Abstract

The prospects, needs and limits in current approaches in catalysis to accelerate the transition to e-chemistry, where this term indicates a fossil fuel-free chemical production, are discussed. It is suggested that e-chemistry is a necessary element of the transformation to meet the targets of net zero emissions by year 2050 and that this conversion from the current petrochemistry is feasible. However, the acceleration of the development of catalytic technologies based on the use of renewable energy sources (indicated as reactive catalysis) is necessary, evidencing that these are part of a system of changes and thus should be assessed from this perspective. However, it is perceived that the current studies in the area are not properly addressing the needs to develop the catalytic technologies required for e-chemistry, presenting a series of relevant aspects and directions in which research should be focused to develop the framework system transformation necessary to implement e-chemistry.
Keywords: e-chemistry, defossilized chemical production, reactive catalysis, fossil fuels beyond, renewable energy, petrochemistry
Introduction
Research interest in catalysis based on the direct use of renewable energy sources—RES (photo-, electro- and plasma-catalysis as the main methodologies)—is rapidly rising.1−10 As an index of the growing of activities, papers with electrocatalysis as a keyword increased from about 30 in year 2000 to nearly 600 in 2021, and those containing the term photocatalysis increased from about 40 to nearly 1000 per year in the same period. However, most of the research activity focused on only a few molecules (CO2, N2, H2O, and few biobased chemicals). On the other hand, meeting the targets fixed at the political level (for example, reach a net zero greenhouse gas emission target by year 2050, as committed by the European Union (EU)) would require a more disruptive effort than developing some catalytic processes driven directly from RES.10 It is necessary to consider the possibility to substitute largely, if not entirely, the use of fossil fuels (FF) as both energy and a carbon source, the latter particularly for chemical production. The concept of e-chemistry indicates that this (almost) FF-free chemical production, which can be identified as part of the overall transformation necessary, can meet the targets of net zero emissions (NZE) for year 2050 and beyond. Implementing the concept of e-chemistry (and associated e-refinery) will address some important societal challenges which are facing the realization of a resilient and sustainable future: (i) overcome intermittency of RES, (ii) implement a world economy based on the distribution and long-distance transport of renewable energy, (iii) develop CO2 neutral or even carbon-negative technologies to supply the goods and energy necessary for the society, and (iv) realize a defossilized chemical industry. e-Chemistry is particularly associated with the last objective but is also closely related to the others.
Realization of e-chemistry requires a combination of actions, such as
the direct electrification of the operations in chemical industry using FF as energy input (for example, most of the furnaces in chemical processes use FF to provide the heat necessary for operations)11−14
the closure of the carbon cycle in chemical production by introducing technologies for the molecular reuse of waste and end-of-life chemicals (from CO2 to end-of-life chemicals as recycled plastics)15−17
the realization of chemical processes using RES as energy input for the process, where electro-, photo- and plasma-catalysis represent the main technological options.8,9,18−21
Catalysis plays a crucial role in several of these technologies allowing not only development of innovative routes with a large decrease in the carbon footprint, up to over 90%, but also realization of process intensification with thus reduction of fixed costs and better suitability to a distributed model of chemical production.8,22−24 This combination of drastic reduction in the carbon footprint, use of alternative raw materials to FF, and process intensification (with associated impacts such as the possibility to develop distributed production modes as well as faster and more flexible industrialization by parallel units) is the key to analyzing the potential of catalytic technologies based on the use of RES, rather than being limited to purely economic considerations.
However, addressing this challenge for catalysis requires also reconsidering the fundamental basis of catalysis science and technology. We introduced the term reactive catalysis to differentiate photo-, electro- and plasma-catalysis from the conventional thermal catalysis.10 In the latter case, energy in the form of heat is provided to overcome the activation energy, while in reactive catalysis already highly energetic species (electron, holes, radicals, vibrationally excited species) are generated by application of an electrical potential, by light irradiation or by generation of a nonthermal plasma. The fundamental modes of operations, and consequently the design aspects for the catalysts, are different. It is thus necessary to use methodologies to understand and develop reactive catalysis, and not just continue to use those developed for thermal catalysis.
e-Chemistry thus requires a revolutionary rather than an evolutionary approach, in terms of capability to integrate, from a holistic perspective, many aspects from fundamental to applied chemistry and engineering for the deep transition required to move to a new FF-free sustainable future. Facing the transformation to an NZE society requires radical system shifts (concerted) in the same direction,25,26 requiring thus new assessment modes for their evaluation.27 A concerted synergy between the development of routes and technologies and the parallel changes in the economic, industrial and societal systems is necessary, implying also the creation of pathways by which this concerted mechanism can be achieved. Therefore, a framework assessment of the transformation is required rather than assessing single specific technologies.28
For example, an analysis of the status of the studies on the economics of CO2 utilization29 clearly reveals the limit of application of economic assessment models that do not properly consider the ongoing deep transition and thus are not based on a framework assessment. This is also one of the reasons why very contrasting opinions exist on the opportunity or not to develop new routes to electrify the chemical production, and how to properly rank the priorities.
For example, a recent paper by Ueckerdt et al.30 discussing the pro and cons of e-fuels (where this term indicates the synthetic fuels produced from electricity and CO2 via water electrolysis) argued that “e-fuels’ versatility is counterbalanced by their fragile climate effectiveness, high costs and uncertain availability”. We feel that this conclusion on the negative aspects of e-fuels, and the preferences for alternative solutions, derives from a series of assumptions, on particular that (i) it is necessary to produce H2 via electrolysis and separate and concentrate/purify CO2, (ii) e-fuels can be produced only by power-to-X technologies (i.e., producing H2 by electrolysis and its use for CO2 conversion by thermocatalytic process), and (iii) renewable electricity should be in excess with respect to other uses and with an intermittent production. We feel that these limitations will be overcome within the next decades, as discussed later in this manuscript. When a deep transition occurs as that ongoing, evaluations strongly depend on the scenario assumptions and the capability to consider the possible technological developments, despite the uncertainty associated with them. Most of the scenario analyses are limited from this capability, especially when system changes are occurring as those ongoing.
On the other hand, making estimations, including the economic aspects, without properly considering the technological developments was proven to lead to uncorrected indications. This is well demonstrated by failing predictions of the cost of electricity produced by photovoltaics (PV) and wind, which about two decades ago was estimated to be about 5–10 higher than the effective cost currently available. Analyzing literature studies on the economics of CO2 utilization29 to produce CH4 and CH3OH (mainly by power-to-X technologies), we noted the presence of a very wide range of calculated costs, much broader than the possible uncertainty in cost estimations. This indicates how predicting that a technology like the production of e-fuels will not have a role in the future scenario, based on only cost estimations, can be very dangerous. On the other hand, this evidences the difficulties in making an estimation on technologies still to be fully developed.
Although all predictions about the future scenarios are strongly dependent on many assumptions, quite difficult to prove and with a high degree of uncertainty, we believe that the conclusions made by Ueckerdt et al.30 on negligible climate mitigation effectiveness of e-fuels depend on adopting a pessimistic scenario strongly affected by cost analysis taken from literature and not considering the right perspective regarding the potential of technological development and innovation. In general, we could remark on the need to (i) account the deep transformation occurring and (ii) identify the scientific and technological gaps to overcome in order to implement this transformation.
From the catalysis perspective, it is thus necessary to understand the directions and trends offering new opportunities, but also to identify properly the limits and gaps as well as the crucial issues to overcome.31−33 Also in terms of catalysis technologies, it is necessary to address the limitations noted above, for example the possibility to have photoelectrocatalytic (PEC) cells which are able at the same time to
-
1.
convert directly CO2 from diluted streams without need for concentration/purification (by integrating suitable membranes, for example,34 or combining with integrated electrochemical retention of CO2),35
-
2.
operate directly with solar light for 24 h (by integrating in the PEC cell a redox storage for a temporal decoupling of the redox processes requiring light and those for the production of e-fuels),36,37
-
3.
produce directly in a single cell the e-fuels/e-chemicals from CO2, water and light.32
Research in these directions as well on the fundamentals aspects which differentiate reactive from thermal catalysis is still quite limited. By a proper intensification and focus of the R&D, we believe that within 10–15 years technologies overcoming the above limitations will become feasible. However, it is essential that research will address the proper gaps and limits, with a better focus on crucial aspects to solve.10 Also scenario analyses, when a deep transformation is involved, should be focused toward identifying future technologies and assessing the possible directions from this perspective, and whether the existing scientific and technological gaps can be bridged.
Scope and Limits
This perspective paper aims to contribute in the analysis of the prospects, limits and gaps from the viewpoint of the needs of scientific and technological developments in the field of catalytic technologies to realize e-chemistry. This analysis is preceded by a short assessment of the feasibility and timing to realize e-chemistry as a necessary element of the transformation to meet the NZE targets by year 2050, when a proper R&D effort will be dedicated. However, the aim is not to discuss in depth the different opinions in literature regarding the need to transform petrochemistry into e-chemistry.
Discussion on the needs to realize e-chemistry will not specifically address the issue of scalability of the technologies discussed. In fact, the scope of this paper is oriented toward a long-term vision of the future technologies needed to realize e-chemistry (unexplored reaction pathways) rather that to analyze gaps in the currently available technologies. It is important to identify these future technologies to prepare all the scientific and technological basis for their realization, whether a deep discussion of technology readiness/development is related to current technologies.
The above discussion was mainly focused on electrocatalysis. Other catalytic technologies using RES, such as photo- and plasma-catalysis, or catalysis with microwave or other radiations such as magnetic heating, will also be important to implement e-chemistry. However, the latter two technologies (microwave or magnetic heating) change the mechanism of heating, but the process remains a thermal process, with all the related intrinsic thermodynamic limits. Photo- and plasma-catalysis instead share with electro-catalysis the presence of quite reactive species, such as electrons, holes, radicals, and for this reason are lumped together as reactive catalysis, in contrast with the traditional thermal catalysis.10 However, from an application perspective, electrocatalysis has a more mature stage of development, as well as advantages of higher productivities, efficiencies and process intensification.8 In addition, it will take advantage of the increasing experience on fuel cells and electrolyzers, as well of commercial electrocatalytic processes (chloro-soda, adiponitrile, etc.). Finally, by using stacks it is possible to obtain high productivities per reactor volume. All these aspects will make the scale-up of the electrocatalytic processes faster. Electrocatalysis likely will be thus the initial technology to be applied industrially for the development of new routes for e-chemistry. We have thus focused discussion here on electrocatalysis, but the relevance of other routes (in particular photo- and plasma-catalysis) need to be taken into account. However, it is also necessary to note that a proper comparative analysis of electro-, photo- and plasma-catalysis, and of pro/cons of these different technologies, is largely missing in literature. Moreover, data are reported in a way that is difficult for proper comparison, for example in terms of productivity, energy efficiency, selectivity. Thus, effort in this direction is necessary.
Note finally that we do not address here the role of biobased chemicals in the development of FFs-free chemical production, because it has been already discussed elsewhere.38−40 However, the bioroutes often still have a significant footprint and are not designed in most cases to integrate RES in the production.41,42 Thus, a transition to a fossil-free e-chemistry for an NZE target would require reconsidering these routes in terms of integration with RES and with technologies, such as electrocatalysis (as discussed later), representing the link to effectively integrate RES in the process.
Biocatalysis instead will certainly play a significant role in the future of e-chemistry, although the challenges of (i) performance, (ii) process costs and (iii) process intensification must be solved to expand from dominant pharmaceutical and fine chemical applications to the production of bulk chemicals.38−40 Also in this case, the synergy and interface with electrocatalysis and other catalytic methods based on the use of RES have to be analyzed to define the optimal paths for future e-chemistry. However, this is a largely unexplored area.
Feasibility and Timing for e-Chemistry
There are many different opinions on whether e-chemistry can be feasible, and when it could be effectively implemented on a large scale. Regarding timing, a question is when would we consider realizing the implementation of the transition from petro- to e-chemistry.
The timing of a transition is when the new technologies (for e-chemistry) prevail over those used traditionally (currently in use) at the stage of planning new investments in industrial chemical production. In fact, every massive change in a production system requires time to be completed and there is an unavoidable period in which the new and old technologies will operate in parallel. The transition is realized when these technologies will start to be introduced for new plants. Additional years are due to the time necessary to complete the switchover.
There are clear worldwide indications that investments in new plants and technologies based on FFs are significantly decreasing. Already large consulting companies such as McKinsey, as commented later, evidence the reduced attractiveness to invest in current petrochemistry processes based on FFs.43 Deloitte, another major consulting company, also advised about the changing petrochemicals landscape and that the petrochemicals industry is at a crossroads of major structural shifts.44
Despite the great uncertainty in predicting the future, these signs and indications from consulting companies allow us to assume that for year 2050 the still likely use of FFs will be mainly a consequence of this switchover period rather than of the profitable use of the current petrochemistry technologies (or slightly improved), especially in investing in new plants. Understanding this difference is crucial for a proper scenario analysis and prediction of the technological landscape in year 2050, particularly in geographical regions pushing the NZE transition, such as Europe. However, a prerequisite to realize this scenario is that the R&D investment should be increased, with the identification also of the implementation mechanisms able to focus the R&D activities on the key fundamental and technological aspects leading to an acceleration of the innovation.
An often-posed question concerns the availability of the needed FFs-free electricity for the transformation of petro- to e-chemistry and its cost. The McKinsey report “Pluggin in: What electrification can do for industry”13 indicates that “renewables could produce more than half of the world’s electricity by 2035, at lower prices than fossil-fuel generation”. Many other reports are in line with this estimation. FFs are no longer considered a privileged energy source in terms of costs, but their still dominating monopoly character determines a market controlled by other factors compared to those associated with a truly competitive economy, as shown in the second half of 2021.
Thus, overcoming the dominant use of FFs is a strategic direction and not only relevant to reduce greenhouse gas emissions.45 Substituting the use of FFs is thus the result of different converging elements, from cost advantage, drastic cut in greenhouse gas (GG) emissions (and associated avoided costs) and improved energy geopolitics. It is the combination of these elements that make the transition irreversible, and thus petrochemistry should also reinvent itself in this direction. McKinsey, a major consulting company, already some years ago indicated that petrochemistry has lost the window of opportunity as an advantaged feedstock provider and therefore needs to reinvent itself.43
In petrochemistry, less than half of the FFs input (accounting for >90% of input of the chemical sector) is used as feedstock (e.g., as carbon source), while the remaining part produces the necessary energy for chemical processes. Global fossil fuel consumption corresponds with about 137,000 TWh (TWh = terawatts per hour), and by considering that around 14% and 8% of total primary demand for oil and gas, respectively, is related to chemical production,46 the latter uses globally account for about 12,000 TWh, which is equivalent ot FFs. The global amount of electricity generated from renewables (in 2021) was estimated to be 8300 TWh,47 but with the introduction of the new technologies for e-chemistry, it is expected to increase the efficiency of energy use, thus reducing the energy consumption.47 Clearly, actual renewable energy production is already in use for different applications, but by year 2050 the production of RES is expected to increase by a factor of 3–4 arriving up to 90% of the renewable share in electricity, along with a reduction in the total final energy consumption from 378 EJ (in 2018) to 348 EJ (in 2050) (EJ = exaJoule).48 In addition, the introduction of technologies such as that (indicated above) of PEC devices with integrated redox storage, to enable their continuous use overcoming intermittency of RES (one of the current main drawbacks), offers an additional path to produce e-fuels/e-chemicals using direct solar light.
In a transition path to 2050, FFs substitution in chemical production can be initially realized by replacing their use as energy source (the so-called electrification of the chemical production) and then as carbon source, by introducing technologies for efficient closure of the carbon cycle coupled with a rational use of biobased resources.49 The carbon recycle introduces energy efficiency, besides a better use of resources. The energy efficiency in recycling CO2 to methanol, for example, is potentially up to over 80%,50 while considering the different steps necessary to produce methanol from oil and the losses related to the extraction and transport of oil, the energy efficiency from fossils is on the average lower than 50%.51,52 This is not the efficiency of the single process of methanol production (what often considered), but the global efficiency which accounts for the many steps necessary to arrive to methanol with the current route.
Converting CO2 directly to acetic acid by an electrochemical process would further reduce the number of steps (a main industrial route involves the carbonylation of methanol) and improve the overall energy efficiency of the system.53 The minimum process energy divided by the total process energy input is about 27% for acetic acid in the conventional route,54 while the electrochemical route potentially can reach energy efficiencies above 60%, drastically lowering the carbon footprint. This concept is exemplified in Figure 1 reporting a Grassmann-type diagram of an indicative comparison of exergy in the multistep conventional process to produce acetic acid using FF sources and the direct electrocatalytic route of CO2 conversion to acetic acid with in situ water electrolysis.55 The difference between energy input (as sum of raw materials and fuel input) and the final work potential of the target product represents the sum of internal and external exergy losses, and those related to steam export, which can be difficult to utilize in distributed approaches. This resulting decrease of the carbon footprint is higher than 70%.
Figure 1.
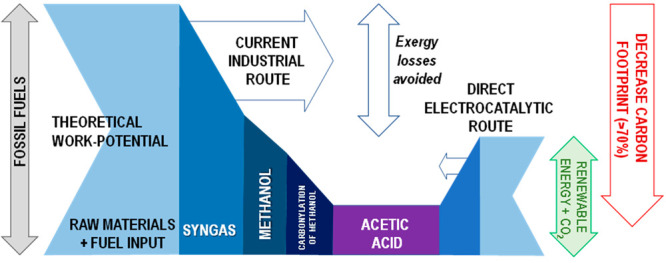
Grassmann-type diagram of indicative comparison of exergy in the multistep process to produce acetic acid via the conventional process starting from fossil fuels and the direct electrocatalytic route of CO2 conversion to acetic acid with in situ water electrolysis. Reproduced from Centi.55 Copyright 2020, The Catalyst Group Resources, Inc.
The great potential of these e-technologies is to introduce process intensification by reducing the number of steps, potentially lowering fixed and operative costs, and introducing new modalities of production with better use of the local resources (distributed production). The full value chain of chemical production and the strong nexus with refinery would be changed in passing to an e-chemistry model. This is the perspective required for the analysis of the feasibility and impact.
We may conclude that in the frame of the proposed high tech scenario, the potential to substitute FFs in the chemical production with renewable energy and alternative C sources exists. This objective would correspond to better use of the resources changing from a linear to a circular economy model. The impact is potentially to lower rather than increase the production costs as instead often claimed. Saygin and Gielen,56 for example, estimated that “achieving full decarbonisation in this sector will increase energy and feedstock costs by more than 35%”. Shreve57 indicated that replacing fossil fuel power systems in the United States could cost up to $4.7 trillion. Markandya et al.58 instead remarked that “the reduction of pollution and climate impact through rapidly increased use of renewable energy by 2030 could save up to USD 4.2 trillion per year worldwide.” These are a few among many other examples of the quite contrasting indications reported in open literature on the feasibility and costs of substituting FFs. In our high tech scenario, where an intensified R&D will allow solving current technological limitations, the innovation which is brought from the transition to an e-chemistry will result in an overall reduction of the costs, in addition to an increased sustainability and reduced impact on the environment.55
In the past, when proper stimulus of R&D was given, for example when the strong limitations on car exhaust emissions (Clean Air Act) were introduced around year 1970, the results were largely beyond expectations. Car exhaust treatment technology realized what was indicated as impossible to achieve. In addition, the current petrochemistry based on olefins was largely realized within a few years, around year 1960, due to a series of converging driving elements.23 Also in this case, transformation occurred much faster than predicted. Therefore, history teaches how major technological changes, as those necessary to implement e-chemistry, may occur despite many negative predictions. The scenarios for 2050 predict completely different situations in terms of reduction of GHG. We believe that it would be better to analyze what the gaps and limits are to implement a possible scenario, rather than to further discuss whether it could be realized, using different assumptions all difficult to prove to be the most correct.
The key question thus remains regarding how to develop the technologies which are needed, accelerating their discovery and industrial implementation. Catalysis, being a key element in most of these technologies, should follow the same trend, and thus the question is how to accelerate the progress necessary in catalysis for e-chemistry, rather than to discuss whether this petro- to e-chemistry transition is feasible. We believe that many elements indicate that an irreversible transition toward the realization of e-chemistry has already begun.
Limits and Gaps in Catalysis for e-Chemistry
The production modes in petrochemistry are rigidly hierarchical with few building blocks (mainly light olefins, aromatics and syngas/methanol), requiring a sequence of steps, often many, to obtain the final chemicals for industrial (polymers, synthetic fibers and rubbers, solvents, etc.) or consumer uses (detergents, drugs, fertilizers and pesticides, paints, etc.). This production scheme is largely associated with the concept of scale economy, e.g., the need to develop large-scale centralized plants and thermal processes.23 This model of chemical production has many limits, from the significant local impact on the environment to the intrinsic low flexibility and adaptability instead required due to an uncertain future. Chemical plants are designed for a utilization factor typically above 90% to operate economically. The global ethylene production plants decreased to an average 82–83% in recent years, and it is predicted to remain lower than 90% in the next decade.60 In these conditions, economic margins for the production are very low, or even negative. This is a general situation for petrochemical production (ending the windows of opportunity),43 and it will be accentuated in the future, requiring change in the production model from centralized (few very large sites) to a distributed model, more flexible and strongly reducing costs and impact of transport/distributions.60 In addition, a distributed model offers integration with the local resources rather than along the value chain (creating also new opportunities for symbiosis and investments). All these elements create a competitive environment based on innovation. Distributed production requires efficient small-medium scale plants well integrated with the territory and the local resources/needs, with a modular plant scheme allowing for a faster time to market and great flexibility in operations. e-Chemistry technologies should have these characteristics.
Therefore, the remark often made that it is not possible to produce large-scale building blocks as ethylene (typical size of steam crackers to produce ethylene goes from 200 to over 1000 ktons/y of ethylene) by electrocatalysis (or other routes based on RESs) is mispresented. In e-chemistry the model of production is changed with the target of small-medium scale productions tailored for the local production needs. It avoids distribution/transport on a large-scale. The aim is the direct conversion to the final product (or at least to strongly reduce the number of steps), avoiding fragmentation of the production in a long sequence of steps. Ethylene is a raw material for polymers (polyethylene), but also the building block for a large series of other chemicals (used mainly for other polymers) such as vinyl chloride, ethylene oxide, vinyl acetate, etc. Thus, technologies for direct ethylene production should be used for polyethylene manufacture, while other chemicals derived from ethylene should be ideally produced directly rather than via ethylene as an intermediate.
Many well-established large-scale chemicals are produced with a sequence of steps which can be potentially drastically reduced. As an example, phenol production is realized commercially with the conversion of benzene to cumene, which is converted to cumene hydroperoxide and then decomposed to phenol and acetone. However, the yield is low, about 8% (on the whole process), due to the critical step of the intermediate cumene hydroperoxide production. The direct one-step synthesis of phenol from benzene and H2O2 is potentially competitive but suffers due to hydrogen peroxide cost.61 H2O2 could be produced electrochemically, and thus an electrochemical route to directly produce phenol from benzene is potentially feasible. It may be a hybrid system with production of H2O2 electrocatalytically combined with in situ catalytic conversion, or better by direct electrocatalytic benzene hydroxylation using hydroxyl species generated at the anode. There are many open questions, from the type of electrolyte and electrode to use to the control of multiple hydroxylation (phenol is more reactive than benzene toward the insertion of further hydroxyl groups, but there are strategies to control this issue by inhibiting the further reactivity of phenol).61 However, studies in the field are extremely limited. The production of H2O2 by electrocatalytic routes is a topic of growing interest,62,63 but not the direct electrocatalytic hydroxylation of benzene, or the hydroxylation of other substrates. However, papers are available on the catalytic hydroxylation of benzene with H2O2.64−67 Benzene direct electrocatalytic hydroxylation is one of the routes to explore for an e-chemistry, but which has been not yet investigated. The benzene current source derives from FFs, mainly as a product of the refinery reforming process. However, in future e-chemistry it could be produced from lignin.68
The example above introduces the concept of extending the use of electrocatalysis by addressing complex syntheses of chemicals by combining electrocatalysis of small molecules (CO2, H2O, N2, CH4, the latter from biogas sources) and of biobased molecules. The integration of these two separate worlds is the grand challenge for e-chemistry and e-refinery.69
Most of the electrocatalysis studies instead address simple reactions as the two-electron reduction of CO2 to CO or to HCOOH.70−73 Also in CO2 electrocatalytic conversion, the challenge is instead to realize complex conversions of CO2 leading to multicarbon (C2+) products and to the intermediates needed to build a petrochemistry-equivalent framework, as commented above. The target is to directly produce both the base chemicals which can be used then as raw materials for the current chemical production (light olefins, for example) or even better produce directly (in one-step) more complex molecules.
The direct synthesis of multicarbon products is a topic of growing interest in CO2 electroreduction,33,74−76 but mainly from an academic perspective rather than as part of a strategy to build new e-chemistry. For example, the electroreduction of CO2 to CO being intrinsically simpler than the formation of C2+ products, a large part of the studies focused on the CO2 electroreduction to CO, with the justification that the performances (Faradaic yield, productivity) are better, and CO could then be used in combination with H2 to make a variety of other chemicals, via methanol or Fischer–Tropsch catalytic processes. In general, the productivity of the CO2 reduction reaction (CO2RR) to C2+ products (especially C2+ hydrocarbons) is still low, although significant progress has been made recently and now Faradaic selectivity and productivity/current density (to ethylene and ethanol, in particular) have reached levels in some cases which allow possible industrialization to be considered.74−76 However, still a gap exists between production of syngas by coelectrolysis on a solid oxide electrolyzer cell (SOEC). The latter is a better solution with respect to the separate production of CO from CO2 and H2 from H2O in polymer electrolyte membrane (PEM)-type electrolyzers, although the SOEC still shows relevant issues in terms of cost/performances, reliability and durability.77 After production of the syngas, with eventual adjustment of the CO/H2 ratio, compression and heating could be necessary, and then one or more thermocatalytic steps of conversion could be necessary. Two routes could be possible to obtain olefin: via the Fischer–Tropsch (FT) process (in the modified version FT to olefins, although performances are still unsatisfactory notwithstanding the recent advances)79 or via intermediate methanol synthesis followed by methanol-to-olefin conversion (followed by further C4+ cracking unit).80 In both routes a broad distribution of products is obtained. If the overall efficiency, especially energetic, accounts for these multistep processes, along with the needs of the complex downstream separation units which also makes the development of small-scale distributed applications costly, the direct electrocatalytic production of olefins appears as a preferable route.81 However, the multistep power-to-olefin route (syngas by coelectrolysis, then thermocatalytic steps) is more mature in terms of implementation. In this case, a decrease of the carbon footprint could be realized through electrification of the thermocatalytic process, e.g. using electricity to provide the heat of reaction.82
A wider range of possibilities exists (than forming C2+ production from CO2) which can be used to build new routes for e-chemistry. An example is the electrocatalytic reductive coupling of CO2 to oxalic acid,83,84 which can be further electro-reduced to a range of valuable chemicals for polymerization like glycolic acid, creating a new C2 value chain from CO2.85−87 Further reduction of glycolic acid may lead to production of ethylene glycol (a main intermediate for polyesters), thus a new path with respect to the current one via ethylene and ethylene oxide. This electrocatalytic chemistry is investigated in the EU project OCEAN (Oxalic acid from CO2 using electrochemistry at demonstration scale, grant 767798). Oxalic acid can be electrocatalytically reduced to glyoxylic acid and further to tartaric acid,88 opening a new path to C4 chemistry from CO2.
The electrocatalytic production of acetate/acetic acid from CO253,89 is also opening new possibilities, not only for the use of acetic acid itself (as solvent) but also to produce electrocatalytically a range of interesting products. One of them is the combination of the electrocatalytic production of acetate from CO2 and its reaction with (bio)ethanol to form ethyl acetate, a solvent of large use. The electrocatalytic reactor is used to produce ethyl acetate both at the anode side, by anodic oxidation of ethanol, and at the cathode side, through in situ catalytic reduction of CO2 to acetate which reacts with ethanol to form ethyl acetate. Therefore, the same product is produced at both electrode sides. This is an interesting example of coupling CO2 electroreduction chemistry to the use of chemicals from biorefinery. This electrocatalytic chemistry is investigated in the frame of EU project DECADE (Distributed chemicals and fuels production from CO2 in photoelectrocatalytic devices, grant 862030). Another possibility is to explore a similar chemistry but finalized to the direct synthesis of vinyl acetate. No studies in this direction are available, but in an old patent90 an electrochemical process to produce vinyl acetate from ethylene and anolyte acetic acid is claimed.
There is a much wider range of possibilities, for example to develop paired or tandem electrocatalytic conversions.91−94 One example is the oxidation of glucose to gluconic acid on the anode side and the hydrodeoxygenation of gluconic acid to adipic acid, allowing the one-step electrosynthesis of adipic acid, a large-scale monomer, from a biobased platform molecule (glucose). This is one of the target reactions of the EU project PERFORM (PowerPlatform: establishment of platform infrastructures for highly selective electrochemical conversions, grant 820723) aiming to develop a small-scale pilot reactor. The TERRA EU project (Tandem electrocatalytic reactor for energy/resource efficiency and process intensification, grant 677471) instead investigated the tandem electrosynthesis from C6 and C5 sugars (respectively) of the two monomers for PEF (PolyEthylene Furanoate): 2,5-Furandicarboxylic acid and ethylene glycol.
These are some examples of the ongoing studies, involving various industries, to build new routes based on electrocatalysis for e-chemistry, and in some cases also at pilot/demo scale. Note that even if the possibility to make a more complex chemistry in the electroreduction of CO2 is known for nearly 15 years,95 only in recent years has a more systematic study in this direction begun. Similarly, while organic electrosynthesis is a well-established scientific area,96−99 and electrochemistry (of fuel cells) has been known for a century,100 the realization of e-chemistry poses new problems and challenges. Electrosynthesis is one of the organic synthesis tools, designed mainly for specialty and fine chemicals, with different typologies of reactions (C–H activation, cyclization, dehalogenation, carboxylation, coupling, etc.). However, it is typically not suited for bulk chemical syntheses.
Now many new possibilities were being explored by recent studies in electrocatalysis. The electrosynthesis of ethylene and propylene oxides is an interesting new area of investigation,101 although already two decades ago the electrosynthesis of alkene oxides (and glycols) from alkenes has been reported.102 Sargent and co-workers101 electrochemically epoxidized ethylene and propylene to the corresponding epoxides (ethylene oxide (EO) and propylene oxide (PO), respectively) at industrially relevant current densities with Faradaic (electron-specific) selectivities of ∼70%. They coupled an electrochemical flow cell to homogeneous reactions for an overall stoichiometry (for ethylene oxide)
| 1 |
although chlorine is used to mediate the process. At the anode, the dissolved olefin is converted to the chlorohydrin, and the hydroxide is produced at the cathode along with H2. Then, in a downstream process the anodic stream containing chlorohydrin [RCH(OH)CH2Cl, where R can be H for ethylene or CH3 for the propylene case] and HCl is mixed with the cathodic stream (containing OH–) to generate the epoxide, Cl– and water. The authors also propose to couple the system to the electrocatalytic conversion of CO2 to ethylene, realizing an integrated CO2-to-ethylene oxide process. Sargent and co-workers101 suggest that this process could be scaled to produce ethylene oxide (EO) at a comparable cost with current industrial practices. Although some open questions exist regarding energy intensity, safety of operations (Cl2 is a very toxic gas, although chloro-alkali technology is one of the oldest electrochemical processes) and stability of materials,19 the above results open new possibilities.
Another example of unconventional application of electrocatalytic routes is in the selective hydrogenation of acetylene.103 Acetylene hydrogenation is an important industrial reaction for purification of ethylene streams. Here the authors103 proposed this technology as a final step in a non-oil route to produce ethylene from natural gas via methane pyrolysis. They reported a high Faradaic efficiency of 83.2% for ethylene with a current density of 29 mA·cm–2 on Cu electrocatalysts, due to strong acetylene chemisorption which limits the side hydrogen formation. This concept of competitive chemisorption to enhance selectivity in electrocatalytic reactions has more general validity in electrocatalysis.
Electrocarboxylation of olefins and diolefins104 (ethylene and butadiene, especially, both which can be produced from bioethanol)105 or of aromatics is another route of interest to build e-chemistry.106,107 A variety of valuable intermediates could be produced by this method.
When other reactants are present, for example nitrate, the spectrum of the possible products further enlarges. One example is the electrochemical synthesis of glycine from oxalic acid and nitrate,108 rather than the electroreduction of oxalic acid to glycolic and glyoxylic acids discussed before. Glycine is one of the base amino acids.
Most of the research in electrocatalysis instead focused on a few limited reactions (CO2 conversion, N2 fixation, water electrolysis or H2O2 production, conversion of few biobased platform molecules), and in particular on the electrocatalysts development, including their design criteria, instead to explore the large range of possibility to build e-chemistry and the interface with biotechnological paths (coupling biorefinery and e-chemistry).109
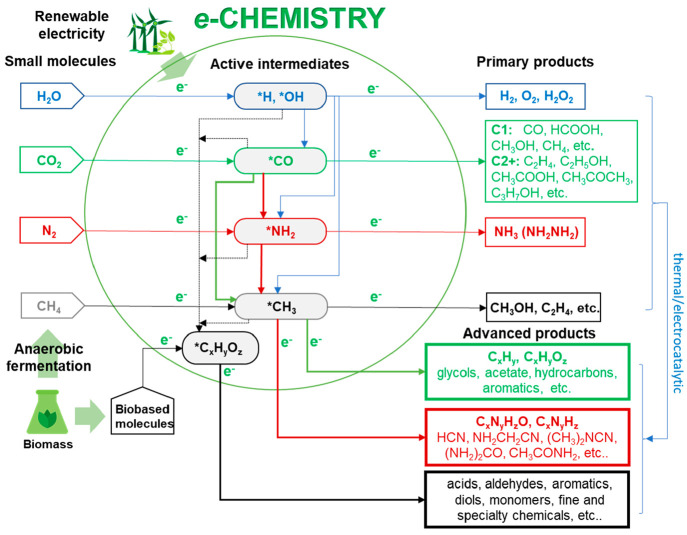
Therefore, there is increasing interest to build new possibilities and routes for e-chemistry, especially at the interface with biobased platform molecules, but on the other hand, research is still rather scattered and a very systematic exploration of all the possibilities is missing. It is necessary to attempt to build a new chemical production framework based on the electrocatalytic framework which combines the conversion of small molecules (CO2, N2, H2O, CH4, the latter derived from biogas) with those obtained from the electrocatalytic conversion of biobased platform molecules. The reactions to explore are regarding both (i) the coupling of in situ generated products with those of biobased molecules (as the cited reaction of in situ generated acetate to form vinyl acetate o ethyl acetate) and (ii) the large range of possibilities by using the in situ generated intermediates in the electro-transformation of the indicated small molecules. Figure 2 summarizes the concept that by combining primary electrocatalytic reactions of small molecules (by using final products and active intermediates, in a combined approach), to the electrocatalytic conversion of bioderived products, it is possible to produce a large range of products forming the skeleton of a new chemical production.69,109 Reactions to explore include (i) direct coupling of in situ generated intermediates, (ii) tandem reactions using in situ generated chemicals, and (iii) the eventual coupling between electro- and heterogeneous catalysis.
Figure 2.
A new framework of electrocatalytically based reactions to develop a new e-chemistry alternative to that based on fossil fuels (petrochemistry). Adapted from Perathoner109 as full re-elaboration of the original concept presented by Tang et al.69 Copyright 2021, The Catalyst Group Resources, Inc.
By properly combining the primary reactions of electro-conversion, a larger range of products can be derived, potentially meeting the needs for e-chemistry in combination with the other paths described before. This approach extends the concept of tandem electrocatalytic processes discussed before.
In tandem or paired reactions, the concept is the valorisation of both cathodic and anodic reactions to form added-value products. In water splitting, but similarly in most studies on CO2 or N2 electroreduction (CO2RR and NRR, respectively), O2 is produced at the anode site: the oxygen in most of the practical cases cannot be used, and is released to the atmosphere, and even when used, it is a low-value chemical. In addition, oxygen evolution reaction (OER) is a four-electron reaction and it is typically the slow step of the electrocatalytic process, which determines most of the energy losses due to overpotential. Moreover, to produce H2O2 by water oxidation instead O2 (a two rather than a four electron reaction) is an interesting target.62,63 H2O2 is a valuable oxidant in many selective oxidation processes (as discussed later) and in environmental applications. There is thus a rising interest in this process which represents not only an alternative to OER but also a synthesis process for H2O2 as an industrial selective oxidant, even if attempts to produce H2O2 electrocatalytically have been known from decades.110 H2O2 is already produced in Mtons scale by the anthraquinone route as the selective oxidant in commercial processes (propylene oxide, caprolactam, catechol and hydroquinone syntheses), for soil and water remediation uses and as a versatile bleaching agent.23
H2O2 formation is half the reaction of the most studied oxygen evolution reaction (OER), the anodic reaction in water electrolysis systems.111 A large gap between current performances and those necessary for industrial exploitability, however, is present. Also, for this electrocatalytic synthesis of H2O2 there is the need to develop more solid bases for the electrocatalysts design. For example, by theoretical modeling the binding strength of the reaction intermediate *OOH to the catalyst surface is indicated as the key parameter for controlling the catalyst performance. However, recent studies, for example on single-atom electrocatalysts, are in contrast with these mechanistic indications.112 Recent advances in the understanding and design of the gas–liquid–solid three-phase architecture have led to significant progress in obtaining high selectivity (83–99% current efficiency) combined with high current density and stability.113 By using a superhydrophobic natural air diffusion electrode (NADE) to improve the oxygen diffusion coefficient at the cathode as compared to the normal gas diffusion electrode (GDE) system, Zhang et al.114 showed that it is possible to largely increase the H2O2 production rate and oxygen utilization efficiency. These examples show how a deep understanding of the engineering aspects at the electrode nanoscale is a crucial factor in determining the behavior, and not only the nature, of the electrode itself.115 Quite similar aspects also strongly determine the behavior in NRR.116 Production of H2O2 rather than O2 in water splitting or other electrocatalytic reduction reactions (CO2RR and NRR) has thus the advantages of making a 2e– rather than a 4e– process (with advantages in terms of rate and reduced overpotential) and obtaining a higher value product. Pdδ+ clusters (Pd3δ+ and Pd4δ+) on mildly oxidized carbon nanotubes (containing controlled defects) were recently shown to allow nearly 100% selectivity in H2O2 formation with low overpotential and high mass activity.117 Therefore, significant advances have been made recently in the electrocatalytic production of H2O2.118−122 However, even if H2O2 is used in many chemical applications, the volume of the potential commercial market is largely lower with respect to that needed for potentially very large-scale reactions as in H2 production by water electrolysis, CO2RR and NRR. In addition, in many applications of H2O2, for example in its industrial use in selective oxidation reactions, the concentration and solvent requirements do not fit well with those produced in the electrocatalytic H2O2 synthesis.23 Finding an alternative optimal reaction to OER thus still remains a challenge. Some further aspects will be discussed later. Note also that the increasing trend to operate in nonprotic solvents to limit the side reaction of H2 formation in CO2RR and NRR further stresses the need to identify valuable anodic substitutes to OER and H2O2 production as well.
Coupling between electro- and heterogeneous catalysis is another area of interest. The development of hybrid catalysts/reactors combining electro- and heterogeneous (or eventually homogeneous) catalysis is an area still largely unexplored which represents a specific opportunity. On the contrary, hybrid electrocatalytic/biocatalytic systems have been more systematically explored.123−125
Figure 3 reports a framework scheme of the possibilities derived by developing hybrid electro- and biosynthetic catalytic pathways in CO2 conversion. The range of possibilities offered from this symbiosis is evident, further enhanced by considering that enzymes for converting other small molecules (N2, H2O and CH4) are also known. A critical challenge would be the transfer of energy and reactive compounds from the electrocatalyst to the cascade (or integrated) biocatalytic process. Three types of configurations can be possible: (i) direct attachment or (ii) indirect attachment of microorganisms on the electrode, and (iii) sequential (physically separated, cascade) reactions at the electrode and the microorganisms. The same configurations can be present also in integrated electro- and heterogeneous/homogeneous catalysis. Each type of configuration has pros and cons.
Figure 3.
Integrated connectivity map of hybrid electro- and biosynthesis catalytic pathways in CO2 conversion. Reproduced from Atanassov and co-workers.123 Copyright 2021, American Chemical Society.
In addition, direct or mediator-assisted electrocatalysis is possible. The use of redox mediators (typically organic compounds, but not limited to them) is common in organic electrosynthesis.126 In the indirect electrocatalysis the electron transfer step is shifted from a heterogeneous process occurring at an electrode to a homogeneous process using an electrochemically generated reagent (“mediator”). The latter is involved in a reversible redox couple initiated at the electrode. The use of these mediators can better control the selectivity and the stability of the electrodes and can overcome kinetic inhibitions. Several new redox mediators have been developed recently, offering a range of possibilities including unconventional types of operation such as double mediatory systems for biphasic media and enantioselective mediators.126 Redox mediators play a crucial role in electroenzymatic reactions (cofactor regeneration). Indirect electrochemical conversions are hybrids between direct electrochemical conversions and homogeneous redox reactions. The electrochemical generation and regeneration of this activated species can either proceed in situ in the same electrochemical cell (in-cell process) or in a separate cell (ex-cell process), allowing also realization of a spatiotemporal decoupling of the processes. Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO) and related N-Oxyl species are widely used as mediators in electrocatalytic reactions.127N-Oxyl compounds undergo facile redox reactions at electrode surfaces, enabling them to mediate a wide range of electrosynthetic reactions. Oxidation of aminoxyls, such as TEMPO, generates oxoammonium species that commonly promote hydride transfer from organic molecules, such as alcohols and amines, to obtain hydroxylamine. While often used in organic electrosynthesis, they are still scarcely explored in larger-scale processes, for issues of separation and stability that often become the discriminating elements for industrial processes.128 On the other hand, by using heterogenized mediators, and by designing the electrocatalytic reactor with integrated membranes, it is possible to overcome, in principle, these issues and exploit the benefits of mediated electrochemical reactions.
Key Directions To Build e-Chemistry
The above discussion evidenced a series of key directions to build the new catalysis required by e-chemistry. It is useful to summarize them to note some of the areas in which R&D should be pushed:
-
1.
The development of PEC devices integrated with redox mediators that allow direct solar-to-fuels/chemicals conversion with continuous (24 h) operations, because the redox mediators store the energy when light is present on one electrode side, allowing operation on the other electrode side in electrocatalytic continuous modes. The spatiotemporal decoupling of the processes in PEC devices overcomes the limits of intermittency (with associated costs and issues) offering also a new possibility to design the devices and to overcome other limits such as insufficient current density for industrial operations.
-
2.
The study of the benzene direct electrocatalytic hydroxylation to phenol, which can be also considered a model for a wider range of e-chemistry routes.
-
3.
Exploring more systematically the electrocatalytic routes by coupling the products of the conversion of small molecules (CO2, N2, H2O) with the in situ functionalization of biobased molecules (or using the products formed in integrated hetero-, homo- or biocatalysis cascade processes). The examples discussed were regarding the reaction of acetate (formed from CO2) with bioethanol or bioethylene to form ethyl acetate (solvent) or vinylacetate (monomer). This is a new area to explore, opening many new possibilities, from the production of glycine (amino acid) to the large range of paths by amination, nitration, selective epoxidation or oxidation, etc. The possibility to revise the old chlorohydrin synthesis to produce EO and PO was discussed as an example for electrosynthesis paths.
-
4.
Investigating the electrocarboxylation of olefins and diolefins, or aromatics, as another route of interest to build an e-chemistry. A variety of valuable intermediates could be produced by this approach.
-
5.
Starting to analyze the even more challenging, but conceptually possible, use of the reactive intermediates formed in the electrocatalytic conversion of the cited small molecules to make new synthetic pathways for e-chemistry. The example of benzene hydroxylation falls within this area. Direct electrocatalytic amination (formation of C–N bonds) is an interesting possibility which can exploit also nitrogen sources as nitrate, also produced, for example, by plasma-catalysis from air.129
-
6.
Exploiting the new possibilities offered by the electrocatalytic conversion of biomethane, which has been at large unexplored up to now.130,131
-
7.
Exploring more systematically the many possible new paths, offering also process intensification, given by tandem and/or paired electrocatalytic reactions.132,133 We have cited the examples of direct (one-step) glucose conversion to adipic acid, or the production of the monomers for PEF polymer synthesis on both sides of the electrocatalytic cell, a greener polymer substituting PET (polyethylene terephthalate), one of the largest in market volume polymers in current use.
-
8.
Intensifying research on the direct synthesis of ethylene or methanol/ethanol by electrocatalytic reduction of CO2, which were not discussed here having been already discussed in many reviews, for example by Zhao et al.2 and Ra et al.16 Ethylene or methanol/ethanol can be then used as such or as a building block for further e-chemistry. A large part of the research in these areas is focused on developing electrocatalysts with improved performances (especially Faradaic efficiency), and to identify the mechanistic features responsible for this improvement. Even if these aspects are relevant, those determining the industrial exploitability are often different from productivity and stability, such as the design of reliable and cost-effective electrodes and electrocatalytic reactors, with continuous operations, at high current densities. Often these aspects also determine the choice of electrode and operation conditions. The development of the electrocatalysts should be made under relevant conditions, yet this is not the case in a large portion of current investigations. A better balance between the fundamental studies and those more relevant for exploitability and integration within a general e-chemistry framework would be necessary.
-
9.
Extending the investigation to the possibilities offered by C2+ (multicarbon) chemistry in the CO2 electroreduction. We have discussed the possibilities by synthesis of oxalic acid, and its electro-conversion to form glycolic acid (for new polyesters) or other valuable chemicals, including ethylene glycol, a large-volume monomer. Oxalic acid can be converted to tartaric acid (via glyoxylic acid), giving rise to a new C4 path. Many other valuable paths are also part of the portfolio of electro-conversion possibilities of CO2 to C2+.
-
10.
Investigating, with a broader approach, the direct synthesis of ammonia (or derivatives, such as the synthesis of amination products) from N2, another emerging electrocatalytic path to both fertilizers (perhaps the largest volume chemical) or N-containing chemicals.134,135 Here it was also earlier evidenced10,31 that the large research effort is improving too slowly from a more practical perspective, in part due to the use of approaches which do not account for the difference between electro- and thermal catalysis. Methodologies (including theoretical) which are not able to catch the intrinsic difference in electrocatalysis are typically used. The result is to “demonstrate” a very large range of (contradictory) mechanistic features for relatively analogous electrocatalytic results. The use of different approaches, including the biomimetic approach based on multielectron/proton simultaneous transfer (requiring a different nature of the active sites), is a direction offering new clues to designing electrocatalysts from a different perspective.136
-
11.
Intensifying the investigation on the electrocatalytic production of H2O2.110 Besides the commercial interest in hydrogen peroxide as commented before, it represents half of the reaction of the most studied OER, the anodic reaction in water electrolysis systems. It is thus a two-electron path alternative to the four-electron path to produce O2 at the anodic part of the cell. It is a lower overpotential reaction, requiring less electrons and giving an added-value product, contrasted, however, from the easier decomposition. A general discrepancy was observed between the simple (H-type) electrochemical cells used in most of the studies and those required for industrial practice. This aspect evidences that the use of the appropriate electrocatalytic cell is crucial to obtain reliable indications. A large gap between current performances and those necessary for industrial exploitability is present, although this aspect is common to most of the current studies in electrocatalysis. The studies can lead to understanding how to generate and use the electrogenerated oxygen active species for different syntheses.
-
12.
Investigating more systematically how to develop electro-synthetic paths for bulk chemicals starting from biobased molecules. Studies in the electrocatalytic conversion of biomass-derived platform chemicals are (i) still limited, (ii) focused on few types of reactions (furfurals conversion, glycerol)137 and especially (iii) still unable to identify the rational bases to design the electrocatalysts. Although our understanding of electrocatalysts is improving,138 with the identification of various factors such as geometric structure of the active sites, presence of surface defects, size and shape of the nanoparticles, solvent, and electrolyte effects determining the behavior, often the indications are specific for a defined catalytic system and results are in contradiction between different materials. Thus, it remains difficult to define general aspects which allow identifying a priori the class of electrocatalysts to be used by hypothesizing the conceptual reaction mechanism. For complex electrocatalytic transformations (multielectron/protons, when biomass-derived chemicals are involved, etc.), exploration of new reactions still remains largely phenomenological. General guidelines for catalyst selection which can restrict the field of investigation to a limited range of materials, as often occurs in the case of thermocatalytic reactions, are also not available. Theoretical approaches for catalyst selection are strongly dependent on having established a precise mechanism of reaction, while more effective electrocatalytic approaches still are yet to be clearly identified. We believe that even if advances have been made, design of in silico electrocatalysts is still not currently possible. A primary research objective should be thus the development of the fundamental framework knowledge to realize this objective. Reviews in the field, attempting to rationalize the research effort in electrocatalysis,139 reported the research status as allowing better identification of the electrocatalysts and more precise identification of the reaction conditions. However, more intense effort, perhaps approaching from a different perspective, would be necessary to properly address the a priori design of efficient electrocatalysts.
Several electrocatalytic classes of reactions were identified by Tang et al.69 to develop the basis for the e-chemistry outlined in Figure 2: (i) C–N coupling, to form a range of N-containing chemicals, such as urea, amides, amines, and nitrile of widespread use as bulk, intermediates or specialty chemicals, for applications ranging from chemical production to fertilizers, agrochemicals, and pharmaceuticals; (ii) selective hydrogenation or hydrogenolysis, with in situ generation of H2 or hydrogen equivalents (H+/e–); and (iii) selective oxidation or oxidative dehydrogenation, also without addition of external oxidation agents, including O2. However, the challenge yet remains to identify enough selective electrocatalysts to make the process industrially feasible. Tang et al.69 provided a series of examples of electrocatalytic reactions for the three classes of the above-cited reactions. However, these examples are still far from industrial exploitability, and even within a homogeneous class of reactions, it is difficult to identify a rationality in the electrocatalysts design. Cardoso et al.140 also discussed a series of redox and acid/base electrocatalytic syntheses, and a series of industrial examples of pilot development, although typically for specific cases and small productions. Moving from these examples related to organic electrosynthesis to the production of bulk chemicals by electrocatalysis is a challenge, which often requires a change in the type of approach used.
Note that, in addition to direct electrocatalytic syntheses, also indirect syntheses via redox mediators (TEMPO being one of the most used) are possible. Thus, various examples of organic electrosynthesis are known, but related to electrochemistry rather than to electrocatalysis. Addressing the challenge given regarding developing a full framework for e-chemistry (Figure 2) requires making a next step in combining catalysis with electrochemistry.
Fundamental Areas for e-Chemistry
Research attention should be focused on specific fundamental areas, still not sufficiently explored, to create the basis for e-chemistry. These areas should complement the development of new electrocatalysts and mechanistic studies, on which research attention is mainly focused currently. Among the different aspects to develop, the following can be highlighted:
The design of advanced electrocatalytic reactors, which strongly determine the performances and behavior, while still most of the studies are based on too simple reactors (like H-cells) that do not allow proper translation of the results to realistic reactors closer to industrial exploitability.
The study of multiphasic electrocatalytic reactors, an area that as outlined before, is crucial to explore electrocatalytic paths, but scarcely investigated.141
Understanding the difference, including mechanistic aspects, between electrocatalysis (in general reactive catalysis) and thermal catalysis,10 as the basis to develop new approaches and explore (complex) pathways. Current studies consider mainly the application of methods used for thermal catalysis to reactive catalysis, with still limited attempts to understand the difference in operations and type of active centers. The dynamics of the polarized interface is crucial, also in determining selectivity,33 and significant reconstruction of active nanoparticles may occur upon application of a potential during electrocatalytic operations.142
Electrocatalytic behavior is determined by an intricate interplay between surface structure (both on the nano- and on the mesoscale), electrolyte effects (pH, buffer strength, ion effects) and mass transport conditions, a complex interplay still far from being completely understood.7 A new electrocatalytic design, for electrolyte-less operations, allows improvement in the behavior by exploiting this interplay, but still systematic knowledge is limited.143,144 A better understanding and a rational design of three-phase boundary in electrocatalysis can lead to enhanced control of the performances.144−148
Addressing the selectivity challenges of electrocatalysts. In several reactions, like CO2 electroreduction, many reactions are possible at the applied potential, which includes the necessary overpotential to drive the reaction at sufficiently high rate. Developing an effective theory of selectivity in electrocatalysis is still at its infancy, even with the advances in the field.149−151
Conclusions
The examples presented show that the potential and feasibility to develop new e-chemistry exist, but it is necessary to push the research to address more specifically this challenge, exploring the various possibilities indicated and integrating in a holistic view the different developments to accelerate the discovery of new paths and the identification of the best options among the different possibilities. The new e-chemistry requires to change the approach in the development of new paths, with a push toward new direct reactions which limit as much as possible the need for multistep processes realizing direct processes in small and efficient devices based on the use of RES. This is a grand challenge, which needs to be addressed to accelerate evolution in this direction. Although attention was given here mainly to electrocatalysis to focus the discussion, the importance of also exploring other routes of direct use of RES, such as photo- and plasma-catalysis, must be noted again. We also evidenced the need for a closer integration and synergy between solar- and biorefineries.152,153
A general indication given is that to properly address the challenge of e-chemistry, there is the need to target complex (direct) syntheses by electrocatalysis. Questions which may be posed is that which is presented above a dream (electro)catalysis and chemistry and would it be better to focus on simpler electrocatalytic reactions (two electrons, such as CO2 to CO), because more complex multielectron/proton transformations are too challenging. The reply is that there was not a sufficient attempt to devise a rational approach to catalysis complexity, identifying the ways to proceed faster in targeting complex (direct) syntheses. Not addressing this question will delay acceleration in progress in this area which is crucial to meet the challenge of converting petro- to e-chemistry. In parallel, e-chemistry requires enhanced efforts in developing new types of electrocatalysts to address the challenges outlined in Figures 2 and 3.154
In conclusion, we believe that e-chemistry (chemistry using RES and based on the closure of the carbon cycle and the use of biomass as key elements to defossilize the industrial chemical production)155 is feasible (assuming a high-tech scenario), and this transformation from current petrochemistry is already started; we suggest it will be largely irreversible. The nexus between refinery and chemistry is already changing, as a sign of this irreversible transformation.156 However, acceleration of this process requires a change in the approach, with a broader vision of the future. For catalysis, this will require revising the fundamental basis and recognize that the new methodologies essential for e-chemistry need to approach catalysis from wider and different perspectives, although based on the extensive background on knowledge on catalysis developed in recent decades.157,158 In deep transitions, a synchronism between R&D and economics/societal changes is necessary. Thus, the development of sustainable process systems requires thinking at a multiscale level, identifying the energy-efficient and highly integrated systems deployed within local and regional contexts.159
While the transformation of the chemical industry to e-chemistry is often considered to be motivated (only) by reducing the carbon-footprint, but at the “expense of higher production costs and unintended environmental burden shifting”,159 we would further remark that instead the push toward necessary innovation will result in lower global costs, with a different model of chemical production, interaction with territory and society.160
For catalysis, e-chemistry is a grand challenge, offering the possibility to rebuild its role as a key technology and develop a new basis for understanding. This will occur only when the significant changes in the approach to catalysis required to face this transformation are understood and introduced in the scientific practice.
Acknowledgments
This manuscript summarizes concepts and discussion emerged from the large EU initiative SUNERGY (fossil-free fuels and chemicals for a climate-neutral Europe; www.sunergy-initiative.eu) which is acknowledged. This work was made in the frame of the ERC Synergy SCOPE (project 810182), EU DECADE project (nr. 862030) and PRIN 2017 project MULTI-e nr. 20179337R7 and CO2 ONLY project nr. 2017WR2LRS, which are gratefully acknowledged. G.C. also thanks the Alexander von Humboldt-Stiftung/Foundation (Humboldt Research Award).
The authors declare no competing financial interest.
References
- Linnemann J.; Kanokkanchana K.; Tschulik K. Design Strategies for Electrocatalysts from an Electrochemist’s Perspective. ACS Catal. 2021, 11, 5318–5346. 10.1021/acscatal.0c04118. [DOI] [Google Scholar]
- Zhao K.; Quan X. Carbon-Based Materials for Electrochemical Reduction of CO2 to C2+ Oxygenates: Recent Progress and Remaining Challenges. ACS Catal. 2021, 11, 2076–2097. 10.1021/acscatal.0c04714. [DOI] [Google Scholar]
- Nguyen H. L.; Alzamly A. Covalent Organic Frameworks as Emerging Platforms for CO2 Photoreduction. ACS Catal. 2021, 11, 9809–9824. 10.1021/acscatal.1c02459. [DOI] [Google Scholar]
- Li C.; Li J.; Qin L.; Yang P.; Vlachos D. G. Recent Advances in the Photocatalytic Conversion of Biomass-Derived Furanic Compounds. ACS Catal. 2021, 11, 11336–11359. 10.1021/acscatal.1c02551. [DOI] [Google Scholar]
- Melchionna M.; Fornasiero P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. 10.1021/acscatal.0c01204. [DOI] [Google Scholar]
- Liu S.; Winter L. R.; Chen J. G. Review of Plasma-Assisted Catalysis for Selective Generation of Oxygenates from CO2 and CH4. ACS Catal. 2020, 10, 2855–2871. 10.1021/acscatal.9b04811. [DOI] [Google Scholar]
- Birdja Y. Y.; Pérez-Gallent E.; Figueiredo M. C.; Göttle A. J.; Calle-Vallejo F.; Koper M. T. M. Advances and Challenges in Understanding the Electrocatalytic Conversion of Carbon Dioxide to Fuels. Nature Energy 2019, 4, 732–745. 10.1038/s41560-019-0450-y. [DOI] [Google Scholar]
- Centi G.; Perathoner S. Catalysis for Solar-Driven Chemistry: the Role of Electrocatalysis. Catal. Today 2019, 330, 157–170. 10.1016/j.cattod.2018.03.005. [DOI] [Google Scholar]
- Bogaerts A.; Tu X.; Whitehead J. C.; Centi G.; Lefferts L.; Guaitella O.; Azzolina-Jury F.; Kim H.-H.; Murphy A. B.; Schneider W. F.; Nozaki T.; Hicks J. C.; Rousseau A.; Thevenet F.; Khacef A.; Carreon M. The 2020 Plasma Catalysis Roadmap. J. Phys. D: Appl. Phys. 2020, 53, 443001. 10.1088/1361-6463/ab9048. [DOI] [Google Scholar]
- Centi G.; Perathoner S. Redesign Chemical Processes to Substitute the Use of Fossil Fuels: A Viewpoint of the Implications on Catalysis. Catal. Today 2022, 387, 216–223. 10.1016/j.cattod.2021.03.007. [DOI] [Google Scholar]
- de Bruyn S.; Jongsma C.; Kampman B.; Görlach B.; Thie J.-E.. Energy-Intensive Industries - Challenges and Opportunities in Energy Transition; European Union, 2020. [Google Scholar]
- Fragkos P.; Fragkiadakis K.; Paroussos L. Reducing the Decarbonisation Cost Burden for EU Energy-Intensive Industries. Energies 2021, 14, 236. 10.3390/en14010236. [DOI] [Google Scholar]
- Roelofsen O.; Somers K.; Speelman E.; Witteveen M.. Plugging in: What Electrification can do for Industry; McKinsey & Co. May 2020. [Google Scholar]
- Bonheure M.; Vandewalle L.; Marin G.; Van Geem K. Dream or reality? Electrification of the Chemical Process Industries. Chem. Eng. Progress 2021, 117, 37–42. [Google Scholar]
- Perathoner S.; Van Geem K. M.; Marin G. B.; Centi G. Chem. Commun. 2021, 57, 10967–10982. 10.1039/D1CC03154F. [DOI] [PubMed] [Google Scholar]
- Ra E. C.; Kim K. Y.; Kim E. H.; Lee H.; An K.; Lee J. S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. 10.1021/acscatal.0c02930. [DOI] [Google Scholar]
- Thunman H.; Berdugo Vilches T.; Seemann M.; Maric J.; Cañete Vela I.; Pissot S.; Nguyen H. N. T. Circular Use of Plastics-Transformation of Existing Petrochemical Clusters into Thermochemical Recycling Plants with 100% Plastics Recovery. Sustainable Materials Techn. 2019, 22, e00124 10.1016/j.susmat.2019.e00124. [DOI] [Google Scholar]
- Schiffer Z. J.; Manthiram K. Electrification and Decarbonization of the Chemical Industry. Joule 2017, 1, 10–14. 10.1016/j.joule.2017.07.008. [DOI] [Google Scholar]
- Barton J. L. Electrification of the Chemical Industry. Science 2020, 368, 1181–1182. 10.1126/science.abb8061. [DOI] [PubMed] [Google Scholar]
- Chen C.; Lu Y.; Banares-Alcantara R. Direct and Indirect Electrification of Chemical Industry using Methanol Production as a Case Study. Appl. Energy 2019, 243, 71–90. 10.1016/j.apenergy.2019.03.184. [DOI] [Google Scholar]
- Navarrete A.; Centi G.; Bogaerts A.; Martín Á.; York A.; Stefanidis G. Harvesting Renewable Energy for CO2 Catalysis. Energy Technol. 2017, 5, 796–811. 10.1002/ente.201600609. [DOI] [Google Scholar]
- Stankiewicz A. I.; Nigar H. Beyond Electrolysis: Old Challenges and new Concepts of Electricity-Driven Chemical Reactors. React. Chem. Eng. 2020, 5, 1005–1016. 10.1039/D0RE00116C. [DOI] [Google Scholar]
- Cavani F.; Centi G.; Perathoner S.; Trifiró F.. Sustainable Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Stankiewicz A.; Van Gerven T.; Stefanidis G.. The Fundamentals of Process Intensification; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Schot J.; Kanger L. Deep transitions: Emergence, Acceleration, Stabilization and Directionality. Research Policy 2018, 47, 1045–1059. 10.1016/j.respol.2018.03.009. [DOI] [Google Scholar]
- Kanger L.; Schot J. Deep transitions: Theorizing the long-term patterns of socio-technical change. Env. Innovation & Soc. Trans. 2019, 32, 7–21. 10.1016/j.eist.2018.07.006. [DOI] [Google Scholar]
- Centi G.; Iaquaniello G.; Perathoner S. Chemical Engineering Role in the Use of Renewable Energy and Alternative Carbon Sources in Chemical Production. BMC Chem. Eng. 2019, 1, 5. 10.1186/s42480-019-0006-8. [DOI] [Google Scholar]
- Wiertzema H.; Svensson E.; Harvey S. Bottom-Up Assessment Framework for Electrification Options in Energy-Intensive Process Industries. Front. Energy Res. 2020, 8, 192. 10.3389/fenrg.2020.00192. [DOI] [Google Scholar]
- Centi G.; Perathoner S.; Salladini A.; Iaquaniello G. Economics of CO2 Utilization: A Critical Analysis. Front. in Energy Res. 2020, 8, 567986. 10.3389/fenrg.2020.567986. [DOI] [Google Scholar]
- Ueckerdt F.; Bauer C.; Dirnaichner A.; Everall J.; Sacchi R.; Luderer G. Potential and Risks of Hydrogen-Based e-Fuels in Climate Change Mitigation. Nat. Climate Change 2021, 11, 384–393. 10.1038/s41558-021-01032-7. [DOI] [Google Scholar]
- Centi G.; Perathoner S. Nanocarbon for Energy Material Applications: N2 Reduction Reaction. Small 2021, 17, 2007055. 10.1002/smll.202007055. [DOI] [PubMed] [Google Scholar]
- Romano V.; D’Angelo G.; Perathoner S.; Centi G. Current Density in Solar Fuel Technologies. Energy & Env. Sci. 2021, 14, 5760–5787. 10.1039/D1EE02512K. [DOI] [Google Scholar]
- Arrigo R.; Blume R.; Streibel V.; Genovese C.; Roldan A.; Schuster M. E.; Ampelli C.; Perathoner S.; Velasco Vélez J. J.; Hävecker M.; Knop-Gericke A.; Schlögl R.; Centi G. Dynamics at Polarized Carbon Dioxide-Iron Oxyhydroxide Interfaces Unveil the Origin of Multicarbon Product Formation. ACS Catal. 2022, 12, 411–430. 10.1021/acscatal.1c04296. [DOI] [Google Scholar]
- Marepally B. C.; Ampelli C.; Genovese C.; Saboo T.; Perathoner S.; Wisser F. M.; Veyre L.; Canivet J.; Quadrelli E. A.; Centi G. Enhanced Formation of > C1 Products in the Electroreduction of CO2 by Adding a Carbon Dioxide Adsorption Component to a Gas Diffusion Layer-Type Catalytic Electrode. ChemSusChem 2017, 10, 4442–4446. 10.1002/cssc.201701506. [DOI] [PubMed] [Google Scholar]
- Carvela M.; Mena I. F.; Raschitor A.; Lobato J.; Rodrigo M. A. Towards the Electrochemical Retention of CO2: Is it Worth it?. ChemElectroChem. 2021, 8, 3947–3953. 10.1002/celc.202101080. [DOI] [Google Scholar]
- Ifkovits Z. P.; Evans J. M.; Meier M. C.; Papadantonakis K. M.; Lewis N. S. Decoupled Electrochemical Water-Splitting Systems: a Review and Perspective. Energy Env. Sci. 2021, 14, 4740–4759. 10.1039/D1EE01226F. [DOI] [Google Scholar]
- Zhang F.; Wang Q. Redox-Mediated Water Splitting for Decoupled H2 Production. ACS Mater. Lett. 2021, 3, 641–651. 10.1021/acsmaterialslett.1c00074. [DOI] [Google Scholar]
- Hauer B. Embracing Nature’s Catalysts: A Viewpoint on the Future of Biocatalysis. ACS Catal. 2020, 10, 8418–8427. 10.1021/acscatal.0c01708. [DOI] [Google Scholar]
- Guajardo N.; Domínguez de María P. Production of Bulk Chemicals with Biocatalysis: Drivers and Challenges Reflected in Recent Industrial Granted Patents (2015–2020). Molecules 2021, 26, 736. 10.3390/molecules26030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley J. M. Towards the Sustainable Production of Bulk-Chemicals using Biotechnology. New Biotechnol. 2020, 59, 59–64. 10.1016/j.nbt.2020.07.002. [DOI] [PubMed] [Google Scholar]
- Lanzafame P.; Abate S.; Ampelli C.; Genovese C.; Passalacqua R.; Centi G.; Perathoner S. Beyond Solar Fuels: Renewable Energy-Driven Chemistry. ChemSusChem 2017, 10, 4409–4419. 10.1002/cssc.201701507. [DOI] [PubMed] [Google Scholar]
- Lanzafame P.; Centi G.; Perathoner S. Evolving Scenarios for Biorefineries and the Impact on Catalysis. Catal. Today 2014, 234, 2–12. 10.1016/j.cattod.2014.03.022. [DOI] [Google Scholar]
- Cetinkaya E.; Liu N.; Simons T. J.; Wallach J.. Petrochemicals 2030: Reinventing The Way to Win in a Changing Industry; McKinsey & Co. Feb. 2018. [Google Scholar]
- Dickson D.; Yankovitz D.; Hussain A.. Building Resilience in Petrochemicals, Deloitte Insights, Oct. 2020. [Google Scholar]
- Perathoner S.; Gross S.; Hensen E. J. M.; Wessel H.; Chraye H.; Centi G. Looking at the Future of Chemical Production through the European Roadmap on Science and Technology of Catalysis the EU Effort for a Long-term Vision. ChemCatChem. 2017, 9, 904–909. 10.1002/cctc.201601641. [DOI] [Google Scholar]
- International Energy Agency - IEA The Future of Petrochemicals; IEA, Paris, 2018. [Google Scholar]
- International Energy Agency - IEA Global Energy Review 2021 - Renewables; IEA, Paris, 2021. [Google Scholar]
- International Renewable Energy Agency - IRENA Renewable Energy Statistics 2021; IRENA; - Abu Dhabi, 2021. [Google Scholar]
- Lange J.-P. Towards Circular Carbo-Chemicals - The Metamorphosis of Petrochemicals. Energy Env. Sci. 2021, 14, 4358–4376. 10.1039/D1EE00532D. [DOI] [Google Scholar]
- Detz R.Technology Factsheet for Methanol Production; TNO (the Netherlands Organisation for applied scientific research), 2019. Available on 28.12.2021 at https://refman.energytransitionmodel.com. [Google Scholar]
- Ayres R. U.; Peiró L. T.; Méndez G. V. Exergy Efficiency in Industry: Where Do We Stand?. Environ. Sci. Technol. 2011, 45, 10634–10641. 10.1021/es202193u. [DOI] [PubMed] [Google Scholar]
- Levi P. G.; Cullen J. M. Mapping Global Flows of Chemicals: From Fossil Fuel Feedstocks to Chemical Products. Environ. Sci. Technol. 2018, 52 (4), 1725–1734. 10.1021/acs.est.7b04573. [DOI] [PubMed] [Google Scholar]
- Genovese C.; Ampelli C.; Perathoner S.; Centi G. Mechanism of C-C Bond Formation in the Electrocatalytic Reduction of CO2 to Acetic Acid. A Challenging Reaction to Use Renewable Energy with Chemistry. Green Chem. 2017, 19, 2406–2415. 10.1039/C6GC03422E. [DOI] [Google Scholar]
- U.S. Department of Energy Exergy Analysis : A Powerful Tool for Identifying Process Inefficiencies in the U.S. Chemical Industry, 2004. [Google Scholar]
- Centi G. Catalysis to Go Beyond the Use of Fossil Fuels: A Dream or an Effective Possibility?. Catalyst Rev. 2020, 10, 6–14. Published by The Catalyst Group. [Google Scholar]
- Saygin D.; Gielen D. Zero-Emission Pathway for the Global Chemical and Petrochemical Sector. Energies 2021, 14, 3772. 10.3390/en14133772. [DOI] [Google Scholar]
- Shreve D.Deep Decarbonisation: The Multi-Trillion-Dollar Question, WoodMackenzie, 2019. Accessible on 28.12.21 at https://www.woodmac.com/news/feature/deep-decarbonisation-the-multi-trillion-dollar-question/.
- Markandya A.; Saygin D.; Miketa A.; Gielen D.; Wagner N.. The True Cost of Fossil Fuels: Saving on the Externalities of Air Pollution and Climate Change, IRENA, Paris, 2016. Available on 28.12.21 at https://www.irena.org/-/media/files/irena/agency/publication/2016/irena_remap_externality_brief_2016.pdf. [Google Scholar]
- Malik D.; Simons T. J.; Manchanda P.; Wallach J.. The impact of COVID-19 on the Global Petrochemical Industry; McKinsey & Co, Oct. 2020. [Google Scholar]
- Lier S.; Grünewald M. Net Present Value Analysis of Modular Chemical Production Plants. Chem. Eng. Technol. 2011, 34, 809–816. 10.1002/ceat.201000380. [DOI] [Google Scholar]
- Centi G.; Perathoner S. One-step H2O2 and phenol syntheses: Examples of Challenges for New Sustainable Selective Oxidation Processes. Catal. Today 2009, 143, 145–150. 10.1016/j.cattod.2008.09.032. [DOI] [Google Scholar]
- Wang N.; Ma S.; Zuo P.; Duan J.; Hou B. Recent Progress of Electrochemical Production of Hydrogen Peroxide by Two- Electron Oxygen Reduction Reaction. Adv. Sci. 2021, 8, 2100076. 10.1002/advs.202100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. C.; Mavrikis S.; Wang L.; Ponce de Leon C. Future Perspectives for the Advancement of Electrochemical Hydrogen Peroxide Production. Current Opinion in Electrochem. 2021, 30, 100792. 10.1016/j.coelec.2021.100792. [DOI] [Google Scholar]
- Yang B.; Zhang S.; Gao Y.; Huang L.; Yang C.; Hou Y.; Zhang J. Unique Functionalities of Carbon Shells Coating on ZnFe2O4 for Enhanced Photocatalytic Hydroxylation of Benzene to Phenol. Appl. Catal., B: Env. 2022, 304, 120999. 10.1016/j.apcatb.2021.120999. [DOI] [Google Scholar]
- ElMetwally A. E.; Eshaq G.; Yehia F. Z.; Al-Sabagh A. M.; Kegnaes S. Iron Oxychloride as an Efficient Catalyst for Selective Hydroxylation of Benzene to Phenol. ACS Catal. 2018, 8, 10668–10675. 10.1021/acscatal.8b03590. [DOI] [Google Scholar]
- Makgwane P. R.; Ray S. S. Development of High- Performance Nanostructured V2O5/SnO2 Catalyst for Efficient Benzene Hydroxylation. Appl. Catal., A: General 2015, 492, 10–22. 10.1016/j.apcata.2014.12.024. [DOI] [Google Scholar]
- Luo Y.; Xiong J.; Pang C.; Li G.; Hu C. Direct Hydroxylation of Benzene to Phenol over TS-1 Catalysts. Catalysts 2018, 8, 49. 10.3390/catal8020049. [DOI] [Google Scholar]
- Meng Q.; Yan J.; Wu R.; Liu H.; Sun Y.; Wu N. N.; Xiang J.; Zheng L.; Zhang J.; Han B. Sustainable Production of Benzene from Lignin. Nat. Commun. 2021, 12, 4534. 10.1038/s41467-021-24780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C.; Zheng Y.; Jaroniec M.; Qiao S.-Z. Electrocatalytic Refinery for Sustainable Production of Fuels and Chemicals. Angew. Chemie Int. Ed. 2021, 60, 19572–19590. 10.1002/anie.202101522. [DOI] [PubMed] [Google Scholar]
- Jin S.; Hao Z.; Zhang K.; Yan Z.; Chen J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chemie, Int. Ed. 2021, 60, 20627–20648. 10.1002/anie.202101818. [DOI] [PubMed] [Google Scholar]
- Al-Tamreh S. A.; Ibrahim M. H.; El-Naas M. H.; Vaes J.; Pant D.; Benamor A.; Amhamed A. Electroreduction of Carbon Dioxide into Formate: A Comprehensive Review. ChemElectroChem. 2021, 8, 3207–3220. 10.1002/celc.202100438. [DOI] [Google Scholar]
- Su X.; Yang X.-F.; Huang Y.; Liu B.; Zhang T. Single- Atom Catalysis toward Efficient CO2 Conversion to CO and Formate Products. Acc. Chem. Res. 2019, 52, 656–664. 10.1021/acs.accounts.8b00478. [DOI] [PubMed] [Google Scholar]
- Centi G.; Quadrelli E. A.; Perathoner S. Catalysis for CO2 Conversion: A Key Technology for Rapid Introduction of Renewable Energy in the Value Chain of Chemical Industries. Energy & Env. Sci. 2013, 6, 1711–1731. 10.1039/c3ee00056g. [DOI] [Google Scholar]
- Zhao K.; Quan X. Carbon- Based Materials for Electrochemical Reduction of CO2 to C2+ Oxygenates: Recent Progress and Remaining Challenges. ACS Catal. 2021, 11, 2076–2097. 10.1021/acscatal.0c04714. [DOI] [Google Scholar]
- Gao D.; Aran-Ais R. M.; Jeon H. S.; Roldan Cuenya B. Rational Catalyst and Electrolyte Design for CO2 Electroreduction towards Multicarbon Products. Nat. Catal. 2019, 2, 198–210. 10.1038/s41929-019-0235-5. [DOI] [Google Scholar]
- Chen J.; Wang T.; Li Z.; Yang B.; Zhang Q.; Lei L.; Feng P.; Hou Y. Recent Progress and Perspective of Electrochemical CO2 Reduction towards C2- C5 Products over Non- Precious Metal Heterogeneous Electrocatalysts. Nano Res. 2021, 14, 3188–3207. 10.1007/s12274-021-3335-x. [DOI] [Google Scholar]
- Schmidt O.; Gambhir A.; Staffell I.; Hawkes A.; Nelson J.; Few S. Future Cost and Performance of Water Electrolysis: An Expert Elicitation Study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. 10.1016/j.ijhydene.2017.10.045. [DOI] [Google Scholar]
- Centi G.; Iaquaniello G.; Perathoner S. Can we Afford to Waste Carbon Dioxide? Carbon Dioxide as a Valuable Source of Carbon for the Production of Light Olefins. ChemSusChem 2011, 4, 1265–1273. 10.1002/cssc.201100313. [DOI] [PubMed] [Google Scholar]
- Lin T.; Gong K.; Wang C.; An Y.; Wang X.; Qi X.; Li S.; Lu Y.; Zhong L.; Sun Y. Fischer–Tropsch Synthesis to Olefins: Catalytic Performance and Structure Evolution of Co2C-Based Catalysts under a CO2 Environment. ACS Catal. 2019, 9, 9554–9567. 10.1021/acscatal.9b02513. [DOI] [Google Scholar]
- Tian P.; Wei Y.; Ye M.; Liu Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. 10.1021/acscatal.5b00007. [DOI] [Google Scholar]
- Wu W.; Hu H.; Ding D. Low-temperature Ethylene Production for Indirect Electrification in Chemical Production. Cell Rep. Phys. Sci. 2021, 2, 100405. 10.1016/j.xcrp.2021.100405. [DOI] [Google Scholar]
- Delikonstantis E.; Igos E.; Theofanidis S.-A.; Benetto E.; Marin G. B.; Van Geem K.; Stefanidis G. D. An Assessment of Electrified Methanol Production from an Environmental Perspective. Green Chem. 2021, 23, 7243–7258. 10.1039/D1GC01730F. [DOI] [Google Scholar]
- Schuler E.; Demetriou M.; Shiju N. R.; Gruter G.-J. M. Towards Sustainable Oxalic Acid from CO2 and Biomass. ChemSusChem 2021, 14, 3636–3664. 10.1002/cssc.202101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Gao H.; Feng J.; Zeng S.; Liu L.; Liu L.; Ren B.; Li T.; Zhang S.; Zhang X. Aromatic Ester- Functionalized Ionic Liquid for Highly Efficient CO2 Electrochemical Reduction to Oxalic Acid. ChemSusChem 2020, 13, 4900–4905. 10.1002/cssc.202001194. [DOI] [PubMed] [Google Scholar]
- Eguchi H.; Kato K.; Juhasz G.; Yamauchi M. Selectivity Enhancement in the Electrochemical Reduction of Oxalic Acid over Titanium Dioxide Nanoparticles achieved by Shape and Energy- State Control. Catal. Sci. & Techn. 2021, 11, 7592–7597. 10.1039/D1CY01239H. [DOI] [Google Scholar]
- Murcia Valderrama M. A.; van Putten R.-J.; Gruter G.-J. M. The Potential of Oxalic - and Glycolic Acid Based Polyesters (Review). Towards CO2 as a Feedstock. Eur. Polym. J. 2019, 119, 445–468. 10.1016/j.eurpolymj.2019.07.036. [DOI] [Google Scholar]
- Abramo F. P.; De Luca F.; Passalacqua R.; Centi G.; Giorgianni G.; Perathoner S.; Abate S. Electrocatalytic Production of Glycolic Acid via Oxalic Acid Reduction on Titania Debris Supported on a Tio2 Nanotube Array. J. Energy Chem. 2022, 68, 669–678. 10.1016/j.jechem.2021.12.034. [DOI] [Google Scholar]
- Garcia A. C.; Sánchez-Martínez C.; Bakker I.; Goetheer E. Sustainable Electrochemical Production of Tartaric Acid. ACS Sustainable Chem. Eng. 2020, 8, 10454–10460. 10.1021/acssuschemeng.0c02493. [DOI] [Google Scholar]
- Guo F.; Liu B.; Liu M.; Xia Y.; Wang T.; Hu W.; Fyffe P.; Tian L.; Chen X. Selective Electrocatalytic CO2 Reduction to Acetate on Polymeric Cu- L (L = Pyridinic N and Carbonyl Group) Complex Core- Shell Microspheres. Green Chem. 2021, 23, 5129–5137. 10.1039/D1GC01464A. [DOI] [Google Scholar]
- Kuder J. E.Electrochemical Production of Vinyl Acetate, US Patent 4383899, 1983, assigned to Celanese Co.
- Liu H.; Li W. Recent Advances in Paired Electrolysis of Biomass- Derived Compounds toward Cogeneration of Value- Added Chemicals and Fuels. Current Opinion in Electrochem. 2021, 30, 100795. 10.1016/j.coelec.2021.100795. [DOI] [Google Scholar]
- Kwon Y.; Schouten K. J. P.; van der Waal J. C.; de Jong E.; Koper M. T. M. Electrocatalytic Conversion of Furanic Compounds. ACS Catal. 2016, 6, 6704–6717. 10.1021/acscatal.6b01861. [DOI] [Google Scholar]
- Chadderdon X. H.; Chadderdon D. J.; Pfennig T.; Shanks B. H.; Li W. Paired Electrocatalytic Hydrogenation and Oxidation of 5- (Hydroxymethyl) Furfural for Efficient Production of Biomass- Derived Monomers. Green Chem. 2019, 21, 6210–6219. 10.1039/C9GC02264C. [DOI] [Google Scholar]
- Zhang P.; Sheng X.; Chen X.; Fang Z.; Jiang J.; Wang M.; Li F.; Fan L.; Ren Y.; Zhang B.; Timmer B. J. J.; Ahlquist M. S. G.; Sun L. Paired Electrocatalytic Oxygenation and Hydrogenation of Organic Substrates with Water as the Oxygen and Hydrogen Source. Angew. Chemie, Int. Ed. 2019, 58, 9155–9159. 10.1002/anie.201903936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centi G.; Perathoner S.; Winè G.; Gangeri M. Electrocatalytic Conversion of CO2 to Long Carbon-Chain Hydrocarbons. Green Chem. 2007, 9, 671–678. 10.1039/b615275a. [DOI] [Google Scholar]
- Dey A.; Gunnoe T. B.; Stamenkovic V. R. Organic Electrosynthesis: When Is It Electrocatalysis?. ACS Catal. 2020, 10, 13156–13158. 10.1021/acscatal.0c04559. [DOI] [Google Scholar]
- Shatskiy A.; Lundberg H.; Kaerkaes M. D. Organic Electrosynthesis: Applications in Complex Molecule Synthesis. ChemElectroChem. 2019, 6, 4067–4092. 10.1002/celc.201900435. [DOI] [Google Scholar]
- Harnisch F.; Schroeder U. Tapping Renewables: A New Dawn for Organic Electrosynthesis in Aqueous Reaction Media. ChemElectroChem. 2019, 6, 4126–4133. 10.1002/celc.201900456. [DOI] [Google Scholar]
- Pletcher D. Organic Electrosynthesis - A road to Greater Application. A Mini Review. Electrochem. Commun. 2018, 88, 1–4. 10.1016/j.elecom.2018.01.006. [DOI] [Google Scholar]
- Sharaf O. Z.; Orhan M. F. An Overview of Fuel Cell Technology: Fundamentals and Applications. Renewable & Sustainable Energy Rev. 2014, 32, 810–853. 10.1016/j.rser.2014.01.012. [DOI] [Google Scholar]
- Leow W. R.; Lum Y.; Ozden A.; Wang Y.; Nam D.-H.; Chen B.; Wicks J.; Zhuang T.-T.; Li F.; Sinton D.; Sargent E. H. Chloride- mediated Selective Electrosynthesis of Ethylene and Propylene Oxides at High Current Density. Science 2020, 368, 1228–1233. 10.1126/science.aaz8459. [DOI] [PubMed] [Google Scholar]
- Scott K.; Odouza C.; Hui W. Pilot Scale Electrosynthesis of Alkene Oxides by Direct and Indirect Oxidation in a Sieve Plate Electrochemical Reactor. Chem. Eng. Sci. 1992, 47, 2957–2962. 10.1016/0009-2509(92)87158-M. [DOI] [Google Scholar]
- Wang S.; Uwakwe K.; Yu L.; Ye J.; Zhu Y.; Hu J.; Chen R.; Zhang Z.; Zhou Z.; Li J.; Xie Z.; Deng D. Highly Efficient Ethylene Production via Electrocatalytic Hydrogenation of Acetylene under Mild Conditions. Nat. Commun. 2021, 12, 7072. 10.1038/s41467-021-27372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann S. N.; Michel C.; Schwiedernoch R.; Wu M.; Sautet P. Electro- Carboxylation of Butadiene and Ethene over Pt and Ni Catalysts. J. Catal. 2016, 343, 240–247. 10.1016/j.jcat.2016.01.008. [DOI] [Google Scholar]
- Lanzafame P.; Centi G.; Perathoner S. Catalysis for Biomass and CO2 use through Solar Energy: Opening new Scenarios for a Sustainable and Low-Carbon Chemical Production. Chem. Soc. Rev. 2014, 43, 7562–7580. 10.1039/C3CS60396B. [DOI] [PubMed] [Google Scholar]
- Matthessen R.; Fransaer J.; Binnemans K.; De Vos D. E. Electrocarboxylation: Towards Sustainable and Efficient Synthesis of Valuable Carboxylic Acids. Beilstein J. Organic Chem. 2014, 10, 2484–2500. 10.3762/bjoc.10.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S.-F.; Horne M. D.; Bond A. M.; Zhang J. Electrocarboxylation in Ionic Liquids. RSC Energy Env. Series 2018, 21, 160–181. 10.1039/9781782623809-00160. [DOI] [Google Scholar]
- Kim J. E.; Jang J. H.; Lee K. M.; Balamurugan M.; Jo Y. I.; Lee M. Y.; Choi S.; Im S. W.; Nam K. T. Electrochemical Synthesis of Glycine from Oxalic Acid and Nitrate. Angew, Chemie, Int. Ed. 2021, 60, 21943–21951. 10.1002/anie.202108352. [DOI] [PubMed] [Google Scholar]
- Perathoner S. Electrocatalysis: The Link for Using Renewable Energy in Chemical and Energy Processes. Catalyst Rev. 2021, 4, 8–13. (Part 1) and 5, 8–13 (Part 2). Published by The Catalyst Group. [Google Scholar]
- Jung E.; Shin H.; Antink W. H.; Sung Y.-E.; Hyeon T. Recent Advances in Electrochemical Oxygen Reduction to H2O2: Catalyst and Cell Design. ACS Energy Lett. 2020, 5, 1881–1892. 10.1021/acsenergylett.0c00812. [DOI] [Google Scholar]
- Fabbri E.; Schmidt T. J. Oxygen Evolution Reaction -The Enigma in Water Electrolysis. ACS Catal. 2018, 8, 9765–9774. 10.1021/acscatal.8b02712. [DOI] [Google Scholar]
- Gao J.; Liu B. Progress of Electrochemical Hydrogen Peroxide Synthesis over Single Atom Catalysts. ACS Mater. Lett. 2020, 2, 1008–1024. 10.1021/acsmaterialslett.0c00189. [DOI] [Google Scholar]
- Cao P.; Quan X.; Zhao K.; Zhao X.; Chen S.; Yu H. Durable and Selective Electrochemical H2O2 Synthesis under a Large Current Enabled by the Cathode with Highly Hydrophobic Three- Phase Architecture. ACS Catal. 2021, 11, 13797–13808. 10.1021/acscatal.1c03236. [DOI] [Google Scholar]
- Zhang Q.; Zhou M.; Ren G.; Li Y.; Li Y.; Du X. Highly Efficient Electrosynthesis of Hydrogen Peroxide on a Superhydrophobic Three-Phase Interface by Natural Air Diffusion. Nat. Commun. 2020, 11, 1731. 10.1038/s41467-020-15597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Quispe-Cardenas E.; Yang S.; Yin L.; Yang Y. Electrosynthesis of >20 g/L H2O2 from Air. ACS ES&T Engg. 2022, 2, 242–250. 10.1021/acsestengg.1c00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X.; Pu M.; Jin Y.; Wessling M. Efficient Electrocatalytic N2 Reduction on Three-Phase Interface Coupled in a Three-Compartment Flow Reactor for the Ambient NH3 Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 21411–21425. 10.1021/acsami.1c03698. [DOI] [PubMed] [Google Scholar]
- Chang Q.; Zhang P.; Mostaghimi A. H. B.; Zhao X.; Denny S. R.; Lee J. H.; Gao H.; Zhang Y.; Xin H. L.; Siahrostami S.; Chen J. G.; Chen Z. Promoting H2O2 production via 2-electron Oxygen Reduction by Coordinating Partially Oxidized Pd with Defect Carbon. Nat. Commun. 2020, 11, 2178. 10.1038/s41467-020-15843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. C.; Mavrikis S.; Wang L.; Ponce de Leon C. Future Perspectives for the Advancement of Electrochemical Hydrogen Peroxide Production. Current Opinion in Electrochem. 2021, 30, 100792. 10.1016/j.coelec.2021.100792. [DOI] [Google Scholar]
- Mavrikis S.; Perry S. C.; Leung P. K.; Wang L.; Ponce de Leon C. Recent Advances in Electrochemical Water Oxidation to Produce Hydrogen Peroxide: A Mechanistic Perspective. ACS Sustainable Chem. & Eng. 2021, 9, 76–91. 10.1021/acssuschemeng.0c07263. [DOI] [Google Scholar]
- Zhang X.; Xia Y.; Xia C.; Wang H. Insights into Practical- Scale Electrochemical H2O2 Synthesis. Trends in Chem. 2020, 2, 942–953. 10.1016/j.trechm.2020.07.007. [DOI] [Google Scholar]
- Perry S. C.; Pangotra D.; Vieira L.; Csepei L.-I.; Sieber V.; Wang L.; Ponce de Leon C.; Walsh F. C. Electrochemical Synthesis of Hydrogen Peroxide from Water and Oxygen. Nat. Rev. Chem. 2019, 3, 442–458. 10.1038/s41570-019-0110-6. [DOI] [Google Scholar]
- Bu Y.; Wang Y.; Han G.-F.; Zhao Y.; Ge X.; Li F.; Zhang Z.; Zhong Q.; Baek J.-B. Carbon- Based Electrocatalysts for Efficient Hydrogen Peroxide Production. Adv. Mater. 2021, 33, 2103266. 10.1002/adma.202103266. [DOI] [PubMed] [Google Scholar]
- Guo S.; Asset T.; Atanassov P. Catalytic Hybrid Electrocatalytic/Biocatalytic Cascades for Carbon Dioxide Reduction and Valorization. ACS Catal. 2021, 11, 5172–5188. 10.1021/acscatal.0c04862. [DOI] [Google Scholar]
- Rabaey K.; Rozendal R. A. Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. 10.1038/nrmicro2422. [DOI] [PubMed] [Google Scholar]
- Claassens N. J.; Sousa D. Z.; dos Santos V. A. P. M.; de Vos W. M.; van der Oost J. Harnessing the Power of Microbial Autotrophy. Nat. Rev. Microbiol. 2016, 14, 692–706. 10.1038/nrmicro.2016.130. [DOI] [PubMed] [Google Scholar]
- Francke R.; Little R. D. Redox Catalysis in Organic Electrosynthesis: Basic Principles and Recent Developments. Chem. Soc. Rev. 2014, 43, 2492–2521. 10.1039/c3cs60464k. [DOI] [PubMed] [Google Scholar]
- Nutting J. E.; Rafiee M.; Stahl S. S. Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions. Chem. Rev. 2018, 118, 4834–4885. 10.1021/acs.chemrev.7b00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriminna R.; Pagliaro M. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Organic Process Res. & Dev. 2010, 14, 245–251. 10.1021/op900059x. [DOI] [Google Scholar]
- Rouwenhorst K. H. R.; Jardali F.; Bogaerts A.; Lefferts L. From the Birkeland-Eyde Process Towards Energy-Efficient Plasma-Based NOx Synthesis: A Techno-Economic Analysis. Energy Env. Sci. 2021, 14, 2520–2534. 10.1039/D0EE03763J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N.; Coco C. A.; Wang Y.; Su T.; Wang Y.; Peng L.; Zhang Y.; Liu Y.; Qiao J.; Zhou X.-D. Electro-Conversion of Methane to Alcohols on “Capsule-Like” Binary Metal Oxide Catalysts. Appl. Catal. B: Env. 2021, 282, 119572. 10.1016/j.apcatb.2020.119572. [DOI] [Google Scholar]
- Meng X.; Cui X.; Rajan N. P.; Yu L.; Deng D.; Bao X. Direct Methane Conversion under Mild Condition by Thermo-, Electro-, or Photocatalysis. Chem. 2019, 5, 2296–2325. 10.1016/j.chempr.2019.05.008. [DOI] [Google Scholar]
- Liu Y.; Qiu H.; Li J.; Guo L.; Ager J. W. Tandem Electrocatalytic CO2 Reduction with Efficient Intermediate Conversion over Pyramid-Textured Cu-Ag Catalysts. ACS Appl. Mater. & Interfaces 2021, 13, 40513–40521. 10.1021/acsami.1c08688. [DOI] [PubMed] [Google Scholar]
- Marken F.; Cresswell A. J.; Bull S. D. Recent Advances in Paired Electrosynthesis. Chem. Rec. 2021, 21, 2585–2600. 10.1002/tcr.202100047. [DOI] [PubMed] [Google Scholar]
- Qing G.; Ghazfar R.; Jackowski S. T.; Habibzadeh F.; Ashtiani M. M.; Chen C.-P.; Smith M. R.; Hamann T. W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. 10.1021/acs.chemrev.9b00659. [DOI] [PubMed] [Google Scholar]
- Shi L.; Yin Y.; Wang S.; Sun H. Rational Catalyst Design for N2 Reduction under Ambient Conditions: Strategies toward Enhanced Conversion Efficiency. ACS Catal. 2020, 10, 6870–6899. 10.1021/acscatal.0c01081. [DOI] [Google Scholar]
- Mallamace D.; Papanikolaou G.; Perathoner S.; Centi G.; Lanzafame P. Comparing molecular mechanisms in solar NH3 production and relations with CO2 reduction. Int. J. Mol. Sci. 2021, 22, 139. 10.3390/ijms22010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L.; Shao Y.; Sun J.; Yin G.; Du C.; Wang Y. Electrocatalytic Valorisation of Biomass Derived Chemicals. Catal. Sci. Technol. 2018, 8, 3216–3232. 10.1039/C8CY00533H. [DOI] [Google Scholar]
- Garlyyev B.; Fichtner J.; Piqué O.; Schneider O.; Bandarenka A. S.; Calle-Vallejo F. Revealing the Nature of Active Sites in Electrocatalysis. Chem. Sci. 2019, 10, 8060–8075. 10.1039/C9SC02654A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masa J.; Andronescu C.; Schuhmann W. Electrocatalysis as the Nexus for Sustainable Renewable Energy: The Gordian Knot of Activity, Stability, and Selectivity. Angew. Chem., Int. Ed. 2020, 59, 15298–15312. 10.1002/anie.202007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso D. S. P.; Šljukić B.; Santos D. M. F.; Sequeira C. A. C. Organic Electrosynthesis: From Laboratorial Practice to Industrial Applications. Org. Process Res. Dev. 2017, 21, 1213–1226. 10.1021/acs.oprd.7b00004. [DOI] [Google Scholar]
- van Ommen J. R.; Nijenhuis J.; Padding J. T. Structured Millichannel Multiphase Reactors. Comput.-Aided Chem. Eng. 2019, 46, 1789–1794. 10.1016/B978-0-12-818634-3.50299-X. [DOI] [Google Scholar]
- Genovese C.; Schuster M. E.; Gibson E. K.; Gianolio D.; Posligua V.; Grau-Crespo R.; Cibin G.; Wells P.; P; Garai D.; Solokha V.; Calderon S. K.; Velasco-Velez J. J.; Ampelli C.; Perathoner S.; Held G.; Centi G.; Arrigo R. Operando Spectroscopy Study of the Carbon Dioxide Electro-Reduction by Iron Species on Nitrogen-Doped Carbon. Nat. Commun. 2018, 9, 935. 10.1038/s41467-018-03138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampelli C.; Genovese C.; Marepally B. C.; Papanikolaou G.; Perathoner S.; Centi G. Electrocatalytic Conversion of CO2 to Produce Solar Fuels in Electrolyte or Electrolyte-Less Configurations of PEC Cells. Faraday Discuss. 2015, 183, 125–145. 10.1039/C5FD00069F. [DOI] [PubMed] [Google Scholar]
- Ampelli C.; Centi G.; Passalacqua R.; Perathoner S. Electrolyte-less Design of PEC Cells for Solar Fuels: Prospects and Open Issues in the Development of Cells and Related Catalytic Electrodes. Catal. Today 2016, 259, 246–258. 10.1016/j.cattod.2015.07.020. [DOI] [Google Scholar]
- Wang Y.; Zou Y.; Tao L.; Wang Y.; Huang G.; Du S.; Wang S. Rational Design of Three- Phase Interfaces for Electrocatalysis. Nano Res. 2019, 12, 2055–2066. 10.1007/s12274-019-2310-2. [DOI] [Google Scholar]
- Melchionna M.; Fornasiero P.; Prato M.; Bonchio M. Electrocatalytic CO2 Reduction: Role of the Cross- Talk at Nano- Carbon Interfaces. Energy Env. Sci. 2021, 14, 5816–5833. 10.1039/D1EE00228G. [DOI] [Google Scholar]
- Li J.; Chen G.; Zhu Y.; Liang Z.; Pei A.; Wu C.-L.; Wang H.; Lee H. R.; Liu K.; Chu S.; Cui Y. Efficient Electrocatalytic CO2 Reduction on a Three- Phase Interface. Nat. Catal. 2018, 1, 592–600. 10.1038/s41929-018-0108-3. [DOI] [Google Scholar]
- Genovese C.; Ampelli C.; Marepally B. C.; Papanikolaou G.; Perathoner S.; Centi G. Electrocatalytic Reduction of CO2 for the Production of Fuels: A Comparison between Liquid and Gas Phase Conditions. Chem. Eng. Trans. 2015, 43, 2281–2286. [Google Scholar]
- Guo X.; Gu J.; Lin S.; Zhang S.; Chen Z.; Huang S. Tackling the Activity and Selectivity Challenges of Electrocatalysts toward the Nitrogen Reduction Reaction via Atomically Dispersed Biatom Catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721. 10.1021/jacs.9b13349. [DOI] [PubMed] [Google Scholar]
- Singh A. R.; Rohr B. A.; Schwalbe J. A.; Cargnello M.; Chan K.; Jaramillo T. F.; Chorkendorff I.; Nørskov J. K. Electrochemical Ammonia Synthesis - The Selectivity Challenge. ACS Catal. 2017, 7, 706–709. 10.1021/acscatal.6b03035. [DOI] [Google Scholar]
- Chen G.-F.; Ren S. Y.; Zhang L. L.; Cheng H.; Luo Y. R.; Zhu K. H.; Ding L.-X.; Wang H. H. Advances in Electrocatalytic N2 Reduction - Strategies to Tackle the Selectivity Challenge. Small Methods 2019, 3, 1800337. 10.1002/smtd.201800337. [DOI] [Google Scholar]
- Abate S.; Lanzafame P.; Perathoner S.; Centi G. New Sustainable Model of Biorefineries: Biofactories and Challenges of Integrating Bio-and Solar Refineries. ChemSusChem 2015, 8, 2854–2866. 10.1002/cssc.201500277. [DOI] [PubMed] [Google Scholar]
- Papanikolaou G.; Lanzafame P.; Giorgianni G.; Abate S.; Perathoner S.; Centi G. Highly Selective Bifunctional Ni Zeo-Type Catalysts for Hydroprocessing of Methyl Palmitate to Green Diesel. Catal. Today 2020, 345, 14–21. 10.1016/j.cattod.2019.12.009. [DOI] [Google Scholar]
- Papanikolaou G.; Lanzafame P.; Perathoner S.; Centi G.; Cozza D.; Giorgianni G.; Migliori M.; Giordano G. High performance of Au/ZTC based catalysts for the selective oxidation of bio-derivative furfural to 2-furoic acid. Catal. Comm. 2021, 149, 106234. 10.1016/j.catcom.2020.106234. [DOI] [Google Scholar]
- Papanikolaou G.; Centi G.; Perathoner S.; Lanzafame P.. Transforming Catalysis to Produce e-Fuels: Prospects and Gaps. Chin. J. Catal. 2022, 43, 10.1016/S1872-2067(21)64016-0. [DOI] [Google Scholar]
- Abate S.; Centi G.; Lanzafame P.; Perathoner S. The Energy-Chemistry Nexus: A Vision of the Future from Sustainability Perspective. J. Energy Chem. 2015, 24, 535–547. 10.1016/j.jechem.2015.08.005. [DOI] [Google Scholar]
- Centi G.; Čejka J. Needs and Gaps for Catalysis in Addressing Transitions in Chemistry and Energy from a Sustainability Perspective. ChemSusChem 2019, 12, 621–632. 10.1002/cssc.201802637. [DOI] [PubMed] [Google Scholar]
- Čejka J.; Nachtigall P.; Centi G. New Catalytic Materials for Energy and Chemistry In Transition. Chem. Soc. Rev. 2018, 47, 8066–8071. 10.1039/C8CS90119H. [DOI] [PubMed] [Google Scholar]
- González-Garay A.; Mac Dowell N.; Shah N. A Carbon Neutral Chemical Industry Powered by the Sun. Discov Chem. Eng. 2021, 1, 2. 10.1007/s43938-021-00002-x. [DOI] [Google Scholar]
- Van Geem K. M.; Weckhuyse B. M. Towards an e-Chemistree: Materials for Electrification of the Chemical Industry. MRS Bull. 2021, 46, 1. [Google Scholar]