Abstract
Nuclear medicine plays an increasingly important role in the management neuroendocrine neoplasms (NEN). Somatostatin analogue (SSA)-based positron emission tomography/computed tomography (PET/CT) and peptide receptor radionuclide therapy (PRRT) have been used in clinical trials and approved by the European Medicines Agency and Food and Drug Administration.
EANM Focus 3 performed a multi-disciplinary Delphi process to deliver a balanced perspective on molecular imaging and radionuclide therapy in well-differentiated neuroendocrine tumours (NET). Several societies’ guidelines address NEN management, however, many issues are still debated, due to both the difficulty in acquiring strong clinical evidence in a rare and heterogeneous disease and the different availability of diagnostic and therapeutic options across countries.
EANM Focus 3 reached consensus on employing [68Ga]Ga-DOTA-SSA PET/CT with diagnostic CT or magnetic resonance imaging for unknown primary NET detection, metastatic NET, NET staging/restaging, suspected extra-adrenal pheochromocytoma/paraganglioma and suspected paraganglioma. Consensus was reached on employing [18F]FDG PET/CT in neuroendocrine carcinoma, G3 NET and in G1–2 NET with mismatched lesions (CT-positive/[68Ga]Ga-DOTA-SSA-negative). PRRT was recommended for second line treatment for gastrointestinal NET with [68Ga]Ga-DOTA-SSA uptake in all lesions, in G1/G2 NET at disease progression, and in a subset of G3 NET provided all lesions are positive at [18F]FDG and [68Ga]Ga-DOTA-SSA. PRRT rechallenge may be used for in patients with stable disease for at least one year after therapy completion.
An international consensus is not only a prelude to a more standardized management across countries but also serves as a guide for the direction to follow when designing new research studies.
Summary
Nuclear medicine imaging and therapies play an increasingly important role in the management of neuroendocrine neoplasms (NEN). EANM Focus 3 reached consensus on the use of [68Ga]Ga-DOTA-SSA in combination with diagnostic computed tomography or magnetic resonance imaging for diagnosis, including unknown primary detection, for staging, for restaging after surgery, following progression, and for known or suspected NET. Consensus was also reached on the use of [18F]FDG in NET G3 and for G1–2 NET with lesions negative on [68Ga]Ga-DOTA-SSA or with rapid progression. [68Ga]Ga-DOTA-SSA is also recommended for suspected extra-adrenal localization of pheochromocytoma/paraganglioma and suspected paraganglioma without evident secondary lesion on morphological imaging. Consensus also supported use of PRRT as second line therapy at first disease progression in all patients with G1–2 [68Ga]Ga-DOTA-SSA positive gastrointestinal NET and in a subset of patients with NET G3 (Ki67 >20%) provided all [18F]FDG positive lesions exhibit [68Ga]Ga-DOTA-SSA uptake. PRRT rechallenge was also supported for prior responders.
Introduction
Neuroendocrine neoplasms (NEN) represent a group of heterogeneous tumours that arise from the disseminated endocrine cell system primarily from gastro-entero-pancreatic (GEP) organs. NEN are classified according to their cells’ morphology and proliferation index (Ki67) as well-differentiated neuroendocrine tumours (NET), including G1 (Ki67≤ 2), G2 (Ki67 3–20%) and well differentiated G3 (Ki67>20%,), showing a more favourable behaviour as compared to poorly differentiated G3 and neuroendocrine carcinomas (NEC, small and large cells). Less common than GEP are bronchopulmonary tract tumours (20–25%), currently classified as typical and atypical carcinoid tumours. Most NET are non-functioning, while a minority present with symptoms related to hypersecretion of bioactive compounds. Delayed diagnosis is common due to asymptomatic presentation or non-specific symptoms. Although the past two decades witnessed both an increased incidence and prevalence of NEN along with a significant improvement in their management, many issues remain openly debated.1,2
NET share many diagnostic commonalities, since they are often hypervascular and > 80% over-express somatostatin receptor (SSTR) on their surface.3 This allows the use of SSTR imaging for staging of these tumours. Moreover, SSTR-imaging can help select patients for specific therapies targeting SSTR. Surgery, when feasible, is the mainstay of therapy for patients with non-metastatic NET, or those who are candidates for cytoreductive operations. Long-acting somatostatin analogues (SSA) including octreotide and lanreotide are first line medical therapy for most patients with advanced NETs. Second line treatments for NET, depending on the primary tumour site, include molecularly targeted therapies such as everolimus and sunitinib, chemotherapy, interferon-alpha, locoregional treatments including transarterial (chemo)embolization, selective internal radiation therapy, and peptide receptor radionuclide therapy (PRRT).4 PRRT, available since the early 1990s, has proved to be a major advance in the therapeutic management of NET based on accurate patient selection through SSTR imaging leading to a long median progression-free survival (PFS).5,6 However, the results obtained with PRRT in early clinical trials were difficult to translate into clinical routine, because they applied different PRRT protocols in terms of injected dose, treatment schedule, and radiopharmaceutical preparations. Patients were often offered PRRT in advanced disease stages after progression on other lines of treatment.4 A randomized phase III trial on standard dose PRRT with 177Lu-DOTATATE in patients with midgut NET recently led to its approval by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA).7 Although the NETTER-1 study included only patients with mid-gut tumours, the subsequent FDA/EMA approval was extended to include pancreatic NET.
Because some NET centres routinely use the most advanced diagnostic (eg, [68Ga]Ga-DOTA-SSA PET/CT) and therapeutic (eg, [177Lu]Lu-DOTA-TATE PRRT) options, and other centres have only recently implemented these techniques, there are differences in the clinical management of NET patients across countries. Many consensus expert panels such as the European Association for Nuclear Medicine (EANM), the European Neuroendocrine Tumour Society (ENETS), the European Society for Medical Oncology (ESMO), and the North American Neuroendocrine Tumour Society (NANETS) have published guidelines concerning molecular imaging and theranostics in NEN.4,8–16,17(p) However, the rarity and heterogeneous presentation of NEN, coupled with the different pace of availability of several procedures (mainly PET/CT with SSTR and PRRT) across countries strongly influenced the proposed diagnostic and therapeutic flow-charts and, therefore, the routine local management of NEN patients and the consequent growth of local expertise.18 Therefore, if on one side these efforts led to an increased detection, awareness and recognition of NEN and to a significant improvement in their management, on the other side many issues still remain openly debated.
Since nuclear medicine plays a central role in both NEN diagnosis and treatment, the EANM promoted an event to integrate nuclear medicine knowledge with other specialities’ expertise and with the voice of a patient advocate. EANM Focus 3 was held January 30 to February 1, 2020 in Athens, Greece, with the aim not to replicate guidelines, but to create a multidisciplinary environment of international NEN experts, recruited in close collaboration with ENETS, to address unresolved NEN management and theranostic issues to develop consensus statements to be applied in clinical practise worldwide.
Discussion on PRRT was not limited to indications and procedural aspects but also included treatment sequencing, patients’ selection, and criteria for response assessment. Although nuclear imaging plays a crucial role for assessing disease extent and patients’ selection for PRRT, imaging protocols are not standardized worldwide. Discussion involved the current controversial role of SSTR-scintigraphy using [111In]In-pentetreotide, which is approved and available in many countries but demonstrably inferior diagnostically compared to [68Ga]Ga-DOTA-SSA PET/CT.19–21 The role of [18F]FDG PET/CT in relation to other diagnostic modalities used in the evaluation of NEN was also discussed,9 and parallel discussions were held on the choice of radiopharmaceutical for nuclear imaging of pheochromocytoma/paraganglioma (PPGL), for which several radiotracers are currently available. Finally, novel and promising preparations (for both diagnosis and therapy), combination treatments, the role of dosimetry, and the future development of nuclear medicine procedures in NET were previewed.
Naturally, invited experts came from high volume centres, with extensive expertise in NEN patients’ management and full availability of both diagnostic ([68Ga]Ga-DOTA-SSA) and therapeutic options (PRRT). Although in a survey performed in 2017 (including 443 respondents from 26 countries), the availability of [68Ga]Ga-DOTA-SSA and PRRT was perceived as limited from the majority of patients, advocates and health care professionals18, this condition is expected to change. In fact, the recent registration of [177Lu]Lu-DOTATATE implies its availability to become a real-life opportunity in a higher number of centres, being therefore only limited by reimbursement national policy. Moreover, among 60 ENETS centres of excellence worldwide, that require PRRT availability in the same centre or in a partner centre, 56/60 are currently located in Europe (the remaining 4 are in USA, Israel and Australia, respectively). Additionally, outside of ENETS centres of excellence, PRRT is performed in others therapy centres, but precise data is not available. Since the availability of diagnostic and therapeutic procedures is expected to rise, the discussion of matters of controversy by expert panellists is a fundamental step to ameliorate and standardise patients’ management worldwide.
Data collection
Panellist selection
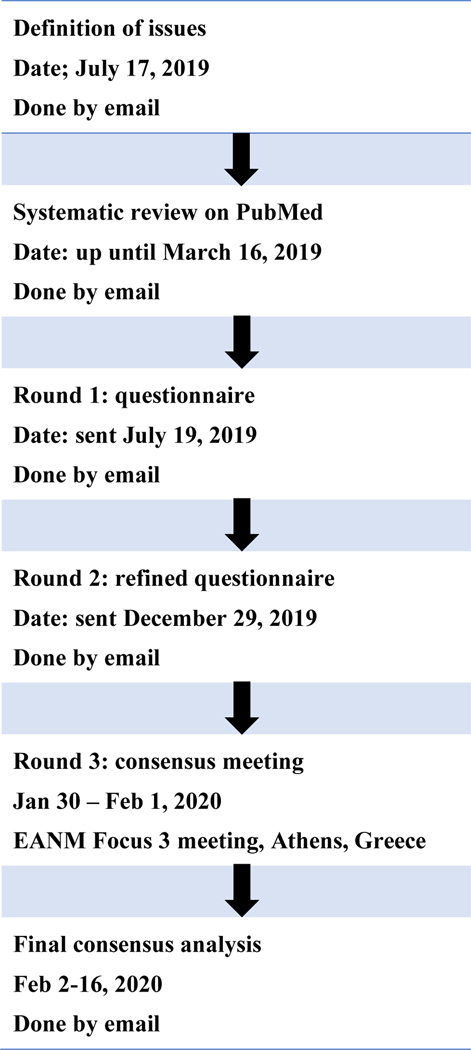
Panellists, 24 in total, were selected on the basis of their expertise and publication record in diagnosis or treatment of NEN with specialties including nuclear medicine (nine panellists), endocrinology (five panellists), medical oncology (three panellists), medical physics (one panellist), surgery (one panellist), radiopharmacy (one panellist), gastroenterology (one panellist), pathology (one panellist), and radiology (two panellists). Panellists were actively involved in all stages of the modified Delphi consensus process (outlined in Figure 1).
Figure 1:
Flow diagram of the modified Delphi process
Search strategy and selection criteria
We first identified the clinical needs in conjunction with the areas where the use of imaging to assess disease status and radiopharmaceuticals for therapy is known to be useful. We performed a comprehensive literature search on PubMed up to March 16, 2019 using medical subject headings vocabulary keywords and free text words for studies published in English. Then, in first instance, systematic reviews were considered. If updated (ie, published since 2017 onwards) systematic reviews were retrieved, and primary studies were not considered. If out-of-date systematic reviews were found, the searches for primary studies were limited to those studies published after the last search date of the most recently published systematic review. In cases where retrieval of many systematic reviews addressed the same subject, only systematic reviews of higher quality according to AMSTAR 2 checklist criteria and most updated were considered. If no systematic reviews were found, a search of primary studies was performed. Five separate bibliographic searches were conducted: imaging of NEN, imaging and therapy of PPGL, genetic testing for PPGL, PRRT of NET, and treatment monitoring. Methodological quality of the included reviews was assessed using AMSTAR 2, diagnostic accuracy of primary studies was assessed using QUADAS-2, uncontrolled case series quality was assessed using a principal component analysis,22 randomized controlled trials using the Cochrane Criteria, and these criteria were listed in the evidence tables and summary documents.22–25 International guidelines were used to discuss the results but were not included in the evidence tables and summaries. All panellists received a summary of the results of the searches and the data taken from the included studies, evidence tables (one per study) containing the main characteristics of each included study/review, the keywords used to build the bibliographic search string, the results of the search, the number of excluded/included studies with reasons for exclusion, and the evaluation of the risk of bias/methodological quality of each included study/review.
Questionnaire
Using the results of the systematic review as a basis, a questionnaire was proposed and agreed among the panellists. A modified Delphi process was then used to gain a structured consensus on each identified and researched topic present in the questionnaire.26 Anonymized summaries of the results of the first two Delphi rounds served as the basis for live presentations and further discussions during EANM Focus 3. For questions which did not achieve consensus during Delphi rounds 1 and 2, the panellists were asked to vote again at the meeting following presentation of these data and moderated discussion (Delphi round 3). For questions designed to reach a single response, a ≥70% cut-off was used to determine consensus; an agreement between 60% and 80% is considered substantial according to the classification of Landis and Koch and is consistent with other consensus procedures.27–32 For questions with a multiple option format, consensus was considered to have been reached, if at least 50% of the panellists preferred at least one answer.
The questionnaire was sent to all 24 panellists. If a panellist did not answer a question, it was either because they abstained, did not feel qualified to answer, or did not provide a response. These panellists did, however, answer other questions. Panellists who responded that they were unqualified to answer or did not answer a given question were not considered for the measurement of agreement for that answer.
Findings
Five topics were identified for the EANM Focus 3 Delphi consensus process: Imaging of NEN; Imaging and Therapy of PPGL; PRRT of NET; Treatment Monitoring; Looking into the Future. The comprehensive literature search identified 22 studies that met the selection criteria.6,7,33–52 Responses to questions where EANM Focus 3 reached consensus are presented by topic in Table 1, and questions where consensus was not reached are reported in Table 2.
Table 1:
Responses from Delphi Rounds
| Imaging of NET | Consensus |
|---|---|
| 1. In cases of patients with suspected of NEN (not pathologically confirmed) based on clinical symptoms and biochemical examination, which imaging technique do you prefer for detection of disease? (choose all that apply) | |
| MRI abdomen or Contrast-enhanced triple phase CT | Round 1: 16 (69.6%) of 231 |
| [68Ga]Ga-DOTA-SSA PET/CT | Round 1: 18 (78.3%) of 231 |
| 2. If you perform SSTR imaging, which tracer and technique do you prefer? | |
| [68Ga]Ga-DOTA-SSA PET/CT | Round 1: 24 (100.0%) of 240 |
| 3. Do you consider SSTR imaging necessary at staging of NET? | |
| Yes, in all patients as complementary to conventional imaging | Round 1: 19 (82.6%) of 230 |
| 4. In addition to SSTR imaging examination, do you consider CT and/or MRI necessary at staging? | |
| Yes, in all patients | Round 1: 16 (69.6%) of 23 |
| Round 2: 20 (87.0%) of 230 | |
| 5. Do you consider [18F]FDG PET/CT necessary/ useful at staging? (choose all that apply) | |
| In selected patients based on grade and correlative imaging, eg, CT/MRI abnormalities without SSTR expression | Round 1: 12 (52.2%) of 231 |
| In patients with NET G3 and NEC | Round 1: 11 (47.8%) of 231 |
| 6. In cases of patients with known metastatic NET disease and unknown primary tumor location, which imaging technique do you prefer? | |
| [68Ga]Ga-DOTA-SSA PET/CT + If not done before CT/MRI | Round 1: 13 (54.2%) of 24 |
| Round 2: 22 (91.7%) of 240 | |
| 7. Do you consider SSTR imaging necessary at re-staging after curative surgery in patients with clinically significant risk of malignant disease? (choose all that apply) | |
| Yes, in all patients as complementary to conventional imaging | Round 1: 10 (43.5%) of 23 |
| Round 2: 14 (60.1%) of 231 | |
| Yes, if no prior SSTR imaging was performed, as complementary to conventional imaging | Round 1: 10 (43.5%) of 23 |
| Round 2: 13 (56.5%) of 231 | |
| 8. Do you consider SSTR imaging necessary at re-staging after non-curative surgery? (choose all that apply) | |
| Yes, in all patients as complementary to conventional imaging | Round 1: 13 (56.5%) of 231 |
| Yes, if no prior SSTR imaging was performed, as complementary to conventional imaging | Round 1: 10 (43.5%) of 231 |
| 9. Do you consider [18F]FDG PET/CT necessary at re-staging? | |
| In a minority of selected patients if positive at baseline or disease trajectory changes | Round 1: 17 (73.9%) of 230 |
| 10. Which imaging technique do you prefer as follow-up for patients with NET who should be followed up? (choose all that apply) | |
| MRI and/or CT | Round 1: 12 (50.0%) of 242 |
| 11. In follow–up, which patients with non-resectable or disseminated NEN qualify for [18F]FDG PET/CT? (choose all that apply) | |
| Patients with NET G3 and NEC | Round 1: 12 (54.5%) of 223 |
| Patients with some anatomical lesions negative for [68Ga]Ga-DOTA-SSA PET/CT | Round 1: 13 (59.1%) of 223 |
| Patients with all grade of tumours and rapid progression | Round 1:14 (63.6%) of 223 |
| 12. In case of clinical or laboratory progression, what would be the proposed first choice imaging test in well-differentiated SSTR positive patients? | |
| SSTR imaging plus CT and or MRI | Round 1: 15 (68.2%) of 22 |
| Round 2: 19 (82.6%) of 230 | |
| 13. Is progression at SSTR imaging together with clinical and laboratory suspicion of progression sufficient to consider the patient in progression notwithstanding a stable CT? | |
| Yes, in most patients | Round 1: 12 (54.5%) of 22 |
| Round 2: 18 (78.3%) of 230 | |
| 16. In cases of patients with non-resectable or disseminated NET and candidates for PRRT treatment, which imaging techniques do you recommend before treatment to confirm target expression? | |
| [68Ga]Ga-DOTA-SSA PET/CT | Round 1: 18 (75.0%) of 240 |
| 17. In patients with non-resectable or disseminated NET and candidates for PRRT treatment, do you recommend [18F]FDG PET/CT before treatment? | |
| Yes, in patients with NET G2 and G3, complementary to SSTR imaging, to exclude patients with mis-match lesion ([18F]FDG-positive/[68Ga]Ga-DOTA-SSA-negative) and as a prognostic factor | Round 1: 12 (52.2%) of 23 |
| Round 2: 19 (82.6 %) of 230 | |
| Imaging and Therapy of Pheochromocytoma and Paraganglioma | Consensus |
| 18. In suspected of adrenal localization of pheochromocytoma, which imaging technique do you recommend? (Choose all that apply) | |
| MRI and/or CT | Round 1: 11 (55.0%) of 202 |
| 19. In suspected of extra-adrenal localization of pheochromocytoma/paraganglioma (positive hormonal test but without abnormality in adrenal gland), which imaging technique do you recommend? (Choose all that apply) | |
| MRI and/or CT | Round 1: 13 (65.0%) of 201 |
| [68Ga]Ga-DOTA-SSA PET/CT | Round 1: 16 (80.0%) of 201 |
| 20. In cases of patients suspected of paraganglioma without evident secondary lesions on CT, which imaging technique do you recommend? | |
| [68Ga]Ga-DOTA-SSA PET/CT | Round 1: 8 (40.0%) of 20 |
| Round 2: 15 (68.2%) of 22 | |
| Round 3: 15 (75.0%) of 200 | |
| PRRT of NET | Consensus |
| 24. In cases of patients with non-resectable or disseminated gastrointestinal NET, what second line treatment (after non-radiolabelled somatostatin analogues) do you recommend? | |
| PRRT if SSTR imaging showed high SSTR expression | Round 1: 20 (95.2%) of 210 |
| 25. Which patients with non-resectable or disseminated NEN qualify for treatment with therapeutic radiopharmaceuticals as a second line? | |
| NET G1, G2 and G3 patients with moderate/high (Krenning 3/4) uptake in all metastases | Round 1: 11 (52.4%) of 21 |
| Round 2: 14 (63.6%) of 22 | |
| Round 3: 19 (86.5%) of 220 | |
| 27. Do you consider PRRT in non-resectable or disseminated NET as a first line of treatment? | |
| Yes, in a minority of selected patients with high SSTR expression in SSTR imaging (based on risk and symptoms, primary tumour location) | Round 1: 11 (52.4%) of 21 |
| Round 2: 17 (77.3%) of 220 | |
| 29. If you refer patients with NET for therapeutic radiopharmaceuticals, which treatment do you prefer? (only 1 answer) | |
| [177Lu]Lu-DOTATATE | Round 1: 15 (71.4%) of 200 |
| 30. In GEP patients (NET G1 and G2, Ki-67<20%) with both elevated SSTR expression and glucose increased uptake in all lesions ([18F]FDG and [68Ga]Ga-DOTA-SSA PET/CT positive, match lesion), would you consider PRRT at first disease progression? | |
| Yes, in all patients with moderate/high (Krenning score ¾) SSTR expression in SSTR imaging | Round 1: 14 (70.0%) of 200 |
| 31. In GEP patients (NET G3, Ki-67>20%) with both elevated SSTR expression and glucose increased uptake in all lesions ([18F]FDG and in [68Ga]Ga-DOTA-SSA PET/CT positive, match lesion), would you consider PRRT at first disease progression? | |
| Yes, in a minority of selected patients with high SSTR expression in SSTR imaging (based on risk and symptoms, primary tumour location) | Round 1: 12 (60.0%) of 20 |
| Round 2: 19 (86.4%) of 220 | |
| 34. In patients who previously responded to PRRT and later progress, do you consider rechallenge with PRRT a feasible option? (choose all that apply) | |
| Yes, in initially responding patients who were stable for at least one year after end of PRRT | Round 1: 12 (54.5%) of 222 |
| 35. Do you consider using PRRT associated with chemotherapy – CAPTEM? (choose all that apply) | |
| Only in patients with Ki-67>20% | Round 1: 2 (9.5%) of 21 |
| Round 2: 18 (85.7%) of 212 | |
| Treatment Monitoring | Consensus |
| 37. Do you consider dosimetry (post-treatment assessment of radiation dose to normal organs and tumour) necessary during PRRT treatment? (choose all that apply) | |
| Yes, in patients in clinical trials | Round 1: 7 (38.9%) of 18 |
| Round 2: 12 (57.1%) of 212 | |
| 38. Which do you believe should be included in PRRT administration protocols of choice for better disease control? (choose all that apply) | |
| Employ PRRT performed following dosimetry estimation or adapting the administered activity to the patient’s clinical data (eg, tumour burden, body mass, comorbidities, lab values) | Round 1: 8 (42.1%) of 19 |
| Round 2: 14 (66.7%) of 212 | |
| Looking into the Future | Consensus |
| 39. Do you consider somatostatin antagonist-based radiopharmaceuticals as a potential future for PRRT? | |
| Yes | Round 1: 14 (70.0%) of 200 |
| 40. In the future, do you believe there will be dedicated imaging for suspected insulinoma, and if so, which imaging method would you prefer? | |
| Yes, [68Ga]Ga- exendin PET/CT | Round 1: 15 (71.4%) of 21 |
| 42. In the future, what do you consider the most important use for PRRT? | |
| Individualized prediction of response and toxicity | Round 1: 9 (42.9%) of 21 |
| Round 2: 15 (65.2%) of 23 | |
| Round 3: 14 (70.0%) of 200 | |
Consensus reached with ≥70% panellists preferring one answer.
Consensus reached in multiple option answer format: at least 50% of the panellists preferred at least one answer, and the majority of patients preferred two options.
Consensus reached in multiple option answer format: at least 50% of the panellists preferred at least one answer, and the majority of panellists preferred one option.
Consensus reached in multiple option answer format: at least 50% of the panellists preferred at least one answer, and the majority of panellists preferred three options.
Table 2:
Questions where consensus was not reached on the molecular imaging and theranostics in neuroendocrine tumours.
| Imaging of NET | Round | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 14. Is progression at SSTR imaging alone (with stable CT, clinical, and laboratory tests) sufficient to consider a patient in progression? 1 | |||
| Yes, in all patients | 1 (4.5%) of 22 | ||
| Yes, in most patients | 6 (27.3%) of 22 | 8 (36.4%) of 22 | 13 (56.5%) of 23 |
| In a minority of patients (based on risk and grading) | 7 (31.8%) of 22 | 7 (31.8%) of 22 | 7 (30.5%) of 23 |
| No | 6 (27.3%) of 22 | 4 (18.2%) of 22 | 3 (13.0%) of 23 |
| Abstain (not sufficient evidence in the literature) | 2 (9.1%) of 22 | 3 (13.6%) of 22 | |
| Unqualified to answer (not an expert in this field of management) | 1 (4.3%) of 23 | 1 (4.3%) of 23 | 1 (4.2%) of 24 |
| 15. Do you consider SSTR imaging relevant for selecting patients with non-functioning NENs for SSA treatment? 2 | |||
| Yes, in all patients | 8 (36.4%) of 22 | 7 (30.4%) of 23 | 7 (29.2%) of 24 |
| Yes, in most patients | 8 (36.4%) of 22 | 12 (52.2%) of 23 | 14 (58.3%) of 24 |
| Yes, but only in patients with NET G3 or NEC) | 1 (4.5%) of 22 | 1 (4.3%) of 23 | |
| No | 4 (18.2%) of 22 | 2 (8.7%) of 23 | 2 (8.3%) of 24 |
| Abstain (not sufficient evidence in the literature) | 1 (4.5%) of 22 | 1 (4.3%) of 23 | 1 (4.2%) of 24 |
| Unqualified to answer (not an expert in this field of management) | 1 (4.3%) of 23 | 1 (4.2%) of 24 | |
| Imaging and Therapy of Pheochromocytoma and Paraganglioma | Responses | ||
| 21. Do you consider genetic examination necessary to choose among the different radiopharmaceuticals to employ? 3 | |||
| Yes, because genetic mutation determines selection of imaging technique | 5 (23.8%) of 21 | 3 (13.6%) of 22 | 1 (4.8%) of 21 |
| Yes, because this has implications for surveillance, prognosis and further family testing | 4 (19.0%) of 21 | 7 (31.8%) of 22 | 9 (42.8%) of 21 |
| No | 8 (38.1%) of 21 | 12 (54.6%) of 22 | 11 (52.4%) of 21 |
| Choice based on availability/cost/clinical symptoms | 2 (9.5%) of 21 | ||
| Abstain (not sufficient evidence in the literature) | 2 (9.5%) of 21 | ||
| Unqualified to answer (not an expert in this field of management) | 2 (8.7%) of 23 | 2 (8.3%) of 24 | 2 (8.7%) of 23 |
| 22. In cases of patients with inoperable or disseminated paraganglioma/pheochromocytoma, what do you recommend as first line treatment? 4 | |||
| Non-radiolabelled somatostatin analogues | 1 (5.9%) of 17 | 2 (10.5%) of 19 | 1 (5.3%) of 19 |
| PRRT if SSTR imaging showed high SSTR expression | 5 (29.4%) of 17 | 9 (47.4%) of 19 | 10 (52.6%) of 19 |
| 131I-mIBG | 3 (17.6%) of 17 | 1 (5.3%) of 19 | |
| Radionuclide therapy guided by molecular imaging phenotype | 5 (29.4%) of 17 | 5 (26.3%) of 19 | 7 (36.8%) of 19 |
| Chemotherapy | 2 (11.7%) of 17 | ||
| Targeted therapy, eg, sunitinib | 1 (5.9%) of 17 | ||
| Abstain (not sufficient evidence in the literature) | 3 (17.6%) of 17 | 2 (10.5%) of 19 | 1 (5.3%) of 19 |
| Unqualified to answer (not an expert in this field of management) | 7 (29.2%) of 24 | 5 (20.8%) of 24 | 4 (17.4%) of 23 |
| PRRT of NET | Responses | ||
| 23. In cases of patients with non-resectable disseminated pancreatic NET, what second line treatment (after non-radiolabelled somatostatin analogues) do you recommend? 5 | |||
| Change non-radiolabelled somatostatin analogues | |||
| Everolimus, Sunitinib or chemotherapy | 8 (38.1%) of 21 | 6 (26.1%) of 23 | 7 (30.4%) of 23 |
| PRRT if SSTR imaging showed high SSTR expression | 14 (66.7%) of 21 |
16 (69.6%) of 23 |
15 (65.2%) of 23 |
| 131I-mIBG | |||
| Selective liver embolization | |||
| Abstain (not sufficient evidence in the literature) | 1 (4.8%) of 21 | 1 (4.4%) of 23 | 1 (4.4%) of 23 |
| Unqualified to answer (not an expert in this field of management) | 3 (12.5%) of 24 | 1 (4.2%) of 24 | 1 (4.2%) of 24 |
| 26. What is the right time point for PRRT in the sequential treatment of NET patients? 5 | |||
| Patients progressed after non-radiolabelled somatostatin analogues | 8 (38.1%) of 21 | 7 (31.8%) of 22 | 4 (17.4%) of 23 |
| Patients progressed after non-radiolabelled somatostatin analogues and kinase inhibitor | 3 (14.3%) of 21 | 1 (4.5%) of 22 | |
| Patients already treated with all other available therapies | 1 (4.8%) of 21 | ||
| PRRT could be consider as a first/second line of treatment depending on SSTR expression and clinical condition of patient | 8 (38.1%) of 21 | 7 (31.8%) of 22 | 12 (52.2%) of 23 |
| It depends on the primary site and grade | 7 (33.3%) of 21 | 6 (27.3%) of 22 | 6 (26.1%) of 23 |
| Abstain (not sufficient evidence in the literature) | 1 (4.5%) of 22 | 1 (4.4%) of 23 | |
| Unqualified to answer (not an expert in this field of management) | 3 (12.5%) of 24 | 2 (8.3%) of 24 | 1 (4.2%) of 24 |
| 28. Do you consider PRRT as a first/second line of treatment in cases of patients with local but non-resectable disease (without metastases)? 2 | |||
| Yes, in all patients with high SSTR expression in SSTR imaging | 5 (25.0%) of 20 | 2 (9.1%) of 22 | 2 (8.7%) of 23 |
| Yes, in a minority of selected patients with high SSTR expression in SSTR imaging (based on risk and symptoms, tumour location) | 6 (30.0%) of 20 | 10 (45.4%) of 22 | 15 (65.2%) of 23 |
| Yes, in patients with high SSTR expression in SSTR imaging if in progression (clinical/imaging) | 6 (30.0%) of 20 | 7 (31.8%) of 22 | 6 (26.1%) of 23 |
| No | 3 (15.0%) of 20 | 2 (9.1%) of 22 | |
| Abstain (not sufficient evidence in the literature) | 1 (5.0%) of 20 | 1 (4.5%) of 22 | |
| Unqualified to answer (not an expert in this field of management) | 3 (13.0%) of 23 | 2 (8.3%) of 24 | 1 (4.2%) of 24 |
| 32. In GEP patients (NET G1 and G2, Ki-67<20%) with several mis-match lesions ([18F]FDG -positive/[68Ga]Ga-DOTA-SSA PET/CT -negative), would you consider PRRT at disease progression? | |||
| No, due to lack of receptors in several lesions | 8 (38.1%) of 21 | 10 (45.5%) of 22 | 10 (43.5%) of 23 |
| Yes, in a minority of selected patients with high SSTR expression in SSTR imaging (based on risk and symptoms, primary tumour location) | 4 (19.0%) of 21 | 1 (4.5%) of 22 | 1 (4.4%) of 23 |
| Yes, but only together with chemotherapy (CAPTEM) or local treatment of discordant lesions | 10 (47.6%) of 21 | 11 (50%) of 22 | 12 (52.2%) of 23 |
| Abstain (not sufficient evidence in the literature) | |||
| Unqualified to answer (not an expert in this field of management) | 3 (12.5%) of 24 | 2 (8.3%) of 24 | 1 (4.2%) of 24 |
| 33. Do you consider using PRRT as a neoadjuvant treatment? 4 | |||
| Yes, only in patients with pancreatic tumour with high SSTR expression in a large tumour with infiltration to surrounding tissue, but without distant metastases | 7 (33.3%) of 21 | 8 (36.4%) of 22 | 11 (50%) of 22 |
| Yes, only in patients with pancreatic tumour with high SSTR expression in a large tumour with infiltration to surrounding tissue, but without distant metastases, with a slowly progressive tumour | 1 (4.8%) of 21 | ||
| Yes, in patients with pancreatic tumour with high SSTR expression in a large tumour with infiltration to surrounding tissue, with distant metastases | 2 (9.5%) of 21 | 1 (4.5%) of 22 | 1 (4.5%) of 22 |
| Yes, in patients with all GEP tumours with high SSTR expression in a large tumour with infiltration to surrounding tissue, but without distant metastases | 4 (19.0%) of 21 | 5 (22.7%) of 22 | 5 (22.7%) of 22 |
| Yes, in patients with all GEP tumours with high SSTR expression in a large tumour with infiltration to surrounding tissue, with distant metastases | 3 (14.3%) of 21 | 1 (4.5%) of 22 | |
| No | 3 (14.3%) of 21 | 1 (4.5%) of 22 | 1 (4.5%) of 22 |
| Abstain (not sufficient evidence in the literature) | 4 (19%) of 21 | 6 (27.3%) of 22 | 4 (18.2%) of 22 |
| Unqualified to answer (not an expert in this field of management) | 3 (12.5%) of 24 | 2 (8.3%) of 24 | 1 (4.3%) of 23 |
| Treatment Monitoring | Responses | ||
| 36. In cases of patients with non-resectable or disseminated NET and treated with PRRT, which imaging techniques do you recommend for monitoring response to therapy? 6 | |||
| Contrast-enhanced triple phase CT and/or MRI | 9 (39.1%) of 23 | 11 (45.8%) of 24 | 10 (41.7%) of 24 |
| 68Ga-DOTA-SSA PET/CT | 4 (17.4%) of 23 | ||
| 68Ga-DOTA-SSA PET/CT + Contrast-enhanced triple phase CT | 10 (43.5%) of 23 | 15 (62.5%) of 24 | 14 (58.3%) of 24 |
| SSTR imaging scintigraphy | 1 (4.3%) of 23 | ||
| The same technique as for qualification for PRRT | 3 (13.0%) of 23 | ||
| No imaging for monitoring | |||
| Choice based on availability/cost | |||
| Abstain (not sufficient evidence in the literature) | |||
| Unqualified to answer (not an expert in this field of management) | |||
| Looking into the Future | Responses | ||
| 41. Which patients with NET do you consider for intraarterial PRRT? 7 | |||
| All patients, to reduce the dose for kidney | |||
| Patients with metastases only in the liver | 1 (5.3%) of 19 | 1 (4.5%) of 22 | 3 (15.8%) of 19 |
| Patients with metastases predominantly in the liver, to reduce dose for kidney | 8 (42.1%) of 19 | 11 (50.0%) of 22 | 10 (52.6%) of 19 |
| Patients with low SSTR expression in liver metastases | 1 (5.3%) of 19 | ||
| No patients | 3 (15.8%) of 19 | 3 (13.6%) of 22 | |
| Abstain (not sufficient evidence in the literature) | 6 (31.6%) of 19 | 7 (31.8%) of 22 | 6 (31.6%) of 19 |
| Unqualified to answer (not an expert in this field of management) | 3 (13.6%) of 22 | 2 (8.3%) of 24 | 2 (9.5%) of 21 |
| 43. What future role do you see for immunotherapy in the treatment of NENs? 1, 5, 8 | |||
| No role at all | 3 (16.7%) of 18 | 1 (5.3%) of 19 | |
| Only last resort after somatostatin, PRRT, TKI | |||
| Potential combination with PRRT (additive effect) in high grade NENs | 7 (38.9%) of 18 | 8 (42.1%) of 19 | 8 (42.1%) of 19 |
| Potential combination with chemotherapy and/or TKI | 3 (16.7%) of 18 | 1 (5.3%) of 19 | |
| Second line after somatostatin | |||
| Abstain (not sufficient evidence in the literature) | 8 (44.4%) of 18 | 11 (57.9%) of 19 | 9 (47.4%) of 19 |
| Unqualified to answer (not an expert in this field of management) | 5 (21.7%) of 23 | 4 (17.4%) of 23 | 2 (9.5%) of 21 |
One panellist did not answer in round 1 and/or 2
One panellist did not answer in round 1
One panellist did not answer in round 1 and/or 3
Some panellists gave more than one answer in round 1; one panellist did not answer in round 3
Some panellists gave more than one answer in round 1
Some panellists gave more than one answer in round 1 and 2; one panellist did not answer in round 1
Two panellist did not answer in round 1 and three did not answer in round 3
Three panellist did not answer in round 3
Imaging of NEN
Consensus was reached on responses to 15 of 17 questions concerning imaging of NEN. Magnetic resonance imaging (MRI) abdomen or contrast-enhanced triple phase CT and PET/CT with [68Ga]Ga-DOTA-SSA (including [68Ga]Ga-DOTA-TOC, [68Ga]Ga-DOTATATE and [68Ga]Ga-DOTA-NOC) were preferred for cases of patients with clinically/biochemically suspected NEN (not pathologically confirmed). [68Ga]Ga-DOTA-SSA PET/CT was preferred for performing SSTR imaging for staging of all NET as complementary to conventional imaging. In addition to SSTR imaging examination, CT and/or MRI were considered necessary in all patients at initial or subsequent staging. [68Ga]Ga-DOTA-SSA PET/CT was the preferred technique for NET patients with known metastatic disease but unknown primary tumour location. SSTR imaging was considered necessary at re-staging after potentially curative surgery in patients with clinically significant risk of residual or development of metastatic disease as complementary to conventional imaging, even if no prior SSTR imaging was performed before surgery to confirm the presence of SSTR expression. SSTR imaging was also considered necessary at re-staging after non-curative surgery in all patients as complementary to conventional imaging.
In case of clinical or laboratory progression, the proposed first choice imaging test in SSTR positive NET was SSTR imaging plus CT and/or MRI. The detection of a new lesion by SSTR PET/CT, associated with a CT demonstrating stable disease, was considered sufficient to define progression only when clinical and laboratory findings also are suggestive for progression.
The panellists recommended [68Ga]Ga-DOTA-SSA PET/CT before PRRT initiation for patients with non-resectable or disseminated NET to confirm target expression.
[18F]FDG PET/CT was considered necessary/useful in selected patients based on grade (particularly for NET G3) and correlative imaging, eg, CT/MRI abnormalities without SSTR expression at staging. [18F]FDG PET/CT was considered necessary at re-staging in a minority of selected patients if positive at baseline or in patients with rapid progression of disease despite earlier low-grade disease on pathology. MRI and/or CT were the preferred imaging techniques for follow-up of patients with NEN. The panellists also recommended [18F]FDG PET/CT for patients with non-resectable or disseminated NET G3 and NEC and for those with some lesions on CT/MRI which are negative on SSTR imaging. For patients with non-resectable or disseminated NET who are candidates for PRRT treatment, the panellists recommended [18F]FDG PET/CT before treatment in patients with NET G2 and G3, complementary to SSTR imaging, to exclude patients with mis-matched lesions ([18F]FDG -positive/[68Ga]Ga-DOTA-SSA -negative) and as a prognostic factor.
Consensus was not reached concerning the determination of progression at SSTR imaging and the relevance of SSTR imaging for selecting patients with non-functioning NEN for SSA treatment.
Imaging and Therapy of PPGL
Five questions addressed imaging and therapy of PPGL, and consensus was reached on responses to three of these. MRI and/or CT were the recommended imaging techniques for suspected adrenal localization, while MRI and/or CT and [68Ga]Ga-DOTA-SSA PET/CT were recommended for suspected extra-adrenal localization (catecholamine hypersecretion but without abnormality in adrenal gland). [68Ga]Ga-DOTA-SSA PET/CT was recommended for clinically or biochemically suspected paraganglioma (PGL) without evident lesions on CT.
Consensus was not reached on the necessity of using genetic examination to choose the appropriate radiopharmaceutical nor the choice of first line treatment for patients with inoperable or disseminated PPGL.
PRRT of NET
PRRT of NET was the topic of 13 questions, and consensus was reached on responses to eight of these. If SSTR imaging showed high SSTR expression, the panellists recommended PRRT as second line treatment (after non-radiolabelled SSAs) for patients with non-resectable or disseminated gastrointestinal NET, and they agreed that NET patients (G1, G2, and G3) with moderate/high uptake (greater than normal liver Krenning score 3 or 4) in all metastases qualify for this treatment.53
[177Lu]Lu-DOTATATE was the preferred radiopharmaceutical of most (15 (71.4%) of 21) panellists for treating patients with NET.
Consensus was not reached concerning second line treatment after non-radiolabelled SSAs for patients with non-resectable disseminated pancreatic NET nor the right time point for PRRT in the sequential treatment of NET patients. Consensus was not reached on whether PRRT should be used as a first/second line of treatment in cases of patients with local but non-resectable primary disease (without metastases). These was no consensus on the use of PRRT in patients with G1/G2 NET with mis-match lesions ([18F]FDG -positive/[68Ga]Ga-SSA -negative) nor on the potential role of PRRT as a neoadjuvant treatment.
Treatment Monitoring
Three questions addressed treatment and monitoring, and consensus was reached on responses to two of these.
Panellists believed dosimetry should be performed only as part of clinical trials and, for better disease control, PRRT should be performed following dosimetry estimation or adapting the administered activity to the patient’s clinical data (eg, tumour burden, body mass, comorbidities, lab values).
Consensus was not reached concerning the choice of imaging technique for monitoring response to therapy in patients with non-resectable or disseminated NET and treated with PRRT.
Looking into the Future
Consensus was reached on responses to three of the five questions addressing future perspectives for nuclear medicine in NEN. Panellists considered SSTR antagonist-based radiopharmaceuticals as a potential future for PRRT, preferred [68Ga]Ga-exendin as having potential as a diagnostic imaging test for suspected insulinoma, and considered individualized prediction of response and toxicity the most important future use for PRRT.
No consensus was reached on whether patients with NET should be considered for intra-arterial PRRT nor on the future role for immunotherapy in the treatment of NENs.
Discussion
The use of PRRT in NET management became a particularly relevant topic following EMA and FDA approval of the use of [177Lu]Lu-DOTATATE in GEP-NET in 2017. Although used mostly in clinical trials and as compassionate use since its first introduction in the mid-1990s, most panellists support the use of PRRT in management of selected cases of NET. This is in line with the results reported here, where most panellists preferred [177Lu]Lu-DOTATATE, while some also considered other options including a combination of 90Y/177Lu together with DOTATATE or DOTATOC, to achieve control of both small and large lesions corresponding to the different tissue penetration of the emitted radiation.54,55,56
In fact, EANM guideline issued in 2013 reports different treatment schemes including both radiolabelled DOTATATE and DOTATOC alone or in association. However, validation studies are needed to confirm the benefits of combination treatment as was done for [177Lu]Lu-DOTATATE in the NETTER-1 trial. It should also be mentioned that from a toxicity point of view, 177Lu is increasingly preferred to 90Y labelling due to its much lower kidney toxicity and the possibility to carry out scintigraphy and thus dosimetry.
Panellists agreed on the use of PRRT as second line treatment in patients with non-resectable or disseminated gastrointestinal NET (after progression or lack of symptomatic control on non-radiolabelled SSTR agonists). This is in line with the results of the NETTER-1 study, where PFS at 20 months was 65.2% for patients treated with [177Lu]Lu-DOTATATE and standard dose of octreotide compared to 10.8% for those treated with off-label high-dose octreotide.7 For pancreatic NET, PRRT was not recommended specifically as a second-line therapy due to the absence of data comparing PRRT to other approved treatments such as sunitinib and everolimus or streptozotocin-based systemic chemotherapy.
Panellists considered PRRT as appropriate second line treatment option in all GI NET patients (NET G1 and G2, Ki-67<20%) with matched [18F]FDG and SSTR-positive uptake in all lesions. Considering the well-known heterogeneous NET behaviour, the employment of PRRT in only G1 and G2 patients may exclude some G3 patients, who might derive benefit from treatment. In fact, good responses to PRRT were also reported in selected G3 NET with lower Ki-67 (reported values of <55%).57,58 EANM Focus 3 panellists were in favour of extending current indication to a subset of patients with GEP G3 (NET G3, Ki-67>20%) showing matched [18F]FDG and SSTR uptake in all lesions (based on risk, symptoms, and primary tumour location).
Panellists also agreed that patients responding to [177Lu]Lu-DOTATATE may benefit from retreatment at disease progression, if partial remission or disease stability was obtained for at least one year after first administration. However, criteria to assess disease stability are not fully standardized and trials are lacking.
In patients with lesions showing heterogeneous (eg, high and low Ki67 values) grade, combining PRRT with capecitabine-temozolomide (CAPTEM) has been reported, but panellists agreed that, based on current evidence, it should only be used within a dedicated protocol considering the potential toxicity of CAPTEM in combination with PRRT.59
Panellists were in favour of considering PRRT as first line treatment in selected cases, eg, patients with high tumour burden and associated symptoms. An ongoing trial (NETTER-2) is currently evaluating first line use of PRRT in advanced NET G2-G3. A few reports indicate a potential use in the neoadjuvant setting but no agreement was reached.60
Use of PRRT with [177Lu]Lu-DOTATATE beyond small bowel has been approved by the EMA and FDA.7,61 However, the comparison of the reported response to PRRT compared to everolimus or sunitinib in pancreatic NET is difficult given differences in study designs, eligibility criteria, and absence of head-to-head comparisons.62,63 Clinical evidence suggests that lesions with mismatched [18F]FDG and somatostatin avidity may develop during NET natural history, particularly in G2 but also in a minority of G1 cases. In the clinical setting of heterogeneous disease and with [18F]FDG positive as the most aggressive component, no consensus was reached on the feasibility of PRRT at disease progression.
To select candidate patients for PRRT, diagnostic nuclear medicine is mandatory for confirming significant SSTR expression in tumour cells. In line with current guidelines,4,8–17 panellists agreed that [68Ga]Ga-DOTA-SSA PET/CT should be preferentially used for NET imaging rather than 99mTc- or 111In-based SSTR scintigraphy. [68Ga]Ga-DOTA-SSA is recommended as the first choice for PET/CT imaging of all NET, by all guidelines as well as EANM Focus 3, 4,8–17 with the exception of adrenal pheochromocytoma (due to physiologic biodistribution to the adrenals), medullary thyroid carcinoma, benign insulinoma, neuroblastoma, and abdominal PGL (all characterized by variable SSTR expression).12 Although EANM guidelines consider using [18F]F-DOPA as additional first choice radiopharmaceutical for small intestine NET,12 both ENETS and EANM Focus 3 do not recommend it,9,13 since it fails to provide data on potential further therapeutic options.
Consensus was reached for using [68Ga]Ga–DOTA-SSA PET/CT in addition to diagnostic contrast-enhanced CT/MRI at disease staging in all patients, including those with metastatic disease and unknown primary tumour site, and at re-staging after surgery (either curative or not). Accurate disease staging is crucial to assess disease extent, detect the site of an occult (mostly small intestine, often multiple) primary, and assess SSTR expression before PRRT.
Consensus was also reached on the employment of both [68Ga]Ga-DOTA-SSA PET/CT and contrast-enhanced CT in cases of suspected NET.
This particular clinical setting is often not covered by societal guidelines, and this omission could lead to performing unnecessary imaging. Indeed, morphologic and functional imaging should only be performed after careful clinical judgement for pre-test probability of disease (with evaluation of presenting symptoms and accurate testing of biochemical markers to reduce their false positivity). In this regard, SSTR positivity is not, per se, diagnostic of NEN (since false positive findings include infection/inflammation and some SSTR expressing non-NEN malignancies).
Panellists agreed to rely on contrast-enhanced CT and/or MRI for disease follow-up according to current guidelines.9
One of the most controversial issues in recent NET management concerns the use of [18F]FDG in addition to [68Ga]Ga-DOTA-SSA to detect the presence of more aggressive tumour cells’ clones. This approach can provide complete biological tumour characterization but, on the other hand, might be unnecessary in NET, particularly in G1 midgut NET. Guidelines (both EANM and ENETS) suggest performing [18F]FDG PET/CT in G3 NET, NEC, and higher grade G2 (eg, Ki-67 10–20%) NET. ESMO guidelines 2020 state, that optimal diagnostic and prognostic information can be achieved by directing all NET G2/G3 patients to PET/CT with both [18F]FDG and [68Ga]Ga-DOTA-SSA, however they also question the utility of such an unconfirmed approach in routine practice.64 Several studies have investigated the impact of double tracer imaging,65,66 however these were mostly retrospective and included NEN with different primary tumours (a factor known to affect the likelihood of [18F]FDG-positivity) and grades. Moreover, for the most part, these did not report the time frame between the detection of [18F]FDG-positivity and assessment of pathological tumour grade. Consequently, the current clinical employment of [18F]FDG may vary among countries and also be influenced by local reimbursement policies.
Consensus was reached on the use of [18F]FDG PET/CT in patients with non-resectable or disseminated NET G3 and NEC, in cases presenting anatomical lesions negative for [68Ga]Ga-DOTA-SSA, and in cases showing rapid progression regardless of tumour grade. For patients with NET G3 and NEC, [18F]FDG PET/CT is recommended if radical surgery is being pursued, or if clarification of equivocal findings on conventional imaging may change the therapeutic approach (although the latter is still not standardized).17(p) For NEC, [18F]FDG PET/CT should not delay the start of chemotherapy. [18F]FDG PET/CT and [68Ga]Ga-DOTA-SSA PET/CT could contribute to prognosis and select a few patients with less aggressive tumours likely to benefit more from PRRT alone or in combination with peptide receptor chemo radionuclide therapy (PRCRT).67
Guidelines published by EANM, ENETS, ESMO, and NANETS recommend, 4,8–17 and EANM Focus 3 supports, using [68Ga]Ga-DOTA-SSA before PRRT to demonstrate in vivo SSTR expression and select the patients who might benefit from treatment. Considering the poorer prognosis to be expected in [18F]FDG positive cases and the relevance of assessing the presence of [68Ga]GA-DOTA-SSA negative/[18F]FDG positive mis-matched lesions, the employment of double tracer ([68Ga]Ga-DOTA-SSA plus [18F]FDG) imaging before PRRT initiation was also recommended in non-resectable or disseminated G2 and G3 NET patients.68–71
The definition of progression is a difficult issue. It is well known, that in many oncological settings, functional changes detected by PET/CT may precede morphological changes on diagnostic CT, especially in case of bone lesions and small lymph nodes (not reaching the CT criteria for positivity).72–74 However, it is also well known that PET/CT may fail to detect very small lesions within the liver due to high adjacent background activity, and that these may be better appreciated by arterial phase or hepatocyte specific MRI/CT.9 Further, Response Evaluation Criteria In Solid Tumours (RECIST), the currently used criteria for response assessment suggested by all guidelines, are not ideal for NEN, since they rely mostly on changes in the dimensions of lesions that may be hard to detect in typically slow growing NET cases and may not capture the full extent of benefits of molecularly targeted treatments.75,76 There is a strong need to define an appropriate and standardized approach to assess tumor response and to define disease progression.63,76,77 Panellists agreed that in case of clinical or biochemical suspicion of progression, detection of new lesions by [68Ga]Ga-DOTA-SSA are sufficient to consider progression notwithstanding stable disease on CT. However, there was no consensus to define progression on the basis of detecting a new lesion by [68Ga]Ga-DOTA-SSA alone (with stable CT, clinical, and laboratory tests). Moreover, panellists did not agree on the imaging technique of choice for monitoring PRRT response in patients with non-resectable or disseminated NET: the majority (14 (58.3%) of 24) voted for both [68Ga]Ga-DOTA-SSA and contrast-enhanced triple phase CT followed by contrast-enhanced triple phase CT and/or MRI (10 (41.7%) of 24) alone. EANM guidelines recommend [68Ga]Ga-DOTA-SSA for treatment monitoring.12 ESMO guidelines recommend CT for follow up15,64, and NCCN recommend [68Ga]Ga-DOTA-SSA only at 12–36 months16
In order to optimize PRRT efficacy while reducing toxicity, panellists agreed that a strong effort should be made towards administering PRRT after dosimetric estimation, or adapting the administered activity to the patient’s clinical data (e.g. tumour burden, mass of organs at risk, comorbidities, and laboratory values) in order to employ patient-tailored treatment schemes. This perception ensues from results using PRRT with [177Lu]Lu-DOTATATE which showed high inter patient variability of tumour and kidney absorbed doses,77,78 the possibility to treat patients with renal insufficiency with lower PRRT cumulative activities adjusted for impaired renal function,77–81 significant correlation between tumour absorbed dose and response,82 higher overall survival (OS) in patients with CR/PR versus SD or PD in a dosimetry-guided prospective study (200 patients), and, especially, doubled mean OS and PFS without renal toxicity in patients who reached a preset absorbed dose to the kidneys (23 Gy).83
EANM Focus 3 was in favour of performing routine dosimetry as part of a clinical trial or retreatment with increased cumulative activities, although not in standard treatment, since the NETTER 1 trial demonstrated the safety of four administrations at fixed doses (7.4 GBq) in most patients. The development of more user-friendly, standardized, accurate, and simplified dosimetry methods may facilitate more routine use of dosimetry and strengthen the evidence-base for or against its utility.84,85
For the assessment of PPGL, the choice of the imaging modality should take into account the physiological biodistribution (SSTR imaging is not ideal for small tumours due to uptake by healthy adrenal cortex), availability ([18F]FDOPA is difficult to synthesize and not available in all centres),12 genetics (eg, succinate dehydrogenase mutation, SDHx),86,87 and clinical need (patients presenting significant SSTR expression may be selected for PRRT for control of catecholamine excess).88 EANM Focus 3 consensus favoured the use of CT/MRI to assess an adrenal mass suspicious for pheochromocytoma, while in cases of extra-adrenal PGL, the majority of panellists preferred two options: [68Ga]Ga-DOTA-SSA PET/CT (16 (80%) of 20), followed by MRI/CT (13 (65%) of 20). The higher detection rate of [68Ga]Ga-DOTA-SSA PET/CT over other radiopharmaceuticals in PPGL was recently reported.42 Nevertheless, EANM guidelines hold that [18F]FDOPA may show higher accuracy for adrenal forms of PGL and for HIF2A/VHL/MAX-related PPGL as compared to [68Ga]Ga-DOTA-SSA. Since tumour size, genotype, and biochemical phenotype strongly influence the risk of malignancy. EANM guidelines also recommend performing nuclear imaging for pheochromocytoma in the following cases: large tumours (>5 cm), SDHB mutated status, noradrenergic biochemical phenotype, and/or high methoxytyramine level. The panellists were not asked about the potential influence of size and secretory profile on pheochromocytoma imaging nor the optimal strategy for imaging metastatic PPGL.
For therapy selection, [123I]mIBG scintigraphy is also recommended for advanced PPLG by guidelines, since it is mandatory to select patients for [131I]mIBG therapy.89 Considering the very complex genetic background of patients presenting with PPLG and the reported higher positivity rate in case of certain gene expressions (eg, [18F]FDG and [68Ga]Ga-DOTA-SSA for SDHx and [18F]FDOPA and [68Ga]Ga-DOTA-SSA for head and neck PGL), it was suggested in the literature to use the genetic expression profile to guide the choice of radiopharmaceutical. On the contrary, consensus was not reached on the need to obtain genetic information prior to PET/CT imaging; a slight majority of panellists found this information unnecessary (11 of 21), while others favoured using these data to guide the choice of radiopharmaceutical (9 of 21). Panellists did not agree on first line treatment for PPGL, although most favoured PRRT in cases presenting with high SSTR expression.90–93
Nuclear medicine is evolving extremely rapidly, change being driven mostly by the introduction of novel radiopharmaceuticals, especially if these have theranostic potential. When asked about the future direction of PRRT, panellists agreed on the promising role of radiolabelled SSTR antagonists.94,95 The higher number of binding sites to SSTR and the reduced uptake in background parenchyma with antagonists as tracers for PET/CT (especially in the liver, spleen, pancreas, and gastrointestinal tract) allows higher lesion detection rates and may also widen the number of conditions to be treated with antagonists-based PRRT.94–98 Moreover, on the labelling side, panellists expect that PRRT will be individualized, and tailored treatment schemes will be routinely used to achieve higher responses while reducing toxicity (see for example NCT03972488, NCT03049189, and NCT03454763).
Due to limited data in the literature, we did not include questions about predictive factors of response to PRRT nor selection criteria on SSTR imaging for PRRT.
Focus 3 stressed the theranostic use of radiopharmaceuticals. We did not focus on [18F] fluoro-3,4-dihydroxyphenylalnine ([18F]F-DOPA), although a useful radiopharmaceutical for NET imaging, since the aim of the paper was to provide a strong indication of what is the most relevant up-to-date and practical choice among different options, also in view of the approval of PRRT, the most effective treatment so far. We recognise, that in some cases [18F]F-DOPA could be used as a diagnostic agent but it is not theranostic.
Although currently used only as part of clinical trials and available in only a few centres, [68Ga]Ga-exendin-4 PET/CT, which targets the glucagon-like peptide 1 receptor (GLP1), is expected by most panellists to become the first choice radiopharmaceuticals for the detection of benign insulinoma.4,99 Insulinoma lesions are clinically challenging (due to often difficult to treat hypoglicemia) and difficult to diagnose, since they often present as small lesions that in the majority of cases do not express SSTR. In insulinoma with significant SSTR expression (GLP-1R-negative, malignant insulinoma), [68Ga]Ga-DOTA-SSA is also a diagnostic and therapeutic option especially in malignant insulinomas which are often GLP-1R negative, while [18F]F-DOPA may still be hampered by physiologic biodistribution to the pancreas.100,101 The limited but extremely promising evidence on the use of [68Ga]Ga-exendin-4 for detection of even small GLP-1R-positive insulinoma lesions, was the basis for panellists’ agreement on an expected increase of its employment in clinical practise.
Imaging biomarkers (such as e.g. dual-tracer imaging with [18F]FDG /[68Ga]Ga-DOTA-SSA) or liquid biomarkers (such as e.g. PPQ) hold promise as predictive biomarkers for PRRT and should be further validated to allow better individualized prediction of response and toxicity when treating patients with neuroendocrine tumours.102,103
The choice for reaching consensus if at least 50% of the panellists preferred at least one answer on questions with a multiple options format limits the conclusions drawn from the affected questions. Further, questions were submitted to a subgroup of reviewers before being rated by panellists, and it was not possible to add or alter questions at a later stage.
Targeted alpha therapy, such as [213Bi]Bi-DOTATOC is a very promising but currently only (if at all) in early clinical development. Published data are limited, small patient cohorts, and mainly preclinical.104 Another problem is the availability of radioisotopes for TAT. Accordingly, TAT was not subsumed under PRRT.
The lack of clinical evidence on the role of immunotherapy in NET accounted for the lack of consensus on the future role of this treatment option in association with PRRT.
Conclusions
The multidisciplinary EANM Focus 3 panel reached consensus on responses to 31 out of 43 questions (72%) concerning molecular imaging and theranostics in NEN. The relevance of achieving a strong consensus is not only a prelude to a more standardized patient management across countries but also serves as a guide for the direction to follow when designing new research studies. The relatively high consensus reached among the panellists indicates the need to refer patients or discuss their clinical cases in multidisciplinary teams, preferably in high volume centres, for better patient management.
EANM Focus 3 reached consensus on:
PRRT as second line for GI-NET, if there is sufficient uptake (modified Krenning 3 or 4) in all lesions;
Consideration of PRRT in GEP-NET patients at first disease progression with all match lesions [68Ga]Ga-DOTA-SSA /[18F]FDG positive in patients if Ki67<20% (G1 and G2) and in a minority of patients with G3 Ki67>20%;
PRRT as a first line of treatment in non-resectable or disseminated NET in a minority of highly selected patients with high SSTR expression (based on risk and symptoms, primary tumour location);
Consideration of PRRT for rechallenge in patients with disease stabilization or remission for at least one year after end of first PRRT;
PRRT in combination with CAPTEM if Ki67>20% only in clinical trials;
[68Ga]Ga-DOTA-SSA PET/CT, in association with diagnostic CT for diagnosis, including unknown primary detection, for staging, for restaging after surgery, in case of progression of known or suspected NET, and for selection for PRRT;
[18F]FDG in G3 NET, in NEC, in cases presenting CT lesions negative for [68Ga]Ga-DOTA-SSA, and in cases showing rapid progression regardless of tumor grade;
[68Ga]Ga-DOTA-SSA for suspected extra-adrenal localization of PPGL with or without CT lesions.
Highlights.
[68Ga]Ga-DOTA-SSA PET/CT and diagnostic CT are the mainstay for NET diagnosis
[18F]FDG in: G3 NET, NEC, CT-pos/SSA-neg lesions, rapidly progressive cases
[68Ga]Ga-DOTA-SSA for suspected extra-adrenal localization of PPGL
PRRT is indicated at first progression of G1-G2 GEP NET and selected NET G3
PRRT as second line for GI-NET,s if there is sufficient uptake in all lesions
Acknowledgements
The authors acknowledge John Bean PhD (Bean Medical Writing, Halle, Belgium) for providing medical writing services, and Hanna Krippl, Susanne Koebe, Petra Neubauer, Barbora Trnena, Jutta Peter, Andreas Felser, and Henrik Silber (all from EANM) for project management. The EANM Focus 3 meeting was funded by the EANM and supported by unrestricted grants from Advanced Accelerator Applications, a Novartis company, ITM Isotopen Technologien München AG, Ipsen, and Siemens Healthineers. These sponsors had no direct or indirect influence on the program and content of the EANM Focus 3 meeting nor the writing and content of this article.
VA reports personal fees from ESMIT and AAA outside the submitted work and is a member of ENETS advisory board, ESMO faculty staff for NET and the scientific board of ITANET; JK reports personal fees from Bayer, outside the submitted work; LB reports non-paid consultant for AAA-Novartis, Ipson, Clovis Oncology, and Curium, and a research grant from AAA-Novaris; JC reports grants and personal fees from Bayer, Eisai, Advanced Accelerator Applications, and Ipsen, and grants from Astrazeneca, Novartis, Pfizer, Merck, Sanofi, Amgen, and Exelixis outside the submitted work; WDH reports personal fees and grants from Ipsen, personal fees from Novartis, and personal fees and grants, from AAA; CD reports consultancy/advisor for Ipsen; MaF reports personal fees from AAA, Novartis, Ipsen, and Celgene outside the submitted work; MeF reports personal fees and research funding from Ipsen; SF reports personal fees from ANMI, Astellas, Bayer, BlueEarth Diagnostics, GE Healthcare, Jenssen, Novartis, Sofie Biosciences, non-financial support from AAA, Bayer, GE Healthcare, Curium, Tema Sinergie, Sanofi, Telix, outside the submitted work; RJH reports holding shares in Telix Pharmaceuticals on behalf of his institution and receiving research funding from Ispen and ITM; VL reports advisory board member of AAA and Ipsen; DOT reports personal fees from Novartis, IPSEN, AstraZeneca and grants from Wyeth Lederle and Ipsen outside the submitted work; MP reports personal fees from AAA, Pfizer, and Riemser and grants and personal fees from Novartis and IPSEN outside the submitted work; RS reports consultancy for AAA, Ipsen, Novartis, ITM and Keocyt and participation in the NETTER 1 and COMPETE Trials; JS reports consultant for Novartis and speaker’s bureau for Ipsen and Lexicon outside the submitted work; DT reports personal fees from AAA/Novartis, IPSEN and Sanofi-Genzyme, outside the submitted work; DW reports personal fees from Ipsen and grants form Siemens Healthineers and Debiopharm International S.A; KH reports personal fees from Bayer, other from Sofie Biosciences, personal fees from SIRTEX, non-financial support from ABX, personal fees from Adacap, personal fees from Curium, personal fees from Endocyte, grants and personal fees from BTG, personal fees from IPSEN, personal fees from Siemens Healthineers, personal fees from GE Healthcare, personal fees from Amgen, personal fees from Novartis, personal fees from ymabs, outside the submitted work; JY reports financial activities with Advanced Accelerated Applications (AAA), Chiasma, Crinetics, Hutchison MediPharma, Ipsen, Merck, Novartis, and Tarvedin.
Footnotes
Declaration of interests
EB, CB, SM, MiC, MC, LK, GK, KO, WO, ASc, ASu, and IV declare no competing interests.
References
- [1].Hallet J, Law CHL, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121(4):589–e97. 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- [2].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3(10): 1335–e42. 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zamora V, Cabanne A, Salanova R, et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis 2010;42(3):220–e5. 10.1016/j.dld.2009.07.018. [DOI] [PubMed] [Google Scholar]
- [4].Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016; 103(2):153–e71. 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatinreceptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Canc 2010;17(1):R53–e73. 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- [6].Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26(13):2124–e30. 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- [7].Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Ludotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376(2):125–e35. 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hicks RJ, Kwekkeboom DJ, Krenning E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology 2017; 105(3):295–e309. 10.1159/000475526. [DOI] [PubMed] [Google Scholar]
- [9].Sundin A, Arnold R, Baudin E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging. Neuroendocrinology 2017;105(3):212–e44. 10.1159/000471879. [DOI] [PubMed] [Google Scholar]
- [10].Taıëb D, Hicks RJ, Hindie E, et al. European association of nuclear medicine practice guideline/society of nuclear medicine and molecular imaging procedure standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imag 2019;46(10):2112–e37. 10.1007/s00259-019-04398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bar-Sever Z, Biassoni L, Shulkin B, et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur J Nucl Med Mol Imag 2018;45(11):2009–e24. 10.1007/s00259-018-4070-8. [DOI] [PubMed] [Google Scholar]
- [12].Bozkurt MF, Virgolini I, Balogova S, et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68GaDOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imag 2017;44(9):1588–e601. 10.1007/s00259-017-3728-y. [DOI] [PubMed] [Google Scholar]
- [13].Niederle B, Pape U-F, Costa F, et al. ENETS consensus guidelines update for neuroendocrine neoplasms of the Jejunum and ileum. Neuroendocrinology 2016;103(2):125–e38. 10.1159/000443170. [DOI] [PubMed] [Google Scholar]
- [14].Giovanella L, Treglia G, Iakovou I, Mihailovic J, Verburg FA, Luster M. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur J Nucl Med Mol Imag 2020; 47(1):61–e77. 10.1007/s00259-019-04458-6. [DOI] [PubMed] [Google Scholar]
- [15].Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and followup. Ann Oncol 2019;30(12):1856–e83. 10.1093/annonc/mdz400. [DOI] [PubMed] [Google Scholar]
- [16].Howe JR, Cardona K, Fraker DL, et al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American neuroendocrine tumor society. Pancreas 2017;46(6):715–e31. 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103(2):186–e94. 10.1159/000443172. [DOI] [PubMed] [Google Scholar]
- [18].Leyden S, Kolarova T, Bouvier C, et al. Unmet needs in the international neuroendocrine tumor (NET) community: assessment of major gaps from the perspective of patients, patient advocates and NET health care professionals. Int J Canc 2020; 146:1316–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med 2010;51: 875–e82. [DOI] [PubMed] [Google Scholar]
- [20].Deppen SA, Blume J, Bobbey AJ, et al. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med 2016; 57:872–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kunikowska J, Lewington V, Krolicki L. Optimizing somatostatin receptor imaging in patients with neuroendocrine tumors: the impact of 99mTc-HYNICTOC SPECT/SPECT/CT versus 68Ga-DOTATATE PET/CT upon clinical management. Clin Nucl Med 2017;42:905–e11. [DOI] [PubMed] [Google Scholar]
- [22].Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol 2015;69:199–e207. 10.1016/j.jclinepi.2015.07.010.e2. [DOI] [PubMed] [Google Scholar]
- [23].Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358. 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155(8):529–e36. 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- [25].Higgins JGS. Cochrane handbook for systematic reviews of interventions. [Accessed 17 January 2020]. https://handbook-5-1.cochrane.org/.
- [26].Delphi method|RAND. [Accessed 22 November 2017]. https://www.rand.org/topics/delphi-method.html.
- [27].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159–e74. [PubMed] [Google Scholar]
- [28].Bruinsma SM, Roobol MJ, Carroll PR, et al. Expert consensus document: semantics in active surveillance for men with localized prostate cancer - results of a modified Delphi consensus procedure. Nat Rev Urol 2017;14(5):312–e22. 10.1038/nrurol.2017.26. [DOI] [PubMed] [Google Scholar]
- [29].Kleynen M, Braun SM, Bleijlevens MH, Lexis MA, Rasquin SM, et al. Using a Delphi technique to seek consensus regarding definitions, descriptions and classification of terms related to implicit and explicit forms of motor learning. PloS One 2014;9(6):e100227. 10.1371/journal.pone.0100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mokkink L, Terwee C, Knol D, et al. BMC medical research methodology protocol of the COSMIN study: COnsensus-based standards for the selection of health measurement INstruments. BMC Med Res Methodol 2006;6:2. 10.1186/14712288-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zafar SY, Currow DC, Cherny N, Strasser F, Fowler R, Abernethy AP. Consensus-based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol 2012; 13(2):e77–e82. 10.1016/S1470-2045(11)70215-7. [DOI] [PubMed] [Google Scholar]
- [32].Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32(4):1008–e15. 10.1046/j.1365-2648.2000.t01-1-01567.x. [DOI] [PubMed] [Google Scholar]
- [33].Barrio M, Czernin J, Fanti S, et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med 2017;58(5):756–e61. 10.2967/jnumed.116.185587. [DOI] [PubMed] [Google Scholar]
- [34].Cheng X, Bao L, Xu Z, Li D, Wang J, Li Y. 18F-FDG-PET and 18F-FDG-PET/CT in the detection of recurrent or metastatic medullary thyroid carcinoma: a systematic review and metaanalysis. J Med Imag Radiat Oncol 2012;56(2):136–e42. 10.1111/j.1754-9485.2012.02344.x. [DOI] [PubMed] [Google Scholar]
- [35].Geijer H akan Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and metaanalysis. Eur J Nucl Med Mol Imag 2013;40(11):1770–e80. 10.1007/s00259-013-2482-z. [DOI] [PubMed] [Google Scholar]
- [36].Graham MM, Gu X, Ginader T, Breheny P, Sunderland JJ. 68Ga-DOTATOC imaging of neuroendocrine tumors: a systematic review and metaanalysis. J Nucl Med 2017;58(9): 1452–e8. 10.2967/jnumed.117.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singh S, Poon R, Wong R, Metser U. 68Ga PET imaging in patients with neuroendocrine tumors: a systematic review and meta-analysis. Clin Nucl Med 2018;43(11):802–e10. 10.1097/RLU.0000000000002276. [DOI] [PubMed] [Google Scholar]
- [38].Skoura E, Michopoulou S, Mohmaduvesh M, et al. The impact of 68Ga-DOTATATE PET/CT imaging on management of patients with neuroendocrine tumors: experience from a national referral center in the United Kingdom. J Nucl Med 2016;57(1): 34–e40. 10.2967/jnumed.115.166017. [DOI] [PubMed] [Google Scholar]
- [39].Treglia G, Rufini V, Salvatori M, Giordano A, Giovanella L. PET imaging in recurrent medullary thyroid carcinoma. Int J Mol Imag 2012;2012. 10.1155/2012/324686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Treglia G, Cocciolillo F, de Waure C, et al. Diagnostic performance of 18F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis. Eur J Nucl Med Mol Imag 2012;39(7):1144–e53. 10.1007/s00259-012-2087-y. [DOI] [PubMed] [Google Scholar]
- [41].Treglia G, Kakhki VRD, Giovanella L, Sadeghi R. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: a systematic review and meta-analysis. Am J Clin Dermatol 2013; 14(6):437–e47. 10.1007/s40257-013-0040-x. [DOI] [PubMed] [Google Scholar]
- [42].Treglia G, Tamburello A, Giovanella L. Detection rate of somatostatin receptor PET in patients with recurrent medullary thyroid carcinoma: a systematic review and a meta-analysis. Hormones (Athens) 2017;16(4):362–e72. 10.14310/horm.2002.1756. [DOI] [PubMed] [Google Scholar]
- [43].van Treijen MJC, van Beek D-J, van Leeuwaarde RS, Vriens MR, Valk GD. Diagnosing nonfunctional pancreatic NETs in MEN1: the evidence base. J Endocr Soc 2018;2(9): 1067–e88. 10.1210/js.2018-00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol 2014;55(4):389–e98. 10.1177/0284185113496679. [DOI] [PubMed] [Google Scholar]
- [45].Han S, Suh CH, Woo S, Kim YJ, Lee JJ. Performance of 68GaDOTAeConjugated somatostatin receptoretargeting peptide PET in detection of pheochromocytoma and paraganglioma: a systematic review and metaanalysis. J Nucl Med 2019;60(3): 369–e76. 10.2967/jnumed.118.211706. [DOI] [PubMed] [Google Scholar]
- [46].Jacobson AF, Deng H, Lombard J, Lessig HJ, Black RR. 123IMeta-Iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysis. J Clin Endocrinol Metab 2010;95(6):2596–e606. 10.1210/jc.2009-2604. [DOI] [PubMed] [Google Scholar]
- [47].Van Der Horst-Schrivers ANA, Jager PL, Boezen HM, Schouten JP, Kema IP, Links TP. Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas–experience and meta-analysis. Anticancer Res 2006; 26(2B):1599–e604. [PubMed] [Google Scholar]
- [48].Brito JP, Asi N, Gionfriddo MR, et al. The incremental benefit of functional imaging in pheochromocytoma/paraganglioma: a systematic review. Endocrine 2015;50(1):176–e86. 10.1007/s12020-015-0544-7. [DOI] [PubMed] [Google Scholar]
- [49].Kan Y, Zhang S, Wang W, Liu J, Yang J, Wang Z. 68Ga-somatostatin receptor analogs and 18F-FDG PET/CT in the localization of metastatic pheochromocytomas and paragangliomas with germline mutations: a meta-analysis. Acta Radiol 2018;59(12):1466–e74. 10.1177/0284185118764206. [DOI] [PubMed] [Google Scholar]
- [50].Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EPM. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol 2014;81(5):642–e51. 10.1111/cen.12542. [DOI] [PubMed] [Google Scholar]
- [51].van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EPM. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol 2014;80(4):487–e501. 10.1111/cen.12341. [DOI] [PubMed] [Google Scholar]
- [52].Gulenchyn KY, Yao X, Asa SL, Singh S, Law C. Radionuclide therapy in neuroendocrine tumours: a systematic review. Clin Oncol 2012;24(4):294–e308. 10.1016/j.clon.2011.12.003. [DOI] [PubMed] [Google Scholar]
- [53].Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med 2002;32:84–e91. [DOI] [PubMed] [Google Scholar]
- [54].Kunikowska J, Królicki L, Hubalewska-Dydejczyk A, Mikołajczak R, Sowa-Staszczak A, Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90YDOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imag 2011;38: 1788–e97. 10.1007/s00259-011-1833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kunikowska J, Zemczak A, Kołodziej M, et al. Tandem peptide receptor radionuclide therapy using 90Y/177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects - polish multicenter experience. Eur J Nucl Med Mol Imag 2020;47(4): 922–e33. 10.1007/s00259-020-04690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Villard L, Romer A, Marincek N, et al. Cohort study of somatostatin-based radiopeptide therapy with [90 Y-DOTA]TOC versus [90 Y-DOTA]-TOC plus [177 Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 2012;30(10):1100–e6. 10.1200/JCO.2011.37.2151. [DOI] [PubMed] [Google Scholar]
- [57].Thang SP, Lung MS, Kong G, et al. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imag 2018;45(2):262–e77. 10.1007/s00259-0173821-2. [DOI] [PubMed] [Google Scholar]
- [58].Nicolini S, Severi S, Ianniello A, et al. Investigation of receptor radionuclide therapy with 177Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imag 2018;45(6):923–e30. 10.1007/s00259-017-3925-8. [DOI] [PubMed] [Google Scholar]
- [59].Pavlakis N, Ransom DT, Wyld D, et al. First results for Australasian Gastrointestinal Trials Group (AGITG) control net study: phase II study of 177Lu-octreotate peptide receptor radionuclide therapy (LuTate PRRT) þ/ capecitabine, temozolomide (CAPTEM) for midgut neuroendocrine tumors (mNETs). J Clin Orthod 2020;38(4_suppl):604. 10.1200/JCO.2020.38.4_suppl.604.604. [DOI] [Google Scholar]
- [60].van Vliet EI, van Eijck CH, de Krijger RR, et al. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med 2015;56(11): 1647–e53. 10.2967/jnumed.115.158899. [DOI] [PubMed] [Google Scholar]
- [61].Brabander T, van der Zwan WA, Teunissen JJM, et al. LongTerm efficacy, survival, and safety of [177Lu-DOTA0,Tyr3] octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Canc Res 2017;23(16): 4617–e24. 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- [62].Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364(6): 514–e23. 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Raymond E, Dahan L, Raoul J-L, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364(6):501–e13. 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- [64].Pavel M, Ö berg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31: 844–e60. [DOI] [PubMed] [Google Scholar]
- [65].Chan DL, Pavlakis N, Schembri GP, et al. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics 2017;7(5):1149–e58. 10.7150/thno.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kayani I, Bomanji JB, Groves A, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68GaDOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008;112(11):2447–e55. 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- [67].Kashyap R, Hofman MS, Michael M, et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imag 2015;42(2):176–e85. 10.1007/s00259-014-2906-4. [DOI] [PubMed] [Google Scholar]
- [68].Severi S, Nanni O, Bodei L, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imag 2013;40(6):881–e8. 10.1007/s00259-013-2369-z. [DOI] [PubMed] [Google Scholar]
- [69].Sansovini M, Severi S, Ianniello A, et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur J Nucl Med Mol Imag 2017;44(3):490–e9. 10.1007/s00259-0163533-z. [DOI] [PubMed] [Google Scholar]
- [70].Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18Ffluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Canc Res 2010;16(3):978–e85. 10.1158/1078-0432.CCR-091759. [DOI] [PubMed] [Google Scholar]
- [71].Zemczak A, Kołodziej M, Gut P, et al. Effect of Peptide Receptor Radionuclide Therapy (PRRT) with tandem isotopes [90Y]Y/[177Lu]Lu-DOTATATE in patients with disseminated neuroendocrine tumours depending on qualification [18F]FDG PET/CT in Polish multicenter experience - do we need [18F] FDG. Endokrynol Pol 2020;71(3):240–e8. 10.5603/EP.a2020.0014. [DOI] [PubMed] [Google Scholar]
- [72].Kroiss A, Shulkin BL, Uprimny C, et al. (68)Ga-DOTATOC PET/CT provides accurate tumour extent in patients with extraadrenal paraganglioma compared to (123)I-MIBG SPECT/CT. Eur J Nucl Med Mol Imag 2015;42:33–e41. [DOI] [PubMed] [Google Scholar]
- [73].Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTATyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48:508–e18. [DOI] [PubMed] [Google Scholar]
- [74].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 2000;92(3):205–e16. http://www.ncbi.nlm.nih.gov/pubmed/10655437. [DOI] [PubMed] [Google Scholar]
- [75].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Canc 2009;45(2):228–e47. 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- [76].Oberg K, Modlin IM, De Herder W, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol 2015; 16(9):e435–e46. 10.1016/S1470-2045(15)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Oberg K, Krenning E, Sundin A, et al. A Delphic consensus assessment: imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect 2016;5(5):174–e87. 10.1530/EC-16-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imag 2015;42(1):5–e19. 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- [79].Cremonesi M, Ferrari M, Bodei L, et al. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur J Nucl Med Mol Imag 2018;45: 2426–e41. 10.1007/s00259-018-4044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sandstrom M, Garske-Roman U, Granberg D, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med 2013; 54(1):33–e41. 10.2967/jnumed.112.107524. [DOI] [PubMed] [Google Scholar]
- [81].Svensson J, Berg G, Wängberg B, Larsson M, ForssellAronsson E, Bernhardt P. Renal function affects absorbed dose to the kidneys and haematological toxicity during 177LuDOTATATE treatment. Eur J Nucl Med Mol Imag 2015;42(6): 947–e55. 10.1007/s00259-015-3001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ilan E, Sandstrom M, Wassberg C, et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med 2015;56(2):177–e82. 10.2967/jnumed.114.148437. [DOI] [PubMed] [Google Scholar]
- [83].Garske-Román U, Sandström M, Fröss Baron K, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imag 2018;45(6):970–e88. 10.1007/s00259-0183945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sundlöv A, Gustafsson J, Brolin G, et al. Feasibility of simplifying renal dosimetry in 177Lu peptide receptor radionuclide therapy. EJNMMI Phys 2018;5(1):12. 10.1186/s40658-018-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chauvin M, Borys D, Botta F, et al. OpenDose: open access resources for nuclear medicine dosimetry. J Nucl Med 2020. 10.2967/jnumed.119.240366.jnumed.119.240366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Elston MS, Meyer-Rochow GY, Conaglen HM, et al. Increased SSTR2A and SSTR3 expression in succinate dehydrogenasedeficient pheochromocytomas and paragangliomas. Hum Pathol 2015;46(3):390–e6. 10.1016/j.humpath.2014.11.012. [DOI] [PubMed] [Google Scholar]
- [87].Kaemmerer D, Sänger J, Arsenic R, et al. Evaluation of somatostatin, CXCR4 chemokine and endothelin A receptor expression in a large set of paragangliomas. Oncotarget 2017; 8(52):89958–e69. 10.18632/oncotarget.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kong G, Grozinsky-Glasberg S, Hofman MS, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab 2017;102(9):3278–e87. 10.1210/jc.201700816. [DOI] [PubMed] [Google Scholar]
- [89].Neumann HPH, William F, Young J, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med 2019. https://www.nejm.org/doi/10.1056/NEJMra1806651. [Accessed 7 February 2020]. [Google Scholar]
- [90].Kolasinska-Cwikła A, Pe ˛czkowska M, Cwikła JB, et al. A clin- ical efficacy of PRRT in patients with advanced, nonresectable, paraganglioma-pheochromocytoma, related to SDHx gene mutation. J Clin Med 2019;8(7). 10.3390/jcm8070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Vyakaranam AR, Crona J, Norlén O, et al. Favorable outcome in patients with pheochromocytoma and paraganglioma treated with 177Lu-DOTATATE. Cancers 2019;11(7). 10.3390/cancers11070909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zandee WT, Feelders RA, Duijzentkunst DAS, et al. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur J Endocrinol 2019;181(1):45–e53. 10.1530/EJE-18-0901. [DOI] [PubMed] [Google Scholar]
- [93].Nicolas GP, Schreiter N, Kaul F, et al. Sensitivity comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase II imaging study. J Nucl Med 2018;59(6):915–e21. 10.2967/jnumed.117.199760. [DOI] [PubMed] [Google Scholar]
- [94].Nicolas GP, Beykan S, Bouterfa H, et al. Safety, biodistribution, and radiation dosimetry of 68Ga-OPS202 in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase I imaging study. J Nucl Med 2018;59(6):909–e14. 10.2967/jnumed.117.199737. [DOI] [PubMed] [Google Scholar]
- [95].Cescato R, Waser B, Fani M, Reubi JC. Evaluation of 177LuDOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med 2011;52(12):1886–e90. 10.2967/jnumed.111.095778. [DOI] [PubMed] [Google Scholar]
- [96].Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med 2017;58(Suppl 2):61S–e6S. 10.2967/jnumed.116.186783. [DOI] [PubMed] [Google Scholar]
- [97].Antwi K, Fani M, Heye T, et al. Comparison of glucagon-like peptide-1 receptor (GLP-1R) PET/CT, SPECT/CT and 3T MRI for the localisation of occult insulinomas: evaluation of diagnostic accuracy in a prospective crossover imaging study. Eur J Nucl Med Mol Imag 2018;45(13):2318–e27. 10.1007/s00259-018-4101-5. [DOI] [PubMed] [Google Scholar]
- [98].Wild D, Christ E, Caplin ME, et al. Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med 2011;52(7):1073–e8. 10.2967/jnumed.110.085142. [DOI] [PubMed] [Google Scholar]
- [99].Chondrogiannis S, Marzola MC, Al-Nahhas A, et al. Normal biodistribution pattern and physiologic variants of 18F-DOPA PET imaging. Nucl Med Commun 2013;34(12):1141–e9. 10.1097/MNM.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bodei L, Kidd MS, Singh A, et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imag 2020;47(4):895–e906. 10.1007/s00259-019-04601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bodei L, Kidd MS, Singh A, et al. PRRT genomic signature in blood for prediction of 177Lu-octreotate efficacy. Eur J Nucl Med Mol Imag 2018;45(7):1155–e69. 10.1007/s00259-018-3967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kunikowska J, Królicki L. Targeted a-emitter therapy of neuroendocrine tumors. Semin Nucl Med 2020;50:171–e6. [DOI] [PubMed] [Google Scholar]