Abstract
To date, the actual prevalence of acute pulmonary embolism (PE) in patients with SARS-CoV-2 infection remains unknown, as systematic screening for PE is cumbersome. We performed a systematic review and meta-analysis on autoptic data to estimate the prevalence of histopathologic findings of acute PE and its relevance as a cause of death on patients with COVID-19. We searched MEDLINE-PubMed and Scopus to locate all articles published in the English language, up to August 10, 2021, reporting the autoptic prevalence of acute PE and evaluating PE as the underlying cause of death in patients with COVID-19. The pooled prevalence for both outcomes was calculated using a random-effects model and presenting the related 95% confidence interval (CI). Statistical heterogeneity was measured using the Higgins I2 statistic. We analyzed autoptic data of 749 patients with COVID-19 (mean age 63.4 years) included in 14 studies. In 10 studies, based on 526 subjects (mean age 63.8 years), a random-effect model revealed that autoptic acute PE findings were present in 27.5% of cases (95% CI 15.0 to 45.0%, I2 89.9%). Conversely, in 429 COVID-19 subjects (mean age 64.0 years) enrolled in 9 studies, acute PE was the underlying cause of death in 19.9% of cases (95% CI 11.0 to 33.3%, I2 83.3%). Autoptic findings of acute PE in patients with COVID-19 are present in about 30% of subjects, whereas a venous thromboembolic event represents the underlying cause of death in about 1 of 4 patients.
COVID-19 is frequently associated with venous thromboembolic events (VTEs),1 especially in patients with severe disease admitted to the intensive care unit (ICU).2 However, diagnosing VTE—especially during the initial phase of the pandemic—was difficult because of overlapping clinical respiratory findings and the limited availability of imaging. VTE prevalence and VTE as a cause of death were, therefore, possibly underestimated, as tissue analysis was also cumbersome. Indeed, during the initial phase of the outbreak, complete autopsy studies were rarely performed because of the risk of infection and biosafety.3 Unfortunately, to date, the actual autoptic prevalence of acute pulmonary embolism (PE) in patients with COVID-19 remains unknown. This study aimed to perform a systematic review and meta-analysis to estimate the autoptic prevalence of histopathologic findings of acute PE and of PE as a cause of death in patients with COVID-19 based on previously published studies.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guidelines (Supplementary File 1). Data were obtained searching MEDLINE-PubMed and Scopus for all autoptic investigations, in the English language, published since inception to August 10, 2021, reporting the occurrence of acute PE as histopathologic findings and/or cause of death in patients with COVID-19.
The prevalence of acute PE in patients with COVID-19, in terms of histopathologic finding, was chosen as the primary outcome. Conversely, the presence of autoptic of acute PE, considered as the underlying cause of death, was selected as the secondary outcome.
The selection of studies to be included in our analysis was independently conducted by 2 authors (GR, MZ) in a blinded fashion. Any discrepancies in study selection were resolved by consulting a third author (LR). The following Medical Subject Headings terms were used for the search: “COVID-19” AND (“Pulmonary embolism” OR “Thrombosis” OR “Venous thromboembolism”) AND “Autopsy.” Moreover, we searched the bibliographies of target studies for additional references. Case reports, review articles, abstracts, editorials/letters, and case series with <20 participants were excluded. Data extraction was independently conducted by 2 authors (MZ, GR). Studies were included in the meta-analysis if they provided (1) autoptic data on patients with confirmed COVID-19 infection and (2) prevalence of acute PE as histopathologic finding and/or as the underlying cause of death. Conversely, we excluded from the meta-analysis those studies not providing autoptic data on acute PE and those enrolling <20 patients because a small sample may have influenced the real prevalence assessment. For all studies reviewed, we extracted and analyzed the location of the study, the number of patients enrolled, the mean age and relative range, male gender, prevalence of obesity, arterial hypertension (HT), diabetes mellitus (DM), dyslipidemia, cancer and deep vein thrombosis (if reported). The quality of included studies was graded using the Newcastle-Ottawa quality assessment scale.4
The cumulative prevalence of autoptic PE findings and/or underlying cause of death (n/N), defined as the ratio between patients who died who experienced acute PE (n) and the number of patients enrolled in each study (N), were pooled using a random-effects model and presented with the corresponding 95% confidence interval (CI). Statistical heterogeneity was measured using the Higgins I2 statistic.5 To evaluate publication bias, both Egger's test (if >10 studies were included) and funnel plots were computed. Conversely, if <10 investigations were analyzed, only the funnel plot inspection was considered. To further appraise the impact of potential baseline confounders, a meta-regression analysis using age, gender, obesity, HT, and DM as moderator variables was performed. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat International, Tampa, Florida).
Results
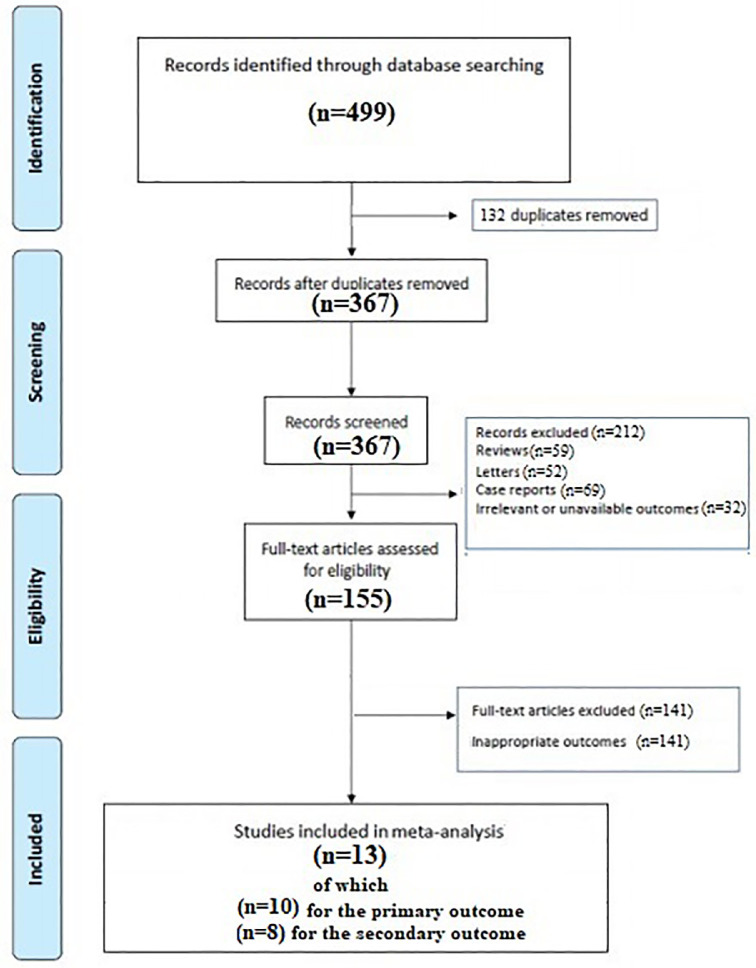
A total of 499 articles were obtained with our search strategy. After excluding duplicates and preliminary screening, 155 full-text articles were assessed for eligibility, and 141 studies were excluded for not meeting the inclusion criteria, leaving 13 studies fulfilling the inclusion criteria (Figure 1 , Supplementary File 2).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
Figure 1.
Flow diagram of selected studies for the meta-analysis according to the PRISMA. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
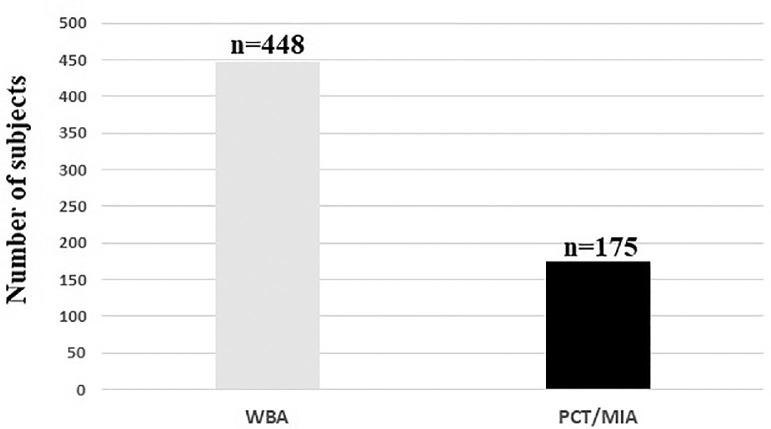
Overall, autoptic data of 623 patients with COVID-19 (mean age 63.4 years) were included in the analysis. The general characteristics of the studies included are listed in Table 1 . Most of the patients received a whole-body autopsy (Figure 2 ). At autopsy, histopathologic findings of deep vein thrombosis were observed in 16.6% of cases in 4 studies (mean age 56.0 years).7 , 9 , 14 , 17 The administration of anticoagulant treatment was not systematically performed in the reviewed studies. Quality assessment showed that all studies were of moderate-high quality according to the Newcastle-Ottawa quality assessment scale (Table 2 ).
Table 1.
General characteristics of the population enrolled
| Author | Country | Population | Age (SD) [range], years | Males | Obesity | HT | DM | Dyslipidaemia | Cancer | DVT N (%)* |
|---|---|---|---|---|---|---|---|---|---|---|
| Sang et al.6 | USA | 50 | 63.5 [31-94] |
36 (72 %) |
25 (50 %) |
45 (90 %) |
28 (56 %) |
16 (32 %) |
NR | NR |
| Himwaze et al.7 | Zambia | 29 | 44 [19-82] |
17 (58.8 %) |
NR | 6 (20.7 %) |
3 (10.3 %) |
NR | NR | 3 (10.3 %) |
| Yao et al.8 | China | 26 | 69.8 (10.3) | 13 (50 %) |
9 (34.6 %) |
NR | 4 (15.4 %) |
NR | 2 (7.7 %) |
NR |
| Mucheleng'anga et al.9 | Zambia | 21 | 40 (12.3) | 14 (66.2 %) |
3 (14.3 %) |
3 (14.3 %) |
1 (4.8 %) |
NR | NR | 3 (14.2 %) |
| Dal Ferro et al.10 | Italy | 40 | 79 (11) | 22 (55 %) |
NR | 15 (37 %) |
10 (24 %) |
NR | NR | NR |
| Romanova et al.11 | Russia | 42 | 69 (15)† 72 (15)‡ |
22 (52.3 %) |
17 (40.4 %) |
38 (90.4 %) |
11 (26.1 %) |
NR | NR | NR |
| Bryce et al.12 | USA | 102 | 68 [29-94] |
NR | 11 (11 %) |
64 (62 %) |
34 (33.3 %) |
NR | 7 (7 %) |
NR |
| Elezkurtaj et al.13 | Germany | 26 | 70 [30-92] |
17 (65.3 %) |
8 (30.7 %) |
17 (65.3 %) |
3 (11.5 %) |
NR | 1 (3.8 %) |
NR |
| Hooper et al.14 | USA | 135 | 61 [64-97] |
81 (60 %) |
46 (34 %) |
87 (64 %) |
71 (52 %) |
NR | NR | 6 (4.4 %) |
| Falasca et al.15 | Italy | 22 | 67 (15.7)§ 48.5 (13.0)∥ |
15 (68.1 %) |
NR | 4 (22.3 %) |
4 (22.3 %) |
NR | 5 (27.8 %) |
NR |
| De Michele et al.16 | USA | 29 | 71.3 [38-93] |
20 (68.9 %) |
NR | 23 (79.3 %) |
16 (55.1 %) |
10 (35.7 %) |
NR | NR |
| Edler et al.17 | Germany | 80 | 79.2 [52-96] |
46 (58 %) |
17 (21.2 %) |
15 (18.7 %) |
17 (21.2 %) |
NR | 13 (16.2 %) |
32 (40.0 %) |
| Menter et al.18 | Switzerland | 21 | 76 [53-96] |
17 (80.9 %) |
6 (28.5 %) |
21 (100 %) |
7 (35.0 %) |
NR | 3 (14.2 %) |
NR |
HT = arterial hypertension; DM = diabetes mellitus. DVT: Deep Vein thrombosis.
Autoptic finding.
Referred to group 1.
Referred to group 2.
With comorbidities.
Without comorbidities.
Figure 2.
Number of patients receiving a WBA or a PCT/MIA.
PCT/MIA = percutaneous/minimally invasive autopsy; WBA = whole-body autopsy.
Table 2.
Quality of the included studies assessed using the Newcastle-Ottawa quality assessment scale (NOS)
| Study | NOS |
|||
|---|---|---|---|---|
| Selection | Comparability | Outcome | Total score | |
| Sang et al.6 |  |
 |
8 | |
| Himwaze et al.7 |  |
 |
 |
8 |
| Yao et al.8 |  |
 |
 |
8 |
| Mucheleng'anga et al.9 |  |
 |
 |
8 |
| Dal Ferro et al.10 |  |
 |
 |
7 |
| Romanova et al.11 |  |
 |
 |
7 |
| Bryce et al.12 |  |
 |
 |
7 |
| Elezkurtaj et al.13 |  |
 |
 |
8 |
| Hooper at al.14 |  |
 |
 |
8 |
| Falasca et al.15 |  |
 |
 |
8 |
| De Michele et al.16 |  |
 |
 |
8 |
| Edler et al.17 |  |
 |
 |
8 |
| Menter et al.18 |  |
 |
 |
8 |
| Satturwar et al.19 |  |
 |
 |
8 |
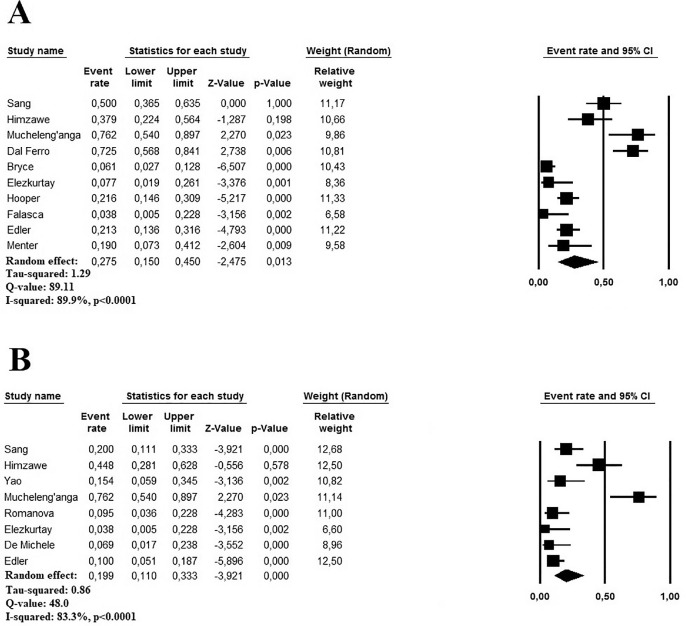
The cumulative autopsy prevalence of acute PE in patients with COVID-19 ranged between 3.8% and 50.0% in 10 studies, enrolling 526 subjects (mean age 63.8 years).6 , 7 , 9 , 10 , 12, 13, 14, 15 , 17 , 18 A random-effect model revealed a pooled prevalence of autoptic acute PE findings in 27.5% of cases (95% CI 15.0 to 45.0%, I2 89.9%) (Figure 3) . Both the Egger's test (t 0.522, p = 0.61) and visual inspection of the relative funnel plot (Supplementary File 3) did not reveal significant evidence of publication bias. Meta-regression analysis showed a direct correlation with age (p = 0.001) and gender (male vs female, p = 0.02), but not with obesity (p = 0.70), HT (p = 0.33) and DM (p = 0.78) (Table 3 ).
Figure 3.
(A) Forest plots investigating the pooled prevalence of autoptic histopathologic findings of acute pulmonary embolism in patients with COVID-19. (B) Forest plots investigating acute pulmonary embolism as underlying cause of death at autopsy in patients with COVID-19.
Table 3.
Meta-regression analysis of the effects of presenting features on the prevalence of histopathologic findings of acute pulmonary embolism
| Moderator | N° of interactions | β | 95% CI | p |
|---|---|---|---|---|
| Age (years) | 10 | 0.021 | -0.101 to 0.011 | 0.001 |
| Males, % | 9 | 0.005 | -0.003 to 0.004 | 0.02 |
| Obesity, % | 6 | 0.011 | -0.072 to 0.048 | 0.70 |
| HT, % | 6 | -0.019 | -0.057 to 0.019 | 0.33 |
| DM, % | 5 | -0.006 | -0.056 to 0.038 | 0.78 |
HT = arterial hypertension; DM = diabetes mellitus.
Autopsy findings reported that the cumulative prevalence of acute PE as an underlying cause of death in patients with COVID-19 ranged between 3.8% and 66.2% reviewing 9 studies based on 303 subjects (mean age 64.0 years).6, 7, 8, 9 , 11 , 13 , 16 , 17 Using a random-effect model, the pooled prevalence of acute PE, as an underlying cause of death was 19.9% (95% CI 11.0 to 33.3%, I2 83.3%) (Figure 3). Because of the low number of the included studies (<10), Egger's test cannot be performed; however, the visual inspection of the relative funnel plot (Supplementary File 3) did not reveal significant evidence of publication bias. Meta-regression analysis showed again a direct correlation with age (p <0.001) and gender (male vs female, p = 0.01) and HT (p <0.001), but no effect when considering obesity (p = 0.13) and DM (p = 0.41) as moderating variables (Table 4 ).
Table 4.
Meta-regression analysis of the effects of presenting features on the autoptic prevalence of acute pulmonary embolism as an underlying cause of death in patients with COVID-19
| Moderator | N° of interactions | β | 95% CI | p |
|---|---|---|---|---|
| Age (years) | 9 | 0.076 | -0.105 to 0.047 | <0.0001 |
| Males, % | 9 | 0.002 | -0.001 to 0.006 | 0.01 |
| Obesity, % | 7 | -0.050 | -0.115 to 0.015 | 0.13 |
| HT, % | 5 | 0.035 | -0.059 to 0.012 | 0.22 |
| DM, % | 5 | 0.027 | -0.095 to 0.039 | 0.41 |
HT = arterial hypertension; DM = diabetes mellitus.
Discussion
In this meta-analysis, the autoptic findings of acute PE in patients with COVID-19 were present in about 30% of subjects, whereas a VTE represented the underlying cause of death in about 1 of 4 patients. Together, these results confirmed that the prevalence of acute PE in patients with COVID-19 is high and significantly increases the risk of death because 20% of subjects died because of a VTE.
Notably, previous data on the incidence and prognostic role of acute PE in patients with SARS-CoV-2 were mainly obtained from clinical and radiologic studies, which generally underestimated the results, because in these patients computed tomography pulmonary angiography was not systematically performed during the infection but only in case of clinical deterioration or worsening of symptoms.1 Previous results on autopsy prevalence of acute PE in patients with COVID-19 were based on isolated case reports and small case series, often including <10 of 15 patients, limiting the accuracy of the results.19 , 20 Moreover, the reasons for performing autopsies were not systematically reported in the original investigations reviewed, so we cannot exclude that autoptic investigations have mainly been performed on these patients with unknown cause of death, potentially distorting our results. Unfortunately, information related to the severity of acute PE at the time of diagnosis was not systematically assessed by original investigations and the clinical scenario (i.e., general ward or ICU) in which the thromboembolic events were diagnosed. These missing data limit the possibility drawing definitive conclusions regarding the real prognostic impact of acute PE on the prognosis of patients with SARS-CoV-2. However, it cannot be neglected to mention that few autopsies were performed during the first part of the pandemic, especially for the risk of viral infection.3 , 21 Therefore, our analysis of autoptic data obtained from mid to large cohorts is likely to have overcome these limitations, also evidencing the proportion of cases in which the thromboembolic event may be retained as the underlying cause of death. The higher prevalence of acute PE obtained in our study compared with that obtained by in-vivo clinical investigations confirmed that the diagnosis of acute PE remains largely underestimated in patients with COVID-19.22, 23, 24, Despite it being reported that the prevalence of acute PE increases with the use of systematic screening based on computed tomography pulmonary angiography,25 this approach cannot be recommended in routine clinical practice. Indeed, it is often not feasible to conduct the imaging studies needed to diagnose VTE in these subjects who are infective, critically ill, intubated, unstable, and often in a prone position.
Our analysis found a marked heterogeneity between studies submitted to meta-regression analysis. In this regard, some of the sources of heterogeneity were represented by age and gender in both outcomes. Indeed, these items represent a major risk factor of developing VTE in the general population and patients with COVID-19 26 , 27 and independent predictors of mortality28 in such subjects. However, it is also true that our results might reflect the prevalence of acute PE in patients with a more severe infection, more relevant comorbidities, or even fewer chances to intensive care treatments, as suggested by age. Moreover, because no studies were performed during the second or third pandemic wave, we cannot evaluate the trend of the autoptic prevalence of acute PE over time and the indirect effect of COVID-19 vaccines, especially in older subjects. We cannot exclude that sampling bias by the competing risk of death may also have led to the underestimation of the real cumulative incidence of thromboembolic events.
We have been unable to assess the impact of ICU hospitalization and the anticoagulant treatment administered, if any because these data were not systematically reported in the reviewed studies. In this regard, further studies are needed to investigate these aspects from an autoptic perspective in patients with COVID-19. Although the enrollment period was not systematically reported by the revised investigations, it clearly emerges how the studies considered was generally performed during the first pandemic wave when the anticoagulants treatments were not largely used. Because of the absence of more recent analyses, when anticoagulants become a cornerstone in the treatment of patients with COVID-19, we cannot compare the autoptic pooled prevalence of acute PE between the 2 periods and therefore assess the indirect effects of anticoagulant treatments in such patients.
As the optimal regimen to prevent VTE in COVID-19 is still largely unknown, this meta-analysis underlines the potential of underdiagnosing VTE; important in designing, performing, and analyzing randomized trials on anticoagulant therapy in patients with COVID-19. Furthermore, our study reinforces the concept that the accurate identification of the unfavorable prognostic factors, as acute PE, is essential in helping clinicians and policymakers tailor the management strategies for patients with COVID-19.
Our study has several limitations related to the observational nature of the reviewed studies with all their inherited biases. In particular, potential underestimation could derive from not systematically searching for PE at autopsy. Similarly, we cannot assess if adequate prophylactic anticoagulation was consistently administered in each study because these data were not systematically provided in the review investigations. Furthermore, few autoptic investigations on the COVID-19 infection have analyzed the prevalence of acute PE as a complication of COVID-19 infection or as the underlying cause of death, limiting the number of the studies included in the meta-analysis and the resulting number of patients. Moreover, considering that most of the autopsies reviewed were legally mandated, our data could be partially distorted because the enrollment of these patients might have determined an involuntary selection bias. Despite these limitations, to the best of our knowledge, this study is the first systematic review and meta-analysis providing a clear estimation on the prevalence of acute PE as both a complication and underlying cause of death in patients with COVID-19.
In conclusion, based on this systematic review and meta-analysis, the prevalence of acute PE in autopsy studies appears higher compared with clinical practice, hinting at an underestimation of acute PE in patients with COVID-19 in clinical practice.
Disclosures
The authors have no conflicts of interest to declare.
Funding
None.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.01.051.
Appendix. Supplementary materials
Supplementary File 1. PRISMA checklist
Supplementary File 2. Period of enrollment and autopsy methodology used in studies reviewed. NR: not reported.
Supplementary File 3. (A) Funnel plot for the pooled autoptic prevalence of histopathologic findings of acute pulmonary embolism. (B) Funnel plot for the pooled autoptic prevalence of acute pulmonary embolism as an underlying cause of death in patients with COVID-19.
References
- 1.Roncon L, Zuin M, Barco S, Valerio L, Zuliani G, Zonzin P, Konstantinides SV. Incidence of acute pulmonary embolism in COVID-19 patients: systematic review and meta-analysis. Eur J Intern Med. 2020;82:29–37. doi: 10.1016/j.ejim.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. The Ottawa Hospital. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed on August 5, 2021.
- 5.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sang CJ, 3rd, Burkett A, Heindl B, Litovsky SH, Prabhu SD, Benson PV, Rajapreyar I. Cardiac pathology in COVID-19: a single center autopsy experience. Cardiovasc Pathol. 2021;54 doi: 10.1016/j.carpath.2021.107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Himwaze CM, Telendiy V, Maate F, Mupeta S, Chitalu C, Chanda D, Julius P, Mumba C, Marimo C, Hamukale A, Mulenga L, Shibemba AL, Zumla A, Mucheleng'anga LA. Post-mortem examination of hospital inpatient COVID-19 deaths in Lusaka, Zambia - a descriptive whole-body autopsy series. Int J Infect Dis. 2021;108:363–369. doi: 10.1016/j.ijid.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang PP, Cai J, Zhou XD, Jiang DP, Fei XC, Huang XQ, Zhao L, Zhang H, Wu HB, Ren Y, Liu ZH, Zhang HR, Chen C, Fu WJ, Li H, Xia XY, Chen R, Wang Y, Liu XD, Yin CL, Yan ZX, Wang J, Jing R, Li TS, Li WQ, Wang CF, Ding YQ, Mao Q, Zhang DY, Zhang SY, Ping YF, Bian XW. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31:836–846. doi: 10.1038/s41422-021-00523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mucheleng'anga LA, Telendiy V, Hamukale A, Shibemba AL, Zumla A, Himwaze CM. COVID-19 and sudden unexpected community deaths in Lusaka, Zambia, Africa - a medico-legal whole-body autopsy case series. Int J Infect Dis. 2021;109:160–167. doi: 10.1016/j.ijid.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dal Ferro M, Bussani R, Paldino A, Nuzzi V, Collesi C, Zentilin L, Schneider E, Correa R, Silvestri F, Zacchigna S, Giacca M, Metra M, Merlo M, Sinagra G. SARS-CoV-2, myocardial injury and inflammation: insights from a large clinical and autopsy study. Clin Res Cardiol. 2021;110(11):1822–1831. doi: 10.1007/s00392-021-01910-2. [published correction appears in Clin Res Cardiol 2021;110:1694] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romanova ES, Vasilyev VV, Startseva G, Karev V, Rybakova MG, Platonov PG. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J Intern Med. 2021;290:655–665. doi: 10.1111/joim.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, AlRasheed MR, Chen J, Li L, Wang D, Corben A, Haines GK, 3rd, Westra WH, Umphlett M, Gordon RE, Reidy J, Petersen B, Salem F, Fiel MI, El Jamal SM, Tsankova NM, Houldsworth J, Mussa Z, Veremis B, Sordillo E, Gitman MR, Nowak M, Brody R, Harpaz N, Merad M, Gnjatic S, Liu WC, Schotsaert M, Miorin L, Aydillo Gomez TA, Ramos-Lopez I, Garcia-Sastre A, Donnelly R, Seigler P, Keys C, Cameron J, Moultrie I, Washington KL, Treatman J, Sebra R, Jhang J, Firpo A, Lednicky J, Paniz-Mondolfi A, Cordon-Cardo C, Fowkes ME. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elezkurtaj S, Greuel S, Ihlow J, Michaelis EG, Bischoff P, Kunze CA, Sinn BV, Gerhold M, Hauptmann K, Ingold-Heppner B, Miller F, Herbst H, Corman VM, Martin H, Radbruch H, Heppner FL, Horst D. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11:4263. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper JE, Padera RF, Dolhnikoff M, da Silva LFF, Duarte-Neto AN, Kapp ME, Lacy JM, Mauad T, Saldiva PHN, Rapkiewicz AV, Wolf DA, Felix JC, Benson P, Shanes E, Gawelek KL, Marshall DA, McDonald MM, Muller W, Priemer DS, Solomon IH, Zak T, Bhattacharjee MB, Fu L, Gilbert AR, Harper HL, Litovsky S, Lomasney J, Mount SL, Reilly S, Sekulic M, Steffensen TS, Threlkeld KJ, Zhao B, Williamson AK. A postmortem portrait of the coronavirus disease 2019 (COVID-19) pandemic: a large multi-institutional autopsy survey study. Arch Pathol Lab Med. 2021;145:529–535. doi: 10.5858/arpa.2020-0786-SA. [DOI] [PubMed] [Google Scholar]
- 15.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, Antinori A, Petrosillo N, Marchioni L, Biava G, D'Offizi G, Palmieri F, Goletti D, Zumla A, Ippolito G, Piacentini M, Del Nonno F. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Michele S, Sun Y, Yilmaz MM, Katsyv I, Salvatore M, Dzierba AL, Marboe CC, Brodie D, Patel NM, Garcia CK, Saqi A. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154:748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satturwar S, Fowkes M, Farver C, Wilson AM, Eccher A, Girolami I, Pujadas E, Bryce C, Salem F, El Jamal SM, Paniz-Mondolfi A, Petersen B, Gordon RE, Reidy J, Fraggetta F, Marshall DA, Pantanowitz L. Postmortem findings associated with SARS-CoV-2: systematic review and meta-analysis. Am J Surg Pathol. 2021;45:587–603. doi: 10.1097/PAS.0000000000001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasquez-Bonilla WO, Orozco R, Argueta V, Sierra M, Zambrano LI, Muñoz-Lara F, López-Molina DS, Arteaga-Livias K, Grimes Z, Bryce C, Paniz-Mondolfi A, Rodríguez-Morales AJ. A review of the main histopathological findings in coronavirus disease 2019. Hum Pathol. 2020;105:74–83. doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salerno M, Sessa F, Piscopo A, Montana A, Torrisi M, Patanè F, Murabito P, Volti GL, Pomara C. No autopsies on COVID-19 deaths: a missed opportunity and the lockdown of science. J Clin Med. 2020;9:1472. doi: 10.3390/jcm9051472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, Sanchez O, Lorut C, Chassagnon G, Revel MP. Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J. 2020;56 doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S, Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost. 2020;4:1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eischer L, Eichinger S, Kyrle PA. Age at first venous thromboembolism and risk of recurrence: a prospective cohort study. Medicine (Baltimore) 2009;88:366–370. doi: 10.1097/MD.0b013e3181c29e31. [DOI] [PubMed] [Google Scholar]
- 27.Henrina J, Santosa Putra IC, Cahyadi I, Lawrensia S, Hadi Gunawan HF, Cahyadi A, Franke J, Suciadi LP. Clinical characteristics and outcomes of venous thromboembolism in patients hospitalized for COVID-19: systematic review and meta-analysis. Thrombosis Update. 2021;2 doi: 10.1016/j.tru.2021.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez CA, Sun CK, Tsai IT, Chang YP, Lin MC, Hung IY, Chang YJ, Wang LK, Lin YT, Hung KC. Mortality and risk factors associated with pulmonary embolism in coronavirus disease 2019 patients: a systematic review and meta-analysis. Sci Rep. 2021;11:16025. doi: 10.1038/s41598-021-95512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1. PRISMA checklist
Supplementary File 2. Period of enrollment and autopsy methodology used in studies reviewed. NR: not reported.
Supplementary File 3. (A) Funnel plot for the pooled autoptic prevalence of histopathologic findings of acute pulmonary embolism. (B) Funnel plot for the pooled autoptic prevalence of acute pulmonary embolism as an underlying cause of death in patients with COVID-19.