Abstract
The activity of LY333328 against Enterococcus faecalis JH2-2, which is susceptible to glycopeptides, and against its transconjugants E. faecalis BM4281 and BM4316, with VanB and VanA phenotypes, respectively, was investigated. LY333328 was active in vitro against the three strains, for which MICs were 2 μg/ml on agar and 0.25 μg/ml in broth. LY333328 was bactericidal in broth against E. faecalis JH2-2 and BM4281 at a concentration of 8 μg/ml and against BM4316 at a concentration of 30 μg/ml. The protein binding of LY333328 to rabbit serum was >99%, and the bactericidal activity of LY333328 in broth was reduced when it was tested in the presence of 90% rabbit serum. Autoradiographic studies performed in rabbits with enterococcal endocarditis showed that 14[C]LY333328 was distributed heterogeneously throughout cardiac vegetations. In rabbits with aortic endocarditis, a regimen of 20 mg of LY333328 per kg of body weight administered intramuscularly twice a day for 5 days after a loading dose of 40 mg/kg was active against the three strains in vivo (P < 0.01), whereas vancomycin was not active against the VanB-type strain and teicoplanin was not active against the VanA-type strain. We conclude that the activity of LY333328 is not significantly modified by acquired resistance to glycopeptides in E. faecalis either in vitro or in experimental endocarditis.
Enterococci are now recognized as major nosocomial pathogens (7), representing the third-most-common cause of hospital-acquired bacteremias (23) and accounting for up to 50% of bacteremias in some centers (5). The major concern with these microorganisms is the emergence in clinical settings of strains which are resistant to penicillins, aminoglycosides, and glycopeptides (18). There is currently no uniformly effective antimicrobial therapy for patients infected with multi-drug-resistant enterococci, emphasizing the need for new therapeutic options.
LY333328 is a semisynthetic N-alkyl derivative of LY264826, a naturally-occurring structural analog of vancomycin. LY333328 has been evaluated in vitro and was highly active against gram-positive cocci, including enterococci with acquired resistance to the available glycopeptides, vancomycin and teicoplanin, in terms of bacteriostatic (13, 16) and bactericidal (18, 20, 26) activities. However, very little is known about its in vivo activity.
Our aim in the present work was to study (i) the activity of LY333328 in vitro and in experimental endocarditis against Enterococcus faecalis susceptible to glycopeptides or against E. faecalis with VanA and VanB phenotypes to investigate the influence of acquired resistance to glycopeptides on the activity of LY333328 and (ii) the pattern of diffusion of radiolabeled LY333328 in vegetations from rabbits with experimental endocarditis.
MATERIALS AND METHODS
In vitro studies. (i) Organisms.
Three strains of E. faecalis (kindly provided by Michel Arthur and Patrice Courvalin, Unité des Agents Antibactériens, Institut Pasteur) were used for in vitro and in vivo experiments. E. faecalis JH2-2 is susceptible to glycopeptides and does not display acquired resistance to high levels of β-lactams or aminoglycosides (15). E. faecalis BM4281, which harbors a 250-kb chromosomal vanB element that confers VanB-type resistance, was obtained by conjugal transfer of vancomycin resistance from clinical isolate E. faecium BM4120 to JH2-2 (4, 22). E. faecalis BM4316 harbors a 50-kb vanA element that confers VanA-type resistance and was obtained by conjugal transfer from clinical isolate E. faecium HM1074 to JH2-2 (3).
(ii) Media and antibiotics.
Antibiotic susceptibility testing was done in brain heart infusion (BHI) broth and agar (Difco Laboratories, Detroit, Mich.) at 37°C. Drugs were supplied by their manufacturers as follows. Vancomycin was obtained from Eli Lilly France, Saint-Cloud, France; teicoplanin was obtained from Marion Merrell Dow, Levallois-Perret, France; and LY333328 and [14C]LY333328 were obtained from Eli Lilly Research Laboratories, Indianapolis, Ind.
(iii) In vitro susceptibility to antibiotics.
The MICs of vancomycin, teicoplanin, and LY333328 were determined by the method of Steers et al. (25) with 105 CFU per spot on BHI agar after 24 h of incubation. The MICs and minimal bactericidal concentrations (MBCs) were also determined by the macrodilution method with an inoculum of 106 CFU/ml in BHI broth alone or supplemented with 50% complement-inactivated rabbit serum. The MIC was defined as the lowest concentration that prevented turbidity after 24 h of incubation. The MBC was defined as the lowest concentration of antimicrobial agent that killed at least 99.9% of the original inoculum (21).
(iv) Study of bactericidal activity.
Time-kill curves were used to test the bactericidal activity of vancomycin, teicoplanin, and LY333328 against the three study strains. Overnight cultures were diluted in glass tubes containing 10 ml of fresh BHI to yield an inoculum of approximately 5 × 106 CFU/ml. Antibiotics were used at concentrations of 30 μg/ml for teicoplanin and vancomycin and 8 and 30 μg/ml for LY333328, which were in the ranges of serum concentrations achieved in treated animals. In order to test the influence of a high inoculum representative of the in vivo inoculum present in cardiac vegetations, the activity of LY333328 was tested against BM4316 with an inoculum of 108 CFU/ml. The influence of the presence of serum was assessed by testing the activity of LY333328 against BM4316 in the presence of 90% rabbit serum (Sigma-Aldrich Chimie, Saint-Quentin Fallvier, France). After 0, 3, 6, and 24 h of incubation at 37°C, serial dilutions of 0.1-ml samples were subcultured onto agar plates with a spiral plater (Spiral System Inc., Cincinnati, Ohio) and incubated for 24 h. Potential antibiotic carryover of LY333328 was assessed by running concurrent samples, which were washed twice to remove antibiotic before plating. After 24 h of incubation, colonies (10 to 100 per plate) were counted, providing a lower limit of detection of 102 CFU/ml. Bactericidal activity was defined by a decrease of the initial inoculum of ≥3 log10 CFU/ml (21). All the in vitro experiments were run at least twice. All points of the time-kill studies were within 1 log of each other on different days.
In vivo studies. (i) Enterococcal experimental endocarditis.
Investigations were performed in female New Zealand White rabbits (weight range, 2.2 to 2.8 kg). Aortic endocarditis was induced in rabbits by insertion of a polyethylene catheter through the right carotid artery into the left ventricle to cause the formation of vegetations, as previously described (12). Twenty-four hours after catheter insertion, each rabbit was inoculated by ear vein with 108 CFU of E. faecalis in 1 ml of 0.9% NaCl. The catheter was left in place throughout the experiment. Forty-eight hours after inoculation, animals were treated intramuscularly twice daily for 5 days with one of the following regimens: vancomycin, 50 mg/kg of body weight; teicoplanin, 20 mg/kg after a loading dose of 40 mg/kg; LY333328, 20 mg/kg after a loading dose of 40 mg/kg. The vancomycin and teicoplanin regimens produced serum levels comparable to those achieved in humans, as previously described (4). Pilot studies showed that serum elimination half-life in rabbit was longer than 12 h for LY333328 (Eli Lilly, data on file), allowing a dosing regimen similar to that for teicoplanin. Control animals were left untreated; they were allowed to die and were sacrificed, if necessary, at the same time as the treated animals. The results obtained with vancomycin and teicoplanin against JH2-2 and BM4281 were previously reported (4). Animals were killed by intravenous injection of pentobarbital 12 h after the last antibiotic injection. All vegetations from each rabbit were excised, rinsed in saline, pooled, and weighed. They were homogenized in 1 ml of sterile saline, and 0.1-ml portions were quantitatively subcultured onto agar plates for 24 h. Colony count results were expressed as log10 CFU per gram of vegetation.
(ii) Serum pharmacokinetic studies.
Antibiotic serum levels were determined in at least three rabbits with experimental endocarditis at peak (1 h) and trough (12 h) during the last day of therapy. The binding of LY333328 to proteins in rabbit plasma was measured with the Centrifree micropartition device (Amicon, Millipore, Saint Quentin-Yveline, France) according to the ultrafiltration method described by Craig and Suh (8). Antibiotic concentrations were determined by high-performance liquid chromatography (HPLC) for teicoplanin and by fluorescence polarization immunoassay for vancomycin (4). LY333328 was assayed by a validated method (14) according to which a solid-phase extraction followed by HPLC with fluorescence detection is used as previously reported (17). Quality control samples (at three concentrations) were assayed with the study samples. Accuracy of the quality control samples ranged from 100.3 to 101.7%, and precision ranged from 0.16 to 0.50%. The lower limit of detection was 4 μg/ml.
Autoradiography studies. (i) Enterococcal endocarditis.
Experimental endocarditis was produced as described above. Animals were infected 24 h after placement of the catheter with 108 CFU of E. faecalis JH2-2.
(ii) [14C]LY333328 administration and assay.
Eight days after bacterial challenge, radiolabeled antibiotic was injected intravenously in a volume of 10 ml over a period of 30 min. Two rabbits received 118 and 352 μCi and were sacrificed 30 min after the end of the infusion, and one rabbit received 457 μCi and was sacrificed 12 h after the end of the infusion so that we could study the influence of time of sacrifice on the diffusion pattern of the antibiotic. Blood and plasma samples were collected at the end of infusion and at the time of sacrifice. Entire vegetations were excised for autoradiography, and others were used for measurement of antibiotic concentrations. Labeled antibiotic concentrations were counted in samples of plasma, blood, cardiac muscle, and vegetations by liquid scintillation counting, as previously described (11), and expressed as disintegrations/minute/gram. Results were expressed as mean radioactivity concentration in vegetation/injected radioactivity and mean vegetation/plasma, vegetation/blood, and vegetation/cardiac tissue concentration ratios.
Quantitative autoradiography.
Frozen vegetation samples were cut on a cryostat, thaw mounted onto gelatin-coated microscope slides, and freeze-dried at −25°C for 24 h, as previously described (11). A radioimager (InstantImager; Packard Instruments, Meriden, Conn.) was used for quantitative autoradiography. The principle of detection has been previously described (10). Vegetation sections were imaged for 20 h, and 14C microscale standards were simultaneously included for quantitation. Digital images were stored in a 400 by 480 pixel matrix (pixel area, 0.5 by 0.5 mm), and regions of interest were then manually drawn in order to obtain the different radioactive concentrations within the diffusion pattern of the antibiotic.
Statistics.
All the results were expressed as means ± standard deviations. Comparisons of the effect of a given antibiotic regimen against each strain of E. faecalis in terms of reduction in log10 CFU per gram of vegetation in relation to control animals (strain effect) and comparisons of bacterial titers in vegetations among different regimens against a given strain (treatment effect) were performed by analysis of variance followed by a multiple comparison of means by Fisher’s least significant difference procedure (22). Ratios of mean antibiotic radioactivity according to the time of sampling were compared by an unpaired t test.
RESULTS
Susceptibility tests.
As expected, vancomycin was not active against the VanB or VanA strains, whereas teicoplanin was not active against the VanA strain (Table 1). Although LY333328 was active against the three study strains, the level of activity depended upon the method used. When LY333328 was tested on agar, its MIC was 2 μg/ml against the three strains and in the range of those of teicoplanin and vancomycin for the susceptible strain JH2-2 (1 and 2 μg/ml, respectively). When LY333328 was tested in broth, its MIC was eightfold lower for the three strains (MIC, 0.25 μg/ml). This difference was not observed for the two other glycopeptides. In the presence of 50% rabbit serum, the MICs of the three antibiotics for the three strains (data not shown) were not significantly increased.
TABLE 1.
MICs and MBCs (in micrograms/milliliter) of LY333328, teicoplanin, and vancomycin for E. faecalis strains with various resistance phenotypes
| Antibiotic |
E. faecalis JH2-2 (susceptible)
|
E. faecalis BM4281 (VanB)
|
E. faecalis BM4316 (VanA)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC in:
|
MBC | MIC in:
|
MBC | MIC in:
|
MBC | ||||
| Agar | Broth | Agar | Broth | Agar | Broth | ||||
| LY333328 | 2 | 0.25 | 1 | 2 | 0.25 | 1 | 2 | 0.25 | 16 |
| Teicoplanin | 1 | 1 | 16 | 1 | 1 | 32 | 64 | 8 | >128 |
| Vancomycin | 2 | 2 | 128 | 256 | 128 | >128 | 256 | >128 | >128 |
In vitro bactericidal activity.
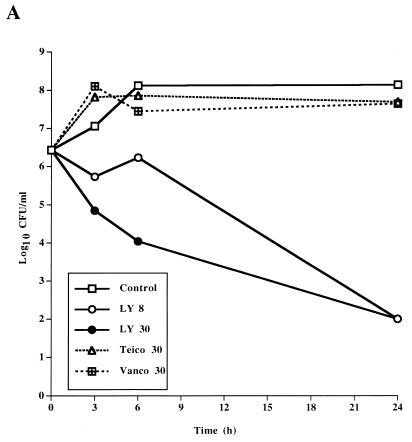
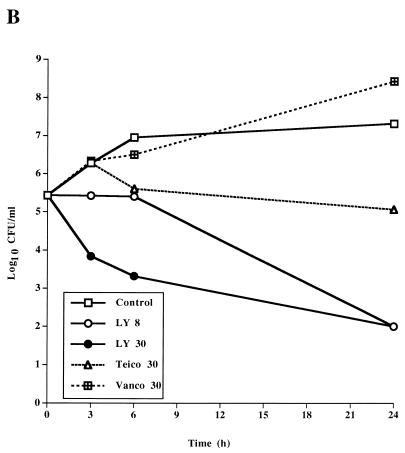
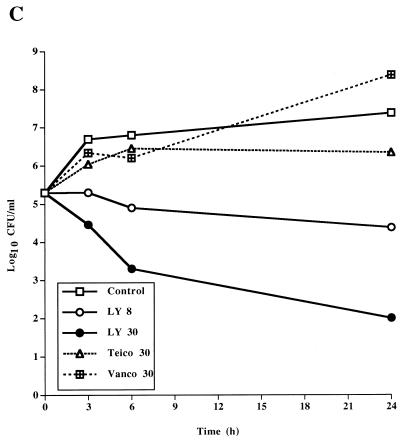
The bactericidal activity of LY333328 differed from those of teicoplanin and vancomycin (Table 1 and Fig. 1). For the susceptible strain JH2-2, teicoplanin and vancomycin had high MBCs and were not bactericidal at concentrations of 30 μg/ml in time-kill studies. In contrast, LY333328 was bactericidal against E. faecalis JH2-2 and BM4281 at a concentration of 8 μg/ml and against BM4316 at a concentration of 30 μg/ml. The higher concentration of LY333328 tested was more rapidly bactericidal than the 8 μg/ml concentration against the three strains and was the only regimen that was bactericidal against BM4316.
FIG. 1.
Time-kill curves determined for a susceptible strain of E. faecalis, JH2-2 (A), a VanB-type strain, BM4281 (B), and a VanA-type strain, BM4316 (C), incubated in BHI broth containing no antibiotic (control), LY333328 at 8 and 30 μg/ml (LY 8 and LY 30, respectively), teicoplanin at 30 μg/ml (Teico 30), or vancomycin at 30 μg/ml (Vanco 30).
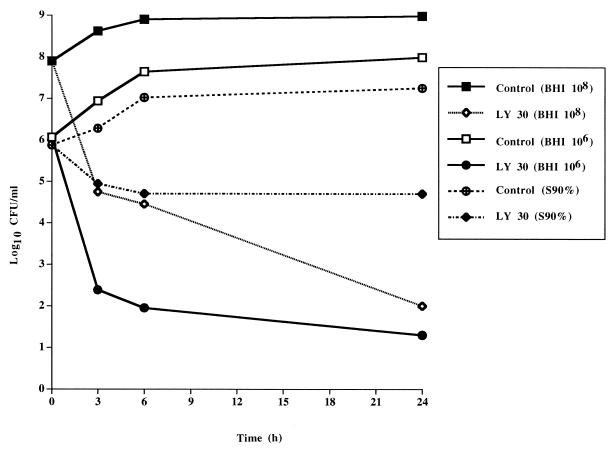
The bactericidal activity of LY333328 tested at 30 μg/ml against BM4316 was markedly reduced in the presence of 90% serum, with an approximately 100-fold reduction in killing at 24 h compared with the activity of LY333328 tested in broth (Fig. 2). In contrast, the presence of a high inoculum did not significantly influence the bactericidal activity of LY333328.
FIG. 2.
Time-kill curves of LY333328 at 30 μg/ml against VanA-type strain E. faecalis BM4316 determined in BHI broth with a standard (106) or high (108) inoculum or in the presence of 90% rabbit serum (S90%).
Antibiotic serum levels and protein binding of LY333328.
Peak and trough serum levels in animals treated for endocarditis were 15.9 ± 1.4 and 10.4 ± 0.9 μg/ml for LY333328 and were previously shown to be 63 ± 23 and 25 ± 10 μg/ml for teicoplanin and 57 ± 6 and 7.0 ± 1.5 μg/ml for vancomycin (4).
Protein binding of LY333328 measured during the 12 h following antibiotic administration ranged between 99.1 and 99.9% (99.7% ± 0.2%; n = 9). However, a similar experiment performed with radiolabeled LY333328 demonstrated that the sum of the free and protein-bound [14C]LY333328 that could be removed from the ultrafiltrator represented only 86% of the total [14C]LY333328 used, indicating that approximately 14% ± 8% (n = 5) of the antibiotic adhered to the filter and/or to the upper or lower part of the tube. The same result was obtained when the filter was preincubated with [14C]LY333328 (18% ± 8% of unrecovered antibiotic).
Autoradiography study.
A quantitative autoradiograph of a vegetation representative of [14C]LY333328 is shown in Fig. 3. [14C]LY333328 was distributed throughout the vegetation without gradient of concentration between the periphery and the core of the vegetation. The ratio between the highest and the lowest radioactivity into the different parts of the vegetation was 1.8. The autoradiographic pattern of [14C]LY333328 was not modified when vegetations were examined 12 h or 30 min after the antibiotic infusion. However, the ratios of mean antibiotic radioactivity of vegetation/plasma, vegetation/blood, and vegetation/cardiac tissue were threefold more 12 h versus 30 min after [14C]LY333328 infusion (P < 0.01 [Table 2]).
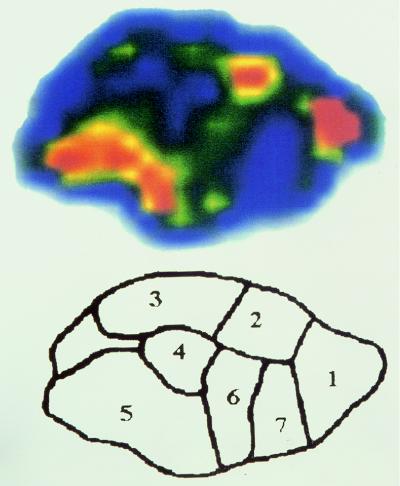
FIG. 3.
Quantitative autoradiography of a cardiac vegetation taken from a rabbit treated with 352 μCi of [14C]LY333328, 30 min after the end of intravenous infusion. The diagram shows the radioactivity, in nanocuries per gram, of labeled antibiotic for each zone indicated on the autoradiograph: 1, 291.6; 2, 229.5; 3, 202.5; 4, 186.3; 5, 275.4; 6, 218.7; 7, 164.7.
TABLE 2.
Radioactively labeled antibiotic concentration in vegetation per injected radioactivity and vegetation/plasma, vegetation/blood, and vegetation/cardiac tissue ratio of [14C]LY333328 concentrations according to time of sampling
| Time of sacrifice after antibiotic infusion | Dose injected (μCi) ± SD (no. of animals) | No. of vegetations | Radioactivity in vegetation/injected radioactivity ± SDa | Ratio of mean antibiotic radioactivity ± SDb
|
||
|---|---|---|---|---|---|---|
| Vegetation/plasma | Vegetation/blood | Vegetation/cardiac tissue | ||||
| 30 min | 235 ± 165 (2) | 8 | 1,108 ± 918 | 0.42 ± 0.24 | 1.20 ± 0.67 | 2.21 ± 1.79 |
| 12 h | 457 (1) | 4 | 1,639 ± 450 | 1.61 ± 0.44c | 4.36 ± 1.20c | 6.30 ± 1.73c |
Disintegrations per minute per gram/disintegrations per minute, 10−6.
The vegetation/plasma and vegetation/blood ratios are expressed as disintegrations per gram/disintegrations per milliliter, and the vegetation/cardiac tissue ratio is expressed as disintegrations per gram/disintegrations per gram.
P < 0.01 versus vegetations from animals sacrificed 30 min after antibiotic infusion.
Experimental endocarditis.
In vivo activities of glycopeptide antibiotics according to study strains are presented in Table 3. Compared with results for control animals, vancomycin was active against JH2-2 (P < 0.0001) but not BM4281 (P = 0.9), as previously reported (4), and teicoplanin was active against JH2-2 (P < 0.0001) and BM4281 (P < 0.001), as previously shown (4), but not against BM4316 (P = 0.20). Therefore, there was a significant strain effect for vancomycin (P < 0.01) and teicoplanin (P < 0.02). In contrast, LY333328 was active against the three strains (P = 0.45) and the activity was not significantly influenced by the phenotype of the strains (P = 0.45). Teicoplanin was the most active antibiotic against JH2-2, whereas LY333328 was the most active against BM4281 and BM4316 (Table 3). No animal retained sterile vegetation.
TABLE 3.
Results of treatment with LY333328, vancomycin, and teicoplanin for 5 days in rabbits with experimental aortic endocarditis due to E. faecalis with various resistance phenotypes
| Regimen | Mean log10 CFU/g of vegetation ± SD (no. of animals) for the indicated E. faecalis strain (phenotype)
|
||
|---|---|---|---|
| JH2-2 (susceptible) | BM4281 (VanB) | BM4316 (VanA) | |
| Control | 9.9 ± 1.1 (8) | 8.5 ± 1.0 (10) | 8.9 ± 0.6 (8) |
| Vancomycin | 7.3 ± 1.1a (6) | 8.4 ± 1.1 (11) | Not done |
| Teicoplanin | 6.5 ± 0.7ab (9) | 6.2 ± 1.6a (13) | 7.9 ± 1.5 (5) |
| LY333328 | 8.1 ± 0.9a (6) | 5.7 ± 1.5bc (8) | 6.8 ± 1.8a (7) |
DISCUSSION
Our in vitro studies performed on isogenic pairs of transconjugants demonstrated that the bacteriostatic and bactericidal activities of LY333328 were not decreased by acquired mechanisms of resistance to vancomycin and teicoplanin. Enterococci with the VanA or VanB phenotypes produce peptidoglycan precursors ending in d-lactate, which bind glycopeptides with reduced affinity (2). Although the mechanism of action of LY333328 is thought to be similar to that of vancomycin, evidence suggests that the high activity of LY333328 against enterococci may be due to factors other than an enhanced affinity for peptidoglycan precursors (1, 19), including a relatively high capacity to dimerize and to bind to bacterial membrane vesicles, which derive from the nature of the hydrophobic side chain of LY333328 (1).
LY333328 has been shown to have potent bactericidal activity against gram-positive bacteria, including vancomycin-resistant enterococci (6, 8, 26). In addition, it was shown that this bactericidal effect is concentration dependent, with maximum killing achieved for concentrations of ca. 16 times the MIC (20). In our study, LY333328 was the only glycopeptide antibiotic to demonstrate bactericidal activity against the study strains, including the VanA strain. A concentration-dependent effect was observed against the three strains in terms of rate of killing of the susceptible and the VanB strains and in terms of bactericidal activity at 24 h against the VanA strain. It is of interest that the maximal bactericidal effect was obtained in vitro with a concentration of 30 μg/ml that indeed corresponded to 15 times the MIC (on agar), as reported by others (20). However, since the 8 μg/ml concentration of LY333328 was bacteriostatic only against the VanA strain, which corresponded to 32 times the MIC in broth and 4 times the MIC on agar, it can be anticipated that 15 to 16 times the MIC measured on agar, not in broth, would be necessary to achieve significant in vitro bactericidal activity (≥3 log reduction in CFU/ml).
Our in vivo studies indicated that LY333328 was the only glycopeptide antibiotic tested to display significant activity in experimental endocarditis regardless of the phenotype of the study strains. Indeed, the in vivo activity of LY333328 was not decreased against the VanB and VanA strains. However, the intensity of the in vivo activity was limited compared to that of its in vitro activity, with a 1.8 to 2.8 reduction in log10 CFU per gram of vegetation compared with control animals after 5 days of therapy, and no animal retained sterile vegetation. Several factors could account for this result. In our study, >99% of LY333328 was bound to plasma proteins in rabbit. Despite major protein binding, MICs were not higher in the presence of 50% rabbit serum, and only a twofold increase in the MIC of LY333328 has been previously reported in the presence of human serum (20). This apparent discrepancy may be due either to an overestimation of protein binding measured in vitro or to the weakness of the binding of LY333328 to protein. Our study with radiolabeled antibiotic showing that 14 to 18% of LY333328 adhered to the filter material itself clearly demonstrates that measuring protein binding by an ultrafiltration method may be confounded by methodological problems for molecules with a high propensity to adhere to surface material. Nevertheless, the bactericidal activity of LY333328 against BM4316 was markedly reduced when it was tested in the presence of 90% serum, even at a concentration of 30 μg/ml. Therefore, protein binding might be a limiting factor for the in vivo activity of LY333328. Diffusion of LY333328 into the vegetation does not appear to be a factor limiting its activity in endocarditis, in contrast to results reported for teicoplanin (9). An autoradiography study showed a heterogeneous diffusion throughout the cardiac vegetations, with a 1.8 ratio between the highest and the lowest concentrations, and no gradient of decreasing concentration between the periphery and the core of the vegetation. The fact that the vegetation/blood ratio of [14C]LY333328 increased threefold after 12 h indicates that the duration of exposure may be critical to ensure maximal diffusion in extravascular infection sites. This result may be related to the large size of the molecule and/or to its high protein binding. The high inoculum present in vegetations may account only in part for the limited level of activity of LY333328 in endocarditis. Indeed, a high inoculum did not significantly reduce the in vitro bactericidal activity of LY333328 against BM4316 when the drug was tested at 30 μg/ml. A twofold MIC increase has been observed with tests with inocula of 107 to 108 CFU/ml (20) compared with MICs achieved with standard inocula. However, a slight increase in LY333328 concentration (from 4 to 16 times the MIC) could easily compensate for the reduced bactericidal activity observed in the presence of proteins or a high bacterial inoculum against vancomycin-resistant enterococci (20). Finally, serum concentrations of LY333328 achieved in rabbits by the intramuscular route might be a limiting factor for in vivo efficacy. Indeed, serum levels in rabbits ranged between 10 and 18 μg/ml, whereas bactericidal activity was achieved in vitro for a concentration of 8 to 30 μg/ml, according to the strain studied. Therefore, a concentration of at least 30 μg/ml would be necessary in serum to provide maximal killing against strains of enterococci, taking into account the high inoculum present in the vegetations, protein binding, and the vegetation/plasma ratio of [14C]LY333328, varying from 0.4 to 1.6. Whether or not those concentrations would be safe in humans is still unknown. Additional animal studies will be required, with different dosing regimens and routes of administration, providing higher serum concentrations, when the results from phase I clinical studies are available.
ACKNOWLEDGMENTS
This work was supported in part by Eli Lilly Laboratories. Agnes Lefort received a grant from La Fondation pour la Recherche Médicale.
We thank Michel Aubier for technical supplies.
REFERENCES
- 1.Allen N E, Le Tourneau D L, Hobbs J N., Jr The role of hydrophobic side chains as determinants of antibacterial activity of semisynthetic glycopeptide antibiotics. J Antibiot. 1997;50:677–684. doi: 10.7164/antibiotics.50.677. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Courvalin P. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993;37:1563–1571. doi: 10.1128/aac.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur M, Depardieu F, Gerbaud G, Galimand M, Leclercq R, Courvalin P. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J Bacteriol. 1997;179:97–106. doi: 10.1128/jb.179.1.97-106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslangul E, Baptista M, Fantin B, Depardieu F, Arthur M, Courvalin P, Carbon C. Selection of glycopeptide-resistant mutants of VanB-type Enterococcus faecalis BM4281 in vitro and in experimental endocarditis. J Infect Dis. 1996;175:598–605. doi: 10.1093/infdis/175.3.598. [DOI] [PubMed] [Google Scholar]
- 5.Awada A, VanderAuwera P, Meunier F, Daneau D, Klastersky J. Streptococcal and enterococcal bacteremia in patients with cancer. Clin Infect Dis. 1992;15:33–48. doi: 10.1093/clinids/15.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Biavasco F, Vignaroli C, Lupidi R, Manso E, Facinelli B, Varaldo P E. In vitro antibacterial activity of LY333328, a new semisynthetic glycopeptide. Antimicrob Agents Chemother. 1997;41:2165–2172. doi: 10.1128/aac.41.10.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Weekly Rep. 1993;42:597–599. [PubMed] [Google Scholar]
- 8.Craig W A, Suh B. Protein binding and the antimicrobial effects: methods for the determination of protein binding. In: Lorian V, editor. Antibiotics in laboratory medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. pp. 265–293. [Google Scholar]
- 9.Crémieux A C, Mazière B, Vallois J M, Ottaviani M, Azancot A, Raffoul H, Bouvet A, Pocidalo J J, Carbon C. Evaluation of antibiotic diffusion into cardiac vegetations by quantitative autoradiography. J Infect Dis. 1989;159:938–944. doi: 10.1093/infdis/159.5.938. [DOI] [PubMed] [Google Scholar]
- 10.Englert E, Roessler N, Jeavons A, Fairless S. Microchannel array detector for quantitative electronic radioautography. Cell Mol Biol. 1995;41:57–64. [PubMed] [Google Scholar]
- 11.Fantin B, Leclercq R, Ottaviani M, Vallois J-M, Mazière B, Duval J, Pocidalo J-J, Carbon C. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob Agents Chemother. 1994;38:432–437. doi: 10.1128/aac.38.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantin B, Leclercq R, Garry L, Carbon C. Influence of inducible cross-resistance to macrolides, lincosamides, and streptogramin B-type antibiotics in Enterococcus faecium on activity of quinupristin/dalfopristin in vitro and in rabbits with experimental endocarditis. Antimicrob Agents Chemother. 1997;41:931–935. doi: 10.1128/aac.41.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraise A P, Andrews J, Wise R. Activity of a new glycopeptide antibiotic ( LY333328) against enterococci and other resistant gram-positive organisms. J Antimicrob Chemother. 1997;40:423–425. doi: 10.1093/jac/40.3.423. [DOI] [PubMed] [Google Scholar]
- 14.Inman E L. Determination of vancomycin related substances by gradient high-performance liquid chromatography. J Chromatogr. 1987;410:363–372. doi: 10.1016/s0021-9673(00)90066-9. [DOI] [PubMed] [Google Scholar]
- 15.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones N R, Barrett M S, Erwin M E. In vitro activity and spectrum of LY333328, a novel glycopeptide derivative. Antimicrob Agents Chemother. 1996;41:488–493. doi: 10.1128/aac.41.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaatz G W, Seo S M, Aeschlimann J R, Houlihan H H, Mercier R C, Rybak M J. Efficacy of LY333328 against experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1998;42:981–983. doi: 10.1128/aac.42.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leclercq R, Courvalin P. Resistance to glycopeptides in enterococci. Clin Infect Dis. 1997;24:545–556. doi: 10.1093/clind/24.4.545. [DOI] [PubMed] [Google Scholar]
- 19.Linsdell H, Toiron C, Bruix M, Rivas G, Menedez M. Dimerization of A82846B, vancomycin and risocetin: influence on antibiotic complexation with cell wall model peptides. J Antibiot. 1996;49:181–193. doi: 10.7164/antibiotics.49.181. [DOI] [PubMed] [Google Scholar]
- 20.Mercier R C, Houlihan H H, Rybak M J. Pharmacodynamic evaluation of a new glycopeptide, LY333328, and in vitro activity against Staphylococcus aureus and Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:1307–1312. doi: 10.1128/aac.41.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson R D, Steigbigel R T, Davis H T, Chapman S W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quintiliani J R, Courvalin P. Conjugal transfer of the vancomycin resistance determinant vanB between enterococci involves the movement of large genetic elements from chromosome to chromosome. FEMS Microbiol Lett. 1994;119:359–364. doi: 10.1111/j.1574-6968.1994.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 23.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(3B):72–76. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 24.Steel R G D, Tovrie J H. Multiple comparisons. In: Napier C, Maisel J W, editors. Principles and procedures of statistics: a biometrical approach. New York, N.Y: McGraw-Hill; 1980. pp. 172–194. [Google Scholar]
- 25.Steers E, Folz E L, Graves B S, Riden J. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot Chemother. 1959;9:307–311. [PubMed] [Google Scholar]
- 26.Zelenitsky S A, Karlowsky J A, Zhanel G G, Hoban D J, Nicas T N. Time-kill curves for a semisynthetic glycopeptide, LY333328, against vancomycin-susceptible and vancomycin-resistant Enterococcus faecium strains. Antimicrob Agents Chemother. 1997;41:1407–1408. doi: 10.1128/aac.41.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]