Summary
Background
Trastuzumab is a monoclonal antibody against HER2 (also known as ERBB2). The primary objective of the NRG Oncology/RTOG-1010 trial was to establish whether trastuzumab improves disease-free survival when combined with trimodality treatment (paclitaxel plus carboplatin and radiotherapy, followed by surgery) for patients with untreated HER2-overexpressing oesophageal adenocarcinoma.
Methods
NRG Oncology/RTOG-1010 was an open label, randomised, phase 3 trial for which patients were accrued from 111 NRG-affiliated institutions in the USA. Eligible patients were adults (aged ≥18 years) with newly diagnosed pathologically confirmed oesophageal adenocarcinoma, American Joint Committee on Cancer 7th edition T1N1–2 or T2–3N0–2 stage disease, and a Zubrod performance status of 0–2. Patients were stratified by adenopathy (no vs yes [coeliac absent] vs yes [coeliac present ≤2 cm] ) and randomly assigned (1:1) to receive weekly intravenous paclitaxel (50 mg/m2 intravenously over 1 h) and carboplatin (area under the curve 2, intravenously over 30–60 min) for 6 weeks with radiotherapy 50·4 Gy in 28 fractions (chemoradiotherapy) followed by surgery, with or without intravenous trastuzumab (4 mg/kg in week one, 2 mg/kg per week for 5 weeks during chemoradiotherapy, 6 mg/kg once presurgery, and 6 mg/kg every 3 weeks for 13 treatments starting 21–56 days after surgery). The primary endpoint, disease-free survival, was defined as the time from randomisation to death or first of locoregional disease persistence or recurrence, distant metastases, or second primary malignancy. Analyses were done by modified intention to treat. This study is registered with Clinicaltrials.gov, NCT01196390; it is now closed and in follow-up.
Findings
606 patients were entered for HER2 assessment from Dec 30, 2010 to Nov 10, 2015, and 203 eligible patients who were HER2-positive were enrolled and randomly assigned to chemoradiotherapy plus trastuzumab (n=102) or chemoradiotherapy alone (n=101). Median duration of follow-up was 2·8 years (IQR 1·4–5·7). Median disease-free survival was 19·6 months (95% CI 13·5–26·2) with chemoradiotherapy plus trastuzumab compared with 14·2 months (10·5–23·0) for chemoradiotherapy alone (hazard ratio 0·99 [95% CI 0·71–1·39], log-rank p=0·97). Grade 3 treatment-related adverse events occurred in 41 (43%) of 95 patients in the chemoradiotherapy plus trastuzumab group versus 52 (54%) of 96 in the chemoradiotherapy group and grade 4 events occurred in 20 (21%) versus 21 (22%). The most common grade 3 or worse treatment-related adverse events for both groups were haematological (53 [56%] of 95 patients in the chemoradiotherapy plus trastuzumab group vs 55 [57%] of 96 patients in the chemotherapy group) or gastrointestinal disorders (28 [29%] vs 20 [21 %]). 34 (36%) of 95 patients in the chemoradiotherapy plus trastuzumab group and 27 (28%) of 96 patients in the chemoradiotherapy only group had treatment-related serious adverse events. There were eight treatment-related deaths: five (5%) of 95 patients in the chemoradiotherapy plus trastuzumab group (bronchopleural fistula, oesophageal anastomotic leak, lung infection, sudden death, and death not otherwise specified), and three (3%) of 96 in the chemoradiotherapy group (two multiorgan failure and one sepsis).
Interpretation
The addition of trastuzumab to neoadjuvant chemoradiotherapy for HER2-overexpressing oesophageal cancer was not effective. Trastuzumab did not lead to increased toxicities, suggesting that future studies combining it with or using other agents targeting HER2 in oesophageal cancer are warranted.
Introduction
Oesophageal cancer is an important cause of cancer death. The ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial showed an improvement in progression-free survival and overall survival with trimodality carboplatin, paclitaxel, and concurrent radiotherapy followed by oesophagectomy compared with surgery alone in patients with oesophageal cancer.1 Unfortunately, the majority of patients who receive this trimodality therapy will still relapse and die from their disease. Therapies that block aberrant growth factor pathways have shown important progress in the treatment of malignancies, such as non-small-cell lung cancer and myelogenous leukaemia. The human epidermal growth factor receptor (HER) family consists of four transmembrane glycoproteins (HER1–HER4).2 HER2 is the preferred dimerisation partner of the other HER family members.3
The HER2 gene encodes a transmembrane glycoprotein receptor, p185HER2, that is targeted by the humanised anti-p185HER2 monoclonal antibody trastuzumab.4 In breast cancer, trastuzumab increases overall survival in the metastatic and adjuvant settings.4,5 HER2 gene amplifi cation by fluorescence in-situ hybridisation has been reported in 19–32% of patients with oesophageal adeno carcinoma.6,7 In 2007, the Brown University Oncology Research Group reported a phase 1–2 study of 19 patients in which trastuzumab was added to standard paclitaxel, carboplatin, and 50·4 Gy radiotherapy.8 No cardiac toxicity of grade 3 or worse was reported.8 The Trastuzumab for Gastric Cancer (ToGA) trial was a randomised phase 3 trial in metastatic gastroesophageal cancer that showed that overall survival was significantly improved with the addition of trastuzumab to chemotherapy. 7 These data provided the rationale for the initiation of an intergroup phase 3 trial, RTOG 1010, led by the Radiation Therapy Oncology Group (RTOG), now NRG Oncology. The primary objective was to establish whether the addition of trastuzumab to trimodality treatment for patients with HER2-overexpressing oesophageal cancer increases disease-free survival.
Methods
Study design and participants
NRG Oncology/RTOG 1010 is an open-label, randomised, phase 3 trial, for which patients were accrued from 111 NRG-affiliated institutions in the USA (appendix pp 1–3).
Eligibility criteria included patients aged 18 years or older with newly diagnosed pathologically confirmed adenocarcinoma of the oesophagus involving the mid (≤25 cm), distal, or oesophagogastric junction with involvement of up to 5 cm of the stomach via endoscopy. Siewert types I–III were eligible. Patients were required to have stage T1N1–2 or T2–3N0–2 disease, according to the American Joint Committee on Cancer 7th edition. Patients could have para-oesophageal, gastric, gastro-hepatic, and coeliac nodes. If coeliac adenopathy was present, it had to be no more than 2 cm in size. All patients were required to have CT scan of the chest, abdomen, and pelvis or a whole-body PET–CT scan at baseline. Endoscopic ultrasound was required for disease staging unless there was obvious adenopathy on CT or PET–CT scan. Patients had to have a white blood cell count of at least 2 × 109 cells per L, absolute neutrophil count of at least 1·5 × 109 cells per L, platelet count of at least 100 × 109 per L, haemoglobin concentration of at least 80 g/L, serum creatinine concentration of up to 2 × the upper limit of normal (ULN), aspartate aminotransferase concentration up to 3 × ULN, and total bilirubin concentration up to 1·5 × ULN.
Eligibility also required surgical consultation to confirm patients were candidates for potentially curative resection, as well as consultations with radiation oncologists and medical oncologists. Medical and radiation oncology consultations were required to confirm patients were appropriate candidates for chemoradiotherapy. Other eligibility included left ventricular ejection fraction (LVEF) of at least the institutional lower limit of normal by cardiac echography or multiple-gated acquisition scan, and a Zubrod performance status of 0–2. No previous treatment for oesophageal cancer was allowed. Key exclusion criteria included previous invasive malignancy (except for non-melanomatous skin cancer) unless disease-free for at least 2 years, previous history of congestive heart failure, or severe active comorbidity. Complete eligibility criteria are provided in the protocol (appendix).
Patients had to provide written, informed consent. The protocol, protocol amendments, and informed consent documents were approved by the National Cancer Institute Central institutional review board and the institutional review board or ethics committee at each site before the start of the study. Participants were not compensated. The NRG/RTOG Data Monitoring Committee evaluated the trial twice per year for accrual and toxic effects, as well as at predefined timepoints for outcome.
Randomisation and masking
Patients were stratified by presence of adenopathy (no vs yes [coeliac absent] vs yes [coeliac present ≤2 cm]) and randomly assigned (1:1) to receive radiotherapy plus paclitaxel plus carboplatin plus trastuzumab (chemoradio therapy plus trastuzumab group) or radiotherapy plus paclitaxel plus carboplatin (chemoradiotherapy group) by means of Zelen9 modified permuted block randomisation with a block size of two.
Treatment assignment was centrally generated at the NRG Oncology/RTOG Oncology Statistics and Data Management Center (Philadelphia, PA, USA). Treatment assignment was not masked to participating site, enrolling physician, or responsible statistician.
Procedures
HER2 overexpression (evaluated at screening) was defined by immunohistochemistry score of 3+ or HER2 amplification by fluorescence in-situ hybridisation (FISH). To determine HER2 status, dual immunohistochemistry and FISH testing were done. The immunohistochemistry scoring system used the modified immunohistochemistry scoring validated in the phase 3 ToGA study7 and as reviewed by Hofmann and colleagues.10 FISH was defined as positive if the HER2-to-chromosome enumeration probe 17 (CEP17) ratio was greater than 2·0. For tumours with immunohistochemistry of 0, 1+, or 2+ by immunohistochemistry, if they were FISH positive they were considered to be HER2 positive. Central pathological review was used for determination of HER2 overexpression.
All patients received weekly chemoradiotherapy (once a week), consisting of concurrent paclitaxel (50 mg/m2 intra venously over 1 h), carboplatin (area under the curve 2, intravenously over 30–60 min), and radiotherapy (5 days a week) 50·4 Gy in 28 fractions (1·8 Gy per fraction) followed by surgery (5–8 weeks after completion of chemoradio therapy). Radiation could be delivered by means of three-dimensional conformal radiotherapy or intensity-modulated radiotherapy. The initial phase of radiotherapy was 45 Gy in 25 fractions. A minimum of 95% of the planning target volume received 45 Gy. The final boost was an additional 5·4 Gy in three fractions. For this phase, a minimum of 95% of the boost planning target volume received a dose of 5·4 Gy.
Paclitaxel and carboplatin were interrupted for grade 3 or worse non-haematological toxicities, absolute neutrophil count less than 1·5 × 109 cells per L, or platelet count less than 75 × 109 per L and resumed at a one doselevel reduction when non-haematology toxicity resolved to a maximum of grade 2 severity, absolute neutrophil count was at least 1·0 × 109 cells per L, and platelet count was at least 75 × 109 per L. Radiotherapy was interrupted for absolute granulocyte counts of 0·5 × 109 cells per L or less, platelet count of 50 × 109 per L or less, and radiation-related, non-haematological toxicity exceeding grade 3 in severity (appendix).
Patients randomly assigned to the trastuzumab group received 4 mg/kg trastuzumab intravenously over 90 min in week one (once a week), then 2 mg/kg per week over 30–60 min for 5 weeks during chemoradiotherapy, then 6 mg/kg for one dose before surgery and 6 mg/kg every 3 weeks for 13 treatments starting 21–56 days after surgery. Dose reductions were not allowed for trastuzumab. The decision to continue or pause trastuzumab treatment was based on the measured LVEF as described in the protocol.
Patients were assessed for toxicity and had laboratory assessments weekly during chemoradiotherapy and before surgery. CT scans were done every 4 months from the end of surgery for 2 years, then annually. Adverse events were graded by means of the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4). Assessment of LVEF was obtained in all patients, before chemoradiotherapy and after chemoradiotherapy. In the trastuzumab group, an echocardiogram was repeated at 3, 6, 9, and 12 months from the start of maintenance treatment. In the chemoradiotherapy only group, an echocardiogram was done at 6 and 12 months after surgery.
Patients were not removed from the study by the investigators; however, patients had the right to withdraw consent at any time. All data up to the date of consent withdrawal are used.
Outcomes
The primary endpoint was disease-free survival, defined as the time from randomisation to death or any of the following events: local or regional disease persistence or recurrence, distant metastases, or second primary malignancy. Radiographic review was done by the treating investigator, and central radiographic review was not utilised. If a patient had an R0 or R1 resection they were considered disease free after surgery. However, patients who had an R2 resection were not considered disease free after surgery. For patients who did not undergo surgery, those patients who had a negative endoscopic biopsy were considered disease free, whereas those patients with a positive biopsy and those not undergoing biopsy were considered to have persistence of disease.
Secondary endpoints included overall survival (time from randomisation to death from any cause), adverse events, predictors of cardiotoxicity, and pathological com plete response at time of surgery (5–8 weeks after completion of radiotherapy and defined as no cancer in the resected oesophageal specimen and accompanying lymph nodes and margins free of tumour). Molecular correlates of efficacy and the patient-reported quality of life secondary endpoints will be reported separately.
Statistical analysis
Per the protocol statistical analysis plan, all randomly assigned patients meeting protocol-specified eligibility were included in this analysis on the basis of the group to which they were randomly assigned (modified intentionto-treat).9 For safety, the population was the same as the efficacy analysis accept that we excluded patients who did not receive any treatment as they could not possibly have any toxicities related to treatment. The sample size was based on the primary objective that the addition of trastuzumab would increase disease-free survival. Assuming a median disease-free survival of 15 months with chemoradio therapy alone, it was hypothesised that the addition of trastuzumab would result in an HR of 0·60, corresponding to a median disease-free survival of 25 months. Assuming an exponential distribution and constant hazards, 183 HER2-positive patients were required for 162 disease-free survival events, with 90% statistical power, a two-sided α of 0·05, 5 years of accrual, 3 years of follow-up, and two interim analyses (after 81 and 122 disease-free survival events; efficacy p values of 0·001 and futility p values of 0·83 and 0·25, respectively). Allowing for a 7% or lower ineligibility rate, the final target randomised accrual was 197 HER2-positive patients. On the basis of a projected 33% HER2-positive rate, it was projected that 591 patients would need to be screened in order to reach this number of HER2-positive patients.
Owing to concerns that trastuzumab could increase cardiac adverse events, the trial included safety interim analyses assessing the rate of cardiomyopathy-related adverse events after the first 25 and 50 evaluable patients in the chemoradiotherapy plus trustuzumab group. These cardiomyopathy-related adverse events were defined as grade 3–4 restrictive cardiomyopathy or incipient congestive heart failure. Incipient congestive heart failure was defined as an asymptomatic greater than 20% decrease in LVEF or a decrease in LVEF greater than 10% below the institutional lower limit of normal that is judged to require treatment according to institutional guidelines. In addition, after 50 evaluable patients had been entered and followed from the start of chemoradiotherapy up to the earlier date of surgery or 6 weeks after completion of chemoradiotherapy, the rates of cardiomyopathy-related adverse events, all grade 3 or worse non-haematological adverse events, and all grade 3 or worse adverse events were compared between the treatment groups.
A χ2 test, with a two-sided α of 0·05, was used to compare pathological complete response rates between treatment groups. The 95% CIs for the rates were calculated using the binomial exact calculation. Disease-free survival and overall survival were estimated by the Kaplan-Meier method.11 The distributions of disease-free survival and overall survival estimates between the two groups were compared by means of the log-rank test. The 95% CIs for the disease-free survival and overall survival rates estimates were calculated using their SEs.12 Disease-free survival time was measured from the date of randomisation to the date of first failure or last follow-up. Overall survival was measured from the date of randomisation to the date of death or last follow-up and tested with a two-sided α of 0·05. The 2-year, 3-year, and 4-year disease-free survival and overall survival estimates are also reported (as post-hoc timepoints). A post-hoc, as-treated sensitivity analysis was done for disease-free survival. In a prespecified analysis, the Cox proportional hazard regression model13 was used to analyse treatment effect for disease-free survival and overall survival (to see if treatment was associated with these outcomes while adjusting for other variables) with the stratification factor (presence of adenopathy [no vs yes—coeliac absent vs yes—coeliac present ≤2 cm]) included as a fixed covariate for disease-free survival and overall survival. Other covariates considered in the multivariable Cox proportional hazard model by means of the backwards selection procedure at a significance level of 0·05 were age (<65 years vs ≥65 years), sex (female vs male), race (White vs non-White), Zubrod performance status (0 vs 1–2), T stage (T1/T2 vs T3), and N stage (N0 vs N1/2). The proportional hazards assumption was met, on the basis of non-random patterns in the graphical diagnostics of the scaled Schoenfeld residuals and p greater than 0·05. Logistic regression was used to evaluate predictors of any cardiac toxicity by means of the same covariates and significance level. Missing data were minimal; therefore, no imputations for missing data were done and analyses were done with the use of all available data.
Statistical analyses were done using SAS statistical software (version 9·4). The trial is registered at Cinicaltrials.gov, NCT01196390.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
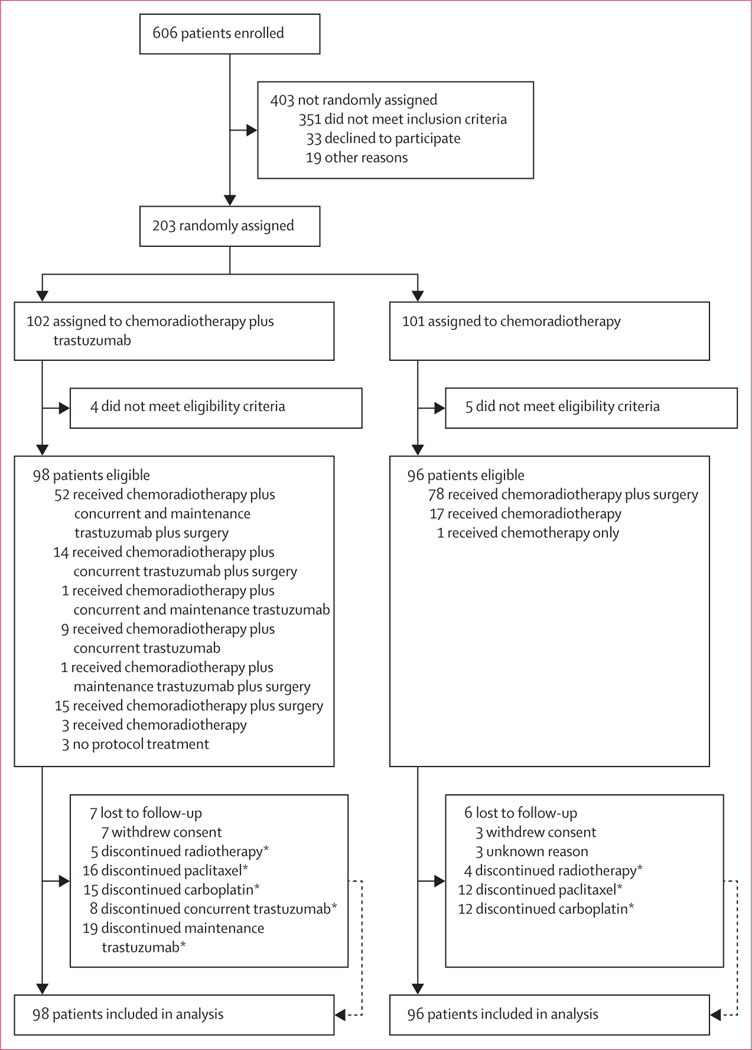
Accrual began on Dec 30, 2010, and was completed on Nov 10, 2015, with 606 patients enrolled and 203 HER2positive patients randomly assigned: 102 to the chemoradiotherapy plus trastuzumab group and 101 to the chemoradiotherapy group (figure 1; appendix pp 4–5). Four patients in the chemoradiotherapy plus trastuzumab group and five in the chemoradiotherapy group were ineligible and were not included in the analysis. Patient characteristics of the 194 eligible patients (98 in the chemoradiotherapy plus trastuzumab group and 96 in the chemoradiotherapy group) are shown in table 1. As of the data cutoff date of Dec 31, 2020, the median duration of follow-up was 2·8 years (IQR 1·4–5·7) and it was 6·0 (4·8–7·0) years for patients who were still alive. Age, T stage, N stage, and Zubrod performance status were well balanced between the treatment groups (table 1). Endoscopic ultrasound was done as part of staging in 90 (92%) of 98 patients in the chemo radiotherapy plus trastuzumab group and 86 (90%) of 96 patients in the chemoradiotherapy group.
Figure 1: Trial profile.
*For reasons for discontinuation see appendix (pp 4–5).
Table 1:
Baseline characteristics
| Chemoradiotherapy plus trastuzumab group (n=98) | Chemoradiotherapy group (n=96) | |
|---|---|---|
| Age, years | ||
| Median | 63 (56–68) | 66 (57–71) |
| Minimum–maximum | 40–80 | 24–83 |
| Sex | ||
| Male | 85 (87%) | 79 (82%) |
| Female | 13 (13%) | 17 (18%) |
| Race | ||
| American Indian or Alaskan Native | 0 | 2 (2%) |
| Asian | 3 (3%) | 0 |
| Black or African American | 1 (1%) | 0 |
| White | 94 (96%) | 92 (96%) |
| Unknown | 0 | 2 (2%) |
| Ethnicity | ||
| Hispanic or Latinx | 1 (1%) | 5 (5%) |
| Not Hispanic or Latinx | 96 (98%) | 89 (93%) |
| Unknown | 1 (1%) | 2 (2%) |
| Zubrod performance status | ||
| 0 | 62 (63%) | 62 (65%) |
| 1 | 34 (35%) | 31 (32%) |
| 2 | 2 (2%) | 3 (3%) |
| T stage, clinical * | ||
| T1 | 1 (1%) | 4 (4%) |
| T2 | 18 (18%) | 17 (18%) |
| T3 | 79 (81%) | 75 (78%) |
| N stage, clinical * | ||
| N0 | 27 (28%) | 29 (30%) |
| N1 | 55 (56%) | 48 (50%) |
| N2 | 16 (16%) | 19 (20%) |
| Presence of adenopathy | ||
| No | 38 (39%) | 38 (40%) |
| Yes—coeliac adenopathy absent | 48 (49%) | 48 (50%) |
| Yes—coeliac adenopathy present ≤2 cm | 12 (12%) | 10 (10%) |
| HER2-positive determination | ||
| Positive by immunohistochemistry | 23 (23%) | 18 (19%) |
| Positive by fluorescence in-situ hybridisation | 34 (35%) | 21 (22%) |
| Positive by both | 41 (42%) | 57 (59%) |
Data are n (%) or median (IQR), unless otherwise stated.
American Joint Committee on Cancer 7th edition.
Per the protocol design, 3 years of follow-up was projected to reach the required 162 disease-free survival events for the final analysis. At the end of that 3 years, in the statistical monitoring of the number of primary endpoint events, it was noted that there had been a drastic drop off in new events, despite having up-to-date follow-up data. This issue was discussed extensively with the NRG Data Monitoring Committee. As the number of events at the time of that discussion (Feb 7, 2019) provided sufficient statistical power (>80%) to test the primary endpoint hypothesis, the recommendation was made to release the trial for reporting. The statistical power for the disease-free survival results reported in this analysis, with 137 disease-free survival events, is 85%.
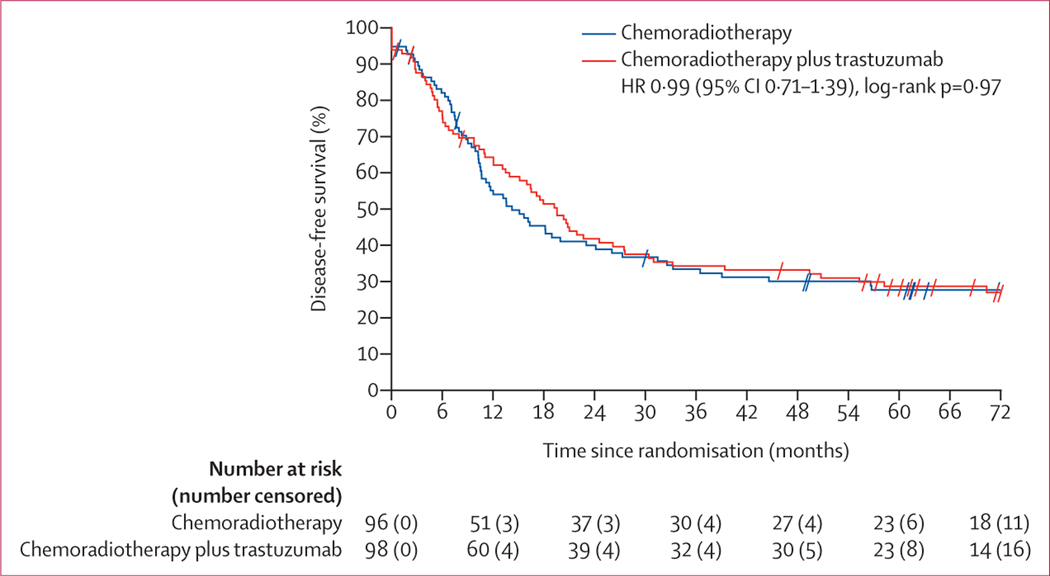
The analysis reported here is the final analysis since neither of the two planned disease-free survival efficacy or futility interim analyses crossed prespecified boundaries. Of the 137 disease-free survival events in the final analysis, 70 were in the chemoradiotherapy plus trastuzumab group and 67 were in the chemoradiotherapy group. Median disease-free survival was 19·6 months (95% CI 13·5–26·2) in the chemoradiotherapy plus trastuzumab group compared with 14·2 months (10·5–23·0) in the chemoradiotherapy group (hazard ratio [HR] 0·99 [95% CI 0·71–1·39], log-rank p=0·97; figure 2). In addition to the protocol intention-to-treat analyses, as-treated sensitivity analyses were run and produced similar disease-free survival results (appendix p 6).
Figure 2: Kaplan-Meier curve of disease-free survival.
Tick marks represent censored patients. HR=hazard ratio.
The estimated 2-year, 3-year, and 4-year disease-free survival estimates for the chemoradiotherapy plus trastuzumab group were 41·8% (95% CI 31·8–51·7), 34·3% (24·7–43·9), and 33·2% (23·7–42·7), respectively, and for the chemoradiotherapy group were 40·0% (30·0–49·9), 33·4% (23·8–43·0), and 30·1% (20·7–39·4), respectively.
Table 2 shows the multivariable Cox proportional hazard models for disease-free survival. After adjusting for other factors, treatment was not a significant factor for an improvement in disease-free survival, when comparing chemoradiotherapy plus trastuzumab with chemoradiotherapy alone. Only age and T stage were associated with disease-free survival, whereas presence of adenopathy, sex, race, Zubrod performance status, and pretreatment nodal involvement were not. Besides the stratification factor of adenopathy, only significant variables are shown in Table 2.
Table 2:
Multivariable analyses for disease-free and overall survival
| Disease-free survival | Overall survival | |||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | p value* | Hazard ratio (95% CI) | p value* | |
| Treatment group | ||||
| Chemoradiotherapy | 1 (ref) | .. | 1 (ref) | .. |
| Chemoradiotherapy plus trastuzumab | 1·10 (0·78–1·55) | 0·59 | 1·14 (0·78–1·66) | 0·51 |
| Age, years | ||||
| <65 | 1 (ref) | .. | 1 (ref) | .. |
| ≥65 | 1·98 (1·40–2·80) | 0·0001 | 2·24 (1·53–3·28) | <0·0001 |
| T stage | ||||
| T1–T2 | 1 (ref) | .. | 1 (ref) | .. |
| T3 | 1·88 (1·19–2·96) | 0·0069 | 2·23 (1·30–3·83) | 0·0035 |
| Presence of adenopathy | ||||
| No | 1 (ref) | .. | 1 (ref) | .. |
| Yes—coeliac adenopathy absent | 1·08 (0·75–1·57) | 0·67 | 0·99 (0·66–1·49) | 0·97 |
| Yes—coeliac adenopathy present ≤2 cm | 1·16 (0·66–2·02) | 0·61 | 0·77 (0·40–1·46) | 0·42 |
χ2 using the Cox proportional hazards model for disease-free and overall survival.
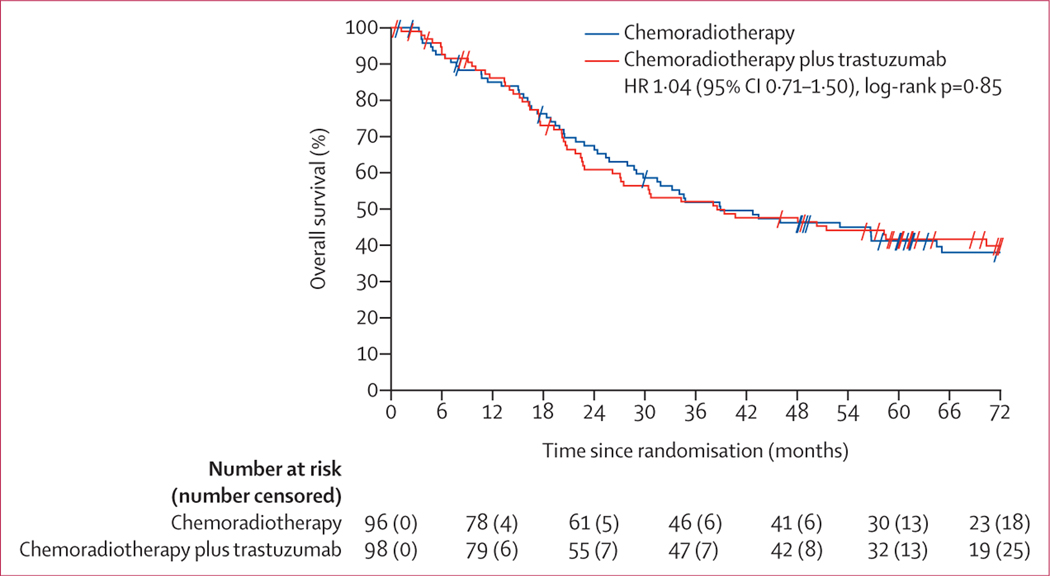
At data cutoff, 111 patients had died: 56 in the chemoradiotherapy plus trastuzumab group and 55 in the chemoradiotherapy group. Causes of death are shown in the appendix (p 7). Median overall survival was 38·5 months (95% CI 26·2–70·4) in the chemoradiotherapy plus trastuzumab group and 38·9 months (29·0–64·5) in the chemoradiotherapy group (HR 1·04 [95% CI 0·71–1·50], log-rank p=0·85; figure 3). The estimated 2-year, 3-year, and 4-year overall survival estimates for the chemoradiotherapy plus trastuzumab group were 60·8% (95% CI 50·8–70·8), 52·0% (41·7–62·2), and 47·6% (37·3–57·8), respectively, and for the chemoradiotherapy group were 67·4% (57·8–77·0), 51·8% (41·5–62·1), and 46·2% (35·9–56·5), respectively. The multivariable Cox analysis for overall survival showed similar results to that for disease-free survival (table 2).
Figure 3: Kaplan-Meier curve of overall survival.
Tick marks represent censored patients. HR=hazard ratio.
82 (84%) of 98 patients underwent surgical resection in the chemoradiotherapy plus trastuzumab group and 78 (81%) of 96 patients underwent surgery in the chemoradiotherapy group. 34 (18%) of 194 patients (16 in the chemoradiotherapy plus trastuzumab group and 18 in the chemoradiotherapy group) did not undergo surgery (figure 1). Patient refusal and progression were the main reasons for no surgery (appendix p 5). Thoracoabdominal oesophagectomy was done in more than half of patients (47 [57%] of 82 in the chemoradiotherapy plus trastuzumab group and 29 [37%] of 78 in the chemoradiotherapy group). Transthoracic oesophagectomy (via the two-stage Ivor Lewis approach was the second most common surgical procedure (appendix p 8). The median time from end of concurrent treatment to surgery was 6 weeks (IQR 4–7) for the chemoradiotherapy plus trastuzumab group and 7 weeks (6–8) for the chemo radiotherapy group. The median number of nodes resected was 15 (IQR 11–20) for the chemoradiotherapy plus trastuzumab group and 13 (9–19) for the chemoradiotherapy group. The number of patients with positive surgical margins was two (2%) of 82 in the chemoradiotherapy plus trastuzumab group and none in the chemoradiotherapy group. The resection rates for patients undergoing surgery in the chemoradiotherapy plus trastuzumab group were as follows R0 80 (98%) of 82 patients, R1 two (2%) patients, and R2 no patients. In the chemoradiotherapy group, all 78 (100%) patients had R0 resection. The pathological complete response rate was 22 (27%) of 82 patients in the chemoradiotherapy plus trastuzumab group versus 23 (29%) of 78 in the chemoradiotherapy group (p=0·71).
The findings of the modality-specific treatment reviews are summarised in the appendix (pp 9–12). Three (2%) of 98 patients (all of whom were in the chemoradiotherapy plus trastuzumab group) did not receive protocol treatment and are excluded from the results of the reviews. Notably, 83 (87%) of 95 patients in the chemoradiotherapy plus trastuzumab group and 89 (93%) of 96 in the chemoradiotherapy group had radiation tumour volume contours scored acceptable according to protocol (appendix p 9).
On concurrent chemotherapy review, there were no notable differences between the groups for paclitaxel and carboplatin. One (1%) of 96 patients in the chemoradiotherapy group had an unacceptable deviation (appendix p 10). 76 (80%) of 95 patients received concurrent trastuzumab per protocol or with acceptable variation, and 65 (68%) of 95 patients received at least 85% of the planned concurrent trastuzumab dose (appendix p 11). 87 (89%) of 95 patients were evaluable for maintenance trastuzumab review. 56 (64%) of these 87 patients were per protocol or with acceptable variation and 41 (47%) of 86 patients received at least 85% of the planned trastuzumab dose (appendix p 11). Of the 95 patients in the chemoradiotherapy plus trastuzumab group who received treatment, 37 (39%) patients had a dose reduction of their systemic therapy, and of the 96 patients in the chemoradiotherapy group who received treatment, 23 (24%) patients had a dose reduction of their systemic therapy.
On surgical review, most patients had surgery per protocol or with acceptable variation. One (1%) of 78 patients in the chemoradiotherapy group had an unacceptable deviation (appendix p 12) due to having a proximal gastrectomy and not a protocol-suggested surgical procedure.
Three of 98 patients in the chemoradiotherapy plus trastuzumab group did not receive any protocol treatment; therefore, 95 patients in the chemoradiotherapy plus trastuzumab group and 96 in the chemoradiotherapy group are included in the safety analyses. There was no increase in the proportion of patients experiencing grade 3 or worse treatment-related adverse events in the trastuzumab group compared with the chemoradiotherapy group (66 [69%] of 95 vs 76 [79%] of 96; table 3). The most common grade 3 or worse adverse events in both groups were haematological (defined in table 3; 53 [56%] of 95 patients in the chemoradiotherapy plus trastuzumab group vs 55 [57%] of 96 patients in the chemotherapy group) and gastrointestinal disorders (28 [29%] vs 20 [21 %]). In the chemo radiotherapy plus trastuzumab group, 20 (21%) of 95 patients had treatment-related grade 4 adverse events and five (5%) patients had treatment-related grade 5 adverse events. In the chemoradiotherapy group, 21 (22%) of 96 patients had treatment-related grade 4 adverse events and three (3%) patients had treatment-related grade 5 adverse events. Seven of the eight grade 5 adverse events occurred within 90 days of surgery and included four (of five) in the chemoradiotherapy plus trastuzumab group (bronchopleural fistula, oesophageal anastomotic leak, lung infection, and sudden death) and three (of three) in the chemoradiotherapy group (multiorgan failure [n=2] and sepsis). One of five patients in the chemoradiotherapy plus trastuzumab group had a death not otherwise specified after completion of chemoradio therapy before surgery.
Table 3:
Adverse events related to treatment
| Chemoradiotherapy plus trastuzumab group (n=95*) | Chemoradiotherapy group (n=96) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| Overall highest grade | 29 (31%) | 41 (43%) | 20 (21%) | 5 (5%) | 20 (21%) | 52 (54%) | 21 (22%) | 3 (3%) |
| Haematological† | 34 (36%) | 36 (38%) | 17 (18%) | 0 | 39 (41%) | 36 (38%) | 19 (20%) | 0 |
| Blood and lymphatic system disorders | 52 (55%) | 7 (7%) | 1 (1%) | 0 | 51 (53%) | 2 (2%) | 0 | 0 |
| Cardiac disorders | 9 (9%) | 4 (4%) | 1 (1%) | 0 | 9 (9%) | 1 (1%) | 2 (2%) | 0 |
| Ear and labyrinth disorders | 4 (4%) | 0 | 0 | 0 | 3 (3%) | 0 | 0 | 0 |
| Eye disorders | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal disorders | 62 (65%) | 25 (26%) | 3 (3%) | 0 | 68 (71%) | 19 (20%) | 1 (1%) | 0 |
| General disorders and administration site conditions | 71 (75%) | 2 (2%) | 1 (1%) | 2 (2%) | 65 (68%) | 11 (11%) | 0 | 2 (2%) |
| Hepatobiliary disorders | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 |
| Infections and infestations | 4 (4%) | 5 (5%) | 5 (5%) | 1 (1%) | 5 (5%) | 5 (5%) | 1 (1%) | 1 (1%) |
| Injury, poisoning, and procedural complications | 11 (12%) | 0 | 1 (1%) | 1 (1%) | 15 (16%) | 1 (1%) | 1 (1%) | 0 |
| Investigations | 36 (38%) | 35 (37%) | 16 (17%) | 0 | 39 (41%) | 37 (39%) | 19 (20%) | 0 |
| Metabolism and nutrition disorders | 51 (54%) | 12 (13%) | 0 | 0 | 39 (41%) | 19 (20%) | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 15 (16%) | 1 (1%) | 0 | 0 | 14 (15%) | 4 (4%) | 0 | 0 |
| Nervous system disorders | 35 (37%) | 0 | 0 | 0 | 33 (34%) | 2 (2%) | 1 (1%) | 0 |
| Psychiatric disorders | 14 (15%) | 2 (2%) | 0 | 0 | 18 (19%) | 0 | 0 | 0 |
| Renal and urinary disorders | 1 (1%) | 0 | 1 (1%) | 0 | 2 (2%) | 0 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 26 (27%) | 3 (3%) | 2 (2%) | 1 (1%) | 26 (27%) | 5 (5%) | 2 (2%) | 0 |
| Skin and subcutaneous tissue disorders | 32 (34%) | 0 | 0 | 0 | 24 (25%) | 2 (2%) | 0 | 0 |
| Surgical and medical procedures | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
| Vascular disorders | 13 (14%) | 3 (3%) | 1 (1%) | 0 | 9 (9%) | 4 (4%) | 0 | 0 |
Data are n (%).
Excludes patients who did not receive any protocol treatment. Adverse events were graded with National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.
Haematological includes the following CTCAE version 4 terms from the investigations system organ class: CD4 lymphocytes decreased, lymphocyte count decreased, lymphocyte count increased, neutrophil count decreased, platelet count decreased, and white blood cell decreased; and all terms from the system organ class of blood and lymphatic system disorders.
34 (36%) of 95 patients in the chemoradiotherapy plus trastuzumab group and 27 (28%) of 96 patients in the chemoradiotherapy only group had treatment-related serious adverse events. The most common serious treatment-related adverse events reported in the chemoradiotherapy plus trastuzumab group were gastro intestinal disorders (13 [14%] of 95 patients) with oesophagitis reported in three (3%) patients. Sepsis was also reported in five (5%) patients in the chemoradiotherapy plus trastuzumab group (appendix pp 13–18). The most common serious treatment-related adverse events reported in the chemoradiotherapy group were gastrointestinal disorders (11 [11%] of 96 patients) with nausea reported in six (6%). Dehydration was also reported in nine (9%) patients in the chemoradiotherapy group (appendix pp 13–18).
Selected common treatment-related adverse events are shown in appendix (p 19). The prevalence of grade 3–4 oesophagitis was seven (7%) of 95 patients in the chemoradiotherapy plus trastuzumab group vs four (4%) of 96 in the chemoradiotherapy group. Five (5%) patients in the chemoradiotherapy plus trastuzumab group had grade 3–4 treatment-related cardiac disorder adverse events compared with three (3%) patients in the chemo radiotherapy group. Neither of the two planned interim analyses for cardiomyopathy-related adverse events crossed the prespecified boundaries for excessive cardiac adverse events in the chemoradiotherapy plus trastuzumab group (≥5 patients out of the first 25 evaluable patients; ≥6 patients out of the first 50 evaluable patients).
One patient in each group with grade 3–4 cardiac disorder adverse events had a greater than 10% decline in LVEF from baseline. All other grade 3–4 cardiac adverse events were related to atrial fibrillation, atrial flutter, sinus tachycardia, supraventricular tachycardia, and left ventricular systolic dysfunction. Logistic regression analysis did not identify any significant covariates associated with grade 3–4 treatment-related cardiac disorder adverse events (data not shown); however, given the small number of these events, there is little statistical power to detect such associations.
Discussion
The hypothesis for this study, based on the improvement of disease-free survival and overall survival with the use of trastuzumab in the adjuvant setting in breast cancer and the positive phase 3 ToGA trial in metastatic gastroesophageal cancer, was that neoadjuvant and adjuvant trastuzumab would improve outcomes in HER2-overexpressing oesophageal cancer. The RTOG-led intergroup trial INT0123/RTOG 94–05 was used to estimate the disease-free survival of 15 months for the control (chemoradiotherapy alone) group.14 The hypothesised HR for improvement of disease-free survival, which was 0·6 with the addition of trastuzumab, was based on the improvement in disease-free survival with trastuzumab in adjuvant breast cancer in the phase 3 studies by Romond and colleagues (HR 0·48, p<0·0001) and Piccart-Gebhart and colleagues (HR 0·54, p<0·0001).5,15 However, the data from NRG Oncology/ RTOG 1010 did not show an improvement in disease-free survival, overall survival, or pathological complete response with the addition of trastuzumab to trimodality treatment in HER2-positive oesophageal adenocarcinoma. However, there was no increase in oesophagitis or cardiac toxicity with the addition of trastuzumab.
Eligibility criteria for this trial included HER2-overexpressing oesophageal cancer on the basis of either FISH or immunohistochemistry per the eligibility of the phase 3 ToGA trial. Trastuzumab is approved by the US Food and Drug Administration for metastatic gastro-oesophageal cancer for patients with HER2 overexpression as determined by immunohistochemistry or FISH. A post-hoc exploratory analysis of the ToGA trial showed a median overall survival of 16 months with tumours with higher HER2 protein expression (immunohistochemistry 2+ or FISH-positive or immunohisto chemistry 3+) versus 11·8 months for patients with immunohistochemistry 0–1 who were FISH positive.16 In breast cancer, it has been strongly established that all patients who are FISH-positive can benefit from adjuvant trastuzumab; therefore, all patients who were FISH positive were eligible for RTOG 1010. However, in gastro-oesophageal cancers, interpretation of immuno histochemistry can be discordant. Haffner and colleagues showed a discordance rate of 22·7% in 458 patients with gastric cancer assessed for HER2 overexpression.17 Patients centrally confirmed as HER2-positive lived significantly longer than patients who received trastuzumab for locally established HER2-positive disease but were assessed as HER2-negative by the central laboratory.17 Therefore NRG/RTOG 1010 used central pathological review for determination of HER2 overexpression. A pathological tumour regression grade scoring system was not used since there are numerous tumour regression grading systems in use and there is no international standardisation.
There are several factors that could account for the results of this trial, including tumour heterogeneity and molecular mechanisms of resistance. Intrapatient tumour heterogeneity has been described between primary tumours and synchronous metastases.18 In a study of 22 pairs of tumour samples taken at baseline and post-progression in patients receiving chemotherapy and trastuzumab for advanced HER2-positive gastro-oesophageal cancer, loss of HER expression was noted in 32% of samples.19
The combination of chemotherapy and trastuzumab might limit the effect of heterogeneity by providing another means of cell killing. Only low-dose weekly radiosensitising chemotherapy for 6 weeks was administered concurrently with trastuzumab in NRG/RTOG 1010. Regimens other than paclitaxel and carboplatin are more effective in patients with metastatic HER2-positive oesophageal cancer and might be more effective in the neoadjuvant setting. For example, Rivera and colleagues assessed perioperative capecitabine, oxaliplatin, and trastuzumab,20 and the PETRARCA trial evaluated trastuzumab and pertuzumab with perioperative fluorouracil, leucovorin, oxaliplatin, and docetaxel.21
Oesophageal cancer is characterised by different molecular alterations to those of breast cancer.22 Molecular determinants of resistance to trastuzumab have been described in breast and gastro-oesophageal cancer. HER2 dimerisation leads to activation signalling via the MAPK and PI3K–AKT–mTOR pathways. Alterations in these pathways are associated with resistance to trastuzumab. For example, activation of the PI3K pathway has been identified as one of the most important mechanisms of resistance to anti-HER2 agents.23 Constitutive activation of PI3K via overexpression of PIK3CA mutants conferred resistance to trastuzumab in preclinical studies of breast cancer and gastro-oesophageal cancer.23 A series of 65 patients with HER2-amplified gastric cancer were assessed for genomic abnormalities in EGFR, MET, KRAS, PI3K, and PTEN.24 None of the trastuzumab-responding patients showed any abnormalities in these genes, whereas 55% of the resistant patients showed abnormalities. Median overall survival of patients without any molecular alterations in the panel was 16·1 months, compared with 7·6 months for those presenting at least one such alteration.24 Mutations in the HER2 gene have also been reported that change the receptor structure, preventing the binding of trastuzumab.25
Clinical trials have shown important differences in targeting HER2 in oesophageal cancer compared with breast cancer. Several agents with efficacy in breast cancer have been ineffective in oesophageal cancer, including lapatinib, pertuzumab, and trastuzumab emtansine.26–28
Limitations of this trial are that the trial protocol did not require biopsy at progression, and therefore data on HER2 expression at recurrence are not available. The administration of trastuzumab was not masked and central radiological review was not used to establish recurrence. This lack of masking and central review could have affected determination of disease-free survival. Trastuzumab was available to all patients at recurrence, which might have reduced the effect of adjuvant or neoadjuvant trastuzumab on overall survival. When the Data Monitoring Committee released the trial for reporting, the available statistical power to test the primary hypothesis was more than 80%, the minimum statistical power for a prospective phase 3 trial. At the time of the analysis reported here, the number of disease-free survival events provided 85% statistical power; therefore, the reporting of this trial before the planned 162 disease-free survival events is not a limitation for interpreting the results.
Laboratory correlative studies are being planned from NRG/RTOG 1010 to establish whether a cohort of patients can be identified that benefit from the addition of trastuzumab to trimodality treatment. To this end, assessing outcomes by the degree of HER2 positivity by FISH and immunohistochemistry is being considered. Genomic alterations in EGFR, MET, KRAS, PI3K, PTEN, and other abnormalities will also be assessed by next-generation sequencing to establish whether patients without these alterations benefit from trastuzumab.
Future strategies could include studying new agents to target HER2 in oesophageal cancer. Trastuzumab deruxtecan (DS-8201), an antibody–drug conjugate, has shown a response rate of 51% in metastatic gastro-oesophageal cancer.29 The addition of PD-1 blockade to trastuzumab in gastro-oesophageal cancer also appears to be a promising treatment approach.30
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles published in English between Jan 1, 2005, and Jan 1, 2020, using the terms “trastuzumab” combined with “esophageal adenocarcinoma”. To our knowledge, this was the first randomised trial assessing the role of trastuzumab with neoadjuvant chemoradiotherapy followed by surgery in patients with HER2 (also known as ERBB2)-overexpressing oesophageal adenocarcinoma. A previous phase 1 study showed that the addition of trastuzumab to chemoradiotherapy in oesophageal cancer was safe. A previous phase 3 study showed that trastuzumab increased overall survival in patients with metastatic HER2-overexpressing gastro-oesophageal cancer. A high percentage of patients with oesophageal adenocarcinoma have recurrent disease after trimodality treatment (carboplatin and paclitaxel with concurrent radiotherapy followed by oesophagectomy), creating a high unmet clinical need. There were no previous randomised studies to provide level 1A evidence for the addition of trastuzumab to trimodality treatment on disease-free survival, overall survival, or pathological complete response in previously untreated oesophageal adenocarcinoma.
Added value of this study
To our knowledge, NRG Oncology/RTOG 1010 is the first study to evaluate the addition of trastuzumab to trimodality treatment of HER2-overexpressing oesophageal cancer. The study results showed that trastuzumab was not effective in preventing disease recurrence, increasing pathological response, or prolonging overall survival. There was no significant increase in cardiac toxicity or other toxicities with the addition of trastuzumab.
Implications of all the available evidence
The addition of HER2-directed therapy with trastuzumab to trimodality treatment was not effective in HER2-overexpressing oesophageal cancer. Efforts to identify predictive biomarkers and molecular mechanisms of resistance are warranted. This study highlights the need for evaluating new combinations of HER2-directed therapies and HER2-targeted antibody–drug conjugates, including combinations with immunotherapy, in the trimodality setting.
Acknowledgments
This project was supported by the following grants from the US NCI: NRG Oncology Operations (grant number U10CA180868), NRG Oncology Statistics and Data Management Center (grant number U10CA180822), NCI Community Oncology Research Program (grant number UG1CA189867), and NRG Specimen Bank (grant number 24CA196067), and by Genentech. A copy of the manuscript was provided to the industry sponsor (Genentech) at the time of submission.
Funding National Cancer Institute and Genentech.
Footnotes
Declaration of interests
LAK reports personal fees from UpToDate and New Beta Innovations, and grants from Varian Medical Systems, outside the submitted work. DHI reports personal fees from Bristol Myers Squibb, Genentech, AstraZeneca, Merck, AMGEN, TAIHO, and Bayer, outside the submitted work. KLL reports personal fees from Elsevier, outside the submitted work. HM reports personal fees from Merck, outside the submitted work, and has received honoraria as chapter co-author for UpToDate (the chapters are all about gastrointestinal malignancies, but not oesophageal cancer). JM and KW report grants from the National Cancer institute (NCI) and other support from Genentech, during the conduct of the study. TD reports a patent for an intraoperative brachytherapy device. AKS, AWK, MGH, HPS, CHC, SS, MR, DADP, TSH, CMA, DW, LPL, XS, JS, and LR declare no competing interests.
Data sharing
Per NCI requirements, the data from this Article will be submitted to the NCI National Clinical Trials Network and the NCI Community Oncology Research Program data archive (https://nctn-data-archive.nci.nih.gov/) no later than 6 months after publication. After the required NCI reviews are completed, the data will be released and available in the data archive for data sharing proposals.
References
- 1.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–98. [DOI] [PubMed] [Google Scholar]
- 2.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2: 127–37. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002; 20: 719–26. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–84. [DOI] [PubMed] [Google Scholar]
- 6.Safran H, DiPetrillo T, Nadeem A, et al. Trastuzumab, paclitaxel, cisplatin, and radiation for adenocarcinoma of the esophagus: a phase I study. Cancer Invest 2004; 22: 670–77. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Cutsem EV, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–97. [DOI] [PubMed] [Google Scholar]
- 8.Safran H, Dipetrillo T, Akerman P, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys 2007; 67: 405–09. [DOI] [PubMed] [Google Scholar]
- 9.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis 1974; 27: 365–75. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008; 52: 797–805. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 12.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 1966; 50: 163–70. [PubMed] [Google Scholar]
- 13.Cox DR. Regression models and life-tables. J R Stat Soc B 1972; 34: 187–202. [Google Scholar]
- 14.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002; 20: 1167–74. [DOI] [PubMed] [Google Scholar]
- 15.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–72. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015; 18: 476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffner I, Schierle K, Raimundez E, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol 2021, 39: 1468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pectasides E, Stachler MD, Derks S, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov 2018; 8: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambardella V, Fleitas T, Tarazona N, et al. Towards precision oncology for HER2 blockade in gastroesophageal adenocarcinoma. Ann Oncol 2019; 30: 1254–64. [DOI] [PubMed] [Google Scholar]
- 20.Rivera F, Izquierdo-Manuel M, García-Alfonso P, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer 2021; 145: 158–67. [DOI] [PubMed] [Google Scholar]
- 21.Hofheinz RD, Haag GM, Ettrich TJ, et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: final results of the PETRARCA multicenter randomized phase II trial of the AIO. Proc Am Soc Clin Oncol 2020; 38 (suppl 15): 4502 (abstr). [Google Scholar]
- 22.Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541: 169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018; 8: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietrantonio F, Fucà G, Morano F, et al. Biomarkers of primary resistance to trastuzumab in HER2-positive metastatic gastric cancer patients: the AMNESIA case–control study. Clin Cancer Res 2018; 24: 1082–89. [DOI] [PubMed] [Google Scholar]
- 25.Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 2007; 99: 628–38. [DOI] [PubMed] [Google Scholar]
- 26.Hecht JR, Bang Y-J, Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC— a randomized phase III trial. J Clin Oncol 2016; 34: 443–51. [DOI] [PubMed] [Google Scholar]
- 27.Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018; 19: 1372–84. [DOI] [PubMed] [Google Scholar]
- 28.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017; 18: 640–53. [DOI] [PubMed] [Google Scholar]
- 29.Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 2020; 382: 2419–30. [DOI] [PubMed] [Google Scholar]
- 30.Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, singlearm, phase 2 trial. Lancet Oncol 2020; 21: 821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.