Abstract
The population pharmacokinetics of nevirapine (NVP), zidovudine (ZDV), and didanosine (ddI) were evaluated in a total of 175 patients infected with human immunodeficiency virus randomized to receive either a double combination of ZDV plus ddI or a triple combination of NVP plus ZDV plus ddI as a substudy of the AIDS Clinical Trials Group Protocol 241. Levels (approximating 3.5 determinations/patient) of the three drugs in plasma were measured during 44 of a total 48 weeks of study treatment, and a set of potential covariates was available for nonlinear mixed-effect modeling analysis. A one-compartment model with zero-order input and first-order elimination was fitted to the NVP data. Individual oral clearance (CL) and volume of distribution (V) averaged 0.0533 liters/h/kg of body weight and 1.17 liters/kg, respectively. Gender was the only covariate which significantly correlated with the CL of NVP. ZDV and ddI data were described by a two-compartment model with zero-order input and first-order elimination. Individual mean oral CL, VSS (volume of distribution at steady state), and V of ZDV were 1.84 liters/h/kg and 6.68 and 2.67 liters/kg, respectively, with body weight and age as correlates of CL and body weight as a correlate of VSS. The average individual oral CL, VSS, and V of ddI were 1.64 liters/h/kg and 3.56 and 2.74 liters/kg, respectively, with body weight as a significant correlate of both CL and VSS. The relative bioavailability (F) of ZDV and ddI in the triple combination compared to that in the double combination was also evaluated. No significant effects of the combination regimens on the F of ddI were detected (FTRIPLE = 1.05 and FDOUBLE = 1 by definition), but the F of ZDV was markedly reduced by the triple combination, being only 67.7% of that of the double combination. Large (>50%) intraindividual variability was associated with both ZDV and ddI pharmacokinetics. Individual cumulative area under the plasma drug level-time curve of the three drugs was calculated for the entire study period as a measure of drug exposure based on the individual data and the final-model estimates of structural and statistical parameters.
Until the early 1990s, monotherapy with zidovudine (ZDV), the first clinically approved nucleoside analog, was the predominant antiretroviral treatment for patients infected with human immunodeficiency virus type I (HIV-1) (15). Significant progress in the field of anti-HIV chemotherapy has been achieved with the development and evaluation of several additional nucleoside analogs inhibiting HIV reverse transcriptase, including didanosine (ddI), zalcitabine, lamivudine, and stavudine (38). With the availability of these drugs, novel strategies entailing combination therapy have become possible. Additive and/or synergistic antiviral effects have been shown in vitro by combining some of these nucleoside analogs (30, 33). Clinical trials involving two nucleoside analogs, such as ZDV with ddI, zalcitabine, or lamivudine have demonstrated more pronounced immunological and virological effects than ZDV monotherapy (12, 24, 37, 40). However, combination regimens of two nucleoside analogs were not highly effective in preventing further disease progression, due in part to the development of multidrug resistance (19, 29, 34). Therefore, it was hypothesized that the addition of a third drug with an independent mechanism of action and a resistance pattern that did not overlap with nucleoside resistance, such as the nonnucleoside reverse transcriptase inhibitor (NNRTI) nevirapine (NVP) (10) or a protease inhibitor (11) (e.g., ritonavir, saquinavir, indinavir, or nelfinavir), might more effectively contain replication of HIV. The combination of ZDV, ddI, and a NNRTI was shown to improve antiviral effect in vitro compared to ZDV and ddI (7). The phase II AIDS Clinical Trial Group (ACTG) Protocol 241 evaluated the safety, tolerability, and anti-HIV activity of the addition of NVP to ZDV and ddI among patients with extensive prior nucleoside treatment (9). This study demonstrated that the addition of NVP to the two nucleoside analogs led to improved antiviral and immunological effects over 48 weeks (9). This triple combination was shown to be even more effective in antiretroviral-naive HIV-infected patients, with the demonstration of undetectable plasma HIV RNA levels being achieved in a large number of patients (8).
As part of ACTG 241, substudy 809 was designed to evaluate the population pharmacokinetics of ZDV, ddI, and NVP. The population pharmacokinetics of NVP are described for the first time, as well as the population pharmacokinetic characteristics of ZDV and ddI when administered in a triple-combination regimen. Drug exposure for each patient and for each study drug was assessed by cumulative area under the plasma drug level-time curve (CAUC). Knowledge of drug exposure is expected to be useful in establishing the relationship of virological endpoints obtained during an intensive virology substudy performed on the same patient population, with drug exposure.
MATERIALS AND METHODS
Study design and patients.
ACTG 241 was a phase II, multicenter, randomized double-blinded clinical trial of a one-oral-dose regimen of NVP in combination with ZDV and ddI compared with ZDV and ddI in HIV-infected patients with CD4+ cell counts of less than 350/mm3 who had been treated with nucleoside analogs for more than 6 months (9). After giving written informed consent, a total of 398 patients were enrolled and received 200 mg of ZDV three times a day and 200 mg of ddI twice a day (b.i.d.) (patients weighing less than 60 kg received 125 mg b.i.d. of ddI) plus either a placebo of NVP b.i.d. or 200 mg b.i.d. of NVP. A cohort of 175 of the 398 patients participated in substudy 809, designed to evaluate the population pharmacokinetics of NVP, ZDV, and ddI. Table 1 summarizes patient characteristics by study drug.
TABLE 1.
Characteristics of patient population
| Characteristic | Value for group
|
||
|---|---|---|---|
| NVP | ZDV | ddI | |
| Patients (no. (%)] | |||
| Total | 82 | 175 | 172 |
| Double-combination arm (ZDV + ddI) | 89 (50.9) | 88 (51.2) | |
| Triple-combination arm (NVP + ZDV + ddI) | 82 | 86 (49.1) | 84 (48.8) |
| Samples | |||
| Total | 273 | 604 | 597 |
| Mean no./patient | 3.3 | 3.5 | 3.5 |
| Absorption population [no. (%)] | |||
| Fast absorbers | 12 (14.6) | 73 (41.7) | 106 (61.6) |
| Slow absorbers | 70 (85.4) | 102 (58.3) | 66 (38.4) |
| Weight [mean (range)] (kg) | 73.9 (51–109) | 73.6 (41–109) | 72.8 (41–109) |
| Age [mean (range)] (yr) | 39.2 (20.5–60.8) | 38.6 (15.7–60.8) | 38.7 (15.7–60.8) |
| Gender [no. (%)] | |||
| Women | 10 (12.2) | 28 (16.0) | 26 (15.1) |
| Men | 72 (87.8) | 147 (84.0) | 146 (84.9) |
| Race [no. (%)] | |||
| White | 69 (84.1) | 137 (78.3) | 139 (80.8) |
| Black | 5 (6.1) | 17 (9.7) | 16 (9.3) |
| Hispanic/Latino | 8 (9.8) | 21 (12.0) | 17 (9.9) |
| HIV acquisition risk [no. (%)] | |||
| HP | 4 (4.9) | 5 (2.9) | 5 (2.9) |
| IVUH | 11 (13.4) | 19 (10.9) | 17 (9.9) |
| HMS | 57 (69.5) | 127 (72.6) | 127 (74.3) |
| AIDS at entry [no. (%)] | 19 (23.2) | 25 (14.3) | 24 (14.0) |
Sampling schedule and analytical methods.
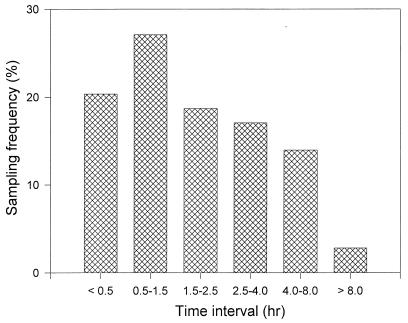
Blood samples were obtained from weeks 8 through 44 according to the following schedule: at week 8, a sample was drawn between 0.25 and 0.5 h and a second sample between 1 and 1.5 h after dosing; at week 24, a sample was obtained at 2.5 h after dosing; at week 32, a sample was collected between 3.5 and 4 h postadministration; and at week 44, a sample was drawn between 7 and 8 h after dosing. Figure 1 depicts the frequency distribution of plasma samples by sampling time interval. Drug administration involved the simultaneous ingestion of all assigned study medication. All blood samples (10 ml) were drawn into heparinized tubes and centrifuged at 2,000 × g for 10 min. The resulting plasma samples were stored at −20°C until analysis. Plasma ZDV levels were determined by a sensitive and specific double-antibody competitive radioimmunoassay (RIA) (ZDV-trac; Instar Corp., Stillwater, Minn.). Plasma ddI levels were measured by a double-antibody competitive RIA with anti-ddI rabbit antiserum, a goat anti-rabbit second antibody, and tritiated ddI (Sigma, St. Louis, Mo.). Both RIAs had a limit of quantitation of 1 ng/ml as well as intra- and interassay coefficients of variation (CVs) of less than 10%. The lowest quality control samples and associated variabilities (CVs) were 10 (5.5%) and 3 ng/ml (4.5%) for ZDV and ddI, respectively. Plasma NVP levels were quantitated by a high-performance liquid chromatographic method previously described (25). This method had a limit of quantitation of 50 ng/ml and intra- and interassay variability of less than 15%.
FIG. 1.
Frequency distribution of plasma samples by collection time interval.
Pharmacokinetic analysis.
All analyses were performed with the nonlinear mixed-effects modeling (NONMEM) program (double precision; version IV; level 1.2) (3). First-order conditional estimation was used. For all three drugs, the absorption phase was modeled as zero-order input (constant-rate infusion), with infusion duration (D) fixed to 0.25 h (first sampling time point) for fast absorbers or to be estimated (>0.25 h) for slow absorbers. For NVP, a one-compartment model with first-order elimination was used. Basic pharmacokinetic parameters were clearance (CL) and volume of distribution (V). Drugs were only administered by the oral route, and therefore the parameters termed “clearance” and “volume of distribution” represent the ratios of these parameters for any given drug to their unknown absolute bioavailability. For ZDV and ddI, a two-compartment model with first-order elimination was used, parameterized in CL, VSS (volume of distribution at steady state), V (volume of central compartment), and Q (intercompartmental clearance). V was modeled as a fraction of VSS: θpV × VSS, where θpV was the fractional volume to be estimated. Possible effects of treatment regimens on the pharmacokinetics of ZDV and ddI were evaluated by estimating the ratio of the bioavailability fraction (F) of the central compartment of drugs administered in the double or triple combination. Incorporating the treatment effect into the models for CL or V did not significantly improve data fit (data not shown). Therefore, it was assumed that the treatment affected only the bioavailability fraction. For the double combination, F was defined to be 1. Therefore, the estimated bioavailability ratio represents the relative bioavailability of the triple combination versus that of the double combination.
The variances of log CL, log VSS (two-compartment model) or log V (one-compartment model), and log D were assumed to be constant and were denoted ω2CL, ω2VSS or ω2V, and ω2D, respectively. Covariances ω2CL-VSS or ω2CL-V were also estimated. When a full covariance matrix of log clearance and log volume of distribution could not be precisely estimated, (a) ω2CL was always retained in the model, since CL was the most important parameter; (b) if the correlation between clearance and volume was low, then ω2VSS or ω2V was fixed to 0; or (c) if the correlation between clearance and volume was high (r2>0.5), it was fixed to unity, and variances of both CL and VSS were estimated. In the case of ZDV, ω2VSS was estimated as θpωVSS × ω2CL, where θpωVSS is the proportionality factor.
Since larger intraindividual variability was likely to be associated with levels drawn closer to the time of dosing, reflecting the variability of the rate of absorption, a mixture proportional-error model was used to describe residual variability as follows: Cij = Ĉij × (1 + θip × ɛij), where Cij and Ĉij are, respectively, the ith measured and model predicted plasma drug concentrations of individual j; ɛij is the residual intraindividual random error with zero mean and variance ς2, and θip is an increment proportion (ip) whose value is to be estimated but is fixed to 1 for a tj of >2 h.
The correlation of various continuous or categorical covariates with the principal pharmacokinetic parameters, including CL, VSS or V, and/or bioavailability fraction (F), were assessed as described by Mandema et al. (32). Briefly, a basic model lacking any covariates was determined and individual a posteriori Bayesian estimates of CL, VSS, or V were calculated. The differences between these individual estimates and the population mean values (residuals) were plotted against covariate values to visualize potential relationships. Such a visual inspection was further refined by evaluating the relationship between residuals and covariates by using a generalized additive model, thereby identifying the most significant covariate. A full model incorporating the covariates selected in the generalized additive model step was then built and further refined to yield a final model by deleting covariates which, when set to their null value, failed to significantly (P > 0.05) increase the minimum value objective function (MVOF) as supplied by NONMEM. Finally, each individual subject kinetic profile was obtained for the entire study period (48 weeks) by using the final-model parameter estimates, and the CAUC was determined, based on the simulated individual kinetic data, according to the trapezoidal rule.
RESULTS
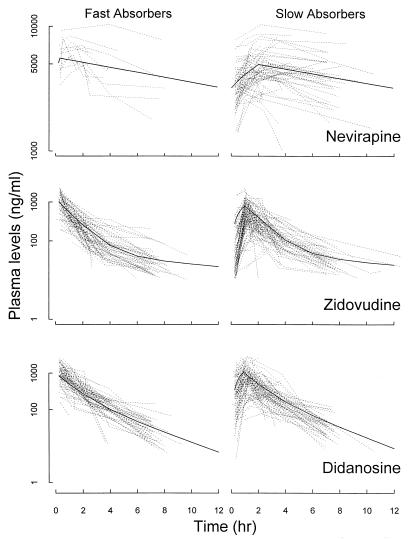
Individual plasma drug concentration-time courses for the study drugs, as depicted in Fig. 2, demonstrate that the patients can be divided into two different populations based on their absorption rates. The fast absorbers exhibited intravenous bolus-like patterns with only elimination and/or distribution phases, while the slow absorbers had typical oral profiles with absorption, elimination, and/or distribution phases.
FIG. 2.
Individual plasma drug level-time courses for NVP, ZDV, and ddI. The solid lines are the simulated curves with population-typical parameter estimates.
NVP.
Eighty-two of the 100 patients randomized to receive the triple combination (ZDV plus ddI plus NVP) had 273 (mean, 3.3 per individual) specimens drawn for quantitation of plasma NVP levels and subsequent NONMEM analysis. As described in Materials and Methods, a model retaining only ω2CL among various terms was selected. Results from the basic model of NVP are detailed in Table 2.
TABLE 2.
NVP model parameter estimates
| Parameter | Estimate | CV (%) | 95% Confidence interval |
|---|---|---|---|
| Basic model | |||
| θCL (liters/h) | 3.84 | 3.83 | 3.55–4.13 |
| θV (liters) | 83.5 | 8.63 | 69.37–97.63 |
| θD (h) | 1.98 | 9.34 | 1.62–2.34 |
| ω2CL | 0.0992 | 21.77 | 0.0568–0.141 |
| ς2 | 0.0440 | 22.39 | 0.0247–0.0633 |
| θip | 0.777 | 17.12 | 0.516–1.038 |
| Final model | |||
| θCL (liters/h) | 3.02 | 9.70 | 2.45–3.59 |
| θGENDER-CL (liters/h) | 0.274 | 37.5 | 0.0721–0.476 |
| θV (liters) | 84.5 | 8.92 | 69.72–99.28 |
| θD (h) | 1.96 | 9.44 | 1.597–2.323 |
| ω2CL | 0.0805 | 20.37 | 0.0484–0.113 |
| ς2 | 0.0476 | 21.64 | 0.0274–0.0678 |
| θip | 0.737 | 16.55 | 0.498–0.976 |
Initial covariate analysis (see Materials and Methods) identified three categorical factors, i.e., RACE, GENDER (1 = men; 0 = women), and AIDS, as possibly significant, but the factors RACE and AIDS failed to significantly increase MVOF and were deleted from the full model. GENDER was the only covariate incorporated into the final model for CL: CL (liters per hour) = 3.02 × e0.274 × GENDER V (liters) = 84.5 D (hours) = 0.25; if slow absorbers, D (hours) = 1.96
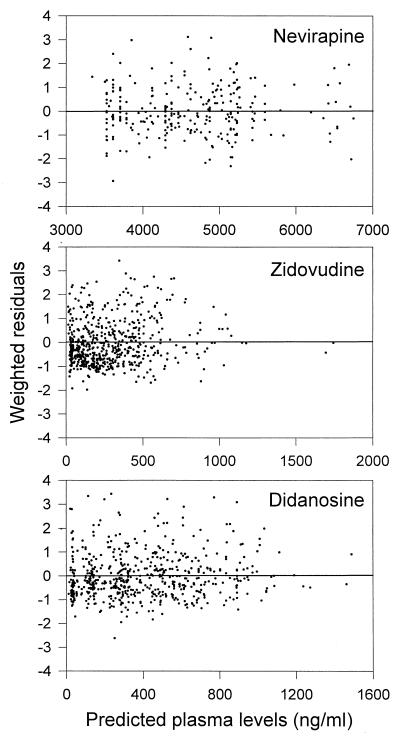
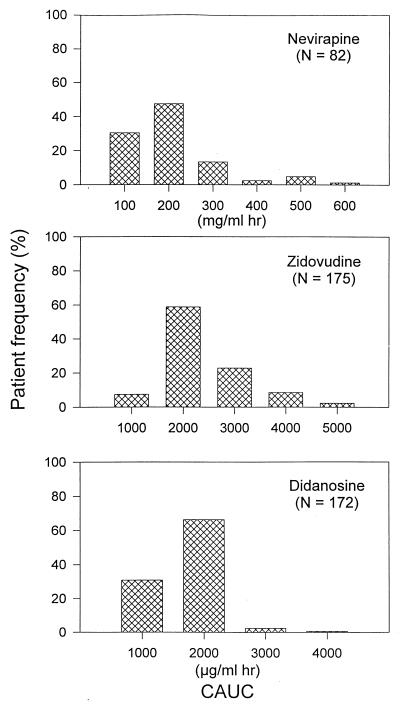
Other final-model estimates are presented in Table 2. Final individual estimates (mean ± standard deviation [SD]) of oral CL were 3.86 ± 0.31 liters/h, or 0.0533 ± 0.008 liters/h/kg of body weight, or 0.88 ± 0.02 ml/min/kg, and estimates of V were 84.5 liters or 1.17 ± 0.18 liters/kg. The residual variabilities as estimated from the basic and final models were comparable, being 21.0 and 21.8%, respectively. Simulated plasma kinetics of NVP with population-typical parameters for fast and slow absorbers are shown in Fig. 2. A plot of weighted residuals versus predicted levels of NVP is displayed in Fig. 3. The mean individual CAUC of NVP was 158.4 ± 102.5 mg/ml × h for the entire study period. The frequency distribution of individual CAUCs of NVP is depicted in Fig. 4.
FIG. 3.
Plots of weighted residuals versus predicted levels of NVP, ZDV, and ddI in plasma.
FIG. 4.
Frequency distribution of estimated individual CAUCs for NVP, ZDV, and ddI.
ZDV.
Among the patients enrolled in the substudy, 175 had a total of 604 (mean, 3.5 per patient) specimens drawn for measurement of plasma ZDV levels, which were used in the NONMEM analysis; 89 patients were from the double-combination arm, and 86 patients were from the triple-combination group. A two-compartment model with zero-order input and first-order elimination, as indicated by the individual plasma ZDV levels-versus-time plot (Fig. 2) and further ascertained by the likelihood ratio test, was used. Initial analysis during the search for the best basic model demonstrated that a full variance-covariance matrix could not be obtained (data not shown). However, the correlation between CL and VSS was significantly high (r2 = 0.73). Therefore the correlation was assumed to be unity. The results from the basic model for the ZDV data are shown in Table 3.
TABLE 3.
ZDV model parameter estimates
| Parameter | Estimate | CV (%) | 95% Confidence interval |
|---|---|---|---|
| Basic model | |||
| θCL (liters/h) | 153.1 | 3.89 | 141.42–164.78 |
| θVSS (liters) | 483.9 | 18.91 | 604.56–663.24 |
| θpV | 0.448 | 17.28 | 0.296–0.599 |
| θQ (liters/h) | 32.9 | 17.51 | 21.61–44.19 |
| θD (h) | 1.38 | 8.48 | 1.15–1.61 |
| ω2CL | 0.114 | 27.28 | 0.053–0.175 |
| θpωVSS | 0.770 | 30.13 | 0.547–0.993 |
| ς2 | 0.308 | 12.08 | 0.235–0.381 |
| θip | 1.51 | 13.05 | 1.12–1.90 |
| Final model | |||
| θCL (liters/h) | 127.2 | 4.9 | 114.9–139.1 |
| θWGT-CL (liters/h/kg) | 0.93 | 35.7 | 0.28–1.58 |
| θAGE-CL (liters/h/yr) | 6.52 | 19.9 | 3.97–9.07 |
| θVSS (liters) | 463.8 | 17.4 | 306.2–625.0 |
| θWGT-VSS (liters/kg) | 9.83 | 28.9 | 4.26–15.40 |
| θpV | 0.374 | 15.1 | 0.263–0.485 |
| θQ (liters/h) | 27.0 | 15.2 | 19.0–35.0 |
| θD (h) | 1.57 | 5.3 | 1.41–1.73 |
| FTRIPLE | 0.677 | ||
| ω2CL | 0.0703 | 28.2 | 0.0315–0.109 |
| θpωVSS | 0.610 | 46.4 | 0.0553–1.165 |
| ς2 | 0.261 | 9.2 | 0.214–0.308 |
| θip | 1.560 | 8.1 | 1.311–1.809 |
Initial covariate analysis identified weight (WGT), AGE, treatment (TRET), hemophiliac (HP), and homosexual (HMS) as possibly significant. The potential effect of treatment regimen on the relative bioavailability fraction of the central compartment (F) was evaluated by incorporating TRET in the F model. During the model-refining processes, the effects of TRET, HP, and HMS on CL and VSS were insignificant (P > 0.05). However, removal of the effect of TRET on the F model resulted in the largest increase in MVOF, and this covariate was included with WGT and AGE in the final model: CL (liters per hour) = 127 + 0.93 × (WGT − 70); If AGE < 30 years old CL (liters per hour) = 127 + 0.93 × (WGT − 70) + 6.52 × (AGE − 25); VSS (liters) = 464 + 9.83 × (WGT − 70) V (liters) = 0.374 × VSS Q (liters per hour) = 27.0 F = 1; if triple combination, F = 0.677 D (hour) = 0.25; if slow absorbers, D (hours) = 1.57
The structural and statistical results of the final model are summarized in Table 3. Compared with that in the double arm, the bioavailability of ZDV in the triple combination decreased by more than 30%. Final individual estimates (mean ± SD) of oral CL, VSS, and V were 132.6 ± 18.4 liters/h or 1.84 ± 0.14 liters/h/kg, 490.9 ± 118.9 liters or 6.68 ± 0.61 liters/kg, and 195.0 ± 47.2 liters or 2.67 ± 0.24 liters/kg, respectively. Using the previously reported value for a mean absolute ZDV bioavailability of 63% (5, 27), these values correspond to 1.16 ± 0.09 liters/h/kg, 4.20 ± 0.38 liters/kg, and 1.67 ± 0.15 liters/kg for CL, VSS, and V, respectively. The residual variability was high, being 55.5 and 51.1% from the basic model and the final model, respectively. Simulated plasma kinetics of ZDV with population-typical parameters for fast and slow absorbers are shown in Fig. 2. A plot of weighted residuals versus predicted levels of ZDV is displayed in Fig. 3. The mean individual CAUC of ZDV was 1,871 ± 794 μg/ml × h during 48 weeks. The frequency distribution of individual CAUCs of ZDV is represented in Fig. 4.
ddI.
From the 172 patients receiving ddI, 597 (mean, 3.5 per subject) plasma ddI levels were obtained and included in the population analysis. Among these 172 patients, 87 were enrolled in the double-combination group and 85 received the triple-combination regimen. A two-compartment model with zero-order input and first-order elimination, as evidenced by the individual level-versus-time plots (Fig. 2) and further justified by the likelihood ratio test, was used. In contrast to those of NVP and ZDV, ddI data supported the estimation with good precision of a full variance-covariance matrix for CL and VSS. Interpatient variability of the slower D (ω2D) was, however, poorly estimated, and therefore this statistical parameter was deleted. The results from the basic model of ddI are presented in Table 4.
TABLE 4.
ddI model parameter estimates
| Parameter | Estimate | CV (%) | 95% Confidence interval |
|---|---|---|---|
| Basic model | |||
| θCL (liters/h) | 116.0 | 3.8 | 107.4–124.6 |
| θVSS (liters) | 250.8 | 8.4 | 209.8–292.2 |
| θpV | 0.777 | 6.2 | 0.682–0.872 |
| θQ (liters/h) | 32.4 | 36.7 | 9.1–55.7 |
| θD (h) | 0.90 | 12.1 | 0.69–1.12 |
| ω2CL | 0.154 | 15.6 | 0.107–0.201 |
| ω2VSS | 0.357 | 20.5 | 0.214–0.500 |
| ω2CL-VSS | 0.221 | 16.3 | 0.150–0.292 |
| ς2 | 0.309 | 15.7 | 0.214–0.404 |
| θip | 0.883 | 17.3 | 0.583–1.183 |
| Final model | |||
| θCL (liters/h) | 115.9 | 3.7 | 107.6–124.4 |
| θWGT-CL (liters/h/kg) | 1.84 | 29.4 | 0.78–2.90 |
| θVSS (liters) | 255.3 | 8.0 | 215.2–294.8 |
| θWGT-VSS (liters/kg) | 5.22 | 27.2 | 2.44–8.00 |
| θpV | 0.769 | 7.1 | 0.689–0.876 |
| θQ (liters/h) | 31.4 | 23.3 | 17.1–45.7 |
| θD (h) | 0.89 | 12.0 | 0.68–1.10 |
| ω2CL | 0.145 | 15.9 | 0.10–0.19 |
| θVSS | 0.334 | 22.2 | 0.189–0.479 |
| θCL-VSS | 0.205 | 17.7 | 0.134–0.276 |
| ς2 | 0.290 | 14.9 | 0.206–0.374 |
| θip | 0.925 | 16.2 | 0.631–1.219 |
The potential effects of the combination regimens on the relative F of ddI in the triple arm as compared with those in the double arm were evaluated by including TRET in the F model. Results demonstrated that relative F with a value of 1.05 in the triple combination was comparable to that in the double combination (F = 1 by definition).
Initial covariate analysis indicated that WGT affected CL while both WGT and AGE, as well as intravenous drug use history (IVUH), significantly influenced VSS, but the effects of AGE and IVUH on VSS were not confirmed in the refining step. Therefore, the final model is as follows: CL (liters per hour) = 115.9 VSS (liters) = 255.3; If WGT (kilograms) < 80 CL (liters per hour) = 115.9 + 1.84 × (WGT − 67) VSS (liters) = 255.3 + 5.22 × (WGT − 67); V (liters) = 0.769 × VSS Q (liters per hour) = 31.4 D (hour) = 0.25; if slower absorbers, D (hour) = 0.891
The final model is detailed in Table 4. Individual final estimates of oral CL, VSS, and V were (mean ± SD) 117.5 ± 14.1 liters/h or 1.64 ± 0.18 liters/h/kg, 256.9 ± 40.1 liters or 3.56 ± 0.44 liters/kg, and 197.6 ± 30.8 liters or 2.74 ± 0.34 liters/kg, respectively. When adjusted for a reported mean absolute ddI bioavailability of 35% (22, 28), CL, VSS, and V were (mean ± SD) 0.57 ± 0.06 liter/h/kg, 1.25 ± 0.15 liters/kg, and 0.96 ± 0.12 liter/kg, respectively. Large intraindividual variability was observed, being 55.6 and 53.9% from the basic model and the final model, respectively. Simulated plasma kinetics of ddI with population-typical parameters for fast and slow absorbers are shown in Fig. 2. A plot of weighted residuals versus predicted levels of ddI is displayed in Fig. 3. The mean individual CAUC of ddI was 1,226 ± 400 μg/ml × h throughout the study period. The frequency distribution of individual CAUCs of ddI is illustrated in Fig. 4.
DISCUSSION
NONMEM analysis has been previously applied to evaluate the population pharmacokinetics of several nucleoside analogs, including ZDV and ddI, using plasma drug levels obtained during monotherapy (18, 35). However, such a pharmacokinetic analysis has not yet been performed after administration of these drugs in combination therapy. Combination treatment with nucleoside analogs and NNRTIs or protease inhibitors results in substantial suppression of HIV replication in vitro and in clinical trials with HIV-infected patients (10, 11, 38). While combination treatments represent the major therapeutic strategy for HIV infection, the identification of the individual characteristics which account for the significant intersubject variabilities in the pharmacokinetic parameters of each drug is of particular importance in defining quantitative relationships between drug exposure and both virological and clinical endpoints.
In this triple-combination study of NVP, ZDV, and ddI, the drugs were administered orally. Due to the fact that too few samples were available during the absorption phase, we were unable to model the absorption phase as a first-order process for any of the three drugs, as this would have led to biased parameter estimates (data not shown). The absorption phase was successfully modeled as a zero-order infusion with the infusion duration (D) being a structural parameter to be estimated for the slow absorbers only. The sparse nature of the data, however, prevented the estimation of the interindividual variability of D.
The individual pharmacokinetics of NVP have previously been reported in HIV-infected patients (6, 23). In these studies, NVP exhibited a long plasma elimination half-life (22 to 77 h) with a low total CL (0.23 to 0.77 ml/min/kg). The plasma kinetic profile of NVP was characterized, for most patients, by a first peak concentration occurring approximately 2 h after dosing, which was followed by steady-state plasma drug levels and then by a rebound approximately 14 h after drug administration (6). This phenomenon may possibly reflect enterohepatic recycling, but no conclusive evidence for this has yet been reported. In the present study, population pharmacokinetics of NVP were assessed at steady state, but the administration regimen restricted any drawing of blood samples beyond 8 h postdosing except for a few late specimens. Therefore, enterohepatic recycling of NVP, if any, was only indirectly addressed insofar as it would yield a downward-biased estimate of total CL. Of particular note, our estimates of typical oral CL were consistent with previously reported data (6). A relationship between body weight and age, covariates that usually correlate with pharmacokinetic parameters, and NVP kinetic parameters was not observed. Although gender significantly correlated with CL, with a typical value of 3.97 liters/h for men compared to 3.02 liters/h for women, this apparent gender effect does not explain substantial CL variability, as the final-model variability is only slightly reduced relative to that of the basic model. Moreover, since women were not well represented in the patient population (10 women of 82 patients), the apparent 25% effect of gender on CL of NVP, if deemed important, will require prospective confirmation.
Oral and intravenous pharmacokinetics of ZDV evaluated individually have been well documented, with reported CL of 1.1 to 1.5 liters/h/kg and a V of 1.3 to 1.4 liters/kg in HIV-infected patients (1, 4, 14, 27, 39). After intravenous infusion, the plasma pharmacokinetics of ZDV are characterized by a biexponential decay pattern. When given orally, ZDV pharmacokinetics exhibited values for CL of 1.78 to 2.6 liters/h/kg and a V of 1.6 to 2.8 liters/kg (2, 5, 16, 41). Oral plasma ZDV kinetics have been described by a one-compartment model, as the distribution phase was easily masked by the absorption phase.
In the present study, during the initial fitting of the basic model, a one-compartment model was first used, but it failed to fit the data (data not shown). In contrast, a two-compartment model resulted in a better fit. Since the sample collection time is correlated with weeks of therapy, the second late or slow phase may reflect a reduction of CL over time. Estimates of CL and V were 1.84 liters/h/kg and 2.67 liters/kg, or 1.16 liters/h/kg and 1.67 liters/kg, assuming an absolute bioavailability of 63%, respectively. These findings are in agreement with previously reported values following oral or intravenous administration of ZDV (2, 4, 5, 16, 27, 41). The effects of covariates potentially affecting ZDV kinetics have been studied previously by population analysis of 103 patients receiving oral ZDV (mean, 4.7 specimens/patient), and body weight was shown to influence CL (18). More recently, the relationships between body weight and body surface area and ZDV pharmacokinetic parameters were investigated in HIV-infected men by using a simple linear regression (36). Body weight significantly correlated with both CL and V of ZDV, but intra- and interindividual variability of the parameters or associated variance and covariance were not assessed. Our study confirmed these previous findings by demonstrating that in all patients, body weight was a covariate for both CL and V. In addition, age correlated with CL in patients less than 30 years old. Of particular note was the demonstration that treatment regimen had an effect on ZDV relative bioavailability. In the presence of NVP, ZDV bioavailability was decreased by approximately 30%, a value consistent with previous preliminary findings (31). This decrease in ZDV relative bioavailability may not be clinically significant, based on the assumption that exposure decrements of only 30% rarely affect pharmacodynamic effects of drugs (17). Wide variations in drug exposure were observed among the patients (Fig. 4), and yet ZDV appeared to be efficacious. However, it would be particularly important to evaluate whether the decrease in ZDV bioavailability observed in the NVP recipients will have some impact on the selection of resistant mutants. Different mutational pathways may occur during combination therapy compared to monotherapy, which may reflect drug exposure differences, additional antiviral activity, or other factors. Comparative analysis of pharmacology and virology data is ongoing to investigate this possibility.
Using a noncompartmental analysis (22, 28), the parameters CL and VSS of ddI were 0.73 to 1.53 liters/h/kg and 0.76 to 1.29 liters/kg, respectively, in HIV-infected patients. The population pharmacokinetics of ddI in HIV-infected patients have been previously described (13, 21, 35). The first study was a phase I trial, with 69 patients receiving ddI either intravenously or orally (35). The data set was information rich, with more than 10 plasma levels available for each patient. Simultaneous analysis of intravenous and oral data led to typical estimates of CL, V, and VSS of 0.77 liters/h/kg and 0.18 and 0.84 liters/kg, respectively. None of the covariates examined, including body weight, correlated with any of these parameters (35). In a more recent report, using 66 plasma levels measured in 33 patients, mean individual parameter estimates of oral CL and V were 238 liters/h or 1.19 liters/h/kg and 438 liters or 2.19 liters/kg, respectively (21), assuming an average body weight of 70 kg and an absolute bioavailability of 35% (22, 28). In the present study, typical population estimates of oral CL and VSS of ddI were comparable with previous data, with the exception of V, which was far larger than the value reported in the first ddI population analysis (35). As expected, body weight was identified as a covariate of both CL and VSS. In contrast to ZDV, concomitant NVP did not interfere with ddI bioavailability, demonstrating that the combination of these two drugs allows the full impact of their respective pharmacodynamic properties.
Data from the present study and previous reports have demonstrated large (>50%) intraindividual variability associated with both ZDV and ddI pharmacokinetics (20, 21, 35). This variability could not be further reduced even with the incorporation of significant covariates into the final model. Since our study involved the use of plasma drug level data gathered over a long period, the large residual variability may be partly attributable to interoccasion variation of individual pharmacokinetic behavior (26) secondary to changes in patients’ physiopathological conditions, including renal and hepatic function; covariates, such as body weight; disease progression; concomitant medications; and compliance. Among the main pharmacokinetic processes, including absorption, distribution, metabolism, and elimination, it has been suggested that variations in bioavailability primarily resulting from changes (saturation) in drug absorption may be a major source of the unexplained variability (13). Our data support this point of view with the demonstration that unexpected drug-drug interactions between ZDV and NVP may lead to bioavailability changes during combination treatment. It is also possible that this wide intraindividual variability may reflect changes in the regularity of drug ingestion. Such apparently time-dependent pharmacokinetics would consequently make it difficult to predict an individual’s parameters, as suggested previously (20). Moreover, such drug level variability may lead to suboptimal concentrations, which will result in a decreased ability of the drugs to sustain durable virologic responses and enhance the likelihood for selection of drug resistance. Therefore, in addition to the static covariates, dynamic factors representing the physiopathological conditions and drug intake behavior of patients may also need to be assessed to ensure an optimized use of anti-HIV drugs, which will maximize their virological and clinical effects.
In conclusion, the population pharmacokinetics of combined NVP, ZDV, and ddI were evaluated. The values of pharmacokinetic parameters, including CL and V, were in agreement with previously reported data. The population models were used to generate plasma pharmacokinetic profiles for each patient and to obtain estimates of the CAUC for each study drug as a quantitative measure of drug exposure (Fig. 4). The availability of individual estimates of drug exposure together with the immunologic and virologic data generated in the same patient population may allow the assessment of potential pharmacokinetic-pharmacodynamic relationships and a better definition of the events critical to therapeutic efficacy in patients with AIDS.
ACKNOWLEDGMENTS
We thank the patients and staff who contributed to the study, G. Drusano for helpful suggestions toward selection of optimum sampling times, the technical staff at Boehringer-Ingelheim Pharmaceuticals, Inc., for NVP drug analysis, Yu-Hua Wang (University of Alabama at Birmingham) for ZDV and ddI analysis, the participating ACTG Unit sites pharmacology laboratory staff, and the ACTG Operations Staff.
This work was supported in part by the AIDS Clinical Trials Group of NIAID. ZDV was provided by GlaxoWellcome, Research Triangle Park, N.C.; ddI was provided by Bristol-Myers Squibb, Wallingford, Conn.; and NVP was provided by Boehringer-Ingelheim Pharmaceuticals, Ridgefield, Conn.
Appendix
The ACTG Protocol 241 investigators who worked on pharmacology substudy 809 included the members of the Protocol 241 Team, investigators at the National Institute of Allergy and Infectious Diseases (NIAID) AIDS Clinical Trials Units, and investigators at the NIAID Division of AIDS (Bethesda, Md.).
Additional members of the Protocol 241 Team were Lyn Costanzo and Sharon Ruben (ACTG Operations Office, Rockville, Md.); Baiba Berzins (Northwestern University, Chicago, Ill.); Ana Martinez and Irene Fishman (NIAID Pharmaceutical and Regulatory Affairs Branch, Bethesda, Md.); Song-Heng Liou (Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, Mass.); Karen Kazial (Statistical and Data Management Center, Frontier Science and Technology Research Foundation, Amherst, N.Y.); Susannah Cort, Patrick Robinson, David Hall, and Heather Macy (Boehringer-Ingelheim Pharmaceuticals, Inc., Ridgefield, Conn.); Colin McLaren (Bristol-Myers Squibb Co., Wallingford, Conn.); James Rooney and John Warwich (GlaxoWellcome Co., Research Triangle Park, N.C.); Marc Cavaille-Coll (Food and Drug Administration, Bethesda, Md.); Nesli Basgoz (Massachusetts General Hospital, Boston, Mass.); Fred Valentine (New York University Medical Center, New York, N.Y.); and David Booth (Clinical Site Monitoring Group, Durham, N.C.).
Additional investigators at NIAID ACTG Units were Christine Wanke, Roy Gulick, Donald Craven, and Carress Grodman (Harvard University and Boston City Hospital, Boston, Mass.); Robert L. Murphy, Harold Kessler, and Joseph Pulvirenti (Northwestern University); Kathleen Squires, Michael Saag, Jill Weingarten, and John Gnann (University of Alabama at Birmingham, Birmingham); Diane Havlir, Chris Fegan, Stephen Spector, and Douglas Richman (University of California, San Diego); Mark Jacobson, Kathy Dybeck, Patrick Joseph, and Kathleen Clanon (University of California at San Francisco, San Francisco); Robert Schooley, Daniel Kuritzkes, Graham Ray, and Beverly Putnam (University of Colorado Health Sciences Center, Denver); Dushyantha Jayaweera, Janie Patrone-Reese, Thomas Tanner, and Jo Moebus (University of Miami, Miami, Fla.); and Nancy Red, Renee St. Jacue, Keith Henry, and Susan Swindells (University of Minnesota, Minneapolis).
Additional investigators at the NIAID Division of AIDS were Lawrence Deyton and Carla Pettinelli.
REFERENCES
- 1.Acosta E P, Page L M, Fletcher C V. Clinical pharmacokinetics of zidovudine, an update. Clin Pharmacokinet. 1996;30:251–262. doi: 10.2165/00003088-199630040-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bareggi S R, Cinque P, Mazzei M, D’Arminio A, Ruggieri A, Pirola R, Nicolin A, Lazzarin A. Pharmacokinetics of zidovudine in HIV-positive patients with liver disease. J Clin Pharmacol. 1994;34:782–786. doi: 10.1002/j.1552-4604.1994.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 3.Beal, S. L., and L. B. Sheiner. NONMEM user’s guide. University of California at San Francisco, San Francisco.
- 4.Blum, M. R., S. Liao, S. S. Good, and P. de Miranda. 1988. Pharmacokinetics and bioavailability of zidovudine in humans. Am. J. Med. 85(Suppl. 2A):189–194. [PubMed]
- 5.Burger D M, Meenhorst P L, ten Napel C H, Mulder J W, Neef C, Koks C H, Bult A, Beijnen J H. Pharmacokinetic variability of zidovudine in HIV-infected individuals: subgroup analysis and drug interactions. AIDS. 1994;8:1683–1689. doi: 10.1097/00002030-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Cheeseman S H, Hattox S E, McLaughlin M M, Koup R A, Andrews C, Bova C A, Pav J W, Roy T, Sullivan J L, Keirns J J. Pharmacokinetics of nevirapine: initial single-rising-dose study in humans. Antimicrob Agents Chemother. 1993;37:178–182. doi: 10.1128/aac.37.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow Y K, Hirsch M S, Merrill D P, Bechtel L J, Eron J J, Kaplan J C, D’Aquila R T. Use of evolutionary limitations of HIV-1 multidrug resistance to optimize therapy. Nature. 1993;361:650–654. doi: 10.1038/361650a0. [DOI] [PubMed] [Google Scholar]
- 8.Conway B, Montaner J S G, Cooper D, Vella S, Reiss P, Lange J. Randomized, double-blind one year study of the immunologic and virologic effects of nevirapine, didanosine and zidovudine combinations among antiretroviral naïve, AIDS-free patients with CD4 300–600. 1996. p. 515. , abstr. OP7.1. In Proceedings of the 3rd International Congress on Drug Therapy in HIV Infection. [Google Scholar]
- 9.D’Aquila R T, Hughes M D, Johnson V A, Fischl M A, Sommadossi J P, Liou S H, Timpone J, Myers M, Basgoz N, Niu M, Hirsch M S. Nevirapine, zidovudine, and didanosine compared with zidovudine and didanosine in patients with HIV-1 infection—a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;124:1019–1030. doi: 10.7326/0003-4819-124-12-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 10.de Clercq E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) for the treatment of human immunodeficiency virus type 1 (HIV-1) infections: strategies to overcome drug resistance development. Med Res Rev. 1996;16:125–157. doi: 10.1002/(SICI)1098-1128(199603)16:2<125::AID-MED1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors—a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 12.Delta Coordinating Committee. Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet. 1996;348:283–291. [PubMed] [Google Scholar]
- 13.Drusano G L, Yuen G J, Morse G, Cooley T P, Seidlin M, Lambert J S, Liebman H A, Valentine F T, Dolin R. Impact of bioavailability on determination of the maximal tolerated dose of 2′,3′-dideoxyinosine in phase I trials. Antimicrob Agents Chemother. 1992;36:1280–1283. doi: 10.1128/aac.36.6.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley, M. N. 1995. Clinical pharmacokinetics of nucleoside antiretroviral agents. J. Infect. Dis. 171(Suppl. 2):99–112. [DOI] [PubMed]
- 15.Fischl M A, Richman D D, Grieco M H, Gottlieb M S, Volberding P A, Laskin O L, Leedom J M, Groopman J E, Mildvan D, Schooley R T, Jackson G G, Durack D T, King D. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher C V, Rhame F S, Beatty C C, Simpson M, Balfour H H., Jr Comparative pharmacokinetics of zidovudine in healthy volunteers and in patients with AIDS with and without hepatic disease. Pharmacotherapy. 1992;12:429–434. [PubMed] [Google Scholar]
- 17.Food and Drug Administration. Report by the Bioequivalence Task Force on recommendations from the bioequivalence hearings conducted by the Food and Drug Administration. Sep 29–Oct 1, 1986. Rockville, Md: Food and Drug Administration; 1988. [Google Scholar]
- 18.Gitterman S R, Drusano G L, Egorin M J, Standiford H C. Population pharmacokinetics of zidovudine. The Veterans Administration Cooperative Studies Group. Clin Pharmacol Ther. 1990;48:161–167. doi: 10.1038/clpt.1990.131. [DOI] [PubMed] [Google Scholar]
- 19.Goulden M G, Cammack N, Hopewell P L, Penn C R, Cameron J M. Selection in vitro of an HIV-1 variant resistant to both lamivudine (3TC) and zidovudine. AIDS. 1996;10:101–102. doi: 10.1097/00002030-199601000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Grasela T H, Walawander C A, Beltangady M, Knupp C A, Martin R R, Dunkle L M, Barbhaiya R H, Pittman K A, Dolin R, Valentine F T, Liebman H A. Analysis of potential risk factors associated with the development of pancreatitis in phase I patients with AIDS or AIDS-related complex receiving didanosine. J Infect Dis. 1994;169:1250–1255. doi: 10.1093/infdis/169.6.1250. [DOI] [PubMed] [Google Scholar]
- 21.Harb G E, Mandema J W, Delahunty T, Benowitz N B, Coleman R, Sheiner L B, Jacobson M A. Population pharmacokinetics of didanosine in patients with human immunodeficiency virus infection. J Infect Dis. 1996;173:273. doi: 10.1093/infdis/173.1.273. [DOI] [PubMed] [Google Scholar]
- 22.Hartman N R, Yarchoan R, Pluda J M, Thomas R V, Marczyk K S, Broder S, Johns D G. Pharmacokinetics of 2′,3′-dideoxyadenosine and 2′,3′-dideoxyinosine in patients with severe human immunodeficiency virus infection. Clin Pharmacol Ther. 1990;47:647–654. doi: 10.1038/clpt.1990.86. [DOI] [PubMed] [Google Scholar]
- 23.Havlir D, Cheeseman S H, McLaughlin M, Murphy R, Erice A, Spector S A, Greenough T C, Sullivan J L, Hall D, Myers M, Lamson M, Richman D D. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis. 1995;171:537–545. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 24.Jablonowski, H. 1995. Studies of zidovudine in combination with didanosine and zalcitabine. J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 10(Suppl. 1):52–56. [PubMed]
- 25.Jayaraj A, Alexander J, Price C, Daly D, Pav J, Hattox S, Keirns J. A rapid and sensitive HPLC-UV method for the quantitation of an anti-HIV agent, nevirapine, and its solid-phase extractable metabolites in biological fluids. Pharm Res. 1992;9:S334. [Google Scholar]
- 26.Karlsson M O, Sheiner L B. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm. 1993;21:735–750. doi: 10.1007/BF01113502. [DOI] [PubMed] [Google Scholar]
- 27.Klecker R W, Jr, Collins J M, Yarchoan R, Thomas R, Jenkins J F, Broder S, Myers C E. Plasma and cerebrospinal fluid pharmacokinetics of 3′-azido-3′-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987;41:407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- 28.Knupp C A, Shyu W C, Dolin R, Valentine F T, McLaren C, Martin R R, Pittman K A, Barbhaiya R H. Pharmacokinetics of didanosine in patients with acquired immunodeficiency syndrome or acquired immunodeficiency syndrome-related complex. Clin Pharmacol Ther. 1991;49:523–535. doi: 10.1038/clpt.1991.63. [DOI] [PubMed] [Google Scholar]
- 29.Kuritzkes D R, Quinn J B, Benoit S L, Shugarts D L, Griffin A, Bakhtiari M, Poticha D, Eron J J, Fallon M A, Rubin M. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS. 1996;10:975–981. doi: 10.1097/00002030-199610090-00007. [DOI] [PubMed] [Google Scholar]
- 30.Larder B A, Kemp S D, Harrigan P R. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995;269:696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- 31.MacGregor T R, Lamson M J, Cort S, Pav J W, Saag M S, Elvin A T. Steady state pharmacokinetics of nevirapine, didanosine, zalcitabine and zidovudine combination therapy in HIV-1 positive patients. Pharm Res. 1995;12:9. [Google Scholar]
- 32.Mandema J W, Verotta D, Sheiner L B. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–528. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 33.Merrill D P, Moonis M, Chou T C, Hirsch M S. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type-1 replication in vitro. J Infect Dis. 1996;173:355–364. doi: 10.1093/infdis/173.2.355. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen C, Bruun L, Mathiesen L R, Pedersen C, Gerstoft J. Development of resistance to zidovudine (ZDV) and didanosine (ddI) in HIV from patients in ZDV, ddI and alternating ZDV/ddI therapy. AIDS. 1996;10:625–633. doi: 10.1097/00002030-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Pai S M, Shukla U A, Grasela T H, Knupp C A, Dolin R, Valentine F T, McLaren C, Liebman H A, Martin R R, Pittman K A, Barbhaiya R H. Population pharmacokinetic analysis of didanosine (2′,3′-dideoxyinosine) plasma concentrations obtained in phase I clinical trials in patients with AIDS or AIDS-related complex. J Clin Pharmacol. 1992;32:242–247. doi: 10.1002/j.1552-4604.1992.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 36.Sahai J, Gallicano K, Ormsby E, Garber G, Cameron D W. Relationship between body weight, body surface area and serum zidovudine pharmacokinetic parameters in adult, male HIV-infected patients. AIDS 1994. 1994;8:793–796. doi: 10.1097/00002030-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Schooley R T, Ramirez-Ronda C, Lange J M, Cooper D A, Lavelle J, Lefkowitz L, Moore M, Larder B A, St. Clair M, Mulder J W, McKinnis R, Pennington K N, Harrigan P R, Kinghorn I, Steel H, Rooney J F. Virologic and immunologic benefits of initial combination therapy with zidovudine and zalcitabine or didanosine compared with zidovudine monotherapy. J Infect Dis. 1996;173:1354–1366. doi: 10.1093/infdis/173.6.1354. [DOI] [PubMed] [Google Scholar]
- 38.Sommadossi J P. Nucleoside analogs: similarities and differences. Clin Infect Dis. 1993;16:S7–S15. doi: 10.1093/clinids/16.supplement_1.s7. [DOI] [PubMed] [Google Scholar]
- 39.Stagg M P, Cretton E M, Kidd L, Diasio R B, Sommadossi J P. Clinical pharmacokinetics of 3′-azido-3′-deoxythymidine (zidovudine) and catabolites with formation of a toxic catabolite, 3′-amino-3′-deoxythymidine. Clin Pharmacol Ther. 1992;51:668–676. doi: 10.1038/clpt.1992.79. [DOI] [PubMed] [Google Scholar]
- 40.Staszewski S, Loveday C, Picazo J J, Dellamonica P, Skinhoj P, Johnson M A, Danner S A, Harrigan P R, Hill A M, Verity L, McDade H. Safety and efficacy of lamivudine-zidovudine combination therapy in zidovudine-experienced patients—a randomized controlled comparison with zidovudine monotherapy. JAMA. 1996;276:111–117. [PubMed] [Google Scholar]
- 41.Zhou X J, Sommadossi J P. Comparative pharmacokinetics of zidovudine and its toxic catabolite 3′-amino-3′-deoxythymidine in HIV-infected patients. AIDS Res Hum Retroviruses. 1996;12:229–233. doi: 10.1089/aid.1996.12.229. [DOI] [PubMed] [Google Scholar]