetic acid (5-HIAA, 14%) and bufotenine (9%) (Agurell et al., 1969; Ahlborg et al., 1968; Sitaram et al., 1987d; Squires, 1975; Suzuki et al., 1981; Yu et al., 2003; see Figure 2).
Figure 2.
Metabolism of 5-MeO-DMT.
When doses of 10 or 20 mg/kg of 5-MeO-DMT (IP and IV) are administered to mice, a 50% decrease in systemic clearance is observed, indicating that MAOA-mediated metabolism becomes saturated. This non-linearity is also reflected in corresponding increases in brain concentration of 5-MeO-DMT (Shen et al., 2010b, 2011).
The extent of O-demethylation depends on the genetic variant of the cytochrome P450 2D6 enzyme (CYP2D6). This enzyme mediates production of the psychoactive metabolite bufotenine from 5-MeO-DMT (Shen et al., 2010b).
In vivo pharmacology
Studies of 5-MeO-DMT have been conducted in mice, rats, gerbils, hamsters, guinea pigs, rabbits, goldfish, cats, dogs, sheep, pigs and primates. The most common route of administration for rodents was subcutaneous or intraperitoneal. For a summary of the doses, routes of administration and behavioural effects by species, see Tables 2 and 3.
Table 2.
Doses and routes of administration in different species.
| Species | References | Threshold dose | Effective dose | High dose | LD50 |
|---|---|---|---|---|---|
| Goldfish | Abramson et al. (1979) | NA | 5 µg IC for a 1.5–3 g fish | NA | NA |
| Mouse* | Jiang et al. (2016a, 2016b); Winter et al. (2011); Martin et al. (1985); Moser and Redfern (1985); Gillin et al. (1976); Ho et al. (1970); Benington et al. (1965) | NA | 0.3–5 mg/kg IP, SC | >8 mg/kg IP, IV >32 mg/kg IP toxicity |
75–115 mg/kg IP 48 mg/kg IV 113 mg/kg SC 278 mg/kg O |
| Rat* | Halberstadt et al. (2008, 2012); Krebs-Thomson (2006); Critchley and Handley (1987); Winter and Petti (1987); Gudelsky et al. (1986); Tricklebank et al. (1985); Trulson and MacKenzie (1981); Bedard and Pycock (1977) | <0.1 mg/kg IP, SC | 0.5–2 mg/kg IP, SC | >3 mg/kg IP | NA |
| Gerbil | Eison and Wright (1992) | 0.5 mg/kg SC | NA | 8 mg/kg SC | NA |
| Guinea-pig | Evenden (1994); Nielsen (1998) | NA | 1–10 mg/kg SC | NA | NA |
| Hamster | Richter and Löscher (1995) | NA | 1–4 mg/kg IP | >5 mg/kg IP | NA |
| Rabbit | Romano et al. (2010) | NA | 0.29 mg/kg | NA | NA |
| Cat | Trulson and Jacobs (1979); Benington et al. (1965) | 0.025 mg/kg IM | 0.25–0.5 mg/kg IM 0.1–0.3 IV |
>1–5 mg/kg IM, IV, | 15 mg/kg IM |
| Dog | Gessner et al. (1961) | NA | 0.1 mg/kg IV | NA | NA |
| Sheep | Bourke et al. (1990); Bourke et al. (1988); Gillin et al. (1976); Gallagher et al. (1964) | 0.02 mg/kg IV >18 mg/kg O |
0.1–0.7 mg/kg IV 40 mg/kg O |
>1 mg/kg IV >40 mg/kg O |
1–5 mg/kg IV 1–2 mg/kg SC 85 mg/kg O |
| Pig | Löscher et al. (1990) | NA | 0.5–1.8 mg/kg IV | NA | NA |
| Grivet monkey | Nielsen (1985) | NA | 0.45 mg/kg SC | NA | NA |
| Stumptail macaque monkey | Schlemmer and Davis (1981, 1981); Schlemmer et al. (1977) | 0.05 mg/kg IM | 0.1–0.25 mg/kg IM | NA | NA |
| Rhesus monkey | Gillin et al. (1976) | >0.1 mg/kg IV | 0.25 mg/kg IV | 8–16 mg/kg IV | NA |
| Human | Erowid (2021); Metzner (2013); Shulgin and Shulgin (1997); Ott (2001); Davis et al. (2018); Uthaug et al. (2020a) | 1–2 mg S 3–5 mg IN 0.25 mg IV |
2–10 mg S 5–15 mg IN 10 mg SL 10–30 mg O 0.5–2 mg IV 1.4–10 mg IM |
10–20 mg S 10–25 mg IN >30 mg O >2 mg IV |
NA |
IC: intracranial; IM: intramuscular; IN: intranasal; IP: intraperitoneal; IV: intravenous; MAOI: monoamine oxidase inhibitor; NA: information not available; O: oral; S: smoked or vapourised; SC: subcutaneous; SL: sublingual.
Minimal (or threshold) dose is defined as the dose after which any difference in behaviour or physiology compared to baseline is observed. Effective dose is defined in a similar way to ED50 and reliably produces hallucinogenic-like and other characteristic behavioural effects in animal models. High dose is the one leading to marked serotonin syndrome or other serious adverse effects. Note that these dose ranges are an approximation, depend on particular behaviour/task, and in some species based on only one or two studies and drug administrations. The species differences could be due to the pharmacokinetics and metabolism differences, as well as the physiology of the specific animal models. It is also likely that direct mg/kg comparison is not appropriate across species and interspecies scaling factor is necessary for the meaningful comparison.
Representative references, selecting studies containing multiple doses.
Table 3.
Behavioural effects of 5-MeO-DMT in different species.
| Species | Effects | References |
|---|---|---|
| Cotton boll weevil (Anthonomus grandis) | Potent anti-feeding effect | Miles et al. (1987) |
| Goldfish (Carassius auratus) | Surfacing behaviour, which is a characteristic hallucinogen response in fish | Abramson et al. (1979) |
| Mouse* (Mus musculus) | • Mice discriminate 5-MeO-DMT from saline • Dose-dependent increase in the head-twitch response. Head-twitch response shows circadian variation • Reduction in locomotor activity and in investigatory behaviour • Increased latency to feed in the novel environment • Inhibition of isolation-induced aggression • Inhibition of memory retention in conditional avoidance task • Facilitation of memory retention in active avoidance task by lower, but not higher doses • Straub tail, forepaw threading, twitching, flat-body posture, hindlimb abduction, tremors • Reduced sensitivity to pain (increased tail flick response latencies) |
Jiang et al. (2016a, 2016b); Winter et al. (2011); Halberstadt et al. (2011); Van den Buuse et al. (2011); Duvvuri et al. (2009); Pavone et al. (1993); Sánchez et al. (1993); Eide and Tjølsen (1988); Quartermain et al. (1988); Martin et al. (1985); Moser and Redfern (1985); Singleton and Marsden (1981); Benington et al. (1965) |
| Rat* (Rattus norvegicus domestica) | • Rats discriminate 5-MeO-DMT from saline, but not from other classic psychedelics • Dose-dependent inhibition of locomotor activity, reduction in investigatory behaviour (increased fear of the open spaces and novel objects) • Dose-dependent increase in forepaw treading, flat-body posture, Straub tail response, hindlimb abduction, tremor, head-twitch response–no sex differences in these effects in response to 5-MeO-DMT • Medium doses: ‘wet-dog’ shakes, head shakes, lower lip retraction • Increased latency to feed in the novel environment (hyponeophagia) and reduced palatability of sucrose drink • High doses: severe tremors, shivering, biting of paws, convulsions, muscle spasms, rocking from side to side, compulsively biting grid floor/cage, walking backwards and abnormal gait, inhibit shock-elicited fighting, respiratory arrest • Non-linear effect on nociception, measured with tail flick latencies (enhanced at low doses, reduced at high doses) • Inhibition of conditioned avoidance, failure to orient towards ‘warning’ stimuli, deficit in aversive conditioning learning • Normal doses stimulate sexual behaviour (mounts, ejaculatory response, a decrease in the number of intromissions to ejaculation and in the ejaculation latency) in male rats • In neonatal rats, 5-MeO-DMT at normal doses had no effect in either 5- or 20-day-old pups. High dose produced increase in locomotion in 5-day-old pups, and hyperlocomotion, tremor, flattened body posture, forepaw threading and head weaving in 20-day-old pups • Chronic administration: tolerance develops to some drug effects, but only with very frequent drug administration (twice/per day or every 30 min) |
Halberstadt et al. (2008, 2012); Krebs-Thomson et al. (2006); Winter et al. (2000); Ahlenius and Larsson (1991); O’Hare et al. (1991); Berendsen et al. (1989); Critchley and Handley (1987); Winter and Petti (1987); Dickinson and Curzon (1986); Rényi (1986a, 1986b); Sills et al. (1985); Tricklebank et al. (1985); Trulson et al. (1985); Archer et al. (1982); Shephard and Broadhurst (1982); Berge (1982); Berge et al. (1980); Walters et al. (1978); Bedard and Pycock (1977); Gillin et al. (1976); Grahame-Smith (1971); Ahlborg et al. (1968); Gessner and Page (1962); Gessner et al. (1961) |
| Mongolian gerbil (Meriones unguiculatus) | • Reciprocal forepaw treading, reciprocal hindleg body scratch, hindleg abduction, body tremors and Straub tail | Eison and Wright (1992) |
| Guinea-pig (Cavia porcellus) | • Dose-dependent increase in the locomotor activity of naïve, unhabituated guinea pigs. Interestingly, there results are opposite to what is commonly observed in rats, that is, decrease in locomotor activity • At higher doses head jerking and whole-body myoclonic jerking. Flat-body posture, tremor and head twist or head shake |
Evenden (1994)
Nielsen (1998) |
| Syrian hamster (Mesocricetus auratus) | • At lower doses, the predominant effect was flat-body posture • Higher doses additionally induced hyperlocomotion and hindlimb abduction, plus salivation, ataxia and piloerection. Unlike in studies with other rodents, no forepaw threading was observed |
Richter and Löscher (1995) |
| Rabbit (Oryctolagus cuniculus domesticus) | • 5-MeO-DMT produced head bobs but not body shakes | Romano et al. (2010) |
| Cat (Felis catus) | • Limb flicking, abortive grooming (starting the motion as if to groom, and stopping it), head shaking, staring, investigatory and hallucinatory-like behaviours similar to those produced by other psychedelics, except for the faster onset and shorter duration in case of 5-MeO • ‘Sham rage’ response, hissing, growling, withdrawal, salivation • Inhibition of conditioned avoidance response (i.e. no reaction to auditory stimulus associated with electric shock) • Chronic effects: no tolerance during once-daily administration |
Trulson and Jacobs (1979); Benington et al. (1965) |
| Sheep (Ovis aries) | • Low doses: urination, tail, ear and lip twitching, lip licking, head shaking, agitation, pupil dilation, mild hind limb paresis and mild ataxia • Medium doses: chewing movements, salivation, head and body tremors, neck extension, hind limb paresis, ataxia, hypermetria, walking backwards or in circles, walking on the knees and intermittent periods of either sitting on the haunches of in sternal recumbency, moderate pelvic and thoracic limb paresis, disturbed equilibrium and laboured breathing • High doses: all of the previous clinical signs plus protracted periods of recumbency accompanied by vigorous attempts to get up, knuckling over in the fore fetlocks, mild cyanosis of the mucous membranes and mild respiratory distress, muscle rigidity, intermittent periods of reduced consciousness when the animals lay down, with necks extended, heads swaying, and eyes staring, tetanic spasms, acute respiratory and heart failure, death |
Bourke et al. (1988, 1990); Gillin et al. (1976); Gallagher et al. (1964) |
| Pig (Sus scrofa domesticus) | • Grimacing, backward locomotion, blank stare, screams, head shakes, generalised tremor followed by lateral recumbency with muscle rigidity | Löscher et al. (1990) |
| Grivet monkey (Chlorocebus aethiops) | • 5-MeO-DMT substitutes completely for LSD in drug discrimination studies | Nielsen et al. (1985) |
| Stumptail macaque monkey (Macaca arctoides) | • Acute effects: increased submissive gestures and hyperactivity, a reduction in social grooming and other social behaviour, an increase in distancing from other monkeys, and increase in checking, limb jerks, body shakes. Animals appear alert and restless • Chronic effects: no tolerance after daily administration. With more frequent drug administration every 30 min for 9 h, and then 26 h later tolerance developed to limb jerks, body shakes and checking behaviour |
Schlemmer and Davis (1981, 1986); Schlemmer (1977); Heinze et al. (1983) |
| Rhesus macaque monkey (Macaca mulatta) | • Medium doses: ataxia, decreased spontaneous movement and climbing, and unresponsiveness to salient external stimuli, slow nystagmoid movements and mydriasis, stringy salivation, jaw clenching, loss of motor coordination and diminished muscle tone • High doses: animals were comatose and could not be aroused • Chronic: no tolerance to 5-MeO-DMT with daily administration |
Gillin et al. (1976) |
LSD: lysergic acid diethylamide.
For rats and mice, representative references are presented.
Behavioural effects
The behavioural effects of 5-MeO-DMT have been best characterised in rodents and are similar to those of other classic hallucinogens, although rodent strain differences have been observed (Cazala and Garrigues, 1983; Gudelsky et al., 1985; Shephard and Broadhurst, 1983; Stoff et al., 1978). Rats quickly learn to discriminate 5-MeO-DMT from saline (Glennon et al., 1979, 1982a, 1982b; Spencer et al., 1987), but not from another classic psychedelic, including partial generalisation with a more selective 5-HT2A, 2B and 2C agonist 2,5-Dimethoxy-4-methylamphetamine (DOM) (Glennon et al., 1979, 1980, 1982a, 1982b; Spencer et al., 1987; Winter et al., 2000; Young et al., 1982). The 5-MeO-DMT discriminative stimulus involves both 5-HT1A- and 5-HT2A-mediated components, although the latter plays a less important role as the discriminative stimuli induces by 5-MeO-DMT are diminished by 5-HT1A antagonists (Schreiber and de Vry, 1993; Spencer et al., 1987; Winter et al., 2000). A signature behavioural response to 5-HT2A receptor stimulation and a behavioural mode of hallucinogenic effect in rodents, the head-twitch, is induced by 5-MeO-DMT over a comparable dose range to 5-HT1A-mediated behaviours, is attenuated by selective 5-HT2A receptor antagonists and is absent in 5-HT2A-knockout mice (Halberstadt, 2016; Halberstadt et al., 2011; Matsumoto et al., 1997).
Other behavioural effects of 5-MeO-DMT are predominantly 5-HT1A mediated, although 5-HT2A receptor activation is also involved (Berendsen et al., 1989; Eison and Wright, 1992; Halberstadt and Geyer, 2011; Krebs-Thomson et al., 2006; Lucki et al., 1984; Smith and Peroutka, 1986; Tricklebank et al., 1985). Activity at the 5-HT2C receptor serves to modify some of the behavioural effects of hallucinogens (Halberstadt et al., 2011). 5-MeO-DMT dose dependently reduces locomotor activity, reduces investigatory behaviour but induces forepaw treading, flat-body posture, Straub tail response and hindlimb abduction. This appears to be mainly mediated through 5-HT1A receptors, with some contribution of 5-HT2A receptors (Bedard and Pycock, 1977; Castellanos et al., 2020; Eide and Tjølsen, 1988; Halberstadt, 2016; Halberstadt et al., 2008, 2011, 2012; Jiang et al., 2016b; Krebs-Thomson et al., 2006; Matsumoto et al., 1997; Matthews and Smith, 1980; Rigdon and Weatherspoon, 1992; Smith and Peroutka, 1986; Tricklebank et al., 1985; Van den Buuse et al., 2011). 5-MeO-DMT at high doses inhibits shock-elicited fighting in rats (Walters et al., 1978). 5-MeO-DMT at medium doses stimulates male sexual behaviour in rats (Ahlenius and Larsson, 1991; Kolbeck and Steers, 1992; Rényi, 1986a, 1986b). See Table 3 for the full list of behavioural effects in different animal models.
Neurobiological effects
In a healthy volunteer field study evaluating EEG and psychedelic experience correlates, Acosta-Urquidi observed that smoked 5-MeO-DMT suppressed alpha frequencies acutely, followed by a rebound increase in alpha-power ~20 min post inhalation. The time course and intensity of the subjective experience correlated with the magnitude of the observed EEG effects (Acosta-Urquidi, 2015). Other effects were an emergent increase in the delta/theta power. The findings are broadly consistent with those from a DMT study (Timmermann et al., 2019).
Riga et al. (2016, 2014, 2018) have investigated the neuropharmacology of 5-MeO-DMT in various rodent models and propose that effects on medial prefrontal cortex (mPFC) oscillatory activity and cortico-thalamic coherence underpin its antidepressant-like effect. 5-MeO-DMT disrupted low-frequency mPFC oscillations in a similar way to other 5-HT2A-mediated classic psychedelics and decreased blood oxygen level–dependent (BOLD) responses in visual cortex (V1) and mPFC. The effects observed in both normal and 5-HT2A knockout mice were reversed by a 5-HT1A receptor antagonist, indicating the importance of 5-HT1A receptors in the effects of 5-MeO-DMT (Riga et al., 2016, 2018). In rats, 5-MeO-DMT altered the frequency and pattern of firing of level V pyramidal neurons in mPFC and reduced the amplitude of low-frequency oscillations (Riga et al., 2014).
Winne et al. (2020) found that pre-treatment with 5-MeO-DMT prevented anxiety-like behaviour (measured in the open field test and elevated plus maze) and abnormal neural activity (increase in theta 2 and slow gamma oscillations in the hippocampus and mPFC) triggered by tinnitus in mice.
Lima da Cruz et al. demonstrated that 5-MeO-DMT increases neuronal progenitor cell proliferation and survival in the mouse hippocampus. A single dose of 5-MeO-DMT increased the number of progenitor cells in the dentate gyrus, which survived better and matured faster (i.e. had more complex dendrites and greater capacity for high-frequency firing) compared to those of saline-treated animals (Lima da Cruz et al., 2018).
Earlier studies examined the effects of 5-MeO-DMT on cat and rat neuron firing in the central and peripheral nervous systems. Generally, 5-MeO-DMT in cats produces a rapid, dose-dependent inhibition of 5-HT neuronal activity (Adrien and Lanfumey, 1986; Fornal et al., 1985, 1994; Heym et al., 1982; Jacobs et al., 1983; Kodama et al., 1989; Rasmussen et al., 1984; Trulson et al., 1984a, 1984b) and antiepileptic effects (Wada et al., 1992). 5-MeO-DMT increases the excitability of several types of spinal neurons, including motoneurons, and consequently influences the locomotor pattern as well as the reflex responsiveness in cats with severed spinal cords (Barbeau and Rossignol, 1990). In rats, 5-MeO-DMT dose dependently increases the activity of motoneurons through 5-HT2 receptors, but it has an inhibitory action on the pathway of the monosynaptic reflex (Yamazaki, 1992).
Cardiovascular effects
Psychedelics may increase heart rate and blood pressure via the sympathomimetic effects of 5-HT2A receptor agonism. 5-HT1A agonists however, decrease blood pressure and heart rate via peripheral vasodilation and vagus nerve stimulation (Dabiré, 1991; Kaumann and Levy, 2006). In healthy anaesthetised dogs and cats, 0.1 mg IV 5-MeO-DMT had a triphasic effect on blood pressure; an immediate rapid fall, followed by a brisk rise and finally a more prolonged fall (Gessner et al., 1961). A modest biphasic blood pressure response, with initial dose-dependent increase followed by a decrease, accompanied by a slight decrease in heart rate has also been demonstrated in rats (Dabiré et al., 1987). Bradycardia was also observed in rhesus monkeys, but otherwise electrocardiography measures were normal with doses up to 8 mg/kg. However, it is important to note that in addition to the direct cardiovascular effects described above, there are also likely to be indirect, psychosomatic effects of anticipating or having an intense psychedelic experience.
5-MeO-DMT, like psilocybin, binds to 5-HT2B receptors (Halberstadt et al., 2012). Some 5-HT2B agonists are associated with valvular heart disease (Roth, 2007; Rothman et al., 2000). However, to date, no research studies link classic psychedelic use and valvular heart disease. Any potential toxicity would likely be dose and frequency dependent.
Thermoregulatory effects
Stimulation of different 5-HT receptors can have opposing effects on thermoregulation: Hypothermia can be triggered by 5-HT1A receptor agonists while 5-HT2A stimulation can cause hyperthermia (Gudelsky et al., 1986a, 1986b). 5-HT2A receptor-related vasoconstriction is thought to be a main effector site of serotonergic thermoregulation (Ootsuka et al., 2004). Using an experimental drug administration and mathematical pharmacokinetic/pharmacodynamic (PK/PD) model, Jiang et al. (2016b) demonstrated that 5-MeO-DMT induces transient hyperthermia in mice. However, another study showed that 3 mg/kg 5-MeO-DMT reduced tail-skin temperature in mice by 1.8°C (Eide and Tjølsen, 1988). In rats, 5-MeO-DMT has a non-linear effect on body temperature: at low (0.5–1.0 mg/kg) doses causing hypothermia but hyperthermia at high doses (3–10 mg/kg). The hyperthermic effect may be completely attenuated or even converted into hypothermia by the 5-HT2A antagonist, ketanserin (Gudelsky et al., 1986a, 1986b). 5-MeO-DMT at 0.5–1.8 mg/kg also caused hyperthermia in pigs. Administration of higher doses to pigs genetically susceptible to malignant hyperthermia was fatal (Löscher et al., 1990).
Effects on nociception
The analgesic effects of 5-MeO-DMT are also non-linear: Nociception in rats is enhanced after very low doses (1.6–25 µg) and then becomes biphasic at medium doses (hyperalgesia followed by analgesia at 50–100 µg) and reduced after higher doses (400 µg) of 5-MeO-DMT (Berge et al., 1980).
Endocrine effects
5-MeO-DMT causes increased prolactin levels, dose dependently in both male and female rats (Carlsson and Eriksson, 1986; Meltzer et al., 1978; Seeman and Brown, 1985), although there is one report of a biphasic response, with initial increase followed by decrease (Simonovic and Meltzer, 1983). Repeated administration of 5-MeO-DMT (5 mg/kg, every 3 h for a total of four injections) potentiated its prolactin-releasing effect (Simonovic and Meltzer, 1979).
A prospective examination of 5-MeO-DMT inhalation in humans demonstrated that a single inhalation of 5-MeO-DMT increases cortisol levels in saliva (Uthaug et al., 2020b).
Immunological effects
5-MeO-DMT can modulate immune responses in human primary immune cell cultures (Szabo et al., 2014). Treatment of immune-challenged, human monocyte-derived dendritic cells with 5-MeO-DMT resulted in a marked decrease in gene expression and secretion of various inflammatory cytokines and chemokines (IL-1β, IL-6, IL-8 and TNF-α), while strongly increasing the levels of the cytokine interleukin-10 (IL-10), an anti-inflammatory cytokine, mediated via the σ-1 receptor. In two different models, 5-MeO-DMT had strong immune modulating effects, with no impact on antibody production, immune homeostasis interleukins IL-4, IL-5 or T helper 2 cells. In a human study, a single inhalation of 5-MeO-DMT decreased the levels of circulating IL-6 (Uthaug et al., 2020b).
Effects on gene expression
Dakic et al. (2017) studied the effects of 5-MeO-DMT on proteins in human brain organoids. Using mass spectrometry and shotgun proteomics, they identified more than 900 proteins (out of ~6700 sampled) differentially expressed after treatment with 5-MeO-DMT. These proteins impact anti-inflammatory effects, long-term potentiation, the formation of dendritic spines, microtubule dynamics and cytoskeletal reorganisation.
Drug interactions
Jiang et al. (2013) examined 5-MeO-DMT interactions with MAOA inhibitors. Coadministration of even a relatively low dose of harmaline (an inhibitor of monoamine oxidase) readily blocks MAOA-dependent elimination in mice, shifting 5-MeO-DMT metabolism to alternative pathways such as O-demethylation. This leads to a greater rate of conversion to bufotenine and significantly extends systemic and central exposure to 5-MeO-DMT (Halberstadt, 2016; Halberstadt et al., 2008, 2012; Jiang et al., 2016b; Shen et al., 2010b). In contrast, chronic treatment with MAO inhibitors suppresses response to 5-MeO-DMT in rodents (Gudelsky et al., 1986b; Lucki and Frazer, 1982).
Potential drug interactions with tetrahydrocannabinol (THC), mitragynine, lithium, haloperidol, benzodiazepines and antidepressants have been investigated in rodents. Small doses of 5-MeO-DMT rescue memory impairments produced by THC (Egashira et al., 2002). Mitragynine suppresses 5-MeO-DMT-induced head-twitch response in mice (Matsumoto et al., 1997). Chronic lithium treatment potentiates the serotonin behavioural syndrome in rats, particularly flat posture and tremor but attenuates head-twitch and ‘wet-dog shake’ response (Goodwin et al., 1986a, 1986b; Harrison-Read, 1979; Kofman and Levin, 1995). Acute benzodiazepine treatment potentiates 5-MeO-DMT-induced head-twitch response (Moser and Redfern, 1988), but attenuates hyponeophagia (Shephard and Broadhurst, 1982). Chronic administration of tricyclic antidepressants consistently attenuates 5-MeO-DMT-induced analgesia (Danysz et al., 1986), head-twitch response (Friedman et al., 1983; Metz and Heal, 1986) and behaviour response (Stolz et al., 1983). However, enhanced responsiveness to 5-MeO-DMT was observed upon 24–48 h withdrawal from the last dose of some tricyclic antidepressants (Friedman et al., 1983; Stolz et al., 1983). Chronic treatment with fluoxetine, a selective serotonin reuptake inhibitor, reduced response to 5-MeO-DMT, which remained attenuated for 3 days following fluoxetine withdrawal (Stolz et al., 1983) and, in a different study, continued to be attenuated until day 9, returning to control levels on day 14 (Rényi et al., 1986b). Citalopram inhibited response to 5-MeO-DMT acutely, but had no effect after 4 h to 7 days (Rényi et al., 1986b). Acute fluoxetine enhanced response to 5-MeO-DMT (Winter, 1999). Chronic haloperidol treatment had no effect on 5-MeO-DMT response (Friedman et al., 1983).
Toxicology
The LD50 in sheep is 1 mg/kg (see Table 2), ranges from 48 to 278 mg/kg in mice (depending on route of administration) (Gillin et al., 1976; Ho et al., 1970) and in cats is 15 mg/kg (Benington et al., 1965).
There have been studies of 5-MeO-DMT toxicity in mice, rats, cats, sheep and monkeys (Benington et al., 1965; Gillin et al., 1976). High doses of 5-MeO-DMT produce ataxia, mydriasis, head nodding, lateral head weaving, tremor, convulsions, shivering, tachycardia and loss of consciousness and in toxic doses respiratory failure (Grahame-Smith, 1971; Lucki et al., 1984).
Tolerance
Tolerance develops to some (but not all) behavioural and physiological effects of 5-MeO-DMT in rats, cats and monkeys. Studies with once-daily dose regimens reported no tolerance to 5-MeO-DMT-induced changes in neuronal activity in the raphe nucleus (Larson, 1984) or ataxia, decrease in movement and unresponsiveness to loud noise/touch in rhesus monkeys (Gillin et al., 1976). No tolerance was observed in behavioural effects in macaque monkeys administered 0.25 mg/kg IM 5-MeO-DMT every day for 8–12 days. With more frequent drug administration of 0.25 mg/kg IM 5-MeO-DMT administered every 30 min for 9 and 26 h subsequently, tolerance developed to limb jerks, body shakes and checking behaviour and persisted for 26 h (Heinze et al., 1983; Schlemmer and Davis, 1986). Likewise, when 5-MeO-DMT was administered every 30 min for 4 h (at 2 mg/kg IP) to rats, tolerance to the serotonergic behavioural syndrome developed and persisted for 4 h (Trulson and Keltch, 1985). Chronic, frequent administration of 5-MeO-DMT diminishes the responsiveness of 5-HT1A receptor-mediated changes in body temperature and corticosterone secretion without altering the responses mediated by 5-HT2 receptors (Nash et al., 1989).
Physical dependence or withdrawal signs have not been reported in any of the repeated dose-administration studies (Gillin et al., 1976; Larson, 1984; Nash et al., 1989; Schlemmer and Davis, 1986; Sills et al., 1985; Trulson and Keltch, 1985).
Abuse potential and prevalence of use
No studies have investigated whether laboratory animals self-administer 5-MeO-DMT. However, similar studies with other classical psychedelics failed to induce self-administration, or did so only marginally and transiently (Fantegrossi et al., 2004; Yanagita, 1986). There is evidence that 5-HT2C receptor agonists possess anti-addictive properties (Canal and Murnane, 2017).
5-MeO-DMT is not specifically mentioned by the United Nations Office on Drugs and Crime (2020) World Drug Report or the European Drug Report of the European Monitoring Centre for Drugs Drug Addiction (EMCDDA, 2019) or the Global Drug Survey (GDS, 2020; Global Drug Survey, 2021). When it is mentioned, it is often subsumed under the moniker of ‘novel psychoactive substances’, rendering estimation of prevalence of use problematic. A large annual cross-sectional population survey in the United States, National Survey on Drug Use and Health (NSDUH), includes data on 5-MeO-DMT (see Supplementary Information). Over the last 18 years (2002–2019) and 722,653 total respondents aged 12 and older, 33 and 13 respondents (0.0046% and 0.0018% unweighted estimate) reported the lifetime use of 5-MeO-DMT or bufotenine/toad secretions, respectively (Substance Abuse Mental Health Services Administration (SAMHSA), 2021a), and the rates of reporting were steady at 2–3 per year (Palamar and Le, 2019). Because of rarity and possible underreporting, it is difficult to accurately extrapolate prevalence in the general population, but the estimate is around 0.003% for 5-MeO-DMT (Palamar and Le, 2019; SAMHSA, 2021a; Sexton et al., 2020). According to a survey of Australian ecstasy users, only 2% have ever tried 5-MeO-DMT (Bruno et al., 2012).
It is likely appropriate to consider 5-MeO-DMT to have limited abuse liability given anecdotal reports of behaviourally impairing effects (i.e. intoxicating effects that could result in harm) similar to other classic psychedelic compounds (Johnson et al., 2018).
Epidemiological studies of human recreational/spiritual use
There are no published human clinical trials of 5-MeO-DMT. The published data include a case report of improved outcome measures following sequential administration of ibogaine and 5-MeO-DMT in a veteran with alcohol use disorder (Barsuglia et al., 2018); epidemiological studies and surveys of recreational/spiritual/medicinal use (Barsuglia et al., 2018; Davis et al., 2018, 2019, 2020; Lancelotta and Davis, 2020; Palamar and Acosta, 2020; Uthaug et al., 2019, 2020a, 2020b); and accounts of self-experimentation and recreational/spiritual use (Erowid, 2021; Metzner, 2013; Ott, 2001; Shulgin and Shulgin, 1997).
Reported recreational dose ranges are inhalation: ~6–20 mg; intravenous injection: ~0.7–3.1 mg; sublingual or intranasal routes: ~10 mg; intramuscular: ~5–10 mg; and oral: ~10–30 mg; although Shulgins report it is inactive without a MAO inhibitor (Erowid, 2021; Ott, 2001; Shulgin and Shulgin, 1997). 5-MeO-DMT has a rapid onset when smoked or vapourised: effects peak in 2–5 min, last 15–20 min and return to baseline by 30 min (Davis et al., 2018a). Insufflated, the experience lasts longer, up to 45 min, and the onset is slower (5–7 min) (Metzner, 2013). Users report that smoking/vaporising 5-MeO-DMT elicits more intense effects compared to most other psychedelics (Barsuglia et al., 2018; Davis et al., 2018). Although no qualitative studies so far directly compared phenomenology of 5-MeO-DMT-elicited experience with other short-lasting psychedelics frequently referred to as intense, such as DMT or Salvia divinorum, anecdotal reports describe that 5-MeO-DMT feels very distinct. The subjective experience is generally described as transcendent, often involving ego-dissolution, non-dual awareness and an increased range and intensity of emotions, spanning the feeling of love, unity and awe to panic and terror. Notable is the frequent absence of visual effects (Erowid, 2021). It is possible that the absence of visual effects is due to 5-HT1A receptor action, as it was demonstrated that 1A receptor agonists reduce visual imagery induced by psilocybin (Pokorny et al., 2016). In contrast to highly detailed DMT or salvia trips, users of 5-MeO-DMT often describe content-free experiences, associate with loss of sense of self and bodily awareness, and sensory deprivation (described as all-white light, or all-black), with common descriptors such as: ‘emptiness’, ‘nothingness’ or ‘void’ (Millière et al., 2018). Dose, set and setting have considerable impact on the perceptual and emotional experience and, in common with all psychedelics, adequate preparation has been reported to be important (Lancelotta and Davis, 2020; Metzner, 2013). Anecdotal reports and surveys indicate that repeated dosing with 5-MeO-DMT is possible, with almost no desensitisation or tolerance to psychedelic effects reported (Davis et al., 2018; Trout, 2007; Uthaug, 2020a, 2020b).
Retrospective surveys examined 5-MeO-DMT patterns of use, motivations for consumption, subjective effects and potential benefits and consequences associated with use. It is worth noting that survey data is likely biased towards positive outcomes due to selection bias.
The main reasons for trying 5-MeO-DMT were spiritual exploration (68%), recreation (18%) or healing (14%); most people used it less than 4 times in their life (59%). 90% reported positive and/or transcendent experiences, 57% fit the criteria for complete mystical experience (scored as reaching ⩾60% on each of the subscales of the Mystical Experience Questionnaire, MEQ-30; Barrett et al., 2015) with around 37% having challenging experiences (measured by the Challenging Experience Questionnaire; Barrett et al., 2016; Davis et al., 2018a).
In a subsequent survey, Davis et al. (2019) collected self-report measures of depression and anxiety in 362 people who took 5-MeO-DMT in a group setting. Of those diagnosed with depression (41%) or anxiety (48%), most reported these conditions were improved (depression = 80%; anxiety = 79%) following 5-MeO-DMT use, and fewer reported they were unchanged (depression = 17%; anxiety = 19%) or worsened (depression = 3%; anxiety = 2%). Associations were reported between improvement in depression/anxiety, and greater intensity of mystical experiences (as measured by MEQ-30) and higher ratings of the spiritual significance/personal meaning of the 5-MeO-DMT experience (Davis et al., 2019). Moreover, supportive setting in a group was associated with much higher ratings of complete mystical experience – 83%, compared to 54% of respondents who had 5-MeO-DMT experience in the recreational setting, and inverse relationship was noted for challenging experiences (Sepeda et al., 2019).
Several countries where 5-MeO-DMT is unregulated offer retreats and treatment programmes. A survey of 51 US Special Operations Forces Veterans from one such retreat, with combined 5-MeO-DMT and ibogaine treatments, indicated the experience was therapeutic for their traumatic experiences, suicidal ideation, depression and anxiety (Davis et al., 2020). Another case study presents brain imaging data from one participant (31-year-old military veteran with alcohol use disorder) of a similar treatment centre in Mexico (Barsuglia et al., 2018). Single-photon emission computed tomography (SPECT) neuroimaging after treatment with ibogaine and 5-MeO-DMT showed increases in brain perfusion in bilateral caudate nuclei, left putamen, right insula, as well as temporal, occipital and cerebellar regions compared to baseline. The patient reported improvement in mood, cessation of alcohol use and reduced cravings at 5 days post-treatment, effects which were sustained at 1 month, with a partial return to mild alcohol use at 2 months (Barsuglia et al., 2018). In a survey of 20 individuals from the same retreat centre, 75% reported a ‘complete mystical experience’, as measured by MEQ-30 (Barsuglia et al., 2018).
Two prospective studies examined the effects of vapourised 5-MeO-DMT inhalation (11 participants) (Uthaug et al., 2020b) and the effects of toad secretions (42 participants) (Uthaug et al., 2019). In both studies, compared to baseline, the ratings of mindfulness facets increased (measured with Five Facets Mindfulness questionnaire, FFMQ-15; Gu et al., 2016), while ratings of depression and anxiety decreased (measured with Depression, Anxiety and Stress scale, DASS-21 (Henry and Crawford, 2005) or with Brief Symptom Inventory, BSI-18 (Derogatis, 2001)) immediately after the session and remained so at follow up. Whether there are any potential clinical implications of this is unclear.
Acute adverse effects of 5-MeO-DMT reported in some of the above studies (Barsuglia et al., 2018; Davis et al., 2018, 2019; Uthaug et al., 2020b) include fear, sadness, anxiety, confusion, profound experience of one’s own death, crying, paranoia, shaking/trembling, vomiting, nausea, transient headache, pressure or weight in the chest or abdomen and loss of body perception (Table 4). Dissociative experiences with memory loss (blackout) have been reported (Metzner, 2013). Delayed adverse effects (up to 1 week) included somatic tension in muscles, difficulties sleeping, ‘flashbacks’ or ‘reactivations’ – re-experiencing some of the effects felt during the drug session (Uthaug et al., 2020a, 2020b), and in rare cases – psychosis (Metzner, 2013; Sauras Quetcuti et al., 2019; Shulgin and Shulgin, 1997). In an online retrospective survey, flashbacks were reported as more common with higher doses and with vaporised rather than intramuscular administration (Uthaug et al., 2020a).
Table 4.
Adverse effects of 5-MeO-DMT from human epidemiological studies and published ‘underground’ reports.
| Study | Acute adverse effects | Delayed adverse effects |
|---|---|---|
| Davis 2020 (retrospective survey) | Participants reported that psychedelic treatment they received (ibogaine plus 5-MeO-DMT) was one of the most psychologically challenging (69%) experiences of their entire lives Adverse events not assessed |
Not assessed |
| Palamar and Acosta (2020; retrospective survey) | Not assessed | Not assessed |
| Uthaug (2020b) (prospective study of synthetic 5-MeO-DMT in naturalistic settings) | 45.5% (N = 5) of the sample reported adverse effects post-session. One participant reported feeling ‘scared and confused’, one participant reported ‘feeling anger, joy love and fear’, one participant vomited shortly after intake, one participant expressed ‘feeling a little shock on the first try, but nothing bad’ and finally one participant reported feeling that their throat was scratching from smoking | On the 7-day follow-up, 27.3% (N = 3) of the sample reported adverse effects in the days following the session. One participant reported some affective symptoms and somatic tension in muscles, one participant reported difficulties sleeping (insomnia), and one participant reported experiencing somatic tension in muscles |
| Uthaug (2020a) (retrospective survey) | Not assessed | ‘Reactivation’ or flashback experiences reported more common with vaporised route of administration compared to the intramuscular (3/14 vs 9/13 participants, respectively) |
| Uthaug (2019) (prospective study of toad secretions in naturalistic setting) | Not assessed | Not assessed |
| Davis 2019 (retrospective survey) | Assessed using CEQ: Mintensity = 0.8 (SD = 0.8), range 0–5. Subscales: Isolation = 0.4(0.9) Fear = 1.0(1.3) Grief = 0.9(1.1) Physical Distress = 0.9(0.9) Insanity = 0.5(1.0) Death/Dying = 1.5(1.7) Paranoia = 0.0(0.3) There were no differences in the intensity of acute challenging experiences between those who did or did not report an improvement in depression or anxiety, which could be because respondents reported only a ‘slight’ intensity of challenging experiences |
Not assessed |
| Barsuglia et al. (2018) (retrospective survey) | Not assessed | Not assessed |
| Barsuglia et al. (2018) (case study) | Physical purging through dry heaving that lasted for several minutes | Not assessed |
|
Davis et al. (2018) (retrospective survey) Lancelotta and Davis (2020) (same survey) |
Assessed using CEQ: Mintensity = 0.95, SD = 0.91; range 0–5. Subscales: Isolation = 0.76(1.23) Fear = 1.22(1.38) Grief = 0.69(1.00) Physical Distress = 1.15(1.09) Insanity = 0.85(1.21) Death/Dying = 1.75(1.90) Paranoia = 0.18(0.60) On average 37% of respondents reported experiencing challenging psychological and somatic experiences. Between 40% and 66% reported experiences of feeling their heart beat, fear, frightened, their body shake/tremble, anxious, as if they were dead or dying, shaky inside, that something horrible would happen, like crying, pressure or weight in their chest or abdomen, and panic, and having the profound experience of their own death |
Not assessed |
| Metzner (2013) (qualitative field report of ‘underground’ use) | ‘Dissociative experiences’, involving losing consciousness and memory of the drug session, psychotic or fear-panic reactions occurred in about 10% of cases. Most dissipate as the drug wears off | Flashback/reactivation experience (of the dissociative, fear or psychotic reactions) |
| Ott (2001) (self-experimentation) | Tinnitus | Not reported |
| Shulgin and Shulgin (1997) (qualitative field reports of ‘underground’ use and self-experimentation) | Nausea, tinnitus, fear, feeling like dying, blackout, purple face and no breathing (in one case with unknown but very large smoked dose) | Psychosis, terror, lack of sleep (one report) |
SD: standard deviation.
In the published mortality and morbidity reports mentioning 5-MeO-DMT, it had been taken as toad secretions, concurrently with other drugs of abuse or together with monoamine oxidase inhibitors (MAOIs). One of the earliest toxicity reports is of a 5-year-old child hospitalised with profuse salivation and continuous seizures after licking Incilius alvarius toad (Hitt and Ettinger, 1986). A 17-year old was hospitalised with extreme agitation, hyperthermia, tachycardia and rhabdomyolysis after consuming 5-MeO-DMT and the MAOI harmaline (Brush et al., 2004). There are reported fatalities, including a 25-year old who ingested ayahuasca with 5-MeO-DMT (Sklerov et al., 2005).
There are no reports of deaths related to 5-MeO-DMT from the Office for National Statistics (2020) (England and Wales), DAWN 2011 report/2020 preliminary data (Drug Abuse Warning Network, SAMHSA, 2021b), the Report of the American Association of Poison Control Centers’ National Poison Data (Gummin et al., 2020) or the National Programme on Substance Abuse Deaths (NPSAD, 2021). This could be because use is still relatively limited, the toxicity is very low, this substance is not routinely tested for and/or because 5-MeO-DMT is not included in most national databases or epidemiological surveys (Palamar and Le, 2019).
Discussion
In this review we have summarised and synthesised the data on 5-MeO-DMT thus far to inform controlled clinical trials of its basic safety, pharmacokinetic and pharmacodynamic profiles in humans.
5-MeO-DMT is a naturally occurring tryptamine derivative found in gland secretions of the Sonoran Desert toad, in a variety of plants, and endogenously in mammals. It is also available as a pure compound. There have been no published laboratory studies in humans on the effects of 5-MeO-DMT, except for a case study with a single individual (Barsuglia et al., 2018).
Animal studies have demonstrated paradoxical (non-linear or biphasic) effects of 5-MeO-DMT on pharmacokinetics, thermoregulation, nociception, heart rate and blood pressure. Dose finding studies with different routes of administration of 5-MeO-DMT in humans are required to establish the therapeutic dose range and safety profile. The pharmacokinetic profile of the therapeutic dose range in humans needs to be determined, as studies with rodents indicate that higher doses result in non-linear PK profile, and subjective effects at higher doses might depend on the CYP2D6 genotype.
The available data indicate that established safety measures for psychedelic research should be implemented for 5-MeO-DMT human clinical trials. Concomitant use of MAOIs and lithium should be avoided. Flashbacks or ‘reactivations’ have been reported in surveys of recreational use of psychedelics including 5-MeO-DMT. Such effects have not been observed in clinical studies of psychedelics to date indicating the importance of screening, monitoring and other safety measures. Hallucinogen-persisting perception disorder (HPPD) describes a nebulous set of symptoms persisting weeks, months or years after psychedelic use that are associated with anxiety or distress. The prevalence of HPPD is estimated to be very rare among classical psychedelic users (Halpern et al., 2018), with one estimate being that it is present in 1 in 50,000 psychedelic users (Grinspoon and Bakalar, 1998). The clinical concept is sufficiently vague to make true estimate of prevalence very difficult. This is further complicated by recreational users taking psychedelics with other drugs including alcohol. An analysis of people reporting symptoms of HPPD found that symptoms were more frequently preceded by use of non-psychedelic substances such as alcohol, tobacco and cannabis than by the use of psychedelics and that some individuals with these symptoms had never taken a psychedelic (Halpern et al., 2018). These data call into question whether HPPD is peculiar to psychedelic use and suggest that it may instead constitute a syndrome aetiologically related to many different psychoactive substances, occurring in those with a pre-existing vulnerability. A direct neurotoxic effect appears unlikely.
The therapeutic potential of 5-MeO-DMT is hypothetical, but intriguing. Surveys of recreational users suggest rapid anxiolytic and antidepressant properties not dissimilar to those being probed in early-phase studies of psilocybin and LSD, as well as later phase studies of ketamine and its analogues. 5-MeO-DMT shares similar pharmacology to other classical psychedelics; however, the specific pharmacokinetic and pharmacodynamic properties of the drug may confer clinical advantages. One of these is a short duration of action, which may require less healthcare resource utilisation and thus increasing access to treatment. Another is the absence of visual effects, which could be distracting. Their absence might lead to higher rates of mystical experiences. As such, it deserves further investigation as a putative rapid-acting antidepressant. A key step will be establishing a pharmacokinetic profile and safety profile of 5-MeO-DMT in healthy volunteers in a controlled trial design.
Conclusion
5-MeO-DMT is a short-lasting psychedelic substance with a unique subjective effect profile making it an intriguing compound to research. The available data indicate the risk profile of 5-MeO-DMT is similar to other classic psychedelics, such as psilocybin and that established safety precautions for psychedelic research be followed. A notable feature of 5-MeO-DMT is the reportedly high rates of the ego-dissolution and mystical experiences, which in studies with other psychedelics are related to long-term positive therapeutic outcomes, calling for clinical exploration.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811211050543 for A narrative synthesis of research with 5-MeO-DMT by Anna O Ermakova, Fiona Dunbar, James Rucker and Matthew W Johnson in Journal of Psychopharmacology
Acknowledgments
We thank Steve Wooding for his comments on the manuscript.
Footnotes
Author contributions: All authors made a substantial contribution to the concept, design and interpretation of data, and made critical revisions to the manuscript. A.O.E. performed systematic literature search and selection of papers, and wrote the first draft of the manuscript.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.O.E., J.R., F.D., and M.W.J. provide paid consulting services to Beckley Psytech. M.W.J. also provides paid consulting services to AJNA Labs, AWAKN Life Sciences, Entheogen Biomedical, Field Trip Psychedelics, MindMed, Otsuka Pharmaceutical Development & Commercialization and Silo Pharma. M.W.J. is a Professor in the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine. M.W.J. engaged in this research as a private advisor and not in his capacity as a Johns Hopkins faculty member. M.W.J. was compensated for the advising service in income and stock options. J.R. leads the Psychedelic Trials Group at King’s College London. King’s College London receives grant funding from Compass Pathways and Beckley Psytech to undertake clinical trials with psychedelics. This work presents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: J.R.’s salary is funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR).
ORCID iDs: Anna O Ermakova  https://orcid.org/0000-0002-1869-0084
https://orcid.org/0000-0002-1869-0084
James Rucker  https://orcid.org/0000-0003-4647-8088
https://orcid.org/0000-0003-4647-8088
Supplemental material: Supplemental material for this article is available online.
References
- Abramson HA, Gettner HH, Carone PA, et al. (1979) The intracranial injection of drugs in goldfish – I: Hallucinogens and their antagonism to smooth muscle activity. Journal of Asthma Research 16(2): 55–61. [DOI] [PubMed] [Google Scholar]
- Acosta-Urquidi J. (2015) QEEG studies of the acute effects of the visionary tryptamine DMT. Cosmos and History: The Journal of Natural and Social Philosophy 11(2): 115–129. [Google Scholar]
- Adrien J, Lanfumey L. (1986) Ontogenesis of unit activity in the raphe dorsalis of the behaving kitten: Its relationship with the states of vigilance. Brain Research 366(1–2): 10–21. [DOI] [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. (1969) Metabolism of 5-methoxy-N,N dimethyltryptamine-14C in the rat. Biochemical Pharmacology 18(12): 2771–2781. [DOI] [PubMed] [Google Scholar]
- Ahlborg U, Holmstedt B, Lindgren JE. (1968) Fate and metabolism of some hallucinogenic indolealkylamines. Advances in Pharmacology 6(Pt. B): 213–229. [DOI] [PubMed] [Google Scholar]
- Ahlenius S, Larsson K. (1991) Opposite effects of 5-methoxy-N, N-di-methyl-tryptamine and 5-hydroxytryptophan on male rat sexual behavior. Pharmacology, Biochemistry, and Behavior 38(1): 201–205. [DOI] [PubMed] [Google Scholar]
- Archer T, Ogren SO, Ross SB. (1982) Serotonin involvement in aversive conditioning: Reversal of the fear retention deficit by long-term p-chloroamphetamine but not p-chlorophenylalanine. Neuroscience Letters 34(1): 75–82. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. (1990) The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Research 514(1): 55–67. [DOI] [PubMed] [Google Scholar]
- Barker SA, Littlefield-Chabaud MA, David C. (2001) Distribution of the hallucinogens N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine in rat brain following intraperitoneal injection: Application of a new solid-phase extraction LC–APcI–MS–MS–isotope dilution method. Journal of Chromatography B: Biomedical Sciences and Applications 751(1): 37–47. [DOI] [PubMed] [Google Scholar]
- Barker SA, McIlhenny EH, Strassman R. (2012) A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955-2010. Drug Testing and Analysis 4(7–8): 617–635. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Bradstreet MP, Leoutsakos J-MS, et al. (2016) The Challenging Experience Questionnaire: Characterization of challenging experiences with psilocybin mushrooms. Journal of Psychopharmacology 30(12): 1279–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett FS, Johnson MW, Griffiths RR. (2015) Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. Journal of Psychopharmacology 29(11): 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsuglia J, Davis AK, Palmer R, et al. (2018) Intensity of mystical experiences occasioned by 5-MeO-DMT and comparison with a prior psilocybin study. Frontiers in Psychology 9: 2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsuglia JP, Polanco M, Palmer R, et al. (2018) A case report SPECT study and theoretical rationale for the sequential administration of ibogaine and 5-MeO-DMT in the treatment of alcohol use disorder. Progress in Brain Research 242: 121–158. [DOI] [PubMed] [Google Scholar]
- Beaton JM, Morris PE. (1984) Ontogeny of N, N-dimethyltryptamine and related indolealkylamine levels in neonatal rats. Mechanisms of Ageing and Development 25(3): 343–347. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. (1977) ‘Wet-dog’ shake behaviour in the rat: A possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology 16(10): 663–670. [DOI] [PubMed] [Google Scholar]
- Benington F, Morin RD, Clark LC. (1965) 5-methoxy-N, N-dimethyltryptamine, a possible endogenous psychotoxin. The Alabama Journal of Medical Sciences 2(4): 397–403. [PubMed] [Google Scholar]
- Berendsen HHG, Jenck F, Broekkamp CLE. (1989) Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacology, Biochemistry, and Behavior 33(4): 821–827. [DOI] [PubMed] [Google Scholar]
- Berge O, Chacho D, Hole K. (1983) Inhibitory effect of 5-methoxy-N,N-dimethyltryptamine on the synaptosomal uptake of 5-hydroxytryptamine. European Journal of Pharmacology 90(2–3): 293–296. [DOI] [PubMed] [Google Scholar]
- Berge O-G. (1982) Effects of 5-HT receptor agonists and antagonists on a reflex response to radiant heat in normal and spinally transected rats. Pain 13(3): 253–266. [DOI] [PubMed] [Google Scholar]
- Berge O-G, Hole K, Dahle H. (1980) Nociception is enhanced after low doses and reduced after high doses of the serotonin receptor agonist 5-methoxy-N,N-dimethyltryptamine. Neuroscience Letters 19(2): 219–223. [DOI] [PubMed] [Google Scholar]
- Berger G, Mazière M, Marazano C, et al. (1978) Carbon-11 labeling of the psychoactive drug O-methyl-bufotenine and its distribution in the animal organism. European Journal of Nuclear Medicine 3(2): 101–104. [DOI] [PubMed] [Google Scholar]
- Blough BE, Landavazo A, Decker AM, et al. (2014) Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology 231(21): 4135–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology 29(3): 289–299. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Podrebarac SK, Duane JH, et al. (2018) Clinical interpretations of patient experience in a trial of psilocybin-assisted psychotherapy for alcohol use disorder. Frontiers in Pharmacology 9: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke C, Carrigan M, Dixon R. (1988) Experimental evidence that tryptamine alkaloids do not cause Phalaris aquatica sudden death syndrome in sheep. Australian Veterinary Journal 65(7): 218–220. [DOI] [PubMed] [Google Scholar]
- Bourke C, Carrigan M, Dixon R. (1990) The pathogenesis of the nervous syndrome of Phalaris aquatica toxicity in sheep. Australian Veterinary Journal 67(10): 356–358. [DOI] [PubMed] [Google Scholar]
- Bruno R, Matthews AJ, Dunn M, et al. (2012) Emerging psychoactive substance use among regular ecstasy users in Australia. Drug and Alcohol Dependence 124(1–2): 19–25. [DOI] [PubMed] [Google Scholar]
- Brush DE, Bird SB, Boyer EW. (2004) Monoamine oxidase inhibitor poisoning resulting from internet misinformation on illicit substances. Journal of Toxicology: Clinical Toxicology 42(2): 191–195. [DOI] [PubMed] [Google Scholar]
- Canada Justice Laws (1996) Consolidated Federal Laws of Canada, Controlled Drugs and Substances Act (S.C. 1996, c. 19). Available at: https://laws-lois.justice.gc.ca/eng/acts/C-38.8/ (accessed 26 February 2021).
- Canal CE, Murnane KS. (2017) The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens. Journal of Psychopharmacology 31(1): 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ. (2010) User perceptions of the benefits and harms of hallucinogenic drug use: A web-based questionnaire study. Journal of Substance Use 15(4): 283–300. [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, et al. (2018) Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 235(2): 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. (2016) Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. The Lancet Psychiatry 3(7): 619–627. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Eriksson E. (1986) A central serotonin receptor agonist, 8-hydroxy-2-(di-n-propylamino)tetralin, has different effects on prolactin secretion in male and female rats. Acta Pharmacologica et Toxicologica 58(4): 297–302. [DOI] [PubMed] [Google Scholar]
- Castellanos JP, Woolley C, Bruno KA, et al. (2020) Chronic pain and psychedelics: A review and proposed mechanism of action. Regional Anesthesia & Pain Medicine 45(7): 486–494. [DOI] [PubMed] [Google Scholar]
- Cazala P, Garrigues AM. (1983) Effects of apomorphine, clonidine or 5-methoxy-NN-dimethyltryptamine on approach and escape components of lateral hypothalamic and mesencephalic central gray stimulation in two inbred strains of mice. Pharmacology, Biochemistry, and Behavior 18(1): 87–93. [DOI] [PubMed] [Google Scholar]
- Christian ST, Benington F, Morin RD, et al. (1975) Gas-liquid chromatographic separation and identification of biologically important indolealkylamines from human cerebrospinal fluid. Biochemical Medicine 14(2): 191–200. [DOI] [PubMed] [Google Scholar]
- Clinicaltrials.gov (2019) Safety of GH001 in Healthy Volunteers (ClinicalTrials.gov Identifier: NCT 04640831). Bethesda, MD: National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT04640831 (accessed 16 August 2021). [Google Scholar]
- Clinicaltrials.gov (2021. a) ClinicalTrials.gov Identifier: NCT 04698603 Clinical Study of GH001 in Depression. Bethesda, MD: National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT04698603 (accessed 16 August 2021). [Google Scholar]
- Clinicaltrials.gov (2021. b) ClinicalTrials.gov Identifier: NCT 05032833 Single Ascending Dose Study with 5-MeO-DMT in Healthy Subjects. Bethesda, MD: National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/show/NCT05032833 (accessed 3 September 2021). [Google Scholar]
- Corbett L, Christian ST, Morin RD, et al. (1978) Hallucinogenic N-methylated indolealkylamines in the cerebrospinal fluid of psychiatric and control populations. The British Journal of Psychiatry 132: 139–144. [DOI] [PubMed] [Google Scholar]
- Critchley MAE, Handley SL. (1987) Effects in the X-maze anxiety model of agents acting at 5-HT1 and 5-HT2 receptors. Psychopharmacology 93(4): 7243. [DOI] [PubMed] [Google Scholar]
- Dabiré H. (1991) Central 5-hydroxytryptamine (5-HT) receptors in blood pressure regulation. Therapie 46(6): 421–429. [PubMed] [Google Scholar]
- Dabiré H, Cherqui C, Fournier B, et al. (1987) Comparison of effects of some 5-HT1 agonists on blood pressure and heart rate of normotensive anaesthetized rats. European Journal of Pharmacology 140(3): 259–266. [DOI] [PubMed] [Google Scholar]
- Dakic V, Minardi Nascimento J, Costa Sartore R, et al. (2017) Short term changes in the proteome of human cerebral organoids induced by 5-MeO-DMT. Scientific Reports 7(1): 12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Minor BG, Post C, et al. (1986) Chronic treatment with antidepressant drugs and the analgesia induced by 5-methoxy-N, N-dimethyltryptamine: Attenuation by desipramine. Acta Pharmacologica et Toxicologica 59(2): 103–112. [DOI] [PubMed] [Google Scholar]
- Davis AK, Averill LA, Sepeda ND, et al. (2020) Psychedelic treatment for trauma-related psychological and cognitive impairment among US special operations forces veterans. Chronic Stress 4: 93956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, Barsuglia JP, Lancelotta R, et al. (2018) The epidemiology of 5-methoxy-N, N-Dimethyltryptamine (use Benefits consequences patterns of use subjective effects and reasons for consumption). Journal of Psychopharmacology 32(7): 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AK, So S, Lancelotta R, et al. (2019) 5-methoxy-N, N-dimethyltryptamine (used in a naturalistic group setting is associated with unintended improvements in depression and anxiety). The American Journal of Drug and Alcohol Abuse 45(2): 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. (2001) Brief Symptom Inventory 18: Administration, Scoring and Procedures Manual. NCS Pearson. Available at: https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Personality-%26-Biopsychosocial/Brief-Symptom-Inventory-18/p/100000638.html (accessed 11 March 2021). [Google Scholar]
- Dickinson SL, Curzon G. (1986) 5-hydroxytryptamine-mediated behaviour in male and female rats. Neuropharmacology 25(7): 771–776. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) Department of Justice (2010) Schedules of controlled substances: Placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act: Final rule. Federal Register 75(243): 79296–79300. [PubMed] [Google Scholar]
- Duvvuri V, Risbrough VB, Kaye WH, et al. (2009) 5-HT1A receptor activation is necessary for 5-MeODMT-dependent potentiation of feeding inhibition. Pharmacology, Biochemistry, and Behavior 93(3): 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Katsurabayashi S, et al. (2002) Involvement of 5-hydroxytryptamine neuronal system in D9-tetrahydrocannabinol-induced impairment of spatial memory. European Journal of Pharmacology 9: 221–229. [DOI] [PubMed] [Google Scholar]
- Eide PK, Tjølsen A. (1988) Effects of serotonin receptor antagonists and agonists on the tail-flick response in mice involve altered tail-skin temperature. Neuropharmacology 27(9): 889–893. [DOI] [PubMed] [Google Scholar]
- Eison AS, Wright RN. (1992) 5-HT1A and 5-HT2 receptors mediate discrete behaviors in the Mongolian Gerbil. Pharmacology, Biochemistry, and Behavior 43(1): 131–137. [DOI] [PubMed] [Google Scholar]
- Erspamer V, Vitali T, Roseghini M, et al. (1967) 5-methoxy- and 5-hydroxyindoles in the skin of Bufo alvarius. Biochemical Pharmacology 16(7): 1149–1164. [DOI] [PubMed] [Google Scholar]
- Erowid (2021) Erowid 5-MeO-DMT vault. Available at: https://erowid.org/chemicals/5meo_dmt/5meo_dmt.shtml (accessed 1 March 2021).
- European Monitoring Centre for Drugs Drug Addiction (EMCDDA) (2021) European drug report 2020. Available at: https://www.emcdda.europa.eu/edr2020 (accessed 1 March 2021).
- Evenden JL. (1994) The effect of 5-HT1A receptor agonists on locomotor activity in the guinea-pig. British Journal of Pharmacology 112(3): 861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G. (2004) Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behavioural Pharmacology 15(2): 149–157. [DOI] [PubMed] [Google Scholar]
- Federal Register of Legislation (2016) Poisons standard, July. Available at: https://www.legislation.gov.au/Details/F2016L01071 (accessed 28 May 2021).
- Fornal C, Auerbach S, Jacobs BL. (1985) Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Experimental Neurology 88(3): 590–608. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Litto WJ, Metzler CW, et al. (1994) Single-unit responses of serotonergic dorsal raphe neurons to 5-HT1A agonist and antagonist drug administration in behaving cats. The Journal of Pharmacology and Experimental Therapeutics 270(3): 1345–1358. [PubMed] [Google Scholar]
- Forsström T, Tuominen J, Karkkäinen J. (2001) Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Scandinavian Journal of Clinical and Laboratory Investigation 61(7): 547–556. [DOI] [PubMed] [Google Scholar]
- Friedman E, Cooper TB, Dallob A. (1983) Effects of chronic antidepressant treatment on serotonin receptor activity in mice. European Journal of Pharmacology 89(1–2): 69–76. [DOI] [PubMed] [Google Scholar]
- Gallagher CH, Koch JH, Moore RM, et al. (1964) Toxicity of phalaris tuberosa for sheep. Nature 204: 542–545. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu A, Davis AK, Erowid F, et al. (2019) Cessation and reduction in alcohol consumption and misuse after psychedelic use. Journal of Psychopharmacology 33(9): 1088–1101. [DOI] [PubMed] [Google Scholar]
- GDS (2020) Global drug survey 2020. Available at: https://www.globaldrugsurvey.com/gds-2020/ (accessed 1 March 2021).
- Gessner PK, Page IH. (1962) Behavioral effects of 5-methoxy-N:N-dimethyltryptamine, other tryptamines, and LSD. American Journal of Physiology 203(1): 167–172. [Google Scholar]
- Gessner PK, Godse DD, Krull AH, et al. (1968) Structure-activity relationships among 5-methoxy-N:N-dimethyltryptamine, 4-hydroxy-N:N-dimethyltryptamine (psilocin) and other substituted tryptamines. Life Sciences 7(5): 267–277. [DOI] [PubMed] [Google Scholar]
- Gessner PK, McIsaac WM, Page IH. (1961) Pharmacological actions of some methoxyindolealkylamines. Nature 190(4771): 179–180. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Tinklenberg J, Stoff DM, et al. (1976) 5-Methoxy-N,N-dimethyltryptamine: Behavioral and toxicological effects in animals. Biological Psychiatry 11(3): 355–358. [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R, et al. (1979) Hallucinogens as a discriminative stimuli: Generalisation of DOM to 5-methoxy-N, N-dimethyltryptamine stimulus. Life Sciences 24(11): 993–997. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Benington F, et al. (1982. a) Hallucinogens as discriminative stimuli: A comparison of 4-OMe and 5-OMe DMT with their methylthio counterparts. Life Sciences 30(5): 465–467. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA, et al. (1980) Hallucinogenic agents as discriminative stimuli: A correlation with serotonin receptor affinities. Psychopharmacology 68(2): 155–158. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA. (1982. b) Discriminative stimulus properties of DOM and several molecular modifications. Pharmacology, Biochemistry, and Behavior 16(4): 553–556. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, et al. (2007) Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 53(3): 439–452. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, DeSouza RJ, Wood AJ, et al. (1986. a) Lithium decreases 5-HT1A and 5-HT2 receptor and α2-adrenoreceptor mediated function in mice. Psychopharmacology 90(4): 74065. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Wood AJ, et al. (1986. b) The enhancement by lithium of the 5-HT1A mediated serotonin syndrome produced by 8-OH-DPAT in the rat: Evidence for a post-synaptic mechanism. Psychopharmacology 90(4): 488–493. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG. (1971) Inhibitory effect of chlorpromazine on the syndrome of hyperactivity produced by l-tryptophan or 5-methoxy-N,N-dimethyltryptamine in rats treated with a monoamine oxidase inhibitor. British Journal of Pharmacology 43(4): 856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of Psychopharmacology 30(12): 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. (1998) Psychedelic Drugs Reconsidered (2. Print. A Drug Policy Classic Reprint from The Lindesmith Center). New York: Lindesmith Center. [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, et al. (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of General Psychiatry 68(1): 71. [DOI] [PubMed] [Google Scholar]
- Gu J, Strauss C, Crane C, et al. (2016) Examining the factor structure of the 39-item and 15-item versions of the Five Facet Mindfulness Questionnaire before and after mindfulness-based cognitive therapy for people with recurrent depression. Psychological Assessment 28(7): 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guchhait RB. (1976) Biogenesis of 5-methoxy-N,N-dimethyltryptamine in human pineal gland. Journal of Neurochemistry 26(1): 187–190. [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. (1985) Altered responses to serotonergic agents in Fawn-Hooded rats. Pharmacology, Biochemistry, and Behavior 22(3): 489–492. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Jackman H, et al. (1986. a) Suppression of the hypo- and hyperthermic responses to 5-HT agonists following the repeated administration of monoamine oxidase inhibitors. Psychopharmacology 90(3): 79199. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. (1986. b) Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Neuropharmacology 25(12): 1307–1313. [DOI] [PubMed] [Google Scholar]
- Gummin DD, Mowry JB, Beuhler MC, et al. (2020) 2019 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual report. Clinical Toxicology 58(12): 1360–1541. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. (2004) The molecular acrobatics of arrestin activation. Trends in Pharmacological Sciences 25(2): 105–111. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. (2016) Behavioral and pharmacokinetic interactions between monoamine oxidase inhibitors and the hallucinogen 5-methoxy-N,N-dimethyltryptamine. Pharmacology, Biochemistry, and Behavior 143: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61(3): 364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, et al. (2008) Modification of the effects of 5-methoxy-N, N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology 201(1): 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, et al. (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. Journal of Psychopharmacology 25(11): 1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Nichols DE, Geyer MA. (2012) Behavioral effects of α,α,β,β-tetradeutero-5-MeO-DMT in rats: Comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology 221(4): 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern JH, Lerner AG, Passie T. (2018) A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Current Topics in Behavioral Neurosciences 36: 333–360. [DOI] [PubMed] [Google Scholar]
- Harrison-Read PE. (1979) Evidence from behavioural reactions to fenfluramine, 5-hydroxytryptophan, and 5-methoxy-N,N-dimethyltryptamine for differential effects of short-term and long-term lithium on indoleaminergic mechanisms in rats. British Journal of Pharmacology 66(1): 144P–145P. [PMC free article] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, et al. (2004) Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology 172(2): 145–156. [DOI] [PubMed] [Google Scholar]
- Heinze WJ, Schlemmer RF, Tyler CB, et al. (1983) The comparative behavioral effects of N,N-dimethyltryptamine and N,N-diethyltryptamine in primate dyads. Biological Psychiatry 18(7): 829–836. [PubMed] [Google Scholar]
- Heller B, Narasimhachari N, Spaide J, et al. (1970) N-dimethylated indoleamines in blood of acute schizophrenics. Experientia 26(5): 503–504. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. (2005) The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. The British Journal of Clinical Psychology 44(Pt. 2): 227–239. [DOI] [PubMed] [Google Scholar]
- Heym J, Steinfels GF, Jacobs BL. (1982) Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Research 251(2): 259–276. [DOI] [PubMed] [Google Scholar]
- Himwich HE, Jenkins RL, Fujimori M, et al. (1972) A biochemical study of early infantile autism. Journal of Autism and Childhood Schizophrenia 2(2): 114–126. [DOI] [PubMed] [Google Scholar]
- Hitt M, Ettinger DD. (1986) Toad toxicity. The New England Journal of Medicine 314(23): 1517–1518. [PubMed] [Google Scholar]
- Ho BT, McIsaac WM, An R, et al. (1970) Biological activities of some 5-substituted N, N-dimethyltryptamines, α-methyltryptamines, and gramines. Psychopharmacologia 16(5): 385–394. [DOI] [PubMed] [Google Scholar]
- Holmstedt B, Lindgren JE, Plowman T, et al. (1980) Indole alkaloids in Amazonian Myristicaceae: Field and laboratory research. Botanical Museum Leaflets, Harvard University 28(3): 215–234. [Google Scholar]
- Holze F, Duthaler U, Vizeli P, et al. (2019) Pharmacokinetics and subjective effects of a novel oral LSD formulation in healthy subjects. British Journal of Clinical Pharmacology 85(7): 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home Office (2019) Controlled drugs list of most commonly encountered drugs currently controlled under the misuse of drugs legislation. Available at: https://www.gov.uk/government/publications/controlled-drugs-list–2/list-of-most-commonly-encountered-drugs-currently-controlled-under-the-misuse-of-drugs-legislation (accessed 28 May 2021).
- Hoshino T, Shimodaira K. (1936) Über Die Synthese Des Bufotenin-Methyl-Äthers (5-Methoxy-N-Dimethyl-Tryptamin) Und Bufotenins (Synthesen In Der Indol-Gruppe. Xv). Bulletin of the Chemical Society of Japan 11(3): 221–224. [Google Scholar]
- Huszka L, Zabek DH, Doust JWL. (1976) Urinary excretion of N,N-dimethylated tryptamines in chronic schizophrenia: A review of the present status of the hypothesis. Canadian Psychiatric Association Journal 21(8): 541–546. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Heym J, Rasmussen K. (1983) Raphe neurons: Firing rate correlates with size of drug response. European Journal of Pharmacology 90(2–3): 275–278. [DOI] [PubMed] [Google Scholar]
- Jiang X-L, Shen H-W, Yu A-M. (2015) Potentiation of 5-methoxy-N,N-dimethyltryptamine-induced hyperthermia by harmaline and the involvement of activation of 5-HT1A and 5-HT2A receptors. Neuropharmacology 89: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X-L, Shen H-W, Yu A-M. (2016. a) Modification of 5-methoxy-N,N-dimethyltryptamine-induced hyperactivity by monoamine oxidase A inhibitor harmaline in mice and the underlying serotonergic mechanisms. Pharmacological Reports 68(3): 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X-L, Shen H-W, Mager DE, et al. (2013) Pharmacokinetic interactions between monoamine oxidase A inhibitor harmaline and 5-methoxy-N, N-dimethyltryptamine, and the impact of CYPD. Status Drug Metabolism and Disposition 41(5): 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X-L, Shen H-W, Mager DE, et al. (2016. b) Development of a mechanism-based pharmacokinetic/pharmacodynamic model to characterize the thermoregulatory effects of serotonergic drugs in mice. Acta Pharmaceutica Sinica B 6(5): 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R. (2008) Human hallucinogen research: Guidelines for safety. Journal of Psychopharmacology 22(6): 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, et al. (2014) Pilot study of the 5-HT 2A R agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology 28(11): 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Johnson PS, et al. (2017) An online survey of tobacco smoking cessation associated with naturalistic psychedelic use. Journal of Psychopharmacology 31(7): 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, et al. (2018) The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 142: 143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaumann AJ, Levy FO. (2006) 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacology & Therapeutics 111(3): 674–706. [DOI] [PubMed] [Google Scholar]
- Kodama T, Mushiake H, Shima K, et al. (1989) Slow fluctuations of single unit activities of hippocampal and thalamic neurons in cats – II: Role of serotonin on the stability of neuronal activities. Brain Research 487(1): 35–44. [DOI] [PubMed] [Google Scholar]
- Kofman O, Levin U. (1995) Myo-inositol attenuates the enhancement of the serotonin syndrome by lithium. Psychopharmacology 118(2): 213–218. [DOI] [PubMed] [Google Scholar]
- Kolbeck SC, Steers WD. (1992) Neural regulation of the vas deferens in the rat: An electrophysiological analysis. American Journal of Physiology-regulatory, Integrative and Comparative Physiology 263(2): R331–R338. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, et al. (2006) The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology 189(3): 319–329. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, et al. (2003) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. Journal of Pharmacology and Experimental Therapeutics 304(1): 229–237. [DOI] [PubMed] [Google Scholar]
- Lancelotta RL, Davis AK. (2020) Use of benefit enhancement strategies among 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) users: Associations with mystical, challenging, and enduring effects. Journal of Psychoactive Drugs 52(3): 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson AA. (1984) Acute and chronic effects of LSD and 5-MeODMT on raphe-evoked dorsal root potentials in the cat. Life Sciences 34(12): 1193–1201. [DOI] [PubMed] [Google Scholar]
- Lima da Cruz RV, Moulin TC, Petiz LL, et al. (2018) A single dose of 5-MeO-DMT stimulates cell proliferation, neuronal survivability, morphological and functional changes in adult mice ventral dentate gyrus. Frontiers in Molecular Neuroscience 11: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Witte U, Fredow G, et al. (1990) Pharmacodynamic effects of serotonin (5-HT) receptor ligands in pigs: Stimulation of 5-HT2 receptors induces malignant hyperthermia. Naunyn-schmiedeberg’s Archives of Pharmacology 341(6): 483–493. [DOI] [PubMed] [Google Scholar]
- Lucki I, Frazer A. (1982) Prevention of the serotonin syndrome in rats by repeated administration of monoamine oxidase inhibitors but not tricyclic antidepressants. Psychopharmacology 77(3): 205–211. [DOI] [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. (1984) Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. The Journal of Pharmacology and Experimental Therapeutics 228(1): 133–139. [PubMed] [Google Scholar]
- Martin P, Frances H, Simon P. (1985) Dissociation of head twitches and tremors during the study of interactions with 5-hydroxytryptophan in mice. Journal of Pharmacological Methods 13(3): 193–200. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Mizowaki M, Takayama H, et al. (1997) Suppressive effect of mitragynine on the 5-methoxy-N,N-dimethyltryptamine-induced head-twitch response in mice. Pharmacology, Biochemistry, and Behavior 57(1–2): 319–323. [DOI] [PubMed] [Google Scholar]
- Matthews WD, Smith CD. (1980) Pharmacological profile of a model for central serotonin receptor activation. Life Sciences 26(17): 1397–1403. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Fessler RG, Simonovic M, et al. (1978) Stimulation of rat prolactin secretion by indolealkylamine hallucinogens. Psychopharmacology 56(3): 255–259. [DOI] [PubMed] [Google Scholar]
- Metz A, Heal DJ. (1986) In mice repeated administration of electroconvulsive shock or desmethylimipramine produces rapid alterations in 5-HT2-mediated head-twitch responses and cortical 5-HT2 receptor number. European Journal of Pharmacology 126(1–2): 159–162. [DOI] [PubMed] [Google Scholar]
- Metzner R. (2013) The Toad and the Jaguar: A Field Report of Underground Research on a Visionary Medicine: Bufo Alvarius and 5-Methoxy-dimethyltryptamine (1st edn). Berkeley, CA: Regent Press. [Google Scholar]
- Miles DH, Ly AM, Randle SA, et al. (1987) Alkaloidal insect antifeedants from Virola calophylla warb. Journal of Agricultural and Food Chemistry 35(5): 794–797. [Google Scholar]
- Millière R, Carhart-Harris RL, Roseman L, et al. (2018) Psychedelics, meditation, and self-consciousness. Frontiers in Psychology 9: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser PC, Redfern PH. (1985) Circadian variation in behavioural responses to central 5-HT receptor stimulation in the mouse. Psychopharmacology 86(1–2): 223–227. [DOI] [PubMed] [Google Scholar]
- Moser PC, Redfern PH. (1988) The effect of benzodiazepines on the 5-HT agonist-induced head-twitch response in mice. European Journal of Pharmacology 151(2): 223–231. [DOI] [PubMed] [Google Scholar]
- Most A. (1984) Bufo Alvarius: The Psychedelic Toad of the Sonoran Desert. Denton, TX: Venom Press. [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. (2007) The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. European Journal of Pharmacology 559(2–3): 132–137. [DOI] [PubMed] [Google Scholar]
- Narasimhachari N, Avalos J, Fujimori M, et al. (1972) Studies of drug-free schizophrenics and controls. Biological Psychiatry 5(3): 311–318. [PubMed] [Google Scholar]
- Narasimhachari N, Baumann P, Pak HS, et al. (1974) Gas chromatographic-mass spectrometric identification of urinary bufotenin and dimethyltryptamine in drug-free chronic schizophrenic patients. Biological Psychiatry 8(3): 293–305. [PubMed] [Google Scholar]
- Narasimhachari N, Heller B, Spaide J, et al. (1971. a) N, N-dimethylated indoleamines in blood. Biological Psychiatry 3(1): 21–23. [PubMed] [Google Scholar]
- Narasimhachari N, Heller B, Spaide J, et al. (1971. b) Urinary studies of schizophrenics and controls. Biological Psychiatry 3(1): 9–20. [PubMed] [Google Scholar]
- Nash JF, Meltzer HY, Gudelsky GA. (1989) Selective cross-tolerance to 5-HT1A and 5-HT2 receptor-mediated temperature and corticosterone responses. Pharmacology, Biochemistry, and Behavior 33(4): 781–785. [DOI] [PubMed] [Google Scholar]
- National Programme on Substance Abuse Deaths (NPSAD) (2021) National Programme on Substance Abuse Deaths. Available at: https://www.sgul.ac.uk/about/our-institutes/population-health/research-themes/health-lifestyle-and-environments/npsad (accessed 8 March 2021). [DOI] [PMC free article] [PubMed]
- New Zealand Legislation (1975) New Zealand Legislation Misuse of Drugs Act, Schedule 1, Class A controlled drugs. Available at: https://www.legislation.govt.nz/act/public/1975/0116/latest/DLM436576.html (accessed 28 May 2021).
- Nielsen CK. (1998) Head and whole-body jerking in guinea pigs are differentially modulated by 5-HT1A, 5-HT1B/1D and 5-HT2A receptor antagonists. European Journal of Pharmacology 361(2–3): 185–190. [DOI] [PubMed] [Google Scholar]
- Nielsen EB. (1985) Discriminative stimulus properties of lysergic acid diethylamide in the monkey. The Journal of Pharmacology and Experimental Therapeutics 234(1): 244–249. [PubMed] [Google Scholar]
- Noorani T, Garcia-Romeu A, Swift TC, et al. (2018) Psychedelic therapy for smoking cessation: Qualitative analysis of participant accounts. Journal of Psychopharmacology 32(7): 756–769. [DOI] [PubMed] [Google Scholar]
- Nutt D, Erritzoe D, Carhart-Harris R. (2020) Psychedelic psychiatry’s brave new world. Cell 181(1): 24–28. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics (2020) Deaths related to drug poisoning by selected substances. 14 October. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsrelatedtodrugpoisoningbyselectedsubstances (accessed 12 March 2021).
- O’Hare E, Tierney KJ, Shephard RA. (1991) Cyclic-ratio schedule analysis of a serotonin agonist and depletor on consummatory behaviour. Physiology & Behavior 49(2): 331–334. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Nalivaiko E, Blessing WW. (2004) Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, ‘Ecstasy’) and its reversal by clozapine. Brain Research 1014(1–2): 34–44. [DOI] [PubMed] [Google Scholar]
- Ott J. (1996) Pharmacotheon: Entheogenic Drugs, Their Plant Sources and History (2nd edn). Kennewick, WA: Natural Products Co. [Google Scholar]
- Ott J. (2001) Pharmepéna-psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N, N-dimethyl-tryptamine. Journal of Psychoactive Drugs 33(4): 403–407. [DOI] [PubMed] [Google Scholar]
- Pachter IJ, Zacharias DE, Ribeiro O. (1959) Indole alkaloids of Acer saccharinum (The Silver Maple), Dictyoloma incanescens, Piptadenia colubrina, and Mimosa hostilis. The Journal of Organic Chemistry 24(9): 1285–1287. [Google Scholar]
- Palamar JJ, Acosta P. (2020) A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines. Human Psychopharmacology 35(1): e2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Le A. (2019) Use of new and uncommon synthetic psychoactive drugs among a nationally representative sample in the United States, 2005–2017. Human Psychopharmacology 34(2): e2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone F, Fagioli S, Castellano C. (1993) Effects of oxotremorine on inhibitory avoidance behaviour in two inbred strains of mice: Interaction with 5-methoxy-NN-dimethyltriptamine. Psychopharmacology 112(2–3): 249–252. [DOI] [PubMed] [Google Scholar]
- PDSP Database (2021) PDSP database-UNC. Available at: https://pdsp.unc.edu/databases/kidb.php (accessed 26 February 2021).
- Pochettino ML, Cortella AR, Ruiz M. (1999) Hallucinogenic snuff from Northwestern Argentina: Microscopical identification of Anadenanthera colubrina var. cebil (fabaceae) in powdered archaeological material. Economic Botany 53(2): 127–132. [Google Scholar]
- Pokorny T, Preller KH, Kraehenmann R, et al. (2016) Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. European Neuropsychopharmacology 26(4): 756–766. [DOI] [PubMed] [Google Scholar]
- PubChem (2021) N, N-dimethyl-5-methoxytryptamine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/1832 (accessed 26 February 2021).
- Quartermain D, Judge ME, Leo P. (1988) Attenuation of forgetting by pharmacological stimulation of aminergic neurotransmitter systems. Pharmacology, Biochemistry, and Behavior 30(1): 77–81. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Heym J, Jacobs BL. (1984) Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats. Experimental Neurology 83(2): 302–317. [DOI] [PubMed] [Google Scholar]
- Rätsch C. (2005) The Encyclopedia of Psychoactive Plants: Ethnopharmacology and Its Applications. Rochester, VT: Park Street Press. [Google Scholar]
- Ray TS. (2010) Psychedelics and the human receptorome. PLoS ONE 5(2): e9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rényi L. (1986. a) The effects of monoamine oxidase inhibitors on the ejaculatory response induced by 5-methoxy-N,N-dimethyltryptamine in the rat. British Journal of Pharmacology 88(4): 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rényi L. (1986. b) The effect of selective 5-hydroxytryptamine uptake inhibitors on 5-methoxy-N,N-dimethyltryptamine-induced ejaculation in the rat. British Journal of Pharmacology 87(4): 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riceberg LJ, Vunakis HV. (1978) Determination of N,N-dimethylindolealkylamines in plasma, blood and urine extracts by radioimmunoassay and high pressure liquid chromatography. Journal of Pharmacology and Experimental Therapeutics 206(1): 158–166. [PubMed] [Google Scholar]
- Richter A, Löscher W. (1995) Behavioural response to pharmacologic manipulation of serotonin receptors in the genetically dystonic hamster. Pharmacology, Biochemistry, and Behavior 52(4): 655–665. [DOI] [PubMed] [Google Scholar]
- Riga MS, Bortolozzi A, Campa L, et al. (2016) The serotonergic hallucinogen 5-methoxy-N,N-dimethyltryptamine disrupts cortical activity in a regionally-selective manner via 5-HT 1A and 5-HT 2A receptors. Neuropharmacology 101: 370–378. [DOI] [PubMed] [Google Scholar]
- Riga MS, Lladó-Pelfort L, Artigas F, et al. (2018) The serotonin hallucinogen 5-MeO-DMT alters cortico-thalamic activity in freely moving mice: Regionally-selective involvement of 5-HT1A and 5-HT2A receptors. Neuropharmacology 142: 219–230. [DOI] [PubMed] [Google Scholar]
- Riga MS, Soria G, Tudela R, et al. (2014) The natural hallucinogen 5-MeO-DMT, component of Ayahuasca, disrupts cortical function in rats: Reversal by antipsychotic drugs. The International Journal of Neuropsychopharmacology 17(08): 1269–1282. [DOI] [PubMed] [Google Scholar]
- Rigdon C, Weatherspoon K. (1992) 5-hydroxytryptaminea receptor agonists block prepulse inhibition of acoustic startle reflex. Journal of Pharmacology and Experimental Therapeutics 263(2): 486–493. [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Li L, et al. (2010) Intrahippocampal LSD accelerates learning and desensitizes the 5-HT2A receptor in the rabbit, Romano et al. Psychopharmacology 212(3): 441–448. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. Journal of Psychopharmacology 30(12): 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. (2007) Drugs and valvular heart disease. The New England Journal of Medicine 356(1): 6–9. [DOI] [PubMed] [Google Scholar]
- Roth BL, Lopez E, Patel S, et al. (2000) The multiplicity of serotonin receptors: Uselessly diverse molecules or an embarrassment of riches? The Neuroscientist 6(4): 252–262. [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, et al. (2000) Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102(23): 2836–2841. [DOI] [PubMed] [Google Scholar]
- Rucker JJH, Iliff J, Nutt DJ. (2018) Psychiatry & the psychedelic drugs: Past, present & future. Neuropharmacology 142: 200–218. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Arnt J, Hyttel J, et al. (1993) The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology 110(1–2): 53–59. [DOI] [PubMed] [Google Scholar]
- Sauras Quetcuti RB, Farre A, Mateu G, et al. (2019) A psychotic episode after ayahuasca and secretion of Bufo alvarius toad consumption: A case report. Institute of neuropsychiatry and addictions INAD, Parc de Salut Mar, 2019. Available at: http://www.postermedic.com/parcdesalutmar/pparcdesalutmar1917758/pdfbaja/pparcdesalutmar1917758.pdf
- Schlemmer RF, Davis JM. (1981) Evidence for dopamine mediation of submissive gestures in the stumptail Macaque monkey. Pharmacology, Biochemistry, and Behavior 14: 95–102. [DOI] [PubMed] [Google Scholar]
- Schlemmer RF, Davis JM. (1986) A primate model for the study of hallucinogens. Pharmacology, Biochemistry, and Behavior 24(2): 381–392. [DOI] [PubMed] [Google Scholar]
- Schlemmer RF, Narasimhachari N, Thompson VD, et al. (1977) The effect of a hallucinogen, 5-methoxy N,N-dimethyltryptamine, on primate social behavior. Communications in Psychopharmacology 1(2): 105–118. [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. (2010) Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a -arrestin2/Src/Akt signaling complex in vivo. Journal of Neuroscience 30(40): 13513–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, de Vry J. (1993) Studies on the neuronal circuits involved in the discriminative stimulus effects of 5-hydroxytryptamine 1A receptor agonists in the rat. The Journal of Pharmacology and Experimental Therapeutics 265(2): 572–579. [PubMed] [Google Scholar]
- Schultes R, Hofmann A. (1980) The Botany and Chemistry of Hallucinogens. Springfield: Charles C Thomas Publisher. [Google Scholar]
- Schultes RE, Hofmann A, Rätsch C. (2001) Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers (Rev. and expanded edn). Rochester, VT: Healing Arts Press. [Google Scholar]
- Seeman G, Brown GM. (1985) Indolealkylamines and prolactin secretion a structure-activity study in the central nervous system of the rat. Neuropharmacology 24(12): 1195–1200. [DOI] [PubMed] [Google Scholar]
- Sepeda ND, Clifton JM, Doyle LY, et al. (2019) Inhaled 5-methoxy-N,N-dimethyltryptamine: Supportive context associated with positive acute and enduring effects. Journal of Psychedelic Studies 4(2): 114–122. [Google Scholar]
- Sexton JD, Nichols CD, Hendricks PS. (2020) Population survey data informing the therapeutic potential of classic and novel phenethylamine, tryptamine, and lysergamide psychedelics. Frontiers in Psychiatry 10: 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Jiang X-L, Yu A-M. (2009) Development of a LC–MS/MS method to analyze 5-methoxy-N, N-dimethyltryptamine and bufotenine: Application to pharmacokinetic study. Bioanalysis 1(1): 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Jiang X-L, Yu A-M. (2011) Nonlinear pharmacokinetics of 5-methoxy-N, N-dimethyltryptamine in mice. Drug Metabolism and Disposition 39(7): 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Jiang X-LC, Winter J, et al. (2010. a) Psychedelic 5-methoxy-N,N-dimethyltryptamine: Metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Current Drug Metabolism 11(8): 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Wu C, Jiang X-L, et al. (2010. b) Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochemical Pharmacology 80(1): 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RA, Broadhurst PL. (1982) Effects of diazepam and of serotonin agonists on hyponeophagia in rats. Neuropharmacology 21(4): 337–340. [DOI] [PubMed] [Google Scholar]
- Shephard RA, Broadhurst PL. (1983) Hyponeophagia in the Roman rat strains: Effects of 5-methoxy-N,N-dimethyltryptamine, diazepam, methysergide and the stereoisomers of propranolol. European Journal of Pharmacology 95(3–4): 177–184. [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. (1997) Tihkal: The Continuation. Berkeley, CA: Transform press. [Google Scholar]
- Sills MA, Lucki I, Frazer A. (1985) Development of selective tolerance to the serotonin behavioral syndrome and suppression of locomotor activity after repeated administration of either 5-MeODMT or mCPP. Life Sciences 36(26): 2463–2469. [DOI] [PubMed] [Google Scholar]
- Simonovic M, Meltzer HY. (1979) Repeated administration of 5-methoxy-N,N-dimethyltryptamine to male rats potentiates stimulation of prolactin secretion by serotonin agonists. European Journal of Pharmacology 58(4): 399–405. [DOI] [PubMed] [Google Scholar]
- Simonovic M, Meltzer HY. (1983) Biphasic effect of 5-methoxy-N,N-dimethyltryptamine on rat prolactin secretion. Brain Research 272(2): 269–275. [DOI] [PubMed] [Google Scholar]
- Singleton C, Marsden CA. (1981) Circadian variation in the head twitch response produced by 5-methoxy-N1,N1-dimethyltryptamine and p-chloroamphetamine in the mouse. Psychopharmacology 74(2): 173–176. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, McLeod WR. (1990) Observations on the metabolism of the psychotomimetic indolealkylamines: Implications for future clinical studies. Biological Psychiatry 28(10): 841–848. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, Blackman GL, et al. (1987. a) Urinary excretion of 5-methoxy-N, N-dimethyltryptamine, N,N-dimethyltryptamine and their N-oxides in the rat. Biochemical Pharmacology 36(13): 2235–2237. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, McLeish M, et al. (1987. b) Gas chromatographic: Mass spectroscopic characterisation of the psychotomimetic indolealkylamines and their in vivo metabolites. Journal of Chromatography B: Biomedical Sciences and Applications 422: 13–23. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, Talomsin R, et al. (1987. c) In vivo metabolism of 5-methoxy-N, N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochemical Pharmacology 36(9): 1509–1512. [DOI] [PubMed] [Google Scholar]
- Sitaram BR, Talomsin R, Blackman GL, et al. (1987. d) Study of metabolism of psychotomimetic indolealkylamines by rat tissue extracts using liquid chromatography. Biochemical Pharmacology 36(9): 1503–1508. [DOI] [PubMed] [Google Scholar]
- Sklerov J, Levine B, Moore KA, et al. (2005) A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. Journal of Analytical Toxicology 29(8): 838–841. [DOI] [PubMed] [Google Scholar]
- Smith LM, Peroutka SJ. (1986) Differential effects of 5-hydroxytryptamine 1A selective drugs on the 5-HT behavioral syndrome. Pharmacology, Biochemistry, and Behavior 24(6): 1513–1519. [DOI] [PubMed] [Google Scholar]
- Smythies JR, Morin RD, Brown GB. (1979) Identification of dimethyltryptamine and O-methylbufotenin in human cerebrospinal fluid by combined gas chromatography/mass spectrometry. Biological Psychiatry 14(3): 549–556. [PubMed] [Google Scholar]
- Spencer DG, Glaser T, Traber J. (1987) Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology 93(2): 158–166. [DOI] [PubMed] [Google Scholar]
- Squires RF. (1975) Evidence that 5-Methoxy-N, N-dimethyltryptamine is a specific substrate for MAO-A in the rat: Implications for the indoleamine dependent behavioural syndrome. Journal of Neurochemistry 24(1): 47–50. [DOI] [PubMed] [Google Scholar]
- Stoff DM, GoreLick DA, Bozewicz T, et al. (1978) The indole hallucinogens, N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), have different effects from mescaline on rat shuttlebox avoidance. Neuropharmacology 17(12): 1035–1040. [DOI] [PubMed] [Google Scholar]
- Stolz JF, Marsden CA, Middlemiss DN. (1983) Effect of chronic antidepressant treatment and subsequent withdrawal on [3H]-5-hydroxytryptamine and [3H]-spiperone binding in rat frontal cortex and serotonin receptor mediated behaviour. Psychopharmacology 80(2): 150–155. [DOI] [PubMed] [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA) (2021. a) National Survey on Drug Use and Health: Concatenated Public Use File (2002 to 2019). Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA. Available at: https://pdas.samhsa.gov/#/survey/NSDUH-2002-2019-DS0001 [Google Scholar]
- Substance Abuse Mental Health Services Administration (SAMHSA) (2021. b) Drug Abuse Warning Network (2011): National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: SAMHSA. Available at: https://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf; https://www.samhsa.gov/data/report/national-estimates-drug-related-emergency-department-visits-2004-2011-illicits-excluding; https://www.samhsa.gov/data/sites/default/files/reports/rpt32809/DAWN%20Profile%20Expanded.pdf [Google Scholar]
- Suzuki O, Katsumata Y, Oya M. (1981) Characterization of eight biogenic indoleamines as substrates for type A and type B monoamine oxidase. Biochemical Pharmacology 30(11): 1353–1358. [PubMed] [Google Scholar]
- Szabo A, Kovacs A, Frecska E, et al. (2014) Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS ONE 9(8): e106533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimukai H. (1967) Modifications of paper and thin layer chromatographic methods to increase sensitivity for detecting n-methylated indoleamines in urine. Journal of Chromatography 30(1): 155–163. [DOI] [PubMed] [Google Scholar]
- Tanimukai H, Ginther R, Spaide J, et al. (1970) Detection of psychotomimetic N,N-dimethylated indoleamines in the urine of four schizophrenic patients. The British Journal of Psychiatry 117(539): 421–430. [DOI] [PubMed] [Google Scholar]
- Timmermann C, Roseman L, Schartner M, et al. (2019) Neural correlates of the DMT experience assessed with multivariate EEG. Scientific Reports 9(1): 51974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CM, Repke DB. (2006) Anadenanthera: Visionary Plant of Ancient South America. New York: Haworth Herbal Press. [Google Scholar]
- Tricklebank MD, Forler C, Middlemiss DN, et al. (1985) Subtypes of the 5-HT receptor mediating the behavioural responses to 5-methoxy-N, N-dimethyltryptamine in the rat. European Journal of Pharmacology 117(1): 15–24. [DOI] [PubMed] [Google Scholar]
- Trout K. (2007) Trout’s Notes on Some Simple Tryptamines: A Brief Overview & Resource Compendium. Mydriatic Productions. [Google Scholar]
- Trulson ME, Jacobs BL. (1979) Effects of 5-methoxy-N,N-dimethyltryptamine on behavior and raphe unit activity in freely moving cats. European Journal of Pharmacology 54(1–2): 43–50. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Keltch GF. (1985) Development of tolerance to repeated administration of 5-methoxy-N, N-dimethyltryptamine in rats. European Journal of Pharmacology 108(1): 33–37. [DOI] [PubMed] [Google Scholar]
- Trulson ME, MacKenzie RG. (1981) Subsensitivity to 5-hydroxytryptamine in agonists occurs in streptozocindiabetic rats with no change in [3H]-5-HT receptor binding. Journal of Pharmacy and Pharmacology 33(1): 472–474. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Crisp T, Trulson VM. (1984. a) Activity of serotonin-containing nucleus centralis superior (raphe medianus) neurons in freely moving cats. Experimental Brain Research 54(1): 816. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Preussler DW, Trulson VM. (1984. b) Differential effects of hallucinogenic drugs on the activity of serotonin-containing neurons in the nucleus centralis superior and nucleus raphe pallidus in freely moving cats. The Journal of Pharmacology and Experimental Therapeutics 228(1): 94–102. [PubMed] [Google Scholar]
- Tyler V, Gröger D. (1964) Investigation of the alkaloids of Amanita species 1-II: Amanita citrina and Amanita porphyria. Planta Medica 12(04): 397–402. [Google Scholar]
- United Nations Convention on Psychotropic Substances (1971) United Nations Convention on Psychotropic Substances. Available at: https://www.unodc.org/pdf/convention_1971_en.pdf (accessed 27 August 2019).
- United Nations Office on Drugs Crime (2020) World drug report. Available at: https://wdr.unodc.org/wdr2020/index.html (accessed 1 March 2021).
- Urban JD, Clarke WP, von Zastrow M, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. The Journal of Pharmacology and Experimental Therapeutics 320(1): 1–13. [DOI] [PubMed] [Google Scholar]
- Uthaug MV, Lancelotta R, Ortiz Bernal AM, et al. (2020. a) A comparison of reactivation experiences following vaporization and intramuscular injection (IM) of synthetic 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting. Journal of Psychedelic Studies 4: 104–113. [Google Scholar]
- Uthaug MV, Lancelotta R, Szabo A, et al. (2020. b) Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: Effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology 237(3): 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaug MV, Lancelotta R, van Oorsouw K, et al. (2019) A single inhalation of vapor from dried toad secretion containing 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology 236(9): 2653–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Buuse M, Ruimschotel E, Martin S, et al. (2011) Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT1A receptor knockout mice: Implications for schizophrenia. Neuropharmacology 61(1–2): 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Hasegawa H, Nakamura M, et al. (1992) Behavioral and electroencephalographic effects of a serotonin receptor agonist (5-methoxy-N, N-dimethyltryptamine) in a feline model of photosensitive epilepsy. Neuroscience Letters 138(1): 115–118. [DOI] [PubMed] [Google Scholar]
- Wallach JV. (2009) Endogenous hallucinogens as ligands of the trace amine receptors: A possible role in sensory perception. Medical Hypotheses 72(1): 91–94. [DOI] [PubMed] [Google Scholar]
- Walters JK, Sheard MH, Davis M. (1978) Effects of N,N-dimethyltryptamine (DMT) and 5-methoxy-N, N-dimethyltryptamine (5-MeODMT) on shock elicited fighting in rats. Pharmacology, Biochemistry, and Behavior 9(1): 87–90. [DOI] [PubMed] [Google Scholar]
- Weil AT, Davis W. (1994) Bufo alvarius: A potent hallucinogen of animal origin. Journal of Ethnopharmacology 41(1–2): 1–8. [DOI] [PubMed] [Google Scholar]
- Weston NM, Gibbs D, Bird CIV, et al. (2020) Historic psychedelic drug trials and the treatment of anxiety disorders. Depression and Anxiety 37: 1261–1279. [DOI] [PubMed] [Google Scholar]
- Winne J, Boerner BC, Malfatti T, et al. (2020) Anxiety-like behavior induced by salicylate depends on age and can be prevented by a single dose of 5-MeO-DMT. Experimental Neurology 326: 113175. [DOI] [PubMed] [Google Scholar]
- Winter J. (1999) The acute effects of monoamine reuptake inhibitors on the stimulus effects of hallucinogens. Pharmacology, Biochemistry, and Behavior 63(3): 507–513. [DOI] [PubMed] [Google Scholar]
- Winter JC, Amorosi DJ, Rice KC, et al. (2011) Stimulus control by 5-methoxy-N, N-dimethyltryptamine in wild-type and CYP2D6-humanized mice. Pharmacology, Biochemistry, and Behavior 99(3): 311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC, Petti DT. (1987) The effects of 8-hydroxy-2-(di-n-propylamino)tetralin and other serotonergic agonists on performance in a radial maze: A possible role for 5-HT1A receptors in memory. Pharmacology, Biochemistry, and Behavior 27(4): 625–628. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, et al. (2000) The paradox of 5-methoxy-N,N-dimethyltryptamine. Pharmacology, Biochemistry, and Behavior 65(1): 75–82. [DOI] [PubMed] [Google Scholar]
- Yamazaki J. (1992) Stimulatory and inhibitory effects of serotonergic hallucinogens on spinal mono-and polysynaptic reflex pathways in the rat. Neuropharmacology 31(7): 635–642. [DOI] [PubMed] [Google Scholar]
- Yanagita T. (1986) Intravenous self-administration of (-)-cathinone and 2-amino-1-(2,5-dimethoxy-4-methyl)phenylpropane in rhesus monkeys. Drug and Alcohol Dependence 17(2–3): 135–141. [DOI] [PubMed] [Google Scholar]
- Young R, Rosecrans JA, Glennon RA. (1982) Comparative discriminative stimulus effects of 5-methoxy-N, N-dimethyltryptamine and LSD. Life Sciences 30(24): 2057–2062. [DOI] [PubMed] [Google Scholar]
- Yu A-M, Idle JR, Herraiz T, et al. (2003) Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics 13(6): 307–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811211050543 for A narrative synthesis of research with 5-MeO-DMT by Anna O Ermakova, Fiona Dunbar, James Rucker and Matthew W Johnson in Journal of Psychopharmacology