Abstract
Background
While microcephaly is a significant adverse outcome of prenatal exposure to the Zika virus (ZIKV), subtle malformations of cortical development (MCD) have been observed in Zika-exposed children (ZEC), including delays in language, cognition, and motor domains, and visual acuity deficits. Interventions within the first 1,000 days of life can significantly improve developmental outcomes. This study examined a 12-week Responsive Caregiving Intervention on neurodevelopmental outcomes in 24-30-month-old ZEC.
Methodology/Principal findings
A randomized controlled trial was implemented in Grenada, West Indies using an existing ZIKV cohort surveillance study. When children in that study turned 24 months, baseline child neurodevelopmental measures and caregiver interviews were administered. Caregivers who agreed to participate in the 12-week Responsive Caregiving Intervention, implemented when children were 24–30 months of age, were randomly assigned to the Intervention or Waitlist Control group. Children in both groups were re-assessed on the neurodevelopmental measures post-intervention.
Conclusions/Significance
233 children from the ZIKV surveillance study met inclusion criteria, of which n = 80 declined participation, n = 42 did not complete the Intervention, and n = 72 missed follow-up assessments given strict timelines in the study design. The final sample for analysis was N = 13 children in the Intervention group and N = 26 children in the Control group. A GEE model analysis showed significantly higher language (p = 0.021) and positive behaviour (p = 0.005) scores for children in the Intervention group compared to the Control group. The Intervention had a medium effect on child language (d = 0.66) and a large effect on positive behaviour (d = 0.83). A 12-week Responsive Caregiving Intervention Programme significantly improves language and positive behaviour scores in 30-month-old normocephalic children who were exposed to ZIKV in utero. The programme provides an option for mothers of ZIKV-exposed children who are seeking an evidence-based neurodevelopmental intervention regardless of known impact of the virus on cortical formation.
Trial registration
The study was registered with clinicaltrials.gov (NCT04697147).
Author summary
The relationship between Zika virus exposure during pregnancy and microcephaly (abnormally small head size) in infants is well known. Prenatal Zika virus exposure can also cause more subtle impacts in infant language, cognition, and motor function, and visual acuity, even in the absence of microcephaly. Interventions in the first 1,000 days of life can significantly improve these outcomes in at-risk children. Few studies have been carried out with the aim of improving neurodevelopment in Zika-exposed children. The present study utilized a randomized controlled trial to examine the impact of a 12-week Intervention on neurodevelopmental outcomes in 24-30-month-old Zika-exposed children. Analysis showed significantly higher language and positive behaviour scores among children in the Intervention group compared to children in the Control group. The Intervention used in this study is relatively low-cost and easy to implement. The application of interventions from the global early child development field has implications for governments, policy makers, public health officials, and parents of Zika-exposed children who are seeking effective interventions to mitigate the risk of neurodevelopment delays in those children. The fact that these much-needed interventions already exist and can be rolled out relatively quickly and effectively is encouraging.
Introduction
On 1 February 2016, the World Health Organization (WHO) declared a Public Health Emergency of International Concern (PHEIC) regarding microcephaly and other neurological disorders associated with the Zika virus (ZIKV). While the PHEIC was lifted on 18 November 2016, the WHO still considers the Zika epidemic and the associated complications a significant public health challenge requiring intense action, [1], and a global consortium has been established to examine pooled data from ZIKV infection [2].
Zika is a vector-borne virus spread via the bite of an infected Aedes spp. mosquito. It was first identified in Uganda in monkeys in 1947 and later in humans in 1952 [3]. The first recorded major ZIKV outbreak occurred in 2007 in the Federated States of Micronesia [3]. In 2015, the virus was identified in the Americas for the first time in Brazil and since then, it has spread to the rest of the region, including the Caribbean [4]. An outbreak of ZIKV was identified in the Caribbean Island of Grenada from April to November 2016 [5]. While existing data suggests that no cases have been reported in the Caribbean region since December 2016 [6], studies continue to examine the potential impact of infection on children born during the 2016 outbreak under the auspices of the original PHEIC.
The first evidence for a negative impact of prenatal ZIKV exposure on neurodevelopment came during the ZIKV outbreak in Brazil when an unusually high proportion of children were born with microcephaly. The presence of microcephaly in ZIKV-exposed children (ZEC) is a clear risk factor for adverse long-term outcomes such as developmental delays and seizures [7]. While microcephaly is a significant and obvious adverse outcome, more subtle malformations of cortical development (MCD) have also been observed in ZEC [8–10]. Even subtle MCDs, when strategically located, can contribute to cognitive deficits, intellectual disabilities, and learning disorders later in life [11,12]. Normocephalic ZEC can show delays in language, cognition, and motor domains [9,13–20]. In some normocephalic ZEC, visual acuity deficits are present, in the absence of any other cognitive, motor, language, or behavioural delays [21].
Interventions within the first 1,000 days of life can significantly improve developmental outcomes during early childhood with effects persisting across the life course (e.g., [22–25]). These interventions are the culmination of decades of global ECD work that initially focused on reducing child mortality and improving maternal health, as outlined by the United Nations (UN) Millennium Declaration [26], and shifted to improving child brain developmental outcomes via nurturing care [27] following a 53% decline in the under-5 mortality rate from 2000 to 2015 [28]. The nurturing care framework includes five components to maximize ECD outcomes: (1) good health; (2) adequate nutrition; (3) responsive caregiving; (4) safety and security; and (5) opportunities for early learning [29]. The nurturing care approach requires a multi-sectoral, collaborative effort that includes public policies, programmes, and services to support parents, caregivers, and communities in improving child development. A series on advancing ECD highlights the importance of interventions that support positive behaviour change among parents to achieve these nurturing care standards. These interventions, which are designed to augment the positive impact of basic health and nutrition, education, and protection interventions on ECD, reflect the Responsive Caregiving component, which is the parent or caregiver’s ability to notice, understand, and respond to the needs of his/her child appropriately [29].
While no studies with the specific aim of improving neurodevelopment among ZEC have been carried out in the Caribbean region, other interventions have shown improved outcomes in children who may be at risk for developmental delay in the absence of exposure to ZIKV. One such study is the Jamaican-developed Reach Up Intervention, a home visiting programme that supports mothers’ engagement with their children, thus promoting their children’s development [30]. The effectiveness of the Reach Up Programme has been measured in varying populations of at-risk children: low-socioeconomic status, severely malnourished, low birth weight, and stunted [30]. Studies have consistently demonstrated improvements in cognitive function and language or overall mental development among children who were exposed to the intervention [30]. In one study, Hamadani and colleagues [31] adapted the Reach Up Intervention for children at high risk of developmental delay in Bangladesh and found post-intervention improvements in children’s neurodevelopment [30,31].
The Reach Up Intervention involves parental education about early child brain development and techniques for child stimulation to improve developmental outcomes [30,31]. The Saving Brains Grenada Programme is another Responsive Caregiving Intervention that focuses on working with parents and caregivers to promote and teach self-regulation, social-emotional connection, and development. Waechter and colleagues [32] examined the impact of the intervention on 24-month-old children whose parents received the community-based training and noted that children whose caregivers were assigned to the Intervention group attained significantly higher post-intervention scores on measures of cognition, fine and gross motor skills, and language at 24 months of age compared to their peers who were assigned to the Waitlist Control group. Further, the intervention contributed the greatest level of variance among all measured ECD factors for fine motor, gross motor, and language development scores; and the second most variance for cognition scores [32].
Given the emerging evidence about the effectiveness of intervention programmes to improve ECD in low-and-middle-income countries and concerns about the potential subtle neurological impact of ZIKV on child development that can manifest as deficits in cognition, intellectual disabilities and learning disorders later in life, more evidence-based interventions are needed to determine whether neurodevelopment can be improved in ZEC. The present study aimed to examine the impact of a 12-week Responsive Caregiving Intervention on neurodevelopmental outcomes in 24-30-month-old ZEC. We hypothesized that ZEC whose parents received the Responsive Caregiving Intervention would show better neurodevelopmental outcomes at 2.5-year follow-up than those who were allocated to the Waitlist Control group.
Methods
Ethics statement
This study was approved by the St. George’s University Institutional Review Board (#16061). Written informed consent was obtained from all participants. The study was registered with clinicaltrials.gov (NCT04697147).
Study design
A randomized controlled trial design was implemented to evaluate the impact of a Responsive Caregiving Intervention on children between the ages of 24–30 months in Grenada, West Indies. The sample was derived from an existing ZIKV cohort study in Grenada (see Blackmon et al. [21]). When the children in this existing surveillance study turned 24 months, baseline neurodevelopmental measures and parent/caregiver interviews were administered at the community health clinic closest to the participant. Once this baseline data was collected, the caregiver was asked if he/she would like to participate in the 12-week Intervention. Those who agreed to participate in the study were randomly assigned to the Intervention, or the Waitlist Control group. The intervention was implemented when the children were between 24 and 30 months of age while the Control group received no intervention. Children were then re-assessed on the neurodevelopmental measures post-intervention.
Participants
All participants in the trial belonged to an ongoing ZIKV cohort study in Grenada, West Indies, with details regarding serum testing and ZIKV status for mothers and infants previously described (see Blackmon et al. [21]). For this trial, participants were recruited for the intervention when the child received his/her 24-month follow-up assessment for the ZIKV cohort study. Inclusion criteria consisted of: (i) active enrollment in the existing ZIKV cohort, (ii) child ≤ 24 months of age at the time of intervention enrollment, and (iii) child classified as ZIKV-exposed (see Blackmon et al. [21]).
Written consent and contact information were obtained from participants who wished to be involved and they were randomized to either Intervention or Control via random numbers table. Participants were contacted by a community health worker (Roving Caregiver) to schedule convenient days/times to complete their intervention sessions. A total of n = 233 parents/caregivers were contacted about participation in the study when their child turned 24-months old and n = 153 agreed and were randomized to either the Intervention or Control group. The intervention was provided to families between 24–29 months of the child’s life.
Responsive caregiving intervention
The Responsive Caregiving Intervention used in the current study draws upon the US-based Conscious Discipline (CD) Programme, which has been adapted for and used in the study site (Grenada, West Indies) for more than 10 years. CD is a brain-based, trauma informed curriculum emphasizing the importance of social-emotional learning and self-regulation for fostering neurodevelopment in children through behavioural and perceptual change in adults. CD focuses on building a strong social-emotional connection between the child and his/her caregivers, both in home and in school environments. For this intervention, community health workers (called Roving Caregivers) were trained in the CD curriculum and visited the homes of the participants over the course of 12 weeks, for a 1-hour session each week, to work with caregiver-child dyads. Both caregiver and child were required to be present for each session. The Roving Caregiver Programme was established in 1992 to deliver stimulation through home visits to at-risk children [33]. In 2014 the Roving Caregivers adopted CD, which focuses on building caregiver-child social-emotional connection rather than stimulation for infants alone. The CD curriculum teaches seven skills associated with caregiver self-regulation and optimal responsive caregiving: Composure, Assertiveness, Encouragement, Choices, Empathy, Positive Intent, and Consequences [34]. In each week’s lesson, the Roving Caregivers demonstrated interactions with children and allowed parents/caregivers to practice these same activities—enjoyable songs and games—to build attunement through safe, predictable interactions using the variables required for connection: eye contact, touch, presence in the moment and sense of play. Caregivers were shown a simplified triune brain model that provides a rationale for safety and connection by describing brain states. More information on this CD-based Responsive Caregiving Intervention is available from the corresponding author who can provide a copy of the Manual for this 12-week Intervention.
The Control group was not exposed to the Responsive Caregiving Intervention. All ZEC in the Control group were scheduled for neurodevelopmental assessments at 24, 27, and 30 months of age to compare their outcomes to the Responsive Caregiving Intervention group.
Outcome measures
Neurodevelopmental outcomes at 2 years were measured via the INTERGROWTH-21st Neurodevelopment Assessment (INTER-NDA) [35,36]. The INTER-NDA is a multi-dimensional, standardized assessment measuring cognition, fine motor, gross motor, language, and positive and negative behaviour outcomes in children aged 22 to 30 months [35]. It was developed for and has been implemented in low-, middle-, and high-income populations [35]. Its 37 items are scored on a 5-point scale, characterizing the child’s performance across a spectrum rather than on the binary (pass/fail) scoring classification. The assessment combines the psychometric methods of direct assessments, reporters’ observation, and caregiver reports. The INTER-NDA takes approximately 20 minutes to complete and can be administered in the field by non-specialists. The INTER-NDA has previously been shown to have good agreement with the BSID III edition (intraclass correlation coefficients between 0.745 and 0.883 [p < 0.001] for all subscales) [36]; a test-retest reliability of k = 50.79 (95% CI: 0.48–0.96) and an interrater reliability of k = 50.70 (95% CI: 0.47–0.88) [35]. The INTER-NDA’s normative ranges for the standardized scores for each of its six subscales are international standards (as opposed to references) of child development and were developed according to the WHO’s prescriptive approach for the construction of a biological standard as applied in the WHO Multicentre Growth Reference Study [37]. It has been piloted and culturally customized for the Grenadian population and has been used in previous studies assessing neurodevelopment in Grenadian children [38]. The INTER-NDA was administered by research assistants who were masked to the child’s ZIKV status and group (i.e., Intervention versus Control).
Parent demographics and social-environmental assessment were measured via standardized surveys and questions that evaluated environmental conditions that could potentially affect the child’s development. These surveys included demographic and socioeconomic status (SES) questions such as primary caregiver, monthly income and number of persons living in the household, and pre- and post-natal behaviours of the mother. Standardized questionnaires included USDA Food Security Questionnaire [39,40], General Health Questionnaire (GHQ-12) [41], Social Support Questionnaire (adapted from Assessment of Parental Well-being and Behaviours) [42], Confusion, Hubbub and Order Scale (CHAOS) [43], and the Home Observation for Measurement of the Environment (HOME) [44]. The parent/caregiver interview was administered by a research assistant at the 24-month visit and took approximately 30 minutes to complete.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences (SPSS) v. 26 (IBM Corp). Mean scores on the outcome measure (INTER-NDA) were converted to standardized scores per the procedure outlined by Fernandes and colleagues [37] and analyzed in SPSS.
T-tests and chi-square analyses were used to compare baseline socio-demographics between Intervention and Control groups. These tests were also used to compare baseline data for those participants who remained in the study and those lost to follow-up. Generalized estimating equation (GEE) [45] analyses were used to examine differences in child neurodevelopment (INTER-NDA scores) between Intervention and Control groups. To investigate the impact of the intervention, endpoint estimated marginal means derived from GEE models were used to calculate standardized effect sizes (Cohen’s d). Cohen’s d values were interpreted using the following reference values: 0.2, 0.5, 0.8 for small, medium, and large, respectively [46].
Results
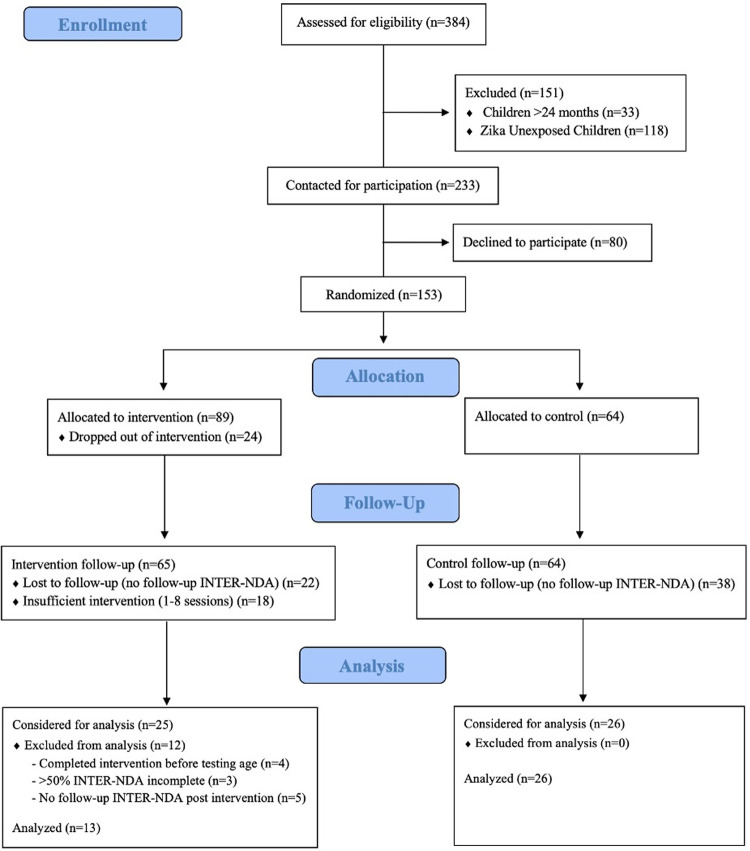
Caregivers of children enrolled in a pre-existing longitudinal ZIKV cohort study (N = 384) were eligible to participate in the CD-based Responsive Caregiving Intervention when their child turned 24 months of age (Fig 1). A total of n = 118 of those children were not exposed to ZIKV in utero and did not meet inclusion criteria. Additionally, n = 33 children were older than 24 months of age when the intervention study was initiated and were excluded. The remaining n = 233 children met inclusion criteria and their caregivers were approached about participating in the intervention when the child turned 24 months of age. A total of n = 80 (34%) declined participation, leaving n = 153 to be randomly assigned to the Intervention group (n = 89) or Control group (n = 64). Of the n = 89 participants assigned to the Intervention group, n = 24 dropped out due to scheduling conflicts or other reasons. A further n = 18 were removed from the Intervention group for completing fewer than 75% of the intervention classes (i.e., 9 of 12 sessions). Finally, n = 60 children across both groups (Intervention n = 22; Control n = 38) missed follow-up assessment sessions, resulting in a lack of neurodevelopmental scores. The remaining n = 51 participants (Intervention n = 25; Control n = 26) were considered for analysis. After data cleaning, n = 12 Intervention participants were excluded from the final analysis due to missing INTER-NDA scores (in these cases, item scores were not collected or were considered invalid due to distractions or child fatigue). No exclusions were made in the Control group. The final sample size for analysis included n = 13 children in the Intervention group and n = 26 children in the Control group (Fig 1).
Fig 1. CONSORT Flow Diagram.
Socio-demographic data collected at baseline for Intervention (n = 13) and Control (n = 26) groups were examined for any significant disparities between the groups. Results showed that the Intervention and Control groups were equivalent across all variables except parental education level (Table 1). A higher level of education was attained by parents in the Control group compared to the Intervention group (p < .001). Further analyses were run with and without parent education level as a covariate. Inclusion of this variable did not significantly change the results. Thus, the results reported below do not include parental education level as a covariate.
Table 1. Baseline Characteristics of the Sample.
| Sample Characteristics | Intervention (N = 13) M (SD) | Control (N = 26) M (SD) | Total (N = 39) M (SD) | p |
| Infant Age | 23.7 (0.59) | 23.8 (0.67) | 23.8 (0.64) | 0.730 |
| Maternal General Health a | 17.2 (3.56) | 19.1 (6.02) | 18.5 (5.36) | 0.211 |
| Social Support b | 39.7 (8.23) | 33.6 (10.56) | 35.6 (10.17) | 0.074 |
| CHAOS c | 26.7 (6.63) | 26.8 (9.71) | 26.8 (8.71) | 0.970 |
| HOME d | 35.6 (3.60) | 31.8 (8.24) | 33.1 (7.21) | 0.061 |
| Sample Characteristics | Intervention (N = 13) N (%) | Control (N = 26) N (%) | Total (N = 39) N (%) | p |
| Infant Gender | ||||
| Female | 7 (54%) | 15 (58%) | 22 (56%) | 0.819 |
| Male | 6 (46%) | 11 (42%) | 17 (44%) | |
| Parent Age | ||||
| 18–30 | 9 (69%) | 14 (54%) | 23 (59%) | 0.618 |
| 31–40 | 3 (23%) | 10 (38%) | 13 (33%) | |
| 41–50 | 1 (8%) | 2 (8%) | 3 (8%) | |
| Parent Education Level | ||||
| Primary | 0 (0%) | 9 (35%) | 9 (23%) | 0.001 |
| Secondary | 10 (77%) | 4 (15%) | 14 (36%) | |
| Tertiary | 3 (23%) | 13 (50%) | 16 (41%) | |
| Monthly Income | ||||
| < $500 XCD | 1 (8%) | 1 (4%) | 2 (5%) | 0.655 |
| $500–1000 XCD | 2 (15%) | 4 (15%) | 6 (15%) | |
| $1001–2000 XCD | 5 (38%) | 7 (27%) | 12 (31%) | |
| $2001–3000 XCD | 0 (0%) | 2 (8%) | 2 (5%) | |
| $3001+ XCD | 2 (15%) | 8 (30%) | 10 (26%) | |
| Marital Status | ||||
| Single | 6 (46%) | 8 (30%) | 14 (36%) | 0.611 |
| Domestic Partnership | 4 (31%) | 9 (35%) | 13 (33%) | |
| Married | 3 (23%) | 9 (35%) | 12 (31%) | |
| Birth Complications | ||||
| Yes | 3 (23%) | 5 (19%) | 8 (21%) | 0.729 |
| No | 9 (69%) | 20 (77%) | 29 (74%) | |
| Complications Post Birth | ||||
| Yes | 5 (38%) | 6 (23%) | 11 (28%) | 0.351 |
| No | 8 (62%) | 19 (70%) | 27 (69%) | |
| Feeding | ||||
| Breastfed | 6 (46%) | 8 (30%) | 14 (37%) | 0.436 |
| Bottle-fed | 2 (15%) | 2 (8%) | 4 (10%) | |
| Both | 5 (38%) | 15 (58%) | 20 (51%) | |
| Food Security | ||||
| Food Secure | 8 (62%) | 14 (54%) | 22 (56%) | 0.843 |
| Food Insecure (Moderate) | 2 (15%) | 6 (23%) | 8 (21%) | |
| Food Insecure (Severe) | 3 (23%) | 6 (23%) | 9 (23%) |
a Higher scores on the GHQ-12 indicate worse mental health
b Higher scores on the SSQ indicate more social support
c Higher scores on the CHAOS indicate a more chaotic home environment
d Higher scores on the HOME indicate a better home environment
An analysis to determine the equivalence of the participants who remained in the study to those who were lost to follow-up in both the Intervention and Control groups was run on baseline data. The results indicated that those lost to follow-up were equivalent to those who remained in the Control group of the study, whereas the Intervention group showed some disparity on child age and monthly income. Those lost to follow-up (M = 24.39) in the Intervention group showed a higher child age than those who remained (M = 24.00, p = 0.027); and those lost to follow-up in the Intervention group showed higher monthly incomes than those who remained (p = 0.041) (S1 Table). Child age and monthly income were included as covariates in the main analysis comparing child neurodevelopment scores between the Control and Intervention groups to ensure that these variables were not driving the outcomes. Neither variable significantly contributed to neurodevelopmental outcomes when comparing the Intervention and Control groups.
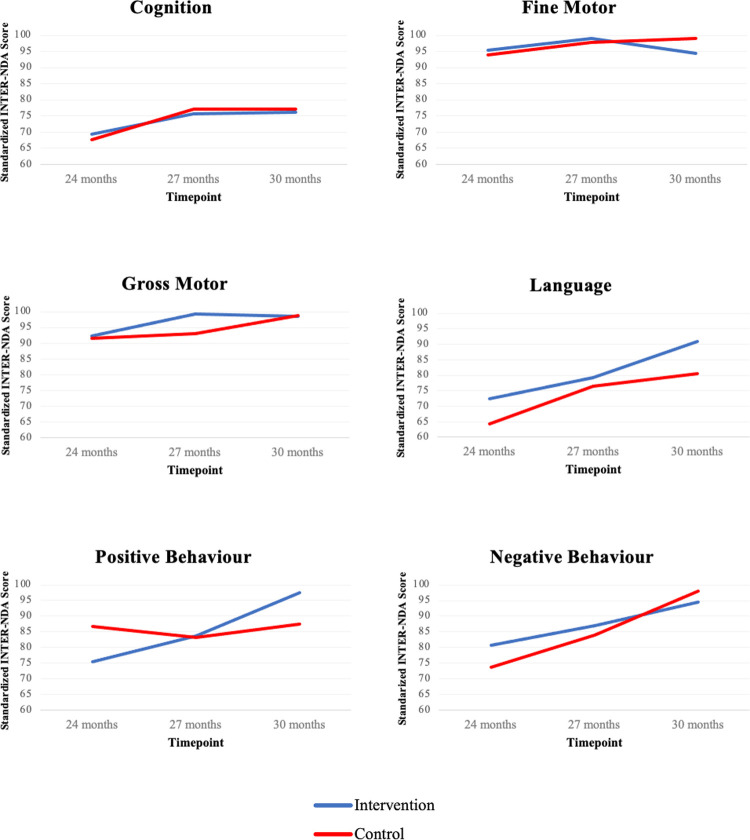
Results of a GEE model analysis showed significantly higher language (p = 0.021) and positive behaviour (p = 0.005) standardized scores on the INTER-NDA for the children in the CD-based Responsive Caregiving Intervention group compared to the children in the Control group (Table 2). The intervention had a medium effect on child language (d = 0.66) and a large effect on positive behaviour (d = 0.83) (Table 3). Both groups scored similarly on the remaining four domains of the INTER-NDA (cognition, gross motor, fine motor, negative behaviour) (Table 3 and Fig 2).
Table 2. Intervention Effects on Child Development Domains Measured by the INTER-NDA.
| INTER-NDA Domain | B | CI Lower | CI Upper | p |
|---|---|---|---|---|
| Cognition | -1.0 | -8.7 | 6.7 | 0.799 |
| Fine Motor | -4.7 | -11.2 | 1.8 | 0.153 |
| Gross Motor | -0.3 | 2.5 | -5.3 | 0.891 |
| Language | 10.5 | 1.6 | 19.3 | 0.021 |
| Positive Behaviour | 9.9 | 3.0 | 16.8 | 0.005 |
| Negative Behaviour | -3.4 | -12.2 | 5.5 | 0.453 |
Table 3. Estimated Marginal Means of Child Development Outcomes Derived from the GEE Models by Group and Effect Sizes.
| INTER-NDA Subscale | Time | Group | M | CI Lower | CI Upper | d |
|---|---|---|---|---|---|---|
| Cognition | 1 (24 months) | Intervention (N = 13) | 69.3 | 61.4 | 77.3 | |
| Control (N = 26) | 67.7 | 63.4 | 71.9 | |||
| 2 (27 months) | Intervention (N = 13) | 75.8 | 69.5 | 82.0 | ||
| Control (N = 26) | 77.1 | 72.5 | 81.7 | |||
| 3 (30 months) | Intervention (N = 13) | 76.2 | 70.0 | 82.3 | 0.085 | |
| Control (N = 26) | 77.2 | 72.6 | 81.8 | |||
| Fine Motor | 1 (24 months) | Intervention (N = 13) | 95.5 | 90.9 | 100.1 | |
| Control (N = 26) | 93.9 | 90.0 | 97.8 | |||
| 2 (27 months) | Intervention (N = 13) | 99.2 | 97.6 | 100.7 | ||
| Control (N = 26) | 97.7 | 95.4 | 100.1 | |||
| 3 (30 months) | Intervention (N = 13) | 94.4 | 88.1 | 100.7 | 0.544 | |
| Control (N = 26) | 99.1 | 97.6 | 100.7 | |||
| Gross Motor | 1 (24 months) | Intervention (N = 13) | 92.3 | 87.3 | 97.3 | |
| Control (N = 26) | 91.5 | 86.8 | 96.1 | |||
| 2 (27 months) | Intervention (N = 13) | 99.3 | 97.9 | 100.7 | ||
| Control (N = 26) | 93.1 | 89.2 | 96.9 | |||
| 3 (30 months) | Intervention (N = 13) | 98.6 | 95.5 | 101.6 | 0.042 | |
| Control (N = 26) | 98.9 | 95.0 | 102.8 | |||
| Language | 1 (24 months) | Intervention (N = 13) | 72.3 | 63.7 | 80.8 | |
| Control (N = 26) | 64.2 | 55.4 | 73.0 | |||
| 2 (27 months) | Intervention (N = 13) | 79.2 | 71.4 | 86.9 | ||
| Control (N = 26) | 76.5 | 69.1 | 83.9 | |||
| 3 (30 months) | Intervention (N = 13) | 90.9 | 88.0 | 93.9 | 0.659 | |
| Control (N = 26) | 80.5 | 72.1 | 88.8 | |||
| Positive Behaviour | 1 (24 months) | Intervention (N = 13) | 75.4 | 68.8 | 82.0 | |
| Control (N = 26) | 86.8 | 79.8 | 93.7 | |||
| 2 (27 months) | Intervention (N = 13) | 83.7 | 76.6 | 90.7 | ||
| Control (N = 26) | 83.1 | 76.6 | 89.6 | |||
| 3 (30 months) | Intervention (N = 13) | 97.5 | 94.2 | 100.8 | 0.833 | |
| Control (N = 26) | 87.6 | 81.5 | 93.6 | |||
| Negative Behaviour | 1 (24 months) | Intervention (N = 13) | 80.8 | 67.5 | 94.0 | |
| Control (N = 26) | 73.7 | 62.7 | 84.7 | |||
| 2 (27 months) | Intervention (N = 13) | 87.1 | 73.6 | 100.6 | ||
| Control (N = 26) | 83.9 | 75.4 | 92.4 | |||
| 3 (30 months) | Intervention (N = 13) | 94.5 | 88.8 | 100.3 | 0.234 | |
| Control (N = 26) | 97.9 | 91.2 | 104.6 |
Fig 2. Fitted GEE Model Plots of Child Development Domains Over Time by Group.
Discussion
We have shown that a 12-week Responsive Caregiving Intervention programme significantly improves language and positive behaviour scores in 30-month-old normocephalic children who were exposed to ZIKV in utero. The findings of our study are consistent with previous reports investigating the impact of home-visiting early childhood interventions including those involving children born to low-income families [47–54], children with or at risk of developmental delays [30,31,48,50,52,53,55–58], and children with other health conditions [50,55]. Our findings are also consistent with a Brazilian study designed to improve developmental outcomes in children with congenital Zika syndrome (CZS) by using the principles of the Goals-Activity-Motor Enrichment (GAME) based Programme. GAME is an intervention that involves: (1) goal-oriented motor training; (2) parental education; and (3) enrichment of the child’s motor learning environment [59]. The 16-week pilot study, which involved infants with CZS between the ages of 3 and 9 months, examined the effect of the GAME Programme on how mothers perceived their children’s achievement of functional goals, (e.g., rolling during play, holding toys, sitting to play, or be fed, and maintaining head/trunk control while being carried) and whether the Programme improved infants’ cognitive and motor abilities [59]. The study further examined whether the GAME protocol would result in enrichment in the child’s home environment and how the mothers perceived the service they were given [59]. A total of 22 infants in the study received the GAME Programme while 10 were in the control group and received traditional care that did not involve active participation by the mother [59]. The results of the study showed an improvement in mothers’ rating of infants’ performance in functional goals and enrichment of the home environment for those that received the GAME Intervention [59]. Neither the GAME group nor the Control group showed any significant improvements in cognitive and motor function [59]. No other known studies have examined the impact of an intervention on neurodevelopmental outcomes specifically in ZEC with and without microcephaly. Our study is unique in that it is the first time an ECD-based Responsive Caregiving Intervention has been shown to improve neurodevelopmental outcomes in ZEC.
The results of this study are consistent with a recent CD-based Responsive Caregiving Intervention study carried out in Grenada, West Indies [32]. Waechter et al. [32] found significantly higher scores across the neurodevelopmental domains of cognition, fine motor, gross motor, and language in 24-month-old children exposed to the Responsive Caregiving Intervention versus children allocated to a Waitlist Control group. The intervention contributed the greatest level of variance among all measured ECD factors for fine motor scores (d = 0.524), gross motor scores (d = 0.238), and language development scores (d = 0.259); and the second most variance for cognition scores (d = 0.216). This previous study differed from the neurodevelopmental outcomes seen in ZEC randomly assigned to the Intervention group in the present study, who did not show improvement in cognition and fine or gross motor neurodevelopment scores. A potential explanation for this difference is that Waechter and colleagues [32] intervened with children prior to 24 months of age and assessed these children at the 24-month timepoint, whereas in the present study, children received the intervention between the ages of 24 and 30 months and were assessed at the 24-, 27-, and 30-month timepoints. This difference in age at the time of the intervention and assessment could account for the differences in the overall effect of the intervention as an earlier start age for intervention could predict a bigger overall effect on neurodevelopment across multiple domains [60].
The main limitations of the current study are its small final sample size and high levels of attrition between initial contact for inclusion in the study and drop out from the intervention. Moreover, some participants were excluded from analysis due to missing neurodevelopment data post-intervention. Study protocol adherence and lost follow-up are significant limitations that are challenging to overcome and are not uncommon in community-based intervention studies with longitudinal follow-ups [50]. Another significant limitation is the lack of blinding to group allocation among both the participants themselves, as well as the Roving Caregivers who provided the intervention. Just by knowing they were receiving an intervention, the parents may have interacted differently with their children, and that may have impacted neurodevelopmental outcomes in the children. There was also a chance of contamination between the Intervention and Control groups given the relatively small size of Grenada, though we believe this was unlikely given the small final sample size of the Intervention group, wide distribution of families across different villages, and the fact that most families do not travel far from their village. In an effort to counteract these weaknesses, we ensured that all personnel who assessed neurodevelopmental outcomes in the children were blinded to the group assignment of the children (i.e., Control vs. Intervention).
All the children in the present study were exposed to ZIKV in utero. While this allowed for a direct comparison of neurodevelopmental outcomes between those who received the Intervention and those in the Control group, it was not possible to determine whether any or all the ZEC in the study had experienced subtle MCDs and/or neurocognitive impairment and/or neurodevelopmental delay because of their exposure to ZIKV. Future studies would need to include ZEC with documented MCDs and/or neurocognitive impairment and/or neurodevelopmental delay to determine whether the intervention can improve neurodevelopmental outcomes in children with ZIKV-related impairment short of microcephaly.
The CD-based Responsive Caregiving Intervention used in the present study prioritizes the building of social-emotional connections between caregiver and child to improve child neurodevelopment. We hypothesize that the nature of this intervention lends itself to the increase in positive behaviour and language abilities seen in the children randomly assigned to the Intervention group. The intervention used in the present study includes several activities that help forge a relationship between caregiver and child. These activities include singing songs and rhymes, reading stories, allowing, and encouraging the child to express themselves verbally, and equipping children with tools for expressing certain needs and wishes. These activities likely explain the improved language scores amongst our Intervention group.
The CD-based Responsive Caregiving Intervention used in the present study and similar interventions can be low-cost and relatively easy to implement. This could lead to more children having access to interventions that can mitigate neurodevelopmental delays in cases of arboviral diseases or otherwise. The cross-translation of evidence-based interventions from the global ECD field has implications for governments, policy makers, public health officials, ECD experts and parents of ZEC who are seeking effective interventions to mitigate the risk of neurodevelopment delays in those children. The fact that these much-needed interventions already exist and can be rolled out relatively quickly and effectively is encouraging. Moreover, the diversity of ECD interventions allows for a selection of programmes that apply best to regional, cultural, and economic variance.
Supporting information
(DOCX)
Acknowledgments
We thank all the Grenadian parents and their children who agreed to participate in the study. We thank the Roving Caregivers and Supervisors for implementing this intervention. We thank our research team Rashida Isaac, Bianca Punch, Kemi Burgen, Toni Murray, Lauren Mohammed, Dane Reid, Lauren Orlando, and intervention project manager Stephanie Holmes. We thank the Grenada Ministry of Health and Chief Medical Officer; without whose support this study would not have been possible.
Data Availability
Data is not publicly available due to patient/participant confidentiality (Grenada is a very small place where people can be identified even with de-identified data) and that the data can be made available by contacting the IRB administrator at SGU - Kareem Coomansingh: kcoomans@sgu.edu.
Funding Statement
This study was supported by funding from the National Institutes of Health; National Institute of Child Health and Human Development grant # 1R21HD093551 (PIs: RW, MF and BL). https://www.nichd.nih.gov The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Control ECoD. WHO ends Zika as a Public Health Emergency of International Concern 2016. [updated 2016-11-23]. Available from: https://www.ecdc.europa.eu/en/news-events/who-ends-zika-public-health-emergency-international-concern. [Google Scholar]
- 2.Consortium ZVIPD. The Zika Virus Individual Participant Data Consortium: A Global Initiative to Estimate the Effects of Exposure to Zika Virus during Pregnancy on Adverse Fetal, Infant, and Child Health Outcomes. Tropical Medicine and Infectious Disease. 2020;5(4):152. doi: 10.3390/tropicalmed5040152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Zika virus 2018 [cited 2020]. Available from: https://www.who.int/news-room/fact-sheets/detail/zika-virus.
- 4.Journel I, Andrécy LL, Metellus D, Pierre JS, Faublas RM, Juin S, et al. Transmission of Zika Virus—Haiti, October 12, 2015-September 10, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(6):172–6. Epub 2017/02/17. doi: 10.15585/mmwr.mm6606a4 ; PubMed Central PMCID: PMC5657860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenciaglia M, Noël TP, Fields PJ, Bidaisee S, Myers TE, Nelson WM, et al. Clinical, Serological, and Molecular Observations from a Case Series Study during the Asian Lineage Zika Virus Outbreak in Grenada during 2016. Canadian Journal of Infectious Diseases and Medical Microbiology. 2018;2018. 10.1155/2018/4635647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization PH. Countries and territories of the Americas with confirmed autochthonous cases of Zika virus (vector-borne transmission), 2015–2017 2017 [cited 2021]. Available from: https://ais.paho.org/phip/viz/ed_zika_countrymap.asp. [Google Scholar]
- 7.Pessoa A, van der Linden V, Yeargin-Allsopp M, Carvalho MDCG, Ribeiro EM, Van Naarden Braun K, et al. Motor Abnormalities and Epilepsy in Infants and Children With Evidence of Congenital Zika Virus Infection. Pediatrics. 2018;141(Supplement 2):S167–S79. doi: 10.1542/peds.2017-2038F [DOI] [PubMed] [Google Scholar]
- 8.Alves LVA, Camila EP, Germanna CS, Júlia GM, João G. Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: a case series study. 2018. doi: 10.1136/bmjopen-2017-021304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulkey SB, Arroyave-Wessel M, Peyton C, Bulas DI, Fourzali Y, Jiang J, et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr. 2020;174(3):269–76. Epub 2020/01/07. doi: 10.1001/jamapediatrics.2019.5204 ; PubMed Central PMCID: PMC6990858 Thrasher Research Fund during the conduct of the study. Drs Mulkey and DeBiasi reported providing technical expertise to the Zika studies by the Centers for Disease Control and Prevention outside the submitted work. Dr Fourzali reported receiving other compensation from the Children’s National Hospital outside the submitted work. No other disclosures were reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, et al. Congenital Zika Virus Infection: Beyond Neonatal Microcephaly. JAMA Neurol. 2016;73(12):1407–16. Epub 2016/10/04. doi: 10.1001/jamaneurol.2016.3720 . [DOI] [PubMed] [Google Scholar]
- 11.Lee J. Malformations of cortical development: genetic mechanisms and diagnostic approach. Korean journal of pediatrics. 2017;60(1):1–9. Epub 2017/02/17. doi: 10.3345/kjp.2017.60.1.1 ; PubMed Central PMCID: PMC5309318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vhp L, Aragão MM, Pinho RS, Hazin AN, Paciorkowski AR, Penalva de Oliveira AC, et al. Congenital Zika Virus Infection: a Review with Emphasis on the Spectrum of Brain Abnormalities. Current Neurology and Neuroscience Reports. 2020;20(11):1–11. doi: 10.1007/s11910-020-01072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Souza R, Anny Beatriz Costa Antony de A, Elijane de Fatima R, Salete Sara F, Marcia da Costa C, Silvana Gomes B, et al. Neurological Findings in Children without Congenital Microcephaly Exposed to Zika Virus in Utero: A Case Series Study. Viruses. 2020;12(11):1335. doi: 10.3390/v12111335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cranston JS, Tiene SF, Nielsen-Saines K, Vasconcelos Z, Pone MV, Pone S, et al. Association Between Antenatal Exposure to Zika Virus and Anatomical and Neurodevelopmental Abnormalities in Children. JAMA Network Open. 2020;3(7):e209303–e. doi: 10.1001/jamanetworkopen.2020.9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Einspieler C, Utsch F, Brasil P, Panvequio Aizawa CY, Peyton C, Hydee Hasue R, et al. Association of Infants Exposed to Prenatal Zika Virus Infection With Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw Open. 2019;2(1):e187235. Epub 2019/01/19. doi: 10.1001/jamanetworkopen.2018.7235 ; PubMed Central PMCID: PMC6431234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen-Saines K, Brasil P, Kerin T, Vasconcelos Z, Gabaglia CR, Damasceno L, et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nature Medicine. 2019;25(8):1213–7. doi: 10.1038/s41591-019-0496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peçanha PM, Gomes Junior SC, Pone SM, Pone MVdS, Vasconcelos Z, Zin A, et al. Neurodevelopment of children exposed intra-uterus by Zika virus: A case series. PLOS ONE. 2020;15(2):e0229434. doi: 10.1371/journal.pone.0229434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdes V, Zorrilla CD, Gabard-Durnam L, Muler-Mendez N, Rahman ZI, Rivera D, et al. Cognitive Development of Infants Exposed to the Zika Virus in Puerto Rico. JAMA Network Open. 2019;2(10):e1914061–e. doi: 10.1001/jamanetworkopen.2019.14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faiçal AV, de Oliveira JC, Oliveira JVV, de Almeida BL, Agra IA, Alcantara LCJ, et al. Neurodevelopmental delay in normocephalic children with in utero exposure to Zika virus. BMJ paediatrics open. 2019;3(1):e000486. Epub 2019/07/25. doi: 10.1136/bmjpo-2019-000486 ; PubMed Central PMCID: PMC6613842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Familiar I, Boivin M, Magen J, Azcorra JA, Phippen C, Barrett EA, et al. Neurodevelopment outcomes in infants born to women with Zika virus infection during pregnancy in Mexico. Child: Care, Health and Development. 2021;47(3):311–8. doi: 10.1111/cch.12842 [DOI] [PubMed] [Google Scholar]
- 21.Blackmon K, Evans R., Fernandes M, Landon B, Noël T, Macpherson C, Cudjoe N, et al. Neurodevelopment in normocephalic children with and without prenatal Zika virus. Archives of Diseases in Childhood. 2021;107(3):244–250. doi: 10.1136/archdischild-2020-321031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eickmann SH, Lima AC, Guerra MQ, Lima MC, Lira PI, Huttly SR, et al. Improved cognitive and motor development in a community-based intervention of psychosocial stimulation in northeast Brazil. Developmental medicine and child neurology. 2003;45(8):536–41. Epub 2003/07/29. doi: 10.1017/s0012162203000987 . [DOI] [PubMed] [Google Scholar]
- 23.Hartinger SM, Lanata CF, Hattendorf J, Wolf J, Gil AI, Obando MO, et al. Impact of a child stimulation intervention on early child development in rural Peru: a cluster randomised trial using a reciprocal control design. Journal of Epidemiology and Community Health. 2017;71(3):217–24. doi: 10.1136/jech-2015-206536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black MM, Walker SP, Fernald LCH, Andersen CT, DiGirolamo AM, Lu C, et al. Early childhood development coming of age: science through the life course. Lancet (London, England). 2017;389(10064):77–90. Epub 2016/10/09. doi: 10.1016/S0140-6736(16)31389-7 ; PubMed Central PMCID: PMC5884058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker SP, Chang SM, Powell CA, Grantham-McGregor SM. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet (London, England). 2005;366(9499):1804–7. Epub 2005/11/22. doi: 10.1016/S0140-6736(05)67574-5 . [DOI] [PubMed] [Google Scholar]
- 26.Organization WH. Millennium Development Goals (MDGs) 2018 [cited 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/millennium-development-goals-(mdgs)#:~:text%20=%20The%20United%20Nations%20Millennium%20Declaration,are%20derived%20from%20this%20Declaration.
- 27.Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, et al. Nurturing care: promoting early childhood development. Lancet (London, England). 2017;389(10064):91–102. Epub 2016/10/09. doi: 10.1016/S0140-6736(16)31390-3 . [DOI] [PubMed] [Google Scholar]
- 28.UNICEF. For every child, a fair chance: the promise of equity 2015. Available from: https://www.unicef.org/media/50421/file/For_every_child_a_fair_chance-ENG.pdf.
- 29.Development NCFfEC. Nurturing care handbook—Nurturing Care Framework for Early Childhood Development 2021 [updated 2021-01-21]. Available from: https://nurturing-care.org/handbook/.
- 30.Grantham-McGregor S, Smith J. Extending the Jamaican early childhood development intervention. Journal of Applied Research on Children: Informing Policy for Children at Risk. 2016;7(2). [Google Scholar]
- 31.Hamadani JD, Mehrin SF, Tofail F, Hasan MI, Huda SN, Baker-Henningham H, et al. Integrating an early childhood development programme into Bangladeshi primary health-care services: an open-label, cluster-randomised controlled trial. The Lancet Global Health. 2019;7(3):e366–e75. doi: 10.1016/S2214-109X(18)30535-7 [DOI] [PubMed] [Google Scholar]
- 32.Waechter R, Evans R, Fernandes M, Bailey B, Holmes S, Murray T, et al. A community-based responsive caregiving program improves neurodevelopment in two-year old children in a middle-income country—Grenada, West Indies. Psychosocial Intervention. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roopnarine JLH, Ziarat. The Roving Caregiver Program. A family-based early intervention programme for enhancing early childhood development and parenting. Online Childhood Matters. 2007 [Google Scholar]
- 34.Bailey B. Conscious Discipline Building Resilient Classrooms Expanded & Updated Edition: Loving Guidance Inc.; 2015. [Google Scholar]
- 35.Fernandes M, Stein A, Newton CR, Cheikh-Ismail L, Kihara M, Wulff K, et al. The INTERGROWTH-21st Project Neurodevelopment Package: A Novel Method for the Multi-Dimensional Assessment of Neurodevelopment in Pre-School Age Children. PLOS ONE. 2014;9(11):e113360. doi: 10.1371/journal.pone.0113360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray E, Fernandes M, Newton CRJ, Abubakar A, Kennedy SH, Villar J, et al. Evaluation of the INTERGROWTH-21st Neurodevelopment Assessment (INTER-NDA) in 2 year-old children. PLOS ONE. 2018;13(2):e0193406. doi: 10.1371/journal.pone.0193406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes M, Villar J, Stein A, Staines Urias E, Garza C, Victora CG, et al. INTERGROWTH-21st Project international INTER-NDA standards for child development at 2 years of age: an international prospective population-based study. BMJ Open. 2020;10(6):e035258. doi: 10.1136/bmjopen-2019-035258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waechter R, Ingraham E, Evans R, Cudjoe N, Krystosik A, Isaac R, et al. Pre and postnatal exposure to Chikungunya virus does not affect child neurodevelopmental outcomes at two years of age. PLOS Neglected Tropical Diseases. 2020;14(10):e0008546. doi: 10.1371/journal.pntd.0008546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbasi N, M. Ghoochani O, Ghanian M, Kitterlin M. Assessment of Households’ Food Insecurity through use of a USDA Questionnaire. Advances in Plants & Agriculture Research. 2016;4. doi: 10.15406/apar.2016.04.00155 [DOI] [Google Scholar]
- 40.Coates J, Swindale A, Bilinsky P. HFIAS for Measurement of Food Access Indicator Guide. Journal of Chemical Information and Modeling. 2013;53(9):1689–99.23800267 [Google Scholar]
- 41.Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychological medicine. 1979;9(1):139–45. Epub 1979/02/01. doi: 10.1017/s0033291700021644 . [DOI] [PubMed] [Google Scholar]
- 42.Ramthal A, Thomas T, Lukose A, Shiny R, Bosch R, Kurpad A, et al. Risk factors for depression in pregnant urban South Indian women. The FASEB Journal. 2011;25(S1):780.14-.14. 10.1096/fasebj.25.1_supplement.780.14. [DOI] [Google Scholar]
- 43.Matheny AP, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the confusion, hubbub, and order scale. Journal of Applied Developmental Psychology. 1995;16(3):429–44. 10.1016/0193-3973(95)90028-4. [DOI] [Google Scholar]
- 44.Bradley RH, Caldwell BM. The HOME Inventory and family demographics. Developmental Psychology. 1984;20:315–20. doi: 10.1037/0012-1649.20.2.315 [DOI] [Google Scholar]
- 45.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 46.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd edition): Routledge; 1988. [Google Scholar]
- 47.Aboud FE, Singla DR, Nahil MI, Borisova I. Effectiveness of a parenting program in Bangladesh to address early childhood health, growth and development. Social Science & Medicine. 2013;97:250–8. doi: 10.1016/j.socscimed.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 48.Muhoozi GKM, Atukunda P, Diep LM, Mwadime R, Kaaya AN, Skaare AB, et al. Nutrition, hygiene, and stimulation education to improve growth, cognitive, language, and motor development among infants in Uganda: A cluster-randomized trial. Maternal & child nutrition. 2018;14(2):e12527. Epub 2017/09/20. doi: 10.1111/mcn.12527 ; PubMed Central PMCID: PMC6866193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olds DL, Holmberg JR, Donelan-McCall N, Luckey DW, Knudtson MD, Robinson J. Effects of Home Visits by Paraprofessionals and by Nurses on Children: Follow-up of a Randomized Trial at Ages 6 and 9 Years. JAMA Pediatrics. 2014;168(2):114–21. doi: 10.1001/jamapediatrics.2013.3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peacock S, Konrad S, Watson E, Nickel D, Muhajarine N. Effectiveness of home visiting programs on child outcomes: a systematic review. BMC Public Health. 2013;13(1):17. doi: 10.1186/1471-2458-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singla DR, Kumbakumba E, Aboud FE. Effects of a parenting intervention to address maternal psychological wellbeing and child development and growth in rural Uganda: a community-based, cluster-randomised trial. The Lancet Global Health. 2015;3(8):e458–e69. doi: 10.1016/S2214-109X(15)00099-6 [DOI] [PubMed] [Google Scholar]
- 52.Tofail F, Hamadani JD, Mehrin F, Ridout DA, Huda SN, Grantham-McGregor SM. Psychosocial Stimulation Benefits Development in Nonanemic Children but Not in Anemic, Iron-Deficient Children. The Journal of Nutrition. 2013;143(6):885–93. doi: 10.3945/jn.112.160473 [DOI] [PubMed] [Google Scholar]
- 53.Worku BN, Abessa TG, Wondafrash M, Lemmens J, Valy J, Bruckers L, et al. Effects of home-based play-assisted stimulation on developmental performances of children living in extreme poverty: a randomized single-blind controlled trial. BMC Pediatrics. 2018;18(1):29. doi: 10.1186/s12887-018-1023-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yousafzai AK, Obradović J, Rasheed MA, Rizvi A, Portilla XA, Tirado-Strayer N, et al. Effects of responsive stimulation and nutrition interventions on children’s development and growth at age 4 years in a disadvantaged population in Pakistan: a longitudinal follow-up of a cluster-randomised factorial effectiveness trial. The Lancet Global Health. 2016;4(8):e548–e58. doi: 10.1016/S2214-109X(16)30100-0 [DOI] [PubMed] [Google Scholar]
- 55.Black MM, Dubowitz H, Hutcheson J, Berenson-Howard J, Starr RH Jr. A randomized clinical trial of home intervention for children with failure to thrive. Pediatrics. 1995;95(6):807–14. Epub 1995/06/01. . [PubMed] [Google Scholar]
- 56.Lederer S. Efficacy of Parent-Child Language Group Intervention for Late-Talking Toddlers. Infant-Toddler Intervention: The Transdisciplinary Journal. 2001;11. [Google Scholar]
- 57.Rajesh V, Venkatesh L. Preliminary evaluation of a low-intensity parent training program on speech-language stimulation for children with language delay. International Journal of Pediatric Otorhinolaryngology. 2019;122:99–104. doi: 10.1016/j.ijporl.2019.03.034 [DOI] [PubMed] [Google Scholar]
- 58.Roberts Megan Y, Kaiser Ann P. The Effectiveness of Parent-Implemented Language Interventions: A Meta-Analysis. American Journal of Speech-Language Pathology. 2011;20(3):180–99. doi: 10.1044/1058-0360(2011/10-0055) [DOI] [PubMed] [Google Scholar]
- 59.Brandão MdB Frota LMdCP, Miranda JL Cavalcante Brasil RM, Mancini MC. Family-Centered Early Intervention Program for Brazilian Infants with Congenital Zika Virus Syndrome: A Pilot Study. Physical & Occupational Therapy In Pediatrics. 2019;39(6):642–54. doi: 10.1080/01942638.2019.1600100 [DOI] [PubMed] [Google Scholar]
- 60.Towle PO, Patrick PA, Ridgard T, Pham S, Marrus J. Is Earlier Better? The Relationship between Age When Starting Early Intervention and Outcomes for Children with Autism Spectrum Disorder: A Selective Review. Autism Research and Treatment. 2020;2020:7605876. doi: 10.1155/2020/7605876 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Data is not publicly available due to patient/participant confidentiality (Grenada is a very small place where people can be identified even with de-identified data) and that the data can be made available by contacting the IRB administrator at SGU - Kareem Coomansingh: kcoomans@sgu.edu.