Abstract
Purpose:
The risk factors for future infertility in adolescents with varicocele are controversial and little is known about the association between hormone levels and semen parameters. Semen analysis is likely the closest marker to fertility but may be difficult to obtain in some boys secondary to personal, familial, or religious reasons. Identifying other clinical surrogates for abnormal semen parameters may offer an alternative for assessing varicocele severity in these boys. Here, we hypothesize that hormone levels and TTV are predictive of abnormal TMSC.
Methods:
We retrospectively reviewed Tanner 5 boys with palpable left varicoceles who underwent a semen analysis (SA) and had serum hormone labs (LH, FSH, inhibinB, AMH, and/or total testosterone) within a 6-month period. TTV was also calculated. Abnormal TMSC was defined as <9 million sperm/ejaculate.
Results:
78 boys (median age 17.2yrs (IQR=16.5–18.0)) were included. LH, AMH, and total testosterone were not correlated with any SA parameter. There was a negative correlation between FSH and TMSC (ρ= −0.35, p=0.004) and positive correlation between inhibin B and TMSC (ρ = 0.50, p<0.001). TTV was significantly positively correlated with TMSC (ρ = 0.35, p=0.01). ROC analyses revealed an optimal FSH cutoff of 2.9, an optimal inhibinB cutoff of 204, and an optimal TTV cutoff of 34.4cc to predict abnormal TMSC.
Conclusions:
TMSC is inversely associated with FSH levels and directly associated with inhibin B levels and TTV. Optimized cutoffs for serum FSH, inhibinB, and TTV may prove to be a reasonable surrogates for TMSC in boys who defer SA for personal or religious/cultural reasons.
Keywords: varicocele, semen analysis, hormones, FSH, inhibin B, total motile sperm count
Introduction
Varicoceles affect 15% of men in the general population and an even higher percentage of infertile men. In pediatric urology practice, varicoceles are found varicoceles in 14–29% of adolescent boys at the end of puberty (1–3). The risk factors for future infertility in adolescents with varicocele and the indications for treatment of varicoceles during adolescence are controversial. Over the past several decades, various clinical markers have been proposed as indications for varicocele severity or treatment. Semen analysis is likely the closest marker to fertility available in adolescent boys in the absence of attempting paternity but even abnormal semen parameters do not necessarily indicate future fertility issues. Furthermore, semen analysis may be difficult to obtain in some boys secondary to personal, familial, or religious reasons. Additionally, physician comfort with asking for a SA and parent/patient knowledge about SA may present additional barriers to obtaining SA (4). Identifying other clinical surrogates for abnormal semen parameters may offer an alternative for assessing varicocele severity in boys unable to provide semen samples. In 2007, Diamond and colleagues found significant correlations between %asymmetry and semen quality (5). Additionally, TTV and TVdiff have been found in other studies to be associated with TMSC (6,7).
Despite these reports, the association between hormone levels and semen parameters in adolescent boys still remains largely unknown. Guarino and associates, in one of the only studies in adolescent boys with varicoceles, noted a significantly increased stimulated FSH in response to GnRH, as well as basal levels of LH and FSH, in boys with varicoceles and abnormal semen parameters compared to boys with varicoceles and normal semen parameters (8). Other studies of adults with varicoceles have analyzed hormone levels (testosterone, FSH, LH, and prolactin) in combination with other factors to determine risk factors for abnormal SA parameters or as markers for improvement in semen quality after varicocelectomy (9–12). Here, we investigate if any such relationship exists in a cohort of post-pubertal boys with varicoceles. We hypothesized that hormone levels (specifically FSH, LH, and inhibin-B), TTV and TVdiff would be associated with abnormal TMSC.
Materials and Methods
We retrospectively reviewed our institutional review board approved database of varicocele patients seen at a single institution from 1/2014–6/2017. All Tanner 5 boys (between 15 and 19 years old) with palpable left varicoceles who underwent a SA and had serum hormone labs obtained within a 6-month period of the SA were included. We excluded any boy with a subclinical left varicocele or bilateral varicoceles. If more than one SA result was available within the 6 month window, the SA with the best results was used. Starting prior to 2014, the Division of Pediatric Urology at the Children’s Hospital of Philadelphia followed a departmental protocol for evaluation and follow up of all patients with varicoceles. As part of this protocol, all boys with palpable left varicoceles who are at least 15 years old and Tanner 5 are recommended to obtain serum hormone studies including LH, FSH, inhibin B, AMH, and total testosterone at time of SA. In patients with scrotal ultrasound or orchidometer measurements within 6 months of the SA, TTV, TVdiff and %asymmetry were calculated. For %asymmetry the following equation was use: [(Right Testis Volume – Left Testis Volume)/Total Testicular Volume]. When both measurements were obtained, ultrasound measurements were used for analysis.
Serum hormone levels, TTV, TVdiff and %asymmetry were correlated with semen parameters including total volume, percent motility, and TMSC using Spearman’s rank correlation. The Mann-Whitney U test was used to compare continuous variables between groups. ROC analysis was used to analysis the ability of hormone levels and/or testicular volume measurements to predict abnormal TMSC (defined as <9 million sperm/ejaculate based on WHO 2010 criteria for minimal reference ranges for total volume [1.5mL], sperm concentration [15million/mL] and percent motility [40%])(13,14). To determine optimal cutoffs for ROC curves, the Youden index was calculated. The Youden index (J) is a statistical method that maximizes the sum of the sensitivity and specificity when determining the optimal cut-off of a diagnostic test (15–17). All statistics were performed with STATA 14.2 (StataCorp, College Station,TX).
Results
In total, 117 Tanner 5 boys with palpable left varicoceles met eligibility criteria during the study period. Of this group, 25 did not obtain a SA. Ninety-two boys underwent SA and of this group 78 boys also underwent serum hormone studies within 6 months of the SA. Baseline characteristics for all 78 boys are shown in Table 1. Median patient age was 17.2 years (interquartile interval [IQR]=16.5–17.9 years) and median TMSC was 14.7 (IQR=3.7–36.2). In total, 29 of the 78 boys (37%) had TMSC less than 9 million/ejaculate and met criteria for abnormal semen analysis. LH and AMH were not significantly correlated with any SA parameter. FSH was weakly negatively correlated with both total sperm count (ρ[rho]=−0.38, p=0.005) and TMSC (ρ=−0.35, p=0.004). Total testosterone level was only negatively correlated with percent sperm motility although the relationship was weak (ρ=−0.29, p=0.01). The strongest positive correlations were noted between inhibin B levels and SA parameters. Inhibin B was moderately correlated with total sperm count (ρ=0.52, p<0.001) and TMSC (ρ=0.50, p<0.001).
Table 1.
Baseline cohort characteristics
| Number of Patients | 78 | |
| Median age at SA (IQR) [years] | 17.2 (16.5 – 17.9) | |
| No. Varicocele grade (%) | ||
| I | 8 (10) | |
| II | 27 (34) | |
| III | 41 (53) | |
| Unknown | 2 (3) | |
| Median testis volume (IQR) (n = 71) | ||
| Right [cc] | 20.0 (15.0 – 25.0) | |
| Left [cc] | 17.2 (13.0 – 22.0) | |
| TTV [cc] | 38.3 (29.6 – 49.7) | |
| Median Semen Parameters (IQR) | 2010 WHO 5th Percentile | |
| Volume [mL] | 1.8 (1 – 2.7) | 1.5 mL |
| Concentration [million/mL] | 20 (10 – 38) | 15 million/mL |
| % total motility | 51 (41 – 60) | 40% |
| TMSC [million/ejaculate] | 14.7 (3.7 – 36.2) | 9 million/ejaculate |
| No. <9 million/ejaculate (%) | 29 (37) | |
| Median serum hormone levels (IQR) | Lab range (male, Tanner 5) | |
| LH [mIU/mL] | 3.8 (2.7 – 4.9) | 1.7 – 8.6 mIU/mL |
| FSH [mIU/mL] | 3.6 (2.2 – 5) | 1.5 – 12.4 mIU/mL |
| Inhibin B [pg/mL] | 188 (142 – 234) | 66.9 – 300 pg/mL |
| AMH [ng/mL] | 8.1 (4.8 – 10.6) | 0.11 – 13.07 ng/mL |
| Total Testosterone [ng/dL] | 524 (386 – 685) | 188 – 882 ng/dL |
In the 71 boys who also had testicular volume measurements, TTV was significantly positively correlated with TMSC (ρ=0.35, p=0.01). No other testicular volume measurements including TVdiff and %asymmetry were correlated with any SA parameters. In addition, no testicular volume parameter was significantly correlated with any serum hormone studies.
When comparing boys with TMSC<9 to boys with TMSC ≥9, serum FSH and inhibin B levels were the only hormone levels that were significantly different between the groups (Table 2). Median TTV was also significantly different between TMSC<9 boys and TMSC ≥9 boys (30.6cc vs. 41.0cc, respectively, p<0.01). All other serum labs including LH, AMH, and total testosterone, as well as varicocele grade were similar between the two groups.
Table 2.
Median TTV and hormone levels based on TMSC group. P-values calculated using Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables (ie, varicocele grade).
| TMSC <9 (Abnormal) | TMSC ≥9 (Normal) | p-value | |
|---|---|---|---|
| Median TTV (IQR) [cc] | 30.6 (25.6 – 41.3) | 41.0 (35.1 – 50.0) | <0.01* |
| Grade III varicocele | 48.3% | 55.6% | 0.79 |
| Median serum hormone levels (IQR) | |||
| LH (mIU/mL) | 3.7 (2.8 – 4.6) | 3.8 (2.7 – 5.3) | 0.59 |
| FSH (mIU/mL) | 4.4 (3 – 6.5) | 2.6 (1.9 – 4.4) | <0.01* |
| Inhibin B (pg/mL) | 163 (103 – 196) | 211 (158 – 264) | <0.01* |
| AMH (ng/mL) | 8.7 (6.1 – 13.5) | 8.1 (4.7 – 10.2) | 0.41 |
| Total Testosterone (ng/dL) | 514 (370 – 719) | 523 (407 – 683) | 0.99 |
Given these findings, we next generated ROC curves to determine optimal cutoffs for FSH, inhibin B, and TTV, separately, in predicting abnormal TMSC. A TMSC <9 million sperm/ejaculate was used as a cutoff for abnormal TMSC as described in the methods. ROC analysis revealed an optimal FSH cutoff of 2.9 to predict abnormal TMSC (AUC=0.69, 95%CI=0.57–0.82). With this cutoff, an FSH >2.9 had 60% specificity and 78% sensitivity, in predicting an abnormal TMSC (Figure 1a). Similar analyses, revealed an optimal cutoff of 204 for inhibin B (AUC=0.76, 95%CI=0.65–0.87, sensitivity 60% and specificity 83%, figure 1b) and a cutoff of 34.4 cc for TTV (AUC=0.70, 95%CI=0.58–0.83, sensitivity 79% and specificity 61%, figure 1c).
Figure 1.


ROC curves. A) ROC curve of FSH as a predictor of abnormal TMSC (defined as <9 million sperm/ejaculate). The AUC was 0.69 (95% CI = 0.57–0.82), and Youden’s index analysis determined an ideal cutoff of 2.9 as a predictor of abnormal TMSC. B) ROC curve of inhibin B as a predictor of abnormal TMSC (defined as <9 million sperm/ejaculate). The AUC was 0.76 (95% CI = 0.65–0.87), and Youden’s index analysis determined an ideal cutoff of 204 as a predictor of abnormal TMSC. C) ROC curve of TTV as a predictor of abnormal TMSC (defined as <9 million sperm/ejaculate). The AUC was 0.70 (95% CI = 0.58–0.83), and Youden’s index analysis determined an ideal cutoff of 34.4 as a predictor of abnormal TMSC.
Table 3 shows the optimized cut-off values for each risk factor, as well as the percentage of patients in the cohort with abnormal or normal TMSC based on the presence of none, 1, 2, or 3 of the factors at time of obtaining SA. For patients with none of the risk factors, all 18 (100%) had normal TMSC, while for patients with all three of the risk factors, only 31% had normal TMSC.
Table 3.
Final risk scoring model using all three cutoffs to predict abnormal TMSC (defined as <9 million sperm/ejaculate) and outcomes based on composite score for 71 patients in current study with all three parameters available.
| Factor in Risk Score | Cut-off (1 point for each) | |
|---|---|---|
| FSH | > 2.9 mIU/ml | |
| Inhibin B | < 220 pg/mL | |
| TTV | < 34.4 cc | |
| Composite Score | Patients with normal TMSC (% of patients with that score) | Patients with abnormal TMSC (% of patients with that score) |
| 0 | 18 (100%) | 0 |
| 1 | 13 (68%) | 6 (32%) |
| 2 | 7 (33%) | 14 (67%) |
| 3 | 4 (31%) | 9 (69%) |
Discussion
The ultimate goal of varicocele treatment is preserving or restoring fertility. In adolescents with varicocele there is controversy over what clinical parameters or findings will be associated with future infertility. The AUA/ASRM “Best Practice Policy” from 2001 and the more recent “Report on varicocele and infertility” from the ASRM and the Society for Male Reproduction and Urology, provide two indications for varicocele treatment in adolescent boys – objective evidence of reduced ipsilateral testicular size or semen abnormality (18, 19). Neither indication provides a cutoff value or definition of what is meant by “reduced…size” or semen “abnormality.”
Semen analysis is likely the closest marker to fertility available in adolescent boys in the absence of attempting paternity but even abnormal semen parameters do not necessarily indicate future fertility issues. Based on the WHO guideline reference ranges for semen parameters can be determined but the ideal values in adolescents is unclear (13). Importantly, Chu and colleagues recently showed that semen parameters can normalize over time in up to 47% of Tanner V boys with initial abnormal parameters, without surgical repair (20).
Despite their importance in evaluating post-pubertal boys with varicoceles, SAs are not always obtained. Difficulty in obtaining SA may be secondary to personal, familial, or religious reasons and even secondary to physician barriers (4). Furthermore, the process of SA acquisition involves time, special facilities, and possible costs to the patient/family. For these reasons, surrogate markers for abnormal semen parameters may obviate the need for SA (or repeat analyses) and provide an alternative to patients unable or unwilling to provide samples.
Testicular volumes can be obtained with orchidometer during physical examination or ultrasonography, although some studies have shown variability with each technique and likely higher accuracy with ultrasound measurements (21–23). In 2007, Diamond and colleagues found significant correlations between %asymmetry and semen quality (5). Boys with greater than 10% asymmetry had significantly lower sperm concentration and TMSC than boys with less than 10% and these decreases were worse for boys with >20% asymmetry (5). Additionally, TTV and TVdiff have been found in other studies to be associated with TMSC (6, 7).
Hormone dysfunction in and of itself has been long considered as a potential marker for varicocele severity and has been proposed as an indicator for surgical correction by some. Kass and associates were the first to perform GnRH stimulation tests in boys with varicoceles and they reported exaggerated LH and FSH responses after stimulation in 31% of boys aged 10–19 years old with palpable varicoceles (24). Baseline LH, FSH and testosterone levels, however, have not been shown to be consistently different between boys with varicoceles and age- or Tanner-matched controls (25, 26). Studies in adult infertile men with varicoceles and hypogonadism show significant improvements in serum testosterone after surgical repair (27). Interestingly, Guarino and colleagues reported significantly increased stimulated FSH in response to GnRH, as well as basal levels of LH and FSH, in boys with varicoceles and abnormal semen parameters compared to boys with varicoceles and normal semen parameters (8).
In addition to FSH, LH, testosterone, and the GnRH stimulation test, serum inhibin B levels have been suggested as an alternate to the use of basal and stimulated gonadotropin levels. Inhibin B is secreted by the Seroli cells and is involved in negative feedback control of FSH production by the pituitary. One study found no differences in basal or GnRH stimulated FSH and LH, or basal testosterone between pubertal boys (Tanner 4 or 5) with varicoceles and age-matched controls, but did find a significant reduction in inhibin B levels in the boys with varicoceles compared to controls (26). This study also showed a significant correlation between inhibin B levels and testes volume, suggesting that inhibin B level may be an early marker for Sertoli cell damage.
Several studies have analyzed hormone dysfunction in adult men with varicoceles and correlated these findings to semen parameters (9–12). Damsgaard and associates performed a cross-sectional, multi-institutional, multi-national study of 7,035 European men (all >18 years old) and noted that worsening severity of varicocele grade was associated with higher serum FSH levels, lower serum inhibin B levels, and higher serum LH levels (10). Interestingly, the authors did not find any significant difference between serum testosterone and free testosterone between men with and without varicocele (10). In another study in adults (mean age 35 years) undergoing varicocelectomy for infertility, serum inhibin B levels increased post-varicocelectomy and were associated with improvement in semen parameters (11). While these studies in adult men are compelling, the generalization of their findings to adolescent varicocele patients not referred for fertility concerns is unclear and the lack of studies in adolescent boys with varicoceles comparing hormone levels and semen parameters was one of the primary driving forces behind this present study.
In this study, we found several significant correlations between hormone levels and semen parameters. Although most correlations were weak (FSH, TTV), inhibin B had a moderate positive correlation with TMSC. Total testosterone was only found to have a weak negative correlation with percent sperm motility. Given that TMSC is used most commonly in the literature and is thought by many to represent the most important semen parameter, we focused on this parameter in our ROC analyses and for designing a risk model. Using the WHO 2010 criteria and recent publications as guidance, an abnormal TMSC was defined as <9 million sperm/ejaculate (13, 14). With this definition, ROC curves were generated for FSH, inhibin B, and TTV.
In our analyses, the optimal cutoffs were FSH >2.9 mIU/mL, inhibin B <204 pg/mL, and TTV <34.4 cc. Interestingly, when we used these cutoffs to evaluate our patients, all 18 boys (100%) with none of these abnormal factors had normal TMSC, while for boys with all three abnormal factors, 31% had normal TMSC and 69% had abnormal TMSC.
We believe that these cutoffs for FSH, inhibin B, and TTV in assessing risk for abnormal TMSC may be helpful in situations in which semen analyses cannot be obtained in Tanner 5 boys with varicoceles; with the understanding that none of the factors are able determine all normal or abnormal TMSC patients. Furthermore, we are not proposing the elimination of semen analysis in the evaluation of the adolescent varicocele, but rather suggesting that serum levels of FSH and inhibin B and TTV, may serve as possible markers for abnormal TMSC and may play a role in the follow up of patients with abnormal semen parameters. Understanding that there are still limitations to these markers and cutoffs, if there are no cultural, religious or personal reasons to not obtain a SA, we continue to recommend obtaining a SA in all Tanner 5 boys who are over 15 years of age with palpable left varicoceles.
This study has several limitations including its retrospective nature and small sample size. Our results are only generalizable to Tanner 5 boys who are over 15 years of age with palpable varicoceles, and should not be used for boys at other stages of puberty given the variability in hormone and semen parameters that can occur during pubertal development. Furthermore, hormone levels at time of semen analysis were included but we did not analyze repeat hormone levels. Another limitation of our study is that we combined orchidometer and ultrasound measurements for testicular volume. It has been our practice to minimize use of ultrasound and not to obtain repeat ultrasounds annually; therefore, combining these measurements is a truer reflection of our clinical practice but may introduce measurement bias. Lastly, use of a single semen analysis may lead to incorrect conclusions about semen parameters and while it is our practice to repeat all abnormal semen analyzes, this was not done in all cases and may limit the generalizability of the results.
Conclusion
TMSC, which is thought to be the most important semen parameter related to fertility, is inversely correlated with serum FSH levels and directly correlated with inhibin B levels and TTV. While semen analysis remains the closest surrogate to future fertility in adolescent boys with varicoceles, serum FSH, serum inhibin B, and TTV may prove to be reasonable surrogates for TMSC in boys who defer SA for personal or religious/cultural reasons.
Figure 2.
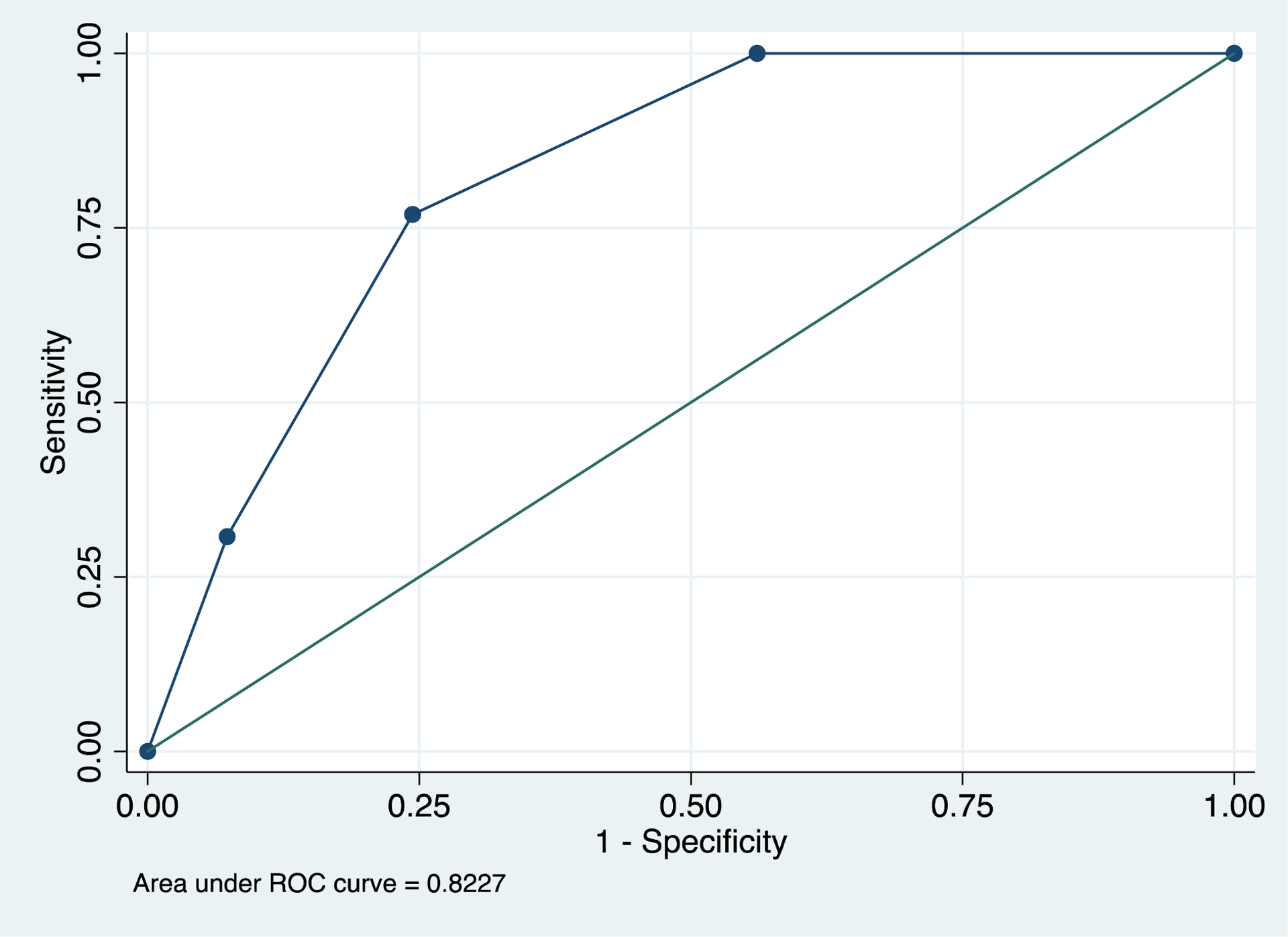
ROC curve of final model using all three cutoffs (FSH > 2.9 mIU/ml, inhibin B <204 pg/mL, and TTV < 34.4 cc) to predict abnormal TMSC (defined as <9 million sperm/ejaculate). The AUC was 0.82, and Youden’s index analysis determined an ideal cutoff of 1.5 points (with 77% sensitivity and 76% specificity).
Source of Funding:
This work was supported in part by the 2016-2017 Urology Care Foundation Research Scholar Award Program (JPV), the Urology Care Foundation Rising Stars in Urology Research Award Program and Frank and Marion Hinman Urology Research Fund (JPV), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number K08DK120934 (JPV), and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001879 (JPV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: None
References:
- 1.Akbay E, Cayan S, Doruk E, Duce MN, & Bozlu M (2000) The prevalence of varicocele and varicocele-related testicular atrophy in Turkish children and adolescents. BJU Int 86(4):490–493. [DOI] [PubMed] [Google Scholar]
- 2.Paduch DA & Skoog SJ (2004) Diagnosis, evaluation and treatment of adolescent varicocele. ScientificWorldJournal 4 Suppl 1:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zampieri N & Cervellione RM (2008) Varicocele in adolescents: a 6-year longitudinal and followup observational study. J Urol 180(4 Suppl):1653–1656; discussion 1656. [DOI] [PubMed] [Google Scholar]
- 4.Fine RG, Gitlin J, Reda EF, Palmer LS (2016) Barriers to use of semen analysis in the adolescent with a varicocele: Survey of patient, parental, and practitioner attitudes. J Pediatr Urol 12(1):41.e1–41.e416. [DOI] [PubMed] [Google Scholar]
- 5.Diamond DA, et al. (2007) Relationship of varicocele grade and testicular hypotrophy to semen parameters in adolescents. J Urol 178(4 Pt 2):1584–1588. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz MP, et al. (2015) Semen parameters in adolescents with varicocele: association with testis volume differential and total testis volume. J Urol 193(5 Suppl):1843–1847. [DOI] [PubMed] [Google Scholar]
- 7.Christman MS, Zderic SA, Canning DA, & Kolon TF (2014) Active surveillance of the adolescent with varicocele: predicting semen outcomes from ultrasound. J Urol 191(5):1401–1406. [DOI] [PubMed] [Google Scholar]
- 8.Guarino N, Tadini B, & Bianchi M (2003) The adolescent varicocele: the crucial role of hormonal tests in selecting patients with testicular dysfunction. J Pediatr Surg 38(1):120–123; discussion 120–123. [DOI] [PubMed] [Google Scholar]
- 9.Chen SS and Chen LK (2012) Risk factors for progressive deterioration of semen quality in patients with varicocele. Urology 79(1):128–132. [DOI] [PubMed] [Google Scholar]
- 10.Damsgaard J, Joensen UN, Carlsen E, et al. (2016) Varicocele Is Associated with Impaired Semen Quality and Reproductive Hormone Levels: A Study of 7035 Healthy Young Men from Six European Countries. Eur Urol 70(6):1019–1029. [DOI] [PubMed] [Google Scholar]
- 11.Pierik FH, Abdesselam SA, Vreeburg JT, Dohle GR, De Jong FH, Weber RF (2001) Increased serum inhibin B levels after varicocele treatment. Clin Endocrinol (Oxf) 54(6):775–780. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa M, Dobashia M, Yamasaki T, et al. (2001) Significance of serum inhibin B concentration for evaluating improvement in spermatogenesis after varicocelectomy. Hum Reprod 16(9): 1945–9. [DOI] [PubMed] [Google Scholar]
- 13.Cooper TG, et al. (2010) World Health Organization reference values for human semen characteristics. Human reproduction update 16(3):231–245. [DOI] [PubMed] [Google Scholar]
- 14.Samplaski MK et al. (2017) Varicolectomy to upgrade semen quality to allow couples to use less invasive forms of assisted reproductive technology. Fertil Steril 108(4): 609–612 [DOI] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35 [DOI] [PubMed] [Google Scholar]
- 16.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159(6):1638–1645 [DOI] [PubMed] [Google Scholar]
- 17.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. [DOI] [PubMed] [Google Scholar]
- 18.“An AUA Best Practice Policy and ASRM Practice Committee Report: Report on varicocele and infertility.” April 2001, accessed from: www.auanet.org
- 19.Practice Committee of the American Society for Reproductive Medicine; Society for Male Reproduction and Urology. Report on varicocele and infertility: a committee opinion. Fertil Steril. 2014;102(6):1556–1560. [DOI] [PubMed] [Google Scholar]
- 20.Chu DI, et al. (2017) The natural history of semen parameters in untreated asymptomatic adolescent varicocele patients: A retrospective cohort study. Journal of pediatric urology 13(1):77.e71–77.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh ML, Huang ST, Huang HC, Chen Y, & Hsu YC (2009) The reliability of ultrasonographic measurements for testicular volume assessment: comparison of three common formulas with true testicular volume. Asian journal of andrology 11(2):261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz MP, Migliozzi M, Rosoklija I, Zurakowski D, & Diamond DA (2015) Accuracy of orchidometry in boys with varicocele. Journal of pediatric urology 11(4):185.e181–185. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto H, et al. (2007) Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology 69(1):152–157. [DOI] [PubMed] [Google Scholar]
- 24.Kass EJ, Freitas JE, Salisz JA, & Steinert BW (1993) Pituitary gonadal dysfunction in adolescents with varicocele. Urology 42(2):179–181. [DOI] [PubMed] [Google Scholar]
- 25.Fideleff HL, et al. (2000) Controversies in the evolution of paediatric-adolescent varicocele: clinical, biochemical and histological studies. Eur J Endocrinol 143(6):775–781. [DOI] [PubMed] [Google Scholar]
- 26.Romeo C, et al. (2007) Altered serum inhibin b levels in adolescents with varicocele. J Pediatr Surg 42(2):390–394. [DOI] [PubMed] [Google Scholar]
- 27.Dabaja A, Wosnitzer M, & Goldstein M (2013) Varicocele and hypogonadism. Curr Urol Rep 14(4):309–314. [DOI] [PubMed] [Google Scholar]


