Abstract
Purpose:
Radiological identification of lung masses in patients with pneumoconiosis is difficult. The aim of the study is to characterize Computed Tomography (CT) findings of Progressive Massive Fibrosis (PMF).
Methods:
The data of pneumoconiosis patients, who were diagnosed with PMF between 2014-2019 in a tertiary hospital, were collected. Demographic data, work-related data, Pulmonary Function Test results and radiological imaging results were gathered. Separate evaluations were made for the right and left lungs, and the CT findings and measurement results were recorded.
Results:
In 90% of our cases, PMF lesions were bilaterally located. Eighty-eight point five percent of the unilateral lesions were located in the upper lobe of the right lung. Enlarged lymph nodes were found in 83.3% and calcification was found in the lymph nodes in 63% of the cases. Band structures extending between the PMF lesion and the adjacent pleura were observed in 86% of the cases, and invagination in the lung parenchyma adjacent to the PMF was observed in 80% of the cases.
Conclusion:
In general, our findings were consistent with the radiologically defined PMF. In addition, pleural findings, which are not frequently studied in the literature except for asbestosis, were also described in the study.
Keywords: Pneumoconiosis, progressive massive fibrosis, computed tomography, silicosis, spirometry
Introduction
Pneumoconiosis is a progressive and irreversible lung disease caused by inhalation of inorganic dust and fibers [1]. The World Health Organization launched a campaign in 1995 with the prediction that pneumoconiosis could be eliminated worldwide by 2030. However, pneumoconiosis still continues to be an important health problem today. According to the 2016 Global Burden of Disease Study, it is estimated that 21,500 deaths and 414,900 years of potential life lost annually are thought to be due to pneumoconiosis [2]. Although it is a preventable disease, it is one of the most important causes of mortality due to occupational diseases. The diagnosis of pneumoconiosis is made by the determination of occupational exposure and the presence of typical radiological findings on standard chest radiographs (CXR).
The use of chest Computed Tomography (CT) has increased in recent years due to the low sensitivity and specificity of CXR in the diagnosis of early-stage pneumoconiosis and the high difference between intraobserver and interobserver evaluations in the ILO international classification of radiographsof pneumoconiosis [3, 4]. In the radiological evaluation of occupational lung disease, chest CT contributes to diagnosis, disease severity, prognosis, and identify comorbidities [4-6].
Progressive massive fibrosis (PMF) is a late-stage chronic pneumoconiosis form that is pathologically defined by clustering of silicotic nodules fused with connective tissue in silicosis and coal macules surrounded by fibrous tissue in coal workers pneumoconiosis [7]. PMF CXR findings have been known for years and are defined in the radiological classification of pneumoconiosis made by the ILO. According to this classification, PMF was defined as a large opacity exceeding 1 cm in diameter. PMF is classified as category A(one or more opacities greater than 10 mm but less than 50 mm in diameter), category B(one or more opacities greater than 50 mm in diameter but not exceeding the upper right zone), category C (one or more opacities in diameter exceeding the right upper zone) [8].
There are few studies in the literature describing the distinctive CT findings of PMF lesions. A limited number of studies were designed to describe CT findings of PMF such as shape, size, location, accompanying lymph node, calcification and other radiological abnormalities. Due to the difficulties in distinguishing large radiological lung masses in patients with pneumoconiosis or lung cancer, the primary aim of this study is to characterize CT findings of PMF. In addition, the relationship between the occupational characteristics, working hours, smoking habits and accompanying diseases of pneumoconiosis cases with the development of PMF and respiratory functions were presented.
Methods
Study Population
The study population of the retrospective cross-sectional study consists of all pneumoconiosis patients followed in the Occupational Diseases Training Clinic, in a tertiary hospital in Turkey outpatient clinic and service between January 1, 2014 and December 31, 2019. The study was started after the approval of the ethics committee (approval number: KAEK/2021-2348). Due to the retrospective design of the study, informed consent could not be obtained from the patients, but necessary permissions were obtained from the medical education board commission. The diagnosis was established by the presence of the occupational history of inorganic dust exposure and radiological findings consistent with pneumoconiosis and exclusion of other diagnoses. Of the 525 pneumoconiosis patients, 130 were diagnosed with PMF (solid pulmonary lesion > 1 cm). Cases without CT were excluded from the study and the remaining 90 PMF patients were included in the study.
Data Collection
The data was collected from patients' hospital files and hospital-based information systems. The pulmonary function test (PFT) results and radiological images obtained at the time of diagnosis were used. The dependent variable of the study was the presence of PMF and its independent variables were demographic data (age, gender), work-related data (duration of work in the profession, exposure time), smoking status, presence of concomitant disease, PFT results (FEV1, FVC, FEV1/FVC), and radiological imaging results (CXR, CT). In our study, smoking status was arranged according to WHO's guidelines for controlling and monitoring the tobacco epidemic [9]. Those who regularly smokecigarettes every day and those who smoke occasionally were included in the smokers group, and those who quit smoking were included in the ex-smokers group and who never smoke were included in the non-smokers group.
PFTs were performed according to the ATS/ERS joint consensus report on pulmonary function testing and by a trained operator. Standard spirometry evaluations were performed using the Zan 100 brand flow-sensitive spirometry device. Spirometry results were analyzed according to the acceptability and reproducibility criteria of the ATS/ERS Spirometry Standardization. The tests were repeated until the acceptability criteria were met. Spirometry measurements of the cases were evaluated according to the percentage of reference values.
CT images of all patients were retrieved from the PACS archive (GE Centricity PACS, Barrington, IL, USA). All the images evaluated were reconstructed images with 1 mm section thickness after the patients were taken with the spiral volumetric technique. Toshiba Alexion 16-section spiral CT device was used for shooting, and the shooting parameters were as follows: tube voltage=120 V, tube current: 80 mA, section thickness: 1 mm, spiral pitch factor:1.4, convolution filter: FC13-H, filter type: large. All CT images were evaluated by a radiologist with more than 20 years of experience in thoracic radiology. Images were viewed on medical monitors via a PACS viewer (GE Centricity PACS Universal Viewer Web Client Ver 6.0) and length measurements were made with the measurement tools in this viewer. After examining the cross-sectional images of the patients in the axial plane, multiplanar reconstruction images were created in the coronal plane for each patient, and these images were used to clarify the localization of the lesions and to measure their vertical length. In order not to cause confusion in the evaluation of calcifications, CT images without intravenous contrast material were preferred.
Separate evaluations were made for the right and left lungs, and the findings and measurement results were recorded in a table. The cases where the short axis of the mediastinal lymph nodes was above 1 cm were considered as lymphadenopathy. When evaluating the shape of the PMF lesions, lesions described as “spherical” or “discoid” were characterized by evaluating both axial and coronal images together. After examining the images in both planes, lesions that could not be classified as spherical or discoid were defined as “irregular” shaped.
PMF is defined as a large opacity exceeding 1 cm in diameter, according to the ILO international classification of pneumoconiosis. Category-A: one or more opacities greater than 10 mm but less than 50 mm in diameter; Category- B: One or more opacities greater than 50 mm in diameter but not exceeding the upper right zone; Category-C: defined as one or more opacities in diameter exceeding the right upper zone. Small opacity profusion was classified into 4 categories (0,1,2,3), each divided into 3 subcategories (0/- to 3/+). CT findings of all cases, previously classified by two ILO-certified Pneumoconiosis readers, were evaluated by an expert radiologist. 171 PMF lesions observed on CT of 90 patients were evaluated in detail.
Statistical analyses were performed by IBM SPSS Statistics V22 (standard version) package program. Frequency, percentage, mean value, and standard deviation were used for descriptive statistics. Chi-square was used for the analysis of categorical data, the Kolmogorov-Smirnov test was used for the normal distribution fit of the variables for the analysis of quantitative data, Student's t-test was used because it conformed to the normal distribution, and One-way Anova, (posthoc Tukey) was used for more than two variables. Pearson correlation analysis was used for correlation analysis. p values less than 0.05 were considered significant.
Results
All of the 90 individuals diagnosed with PMF were male. The mean age was 56.1±13.5 (min-max:26-79) years. The characteristics of the study population such as occupation, smoking status, exposure time, type of large opacity, and ILO category are given in Table 1. Respiratory diseases accompanying PMF were COPD (66.7%), pulmonary tuberculosis (21.2%) and asthma (5.6%). The most common small opacity was q (39.9%) and the most common large opacity was A (41.1%).
Table 1.
Characteristics of the study population
| Variable | n | % | |
|---|---|---|---|
| Occupation | Dental Technician | 16 | 17,8 |
| Foundry Worker | 10 | 11,1 | |
| Construction Worker | 2 | 2,2 | |
| Welder | 2 | 2,2 | |
| Sandblaster | 6 | 6,7 | |
| Coal Miner | 35 | 38,9 | |
| Marble worker | 5 | 5,6 | |
| Ceramic Worker | 4 | 4,4 | |
| Quarry mill worker | 6 | 6,6 | |
| Tunnel Worker | 4 | 4,4 | |
| Smoking Status | Smoker | 32 | 35,6 |
| Non-smoker | 21 | 23,3 | |
| Ex|-smoker | 37 | 41,1 | |
| Exposure time (year) | 0-3 | 5 | 5,6 |
| 4-9 | 11 | 12,2 | |
| 10-19 | 26 | 28,9 | |
| ≥20 | 48 | 53,3 | |
| Large Opacity | A | 37 | 41,1 |
| B | 26 | 28,9 | |
| C | 27 | 30,0 | |
| ILO Category | Category 1 | 9 | 10,0 |
| Category 2 | 46 | 51,1 | |
| Category 3 | 35 | 38,9 | |
| Dominant Small Opacity | p | 3 | 3,3 |
| q | 39 | 43,3 | |
| r | 36 | 40 | |
| s | 3 | 3,3 | |
| t | 9 | 10 | |
The mean value of FEV1, FVC, FEV1/FVC were 58.7±21.3, 66.8±19.9 and 68.9±13.5 respectively. When some variables were compared with pulmonary function test results, a statistically significant decrease was found in FEV1 and FVC as the large opacity size increased (p<0.05). Statistically significant decrease was found FEV1 and FEV1/FVC in the presence of emphysema (p<0.05). No statistically significant difference was found in ILO small opacity type (p,q,r,s,t) and profusion category with pulmonary function test results (p>0.05). The relationship between pulmonary function test results and some variables is reported in Table 2.
Table 2.
The relationship between pulmonary function test results and some variables
| Variables | PFT Results | ||||
|---|---|---|---|---|---|
| n | FEV1 Mean/sd | FVC Mean/sd | FEV1/FVC Mean/sd | ||
| Smoking Status* | Smoker | 32 | 54,9±21,3 | 63,1±20,4 | 69,3±15,9 |
| Non-smoker | 21 | 65,8±20,6 | 72,2±18,4 | 70,6±14,5 | |
| Ex-smoker | 37 | 57,9±21,1 | 67,1±20,0 | 67,6±10,4 | |
| p | 0,183 | 0,264 | 0,703 | ||
| Large Opacity** | A | 37 | 67,8±16,5a | 74,8±15,3a | 71,4±11,0 |
| B | 26 | 62,8±22,0a | 71,5±20,2a | 68,8±17,2 | |
| C | 27 | 42,3±17,3b | 51,6±16,8b | 65,5±12,1 | |
| p | <0,001 | <0,001 | 0,219 | ||
| Emphysema* | Yes | 35 | 51,5±22,0 | 64,5±22,5 | 63,0±15,69 |
| No | 55 | 63,3±19,7 | 68,4±18,0 | 72,7±10,1 | |
| p | 0,010 | 0,372 | 0,001 | ||
* Student t Test
** One Way ANOVA (post hoc Tukey), a,b: The difference between groups that do not have the same letter in each line is significant (p<0.05)
PMF lesions were bilaterally located in 81 (90%) of the cases. In 95.6% of the cases all lesions were located in the superior lobes; only 4 (4.4%) cases had PMF lesions located in the middle and lower lobes. Eight of the unilateral lesions were located in the right upper lobe, and one was located in the left upper lobe. The shape of the 171 PMF lesions were irregular in 134 (78.3%) spherical in 26 (15.2%) and discoid in 11 (6.4%).
The number of lesions with regular peripheral contour and irregular central contour was 55 (32.2%). Calcification was detected in 107 (62.6%) of the PMF lesions, and the type of calcification was mostly punctate calcification. Multiple lymphadenopathies were observed in 75 (83.3%) of the cases, and calcification was observed in 57 (63.3%) of them. Eggshell calcification was found in 24.4% of the lymph nodes. Figures 1 and 2 show representative CT findings in pneumoconiosis patients with PMF. Multiple small nodules, thick band appearance extending from the adjacent pleura to the PMF, and paracicatricial emphysematous lung tissue between the pleura and the PMF lesion were seen in figures. Accompanying radiological findings with PMF lesions includes: round atelectasis in one patient, subpleural line in one patient, ground-glass opacity in two patients, interlobular septal thickening in 3 patients, consolidation in 3 patients, and atelectasis in 7 patients. Honeycomb and satellite nodules were not detected in any patient. The frequency of CT findings of the patients is given in Table 3.
Figure 1.
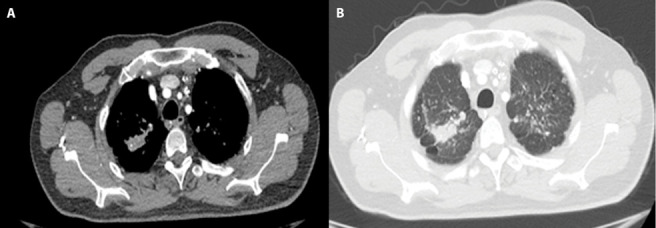
42 years old man who has done sandblasting on glass for 20 years diagnosed as PMF.
A-B. Chest HRCT image shows irregularly shape with punctate calcification PMF lesion in the right upper lobe. Multiple small nodules indicative of silicosis are also seen in lung tissue bilaterally. Thick band appearance extending from the adjacent pleura to the PMF, and paracicatricial emphysematous lung tissue between the pleura and the PMF lesion was seen.
Figure 2.
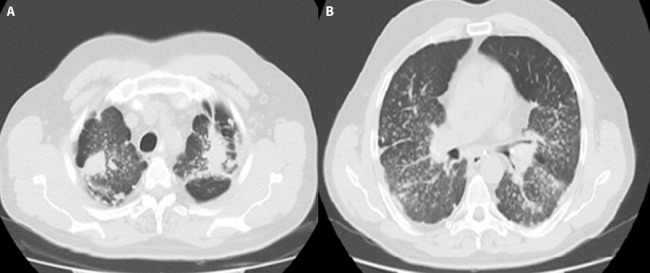
54 years old man who worked as a miner in a lead mine for 22 years diagnosed PMF.
A-B. Chest HRCT image shows bilaterally located, discoid shaped in right upper lobe and irregular shaped in left upper lobe with punctate calcification PMF lesions. Multiple small nodules indicative of silicosis are also seen in surrounding lung tissue. Thick band appearance extending from the adjacent pleura to the PMF, and paracicatricial emphysematous lung tissue between the pleura and the PMF lesion was seen.
Table 3.
The frequency of CT findings of the patients
| CT Findings | n | % | |
|---|---|---|---|
| Mass Calcification Type (n:107) | Amorphous | 12 | 11,2 |
| Punctat | 70 | 65,4 | |
| Diffuse | 13 | 12,2 | |
| Eggshell | 12 | 11,2 | |
| Type of Lymph node Calcification (n: 57) | Amorphous | 1 | 1,1 |
| Punctat | 26 | 28,8 | |
| Eggshell | 22 | 24,4 | |
| Diffuse | 8 | 8,8 | |
| Pleural Band | Non-exists | 12 | 13,3 |
| Exists | 78 | 86,7 | |
| Pleural pseudoplaque | Non-exists | 85 | 94,4 |
| Exists | 5 | 5,6 | |
| Bronchiectasis | Non-exists | 61 | 67,8 |
| Exists | 29 | 32,2 | |
| Pleural Thickening | Non-exists | 79 | 87,8 |
| Exists | 11 | 12,2 | |
| Pleural Shrinking | Non-exists | 18 | 20,0 |
| Exists | 72 | 80,0 | |
| Pleural Fluid | Non-exists | 85 | 94,4 |
| Exists | 5 | 5,6 | |
| Fine Reticular Density | Non-exists | 86 | 95,6 |
| Exists | 4 | 4,4 | |
| Millimetric nodule | Non-exists | 27 | 30 |
| Exists | 63 | 70 | |
| Emphysema | Non-exists | 55 | 51 |
| Exists | 35 | 39 | |
When the sequential correlation between small opacity profusion category and large opacity is evaluated, the profusion category increased as the large opacity size increased (r: 0.265, p: 0.012). When the dominant small opacities were categorized according to their size (<1.5 mm, 1.5-3mm, and >3mm), it was found that the large opacity size increased as the dominant small opacity size increased (r: 0.409, p <0.001).
When the exposure time is categorized in two groups (≤ 10 years and >10 years) there was no significant difference between the groups in terms of large opacity size, small opacity profusion category, and concomitant pulmonary disease (p>0.05). When the relationship between smoking status and ILO profusion category and large opacity was evaluated, no significant difference was found (p>0.05). When the relationship between smoking status and presence of emphysema was evaluated, a significant difference was found (p: 0.011). Emphysema existence in smokers, ex-smokers, and non-smokers were 59.4%, 29.7%, and 23.8% respectively. In the presence of concomitant tuberculosis, only pleural retraction and fine reticular density were higher, no correlation was found with other findings (X2: 4,270, p: 0,050 & X2: 7,299, p: 0,028).
Discussion
Pneumoconiosis is a preventable occupational disease that can be diagnosed with occupational history, clinical and radiological findings [1]. However, it is not always possible to distinguish PMF lesions from other lung mass lesions radiologically. In the literature, the number of studies describing the tomographic findings of complicated pneumoconiosis in detail and revealing the relationship with occupational characteristics is limited. Due to the difficulties in radiological identification of lung masses in patients with pneumoconiosis, this study aimed to characterize CT findings of PMF.
In general, our findings were consistent with the location data of PMF lesions defined in the literature. In 90% of our cases, PMF lesions were bilaterally located. Eighty-eight point eight percent of the unilateral lesions were located in the upper lobe of the right lung. The outer surface was regular and the central surface was irregular in 32% of the PMF lesions. Contrary to the information previously described in the literature that the outer surface of the PMF lesions is regular and the central surface is irregular, both the outer surface and the central surface of the PMF lesions were found to be irregular in most of our cases. Generally, PMF lesions are thought to be elongated (aspect ratio greater than 3) and less spherical than other lesions. However, in our study, only 5% (9/171) of the lesions had elongated shape. There was positive correlation between the size of large opacity and the profusion category and size of small opacity. In 51.1% of the cases, the small opacity profusion category was 2 (2/1, 2/2, 2/3), and the median profusion score was 2/2.
Associated with normal airway anatomy, inhaled particles are accumulated in the upper lobes, especially the right upper lob. Additionally, with slow lymphatic drainage in the upper lobes, PMF lesions are usually located in the upper lobes [10-12].In our study, the lesions were generally located in the upper lobe, consistent with the literature, and the lesions were located in the middle and lower lobes in only 4.4% of the cases. Similar to our study, PMF lesions were observed in the middle and lower lung areas in only 6.8% of the cases in the study of CN Halldin et al. [13].In the study of Ferreira AS et al., PMF lesions were reported to be located in the upper and posterior lobes in 88.6% of the cases [14].
The data obtained from studies evaluating the relationship between the small opacity profusion category and the incidence, number and size of large opacity in the literature are contradictory. In our study, a positive correlation was found between small opacity profusion category, small opacity size and large opacity size. Similar to our study, a positive correlation was reported between the small opacity profusion category and the incidence of PMF in the study of Ng et al., no correlation was found between the small opacity profusion score and the frequency and size of PMF in the study of CN Haldin et al. [13, 15].
Studies that examine PMF lesions in coal workers retrospectively, a small opacity profusion score was found to be stage 1 in 37-40% of cases with PMF [13, 16]. In our study, the small opacity profusion score was evaluated as Stage 1 (1/1, 1/2) in 10% of the cases. The low number of cases with low small opacity profusion scores in our study may be due to the lack of a surveillance system in pneumoconiosis cases who retired in our country, that is, the fact that these cases could not be detected. This uncertainty regarding the progression of the disease reminds the importance of the need for surveillance of pneumoconiosis cases with low profusion scores even after exposure ends.
It has been suggested that hilar and mediastinal lymphadenopathy precede the parenchymal findings of silicosis [17-19]. Cox-Grasner et al. found that 90% of cases with silicosis were accompanied by fibrotic lymph nodes [20]. In a study of necrobiopsy materials of 849 miners, fibrotic lymph nodes were found in 57% of all cases and fibrosis in lymph nodes was found in 88% of cases with silicosis [21]. In our study, enlarged lymph nodes were found in 83.3% of the cases. These findings support the hypothesis that the development of fibrotic nodules in the lymph nodes impairs the removal of silica dust from the lungs by causing impaired lymphatic drainage in the lung, increasing the dust load in the lung and reflecting an additional risk for parenchymal silicosis.
Although lymph node calcification is often caused by primary granulomatous infections such as tuberculosis, it can also be detected less frequently in diseases such as silicosis, sarcoidosis and amyloidosis [22]. In our study, calcification was found in the lymph nodes in 63% of the cases. Similar to our study, Antao et al.'s study showed that 74% of patients with silicosis had enlarged mediastinal lymph nodes, and 66% of them had lymph node calcification [3]. Eggshell calcification is the most common pattern defined in silicosis [14, 18, 23]. However, some authors reported that the punctate pattern was detected more frequently than eggshell calcification [3, 24]. In our study, 29% of lymph node calcifications were punctate, and eggshell calcification was found in 24% of the cases.
It has been reported that cavitation rarely develops in PMF lesions due to concomitant tuberculosis infection and ischemic necrosis [7, 25]. In our study, cavitation was seen in 5 cases and was an uncommon finding. There was concomitant tuberculosis infection in 3 of the 5 cases with cavitation. The exposure time of the other 2 cases was shorter than 10 years, and it was thought that cavitation developed due to ischemic necrosis due to accelerated silicosis. In the study of Ferreira et al., cavitation was found in 8 out of 75 PMF cases, similar to our study, and concomitant tuberculosis was reported in 6 of them [14].
Tuberculosis is one of the main complications of silicosis. The risk of developing tuberculosis in patients diagnosed with silicosis is 2.8-39 times higher than in healthy volunteers [26-28].The coexistence of silicosis and tuberculosis accelerates the progression of pneumoconiosis. In the literature, it has been reported that the incidence of tuberculosis is higher in complicated silicosis than in simple silicosis. In the study of Ferreira et al., coexistence of silicosis and tuberculosis was found in 52% of the cases [14]. In another study, tuberculosis was found in 34% of sandblasting workers who developed PMF [29]. In our study, concomitant pulmonary tuberculosis was found in 22% of the cases, and the importance of tuberculosis infection as a risk factor for the development of PMF was confirmed. Susceptibility to tuberculosis in PMF is thought to increased due to multifactorial factors such as the possible chemical effect of silica on bacillus proliferation, as well as macrophage toxicity and long-term retention of the bacillus in the lung tissue due to inadequate lymphatic drainage [30].
Although pleural diseases such as pleural plaque, pleural effusion, and pleural thickening are well-known consequences of asbestos exposure, other pneumoconiosis has also been rarely reported to cause pleural diseases [31-33]. In a study evaluating the pleural findings in patients with silicosis, pleural thickening was found in 58% of the patients, and in another study in which tuberculosis patients were excluded, pleural thickening was found in 32% of the patients [34, 35].
Pleural thickening in the advanced stage silicosis develops in association with scarring and fibrosis in the pulmonary parenchyma and it is thought that pulmonary tuberculosis contributes to its development [36]. In our study, pleural thickening was found in 12% of the cases. The reason for the lower rate of pleural thickening in our study may be due to the presence of patients with anthracosis and silicosis together and the lower rate of pulmonary tuberculosis in our study than in other studies.
In addition to pleural thickening, some studies have reported that patients with silicosis may develop PMF-related pleural invagination. Arakawa et al. was reported that a thick band appearance extending from the adjacent pleura to the PMF was found in 17 cases (44%) with PMF lesions on CT. It has also been confirmed pathologically that the thickened band structures represent the pleural invagination [34]. In our study, band structures extending between the PMF lesion and the adjacent pleura were observed in 86% of the cases, and shrinking in the lung parenchyma adjacent to the PMF was observed in 80% of the cases. There is no definite knowledge as to why the thickened pleura invaginates towards the parenchyma but some hypothesis exists. PMF causes fibrosis and recession in the adjacent lung parenchyma by coalescing of small opacities, resulting in volume loss and emphysema in the lung tissue surrounding PMF. Therefore, the thickened pleura is assumed to proliferate and fold towards the contracting PMF, especially when the intervening distance is small [4].
Although pulmonary dysfunction detected in patients with pneumoconiosis was associated with small opacity profusion score and large opacity size in some studies, this correlation could not be demonstrated in other studies [3, 35-38]. In our study no relationship was found between pulmonary functions and small opacity profusion, however a statistically significant decrease was found in FEV1 and FVC as the PMF size increased. Furthermore, statistical significance did not change when smokers were excluded.
PMF lesions tend to occur peripherally and extend towards the hilum, forming paracicatricial emphysematous lung tissue between the pleura and the PMF lesion. In our study, an emphysematous lung area was found between the lesion and the visceral pleura in 39% of the cases. Pulmonary emphysema is associated with alveolar destruction, increased dead space, and obstructive-type respiratory dysfunction. However, it is debated whether emphysema occurs as a complication of pneumoconiosis or as a result of smoking. There was an association between smoking and emphysema in our study. Contrary to the publications reporting that the effect of smoking on lung functions is greater than that of coal mining, there are many studies showing that smoking is not associated with lung functional changes detected in CWP [39-43]. It was reported that emphysema was associated with PMF and silica exposure in nonsmoker silicosis patients [35, 36, 38, 44]. In the study of Lyons et al., emphysema was reported similarly in autopsy tissues of smokers and non-smokers, and it was reported that emphysema developed as a complication of pneumoconiosis independent of smoking [45].
Inconclusion, our study describes, for the first time in Turkey, the occupational characteristics of PMF cases together with pulmonary function test findings and detailed radiological evaluation results. In general, our findings were consistent with the radiologically defined PMF location, shape, calcification and accompanying emphysema data in the literature. In addition, pleural findings in pneumoconiosis, which are not frequently studied in the literature except for asbestosis, were described in our study. Band structures extending between the PMF lesion and adjacent pleura and shrinking in the adjacent lung parenchyma were observed as pleural invagination findings in the vast majority of our cases, and the importance of pleural findings in the radiological definition of PMF was also noted.
However, there were some limitations of the study. Workers who applied to our hospital with symptoms were included in the study and this feature may have affected our results due to the characteristics of the participants. Conducting studies with larger participation may enable the development of appropriate strategies for the eradication of pneumoconiosis by enabling the early identification of cases and the determination of risk factors to be considered in order to prevent the development of severe disease. Another limitation of our study is the lack of a nationwide surveillance system for those working in pneumoconiosis-inducing jobs, which precludes providing precise data to characterize the pneumoconiosis burden in Turkey.
Conflict of Interest:
No conflict of interest was declared by the authors.
References
- 1.Organization IL. Fourth International Pneumoconiosis Conference; Geneva, Switzerland; 1971. Report of the Working Party on the Definition of Pneumoconiosis. [Google Scholar]
- 2.Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.dos Santos Antao VC, Pinheiro GA, Terra-Filho M, Kavakama J, Müller NL. High-resolution CT in silicosis: correlation with radiographic findings and functional impairment. J Comput Assist Tomogr. 2005;29(3):350–6. doi: 10.1097/01.rct.0000160424.56261.bc. [DOI] [PubMed] [Google Scholar]
- 4.Bergin C, Muller N, Vedal S, Chan-Yeung M. CT in silicosis: correlation with plain films and pulmonary function tests. Am J Roentgenol. 1986;146(3):477–83. doi: 10.2214/ajr.146.3.477. [DOI] [PubMed] [Google Scholar]
- 5.Bégin R, Ostiguy G, Fillion R, Colman N. Computed Tomography Scan in the Early Detection of Silicosis1, 2. Am Rev Respir Dis. 1991;144:697–705. doi: 10.1164/ajrccm/144.3_Pt_1.697. [DOI] [PubMed] [Google Scholar]
- 6.Talini D, Paggiaro PL, Falaschi F, et al. Chest radiography and high resolution computed tomography in the evaluation of workers exposed to silica dust: relation with functional findings. Occup Environ Med. 1995;52(4):262–7. doi: 10.1136/oem.52.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Pneumoconiosis: Comparison of Imaging and Pathologic Findings. RadioGraphics. 2006;26(1):59–77. doi: 10.1148/rg.261055070. [DOI] [PubMed] [Google Scholar]
- 8.Organization IL. guidelines for the use of the ilo international classification of radiographs of pneumoconioses: occupational safety and health series no. 22 (rev. 2011) 2011 [Google Scholar]
- 9.Organization WH. Guidelines for controlling and monitoring the tobacco epidemic: World Health Organization. 1998 [Google Scholar]
- 10.Egashira R, Tanaka T, Imaizumi T, et al. Differential distribution of lymphatic clearance between upper and lower regions of the lung. Respirology. 2013;18(2):348–53. doi: 10.1111/resp.12006. [DOI] [PubMed] [Google Scholar]
- 11.Asgharian B, Hofmann W, Bergmann R. Particle deposition in a multiple-path model of the human lung. Aerosol Sci Technol. 2001;34(4):332–9. [Google Scholar]
- 12.Subramaniam RP, Asgharian B, Freijer JI, Miller FJ, Anjilvel S. Analysis of lobar differences in particle deposition in the human lung. Inhal Toxicol. 2003;15(1):1–21. doi: 10.1080/08958370304451. [DOI] [PubMed] [Google Scholar]
- 13.Halldin CN, Blackley DJ, Markle T, Cohen RA, Laney AS. Patterns of progressive massive fibrosis on modern coal miner chest radiographs. Arch Environ Occup Health. 2020;75(3):152–8. doi: 10.1080/19338244.2019.1593099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira ÁS, Moreira VB, Ricardo HMV, Coutinho R, Gabetto JM, Marchiori E. Progressive massive fibrosis in silica-exposed workers: high-resolution computed tomography findings. J Bras Pneumol. 2006;32(6):523–8. doi: 10.1590/s1806-37132006000600009. [DOI] [PubMed] [Google Scholar]
- 15.Ng TP, Chan SL. Factors associated with massive fibrosis in silicosis. Thorax. 1991;46(4):229–32. doi: 10.1136/thx.46.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almberg KS, Halldin CN, Blackley DJ, et al. Progressive massive fibrosis resurgence identified in US coal miners filing for black lung benefits, 1970–2016. Ann Am Thorac Soc. 2018;15(12):1420–6. doi: 10.1513/AnnalsATS.201804-261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nin CS, de Souza VVS, do Amaral RH, et al. Thoracic lymphadenopathy in benign diseases: A state of the art review. Respir Med. 2016;112:10–7. doi: 10.1016/j.rmed.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Satija B, Kumar S, Ojha UC, Gothi D. Spectrum of high-resolution computed tomography imaging in occupational lung disease. Indian J Radiol Imaging. 2013;23(4):287. doi: 10.4103/0971-3026.125564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaton A, Cherrie JW. Quartz exposures and severe silicosis: a role for the hilar nodes. Occup Environ Med. 1998;55(6):383–6. doi: 10.1136/oem.55.6.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox-Ganser JM, Burchfiel CM, Fekedulegn D, Andrew ME, Ducatman BS. Silicosis in lymph nodes: the canary in the miner? JOEM/ACOEM. 2009;51(2):164. doi: 10.1097/JOM.0b013e31818f6a0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray J, Webster I, Reid G, Kielkowski D. The relation between fibrosis of hilar lymph glands and the development of parenchymal silicosis. Occup Environ Med. 1991;48(4):267–9. doi: 10.1136/oem.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchiori E, Hochhegger B, Zanetti G. Lymph node calcifications. J Bras Pneumol. 2018;44(2):83. doi: 10.1590/S1806-37562018000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown K, Mund DF, Aberle DR, Batra P, Young DA. Intrathoracic calcifications: radiographic features and differential diagnoses. Radiographics. 1994;14(6):1247–61. doi: 10.1148/radiographics.14.6.7855339. [DOI] [PubMed] [Google Scholar]
- 24.Ooi C, Khong P, Cheng R, et al. The relationship between mediastinal lymph node attenuation with parenchymal lung parameters in silicosis. Int J Tuberc Lung Dis. 2003;7(12):1199–206. [PubMed] [Google Scholar]
- 25.Cox CW, Rose CS, Lynch DA. State of the art: imaging of occupational lung disease. Radiology. 2014;270(3):681–96. doi: 10.1148/radiol.13121415. [DOI] [PubMed] [Google Scholar]
- 26.Corbett EL, Churchyard GJ, Clayton T, et al. Risk factors for pulmonary mycobacterial disease in South African gold miners: a case-control study. Am J Respir Crit Care Med. 1999;159(1):94–9. doi: 10.1164/ajrccm.159.1.9803048. [DOI] [PubMed] [Google Scholar]
- 27.Barboza CEG, Winter DH, Seiscento M, Santos UdP, Terra Filho M. Tuberculosis and silicosis: epidemiology, diagnosis and chemoprophylaxis. J Bras Pneumol. 2008;34(11):959–66. doi: 10.1590/s1806-37132008001100012. [DOI] [PubMed] [Google Scholar]
- 28.Cowie RL. The epidemiology of tuberculosis in gold miners with silicosis. Am J Respir Crit Care Med. 1994;150(5):1460–2. doi: 10.1164/ajrccm.150.5.7952577. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira A, Moreira V, Souza A, Gabetto J, Clemente C, Aidé M. Silicotuberculose: análise de 82 casos. J Pneumol. 2000;26(S3):43–4. [Google Scholar]
- 30.Algranti E. Slateworkers’ pneumoconiosis: MSc thesis. University of Wales. 1982 [Google Scholar]
- 31.Al-Kassimi FA. Pleural effusion in silicosis of the lung. Br J Ind Med. 1992;49(6):448. doi: 10.1136/oem.49.6.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazziotti S, Costa C, Ascenti G, Lamberto S, Scribano E. Unusual pleural involvement after exposure to amorphous silicates (Liparitosis): report of two cases. Eur Radiol. 2002;12(5):1058–60. doi: 10.1007/s003300101046. [DOI] [PubMed] [Google Scholar]
- 33.Xeren EH, Colby TV, Roggli VL. Silica-induced pleural disease: an unusual case mimicking malignant mesothelioma. Chest. 1997;112(5):1436–8. doi: 10.1378/chest.112.5.1436. [DOI] [PubMed] [Google Scholar]
- 34.Arakawa H, Honma K, Saito Y, et al. Pleural disease in silicosis: pleural thickening, effusion, and invagination. Radiology. 2005;236(2):685–93. doi: 10.1148/radiol.2362041363. [DOI] [PubMed] [Google Scholar]
- 35.Lopes AJ, Mogami R, Capone D, Tessarollo B, Melo PLd, Jansen JM. High-resolution computed tomography in silicosis: correlation with chest radiography and pulmonary function tests. J Bras Pneumol. 2008;34(5):264–72. doi: 10.1590/s1806-37132008000500004. [DOI] [PubMed] [Google Scholar]
- 36.Ooi GC, Tsang KW, Cheung TF, et al. Silicosis in 76 men: qualitative and quantitative CT evaluation—clinical-radiologic correlation study. Radiology. 2003;228(3):816–25. doi: 10.1148/radiol.2283020557. [DOI] [PubMed] [Google Scholar]
- 37.Bégin R, Ostiguy G, Cantin A, Bergeron D. Lung function in silica-exposed workers: a relationship to disease severity assessed by CT scan. Chest. 1988;94(3):539–45. doi: 10.1378/chest.94.3.539. [DOI] [PubMed] [Google Scholar]
- 38.Arakawa H, Gevenois PA, Saito Y, et al. Silicosis: expiratory thin-section CT assessment of airway obstruction. Radiology. 2005;236(3):1059–66. doi: 10.1148/radiol.2363041611. [DOI] [PubMed] [Google Scholar]
- 39.Gross P, de Treville RT. Black lungs. Taylor & Francis; 1970. [DOI] [PubMed] [Google Scholar]
- 40.Morgan WKC, Burgess DB, Jacobson G, et al. The prevalence of coal workers’ pneumoconiosis in US coal miners. Arch Environ Health. 1973;27(4):221–6. doi: 10.1080/00039896.1973.10666356. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh Y, Wang D, Shen C, Chiang C. The diffusing capacity blood gases analysis and ventilatory functions in coal workers' pneumoconiosis. Chin Med J. 1981;28:103–16. [Google Scholar]
- 42.Rasmussen D, Nelson C. Respiratory function in southern Appalachian coal miners. American Review of Respiratory Disease. 1971;103(2):240–8. doi: 10.1164/arrd.1971.103.2.240. [DOI] [PubMed] [Google Scholar]
- 43.Yeoh C-I, Yang S-C. Pulmonary function impairment in pneumoconiotic patients with progressive massive fibrosis. Chang Gung medical journal. 2002;25(2):72–80. [PubMed] [Google Scholar]
- 44.de Castro MCS, Ferreira AS, Irion KL, et al. CT quantification of large opacities and emphysema in silicosis: correlations among clinical, functional, and radiological parameters. Lung. 2014;192(4):543–51. doi: 10.1007/s00408-014-9590-9. [DOI] [PubMed] [Google Scholar]
- 45.Lyons J, Ryder R, Seal R, Wagner J. Emphysema in smoking and non-smoking coalworkers with pneumoconiosis. Bull Eur Physiopathol Respir. 1981;17(1):75–85. [PubMed] [Google Scholar]


