Abstract
Objective
Continuous glucose monitoring (CGM) has demonstrated benefits in managing inpatient diabetes. We initiated this single-arm pilot feasibility study during the COVID-19 pandemic in 11 patients with diabetes to determine the feasibility and accuracy of real-time CGM in patients who underwent cardiac surgery and whose care was being transitioned from the intensive care unit.
Methods
A Clarke error grid analysis was used to compare CGM and point-of-care measurements. The mean absolute relative difference (MARD) of the paired measurements was calculated to assess the accuracy of CGM for glucose measurements during the first 24 hours on CGM, the remaining time on CGM, and for different chronic kidney disease (CKD) strata.
Results
Overall MARD between point-of-care and CGM measurements was 14.80%. MARD for patients without CKD IV and V with an estimated glomerular filtration rate (eGFR) of ≥20 mL/min/1.73 m2 was 12.13%. Overall, 97% of the CGM values were within the no-risk zone of the Clarke error grid analysis. For the first 24 hours, a sensitivity analysis of the overall MARD for all patients and those with an eGFR of ≥20 mL/min/1.73 m2 was 15.42% ± 14.44% and 12.80% ± 7.85%, respectively. Beyond the first 24 hours, overall MARD for all patients and those with an eGFR of ≥20 mL/min/1.73 m2 was 14.54% ± 13.21% and 11.86% ± 7.64%, respectively.
Conclusion
CGM has shown great promise in optimizing inpatient diabetes management in the noncritical care setting and after the transition of care from the intensive care unit with high clinical reliability and accuracy. More studies are needed to further assess CGM in patients with advanced CKD.
Key words: cardiac surgery, chronic kidney disease (CKD), Clark error grid (CEG) analyses, continuous glucose monitoring (CGM), mean absolute relative difference (MARD), noncritically ill
Abbreviations: ARD, absolute relative difference; CABG, coronary artery bypass grafting; CEG, Clarke error grid; CGM, continuous glucose monitoring; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; ICU, intensive care unit; MARD, mean absolute relative difference; POC, point-of-care; TIR, time in range
Introduction
The use of continuous glucose monitoring (CGM) in hospitalized patients has demonstrated benefits over the traditional point-of-care (POC) capillary blood glucose testing in the prevention of both severe hypoglycemia and hyperglycemia and in reducing the burden of care associated with POC blood glucose monitoring on the nursing staff.1, 2, 3, 4 The U.S. Food and Drug Administration’s decision to allow the use of CGM in hospitalized patients to support health care efforts during the COVID-19 pandemic has helped initiate several studies investigating the use of CGM in the inpatient setting.5 These studies have demonstrated the feasibility, safety, reliability, and accuracy of CGM in the hospital setting.6, 7, 8, 9 Davis et al10 recently reported on the accuracy of CGM use in the largest clinical study to date on a diverse population of noncritically ill patients with diabetes.
A limited number of studies have assessed the use of CGM in non–intensive care unit (ICU) patients,1 , 6, 7, 8, 9, 10, 11, 12 and none of the studies have focused solely on hospitalized non-ICU patients who have undergone cardiac surgery, have diabetes, and are transferred to the surgery ward from the ICU after undergoing cardiovascular surgery (primarily coronary artery bypass grafting [CABG]). It is well established that patients with diabetes are at a higher risk of coronary artery disease than the general population and that approximately two thirds of these patients have multivessel disease.13 For most patients with diabetes and multivessel disease, CABG has been demonstrated to be the optimal revascularization strategy.14 Approximately 30% to 40% of the patients undergoing CABG have diabetes, and 60% to 90% of these patients have been reported to develop hyperglycemia in the perioperative period.15, 16, 17, 18, 19 Perioperative hyperglycemia in patients with and without diabetes has been associated with complications such as an increased rate of wound infections, acute kidney injury, prolonged hospitalization, and an increase in perioperative mortality compared with those without hyperglycemia. Randomized controlled trials have demonstrated that glycemic control in patients undergoing cardiac surgery is associated with an improvement in clinical outcomes and mortality in the ICU settings,20, 21, 22 although little published data exist on the outcomes after these patients transition to the noncritical care settings. A key study by Krinsley et al23 addressed this important clinical issue and validated the importance of glycemic control across the entire trajectory of the hospitalization to achieve optimal clinical outcomes.
Accordingly, given that CGM has demonstrated success in preventing both hyperglycemia and hypoglycemia, we initiated this prospective pilot feasibility study during the COVID-19 pandemic to determine the feasibility of real-time CGM use within our hospital and assess the accuracy of CGM in high-risk hospitalized noncritically ill patients with diabetes and cardiovascular disease after cardiac surgery.1 To our knowledge, this is the first published report of the use of CGM in a sole population of hospitalized non-ICU patients who have undergone cardiac surgery, have diabetes, and are transferred to the surgery ward from the ICU after undergoing primarily CABG.
Methods
Eligible patients included adults (aged 18-80 years) with diabetes receiving treatment with subcutaneous insulin who were hospitalized for cardiac surgery, primarily CABG, with a planned hospital stay of at least 3 days. We excluded patients who required transfer from the ward for a procedure or were required to be transferred back to the ICU. Patients who required >4 g of acetaminophen in 24 hours were also excluded. The study was approved by our local institutional review board at St. Elizabeth's Medical Center.
In this prospective pilot study, we recruited 11 consecutive patients from our endocrine consultation service who had undergone cardiac surgery, been discharged from the ICU, were transferred to the cardiac transition unit, and were receiving treatment with subcutaneous insulin. The primary study outcomes were to assess the feasibility and accuracy of CGM. A secondary outcome was the percent time in range (TIR), defined as the proportion of glucose levels between 70 mg/dL and 180 mg/dL. After patients were transferred to the cardiac transition unit, a G6 CGM sensor and transmitter (Dexcom, Inc) were placed on the upper portion of the outer part of their arms. A smartphone in the patients’ rooms functioned as a receiver and relayed the glucose concentration estimates and trending information to a tablet at the nurses’ station, thereby creating a glucose telemetry system as described by Spanakis et al.1 The data were also stored in a cloud-based platform to allow remote monitoring via smartphones for study investigators, including nurses, residents, and attending physicians. Patients were also on a standard POC protocol with fingerstick blood glucose levels obtained before meals and at 10 pm. Insulin doses were adjusted daily per the modified RABBIT 2 protocol.24 CGM readings from a maximum of 76 hours of monitoring were used for analysis.
Statistical Analyses
Summary statistics of patients’ baseline characteristics and CGM outcomes over a maximum of 76 hours were described using mean, standard deviation, frequency, and percentage. A Clarke error grid (CEG) analysis25 , 26 was used to compare the matched CGM and POC measurements. The mean absolute relative difference (MARD) and median absolute relative difference (ARD) of the paired measurements were calculated to assess the accuracy of CGM, with mean, median, standard deviation, and the minimum and maximum values reported for both metrics.27 , 28 The MARD was also calculated for glucose measurements during the first 24 hours on CGM and the remaining time on CGM. The MARD values for different CKD strata (CKD stages IV and V [with an estimated glomerular filtration rate {eGFR} of <20 mL/min/1.73 m2] vs without CKD stages IV and V [with an eGFR of ≥20 mL/min/1.73 m2]) were also calculated. All analyses were conducted using R software (R version 4.0.3). The error grid analysis (EGA) package (version 2.0.0) was used for the CEG analysis (https://cran.rproject.org/web/packages/ega/vignettes/ega.html).
Results
Eleven adult patients with type 2 diabetes (8 receiving insulin as outpatients and all receiving subcutaneous insulin during their hospitalization at enrollment) were enrolled. Their baseline characteristics are listed in Table 1 . Their mean age (±SD) was 72.5 (±4.3) years, with a mean body mass index of 30.6 ± 5.2 kg/m2. Three patients were women and 3 were minorities. The median HbA1c level was 7.8% (62 mmol/mol) (interquartile range, 7.4%, 10.3% [57 mmol/mol, 89 mmol/mol]). All patients required basal-bolus insulin therapy before and after surgery. Nine patients (81%) had chronic kidney disease (CKD): stage II (n = 1), stage III (n = 5), stage IV (n = 2), and stage V (n = 1). Outcomes over 3 days of hospitalization are summarized in Table 2 . The mean CGM glucose level was 179 mg/dL and the TIR (percentage TIR of 70-180 mg/dL) was 59.8%. The maximum duration of CGM per patient was 76 hours.
Table 1.
Summary Statistics of Demographic Characteristics and Risk Factors
| Overall (n = 11) | |
|---|---|
| Demographic characteristics | … |
| Age, y, mean (SD) | 72.5 (4.3) |
| Male | 8 (72.7) |
| Minority race/ethnicity | … |
| African American | 1 (9.1) |
| Asian | 1 (9.1) |
| Hispanic | 1 (9.1) |
| Weight, kg, mean (SD) | 84.8 (17.9) |
| BMI, kg/m2, mean (SD) | 30.6 (5.2) |
| HbA1c, median (IQR) | 7.8% (62 mmol/mol) (7.4%, 10.3% [57 mmol/mol, 89 mmol/mol]) |
| DM complications | … |
| Retinopathy (%) | 0 (0.0) |
| Nephropathy eGFR (%) | 9 (81.8) |
| CKD stage (%) | … |
| II | 1 (9.1) |
| IIIA | 2 (18.2) |
| IIIB | 3 (27.3) |
| IV | 2 (18.2) |
| V | 1 (9.1) |
| Neuropathy (%) | 3 (27.3) |
| CAD (%) | 11 (100.0) |
| CVA (%) | 0 (0.0) |
| PVD (%) | 2 (18.2) |
| Risk factors for hypoglycemia | … |
| Age of >67 y (%) | 9 (81.8) |
| BMI of <27 kg/m2 (%) | 4 (36.4) |
| Renal failure: eGFR of <60 mL/min/1.73 m2 | 7 (63.6) |
| Malnutrition (%) | 0 (0.0) |
| History of recent hypoglycemia (6-8 wk) (%) | 0 (0.0) |
| Long DM duration >20 y (%) | 7 (63.6) |
| CHF (%) | 7 (63.6) |
| After cardiac surgery (%) | … |
| CABGX2 | 2 (18.2) |
| CABGX3 | 1 (9.1) |
| CABGX4 | 7 (63.6) |
| MVR/TVR | 1 (9.1) |
Abbreviations: BMI = body mass index; CABG = coronary artery bypass graft; CAD = coronary artery disease; CHF = congestive heart failure; CKD = chronic kidney disease; CVA = cerebrovascular accident; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HbA1c = glycated hemoglobin; IQR = interquartile range; MVR = mitral valve replacement; PVD = peripheral vascular disease; TVR = tricuspid valve replacement.
Table 2.
Summary Statistics of Outcomes Over 3 Days
| Outcomes | Overall (n = 10a) |
|---|---|
| % Time in range 70-180 mg/dL, mean (SD) | 59.8 (22.7) |
| % Time hypoglycemia of <70 mg/dL, mean (SD) | 1.4 (2.0) |
| % Time hypoglycemia of <54 mg/dL, mean (SD) | 0.3 (0.9) |
| % Time hyperglycemia of >180 mg/dL, mean (SD) | 38.8 (22.1) |
| Number of patients with any hypoglycemic excursion glucose level of <70 mg/dL for >20 min (%)b | 1 (10.0) |
| The 3-day rate of hypoglycemic excursion glucose level of <70 mg/dL for >20 min (number of events per person-time) | 1/30 person-days |
| Total daily insulin dose on the final day, units/kg/d, mean (SD) | 0.6 (0.4) |
| Mean basal, units (SD) | 26.0 (16.8) |
| Mean bolus, units (SD) | 21.2 (16.0) |
One patient remained in hospital for <3 days.
There was only 1 patient with 1 incidence of hypoglycemic excursion (glucose level of <70 mg/dL) among 10 patients within the 3-day window.
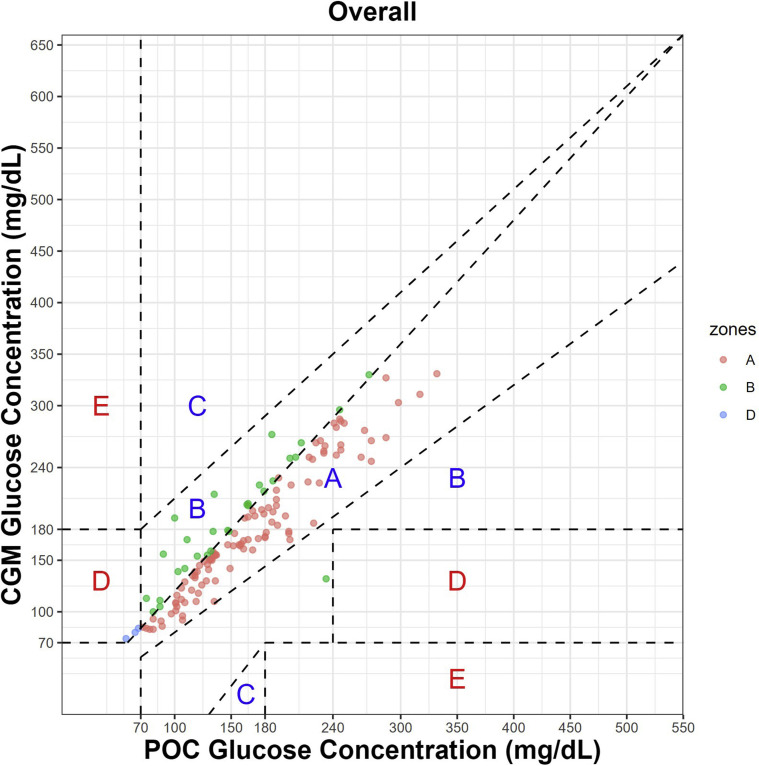
The CEG analysis and MARD are illustrated in Figure 1 . A total of 137 paired POC-CGM measurements were used for analysis. The MARD statistics between CGM and POC blood glucose levels as well as CKD strata are listed in Table 3 . The overall MARD and median ARD between POC and CGM measurements was 14.80% ± 13.53% and 13.20% [interquartile range: 5.22%, 18.52%] respectively. The MARD for the patients with an eGFR of ≥20 mL/min/1.73 m2 was 12.13% ± 7.67% and for those with an eGFR of <20 mL/min/1.73 m2 was 21.27% ± 20.81%. A further sensitivity analysis of the overall MARD for all patients for the first 24 hours and those with an eGFR of ≥20 mL/min/1.73 m2 was 15.42% ± 14.44% and 12.80% ± 7.85%, respectively (Tables 4 and 5 ). Overall MARD for all patients beyond the first 24 hours and MARD for those with an eGFR of ≥20 mL/min/1.73 m2 was 14.54% ± 13.21% and 11.86% ± 7.64%, respectively.
Fig. 1.
| N | A (%) | B (%) | D (%) |
|---|---|---|---|
| 137 | 76.6 | 21.2 | 2.2 |
Table 3.
Mean Absolute Relative Difference Between Point-of-Care and Continuous Glucose Monitoring
| ARD | Overall (n = 137) | eGFR ≥ 20 mL/min/1.73 m2 (n = 97) | eGFR < 20 mL/min/1.73 m2 (n = 40) |
|---|---|---|---|
| Mean (SD) | 14.80 (13.53) | 12.13 (7.67) | 21.27 (20.81) |
| Median (IQR) | 13.20 (5.22, 18.52) | 12.71 (5.35, 17.16) | 16.37 (5.09, 25.08) |
| (Minimum, maximum) | (0, 91.00) | (0, 34.95) | (0, 91.00) |
Abbreviations: ARD = absolute relative difference; eGFR = estimated glomerular filtration rate; IQR = interquartile range.
Mean absolute relative difference was calculated on the basis of 137 matched glucose pairs of 11 patients during their hospital stay; the maximum hospital stay is 76 hours. Mean absolute relative difference has also been shown for patients in different chronic kidney disease strata (those with eGFR of ≥20 mL/min/1.73 m2 and those with eGFR of <20 mL/min/1.73 m2).
Table 4.
Sensitivity Analysis: Mean Absolute Relative Difference for Glucose Measurement Within the First 24 Hours During Hospital Stay and Rest of Hospital Stay
| ARD | Overall (n = 40) | eGFR ≥ 20 mL/min/1.73 m2 (n = 28) | eGFR < 20 mL/min/1.73 m2 (n = 12) |
|---|---|---|---|
| Mean (SD) | 15.42 (14.44) | 12.80 (7.85) | 21.55 (23.02) |
| Median (IQR) | 14.78 (7.38, 20.18) | 12.65 (6.37, 17.63) | 18.32 (12.94, 20.96) |
| (Minimum, maximum) | (0.30, 91.00) | (0.30, 30.28) | (1.03, 91.00) |
Abbreviations: ARD = absolute relative difference; eGFR = estimated glomerular filtration rate; IQR = interquartile range.
Mean absolute relative difference has also been calculated in different chronic kidney disease strata (patients with eGFR of >20 mL/min/1.73 m2 and those with <20 mL/min/1.73 m2).
Table 5.
Sensitivity Analysis: Mean Absolute Relative Difference for Glucose Measurement After the First 24 Hours During Hospital Stay and Rest of Hospital Stay
| ARD | Overall (n = 97) | eGFR ≥ 20 mL/min/1.73 m2 (n = 69) | eGFR < 20 mL/min/1.73 m2 (n = 28) |
|---|---|---|---|
| Mean (SD) | 14.54 (13.21) | 11.86 (7.64) | 21.15 (20.23) |
| Median (IQR) | 13.14 (5.10, 18.06) | 12.71 (5.35, 16.67) | 14.71 (4.41, 29.46) |
| (Minimum, maximum) | (0, 73.33) | (0, 34.95) | (0, 73.33) |
Abbreviations: ARD = absolute relative difference; eGFR = estimated glomerular filtration rate; IQR = interquartile range.
Mean absolute relative difference has also been calculated in different chronic kidney disease strata (patients with eGFR of >20 mL/min/1.73 m2 and those with <20 mL/min/1.73 m2).
The CEG analysis (Fig. 1) of all matched pair data demonstrated good clinical reliability, as we observed that 76.6% of the values were within zone A, in which the POC-CGM values were within 20%; 21.2% were within zone B, in which the POC-CGM values differed by >20% but with no effect on the clinical outcome; and 2.2% were within upper zone D, in which there was undetected hypoglycemia. One study patient had a hypoglycemic excursion glucose level of <70 mg/dL, which was captured on CGM but not on the POC blood glucose level. In this instance, the physician noted the CGM alarm and alerted the assigned nurse.
Discussion
In this small, heterogeneous population of elderly patients with type 2 diabetes and cardiovascular disease, with complications, the Dexcom G6 CGM demonstrated good overall accuracy in patients with eGFR of ≥20 mL/min/1.73 m2 with a MARD of 12.13% ± 7.67% and with 97% of the CGM values within zones A and B of the CEG analysis compared with the standard POC blood glucose levels. We attribute our higher overall MARD of 14.80% (compared with other reported values [range, 9.4%-12.7%])6 , 7 , 25 and median ARD of 13.2% to our small, heterogeneous population and particularly the inclusion of patients with an eGFR of <20 mL/min/1.73 m2. Two patients, 1 with advanced stage IV (eGFR of <20 mL/min/1.73 m2) and 1 with stage V CKD (eGFR of <15 mL/min/1.73 m2; receiving dialysis) had the most discordance between their POC and CGM blood glucose levels, with each having 7 values differing by >20% and, therefore, having a higher MARD, whereas 1 patient with CKD IV and an eGFR of >20 mL/min/1.73 m2 had a MARD of <5%. There are little published data on the MARD in patients with CKD, although a recent study by Davis et al10 that included 900 to 1100 matched pairs across different CKD strata showed comparable accuracy metrics. Because this study did not further substratify the MARD for patients with an eGFR of <30 mL/min/1.73 m2, the number of patients with an eGFR of <20 mL/min/1.73 m2 and those with end-stage renal disease (ESRD) receiving hemodialysis who were included is unclear.10 A recent small study in patients with type 2 diabetes on dialysis found the MARD between CGM and POC capillary blood glucose levels to be significantly higher for hypoglycemia (31.9 ± 25 mg/dL) and euglycemia (22.8 ± 14.6 mg/dL) than hyperglycemia (13 ± 8.5 mg/dL) (P < .001 for both).29 Other studies have also demonstrated a higher MARD in patients with type 2 diabetes receiving dialysis.30 , 31 In addition, some studies assessing CGM accuracy intentionally excluded patients with severely impaired renal function, presumably owing to lower accuracy.32 The patients with advanced CKD IV and V in our study were more prone to hypoglycemia (glucose levels of <70 mg/dL) and significant anemia (mean hemoglobin levels of 8.2 g/dL)—2 circumstances associated with a higher MARD.2 , 3 , 9 , 10 Inclusion of CGM levels from the first 24 hours after CGM placement also contributed to the higher overall MARD because CGM is known to be less accurate during this period.7 , 10 The higher MARD in our study was impacted by our limited sample size of 137 matched pairs to assess CGM accuracy. In addition, although the Dexcom G6 CGM is a factory-calibrated device, it can be calibrated if the CGM and POC blood glucose levels differ by >20%. Future studies that explore clinical scenarios and protocols in which it is beneficial to manually calibrate CGM devices would be helpful.
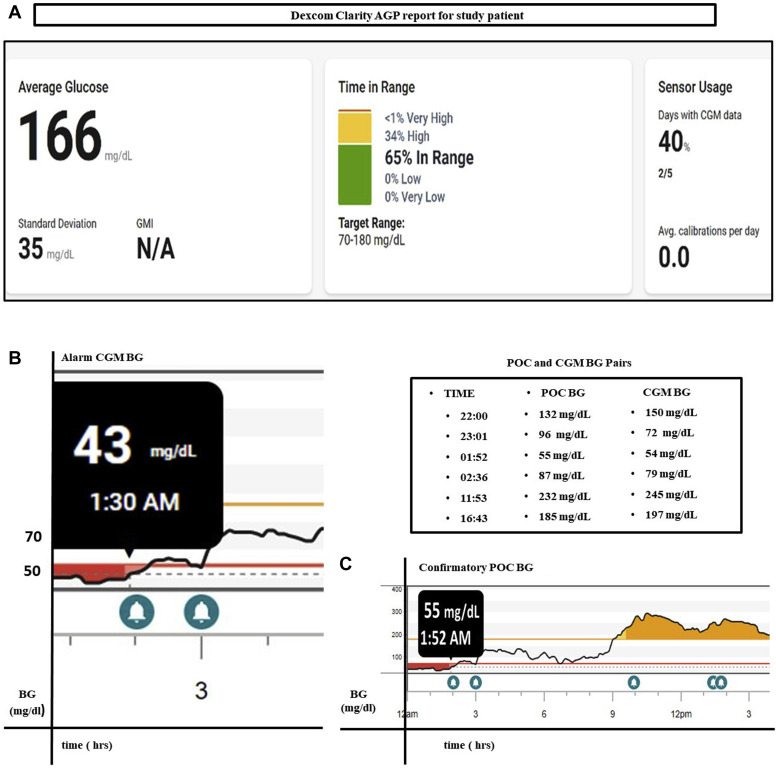
Hypoglycemia in hospitalized patients is associated with an increased risk of morbidity and mortality as well as an increase in the length of stay in the hospital.33 The superior detection of hypoglycemia in hospitalized patients using CGM compared with POC capillary blood glucose levels particularly prolonged nocturnal hypoglycemia, as demonstrated by Galindo et al3 and as we found in one of our patients, highlight the use of CGM as a very helpful comprehensive tool to elucidate glycemic patterns (as shown in Fig. 2 A ambulatory glucose profile [AGP] report) and thereby optimize and safely provide inpatient diabetes care.2, 3, 4 Importantly, studies by Galindo et al3 and others demonstrate that the prevention of hypoglycemia occurs without an increase in the frequency of hyperglycemia. The occurrence of unrecognized hypoglycemia by the nurse assigned to the patient in our study (shown in Figs. 2 B and C) highlights the critical importance of thoroughly educating all nursing staff to successfully and safely implement CGM as outlined in the recent 2020 CGM hospital consensus guidelines.34 The use of trends and alarms has been shown to prevent hypoglycemia, and staff training regarding the recognition of these alarms is essential.2
Fig. 2.
A, Summary data of CGM study patient, illustrating 65% time in range vs 0% hypoglycemia and 34% hyperglycemia. B, CGM data from a study patient, illustrating how overnight hypoglycemia and prolonged postprandial hyperglycemia are captured using CGM but C, are not detected via the standard fingerstick POC blood glucose level protocol. AGP = ambulatory glucose profile; BG = blood glucose; CGM = continuous glucose monitoring; GMI = glucose management indicator; POC = point-of-care.
Hypoglycemia is a particularly common issue in hospitalized patients with advanced CKD due to strong disturbances in insulin and glucose metabolism (changes in insulin clearance, degradation, and secretion as well as a decrease in glucose filtration and gluconeogenesis) that can vary greatly between individuals and contribute to significant glucose variability.35 , 36 Patients with diabetes and advanced CKD who have an eGFR of <30 mL/min/1.73 m2 are at a high risk of death and have a similar risk of complications compared with those with ESRD receiving dialysis.36 Notably, patients with diabetes and ESRD receiving hemodialysis are at the highest risk of mortality within the entire population of patients with ESRD.36 Thus, there is a great impetus to achieve optimal glycemic control and to prevent hypoglycemia in this complex population. The DIALYDIAB trial conducted in patients with diabetes and ESRD receiving dialysis demonstrated that CGM was associated with more frequent changes in patients’ glycemic regimen and improved glycemic control without hypoglycemia.37
CGM has also been shown to help improve glycemic monitoring, facilitate glucose management, and prevent hyperglycemia in non-ICU hospitalized patients.34 Fortmann et al12 recently published the first randomized controlled trial using real-time CGM versus standard hospital glucose management in a non-ICU hospital setting. Their data demonstrated that the use of real-time CGM, along with hospital protocols to manage hypoglycemia and hyperglycemia, improved mean glucose and TIR without increasing the frequency of hypoglycemia in patients with type 2 diabetes.12 The comprehensive and continuous glucose data that CGM provides over time assists in discerning glycemic patterns and aids in treatment adjustments. Figures 2 B and C also illustrate an episode of prolonged hyperglycemia detected using CGM in a study patient that would not be apparent with POC fingerstick capillary blood glucose measurements alone.
Our lower mean TIR of 59.8% was impacted by enrolling a patient with a baseline HbA1c level of 13.2% (121 mmol/mol) who exhibited considerable insulin resistance, as despite titrating his insulin dose to 1.2 units/kg/24 h, he remained in poor glycemic control. The median titrated dose of insulin received by study patients on day 3 was relatively modest, at 0.6 units/kg/d, and more aggressive insulin titration in select patients would have achieved a superior TIR percentage. A study similar to ours excluded patients with entry POC blood glucose levels of >350 mg/dL.6
Recent studies have highlighted issues with implementing CGM in hospitalized ICU patients. Perez-Guzman et al32 studied ICU patients with type 2 diabetes undergoing urgent CABG and reported that CGM technology is less reliable owing to sensor signal loss, which commonly occurs intraoperatively; however, they found that sensors that recovered immediately after surgery had sustained accuracy. They advised “avoiding clinical treatment decisions after surgery based on CGM readings until accuracy can be confirmed (within 20% of reference values) with POC testing or laboratory tests.”32 Davis et al38 studied a small population of ICU patients with diabetes during the COVID-19 pandemic and found that sensor signal loss occurred commonly during hypoperfusion, cardiac arrest, defibrillator use, and position changes during pronation or hypothermia protocols. They successfully implemented and linked a hybrid real-time CGM and POC glucose testing protocol through a computerized decision support algorithm (Glucommander) and integrated a validation system for sensor glucose values into their electronic medical record.38 This approach was helpful in achieving and maintaining TIR in a critically ill population managed on mechanical ventilation and treated with glucocorticoids. Importantly, their well-designed integration of a validation system for sensor values also helps overcome the challenge of CGM implementation in ICU patients. More studies evaluating this approach and other innovative methods of integrating hybrid systems into a validation method are needed.
Studies have demonstrated the need for improved transitions of care in hospitalized patients.39 Glycemic management in the medical and surgical wards after an ICU transfer is an important transition of care that is high-risk and could potentially be associated with gaps in care that could negatively impact patients’ safety and the length of stay. In comparison to the ICU, patients receive less monitoring in the wards, and the nurse-to-patient ratios are higher. Our study validated the feasibility and accuracy during this transition of care from the ICU to a cardiac transition unit (or surgical ward) in elderly patients with type 2 diabetes, with complications. The use of CGM in the noncritical care setting is also an opportunity to introduce this technology to patients with a view of potential use in the home setting. Diabetic patients with hyperglycemia who are hospitalized have higher 30-day hospital readmission rates than patients without diabetes and hyperglycemia.40 These readmitted patients have been shown to have higher mean blood glucose levels, more extreme glucose excursions, and high glycemic variability during their initial hospitalization.40 More studies are needed to further explore the role of CGM in the transitions of care for hospitalized patients.
Conclusions
In summary, CGM technology was successfully implemented during the COVID-19 pandemic in our high-risk patients after cardiac surgery and after their transition of care from the ICU with high clinical reliability, as 97% of the CGM values were within zones A and B of the CEG analysis compared with the standard POC capillary blood glucose levels. CGM has been shown to improve inpatient diabetes care by preventing both hypoglycemia and hyperglycemia and by facilitating therapeutic insulin management. Our study was limited by its small size and the heterogeneity of patients. Larger randomized controlled trials are needed to validate and further explore the use of CGM in hospitalized non-ICU patients, particularly those with advanced CKD receiving dialysis. In conclusion, CGM holds great promise to optimize inpatient diabetes care with high clinical reliability and accuracy.
Acknowledgment
We thank our dedicated study coordinator, Ms Aimee Jovino. We are also grateful to Dexcom as they provided the continuous glucose monitoring system and sensors as well as funding for statistical support. Dexcom played no role in statistical analysis, manuscript preparation, or the study findings.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.Spanakis E.K., Levitt D.L., Siddiqui T., et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol. 2018;12(1):20–25. doi: 10.1177/1932296817748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh L.G., Satyarengga M., Marcano I., et al. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care. 2020;43(11):2736–2743. doi: 10.2337/dc20-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galindo R.J., Migdal A.L., Davis G.M., et al. Comparison of the freestyle libre pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43(11):2730–2735. doi: 10.2337/dc19-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez A.M., Umpierrez G.E., Muñoz O.M., et al. Continuous glucose monitoring versus capillary point-of-care testing for inpatient glycemic control in type 2 diabetes patients hospitalized in the general ward and treated with a basal bolus insulin regimen. J Diabetes Sci Technol. 2015;10(2):325–329. doi: 10.1177/1932296815602905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fact sheet for healthcare providers: use of Dexcom continuous monitoring systems during the COVID-19 pandemic. Dexcom. https://www.dexcom.com/hospitalfacts
- 6.Reutrakul S., Genco M., Salinas H., et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care. 2020;43(10):e137–e138. doi: 10.2337/dc20-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair B.G., Dellinger E.P., Flum D.R., Rooke G.A., Hirsch I.B. A pilot study of the feasibility and accuracy of inpatient continuous glucose monitoring. Diabetes Care. 2020;43(11):e168–e169. doi: 10.2337/dc20-0670. [DOI] [PubMed] [Google Scholar]
- 8.Singh L.G., Levitt D.L., Satyarengga M., et al. Continuous glucose monitoring in general wards for prevention of hypoglycemia: results from the glucose telemetry system pilot study. J Diabetes Sci Technol. 2020;14(4):783–790. doi: 10.1177/1932296819889640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galindo R.J., Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. doi: 10.1016/j.diabres.2020.108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis G.M., Spanakis E.K., Migdal A.L., et al. Accuracy of Dexcom G6 continuous glucose monitoring in non-critically ill hospitalized patients with diabetes. Diabetes Care. 2021;44(7):1641–1646. doi: 10.2337/dc20-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaupp L., Donsa K., Neubauer K.M., et al. Taking a closer look—continuous glucose monitoring in non-critically ill hospitalized patients with type 2 diabetes mellitus under basal-bolus insulin therapy. Diabetes Technol Ther. 2015;17(9):611–618. doi: 10.1089/dia.2014.0343. [DOI] [PubMed] [Google Scholar]
- 12.Fortmann A.L., Spierling Bagsic S.R., Talavera L., et al. Glucose as the fifth vital sign: a randomized controlled trial of continuous glucose monitoring in a non-ICU hospital setting. Diabetes Care. 2020;43(11):2873–2877. doi: 10.2337/dc20-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donahoe S.M., Stewart G.C., McCabe C.H., et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 14.BARI 2D Study group. Frye R.L., August P., et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National diabetes statistics report, 2015 Centers for Disease Control and Prevention, US Department of Health and Human Services. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 16.Lauruschkat A.H., Arnrich B., Albert A.A., et al. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation. 2005;112(16):2397–2402. doi: 10.1161/CIRCULATIONAHA.105.534545. [DOI] [PubMed] [Google Scholar]
- 17.Raza S., Sabik J.F., III, Ainkaran P., Blackstone E.H. Coronary artery bypass grafting in diabetics: a growing health care cost crisis. J Thorac Cardiovasc Surg. 2015;150(2) doi: 10.1016/j.jtcvs.2015.03.041. 304-2.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAlister F.A., Man J., Bistritz L., Amad H., Tandon P. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26(5):1518–1524. doi: 10.2337/diacare.26.5.1518. [DOI] [PubMed] [Google Scholar]
- 19.Schmeltz L.R., DeSantis A.J., Thiyagarajan V., et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30(4):823–828. doi: 10.2337/dc06-2184. [DOI] [PubMed] [Google Scholar]
- 20.van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 21.Furnary A.P., Gao G., Grunkemeier G.L., et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 22.Umpierrez G., Cardona S., Pasquel F., et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care. 2015;38(9):1665–1672. doi: 10.2337/dc15-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krinsley J.S., Maurer P., Holewinski S., et al. Glucose control, diabetes status, and mortality in critically ill patients: the continuum from intensive care unit admission to hospital discharge. Mayo Clin Proc. 2017;92(7):1019–1029. doi: 10.1016/j.mayocp.2017.04.015. Published correction appears in Mayo Clin Proc. 2019;94(6):1121. [DOI] [PubMed] [Google Scholar]
- 24.Umpierrez G.E., Smiley D., Zisman A., et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial) Diabetes Care. 2007;30(9):2181–2186. doi: 10.2337/dc07-0295. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal S., Mathew J., Davis G.M., et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care. 2021;44(3):847–849. doi: 10.2337/dc20-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke W.L. The original Clarke error grid analysis (EGA) Diabetes Technol Ther. 2005;7(5):776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 27.Freckmann G., Pleus S., Grady M., Setford S., Levy B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol. 2019;13(3):575–583. doi: 10.1177/1932296818812062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiterer F., Polterauer P., Schoemaker M., et al. Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol. 2017;11(1):59–67. doi: 10.1177/1932296816662047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yajima T., Takahashi H., Yasuda K. Comparison of interstitial fluid glucose levels obtained by continuous glucose monitoring and flash glucose monitoring in patients with type 2 diabetes mellitus undergoing hemodialysis. J Diabetes Sci Technol. 2020;14(6):1088–1094. doi: 10.1177/1932296819882690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hissa M.R.N., Hissa P.N.G., Guimarães S.B., Hissa M.N. Use of continuous glucose monitoring system in patients with type 2 mellitus diabetic during hemodialysis treatment. Diabetol Metab Syndr. 2021;13(1):104. doi: 10.1186/s13098-021-00722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Y.P., Su X.F., Yin G.P., et al. Blood glucose fluctuations in hemodialysis patients with end stage diabetic nephropathy. J Diabetes Complications. 2015;29(3):395–399. doi: 10.1016/j.jdiacomp.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Guzman M.C., Duggan E., Gibanica S., et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care. 2021;44(3):e50–e52. doi: 10.2337/dc20-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turchin A., Matheny M.E., Shubina M., Scanlon J.V., Greenwood B., Pendergrass M.L. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care. 2009;32(7):1153–1157. doi: 10.2337/dc08-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galindo R.J., Umpierrez G.E., Rushakoff R.J., et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol. 2020;14(6):1035–1064. doi: 10.1177/1932296820954163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahhal M.N., Gharaibeh N.E., Rahimi L., Ismail-Beigi F. Disturbances in insulin-glucose metabolism in patients with advanced renal disease with and without diabetes. J Clin Endocrinol Metab. 2019;104(11):4949–4966. doi: 10.1210/jc.2019-00286. [DOI] [PubMed] [Google Scholar]
- 36.Galindo R.J., Beck R.W., Scioscia M.F., Umpierrez G.E., Tuttle K.R. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;41(5):756–774. doi: 10.1210/endrev/bnaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joubert M., Fourmy C., Henri P., Ficheux M., Lobbedez T., Reznik Y. Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract. 2015;107(3):348–354. doi: 10.1016/j.diabres.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 38.Davis G.M., Faulds E., Walker T., et al. Remote continuous glucose monitoring with a computerized insulin infusion protocol for critically ill patients in a COVID-19 medical ICU: proof of concept. Diabetes Care. 2021;44(4):1055–1058. doi: 10.2337/dc20-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pichardo-Lowden A., Umpierrez G., Lehman E.B., et al. Clinical decision support to improve management of diabetes and dysglycemia in the hospital: a path to optimizing practice and outcomes. BMJ Open Diabetes Res Care. 2021;9(1) doi: 10.1136/bmjdrc-2020-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eiland L.A., Luo J., Goldner W.S., Drincic A. The association of diabetes and hyperglycemia on inpatient readmissions. Endocr Pract. 2021;27(5):413–418. doi: 10.1016/j.eprac.2021.01.008. [DOI] [PubMed] [Google Scholar]