Abstract
The Omicron variant has been detected in nearly 150 countries. We analyzed the mutational landscape of Omicron throughout the genome, focusing the S-glycoprotein. We also evaluated mutations in the antibody-binding regions and observed some important mutations overlapping those of previous variants including N501Y, D614G, H655Y, N679K, and P681H. Various new receptor-binding domain mutations were detected, including Q493K, G496S, Q498R, S477N, G466S, N440K, and Y505H. New mutations were found in the NTD (Δ143-145, A67V, T95I, L212I, and Δ211) including one new mutation in fusion peptide (D796Y). There are several mutations in the antibody-binding region including K417N, E484A, Q493K, Q498R, N501Y, and Y505H and several near the antibody-binding region (S477N, T478K, G496S, G446S, and N440K). The impact of mutations in regions important for the affinity between spike proteins and neutralizing antibodies was evaluated. Furthermore, we examined the effect of significant antibody-binding mutations (K417N, T478K, E484A, and N501Y) on antibody affinity, stability to ACE2 interaction, and possibility of amino acid substitution. All the four mutations destabilize the antibody-binding affinity. This study reveals future directions for developing neutralizing antibodies against the Omicron variant.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00532-4.
Keywords: Mutations, Genome, S-glycoprotein, Omicron, Ab binding region
Introduction
A new variant of SARS-CoV-2 was detected in South Africa in late November 2021 and as Omicron by the WHO. Omicron was categorized as a VOC on November 26, 2021. After the first Omicron case was identified in Botswana, in Southern Africa, the virus sequence was determined [1, 2]. The variant has created a huge concern among scientist and policymakers worldwide because of its large number of mutations [3]. After detection in South Africa, the new variant spreaded rapidly, and an Omicron-infected case was observed in Hong Kong within few days [1]. The first confirmed case of Omicron was identified in the USA on December 1, 2021. The US-CDC COVID-19 response team reported at least one Omicron variant case in each of the 22 states across the country [4]. Simultaneously, Brandal et al. revealed that 117 individuals were infected with Omicron in Norway and that no patients with this variant were hospitalized until December 13, 2021 [5].
Similarly, Espenhain et al. reported 785 Omicron-positive cases in Denmark, among which 76% patients were vaccinated and 7.1% patients were booster-vaccinated. Additionally, 4.3% of the patients had previously been infected with SARS-CoV-2 [6]. Later, Thakur and Kanta Ratho observed that the variant had been transmitted to over 77 countries within a month with the largest number of cases observed in the UK, USA, and South Africa. The spread of Omicron variant has created high health concerns and global panic [7]. Approximately 128 countries have submitted genome sequences to the GISAID database. A study of this data revealed that Omicron rapidly replaced Delta as the dominant variant [8, 9], Through sequence analysis, Wang and Cheng observed that the Omicron variant evolved rapidly and confirmed that it had replaced the Delta variant as the dominant strain [10].
Notably, the appearance of new variants of SARS-CoV-2, particularly VOCs such as Delta, Beta, and Alpha, have been associated with new disease waves in many countries leading to increased infections and, some cases, worldwide dissemination [11]. For example, increased infection with the Delta variant (B.1.617.2) in India resulted in a serious second wave that killed large numbers of people [12]. Similarly, the emergence of the Alpha variant initiated a second wave in the UK [13]. The emergence of the variant has created new concerns related to the pandemic. Many countries are facing new waves because of the sudden appearance of the Omicron variant and are preparing countermeasures. Preliminary reports revealed the high transmissibility of Omicron. However, Mahase reported that hospital admission is less (50–70% lower) for Omicron infected cases compared to Delta infected cases [14], which relieves some concerns.
The Omicron variant is highly mutated [3, 15], containing more mutations than to all previous variants of SARS-CoV-2 [16]. Omicron variant contains approximately 50 mutations throughout its genome among which approximately, 32 mutations are localized to the coding sequence for the S-glycoprotein [3, 15, 17, 18]. Some mutations including T478K, N501Y, N655Y, N679K, and P681H overlap with those in other VOCs or VOIs such as Delta, Gamma, Alpha, and Beta [1, 19–21]. However, Omicron has been described as an immensely mutated variant containing an “unusual constellation of mutations” [22]. Therefore, understanding the mutation landscape of the Omicron variant is crucial, particularly the effect of mutations on antibody escape which has commonly been reported for SARS-CoV-2 variants [23]. A recent study revealed mutations in the RBD and NTD. V445E and K444 Q/R/N in the RBD and K150 T/Q/R/E, and N148S in NTD may be responsible for antibody escape [24]. Another study observed that the Omicron variant decreased neutralization ability using post-immunization serum from individuals administered two doses of Oxford–AstraZeneca vaccine or two doses of the Pfizer–BioNTech vaccine [25], indicating a nAb escape event. Studies are needed to understand the immune escape and antibody escape by the Omicron variant based on the large number of mutations in its spike protein.
This study was performed to understand the mutational landscape of the Omicron variant throughout the genome, particularly in S-glycoprotein, and identify different mutations in the antibody-binding regions that may affect nAb binding.
Materials and methods
Collection of data to analyze mutations in Omicron variant
Literature were collected on Omicron variant from several databases and search engines such as PubMed [26, 27], Google Scholar [28], and Web of Science [29]. Data on the Omicron variant were obtained from the eCDC [30]; WHO [31], and US CDC [32]. In addition, a keyword search was performed for the Omicron variant using different significant or relative keywords and genomic information was obtained from the GISAID database [33, 34]. PDB files were retrieved from the RCSBPDB database for S-glycoprotein/S- glycoprotein-antibody interactions [35].
Analysis of key mutations and their properties
Overall mutational landscape analysis and 3D model development
We collected the data from the GISAID database [33, 34] to illustrate the mutational landscape both for the S-glycoprotein and throughout the genome. Mutation data were also collected or fetched from different websites such as eCDC [30]; WHO [31], and CDC [32]. To understand the mutations and their properties, we collected data from PubMed [26, 27], Google Scholar [28], and Web of Science [29]. We used PDB files for 3D model development, and the PDB files were retrieved from the RCSBPDB database for S-glycoprotein [35].
Mutational event diversity of Omicron and all SARS-CoV-2 lineages in the genome and S-glycoprotein
The mutational event diversity of SARS-CoV-2 and S-glycoprotein of Omicron and another lineage were analyzed to reveal the number of mutations in specific positions using Nextstrain server [36, 37]. The Nextstrain server, a database used to analyzed viral genomes, fetched the sequence of SARS-CoV-2 from the GISAID database to plot the mutational event diversity.
Mutations in antibody-binding region and 3D model development
We comprehensively evaluated antibody-binding region and its interaction positions using four classes of antibodies (class-1 antibody to class-IV antibody). We developed a 3D model to show the interactions of antibodies with the RBD. Furthermore, we mapped mutations in the RBD of Omicron, which were compared with mutations in the antibody binding positions, and developed 3D model of RBD mutations in or near the antibody-binding region.
Risk analysis of Omicron variant (regarding the affinity between spike proteins and neutralizing antibodies) and significant four antibody-binding mutations (K417, T478, E484, and N501)
The risk level of each virus variant is associated with antibody-binding and neutralization of the variant. Sun et al. developed a tool based on a machine learning model to analyzed the risk [38]. We determined various parameters using the machine learning model, such as the stability of binding with ACE2, binding affinity of antibody, and substitution risk of amino acids in a specific position of S-glycoprotein.
We used VarEPS to analyze critical mutations related to antibody-binding sites and their significant features, such as the affinity between spike protein and neutralizing antibodies and risk analysis of important mutations [38]. We used the same machine learning model risk analysis for four significant antibody-binding mutations (K417, T478, E484, and N501).
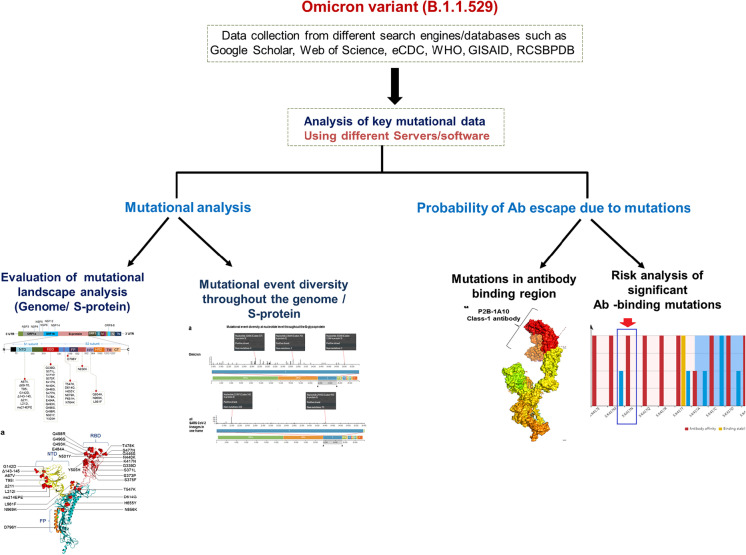
MATLAB was used to draw the pie chart, graphs, and plots [39]. A flowchart of the method is presented in Fig. 1.
Fig. 1 .
Flowchart describes the adopted methodology
Results
Overall understanding the mutational landscape and 3D model development of S-glycoprotein
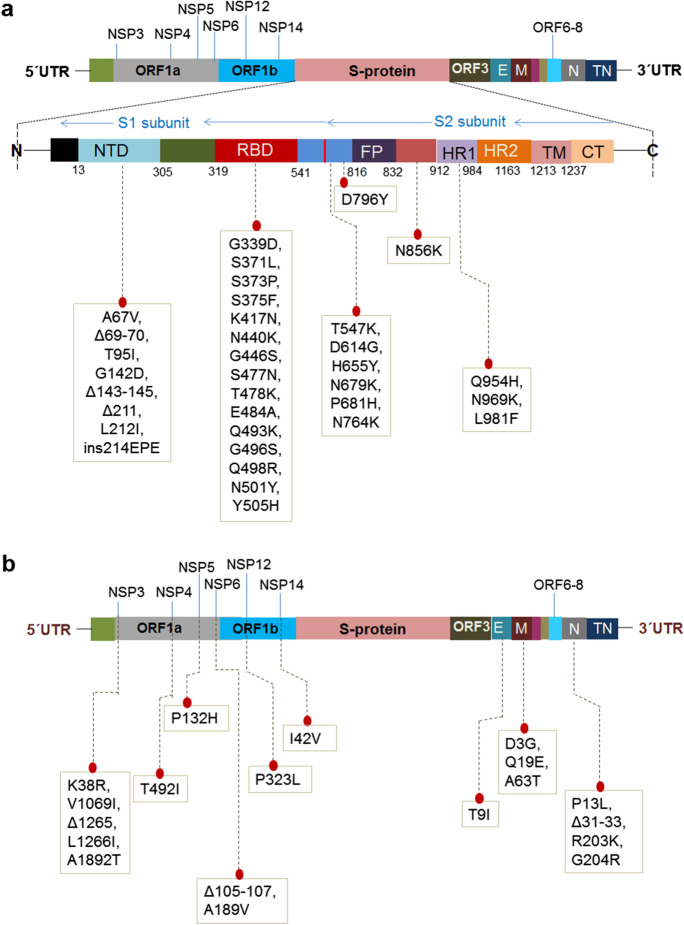
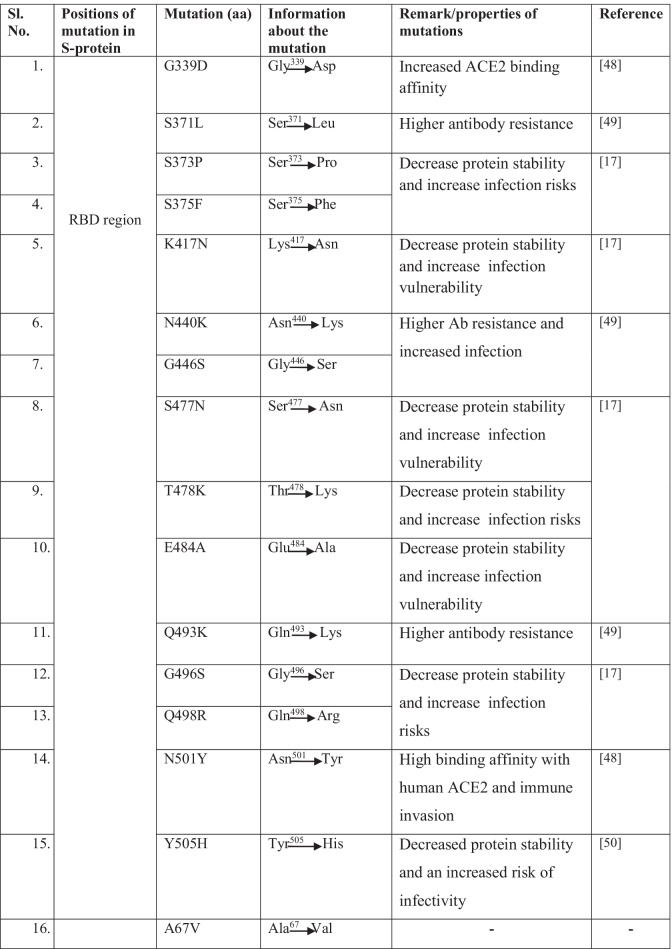
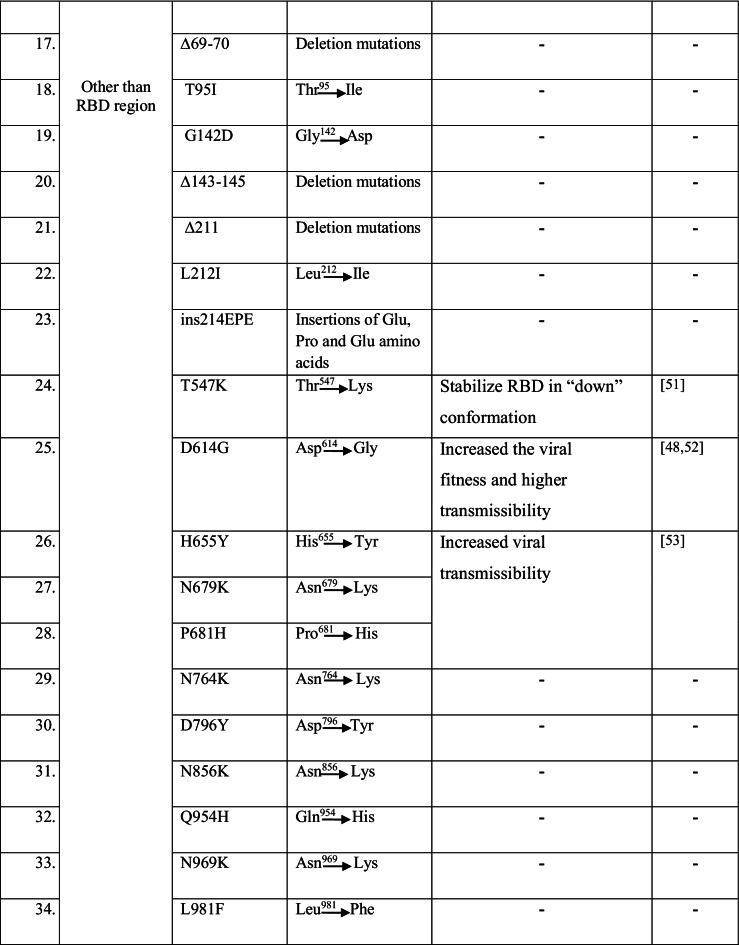
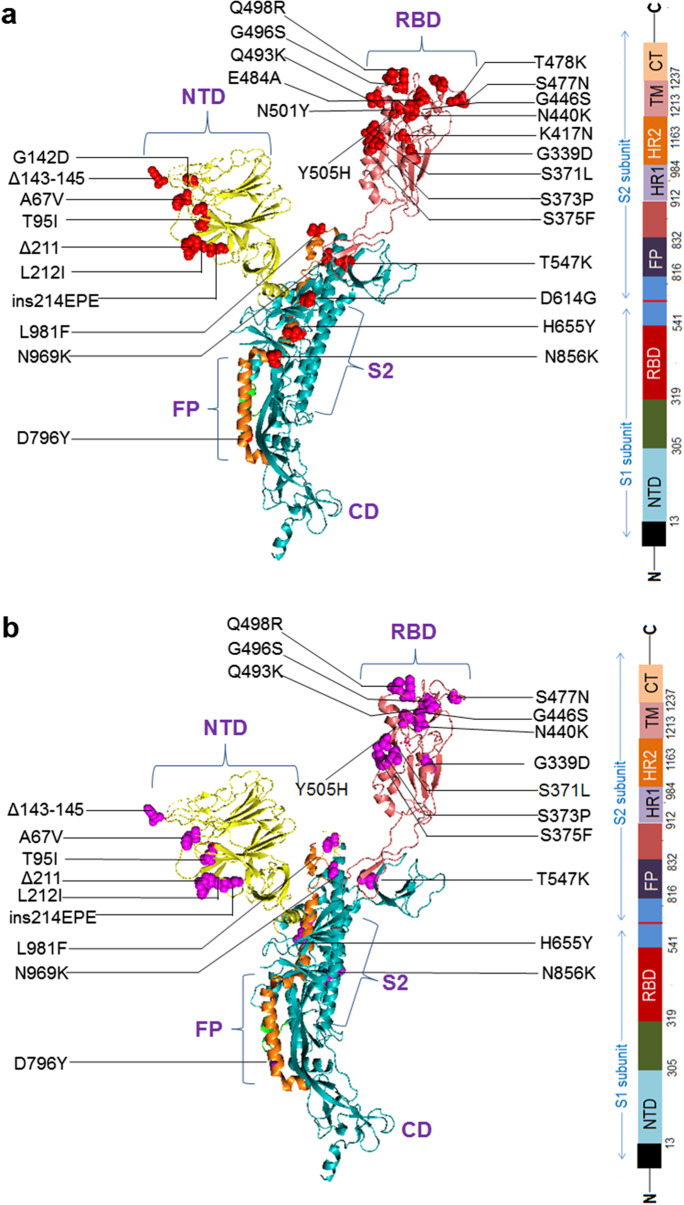
The two schematic diagrams represent the mutational landscape of the Omicron variant. We have drawn a diagram to show different mutations in S-glycoprotein (Fig. 2a). At the same time, analysis of significant mutations in S-glycoprotein revealed the largest number of mutations in the RBD (Fig. S1). Similarly, we have drawn a schematic diagram for all significant mutations in non-structural and structural proteins other than S-glycoprotein (Fig. 2b). Analysis of non-structural proteins showed that NSP3 contains the largest number of significant mutations (Fig. S2). Similarly, the analysis of structural proteins showed that S-glycoprotein has the largest number of significant mutations (Fig. S3). The different mutations in S-protein and their important properties such as antibody resistance, higher binding affinity, transmutability, and higher infectivity from different published scientific articles are listed in Table 1.
Fig. 2 .
A schematic diagram that illustrates the mutational landscape of the Omicron variant. a Different mutations in several regions in the S-glycoprotein. The figure also illustrates the deletions and insertion along with the point mutations. b All significant mutations of the non-structural and structural proteins other than the S-glycoprotein. The figure also illustrates the deletions in the NSP3, NSP6, and N proteins
Table 1.
Significant mutations and their properties in S-protein of Omicron variant
We developed a 3D model of S-glycoprotein from Omicron covering all mutations in the protein (Fig. 3a). We identified several overlapping mutations in Omicron, and VOCs and VOIs such as Delta, Gamma, Alpha, and Beta. We detected several overlapping significant mutations with characteristic features that are similar to those in VOCs or VOIs (Table 2). Mutations previously reported as important in different variants were N501Y, D614G, H655Y, N679K, and P681H. However, we observed several novel mutations in different parts of S-glycoprotein (Fig. 3b). Significant mutations in the RBD include Q493K, G496S, Q498R, S477N, G466S, N440K, and Y505H, whereas those in the NTD included Δ143-145, A67V, T95I, L212I, and Δ211. Similarly, D796Y was identified as a new mutation in fusion protein (FP).
Fig. 3 .
The diagram illustrates all significant mutations in the different parts (3D model) of an S-glycoprotein. a Significant mutations in the different parts (3D model) of an S-glycoprotein. b All new mutations in the different parts of the 3D model of an S-glycoprotein. These mutations are not found in the other VOCs and VOIs. The new mutation noted the deletions (Δ143-145, Δ211) and insertion (ins214EPE) and the different point mutations in the NTD region
Table 2.
Different overlapping significant mutations that are present in other VOCs and noted in Omicron. Important characteristic features of these mutations are also illustrated
| Sl. No | Known mutations for other SARS-CoV-2 variants which present in Omicron | Characteristics of mutations | Reference |
|---|---|---|---|
| 1 | N501Y |
Augmenting the binding capability of S-protein with the ACE2 receptor also increased viral transmission The mutations N501Y and Q498R combination significantly increased the ACE2 binding affinity |
[48, 49] |
| 2 | D614G |
High infectivity rate with greater binding affinity of S-protein to the ACE2 receptor Mutation added viral fitness to augment viral replication and transmission |
[50, 51] |
| 3 | H655Y | Mutation in the proximal part of the furin cleavage site could increase the spike cleavage and accelerate the transmission | [52] |
| 4 | N679K | Boost up the spike cleavage pattern and significantly increase the viral transmission | [53] |
| 5 | P681H |
Mutation results in a conformational change to enhance the binding affinity of furin to S-protein and support entry of virus within host cells, also increasing the infectivity Such mutation (P681H) found in Alpha variant, in Delta, is shown as alternate mutation (P681R) |
[54] |
Mutational event diversity throughout the genome of Omicron and all SARS-CoV-2 lineages
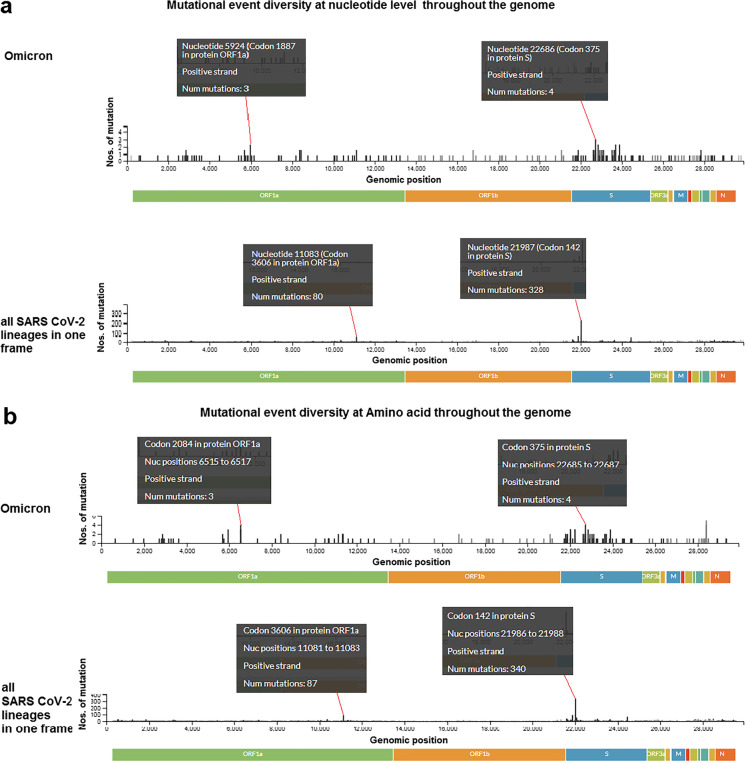
We analyzed mutational event diversity in the genome at both the nucleotide and amino acid levels. First, we depicted the mutational event diversity in the genome at the nucleotide level for Omicron and all SARS-CoV-2 lineages (Fig. 4a), revealing several mutations in the Omicron genome. Our detected three mutations at nucleotide 5924 (codon position 1887 at the protein level) in ORF1a and four mutations nucleotide position 22,686 (codon position 375 at the protein level) in S-glycoprotein in Omicron. Similar results were observed throughout the genome.
Fig. 4 .
Mutational event diversity throughout the genome of Omicron and all SARS-CoV-2 lineages in a single plot. Mutational event diversity describes the number of mutations or the occurrence of mutation in a particular genomic position. a Mutational event diversity at nucleotide level throughout the genome of Omicron and all SARS-CoV-2 lineages in a single plot. Some mutational event or occurrence was pointed out in the particular position of the genome. The first black box noted a mutational event in the 5924 nucleotide position in the genome. The noted mutational number is 3 in the nucleotide position of 5924 in the genome. On the other hand, the mutational number is 80 in the nucleotide position of 11,083 in the genome. b Mutational event diversity at amino acid level throughout the genome of Omicron and all SARS-CoV-2 lineages in a single plot. The first black box noted a mutational event in 2084 in codon position in the ORF1a. The noted mutational number is 3 in 2084 in the codon position. On the other hand, the mutational number is 87 in the codon position of 3606 in the ORF1a for all SARS-CoV-2 lineages in a single plot. We have used the Nextstrain server [36] for the analysis
Together, analysis of the mutational event diversity in all SARS-CoV-2 lineages showed the largest number of mutations in some parts of the genome at the nucleotide level. We observed 80 mutations at nucleotide position 11,083 (codon position 3606 at the protein level) in ORF1a and 328 mutations in S-glycoprotein at nucleotide position 21,987 (codon position 142 at the protein level).
We analyzed the mutational event diversity at the amino acid level in Omicron and all SARS-CoV-2 (Fig. 4b) and found mutational events in several positions in the genome of Omicron. We found three mutations at codon position 2084 at the protein level in ORF1a and four mutations at codon position 375 in protein, the S-glycoprotein in Omicron.
The mutational event diversity in all SARS-CoV-2 lineages showed the largest number of mutations in few parts at the amino acid level. We observed 87 mutations in codon 3606 in ORF1a and 340 mutations at codon 142 in the S-glycoprotein.
Mutational event diversity in S-glycoprotein of Omicron and all SARS-CoV-2 lineages
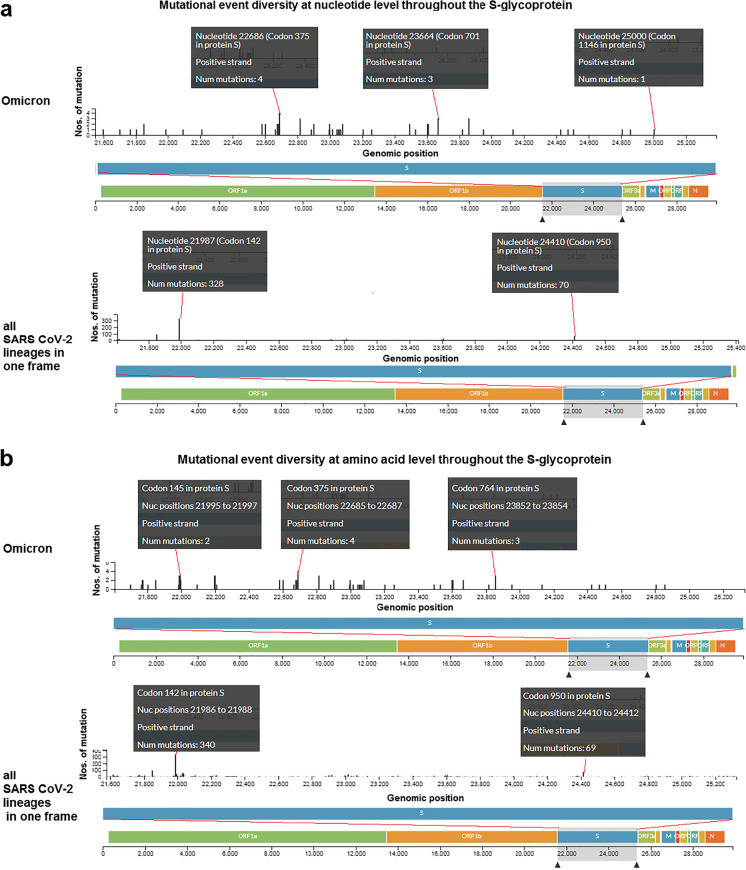
Similar to previous analysis, we observed mutational event diversity in S-glycoprotein at both the nucleotide and amino acid levels. We analyzed this diversity at the nucleotide level in Omicron and all SARS-CoV-2 lineages (Fig. 5a). Diverse mutations were found at the nucleotide level in the S-glycoprotein of Omicron, including four mutations in S-glycoprotein at nucleotide position 22,686 (codon position 375 at the protein level), three mutations at nucleotide position 23,664 (codon position 701 at the protein level) and one mutation at nucleotide position 25,000 (codon position 1146 at the protein level).
Fig. 5 .
Mutational event diversity throughout the S-glycoprotein of Omicron and all SARS-CoV-2 lineages in a single plot. a Mutational event diversity at nucleotide level throughout the S-glycoprotein of Omicron and all SARS-CoV-2 lineages in a single plot. The first black box noted a mutational event in the 22,686 nucleotide position in the S-glycoprotein. The noted mutational number is 4 in the nucleotide position of 22,686 in the S-glycoprotein. On the other hand, the mutational number is 328 in the nucleotide position of 21,987 in the S-glycoprotein. b Mutational event diversity at amino acid level throughout the S-glycoprotein of Omicron and all SARS-CoV-2 lineages in a single plot. The first black box noted a mutational event in the 145 codon position in the S-glycoprotein. The noted mutational number is 2 in the 145 in codon position. On the other hand, the mutational number is 340 in the codon position of 142 in the S-glycoprotein for all SARS-CoV-2 lineages in in a single plot. We have used the Nextstrain server [36] for the analysis
Analysis of mutational event diversity in all SARS-CoV-2 lineages revealed more mutations in some regions than in other regions of S-glycoprotein at the nucleotide level. We observed 328 mutations at nucleotide position 21,987 (codon position 142 at the protein level) in the S-glycoprotein and observed 70 mutations at 24,410 nucleotide position (codon position 910 at the protein level).
Similarly, we analyzed the mutational event diversity of S-glycoprotein at the amino acid level in Omicron and all SARS-CoV-2 lineages (Fig. 5b). We detected mutational events in several positions throughout S-glycoprotein in Omicron with two mutations at codon 145 four mutations at codon 375 and three mutations at codon position of 764 at the protein level, of S-glycoprotein in Omicron.
Analysis of the mutational event diversity in all SARS-CoV-2 lineages revealed a large number of mutations in some regions at the amino acid level in the S-glycoprotein. We identified 340 mutations at codon position 142 and 69 mutations at codon position 950 in S-glycoprotein.
Mutations in antibody-binding region and 3D model development of S-glycoprotein
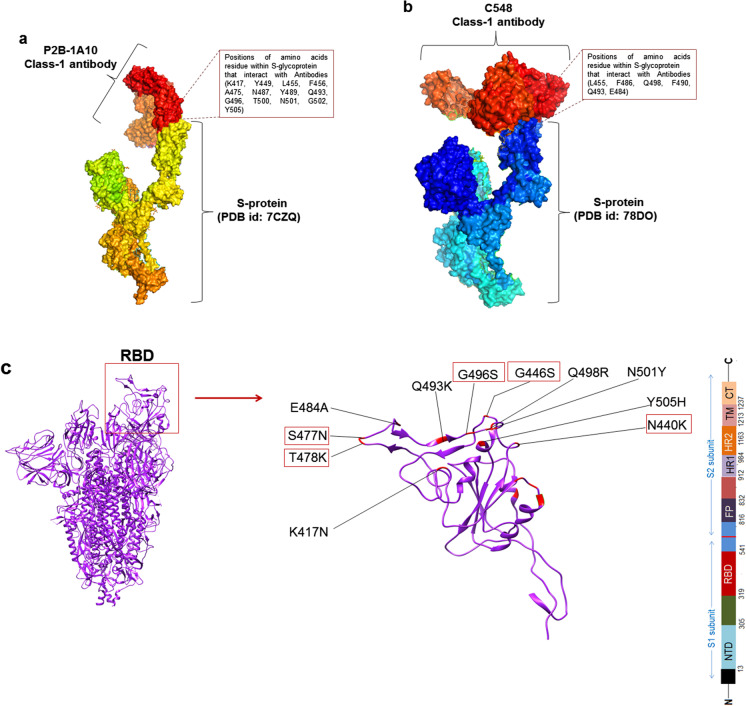
We found mutations in the antibody-binding region of the Omicron variant. We used four classes of antibodies (Class-1 antibody to Class-IV antibody) to examine the amino acid residue positions involved in S-glycoprotein binding to the antibodies. Here, we show the results for two Class-1 antibody-binding regions from two PDB entries (PDB ID: 7CZQ and 78DO). In the first interaction, P2B-1A10 nAb (Class-1 antibody) interacted with the RBD of S-glycoprotein (Fig. 6a) through interacting residues K417, Y449, L455, F456, A475, N487, N489, Q493, G496, T500, N501, G502, and Y505. In the second interaction, we found that C548 nAb (Class-1 antibody) interacted with the S-glycoprotein RBD region (Fig. 6b) at L455, F486, Q498, F490, Q493, and E484. All interacting residues were compared with mutated residues in S-glycoprotein in Omicron. We found several RBD mutations in or near the antibody-binding region (Fig. 6c). K417N, E484A, Q493K, Q498R, N501Y, and Y505H were in the antibody-binding region, and S477N, T478K, G496S, G446S, and N440K were near the antibody-binding region.
Fig. 6 .
Mutations in antibody-binding region in the S-glycoprotein of Omicron. a 3D model shows the interaction between P2B-1A10 nAb (Class-1 antibody) and the RBD region of the S-glycoprotein. b 3D model shows the interaction between C548 nAb (Class-1 antibody) and the S-glycoprotein’s RBD region. c 3D model shows RBD mutations located in the binding region or near the antibody-binding region
Omicron variant: its risk mutations regarding the affinity between spike proteins and neutralizing antibodies
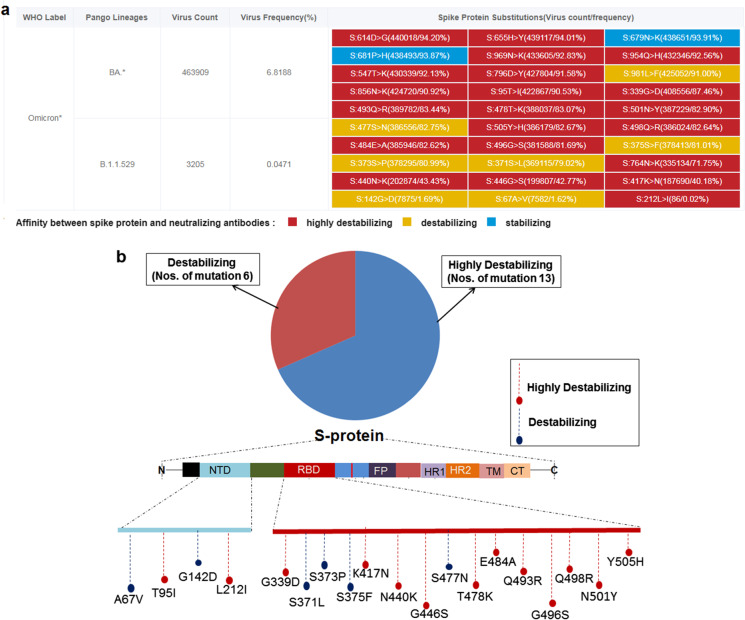
We analyzed the risk of mutation in S-glycoprotein in the Omicron variant and categorized the mutations affecting the affinity between spike protein and neutralizing antibodies. We identified three categories of mutations in S-glycoprotein, highly destabilizing, destabilizing, and stabilizing. We detected 19 mutations in the RBD and NTD regions of S-glycoprotein of B.1.1.529 that can interact with neutralizing antibodies. These mutations were categorized as highly destabilizing, destabilizing and stabilizing (Fig. 7a). Among the 19 mutations (RBD and NTD), 13 were highly destabilizing, and six mutations were destabilizing (Fig. 7b).
Fig. 7 .
The mutational risk of the S-glycoprotein of B.1.1.529 that can interact with neutralizing antibodies and categorizations of the mutations. a Mutational risk of the S-glycoprotein of B.1.1.529 that can interact with neutralizing antibodies, and ranking of the mutations. With reference to interaction, the mutational risk has been illustrated in three categories in respect of antibody affinity/interaction, which is highly destabilizing, destabilizing, and stabilizing. b Number of highly destabilizing and destabilizing mutations in the S-glycoprotein of B.1.1.529. We have 19 mutations related to Ab binding (both in NTD and RBD region), 13 are highly destabilizing, and six mutations are destabilizing. We also pointed out the locations of the highly destabilizing, and destabilizing mutation in the schematic diagram of the S-glycoprotein. We have used the VarEPS server for the analysis
Risk analysis of significant four antibody-binding mutations (K417, T478, E484, and N501)
The risk level of mutation analysis in four positions of the RBD regions (K417, T478, E484, and N501) showed four residues that were significant for the interaction with the antibody.
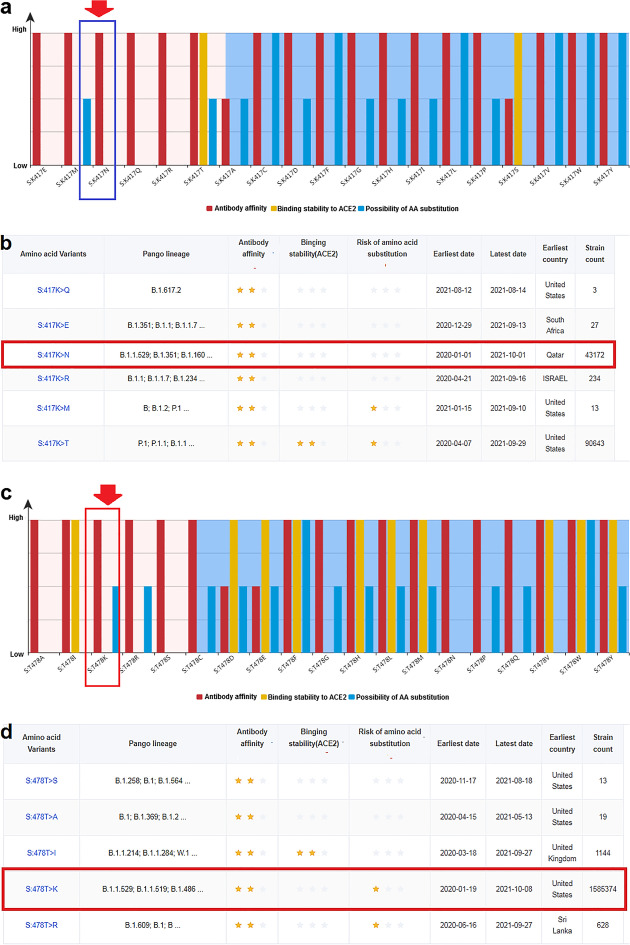
Mutation in amino acid residue K417
Risk analysis of amino acid mutation at the significant K417 position revealed the risk level of 19 types of mutations in different variants (Fig. 8a). The K417N mutation destabilized the S-glycoprotein. However, some variants contained a mutation at residue K417 (Fig. 8b). At the same time, the strain count for K417N mutation was 43,172; this mutation was first reported in Qatar.
Fig. 8 .
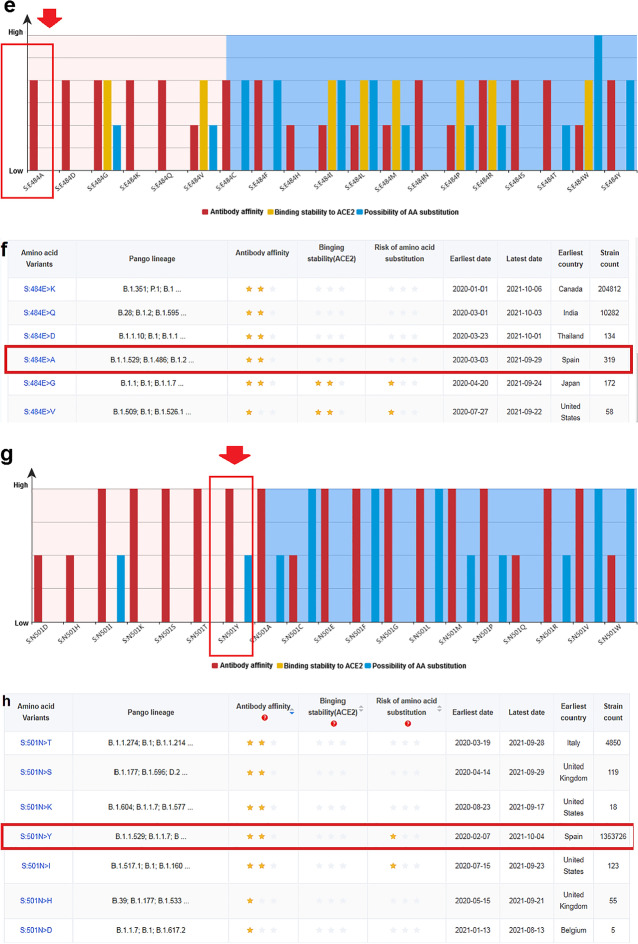
Risk analysis of significant four mutations in antibody-binding site (K417, T478, E484, and N501). a Risk level of different 19 types of mutations in different variants in antibody-binding site K417. b Different amino acid variants of K417, their antibody-binding, their binding stability with ACE2, and risk of amino acid substitution. c The risk level of 19 types of mutations in different variants in antibody-binding site T478. d Different amino acid variants of T478, their antibody-binding, their binding stability with ACE2, and risk of amino acid substitution. e The risk level of 19 types of mutations in different variants in antibody-binding site E484. f Different amino acid variants of E484, their antibody-binding, their binding stability with ACE2, and risk of amino acid substitution. g The risk level of 19 types of mutations in different variants in antibody-binding site N501. h Different amino acid variants of N501, their antibody-binding, their binding stability with ACE2, and risk of amino acid substitution. In the figure, red arrows in a, c, e, and g show the K417N, T478K, E484A, and N501Y mutations found in the Omicron variant. Other amino acid changes are also noted in other SARS-CoV-2 variants in those figures. Stars in b, d, f, and h illustrates risk level of antibody-binding, binding stability with ACE2, and risk of amino acid substitution. Red box in b, d, f, and h represents the county of origin of the K417N, T478K, E484A, and N501Y mutations
Mutation in amino acid residue T478
Similarly, amino acid mutation risk analysis for the T478 position was performed 19 types of mutation risks were found in 19 types of variants (Fig. 8c). The T478K mutation destabilized the interaction between the antibody and S-glycoprotein. However, some variants with the T478 residue were observed (Fig. 8d). The strain count for the T478K mutation was observed as 1,585,374, and this variant (K417N) was first reported in the USA.
Mutation in the amino acid residue E484
We analyzed the risk of amino acid mutation at position E484, and the risk level was determined for different 19 types of mutations in different 19 variants (Fig. 8e). The E484A mutation destabilized binding between the antibody and S-glycoprotein RBD. Moreover, some variants showed mutation at residue E484 (Fig. 8f). The strain count for the E484A was 319, and this variant was first reported in Spain.
Mutation in amino acid residue N501
Risk analysis of mutation at amino acid position N501 revealed the risk level of 19 types of mutations in 19 variants (Fig. 8g). The N501 mutation also destabilized the binding between the antibody and S-glycoprotein' RBD. Moreover, some variants with the mutation at residue N501 were detected (Fig. 8h). The strain count for the N501Y mutation was 1,353,726, and this variant was first recorded in Spain.
Discussion
The Omicron variant is highly concerned globally because of its large number of mutations [40, 41]. Several overlapping mutations have been observed in previous VOCs and VOIs. The characteristic features of these mutations are known and have also been observed in the Omicron variant. The significant mutations and features are N501Y (augments the binding between of S-protein and ACE2); D614G (increase infectivity); H655Y (accelerate transmission); N679K (increase viral transmission); and P681H (enhance binding affinity of S-protein) (Table 2). The variant acquired some new mutations and the characteristic features of these new mutations are unknown. Lupala et al. found that the RBD region of Omicron can bind to the hACE2 receptor more strongly through increased hydrogen bonding interface compare to other variants [42].
In addition to point mutations, deletions and insertion are clustered in the NTD region of the S1 subunit. Deletions were also found randomly in the NSP3, NSP6, and N genes. The biological consequences of deletions and insertions in the Omicron variant are unclear. However, ΔH69/V70 has been observed in different lineages, including in the B.1.1.7 lineage (Alpha variant) and is associated with cleaved spike integration, which increases the infectivity of the Alpha variant. The rapid syncytium formation of B.1.1.7 variant S-glycoprotein requires the deletion of H69/V70; however, this deletion does not affect antibodies escape [43]. Additionally, insertions associated with the NTD and these insertions are linked with the immune or antibody escape of different variants. For example, insertion of an 11-residue fragment in between Y248 and L249 in the N5 loop of the NTD is associated with the prevention of neutralization [44]. However, in Omicron deletions and insertions in the NTD may result in conformational changes that potentially affect the RBD. Therefore, additional studies are needed to evaluate deletions and insertions in different genome positions, including the S gene of the Omicron variant.
We examined the total mutational landscape throughout the genome, particularly in S-glycoprotein of the Omicron variant, and the critical mutations in antibody-binding regions can hinder nAb binding and enhance the potentiality of nAb/mAb escape or resistance.
To understand the mutational landscape, we analyzed the mutation event diversity in the genome of Omicron and all SARS-CoV-2 lineages. We also analyzed the mutational event diversity to understand the mutation pattern throughout the S-glycoprotein of Omicron and all SARS-CoV-2 lineages. This analysis improves the understanding of different mutations in the antibody-binding regions. Several mutations in antibody-binding regions were detected, and bioinformatics analysis showed that most of the mutations were highly destabilizing or destabilizing. The destabilized interface may facilitate in nAb/mAb escape or resistance.
Mutations in the antibody-binding region of the Omicron variant and its antibody escape ability have been evaluated previously. For example, Kannan et al. observed several significant mutations in the antibody-binding regions [45]. Coa et al. illustrated an nAb escape event by the Omicron variant, with the K417N, G446S, E484A, Q493K/R, and N440K mutations, involved in antibody escape [46]. Some studies showed that a booster vaccine dose may increase the potency of Omicron neutralization. A survey by Lippi et al. suggested that the booster vaccine dose effectively neutralize Omicron, providing continuous augmentation of neutralizing potency against the variant [47]. Our study also revealed all the mutations in the antibody-binding region of the Omicron variant and antibody escape event by the variant.
Conclusion
We analyzed the entire mutational landscape of the genome and S-glycoprotein of the Omicron variant. We also identified the significant mutations in the antibody-binding region and antibody escape event by the newly emerging Omicron variant. Our study will help future researchers to develop methods for restoration of neutralizing antibodies against the variant and design antibody-based therapeutics against Omicron variant, as per new mutations. At the same time, these results provide a foundation for further studies of neutralizing antibody activity against all current circulating variants.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ACE2
Angiotensin-converting enzyme 2
- VOC
Variant of concern
- VOI
Variant of interest
- RBD
Receptor-binding domain
- NTD
N-terminal domain
- GISAID
Global initiative on sharing all influenza data
- PDB
Protein Data Bank
- MATLAB
MATrix LABoratory
- ORF
Open reading frame
- S-protein
Spike protein
- nAb
Neutralizing antibody
Author contribution
The study design, data collection, analysis, and drafting the manuscript by Chiranjib Chakraborty; formal analysis and figure developed by Manojit Bhattacharya; editing and revising of manuscript by Ashish Ranjan Sharma; editing and revising of manuscript performed by Kuldeep Dhama; manuscript editing and revising of manuscript content performed by Govindasamy Agoramoorthy. All authors approving the final version of manuscript.
Data availability
The data that supports the finding of the study is included within the manuscript.
Declarations
Ethics approval
Not required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manojit Bhattacharya and Chiranjib Chakraborty contributed equally.
References
- 1.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferré VM, Peiffer-Smadja N, Visseaux B, Descamps D, Ghosn J, Charpentier C. Omicron SARS-CoV-2 variant: what we know and what we don’t. Anaesth Crit Care Pain Med. 2022;41(1):100998. doi: 10.1016/j.accpm.2021.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021;600(7887):21. doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- 4.COVID C, Team R. SARS-CoV-2 B. 1.1. 529 (Omicron) Variant—United States, December 1–8, 2021. MMWR Morbidity and mortality weekly report. 2021;70(50):1731–4. [DOI] [PMC free article] [PubMed]
- 5.Brandal LT, MacDonald E, Veneti L, Ravlo T, Lange H, Naseer U, et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Eurosurveillance. 2021;26(50):2101147. doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espenhain L, Funk T, Overvad M, Edslev SM, Fonager J, Ingham AC, et al. Epidemiological characterisation of the first 785 SARS-CoV-2 Omicron variant cases in Denmark, December 2021. Eurosurveillance. 2021;26(50):2101146. doi: 10.2807/1560-7917.ES.2021.26.50.2101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur V, Kanta Ratho R. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. Journal of Medical Virology. 2020. 10.1002/jmv.27541. [DOI] [PubMed]
- 8.Rio C, Omer S, Malani P. Winter of Omicron—the evolving COVID-19 pandemic. JAMA. 2021. 10.1001/jama.2021.24315 [DOI] [PubMed]
- 9.Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee S-S. A paradigm shift in the combination changes of SARS-CoV-2 variants and increased spread of delta variant (B.1.617.2) across the world. Aging and disease. 2021: doi:10.14336/ad.2021.1117. [DOI] [PMC free article] [PubMed]
- 10.Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant omicron in South Africa. Journal of medical virology. 2021: 10.1002/jmv.27516 [DOI] [PubMed]
- 11.Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397(10278):952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee S-S. The current second wave and COVID-19 vaccination status in India. Brain Behav Immun. 2021;96:1–4. doi: 10.1016/j.bbi.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cusinato M, Gates J, Jajbhay D, Planche T, Ong Y-E. Increased risk of death in covid-19 hospital admissions during the second wave as compared to the first epidemic wave. A prospective dynamic cohort study in South London, UK. medRxiv. 2021. 10.1007/s15010-021-01719-1 [DOI] [PMC free article] [PubMed]
- 14.Mahase E. Covid-19: Hospital admission 50–70% less likely with omicron than delta, but transmission a major concern. BMJ. 2021;375:n3151. doi: 10.1136/bmj.n3151. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Masaki F. Preparation for the challenge of heavily mutated Omicron variant. Clinical and Translational Medicine. 2021;11(12). [DOI] [PMC free article] [PubMed]
- 16.Zhang L, Li Q, Liang Z, Li T, Liu S, Cui Q, et al. The significant immune escape of pseudotyped SARS-CoV-2 Variant Omicron. Emerg Microbes & Infect. 2022;11:1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. Journal of Medical Virology.. 10.1002/jmv.27526. [DOI] [PubMed]
- 18.Miller NL, Clark T, Raman R, Sasisekharan R. Insights on the mutational landscape of the SARS-CoV-2 Omicron variant. bioRxiv. 2021. 10.1101/2021.12.06.471499 [DOI] [PMC free article] [PubMed]
- 19.Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee S-S. Evolution, mode of transmission, and mutational landscape of newly emerging SARS-CoV-2 variants. MBio. 2021;12(4):e01140–e1221. doi: 10.1128/mBio.01140-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty C, Bhattacharya M, Sharma AR. Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: their significant mutations in S‐glycoprotein, infectivity, re‐infectivity, immune escape and vaccines activity. Reviews in Medical Virology. 2021:e2270.
- 21.Chakraborty C, Bhattacharya M, Sharma AR, Lee S-S, Agoramoorthy G. SARS-CoV-2 Brazil variant in Latin America: more serious research urgently needed on public health and vaccine protection. Annals of Medicine and Surgery. 2021:102428. [DOI] [PMC free article] [PubMed]
- 22.Islam R, Hossain J. Detection of Omicron (B.1.1.529) variant has created panic among the people across the world: what should we do right now? Journal of Medical Virology. 10.1002/jmv.27546. [DOI] [PubMed]
- 23.Chakraborty C, Bhattacharya M, Sharma AR. Emerging mutations in the SARS-CoV-2 variants and their role in antibody escape to small molecule-based therapeutic resistance. Current Opinion in Pharmacology. 2021. [DOI] [PMC free article] [PubMed]
- 24.Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, et al. Reduced neutralisation of SARS-CoV-2 omicron B. 1.1 529 variant by post-immunisation serum. Lancet. 2022;399(10321):234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo X, Chen Y, Ohno-Machado L, Xu H. How do we share data in COVID-19 research? A systematic review of COVID-19 datasets in PubMed Central Articles. Brief Bioinform. 2021;22(2):800–811. doi: 10.1093/bib/bbaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan J, Oo S, Chor CYT, Yim D, Chan JSK, Harky A. COVID-19 and literature evidence: should we publish anything and everything? Acta Bio Medica: Atenei Parmensis. 2020;91(3):e2020020. doi: 10.23750/abm.v91i3.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoma B, Chan TM. Using Google Scholar to track the scholarly output of research groups. Perspect Med Educ. 2019;8(3):201–205. doi: 10.1007/s40037-019-0515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farooq RK, Rehman SU, Ashiq M, Siddique N, Ahmad S. Bibliometric analysis of coronavirus disease (COVID-19) literature published in Web of Science 2019–2020. J Fam Community Med. 2021;28(1):1. doi: 10.4103/jfcm.JFCM_332_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Centre for Disease Prevention and Control. Implications of the spread of the SARS-CoV-2 B.1.1.529 variant of concern (Omicron) for the EU/EEA-– first update. ECDC: Stockholm. 2021. (Acessed on 27 December 2021).
- 31.WHO. Update on Omicron. 2021. (Accessed on 27 December 2021). https://www.who.int/news/item/28-11-2021-update-on-omicron
- 32.CDC. Science Brief: Omicron (B.1.1.529) Variant. 2021. (Acessed on 27 December 2021). https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html
- 33.Shu Y, McCauley J. GISAID: Global initiative on sharing all influenza data–from vision to reality. Eurosurveillance. 2017;22(13):30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Global Chall. 2017;1(1):33–46. doi: 10.1002/gch2.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velankar S, Burley SK, Kurisu G, Hoch JC, Markley JL. The Protein Data Bank Archive. Methods Mol Biol (Clifton, NJ) 2021;2305:3–21. doi: 10.1007/978-1-0716-1406-8_1. [DOI] [PubMed] [Google Scholar]
- 36.Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C et al. A global database of COVID-19 vaccinations. Nature human behaviour. 2021:1–7. [DOI] [PubMed]
- 37.Nextstrain. Nextstrain SARS-CoV-2 resources, on Nextstrain. 2021. https://nextstrain.org/sars-cov-2/ (Acessed on 27 December 2021).
- 38.Sun Q, Shu C, Shi W, Luo Y, Fan G, Nie J, et al. VarEPS: an evaluation and prewarning system of known and virtual variations of SARS-CoV-2 genomes. Nucleic Acids Res. 2022;50(D1):D888–D897. doi: 10.1093/nar/gkab921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MathWorks I. MATLAB, High-performance numeric computation and visualization software: user’s guide: for UNIX workstations. MathWorks; 1992. The University of Michigan, United Kingdom (1–548), ASIN : B001VFE5MC..
- 40.Saxena SK, Kumar S, Ansari S, Paweska JT, Maurya VK, Tripathi AK et al. Characterization of the novel SARS‐CoV‐2 Omicron (B. 1.1. 529) variant of concern and its global perspective. Journal of Medical Virology. 10.1002/jmv.27524. [DOI] [PubMed]
- 41.Kazybay B, Ahmad A, Mu C, Mengdesh D, Xie Y. Omicron N501Y mutation among SARS-CoV-2 lineages: In-silico analysis of potent binding to tyrosine kinase and hypothetical repurposed medicine. Travel Medicine and Infectious Disease. 2021:102242. [DOI] [PMC free article] [PubMed]
- 42.Lupala CS, Ye Y, Chen H, Su X-D, Liu H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem Biophys Res Commun. 2021. 10.1016/j.bbrc.2021.12.079 [DOI] [PMC free article] [PubMed]
- 43.Meng B, Kemp SA, Papa G, Datir R, Ferreira IA, Marelli S, Harvey WT, Lytras S, Mohamed A, Gallo G, Thakur N. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B. 1.1. 7. Cell reports. 2021;35(13):109292. doi: 10.1016/j.celrep.2021.109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kannan SR, Spratt AN, Sharma K, Chand HS, Byrareddy SN, Singh K. Omicron SARS-CoV-2 variant: unique features and their impact on pre-existing antibodies. J Autoimmun. 2022;126:102779. doi: 10.1016/j.jaut.2021.102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao YR, Wang J, Jian F, Xiao T, Song W, Yisimayi A et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. RESEARCH BRIEFINGS, Nature. 2021. 10.1038/d41586-021-03796-6. [DOI] [PMC free article] [PubMed]
- 47.Lippi G, Mattiuzzi C, Henry BM. Neutralizing potency of COVID-19 vaccines against the SARS-CoV-2 Omicron (B.1.1.529) variant. Jour of Medical Virology. 2022; 10.1002/jmv.27575. [DOI] [PMC free article] [PubMed]
- 48.Quarleri J, Galvan V, Delpino M. Omicron variant of the SARS-CoV-2: a quest to define the consequences of its high mutational load. GeroScience, 2021. 10.1007/s11357-021-00500-4 [DOI] [PMC free article] [PubMed]
- 49.Ali F, Kasry A, Amin M. The new SARS-CoV-2 strain shows a stronger binding affinity to ACE2 due to N501Y mutant. Med Drug Discov. 2021;10:100086. doi: 10.1016/j.medidd.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakraborty C, Saha A, Sharma AR, Bhattacharya M, Lee S-S, Agoramoorthy G. D614G mutation eventuates in all VOI and VOC in SARS-CoV-2: is it part of the positive selection pioneered by Darwin? : Molecular Therapy - Nucleic Acids, 2021: 26:237–241. [DOI] [PMC free article] [PubMed]
- 51.Bhattacharya M, Chatterjee S, Sharma AR, Agoramoorthy G, Chakraborty C. D614G mutation and SARS-CoV-2: impact on S-protein structure, function, infectivity, and immunity. Applied microbiology and biotechnology. 2021:1–11. 10.1007/s00253-021-11676-2. [DOI] [PMC free article] [PubMed]
- 52.Gupta D, Sharma P, Singh M, Kumar M, Ethayathulla A, Kaur P. Structural and functional insights into the spike protein mutations of emerging SARS-CoV-2 variants. Cell Mol Life Sci. 2021;78(24):7967–7989. doi: 10.1007/s00018-021-04008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naveca F, Nascimento V, Souza V, Corado A, Nascimento F, Silva G et al. Emergence and spread of SARS-CoV-2 P. 1 (Gamma) lineage variants carrying Spike mutations image. 2021;420(784):75.7.
- 54.Qin S, Cui M, Sun S, Zhou J, Du Z, Cui Y et al. Genome characterization and potential risk assessment of the novel SARS-CoV-2 variant Omicron (B. 1.1. 529). Zoonoses. 2021. 10.15212/ZOONOSES-2021-0024
- 55.Zhang X, Wu S, Wu B, Yang Q, Chen A, Li Y, Zhang Y, Pan T, Zhang H, He X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct Target Ther. 2021;6:430. doi: 10.1038/s41392-021-00852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L, Iketani S, Guo Y, Chan JWF, Wang M, Liu L, Luo Y, Chu H, Huang Y, Nair MS, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2021. 10.1038/s41586-021-04388-0 [DOI] [PubMed]
- 57.Poudel S, Ishak A, Perez-Fernandez J, Garcia E, León-Figueroa DA, Romaní L, Bonilla-Aldana DK, Rodriguez-Morales AJ. Highly mutated SARS-CoV-2 Omicron variant sparks significant concern among global experts–what is known so far? Travel Med Infect Dis. 2022;45:102234. doi: 10.1016/j.tmaid.2021.102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng C, Evans JP, Qu P, Faraone J, Zheng YM, Carlin C, Bednash JS, Zhou T, Lozanski G, Mallampalli R, et al. Neutralization and stability of SARS-CoV-2 Omicron variant. bioRxiv: the preprint server for biology. 2021. 10.1101/2021.12.16.472934.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the finding of the study is included within the manuscript.