Abstract
Involvement of extracellular matrix (ECM) components in aging and age-related neurodegeneration is not well understood. The role of hyaluronan (HA), a major extracellular matrix glycosaminoglycan, in malignancy and inflammation is gaining new understanding. In particular, the differential biological effects of high molecular weight (HMW-HA) and low molecular weight hyaluronan (LMW-HA), and the mechanism behind such differences are being uncovered. Tightly regulated in the brain, HA can have diverse effects on cellular development, growth and degeneration. In this review, we summarize the homeostasis and signaling of HA in healthy tissue, discuss its distribution and ontogeny in the central nervous system (CNS), summarize evidence for its involvement in age-related neurodegeneration and Alzheimer Disease (AD), and assess the potential of HA as a therapeutic target in the CNS.
Keywords: hyaluronic acid, hyaluronan, HA, aging, CNS aging, neurodegeneration, HA therapeutics
1. Introduction
As evidence about individual components of the extracellular matrix (ECM) accumulates, the view of ECM as an architectural scaffold is gradually changing. Though recognized as an important factor for cellular and organ architecture early on (Steinberg, 1963), the importance of ECM components in growth, development, and aging is gaining more appreciation. ECM is composed primarily of proteins, proteoglycans (PGs) and glycosaminoglycans (GAGs); however, the exact distribution of these constituents varies greatly with tissue type (Theocharis, Skandalis, Gialeli, & Karamanos, 2016).
The relatively simple composition of one of the GAGs, hyaluronan (HA), contrasts with its complex roles in development, disease, and aging. This review examines existing evidence on HA homeostasis and signaling, its role in neural ontogeny and aging, and its potential as a diagnostic and therapeutic target for age-related disease in the central nervous system (CNS). Special attention will be given to size-dependent divergence of polymer effects on CNS health and age-related pathology.
2. Hyaluronan Homeostasis
Glycosaminoglycans (GAGs) are a class of extracellular matrix (ECM) molecules consisting primarily of sugar polymers. Most GAGs are further sulfated and are covalently attached to a core protein, forming a proteoglycan molecule (Imberty, Lortat-Jacob, & Pérez, 2007). Hyaluronic acid, or hyaluronan (HA), is a class of GAG that is ubiquitous in most tissues. A polymer of alternating D-glucuronic acid and N-acetyl-D-glucosamine units linked via β-(1–4) and β-(1–3) glycosidic bonds (Weissmann, Meyer, Sampson, & Linker, 1954), HA can be millions of Daltons (Da) in size and, in contrast to other GAGs, is not modified by sulfation or covalent attachment to amino groups (Almond, 2007). Unlike other GAGs, which are synthesized by Golgi enzymes, HA synthesis takes place near the cell membrane (Ng & Schwartz, 1989) by one of three hyaluronic acid synthases, (HAS’s), that are differentially expressed in different tissues at different stages of an organism’s development (Tien & Spicer, 2005).
2.1. HA anabolism.
HA synthesis depends on substrate availability, as uridine diphosphate (UDP)-sugars are the building blocks of the polymer. Inhibition of cell growth regulators protein kinase B, PKB aka AKT, and mechanistic target of Rapamycin (mTOR), a major nutrient sensor, by C2–ceramide and rapamycin, respectively, resulted in decreased HA levels in the media of treated fibroblasts (Qin, Berdyshev, Poirer, Schwartz, & Dawson, 2012). Activation of mTOR/AKT pathways appears to enhance HA anabolism, as demonstrated by increased expression of one of the primary HA synthases, HAS2 (Figure 1). Therefore, HA synthesis appears to be a metabolic state-dependent process.
Figure 1. Homeostasis of HA, major constituent of ECM GAG.
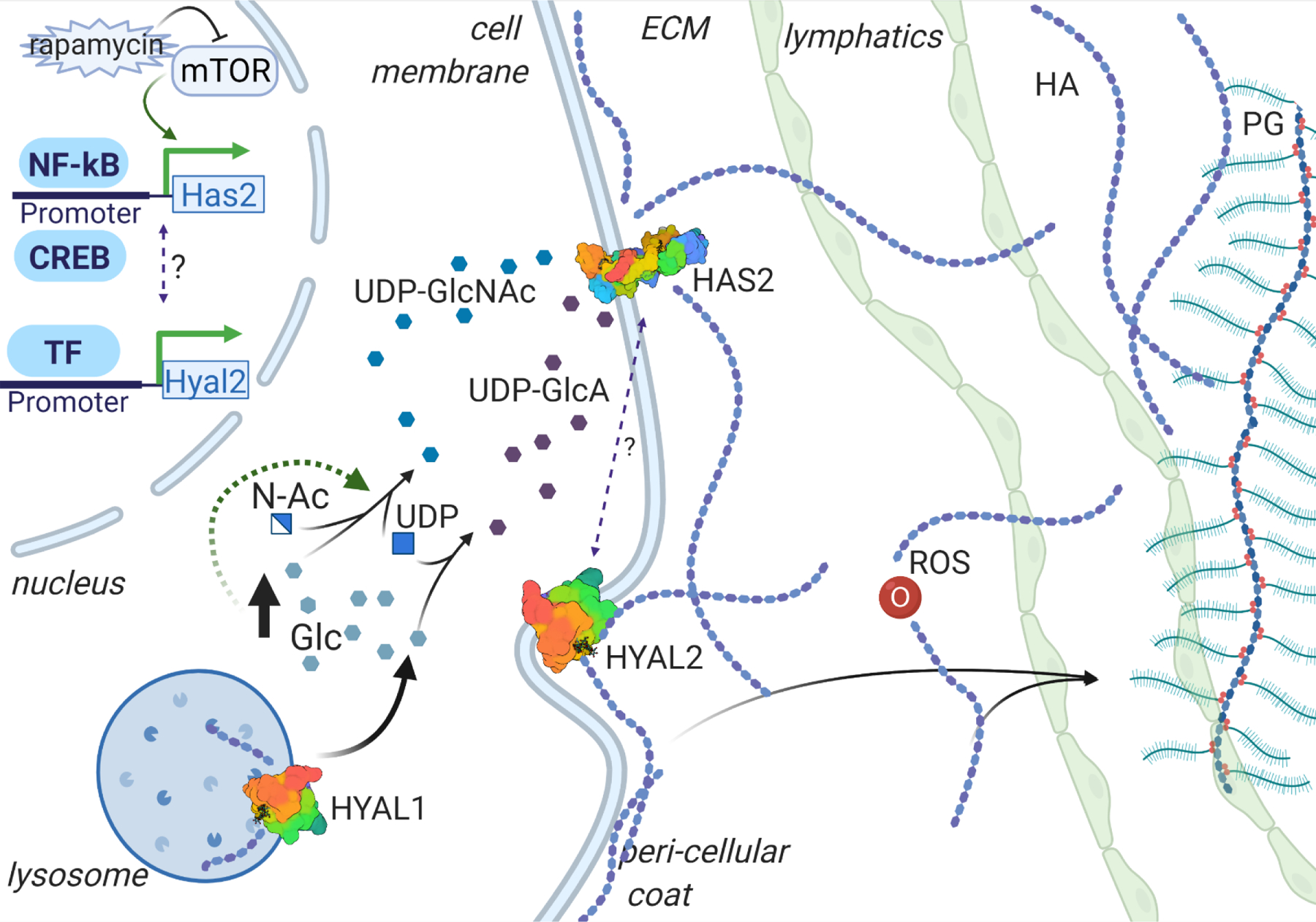
Hyaluronan synthases (HASs) are the enzymes responsible for hyaluronan (HA) anabolism, while hyaluronidases (HYALs) degrade the polymer extra- and intra-cellularly. Reactive Oxidative Species (ROS) are capable of degrading the HA molecules into polymers of random size. The regulation of HA synthesis and degradation is regulated by multiple pathways, including metabolic, growth, and inflammatory signaling. HA turnover involves uptake of the polymers of certain size into lymphatic tissue, and subsequent degradation in lymphatic and hepatic tissue. PG = Proteoglycan; Glc = glucose; N-Ac = N-acetyl; UDP-GlcNAc = UDP-N-acetyl-D-glucosamine, UDP-GlcA = UDP-D-glucuronic acid
Hyaluronan synthases (HASs) comprise three transmembrane glycosyltransferase isoenzymes, that share 50% to 71% homology. The HAS1 gene is found on human chromosome 19, while HAS2 and HAS3 are found on chromosome 8 and 16, respectively. Despite the similarity in structure, HAS1 and HAS3 generate HA polymers of smaller sizes (2×10^5 to 2×10^6 Da), while the average size of the polymer produced by HAS2 is greater than 2×10^6 Da (Itano et al., 1999). Furthermore, the Km for UDP-sugars is higher for HAS1 in comparison to HAS2 and HAS3. Cross-species comparison of HAS2 genes shows high conservation of amino acid sequence. For example, major differences in HAS2 enzymatic activity were proposed to be driven by a few amino acid substitutions, particularly in sites 178 and 301, which appear to be important for production of very high molecular weight HA (HMW-HA) in naked mole rats (NMR) (Faulkes, Davies, Rossiter, & Bennett, 2015; Tian et al., 2014).
As previously mentioned, HA synthesis takes place at the cell membrane. Hyaluronan synthase builds the polymer by adding to the growing chain on the cytoplasmic side of the cell membrane, after which the polymer is directly secreted into the extracellular space (L. H. Philipson & Schwartz, 1984; Prehm, 1984) (Figure 1). This mode of synthesis is thought to underlie the ability of HA polymers to attain megadalton size (Weigel, Hascall, & Tammi, 1997). HA can be deposited pericellularly, forming negatively charged hyaluronan matrix around the cells, or coats (Heldin & Pertoft, 1993), as a component of the glycocalyx, or can be released into the ECM and act as an anchoring molecule for other GAGs and PGs in non-covalent interactions (T. C. Laurent & Fraser, 1992) (Figure 1).
2.2. HA catabolism.
Similar to synthesis of hyaluronan, the degradation of HA is complex. HA turnover is surprisingly rapid, as at least a third of the body’s total content undergoes turnover daily (U. B. G. G. Laurent & Reed, 1991). Local cutting of the polymer can be driven by an enzymatic or a free radical reaction (Figure 1).
Enzymatic degradation is performed by either endo- or exoglycosidases. While exoglycosidases are more abundant than endoglycosidases in tissue, it is unclear if one class of enzymes contributes to overall turnover of HA greater than the other (Stern, Kogan, Jedrzejas, & Šoltés, 2007). Among endoglycosidases, a widely investigated class is endo-β–acetylhexosaminidases. There are six hyaluronidase-like genes (HYALs), found as groups of three on human chromosomes 3 and 7 that share about 40% sequence homology (Csóka, Scherer, & Stern, 1999). HYAL1 and HYAL2, which possess the enzymatic machinery for both hydrolysis and transglycosylation, carry out hyaluronidase activity in somatic tissues (Hoffman, Meyer, & Linker, 1956). While there is some evidence to suggest lysosomal localization of HYAL2 (Chow, Knudson, & Knudson, 2006), another report demonstrates that the enzyme is present on lipid rafts of the cell membrane, where it can associate with cluster of differentiation 44 (CD44) (Andre et al., 2011), one of the major receptors of HA signaling. HYAL2 cleaves large HA into 20 kDa fragments, has a pH optimum of 6.0–7.0 (Harada & Takahashi, 2007) and a relatively low catalytic activity (Lepperdinger, Strobl, & Kreil, 1998). HA degradation by HYAL2 requires formation of an extracellular acidic pocket via interaction of CD44 with the Na+- H+ exchanger at the cell membrane (Andre et al., 2011; L. Y. W. W. Bourguignon, Singleton, Diedrich, Stern, & Gilad, 2004). HYAL1, which is associated with lysosomes and has a pH optimum of 3.7, has the potential to hydrolyze endocytosed HAs with a wider range of sizes as well as the ability to cleave the polymer into oligomers (Takagaki et al., 1994). Hence, enzymatic degradation of HA is driven by HYAL2 at extracellular pockets and is further continued by lysosomal HYAL1 intracellularly (V. Bourguignon & Flamion, 2016) (Figure 1). Though independent from one another, both catabolic processes are CD44 receptor-dependent (Duterme, Mertens-strijthagen, Tammi, & Flamion, 2009; Harada & Takahashi, 2007).
An alternative pathway of tissue HA degradation is reactive oxidative species (ROS)-driven polymer hydrolysis. Superoxide radicals produced by polymorphonuclear cells (PMNs, i.e. granulocytes) (Rees, Hawkins, & Davies, 2004) and nitric oxide (NO) produced by local epithelial cells (Vilar et al., 1997) lead to sporadic cleavage of HA, yielding randomly sized polymers and oligomers. At the same time, a major contributor of tissue HA turnover is lymphatic clearance of GAG with subsequent degradation in the lymphatic system and the liver (Fraser, Kimpton, Laurent, Cahill, & Vakakis, 1988; U. B. G. Laurent & Reed, 1991).
2.3. Homeostatic balance.
There appears to be a complex balance between HASs and HYALs, as evident by extended investigation of HA signaling and homeostasis in the setting of different cancers. Treatment with exogenous HYAL increases HAS activity, as evident by the increase in anabolism of HA in oligodendroglioma cells (Louis H. Philipson, Westley, & Schwartz, 1985). Overexpression of HYAL2, however, does not change total HA levels or HAS expression, as demonstrated in fibroblastic BB16 cell lines (Duterme et al., 2009). This suggests an enzyme type-, localization- and interaction-dependent balancing of synthesis and degradation of HA, which is subject to malignancy-related dysregulation in cellular processes, such as mTOR and extracellular signal-regulated kinase (ERK) signaling.
Total levels of HA in serum and tissues appear to increase as a function of age (Holmes, Bayliss, & Muir, 1988; Inoue et al., 2011a; Yannariello-Brown, Chapman, Ward, Pappas, & Weigel, 1995). The factors contributing to HA accumulation could be decreased activity of hyaluronidases in tissues, including the liver, or dysregulation of lymphatic flow, such as occurs in aging (Zolla et al., 2015). In an aging organism the turnover and homeostasis of HA can be jeopardized, such as due to increased activity of hyaluronidases (Chajara et al., 1998), and lead to an accumulation of random sized HAs. This in turn can lead to deregulated production and degradation of the polymer. Assessment of human cartilage revealed an age-dependent increase in total amount of hyaluronan and a decrease in average HA polymer size, while no change in the size of newly synthesized polymers were observed (Holmes et al., 1988). A potential explanation for these findings is the diminished integrity of lymphatic outflow with age (Zolla et al., 2015), resulting in accumulation of HA that is cleaved by local hyaluronidases. Additionally, age-related myeloid bias, a relative increase of ratio of myeloid to lymphoid cells (Pang et al., 2011), and increased granulocyte populations in peripheral blood (Valiathan, Ashman, & Asthana, 2016) and tissue (Gomez et al., 2007), could result in increased non-specific cleavage of HA by the ROS released by activated immune cells. This increase in depolymerization and reduction in clearance could result in increased HA production by HAS2, to potentially compensate for shift in average HA size, which would further perpetrate a cycle of accumulation and HA degradation. The reduction in average HA size with age and chronic inflammation, in turn, could result in reduced protection from processes caused by advanced glycation end products (AGEs) and activation of local inflammatory responses. Conversely, larger HA polymers are protective against inflammation (Neumann, Schinzel, Palm, Riederer, & Münch, 1999), suggesting that changes in HA size may be a cause rather than a consequence of inflammation.
3. Molecular pathways involved in HA signaling
There appear to be several proteins that can serve as HA receptors to transduce HA signaling, including lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), hyaluronic acid receptor for endocytosis (HARE), toll-like receptors 2 and 4 (Tlr2 and 4), hyaluronan-mediated motility receptor (RHAMM), and CD44. HA signaling through these receptors is discussed by Vigetti et al. (Vigetti et al., 2014) and is summarized in Figure 2. Reports on downstream pathways of these receptors, including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), ERK1/2, Akt, cyclic AMP response element-binding protein B (CREB), and Hippo signaling, suggest overlaps in HA signaling through its receptors, and different outcomes depending on the size of HA polymer. In discussion of size differences, it is important to keep in mind that increase in total HA results in the increase in absolute amount of polymers of both larger and smaller size and the phenotypic outcome is dependent on the balance of the differentially sized HA (Figure 2).
Figure 2. Balanced effects of different sized HA polymers.
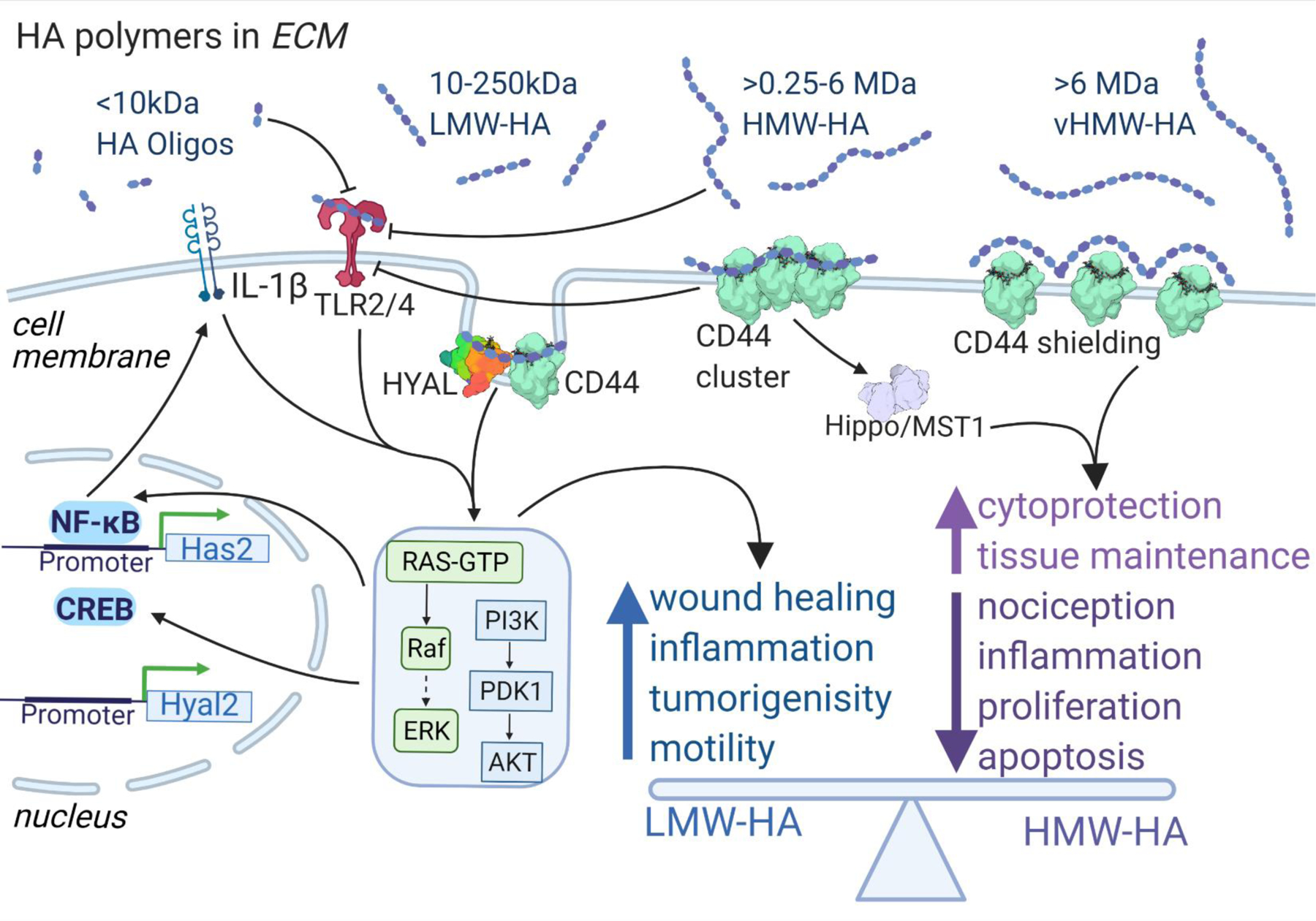
While LMW-HA activates pro-inflammatory and proliferative signaling cascades effects via TLR2/4 and CD44, HMW-HA has anti-hyperalgesic, anti-proliferative and anti-apoptotic activities, potentially by causing a different conformation of its major receptor, CD44. vHMW-HA, additionally, has superior cytoprotective properties. Both LMW-HA and HMW-HA are important for wound healing and tissue maintenance. Disparity of differentially sized HA effects happens on receptor level.
3.1. Inflammatory mediators.
HA’s status in synovial tissue has been associated with inflammatory processes, especially in rheumatologic disease. Serum analysis of osteoarthritis (OA) patients showed an increase in HA levels that correlated with disease severity (Inoue et al., 2011b). Levels of tumor necrosis factor-stimulated gene-6 (TSG-6), an HA binding protein associated with inflammation, were also elevated in synovium and cartilage from arthritis patients, displaying intense perivascular staining (Bayliss et al., 2001). The average molecular weight of HA molecules in the synovial fluid was shown to be lower in patients with arthropathies when compared to normal synovial fluid of age-matched controls (Dahl, Dahl, Engstrom-Laurent, & Granath, 1985). HA polymers present in healthy synovial fluid exerted anti-proliferation effects, whereas those found in inflamed milieus did not exert such an effect on immortalized 3T3 cells (Goldberg & Toole, 1987). Analysis of CD44 expression in synovial tissue of rheumatoid arthritis (RA) patients revealed an increase in receptor expression in RA compared to trauma patients without immune-mediated damage; moreover, CD44 expression was reduced in synovial fluids with higher cell counts, while CD44 liposomes suppressed T cell activation (Haynes, Hale, Patton, Martin, & McCallum, 1991). Interaction through intercellular adhesion molecule-1 (ICAM-1) was also suggested to play a role in an anti-proliferative and anti-inflammatory role of the soluble form of HA receptor in RA (Hiramitsu et al., 2006). Interestingly, these studies highlight the disparity among HA size in inflamed and healthy tissues: the former appear to have an abundance of smaller, or low molecular weight HA (LMW-HA), while healthy tissues have relatively larger, or high molecular weight HA (HMW-HA). Additionally, HA polymers 4 to 25 disaccharides in length have been reported to possess angiogenic properties in a chicken chorioallantoic membrane assay, which was not observed after treatment with HMW or very small HA oligomers (West, Hampson, Arnold, & Kumar, 1985). Therefore, depending on their size, HA polymers can have diverse effects on tissue homeostatic and disease states (Figure 2).
A mechanism directly bridging inflammation and LMW-HA is signaling through the toll-like receptor 2 and 4 (TLR2, TLR4) and subsequent activation of NF-κB (Campo et al., 2010). Introduction of LMW-HA to pyrogenic bacterial lipopolysaccharide (LPS)-treated chondrocytes resulted in a synergistic increase in NF-κB signaling, which was blocked by TLR4 receptor antibody. In contrast, introduction of HMW-HA reduced TLR-4 dependent inflammation. As mentioned earlier, treatment with HMW-HA, and not LMW-HA, resulted in protection from AGE induced NF-kB activation (Neumann et al., 1999).
Reciprocally, interleukin (IL)-1β signaling resulted in elevation of HAS2 expression in an ERK1/2-dependent manner; this elevation was further boosted synergistically by tumor necrosis factor (TNF)-α treatment and resulted in enrichment of short HA polymers 0.85–1.1 kDa in size (Ducale, Ward, Dechert, & Yager, 2005). The observed effect was caused by activation of the NF-kB pathway, as well as stabilization of HAS2 transcripts by IL-1β activation of MAPK (Figure 2). NF-kB appears to directly induce HAS2 transcription by binding HAS promoter transcription factor response elements (RE) (Saavalainen, Tammi, Bowen, Schmitz, & Carlberg, 2007).
A curious effect of differentially sized HA molecules was seen in their regulation of nociception. While intradermal injection of LMW-HA induced a hyperalgesic response in rats to mechanical stimulation, HMW-HA blocked that response in a CD44-dependent manner (Ferrari, Khomula, Araldi, & Levine, 2018). Furthermore, the anti-hyperalgesic effect of HMW-HA was observed in concomitant treatment with other pro-nociceptive mediators such as prostaglandin E2 (PGE2), epinephrine, TNF-α, IL-6 and the chemotherapy drug paclitaxel, which causes severe neuropathies, especially in older patients (Tanabe et al., 2013). The activation of kappa opioid receptors by HA seen in Chinese hamster ovary (CHO) cells expressing a panel of opioid receptors (Zavan et al., 2013) could suggest a possible mechanism for the observed hyperalgesic effect in vivo. These findings suggest that nociception modulation in the setting of inflammation by LMW-HA and HMW-HA can be regulated not only through inflammation associated receptors, such as TRL2/4, but also via signaling through neuron-specific receptors. The nociceptive role of differentially sized HA becomes especially important in aging patients with chronic pain and arthralgias, where enrichment of HMW-HA could help alleviate the chronic symptoms.
3.2. Stress resistance and longevity mediator.
HA has been reported to be important in multiple stress response pathways. Treatment of human corneal epithelial cells with HMW-HA protects against the oxidative damage of ultraviolet (UV)-B radiation, potentially acting as a ROS scavenger in the tissue (Pauloin, Dutot, Joly, Warnet, & Rat, 2009). In studying the role of very high molecular weight HA (vHMW-HA, ~6000 kDa), the type of HA enriched in long-lived rodent, the naked mole rat (NMR), our lab has shown that vHMW-HA was superior to shorter HAs in protecting against oxidative stress caused by treatment with tert-butyl hydroperoxide (Takasugi et al., 2020). The protective effect was dependent on CD44 (Takasugi et al., 2020). Therefore, HA molecules have a potential to protect against ROS as a scavenger molecule or through more complex signaling pathways.
Treatment of epidermal keratinocytes with extracellular adenosine triphosphate (ATP), a local marker of stress and injury, transiently increased the expression of HAS2, and to a lesser extent, HAS3 and CD44, and increased pericellular and ECM hyaluronan (Rauhala et al., 2018). The mechanism of activation involves purinoceptor 2 (P2Y2) stimulation and induction of HAS2 transcription via CREB, which has functional binding sites in proximity to the HAS2 promoter (Makkonen, Pasonen-Seppänen, Törrönen, Tammi, & Carlberg, 2009) (Figure 2). The increase in HAS2 expression in skin fibroblasts also correlated with a reduction in apoptotic responses in cells subjected to an environmental stress (Y. Wang, Lauer, An, Mack, & Maytin, 2014).
In addition to a catalytic domain that cleaves GAGs, HYAL 1 and 2 possess an epidermal growth factor (EGF)-like domain which could aid in protein-protein interaction and play a role in Hyal-CD44-HA complex signaling (Chao, Muthukumar, & Herzberg, 2007). In fact, CD44-HYAL2-ezrin-radixin-moesin (ERM) proteins can be co-immunoprecipitated, this interaction was disrupted by an intact pericellular coat and has been implicated in reduction of cellular motility and wound healing (Duterme et al., 2009). The recently identified hyaluronidase transmembrane protein 2 (TMEM2) appears to regulate the angiogenic effect of LMW-HA via vascular endothelial growth factor (VEGF) signaling, and is essential for primary and secondary angiogenesis in zebrafish (De Angelis et al., 2017). The cell surface hyaluronidase was also shown to act as a potent modulator of endoplasmic reticulum (ER) stress resistance in C. elegans and ectopic expression of human TMEM2 resulted in increase of lifespan in this model (Schinzel et al., 2019). The observed ER resistance was CD44-dependent, further underscoring the importance of the HYAL-CD44 complex interaction for development and stress resistance (Schinzel et al., 2019). Interestingly, treatment of proximal tubule epithelial cells (PTEC) with bone morphogenic protein 7 (BMP7) resulted in HYAL2 nuclear translocation and differential splicing of CD44 mRNA, which resulted in enrichment of the antifibrotic CD44 isoform at the cell surface, where it promoted HA internalization and blocked myofibroblast differentiation (Midgley et al., 2017). HYAL-CD44 interaction in multiple processes lends credence to the idea that their interaction, through HA and without it, is a complex regulator of development, disease, and longevity.
3.3. Anti-cancer mediator.
As mentioned earlier, HA appears to be involved in multiple disease processes, including cancers. There is a complex push-pull relationship between HA synthases and hyaluronidases either by direct interaction or through HA homeostasis feedback. The relationship appears to be important for the cancer-related profiles of HA. For example, transfection of human fibrosarcoma with human HAS2 resulted in increased HA production and increased tumorigenicity of the cells (Kosaki, Watanabe, & Yamaguchi, 1999), while silencing of HAS2 suppressed the malignant phenotype of breast cancer cells in vivo (Li, Li, Brown, & Heldin, 2007). Inhibition with 4-methylumbelliferone, which reduces HA synthesis indirectly (Qin, Kilkus, & Dawson, 2016), appeared to reduce oligodendrioma tumor growth. In a study of the effect of HAS2 expression by glioma cells, HYAL2 status was important for tumor growth, where increased hyaluronidase activity resulted in increased migration of malignant cells (Enegd et al., 2002). The parallel overactivation of both HAS2 and HYAL2 appears to predispose tumors to increased aggressiveness: increased production of HA which is cleaved by locally expressed hyaluronidases results in accumulation of LMW-HA, which in turn promotes inflammation, angiogenesis, and cell migration (i.e., extravasation and metastasis).
HMW-HA, on the other hand, appears to confer an anti-malignant phenotype. Xenograft transplantation of naked mole rat (NMR) skin fibroblasts, which endogenously express NMR-HAS2 capable of synthesizing HA polymers 10x greater in size than human HAS2, yielded no growth in NIH III nude mice, while interference with NMR-HAS2 expression or over-expression of human HYAL2 resulted in malignant transformation of the graft (Tian et al., 2014). The resistance of the NMR cells to transformation appears to be mediated by early contact inhibition (ECI) dependent on p16 signaling (Seluanov et al., 2009). The report by Tian et al. (Tian et al., 2014) further emphasizes that differently sized HA molecules could be exerting opposing effects on cell proliferation and tissue integrity. Additionally, a recent study has shown involvement of the Hippo pathway, responsible for control of organ size via regulation of cell proliferation and apoptosis, in HMW-HA’s cell-density-dependent growth inhibition (Ooki, Murata-Kamiya, Takahashi-Kanemitsu, Wu, & Hatakeyama, 2019). HMW-HA-driven clustering of CD44 receptors results in binding to partitioning-defective 1b (PAR1b), which in turn releases mammalian serine/threonine protein (STE20)-like kinases, mammalian Ste20 (MST) kinases and results in Hippo activation (Figure 2). LMW-HA can interfere with this activation by competitively binding CD44 receptors and preventing receptor clustering, thereby blocking HMW-HA driven cell density-dependent growth inhibition. The enrichment of LMW-HA in aging could be one of the factors that generates a pro-oncogenic microenvironment in aging tissue, and the potential of HMW-HA enrichment to rescue this phenotype needs to be further investigated.
3.4. HA size disparity.
A recurring theme in HA-related literature is opposing biological outcomes based on molecular weight: LMW-HA is involved in inflammation, proliferation, and malignancy, while HMW-HA is involved in tissue maintenance, growth inhibition and recovery (Figure 2). The biochemical mechanisms underlying these size differences are still poorly understood. However, a recent report on HA conformation has shed light on the differences in biological effects of different sized polymers. While proteins appear to interact with HA via a link module, a motif structurally similar to C-type lectin domain (Kohda et al., 1996), a recent study has shown that short HAs appear to behave like a rod while large polymers behave like a random coil (Weigel & Baggenstoss, 2017). This rod-to-coil transition happens at the 150–300 kDa range. Different affinity or binding of the link module to rod versus coil conformation, or the steric hindrance that the larger HA molecules could introduce might underlie this size-dependent differences in protein interactions and, therefore, lead to differences in function. Consideration of conformational differences might be a useful guide in categorizing high molecular weight HA as polymers of >300 kDa and intermediate/low molecular weight HA polymers as molecules <300 kDa in size, keeping in mind that very high molecular weight HA and HA oligomers should be a separate category due to effects that deviate from those of HMW- and LMW-HA polymers. (West et al., 1985). Additionally, as was demonstrated by Takasugi et al. (Takasugi et al., 2020), HA polymers of very high molecular weight greater than 6 mega Dalton (MDa) (vHMW-HA), which are synthetized by NMR fibroblasts, allow for cytoprotection superior to that of HMW-HA. This stronger cytoprotective results from differential interaction of vHMW-HA with CD44, where the larger polymers attenuate CD44 protein-protein interaction, which demands for separate category for the larger polymers. Setting a consensus on the size- and three-dimensional structure- dependent categorization of HA polymers can help parse apart the complexity of HA signaling.
4. Animal models with modulated HA homeostasis
To better understand the role of HA in development and disease, several HA metabolism related genes have been deleted in mice (Table 1). Multiple studies reported HAS2 to be the major HA producing enzyme (Camenisch et al., 2000; Kessler, Obery, & De La Motte, 2015) and Has2 global knockout (KO) was reported to be incompatible with normal embryonic development due to severe cardiac and vascular abnormalities (Camenisch et al., 2000). Targeted knockout of the HAS2 gene in mesenchymal tissue using the paired related homeobox 1 – Cre recombinase (Prx1-Cre) system resulted in severe impairments of limb development, such as phocomelia, and defective joints, further underscoring the role of HAS2 in development. (Matsumoto et al., 2009). Conditional knockout of the Has2 gene in brain using a Nestin-Cre transgene (Has2Nestin-cKO mice) in mice resulted in a seizure phenotype, which was milder than that seen with the Has3 knockout animals (Arranz et al., 2014). Though hippocampal distribution of HA did not display major differences in Has2Nestin-cKO when compared to wild type brains, some areas of the brain, such as the corpus callosum and cingulum bundle, completely lacked HA staining. Therefore, HAS2 appears to be involved in CNS development as well as maintenance of HA content in healthy adult tissue.
Table 1.
Genetically modified mouse models
| Genetic manipulation | Peripheral Phenotype | CNS Phenotype (if reported) | Authors |
|---|---|---|---|
| HAS1 KO | Impedes formation of retrocalcaneal bursa | Mild seizure phenotype | Sikes et al., 2018; Arranz et al., 2014 |
| HAS2 KO | Severe cardiac and vascular abnormalities; incompatible with normal embryonic development | Camenisch et al., 2000 | |
| Conditional HAS2 KO | Under Prx1-Cre: shortened limbs, duplicated proximal phalanges, abnormal growth plates, lack of formation of secondary ossification centers, defective synovial cavities | Under Nestin-Cre: mild seizure phenotype, reduction in cortical HA and lack of HA deposition in major fiber tracts | Matsumoto et al., 2009; Arranz et al., 2014 |
| HAS3 KO | Protection from ventilator induced lung inflammation; protection from dextran sodium sulfate induced colitis | Seizures, reduction in HA content in hippocampus | Bai et al., 2005; Kessler et al., 2015; Arranz et al., 2014 |
| HAS1/3 KO | Abnormal and accelerated wound healing and enhanced inflammation around induced lesions | Mack et al., 2012; | |
| HYAL1 KO | Mild mucopolysaccharidosis | Martin et al., 2008; Bourguignon et al., 2016 | |
| HYAL2 KO | Cranio-vertebral skeletal abnormalities, thrombocytopenia, hemolysis, HA content increase in plasma and liver | Jadin et al., 2008; Bourguignon et al., 2016 | |
| HYAL3 KO | No gross phenotypic change, normal HA levels in serum, but mild changes in alveolar structure | Atmuri et al., 2008 |
Constitutive knockout of the Has1 gene resulted in milder peripheral and CNS phenotypes. HAS1 appears to be important in formation of retrocalcaneal bursa (Sikes et al., 2018), while knockout of the gene resulted in a seizure phenotype akin to Has2 conditional knock out (Has2Nestin-cKO) mice (Arranz et al., 2014). In the same study, lack of Has3 resulted in the most severe epileptic phenotype, which was rescued by restoration of extracellular space with application of a hypertonic solution and could be mimicked by application of hypotonic solution onto wild type brain slices. Global knockout of Has3 resulted in diminished inflammatory reaction after high ventilator volume-induced lung injury (Bai et al., 2005) and after dextran sodium sulfate-induced colitis (Kessler et al., 2015). Finally, double knockout of Has1 and Has3 resulted in abnormal wound healing and enhanced inflammation after 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin injury (MacK et al., 2012). Hence, HAS1 appears to play a minor role in development or disease, while HAS3 function is linked most closely to inflammation and disease phenotype. Therefore, modulation of HAS2 and HAS3 activity could be of interest to understand the role of HA in healthy and pathologic aging, respectively.
To target HA catabolism, mouse with KO of Hyal1, Hyal2, and Hyal3 were generated. Constitutive knockout of Hyal1 resulted in a mouse model with no overt abnormalities, but with mild, but progressing mucopolysaccharidosis, evident by accumulation of HA in multiple joints (Martin et al., 2008). Mice lacking the Hyal2 gene displayed cranial and vertebral bone abnormalities, thrombocytopenia and hemolysis, and elevated levels of HA in plasma (Jadin et al., 2008). Accumulation of HA in liver tissue was also noted. In contrast, Hyal3 KO mice displayed no gross phenotypic changes, with no change in GAG content in the ECM of organs, no apparent vacuolization, no histological changes in joints and no change in HA content in serum (Atmuri et al., 2008). There were, however, structural changes in the lungs of the Hyal3-deficient animals, with enlarged alveoli and thickened ECM. Therefore, it appears that HYAL2 is the major enzyme responsible for local HA degradation, uptake into the lymphatic system and further catabolism in the liver. HYAL1 is most active in HA catabolism in joints, while HYAL3 does not substantively contribute to HA degradation. No pathologic CNS or aging phenotypes, apart from progressive accumulation of HA in joint in Hyal1 KO mice, were reported in these models.
An interesting natural model (without the need for genetic modification) to study effects of HMW-HA in aging is the naked mole rat (NMR). This species shows high enrichment of total HA when compared to mice, and the NMR HAS2 produces polymers that are millions of Daltons in size (Tian et al., 2014), aka vHMW-HA. Interestingly, NMRs show little cognitive decline with aging (Buffenstein, 2008), and are able to maintain roles in their complex social hierarchy even at older age. Additionally, though harboring CNS amyloid beta (Aβ) levels comparable to Alzheimer’s Disease (AD) transgenic mouse models, Aβ in NMR is resistant to aggregation, as evidenced by the absence of plaques in 20+ year old animals (Edrey et al., 2013). A recent report by Kulaberoglu et al. highlighted the structural intricacies of HA in different NMR tissues (Kulaberoglu et al., 2019). Using atomic force microscopy (AFM), the group was able to detect different HA polymer structures formed in vivo, from a gyrated appearance in the brain to “snowman” structures in the lungs. This study emphasized the high affinity of NMR skin HA to water and its capacity to transition from viscoelastic to elastic material upon dehydration, physicochemical functions implicated in the enhanced elasticity of NMR skin. The study of NMR femoral cartilage, which was shown to have superior compression resistance, revealed enrichment with vHMW-HA which correlated with reduced death of chondrocytes post mechanistic stress compared to mouse tissue (Taguchi et al., 2020). Furthermore, compared to mice, NMRs showed remarkable resistance to injury-induced osteoarthritis, suggesting that vHMW-HA protects the joints from injury and inflammation (Taguchi et al., 2020). These findings not only underscore the utility of NMRs as a model of resistance to the deleterious effects of aging, but also suggest that the link between HA and aging needs to be investigated further for insights into potential therapeutic avenues.
5. HA in CNS ontogeny
As evident by complex processes of synthesis and metabolism, as well as complex signaling pathways of differently sized HA molecules, the role of the HA polymer varies depending on the organism’s stage of development (Figure 3).
Figure 3. HA amount and size in CNS ontogeny.
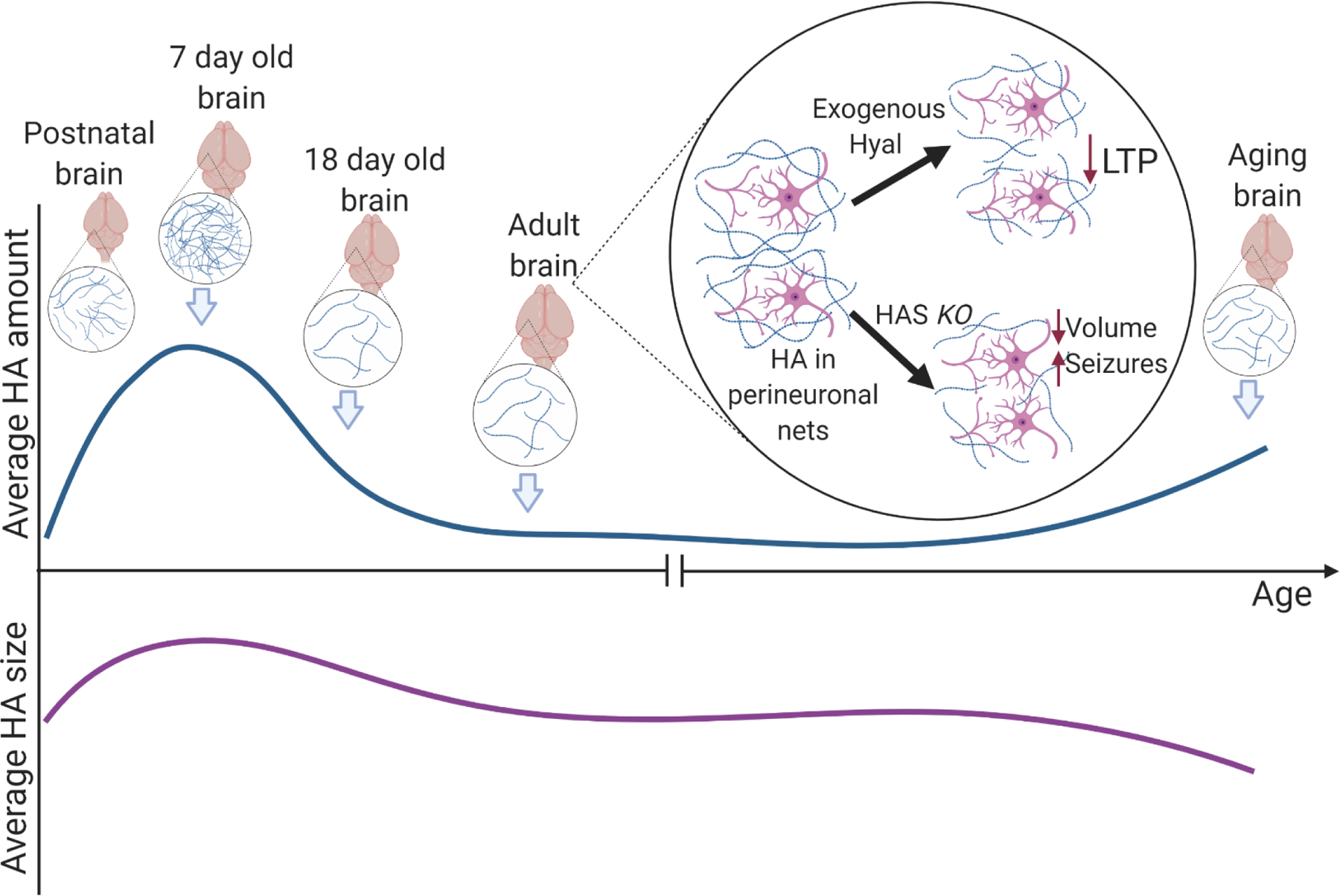
HA is important in maintaining spatial separation of neurons for normal function. In early mouse brain development HA increase is favorable for cell development and maturation. Around day 18 after birth, reduction in HA is important for brain development. HA levels in adult brains undergo minute, quickly amended fluctuations, subject to health state. In aging brain there is an increase in total HA and decrease in average HA size, without change in size of newly synthesized HA - suggestive of inflammation and degradation.
Hyaluronan appears to be present in embryonic tissue at very early developmental stages (Brown & Papaioannou, 1993). Potential roles of HA in developing tissue include mechanical protection, maturation regulation, as well as protection from the maternal immune system, dysregulation of which leads to preterm birth (Vora et al., 2018). Interactions between CD44 and HMW-HA support decidual stromal cells (DSCs), which maintain a balanced cytokine milieu at the maternal-fetal interface in early pregnancy, inhibiting apoptosis in the uterine cells via AKT and ERK1/2 signaling (Zhu et al., 2013). In patients with pre-eclampsia, HAs of undetermined size were elevated in maternal blood, and are thought to contribute to development of fibrinoid deposits in the placentas of patients with this condition (Matejevic, Neudeck, Graf, Müller, & Dietl, 2001). In vivo oviductal infusion of HA polymers 500–750 kDa in size interfered with embryonic development in ewes, while addition of Hyal2 resulted in increase of blastocyst quality, increase in cell division, proliferation and migration (Marei et al., 2016), processes that are critical for embryonic development. While relative mRNA expression of HAS2 decreased closer to blastocysts stage of bovine embryonic development (Marei, Salavati, & Fouladi-Nashta, 2013), it still plays an important role in embryogenic development, as embryonic cardiac organogenesis is dependent on HAS2 expression and signaling of HA via Ras pathway (Camenisch et al., 2000). Moreover, Has2 knockout mouse embryos die mid-gestation, due to impaired organogenesis (Camenisch et al., 2000). Therefore, HA performs multiple roles in an organism’s development.
5.1. HA in early development and postnatal CNS.
Expression of the major HA receptor CD44 has a very limited distribution in embryos. In mouse embryos it appears mid-gestation, primarily in the head mesenchyme and in the marginal zone (Fenderson, Stamenkovic, & Aruffo, 1993), suggesting a tight regulation of expression of HA in developing brain, similar to peripheral organs. This might be an important adaptive mechanism for prevention of inadvertent activation and subsequent inhibition of neuronal proliferation through TLR2, which is widely expressed in embryonic cortex (Okun et al., 2010). HA, however, is important for embryonic CNS development, as there is a CD44-dependent neuronal maturation in the sub-granular zone of embryonic murine dentate gyrus (Su et al., 2017). HA’s role in neuronal development was also recapitulated in vitro, where disruption of HA synthesis interfered with neurite outgrowth in mouse primary neuronal cultures (Takechi et al., 2020). The role of receptor of hyaluronan-mediated motility, RHAMM, in neocortical development has been delineated in studies of fetal human neocortex organotypic slice cultures, where HA-receptor dependent Erk1/2 activation was required for cortical plate folding (Long et al., 2018). Therefore, HA appears to be important for orchestrating brain growth and localized neuronal maturation during CNS development.
Concentrations of HA in the brain increase drastically during the first days of postnatal development (Figure 3). Hyaluronic acid peaks within the first week after birth, and then sharply declines reaching adult levels when measured in whole brain of rat at postnatal day 18 (Margolis, Margolis, Chang, & Preti, 1975). The naturally occurring neuronal death in postnatal rat cortex happens at approximately the same time (Ferrer, Bernet, Soriano, Del Rio, & Fonseca, 1990). Importantly, the volume expanding effect of HA polymer cannot be ignored as an important factor in neuronal development. In HAS3 knockout mice there was a substantial reduction in total extracellular space in hippocampi of affected mice, which displayed an epileptiform phenotype (Arranz et al., 2014). Restoration of volume using hypertonic artificial cerebrospinal fluid (ACSF) in slice preparation alleviated these effects. When exogenous HYAL was injected into the CA1 region of hippocampi of young mice (postnatal day 17, P17) a similar seizure phenotype was observed (Balashova et al., 2019). RNA sequencing analysis also revealed elevation of pro-inflammatory genes, including Tlr2 and c-c motif chemokine ligand (Ccl)2,3,5 suggesting an inflammation driven pathology upon digestion of HA. Additionally, a recent report by Wilson et al. has shown that HYAL treatment resulted in increase in the number of excitatory synapses and of spontaneous activity, offering another mechanism for HA-dependent regulation of neuronal activity (Wilson, Knudson, & Newell-Litwa, 2020). These studies underscore the importance of HA in the CNS ECM and its role as a microenvironment regulating biomolecule.
5.2. HA in adult CNS.
In adult rat brain, similar to human brain, HA appears to be distributed throughout numerous brain regions and associates primarily with parvalbumin positive neurons (Takechi et al., 2020; Ueno et al., 2018; Yasuhara, Akiyama, McGeer, & McGeer, 1994). Adult neurogenesis appears to be regulated by HA via CD44 signaling, since HA (106 Da) treatment of subgranular zone derived neural stem cells inhibited their proliferation (Su et al., 2017). This inhibition of neural stem cell proliferation is in line with anti-proliferative properties of HMW-HA, suggesting that HA signaling pathways are conserved in the adult CNS. Pericellular HA is especially important for normal maturation of neurons, as interference with neuronal HA synthesis resulted in poor neurite development (Takechi et al., 2020). Therefore, HA plays an important role in both adult neurogenesis, neuronal maturation, and normal synaptic function, in particular of a subset of parvalbumin positive cells.
There appears to be an age-related accumulation of HA, driven primarily by HAS1 upregulation (Cargill et al., 2012; Su et al., 2017) (Figure 3). Cortical and cerebellar parenchyma are primary brain regions with increased accumulation of HA (Reed et al., 2018). This accumulation could be driven by excess production of HA by aged astrocytes, with apparent increase of HAS1 and CD44 expression in these cells (Cargill et al., 2012). In contrast, age-related perivascular HA accumulation appears to be dependent on HAS2 up-regulation (Reed et al., 2016). The average size of HA in the parenchyma of aged CNS has not been well characterized. The abovementioned studies report increases in synthases as the inciting event in HA accumulation, yet other factors, such as increased inflammation, ROS-driven degradation, and reduction in lymphatic turnover, could affect size and content of HA in the aging CNS. Importantly, it is not clear whether LMW-HA or HMW-HA are the dominant species in the aging CNS, and the effect of enriching one or the other in aggravation or resolution of age-related neurodegenerative disease remains to be understood.
6. HA in aging and neurodegeneration
Though many questions remain unanswered, HA has been shown to be critical in CNS development and normal functioning. HA’s role in aging and neurodegeneration remains a topic of scrutiny. In order to better understand HA’s role in neurodegenerative conditions, we will first consider its effect on different cell types implicated in CNS aging and disease.
6.1. HA and OPC/oligodendrocytes.
A deregulatory effect of accumulated HA in CNS was reported in studies of demyelinating lesions. In mice with experimental autoimmune encephalomyelitis (EAE) as a surrogate of multiple sclerosis (MS), accumulation of HMW-HA secreted by local astrocytes, which is also seen as part of normal aging (Cargill et al., 2012), was observed in regions of demyelination (Back et al., 2005). Similarly, assessment of postmortem brains of patients with the progressive encephalopathy of vanishing white matter disease showed perilesional HA accumulation (Bugiani et al., 2013). The accumulated polymer prevented oligodendrocyte precursors (OPCs) from maturing, limiting the myelination potential of the precursor cells. TLR2 activation by hyaluronan was implicated in blocking OPC maturation (Sloane et al., 2010), suggesting the possibility that the hyaluronan accumulated in demyelinated lesions undergoes fragmentation, which results in LMW-HA-TLR2 pathway activation to inhibit maturation (Figure 4). Considering the correlation of demyelination and mild cognitive impairment in humans (Carmeli et al., 2013), and the focal demyelinating lesions seen in AD mouse models (Mitew et al., 2010), the status of HA enrichment around OPCs is important, as uncontrolled enrichment of HA around OPCs would interfere with restorative potential of these cells.
Figure 4. Diverging effects of LMW-HA and HMW-HA in neurodegeneration and AD.
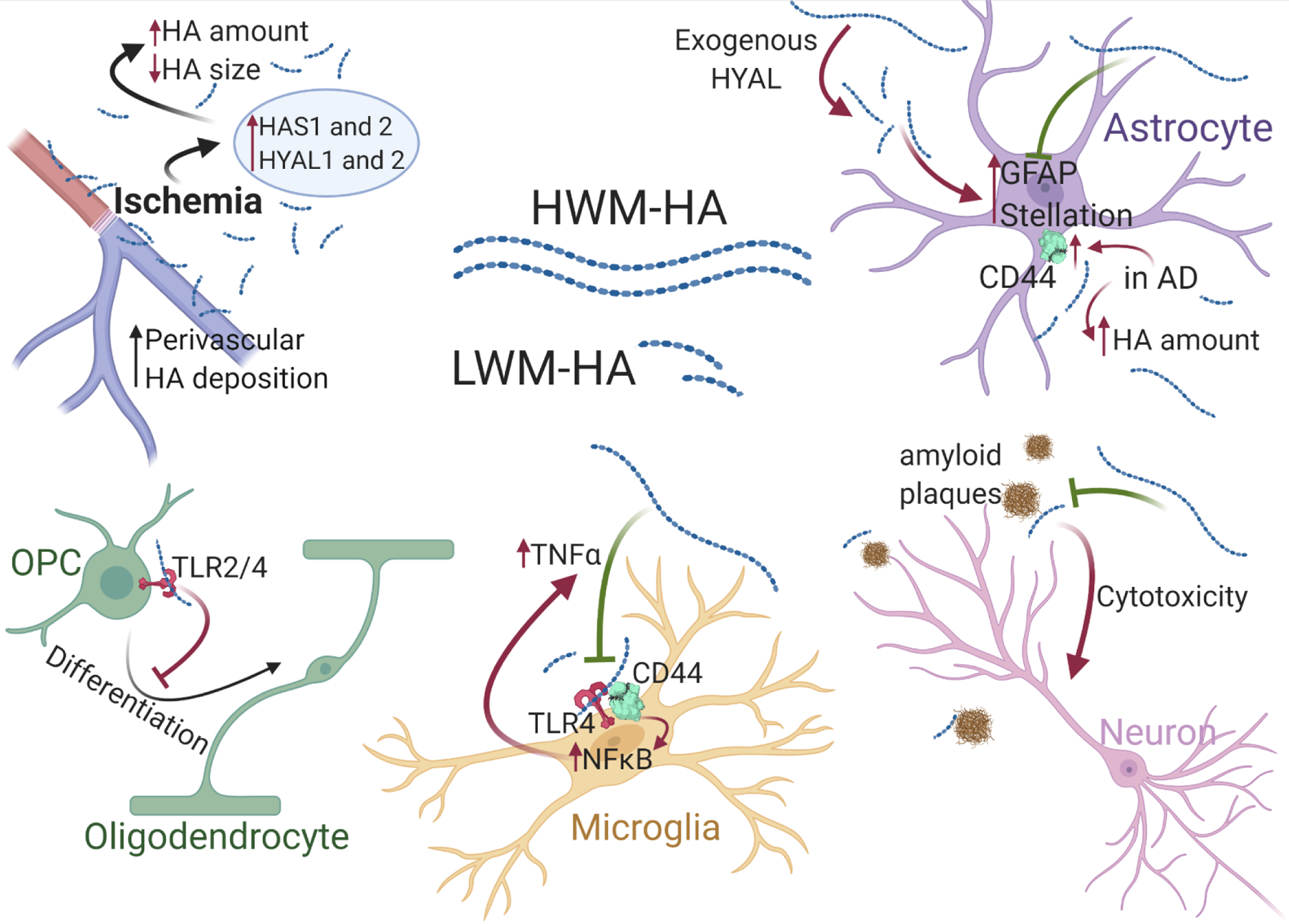
In situations of ischemia, there is an increase in perivascular deposition of short HA polymers. HA interferes with OPC differentiation and remyelination. LMW-HA activates microglia via TLR4 and NFκB pathway, while HMW-HA blocks this activation. HMW-HA blocks cytotoxic effect of LMW-HA and is considered protective against neurodegeneration. Exogenous hyaluronidase (HYAL) treatment results in HA degradation and increased levels of GFAP and astrocyte stellation. Increased levels of astrocytic CD44 and overall HA amount have been reported in AD patients.
6.2. HA and microglia.
CD44-TLR4 interaction in response to treatment with 500–800 kDa HA resulted in increased NF-kB activation and TNF-α production by microglia and peritoneal macrophages in vitro (M. J. Wang et al., 2006). This signaling pathway is thought to be responsible for a prolonged inflammatory response in brain ischemia, where increased HAS1 and 2, as well as Hyal1 and 2 expression, and HA accumulation has been reported (Al’Qteishat et al., 2006). In contrast, in a primary rat microglial culture stimulated with LPS, co-treatment with HMW-HA (1700 kDa) reduced production of IL-1β and IL-6 via Erk 1/2 and Akt pathway inhibition (Austin, Gilchrist, & Fehlings, 2012). Here the authors concluded that due to the early effects of HMW-HA inhibition of inflammatory pathways and intersections with pathways involved in TRL-4 signaling, the anti-inflammatory effects could be driven by direct HMW-HA inhibition of TLR-4 (Figure 4). Their conclusions are in line with earlier findings of TLR-4 signaling suppression by HMW-HA (Campo et al., 2010). Blocking TLR signaling to reduce inflammation has been suggested as a treatment for spinal cord injury (SCI), as inhibition of MyD88-dependent pathway, a pathway downstream of TLR signaling, improved locomotion in a rat SCI model (Yao et al., 2012). Further investigation of the differential roles of HMW-HA and LMW-HA in microglial activation after injury is needed to understand the implication of HA accumulation in CNS injury and the resolution of responses that occur with healing.
6.3. HA and astrocytes.
Another glial subtype targeted in an SCI model was astrocytes, where injection of hyaluronidase resulted in increase of glial fibrillary acidic protein (GFAP) positive cells as well as GFAP- and BrdU-positive cells, while HMW-HA (~1000 kDa) inhibited such proliferation (Struve et al., 2005). Astrocyte stellation, a process observed in stress or cells challenged by inflammation (Acaz-Fonseca, Ortiz-Rodriguez, Azcoitia, Garcia-Segura, & Arevalo, 2019), was increased in vitro when cultured astrocytes were treated with hyaluronidase or transfected with sh-CD44 RNA (Konopka et al., 2016) (Figure 4). The morphological changes occurred because of CD44-dependent regulation of morphology and Rac1 activation upon treatment with Hyaluronidase. The implications of reduced astroglial activation are varied. On the one hand, diminished astrocyte proliferation could reduce scar formation, which is non-permissive for neuronal growth and regeneration. On the other hand, inhibition of astrocyte activation could be detrimental for tissue restoration and remodeling, as well as the astrocytes’ role in normal neuronal signaling. These considerations emphasize the importance of balancing HA expression at an injury-specific location within a critical temporal window.
6.4. HA and neurons.
Though astrocytes are thought to be the primary source of ECM HA in the brain, neurons express both HAS2 and HAS3 in vitro (Fowke et al., 2017). FACS assisted total RNA sequencing in mature mouse forebrain did not reveal an enrichment of HAS2 in any one cell type when comparing expression in mature astrocytes, oligodendrocytes, or neurons, suggesting that HAS2 is expressed at similar levels by these cell types (Cahoy et al., 2008). Moreover, imaging studies of rat neuronal culture produced HA in vitro showed apposition without co-localization of HA and the synaptic markers synaptophysin and postsynaptic density protein (PSD)-95, underscoring perineuronal net (PNN) localization of the polymer (Fowke et al., 2017). This patterning of HA around PNNs could allow spatial segregation of synaptic units, providing a scaffold and a physical barrier between different PNNs. Additionally, HA in adult mouse brain is highly associated with a subset of parvalbumin (PV) positive GABAergic cells (Ueno et al., 2018). The presence of HA in PNNs suggests its potential importance for establishment of a synapse and its plasticity and maintenance of neuronal networks in general (Sun et al., 2018). Digestion of HA using exogenous hyaluronidase resulted in reduced fear conditioning in mice and diminished long-term potentiation in hippocampal slices, while introduction of exogenous HA rescued the phenotype (Kochlamazashvili et al., 2010). In addition to disrupting the normal morphology of peri-neuronal ECM, the observed interference with long term potentiation (LTP) after hyaluronidase treatment could be caused by an increase in LMW-HA locally, which would lead to an increase in inflammatory signaling and dysregulation of glial function, processes strongly implicated in neurodegenerative conditions. The effect of targeted enrichment of HMW-HA around neurons in neurodegeneration remains to be elucidated.
6.5. HA in age-related neurodegeneration.
As mentioned before, the role of HA in aging and disease has been long debated. In the brains of AD patients cellular accumulation of HA was spatially segregated from amyloid deposits, suggesting that those cells were either not yet affected or that HA conferred a local protective effect from perineuronal amyloid aggregation (Yasuhara et al., 1994). A histopathological analysis of brains of AD patients revealed an increase in HA content, without other GAGs in temporal lobes of patients (Jenkins & Bachelard, 1988). This observation is in line with an increased number of astrocytes expressing CD44 that displayed altered morphology in AD patients (Akiyama, Tooyama, Kawamata, Ikeda, & McGeer, 1993). In an accelerated senescence murine model, concomitant decreases in HA and chondroitin sulfate (CS) were observed in the brains of affected animals compared to resistant littermates (Morita & Kamada, 1993). The finding suggests that the accumulation of HA in AD could not be explained just by an early senescence phenotype, as only HA and no other GAGs were affected in AD (Jenkins & Bachelard, 1988). At the same time, evidence of neuroprotection by HA (Yasuhara et al., 1994) and failure of treatment with HA to cause AD-like pathology, which was observed following treatment with sulfated GAGs (Hasegawa et al. 1997), suggests that HA accumulation could be an indicator of a compensatory protective mechanism in neurodegenerative conditions rather than a driver of the pathology. Additionally, intrastriatal injection of amyloid in rats reduced HA content in the tissue (Genedani et al., 2010), which suggests either a steric competition between HA and amyloid aggregates or a more complex intricate relationship, which could involve neuronal, glial, and peripheral immune cells.
Analysis of cerebrospinal fluid (CSF) HA content in patients with AD revealed a gender difference between affected individuals, but failed to demonstrate a significant difference between the control group and AD patients (M. Nielsen, Palmqvist, Minthon, Londos, & Wennstrom, 2012). In a subsequent study to find a better correlate to the observed HA up-regulation, the same investigators examined the correlation of CSF HA content with the severity of vascular dementia (Nägga, Hansson, Van Westen, Minthon, & Wennström, 2014). Indeed, levels of HA were significantly increased in patients of both sexes with vascular disease, which is supported by previous reports of HA dysregulation concomitant with vascular inflammatory processes (Evanko, Raines, Ross, Gold, & Wight, 1998). Interestingly, perivascular HA accumulation with a general decrease in microvascular density has also been reported to be a part of normal aging in mice (Reed et al., 2018, 2016), potentially indicative of degradation of normal vascular architecture, increased inflammation, and failure of HA turnover in aged tissue, or hinting at increased incidence of ischemic events with aging and a consequent aggregation of HA (Al’Qteishat et al., 2006).
To better understand the role of HA size in AD pathology, Reed et al. assessed the extent of HA accumulation in the postmortem brain parenchyma of both male and female AD patients and compared the average polymer sizes in control patients and in patients with AD (Reed et al., 2019). Though the total amount of HA in AD patient brains was significantly elevated, there was no significant difference in average size between the control and AD group, averaging at just above 200 kDa in both groups. In AD patients there was significantly increased expression of TSG-6 mRNA as well as increased parenchymal and perivascular staining. Considering the lack of increased HA in the CSF of AD patients (M. Nielsen et al., 2012), HA aggregation in parenchyma, and with HA turnover occurring in the meningeal lymphatic system, the net aggregation in HA could be due to the gradual failure of the system with age. Indeed, CSF perfusion of the brain was reduced in aged mice when compared to younger counterparts (Da Mesquita et al., 2018). This study also showed that this disruption could underlie worsening of Alzheimer’s phenotype in 5xFAD murine model. This finding underscores the diagnostic potential of HA, which could be acting as a canary in the coalmine: as meningeal lymphatics deteriorate with age, HA would be more likely to aggregate in the parenchyma. We do not, however, possess sufficient evidence to implicate HA as the perpetrator or the victim in this scenario. Though HA accumulation could be an aggravating process worsening the AD pathology, there is evidence to suggest a compensatory and protective response to progressive degeneration and chronic inflammation. A more definitive answer to this question may be provided following further investigation of the roles of HMW-HA and LMW-HA in animal models of aging and neurodegeneration.
7. HA based therapeutics
As our wealth of knowledge on the non-sulfated GAG increases, so have efforts to modulate its presence in different tissues. HMW-HA has been recognized as a protective factor in rheumatologic disease and age-related joint pathologies; thus, efforts have been made to either prevent its degradation or to deliver preformed polymer locally. One proposed approach has been to introduce an inhibitor to HA degradation by exploiting the affinity of hyaluronidases to other GAGs. In their screening of GAGs, Toida et al. observed a noncompetitive inhibitory potential of heparin and strong competitive and non-competitive inhibitory effects of synthetic O-sulfonated HA, by binding at and outside of the active domain, respectively (Toida, Ogita, Suzuki, Toyoda, & Imanari, 1999). Though showing promising effects in vitro and being used as injectable treatments for OA, the bioavailability of GAG polymers and the mode of delivery has brought up reservations about the utility of such molecules for treating other organs, especially the CNS, where application would involve invasive procedures. Thus, alternatives from small molecules to hydrogels have been investigated in both in vitro and in vivo settings (Table 2).
Table 2.
Summary of HA therapeutic approaches
| Name | Type of compound | IC50 | Source | Tested on | Authors |
|---|---|---|---|---|---|
| Heparin | Non-competitive inhibitor of Hyal | 1.14 μg/ml | Porcine intestine | Mammalian testicular Hyal in vitro (Flow Injection Assay) | Toida et al., 1999 |
| O-sulfonated HA | Competitive and non-competitive inhibitor of Hyal | 0.78 μg/ml | HA from Streptococcus zooepidemicus | ||
| L-ascorbic acid 6-hexadecanoate (Vcpal) | Synthetic Hyal inhibitor | 4 and 56 μM | Synthetic | Streptococcal and bovine testicular hy- aluronidase in vitro (turbidimetric assay) | Botzki et al., 2004 |
| Clinopodic acid C | Phenylpropanoid SM Hyal inhibitor | 80.1 μM | Lycopus lucidus | Bovine testicular hy- aluronidase in vitro | Murata et al., 2010 |
| Rashomonic acid D | 183 μM | Meehania urticifolia | Murata et al., 2011 | ||
| Acacenin 7-O-β-D-glucuronopyranoside | 267 μM | Keiskea japonica | Murata et al., 2012 | ||
| Rosmarinic acid | SM Hyal inhibitor | 0.2 mM | Melissa officinalis | Bovine testicular hyaluronidase in vitro | Ippoushi et al., 2000 |
| Esculeoside A | Spirosolane-type glycoside SM competitive Hyal inhibitor | 6.5 μM | Lycopersicon esculentum | Bovine testicular hyaluronidase in vitro | Zhou et al., 2018 |
| Hyaluromycin | Rubromycin family SM Hyal inhibitor | 14 μM | Streptomyces species | Bovine testicular hyaluronidase in vitro (turbidimetric assay) | Harunari et al., 2014 |
| HA oligomers | Oligosaccharides (10 monosaccharide units) | N/A | In vitro synthesis using recombinant Pasteurella multocida hyaluronan synthase; Hyalose | Rabbit model of intraventricular hemorrhage | Vinukonda et al., 2016 |
| HA polymer | HA hydrogel (360kDa) | N/A | Lifecore Biomedical | Organotypic spinal cord slice preparations | Schizas et al., 2014 |
| Chitosan-hyaluronan nanoparticles | HA sodium salt (MW>1x106) | N/A | Aladdin | Viability of SH-SY5Y cells | Jiang et al., 2018 |
| HA | N/A | Sigma | Human induced pluripotent cell (h-iPSCc) spheroids | Bejoy et al., 2018 |
7.1. Small molecules.
Natural products and plant extracts have been investigated as a source of the lead for a potential hyaluronidase inhibitor. L-ascorbic acid 6-hexadecanoate (Vcpal) has been shown to act as a potent bacterial Hyal inhibitor, with reduced potency for bovine testicular Hyal (Botzki et al., 2004), making it a potential anti-microbial agent. Murata et al. identified a number of phenylpropanoid compounds from Lycopus lucidus, Meehania urticifolia, and Keiskea japonica as potential inhibitors of the mammalian Hyal enzymes (Murata, Miyase, & Yoshizaki, 2011, 2012; Murata, Watahiki, Tanaka, Miyase, & Yoshizaki, 2010), with comparable or greater potency than a previously reported Melissa officinalis extract, rosmarinic acid (Ippoushi, Yamaguchi, Itou, Azuma, & Higashio, 2000). With some of the phenylpropanoids having IC50’s in double digit micromolar concentrations, these compounds could be potential leads for a therapeutically applicable hyaluronidase inhibitor. Esculeoside A, a saponin derivative of tomato fruit, was shown to have a competitive inhibition potential with 6.5 μM IC50, making it an intriguing compound for further investigation (Zhou et al. 2018).
Hyaluromycin, a hyaluronidase inhibitor derived from marine Streptomyces species (Harunari et al., 2014), was shown to possess anti-proliferative and anti-migratory activity in pancreatic ductal adenocarcinoma cells (PDACs) (Kohi et al., 2016). Hyaluromycin treatment resulted in reduced amounts of LMW-HA in cell culture medium and diminished proliferation and migration of PDACs. With a reported IC50 of 14 μM and significant inhibition of proliferation at 50 μM, hyaluromycin is an enticing new compound with antineoplastic potential.
As new leading structures of small molecules for modulation of HA content and size are being discovered, their use for treatment of neurodegenerative conditions will need to be tested, subject to their BBB penetrability and bioavailability in neural tissue.
7.2. Oligomers, polymers, and hydrogels.
While inhibition of the degrading enzyme is one approach to modulate HMW-HA – LMW-HA balance, direct introduction of the differently sized polymers is an alternative. In a rabbit model of intraventricular hemorrhage (IVH), where elevated CD44, TLR2 and 4 were observed, treatment with hyaluronidase or hyaluronan oligosaccharides reduced expression of CD44 and TLR4, as well as levels of pro-inflammatory cytokines and of markers of microglial activation (Vinukonda et al., 2016). Degradation of HA with hyaluronidases cleaves the HA polymer to smaller oligosaccharides which interfere with interactions of endogenous HA and pro-inflammatory proteins, including TNF-stimulated gene 6, TSG-6, a situation that is mimicked by addition of exogenous HA oligomers, resulting in reduced inflammatory response. Therefore, HA oligomers can be used as competitive inhibitors of HA interaction with its associated proteins. Their interference with LWM-HA signaling versus HMW-HA signaling remains to be understood.
Polymer application in models of spinal cord injury have been showing some promise as a therapeutic approach. In organotypic spinal cord slice preparations, introduction of an HA hydrogel, but not of soluble HA, resulted in improved neuronal survival (Schizas et al., 2014). The authors suspected a structural scaffolding effect that allowed for improved tissue preservation, while the soluble form was not therapeutic. It would be interesting to determine if local pro- or anti-inflammatory signals were perturbed by either condition. A similar neuroprotective effect was observed in an in vivo hemisection study in rats, where implantation of an HA hydrogel resulted in increased tissue alignment and reduction in lesion size (Kushchayev et al., 2016). Interestingly, the authors noted a decrease in inflammatory cell presence in the area of injury, potentially due to partial blood brain barrier (BBB) restoration in the HA hydrogel treated condition, yet no difference in behavioral outcome was observed. This points out an important consideration that neuronal preservation without a certain amount of inflammation-driven regeneration might not be sufficient to restore functionality of the injured tissue.
7.3. HA as a vehicle.
There have been a number of recent reports applying HA containing formulations as inhibitors of amyloid aggregation. Chitosan-hyaluronan nanoparticles applied to Aβ solution in vitro resulted in disruption of aggregate formation (Jiang, Dong, & Sun, 2018). Introduction of dual inhibitors of amyloid aggregation, epigallocatechin-3-gallate and curcumin, loaded onto HA hydrogel resulted in significant dose dependent inhibition of aggregation (Jiang, Dong, Yan, et al., 2018). In both cases introduction of HA alone did not result in significant effect on Aβ aggregation, suggesting that, though HA can serve as a vehicle for drug delivery, it is unlikely to be involved in amyloid aggregation. However, co-treatment of human induced pluripotent cell (h-iPSCc) spheroids with amyloid and HA resulted in increased cell viability and reduced markers of cytotoxicity (Bejoy et al., 2018). How HA confers neuroprotection and the impact of differently sized HAs on biologic outcomes related to AD remain unanswered questions.
There are several caveats to current therapeutic approaches of HA delivery. Though HA polymers and 3D matrices show some promise as a therapeutic tool for restoration of an open wound or for post-operative recovery, there is currently no procedure to deliver them in a non-invasive manner. Additionally, though often affecting entorhinal and hippocampal regions first, age-related dementias are more distributed diseases, and involve different brain regions, including medial parietal lobe and posterior cingulate (Scahill, Schott, Stevens, Rossor, & Fox, 2002). Therefore, a small molecule modulator of HA size and content in CNS with temporally limited bioavailability would be the most promising therapeutic strategy for future drug development efforts. Local clearance of the compound will have a role in efficiency of therapy, though the cause-outcome relationship of HA of particular size and local vascular and lymphatic flow remains to be elucidated.
8. Conclusions
Hyaluronic acid is a ubiquitous molecule that appears to have numerous and diverse effects on tissue homeostasis. As a biomolecule strongly involved in tissue structure and composition, factors dysregulated in aging, HA function in aging is hard to ignore, considering that despite regional variabilities, there is a trend towards increased HA accumulation with age. First implicated in articular and rheumatological disease, the involvement of HA and its catabolic and anabolic enzymes in cancer and inflammatory conditions has become more evident. Lately, HA involvement in brain injury and neurodegeneration has been interrogated, and appears to have conflicting effects on tissue degradation and regeneration. The intricacies of its homeostasis and signaling pathways add another layer of complexity to the understanding of its role in development, maintenance, and disease. However, a significant point has been underscored by multiple reports: HA cannot be viewed as a simple target, up- or down- regulation of which results in a desired outcome. When considering HA as a target for therapeutic approaches, the modulation of HA size, the type and timing of intervention, the location of application and duration of treatment, and the potential homeostatic compensations must all be kept in mind to achieve beneficial results.
Acknowledgements
The work in the authors’ laboratories is supported by grants from US national Institutes of Health. Frances Tolibzoda Zakusilo is a Medical Scientist Training Program student at University of Rochester. Figures were created with BioRender.com
Footnotes
Declaration of competing interest
The authors declare that there is no conflict of interests.
References
- Acaz-Fonseca E, Ortiz-Rodriguez A, Azcoitia I, Garcia-Segura LM, & Arevalo MA (2019). Notch signaling in astrocytes mediates their morphological response to an inflammatory challenge. Cell Death Discovery, 5(1). 10.1038/s41420-019-0166-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Tooyama I, Kawamata T, Ikeda K, & McGeer PL (1993). Morphological diversities of CD44 positive astrocytes in the cerebral cortex of normal subjects and patients with Alzheimer’s disease. Brain Research, 632(1–2), 249–259. 10.1016/0006-8993(93)91160-T [DOI] [PubMed] [Google Scholar]
- Al’Qteishat A, Al’Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, … Slevin M (2006). Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain, 129(8), 2158–2176. 10.1093/brain/awl139 [DOI] [PubMed] [Google Scholar]
- Almond AH (2007). Hyaluronan. Cellular and Molecular Life Sciences, 64(13), 1591–1596. 10.1007/s00018-007-7032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre B, Duterme C, Van Moer K, Mertens-Strijthagen J, Jadot M, & Flamion B (2011). Hyal2 is a glycosylphosphatidylinositol-anchored, lipid raft-associated hyaluronidase. Biochemical and Biophysical Research Communications, 411(1), 175–179. 10.1016/j.bbrc.2011.06.125 [DOI] [PubMed] [Google Scholar]
- Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, … Yamaguchi Y (2014). Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. Journal of Neuroscience, 34(18), 6164–6176. 10.1523/JNEUROSCI.3458-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmuri V, Martin DC, Hemming R, Gutsol A, Byers S, Sahebjam S, … Triggs-Raine B (2008). Hyaluronidase 3 (HYAL3) knockout mice do not display evidence of hyaluronan accumulation. Matrix Biology, 27(8), 653–660. 10.1016/j.matbio.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Austin JW, Gilchrist C, & Fehlings MG (2012). High molecular weight hyaluronan reduces lipopolysaccharide mediated microglial activation. Journal of Neurochemistry, 122(2), 344–355. 10.1111/j.1471-4159.2012.07789.x [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TMFF, Chen H, Wallingford N, Craig A, Struve J, … Sherman LS (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nature Medicine, 11(9), 966–972. 10.1038/nm1279 [DOI] [PubMed] [Google Scholar]
- Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, & Quinn DA (2005). The role of hyaluronan synthase 3 in ventilator-induced lung injury. American Journal of Respiratory and Critical Care Medicine, 172(1), 92–98. 10.1164/rccm.200405-652OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashova A, Pershin V, Zaborskaya O, Tkachenko N, Mironov A, Guryev E, … Gainullin M (2019). Enzymatic Digestion of Hyaluronan-Based Brain Extracellular Matrix in vivo Can Induce Seizures in Neonatal Mice, 13(September), 1–12. 10.3389/fnins.2019.01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss MT, Howat SLT, Dudhia J, Murphy JM, Barry FP, Edwards JCW, & Day AJ (2001). Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis and Cartilage, 9(1), 42–48. 10.1053/joca.2000.0348 [DOI] [PubMed] [Google Scholar]
- Bejoy J, Song L, Wang Z, Sang Q-X, Zhou Y, & Li Y (2018). Neuroprotective Activities of Heparin, Heparinase III, and Hyaluronic Acid on the Aβ42-Treated Forebrain Spheroids Derived from Human Stem Cells. ACS Biomaterials Science & Engineering, 4(8), 2922–2933. 10.1021/acsbiomaterials.8b00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzki A, Rigden DJ, Braun S, Nukui M, Salmen S, Hoechstetter J, … Buschauert A (2004). L-ascorbic acid 6-hexadecanoate, a potent hyaluronidase inhibitor. X-ray structure and molecular modeling of enzyme-inhibitor complexes. Journal of Biological Chemistry, 279(44), 45990–45997. 10.1074/jbc.M406146200 [DOI] [PubMed] [Google Scholar]
- Bourguignon LYWW, Singleton PA, Diedrich F, Stern R, & Gilad E (2004). CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. Journal of Biological Chemistry, 279(26), 26991–27007. 10.1074/jbc.M311838200 [DOI] [PubMed] [Google Scholar]
- Bourguignon V, & Flamion B (2016). Respective roles of hyaluronidases 1 and 2 in endogenous hyaluronan turnover. FASEB Journal, 30(6), 2108–2114. 10.1096/fj.201500178R [DOI] [PubMed] [Google Scholar]
- Brown JJGG, & Papaioannou VE (1993). Ontogeny of hyaluronan secretion during early mouse development. Development, 117(2), 483–492. [DOI] [PubMed] [Google Scholar]
- Buffenstein R (2008). Negligible senescence in the longest living rodent, the naked mole-rat: Insights from a successfully aging species. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol, 178(4), 439–445. 10.1007/s00360-007-0237-5 [DOI] [PubMed] [Google Scholar]
- Bugiani M, Postma N, Polder E, Dieleman N, Scheffer PG, Sim FJ, … Boor I (2013). Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain, 136(1), 209–222. 10.1093/brain/aws320 [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, … Barres BA (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci, 28(1), 264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine M Lou, Calabro A, … McDonald JA (2000). Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. Journal of Clinical Investigation, 106(3), 349–360. 10.1172/JCI10272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, & Calatroni A (2010). Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie, 92(2), 204–215. 10.1016/j.biochi.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Cargill R, Kohama SG, Struve J, Su W, Banine F, Witkowski E, … Sherman LS (2012). Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiology of Aging, 33(4), 830.e13–830.e24. 10.1016/j.neurobiolaging.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli C, Donati A, Antille V, Viceic D, Ghika J, von Gunten A, … Knyazeva MG (2013). Demyelination in Mild Cognitive Impairment Suggests Progression Path to Alzheimer’s Disease. PLoS ONE, 8(8). 10.1371/journal.pone.0072759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajara A, Delpech B, Courel MN, Marcelle Leroy, Basuyau JP, & Lévesque H (1998). Effect of aging on neointima formation and hyaluronan, hyaluronidase and hyaluronectin production in injured rat aorta. Atherosclerosis, 138(1), 53–64. 10.1016/S0021-9150(98)00004-5 [DOI] [PubMed] [Google Scholar]
- Chao KL, Muthukumar L, & Herzberg O (2007). Structure of human hyaluronidase-1, a hyaluronan hydrolyzing enzyme involved in tumor growth and angiogenesis. Biochemistry, 46(23), 6911–6920. 10.1021/bi700382g [DOI] [PubMed] [Google Scholar]
- Chow G, Knudson CBB, & Knudson W (2006). Expression and cellular localization of human hyaluronidase-2 in articular chondrocytes and cultured cell lines. Osteoarthritis and Cartilage, 14(9), 849–858. 10.1016/j.joca.2006.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csóka AB, Scherer SW, & Stern R (1999). Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics, 60(3), 356–361. 10.1006/geno.1999.5876 [DOI] [PubMed] [Google Scholar]
- Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, … Kipnis J (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature, 560(7717), 185–191. 10.1038/s41586-018-0368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl LB, Dahl IMS, Engstrom-Laurent A, & Granath K (1985). Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Annals of the Rheumatic Diseases, 44(12), 817–822. 10.1136/ard.44.12.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis JE, Lagendijk AK, Chen H, Tromp A, Bower NI, Tunny KA, … Smith KA (2017). Tmem2 Regulates Embryonic Vegf Signaling by Controlling Hyaluronic Acid Turnover. Developmental Cell, 40(2), 123–136. 10.1016/j.devcel.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Ducale AE, Ward SI, Dechert T, & Yager DR (2005). Regulation of hyaluronan synthase-2 expression in human intestinal mesenchymal cells: Mechanisms of interleukin-1β-mediated induction. American Journal of Physiology - Gastrointestinal and Liver Physiology, 289(3 52–3), 462–470. 10.1152/ajpgi.00494.2004 [DOI] [PubMed] [Google Scholar]
- Duterme C, Mertens-strijthagen J, Tammi M, & Flamion B (2009). Two Novel Functions of Hyaluronidase-2 ( Hyal2 ) Are Formation of the Glycocalyx and Control of CD44-ERM Interactions. □, 284(48), 33495–33508. 10.1074/jbc.M109.044362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Medina DX, Gaczynska M, Osmulski PA, Oddo S, Caccamo A, & Buffenstein R (2013). Amyloid beta and the longest-lived rodent: The naked mole-rat as a model for natural protection from alzheimer’s disease. Neurobiology of Aging, 34(10), 2352–2360. 10.1016/j.neurobiolaging.2013.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enegd B, King JAJ, Stylli S, Paradiso L, Kaye AH, Novak U, … Piepmeier JM (2002). Overexpression of hyaluronan synthase-2 reduces the tumorigenic potential of glioma cells lacking hyaluronidase activity. Neurosurgery, 50(6), 1311–1318. 10.1097/00006123-200206000-00023 [DOI] [PubMed] [Google Scholar]
- Evanko SP, Raines EW, Ross R, Gold LI, & Wight TN (1998). Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet- derived growth factor and transforming growth factor-β. Am. J. Pathol, 152(2), 533–546. [PMC free article] [PubMed] [Google Scholar]
- Faulkes CG, Davies KTJJ, Rossiter SJ, & Bennett NC (2015). Molecular evolution of the hyaluronan synthase 2 gene in mammals: Implications for adaptations to the subterranean niche and cancer resistance. Biology Letters, 11(5). 10.1098/rsbl.2015.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenderson BA, Stamenkovic I, & Aruffo A (1993). Localization of hyaluronan in mouse embryos during implantation, gastrulation and organogenesis. Differentiation, 54(3), 85–98. 10.1111/j.1432-0436.1993.tb01591.x [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Khomula EV, Araldi D, & Levine JD (2018). CD44 signaling mediates high molecular weight hyaluronan-induced antihyperalgesia. Journal of Neuroscience, 38(2), 308–321. 10.1523/JNEUROSCI.2695-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Bernet E, Soriano E, Del Rio T, & Fonseca M (1990). Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience, 39(2), 451–458. 10.1016/0306-4522(90)90281-8 [DOI] [PubMed] [Google Scholar]
- Fowke TM, Karunasinghe RN, Bai JZ, Jordan S, Gunn AJ, & Dean JM (2017). Hyaluronan synthesis by developing cortical neurons in vitro. Scientific Reports, 7, 1–13. 10.1038/srep44135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JREE, Kimpton WG, Laurent TC, Cahill RNPP, & Vakakis N (1988). Uptake and degradation of hyaluronan in lymphatic tissue. Biochemical Journal, 256(1), 153–158. 10.1042/bj2560153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genedani S, Agnati LF, Leo G, Buzzega D, Maccari F, Carone C, … Volpi N (2010). β-Amyloid Fibrillation and/or Hyperhomocysteinemia Modify Striatal Patterns of Hyaluronic Acid and Dermatan Sulfate: Possible Role in the Pathogenesis of Alzheimer’s Disease. Current Alzheimer Research, 999(999), 1–8. 10.2174/1567210198607222050 [DOI] [PubMed] [Google Scholar]
- Goldberg RL, & Toole BP (1987). Hyaluronate inhibition of cell proliferation. Arthritis & Rheumatism, 30(7), 769–778. 10.1002/art.1780300707 [DOI] [PubMed] [Google Scholar]
- Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, & Kovacs EJ (2007). Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Critical Care Medicine, 35(1), 246–251. 10.1097/01.CCM.0000251639.05135.E0 [DOI] [PubMed] [Google Scholar]
- Harada H, & Takahashi M (2007). CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and −2. Journal of Biological Chemistry, 282(8), 5597–5607. 10.1074/jbc.M608358200 [DOI] [PubMed] [Google Scholar]
- Harunari E, Imada C, Igarashi Y, Fukuda T, Terahara T, & Kobayashi T (2014). Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. Marine Drugs, 12(1), 491–507. 10.3390/md12010491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Crowther RA, Jakes R, & Goedert M (1997). Alzheimer-like Changes in Microtubule-associated Protein Tau Induced by Sulfated Glycosaminoglycans. Journal of Biological Chemistry, 272(52), 33118–33124. 10.1074/jbc.272.52.33118 [DOI] [PubMed] [Google Scholar]
- Haynes BF, Hale LP, Patton KL, Martin ME, & McCallum RM (1991). Measurement of an adhesion molecule as an indicator of inflammatory disease activity: Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis & Rheumatism, 34(11), 1434–1443. 10.1002/art.1780341115 [DOI] [PubMed] [Google Scholar]
- Heldin P, & Pertoft H (1993). Synthesis and assembly of the hyaluronan-containing coats around normal human mesothelial cells. Experimental Cell Research, 208(2), 422–429. 10.1006/excr.1993.1264 [DOI] [PubMed] [Google Scholar]
- Hiramitsu T, Yasuda T, Ito H, Shimizu M, Julovi SM, Kakinuma T, … Nakamura T (2006). Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1β-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-κB and p38. Rheumatology, 45(7), 824–832. 10.1093/rheumatology/kel026 [DOI] [PubMed] [Google Scholar]
- Hoffman P, Meyer K, & Linker A (1956). Transglycosylation during the mixed digestion of hyaluronic acid and chondroitin sulfate by testicular hyaluronidase. J. Biol. Chem, 219(2), 653–63. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13319286 [PubMed] [Google Scholar]
- Holmes MWAA, Bayliss MT, & Muir H (1988). Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochemical Journal, 250(2), 435–441. 10.1042/bj2500435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imberty A, Lortat-Jacob H, & Pérez S (2007). Structural view of glycosaminoglycan-protein interactions. Carbohydrate Research, 342(3–4), 430–439. 10.1016/j.carres.2006.12.019 [DOI] [PubMed] [Google Scholar]
- Inoue R, Ishibashi Y, Tsuda E, Yamamoto Y, Matsuzaka M, Takahashi I, … Toh S (2011a). Knee osteoarthritis, knee joint pain and aging in relation to increasing serum hyaluronan level in the Japanese population. Osteoarthritis and Cartilage, 19(1), 51–57. 10.1016/j.joca.2010.10.021 [DOI] [PubMed] [Google Scholar]
- Inoue R, Ishibashi Y, Tsuda E, Yamamoto Y, Matsuzaka M, Takahashi I, … Toh S (2011b). Knee osteoarthritis, knee joint pain and aging in relation to increasing serum hyaluronan level in the Japanese population. Osteoarthritis and Cartilage, 19(1), 51–57. 10.1016/j.joca.2010.10.021 [DOI] [PubMed] [Google Scholar]
- Ippoushi K, Yamaguchi Y, Itou H, Azuma K, & Higashio H (2000). Evaluation of Inhibitory Effects of Vegetables and Herbs on Hyaluronidase and Identification of Rosmarinic Acid as a Hyaluronidase Inhibitor in Lemon Balm (Melissa officinalis L.). Food Science and Technology Research, 6(1), 74–77. 10.3136/fstr.6.74 [DOI] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, … Kimata K (1999). Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. Journal of Biological Chemistry, 274(35), 25085–25092. 10.1074/jbc.274.35.25085 [DOI] [PubMed] [Google Scholar]
- Jadin L, Wu X, Ding H, Frost GI, Onclinx C, Triggs-Raine B, & Flamion B (2008). Skeletal and hematological anomalies in HYAL2-deficient mice: a second type of mucopolysaccharidosis IX? The FASEB Journal, 22(12), 4316–4326. 10.1096/fj.08-111997 [DOI] [PubMed] [Google Scholar]
- Jenkins HG, & Bachelard HS (1988). Glycosaminoglycans in Cortical Autopsy Samples from Alzheimer Brain. Journal of Neurochemistry, 51(5), 1641–1645. 10.1111/j.1471-4159.1988.tb01135.x [DOI] [PubMed] [Google Scholar]
- Jiang Z, Dong X, & Sun Y (2018). Charge effects of self-assembled chitosan-hyaluronic acid nanoparticles on inhibiting amyloid b -protein aggregation. Carbohydrate Research, 461, 11–18. 10.1016/j.carres.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Dong X, Yan X, Liu Y, Zhang L, & Sun Y (2018). Nanogels of dual inhibitor-modified hyaluronic acid function as a potent inhibitor of amyloid β -protein aggregation and cytotoxicity. Scientific Reports, 8(1), 1–11. 10.1038/s41598-018-21933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SP, Obery DR, & De La Motte C (2015). Hyaluronan Synthase 3 Null Mice Exhibit Decreased Intestinal Inflammation and Tissue Damage in the DSS-Induced Colitis Model. International Journal of Cell Biology, 2015. 10.1155/2015/745237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PMJ, … Dityatev A (2010). The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels. Neuron, 67(1), 116–128. 10.1016/j.neuron.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, & Day AJ (1996). Solution structure of the link module: A hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell, 86(5), 767–775. 10.1016/S0092-8674(00)80151-8 [DOI] [PubMed] [Google Scholar]
- Kohi S, Sato N, Koga A, Hirata K, Harunari E, & Igarashi Y (2016). Hyaluromycin, a Novel Hyaluronidase Inhibitor, Attenuates Pancreatic Cancer Cell Migration and Proliferation. Journal of Oncology, 2016. 10.1155/2016/9063087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A, Zeug A, Skupien A, Kaza B, Mueller F, Chwedorowicz A, … Dzwonek J (2016). Cleavage of hyaluronan and CD44 adhesion molecule regulate astrocyte morphology via Rac1 signalling. PLoS ONE, 11(5). 10.1371/journal.pone.0155053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaki R, Watanabe K, & Yamaguchi Y (1999). Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Research, 59(5), 1141–1145. [PubMed] [Google Scholar]
- Kulaberoglu Y, Bhushan B, Hadi F, Chakrabarti S, Khaled WT, Rankin KS, … Frankel D (2019). The material properties of naked mole-rat hyaluronan. Scientific Reports, 9(1), 1–14. 10.1038/s41598-019-43194-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushchayev SV, Giers MB, Hom Eng D, Martirosyan NL, Eschbacher JM, Mortazavi MM, … Preul MC (2016). Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. Journal of Neurosurgery: Spine, 25(1), 114–124. 10.3171/2015.9.SPINE15628 [DOI] [PubMed] [Google Scholar]
- Laurent TC, & Fraser JREHRE (1992). Hyaluronan. The FASEB Journal, 6(7), 2397–2404. 10.1096/fasebj.6.7.1563592 [DOI] [PubMed] [Google Scholar]
- Laurent UBGG, & Reed RK (1991). Turnover of hyaluronan in the tissues. Adv. Drug Deliv. Rev, 7(2), 237–256. 10.1016/0169-409X(91)90004-V [DOI] [Google Scholar]
- Laurent UBG, & Reed RK (1991). Turnover of hyaluronan in the tissues. Advanced Drug Delivery Reviews, 7(2), 237–256. 10.1016/0169-409X(91)90004-V [DOI] [Google Scholar]
- Lepperdinger G, Strobl B, & Kreil G (1998). HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. Journal of Biological Chemistry, 273(35), 22466–22470. 10.1074/jbc.273.35.22466 [DOI] [PubMed] [Google Scholar]
- Li Y, Li L, Brown TJ, & Heldin P (2007). Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. International Journal of Cancer, 120(12), 2557–2567. 10.1002/ijc.22550 [DOI] [PubMed] [Google Scholar]
- Long KR, Newland B, Florio M, Kalebic N, Langen B, Kolterer A, … Huttner WB (2018). Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron, 99(4), 702–719.e7. 10.1016/j.neuron.2018.07.013 [DOI] [PubMed] [Google Scholar]
- Nielsen, H. M, Palmqvist S, Minthon L, Londos E, & Wennstrom M (2012). Gender-Dependent Levels of Hyaluronic Acid in Cerebrospinal Fluid of Patients with Neurodegenerative Dementia. Current Alzheimer Research, 9(3), 257–266. 10.2174/156720512800107537 [DOI] [PubMed] [Google Scholar]
- MacK JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, & Maytin EV (2012). Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. Journal of Investigative Dermatology, 132(1), 198–207. 10.1038/jid.2011.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen KM, Pasonen-Seppänen S, Törrönen K, Tammi MI, & Carlberg C (2009). Regulation of the hyaluronan synthase 2 gene by convergence in cyclic AMP response element-binding protein and retinoid acid receptor signaling. Journal of Biological Chemistry, 284(27), 18270–18281. 10.1074/jbc.M109.012492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei WFAA, Raheem KA, Salavati M, Tremaine T, Khalid M, & Fouladi-Nashta AA (2016). Hyaluronan and hyaluronidase, which is better for embryo development? Theriogenology, 86(4), 940–948. 10.1016/j.theriogenology.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Marei WFAA, Salavati M, & Fouladi-Nashta AA (2013). Critical role of hyaluronidase-2 during preimplantation embryo development. Molecular Human Reproduction, 19(9), 590–599. 10.1093/molehr/gat032 [DOI] [PubMed] [Google Scholar]
- Margolis RKUK, Margolis RKUK, Chang LB, & Preti C (1975). Glycosaminoglycans of Brain during Development. Biochemistry, 14(1), 85–88. 10.1021/bi00672a014 [DOI] [PubMed] [Google Scholar]
- Martin DC, Atmuri V, Hemming RJ, Farley J, Mort JS, Byers S, … Triggs-raine BL (2008). A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Human Molecular Genetics, 17(13), 1904–1915. 10.1093/hmg/ddn088 [DOI] [PubMed] [Google Scholar]
- Matejevic D, Neudeck H, Graf R, Müller T, & Dietl J (2001). Localization of hyaluronan with a hyaluronan-specific hyaluronic acid binding protein in the placenta in pre-eclampsia. Gynecologic and Obstetric Investigation, 52(4), 257–259. 10.1159/000052986 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, … Kosher RA (2009). Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development, 136(16), 2825–2835. 10.1242/dev.038505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley AC, Oltean S, Hascal V, Woods EL, Steadman R, Phillips AO, & Meran S (2017). Nuclear hyaluronidase 2 drives alternative splicing of CD44 pre-mRNA to determine profibrotic or antifibrotic cell phenotype. Science Signaling, 10(506). 10.1126/scisignal.aao1822 [DOI] [PubMed] [Google Scholar]
- Mitew S, Kirkcaldie MTK, Halliday GM, Shepherd CE, Vickers JC, & Dickson TC (2010). Focal demyelination in Alzheimer’s disease and transgenic mouse models. Acta Neuropathologica, 119(5), 567–577. 10.1007/s00401-010-0657-2 [DOI] [PubMed] [Google Scholar]
- Morita T, & Kamada A (1993). Age-related changes in central nervous system hyaluronic acid and chondroitin sulfate in senescence-accelerated mice. Journal of Osaka Dental University, 27(1), 37–49. 10.18905/jodu.27.1_37 [DOI] [PubMed] [Google Scholar]
- Murata T, Miyase T, & Yoshizaki F (2011). Hyaluronidase inhibitory rosmarinic acid derivatives from Meehania urticifolia. Chemical and Pharmaceutical Bulletin, 59(1), 88–95. 10.1248/cpb.59.88 [DOI] [PubMed] [Google Scholar]
- Murata T, Miyase T, & Yoshizaki F (2012). Hyaluronidase inhibitors from Keiskea japonica. Chemical and Pharmaceutical Bulletin, 60(1), 121–128. 10.1248/cpb.60.121 [DOI] [PubMed] [Google Scholar]
- Murata T, Watahiki M, Tanaka Y, Miyase T, & Yoshizaki F (2010). Hyaluronidase inhibitors from Takuran, Lycopus lucidus. Chemical and Pharmaceutical Bulletin, 58(3), 394–397. 10.1248/cpb.58.394 [DOI] [PubMed] [Google Scholar]
- Nägga K, Hansson O, Van Westen D, Minthon L, & Wennström M (2014). Increased levels of hyaluronic acid in cerebrospinal fluid in patients with vascular dementia. Journal of Alzheimer’s Disease, 42(4), 1435–1441. 10.3233/JAD-141200 [DOI] [PubMed] [Google Scholar]
- Neumann A, Schinzel R, Palm D, Riederer P, & Münch G (1999). High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-κB activation and cytokine expression. FEBS Letters, 453(3), 283–287. 10.1016/S0014-5793(99)00731-0 [DOI] [PubMed] [Google Scholar]
- Ng KF, & Schwartz NB (1989). Solubilization and partial purification of hyaluronate synthetase from oligodendroglioma cells. Journal of Biological Chemistry, 264(20), 11776–11783. [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Gen Son T, Lee J-H, Roberts NJ, Mughal MR, … Mattson MP (2010). TLR2 activation inhibits embryonic neural progenitor cell proliferation. Journal of Neurochemistry, 114(2), 462–474. 10.1111/j.1471-4159.2010.06778.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooki T, Murata-Kamiya N, Takahashi-Kanemitsu A, Wu W, & Hatakeyama M (2019). High-Molecular-Weight Hyaluronan Is a Hippo Pathway Ligand Directing Cell Density-Dependent Growth Inhibition via PAR1b. Developmental Cell, 49(4), 590–604.e9. 10.1016/j.devcel.2019.04.018 [DOI] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, … Weissman IL (2011). Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proceedings of the National Academy of Sciences of the United States of America, 108(50), 20012–20017. 10.1073/pnas.1116110108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauloin T, Dutot M, Joly F, Warnet JM, & Rat P (2009). High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Molecular Vision, 15(March), 577–583. [PMC free article] [PubMed] [Google Scholar]
- Philipson LH, & Schwartz NB (1984). Subcellular localization of hyaluronate synthetase in oligodendroglioma cells. Journal of Biological Chemistry, 259(8), 5017–5023. [PubMed] [Google Scholar]
- Philipson Louis H., Westley J, & Schwartz NB (1985). Effect of Hyaluronidase Treatment of Intact Cells on Hyaluronate Synthetase Activity. Biochemistry, 24(27), 7899–7906. 10.1021/bi00348a008 [DOI] [PubMed] [Google Scholar]
- Prehm P (1984). Hyaluronate is synthesized at plasma membranes. Biochemical Journal, 220(2), 597–600. 10.1042/bj2200597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Berdyshev E, Poirer C, Schwartz NB, & Dawson G (2012). Neutral sphingomyelinase 2 deficiency increases hyaluronan synthesis by up-regulation of hyaluronan synthase 2 through decreased ceramide production and activation of Akt. Journal of Biological Chemistry, 287(17), 13620–13632. 10.1074/jbc.M111.304857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Kilkus J, & Dawson G (2016). The hyaluronic acid inhibitor 4-methylumbelliferone is an NSMase2 activator—role of Ceramide in MU anti-tumor activity. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1861(2), 78–90. 10.1016/j.bbalip.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhala L, Jokela T, Kärnä R, Bart G, Takabe P, Oikari S, … Tammi RH (2018). Extracellular ATP activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes via P2Y2, Ca2+ signaling, and MAPK pathways. Biochemical Journal, 475(10), 1755–1772. 10.1042/BCJ20180054 [DOI] [PubMed] [Google Scholar]
- Reed MJ, Damodarasamy M, Pathan JL, Chan CK, Spiekerman C, Wight TN, … Keene CD (2019). Increased Hyaluronan and TSG-6 in Association with Neuropathologic Changes of Alzheimer’s Disease. Journal of Alzheimer’s Disease, 67(1), 91–102. 10.3233/JAD-180797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Damodarasamy M, Pathan JL, Erickson MA, Banks WA, & Vernon RB (2018). The Effects of Normal Aging on Regional Accumulation of Hyaluronan and Chondroitin Sulfate Proteoglycans in the Mouse Brain. Journal of Histochemistry & Cytochemistry, 66(10), 697–707. 10.1369/0022155418774779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MJ, Vernon RB, Damodarasamy M, Chan CK, Wight TN, Bentov I, & Banks WA (2016). Microvasculature of the Mouse Cerebral Cortex Exhibits Increased Accumulation and Synthesis of Hyaluronan With Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 72(6), glw213. 10.1093/gerona/glw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees MD, Hawkins CL, & Davies MJ (2004). Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochemical Journal, 381(1), 175–184. 10.1042/BJ20040148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavalainen K, Tammi MI, Bowen T, Schmitz ML, & Carlberg C (2007). Integration of the activation of the human hyaluronan synthase 2 gene promoter by common cofactors of the transcription factors retinoic acid receptor and nuclear factor κB. Journal of Biological Chemistry, 282(15), 11530–11539. 10.1074/jbc.M607871200 [DOI] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, & Fox NC (2002). Mapping the evolution of regional atrophy in Alzheimer’s disease: Unbiased analysis of fluid-registered serial MRI. Proceedings of the National Academy of Sciences of the United States of America, 99(7), 4703–4707. 10.1073/pnas.052587399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel RT, Higuchi-Sanabria R, Shalem O, Moehle EA, Webster BM, Joe L, … Dillin A (2019). The hyaluronidase, TMEM2, promotes ER homeostasis and longevity independent of the UPRER. Cell, 179(6), 1306–1318.e18. 10.1016/j.cell.2019.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizas N, Rojas R, Kootala S, Andersson B, Pettersson J, Hilborn J, & Hailer NP (2014). Hyaluronic acid-based hydrogel enhances neuronal survival in spinal cord slice cultures from postnatal mice. Journal of Biomaterials Applications, 28(6), 825–836. 10.1177/0885328213483636 [DOI] [PubMed] [Google Scholar]
- Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, … Gorbunova V (2009). Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proceedings of the National Academy of Sciences, 106(46), 19352–19357. 10.1073/pnas.0905252106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes KJ, Renner K, Li J, Grande-Allen KJ, Connell JP, Cali V, … Wang VM (2018). Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa. Journal of Orthopaedic Research®, 36(10), 2622–2632. 10.1002/jor.24027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, & Vartanian T (2010). Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proceedings of the National Academy of Sciences of the United States of America, 107(25), 11555–11560. 10.1073/pnas.1006496107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS (1963). “ECM”: Its nature, origin and function in cell aggregation. Experimental Cell Research, 30(2), 257–279. 10.1016/0014-4827(63)90299-4 [DOI] [PubMed] [Google Scholar]
- Stern R, Kogan G, Jedrzejas MJ, & Šoltés L (2007). The many ways to cleave hyaluronan. Biotechnology Advances, 25(6), 537–557. 10.1016/j.biotechadv.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Struve J, Maher PC, Li YQ, Kinney S, Fehlings MG, Iv CK, … Sherman LS (2005). Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia, 52(1), 16–24. 10.1002/glia.20215 [DOI] [PubMed] [Google Scholar]
- Su W, Foster SC, Xing R, Feistel K, Olsen RHJJ, Acevedo SF, … Sherman LS (2017). CD44 transmembrane receptor and hyaluronan regulate adult hippocampal neural stem cell quiescence and differentiation. Journal of Biological Chemistry, 292(11), 4434–4445. 10.1074/jbc.M116.774109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun ZY, Bozzelli PL, Caccavano A, Allen M, Balmuth J, Vicini S, … Conant K (2018). Disruption of perineuronal nets increases the frequency of sharp wave ripple events. Hippocampus, 28(1), 42–52. 10.1002/hipo.22804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Kotelsky A, Takasugi M, Chang M, Ke Z, Betancourt M, … Gorbunova V (2020). Naked mole-rats are extremely resistant to post-traumatic osteoarthritis. Aging Cell, 19(11), 1–8. 10.1111/acel.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki K, Nakamura T, Izumi J, Saitoh H, Endo M, Kojima K, … Majima M (1994). Characterization of Hydrolysis and Transglycosylation by Testicular Hyaluronidase Using Ion-Spray Mass Spectrometry. Biochemistry, 33(21), 6503–6507. 10.1021/bi00187a017 [DOI] [PubMed] [Google Scholar]
- Takasugi M, Firsanov D, Tombline G, Ning H, Ablaeva J, Seluanov A, & Gorbunova V (2020). Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nature Communications, 11(1), 1–10. 10.1038/s41467-020-16050-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi M, Oshima K, Nadano D, Kitagawa H, Matsuda T, & Miyata S (2020). A pericellular hyaluronan matrix is required for the morphological maturation of cortical neurons. Biochimica et Biophysica Acta - General Subjects, 1864(10), 129679. 10.1016/j.bbagen.2020.129679 [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Hashimoto K, Shimizu C, Hirakawa A, Harano K, Yunokawa M, … Fujiwara Y (2013). Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. International Journal of Clinical Oncology, 18(1), 132–138. 10.1007/s10147-011-0352-x [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, & Karamanos NK (2016). Extracellular matrix structure. Advanced Drug Delivery Reviews, 97, 4–27. 10.1016/j.addr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Tian X, Azpurua J, Hine C, Vaidya A, Myakishev M, Ablaeva J, … Gorbunova V (2014). High molecular weight hyaluronan mediates the cancer resistance of the naked mole-rat. Nature, 499(7458), 346–349. 10.1038/nature12234.High [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien JYLL, & Spicer AP (2005). Three vertebrate hyaluronan synthases are expressed during mouse development in distinct spatial and temporal patterns. Developmental Dynamics, 233(1), 130–141. 10.1002/dvdy.20328 [DOI] [PubMed] [Google Scholar]
- Toida T, Ogita Y, Suzuki A, Toyoda H, & Imanari T (1999). Inhibition of hyaluronidase by fully O-sulfonated glycosaminoglycans. Archives of Biochemistry and Biophysics, 370(2), 176–182. 10.1006/abbi.1999.1395 [DOI] [PubMed] [Google Scholar]
- Ueno H, Suemitsu S, Murakami S, Kitamura N, Wani K, Matsumoto Y, … Ishihara T (2018). Hyaluronic acid is present on specific perineuronal nets in the mouse cerebral cortex. Brain Research, 1698(June), 139–150. 10.1016/j.brainres.2018.08.011 [DOI] [PubMed] [Google Scholar]
- Valiathan R, Ashman M, & Asthana D (2016). Effects of Ageing on the Immune System: Infants to Elderly. Scandinavian Journal of Immunology, 83(4), 255–266. 10.1111/sji.12413 [DOI] [PubMed] [Google Scholar]
- Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, & Passi A (2014). Hyaluronan: Biosynthesis and signaling. Biochimica et Biophysica Acta - General Subjects, 1840(8), 2452–2459. 10.1016/j.bbagen.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Vilar RE, Ghael D, Li M, Bhagat DD, Arrigo LM, Cowman MK, … Rosenfeld L (1997). Nitric oxide degradation of heparin and heparan sulphate. Biochemical Journal, 324(2), 473–479. 10.1042/bj3240473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinukonda G, Dohare P, Arshad A, Zia MT, Panda S, Korumilli R, … Ballabh P (2016). Hyaluronidase and Hyaluronan oligosaccharides promote neurological recovery after intraventricular hemorrhage. Journal of Neuroscience, 36(3), 872–889. 10.1523/JNEUROSCI.3297-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora B, Wang A, Kosti I, Huang H, Paranjpe I, Woodruff TJ, … Sirota M (2018). Meta-analysis of maternal and fetal transcriptomic data elucidates the role of adaptive and innate immunity in preterm birth. Frontiers in Immunology, 9(MAY), 1–20. 10.3389/fimmu.2018.00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MJ, Kuo JS, Lee WW, Huang HY, Chen WF, & Lin SZ (2006). Translational event mediates differential production of tumor necrosis factor-α in hyaluronan-stimulated microglia and macrophages. Journal of Neurochemistry, 97(3), 857–871. 10.1111/j.1471-4159.2006.03776.x [DOI] [PubMed] [Google Scholar]
- Wang Y, Lauer ME, An S, Mack JA, & Maytin EV (2014). Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. Journal of Biological Chemistry, 289(46), 32253–32265. 10.1074/jbc.M114.578377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, & Baggenstoss BA (2017). What is special about 200 kDa hyaluronan that activates hyaluronan receptor signaling? Glycobiology, 27(9), 868–877. 10.1093/glycob/cwx039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Hascall VC, & Tammi M (1997). Hyaluronan synthases. Journal of Biological Chemistry, 272(22), 13997–14000. 10.1074/jbc.272.22.13997 [DOI] [PubMed] [Google Scholar]
- Weissmann B, Meyer K, Sampson P, & Linker A (1954). Isolation of oligosaccharides enzymatically produced from hyaluronic acid. The Journal of Biological Chemistry, 208(1), 417–429. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13174551 [PubMed] [Google Scholar]
- West DC, Hampson IN, Arnold F, & Kumar S (1985). Angiogenesis induced by degradation products of hyaluronic acid. Science, 228(4705), 1324–1326. 10.1126/science.2408340 [DOI] [PubMed] [Google Scholar]
- Wilson E, Knudson W, & Newell-Litwa K (2020). Hyaluronan regulates synapse formation and function in developing neural networks. Scientific Reports, 10(1), 1–14. 10.1038/s41598-020-73177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannariello-Brown J, Chapman SH, Ward WF, Pappas TC, & Weigel PH (1995). Circulating hyaluronan levels in the rodent: Effects of age and diet. American Journal of Physiology - Cell Physiology, 268(4 37–4). 10.1152/ajpcell.1995.268.4.c952 [DOI] [PubMed] [Google Scholar]
- Yao AH, Jia LY, Zhang YK, Ma QR, Cheng P, Liu L, … Kuang F (2012). Early blockade of TLRs MyD88-dependent pathway may reduce secondary spinal cord injury in the rats. Evidence-Based Complement. Altern. Med, 2012. 10.1155/2012/591298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara O, Akiyama H, McGeer EG, & McGeer PL (1994). Immunohistochemical localization of hyaluronic acid in rat and human brain. Brain Research, 635(1–2), 269–282. 10.1016/0006-8993(94)91448-6 [DOI] [PubMed] [Google Scholar]
- Zavan B, Ferroni L, Giorgi C, Calò G, Brun P, Cortivo R, … Pinton P (2013). Hyaluronic Acid Induces Activation of the κ-Opioid Receptor. PLoS ONE, 8(1), 1–8. 10.1371/journal.pone.0055510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JR, Kimura S, Nohara T, & Yokomizo K (2018). Competitive inhibition of mammalian hyaluronidase by tomato saponin, esculeoside A. Natural Product Communications, 13(11), 1461–1463. [DOI] [Google Scholar]
- Zhu R, Wang SC, Sun C, Tao Y, Piao HL, Wang XQ, … Da-Jin Li. (2013). Hyaluronan-CD44 Interaction Promotes Growth of Decidual Stromal Cells in Human First-Trimester Pregnancy. PLoS ONE, 8(9), 1–10. 10.1371/journal.pone.0074812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla V, Nizamutdinova IT, Scharf B, Clement CC, Maejima D, Akl T, … Santambrogio L (2015). Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell, 582–594(January), 582–594. 10.1111/acel.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]


