Abstract
Background
The immunopathological pathways enabling post-coronavirus disease 2019 (COVID-19) syndrome (PCS) development are not entirely known. We underwent a longitudinal analysis of patients with COVID-19 who developed PCS aiming to evaluate the autoimmune and immunological status associated with this condition.
Methods
Thirty-three patients were included for longitudinal clinical and autoantibody analyses, 12 of whom were assessed for cytokines and lymphocyte populations. Patients were followed for 7–11 months after acute COVID-19. Autoimmune profile and immunological statuses were evaluated mainly by enzyme-linked-immunosorbent assays and flow cytometry.
Results
Latent autoimmunity and overt autoimmunity persisted over time. A proinflammatory state was observed in patients with PCS characterized by up-regulated interferon-α, tumor necrosis factor-α, granulocyte colony-stimulating factor (G-CSF), interleukin (IL)-17A, IL-6, IL-1β, and IL-13, whereas interferon-γ-induced protein 10 (IP-10) was decreased. In addition, PCS was characterized by increased levels of Th9, CD8+ effector T cells, naive B cells, and CD4+ effector memory T cells. Total levels of immunoglobulin G S1-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies remained elevated over time.
Conclusions
The clinical manifestations of PCS are associated with the persistence of a proinflammatory and effector phenotype induced by SARS-CoV-2 infection. This long-term persistent immune activation may contribute to the development of latent and overt autoimmunity. Results suggest the need to evaluate the role of immunomodulation in the treatment of PCS.
Keywords: autoimmunity, COVID-19, long COVID, naive B cells, post-COVID syndrome
Post-COVID syndrome is characterized by the persistence of latent autoimmunity up to 11 months after recovery. This phenomenon could be driven by a persistent proinflammatory state and an altered effector phenotype.
During acute infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the coronavirus disease 2019 (COVID-19), clinical manifestations vary from mild forms to critical and more severe cases [1]. Symptoms include dry cough, fatigue, anosmia, and fever. However, some patients may worsen and require intensive care unit admission, mechanical ventilation, and vasopressor support [1].
Although most of the COVID-19 patients recover entirely, without sequelae, more than 20% of patients may keep experiencing symptoms after 4 weeks of acute disease and others may even develop new symptoms [2]. This clinical spectrum is called post-COVID syndrome (PCS) [2]. One third of patients with PCS present with at least 1 musculoskeletal, respiratory, gastrointestinal, and neurological symptom [2].
The causes of PCS are under study. Viral persistence [3], endocrine dysregulation [4], endotheliopathy [5], autoimmune response [6], and a persistent inflammatory state [7] have been advocated. However, the precise mechanisms associated with its appearance and the influence of biological alterations on clinical phenotypes remain to be elucidated. In this study, we present a longitudinal analysis of a case series of patients with COVID-19 who developed PCS, and we aim to evaluate the autoimmune and immunological pathways associated with this condition and the likely targets for novel treatments.
METHODS
Study Design
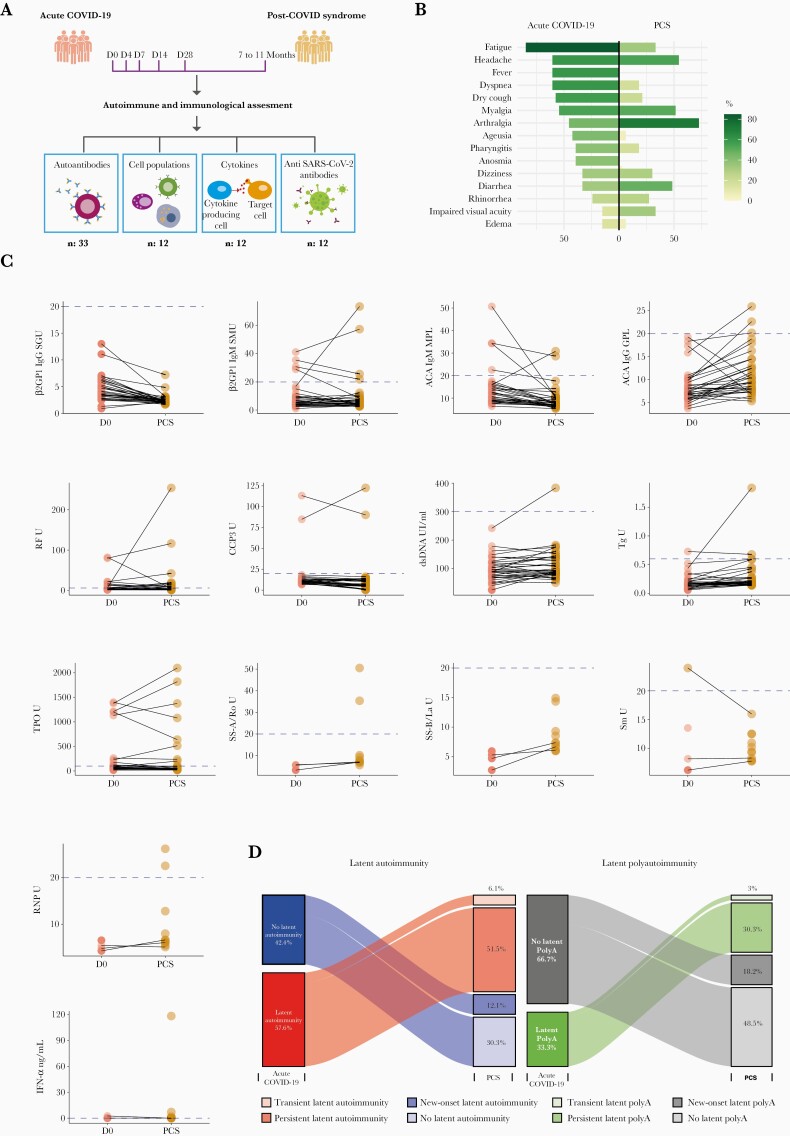
Coronavirus disease 2019 patients who participated in a previous study [8] were contacted by telephone and reassessed for possible post-COVID symptoms, as described in detail elsewhere [2]. Those who were suspected to present PCS were invited to attend the post-COVID unit at the Clínica del Occidente, in Bogota, Colombia, for clinical and immunological evaluation. A final sample size of 33 patients was included for longitudinal autoantibody analyses, and 12 were assessed for cytokines, lymphocyte populations, and anti-SARS-CoV-2 antibodies (Figure 1A). Serological data of 100 healthy controls, previously reported, served as historical control group for autoimmune comparisons [9], and a group of 8 prepandemic healthy individuals was the control group for the immunological assessment [8].
Figure 1.
Autoimmune assessment of post-coronavirus disease 2019 (COVID-19) syndrome (PCS). (A) Study design. Patients assessed for autoantibodies were followed 7–11 months postinfection (n: 33). Patients evaluated for cytokines, lymphocytes, immunoglobulin (Ig)G, and IgA S1-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were followed 7–9 months postinfection (n: 12). (B) Mirrored bar plot for symptoms on acute COVID-19 and PCS (n: 33). (C) Paired dot plot for concentration of autoantibodies. Dashed blue line represents cutoff values for positivity of each autoantibody (by their respective enzyme-linked-immunosorbent assay thresholds). (D) Alluvial diagrams for latent autoimmunity and overt autoimmunity. ACAs, Anti-cardiolipin antibodies; β2GPI, β2 glycoprotein-1; CCP3, Cyclic citrullinated peptide third-generation; dsDNA, Double-stranded DNA; IFN-α, Interferon-α; PolyA, Polyautoimmunity; RF, Rheumatoid factor; RNP, Ribonucleoprotein; Sm, Smith; Tg, Thyroglobulin.
Patient Monitoring and Clinical Evaluation
Patients were systematically evaluated for acute COVID-19 and post-COVID clinical manifestations, as previously described [2]. Of 33 patients with PCS evaluated for autoantibodies, 5 were vaccinated during the PCS. This study was done in compliance with Act 008430/1993 of the Ministry of Health of the Republic of Colombia, which classified it as minimal-risk research. All the patients were asked for their consent and were informed about the Colombian data protection law (1581 of 2012). The institutional review board of the CES University approved the study design.
Autoantibodies
Detection of immunoglobulin (Ig)M rheumatoid factor (RF), IgG anti-cyclic citrullinated peptide third generation (CCP3) antibodies, IgM and IgG anti-cardiolipin antibodies (ACAs), IgM and IgG anti-β2 glycoprotein-1 (β2GP1) antibodies, IgG anti-double-stranded deoxyribonucleic acid (dsDNA) antibodies, IgG anti-thyroglobulin (Tg) antibodies, and anti-thyroid peroxidase (TPO) antibodies were all quantified by enzyme-linked-immunosorbent assay (ELISA). In addition, antinuclear antibodies (ANAs) were evaluated by using an indirect immunofluorescence assay. Positive results were considered from dilution 1/80. In case of ANAs positivity, anti-SSA/Ro, anti-SSB/La, anti-ribonucleoprotein (RNP), and anti-smith (Sm) antibodies were further evaluated by a commercial ELISA. All the assay kits were from Inova Diagnostics, Inc. (San Diego, CA) as previously reported [9]. Assessment of anti-interferon (IFN)-α antibodies was done by ELISA (Thermo Fisher Scientific, Waltham, MA) following manufacturer’s specifications.
Cytokine Assay and Lymphocytes Immunophenotype
Serum concentration of 20 cytokines (interleukin [IL]-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-17A, tumor necrosis factor [TNF]-α, granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage CSF [GM-CSF], RANTES, monocyte chemoattractant protein [MCP]-1, interferon-γ-induced protein 10 [IP-10], IFN-γ, IFN-α) was assessed by Cytometric Bead Array ([CBA] Becton Dickinson Biosciences, San Diego, CA). The test was done according to the manufacturer’s protocols. Concentration of the cytokines was calculated using the FCAP Array Software (BD Bioscience) as reported elsewhere [10].
Thirty cell subsets (Supplementary Appendix) were also assessed. A minimum of 100 000 lymphocytes per sample were acquired on a FACSCanto II flow cytometer (BD Biosciences) and data were analyzed with FlowJo software version 9 (BD Biosciences) as reported elsewhere [8].
Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies
The Euroimmun anti-SARS-CoV-2 ELISA (Euroimmun, Luebeck, Germany) was used for serological detection of human IgG and IgA antibodies against the SARS-CoV-2 S1 structural protein, in accordance with the manufacturer’s instructions, as previously described [2]. The ratio interpretation was <0.8 = negative, ≥0.8 to <1.1 = borderline, and ≥1.1 = positive. Antibody positivity was performed using a 1:100 dilution.
Statistical Analysis
Univariate descriptive statistics were performed. Categorical variables were analyzed using frequencies, and quantitative continuous variables were expressed in the median and interquartile range (IQR). Fisher’s exact or Mann-Whitney U tests were used based on the results. Cytokine concentrations were analyzed after log transformation; all other parameters were analyzed without any additional data transformation.
Initially, generalized linear models were used to evaluate longitudinal changes in IgA and IgG SARS-CoV-2 antibodies ratios, cytokines, and lymphocyte populations, as previously described [8]. In addition, linear regression models were fitted to estimate the differences in cytokines and lymphocyte populations between PCS and prepandemic controls. For these models, post hoc comparison of means was based on both adjusted Bonferroni P values and Fisher’s protected least significant differences procedure using t statistics based on Satterthwhaite’s approximation.
Next, we evaluated whether levels and positivity of autoantibodies changed from acute COVID-19 to PCS. The McNemar test with continuity correction and paired t test were performed for positivity and autoantibodies levels, respectively. The significance level of the study was set to 0.05. Statistical analyses were done using R software version 4.0.2.
RESULTS
Autoimmune Assessment
The main clinical characteristics of the 33 patients are shown in Figure 1B. Most of them were male (19 of 33, 57.6%) with a median age of 55 years (IQR, 50 to 63). The median post-COVID time was 266 (IQR, 253 to 288) days. Despite the variation in concentration (Figure 1C), autoantibodies positivity did not change from acute disease (D0) to PCS (McNemar test, P > .0500), except for ANAs, which showed a slight increase in its frequency at 1/80 dilution (McNemar test, P = .0455).
Compared with prepandemic controls, frequency of β2GP1 IgM autoantibodies was higher in PCS (Fisher’s exact test, P = .0135). The remaining autoantibodies did not differ from healthy subjects at baseline (D0) or at the time of PCS (Fisher’s exact test, P > .0500). Despite differences in ages (55 years in PCS vs 35 years in controls, Mann-Whitney U test, P < .0001), there were no differences in sex frequencies (Fisher’s exact test, P = .1632).
During the acute phase of illness, there were 19 of 33 (57.6%) patients presenting with at least 1 autoantibody (ie, latent autoimmunity), and 11 of 33 (33.3%) patients presented with 2 or more autoantibodies (ie, latent polyautoimmunity), whereas during the PCS there were 21 of 33 (63.6%) patients presenting with at least 1 autoantibody (Fisher’s exact test, P = .0006; McNemar test, P = .6831), of whom 16 of 33 (48.5%) presented with 2 or more autoantibodies (Fisher’s exact test, P = .0007; McNemar test, P = .1306) (Figure 1D). These results indicate that there was not only a persistence of latent autoimmunity during the PCS but also an increased prevalence of both latent autoimmunity and latent polyautoimmunity (Figure 1D).
At the acute COVID-19, 5 of 33 (15.2%) patients disclosed non-autoimmune hypothyroidism, and 3 of 33 (9.1%) had autoimmune thyroid disease (AITD). During the PCS, there was a patient who developed AITD, and another in whom anti-TPO antibodies became negative.
Immunological Assessment
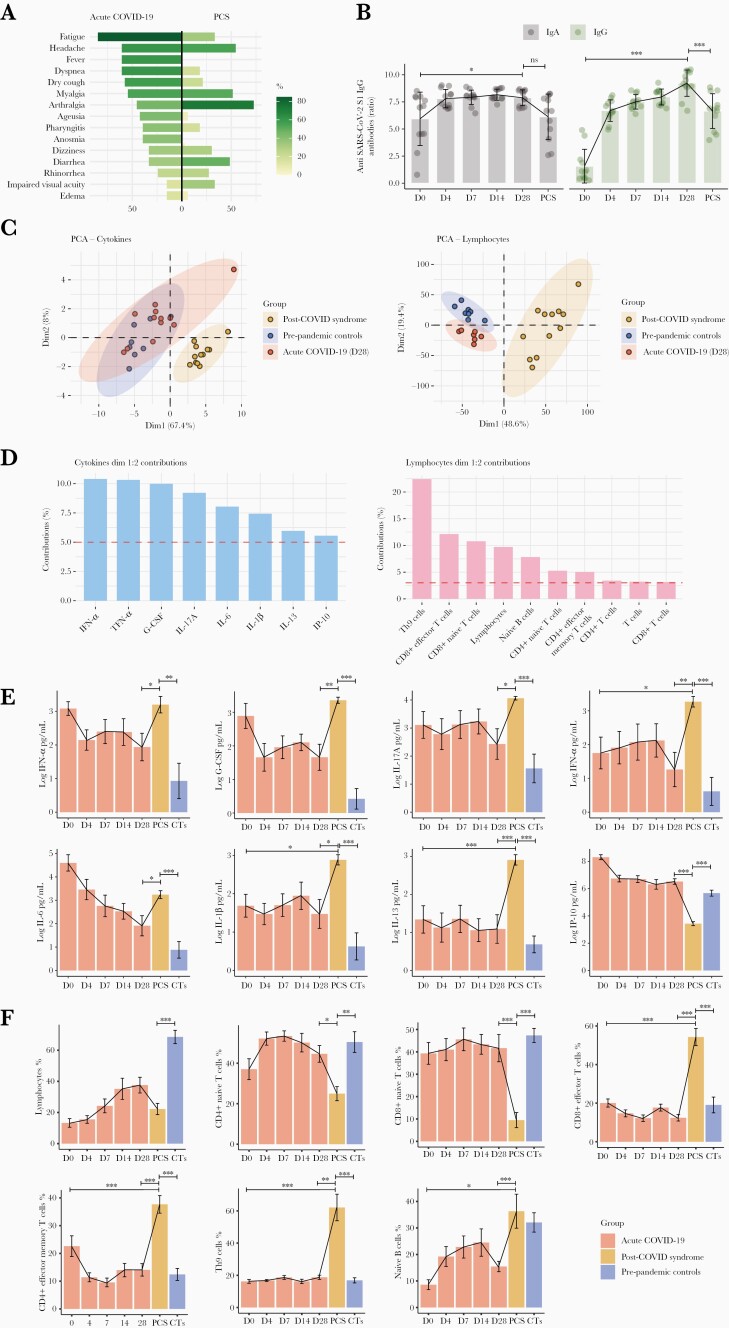
The main clinical characteristics of the 12 patients are shown in Figure 2A. Half were male (6 of 12, 50%) with a median age of 50.5 years (IQR, 49.75 to 55.5). The median post-COVID time was 259 (IQR, 235.5 to 271.5) days. During acute COVID-19, patients presented a progressive increase of IgA (from day 0 to day 28, P = .0257) and IgG (from day 0 to day 28, P < .0001) anti-SARS-CoV-2 S1 antibodies, and a reduction of 22.3% and 33.6% was observed at the PCS evaluation, respectively. However, IgA antibodies returned to similar levels from baseline (P = 1.0000), and IgG antibodies remained high (P < .0001) (Figure 2B). None of these patients were vaccinated during the follow-up.
Figure 2.
Immunological assessment of post-coronavirus disease 2019 (COVID-19) syndrome (PCS). (A) Mirrored bar plot for symptoms of acute COVID-19 and post-COVID syndrome (n: 12). (B) Dynamics of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody response and the change in anti-SARS-CoV-2 immunoglobulin (Ig)A and IgG ratios (OD sample/OD calibrator) were plotted against days of follow-up. None of these patients were vaccinated during the follow-up. (C) Principal component analysis (PCA) of 20 cytokines and 30 cell subsets that were analyzed in prepandemic controls (n: 8), acute COVID-19 (n: 12), and PCS (n: 12). (D) Contribution of cytokines and lymphocytes on dimensions 1 and 2 from principal component analyses. Thresholds for cytokines 5% (1/20 cytokines = 5%), and lymphocytes (1/30 populations = 3.3%). (E) Longitudinal bar plots for selected cytokines in prepandemic controls (n: 8), acute COVID-19 (n: 12), and PCS (n: 12). Longitudinal analyses were done by generalized linear models with post hoc comparisons adjusted by Bonferroni correction. Comparisons between prepandemic controls and PCS were analyzed by means of linear regression with post hoc comparison. (F) Longitudinal bar plots for selected lymphocyte populations in prepandemic controls (n: 8), acute COVID-19 (n: 12), and PCS (n: 12). Longitudinal analyses were done by generalized linear models with post hoc comparisons adjusted by Bonferroni correction. Comparisons between prepandemic controls and PCS were analyzed by means of linear regression with post hoc comparison. ∗∗∗, P < .0001; ∗∗, P < .0010; ∗P < .0500. CTs, Prepandemic controls; D, Days; G-CSF, Granulocyte colony-stimulating factor; IFN, Interferon; IL, Interleukin; IP-10, Interferon-γ-induced protein 10; ns, Not significant; TNF-α, Tumor necrosis factor-alpha.
Cytokine and Lymphocyte Profiles in Post-Coronavirus Disease 2019 Syndrome
Principal component analysis of a panel of cytokine and lymphocyte populations contributing to PCS pathophysiology showed that PCS patients were segregated from the other 2 groups of patients (ie, day 28 and prepandemic controls) (Figure 2C). Then, the most critical factors for such distinction were assessed. We found that IFN-α, TNF-α, G-CSF, IL-17A, IL-6, IL-1β, IL-13, and IP-10 were the most contributing cytokines (ie, average contribution cutoff >5%) (Figure 2D). The most contributing lymphocyte immunophenotypes were Th9, CD8+ effector T cells, CD8+ naive T cells, total lymphocytes, naive B cells, CD4+ naive T cells, CD4+ effector memory T cells, CD4+ and CD8+ T cells, and total T cells (average contribution cutoff >3.33%) (Figure 2D).
Longitudinal evaluation of selected biomarkers disclosed an increase of most of the cytokines during PCS compared with levels measured at day 28 and with prepandemic controls, whereas IP-10 decreased (Figure 2E). The TNF-α (P = .0118), IL-1β (P = .0166), and IL-13 (P < .0001) levels were higher during PCS than at day 0 (Figure 2E).
Regarding lymphocytes, Th9, CD8+ effector T cells, naive B cells, and CD4+ effector memory T cells were increased in PCS compared with levels measured at day 28 and with prepandemic controls (Figure 2F). On the other hand, lower levels of CD8+ and CD4+ naive T cells and total lymphocytes were observed (Figure 2F). There was no influence of age (50.5 years in PCS vs 46 years in controls, Mann-Whitney U test, P = .5614) or sex (Fisher's exact test, P = .3729) that could have biased the differences between PCS and prepandemic controls on cytokines and lymphocytes. Representative flow cytometry plots for each reported cellular population are provided in Supplementary Figure 1.
DISCUSSION
This study shows the development of autoimmunity during PCS (Figure 1D) together with a persistent proinflammatory state and a dysregulated cellular immune response. Levels of IgG anti-SARS-CoV-2 S1 antibodies remained high since the acute phase of illness to the PCS, whereas IgA antibodies tend to return to the basal state. Long-term antibody responses to SARS-CoV-2 and a high interindividual variability has been reported [2]. Other longitudinal studies have shown small or no change in neutralizing antibody titers at 5 months postinfection [11, 12]. Chen et al [13] showed that 92.5% of patients had detectable neutralizing antibodies approximately 7 months after infection, despite a decrease in antibody titers. This is in line with our study, in which IgG and IgA antibodies against S1 SARS-CoV-2 decreased slightly 7 to 9 months of evaluation. Likewise, a study conducted by Ivanov and Semenova [14], in which asymptomatic or mild COVID-19 patients were evaluated, showed that levels of IgA antibodies were persistently high for more than 6 months after recovery from COVID-19.
Latent polyautoimmunity is associated with deleterious outcomes in patients with acute COVID-19 [9], and persistence of autoantibodies could influence clinical phenotypes in PCS, and the development of overt autoimmunity [15]. Our results demonstrate that latent polyautoimmunity observed during the acute phase of disease persists during PCS. In other words, latent polyautoimmunity in COVID-19 is not transient. Persistent ACA IgG positivity has been reported in a patient developing PCS [16], as well as an increase in the positivity of ANAs antibodies during PCS [17]. Nevertheless, because no information of autoantibodies before SARS-CoV-2 infection exist, a causal effect of virus infection in the development of autoimmunity during the acute phase of disease is precluded.
Cañas [6] proposed that the development of autoimmune conditions subsequent to COVID-19 infection could be associated (1) with transient immunosuppression of innate and acquired immunity leading to a loss of self-tolerance and (2) with a form of inappropriate immune reconstitution in genetically susceptible individuals. Our results show that patients with PCS have high levels of naive B cells, which are known to be a source of autoantibodies [18, 19].
We observed an increase of proinflammatory cytokines in PCS (ie, IFN-α, TNF- α, G-CSF, IL17A, IL-6, IL1-β, and IL-13), which confirmed previous observations [20, 21]. In addition, a significant decrease in IP-10 was noticed. This cytokine is a biomarker associated with severity and risk of death in COVID-19 patients. Interferon-γ-induced protein 10 is implicated in the acute phase of COVID-19 as promoting viral clearance and as an effector of immune-mediated acute lung injury [22]. Our results suggest that IP-10 plays a critical role in the acute phase of illness but not in PCS.
Studies based on the general population have shown associations between high concentrations of circulating inflammatory markers, such as C-reactive protein (CRP), IL-6, and TNF-α with depressive and cognitive symptoms [23, 24]. In PCS, persistent IL-6 dysregulation may contribute to fatigue, sleeping difficulties, depression, and anxiety, suggesting that a maintained inflammation is associated with these symptoms [25, 26]. Other biomarkers such as IL-1β, TNF-α, IFN-γ, IL-10, IL-2, CRP, MCP-1, serum amyloid A, and metabolites of the kynurenine pathway may contribute to this symptomatology in PCS [27]. Identification of these biomarkers, which are associated with chronic inflammation, may help us understand how depression develops in PCS. Moreover, IL-6 could drive autoinflammatory reactions and autoimmunity, via pre-existing natural B cell clones [28].
Regarding cellular immune response, our results reveal that 7 to 9 months after SARS-CoV-2 infection, most of the components of cellular immunity do not return to normal baseline in patients with PCS. An increase in CD4+ effector memory T cells, CD8+ effector T cells, Th9, and naive B cells was observed.
Studies in acute COVID-19 have shown a functional depletion and decrease in CD4+ and CD8+ T lymphocytes and natural killer cell numbers [29]. The apoptosis induced by SARS-CoV-2 in lymphocytes expressing the angiotensin-converting enzyme 2 receptor and the cytokine storm explain this phenomenon [29]. In our study, we found that lymphopenia persists in PCS, affecting total lymphocytes and CD8+ and CD4+ naive T cells. In addition to the apoptosis process, this lymphopenia can be caused by a change of the CD4+ and CD8+ T-cell effector phenotype. Townsend et al [30] demonstrated an expansion of effector CD8+ T cells and activated CD4+ and CD8+ T cells, with a reduction in naive CD4+ and CD8+ T cells at 101 days postinfection. Varghese et al [31] showed that lymphopenia persisted in 14% of patients 102 days postinfection.
A significant increase in Th9 cells compared with prepandemic controls and COVID-19 acute phase (ie, D28) was observed in our study. Previous studies described that Th9 responses mediated by IL-9 production are involved in tissue inflammation and immune-mediated diseases such as autoimmunity and asthma [32]. Orologas-Stavrou et al [33] showed a high Th9/Th17 ratio associated with persistence of a generalized inflammatory reaction (by Th17 cells), particularly in lungs (by Th9 cells) 2 months after infection.
Our study has several strengths. The longitudinal follow-up allows studying the causality of SARS-CoV-2 infection on the clinical and immunological profiles evaluated several months after infection. This allowed us to compare clinical and immunological phenotypes during PCS. In addition, prepandemic controls guarantee the lack of influence of unrecognized/asymptomatic infections on the autoimmune and immunological profiles. None of the included patients received immunomodulatory treatments that may have influenced the immune response during PCS.
Study limitations are acknowledged. A broader panel of B and T cells, as well as their functional analyses, was not possible. Genetic analysis was not done. The lack of polymerase chain reaction testing hindered the evaluation of viral persistence or reinfection during the follow-up. In addition, all patients presented PCS, and this did not allow us to compare inflammation/autoimmunity with recovered patients without PCS. The IgM RF antibodies are the most commonly used autoantibodies for the evaluation of rheumatoid arthritis in the clinical setting. Thus, identifying them is of clinical relevance in real-world scenarios. However, we acknowledge that IgA and IgG RF would be required to increase the understanding of autoimmune response in PCS. Another potential shortcoming of the present study is that the observed results might be due to chance alone or to the moderate sample size. However, this is unlikely because of the highly significant results observed after adjustments and corrections as well as their consistent direction and magnitude within the different analyses.
CONCLUSIONS
The clinical manifestations in PCS could be associated with the persistence of a proinflammatory and effector phenotype induced by SARS-CoV-2 infection. Results advocate for therapeutic targets for management of PCS, including TNF or IL-6 blockade, and immunomodulatory drugs. In addition, this long-term persistent immune activation could contribute to reactivity and the development of latent and overt autoimmunity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the members of the Center for Autoimmune Diseases Research for contributions and fruitful discussions during the preparation of the manuscript.
Disclaimer. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial support. This work was funded by the Universidad del Rosario Grant Numbers IV-FBG001 and ABN-011.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Dhama K, Khan S, Tiwari R, et al. . Coronavirus disease 2019–COVID-19. Clin Microbiol Rev 2020; 33:e00028–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anaya J-M, Rojas M, Salinas ML, et al. . Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev 2021; 20:102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobs JJL. Persistent SARS-2 infections contribute to long COVID-19. Med Hypotheses 2021; 149:110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mongioì LM, Barbagallo F, Condorelli RA, et al. . Possible long-term endocrine-metabolic complications in COVID-19: lesson from the SARS model. Endocrine 2020; 68:467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fogarty H, Townsend L, Morrin H, et al. . Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 2021; 19:2546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cañas CA. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses 2020; 145:110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tserel L, Jõgi P, Naaber P, et al. . Long-term elevated inflammatory protein levels in asymptomatic SARS-CoV-2 infected individuals. Front Immunol 2021; 12:709759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Acosta-Ampudia Y, Monsalve DM, Rojas M, et al. . COVID-19 convalescent plasma composition and immunological effects in severe patients. J Autoimmun 2021; 118:102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anaya J-M, Monsalve DM, Rojas M, et al. . Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J Transl Autoimmun 2021; 4:100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pacheco Y, Barahona-Correa J, Monsalve DM, et al. . Cytokine and autoantibody clusters interaction in systemic lupus erythematosus. J Transl Med 2017; 15:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wajnberg A, Amanat F, Firpo A, et al. . Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 370:1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iyer AS, Jones FK, Nodoushani A, et al. . Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol 2020; 5:eabe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Xiaomin L, Zhang X, et al. . Decline in neutralising antibody responses, but sustained T-cell immunity, in COVID-19 patients at 7 months post-infection. Clin Transl Immunol 2021; 10:e1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivanov A, Semenova E.. Long-term monitoring of the development and extinction of IgA and IgG responses to SARS-CoV-2 infection. J Med Virol 2021; 93:5953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knight JS, Caricchio R, Casanova J-L, et al. . The intersection of COVID-19 and autoimmunity. J Clin Invest 2021; 131:e154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertin D, Kaphan E, Weber S, et al. . Persistent IgG anticardiolipin autoantibodies are associated with post-COVID syndrome. Int J Infect Dis 2021; 113:23–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seeßle J, Waterboer T, Hippchen T, et al. . Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis 2021:ciab611. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tipton CM, Fucile CF, Darce J, et al. . Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015; 16:755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jenks SA, Cashman KS, Zumaquero E, et al. . Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 2018; 49:725–39.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ong SWX, Fong S-W, Young BE, et al. . Persistent symptoms and association with inflammatory cytokine signatures in recovered coronavirus disease 2019 patients. Open Forum Infect Dis 2021; 8:ofab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phetsouphanh C, Darley DR, Wilson DB, et al. . Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 2022; 23:210–6. [DOI] [PubMed] [Google Scholar]
- 22. Chen Y, Wang J, C L, et al. . IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol Med 2020; 26:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB.. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatr 2014; 71:1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wium-Andersen MK, Ørsted DD, Nielsen SF, Nordestgaard BG.. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatr 2013; 70:176–84. [DOI] [PubMed] [Google Scholar]
- 25. Ortelli P, Ferrazzoli D, Sebastianelli L, et al. . Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: insights into a challenging symptom. J Neurol Sci 2021; 420:117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koralnik IJ, Tyler KL.. COVID-19: a global threat to the nervous system. Ann Neurol 2020; 88:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lorkiewicz P, Waszkiewicz N.. Biomarkers of post-COVID depression. J Clin Med 2021; 10:4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vlachoyiannopoulos PG, Magira E, Alexopoulos H, et al. . Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann Rheum Dis 2020; 79:1661–3. [DOI] [PubMed] [Google Scholar]
- 29. Diao B, Wang C, Yingjun T, et al. . Reduction and functional exhaustion of T cells in patients with Coronavirus disease 2019 (COVID-19). Front Immunol 2020; 11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Townsend L, Dyer AH, Naughton A, et al. . Longitudinal analysis of COVID-19 patients shows age-associated T cell changes independent of ongoing ill-health. Front Immunol 2021; 12:676932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varghese J, Sandmann S, Ochs K, et al. . Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep 2021; 11:12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jabeen R, Kaplan MH.. The symphony of the ninth: the development and function of Th9 cells. Curr Opin Immunol 2012; 24:303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orologas-Stavrou N, Politou M, Rousakis P, et al. . Peripheral blood immune profiling of convalescent plasma donors reveals alterations in specific immune subpopulations even at 2 months post SARS-CoV-2 infection. Viruses 2020; 13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.