Abstract
The effect of a novel consensus bacterial 6-phytase variant (PhyG) on apparent ileal digestibility (AID) of amino acids (AA) and phosphorus (P) utilization in young broilers when added to diets with high phytate-P (PP) content without added inorganic phosphate (Pi) and deficient in digestible (dig) AA and metabolizable energy (ME) was investigated. A total of 256 Ross 308 male broilers were assigned to 4 treatments (8 birds/cage, 8 cages/treatment) in a completely randomized design. Treatments comprised a positive control (PC, 2,975 kcal/kg ME, 3.7 g/kg dig P, 2.83 g/kg PP, 8.4 g/kg Ca, 10.6 g/kg dig lysine), a negative control (NC) without added Pi (ME −68 kcal/kg, crude protein −10 g/kg, dig AA −0.1 to −0.4 g/kg, Ca −2.0 g/kg, dig P −2.2 g/kg, Na −0.4 g/kg vs. PC), and NC plus 500 or 1,000 FTU/kg of PhyG. Test diets were corn/soy/rapeseed-meal/rice-bran-based and fed from 5 to 15 d of age. Ileal digesta and tibias were collected on day 15. Excreta was collected during days 12 to 15 to determine P retention. The NC (vs. PC) reduced (P < 0.05) P retention (−10.4% units), tibia ash (−14.3% units), weight gain (−109 g), feed intake (−82 g) and increased FCR (from 1.199 to 1.504), confirming that the NC was extremely deficient in nutrients and energy. Phytase addition to the NC linearly (P < 0.001) improved performance, but did not fully recover it to the level of the PC due to the severe nutrients/energy reduction in NC. Phytase linearly increased P retention (P < 0.001), tibia ash (P < 0.001), AID of dry matter (P < 0.05), nitrogen (P < 0.01), gross energy (P < 0.05), and all 17 individual AA (P < 0.01). At 1,000 FTU/kg, phytase increased (P < 0.05) P retention vs. PC and NC (+14.5 and +24.9% units, respectively) and increased tibia ash vs. NC (+13.8% units), equivalent to PC. The NC decreased AID of Cys, Gly, Thr, and Met vs. PC (P < 0.05). At 1,000 FTU/kg, phytase increased AID of all 17 AA vs. NC (P < 0.01), equivalent to PC. At 1,000 FTU/kg, AID AA responses (above NC) ranged from +4.5% (Met) to +15.0% (Cys), being maximal for essential Thr (+10.4%) and Val (+8.2%) and non-essential Cys (+15.0%) and Gly (+10.4%). The results highlight the efficacy of PhyG at a dose level of 500 to 1,000 FTU/kg in young broilers for improving the ileal digestibility of nitrogen, AA, and energy alongside P retention and tibia ash. The performance data emphasize the need to consider digestible nutrient intake as a response variable in exogenous enzyme studies.
Keywords: amino acids, broilers, growth performance, phytase, tibia ash
Lay Summary
Microbial phytase is widely used in commercial broiler diets to improve digestion of phosphorus (P) and reduce its excretion into the environment. Phytase improves the digestion of phosphorus and other nutrients including amino acids (AA). This study evaluated the effect of a novel consensus bacterial 6-phytase variant (PhyG) added to a nutrient-reduced diet without any added inorganic P on the digestibility of nutrients including P and AA in the ileum of young broilers. Effects on P retention and bone mineralization were also assessed. Compared to an unsupplemented negative control diet, PhyG improved growth performance, P retention, bone mineralization (tibia ash), digestibility of dry matter, nitrogen, gross energy, and all 17 individual AA during 5 to 15 d post-hatch, in a dose-dependent manner (dose range 0 to 1,000 phytase units [FTU] per kilogram of feed). For some AA, the increases in digestibility with PhyG at 1,000 FTU/kg were substantial (cysteine: +15.0%, threonine:+10.4%), and for all AA were equivalent to the responses produced by a nutritionally adequate positive control (unsupplemented) diet. The results demonstrate the efficacy of PhyG to improve AA digestibility alongside growth performance, P retention, and bone mineralization in young broilers.
A novel microbial 6-phytase added to a phytate-rich diet containing no added inorganic phosphate improved growth performance, amino acid and energy digestibility, phosphorus retention, and tibia ash in a dose-dependent manner in young broilers.
Introduction
Supplementation of commercial broiler diets with microbial phytase is a common practice for improving phosphorus (P) availability from plant-derived phytate whilst reducing the excretion of undigested P into the environment (Selle and Ravindran 2007; Humer et al., 2015). Substantial evidence exists demonstrating that phytase increases the utilization of phytate-bound P and P retention in a wide variety of diets and settings (Leske and Coon, 1999; Selle and Ravindran, 2007; Dersjant-Li et al., 2015) with a clear dose–response effect (Kiarie et al., 2015; Walters et al., 2019; Dersjant-Li et al., 2020). Phytase-related improvements in calcium (Ca) digestibility have also been reported, but less universally (Tamim et al., 2004; Selle et al., 2009a, b; Amerah et al., 2014) and are thought to be mediated via a reduction in the availability of phytate to form complexes with Ca that precipitate at or above pH 5.0 (Selle et al., 2009a) and are highly resistant to digestion.
Phytase effects on the digestibility of other nutrients, including amino acids (AA), are variable across studies and with different phytases (Sebastian et al., 1997; Ravindran et al., 1999; Selle et al., 2012, Cowieson et al., 2017), with underlying mechanisms being more complex (Selle et al., 2012) and less well understood. Responses of individual AA to phytase are not equal (Ravindran et al., 1999; Cowieson et al., 2006), appear to differ in male and female broilers (Sebastian et al., 1997), with bird strain and age (Li et al., 2015), and can vary among different phytases even when tested under the same conditions at equivalent dose-level (Dersjant-Li and Kwakernaak, 2019). The AA response to phytase may also be influenced by a range of dietary related factors including the inherent AA and protein digestibility of feedstuffs in the diet (Ravindran et al., 1999; Krieg et al., 2020), phytate content (Cabahug et al., 1999; Walk and Rao, 2020), and Ca content or ratio to digestible P (Sebastian et al., 1997; Amerah et al., 2014). A key step in understanding the effects of a new phytase on AA nutrition is to evaluate and characterize the dose-response of AA digestibility under controlled conditions using diet compositions relevant to commercial practice.
Evaluating AA responses to phytase in young birds is of particular importance because the starter phase (days 0 to 10) is a period of rapid growth when bones and other tissues are developing and mineral and AA requirements are the highest (Ravindran and Abdollahi, 2021). Endogenous amino acid losses are also higher in week 1 posthatch and decrease with advancing age (Adedokun et al., 2007; Barua et al., 2021). The risk of AA deficiency is therefore greater in young birds and deficiency in key limiting AA such as Lys can severely affect muscle development and body weight (Tesseraud et al., 1996). On the other hand, the potential for phytase to improve AA digestibility is relatively high in young birds as ileal digestibility of protein and AA is inherently lower in younger than in older birds (Batal and Parsons, 2002; Cowieson et al., 2006; Li et al., 2015).
Earlier studies have demonstrated the efficacy of PhyG, with a full or partial nutrient matrix, in totally replacing Pi during all growth phases in broiler diets containing a high PP content (>0.3% PP) based on growth performance and bone mineralization outcomes (Marchal et al., 2021). However, AA digestibility responses to this novel phytase in Pi-free diets have not been previously evaluated. In the present study, ileal AA digestibility responses of young broilers (15 d of age) fed nutrient-reduced diets containing no added Pi and supplemented with PhyG at dose level of 500 and 1,000 FTU/kg were examined, together with P retention and tibia ash. The test hypothesis was that, under these conditions, increasing phytase dose level will lead to a linear increase in ileal AA digestibility in young broilers at 15 d of age.
Materials and Methods
The experimental protocol was reviewed and approved by the Massey University Animal Ethics Committee and complied with the New Zealand Code of Practice for the Care and Use of Animals for Scientific Purposes.
Experimental design, birds, and housing
A total of 256-d-old male broilers (Ross 308) were obtained from a commercial hatchery and fed a pre-starter diet until 5 d of age. On day 5, chicks were individually weighed and assigned to cages (8 birds/cage, 8 cages/treatment) on the basis of body weight (BW), so that each cage contained birds of equal average BW, in a completely randomized design. Cages were located in an environmentally controlled house. The temperature was maintained at 32 °C for the first 7 d and then gradually reduced to 27 °C by day 14. The birds received 20 h of fluorescent illumination per day. Diets and water were provided ad libitum for the duration of the study (5–15 d posthatch).
Diets and the enzyme
The composition of diets is provided in Table 1. The pre-starter diet was formulated to meet the nutritional requirements of birds during days 1 to 5 in accordance with recommendations set by the breeder (Aviagen Inc., 2019). From day 5, birds were fed one of four experimental diets, in mash form. The positive control (PC) diet was based on corn, soybean meal, rapeseed meal, and rice bran and formulated to meet the minimum requirements for nutrients (Aviagen Inc., 2019). The negative control (NC) was formulated without Pi and reduced in ME (−68 kcal/kg), crude protein (CP, −10 g/kg), digestible (dig) AA (dig Lys, Met and Cys, Thr, Arg, Val, and Ile, −0.1 to −0.4 g/kg; Table 1), Ca (−2.0 g/kg), dig P (−2.2 g/kg, without inorganic P), and sodium (−0.4 g/kg). The other two treatments comprised of the NC supplemented with 500 or 1,000 FTU/kg of PhyG, a commercial consensus bacterial 6-phytase variant produced in Trichoderma reesei [Axtra PHY GOLD, Danisco Animal Nutrition & Health, IFF, the Netherlands]. The development and biochemical and enzymatic characteristics of this novel phytase have been described previously (Christensen et al., 2020; Ladics et al., 2020). The phytase was premixed with a portion (10 kg) of the NC diet before mixing with the main batch in a horizontal mixer to ensure homogenous distribution of the enzyme. Titanium dioxide (5 g/kg) was added to all diets as an indigestible marker.
Table 1.
Ingredient and calculated nutrient contents of the basal diets
| Pre-starter diet (days 1 to 5) | PC (days 5 to 15) | NC (days 5 to 15)1 | |
|---|---|---|---|
| Ingredient, g/kg (as-fed basis) | |||
| Corn | 540.3 | 602.7 | 628.7 |
| Soybean meal 480 g/kg | 330.0 | 281.0 | 244.8 |
| Rapeseed meal | 50.0 | 50.0 | 50.0 |
| Rice bran | 30.0 | 10.0 | 47.7 |
| Dicalcium phosphate | 18.2 | 15.7 | 0.00 |
| Soybean oil | 10.5 | 16.4 | 0.0 |
| Limestone2 | 9.0 | 7.9 | 13.3 |
| l-lysine HCl | 2.1 | 1.9 | 2.3 |
| dl-methionine | 3.1 | 2.8 | 2.8 |
| l-threonine | 1.1 | 0.9 | 1.2 |
| Salt | 2.5 | 2.5 | 2.4 |
| Sodium bicarbonate | 1.5 | 1.5 | 0.10 |
| Vitamin-mineral premix3 | 1.7 | 1.7 | 1.7 |
| Titanium dioxide | 0 | 5.0 | 5.0 |
| Nutrient composition, g/kg | |||
| ME, kcal/kg | 2,875 | 2,975 | 2,907 |
| Crude protein | 225.0 | 204.0 | 194.0 |
| Calcium | 9.6 | 8.4 | 6.4 |
| Total phosphorus | 7.8 | 7.0 | 4.7 |
| Digestible phosphorus | 4.2 | 3.7 | 1.5 |
| Phytate-P | 3.25 | 2.83 | 3.33 |
| Sodium | 1.8 | 1.8 | 1.4 |
| Digestible lysine | 11.9 | 10.6 | 10.2 |
| Digestible methionine + cysteine | 8.8 | 8.1 | 7.8 |
| Digestible methionine | 5.9 | 5.4 | 5.3 |
| Digestible threonine | 8.0 | 7.1 | 7.0 |
| Digestible valine | 8.9 | 8.1 | 7.7 |
| Digestible isoleucine | 7.9 | 7.1 | 6.7 |
| Digestible arginine | 13.4 | 12.0 | 11.1 |
Phytase enzyme was added on top of the NC diet.
Limestone mean particle size, 380 μm GMD; in vitro solubility 45%, 75%, and 90% after incubation for 5, 15, and 30 min, pH 3.0, determined according to the method of Kim et al. (2019).
Supplied per kg diet: vitamin A (E 672), 10,000 IU; vitamin D3 (E 671), 2,000 IU; vitamin E (a-tocopherol), 30.0 mg; vitamin K3, 2.0 mg; vitamin B1, 1.0 mg; vitamin B2, 5.0 mg; vitamin B6, 3.0 mg; vitamin B12, 12.0 µg; nicotinic acid, 40.0 mg; calcium pantothenate, 10.0 mg; Folic acid, 1.0 mg; biotin, 0.1 mg; choline chloride, 400 mg; copper as CuSO4·5H2O, 8.0 mg; iron as FeCO3, 60.0 mg; iodine as IK, 2.0 mg; manganese as MnO, 70.0 mg; selenium as Na2SeO3, 0.15 mg; zinc as ZnO, 35.0 mg; butylated hydroxytoluene, 4 mg; citric acid, 13,8 mg; sodium citrate, 0.4 mg; sepiolite, 0.4 g; calcium carbonate, 2.34 g.
Measurements and sample collection
Birds were weighed individually on days 5 and 15. Feed intake was monitored by weighing feed on day 5 and that remaining on day 15. Mortality was recorded daily, and the BW of any dead birds used to correct the feed conversion ratio, calculated as total feed intake divided by total weight gain of live plus dead birds.
During days 12 to 15 posthatch, feed intake was monitored, and excreta was collected daily, weighed, and pooled per cage. Pooled excreta were mixed well in a blender, sub-sampled, freeze-dried, ground to pass through a 0.5-mm sieve, and stored at 4 °C for the analysis of dry matter (DM) and P.
On day 15, all birds were euthanized by intravenous injection of sodium pentobarbitone (Provet NZ Pty Ltd., Auckland, New Zealand). Digesta was collected from the terminal ileum, with ileum defined as the length of the small intestine from Meckel’s diverticulum to 4 cm anterior of the ileocecal junction. Digesta was pooled per cage and stored at −20 °C until the determination of DM, AA, nitrogen (N), and gross energy (GE). The right tibia from six birds per cage was extracted and pooled per cage. Fibula, muscle, and connective tissues were removed and tibias were dried at 70 °C and de-fatted through multiple passes of ethyl ether in a Soxhlet apparatus. De-fatted tibias were dried at 100 °C to obtain the DM and then ashed at 600 °C for 24 h. Ash content was reported as % fat-free DM.
Phytase activities and phytate in the diets were analyzed by Danisco Animal Nutrition Research Centre, Brabrand, Denmark.
Chemical analysis
Dry matter was determined in a convection oven at 105 °C according to AOAC method 930.15 (AOAC, 2005). Nitrogen was determined using a FP-428 nitrogen determinator (LECO Corporation, St. Joseph, MI). Gross energy was determined using an adiabatic bomb calorimeter (Gallenkamp Autobomb, Weiss Gallenkamp Ltd, Loughborough, UK) standardized with benzoic acid. Amino acids were determined by HPLC with post-column derivatization and fluorometric detection of AA using 0-phthaldialdehyde, according to the methods described by Ravindran et al. (2009). Ash was determined according to AOAC method 942.05 (AOAC, 2005). Calcium and P were determined by colorimetric methods after ashing the samples at 550 °C and acid digestion in 6.0M HCl according to AOAC method 968.08(D) (AOAC, 2005). Phytase activity was determined according to a modified version of the 2000.12 AOAC method (Engelen et al., 2001). One phytase unit (FTU) was defined as the amount of enzyme that liberated 1 μmol of inorganic phosphate per minute from a sodium phytate substrate at a pH of 5.5 and a temperature of 37 °C. Phytate-P concentrations were determined using a modified version of the HPLC method described by Skoglund et al. (1998). Titanium dioxide was determined according to the method described by Short et al. (1996).
Calculations
The AID coefficients of DM, N, GE, and AA were calculated from the dietary ratio of nutrient to titanium relative to the corresponding ratio in the ileal digesta, according to the following formula:
where (Nutrient/Ti)diet is the ratio of the nutrient and titanium dioxide marker in the diet, and (Nutrient/Ti)digesta is the ratio of the nutrient and titanium dioxide marker in the ileal digesta.
The total tract retention (TTR) of P, as a percentage of intake, was determined according to the following formula:
where feed intake is the amount of feed consumed in g/bird, Excreta ouptut is the determined amount of excreta in g/bird, Phosphorusdiet is the analyzed concentration of P in the diet (g/kg), and Phosphorusexcreta is the measured concentration of P in the excreta (g/kg).
Statistical analysis
Cage was the experimental unit in all analyses. Data were analyzed by one-way ANOVA. Means separation was achieved using Tukey’s HSD test. All statistical analyses were performed in the Fit Model platform of JMP 14.0 (JMP, 2019). Phytase dose-response was analyzed by linear regression of y (response paramater) on x (phytase dose level) within the dose range of 0 (NC) to 1,000 FTU/kg. Effects and differences were considered significant at P < 0.05.
Results
Analyzed levels of Ca and PP in the basal PC and NC diets and of P in the PC were in close agreement with formulated values (≤10% variation from expected values; Table 2). Analyzed total P in the NC was determined to be lower (−20%) than expected. Calcium to P ratios were 1.28:1 and 1.89:1 in the PC and NC diets, respectively. Amino acid levels varied from formulated values by < 20%. Phytase activities were very low in the NC and in close alignment (<15% variation) with target dose levels in phytase-supplemented diets.
Table 2.
Analyzed composition of experimental diets (g/kg as received)1,2
| PC (days 5 to 15) | NC (days 5 to 15) | |
|---|---|---|
| Dry matter | 914 | 912 |
| Nitrogen | 31.3 | 31.1 |
| Gross energy, kcal/kg | 4054 | 4023 |
| Total phosphorus | 6.48 | 3.75 |
| Phytate-P | 2.89 | 3.32 |
| Total calcium | 8.32 | 7.08 |
| Ca:P ratio | 1.28 | 1.89 |
| Amino acids | ||
| Ala | 10.04 | 9.62 |
| Arg | 12.84 | 11.94 |
| Asp | 19.67 | 18.21 |
| Cys | 3.16 | 2.99 |
| Glu | 36.72 | 34.12 |
| Gly | 7.93 | 7.48 |
| His | 5.29 | 4.85 |
| Ile | 7.87 | 7.34 |
| Leu | 16.50 | 15.62 |
| Lys | 12.08 | 11.59 |
| Met | 5.58 | 5.39 |
| Phe | 9.60 | 8.91 |
| Pro | 11.93 | 11.27 |
| Ser | 8.99 | 8.42 |
| Thr | 8.47 | 7.94 |
| Tyr | 7.27 | 6.99 |
| Val | 9.38 | 8.93 |
NC, negative control; PC, positive control. NC was formulated without Pi and reduced in ME (−68 kcal/kg), crude protein (CP, −10 g/kg), select AA (digestible Lys, Met and Cys, Thr, Arg, Val, and Ile, −0.1 to −0.4 g/kg; Table 1), Ca (−2.0 g/kg), digestible P (−2.2 g/kg), and sodium (−0.4 g/kg).
Analyzed phytase activities, NC = 45 FTU/kg; NC+PhyG 500 FTU/kg = 586 FTU/kg; NC+PhyG 1,000 FTU/kg = 1,036 FTU/kg.
Severe reductions of nutrient and energy contents together with total removal of inorganic P in the NC markedly reduced (P < 0.05) the weight gain (−40%), feed intake (−25%) and increased (P < 0.05) the FCR, compared to the PC (Table 3). Addition of phytase to the NC linearly (P < 0.001) improved the broiler performance. Phytase at both dose levels improved weight gain, feed intake, and FCR compared to NC (P < 0.05), but not to the level of the PC.
Table 3.
Treatment effects on the growth performance of young broilers, 5 to 15 d posthatch
| Weight gain (g/bird) | Feed intake (g/bird) | Feed conversion ratio (g/g) | |
|---|---|---|---|
| PC1 | 275a | 330a | 1.199c |
| NC1 | 166c | 248c | 1.504a |
| NC+ 500 FTU/kg | 218b | 283b | 1.302b |
| NC+ 1,000 FTU/kg | 237b | 307ab | 1.294b |
| SEM2 | 7.07 | 7.09 | 0.017 |
| P-value, ANOVA | <0.0001 | <0.0001 | <0.0001 |
| P-value, linear3 | <0.0001 | <0.0001 | <0.0001 |
NC, negative control; PC, positive control. NC formulated without Pi and reduced in ME (−68 kcal/kg), crude protein (−10 g/kg), select AA (digestible Lys, Met and Cys, Thr, Arg, Val, and Ile, −0.1 to −0.4 g/kg; Table 1), Ca (−2.0 g/kg), digestible P (−2.2 g/kg), and sodium (−0.4 g/kg).
SEM, pooled standard error of the mean.
Excluding PC from the analysis.
Mean values in the same column bearing different superscript letters are significantly different (P < 0.05).
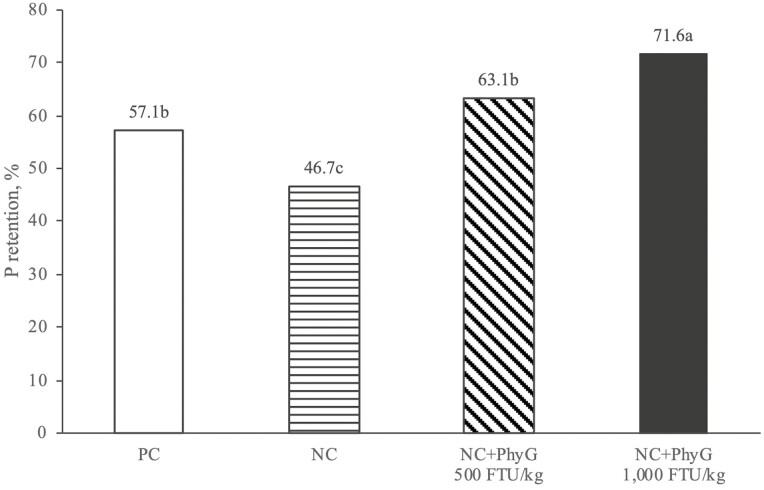
Phosphorus retention (%) at 15 d of age was reduced in the NC compared to PC (−10.4% units, P < 0.05) and increased linearly (P < 0.001) with increasing phytase dose level (Figure 1). At 500 FTU/kg, P retention was increased (P < 0.05) by 6.0% units vs. NC, equivalent to PC. At 1,000 FTU/kg, P retention was increased vs. both NC and PC (+ 24.9 and +14.5% units, respectively; P < 0.05).
Figure 1.
Effect of dietary treatments on total tract P retention (% intake), measured during day 12–15 posthatch.
a–cColumns bearing different letters are significantly different at P ≤ 0.05 (based on one-way ANOVA, followed by Tukey’s HSD test for means separation). Standard error of the mean (SEM): 2.05; P-value, ANOVA < 0.001; P-value, linear (excluding PC) < 0.001.
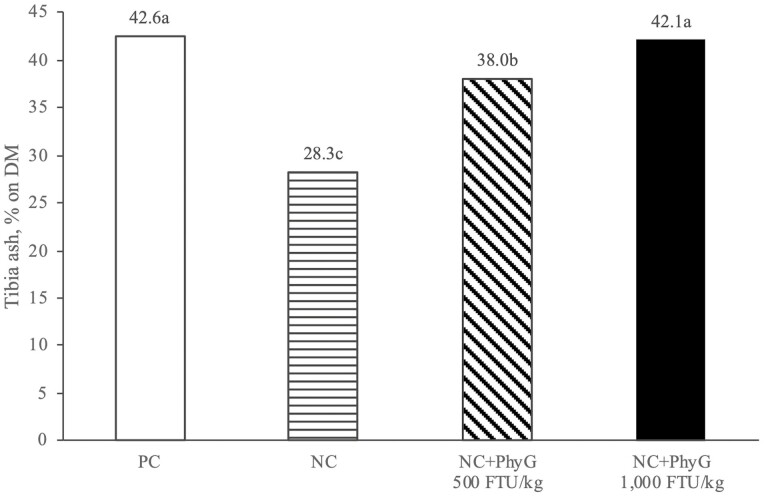
Effects of phytase on tibia ash followed a trend similar to those on P retention. Tibia ash was reduced in the NC vs. PC (−14.3% units, P < 0.05) and increased linearly (P < 0.001) with increasing phytase dose level (Figure 2). At 500 FTU/kg, phytase increased (P < 0.05) tibia ash vs. NC (+9.7% units) but did not reach the level of the PC, whereas phytase at 1,000 FTU/kg increased (P < 0.05) tibia ash by 13.8% units vs. NC, equivalent to PC.
Figure 2.
Effect of dietary treatments on tibia ash (% defatted dry matter), measured at day 15 posthatch.
a–cColumns bearing different letters are significantly different at P ≤ 0.05 (based on one-way ANOVA, followed by Tukey’s HSD test for means separation). Standard error of the mean (SEM) = 0.77, P-value, ANOVA < 0.001; P-value, linear (excluding PC) < 0.001.
The AID coefficients of DM, N, and GE were not different (P > 0.05) between the NC and PC (Table 4) but were all improved with phytase supplementation in a linear manner (P < 0.05). At 1,000 FTU/kg, AID coefficients of DM, N and GE were similar (P < 0.05) to those of the PC.
Table 4.
Treatment effects on the apparent ileal digestibility (AID) coefficients of dry matter (DM), nitrogen (N), and gross energy (GE) in young broilers, measured on day 15 posthatch
| AID DM | AID N | AID GE | |
|---|---|---|---|
| PC1 | 0.69 | 0.77ab | 0.72 |
| NC1 | 0.66 | 0.74b | 0.68 |
| NC + 500 FTU/kg | 0.71 | 0.79a | 0.72 |
| NC + 1,000 FTU/kg | 0.71 | 0.78ab | 0.72 |
| SEM2 | 0.016 | 0.013 | 0.015 |
| P-value, ANOVA | 0.14 | 0.02 | 0.15 |
| P-value, linear3 | 0.03 | 0.002 | 0.04 |
NC, negative control; PC, positive control. NC formulated without Pi and reduced in ME (−68 kcal/kg), crude protein (−10 g/kg), select AA (digestible Lys, Met and Cys, Thr, Arg, Val, and Ile, −0.1 to −0.4 g/kg; Table 1), Ca (−2.0 g/kg), digestible P (−2.2 g/kg), and sodium (−0.4 g/kg).
SEM, pooled standard error of the mean.
Excluding PC from the analysis.
Mean values in the same column bearing different superscript letters are significantly different at P < 0.05.
Treatment effects on AID AA coefficients are summarized in Table 5. In the PC, AID coefficients ranged from 0.68 for Cys to 0.91 for Met. The AID coefficients were numerically reduced for most AA in the NC compared to PC, except for Cys (−11.8%), Gly (−8.2%), Thr (−8.2%), and Met (−3.3%) that were significantly (P < 0.05) lower. Increasing phytase dose level linearly (P < 0.01) increased the AID of all 17 AA. At 1,000 FTU/kg, the AID of all AA was increased vs. NC (P < 0.05) and was similar (P > 0.05) to those of the PC. Among the essential AA, increases in AID by phytase at 1,000 FTU/kg vs. NC were greatest for Thr (+10.4%), Val (+8.2%) and Ile (+8.0%) and minimal for Met (+4.5%). Among the non-essential AA, the increases were greatest for Cys (+15.0%), Gly (+10.4%), and Ser (+10.0%) and minimal for Arg (+4.8%) and Glu (+4.8%). At 500 FTU/kg, increases in AID coefficients were maximal in the same individual AA as at 1,000 FTU/kg, but the increases were of lower magnitude. The average increase (above NC) in AID across all AA with phytase at 500 or 1,000 FTU/kg was +6.7% and +8.0%, respectively.
Table 5.
Effect of dietary treatments on the apparent ileal digestibility coefficients of amino acids in young broilers, measured on day 15 posthatch
| PC1 | NC1 | Phytase supplemented treatments | SEM2 | ANOVA P-value | Linear P-value3 | ||
|---|---|---|---|---|---|---|---|
| NC + 500 FTU/kg | NC + 1,000 FTU/kg | ||||||
| Ala | 0.80ab | 0.76b | 0.81ab | 0.82a | 0.011 | 0.019 | 0.006 |
| Arg | 0.85ab | 0.83b | 0.86ab | 0.87a | 0.009 | 0.021 | 0.005 |
| Asp | 0.76ab | 0.73b | 0.78a | 0.79a | 0.012 | 0.009 | 0.004 |
| Cys | 0.68a | 0.60b | 0.67a | 0.69a | 0.005 | 0.003 | 0.001 |
| Glu | 0.85ab | 0.83b | 0.86ab | 0.87a | 0.009 | 0.036 | 0.008 |
| Gly | 0.73a | 0.67b | 0.72a | 0.74a | 0.013 | 0.004 | 0.002 |
| His | 0.82ab | 0.78b | 0.82ab | 0.83a | 0.011 | 0.018 | 0.004 |
| Ile | 0.78ab | 0.75b | 0.79ab | 0.81a | 0.012 | 0.017 | 0.004 |
| Leu | 0.81ab | 0.78b | 0.82ab | 0.83a | 0.011 | 0.027 | 0.007 |
| Lys | 0.84ab | 0.82b | 0.85ab | 0.86a | 0.009 | 0.024 | 0.006 |
| Met | 0.91a | 0.88b | 0.91a | 0.92a | 0.006 | 0.004 | 0.004 |
| Phe | 0.81ab | 0.78b | 0.82ab | 0.83a | 0.011 | 0.012 | 0.003 |
| Pro | 0.78ab | 0.74b | 0.78ab | 0.79a | 0.012 | 0.025 | 0.006 |
| Ser | 0.74ab | 0.70b | 0.76a | 0.77a | 0.014 | 0.010 | 0.002 |
| Thr | 0.73a | 0.67b | 0.73a | 0.74a | 0.014 | 0.009 | 0.002 |
| Tyr | 0.78ab | 0.74b | 0.79a | 0.81a | 0.012 | 0.004 | 0.001 |
| Val | 0.77ab | 0.73b | 0.77ab | 0.79a | 0.013 | 0.033 | 0.007 |
| Average of all amino acids | 0.79ab | 0.75b | 0.80ab | 0.81a | 0.011 | 0.011 | 0.003 |
NC, negative control; PC, positive control. NC formulated without Pi and reduced in ME (−68 kcal/kg), crude protein (−10 g/kg), select AA (digestible Lys, Met and Cys, Thr, Arg, Val, and Ile, −0.1 to −0.4 g/kg; Table 1), Ca (−2.0 g/kg), digestible P (−2.2 g/kg), and sodium (−0.4 g/kg).
SEM, standard error of the mean.
Excluding PC from the analysis.
Mean values in the same row bearing different superscript letters are significantly different at P < 0.05.
Discussion
As newer generations of phytase with enhanced efficacy to release P and other phytate-bound nutrients continue to become available, the potential for formulating broiler diets without the addition of Pi and for reducing the content of other costly components such as essential AA is attracting research attention. Diet formulations based on digestible AA to account for the expected contribution of a given phytase are of interest both from the perspective of feed cost and environment. Any reduction in the amount of digestible AA added to the diet is likely to reduce the excretion of undigested N, which can have detrimental effects on ground and surface water ecosystems.
Previous studies on PhyG have demonstrated its efficacy in nutrient-reduced broiler diets to improve phytate degradation, increase the digestible P contents, and maintain or improve growth performance and bone mineralization over an entire growth cycle when compared with a nutritionally adequate diet with no added phytase (Dersjant-Li et al., 2020; Marchal et al., 2021; Dersjant-Li et al., 2022). The primary aim of the present study was to evaluate the dose-response effect of this phytase, when applied to Pi-free and high PP diets, on AA digestibility responses of young broilers. Effects on P retention, digestibility of other key nutrients, and tibia ash were also examined to provide wider indication of the capacity of the phytase to compensate for reductions in energy, crude protein, dig AA, and dig P.
The reduced P retention in birds fed the NC diet formulated without Pi (dig P content 1.5 g/kg) and with a high phytate content (3.33 g/kg PP) is reflective of reduced P availability. This is consistent with the previous literature describing PP as a poor source of P in the absence of phytase and demonstrating its negative effects on P digestibility and utilization in young broilers (Selle and Ravindran, 2007; Dersjant-Li et al., 2015; Inglemann et al., 2019). Supplementation of PhyG at 1,000 FTU/kg improved P retention (+14.5% units vs. PC). Given the capacity of PP to complex with mineral cations in the digestive tract (Selle et al., 2012), which limits its availability for digestion, the hypothesized mode of action for this phytase is the early, rapid, and extensive hydrolysis of phytate to lower IP esters in the upper digestive tract. The low pH optima (pH 3.5–4.5 in vitro), wide pH range of activity (pH 2.0–5.0) (Christensen et al., 2020), and extensive hydrolysis of IP6 to IP2 observed in vitro and in vivo (Christensen et al., 2020; Dersjant-Li et al., 2021) of PhyG support this thesis. The degree of improvement (above NC) in P retention with 1,000 FTU/kg (+24.9% units) is consistent with the degree of improvement in ileal P digestibility (+25% units) recently observed in 21-d-old broilers fed Pi-free diets containing similar PP content (3.45 g/kg) with 1,000 FTU/kg of the same phytase (Dersjant-Li et al., 2021). Although total tract retention does not equate to digestibility as it includes P excreted via urine, comparative studies of the two measures for evaluating P availability from Pi sources have suggested comparable results (Shastak et al., 2012).
Early studies have established tibia ash as a useful criterion to determine P availability in Pi sources (Nelson and Peeler, 1961) and as an indicator of phytase effects on dietary P availability (Denbow et al., 1995; Qian et al., 1996). In the current study, PhyG at 1,000 FTU/kg was required to restore tibia ash to the level of the PC. Given the low P retention in the basal NC diet, this observation supports that the improvement in tibia ash versus NC with phytase would largely have resulted from the degradation of phytate by the phytase, confirming the high phosphoric effect of the phytase.
Extra-phosphoric effects of PhyG on the ileal digestibility of DM, N, and GE were indicated by the significant linear dose–response relationships for these components. For the ileal digestibility of AA, there were substantial negative effects of the applied AA reductions in the NC diet when compared with the nutritionally adequate PC diet; the average AID AA was reduced by 5.1%, with a marked reduction of −11.8% for Cys. These reductions in AID AA are consistent with those observed by Ravindran et al. (2001) in Lys-deficient diets. PhyG supplementation of the NC diet resulted in linear increases in the AID of all 17 AA with increasing PhyG dose level between 0 and 1,000 FTU/kg. It was notable that the AID responses varied depending on the individual AA: For some AA, the magnitude of improvement was substantial (+15.0% above NC for Cys at 1,000 FTU/kg), whereas for others the effects were modest (+4.5% for Met at 1,000 FTU/kg). The probable mechanistic bases for antinutritive effects of PP on protein and AA digestibility have been discussed by Selle et al. (2012). The underlying modes of action are thought to include 1) decreased AA absorption due to the pH dependent formation of binary (direct) or ternary (via cationic bridges) binding of PP with AA residues on proteins, 2) upregulation of mucin production by phytate leading to increased endogenous losses of some AA, and/or 3) disruption of AA transport across the intestinal membrane via effects on the Na+, K+-ATPase pump. Reduction of these negative effects as a result of the hydrolysis of phytate by phytase is the major factor contributing to the improvements in AA digestibility, as evidenced by the reports of strong correlations between AA digestibility and the degree of phytate degradation by phytase (Amerah et al., 2014; Cowieson et al., 2017). Previous studies indicate that the AID AA in the absence of phytase is a function of type of protein source, inherent AA digestibility, and dietary phytate content (Ravindran et al., 1999; Cowieson et al., 2017). Direct comparison of phytase on AA digestibility between individual studies involving different protein sources/content in the test diets and different methodologies is therefore problematic but may be resolved by meta-analyses of modeled data from multiple studies.
The improvements in AID coefficients with phytase were dose-related and differed in magnitude for individual AA. The greatest increases were evident for the essential AA, Thr, Val, and Ile, and for the non-essential AA, Ala, Cys, and Gly. It is noteworthy that these AA are among the abundant AA in endogenous proteins in poultry (Ravindran, 2021), lending support to the reduced endogenous AA loss mechanism of added phytase. PhyG addition at 500 or 1,000 FTU/kg to the nutrient-reduced NC diet resulted in AID coefficients that were equivalent to those of the nutritionally adequate diet, indicating that PhyG at either dose level was able to fully compensate for the AA reductions applied to the NC diet.
Among the few other studies to have evaluated AA responses to phytase specifically in young broilers, Cowieson et al. (2006) similarly observed the highest response levels for Thr, Val, and Ile and a low response level for Arg in broilers at 16 d of age, whereas Namkung and Leeson (1999) observed greater increases for Val and Ile but not for Thr. Studies in older birds have also reported Thr, Val, and Ile to be among the AA most improved by phytase (Ravindran et al., 1999). As also observed by Ravindran et al. (1999), the AA most improved by phytase in the present study appeared to be those with the lowest inherent digestibility in the basal diet. As one of the most limiting AA in poultry diets (Warnick and Anderson, 1968), the substantial improvement in the digestibility of Cys by PhyG is particularly notable, as is the improvement in the digestibility of Thr, the third limiting AA. A recent meta-analysis of 24 studies carried out between 1996 and 2015 comprising phytase dose levels from 500 to 2,000 FTU/kg in 21- to 28-d-old broilers reported an average improvement in the AID across all AA of 4.1% above the respective NC diets with greatest improvements being in the digestibility of Cys (+7.2%) and least for Met (+1.3%) (Cowieson et al., 2017). Similar trends were observed in the present study, wherein the average improvement (above NC) in AID of all AA by PhyG at 500 and 1,000 FTU/kg was 6.7% and 8.0%, respectively, and improvements at 1,000 FTU/kg were also greatest for Cys (+15.0%) and lowest for Met (+4.5%). However, the degree of response was greater in the present study, possibly due in part to the phytase source and dietary settings. In both the meta-analysis (Cowieson et al., 2017) and an earlier study in young broilers (Cowieson et al., 2006), a plateauing effect was observed with increasing phytase dose levels beyond 1,000 FTU/kg. Amino acid responses to PhyG phytase at dose levels above 1,000 FTU/kg are yet to be evaluated.
The NC diet was formulated without Pi and with reduced ME (−68 kcal/kg), CP (−10 g/kg), digestible AA (digestible Lys, Met and Cys, Thr, Arg, Val and Ile, −0.1 to −0.4 g/kg), Ca (−2.0 g/kg), digestible P (−2.2 g/kg), and sodium (−0.4 g/kg). Because of the severe nutrient reductions imposed, the markedly lower weight gain of broilers fed the NC diet was to be expected. Although PhyG was found to improve the AID coefficient of AA, P retention and tibia ash to levels equivalent to or above those of the PC diet indicating that the reductions in P, CP, and digestible AA were ameliorated, it was perplexing that the added phytase, even at 1,000 FTU/kg, did not fully recover performance to the level of the PC. Feed intake is the major factor driving weight gain and modern broilers are more than ever susceptible to feed intake stressors (Abdollahi et al., 2018). In the current study, feeding the NC diet, compared to the PC diet, reduced feed intake by 82 g/bird (25% reduction). Although inclusion of PhyG at 500 and 1,000 FTU/kg recovered the feed intake by 35 and 59 g, respectively, above the NC, this was not sufficient to restore weight gain to a level equivalent to that of birds fed the PC diet. This finding highlights the importance of measuring feed intake in exogenous phytase studies and the fact that growth performance is essentially a result of digestible nutrient intake rather than nutrient digestibility per se. To restore the weight gain and feed efficiency in severely nutrient/energy-deficient diets, such as the one used in the current study, besides enhancing the nutrient digestibility, restoration of the feed intake is also required (Kelly et al., 1991; Abdollahi et al., 2013). The intake of digestible nutrients, therefore, might need to be considered as a more reliable response variable in exogenous enzyme studies (Abdollahi et al., 2018; Walk and Bedford, 2020). In addition, it should be noted that total removal of inorganic P (−2.2g/kg dig P reduction) together with the severe reduction of other nutrients/energy in the diet will require a higher phytase dose to fully recover performance back to the level of the PC. In other words, the down specifications of ME, crude protein, digestible AA, Ca, and dig P without inorganic P applied in the present study were greater than the contribution of the phytase at 1,000 FTU/kg, explaining why the phytase at 1,000 FTU/kg did not fully recover performance to the level of the PC.
Conclusions
A novel consensus bacterial 6-phytase variant, PhyG, at 500 or 1,000 FTU/kg, improved the ileal digestibility of AA in 15-d-old broilers fed a high PP, Pi-free, nutrient-reduced diet and the responses were equivalent to those produced by a nutritionally adequate diet that contained no phytase. Amino acid digestibility responses to the phytase were dose-dependent within the tested dose range of 0 to 1,000 FTU/kg. Dose-related increases in P-retention, tibia ash, and ileal digestibility of DM, N, and GE were also observed. PhyG at 1,000 FTU/kg resulted in an average improvement in AID AA of 8.0% above the NC diet, reflecting the high capacity of this phytase to improve AA utilization. This is the first study to evaluate dose-related digestible AA responses to this novel bacterial 6-phytase. Additional studies under different conditions with varied diet compositions and bird ages are needed to generate a robust digestible AA matrix that is applicable across multiple settings. The relevance of digestible nutrient intake as a response variable in exogenous enzyme studies is highlighted.
Acknowledgments
We would like to thank Dr Joelle Buck (Newbury, UK) for her assistance with the compilation of this manuscript and this was sponsored by Danisco Animal Nutrition & Health, IFF, the Netherlands, in accordance with Good Publication Practice guidelines.
Glossary
Abbreviations
- AA
amino acid
- ME
metabolizable energy
- AID
apparent ileal digestibility
- BW
body weight
- Dig
digestible
- DM
dry matter
- FCR
feed conversion ratio
- FTU
phytase unit
- GIT
gastrointestinal tract
- NC
negative control
- MCP
monocalcium phosphate
- PC
positive control
- PP
phytate phosphorus
- PhyG
novel consensus bacterial 6-phytase variant
- Pi
inorganic phosphate
- TTR
total tract retention
Conflict of Interest Statement
Y. Dersjant-Li, A. Bello, K. Waller, and L. Marchal are employees of Danisco Animal Nutrition & Health, IFF.
Literature Cited
- Abdollahi, M. R., Ravindran V., and Svihus B.. . 2013. Influence of grain type and feed form on performance, apparent metabolisable energy and ileal digestibility of nitrogen, starch, fat, calcium and phosphorus in broiler starters. Anim. Feed Sci. Technol. 186:193–203. doi: 10.1016/j.anifeedsci.2013.10.015 [DOI] [Google Scholar]
- Abdollahi, M. R., Zaefarian F., and Ravindran V.. . 2018. Feed intake response of broilers: impact of feed processing. Anim. Feed Sci. Technol. 237:154–165. doi: 10.1016/j.anifeedsci.2018.01.013 [DOI] [Google Scholar]
- Adedokun, S. A., Parsons C. M., Lilburn M. S., Adeola O., and Applegate T. J.. . 2007. Endogenous amino acid flow in broiler chicks is affected by the age of birds and method of estimation. Poult. Sci. 86:2590–2597. doi: 10.3382/ps.2007-00096 [DOI] [PubMed] [Google Scholar]
- Amerah, A. M., Plumstead P. W., Barnard L. P., and Kumar A.. . 2014. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult. Sci. 93:906–915. doi: 10.3382/ps.2013-03465 [DOI] [PubMed] [Google Scholar]
- AOAC. 2005. Official methods of analysis. 18th ed. Arlington (VA): Association of Official Analytical Chemists. [Google Scholar]
- Aviagen Inc. 2019. Ross 308 Broiler: Nutrition Specifications. Huntsville (AL)—[accessed January 20, 2020]. http://tmea.staging.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-308-Broiler-Nutrition-Specs-2014r17-EN.pdf [Google Scholar]
- Barua, M., Abdollahi M. R., Zaefarian F., Wester T. J., Girish C. K., Chrystal P. V., and Ravindran V.. . 2021. Basal ileal endogenous amino acid flow in broiler chickens as influenced by age. Poult. Sci. 100:101269. doi: 10.1016/j.psj.2021.101269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batal, A. B., and Parsons C. M.. . 2002. Effects of age on nutrient digestibility in chicks fed different diets. Poult. Sci. 81:400–407. doi: 10.1093/ps/81.3.400 [DOI] [PubMed] [Google Scholar]
- Cabahug, A., Ravindran V., Selle P. H., and Bryden W. L.. . 1999. Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorus contents. I. Effects on bird performance and toe ash. Br. Poult. Sci. 40:660–666. doi: 10.1080/00071669987052 [DOI] [PubMed] [Google Scholar]
- Christensen, T., Dersjant-Li Y., Sewalt V., Mejldal R., Haaning S., Pricelius S., Nikolaev I., Sort R. A., and de Kreij A.. . 2020. In vitro characterization of a novel consensus bacterial 6-phytase and one of its variants. Current Biochem. Eng. 6:156–171. doi: 10.2174/221271190699920201710 [DOI] [Google Scholar]
- Cowieson, A.J., Acamovic T., and Bedford M. R.. . 2006. Supplementation of corn-soy-based diets with an Escherichia coli-derived phytase: effects on broiler chick performance and the digestibility of amino acids and metabolizability of minerals and energy. Poult. Sci. 85:1389–1397. doi: 10.1093/ps/85.8.1389. [DOI] [PubMed] [Google Scholar]
- Cowieson, A. J., Rickebusch J. -P., Sorbara J. O. B., Wilson J. W., Guggenbuhl P., and Roos F. F.. . 2017. A systematic view on the effect of phytase on ileal amino acid digestibility in broilers. Anim. Feed Sci. Technol. 225:182–194. doi: 10.1017/j.anifeesci.2017.01.008 [DOI] [Google Scholar]
- Denbow, L. M., Ravindran V., Kornegay E. T., Yi Z., and Hulet R. M.. . 1995. Improving phosphorus availability in soybean meal for broilers by supplemental phytase. Poult. Sci. 74:1831–1842. doi: 10.3382/ps.0741831 [DOI] [PubMed] [Google Scholar]
- Dersjant-Li, Y., Archer G., Stiewert A. M., Brown A. A., Sobotik E. G., Jasek A., Marchal L., Bello A., Sort R. A., Christensen T., Kim H. -S., Mejldal R., Nkolaev I., Pricelius S., Haaning S., Søresnesn J. F., de Kreij A., and Sewalt V.. . 2020. Functionality of a next generation biosynthetic bacterial 6-phytase in enhancing phosphorus availability to broilers fed a corn-soybean meal-based diet. Anim. Feed Sci. and Technol. 264:114481. doi: 10.1016/j.anifeedsci.2020.114481 [DOI] [Google Scholar]
- Dersjant-Li, Y., Awati A., Schulze H., and Partridge G.. . 2015. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 95:878–896. doi: 10.1002/jsfa.6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li, Y., Bello A., Esteve-Garcia E., Ramírez Creus C., and Marchal L.. . 2022. A novel consensus bacterial 6-phytase variant added to phytate-rich diets totally replaced inorganic phosphate in broilers. J. Appl. Anim. Nutr. In press. [Google Scholar]
- Dersjant-Li, Y., Christensen T., Knudsen S., Bello A., Toghyani M., Liu S. Y., Selle, P. H. and Marchal L.. . 2021. Effect of increasing dose level of a novel consensus bacterial 6-phytase variant on phytate degradation in broilers fed diets containing varied phytate levels. Br. Poult. Sci. doi: 10.1080/00071668.2021.2000586. Epub ahead of print. PMID: [DOI] [PubMed] [Google Scholar]
- Dersjant-Li, Y., and Kwakernaak C.. . 2019. Comparative effects of two phytases versus increasing the inorganic phosphorus content of the diet, on nutrient and amino acid digestibility in broilers. Anim. Feed Sci. Technol. 253:166–180. doi: 10.1016/j.anifeedsci.2019.05.018 [DOI] [Google Scholar]
- Engelen, A., van der Heeft F., Randsdorp P., Somers W., Schaefer J., and van der Vat B.. . 2001. Determination of phytase activity in feed by a colorimetric enzymatic method: collaborative interlaboratory study. J. AOAC Int. 84:629–633. doi: 10.1093/jaoac/84.3.629 [DOI] [PubMed] [Google Scholar]
- Humer, E., Schwarz C., and Schedle K.. . 2015. Phytate in pig and poultry nutrition. J. Anim. Phys. Anim. Nutr. 99:605–625. doi: 10.1111/jpn.12258 [DOI] [PubMed] [Google Scholar]
- Inglemann, C-J., Witzig M., Möhring J., Schollenberger M., Kühn I., and Rodehutscord M.. . 2019. Phytate degradation and phosphorus digestibility in broilers and turkeys fed different corn sources with or without added phytase. Poult. Sci. 98:912–922. doi: 10.3382/ps/pey438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- JMP. 2019. Version 14. Cary (NC): SAS Institute Inc.; 1989–2019. [Google Scholar]
- Kelly, D., Smyth J. A., and McCracken K. J.. . 1991. Digestive development of the early-weaned pig. 2. Effect of level of food intake on digestive enzyme activity during the immediate post-weaning period. Br. J. Nutr. 65:181–188. doi: 10.1079/BJN19910079 [DOI] [PubMed] [Google Scholar]
- Kiarie, E., Woyengo T., and Nyachoti C. M.. . 2015. Efficacy of a new 7-phytase from buttiauxella spp. On growth performance and nutrient retention in broiler chickens fed corn soybean meal-based diets. Asian Austr. J. Anim. Sci. 28:1479–1487. doi: 10.5713/ajas.15.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. -W., Li W., Angel R., and Plumstead P. W.. . 2019. Modification of a limestone solubility method and potential to correlate with in vivo limestone calcium digestibility. Poult. Sci. 98:6837–6848. doi: 10.3382/ps/pez423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg, J., Siegert W., Berghaus D., Bock J., Feuerstein D., and Rodehutscord M.. . 2020. Phytase supplementation effects on amino acid digestibility depend on the protein source in the diet but are not related to InsP6 degradation in broiler chickens. Poult. Sci. 99:3251–3265. doi: 10.1016/j.psj.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladics, G. S., Han K. -H., Jang M. S., Park H., Marshall V., Dersjant-Li Y., and Sewalt V. J.. . 2020. Safety evaluation of a novel variant of consensus bacterial phytase. Toxicol. Lett. 7:844–851. doi: 10.1016/j.toxrep.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leske, K. L., and Coon C. N.. . 1999. A bioassay to determine the effect of phytase on phytate phosphorus hydrolysis and total phosphorus retention of feed ingredients as determined with broilers and laying hens. Poult. Sci. 78:1151–1157. doi: 10.1093/ps/78.8.1151 [DOI] [PubMed] [Google Scholar]
- Li, W., Angel R., Kim S. -W., Jiménez-Moreno E., Proszkowiec-Weglarz M., and Plumstead P. W.. . 2015. Age and adaptation to ca and P deficiencies. 2. Impacts on amino acid digestibility and phytase efficacy in broilers. Poult. Sci. 94:2917–2931. doi: 10.3382/ps/pev273 [DOI] [PubMed] [Google Scholar]
- Marchal, L., Bello A., Sobotik E. B., Archer G., and Dersjant-Li Y.. . 2021. A novel consensus bacterial 6-phytase variant completely replaced inorganic phosphate in broiler diets, maintaining growth performance and bone quality: data from two independent trials. Poult. Sci. 100:1–14. doi: 10.1016/j.psj.2020.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung, H., and Leeson S.. . 1999. Effect of phytase enzyme on dietary nitrogen-corrected apparent metabolizable energy and the ileal digestibility of nitrogen and amino acids in broiler chicks. Poult. Sci. 78:1317–1319. doi: 10.1093/ps/78.9.1317 [DOI] [PubMed] [Google Scholar]
- Nelson, T. S., and Peeler H. T.. . 1961. The availability of phosphorus from single and combined phosphates to chicks. Poult. Sci. 40:1321–1328. doi: 10.3382/ps.0401321 [DOI] [Google Scholar]
- Qian, H., Veit H. P., Kornegay E. T., Ravindran V., and Denbow D. M.. . 1996. Effects of supplemental phytase and phosphorus on histological and other tibial bone characteristics and performance of broilers fed semi-purified diets. Poult. Sci. 75:618–626. doi: 10.3382/ps.0750618 [DOI] [PubMed] [Google Scholar]
- Ravindran, V. 2021. Progress in ileal endogenous amino acid flow research in poultry. J. Anim. Sci. Biotechnol. 12:5. doi: 10.1186/s40104-020-00526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, V., and Abdollahi M. R.. . 2021. Nutrition of the newly hatched broiler chick: state of the art and outlook. Animals 11:2795. doi: 10.3390/ani11102795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, V., Cabahug S., Ravindran G., and Bryden W. L.. . 1999. Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult. Sci. 78:699–706. doi: 10.1093/ps/78.5.699 [DOI] [PubMed] [Google Scholar]
- Ravindran, V., Morel P. C. H., Rutherfurd S. M., and Thomas D. V.. . 2009. Endogenous flow of amino aids in the avian ileum is increased by increasing dietary peptide concentrations. Br. J. Nutr. 101:822–828. doi: 10.1017/S0007114508039974 [DOI] [PubMed] [Google Scholar]
- Ravindran, V., Selle P. H., Ravindran G., Morel P. C. H., Kies A. K., and Bryden W. L.. . 2001. Microbial phytase improves performance, apparent metabolizable energy and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poult. Sci. 80:338–344. doi: 10.1093/ps/80.3.338 [DOI] [PubMed] [Google Scholar]
- Sebastian, S., Touchburn S. P., Chavez E. R., and Laguë P. C.. . 1997. Apparent digestibility of protein and amino acids in broiler chickens fed a corn-soybean diet supplemented with microbial phytase. Poult. Sci. 76:1760–1769. doi: 10.1093/ps/76.12.1760 [DOI] [PubMed] [Google Scholar]
- Selle, P. H., Cowieson A. J., Cowieson N. P., and Ravindran V.. . 2012. Protein-phytate interactions in pig and poultry nutrition: a reappraisal. Nutr. Res. Rev. 25:1–17. doi: 10.1017/S0954422411000151 [DOI] [PubMed] [Google Scholar]
- Selle, P. H., Cowieson A. J., and Ravindran V.. . 2009a. Consequences of calcium interactions with phytate and phytase for poultry and pigs. Livest. Sci. 124:126–141. doi: 10.1016/j.livsci.2009.01.006 [DOI] [Google Scholar]
- Selle, P. H., and Ravindran V.. . 2007. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 135:1–41. doi: 10.1017/j.anifeedsci.2006.06.010 [DOI] [Google Scholar]
- Selle, P. H., Ravindran V., and Partridge G. G.. . 2009b. Beneficial effects of xylanase and/or phytase inclusions on ileal amino acid digestibility, energy utilisation, mineral retention and growth performance in wheat-based broiler diets. Anim. Feed Sci. Technol. 153:303–313. doi: 10.1016/j.anifeedsci.2009.06.011 [DOI] [Google Scholar]
- Shastak, Y., Witzig M., Hartung K., and Rodehutscord M.. . 2012. Comparison of retention and prececal digestibility measurements in evaluating mineral phosphorus sources in broilers. Poult. Sci. 91:2201–2209. doi: 10.3382/ps.2011-02063 [DOI] [PubMed] [Google Scholar]
- Short, F. J., Gorton P., Wiseman J., and Boorman K. N.. . 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 59:215–221. doi: 10.1016/0377-8401(95)00916-7 [DOI] [Google Scholar]
- Skoglund, E., Carlsson N. G., and Sandberg A. S.. . 1998. High performance chromatographic separation of inositol phosphate isomers on strong anion exchange columns. J. Agric. Food Chem. 46:1877–1882. doi: 10.1021/jf97009257 [DOI] [Google Scholar]
- Tamim, N. M., Angel R., and Christman M.. . 2004. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 83:1358–1367. doi: 10.1093/ps/83.8.1358 [DOI] [PubMed] [Google Scholar]
- Tesseraud, S., Peresson R., Lopes J., and Chagneau A. M.. . 1996. Dietary lysine deficiency greatly affects muscle and liver protein turnover in growing chickens. Br. J. Nutr. 75:853–865. doi: 10.1079/BJN19960191 [DOI] [PubMed] [Google Scholar]
- Walk, C. L., and Bedford M. R.. . 2020. Application of exogenous enzymes: is digestibility an appropriate response variable? Anim. Prod. Sci. 60:993–998. doi: 10.1071/AN19437 [DOI] [Google Scholar]
- Walk, C. L., and Rao S. V.. . 2020. Increasing dietary phytate has a significant anti-nutrient effect on apparent ileal amino acid digestibility and digestible amino acid intake requirement increasing dose level of phytase as evidence by prediction equations in broilers. Poult. Sci. 99:290–300. doi: 10.3382/ps/pez489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, H. G., Coelho M., Coufal C. D., and Lee J. T.. . 2019. Effects of increasing phytase inclusion levels on broiler performance, nutrient digestibility, and bone mineralization in low-phosphorus diets. J. Appl. Poult. Res. 28:1210–1224. doi: 10.3382/japr/pfz087 [DOI] [Google Scholar]
- Warnick, R. E., and Anderson J. O.. . 1968. Limiting essential amino acids in soybean meal for growing chickens and the effects of heat upon availability of the essential amino acids. Poult. Sci. 47:281–287. doi: 10.3382/ps.0470281 [DOI] [PubMed] [Google Scholar]