Abstract
STUDY QUESTION
Does the endometrial preparation protocol (artificial cycle (AC) vs natural cycle (NC) vs stimulated cycle (SC)) impact the risk of early pregnancy loss and live birth rate after frozen/thawed embryo transfer (FET)?
SUMMARY ANSWER
In FET, ACs were significantly associated with a higher pregnancy loss rate and a lower live birth rate compared with SC or NC.
WHAT IS KNOWN ALREADY
To date, there is no consensus on the optimal endometrial preparation in terms of outcomes. Although some studies have reported a higher pregnancy loss rate using AC compared with NC or SC, no significant difference was found concerning the pregnancy rate or live birth rate. Furthermore, no study has compared the three protocols in a large population.
STUDY DESIGN, SIZE, DURATION
A multicenter retrospective cohort study was conducted in nine reproductive health units in France using the same software to record medical files between 1 January 2012 and 31 December 2016. FET using endometrial preparation by AC, modified NC or SC were included. The primary outcome was the pregnancy loss rate at 10 weeks of gestation. The sample size required was calculated to detect an increase of 5% in the pregnancy loss rate (21–26%), with an alpha risk of 0.5 and a power of 0.8. We calculated that 1126 pregnancies were needed in each group, i.e. 3378 in total.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Data were collected by automatic extraction using the same protocol. All consecutive autologous FET cycles were included: 14 421 cycles (AC: n = 8139; NC: n = 3126; SC: n = 3156) corresponding to 3844 pregnancies (hCG > 100 IU/l) (AC: n = 2214; NC: n = 812; SC: n = 818). Each center completed an online questionnaire describing its routine practice for FET, particularly the reason for choosing one protocol over another.
MAIN RESULTS AND THE ROLE OF CHANCE
AC represented 56.5% of FET cycles. Mean age of women was 33.5 (SD ± 4.3) years. The mean number of embryos transferred was 1.5 (±0.5). Groups were comparable, except for history of ovulation disorders (P = 0.01) and prior delivery (P = 0.03), which were significantly higher with AC. Overall, the early pregnancy loss rate was 31.5% (AC: 36.5%; NC: 25.6%; SC: 23.6%). Univariable analysis showed a significant association between early pregnancy loss rate and age >38 years, history of early pregnancy loss, ovulation disorders and duration of cryopreservation >6 months. After adjustment (multivariable regression), the early pregnancy loss rate remained significantly higher in AC vs NC (odds ratio (OR) 1.63 (95% CI) [1.35–1.97]; P < 0.0001) and in AC vs SC (OR 1.87 [1.55–2.26]; P < 0.0001). The biochemical pregnancy rate (hCG > 10 and lower than 100 IU/l) was comparable between the three protocols: 10.7% per transfer.
LIMITATIONS, REASONS FOR CAUTION
This study is limited by its retrospective design that generates missing data. Routine practice within centers was heterogeneous. However, luteal phase support and timing of embryo transfer were similar in AC. Univariable analysis showed no difference between centers. Moreover, a large number of parameters were included in the analysis.
WIDER IMPLICATIONS OF THE FINDINGS
Our study shows a significant increase in early pregnancy loss when using AC for endometrial preparation before FET. These results suggest either a larger use of NC or SC, or an improvement of AC by individualizing hormone replacement therapy for patients in order to avoid an excess of pregnancy losses.
STUDY FUNDING/COMPETING INTEREST(S)
The authors declare no conflicts of interest in relation to this work. G.P.-B. declares consulting fees from Ferring, Gedeon-Richter, Merck KGaA, Theramex, Teva; Speaker’s fees or equivalent from Merck KGaA, Ferring, Gedeon-Richter, Theramex, Teva. N.C. declares consulting fees from Ferring, Merck KGaA, Theramex, Teva; Speaker’s fees or equivalent from Merck KGaA, Ferring. C.R. declares a research grant from Ferring, Gedeon-Richter; consulting fees from Gedeon-Richter, Merck KGaA; Speaker’s fees or equivalent from Merck KGaA, Ferring, Gedeon-Richter; E.M.d’A. declares Speaker’s fees or equivalent from Merck KGaA, MSD, Ferring, Gedeon-Richter, Theramex, Teva. I.C-D. declares Speaker’s fees or equivalent from Merck KGaA, MSD, Ferring, Gedeon-Richter, IBSA. N.M. declares a research grant from Merck KGaA, MSD, IBSA; consulting fees from MSD, Ferring, Gedeon-Richter, Merck KGaA; Speaker’s fees or equivalent from Merck KGaA, MSD, Ferring, Gedeon-Richter, Teva, Goodlife, General Electrics.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: miscarriage, HRT, embryo transfer, natural cycle, IVF/ICSI outcome
WHAT DOES THIS MEAN FOR PATIENTS?
There are three different approaches that can be used to prepare the uterus before frozen embryo transfer. These are known as the artificial cycle, the natural cycle and the stimulated cycle. To date, there is no consensus on which approach should be used. Some studies have reported that artificial cycle led to higher pregnancy loss rates, but no clear difference in pregnancy rate or live birth rate has been established. Furthermore, no study has compared the three approaches in a large population.
This study analyzed 14 421 cycles of frozen embryo transfer, corresponding to 3844 pregnancies. It shows that the artificial cycle is associated to significantly higher early pregnancy loss rates and significantly lower live birth rates compared to the other approaches. These results suggest either a greater use of the other approaches (natural cycle or stimulated cycle), or that the artificial cycle protocol should be improved.
Introduction
Since the first birth resulting from frozen embryo transfer (FET) in 1984 in Australia, the number of FET cycles has progressively increased. The practice of FET has been enhanced by significant improvements in the field of cryopreservation (vitrification) and by the development of elective single embryo transfers to limit multiple pregnancies (de Mouzon et al., 2020). In Europe in 2016, FET accounted for 44.1% of all transfers (Wyns et al., 2020). Obstetrical and perinatal outcomes of pregnancies after FET are reassuring (Wong et al., 2017). The proportion of children born after FET is increasing. In France in 2017, FET represented 29.2% of live births obtained with ART and 26.3% in Europe in 2014 (‘Agence de la Biomédecine,’ n.d.).
The success of FET relies on embryo quality, uterine receptivity and synchronization between the endometrium and the embryo. FET should be performed at a time when the endometrium is the most receptive, known as the ‘implantation window’. Three types of endometrial preparation protocols are used in current practice. The artificial cycle (AC) consists in exogenous supplementation by estradiol and progesterone. It is the most commonly used protocol worldwide as it enables flexibility and regulation of embryo transfers for IVF centers. Stimulated cycle (SC) consists in ovarian stimulation by exogenous treatments (gonadotropins, letrozole or clomifene citrate for example) followed by ovulation triggering by hCG. The natural cycle (NC), which is increasingly used, consists in the monitoring of a physiological cycle. It can be ‘modified’ by using hCG to trigger ovulation and/or associated to a luteal phase support by progesterone.
Previous studies have reported that the early pregnancy loss rate in FET was higher when using the AC protocol compared to NC or SC (Veleva et al., 2008; Tomás et al., 2012; Cerrillo et al., 2017; Peigné et al., 2019; Liu et al., 2020; Levi Setti et al., 2020). A 7-year study of vitrified blastocyst transfers found that the risk of early pregnancy loss was higher using AC compared to NC or modified NC with ovulation triggering (Levi Setti et al., 2020). Similarly, a retrospective study of 1846 normally ovulating patients also reported that the early pregnancy loss rate was significantly higher with AC than with NC (Liu et al., 2020). However, so far, no consensus exists concerning the use of one protocol over another. Furthermore, none of these protocols has shown its superiority in terms of live birth rate (Groenewoud et al., 2013; Ghobara et al., 2017).
The aim of our study was to determine in a large cohort whether the type of protocol for endometrial preparation has an impact on the risk of early pregnancy loss and live birth rate after FET, as well as the risk factors associated to early pregnancy loss after FET.
Materials and methods
Design
Our study is a multicenter retrospective study using data collected prospectively from January 2012 to December 2016. Participating centers were selected if they used the same electronic health record software (MediFirst-AMP®) and if the three types of endometrial preparation protocols were performed.
Data collection and ethics
Data were obtained from registries of nine French IVF centers: Angers, Bondy, Creteil, Marseille, Montpellier, Strasbourg and three in Paris (Diaconesses, Institut Mutualiste Montsouris, Tenon). In each center, the registry was continuously updated using MediFirst-AMP® (www.medifirst.net), with a format that meets requirements of the French Biomedicine Agency (Agence de la Biomédecine). The use of data from these registries for purposes of observational studies complies with national regulations (French Data Protection Authority; Commission Nationale de l’Informatique et des Libertés or CNIL). According to French law (2012-300), patients are aware that their data can be used for anonymous clinical studies unless they specifically object. Individual registries were integrated into a global database and processed in full conformity with the reference methodology (MR-003) of the French Data Protection Authority (CNIL 2016). Data on each frozen cycle of each center were merged in a final database. For each cycle, baseline characteristics, treatment-related data and reproductive outcomes were reported up to live birth. Data were included for all cycles performed between 2012 and 2016. According to French law, approval of an ethical committee is not required for research on previously collected data.
An online questionnaire was also sent to each participating center to collect information on routine practice regarding endometrial preparation before FET (choice of preparation, patient profile, type of drugs used, etc.).
Endometrial preparation protocols
In AC protocols, estrogen supplementation was performed by using Provames® (between 4 mg and 6 mg by oral route or vaginal route) and Vivelledot® (skin patch 100 μg ± 50 μg every 2–4 days). Three centers performed prior hypophyseal block by injection of a GnRH agonist (Decapeptyl® 3.75 mg) after checking endometrial thickness by ultrasound. Natural micronized progesterone (Utrogestan® or Progestan®) was introduced vaginally, between 400 mg and 800 mg, depending on the center. FET was performed after 2 or 3 days of progesterone for Day 2 or Day 3 embryos and after 5 days of progesterone for blastocysts. Administration of progesterone was continued until at least 9 weeks of gestation in all centers.
All NC were modified NC protocols. Modified NC protocols had luteal phase support by progesterone. According to centers, ovulation was triggered by recombinant hCG. In modified NC, monitoring by ultrasound and hormone assay (estrogen, LH and progesterone serum levels) was performed between Day 9 and Day 12.
In SC, recombinant or urinary gonadotropin (50–75 IU) was used and ovulation was triggered when a dominant follicle (>16–17 mm) and an adequate endometrial thickness were present on ultrasound. Luteal phase support by progesterone was added.
Endpoints
The main endpoint was the early pregnancy loss rate before 10 weeks of gestation among the total number of pregnancies (defined by hCG > 100 IU/ml). Secondary endpoints were the pregnancy rate per FET (number of pregnancies with hCG > 100 IU/ml/number of FET), the biochemical pregnancy rate per pregnancy (number of pregnancies with hCG > 10 and lower than 100 IU/l/number of pregnancies) and the live birth rate per FET (number of liveborn infants after 22 weeks of gestation/number of FET).
Inclusion/exclusion criteria
Criteria for inclusion of patients were age between 18 and 43 years and at least one frozen/thawed embryo transfer performed, whatever the embryo stage and technique used (IVF or ICSI). Exclusion criteria were gamete donation, the use of sperm obtained by surgical extraction and preimplantation genetic testing.
Calculation of the number of subjects needed
The average rate of early pregnancy loss in FET is 25% (‘Agence de la Biomédecine,’ n.d.). To show a significant increase of 5% with AC compared with NC or SC (21% vs 26%) with an alpha risk of 5% and a power of 80%, it was necessary to analyze 1126 pregnancies per group, i.e. a total of 3378 pregnancies obtained after FET. Given the pregnancy rate after FET (26%), it was necessary to include a total of ∼ 13 000 FET cycles.
Statistical analyses
Qualitative variables are described in numbers and percentages. Proportions were compared using Chi-squared tests. Continuous variables were compared by ANOVA. Continuous variables were included in univariable or multivariable analysis in the form of continuous variables or clinically relevant classes.
Logistic regression analysis was used to identify factors associated with the risk of early pregnancy loss. Variables were analyzed according to their relevance or to the degree of significance in the different comparative analyses performed. Variables associated with the risk of early pregnancy loss in univariable analysis with a P ≤ 0.20 were selected for inclusion in the multivariable model. The threshold of significance was defined by a P-value ≤ 0.05. Statistical analyses were performed using SAS software version 9.4.
Results
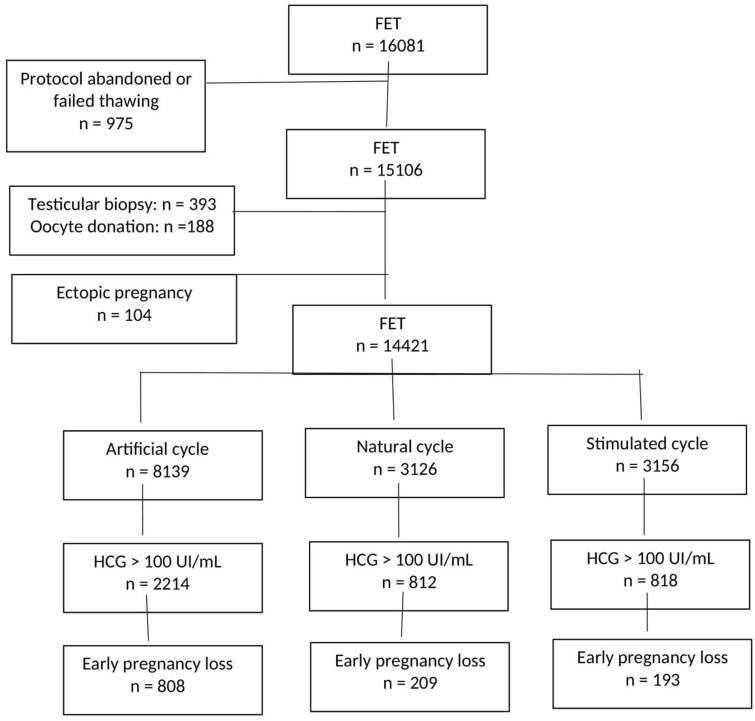
We collected data from 16 081 FET cycles. In total, 14 421 cycles comprising 3844 pregnancies (hCG > 100 IU/ml) were included in the study. No patient underwent more than one cycle. Ectopic pregnancies (n = 104, 2.6%) were excluded (1.4% in AC, 0.6% in NC and 0.4% in SC) (Fig. 1). Patient characteristics and number of frozen–thawed embryo transfers according to endometrial preparation protocol are shown in Supplementary Table SI.
Figure 1.
Flow chart of frozen embryo transfer (FET), endometrial preparation protocols and early pregnancy loss.
In the whole study population, the live birth rate after FET was 17.8% and the pregnancy rate after FET was 26.6%. The early pregnancy loss rate was 31.5% (range: 27.3–35.1%) and did not statistically differ between centers (P = 0.1). No significant difference was found between the years during which FET were performed (2012, 2013, 2014, 2015 and 2016) (P = 0.63). Distribution of cycles, pregnancies and early pregnancy loss according to the three protocols is shown in Fig. 1.
Use of endometrial preparation protocols according to the study center
Overall, AC was the most common protocol (56.5% of cycles) and was used first line in five of the nine study centers. SC and NC were used to a similar degree (21.8% vs 21.7%, respectively).
NC was used first line by three centers. All centers used modified NC. SC was used first line in two centers. In NC and SC, FET was performed according to hormonal monitoring and embryo stage (Day 2, Day 3 or blastocysts) (Labrosse et al., 2020).
For all FET, blood samples to measure serum hCG levels were performed at Days 12, 14 and 16 after embryo transfer. Vitrification was the method of freezing used for all embryos.
Outcomes of FET cycles
Results are reported in Table I. The early pregnancy loss rate was significantly higher for AC in comparison with NC and SC (36.5% vs 25.7% vs 23.6%, respectively, P < 0.005). The early pregnancy loss rate was also significantly higher for NC compared with SC, but the threshold of significance was lower (P = 0.03). The live birth rate was significantly lower for AC compared with NC [odds ratio (OR): 0.88 [0.79–0.98] (95% confidence interval)] and SC [OR: 0.85 [0.76–0.94] (95% confidence interval)] (16.9% vs 18.8% vs 19.3%, respectively, P < 0.003). No difference between the different types of protocol was found in terms of biochemical pregnancies (hCG > 10 and lower than 100 IU/l) and rates of pregnancies with hCG > 100 IU/ml.
Table I.
Patient characteristics and outcomes of frozen–thawed embryo transfer according to endometrial preparation protocol.
| Total population | Artificial cycle (AC) | Natural cycle (NC) | Stimulated cycle (SC) | P | |
|---|---|---|---|---|---|
| Number of FET | 14 421 | 8139 | 3126 | 3156 | |
| Age (years), mean ± SD | 34.2 | 34.1 ± 4.7 | 34.3 ± 4.5 | 34.2 ± 4.4 | 0.70 |
| Primary infertility, n (%) | 7326 (50.8) | 4182 (51.4) | 1513 (48.4) | 1541 (48.8) | 0.004 |
| Length of infertility (days), mean ± SD | 2042 ± 1054 | 2054 ± 1,059 | 2015 ± 1004 | 2035 ± 1088 | 0.92 |
| History of early pregnancy loss, n (%) | 2064 (14.3) | 1134 (13.9) | 476 (15.2) | 454 (14.4) | 0.21 |
| BMI (kg/m2), mean ± SD | 23.4 ± 5.4 | 23.8 ± 5.2 | 22.7 ± 5.8 | 23.6 ± 5.2 | 0.07 |
| Smoking, n (%) | 3390 (23.5) | 1923 (23.6) | 736 (23.5) | 731 (23.2) | 0.87 |
| ICSI, n (%) | 8321 (57.7) | 4638 (57.0) | 2036 (65.1) | 1647 (52.2) | <0.0001 |
| Endometrial thickness (mm), mean ± SD | 9.3 ± 2.5 | 9.1 ± 2.6 | 9.8 ± 2.3 | 9.1 ± 2.2 | 0.02 |
| Number of embryos transferred, mean ± SD | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 | 0.09 |
| Biochemical pregnancy (hCG 10–100 IU/ml) per pregnancy, n (%) | 461 (10.7) | 251 (10.2) | 95 (10.5) | 115 (12.2) | 0.20 |
| Pregnancy with HCG >100 IU/ml per FET, n (%) | 3844 (26.7) | 2214 (27.2) | 812 (26.0) | 818 (25.9) | 0.20 |
| Early pregnancy loss per pregnancy with HCG >100 IU/ml, n (%) | 1210 (31.5) | 808 (36.5) | 209 (25.7) | 193 (23.6) | <0.005 |
| Live birth per FET, n (%) | 2571 (17.8) | 1375 (16.9) | 588 (18.8) | 608 (19.3) | <0.003 |
FET, frozen embryo transfer.
Risk factors for early pregnancy loss: logistic regression
Characteristics of patients having obtained a pregnancy with hCG >100 IU/ml are shown in Table II. Patients were comparable between the three groups, except for higher BMI and more patients with ovulation disorder in AC and SC. These differences are related to indications reported by the centers (NC was not used for patients with polycystic ovary syndrome). A history of childbirth was significantly more frequent in the AC group and endometrial thickness was significantly greater in the NC group.
Table II.
Characteristics of patients with pregnancy with HCG >100 IU/ml after FET according to endometrial preparation protocol.
| Total cycles | Artificial cycle (AC) | Natural cycle (NC) | Stimulated cycle (SC) | P | |
|---|---|---|---|---|---|
| Number of pregnancies with HCG >100 IU/ml | 3844 | 2214 | 812 | 818 | |
| Age (years), mean ± SD | 33.5 ± 4.3 | 33.3 ± 4.4 | 33.7 ± 4.3 | 33.8 ± 4.2 | 0.40 |
| BMI (kg/m2), mean ± SD | 21.9 ± 4.8 | 22.4 ± 4.5 | 21.4 ± 5.1 | 21.1 ± 5.6 | 0.01 |
| Smoking (%) | 879 (22.9) | 517 (23.4) | 188 (23.2) | 174 (21.3) | 0.50 |
| Ovulation disorder* (%) | 937 (24.4) | 682 (30.8) | 89 (11) | 166 (20.3) | 0.01 |
| History of early pregnancy loss (%) | 591 (13.4) | 335 (15.1) | 131 (16.1) | 125 (15.3) | 0.80 |
| History of childbirth (%) | 1919 (49.9) | 1137 (51.5) | 373 (48.4) | 409 (48.8) | 0.03 |
| Uterine disease (%) | 261 (6.8) | 152 (6.9) | 65 (8) | 44 (5.4) | 0.10 |
| Endometriosis (%) | 383 (10) | 228 (10.3) | 69 (8.5) | 86 (10.5) | 0.30 |
| Endometrial thickness (mm), mean ± SD | 9.3 ± 2.6 | 9 ± 2.8 | 9.9 ± 2.4 | 9.2 ± 2.2 | <0.01 |
| Number of embryos transferred, mean ± SD | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.5 ± 0.5 | 0.09 |
| Duration of freezing (days), mean ± SD | 363 ± 470 | 354 ± 467 | 382 ± 465 | 371 ± 484 | 0.60 |
Ovulation disorders include central anovulation, polycystic ovary syndrome and primary ovarian insufficiency.
FET, frozen embryo transfer.
A logistic regression model with adjustment was used to identify factors significantly correlated with the risk of early pregnancy loss. Results of the univariable and multivariable analyses are shown in Table III. Smoking and BMI were included in the multivariable analysis despite the lack of significance in univariable analysis, as previous studies have shown these variables to be associated with the risk of early pregnancy loss (Boots and Stephenson, 2011; Pineles et al., 2014; Cavalcante et al., 2019; Ghimire et al., 2020). After adjustment for confounding variables (age, BMI, smoking, history of childbirth or of early pregnancy loss, ovulation disorder, endometriosis, uterine disease and date of embryo freezing), the multivariable analysis showed a greater risk of early pregnancy loss with AC compared with NC, with an OR of 1.63 (1.35–1.97; P < 0.0001). There was no significant difference between NC and SC. Other factors significantly associated to the risk of early pregnancy loss after FET were maternal age over 38 years at the time of embryo freezing, history of early pregnancy loss, ovulation disorder and length of embryo storage superior to 6 months. Early pregnancy loss per pregnancy with hCG >100 IU/ml according to centers is shown in Supplementary Table SII.
Table III.
Risk of early pregnancy loss per pregnancy with HCG >100 IU/ml according to endometrial preparation protocol in univariable and multivariable analysis.
| Early pregnancy loss |
Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| N | % | Odds ratio (CI 95%) | Odds ratio (CI 95%) | P | |
| Endometrial preparation protocol | |||||
| Natural cycle | 209 | 25.7 | 1 | 1 | – |
| Stimulated cycle | 193 | 23.6 | 0.89 (0.71–1.12) | 0.88 (0.69–1.10) | 0.26 |
| Artificial cycle | 808 | 36.5 | 1.66 (1.38–1.78) | 1.63 (1.35–1.97) | <0.0001 |
| Age (years) | |||||
| 25–34 | 626 | 28.9 | 1 | 1 | – |
| <25 | 27 | 37.5 | 1.48 (0.91–2.41) | 1.44 (0.88–2.37) | 0.15 |
| 35–37 | 264 | 30.3 | 1.07 (0.90–1.27) | 1.05 (0.88–1.25) | 0.62 |
| 38–40 | 204 | 38 | 1.51 (1.24–1.84) | 1.50 (1.22–1.84) | 0.0001 |
| >40 | 89 | 45.9 | 2.09 (1.55–2.81) | 2.01 (1.48–2.74) | <0.0001 |
| BMI (kg/m2) | |||||
| 18–24 | 687 | 31.2 | 1 | 1 | – |
| <18 | 45 | 37.2 | 1.30 (0.89–1.91) | 1.34 (0.91–1.98) | 0.14 |
| 25–29 | 210 | 31.4 | 1.01 (0.84–1.22) | 1.04 (0.85–1.26) | 0.72 |
| ≥30 | 125 | 33.7 | 1.12 (0.89–1.41) | 1.09 (0.85–1.39) | 0.49 |
| Missing data | 143 | 29.5 | 0.92 (0.74–1.14) | 1.32 (0.97–1.79) | 0.08 |
| Smoking | |||||
| No | 779 | 31.6 | 1 | 1 | – |
| Yes | 295 | 33.6 | 1.09 (0.93–1.29) | 1.13 (0.95–1.34) | 0.16 |
| Missing data | 136 | 27.4 | 0.82 (0.66–1.01) | 0.88 (0.63–1.19) | 0.37 |
| History of early pregnancy loss | |||||
| No | 1 | 1 | |||
| Yes | 277 | 22.9 | 2.19 (1.83–2.62) | 2.13 (1.72–2.62) | <0.0001 |
| Ovulation disorder* | |||||
| No | 738 | 30.1 | 1 | 1 | – |
| Yes | 342 | 36.5 | 1.33 (1.14–1.56) | 1.23 (1.04–1.45) | 0.01 |
| Missing data | 130 | 28.4 | 0.92 (0.74–1.14) | 1.10 (0.82–1.48) | 0.52 |
| Uterine disease | |||||
| No | 1108 | 30.9 | 1 | 1 | |
| Yes | 102 | 39.1 | 1.43 (1.11–1.86) | 1.20 (0.91–1.57) | 0.19 |
| Endometriosis | |||||
| No | 1069 | 30.9 | 1 | ||
| Yes | 141 | 36.8 | 1.30 (1.05–1.62) | 1.24 (0.99–1.56) | 0.07 |
| Length of embryo storage | |||||
| <3 months | 272 | 27.4 | 1 | 1 | – |
| 3–6 months | 368 | 32.5 | 1.27 (1.05–1.53) | 1.20 (0.99–1.45) | 0.06 |
| 6–12 months | 240 | 34.2 | 1.37 (1.14–1.69) | 1.29 (1.04–1.60) | 0.02 |
| >12 months | 326 | 32.4 | 1.26 (1.04–1.53) | 1.24 (1.01–1.51) | 0.04 |
| Missing data | 4 | 36.4 | 1.51 (0.44–5.20) | 1.22 (0.34–4.32) | 0.76 |
| Embryo stage | |||||
| Day 2 | 164 | 35.3 | 1 | 1 | – |
| Day 3 | 499 | 30.4 | 0.80 (0.64–0.99) | 0.83 (0.66–1.05) | 0.11 |
| Blastocyst | 543 | 31.5 | 1.00 (0.87–1.15) | 0.88 (0.70–1.11) | 0.28 |
| Zygote | 4 | 28.6 | 0.73 (0.23–2.37) | 0.71 (0.21–2.37) | 0.57 |
Ovulation disorders include central anovulation, polycystic ovary syndrome, and primary ovarian insufficiency.
Discussion
This multicenter cohort study of 14 421 FET cycles and 3844 pregnancies shows that endometrial preparation by AC is associated to a significantly higher risk of early pregnancy loss and significantly lower live birth rate compared with NC and SC. The rate of pregnancies with hCG >100 IU/ml was comparable between the three types of protocols.
To our knowledge, our study is the first to compare the early pregnancy loss rate between the three types of endometrial preparation protocols for FET. So far, no consensus exists on which protocol leads to the best pregnancy rates and clinical outcomes (Groenewoud et al., 2013; Peeraer et al., 2015; Yu et al., 2015; Cerrillo et al., 2017; Ghobara et al., 2017). This lack of significant difference between protocols may relate to insufficient sample sizes and an overall low level of proof. The power of our study was high, as it included a large number of FET cycles and considered various parameters affecting the likelihood of childbirth. However, the fact that the AC group comprised more patients than the NC and SC group (n = 2214 vs 812 vs 818, respectively) might limit the validity of our findings. Furthermore, the AC group comprised a significantly higher proportion of patients with ovulatory disorders (31% for AC vs 11% for NC vs 20% for SC, respectively, P < 0.01), which is explained by the fact that endometrial preparation by AC was more prescribed to patients with ovulatory disorders such PCOS, central anovulation and primary ovarian insufficiency compared to NC and SC protocols. Since PCOS patients are associated to higher risks of early pregnancy loss, this fact may also increase the proportion of pregnancy losses in the AC group compared to the other groups. Moreover, the risk of early pregnancy loss considering clinical pregnancies has not been evaluated, as cut-offs and definitions used in our study were determined in accordance with recommendations of the French Biomedicine Agency (Agence de la Biomédecine). Further studies using more standardized definitions are warranted (Kolte et al., 2015). After adjustment on potential risk factors, we found that the different types of protocol were similar in terms of biochemical pregnancies (hCG > 10 and lower than 100 IU/l) and rates of pregnancies with hCG >100 IU/ml. Hence, defective implantation might not be the only explanation of the increased risk of early pregnancy loss with AC, which has a significant impact on the likelihood of childbirth. Altogether, the exact mechanisms underlying the enhanced rates of early pregnancy losses reported in our study remain to be established. Although AC was significantly associated to lower rates of live birth compared to NC and SC (16.9% vs 18.8% vs 19.3%, respectively, P < 0.05), the variation among the three groups was not as distinct as the variation observed for early pregnancy loss.
These results are in line with findings of several earlier and smaller studies. The randomized prospective study of Cerrillo et al. led in 2011–2012 on 570 FET cycles found a first-trimester early pregnancy loss rate of 21.2% with AC, 12.9% with NC and 11.1% with modified NC (P = 0.01) (Cerrillo et al., 2017). These rates are lower than those reported in our study but exclusion criteria differed: patients were under 39 years of age and had regular cycles, and patients with stage III/IV endometriosis and polycystic ovary syndrome were excluded. In their retrospective study of 4470 FET, Tomás et al. (2012) also reported a higher rate of early pregnancy loss with AC compared with modified NC and NC with luteal phase support by progesterone (41.5% vs 33.6% vs 22.4%, respectively, P < 0.001). In a retrospective analysis of 1132 FET, Veleva et al. (2008) showed that the early pregnancy loss rate was 1.7 times higher in AC than in the NC and fresh embryo transfer cycles. A more recent French retrospective study analyzing 1926 FET also found an increase of over 20% of early pregnancy loss with AC compared with SC (Hatoum et al., 2018). Live birth rate was also significantly lower with AC (29.6%) than SC (29.6% vs 59.9%; P < 0.001) (Hatoum et al., 2018). Similarly to our study, there was no difference in terms of clinical pregnancies. Overall, the higher rates of early pregnancy loss reported with AC might be explained by defective placentation. In addition to early pregnancy loss, AC has also been associated to adverse obstetrical and perinatal outcomes. A retrospective study of 9726 singletons born after FET, of which 6297 NC, 1983 SC and 1446 AC showed that AC was associated with a higher risk of hypertensive disorders during pregnancy, postpartum hemorrhage, post-term birth and macrosomia compared to SC and NC (Ginström Ernstad et al., 2019). Similarly, a retrospective cohort study of FET, of which 29 760 performed with NC and 75 474 performed with AC found increased risks of hypertensive disorders of pregnancy and placenta accreta compared to NC (Saito et al., 2019). Moreover, risk factors associated to early pregnancy loss after FET reported in previous literature were confirmed in our study: maternal age, history of early pregnancy loss and ovulation disorder. The rate of blastocyst transfers was significantly higher in the AC group (40.9% for AC vs 26.3% for NC vs 38.1% for SC, respectively, P < 0.05), but was not significantly associated to early pregnancy loss in multivariable analysis.
A real or relative deficiency in progesterone during early pregnancy might increase the risk of early pregnancy loss. Indeed, progesterone plays an essential role in the secretory transformation of the endometrium to enable synchronization with embryonic development and maintain pregnancy. An insufficient progesterone concentration at the time of implantation or at the start of pregnancy may result in early pregnancy loss or defective placentation. Progesterone also reduces the contractility of the myometrium (Hill et al., 1990). For FET with AC, sufficient exogenous supplementation with progesterone is mandatory since no functional corpus luteum is present. In our study, luteal phase support by progesterone was homogeneous overall. In our study, the differences between centers concerning the dose of progesterone used did not affect the results. Indeed, no significant difference was observed in early pregnancy loss rates between the nine centers, whatever the dose used, which ranged from 400 to 800 mg per day (micronized progesterone by vaginal route in all centers). The dosage and route of administration of progesterone supplementation is currently discussed. The 2010 Cochrane meta-analysis (Glujovsky et al., 2010) and the 2017 meta-analysis of Mackens et al. (2017) agree that there is no superior route of administration. A more recent American prospective study (Devine et al., 2018) suggests that the intramuscular route results in fewer early pregnancy loss and hence more live births. Pharmacokinetics is known to differ between the different routes (Miles et al., 1994). Although several studies (Miles et al., 1994; Kofinas et al., 2015; Yovich et al., 2015) have investigated the association between progesterone levels on the day of embryo transfer and the outcome of FET in AC, no clear guideline concerning a progesterone level cut-off exists so far. Serum progesterone levels <10 ng/ml on transfer day were reported to be significantly associated to lower pregnancy rates (P = 0.04) and live birth rates (P = 0.01) (Cédrin-Durnerin et al., 2019), which is consistent with findings of Gaggiotti-Marre et al. (2019). Furthermore, increasing the dose of progesterone administered in case of levels <10 ng/ml did not improve pregnancy rates. Recently, serum progesterone levels <8.8 ng/ml on the day of embryo transfer was associated to lower ongoing pregnancy rate in both autologous and donated oocyte cycles (Labarta et al., 2021).
The association between serum progesterone levels and outcomes in autologous FET using AC have also been described whether progesterone was administered using the vaginal route (Yovich et al., 2015; Alsbjerg et al., 2018; Basnayake et al., 2018) or the intramuscular route (Kofinas et al., 2015; Liu et al., 2018; Boynukalin et al., 2019). Moreover, a prospective study combining vaginal and rectal progesterone in AC cycles reported a non-linear relationship between serum progesterone levels and ongoing pregnancy rates, and that there might be an upper threshold above which pregnancy rates are decreased (Alsbjerg et al., 2020). The same relationship has been observed in the specific context of oocyte donation (Brady et al., 2014; Labarta et al., 2017; Labrosse et al., 2021). Nonetheless, important interindividual variabilities of serum progesterone levels have been described after either route of progesterone administration and various parameters might affect progesterone levels, thus making it particularly difficult to predict serum concentrations after a given dosage (Nahoul et al., 1993; Brady et al., 2014; Kofinas et al., 2015; Merriam et al., 2015). Notably, large variations of serum progesterone levels were reported despite all women receiving the same AC protocol (Yovich et al., 2015). Ultimately, no consensus exists on the optimal threshold for progesterone levels. Systematic assessment of serum progesterone levels during AC, so as to adapt supplementation by vaginal progesterone or to use other routes of administration (subcutaneous, oral) in order to avoid low progesterone levels in AC appears essential (Labarta et al., 2017; Cédrin-Durnerin et al., 2019; Alsbjerg et al., 2020). Furthermore, the use of dydrogesterone might also be an option. A prospective cohort study reported that the addition of dydrogesterone to vaginal progesterone compared to vaginal progesterone only was significantly associated to a lower risk of early miscarriage and to a significantly higher live birth rate in multivariate analysis (Vuong et al., 2021). A retrospective study of 9726 singletons born after FET, of which 6297 NC, 1983 SC and 1446 AC showed that AC was associated with a higher risk of hypertensive disorders during pregnancy, postpartum hemorrhage, post-term birth and macrosomia compared to SC and NC (Ginström Ernstad et al., 2019). Similarly, a retrospective cohort study of FET, of which 29 760 performed with NC and 75 474 performed with AC found increased risks of hypertensive disorders of pregnancy and placenta accreta compared to NC (Saito et al., 2019).
In all, AC was the most used protocol in the nine centers, in line with current practice worldwide. This might be explained by the fact that AC has the advantage of enabling flexibility and simple regulation of the number and timing of transfers for IVF centers. As our study reports that AC is associated to higher rates of early pregnancy loss, therapeutic adaptation and the combination of routes of administration and/or monitoring of serum progesterone in patients undergoing FET with AC might be options to avoid the excess of early pregnancy loss. Routine measurements of serum progesterone levels in AC should be considered. Future studies exploring the efficiency of different routes of progesterone administration and prospective randomized studies analyzing strategies to obtain optimal serum progesterone levels in AC are warranted.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
L.V. was responsible for data collection, data analysis and writing of the first draft of the manuscript. F.P. was responsible for study design and data collection. G.P-B., N.C., J.G., N.A., J.P.A., C.R., P.E.B., E.M.d’A. and I.C.-D. contributed to the execution of the study and critical discussion. J.L. was responsible for the execution and manuscript drafting. N.M. was responsible for study design, execution, data analysis and manuscript drafting.
Funding
None.
Conflict of interest
The authors declare no conflicts of interest in relation to this work. G.P.-B. declares consulting fees from Ferring, Gedeon-Richter, Merck KGaA, Theramex, Teva; Speaker’s fees or equivalent from Merck KGaA, Ferring, Gedeon-Richter, Theramex, Teva. N.C. declares consulting fees from Ferring, Merck KGaA, Theramex, Teva; Speaker’s fees or equivalent from Merck KGaA, Ferring. C.R. declares a research grant from Ferring, Gedeon-Richter; consulting fees from Gedeon-Richter, Merck KGaA; Speaker’s fees or equivalent from Merck KGaA, Ferring, Gedeon-Richter. E.M.d’A. declares Speaker’s fees or equivalent from Merck KGaA, MSD, Ferring, Gedeon-Richter, Theramex, Teva. I.C-D. declares Speaker’s fees or equivalent from Merck KGaA, MSD, Ferring, Gedeon-Richter, IBSA. N.M. declares a research grant from Merck KGaA, MSD, IBSA; consulting fees from MSD, Ferring, Gedeon-Richter, Merck KGaA; Speaker’s fees or equivalent from Merck KGaA, MSD, Ferring, Gedeon-Richter, Teva, Goodlife, General Electrics.
Supplementary Material
Contributor Information
L Vinsonneau, Department of Reproductive Medicine, Hopital Tenon, Paris, France.
J Labrosse, Department of Reproductive Medicine and Fertility Preservation, CHU Jean-Verdier, Bondy, France.
G Porcu-Buisson, Department of Reproductive Medicine, Institut de Médecine de la Reproduction, Marseille, France.
N Chevalier, Department of Reproductive Medicine, Polyclinique Saint-Roch, Montpellier, France.
J Galey, Department of Reproductive Medicine, Institut Montsouris, Paris, France.
N Ahdad, Department of Reproductive Medicine, Hopital Tenon, Paris, France; Department of Reproductive Medicine, Grand Hôpital de l'Est Francilien, Meaux, France.
J P Ayel, Department of Reproductive Medicine, Groupe Hospitalier Diaconesses Croix Saint-Simon, Paris, France.
C Rongières, Department of Reproductive Medicine, Centre Médico-Chirurgical Obstétrique, Strasbourg, France.
P E Bouet, Department of Reproductive Medicine, CHU Angers, Angers, France.
E Mathieu d’Argent, Department of Reproductive Medicine, Hopital Tenon, Paris, France.
I Cédrin-Durnerin, Department of Reproductive Medicine and Fertility Preservation, CHU Jean-Verdier, Bondy, France.
F Pessione, Department of Procreation, Embryology and Human Genetics, Agence de la Biomédecine, Paris, France.
N Massin, Department of Reproductive Medicine, Intercommunal Hospital—University Paris Est, Créteil, France.
References
- Agence de la Biomédecine. https://rams.agence-biomedecine.fr/assistance-medicale-la-procreation (15 January 2022, date last accessed).
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, Haahr T, Humaidan P. Can combining vaginal and rectal progesterone achieve the optimum progesterone range required for implantation in the HRT-FET model? Reprod Biomed Online 2020;40:805–811. [DOI] [PubMed] [Google Scholar]
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, Haahr T, Humaidan P. Progesterone levels on pregnancy test day after hormone replacement therapy-cryopreserved embryo transfer cycles and related reproductive outcomes. Reprod Biomed Online 2018;37:641–647. [DOI] [PubMed] [Google Scholar]
- Basnayake SK, Volovsky M, Rombauts L, Osianlis T, Vollenhoven B, Healey M. Progesterone concentrations and dosage with frozen embryo transfers—what’s best? Aust N Z J Obstet Gynaecol 2018;58:533–538. [DOI] [PubMed] [Google Scholar]
- Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med 2011;29:507–513. [DOI] [PubMed] [Google Scholar]
- Boynukalin FK, Gultomruk M, Turgut E, Demir B, Findikli N, Serdarogullari M, Coban O, Yarkiner Z, Bahceci M. Measuring the serum progesterone level on the day of transfer can be an additional tool to maximize ongoing pregnancies in single euploid frozen blastocyst transfers. Reprod Biol Endocrinol 2019;17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady PC, Kaser DJ, Ginsburg ES, Ashby RK, Missmer SA, Correia KF, Racowsky C. Serum progesterone concentration on day of embryo transfer in donor oocyte cycles. J Assist Reprod Genet 2014;31:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante MB, Sarno M, Peixoto AB, Araujo Júnior E, Barini R. Obesity and recurrent miscarriage: a systematic review and meta-analysis. J Obstet Gynaecol Res 2019;45:30–38. [DOI] [PubMed] [Google Scholar]
- Cédrin-Durnerin I, Isnard T, Mahdjoub S, Sonigo C, Seroka A, Comtet M, Herbemont C, Sifer C, Grynberg M. Serum progesterone concentration and live birth rate in frozen-thawed embryo transfers with hormonally prepared endometrium. Reprod Biomed Online 2019;38:472–480. [DOI] [PubMed] [Google Scholar]
- Cerrillo M, Herrero L, Guillén A, Mayoral M, García-Velasco JA. Impact of endometrial preparation protocols for frozen embryo transfer on live birth rates. Rambam Maimonides Med J 2017;8:e0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mouzon J, Chambers GM, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S, Kupka M, Adamson GD. International Committee for Monitoring Assisted Reproductive Technologies world report: assisted reproductive technology 2012. Hum Reprod 2020;35:1900–1913. [DOI] [PubMed] [Google Scholar]
- Devine K, Richter KS, Widra EA, McKeeby JL. Vitrified blastocyst transfer cycles with the use of only vaginal progesterone replacement with Endometrin have inferior ongoing pregnancy rates: results from the planned interim analysis of a three-arm randomized controlled noninferiority trial. Fertil Steril 2018;109:266–275. [DOI] [PubMed] [Google Scholar]
- Gaggiotti-Marre S, Martinez F, Coll L, Garcia S, Álvarez M, Parriego M, Barri PN, Polyzos N, Coroleu B. Low serum progesterone the day prior to frozen embryo transfer of euploid embryos is associated with significant reduction in live birth rates. Gynecol Endocrinol 2019;35:439–442. [DOI] [PubMed] [Google Scholar]
- Ghimire PR, Akombi-Inyang BJ, Tannous C, Agho KE. Association between obesity and miscarriage among women of reproductive age in Nepal. PLoS One 2020;15:e0236435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev 2017;7:CD003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginström Ernstad E, Wennerholm U-B, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol 2019;221:126.e1–126.e18. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev 2010;10:CD006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewoud ER, Cantineau AEP, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 2013;19:458–470. [DOI] [PubMed] [Google Scholar]
- Hatoum I, Bellon L, Swierkowski N, Ouazana M, Bouba S, Fathallah K, Paillusson B, Bailly M, Boitrelle F, Alter L et al. Disparities in reproductive outcomes according to the endometrial preparation protocol in frozen embryo transfer: the risk of early pregnancy loss in frozen embryo transfer cycles. J Assist Reprod Genet 2018;35:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NC, Selinger M, Ferguson J, López Bernal A, Mackenzie IZ. The physiological and clinical effects of progesterone inhibition with mifepristone (RU 486) in the second trimester. Br J Obstet Gynaecol 1990;97:487–492. [DOI] [PubMed] [Google Scholar]
- Kofinas JD, Blakemore J, McCulloh DH, Grifo J. Serum progesterone levels greater than 20 ng/dl on day of embryo transfer are associated with lower live birth and higher pregnancy loss rates. J Assist Reprod Genet 2015;32:1395–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolte AM, Bernardi LA, Christiansen OB, Quenby S, Farquharson RG, Goddijn M, Stephenson MD; ESHRE Special Interest Group, Early Pregnancy. Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod 2015;30:495–498. [DOI] [PubMed] [Google Scholar]
- Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, Bosch E. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod 2017;32:2437–2442. [DOI] [PubMed] [Google Scholar]
- Labarta E, Mariani G, Paolelli S, Rodriguez-Varela C, Vidal C, Giles J, Bellver J, Cruz F, Marzal A, Celada P et al. Impact of low serum progesterone levels on the day of embryo transfer on pregnancy outcome: a prospective cohort study in artificial cycles with vaginal progesterone. Hum Reprod 2021;36:683–692. [DOI] [PubMed] [Google Scholar]
- Labrosse J, Lobersztajn A, Pietin-Vialle C, Villette C, Dessapt AL, Jung C, Brussieux M, Bry-Gauillard H, Pasquier M, Massin N. Comparison of stimulated versus modified natural cycles for endometrial preparation prior to frozen embryo transfer: a randomized controlled trial. Reprod Biomed Online 2020;40:518–524. [DOI] [PubMed] [Google Scholar]
- Labrosse J, Peigné M, Eustache F, Sifer C, Grynberg M, Cedrin-Durnerin I. Women utilizing oocyte donation have a decreased live birth rate if they displayed a low progesterone level in a previous hormonal replacement mock cycle. J Assist Reprod Genet 2021;38:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi Setti PE, Cirillo F, De Cesare R, Morenghi E, Canevisio V, Ronchetti C, Baggiani A, Smeraldi A, Albani E, Patrizio P. Seven years of vitrified blastocyst transfers: comparison of 3 preparation protocols at a single ART center. Front Endocrinol (Lausanne) 2020;11:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shi W, Shi J. Natural cycle frozen-thawed embryo transfer in young women with regular menstrual cycles increases the live-birth rates compared with hormone replacement treatment: a retrospective cohort study. Fertil Steril 2020;113:811–817. [DOI] [PubMed] [Google Scholar]
- Liu Y-F, Wang F, Huang G-Y, Mao X-G. The relationship between the hormone levels before transplantation and the outcomes of hormone replacement therapy frozen embryo transfer. Minerva Endocrinol 2018;43:406–412. [DOI] [PubMed] [Google Scholar]
- Mackens S, Santos-Ribeiro S, Vijver A, Racca A, Van Landuyt L, Tournaye H, Blockeel C. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod 2017;32:2234–2242. [DOI] [PubMed] [Google Scholar]
- Merriam KS, Leake KA, Elliot M, Matthews ML, Usadi RS, Hurst BS. Sexual absorption of vaginal progesterone: a randomized control trial. Int J Endocrinol 2015;2015:685281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril 1994;62:485–490. [DOI] [PubMed] [Google Scholar]
- Nahoul K, Dehennin L, Jondet M, Roger M. Profiles of plasma estrogens, progesterone and their metabolites after oral or vaginal administration of estradiol or progesterone. Maturitas 1993;16:185–202. [DOI] [PubMed] [Google Scholar]
- Peeraer K, Couck I, Debrock S, De Neubourg D, De Loecker P, Tomassetti C, Laenen A, Welkenhuysen M, Meeuwis L, Pelckmans S et al. Frozen-thawed embryo transfer in a natural or mildly hormonally stimulated cycle in women with regular ovulatory cycles: a RCT. Hum Reprod 2015;30:2552–2562. [DOI] [PubMed] [Google Scholar]
- Peigné M, Devouche E, Ferraretto X, Gricourt S, Luton D, Patrat C, Epelboin S. Higher live birth rate with stimulated rather than artificial cycle for frozen-thawed embryo transfer. Eur J Obstet Gynecol Reprod Biol 2019;243:144–149. [DOI] [PubMed] [Google Scholar]
- Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol 2014;179:807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, Fukami M, Miyasaka N, Ishihara O, Irahara M et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod 2019;34:1567–1575. [DOI] [PubMed] [Google Scholar]
- Tomás C, Alsbjerg B, Martikainen H, Humaidan P. Pregnancy loss after frozen-embryo transfer–a comparison of three protocols. Fertil Steril 2012;98:1165–1169. [DOI] [PubMed] [Google Scholar]
- Veleva Z, Tiitinen A, Vilska S, Hydén-Granskog C, Tomás C, Martikainen H, Tapanainen JS. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum Reprod 2008;23:878–884. [DOI] [PubMed] [Google Scholar]
- Vuong LN, Pham TD, Le KTQ, Ly TT, Le HL, Nguyen DTN, Ho VNA, Dang VQ, Phung TH, Norman RJ et al. Micronized progesterone plus dydrogesterone versus micronized progesterone alone for luteal phase support in frozen-thawed cycles (MIDRONE): a prospective cohort study. Hum Reprod 2021;36:1821–1831. [DOI] [PubMed] [Google Scholar]
- Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2017;3:CD011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, Motrenko T, Rugescu I, Smeenk J, Tandler-Schneider A, Vidakovic S. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovich JL, Conceicao JL, Stanger JD, Hinchliffe PM, Keane KN. Mid-luteal serum progesterone concentrations govern implantation rates for cryopreserved embryo transfers conducted under hormone replacement. Reprod Biomed Online 2015;31:180–191. [DOI] [PubMed] [Google Scholar]
- Yu J, Ma Y, Wu Z, Li Y, Tang L, Li Y, Deng B. Endometrial preparation protocol of the frozen-thawed embryo transfer in patients with polycystic ovary syndrome. Arch Gynecol Obstet 2015;291:201–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.