Abstract
Annual repeat influenza vaccination raises concerns about protective efficacy against mismatched viruses. We investigated the impact of heterologous prime-boost vaccination on inducing cross protection by designing recombinant influenza viruses with chimeric hemagglutinin (HA) carrying M2 extracellular domains (M2e-HA). Heterologous prime-boost vaccination of C57BL/6 mice with M2e-HA chimeric virus more effectively induced M2e and HA stalk specific IgG antibodies correlating with cross protection than homologous prime-boost vaccination. Induction of M2e and HA stalk specific IgG antibodies was compromised in 1-year old mice, indicating significant aging effects on priming subdominant M2e and HA stalk IgG antibody responses. This study demonstrates that a heterologous prime-boost strategy with recombinant influenza virus expressing extra M2e epitopes provides more effective cross protection than homologous vaccination.
Keywords: Recombinant influenza virus, Live attenuated virus, M2e, Cross protection
1. Introduction
Influenza A virus belongs to the Orthomyxoviridae family, a negativesense single-stranded RNA virus containing 8 segmented genomes. It has a wide variety, originating from 18 hemagglutinin (HA) subtypes (H1–H18) and 11 neuraminidase (NA) subtypes (N1–N11), with antigenically diverse strains isolated in each subtype (Tong et al., 2013). Hundreds of millions of people are infected yearly with influenza viruses, which leads to 290,000 to 646,000 deaths globally, with young children and the elderly being the most vulnerable (Iuliano et al., 2018; Lee et al., 2018; Thompson et al., 2003).
Due to the emergence of drifting mutations and pandemics, overall vaccine effectiveness is in a wide range of low efficacy between 10% and 60% (CDC). Low vaccine effectiveness comes from multiple factors such as aging, health and pre-existing immune status, antigenic mismatches, and poor immunogenicity of vaccines. Vaccine strains are annually updated to better reflect the circulating influenza strains and vaccination is recommended every year. While annually repeated influenza vaccination was effective with variable efficacy (Casado et al., 2018; Mastalerz-Migas et al., 2015; Beyer et al., 1999; Smith et al., 1999; de Bruijn et al., 1999; Keitel et al., 1997), several recent studies have indicated that repeated annual vaccination did not result in improving vaccine effectiveness particularly when circulating strains are mismatched (Mclean et al., 2014; Leung et al., 2017; Morimoto and Takeishi, 2018; Song et al., 2020). It has been a high priority to enhance the vaccine efficacy and develop broadly cross protective vaccines.
Influenza virus HA proteins consist of the immune-dominant but highly variable head domain, which provides a strain-specific neutralizing target, as well as the relatively conserved stalk domain, which mediates the viral membrane fusion (Krammer et al., 2018). To overcome the immune-subdominant nature of HA stalk domains, influenza viruses were reverse-genetically engineered to contain chimeric HA where the variable head domain was replaced with a corresponding domain from the antigenically far distant strains without changing the stalk domain (Nachbagauer et al., 2021; Liao et al., 2020). Recombinant chimeric HA influenza virus vaccines were reported to induce high levels of stalk specific IgG responses leading to enhanced cross protection in animal models (Krammer et al., 2013a). In phase I clinical studies, AS03-adjuvanted chimeric HA-based influenza virus vaccine induced durable IgG responses to the HA stalk domain (Nachbagauer et al., 2021; Bernstein et al., 2020). While these results provide a proof-of concept for developing stalk-based cross protective vaccines, they would not confer sufficient protection against currently circulating strains due to their antigenically unrelated HA head domain.
Influenza A virus contains ion channel protein M2 extracellular epitopes (M2e) which are highly conserved but poorly immunogenic, despite being a promising universal antigenic target (Saelens, 2019). To induce immunity to both M2e and circulating HA, replication competent influenza viruses were genetically modified to retain and express chimeric HA molecules with tandem repeat 4xM2e in the N-terminus HA (4xM2e-HA) from H1N1 (Kim et al., 2017), H3N2 (Park et al., 2021), and H7N9 virus (Mezhenskaya et al., 2021). The live recombinant 4xM2e-HA influenza virus vaccines were immunogenic in inducing strain specific neutralizing antibodies and M2e immunity, conferring differential cross protection in BALB/c mice. However, it is likely that the efficacy of homologous prime-boost vaccination would be limited due to pre-existing immunity.
The impact of heterologous prime-boost vaccination with recombinant 4xM2e-HA influenza virus vaccines and pre-existing immunity on inducing cross protection against influenza viruses remains unknown. In this study, we investigated the efficacy of cross protection by heterosubtypic prime-boost vaccination with live recombinant 4xM2e-HA H1N1 and H3N2 influenza virus vaccines in C57BL/6 mice that are known to be a less responder to low immunogenic conserved epitopes. Heterologous prime-boost strategies using recombinant 4xM2e-HA influenza virus vaccines were found to be more effective in inducing cross protection against antigenically different viruses in C57BL/6 mice compared to homologous prime-boost vaccination strategies. M2e and HA stalk immunity might have played a role in cross protection.
2. Results
2.1. In vitro and in vivo virological characterization of recombinant influenza viruses containing chimeric 4xM2e-HA
The rescued recombinant influenza viruses containing chimeric HA where foreign gene fragments were genetically linked to the N-terminus of HA were reported to be highly stable even after 10 passages (Kim et al., 2017; Park et al., 2021; Lee et al., 2015; Mezhenskaya et al., 2021). The rescued recombinant live attenuated influenza viruses H1N1 (A/South Africa/3626/2013), H3N2 (A/Switzerland/9715293/2013), and H7N9 (A/Anhui/1/2013) containing 4xM2e-HA were confirmed by sequencing the full-length HA in previous studies (Kotomina et al., 2020; Mezhenskaya et al., 2021). To determine the impact of heterologous prime-boost vaccination on cross protection, we generated three different recombinant influenza A viruses containing tandem repeat 4xM2e-HA (Fig. 1A). The attPR8 4xM2e is an A/PR8 reassortant virus with an attenuated backbone and chimeric 4xM2e-HA H1 of A/PR8. The Cal 4xM2e virus contains the A/PR8 wild type backbone and 4xM2e-HA where the head domain (C52–C277) of A/PR8 H1 HA was replaced with that of A/California/2019 (A/Cal). The reassortant H3N2 and H3N2 4xM2e viruses contains HA and 4xM2e-HA respectively, H3 HA and N2 NA genes from A/Switzerland/2013 (A/SW, H3N2) and the internal and A/PR8 wild type backbone as previously described (Park et al., 2021). The prediction of structural modeling of 4xM2e-HA chimera indicates similarity in displaying the globular head domain, stalk domain, and tandem repeat 4xM2e linked to the N-terminus of HA and juxtaposed to the bottom of the stalk (Fig. 1B). These recombinant viruses propagated in embryonated chicken eggs retained high infectious titers (Fig. 1C). The Cal 4xM2e, A/SW H3N2, and H3N2 4xM2e viruses showed approximately 4 folds lower hemagglutination activity units (HAU), compared to those of A/PR8, attPR8, and attPR8 4xM2e viruses respectively (Fig. 1D). M2e epitopes in attPR8 4xM2e, H3N2 4xM2e, and Cal 4xM2e viruses were detected at significantly higher levels compared to those of corresponding viruses with unmodified HA (Fig. 1E).
Fig. 1.
In vitro characterization of recombinant influenza viruses containing M2e epitopes (4xM2e) in chimeric HA conjugates. (A) Constructs of chimeric hemagglutinin (HA) carrying 4xM2e in its N-terminus. 4xM2e-HA H1: A/PR8 H1 HA containing 4xM2e, 4xM2e-HACal head: A/PR8 4xM2e-HA head domain (Cys52-Cys277) was replaced with A/Cal HA head domain, 4xM2e-HA H3: A/SW H3 HA carrying 4xM2e. SP: signal peptide, tandem repeat 4xM2e is composed of human M2e (SLLTEVETPIRNEWGSRSNDSSD), swine M2e (SLLTEVETPTRSEWESRSSDSSD), and avian M2e (SLLTEVETPTRNEWESRSSDSSD). L and C represent linker (AAAGGAA) and connector (AAAPGAA). (B) Predictive three-dimensional structure of the chimeric HA monomers for 4xM2e-HA H1, 4xM2e-HACal head and 4xM2e-HA H3 respectively, utilizing PyMol program. (C) Titration of infectious viruses in allantoic fluids of embryonated eggs inoculated with A/PR8, attPR8, attPR8 4xM2e, Cal 4xM2e, H3N2, or H3N2 4xM2e virus. (D) Hemagglutination activity in recombinant virus stocks using chicken RBC. (E) Reactivity of recombinant virus to M2e specific mAb 14C2. Error bars indicate mean ± SEM.
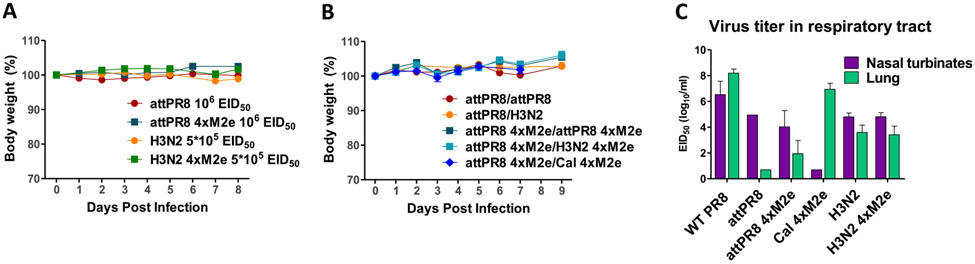
To test the pathogenicity of recombinant viruses, body weight changes were monitored in C57BL/6J mice after intranasal inoculation with attPR8 (106 EID50), attPR8 4xM2e (106 EID50), H3N2 (5 × 105 EID50) or H3N2 4xM2e (5 × 105 EID50) at the doses used as prime vaccination. No body weight loss was detected in all groups (Fig. 2A). In addition, no weight loss was observed in the mice intranasally primed with 106 EID50 of attPR8 or attPR8 4xM2e and homo- or heterologous boost with 106 EID50 of attPR8, H3N2, attPR8 4xM2e, or H3N2 4xM2e, or 105 EID50 of Cal 4xM2e (Fig. 2B).
Fig. 2.
Pathogenicity of recombinant influenza viruses in mice. (A) Body weight changes of C57BL/6J mice (n = 8–24/group) after intranasal inoculation with live attPR8 (106 EID50), attPR8 4xM2e (106 EID50), H3N2 (5 × 105 EID50) or H3N2 4xM2e (5 × 105 EID50). (B) Body weight changes in C57BL/6J mice (n = 5–9/group) prime boost immunized with attPR8/attPR8, attPR8/H3N2, attPR8 4xM2e/attPR8 4xM2e, attPR8 4xM2e/H3N2 4xM2e and attPR8 4xM2e/Cal 4xM2e. 106 EID50 of these viruses were inoculated except for Cal 4xM2e (105 EID50). (C) Virus replications in mouse lung and nasal turbinate (n = 4–5) at day3 post challenge with A/PR8 (104 EID50), attPR8 (106 EID50), attPR8 4xM2e (106 EID50), Cal 4xM2e (104 EID50), H3N2 (106 EID50) and H3N2 4xM2e (106 EID50). Each individual animal is analyzed. Error bars indicate mean ± SEM.
Infectious titers of virus replication in upper (nose) and lower (lung) respiratory tracts were determined at 3 days after intranasal inoculation of mice with A/PR8, attPR8, attPR8 4xM2e, Cal 4xM2e, H3N2 or H3N2 4xM2e (Fig. 2C). The wild type A/PR8 and Cal 4xM2e viruses replicated at higher titers in lung lysates than in nasal turbinates by 102 and 106 folds respectively. In contrast, attPR8, attPR8 4xM2e, H3N2, and H3N2 4xM2e viruses were detected at higher virus titers in the nasal turbinates than in the lung by approximately 104, 102, 10 and 102 folds respectively (Fig. 2C), confirming their attenuated phenotypes.
2.2. H1N1 virus prime and heterologous boost with recombinant viruses carrying 4xM2e-HA induces enhanced M2e specific IgG and differential levels of HA stalk specific IgG antibodies
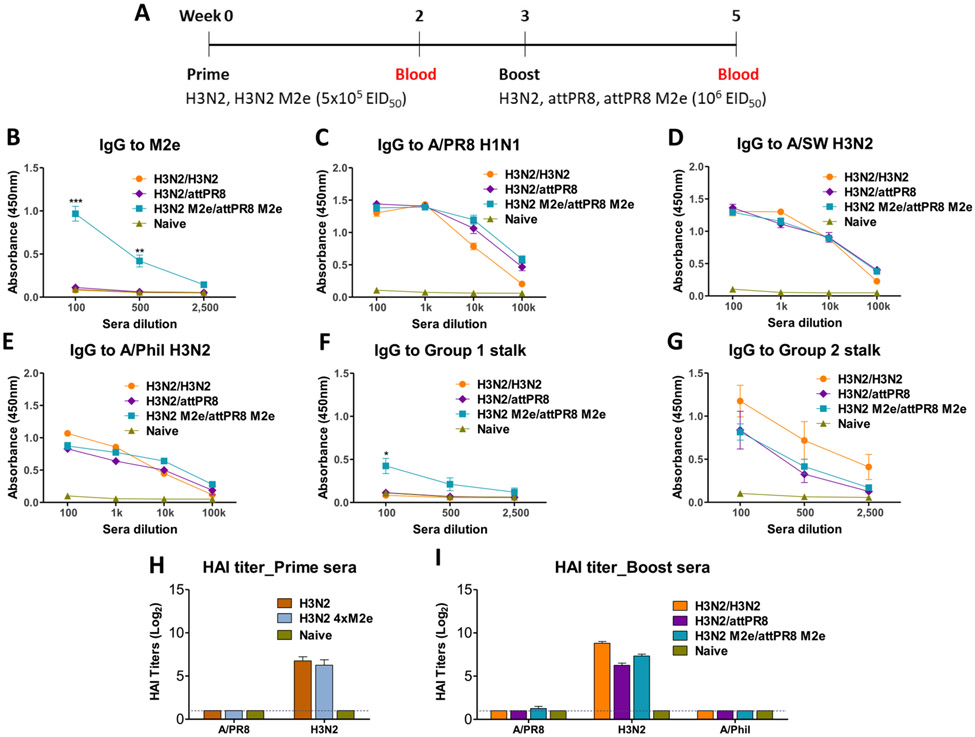
We determined the impact of heterologous boost in mice primed with H1N1 virus (attPR8 ± M2e) on inducing a profile of IgG antibodies for homo and hetero viruses, M2e, and HA stalk domains, in a set of five groups. The groups consisted of attPR8/attPR8, attPR8/H3N2, attPR8 M2e/attPR8 M2e, attPR8 M2e/H3N2 M2e and attPR8 M2e/Cal M2e where M2e indicates 4xM2e in the vaccine groups (Fig. 3A). Intranasal prime inoculation of C57BL/6J mice with attPR8 or attPR8 M2e induced IgG antibodies to homologous A/PR8 at substantial levels and cross-reactive IgG antibodies to A/SW H3N2 and rgH5N1 reassortant viruses at lower levels (Supplementary Fig. S1). M2e and group 1 stalk specific IgG antibodies were induced at very low levels after prime dose attPR8 M2e, suggesting that M2e and HA stalk domains are immuno-subdominant (Supplementary Fig. S1). Heterosubtypic boost (attPR8 M2e/H3N2 M2e) induced highest levels of IgG antibodies specific for M2e followed by the heterologous boost group (attPR8 M2e/Cal M2e) (Fig. 3B). Homo- and heterologous boost groups showed similar levels of IgG antibodies to A/PR8, A/Cal H1N1, and A/Viet rgH5N1 viruses (Fig. 3C, E, F). The attPR8/H3N2 and attPR8 M2e/H3N2 M2e vaccinated groups induced an increased level of IgG antibodies to A/SW H3N2 virus than other groups (Fig. 3D). Meanwhile the heterologous attPR8 M2e/Cal M2e group induced the highest levels of consensus group 1 stalk IgG responses (Fig. 3G). Also, the group boosted with H3N2 or H3N2 M2e showed highest IgG antibody reactivities to consensus group 2 stalk domain as expected (Fig. 3H). These results suggest that a strategy of hetero boost vaccination is more effective in inducing IgG antibodies specific for M2e and HA stalk immuno-subdominant epitopes.
Fig. 3.
IgG antibodies specific for M2e, different viruses, and HA stalk domains after hetero boost with recombinant viruses in H1N1 primed. (A) Timeline strategy of hetero prime boost intranasal immunization with recombinant viruses. C57BL/6J mice received prime vaccination with 106 EID50 of attPR8 H1N1 or attPR8 M2e H1N1 were inoculated with 106 EID50 of attPR8, attPR8 M2e, H3N2 M2e, or 105 EID50 of Cal M2e in a 3-week interval (n = 8/each group). M2e indicates chimeric 4xM2e-HA. Serum IgG antibody responses specific for M2e peptide (B), A/PR8 virus (C), H3N2 virus (D), A/Cal virus (E), A/Viet virus (F), consensus group 1 stalk (G), and consensus group 2 stalk (H). Mouse sera were collected at 2 weeks after prime-boost immunization. attPR8/attPR8: homologous prime boost attPR8 H1N1, attPR8/H3N2: heterosubtypic attPR8 prime and A/SW H3N2 boost, attPR8 M2e/attPR8 M2e: heterosubtypic prime boost with chimeric 4xM2e-HA viruses, and attPR8 M2e/Cal M2e: heterologous H1N1 prime boost with chimeric 4xM2e-HA viruses. M2e in the vaccine groups indicates tandem repeat 4xM2e. Each individual animal is analyzed. Error bars indicate mean ± SEM.
The immunogenicity of hetero boost attPR8 M2e/H3N2 M2e was tested in one year old C57BL/6J mice after intranasal vaccination (Supplementary Fig. S2). Prime dose of attPR8 M2e induced substantial levels of IgG antibodies for A/PR8 H1N1, H3N2 (A/SW), and rgH5N1, but M2e and HA stalk IgG antibodies were detected at very low levels (Supplementary Fig. S2). After H3N2 M2e boost in aged C57BL/6J mice (attPR8 M2e/H3N2 M2e, Fig. 4), IgG antibodies to M2e, A/PR8, H3N2, and rgH5N1 viruses but not to HA stalk domain were induced at increased levels despite lower levels being induced compared to those in young adult mice (Fig. 3). These data implicate that priming vaccination at young ages might be required for effective induction of HA stalk IgG antibodies.
Fig. 4.
IgG antibodies specific for M2e, different viruses, and HA stalk domains in 1 year old C57BL/6J mice after attPR8 M2e prime and H3N2 M2e boost. IgG antibody immune responses to M2e peptide (A), A/PR8 virus (B), H3N2 virus (C), A/Viet virus (D), consensus group 1 stalk protein (E), and consensus group 2 stalk protein (F). One year old C57BL/6J mice (n = 4) were inoculated with 106 EID50 of attPR8 M2e and H3N2 M2e in a 3-week interval and bled at 2 weeks after boost. Each individual animal is analyzed. Error bars indicate mean ± SEM.
2.3. Strain specific HAI titers were induced by heterosubtypic prime boost chimeric recombinant influenza virus vaccination
Prime dose of attPR8 M2e induced A/PR8 specific hemagglutination inhibition (HAI) titers at approximately 2 folds lower than attPR8 in young adult mice and 4 folds higher than that in 1-year old mice (Fig. 5A). Homologous boost with attPR8 and attPR8 M2e increased HAI titers against A/PR8 virus by 4 folds in young adult mice whereas hetero boost with H3N2 M2e (attPR8 M2e/H3N2 M2e) induced 2 folds increases in A/PR8 HAI titers, but not by Cal M2e boost or in 1-year old mice (Fig. 5A and B). High HAI titers against H3N2 (A/SW) were induced by hetero boost with H3N2 or H3N2 M2e in young adult mice, which were approximately 20 folds higher than in 1-year-old mice with H3N2 M2e boost. No cross HAI titers against rgH5N1 virus were detected in young adult and aged mice after vaccination (Fig. 5B).
Fig. 5.
HAI titers against hetero prime and boost viruses. (A) HAI titers against A/PR8 virus in young adult and 1-year old mouse sera after prime dose (106 EID50) of attPR8 or attPR8 M2e. (B) HAI titers against A/PR8 H1N1, A/SW H3N2, and A/Viet rgH5N1 viruses after hetero boost in young adult and 1-year-old mice. Mouse sera were collected at day 14 following immunization. Group labels are the same as in Fig. 3 legend. Each individual mouse sera were analyzed (n = 8/group). Error bars indicate mean ± SEM.
2.4. Hetero boost with recombinants 4xM2e-HA viruses in H1N1 M2e primed mice improves cross protection against rgH5N1 virus
To investigate cross protective efficacy, mice were challenged with A/Viet rgH5N1 virus at a lethal dose 3 weeks after boost immunization (Fig. 6A). The hetero boost groups (attPR8 M2e/H3N2 M2e and attPR8 M2e/Cal M2e) presented similarly least weight loss (~4.2%) whereas the homo boost groups (attPR8/attPR8 and attPR8 M2e/attPR8 M2e) displayed substantial weight loss, 10% and 12% respectively (Fig. 6A). In an additional experimental set to determine lung viral titers, the hetero boost attPR8 M2e/H3N2 M2e group showed slight weight loss (~4%) by day 3 post challenge, they began to recover by day 4 when lung viral loads were reduced to a minimum of 105 folds lower than the titer in naïve mice (107 EID50/ml) after infection with rgH5N1 virus (Fig. 6B and C). Meanwhile, the homo boost attPR8 M2e/attPR8 M2e group manifested moderate weight loss (~9%) and lung viral loads (103.8 EID50/ml), which was 100 folds higher than hetero boost with H3N2 M2e. The attPR8/attPR8 and attPR8/H3N2 groups did not induce M2e specific IgG antibodies, and they showed more weight loss and higher lung viral titers, 105.2 EID50/ml and 104.3 EID50/ml respectively than 4xM2e-HA chimeric viruses (Fig. 6B and C), correlating the protective efficacy with M2e immunity. Hetero vaccination with attPR8 M2e/H3N2 M2e prevented severe weight loss and reduced viral loads in 14 months old mice by the time of challenge with rgH5N1 virus (Supplementary Fig. S3).
Fig. 6.
Hetero boost immunization with 4xM2e-HA chimeric virus enhances cross protection against A/Viet rgH5N1 virus. C57BL/6J mice were challenged with A/Viet rgH5N1 virus (reassortant A/PR8-A/Vietnam/1203/2004, 10 LD50, 1.6 × 106 EID50). (A) Body weight changes after challenge in young adult mice (n = 5/group). Group labels are the same as in Fig. 3 legend. (B–E) Young adult mice (n = 4/group) were intranasally prime boost immunized with attPR8/attPR8, attPR8/H3N2, attPR8 M2e/attPR8 M2e, and attPR8 M2e/H3N2 M2e (n = 5/group) and then challenged with A/Viet rgH5N1 virus. (B) Body weight changes for 4 days after challenge until mice were sacrificed. (C) Virus titers in lung lysates at 4 days after A/Viet challenge. (D) Inflammatory cytokine IL-6 levels in BALF or lung lysates day 4 post challenge with rgH5N1. (E) IgG antibodies specific for M2e and M2e-H1 stalk (HA2 aa1-117 from A/PR8) in lung lysates day 4 post challenge. Error bars indicate mean ± SEM. The statistical significances were determined using one-way (C) or two-way (A, D, E) ANOVA and indicated in *, P < 0.001; **, P < 0.01; ***, P < 0.001. The statistical significances between attPR8 M2e/attPR8 M2e group versus attPR8 M2e/H3N2 M2e group were compared (A).
Consistent with the levels of lung viral titers, hetero prime boost attPR8 M2e/H3N2 M2e vaccination protected against lung inflammation as shown by the lowest levels of inflammatory cytokine IL-6 in bronchoalveolar lavage fluid (BALF) and lung at day 4 after rgH5N1 infection (Fig. 6D). The homologous prime-boost attPR8 M2e/attPR8 M2e and attPR8/H3N2 groups showed high levels of inflammatory IL-6 in lung while low levels in BALF (Fig. 6D). The homo prime boost attPR8/attPR8 group with least protection showed higher IL-6 levels in BALF and lung than other more protective vaccine groups.
We also determined whether rapid induction of mucosal IgG antibodies specific for M2e and HA stalk domains would correlate with heterosubtypic cross protection against rgH5N1 virus (Fig. 6E). The attPR8 M2e/H3N2 M2e group showed the highest levels of lung IgG antibodies specific for M2e and M2e-H1 stalk domains at day 4 after rgH5N1 challenge in young adult mice (Fig. 6E). which was 2 and 7 folds higher respectively than those in 14 months old mice (Supplementary Fig. S3C). In contrast, attPR8/attPR8 and attPR8/H3N2 groups induced M2e and HA stalk IgG antibodies at non-detectable levels, similar to naïve mice after infection (Fig. 6E). Three to four folds lower levels of lung IgG antibodies for M2e and HA stalk antibodies were observed in the homo attPR8 4xM2e/attPR8 4xM2e group than those in the hetero prime boost attPR8 M2e/H3N2 M2e group (Fig. 6E). Taken together, these results indicate the effectiveness of heterologous prime-boost vaccination in conferring cross protection against rgH5N1 virus.
2.5. Hetero boost in H3N2-primed mice with attPR8 M2e virus effectively induce M2e IgG antibodies
Conversely, we determine the impact of hetero boost in H3N2-primed mice by comparing the following three prime-boost combinations, H3N2/H3N2, H3N2/attPR8, and H3N2 M2e/attPR8 M2e (Fig. 7A). Significantly enhanced levels of IgG antibodies specific for M2e were induced in the hetero boost H3N2 M2e/attPR8 M2e group (Fig. 7B) compared to prime dose of H3N2 M2e (Supplementary Fig. S4). Similar levels of IgG antibodies specific to homologous and heterosubtypic viruses such as A/PR8 (H1N1), A/SW (H3N2), and A/Phil (H3N2) were detected in all prime-boost immunized mouse groups (Fig. 7C-E).
Fig. 7.
Intranasal heterosubtypic boost with chimeric 4xM2e-HA A/PR8 virus enhances M2e IgG responses in H3N2 primed mice. (A) Timeline for a reverse order of H3N2 prime and H1N1 boost vaccination strategy. Mice (n = 5/group) were intranasally inoculated in prime-boost regimens with H3N2/H3N2, H3N2/attPR8 or H3N2 M2e/attPR8 M2e (prime dose: 5 × 105 EID50, boost dose: 106 EID50) and immune sera were collected at 3 weeks after boost. IgG antibody responses to M2e (B), A/PR8 virus (C), A/SW H3N2 virus (D), A/Phil H3N2 virus (E), consensus group 1 stalk protein (F) and consensus group 2 stalk protein (G). HAI titers against influenza viruses were measured from immune sera after prime (H) and prime-boost (I) immunization. Each individual animal was analyzed. Error bars indicate mean ± SEM.
Consensus group 1 stalk specific IgG antibodies were induced to a moderate level in the H3N2 M2e/attPR8 M2e group but not in the H3N2/attPR8 group (Fig. 7F). IgG antibodies to consensus group 2 stalk were induced at the highest levels in the homo prime-boost H3N2/H3N2 group and substantial levels of consensus group 2 stalk IgG antibodies were similarly observed in the hetero prime-boost H3N2/attPR8 and H3N2 M2e/attPR8 M2e groups (Fig. 7G).
Homologous strain (H3N2) specific HAI titers were induced at protective levels (~128) by prime dose of H3N2 or H3N2 4xM2e (Fig. 7H). HAI titers against H3N2 virus were further enhanced by 4 folds by homo boost H3N2/H3N2 vaccination (Fig. 7H and I). Heterosubtypic attPR8 or attPR8 M2e boost immunization (H3N2/attPR8 and H3N2 M2e/attPR8 M2e) retained HAI titers induced by prime H3N2 dose (Fig. 7H and I). Induction of HAI titers against A/PR8 might have been suppressed in H3N2 primed mice after attPR8 boost (Fig. 7I). No HAI responses were observed against A/Phil from all groups (Fig. 7I).
2.6. Hetero boost vaccination in H3N2-primed mice induces effective protection against heterologous H3N2 virus
We evaluated whether heterosubtypic boost in H3N2 primed mice would be more effective in inducing cross protection against A/Phil H3N2 virus challenge (Fig. 8). The hetero prime-boost H3N2/attPR8 and H3N2 M2e/attPR8 M2e groups displayed only ~4% weight loss and then all mice recovered (Fig. 8A). In contrast, the homo prime-boost H3N2/H3N2 group showed approximately 10% weight loss at day 2 after A/Phil infection before recovering weight to a normal level (Fig. 8A). Naïve mice continued to display weight loss after A/Phil virus infection until day 5; at this point all mice were sacrificed to collect lung tissues for viral titration. Reduced lung virus titers were detected in the hetero prime-boost H3N2 M2e/attPR8 M2e group after A/Phil challenge, which were ~10 folds lower than those of the homo or hetero prime-boost H3N2/H3N2 and H3N2/attPR8 groups and approximately 105 folds lower than those in naïve mice with infection (Fig. 8B). Significantly higher levels of IgG antibodies specific for M2e, M2e-H1 stalk, and M2e-H3 stalk were measured in lung lysates from the hetero prime-boost H3N2 M2e/attPR8 M2e group (Fig. 8C), likely correlating with lowest lung viral titers (Fig. 8B). Despite the highest levels of group 2 stalk specific IgG responses (Fig. 7G), the H3N2/H3N2 group did not show enhanced cross protection against A/Phil virus (H3N2) (Fig. 8A and B).
Fig. 8.
H3N2 M2e/attPR8 M2e prime-boost vaccination provides effective lung viral control after heterologous A/Phil H3N2 virus challenge. The groups (n = 5/group) of mice immunized with H3N2/H3N2, H3N2/attPR8 or H3N2 M2e/attPR8 M2e were challenged with A/Phil virus (100x LD50, 1.4 × 104 EID50). (A) Body weight changes after A/Phil H3N2 virus challenge for 5 days until mice were sacrificed. (B) Lung virus titers at day 5 after A/Phil virus challenge. (C) Lung IgG antibodies specific for M2e, chimeric M2e-H1 stalk (HA2 aa1-117 from A/PR8), and chimeric M2e-H3 stalk (HA2 aa1-117 from A/Aichi/1968H3N2). Each individual animal is analyzed. Error bars indicate mean ± SEM. The statistical significances were determined using one-way (B) or two-way (C) ANOVA and indicated in **, P < 0.01; ***, P < 0.001.
3. Discussion
Clinical data suggest that repeat seasonal influenza vaccination might not be highly effective in inducing protective antibody responses (Mclean et al., 2014; Thompson et al., 2016; Leung et al., 2017; Morimoto and Takeishi, 2018; Song et al., 2020) or lead to substantial heterogeneity in vaccine effectiveness (Belongia et al., 2017). Our previous studies showed that homologous prime-boost with A/PR8 4xM2e-HA or prime dose of attenuated rgH3N2 4xM2e-HA virus vaccination could be more effective in conferring broader cross protection in BALB/c mice than the wild type HA counterpart virus (Kim et al., 2017; Park et al., 2021). Accordingly, we determine whether heterologous prime-boost vaccination with recombinant influenza viruses containing chimeric 4xM2e-HA would induce more effective cross protection in different genetic background C57BL/6 mice known to be less responsive to influenza vaccination than BALB/c mice (Petrovic et al., 2018). We found that heterosubtypic prime-boost provided more effective in conferring cross protection than homologous vaccination. Hetero prime-boost with recombinant influenza viruses containing 4xM2e-HA induced high levels of IgG antibodies specific for M2e and reduced lung viral loads more effectively after heterosubtypic or heterologous virus challenge.
Prime dose vaccination induced low levels of IgG antibodies for M2e and HA stalk epitopes in 4xM2e-HA chimeric virus, suggesting their immune-subdominance (Supplementary Fig. S1). Boost exposure to antigenically more distant chimeric virus H3N2 4xM2e-HA effectively generated M2e specific IgG antibodies. In the attenuated A/PR8 H1N1 (4xM2e-HA) prime and antigenically different virus boost groups, the levels of M2e antibodies were correlated with lowering lung viral titers and inflammatory cytokine (IL-6) against rgH5N1 virus challenge. The contribution of M2e antibodies to lowering lung viral loads was also observed in the attenuated H3N2 4xM2e-HA/PR8 4xM2e-HA group after A/Phil (H3N2) challenge. Importantly, Cal-head 4xM2e-HA boost induced higher levels of IgG binding to group 1 stalk proteins; the group 1 stalk IgG antibody levels were likely correlated with prevention against severe weight loss against rgH5N1 virus challenge as shown by the PR8–4xM2e/Cal-head 4xM2e group. These immunologic outcomes were consistent with the chimeric HA based vaccination strategy where multiple vaccinations were administered with heterosubtypic modified HA-head domains (Krammer et al., 2013b; Hai et al., 2012). Consistently, a prior study demonstrating heterosubtypic immunity by sequential vaccination with live attenuated chimeric HA head-switched viruses in ferrets (Liu et al., 2019). The correlative contribution of M2e specific IgG antibodies to lowering lung viral loads was also observed in the attenuated H3N2 4xM2e-HA/PR8 4xM2e-HA group after A/Phil (H3N2) challenge. Thus, in the attenuated A/PR8 H1N1 prime and antigenically different boost groups, the combined M2e and HA stalk specific IgG levels might have contributed to cross protection against weight loss and to clearing lung viral loads. Fc receptors (FcR) are known to be required for mediating protection by M2e and HA stalk specific antibodies (El Bakkouri et al., 2011; Kim et al., 2013a; Dilillo et al., 2016; Dilillo et al., 2014), suggesting a significant role of antibody-dependent cell-mediated cytotoxicity (ADCC) in conferring protection. Not only natural killer cells from human donors (FcyγIIIA + primary NK) but also FcγRIIIA engineered NK-92 cells and FcγRIIIA/NFAT-RE/luc2 engineered Jurkat T cells have been used as effector cells in bioassay evaluation of ADCC (Hsieh et al., 2017). We demonstrated that immune sera from the H3N2 4xM2e-HA/PR8 4xM2e-HA group were found to activate FcR in Jurkat cells via the target MDCK cells infected with A/Phil H3N2 or A/Viet rgH5N1 viruses, supporting a possible role of ADCC in mediating protection (Supplementary Fig. S5).
Repeat vaccination was shown to induce lower antibody titers than current seasonal vaccination only (Thompson et al., 2016; Sanyal et al., 2019; Leung et al., 2017) and might lead to reduced antibody-affinity maturation (Khurana et al., 2019). In addition, repeated homologous vaccination was less effective in protection against H3N2 virus than single vaccination in ferrets despite no significant differences in antibody levels (Music et al., 2019). It should be noted that compared to the heterosubtypic H3N2/H1N1 (A/PR8) vaccination, prime-boost with H3N2 virus (2013 isolate) resulted in more weight loss against A/Phil virus (1982 isolate) at days 2–3 post challenge, while it induced higher levels of homologous HAI titers and IgG antibodies for group 2 stalk protein and similar outcomes in lung viral loads. Similarly, higher levels of group 1 stalk IgG antibodies induced by attenuated PR8/PR8 prime-boost did not improve cross protection against rgH5N1 virus compared to the PR8/H3N2 group, which induced near background levels of group 1 stalk specific antibodies. High levels of anti-HA stalk antibodies were reported in the elderly individuals (Nachbagauer et al., 2016), in which population influenza-related morbidity and mortality are mostly associated. Thus, caution should be given in correlating HA stalk specific antibody levels with cross protection. Therefore, inducing extra M2e immunity might provide a safeguard, protecting from severe disease and mortality during a pandemic to which individuals are naïve.
The elderly populations display poor responses to vaccination because of immuno-senescence, limited naïve B cells and IgG repertoire (Crooke et al., 2019), reduced capacity to target antigenically distinct epitopes, and less somatic hypermutations (Henry et al., 2019). One year old C57BL/6 mice were less effective compared to young adult mice in inducing HAI titers, IgG antibodies to M2e and particularly to HA stalk domain. They were also poorly responsive to boost dose in the PR8 4xM2e/H3N2 4xM2e group. To overcome poor responses to low immunogenic but conserved epitopes, immunizing younger age populations with vaccines targeting M2e and HA stalk proteins would be an approach for future studies.
A strategy of heterosubtypic prime-boost with vaccines inducing strain specific HAI titers and IgG antibody responses to conserved M2e and HA stalk domains would improve the breadth of cross protection. In the attenuated A/PR8 prime set with A/SW H3N2 boost, HAI titers against both vaccine strains were induced without significant compromise. However, in the reverse A/SW H3N2 prime and attenuated A/PR8 boost, HAI responses to A/PR8 were compromised although both strains are antigenically unrelated. This might be because attenuated A/PR8 viruses would not replicate at sufficient levels under pre-existing immunity against A/SW H3N2 virus whereas A/SW H3N2 virus could replicate both in the upper and lower respiratory tracts (Fig. 2B). Hence, further studies will be needed to better understand heterosubtypic prime-boost strategies. One caveat of this study is that live attenuated influenza vaccines are not generally recommended in adults and elderly populations probably due to pre-existing immunity negatively affecting vaccine effectiveness. Live attenuated vaccine platforms of head-domain switched chimeric HA-based universal influenza virus vaccine candidates were not effective inducing anti-H1 stalk IgG responses whereas AS03-adjuvanted inactivated virus vaccines were immunogenic in raising durable HA stalk specific antibodies at several folds higher levels in phase 1 studies (Bernstein et al., 2020; Nachbagauer et al., 2021). Inactivated virus platforms of recombinant virus vaccines with 4xM2e-HA were not effective in inducing M2e specific IgG antibody responses after intramuscular immunization of mice (data not shown). In contrast, previous studies reported alternative strategies of recombinant influenza viruses where the foreign epitopes (M2e, conserved NA or neutralizing epitopes of respiratory syncytial virus fusion protein) were inserted in the immunodominant HA head domain, which induced epitope specific antibodies after vaccination of mice with inactivated vaccine platforms and conferred improved protection against challenge with antigenically different viruses (Kim et al., 2020; Lee et al., 2016). A universal vaccine that effectively induces both protective M2e and HA stalk immune responses remains to be developed.
4. Materials and methods
4.1. Cells and viruses
293T cells (ATCC Cat# CRL-3216) were cultured in Dulbecco’s Modified Eagle Medium media (DMEM) and used for plasmid DNA transfection. Influenza viruses such as A/Puerto Rico/8/34 (A/PR8, H1N1), A/California/04/09 (A/Cal, H1N1), and A/Philippines/2/82 (A/Phil, H3N2) were amplified in 10-day old embryonated chicken eggs (Hy-Line N.A., Mansfield, GA) in the BSL2 laboratory facility. A/Vietnam rgH5N1 (A/Viet, rgH5N1) virus is a reverse genetically reassortant virus possessing H5 HA where polybasic residues removed to make the virus attenuated and N1 NA from A/Vietnam/1203/2004 and 6 other backbone genes of A/PR8 as previously described (Song et al., 2011). The rgH5N1 virus was grown in 10-day old embryonated chicken eggs in the BSL2+ laboratory. H3N2 recombinant virus was a reassortant virus with A/PR8 backbone (X-247 rgH3N2, International Reagent Resource, FR-1366) carrying HA and NA genes from A/Switzerland/9715293/2013. The influenza viruses were inactivated by treatment of 37% formalin at 1:4000 (v/v) dilutions as described previously (Quan et al., 2008).
4.2. Construction of attenuated A/PR8 backbone H1N1 and H3N2 viruses expressing chimeric 4xM2e-HA
Recombinant influenza viruses carrying wild type or chimeric hemagglutinin (HA) were generated using reverse genetics with PR8 backbone genes within the pHW2000 plasmids (kindly provided by Dr. Robert G. Webster, St. Jude Children’s Research Hospital). The attenuated PR8 backbone (attPR8) was generated by introducing mutations in the PB1 (K391E, E581G, A661T) and PB2 (N265S) polymerase genes granting temperature-sensitive (ts) attenuation as described (Jung et al., 2020). Reassorted H3N2 virus contained HA and NA genes derived from A/Switzerland/9715293/2013 (H3N2). The chimeric 4xM2e-HA genes encoded for four tandem repeats of M2e (4xM2e composed of 2x human M2e, swine M2e and avian M2e) inserted into the N-terminus of A/PR8 H1 HA (4xM2e-HA H1) (Kim et al., 2017) or A/Switzerland H3 HA (4xM2e-HA H3, Fig. 1A) as previously described (Park et al., 2021). The 4xM2e-HACal head construct contained A/Cal HA head domain (Cys52--Cys277) in the 4xM2e-HA H1 context replacing the A/PR8 HA head domain (Fig. 1A and B). Eight plasmids encoding A/PR8 backbone plus each gene segment, including the desired subtype 4xM2e-HA construct and NA were co-transfected into 293T cells and incubated for 2 days. The sequences of these constructs (4xM2e-HA) were confirmed before transfecting into 293T cells. Chicken embryonated eggs were inoculated with transfection supernatants to rescue different subtype chimeric 4xM2e-HA recombinant viruses. The rescue of recombinant influenza virus was initially confirmed by measuring hemagglutination activity of egg allantoic fluids. We further determined the construct stability in the rescued recombinant viruses by M2e specific ELISA, hemagglutination activity units, and HA antigen specific antibody reactivity.
4.3. Characterization and pathogenicity assessment of recombinant viruses
Recombinant viruses rescued were characterized by measuring replication titers in embryonated chicken eggs (50% egg infectious dose, EID50) and hemagglutination activity using 0.5% chicken red blood cells (RBC, Lampire Biological Laboratories). The existence of 4xM2e in HA was confirmed with enzyme-linked immunosorbent assay (ELISA) using M2e specific monoclonal antibody (14C2 mAb) (Abcam Inc., Cambridge, MA). To assess the pathogenicity of recombinant viruses, C57BL/6J mice were intranasally infected with 106 EID50 of attPR8, attPR8 with 4xM2e-HA H1 (attPR8 4xM2e) or 5 × 105 of EID50 of H3N2 or H3N2 with 4xM2e-HA H3 (H3N2 4xM2e), and body weight changes were monitored. Moreover, nasal turbinates and lung tissue samples were collected and homogenized in RPMI media at 3 days after intranasal infection with 104 EID50 of A/PR8, PR8 with 4xM2e-HACal head (Cal 4xM2e) or 106 EID50 of attPR8, attPR8 4xM2e H3N2 or H3N2 4xM2e. Serially diluted supernatants were inoculated in the embryonated eggs to determine virus titers.
4.4. Immunization and influenza virus challenge in mice
C57BL/6J mice received sequential prime-boost intranasal immunizations with recombinant live viruses in an interval of 3 weeks. The first set of mice groups (n = 20/group, 6–8 weeks old, Jackson Laboratories) were intranasally primed with 106 EID50 of attPR8 or attPR8 4xM2e H1N1 viruses and boosted with 106 EID50 of attPR8, attPR8 4xM2e, H3N2, H3N2 4xM2e or 105 EID50 of Cal 4xM2e H1N1 viruses. One-year-old C57BL/6J mice (n = 5) were primed with attPR8 4xM2e (106 EID50) and boosted with H3N2 4xM2e (106 EID50). For reverse order immunization, mice (n = 20/group, 6–8 weeks old, Jackson Laboratories) were intranasally vaccinated with 5 × 105 EID50 of H3N2 or H3N2 4xM2e and boosted with 106 EID50 of H3N2, attPR8 or attPR8 4xM2e H1N1 viruses. Mouse blood samples were collected through retro-orbital bleeding 2 weeks after each immunization. Mouse groups, including naïve group (unvaccinated mice) from the H1N1 primed set and H3N2 primed another set of hetero prime boost immunizations, were challenged with A/Viet rgH5N1 (10 LD50, 1.6 × 106 EID50) and A/Phil (100 LD50, 1.4 × 104 EID50) in the ABSL2+ and ABSL2 facility, respectively. All animal experiments in this study were approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC) review boards. Mouse animal experiments including virus infection, blood collection, and tissue collections were performed in accordance with the approved IACUC protocol (A21004) and regulations.
4.5. Enzyme-linked immunosorbent assay (ELISA)
IgG antibodies specific for the viruses were measured by ELISA with coating antigens (4 μg/ml) from inactivated A/PR8 H1N1, A/Cal H1N1, A/SW H3N2, A/Phil H3N2, and A/Viet rgH5N1 viruses. M2e-specific IgG antibody responses were determined using M2e peptide antigen (23 amino acids) as described previously (Kim et al., 2013b, Kim et al., 2014). Construction and preparation of the consensus group 1 stalk and group 2 stalk proteins were previously reported (Chae et al., 2019) and used as coating antigens for measuring HA stalk specific IgG antibodies. To determine IgG antibodies to both M2e and HA stalk domains, chimeric M2e-H1 HA stalk protein (aa1-117 of HA2 domain from A/PR8 virus) and M2e-H3 stalk protein (aa1-117 of HA2 domain from A/Aichi/1968H3N2 virus) were expressed in E. Coli and purified (Jeeva at al., unpublished data) and used as an ELISA coating antigen. HRP (Horseradish Peroxidase) conjugated goat anti-mouse IgG (Southern Biotechnology) was used as a secondary antibody. 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Invitrogen™) was utilized for color development. IgG antibody levels were read by BioTek ELISA plate reader at 450 nm.
4.6. Hemagglutination inhibition (HAI) titers
Mouse sera were treated with receptor destroying enzymes (RDE, Sigma) with 1:4 ratio, incubated at 37 °C overnight, and inactivated at 56 °C for 30 min. RDE-treated serum samples were serially diluted (twofold) and incubated with an equal volume of viruses (4 hemagglutination activity units). HAI titers were measured with 0.5% RBC.
4.7. Preparation of lung samples
Lung lysates and bronchoalveolar lavage fluids (BALFs) were collected at day 5 after challenge. Lung extracts were obtained from the lung homogenates in 1.5 ml of RPMI media after centrifugation and used to determine virus titers in embryonated chicken eggs, IgG antibody responses specific for M2e, M2e-H1 stalk and M2e-H3 stalk were measured. BALFs were harvested by infusing 1 ml of phosphate-buffered saline (PBS) into the trachea. IL-6 cytokine levels from BALFs and lung extracts were determined by Ready-SET-Go kits with IL-6 specific antibodies (eBioscience, San Diego, CA) according to the manufacturer’s instructions.
4.8. Statistical analysis
Statistically significant differences were determined among groups using two- or one-way ANOVA. A ρ-values that were less than or equal to 0.05 were considered statically significant. Data were analyzed using Prism software (GraphPad software Inc., San Diego, CA).
Supplementary Material
Acknowledgements
This study was supported by NIH/NIAID grants AI093772 (S.M.K.), AI154656 (S.M.K.), and AI147042 (S.M.K). The following reagent was obtained from International Reagent Resource/CDC: Reassortant A/Switzerland/9715293/2013 virus (X-247 rgH3N2, FR-1366) which carries the A/PR8 backbone genes, and H3 HA and N2 NA genes from A/Switzerland/9715293/2013.
Footnotes
Credit authorship contribution statement
Sang-Moo Kang: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Writing – review & editing. Bo Ryoung Park: Methodology, Investigation, Experiments, Resources, Writing – review & editing. Jeeva Subbiah: Structural modeling of constructs and Experiments. Ki-Hye Kim: Experiments, Writing – review & editing. Young-Man Kwon: Construct resources. Judy Oh: Experiments. Min-Chul Kim: Construct resources. Chong-Hyun Shin: Experiments, Writing – review & editing. Baik Lin Seong: Resources. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2021.12.003.
References
- Belongia EA, Skowronski DM, Mclean HQ, Chambers C, Sundaram ME, DE Serres G, 2017. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev. Vaccines 16, 1–14. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Guptill J, Naficy A, Nachbagauer R, Berlanda-Scorza F, Feser J, Wilson PC, Solorzano A, VAN DER Wielen M, Walter EB, Albrecht RA, Buschle KN, Chen YQ, Claeys C, Dickey M, Dugan HL, Ermler ME, Freeman D, Gao M, Gast C, Guthmiller JJ, Hai R, Henry C, Lan LY, Mcneal M, Palm AE, Shaw DG, Stamper CT, Sun W, Sutton V, Tepora ME, Wahid R, Wenzel H, Wohlbold TJ, Innis BL, Garcia-Sastre A, Palese P, Krammer F, 2020. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis 20, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer WE, DE Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD, 1999. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch. Intern. Med 159, 182–188. [DOI] [PubMed] [Google Scholar]
- Casado I, Dominguez A, Toledo D, Chamorro J, Astray J, Egurrola M, Fernandez-Sierra MA, Martin V, Morales-Suarez-Varela M, Godoy P, Castilla J, Project PIWG, 2018. Repeated influenza vaccination for preventing severe and fatal influenza infection in older adults: a multicentre case-control study. CMAJ (Can. Med. Assoc. J.) 190, E3–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae W, Kim P, Hwang BJ, Seong BL, 2019. Universal monoclonal antibody-based influenza hemagglutinin quantitative enzyme-linked immunosorbent assay. Vaccine 37, 1457–1466. [DOI] [PubMed] [Google Scholar]
- Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB, 2019. Immunosenescence and human vaccine immune responses. Immun. Ageing 16, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn IA, Remarque EJ, Jol-VAN DER Zijde CM, VAN Tol MJ, Westendorp RG, Knook DL, 1999. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J. Infect. Dis 179, 31–36. [DOI] [PubMed] [Google Scholar]
- Dilillo DJ, Palese P, Wilson PC, Ravetch JV, 2016. Broadly neutralizing antiinfluenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Invest 126, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilillo DJ, Tan GS, Palese P, Ravetch JV, 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat. Med 20, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bakkouri K, Descamps F, DE Filette M, Smet A, Festjens E, Birkett A, VAN Rooijen N, Verbeek S, Fiers W, Saelens X, 2011. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: fc receptors and alveolar macrophages mediate protection. J. Immunol 186, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P, 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J. Virol 86, 5774–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C, Zheng NY, Huang M, Cabanov A, Rojas KT, Kaur K, Andrews SF, Palm AE, Chen YQ, Li Y, Hoskova K, Utset HA, Vieira MC, Wrammert J, Ahmed R, Holden-Wiltse J, Topham DJ, Treanor JJ, Ertl HC, Schmader KE, Cobey S, Krammer F, Hensley SE, Greenberg H, He XS, Wilson PC, 2019. Influenza virus vaccination elicits poorly adapted B cell responses in elderly individuals. Cell Host Microbe 25, 357–366 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YT, Aggarwal P, Cirelli D, Gu L, Surowy T, Mozier NM, 2017. Characterization of FcgammaRIIIA effector cells used in in vitro ADCC bioassay: comparison of primary NK cells with engineered NK-92 and Jurkat T cells. J. Immunol. Methods 441, 56–66. [DOI] [PubMed] [Google Scholar]
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, VAN Asten L, Pereira Da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS, GLOBAL SEASONAL INFLUENZA-ASSOCIATED MORTALITY COLLABORATOR, N, 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Lee YN, Kim KH, Lee Y, Jeeva S, Park BR, Kang SM, 2020. Recombinant live attenuated influenza virus expressing conserved G-protein domain in a chimeric hemagglutinin molecule induces G-specific antibodies and confers protection against respiratory syncytial virus. Vaccines (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR, 1997. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 15, 1114–1122. [DOI] [PubMed] [Google Scholar]
- Khurana S, Hahn M, Coyle EM, King LR, Lin TL, Treanor J, Sant A, Golding H, 2019. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat. Commun 10, 3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Jung YJ, Lee Y, Park BR, Oh J, Lee YN, Kim MC, Jeeva S, Kang SM, 2020. Cross protection by inactivated recombinant influenza viruses containing chimeric hemagglutinin conjugates with a conserved neuraminidase or M2 ectodomain epitope. Virology 550, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee JS, Kwon YM, Lee OE, Choi YJ, G J, Wang BZ, Compans RW, Kang SM, 2013a. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antivir. Res 99, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee YN, Kim YJ, Choi HJ, Kim KH, Lee YJ, Kang SM, 2017. Immunogenicity and efficacy of replication-competent recombinant influenza virus carrying multimeric M2 extracellular domains in a chimeric hemagglutinin conjugate. Antivir. Res 148, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Lee YN, Lee KOEJ, Kwon JS, Hwang YM, Song HS, Song JM, Lee BM, Choi YJ, Kang JG, H M, Quan FS, Compans RW, Kang SM, 2014. Supplementation of influenza split vaccines with conserved M2 ectodomains overcomes strain specificity and provides long-term cross protection. Mol. Ther 22, 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC, Song JM, Kwon OE, Y M, Lee YJ, Compans RW, Kang SM, 2013b. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol. Ther 21, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotomina T, Isakova-Sivak I, Kim KH, Park BR, Jung YJ, Lee Y, Mezhenskaya D, Matyushenko V, Kang SM, Rudenko L, 2020. Generation and Characterization of Universal Live-Attenuated Influenza Vaccine Candidates Containing Multiple M2e Epitopes. Vaccines (Basel) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Garcia-Sastre A, Palese P, 2018. Is it possible to develop a "universal" influenza virus vaccine? Potential target antigens and critical aspects for a universal influenza vaccine. Cold Spring Harb Perspect Biol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Pica N, Hai R, Margine I, Palese P, 2013a. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol 87, 6542–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Pica N, Hai R, Margine I, Palese P, 2013b. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol 87, 6542–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VJ, Ho ZJM, Goh EH, Campbell H, Cohen C, Cozza V, Fitzner J, Jara J, Krishnan A, Bresee J, Disease WH, 2018. Advances in measuring influenza burden of disease. Influenza Other Respir Viruses 12, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Hwang HS, Kim MC, Lee YT, Kim YJ, Lee FE, Kang SM, 2016. Protection against respiratory syncytial virus by inactivated influenza virus carrying a fusion protein neutralizing epitope in a chimeric hemagglutinin. Nanomedicine 12, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Suk Hwang H, Kim MC, Lee YT, Cho MK, Kwon YM, Seok Lee J, Plemper RK, Kang SM, 2015. Recombinant influenza virus carrying the conserved domain of respiratory syncytial virus (RSV) G protein confers protection against RSV without inflammatory disease. Virology 476, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung VKY, Carolan LA, Worth LJ, Harper SA, Peck H, Tilmanis D, Laurie KL, Slavin MA, Sullivan SG, 2017. Influenza vaccination responses: evaluating impact of repeat vaccination among health care workers. Vaccine 35, 2558–2568. [DOI] [PubMed] [Google Scholar]
- Liao HY, Wang SC, Ko YA, Lin KI, Ma C, Cheng TR, Wong CH, 2020. Chimeric hemagglutinin vaccine elicits broadly protective CD4 and CD8 T cell responses against multiple influenza strains and subtypes. Proc. Natl. Acad. Sci. U. S. A 117, 17757–17763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Nachbagauer R, Stadlbauer D, Solorzano A, Berlanda-Scorza F, Garcia-Sastre A, Palese P, Krammer F, Albrecht RA, 2019. Sequential immunization with live-attenuated chimeric hemagglutinin-based vaccines confers heterosubtypic immunity against influenza A viruses in a preclinical ferret model. Front. Immunol 10, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastalerz-Migas A, Bujnowska-Fedak M, Brydak LB, 2015. Immune efficacy of first and repeat trivalent influenza vaccine in healthy subjects and hemodialysis patients. Adv. Exp. Med. Biol 836, 47–54. [DOI] [PubMed] [Google Scholar]
- Mclean HQ, Thompson MG, Sundaram ME, Meece JK, Mcclure DL, Friedrich TC, Belongia EA, 2014. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America 59, 1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezhenskaya D, Isakova-Sivak I, Kotomina T, Matyushenko V, Kim MC, Bhatnagar N, Kim KH, Kang SM, Rudenko L, 2021. A Strategy to Elicit M2e-Specific Antibodies Using a Recombinant H7N9 Live Attenuated Influenza Vaccine Expressing Multiple M2e Tandem Repeats. Biomedicines 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto N, Takeishi K, 2018. Change in the efficacy of influenza vaccination after repeated inoculation under antigenic mismatch: a systematic review and meta-analysis. Vaccine 36, 949–957. [DOI] [PubMed] [Google Scholar]
- Music N, Tzeng WP, Liaini Gross F, Levine MZ, Xu X, Shieh WJ, Tumpey TM, Katz JM, York IA, 2019. Repeated vaccination against matched H3N2 influenza virus gives less protection than single vaccination in ferrets. NPJ Vaccines 4, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachbagauer R, Choi A, Izikson R, Cox MM, Palese P, Krammer F, 2016. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. mBio 7 e01996–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachbagauer R, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, Berlanda-Scorza F, Stadlbauer D, Wilson PC, Aydillo T, Behzadi MA, Bhavsar D, Bliss C, Capuano C, Carreno JM, Chromikova V, Claeys C, Coughlan L, Freyn AW, Gast C, Javier A, Jiang K, Mariottini C, Mcmahon M, Mcneal M, Solorzano A, Strohmeier S, Sun W, VAN DER Wielen M, Innis BL, Garcia-Sastre A, Palese P, Krammer F, 2021. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med 27, 106–114. [DOI] [PubMed] [Google Scholar]
- Park BR, Kim KH, Kotomina T, Kim MC, Kwon YM, Jeeva S, Jung YJ, Bhatnagar N, Isakova-Sivak I, Mezhenskaya D, Rudenko L, Wang BZ, Kang SM, 2021. Broad cross protection by recombinant live attenuated influenza H3N2 seasonal virus expressing conserved M2 extracellular domain in a chimeric hemagglutinin. Sci. Rep 11, 4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic R, Bufan B, Arsenovic-Ranin N, Zivkovic I, Minic R, Radojevic K, Leposavic G, 2018. Mouse strain and sex as determinants of immune response to trivalent influenza vaccine. Life Sci. 207, 117–126. [DOI] [PubMed] [Google Scholar]
- Quan FS, Compans RW, Nguyen HH, Kang SM, 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol 82, 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelens X, 2019. The role of matrix protein 2 ectodomain in the development of universal influenza vaccines. J. Infect. Dis S68–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal M, Holmes TH, Maecker HT, Albrecht RA, Dekker CL, He XS, Greenberg HB, 2019. Diminished B-cell response after repeat influenza vaccination. J. Infect. Dis 219, 1586–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Forrest S, Ackley DH, Perelson AS, 1999. Variable efficacy of repeated annual influenza vaccination. Proc. Natl. Acad. Sci. U. S. A 96, 14001–14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, VAN Rooijen N, Bozja J, Compans RW, Kang SM, 2011. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc. Natl. Acad. Sci. U. S. A 108, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Noh JY, Lee JS, Wie SH, Kim YK, Lee J, Jeong HW, Kim SW, Lee SH, Park KH, Choi WS, Cheong HJ, Kim WJ, 2020. Effectiveness of repeated influenza vaccination among the elderly population with high annual vaccine uptake rates during the three consecutive A/H3N2 epidemics. Vaccine 38, 318–322. [DOI] [PubMed] [Google Scholar]
- Thompson MG, Naleway A, Fry AM, Ball S, Spencer SM, Reynolds S, Bozeman S, Levine M, Katz JM, Gaglani M, 2016. Effects of repeated annual inactivated influenza vaccination among healthcare personnel on serum hemagglutinin inhibition antibody response to A/Perth/16/2009 (H3N2)-like virus during 2010-11. Vaccine 34, 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K, 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. J. Am. Med. Assoc 289, 179–186. [DOI] [PubMed] [Google Scholar]
- Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, Yang H, Chen X, Recuenco S, Gomez J, Chen LM, Johnson A, Tao Y, Dreyfus C, Yu W, Mcbride R, Carney PJ, Gilbert AT, Chang J, Guo Z, Davis CT, Paulson JC, Stevens J, Rupprecht CE, Holmes EC, Wilson IA, Donis RO, 2013. New world bats harbor diverse influenza A viruses. PLoS Pathog. 9, e1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.