Abstract
Objective
Safe, effective, long-term oral therapies are needed for plaque psoriasis. This study aimed to assess the safety and effectiveness of tepilamide fumarate (a fumaric acid ester) extended-release tablets.
Methods
This Phase IIb, randomized, double-blind, placebo-controlled, 24-week, multicenter study treated adults with moderate-to-severe plaque psoriasis with tepilamide fumarate 400 mg once (QD) or twice daily (BID), 600 mg BID, or placebo. Coprimary endpoints were the proportion of patients achieving ≥75% reduction in the Psoriasis Area and Severity Index (PASI-75) and Investigator’s Global Assessment (IGA) of clear or almost clear (≥2 points’ reduction).
Results
A total of 426 patients were randomized (mean age 49.6 [±13.0] years). There was a ≥75% PASI reduction in 39.7%, 47.2%, 44.3%, and 20.0% in the 400 mg QD, 400 mg BID, 600 mg BID, and placebo groups, respectively; IGA treatment success was 35.7%, 41.4%, 44.4%, and 22.0%, respectively. Between 50%-66% of tepilamide fumarate and 48% of placebo patients experienced ≥1 treatment-emergent adverse event. Gastrointestinal intolerance (20%-42%), infection (6%-18%), and decreased lymphocyte count (4%-9%) were more common with tepilamide fumarate.
Limitations
High placebo response somewhat limits the utility of these findings.
Conclusion
Patients with moderate-to-severe plaque psoriasis treated with oral tepilamide fumarate demonstrated positive response.
Keywords: Body surface area (BSA) dermatology, Dermatology Life Quality Index (DLQI), dimethyl fumarate, fumaric acid esters, gastrointestinal, immunomodulating, inflammatory cytokine, Investigator’s Global Assessment (IGA), monomethyl fumarate, Nail Psoriasis Severity Index (NAPSI), non-biologic, oral, plaque psoriasis, PPC-06, prodrug, psoriasis, Psoriasis Area and Severity Index (PASI), Psoriasis Scalp Severity Index (PSSI), systemic, tepilamide fumarate, XP23829
Psoriasis is a chronic T-cell mediated autoimmune skin disease affecting 3.2 percent of the U.S. population.1,2 Treatment aims to reduce disease burden and improve signs and symptoms.1,3 According to the American Academy of Dermatology, topical therapies (with or without phototherapy) may be insufficient for patients with moderate-to-severe disease, and systemic therapy, including nonbiologics and biologics, may be necessary.1 Over time, however, the use of the nonbiologic agents (acitretin, cyclosporine, and methotrexate) can lead to target organ toxicity and drug interactions.3 Treatment with apremilast, a phosphodiesterase 4 (PDE4) inhibitor, is associated with an approximate 15 percent risk of upper respiratory tract infection (URTI).4 Long-term safety complications associated with biologic therapies (tumor necrosis factor-[TNF]α antagonists, interleukin [IL]-12/23, and IL-17 monoclonal antibody inhibitors) include immunosuppression and the potential for infection, opportunistic disease, or malignancy.1,3
Drugs in the fumaric acid esters (FAE) class frequently contain the primary active ingredient dimethyl fumarate (DMF); this oral pro-drug generates the active metabolite monomethyl fumarate (MMF),5 which has immunomodulatory and neuroprotective effects in cell-based systems.6,7 Due to its clinical efficacy,8-12 a fixed-dose combination of DMF and MMF salts (Fumaderm®) has been a first-line systemic treatment for moderate-to-severe psoriasis in Germany for decades.7,13 This formulation and other DMF’s are fairly well-tolerated,9,11,14 but associated with high rates of gastrointestinal (GI) side effects and flushing over both short- and long-term follow-up.9,12,15,16
Lymphopenia (sometimes severe), reversible leucopenia, and transient eosinophilia are also common. In rare cases, progressive multifocal leukoencephalopathy (PML) has occurred; however, periodic blood lymphocyte count monitoring and clear treatment discontinuation criteria can mitigate this complication.16,17
Oral treatment options are limited for patients with psoriasis.3 Tepilamide fumarate (PPC-06), a MMF pro-drug, is a patented extended release tablet formulation absorbed throughout the GI tract and rapidly hydrolyzed to release the active moiety MMF.6,7 Unpublished Phase I data suggest that PPC-06 provides more efficient and sustained MMF exposure than marketed DMF formulations.6 This study assessed the safety and efficacy of tepilamide fumarate in patients with moderate-to-severe plaque psoriasis.
METHODS
Participants. This 24-week, Phase IIb, randomized, double-blind, placebo-controlled study was conducted at 74 sites in the United States, primarily dermatology research clinics. Eligible patients were males and non-pregnant females aged ≥18 years with stable, moderate-to-severe plaque psoriasis diagnosed for ≥6 months. Patients were required to be candidates for phototherapy and/or systemic therapy, with no morphology changes or significant disease flares in 6 months. Disease severity had to meet the following criteria: Psoriasis Area and Severity Index (PASI) score ≥12; total body surface area (BSA) ≥10% affected by plaque psoriasis; and Investigator’s Global Assessment (IGA) score ≥3. Patients needed to avoid excessive sun exposure/tanning during the study.
Patients were excluded if they had failed ≥3 systemic therapies or received additional psoriasis treatments within prespecified washout or time periods. Study treatment was permanently discontinued if lymphocytes were <500/mm3 at any visit or <800/mm3 on 3 consecutive visits. Additional inclusion, exclusion, and discontinuation criteria are available in the Supplemental Appendix. All supplemental materials for this article are available on the online version on jcadonline.com.
This clinical trial was registered on February 5, 2018 (NCT03421197, clinicaltrials.gov) and performed in compliance with International Council for Harmonisation Good Clinical Practices, as well as the ethical principles of the Declaration of Helsinki. All patients provided written informed consent.
Randomization. After a 4-week screening, patients were allocated 1:1:1:1 to tepilamide fumarate 400mg once daily (QD; evening); tepilamide fumarate 400 mg twice daily (BID; morning and evening), tepilamide fumarate 600mg BID; or placebo BID. Random allocation sequencing was generated by Medidata Solutions (Iselin, NJ), and patients were stratified by prior biologic use (a maximum of 30% of patients with prior, washed-out biologic use were permitted to enroll). The investigator and all study representatives were blinded to treatment assignments. After randomization, treatment continued for 24 weeks; this included an initial 5-week dose titration period (Supplemental Figure 1).
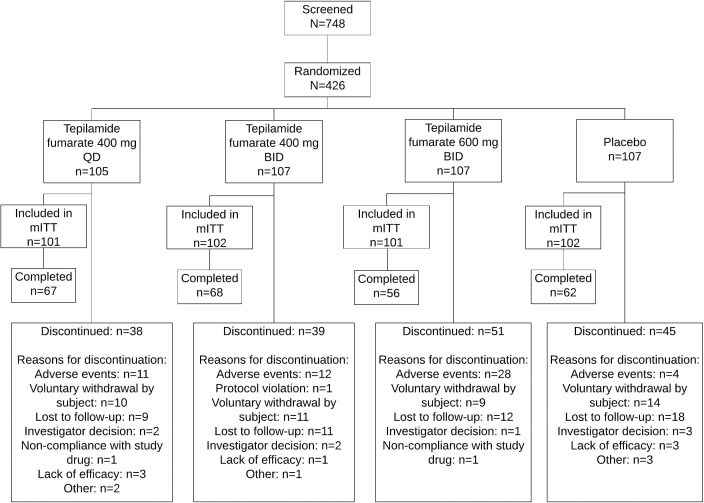
FIGURE 1.
Disposition of study participants; mITT: modified intent-to-treat
Endpoints. Coprimary efficacy endpoints, evaluated at the end of Week 24, included the proportion of patients with a ≥75% reduction from baseline in PASI score (PASI-75) and the proportion who achieved an IGA score of clear or almost clear (0 or 1; i.e., a ≥2-point reduction).
Secondary endpoints included mean absolute change from baseline and proportion of patients achieving: ≥50% and ≥90% reductions in total PASI score (PASI-50 and PASI-90); ≥50% reduction in target fingernail Nail Psoriasis Severity Index score (NAPSI-50); ≥75% reduction in Psoriasis Scalp Severity Index score (PSSI-75); a ≥5-point decrease in Dermatology Life Quality Index (DLQI) score in patients with a baseline score ≥5; and BSA percentage reductions. The NAPSI is a numeric, objective tool used to evaluate nail psoriasis.18 The PSSI is scalp-specific modification of the PASI.19 The DLQI is a validated 10-item questionnaire measuring the impact of skin conditions on daily activities.20
Patients returned at Week 25 for safety follow-up, with safety assessed through AE monitoring, clinical laboratory tests, vital signs, physical examinations, pregnancy testing, and electrocardiograms. Supplemental Table 1 summarizes the assessment schedule.
TABLE 1.
Summary of Patient Baseline Characteristics
| CHARACTERISTIC | TEPILAMIDE FUMARATE | Placebo (N=102) | Total (n=406) | ||
|---|---|---|---|---|---|
| 400mg QD (N=101) | 400mg BID (N=102) | 600mg BID (N=101) | |||
| Age, years | 48.0 (13.2) | 51.0 (12.3) | 50.2 (13.7) | 49.3 (12.6) | 49.6 (12.7) |
| Male, n (%) | 64 (63) | 62 (61) | 61 (60) | 60 (59) | 247 (61) |
| Race, n (%) | |||||
| White | 93 (93) | 91 (89) | 87 (86) | 92 (90) | 363 (90) |
| Black/AfricanAmerican | 6 (6) | 4 (4) | 10 (10) | 5 (5) | 25 (6) |
| Asian | 1 (1) | 3 (3) | 2 (2) | 1 (1) | 7 (2) |
| American Indian, Alaska Native | 0 (0) | 2 (2) | 0 (0) | 1 (1) | 3 (1) |
| Native Hawaiian, Pacific Islander | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (< 1) |
| Other | 0 (0) | 1 (1) | 2 (2) | 3 (3) | 6 (1) |
| Ethnicity, n (%) | |||||
| Hispanic/Latino | 62 (61) | 61 (60) | 60 (59) | 55 (54) | 238 (59) |
| Not Hispanic/Latino | 39 (39) | 41 (40) | 41 (41) | 47 (46) | 168 (41) |
| Body mass index, kg/m2 | 30.4 (5.0) | 30.2 (4.7) | 30.7 (4.7) | 30.3 (5.0) | 30.4 (4.8) |
| Baseline Psoriasis Area and Severity Index | 18.4 (6.4) | 18.6 (8.0) | 18.0 (6.7) | 17.3 (6.2) | 18.1 (6.8) |
| Baseline body surface area affected, % | 26 (15) | 25 (15) | 25 (14) | 24 (13) | 25 (14) |
| Baseline Investigator’s Global Assessment, n (%) | |||||
| 3–Moderate | 75 (74) | 77 (75) | 71 (70) | 73 (72) | 296 (73) |
| 4–Severe | 26 (26) | 25 (25) | 30 (30) | 29 (28) | 110 (27) |
| Prior conventional systemic therapy†, n (%) | 64 (63) | 70 (69) | 67 (66) | 64 (63) | 265 (65) |
| Prior biologic therapy, n (%) | 24 (24) | 20 (20) | 21 (21) | 23 (23) | 88 (22) |
BID=twice daily; QD=once daily; SD=standard deviation.
* Data are presented as mean (SD) unless otherwise noted.
† Patients who received phototherapy, systemic psoriasis medical therapy, or a combination.
Statistical methods. A sample of 400 was estimated to yield ≥84% power to test the coprimary efficacy endpoints. Each dichotomized coprimary endpoint was analyzed using a logistic regression model adjusted for treatment group, baseline body mass index, and pooled analysis center. Multiple imputation was used to address missing data for the primary efficacy analysis. Modified non-responder imputation (m-NRI) and last observation carried forward (LOCF) were also analyzed and used as sensitivity analyses to assess the robustness of efficacy findings. Each tepilamide fumarate dose was compared to placebo. Hypothesis testing for all dose groups was conducted sequentially using a 2-sided type I error rate of 0.05; PASI and IGA response rates were rejected at the 0.05 significance level.
The modified intent-to-treat (mITT) population was used for the primary efficacy analysis and included all randomized patients with ≥1 treatment dose and ≥1 post-dose efficacy assessment. The safety population included all patients who received ≥1 treatment dose. All analyses were performed using SAS (Version 9.4).
RESULTS
A total of 426 patients were randomized, 406 were included in the mITT population (tepilamide fumarate 400 mg QD [n=101]; 400 mg BID [n=102]; 600 mg BID [n=101]; placebo BID [n=102]), and 253 (59%) completed the study (Figure 1). Most were male (n=247/61%) and white (n=363/90%), with a mean age of 49.6 (±13.0) years (Table 1). At baseline, mean (SD) PASI was 18.1 (6.8); 73% and 27% of patients had an IGA score of 3 (moderate) or 4 (severe), respectively; mean (SD) BSA involvement was 25% (14%); and 88 (22%) patients had received prior biologic therapy.
At Week 24, all treatment groups showed higher PASI-75 response and IGA treatment success rates (Table 2, Supplemental Figures 2–3). PASI-75 response rates and odds ratios (OR, 95% CI) were 39.7% (3.1 [1.5-6.8]), 47.2% (3.6 [1.6-8.2]), and 44.3% (3.2 [1.5-7.0]), respectively, with tepilamide fumarate 400 mg QD, 400 mg BID, and 600 mg BID, versus 20.0% (P<0.05, all comparisons) with placebo. PASI-75 response was not significantly different between treatment groups. IGA treatment success rates and ORs (95% CI) were 35.7%, (2.2 [1.0-4.6]), 41.4% (2.6 [1.2-5.8]), and 44.4% (2.9 [1.3-6.5]), respectively, with tepilamide fumarate 400 mg QD, 400 mg BID, and 600 mg BID, versus 22.0% (P<0.05, all comparisons) with placebo. IGA treatment success was not significantly different between treatment groups. Similar PASI-75 and IGA success rates were observed with alternative imputations and in case analyses.
TABLE 2.
Analysis of Coprimary Efficacy Outcomes At Week 24
| MODIFIED INTENT-TO-TREAT POPULATION | TEPILAMIDE FUMARATE | PLACEBO (N=102) | ||
|---|---|---|---|---|
| 400mg QD (N=101) | 400mg BID (N=102) | 600mg BID (N=101) | ||
| PASI-75 | ||||
| MI | 39.7% | 47.2% | 44.3% | 20.0% |
| OR (95% CI)* | 3.1 (1.5-6.8) P=0.004 |
3.6 (1.6-8.2) P=0.002 |
3.2 (1.5-7.0) P=0.004 |
|
| LOCF | 34.4% P=0.003 |
39.0% P=0.003 |
36.0% P=0.011 |
17.0% |
| m-NRI | 44.3% P=0.008 |
50.7% P=0.005 |
51.7% P=0.010 |
24.2% |
| OC | 46.3% P=0.008 |
51.5% P=0.009 |
52.5% P=0.014 |
25.4% |
| IGA IMPROVEMENT | ||||
| MI | 35.7% | 41.4% | 44.4% | 22.0% |
| OR (95% CI)* | 2.2 (1.0-4.6) P=0.044 |
2.6 (1.2-5.8) P=0.021 |
2.9 (1.3-6.5) P=0.010 |
|
| LOCF | 26.5% P=0.024 |
31.0% P=0.009 |
31.0% P=0.011 |
14.0% |
| m-NRI | 37.1% P=0.029 |
42.0% P=0.012 |
43.3% P=0.014 |
19.7% |
| OC | 38.8% P=0.026 |
42.6% P=0.018 |
44.1% P=0.018 |
20.6% |
| Per protocol population | n=62 | >n=60 | n=57 | n=56 |
| PASI-75, m-NRI | 46.8% P=0.002 |
50.0% P=0.012 |
52.6% P=0.009 |
23.2% |
| IGA improvement, m-NRI | 38.7% P=0.027 |
41.7% P=0.027 |
43.9% P=0.023 |
19.6% |
BID=twice daily; CI=confidence interval; IGA=Investigator’s Global Assessment; LOCF=last observation carried forward; MI=multiple imputation; m-NRI=modified-nonresponder imputation; OC=observed cases; OR=odds ratio; PASI=Psoriasis Area and Severity Index; QD=once daily.
All treatment groups showed a gradual, progressive improvement in mean total PASI scores and PASI-50 and PASI-90 response rates from baseline to Week 24 (Table 3).
TABLE 3.
Analysis of Secondary Endpoints at Week 24, Modified Intent-to-Treat Population
| TEPILAMIDE FUMARATE | PLACEBO (N=102) |
|||
|---|---|---|---|---|
| 400mg QD (N=101) |
400mg BID (N=102) |
600mg BID (N=101) |
||
| Total PASI mean absolute change, MI | -10.7 | -12.0 | -11.7 | -6.7 |
| LSM (SE) differences* | -4.6 (1.1) P<0.001 |
-5.3 (1.1) P<0.001 |
-5.4 (1.1) P<0.001 |
0 |
| PASI-50 response, MI | 68.8% | 75.0% | 72.6% | 40.8% |
| OR (95% CI)* | 3.6 (1.8-7.5) P<0.00001 |
4.6 (2.1-9.7) P<0.00001 |
4.0 (1.9-8.6) P<0.00001 |
|
| PASI-90 response, MI | 17.3% | 20.9% | 18.8% | 5.5% |
| OR (95% CI)* | 3.7 (1.2-11.1) P=0.019 |
3.9 (1.3-11.8) P=0.017 |
3.4 (1.1-9.8) P=0.027 |
|
| NAPSI-50 response, n/N (%), LOCF | 10/26 (38.5%) | 8/23 (34.8%) | 12/24 (50.0%) | 6/31 (19.4%) |
| OR (95% CI)* | 2.6 (0.8-8.5) P=0.118 |
2.3 (0.7-7.8) P=0.197 |
4.2 (1.3-13.8) P=0.020 |
|
| PSSI-75 response, n/N (%), MI | 33/85 (38.9%) | 37/79 (47.7%) | 43/81 (53.8%) | 20/77 (26.1%) |
| OR (95% CI)* | 1.9 (0.9-4.0) P=0.120 |
2.6 (1.2-5.6) P=0.019 |
3.1 (1.4-7.2) P=0.007 |
|
| DLQI response, n/N (%), LOCF | 38/60 (63.3%) | 41/65 (63.1%) | 47/67 (70.1%) | 29/64 (45.3%) |
| OR (95% CI)* | 2.5 (1.1-5.4) P=0.023 |
2.4 (1.1-5.2) P=0.024 |
3.5 (1.6-7.6) P=0.002 |
|
| BSA, mean % change | -40.3 | -46.3 | -48.7 | -27.2 |
| LSM (SE) differences* | -14.4 (6.2) P=0.020 |
-18.8 (6.2) P=0.003 |
-21.3 (6.2) P<0.001 |
|
BID=twice daily; DLQI=Dermatology Life Quality Index; LSM=least squares mean; MI=multiple imputation; NAPSI=Nail Psoriasis Severity Index=OR, odds ratio; PASI=Psoriasis Area and Severity Index=PSSI, Psoriasis Scalp Score Index; QD=once daily; SE=standard error.
* Treatment versus placebo.
Mean PASI improvement over baseline at Week 24 ranged from 58.6%-65.5% with tepilamide fumarate (10.7-12.0-point reduction) versus 37.9% with placebo (6.7-point reduction) (P≤0.001, all doses). At Week 24, PASI-50 and PASI-90 response rates ranged from 68.8%-75.0% and 17.3%-20.9% with tepilamide fumarate, respectively, versus 40.8% (P<0.001, all doses) and 5.5% (P<0.05, all doses) with placebo (Table 3, Supplemental Figures 4–5).
A greater proportion of patients receiving tepilamide fumarate achieved fingernail NAPSI-50, PSSI-75, DLQI, and BSA response. At Week 24, NAPSI-50, PSSI-75, and DLQI, rates ranged from 34.8%–50.0%, 38.9%–53.8%, and 63.1%–70.1% with tepilamide fumarate, respectively, versus 19.4%, 26.1%, and 45.3% with placebo (Table 3, Supplemental Figures 6–8). Mean percent change in BSA at Week 24 ranged from -40.3% to -48.7% in patients treated with tepilamide fumarate versus -27.2% change with placebo.
Safety results. The following number of patients experienced ≥1 treatment-emergent adverse event (TEAE) of any severity: 400 mg QD (53 [50.5%]); 400 mg BID (56 [52.3%]); 600 mg BID (70 [66.0%]); and placebo (51 [47.7%]). Among tepilamide fumarate-treated patients, the most frequently reported TEAEs were due to diarrhea (7%-23%), nausea (7%-10%), abdominal pain (8%-20%), lymphopenia (4%-9%), decreased lymphocyte count (4%-8%), and eosinophilia/increased eosinophil count (5%-6%) (Supplemental Table 2).
Treatment-related TEAEs were higher with tepilamide fumarate 600 mg BID (146 events in 53 [50.0%] patients) and 400 mg BID (113 events in 49 [45.8%] patients) than in the 400 mg QD (84 events in 34 [32.4%] patients) and placebo groups (27 events in 17 [15.9%] patients). Eight serious adverse events were reported in 7 patients: 3 events (3 patients) in the 400 mg BID group; 3 events (2 patients) in the 400 mg QD group; and 2 events (2 patients) in the placebo group. Of these, 4 were probably not related to study treatment, 2 were not related, and 2 were possibly related. No deaths were reported.
The number of patients who discontinued the study due to an AE was 10 (9.5%), 12 (11.2%), 29 (27.3%), and 4 (3.7%) in the tepilamide fumarate 400 mg QD, 400 mg BID, 600 mg BID, and placebo groups, respectively. Of these discontinuations, 2 (1.9%), 7 (6.5%), 18 (17.0%), and 1 (0.9%) occurred during the titration period (first 35 days), respectively, while 8 (7.6%), 5 (4.7%), 11 (10.4%), and 3 (2.8%) occurred after (Day 36 through Week 24). The most common AEs leading to discontinuation were diarrhea and abdominal pain. In the tepilamide fumarate and placebo groups, respectively, 1 (1.0%), 5 (4.7%), 10 (9.4%), and 0 patients discontinued due to diarrhea, and 2 (1.9%), 2 (1.9%), 6 (5.7%), and 0 discontinued due to abdominal pain. There were no clinically important changes in vital signs or laboratory results.
A dose-dependent trend towards reduction in mean absolute lymphocyte count was observed with tepilamide fumarate at approximately Week 4 and stabilized around Week 12. Four (3.8%) patients in the tepilamide fumarate 600 mg BID group and 1 (0.9%) in the 400 mg BID group discontinued due to lymphopenia or decreased lymphocyte count (Table 4); lymphopenia reversed with treatment cessation.
TABLE 4.
TEAEs of Low Lymphocyte Count
| TEAE, NUMBER OF PATIENTS (%) | PLACEBO | TEPILAMIDE FUMARATE | ||
|---|---|---|---|---|
| 400mg QD | 400mg BID | 600mg BID | ||
| Lymphopenia | 0 (0%) | 4 (4%) | 4 (4%) | 10 (9%) |
| Lymphocyte Count Decreased | 1 (1%) | 4 (4%) | 9 (8%) | 6 (6%) |
| Total* | 1 (0.94%) | 7 (6.67%) | 12 (11.22%) | 16 (15.09%) |
| Discontinuations for TEAEs of low lymphocyte count, number of patients (%) | ||||
| Lymphopenia | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) |
| Lymphocyte Count Decreased | 0 (0%) | 0 (0%) | 1 (0.94%) | 1 (0.94%) |
| Total | 0 (0%) | 0 (0%) | 1 (0.94%) | 4 (3.77%) |
| TEAE=treatment-emergent adverse event. | ||||
* Includes patients in whom ‘lymphopenia’ or ‘lymphocyte count decreased’ were reported ≥1 time, or ‘lymphopenia’ and ‘lymphocyte count decreased’ were both reported.
BID=twice daily; QD=once daily; TEAE=treatment-emergent adverse event.
DISCUSSION
Over 24 weeks of treatment, tepilamide fumarate showed positive effect in patients with moderate-to-severe plaque psoriasis. Treatment typically took effect within 8-12 weeks and showed progressive benefit. PASI-75 results for the active tepilamide fumarate doses (400 mg QD/400 mg BID/600 mg BID) were 39.7%/47.2%/44.3%, versus 20.0% with placebo, while IGA success rates were 35.7%/41.4%/44.4%, versus 22.0% with placebo. These results are similar to a 2017 randomized, 16-week, double-blind evaluation of two FAEs (DMF and Skilarence® [DMF]), in which 37.5% and 40.3% of patients achieved PASI-75, and 33% were “clear” or “almost clear” using the Physician’s Global Assessment (a precursor to the IGA).12,21
In the current study, 50%/52%/62% of patients treated with tepilamide fumarate and 48% of placebo patients experienced TEAEs. A similarly high placebo TEAE rate has been observed in randomized trials of other DMF formulations. Patients treated with tepilamide fumarate (in particular 400 mg QD and BID) had lower TEAE incidence than clinical trials of other DMF formulations (range: 69%-84%). The most common events observed with other DMF formulations were GI intolerance (range, 56%-63%) and abnormal flushing (16%-31%).9,12,15 In this study, 20%/32%/42% of patients treated with tepilamide fumarate had TEAEs related to GI intolerance and ≤2% experienced flushing. The proportion of tepilamide fumarate patients who discontinued due to AEs was 10%/11%/26%, again lower for 400 mg QD and BID than rates reported in prior DMF trials (24%-31%).9,12 No events of PML or deaths occurred. Last, compared to prior DMF research,12 this study showed similar rates of low lymphocyte (7%/11%/15%) and increased eosinophil count (5%/5%/6%).
In general, FAEs offer some safety advantages over other psoriasis therapies. Treatment with certain biologic drugs increases risk for serious infection (such as tuberculosis reactivation). In a 3-year French registry analysis, patients receiving infliximab or adalimumab had an elevated opportunistic infection risk (OR, 17.6 [95% CI, 4.3-72.9] and 10.0 [2.3-44.4], respectively).22,23 Additionally, antibodies develop in patients receiving biologic therapies, which may reduce treatment efficacy over time.1,3 Patients treated with tyrosine kinase 2 inhibitors and the PDE4 inhibitor apremilast are at elevated risk for URTI.4,24,25 Last, methotrexate and ciclosporin are associated with cumulative liver and kidney toxicity, respectively.3
No evidence suggests increased risk for systemic infection, tuberculosis reactivation, or organ toxicity with FAEs.7,17 An evaluation of German registry data (2008-2012) showed that FAEs had the lowest infection rate among all drugs licensed for systemic psoriasis.26 A recent European consensus document recommends DMF for patients who are candidates for acitretin, ciclosporin, and methotrexate, as well as those previously treated with other systemic agents. This consensus prioritizes DMF treatment before biologics, and in biologics non-responders.13
Oral tepilamide fumarate is likely to be better tolerated than available DMF formulations and may be a promising treatment addition for moderate-to-severe plaque psoriasis. Of the 3 doses evaluated, tepilamide fumarate 400 mg BID strikes a balance between efficacy and tolerability and has been selected for further clinical development.
Study limitations. Treatment efficacy was not confounded by the use of supplemental therapies; however, a high placebo response was seen after Week 16. There was also a relatively high dropout rate due to AEs (10%-26%), voluntary withdrawals (13%), and loss to follow-up (12%). A 35%-40% dropout rate is common in DMF clinical studies.9,12 Furthermore, dropouts in this study due to AEs in the 400 mg BID group were highest during the 35-day titration period (n=7) and decreased thereafter (n=5 over 133 days), suggesting tepilamide may be better tolerated after initial titration.
The long-term efficacy and safety of tepilamide fumarate requires confirmatory research. Since this study focused on patients with plaque psoriasis, results may not be generalizable to individuals with non-plaque psoriasis or excluded comorbidities.
CONCLUSION
Patients with moderate-to-severe plaque psoriasis treated with oral tepilamide fumarate demonstrated positive response and treatment was well-tolerated. The most common AEs were dose-dependent and associated with GI tolerability and decreased lymphocyte count. Oral tepilamide fumarate appears to have similar effectiveness to existing DMF formulations, with lower rates of TEAEs. The clinical development of tepilamide fumarate 400 mg BID may help to address unmet treatment needs in this patient population.
ACKNOWLEDGMENTS
The authors thank the study manager, Carmela Fritz at Dr Reddys for project oversight and and Preeti Singh at Dr Reddys for content review and; Caitlin Rothermel (HLG Health Communications) for editorial support; and Saurabh Srivastava and Promius Chemistry, Manufacturing and Controls.
REFERENCES
- Menter A, Strober BE, Kaplan DH et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58(6):649–658. doi: 10.1111/ijd.14246. [DOI] [PubMed] [Google Scholar]
- Crowley J, Thaci D, Joly P et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/=156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77(2):310–317 e311. doi: 10.1016/j.jaad.2017.01.052. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Morrison PJ, Suhrkamp I, Kumanova M, Clement B. The pharmacokinetics of fumaric acid esters reveal their in vivo effects. Trends Pharmacol Sci. 2018;39(1):1–12. doi: 10.1016/j.tips.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Cambridge, MA: Biogen, Inc; 2017. Tecfidera® (dimethyl fumarate) delayed-release capsules [prescribing information]. [Google Scholar]
- Balak DM. Fumaric acid esters in the management of psoriasis. Psoriasis (Auckl). 2015;5:9–23. doi: 10.2147/PTT.S51490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner A, Spellman MC. P2787 Results of a phase 2 dose-ranging and safety extension study of a novel oral fumarate, BG00012, in patients with severe psoriasis. J Am Acad Dermatol. 2005.
- Mrowietz U, Christophers E, Altmeyer P. Treatment of psoriasis with fumaric acid esters: results of a prospective multicentre study. German Multicentre Study. Br J Dermatol. 1998;138:456–460. doi: 10.1046/j.1365-2133.1998.02124.x. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol. 1999;141(3):424–429. doi: 10.1046/j.1365-2133.1999.03034.x. [DOI] [PubMed] [Google Scholar]
- Ormerod AD, Mrowietz U. Fumaric acid esters, their place in the treatment of psoriasis. Br J Dermatol. 2004;150(4):630–632. doi: 10.1111/j.0007-0963.2004.05903.x. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Szepietowski JC, Loewe R et al. Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque psoriasis: a randomized, double-blind, Fumaderm((R)) - and placebo-controlled trial (BRIDGE). Br J Dermatol. 2017;176(3):615–623. doi: 10.1111/bjd.14947. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Barker J, Boehncke WH et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(3):3–14. doi: 10.1111/jdv.15218. [DOI] [PubMed] [Google Scholar]
- Mrowietz U, Spellman MC. Efficacy and safety of a novel formulation of an oral fumarate, BG-12, in patients with moderate to severe plaque psoriasis: results of a phase III study. J Am Acad Dermatol. 2005. p. 182.
- Carboni I, De Felice C, De Simoni I, Soda R, Chimenti S. Fumaric acid esters in the treatment of psoriasis: an Italian experience. J Dermatolog Treat. 2004;15(1):23–26. doi: 10.1080/09546630310019346. [DOI] [PubMed] [Google Scholar]
- Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003;149(2):363–369. doi: 10.1046/j.1365-2133.2003.05433.x. [DOI] [PubMed] [Google Scholar]
- Smith D. Fumaric acid esters for psoriasis: a systematic review. Ir J Med Sci. 2017;186(1):161–177. doi: 10.1007/s11845-016-1470-2. [DOI] [PubMed] [Google Scholar]
- Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol. 2003;49(2):206–212. doi: 10.1067/s0190-9622(03)00910-1. [DOI] [PubMed] [Google Scholar]
- Bagel J, Lynde C, Tyring S, Kricorian G, Shi Y, Klekotka P. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67(1):86–92. doi: 10.1016/j.jaad.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5-point Investigator’s Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26(1):23–31. doi: 10.3109/09546634.2013.865009. [DOI] [PubMed] [Google Scholar]
- Rademaker M, Agnew K, Anagnostou N et al. Psoriasis and infection. A clinical practice narrative. Australas J Dermatol. 2019;60(2):91–98. doi: 10.1111/ajd.12895. [DOI] [PubMed] [Google Scholar]
- Salmon-Ceron D, Tubach F, Lortholary O et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70(4):616–623. doi: 10.1136/ard.2010.137422. [DOI] [PubMed] [Google Scholar]
- Summit, NJ: Celgene Corporation; 2015. Otezla ® (apremilast) tablets [prescribing information]. [Google Scholar]
- Papp K, Gordon K, Thaci D et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321. doi: 10.1056/NEJMoa1806382. [DOI] [PubMed] [Google Scholar]
- Reich K, Mrowietz U, Radtke MA et al. Drug safety of systemic treatments for psoriasis: results from The German Psoriasis Registry PsoBest. Arch Dermatol Res. 2015;307(10):875–883. doi: 10.1007/s00403-015-1593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]