Abstract
The mechanisms whereby obesity differentially affects males and females are unclear. Since macrophages are functionally the most important cells in obesity-induced inflammation, we sought to determine reasons for male-specific propensity in macrophage migration. Whereas we previously determined that male mice fed high fat diet (HFD) exhibit macrophage infiltration into the hypothalamus, while females were protected irrespective of ovarian estrogen, here we show that males accumulate more macrophages in adipose tissues that are also more inflammatory. Using bone marrow cells or macrophages, differentiated in vitro, from male and female mice fed control and HFD, we demonstrated that macrophages derived from male mice are intrinsically more migratory. We determined that males have higher levels of leptin in serum and adipose tissue. Serum CCL2 levels, however, are the same in males and females, although increased in obese mice compared to lean mice of both sexes. Leptin receptor and free fatty acid receptor, GPR120, are upregulated only in macrophages derived from male mice, when cultured in the presence of free fatty acids (FFA) to mimic hyperlipidemia of obesity. Unless previously stimulated with LPS, CCL2 did not cause migration of macrophages. Leptin, however, elicited migration of macrophages from both sexes. Macrophages from male mice maintained migratory capacity when cultured with FFA, while female macrophages failed to migrate. Therefore, both hyperlipidemia and hyperleptinemia contribute to male macrophage specific migration, since increased FFA induce leptin receptors, while higher leptin causes migration. Our results may explain sex differences in obesity-mediated disorders caused by macrophages infiltration.
Keywords: macrophage, obesity, leptin, high fat diet
Introduction
The prevalence of obesity has increased steadily over the last 30 years (1). Currently, over half of the U.S. population is classified as overweight and a third is classified as obese (2). This obesity epidemic caused an increase in associated diseases, such as type 2 diabetes, cardiovascular disease, stroke, and dementia. Obesity is characterized by chronic inflammation and altered metabolic markers, such as hyperinsulinemia, hyperglycemia and hyperlipidemia (3). Increased adiposity causes an increase in inflammatory cytokines in the circulation, primarily due to infiltration and activation of macrophages in adipose tissue (4–9). The vast majority of studies that analyzed macrophages in adipose tissues during diet induced obesity (DIO) were conducted using only male mice. However, there are profound sex differences in adiposity and obesity-associated diseases (10). To adddress the gap, we analyzed sex differences in this study.
In men, obesity is associated with heart disease and myocardial infarction, while obese women are more prone to ischemic stroke. Although normal weight females have higher proportion of fat than normal weight males, when fed high fat diet (HFD), males accrue more adipose tissue (11). Differential accumulation of adipose tissue may contribute to sex differences in obesity-associated disease, but mechanisms that contribute to higher risk for diseases, such as heart disease and metabolic syndrome, in men are not clear (12). The presence of sex steroid hormones, specifically estrogen, was postulated to contribute to these sex differences (11, 13, 14). Alternatively, sex chromosome complement, in particular X-inactive specific transcript (XIST) expression in females, was shown to be critical for some sex differences (15). In support for the role of estrogen are observations that post-menopausal weight gain in women coincides with a loss of estrogen. Similarly, an increase in adiposity following ovariectomy and removal of ovarian estrogen was observed in rodents (16, 17) and in monkeys (18). Our previous results concur that ovarian estrogen is protective from obesity, but do not support the assumption that ovarian estrogen is necessary for protection from inflammation (19). We demonstrated that females are protected from immunological changes, in cytokine levels, and neuroendocrine changes, in hormone levels, regardless of the gonadal status. For example, males on HFD have increased levels of tumor necrosis factor (TNF)-α, leukemia inhibitory factor (LIF) and interleukin (IL)-6, while in females, unmodified or ovariectomized, TNFα and LIF are unchanged (19, 20). This may indicate that chromosomal differences contribute to intrinsic sex differences in the immune system.
Intrinsic sex differences in immune system and macrophages may contribute to sex differences in obesity-associated diseases, which were reported in human population and in response to DIO in rodent animal models (13, 21). Macrophages, particularly, demonstrate profound sex differences (22). Intrinsic differences in myeloid cells, irrespective of sex-steroid hormones, include enhanced myelopoiesis leading to proinflammatory responses in obese males (23). Macrophage infiltration into insulin-target tissues causes insulin resistance in obesity (8, 9), and insulin resistance maintains a greater incidence in men than women, after adjusting for age and adiposity (10). Male mice fed HFD have a higher propensity for insulin resistance than females, regardless of the strain (24). Myeloid-/ macrophage-specific knock-outs of inflammatory pathway signals ameliorate hyperglycemia and hyperinsulinemia, without preventing obesity when mice are fed HFD (9, 25, 26). Additionally, macrophage-derived molecules that are increased in obesity, such as resistin, contribute to insulin resistance (27). Correspondingly, neutralization of the elevated levels of proinflammatory cytokine TNFα improves insulin resistance (28). Therefore, we postulate that intrinsic sex differences in macrophages contribute to sex differences in macrophage infiltration to various tissues in obesity.
Macrophages infiltrate insulin-target tissues and other tissues in obesity (29). Our recent study demonstrated that peripheral macrophages infiltrate the hypothalamus of obese male mice, contributing to neuroinflammation that is associated with obesity (19). Infiltration of peripheral, circulatory macrophages occurs in addition to activation of resident immune cells, microglia (30, 31). Macrophages infiltrate muscle in mice and humans, where they contribute to insulin resistance and impede muscle regeneration and myogenesis (32, 33). Although the liver contains a resident macrophage population known as Kupffer cells, peripheral monocytes infiltrate the liver and give rise to proinflammatory macrophages, contributing to non-alcoholic fatty liver disease (NAFLD) (34). Several cytokines and chemokines are postulated to serve as chemoattractants for macrophages in obesity. Increase in free fatty acids or leptin in the circulation in obesity, may lead to recruitment of circulatory monocytes to tissues and lead to their activation (8, 35). Monocyte chemoattractant protein-1 (MCP-1, or CCL2 chemokine) is expressed by a variety of cell types upon stimulation with inflammatory signals and stimulates extravasation across the endothelium (36). In obesity, following increase in their size, adipose tissues produce CCL2, and recruit monocytes (37, 38). Another study demonstrated that CCL5, but not CCL2, contributes to macrophage accumulation and survival in adipose tissue (39). CCL5 is also produced by hepatocytes in obesity to stimulate immune cell infiltration and NAFLD (40). Therefore, it is still not clear which adipokine, cytokine or chemokine causes macrophage accumulation in adipose tissue. Furthermore, whether there are sex differences in macrophage response to these stimuli is not known. Herein, we provide answers to both of these questions.
To analyze sex differences and diet-induced changes in monocytes and macrophages, we compared chemoattractive molecules and macrophages in adipose tissues in vivo; and macrophages from male and female, control and diet-induced obese (DIO) mice that were differentiated in culture. We used C57BL/6J mice, the most commonly used strain to study DIO and metabolic syndrome because mice become obese, insulin resistant, and hypertensive, when fed HFD (41, 42). We used bone marrow cells and bone marrow derived macrophages (BMDM) from control-fed and HFD-fed males and females, and analyzed them separately to determine sex differences and diet-induced differences. BMDM from males and females were differentiated using standard protocol in the same medium, without the presence of sex steroids to analyze intrinsic sex differences in macrophages. Since obesity is a state of low grade chronic inflammation, we determined sex differences in obesity, without prior infection or activation by LPS (43–45), which may mask obesity-mediated changes. Herein, we demonstrate profound sex differences in macrophage migration and in their response to increased free fatty acids (FFA). Male macrophages express more inflammatory cytokines and are more migratory in obesity, due to upregulation of both leptin and free fatty acid receptors. These intrinsic differences may contribute to higher infiltration of macrophages in peripheral tissues in obesity that leads to insulin resistance and pathophysiologies of obesity. A better understanding of sex differences with respect to macrophage responses may contribute to better prevention and treatment for obesity and related diseases.
Materials and Methods
Animals
C57BL/6J mice were maintained under a 12-h light, 12-h dark cycle and received food and water ad libitum. All experiments were performed with approval from the University of California (Riverside, CA) Animal Care and Use Committee and in accordance with the National Institutes of Health Animal care and Use Guidelines. C57BL/6J mice were placed on either high fat diet (HFD, D12492, 60% kcal from fat; 5.21 kcal/g; protein 20% kcal; carbohydrate 20% kcal; fat 60% kcal (lard 0.32 g/g diet, soybean oil 0.03 g/g) Research Diet, New Brunswick, NJ) or control diet (CTR, D12450J, 10% kcal from fat; matching sucrose levels to HFD; 3.82 kcal/g; protein 20% kcal; carbohydrate 70% kcal; fat 10% kcal (lard 0.02 g/g diet, soybean oil 0.025 g/g) Research Diet, New Brunswick, NJ) from weaning age for 15 weeks. We reported before that females on HFD have irregular estrous cycles (19). Females were collected in diestrus after 15 weeks on control or HFD. Each animal was processed independently.
Cytokine assay
For serum collection, mice were sacrificed by isoflurane inhalation and blood was obtained from the inferior vena cava. The blood was left to coagulate for 15 min at room temperature, and then centrifuged at 2000 RCF for 15 min for serum separation. Adipose tissue was collected from gonadal fat pads and processed for protein extraction. Fat tissue was homogenized using a bead beater in lysis buffer (PBS with 0.1% NP-40 and protease inhibitors, Sigma Cocktail P8340), centrifuged at 12,000g for 20 minutes 4°C. Protein lysate was separated from fat layer using a 27 gauge needle (repeated twice). Protein concentration was determined using Bradford assay, and 50 ug of protein was used in the Luminex assay. Levels of leptin and CCL2 in serum and gonadal fat tissue protein lysates were measured using Luminex MagPix instrument and mouse Adipokine or Chemokine panel (Millipore, Burlington MA).
Flow cytometry
Tissues from each mouse were processed separately as part of a 5-mouse cohort per group, with each experiment repeated 3 times. In brief, mice were perfused with ice cold PBS, adipose tissue was collected from gonadal fat pads, rinsed in cold PBS, weighted, minced with razor blade and digested enzymatically with 3 mg/mL collagenase at 37 C for 2 hour. Suspension was passed through 40um cell strainer, centrifuged to pellet stromal vascular fraction, cells collected and 2 million cells labeled for flow cytometry analyses. Cells were Fc-blocked with anti-CD16/CD32 (1:100, 553141, BD Biosciences, San Jose, CA) followed by surface marker staining with antibodies to F4/80 (Brilliant Violet 650; 1:400, 123149, Biolegend, San Diego, CA) and CD11b (Brilliant Violet 605; 1:400, Biolegend, San Diego, CA). Flow analysis was performed with BD LSR II Flow Cytometer using the following gating strategy: cells, singlets, live cells, F4/80+CD11b+. For intracellular staining, cells were pretreated with 0.0026% Golgistop (BD Biosciences, CA) for 4 hours to block cytokine release, fixed, permeabilized with Cytofix/Cytoperm Fixation/Permeabilization solution (BDB555028, BD Biosciences, San Jose, CA) and stained with fluorophore-conjugated antibodies specific for IL-6 (anti-IL6 APC, 1:400, 504507, Biolegend, San Diego, CA ), IL-10 (anti-IL10 PerCP/Cy5.5, 1:400, 505027, Biolegend, San Diego, CA) and TNFα (anti-TNFα FITC, TN3–19.12, 11–7423-82 eBioscience, San Diego, CA). Results were analyzed using FlowJo software (Tree Star, Inc.).
Collection of bone marrow cells and adipocytes, and ex vivo derivation of bone marrow derived macrophages (BMDM)
For cell collection, animals were perfused with ice cold phosphate buffer saline (PBS). Bone marrow cells were collected from hind limb long bones as previously described (46). Briefly, the femurs were dissected using sterile surgical scissors. Muscles connected to the bone were removed, and the femurs then were placed in sterile PBS with 1% Penicillin-Streptomycin on ice. In a tissue culture hood, epiphyses were removed using sterile scissors and forceps and the femurs were then placed in sterile 200ul pipet tips. A brief centrifugation was performed using table top centrifuge to collect the bone marrow cells from the femurs. For gene expression analyses, cells were lysed using Trizol. Bone marrow derived macrophages (BMDM) are differentiated from femoral bone marrow cells for 7 days in RPMI 1640 with 10% FBS, in the presence of 30% conditioned medium from the L929 cell cultures that produce macrophage colony-stimulating factor (M-CSF). When indicated, cells were treated with 5% free fatty acid supplement (Sigma, MO) for 7 days, or co-cultured with adipocytes. Matured adipocytes were collected from visceral fat pad as described by (47). Briefly, after fat depot digestion as described above, the supernatants that contain adipocytes were cultured in an inverted completely filled flask. Adipocytes and bone marrow cells (and later their derivatives to BMDM) for co-cultures were collected from the same mouse to ensure isogenic conditions. To analyze a role of estrogen in sex differences, BMDM with and without free fatty acids were also cultured in the presence of 1μM ICI 182,780 (48, 49) for the duration of free fatty acid treatment.
qPCR Analysis
Total RNA was extracted using Trizol (Invitrogen, CA), quantified using Nanodrop and the same amount per sample reverse transcribed using Superscript IV (Invitrogen, CA). qPCR was performed using an iQ SYBR Green supermix and an IQ5 real-time PCR machine (Bio-Rad Laboratories, Hercules, CA), with primers listed in Table 1, under the following conditions: 95 °C for 15 min, followed by 40 cycles at 95 °C for 20 s, 56 °C for 30 s, and 72 °C for 30 s. The amount of the gene of interest was calculated by comparing the threshold cycle obtained for each sample with the standard curve generated in the same run and normalized to the TATA-binding protein (TBP) or GAPDH housekeeping genes, as indicated, in the same sample using ΔΔCt method. Preliminary experiments analyzed several housekeeping genes and determined that these housekeeping genes do not change with treatments. Replicates were averaged. After each run, a melting curve analysis was performed to confirm that a single amplicon was generated in each reaction.
Table 1.
Primers
| Primers | Forward | Reverse |
|---|---|---|
| CCL2 / MCP-1 | CACTCACCTGCTGCTACTCA | CAGCACAGACCTCTCTCTTGA |
| CCL5 / RANTES | GCAAGTGCTCCAATCTTGCA | CTTCTCTGGGTTGGCACACA |
| Leptin | TTCCTGTGGCTTTGGTCCTAT | TGCAGCACATTTTGGGAAGG |
| CCR2 | TCAACTTGGCCATCTCTGACC | AGACCCACTCATTTGCAGCAT |
| CCR5 | AGACATCCGTTCCCCCTACA | GCAGGGTGCTGACATACCAT |
| LEPR | GGTCCTCTTCTTCTGGAGCCT | AGAACTGCTTTCAGGGTCTGG |
| IL10 | GCTGGACAACATACTGCTAACC | ATTTCCGATAAGGCTTGGCAA |
| TNFa | ATGTCTCAGCCTCTTCTCATTCC | GCTTGTCACTCGAATTTTGAGAA |
| TBP | CAAACTCTGACCACTGCACCGTTG | GAAGCTGGTGTGGCAGGAGT |
| GAPDH | TGCACCACCAACTGCTTAG | GGATGCAGGGATGATGTTC |
Table 1. List of primers used to analyze expression by quantitative RT-PCR. Primers were used at 400 nM final concentration in the PCR reaction, as described in Materials and Methods section.
Transwell™ Migration Assay
Two million BMDM, collected using accutase (Innovative Cell Technologies, CA), in 300 uL were placed in the upper chamber of Boyden chamber / Transwell™ dish (5 μm pore size, Corning 3421, NY) and 1mL medium containing chemoattractant (either 50 ug/mL MCP-1 (479-JE, R&D Systems, Tustin, CA) or 100 ng/mL Leptin (ab9750, Abcam, Cambridge, MA)) or medium with vehicle control was added to the lower chamber. Cells that migrated into the lower chamber during 2 hour were collected and counted.
Statistical analyses.
We focused on differences between control and HFD within males and females, and sex differences between males and females. Statistical differences (p < 0.05) were determined by ANOVA followed by Tukey’s test for multiple comparisons using Prism software (GraphPad, CA). Interaction between sex and diet was analyzed by two-way ANOVA using Prism software (GraphPad, CA).
Results
Males accumulate more macrophages in adipose tissue
Chronic inflammation, elicited by high fat diet (HFD), is a hallmark of obesity. Macrophages that are activated in obesity infiltrate not only the adipose tissue but other tissues as well and contribute to insulin resistance and potentially other negative effects. We demonstrated profound sex differences in the diet-induced inflammation in obese mice, that were independent of ovarian estrogen (19). To investigate if these sex differences stem from intrinsic differences in the immune system, we initiated our studies with an analysis of macrophage numbers in the adipose tissue of C57BL/6 male and female mice, fed control and HFD for 15 weeks. Male mice weighed 47.94 grams after 15 weeks on HFD, compared to 27.53 grams for mice on control diet. HFD female mice weighed 29.28 grams, while control-fed female mice weighed 21.2 grams. Macrophages accumulate in adipose tissue in male mice in agreement with previously published reports. Earlier studies that determined macrophage numbers used a single marker to characterize adipose tissue macrophages in male mice (7, 50); here we analyzed double positive F4/80+CD11b+ population in both males and females using flow cytometry. The proportion of macrophages in the stromal vascular fraction of gonadal fat tissue reached 14.2% in HFD male mice (M hfd, black squares), compared to 5% in control male mice (M ctrl, white squares, Fig. 1A, each square represents one animal; a bar represents group mean; star indicates statistical significance between groups). Despite the weight increase, females did not exhibit significant increase in the proportion of macrophages in the adipose tissue (circles, Fig. 1A). We then determined the absolute number of macrophages (Fig. 1B). Male mice fed HFD had 5-fold more macrophages than lean male mice fed control diet, while in females the number doubled. These cohorts of mice exhibited the same increase in fat pads’ weight as we reported before (19). In both male and female mice, fat pad weight increased by ~2.5 fold with HFD. When macrophage numbers were normalized to milligrams of fat tissue, HFD males had 10 times more macrophages than HFD females, while control males had 2 times more macrophages than control females. Therefore, male mice accumulate more macrophages in adipose tissues when exposed to HFD.
Figure 1.
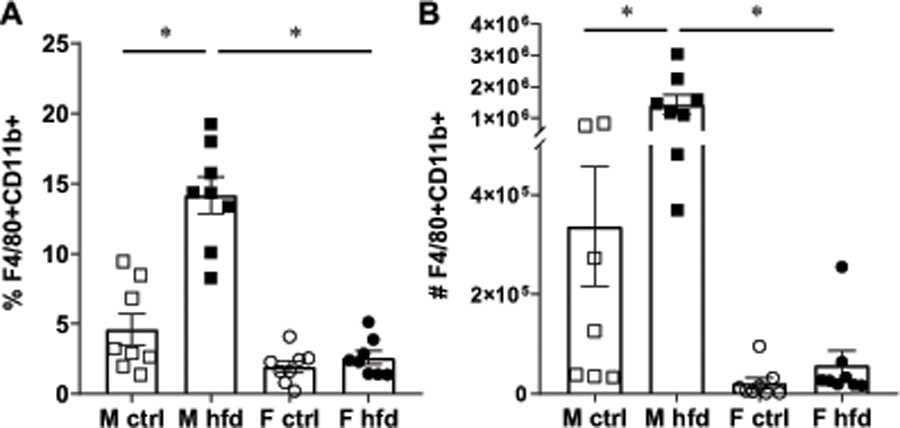
Males accumulate more macrophages in adipose depots. Macrophages identified as F4/80+ and CD11b+ positive cells using flow cytometry in the stromal vascular fraction from gonadal fat tissues from male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles) for 15 weeks. Each point represents one animal, processed independently, while bars represent group means with standard error; n=8. Statistical significance (*, p < 0.05) was determined with ANOVA followed by Tukey’s post hoc test. A, percentage of F4/80+ CD11b+ positive cells of all cell in stromal vascular fraction. B, absolute cell numbers of F4/80+CD11b+ positive in one gonadal fat depot.
Macrophages from male mice remain more inflammatory and more migratory after in vitro differentiation
Previously, we reported that females synthesize higher levels of anti-inflammatory IL-10 than males, in the hypothalami of control diet fed mice, a difference that is exacerbated in HFD mice. That led us to postulate IL-10 as a reason for female protection from neuroinflammation (19). Here, we analyzed sex differences in the expression of proto-typical inflammatory cytokine, TNFα, and anti-inflammatory cytokine, IL-10, in the adipose tissues of control and HFD males and females (Fig. 2A, B). We determined that HFD males increased the expression of TNFα in fat tissue, while the increase in TNFα in females was not significant. Thus, males on HFD expressed a higher level of TNFα than females on HFD (Fig. 2A). IL-10 expression in adipose tissue, on the other hand, increased in female mice on HFD, coinciding with our previous observation in the hypothalamus (Fig. 2B, (19)). We then analyzed expression of these cytokines in the bone marrow cells from males and females following control and HFD (Fig. 2C, D). Similar to adipose tissue, bone marrow cells from male mice fed HFD expressed higher levels of TNFα than control diet males, while in female cells, diet did not cause a change in TNFα expression (Fig. 2C). Expression in control males was higher than in control females, indicating that males expressed higher level of TNFα pro-inflammatory cytokines in basal conditions. Bone marrow cells from HFD males expressed more TNFα than HFD females. IL-10 expression, on the other hand, was higher in control female bone marrow cells than in control male bone marrow cells (Fig. 2D). Males on HFD increased IL-10 expression in bone marrow cells compared to males on control diet, while in females this trend was not significant. We utilized flow cytometry to analyze intracellular TNFα and IL-10 to assess a percentage of adipose tissue macrophages that synthesized these cytokines (Fig 2E, F, G). Adipose tissue macrophages were identified as double positive F4/80+CD11b+ cells and from that population, percentages of adipose tissue macrophages that contained intracellular TNFα and IL-10 were determined. As described in Fig 1, males have an increased percentage of F4/80+CD11b+ positive cells in the stromal vascular fraction (Fig. 2G top panels). To analyze percentages of these cells that synthesize cytokines, we prevented the secretion of cytokines using Golgi block in order to successfully stain for intracellular cytokines. A larger percentage of male macrophages contained TNFα than female macrophages (Fig. 2E and middle panels in Fig. 2G), while higher proportion of female macrophages contained IL-10 (Fig. 2F and bottom panels in Fig. 2G). However, there were no differences in the proportions of positive cells between diets (Fig. 2E, F). There was also no difference in the intensity of staining between sexes or between diets (MFI, data not shown), indicating lack of differences in the amount of either cytokine in each macrophage. Our results demonstrate that the increase in TNFα mRNA in adipose tissue in HFD males (Fig. 2A) stems from the influx of macrophages (Fig 1A and Fig. 2G, top panel), since there is no increase with HFD in the proportion of macrophages in the adipose tissue that contain the cytokine (Fig. 2E) or in the cytokine level per cell (not shown). Significantly, our data also show that male macrophages are more inflammatory than female macrophages.
Figure 2.
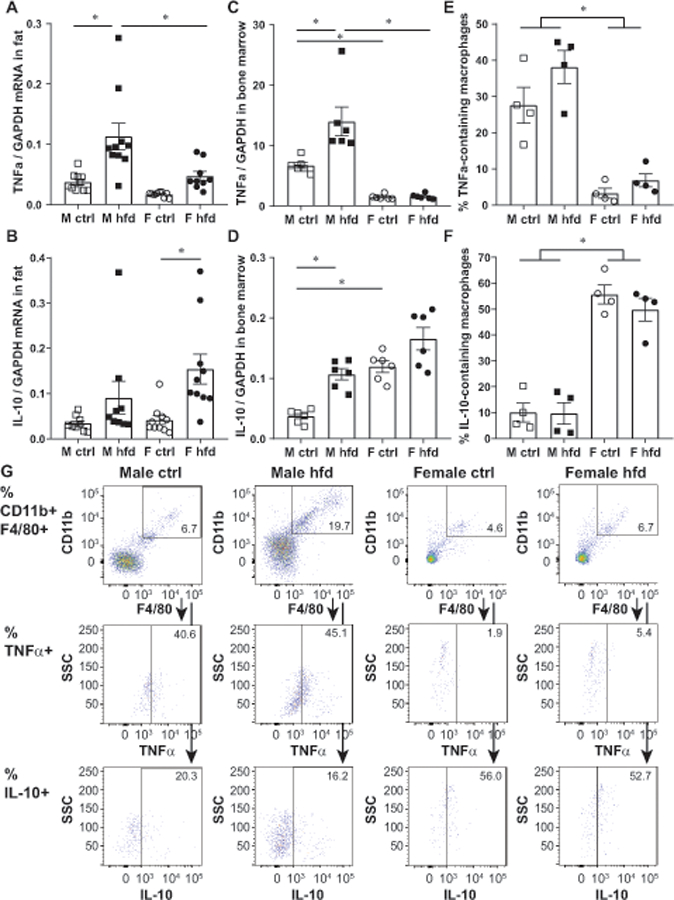
Fat-infiltrated macrophages from male mice are more inflammatory. A, TNFα mRNA expression in gonadal fat tissues from male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles) for 15 weeks. 2 ug of total mRNA was reverse transcribed and qPCR performed with primers listed in Table 1. B, IL-10 mRNA expression in the same tissues as A. C, TNFα expression in bone marrow cells from the same mice as above. D, IL-10 expression in bone marrow cells. E-G, stromal vascular fraction cells from adipose tissue were stained as described in materials and methods, and analyzed by flow cytometry. Macrophages were identified as F4/80+CD11b+ cells as illustrated in G, top panels, and proportions of these double positive cells that additionally stained with TNFα (C, and G, middle panels) and IL-10 (D and G, bottom panels) antibodies determined. A-F, Each point represents one animal, processed and analyzed separately (A-D, n=6–10; E-F, n=4). G, representative example of one animal per group, which was included in E and F. Statistical significance, indicated with a * (p < 0.05) was determined with ANOVA followed by Tukey’s post hoc test.
Since macrophages in obesity infiltrate not only adipose tissue, but other tissues such as liver, muscle and hypothalamus, we then determined the migratory capacity of bone marrow derived macrophages (BMDM) from female and male mice fed control and HFD. Bone marrow cells were differentiated into macrophages according to the published protocol (46) and transferred into Transwell Boyden chamber dishes and their migration monitored after 2 hours. Naïve macrophages derived from male mice were 4 times more migratory than those derived from female mice (Fig. 3). There was no difference between macrophages derived from lean or obese mice in either sex. Taken together, our results indicate that male mice have more macrophages in their adipose tissues than females, likely due to males having macrophages that are more inflammatory and more migratory.
Figure 3.
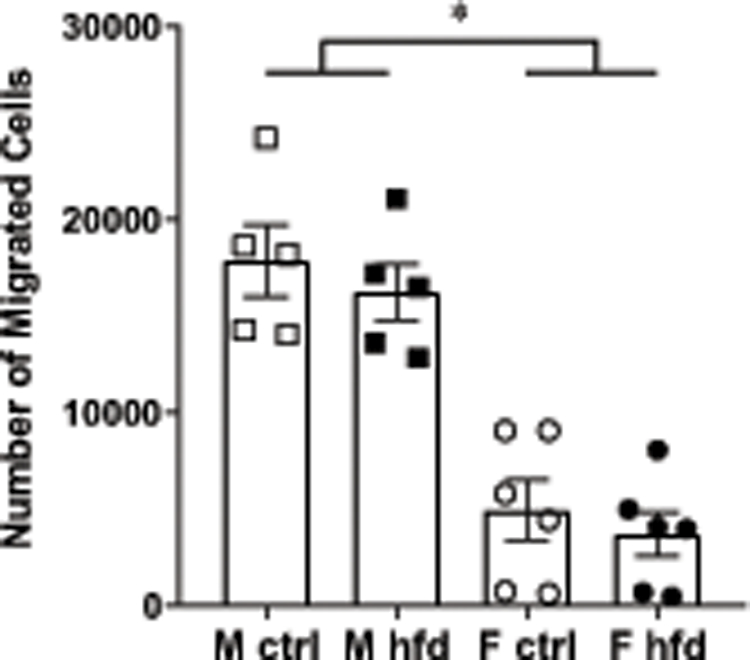
Bone marrow derived macrophages (BMDM) from male mice are more migratory. BMDM differentiated from bone marrow cells for 7 days, as described in materials and methods, from male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles) for 15 weeks. Each point represents cells isolated from one animal (n=5–6), and differentiated into BMDM in vitro, in the same medium using standard conditions. After differentiation, cells were placed in Transwell Boyden chambers in the top well, and migration to the bottom counted after 2 hours. Statistical significance, indicated with a * (p < 0.05) was determined with ANOVA followed by Tukey’s post hoc test.
Males have higher levels of leptin that may attract macrophages
We previously reported that males on HFD have higher levels of inflammatory cytokines than control-fed lean male mice. Female mice, on the other hand, although gained weight when fed HFD, did not show an increase in serum levels of TNFα or LIF (19). Females were protected irrespective of ovarian estrogen, and neither unmodified nor ovariectomized females on HFD had elevated cytokines. This prompted us to investigate sex differences in adipokines and/or cytokines that were postulated to contribute to macrophage recruitment to adipose tissue. We measured leptin, CCL2 / MCP-1 and CCL5 / RANTES in the serum and adipose tissue of control and HFD male and female mice (Fig. 4). There was a significant increase in serum leptin levels in HFD males (6.2 ng/ml) compared to control males (1.3 ng/ml; Fig. 4A). Importantly, HFD males had higher serum leptin (6.2 ng/ml) than HFD females (3.1 ng/ml). In fat tissues, leptin also exhibited sex differences. At the mRNA levels, on control diet, males express higher levels of leptin mRNA compared to females on control diet (Fig. 4B). Both males and females up-regulate leptin expression on HFD. At the protein level, only in males did the increase in leptin reach significance following HFD (35.3 μg/g in HFD compared to 10.6 μg/g in controls), while in females there was no statistical difference in leptin levels between controls and HFD (3.2 μg/g and 6.2 μg/g, respectively) (Fig. 4C). Our results demonstrate that male adipose tissue in control conditions synthesizes higher levels of leptin than female control adipose tissue. Furthermore, HFD males have higher levels of leptin than HFD females in fat tissue.
Figure 4.
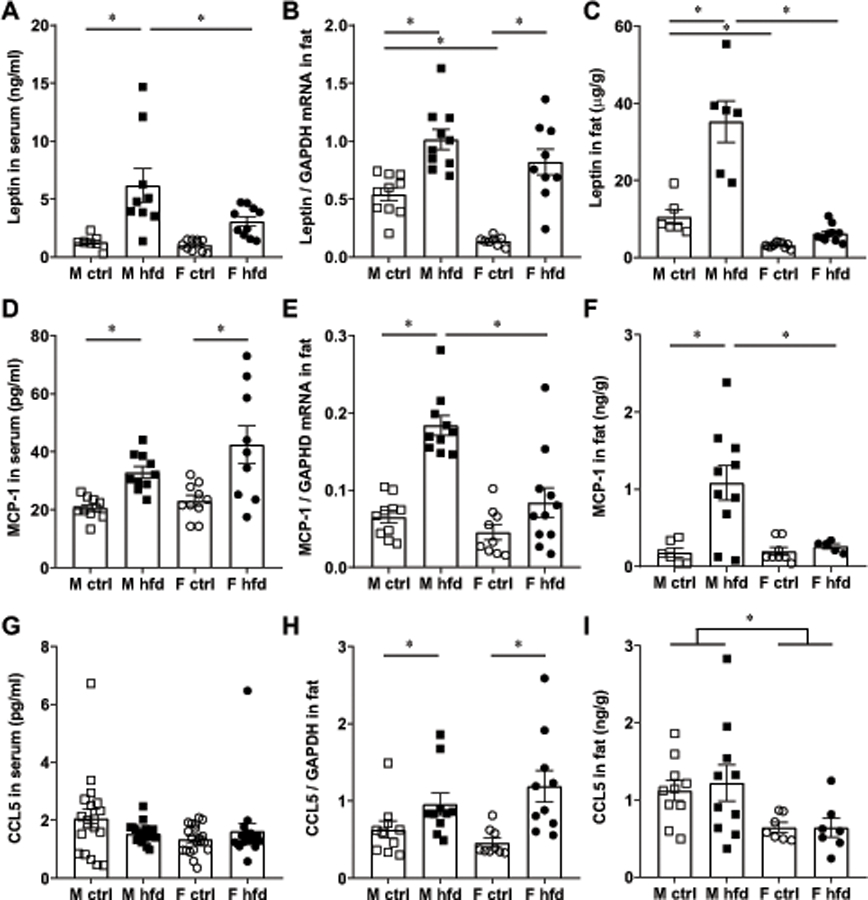
Male adipose tissues have higher levels of leptin and MCP-1 / CCL2. A, D, G, levels in serum of male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles) determined by Luminex assay; n=10–20. B, E, H, mRNA levels normalized to GAPDH in the gonadal depot determined by RT-qPCR, n=9–12. C, F, I, protein levels normalized to grams of total protein in gonadal adipose tissue determined by Luminex assay, n=6–10. Each point represents one animal, while bars represent group means. Statistical significance (p < 0.05), determined with ANOVA followed by Tukey’s post hoc test, is indicated with a *.
CCL2 / MCP-1, as expected, increases in serum in HFD males compared to control males from 20.6 pg/ml to 32.9 pg/ml, and in HFD female mice compared to control female mice from 23.1 pg/ml to 42.4 pg/ml, but there was no difference between control diet females and control diet males, or HFD females and HFD males (Fig. 4D). Thus, there is no sex difference in serum levels of CCL2. In the fat tissue, at mRNA (Fig 4E) and protein levels (Fig. 4F), males increased CCL2 levels 3-fold and 6-fold on HFD, respectively, compared to control diet, while females increased CCL2 levels in fat 1.3-fold on HFD which was not significant (Fig. 4E, F). Thus, males on HFD had significantly higher levels of CCL2 in adipose tissues than females on HFD, while there was no sex difference in the control mice.
Serum CCL5 / RANTES on the other hand, is the same in both males and females on HFD compared to control diet and was the same between sexes (Fig. 4G). In the adipose tissue, both males and females increased expression of CCL5 mRNA on HFD, but there were no sex differences (Fig. 4H). CCL5 protein in adipose tissues of control males was higher than in control females and HFD males had higher levels than HFD females (Fig. 4I). Although there was no difference between control and HFD in either males or females, males had 2-fold higher levels than females.
Next, we assessed receptor levels for the ligands analyzed above, in bone marrow cells and bone marrow derived macrophages (BMDM) from males and females fed control or HFD. There was no difference in the expression level of leptin receptor (LEP-R) in bone marrow cells between diets, however female mice had higher expression than males (Fig. 5A). This sex difference was lost during differentiation into BMDM (Fig. 5B). Thus, although leptin concentration is elevated in the serum and adipose tissue in males fed HFD, the levels of leptin receptor in bone marrow cells or macrophages do not change with diet.
Figure 5.

Expression of leptin receptor mRNA (A, B), CCR2 mRNA (C, D) and CCR5 mRNA (E, F) in bone marrow cells (A, C, E,) and BMDM (B, D, F) from male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles), for 15 weeks, determined by RT-qPCR. Each point represents cells isolated from one animal, n=5–6, and bars represent group means. Statistical significance (p < 0.05), determined with ANOVA followed by Tukey’s post hoc test, is indicated with a *.
CCR2 receptor is expressed on macrophages and binds MCP-1 / CCL2 ligand. CCR2 expression was higher in bone marrow cells from HFD males than control males and in cells from HFD females than control females, but there were no sex differences (Fig. 5C). Therefore, both the ligand, CCL2, and its receptor CCR2, increase with HFD in both sexes. The difference with diet was lost during differentiation in vitro and BMDM expressed similar levels of CCR2 regardless of sex or diet (Fig. 5D). Expression of CCR5 that binds CCL5 / RANTES was higher in bone marrow cells from female mice than in bone marrow cells from male mice, however there was no difference between diets (Fig. 5E). In BMDM this sex difference was lost, and there was no difference between sexes or between diets in CCR5 expression (Fig. 5F). These results indicate that, as expected, leptin is increased in obesity, and that this increase is much higher in males than in females.
Hyperlipidemia of obesity increases leptin receptor levels in male macrophages
Increase in leptin concentration in obese males is not accompanied with increased levels of receptors (Fig. 5A). To determine how macrophages respond to increased leptin concentration, we cultured BMDM in the presence or absence of free fatty acids (FFA) to mimic hyperlipidemia present in obesity. Macrophages derived from male mice exhibited 6-fold increase in the expression of leptin receptor in response to FFA, compared to controls (LEP-R, Fig. 6A). Macrophages from female mice increased LEP-R 1.7-fold in response to FFA, but changes from control culture conditions did not reach significance (Fig. 6A). Moreover, macrophages derived from male mice fed HFD and in the presence of FFA had higher levels of LEP-R than macrophages derived from male mice fed control diet in the presence FFA. When cultured with FFA, macrophages from male mice on HFD exhibited increased expression of CCR2, compared to macrophages cultured in the vehicle control or those derived from male mice on control diet (Fig. 6B). Female-derived BMDM did not increase levels of CCR2 expression compared to controls.
Figure 6.
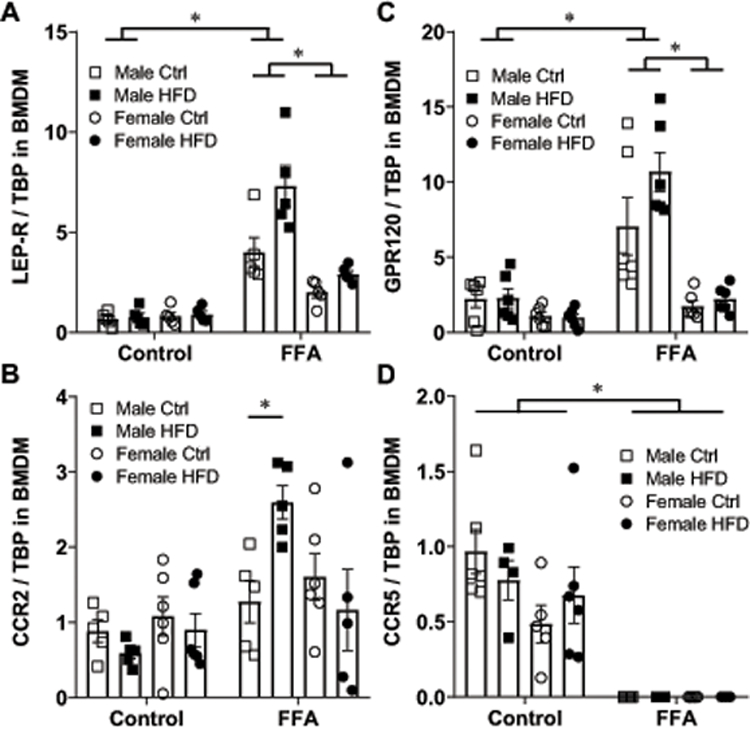
BMDM from male mice cultured in the presence of free fatty acids (FFA) up-regulate expression of leptin receptor, GPR120 and CCR2. A, leptin receptor mRNA; B, CCR2 mRNA; C, GPR120 mRNA, and D, CCR5 mRNA in BMDM from male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles) determined by RT-qPCR. Cells derived from one animal, n=5–6, were split, and one half cultured under control conditions in differentiation medium, and the other half in the same differentiation medium, with addition of FFA. Each point represents cells from one animal that was processed independently. Statistical significance (p < 0.05), determined with ANOVA followed by Tukey’s post hoc test, is indicated with a *.
We also analyzed expression levels of GPR120 (FFAR4), which is a receptor for saturated and unsaturated long chain free fatty acids (51). Expression of GPR120 increased 3.5-fold in the male BMDM cultured in the presence of FFA, but not in female-derived BMDM (Fig. 6C). There were no differences in the GPR120 expression in BMDM from control or HFD fed mice regardless of sex.
Surprisingly, when cultured in the presence of FFA, regardless of sex or diet, BMDM lost expression of CCR5 (Fig. 6D). We further analyzed insulin receptor and IGF-1 receptor. Expression of insulin or IGF-1 receptors in BMDM was the same regardless of sex, diet or culture conditions (data not shown). To determine if increases in LEP-R and GPR120 in male BMDM occur due to hyperlipidemia or adipocyte hypertrophy, which are hallmarks of obesity, we co-cultured BMDM with adipocytes. Adipocytes were purified from the same mouse as BMDM to assure isogenic conditions. There were no changes in the expression in LEP-R or GPR120 receptors when BMDM were co-cultured with adipocytes (data not shown). Therefore, BMDM from male mice in particular those fed HFD, increase expression of leptin receptor, free fatty acid receptor GPR120 and chemoattractant receptor CCR2 when cultured with FFA, while female derived BMDM do not. These results also demonstrate that hyperleptinemia of obesity is not sufficient to increase leptin receptor levels in macrophages, but increased free fatty acids lead to elevated leptin receptor levels particularly in male macrophages.
To determine if estrogen plays a role in observed sex differences, we cultured BMDM with ICI 182,780 (ICI), an estrogen receptor (ER) α antagonist (48, 49). We focused on ERα, since ERα has a larger role in myeloid cells responses to obesity (52) and is expressed at a higher level in mouse and human macrophages than ERβ (53). When cultured with FFA, both male and female BMDM increase expression of TNFα, regardless of the presence of ICI (Fig. 7A). However, cells derived from different animals had varied degree of response, eliciting a lack of significant changes in expression. IL-10 was expressed at a higher level in female BMDM than male BMDM, consistent with results using bone marrow cells presented in Fig. 2D (Fig. 7B). After exposure to FFA, IL-10 expression in male BMDM increased 5.5-fold, while IL-10 expression in female BMDM increased 23-fold. Higher increase in response to FFA in female-derived BMDM than in male-derived BMDM, corresponds to higher IL-10 induction in female adipose tissue than in male adipose tissue in obesity in vivo, presented in Fig. 2B. In the presence of ICI, inhibiting estrogen signaling, increase in IL-10 expression in response to FFA was 40% lower in both males and females (Fig. 7B). Thus, estrogen contributes to maximal induction of IL-10 by FFA, but sex differences in IL-10 expression remain. As presented in Figs 6A and 6B, expression of LEP-R and GPR120 increased in male BMDM in response to FFA, and ICI treatment didn’t cause significant differences (Figs. 7C, 7D). Therefore, estrogen does not play a role in male macrophage induction of leptin and GPR120 in response to FFA, but it may partially contribute to IL-10 expression in females, which may provide protection.
Figure 7.
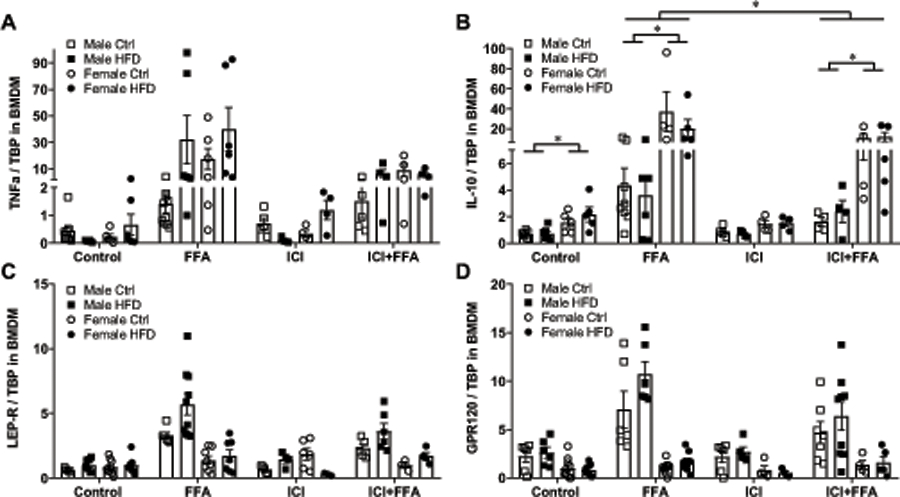
Estrogen is necessary for the maximal induction of IL-10 by FFA, but not for sex differences. A, TNFα mRNA, B, IL-10 mRNA C, leptin receptor (LEP-R mRNA; D, GPR120 mRNA, produced by BMDM from male mice fed control diet (white squares), male mice fed HFD (black squares), female mice fed control diet (white circles) and female mice fed HFD (black circles) determined by RT-qPCR and normalized to TATA-binding protein (TBP). Cells derived from one animal, n=5–6, were split, and one quarter cultured under control conditions in differentiation medium, and second quarter in the same differentiation medium, with addition of FFA for 7 days, third quarter in the presence of 1μM ICI 182,780 (Fulvestrant) estrogen receptor antagonist, and the final quarter with ICI and FFA. Each point represents cells from one animal that was processed independently. Statistical significance (p < 0.05), determined with ANOVA followed by Tukey’s post hoc test, is indicated with a *.
Macrophages from male mice are more migratory in response to leptin
Given that we determined that naïve macrophages from male mice are intrinsically more migratory than macrophages from female mice, we wanted to compare migration in the presence of FFA. Since both leptin and MCP-1 / CCL2 are increased in obese males, and macrophages from male mice cultured in the presence of FFA increase expression levels of leptin receptor, GPR120 and CCR2, we analyzed migration in response to leptin and CCL2 with and without FFA. In agreement with previous studies, CCL2 causes macrophage migration after their activation: we observed BMDM migration in response to CCL2 when cells were pretreated with LPS (Fig. 8A). Given that LPS is not normally present in the adipose tissue in the absence of infection, while increase in FFA is a hallmark of obesity, we assessed if macrophages are migratory in response to CCL2 in the presence of FFA (Fig. 8B). CCL2 failed to cause migration in the absence of LPS, with or without FFA.
Figure 8.
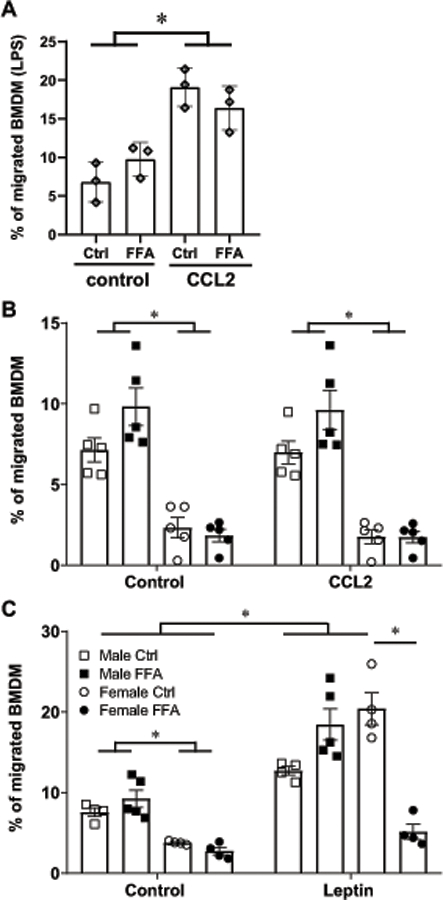
Male macrophages are more migratory in response to leptin. A, percent of BMDM, cultured in control conditions or in the presence of FFA, activated by LPS overnight, that migrate in response to vehicle or CCL2. B, percent of BMDM from male mice cultured in control conditions (white squares), male mice cultured with FFA (black squares), female mice cultured in control conditions (white circles) and female mice cultured with FFA (black circles) that migrated in response to vehicle control or CCL2 in 2 hours. C, percent of BMDM that migrated in response to vehicle control or leptin in 2 hours. Cells derived from one animal were split, and one half cultured under control conditions in differentiation medium, and the other half in the same differentiation medium with FFA. After differentiation, cells were again divided, counted, and 2x106 cells placed in the Boyden chamber with vehicle control, and 2 x106 in the chamber containing CCL2 or leptin to monitor migration after 2 hours. Each point represents cells from one animal, n=5, that was processed and analyzed independently. Statistical significance, p<0.05, indicated with a *, was determined with ANOVA followed by Tukey’s post hoc test.
We then analyzed leptin-stimulated migration and observed an increased migration in response to leptin (Fig. 8C). Presence of FFA prevented migration of macrophages in response to leptin from female mice, but not from males (Fig. 8C). Therefore, male macrophages maintain migratory capacity in culture conditions mimicking hyperleptinemia and hyperlipidemia of obesity, while female macrophages do not. Taken together, our results demonstrate that 1) male mice have higher leptin in adipose tissue than female mice and further increase leptin synthesis in obesity, to a higher level than females; 2) macrophages derived from male mice increase the expression of leptin receptor when cultured in the presence of FFA and 3) male macrophages are intrinsically more migratory, migrate in response to leptin and maintain migration in the presence of FFA. Our results may explain higher infiltration of macrophages in obese adipose tissue in males.
Discussion
We sought out to uncover mechanisms of sex differences in obesity-mediated inflammation that contribute to male propensity for insulin resistance. While it is known that males on HFD gain more adipose tissue than females, that recruits and activates macrophages, the reasons for these differences are only beginning to emerge. Our previous study determined that major obesity-mediated sex differences in inflammatory and endocrine changes occur irrespective of ovarian estrogen (19). Herein, we identify several differences between male and female macrophages and in the expression of cytokines in male and female adipose tissues. We determined that male adipose tissues accumulate more macrophages even when the numbers are normalized to the adipose tissue weight. We also demonstrate that male macrophages are more inflammatory and more migratory, while females may be protected due to higher expression of anti-inflammatory cytokines. Significantly, obese males have much higher levels of leptin than obese females, and macrophages from male mice increase migration in response to leptin. However, this elevated leptin is not sufficient to induce leptin receptor in male macrophages. When cultured in the presence of free fatty acids, which mimics hypelipidemia of obesity, male macrophages increase expression of leptin receptor, which may allow macrophage migration to various tissues in response to higher leptin in males. A previous study that analyzed immune cell autonomous response to obesity determined intrinsic sex differences in myeloid cell, and posited that males increase myelopoiesis leading to inflammation (23). We postulate that males exhibit higher leptin levels, due to differential deposition to fat depots as we demonstrated before (19). Higher leptin, combined with intrinsically higher migratory capacity and higher leptin receptor induction in response to free fatty acids in male macrophages, leads to more macrophage accumulation in male adipose tissues and higher inflammation.
Leptin is produced by adipocytes and its expression increases in response to adiposity and adipocyte hypertrophy. In humans, elevated leptin leads to monocyte activation and proliferation (54). Males especially increase levels of leptin in the serum following exposure to HFD, corresponding to the increase in weight. In the females, 2-fold increase in leptin didn’t reach significant levels. It is possible that the increase in leptin, in the circulation and in adipose tissue, is higher in males than in the females, because males on HFD gain more weight (175% compared to males on control diet) than females on HFD (140% compared to females on control diet). Bone marrow cells of female mice express higher levels of leptin receptor, and in neither sex the expression changes in response to diet. This difference was lost during differentiation into BMDM and there was no difference in leptin receptor expression in BMDM. This also shows that higher leptin in obese males is insufficient to increase leptin receptor levels in male bone marrow cells or BMDM. However, when BMDM were cultured with free fatty acids (FFA) to mimic hyperlipidemia of obesity, macrophages derived from male mice increased expression of leptin receptor. Induction of leptin receptor by FFA was larger than the induction of CCR2 expression. This may indicate that male macrophages may be more responsive to the increase in leptin during obesity. Indeed, male macrophages are more migratory in response to leptin when cultured with FFA. This may be a reason that males accumulate more macrophages in the adipose tissues in obesity. Leptin was postulated to contribute to macrophage accumulation by promoting adhesion of macrophages to endothelial cells and their transport to adipose tissue (8). Although males and females increase adipose tissue weight approximately 2.5-fold, males accumulate 10 times more macrophages, and increase responsiveness of male macrophages to leptin may be one of the reasons. A role for leptin receptor in macrophages was analyzed in the presence of active infection. In response to Salmonella infection, leptin receptor was necessary for maximal cytokine expression, while macrophage-specific leptin signaling inhibited their bactericidal functions (45). On the other hand, lack of leptin receptor, though it had no effect on cytokine production, impaired phagocytosis of Streptococcus (44). We analyzed a role for leptin in sex differences in the naïve physiology of obesity-induced low grade chronic inflammation, without infections; and in macrophage migration. We determined that male macrophages, in the state of hyperlipidemia, augment expression of leptin receptor, which leads to increased migration in response to leptin. This implies that in obesity, both increased leptin and increased fatty acids contribute to certain male-specific pathologies. Since infiltration of macrophages to the liver contributes to insulin resistance (29), our findings may explain higher incidence of insulin resistance in males than females (10, 24).
CCL2, identified as monocyte chemotactic protein (MCP) 1 (55), increases in obesity (7) and contributes to insulin resistance, hepatic steatosis, and macrophage accumulation in adipose tissue (50, 56). In agreement with these studies, concentration of CCL2 increases in serum in both males and females with HFD in our study. On the other hand, we determined that only males had significant increase in CCL2 in adipose tissue. This may cause higher accumulation of macrophages in male adipose tissues as discussed above. It is unlikely, given that CCL2 is not necessary for macrophage recruitment into the adipose tissue of obese mice, since CCL2 null mice had the same proportion of adipose tissue macrophages as controls, following HFD (57). We further show that increased concentration of the ligand in the serum corresponds to increased expression of the receptor, CCR2 in bone marrow cells with HFD in both males and females. After differentiation to BMDM, there was no difference in CCR2 expression, however, HFD male-derived BMDM increased CCR2 expression when they were cultured in the presence of FFA. Significantly, there was a lack of migration in response to CCL2 with and without FFA, if BMDM were not activated previously with LPS, consistent with previous studies (58). Cells that normally express high levels of CCR2, such as THP-1 human immortalized monocytic cell line (59), migrate in response to CCL2 (35). However, these cells shows substantial differences from circulating monocytes or macrophages (60). Bone marrow cells, in obese or genetically modified mice, migrate in response to CCL2 (56), likely because they upregulate CCR2 in response to HFD as we show here. We used BMDM, which as we show lose induction of CCR2 during differentiation, and thus need to be stimulated prior to migration in response to CCL2 (61). LPS activates macrophage migration in response to CCL2, but LPS is not present in normal, physiological conditions in the absence of infection. Given that FFA levels are increased in obesity, we wanted to assess if FFA can activate macrophage migration in response to CCL2. However, neither macrophages from female nor male mice migrated in response to CCL2, with and without FFA, which may indicate that FFA does not crosstalk with CCL2-CCR2 pathway.
Previously a study demonstrated that CCL5 and its receptor, CCR5, expression increased in adipose tissue following HFD (62). Further studies demonstrated that CCL5, rather than CCL2, contributes to macrophage accumulation in adipose tissue in obesity (39). Our results do not support a role for CCL5. CCL5 levels in serum do not change with diet, nor do they exhibit sex differences. In our study, in agreement with previous studies (62, 63), expression of CCL5 mRNA in adipose tissues is increased following HFD. However, this increase is not reflected at the protein level. While in the adipose tissues, males have higher levels of CCL5 than females, which may correspond to higher accumulation of macrophages in males, there is no difference with diet or sex in the expression of its receptor, CCR5, in BMDM. Moreover, the CCR5 expression is completely lost in the presence of FFA that mimic conditions of obesity. It is possible that CCL5 may play a role in the liver and NAFLD (40), but our results indicate that it doesn’t play a role in sex differences in macrophage accumulation to adipose tissue.
FFA increase in the circulation is a hallmark of obesity. Long chain FFA bind GPR40 and GPR120 (64). Since neither macrophages nor adipocytes express GPR40, GPR120 is the relevant receptor that conveys increase in FFA in obesity to these tissues (65). GPR120 binds both unsaturated and saturated long chain FFA (51). Since GPR120 binds omega-3 polyunsaturated fatty acids that have an anti-inflammatory role, GPR120 signaling was also postulated to be anti-inflammatory. However, some studies dispute a role for GPR120 in an anti-inflammatory function of unsaturated fatty acids (66). In human adipose tissues, GPR120 is involved in the induction of both pro-inflammatory and anti-inflammatory cytokines by fatty acids (67). GPR120 levels increase following HFD in adipose tissue stromal vascular fraction, which contains macrophages, and GPR120 activation decreases expression of inflammatory cytokines in response to LPS (65). Similarly to CCL2, macrophages had to be stimulated with LPS to observe the effect of GPR120 on inflammatory cytokine expression (65). We analyzed the levels of GPR120 expression in cultured BMDM in the presence of FFA, and determined that only male-derived macrophages increase the expression of GPR120. Thus, we postulate that male-specific increase in GPR120 may contribute to higher migration of male macrophages in the presence of hyperlipidemia of obesity. Increased leptin receptor and increased free fatty receptor may work together to facilitate larger macrophages infiltration into male adipose tissue.
Some of identified sex differences may depend on sex steroid hormones (11, 13, 14), while others may depend on sex chromosomes (15) or other intrinsic differences (23). Since estrogen is protective from DIO, but is not necessary for protection from neuroinflammatory changes (19), to investigate the contribution of estrogen in sex differences observed here, we treated cells with estrogen receptor antagonist. Consistent with previous studies in which estrogen may either induce or repress TNFα, depending on the duration or concentration of treatment, model or design, or inter-animal variability (30, 49, 68–75), our results with estrogen inhibition of TNFα induction in response to FFA were inconclusive. Since cells from different animals, males or females or control or HFD, were processed and differentiated into BMDM separately, this may have contributed to variability. On the other hand, induction of IL-10 in response to FFA was higher in female-derived BMDM, consistent with IL-10 increase in adipose tissues of obese females in vivo. Disruption of estrogen signaling, prevented maximal induction of IL-10 in both male and female BMDM, but the sex differences remained. Role of estrogen in IL-10 production was shown before (71, 76); as was protection by IL-10 in obesity-mediated inflammation (77). Here we show that female macrophages synthesize more IL-10 than male macrophages, which may contribute to female protection in obesity and relatively fewer pathophysiological outcomes. Even in the absence of estrogen signaling, in the presence of ICI estrogen antagonist, females synthesized more IL-10 than males, which may account for female protection. Since the induction of leptin receptor or GPR120 free fatty acid receptor by FFA was specific for male BMDM, estrogen does not play a role in leptin receptor or GPR120 increases in hyperlipidemic conditions.
Leptin has been shown to promote macrophage migration (35, 78), and our results agree with these reports. Leptin knockout mice and leptin receptor null mice exhibit reduced macrophage infiltration and lower inflammatory gene induction in adipose tissues, despite higher obesity (79). Studies using bone marrow transplants from leptin receptor null mice obtained conflicting results, and thus, the role of leptin receptor specifically in bone marrow cell recruitment into adipose tissues remains unclear. One study that transplanted leptin receptor-deficient bone marrow cells into irradiated wild-type recipient mice observed no differences in immune cell infiltration into adipose tissue following HFD (80). Another study performed the same transplant but observed significantly fewer CCL+ macrophages in adipose tissues and reduced macrophage-forming crown like structures around adipocytes (81). Studies that showed macrophage migration in response to leptin in the transwell dishes used THP-1 cell line that, as discussed above, constitutively expresses high CCR2 levels and was derived from a male (82), while herein, we compared male and female macrophage migration in response to leptin. Furthermore, a role of leptin receptor in macrophages was analyzed before in the context of infection (44, 45). We compared side by side unstimulated BMDM derived from males and females, which were differentiated using the same conditions without the presence of sex steroid hormones, and determined that male BMDM migrate more in response to leptin, especially in the hyperlipidemic conditions. BMDM may differ from resident or recruited tissue macrophages in situ (83–85), since during differentiation, BMDM lose sex differences observed in bone marrow cells, as we show here. However, although differentiated in vitro, BMDM are primary cells, as opposed to transformed cell lines mentioned above. Macrophages are, at any rate, a heterogeneous cell population, which is further confounded with their high plasticity in response to the changing environment in tissues or varied stimuli under different conditions (86). Utilizing BMDM allowed us to control culture conditions and focus on the roles of free fatty acids and leptin in macrophage migration, in addition to differences we determined in bone marrow cells and adipose tissue macrophages in vivo.
Intrinsic sex differences in macrophage migration or sex differences in response to leptin were not analyzed before. Additionally, a role of FFA in macrophage migration or expression of receptors was not analyzed in detail before, although FFA are increased in obesity. We determined that male macrophages increase expression of GPR120 FFA receptor 4, and that the presence of FFA contributes to sex differences in macrophage migration. Our results demonstrated that 1) males have higher leptin levels, 2) male macrophages increase leptin receptor expression in response to free fatty acids, and 3) male macrophages migrate in response to leptin. These results may explain male propensity for insulin resistance that is a hallmark of metabolic syndrome and is elicited by macrophage infiltration. Therefore, our results indicate that hyperleptinemia combined with hyperlipidemia may be the critical condition in obesity that leads to accumulation of macrophages in adipose tissue. Since males increase leptin to much higher levels than females, this causes disproportionally more macrophages in male adipose tissue, which in turn, leads to elevated levels of inflammatory cytokines, such as TNFα. Female macrophages, however, express higher levels of anti-inflammatory IL-10, which may provide protection to females. Together, these differences that lead to chronic inflammation in males, may contribute to disproportionally increased risk of cardiovascular diseases and insulin resistance in obese males than females.
Key points.
Male macrophages are intrinsically more migratory in the same culture conditions
Male bone marrow cells and male adipose tissue macrophages are more inflammatory
Male macrophages induce leptin receptor and GPR120 in response to free fatty acids
Funding Sources
This study was supported by R01 HD091167 from NIH NICHD to D. Coss.
Footnotes
Statement of Ethics
All experiments were performed with approval from the University of California (Riverside, CA) Animal Care and Use Committee and in accordance with the National Institutes of Health Animal care and Use Guidelines.
Disclosure Statement
The authors have no conflicts of interest to declare
References
- 1.2016. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet (London, England) 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Public, H. 2018. Tackling obesity seriously: the time has come. The Lancet. Public health 3: e153. [DOI] [PubMed] [Google Scholar]
- 3.Odegaard JI, and Chawla A 2013. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, Bodzin JL, and Saltiel AR 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla A, Nguyen KD, and Goh YP 2011. Macrophage-mediated inflammation in metabolic disease. Nature reviews. Immunology 11: 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanneganti TD, and Dixit VD 2012. Immunological complications of obesity. Nat Immunol 13: 707–712. [DOI] [PubMed] [Google Scholar]
- 7.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olefsky JM, and Glass CK 2010. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246. [DOI] [PubMed] [Google Scholar]
- 9.de Luca C, and Olefsky JM 2008. Inflammation and insulin resistance. FEBS Lett 582: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link JC, and Reue K 2017. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annual review of nutrition 37: 225–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camporez JP, Lyu K, Goldberg EL, Zhang D, Cline GW, Jurczak MJ, Dixit VD, Petersen KF, and Shulman GI 2019. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. The Journal of physiology 597: 3885–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerdts E, and Regitz-Zagrosek V 2019. Sex differences in cardiometabolic disorders. Nature medicine 25: 1657–1666. [DOI] [PubMed] [Google Scholar]
- 13.Palmer BF, and Clegg DJ 2015. The sexual dimorphism of obesity. Mol Cell Endocrinol 402: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keselman A, Fang X, White PB, and Heller NM 2017. Estrogen Signaling Contributes to Sex Differences in Macrophage Polarization during Asthma. Journal of immunology 199: 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shenoda BB, Ramanathan S, Gupta R, Tian Y, Jean-Toussaint R, Alexander GM, Addya S, Somarowthu S, Sacan A, and Ajit SK 2020. Xist attenuates acute inflammatory response by female cells. Cellular and molecular life sciences : CMLS [DOI] [PMC free article] [PubMed]
- 16.Grove KL, Fried SK, Greenberg AS, Xiao XQ, and Clegg DJ 2010. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. International journal of obesity (2005) 34: 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stubbins RE, Holcomb VB, Hong J, and Nunez NP 2012. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. European journal of nutrition 51: 861–870. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan EL, Daniels AJ, Koegler FH, and Cameron JL 2005. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obesity research 13: 2072–2080. [DOI] [PubMed] [Google Scholar]
- 19.Lainez NM, Jonak CR, Nair MG, Ethell IM, Wilson EH, Carson MJ, and Coss D 2018. Diet-Induced Obesity Elicits Macrophage Infiltration and Reduction in Spine Density in the Hypothalami of Male but Not Female Mice. Frontiers in immunology 9: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lainez NM, and Coss D 2019. Leukemia Inhibitory Factor Represses GnRH Gene Expression via cFOS during Inflammation in Male Mice. Neuroendocrinology 108: 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubbels Bupp MR 2015. Sex, the aging immune system, and chronic disease. Cellular immunology 294: 102–110. [DOI] [PubMed] [Google Scholar]
- 22.Gal-Oz ST, Maier B, Yoshida H, Seddu K, Elbaz N, Czysz C, Zuk O, Stranger BE, Ner-Gaon H, and Shay T 2019. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun 10: 4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer K, Maley N, Mergian T, DelProposto J, Cho KW, Zamarron BF, Martinez-Santibanez G, Geletka L, Muir L, Wachowiak P, Demirjian C, and Lumeng CN 2015. Differences in Hematopoietic Stem Cells Contribute to Sexually Dimorphic Inflammatory Responses to High Fat Diet-induced Obesity. The Journal of biological chemistry 290: 13250–13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks BW, Sallam T, Mehrabian M, Psychogios N, Hui ST, Norheim F, Castellani LW, Rau CD, Pan C, Phun J, Zhou Z, Yang WP, Neuhaus I, Gargalovic PS, Kirchgessner TG, Graham M, Lee R, Tontonoz P, Gerszten RE, Hevener AL, and Lusis AJ 2015. Genetic architecture of insulin resistance in the mouse. Cell metabolism 21: 334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborn O, and Olefsky JM 2012. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine 18: 363–374. [DOI] [PubMed] [Google Scholar]
- 26.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, and Karin M 2007. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell metabolism 6: 386–397. [DOI] [PubMed] [Google Scholar]
- 27.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, and Lazar MA 2009. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. The Journal of clinical investigation 119: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, and Spiegelman BM 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation 95: 2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daemen S, and Schilling JD 2020. The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity. Frontiers in immunology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lainez NM, and Coss D 2019. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 160: 2719–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, and Schwartz MW 2012. Obesity is associated with hypothalamic injury in rodents and humans. The Journal of clinical investigation 122: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, Gurley C, Simpson P, McGehee RE Jr., Kern PA, and Peterson CA 2009. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 296: E1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Zhao D, Cui Y, Lu S, Gao D, and Liu J 2019. Proinflammatory macrophages impair skeletal muscle differentiation in obesity through secretion of tumor necrosis factor-alpha via sustained activation of p38 mitogen-activated protein kinase. J Cell Physiol 234: 2566–2580. [DOI] [PubMed] [Google Scholar]
- 34.Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z, Shah SV, Zhang S, Zhang L, Zhuang X, Michalek S, Grizzle WE, and Zhang HG 2009. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology (Baltimore, Md.) 50: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruen ML, Hao M, Piston DW, and Hasty AH 2007. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. American Journal of Physiology-Cell Physiology 293: C1481–C1488. [DOI] [PubMed] [Google Scholar]
- 36.Gschwandtner M, Derler R, and Midwood KS 2019. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Frontiers in immunology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K.-i., Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, and Kasuga M 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation 116: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan JL, Marshall MA, C. M. C, Harmon DB, Garmey JC, Oldham SN, Hallowell P, and McNamara CA 2015. Adipocyte progenitor cells initiate monocyte chemoattractant protein-1-mediated macrophage accumulation in visceral adipose tissue. Molecular metabolism 4: 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keophiphath M, Rouault C, Divoux A, Clement K, and Lacasa D 2010. CCL5 promotes macrophage recruitment and survival in human adipose tissue. Arteriosclerosis, thrombosis, and vascular biology 30: 39–45. [DOI] [PubMed] [Google Scholar]
- 40.Roh Y-S, and Seki E 2018. Chemokines and Chemokine Receptors in the Development of NAFLD. In Obesity, Fatty Liver and Liver Cancer. Yu J, ed. Springer Singapore, Singapore. 45–53. [DOI] [PubMed] [Google Scholar]
- 41.Collins S, Martin TL, Surwit RS, and Robidoux J 2004. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiology & behavior 81: 243–248. [DOI] [PubMed] [Google Scholar]
- 42.Wang C-Y, and Liao JK 2012. A Mouse Model of Diet-Induced Obesity and Insulin Resistance. Methods in molecular biology (Clifton, N.J.) 821: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acedo SC, Gambero S, Cunha FG, Lorand-Metze I, and Gambero A 2013. Participation of leptin in the determination of the macrophage phenotype: an additional role in adipocyte and macrophage crosstalk. In vitro cellular & developmental biology. Animal 49: 473–478. [DOI] [PubMed] [Google Scholar]
- 44.Mancuso P, Curtis JL, Freeman CM, Peters-Golden M, Weinberg JB, and Myers MG Jr. 2018. Ablation of the leptin receptor in myeloid cells impairs pulmonary clearance of Streptococcus pneumoniae and alveolar macrophage bactericidal function. American journal of physiology. Lung cellular and molecular physiology 315: L78–l86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer J, Gutièrrez S, Ganesan R, Calabrese C, Ranjan R, Cildir G, Hos NJ, Rybniker J, Wolke M, Fries JWU, Tergaonkar V, Plum G, Antebi A, and Robinson N 2019. Leptin signaling impairs macrophage defenses against Salmonella Typhimurium. Proceedings of the National Academy of Sciences of the United States of America 116: 16551–16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amend SR, Valkenburg KC, and Pienta KJ 2016. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. Journal of visualized experiments : JoVE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akita D, Kano K, Saito-Tamura Y, Mashimo T, Sato-Shionome M, Tsurumachi N, Yamanaka K, Kaneko T, Toriumi T, Arai Y, Tsukimura N, Matsumoto T, Ishigami T, Isokawa K, and Honda M 2016. Use of Rat Mature Adipocyte-Derived Dedifferentiated Fat Cells as a Cell Source for Periodontal Tissue Regeneration. Frontiers in physiology 7: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang L, and Wang Z-Y 2010. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. J Cell Mol Med 14: 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karam M, Bièche I, Legay C, Vacher S, Auclair C, and Ricort J-M 2014. Protein kinase D1 regulates ERα-positive breast cancer cell growth response to 17β-estradiol and contributes to poor prognosis in patients. J Cell Mol Med 18: 2536–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, and Kasuga M 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation 116: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura I, Ichimura A, Ohue-Kitano R, and Igarashi M 2020. Free Fatty Acid Receptors in Health and Disease. Physiological reviews 100: 171–210. [DOI] [PubMed] [Google Scholar]
- 52.Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, and Hevener AL 2011. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proceedings of the National Academy of Sciences of the United States of America 108: 16457–16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy AJ, Guyre PM, Wira CR, and Pioli PA 2009. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS One 4: e5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos-Alvarez J, Goberna R, and Sánchez-Margalet V 1999. Human leptin stimulates proliferation and activation of human circulating monocytes. Cellular immunology 194: 6–11. [DOI] [PubMed] [Google Scholar]
- 55.Baggiolini M 1998. Chemokines and leukocyte traffic. Nature 392: 565–568. [DOI] [PubMed] [Google Scholar]
- 56.Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, Kobayashi Y, Nitta N, Yasuda K, Hirata Y, Kuziel WA, Takeya M, Kanegasaki S, Kamei Y, and Ogawa Y 2008. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. The Journal of biological chemistry 283: 35715–35723. [DOI] [PubMed] [Google Scholar]
- 57.Inouye KE, Shi H, Howard JK, Daly CH, Lord GM, Rollins BJ, and Flier JS 2007. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes 56: 2242–2250. [DOI] [PubMed] [Google Scholar]
- 58.Hollingsworth JW, Li Z, Brass DM, Garantziotis S, Timberlake SH, Kim A, Hossain I, Savani RC, and Schwartz DA 2007. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. American journal of respiratory cell and molecular biology 37: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macanas-Pirard P, Quezada T, Navarrete L, Broekhuizen R, Leisewitz A, Nervi B, and Ramirez PA 2017. The CCL2/CCR2 Axis Affects Transmigration and Proliferation but Not Resistance to Chemotherapy of Acute Myeloid Leukemia Cells. PLoS One 12: e0168888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bosshart H, and Heinzelmann M 2016. THP-1 cells as a model for human monocytes. Ann Transl Med 4: 438–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGonigle TA, Dwyer AR, Greenland EL, Scott NM, Carter KW, Keane KN, Newsholme P, Goodridge HS, Pixley FJ, and Hart PH 2018. Reticulon-1 and Reduced Migration toward Chemoattractants by Macrophages Differentiated from the Bone Marrow of Ultraviolet-Irradiated and Ultraviolet-Chimeric Mice. The Journal of Immunology 200: 260–270. [DOI] [PubMed] [Google Scholar]
- 62.Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, Takamura T, Yamamoto H, Miyamoto K, Ginsberg HN, Mukaida N, Kaneko S, and Ota T 2012. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 61: 1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopasov AE, Blokhin SN, Volkova EN, and Morozov SG 2019. Chemokine Expression in Neutrophils and Subcutaneous Adipose Tissue Cells Obtained during Abdominoplasty from Patients with Obesity and Normal Body Weight. Bulletin of experimental biology and medicine 167: 728–731. [DOI] [PubMed] [Google Scholar]
- 64.Talukdar S, Olefsky JM, and Osborn O 2011. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends in pharmacological sciences 32: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, and Olefsky JM 2010. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pærregaard SI, Agerholm M, Serup AK, Ma T, Kiens B, Madsen L, Kristiansen K, and Jensen BA 2016. FFAR4 (GPR120) Signaling Is Not Required for Anti-Inflammatory and Insulin-Sensitizing Effects of Omega-3 Fatty Acids. Mediators of inflammation 2016: 1536047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez-Pacheco F, Gutierrez-Repiso C, Garcia-Serrano S, Alaminos-Castillo MA, Ho-Plagaro A, Valdes S, Garcia-Arnes J, Gonzalo M, Andrade RJ, Moreno-Ruiz FJ, Rodriguez-Cañete A, Martinez-Ferriz A, and Garcia-Fuentes E 2017. The pro-/anti-inflammatory effects of different fatty acids on visceral adipocytes are partially mediated by GPR120. European journal of nutrition 56: 1743–1752. [DOI] [PubMed] [Google Scholar]
- 68.Diebel ME, Diebel LN, and Liberati DM 2016. Gender dimorphism in adipose tissue response to stress conditions: A plausible mechanism to explain the conflicting data regarding trauma and obesity. The journal of trauma and acute care surgery 81: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 69.Bouman A, Heineman MJ, and Faas MM 2005. Sex hormones and the immune response in humans. Human reproduction update 11: 411–423. [DOI] [PubMed] [Google Scholar]
- 70.Bolego C, Cignarella A, Staels B, and Chinetti-Gbaguidi G 2013. Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arteriosclerosis, thrombosis, and vascular biology 33: 1127–1134. [DOI] [PubMed] [Google Scholar]
- 71.Pepe G, Braga D, Renzi TA, Villa A, Bolego C, D’Avila F, Barlassina C, Maggi A, Locati M, and Vegeto E 2017. Self-renewal and phenotypic conversion are the main physiological responses of macrophages to the endogenous estrogen surge. Scientific reports 7: 44270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trenti A, Tedesco S, Boscaro C, Trevisi L, Bolego C, and Cignarella A 2018. Estrogen, Angiogenesis, Immunity and Cell Metabolism: Solving the Puzzle. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Straub RH 2007. The Complex Role of Estrogens in Inflammation. Endocrine reviews 28: 521–574. [DOI] [PubMed] [Google Scholar]
- 74.Soucy G, Boivin G, Labrie F, and Rivest S 2005. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. Journal of immunology 174: 6391–6398. [DOI] [PubMed] [Google Scholar]
- 75.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, Guéry JC, Bayard F, Arnal JF, and Gourdy P 2008. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. Journal of immunology 180: 7980–7988. [DOI] [PubMed] [Google Scholar]
- 76.Villa A, Rizzi N, Vegeto E, Ciana P, and Maggi A 2015. Estrogen accelerates the resolution of inflammation in macrophagic cells. Scientific reports 5: 15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, and Tobe K 2009. Regulatory Mechanisms for Adipose Tissue M1 and M2 Macrophages in Diet-Induced Obese Mice. Diabetes 58: 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conde J, Scotece M, Gómez R, Gómez-Reino JJ, Lago F, and Gualillo O 2010. At the crossroad between immunity and metabolism: focus on leptin. Expert Rev Clin Immunol 6: 801–808. [DOI] [PubMed] [Google Scholar]
- 79.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, and Chen H 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation 112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutierrez DA, and Hasty AH 2012. Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity. J Endocrinol 212: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dib LH, Ortega MT, Fleming SD, Chapes SK, and Melgarejo T 2014. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology 155: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, and Tada K 1980. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). International journal of cancer. Journal international du cancer 26: 171–176. [DOI] [PubMed] [Google Scholar]
- 83.Vereyken EJF, Heijnen PDAM, Baron W, de Vries EHE, Dijkstra CD, and Teunissen CE 2011. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. Journal of neuroinflammation 8: 58-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bisgaard LS, Mogensen CK, Rosendahl A, Cucak H, Nielsen LB, Rasmussen SE, and Pedersen TX 2016. Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression – implications for atherosclerosis research. Scientific reports 6: 35234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C, Yu X, Cao Q, Wang Y, Zheng G, Tan TK, Zhao H, Zhao Y, Wang Y, and Harris DCH 2013. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunology 14: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gordon S, and Plüddemann A 2017. Tissue macrophages: heterogeneity and functions. BMC Biology 15: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]


