Abstract
Objectives
Successful vaccination is key to overcoming the COVID-19 pandemic. Immunosuppressive medication is known to potentially compromise vaccination responses, and expansion of our knowledge on vaccination efficacy in patients with autoimmune inflammatory rheumatic diseases (AIIRD) is therefore of utmost importance.
Methods
We conducted a single-centre observational study and evaluated the efficacy of approved COVID-19 vaccines in 303 adult AIIRD patients. Serum levels of IgG antibodies against the S1 subunit of SARS-CoV-2 spike proteins (anti-S IgG) were measured at least two weeks after vaccination. In a subgroup of patients without humoral response, T-cell responses were determined using an interferon-γ gamma release assay.
Results
Overall seropositivity rate was 78.5% and was significantly lower in patients under immunosuppressive therapy (75.7 vs 93.2%, P = 0.009). No difference regarding the vaccination type was observed. Glucocorticoids, mycophenolate-mofetil, TNF inhibitors, tocilizumab, abatacept and rituximab were all associated with non-response after proper vaccination. The risk was highest under RTX therapy (OR 0.004, 95% CI 0.001, 0.023, P < 0.0001). A strong negative correlation was observed between time since vaccination with an mRNA vaccine and anti-S antibody levels (r=–0.6149, P < 0.0001). In patients without humoral response, a T-cell response was found in 50%.
Conclusions
COVID-19 vaccination in patients with AIIRD is effective using any approved vaccine. Humoral response might be impaired depending on the individual immunosuppressive medication. The risk of non-response is highest under rituximab therapy. Anti-S IgG antibody levels wane over time after mRNA vaccination. Importantly, 50% of humoral non-responders showed a T-cellular response, suggesting T-cell-mediated protection to a certain extent.
Keywords: COVID-19, vaccination, humoral response, cellular T-cell response, rheumatic diseases, immunosuppression
Rheumatology key messages.
Glucocorticoids, mycophenolate-mofetil, TNF inhibitors, tocilizumab, abatacept and rituximab were associated with non-response after proper vaccination.
The risk for serological non-response was highest under rituximab therapy (OR 0.004, 95% CI 0.001, 0.023, P<0.0001).
Fifty per cent of the humoral non-responders had a detectable T-cell response.
Introduction
Since the beginning of the COVID-19 pandemic, patients with autoimmune inflammatory rheumatic diseases have been considered to be at an increased risk for hospitalization and severe or even fatal outcomes [1–3]. Distinct therapies such as the B-cell depleting rituximab or the use of high glucocorticoid doses (≥10 mg prednisone equivalent) are main contributors to that risk [4–6]. On the other hand, some biologic agents (e.g. TNF inhibitors) are not associated with an increased risk of severe COVID-19 and might even reduce the risk of hospitalization [7–9]. Nevertheless, a proper vaccination against COVID-19 is crucial to protect patients with autoimmune inflammatory rheumatic diseases. While randomized trials report high vaccine efficacy, the major drawback of these trials from a rheumatologic perspective is the (understandable) exclusion of immunocompromised patients [10–12]. Unfortunately, as we know from other vaccines (e.g. influenza, pneumococcal), humoral response to vaccination can be impaired under immunosuppressive therapy [13–15]. So far, while first data on COVID-19 vaccine response in immunocompromised patients with rheumatic diseases have been published, our knowledge remains limited and focused on messenger RNA vaccines [16–18].
The aim of this study was to evaluate the humoral response to any valid COVID-19 vaccination in patients with various autoimmune inflammatory rheumatic conditions under different kinds of immunosuppressive medication including conventional synthetic, biologic and targeted synthetic DMARDs. In humoral non-responders, the vaccine-induced T-cell response was determined.
Methods and study design
Study participants
Consecutive adult patients with various autoimmune inflammatory rheumatic diseases have been recruited from the outpatient clinic at Leipzig University during their regular visits between July and September 2021. They have been asked for their COVID-19 vaccination status and valid vaccination was double-checked by either reviewing the individual vaccination cards or using the CovPass smart phone app. CovPass is an application from the Robert Koch Institute for German residents, allowing to store and view European Union Digital COVID Certificates for their valid COVID-19 vaccination [19]. Details on the used vaccine and the application dates were documented. Any approved vaccine [i.e. Comirnaty® (Biontech/Pfizer), Spikevax® (Moderna), Vaxzevria® (AstraZeneca) and COVID-19 vaccine Janssen® (Janssen-Cilag/Johnson&Johnson)] was considered. The individual patient’s characteristics including the medication at the time of vaccination were obtained from the medical record. Patients with a previous SARS-CoV-2 infection were excluded.
Serological testing
To evaluate humoral immunity, IgG antibody levels against the receptor binding domain (RBD) of the S1 subunit of the SARS-CoV-2 spike protein (anti-S IgG) were measured in the patient’s sera if at least two weeks had passed after documented vaccination using a chemiluminescent immunoassay (SARS-CoV-2-IgG-II-Quant-Assay, Architect i2000SR, Abbott, Abbott Park, IL, USA). According to the manufacturer’s instructions, an anti-S IgG ≥7.1 BAU/ml was considered as positive.
T-cell interferon-γ gamma release (IGRA) assay
In some of the patients without humoral immunity (negative anti-S IgG antibody test), the T-cell response against SARS-CoV-2 was evaluated using an IGRA in order to determine if possibly protective cytotoxic T cells exist.
From each patient, peripheral blood mononuclear cells (PBMCs) were isolated from 9 ml heparinized whole blood by density gradient centrifugation (1000×g, 10 min). PBMCs were washed with PBS (500×g, 10 min), counted using an automatic blood counter (Sysmex XP-300, Sysmex, Norderstedt, Germany) and diluted in serum-free AIM-V-Medium (Fisher Scientific, Suisse) to a final cell concentration of 2.5× 105 cells/100 µl. For IGRA we used a T-Spot COVID ELIspot system (Oxford Immunotech, Oxford, UK); 50 µl AIM-V-medium served as the negative control, 50 µl PHA as the positive control and 50 µl Spike-protein mix as the target antigen. To each of the wells, we added 100 µl of the diluted cell suspension and incubated the plate for 20 h at 37°C, 5% CO2. Supernatant was discarded, and wells were washed three times with PBS. Afterwards, 50 µl of conjugate reagent (Oxford Immunotech, Oxford, UK) were added to each well and incubated for one h at 4°C in the dark. The conjugates were discarded and each well was washed 3× with PBS. We added 50 µl of substrate solution (Oxford Immunotech, Oxford, UK) to each well at room temperature for 7 min. The plate was washed thoroughly with distilled water and dried. Finally, the spots were counted by an ELIspot-reader (AID, Straßberg, Germany). Test results were reactive if negative control were <10 spots, positive control >20 spots, and Spike-Mix >10 spots.
Biostatistical analysis
To describe continuous data, mean and S.d. or median and interquartile range (IQR) were used where appropriate. Categorical data were described with absolute or relative frequencies. To compare the frequencies of categorical variables, chi squared test or fisher’s exact test was performed. For comparison of continuous data, student’s t test or Mann–Whitney U test, as appropriate, was applied. To assess the difference of three or more groups, analysis of variance or Kruskal–Wallis test with correction for multiple comparisons was done. Multivariable analysis was carried out using multiple logistic regression. A significant statistical difference was assumed when the P-value was <0.05. All analyses were conducted using GraphPad PRISM Version 8.4 for Mac (GraphPad Software Inc., San Diego, CA, USA).
Ethical approval
All procedures performed in this survey were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The design of the study was approved by the ethics committee of the University of Leipzig (282/21-ek). Data obtained in this study did not interfere with the course of treatment for patients included. Informed consent was obtained from all individual participants included in the study.
Results
Study population
In total, 303 patients with various rheumatic diseases [66% female, mean age 61.4 (14.6) years] have been included in this study. The majority of patients had rheumatoid arthritis (41.9%), followed by spondyloarthritis (24.8%) and connective tissue diseases (23.4%, with 80.3% systemic lupus erythematosus). The mean time since documented vaccination in all patients was 80.3 (50.8) days. See Table 1 for further details on the patient population and the immunosuppressive medication.
Table 1.
Clinical characteristics of the study patients (n = 303); shown are numbers (%) or mean with (S.d.)
| Patients | n (%) | Female, n (%) | Mean age, years | No medication, n (%) | GC, n (%) | MTX, n (%) | AZA, n (%) | MMF, n (%) | TNFi, n (%) | JAKi, n (%) | TOZ, n (%) | IL-17i, n (%) | ABA, n (%) | RTX, n (%) | BEL, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients | 303 | 200 (66) | 61.4 (14.6) | 24 (7.9) | 149 (49.2) | 111 (37) | 19 (6.3) | 24 (7.9) | 70 (23.1) | 22 (7.3) | 7 (2.3) | 19 (6.3) | 17 (5.6) | 29 (9.6) | 5 (1.7) |
| Rheumatoid arthritis | 127 (41.9) | 91 (71.7) | 67 (13.1) | 5 (3.9) | 76 (59.8) | 70 (55.1) | 2 (1.6) | — | 35 (27.6) | 13 (10.2) | 2 (1.6) | — | 17 (13.4) | 20 (15.7) | — |
| Spondyloarthritisa | 75 (24.8) | 37 (49.3) | 56 (14.1) | 6 (8) | 11 (14.7) | 25 (33.3) | — | — | 35 (46.7) | 3 (4) | — | 19 (25.3) | — | — | — |
| Connective tissue diseases | 71 (23.4) | 57 (83.1) | 56.3 (14.6) | 11 (15.5) | 39 (54.9) | 7 (9.9) | 15 (20.8) | 23 (31.9) | — | 6 (8.3) | — | — | — | 1 (1.4) | 5 (7.0) |
| Systemic lupus erythematosus | 57 (18.8) | 47 (82.5) | 53.3 (13.7) | 5 (8.8) | 34 (59.6) | 5 (8.8) | 13 (22.8) | 21 (36.8) | — | 6 (10.5) | — | — | — | 1 (1.8) | 5 (8.8) |
| ANCA-associated vasculitis | 17 (5.6) | 7 (41.2) | 63.7 (12.9) | 1 (5.9) | 16 (94.1) | 5 (29.4) | 2 (11.8) | — | — | — | — | — | — | 7 (41.2) | — |
| Large-vessel vasculitis | 7 (2.3) | 3 (42.9) | 69 (13.2) | — | 6 (85.7) | 2 (28.6) | — | 1b (14.3) | — | — | 4 (57.1) | — | — | — | — |
| Adult-onset Still’s disease | 3 (1) | 1 (33.3) | 59 (15.1) | — | — | 2 (66.7) | — | — | — | — | 1 (33.3) | — | — | 1 (33.3) | — |
| Idiopathic juvenile arthritis | 2 (0.7) | 2 (100) | 46 (14.1) | 1 (50) | 1 (50) | — | — | — | — | — | — | — | — | — | — |
Including axial spondyloarthritis and psoriatic arthritis.
MMF is prescribed for a coincidental autoimmune hepatitis.
ABA: abatacept; BEL: belimumab; GC: glucocorticoids; IL-17i: IL17 inhibitor; JAKi: JAK inhibitor; RTX: rituximab; TNFi: TNF inhibitor; TOZ: tocilizumab.
Overall seropositivity rate after complete COVID-19 vaccination was 78.5% with a lower mean age among the responders [60 (14.6) vs 66.1 (14) years, P = 0.003].
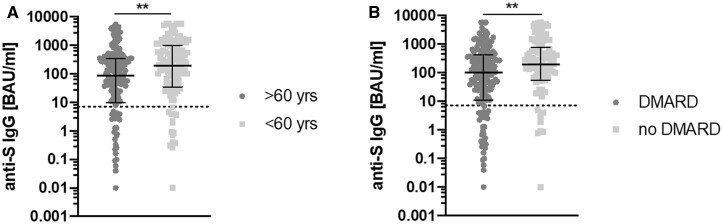
Excluding the patients under B-cell-depleting therapy, response rate was 85.4%. No difference regarding the time since vaccination between responders and non-responders was observed. Seroconversion rate was significantly lower in patients under immunosuppressive therapy than in patients without [75.7 vs 93.2%, odds ratio (OR) 0.228, 95% CI 0.068, 0.760, P = 0.009]. The yielded anti-S IgG levels were higher in patients under 60 years (P = 0.0017) and without DMARD therapy (P = 0.0048), see Fig. 1. Further details on seropositivity in the different patient subgroups can be obtained from Table 2.
Fig. 1.
Comparison of IgG antibodies against the spike protein of SARS-CoV-2 (anti-S IgG)
(A) Patients >60 vs <60 years, (B) Patients with and without DMARD therapy. Shown are individual values, median and interquartile range. The dotted line represents the seropositivity cut-off (7.1 BAU/ml).
Table 2.
Seropositivity rate, stratified by disease and medication (n = 303)
| Patient group | Seropositivity, n (%) |
|---|---|
| All patients | 238/303 (78.5) |
| RA | 85/127 (66.9) |
| Spondyloarthritisa | 71/75 (94.7) |
| CTD | 60/71 (84.5) |
| SLE | 47/57 (82.5) |
| ANCA-associated vasculitis | 11/17 (64.7) |
| Large-vessel vasculitis | 7/7 (100.0) |
| Adult-onset Still’s disease | 2/3 (66.7) |
| Idiopathic juvenile arthritis | 2/2 (100.0) |
| Medication (any combination) | |
| Glucocorticoids | 98/149 (65.8) |
| MTX | 84/111 (75.7) |
| AZA | 18/19 (94.7) |
| MMF | 18/24 (75) |
| TNF inhibitor | 59/70 (84.3) |
| JAK inhibitor | 19/22 (86.4) |
| IL17 inhibitor | 19/19 (100) |
| Abatacept | 6/17 (35.3) |
| Tocilizumab | 5/7 (71.4) |
| Belimumab | 4/5 (80) |
| Rituximab | 3/29 (10.3) |
Including axial spondyloarthritis and psoriatic arthritis.
Humoral response under distinct disease-modifying anti-rheumatic and immunosuppressive drugs
Treatment with AZA and MTX monotherapy was associated with a high seropositivity of 94.7% and 95.5%, respectively. Combination of MTX with other therapies reduced that rate to 60.9% (P < 0.0001). While combination with a tumor necrosis factor inhibitor (TNFi) led to a reduced seroconversion rate of 75% (P < 0.0001), combination with abatacept (ABA) decreased that rate even further (33.3%, P < 0.0001). Seroconversion was 0% in combination with tocilizumab (TOZ, P < 0.0001).
Cytokine inhibiting therapy [TNFi, TOZ, interleukin (IL) 17 inhibitors] was associated with a seroconversion rate of 85.6%. While seroconversion was 83.3% under TOZ monotherapy, TNFi monotherapy led to a humoral response in 90.7%. Importantly, none of the patients under IL-17i treatment failed to mount a humoral response (seroconversion rate 100%, P = 0.0021 and P < 0.0001 vs TNFi and TOZ, respectively).
Patients under glucocorticoid (GC) monotherapy showed a humoral response in 85.2%, and patients receiving GC in combination with either DMARD had a significantly reduced seroconversion rate of 65.8% (P = 0.0018). Patients with janus kinase inhibitor (JAKi) treatment had a seropositivity in 86.4%, independently of combination with MTX. Both MMF and ABA therapy were associated with low seroconversion rates (75 and 35.3%, respectively). Of interest, treatment with belimumab (BEL) was associated with a seroconversion rate of 80%.
Seropositivity was lowest under B-cell-depleting treatment with rituximab (RTX, 10.3%). The low response under RTX was independent of MTX co-medication. Median distance to the last RTX application was 215 days (IQR 190-293) and the median B-cell count was 0 cells/γl (IQR 0–21.5). While the B-cell count was higher in responders without reaching significance (9 vs 0 cells/γl, P = 0.1842), the anti-S IgG titre correlated positively with the peripheral B-cell count (r = 0.5348, P = 0.0125).
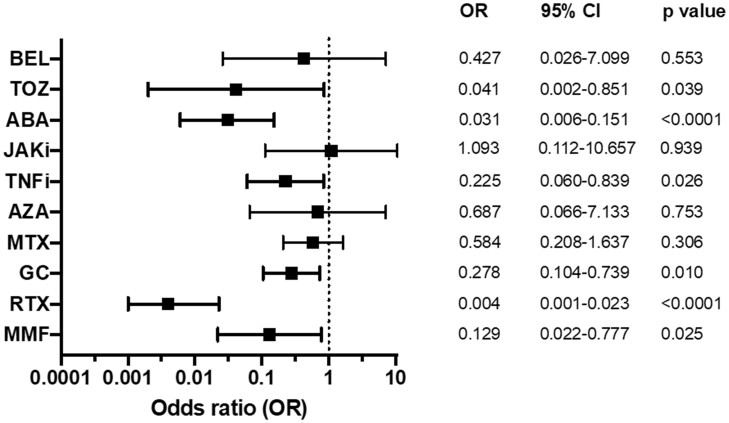
The conducted logistic regression analysis (model a priori adjusted for age, gender, diagnose, vaccine type and time since vaccination) revealed treatment with GC, MMF, TNFi, TOZ, ABA and RTX to be associated with non-seroconversion after proper vaccination (for details and OR see Fig. 2). The risk was highest among patients under B-cell-depleting therapy with rituximab (OR 0.004, 95% CI 0.001, 0.023, P < 0.0001).
Fig. 2.
Odds ratios (OR) for seroconversion after COVID-19 vaccination as determined by multiple logistic regression
ABA: abatacept; BEL: belimumab; GC: glucocorticoids; JAKi: JAK inhibitor; RTX: rituximab; TNFi: TNF inhibitor; TOZ: tocilizumab.
Differences in humoral immunity depending on the used vaccine
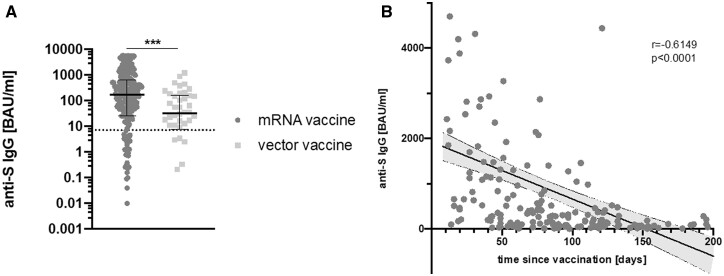
The majority of the included patients (85.1%) have been vaccinated with mRNA vaccines, while 14.9% were immunized with vector vaccines. Seropositivity rate did not differ between the two vaccine types (80.3% after mRNA and 78% after vector-based vaccination, respectively). The humoral responses differed significantly, however. Anti-S IgG levels were higher in patients vaccinated with an mRNA vaccine compared with vector vaccine (168 BAU/ml vs 32.5 BAU/ml, P = 0.0002, Fig. 3A).
Fig. 3.
Difference of anti-S IgG titre and antibody time course depending on the used vaccine
(A) Anti-S IgG titre comparison after vaccination with either mRNA or vector vaccine. Shown are individual values, median and interquartile range. The dotted line represents the seropositivity cut-off (7.1 BAU/ml). (B) Relationship between anti-S IgG titre and time after vaccination with an mRNA-based vaccine. The shaded area represents the 95% CI. mRNA: messenger ribonucleic acid. ***P<0.001.
Anti-S IgG antibody titres after mRNA vaccine were found to correlate strongly with the elapsed time between complete vaccination and analysis in the study (r =–0.6149, P < 0.0001, see Fig. 3B). For vector vaccines, in contrast, no such correlation was seen (r =–0.1255, P = 0.4660, data not shown). The lower titres achieved by vector vaccine were not due to waning antibody titres, because the mean time since complete vaccination was 83.7 (51.4) days for mRNA vaccines and 58.2 (35.4) days for vector vaccines (P = 0.003).
T-cell response in patients without humoral immunity
Sixty-five patients (21.5%) did not mount a humoral response after valid vaccination. T-cell response was assessed in 20 of these patients. The majority of the evaluated patients (45%) were treated with the B-cell-depleting agent RTX. ABA, MMF and TNFi were each used in three patients (15%). Only one patient was treated with belimumab.
In total, 50% of the patients had a detectable T-cell response towards the SARS-CoV-2 spike protein. The rate of T-cell response was highest in patients under RTX treatment (55.6% vs 33.3% in patients with ABA, MMF or TNFi therapy). There was no correlation between anti-S IgG titres and ELIspot numbers (data not shown) and the peripheral B-cell count was higher in patients without T-cell response (20.5 vs 4 cells/γl, P = 0.7863).
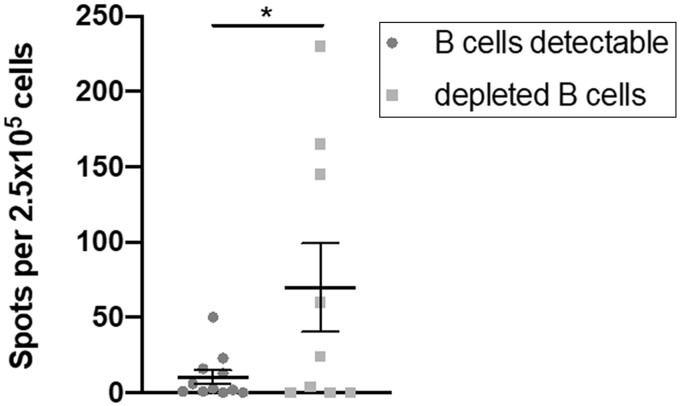
Of particular interest, absolute ELIspot count though was increased in patients with totally depleted B cells (P = 0.0398, Fig. 4), with 88.9% of them under RTX treatment.
Fig. 4.
Comparison of ELIspot count between patients with detectable and depleted B cells
Shown are individual values, mean and standard error of the mean.
Cellular immunity was impaired by the use of glucocorticoids (OR 0.062, 95% CI 0.003, 1.329, P = 0.0379).
Discussion
This study reports a seropositivity rate of 78.5% after valid COVID19 vaccination for patients with various autoimmune inflammatory rheumatic diseases and different anti-rheumatic drugs. Response to vaccination was significantly higher in patients without immunosuppressive therapy (93.2 vs 76.1%). Importantly, we saw no difference in seroconversion between the used vaccine types.
In the current literature, reported rates of seroconversion in immunocompromised patients with autoimmune inflammatory rheumatic diseases vary from 49% to 100% [16–18, 20–22], most likely due to a great variability in study design, the used vaccines, medication and the patient population enrolled. While a smaller investigation (n = 84) showed a seropositivity rate of 94% (none of the patients had been under RTX therapy nor treatment with abatacept or mycophenolate-mofetil) [18], larger studies with a greater treatment variety reported up to 86% response rate [16]. Investigations focusing on RTX treatment demonstrated lower rates of humoral response (49–59.3%) [20, 22]. Most of the studies investigated vaccination with the mRNA vaccine BNT162b2 (Comirnaty®).
Our results indicate that not only medication with rituximab, abatacept, glucocorticoids and mycophenolate-mofetil, but also with TNFi hampers humoral immunity. Interestingly, while Furer et al. reported no negative impact of TNFi on seroconversion from their trial [16], colleagues from the Netherlands found reduced odds for seroconversion after one vaccination shot, particularly in combination with MTX [23]. Because we did not find a difference in seropositivity rate after vaccination with mRNA or vector vaccine, the exclusive use of Comirnaty® in the trial from Furer et al. cannot explain this difference. A large investigation from the UK in patients with inflammatory bowel diseases and therapy with infliximab reports a reduced seroconversion rate of 85% after vaccination, comparable to our findings [24]. In general, combination therapy (e.g. MTX+TNFi) seems to be more immunosuppressive than monotherapy. Concordantly, a recent small investigation showed that functional humoral immunity was not impaired in patients with psoriasis under monotherapy with MTX or cytokine inhibitors after mRNA-based vaccination [25].
Of particular importance is the finding that none of the patients receiving anti-IL-17 therapy, neither in monotherapy nor in combination, failed to seroconvert. Because IL-17i treatment is particularly used in patients with spondyloarthritis, this finding might contribute to the high seropositivity rate (94.7%) in that patient group. High seropositivity rates were also found in patients treated with AZA, MTX monotherapy and JAK inhibitors. These results suggest that withholding therapy prior to vaccination might not be necessary with these immunosuppressive medications. Importantly, when MTX is used in combination with other DMARDs, suspending the combination partners for vaccination should be considered (particularly TNFi, TOZ or ABA).
B-cell-depleting therapy was the strongest predictor of failing humoral response after complete vaccination. The very low response rate of 10.3% is likely to be explained by the short median distance to the last RTX application (215 days, IQR 190–293) and the almost total depletion of the B-cell compartment. Other trials with a higher seroconversion rate under RTX treatment reported longer median distances (>1 year) to the last application and at least partial B-cell reconstitution [22, 26]. Humoral response seems to correlate with the time to the prevaccination RTX administration, with a more than 2-fold increase when vaccination is performed 12 months rather than 6 months after RTX treatment [16]. Furthermore, a distinct amount of circulating B cells (10 cells/γl, [27]) might be required for seroconversion. In our cohort, seropositive patients show higher B-cell counts than seronegative patients without reaching significance, very likely due to the low rate of seroconversion and therefore low numbers in the responder arm. The correlation of anti-S IgG titre with the peripheral B-cell count emphasizes the critical role of B cells in eliciting a humoral response after vaccination of RTX-treated patients.
RTX is predominantly used for the treatment of RA and ANCA-associated vasculitis (AAV), which most probably contributes to the reduced seroconversion rates in these patient subgroups in our cohort (seroconversion rate in RA patients without RTX 78.5%, in AAV patients without RTX 100%, accordingly).
Compared with vaccination with an mRNA vaccine, the induced antibody titres were significantly lower after vaccination with a vector vaccine. In contrast, while anti-S IgG titres showed a strong negative correlation with time since vaccination in mRNA-vaccinated patients, such a relationship was not seen in patients after vector-based vaccination. The negative correlation mirrors waning antibody titres that have already been predicted at the beginning of the worldwide vaccination campaign and could be confirmed just recently for mRNA vaccines [28, 29]. Although the implications of waning antibody levels are still a matter of debate, higher antibody levels are likely associated with an increased protection particularly against virus variants [30]. Furthermore, anti-S IgG have been demonstrated to correlate with neutralizing antibody levels [28] and a publication from Israel reports an impaired protection 6 months after vaccination [31]. Lower antibody levels after vector vaccination consequently raise concerns about actual protection, especially in immunocompromised patients. Nevertheless, a protective threshold has yet to be defined. Therefore, the recently approved mRNA booster vaccination for patients at risk (including patients with autoimmune inflammatory rheumatic diseases) in Germany [32] therefore meets concerns in this direction and is particularly needed for patients under RTX and ABA treatment. Because humoral response most likely depends on the elapsed time since the last RTX application and the accompanied B-cell repopulation, that interval should be lengthened when clinically feasible. Likewise, ABA administration should be postponed to improve vaccine-induced immunity. CYC was not used in any of our patients but is a potent immunosuppressive with depleting effects on B and T cells. Consequently, a hampering effect comparable to RTX or MMF may be presumed. While specific literature on the impact of CYC on COVID19 vaccination response in humans is missing, CYC is known to impair influenza vaccine responses. A recent investigation in mice suggests an impaired humoral immunity against SARS-CoV-2 spike protein [33]. The American College of Rheumatology recommends timing of IV administration approximately one week after each vaccine dose when feasible [34].
At least 50% of the non-responders in our cohort showed a detectable T-cell response. Other studies reported cellular response rates ranging from 20% in a mixed cohort of patients receiving RTX for various indications [22] up to 82.6% in patients with AAV and podocytopathies [20]. It must be taken into consideration, however, that T-cell response has been determined independently of seroconversion in these studies [20, 22]. Focusing on SLE patients, two recent investigations showed a positive correlation between anti-S IgG and T-cell response [35, 36]. Both patients with and without humoral response were included. Because our T-cell substudy only considered patients without humoral response, these results could not be compared with our finding of a missing correlation between humoral and cellular response. Important, though, is our finding of an increased ELIspot count in humoral non-responders with totally depleted B cells when being compared with patients with measurable B cells. The pre-print publication of the OCTAVE trial [37] reported comparable T-cell responses between healthy controls and diseases such as inflammatory arthritis or AAV. Lacking further comparative analyses (e.g. differentiation after serostatus or B-cell count), the interpretation is difficult. Taking our finding of an increased ELIspot count in non-seroconverted, B-cell-depleted patients into account, we suggest that the missing serological response due to lack of B cells might be counteracted by an increased T-cell response.
T-cell response is important for recovery from COVID-19 and provides increased immunity against re-infection [38]. Although T cells might not be able to prevent infection, they can reduce the viral load and therefore the pathogenicity of the virus [39]. Investigations in rhesus macaques suggest a protective role of CD8-driven cellular immunity against SARS-CoV-2 even in the context of waning or subprotective antibody titres [40].
The detection of a positive T-cell response against the spike protein in 50% of patients without seroconversion is encouraging and might confer protection even in the absence of specific antibodies.
The limitations of our study include the observational design, the small size of some medication subgroups, and the variable time since vaccination. Our logistic regression model was adjusted accordingly for the time elapsed since vaccination. In future studies, longitudinal determination of anti-S IgG antibody levels will also be required to investigate the individual course of the waning of antibody responses. As in any COVID-19 vaccination-related investigations, a protective antibody level threshold is currently missing due to lacking data.
Conclusions
Vaccination against COVID-19 in patients with inflammatory rheumatic conditions is effective using both mRNA and vector vaccines. Our results indicate that seroconversion is highest in patients without immunosuppression and that humoral response might be impaired depending on the individual immunosuppressive therapy. The risk of non-response is highest in patients under B-cell-depleting therapy, but abatacept, glucocorticoids, mycophenolate-mofetil, TNF inhibitors and tocilizumab were also identified as risk factors for non-response. If clinically feasible, postponing of RTX and ABA therapy is likely to improve vaccine-induced humoral immunity. Anti-S IgG shows a strong negative correlation with the time since complete mRNA-based vaccination, indicating waning antibody levels. Booster vaccinations are probably needed to maintain high antibody levels and to ensure protection particularly in patients with B-cell-depleting therapy. Encouraging, is that 50% of the humoral non-responders show a cellular response, which suggests protection to some extent.
Acknowledgements
M.K.: conceptualization, data collection, statistical analysis, data interpretation and manuscript drafting. U.W.: data collection and contribution to manuscript preparation. P.N.: data collection, statistical analysis and contribution to manuscript preparation. C.P.: anti-S IgG antibody measurement, data collection and contribution to manuscript preparation. A.B.: IGRA measurement, data collection and contribution to manuscript preparation. C.B.: data collection, contribution to data interpretation and manuscript drafting. M.P.: data collection and statistical analysis. O.S.: data collection and contribution to manuscript preparation. All authors read the final version of the manuscript and agreed to publication.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure statement: C.B. received lecture fees from Merck, MSD, Mundipharma and Pfizer. The other authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hyrich KL, Machado PM.. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol 2021;17:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu C, Yi Z, Cai R. et al. Clinical outcomes of COVID-19 in patients with rheumatic diseases: a systematic review and meta-analysis of global data. Autoimmun Rev 2021;20:102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avouac J, Drumez E, Hachulla E. et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutherford MA, Scott J, Karabayas M, Antonelou M. et al. Risk factors for severe outcomes in patients with systemic vasculitis and COVID-19: a Binational, Registry-Based Cohort Study. Arthritis Rheumatol 2021;73:1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gianfrancesco M, Hyrich KL, Al-Adely S. et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner EJ, Ungaro RC, Gearry RB. et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–91.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leipe J, Hoyer BF, Iking-Konert C. et al. SARS-CoV-2 & rheumatic disease: consequences of the SARS-CoV-2 pandemic for patients with inflammatory rheumatic diseases. A comparison of the recommendations for action of rheumatological societies and risk assessment of different antirheumatic treatments. Z Rheumatol 2020;79:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahil SK, Dand N, Mason KJ. et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. J Allergy Clin Immunol 2021;147:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson EJ, Rouphael NG, Widge AT. et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sadoff J, Gray G, Vandebosch A. et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahin U, Muik A, Derhovanessian E. et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020;586:594–9. [DOI] [PubMed] [Google Scholar]
- 13. Hua C, Barnetche T, Combe B, Morel J.. Effect of methotrexate, anti-tumor necrosis factor alpha, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:1016–26. [DOI] [PubMed] [Google Scholar]
- 14. Park JK, Lee YJ, Shin K. et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Assen S, Holvast A, Benne CA. et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75–81. [DOI] [PubMed] [Google Scholar]
- 16. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 17. Geisen UM, Berner DK, Tran F. et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon D, Tascilar K, Fagni F. et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2021;80:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robert-Koch-Institut (RKI). 2021. https://www.digitaler-impfnachweis-app.de/en/ (10 September 2021, date last accessed).
- 20. Prendecki M, Clarke C, Edwards H. et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seror R, Camus M, Salmon J-H. et al. Do JAK inhibitors affect immune response to COVID-19 vaccination? Data from the MAJIK-SFR Registry. Lancet Rheumatol 2022;4:e8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moor MB, Suter-Riniker F, Horn MP. et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021;3:e789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boekel L, Steenhuis M, Hooijberg F. et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021;3:e778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy NA, Lin S, Goodhand JR. et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021;70:1884–93. [DOI] [PubMed] [Google Scholar]
- 25. Mahil SK, Bechman K, Raharja A. et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol 2021;3:e627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spiera R, Jinich S, Jannat-Khah D.. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021;80:1357–9. [DOI] [PubMed] [Google Scholar]
- 27. Stefanski AL, Rincon-Arevalo H, Schrezenmeier E. et al. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. Arthritis Rheumatol 2021; doi: 10.1002/art.42060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levin EG, Lustig Y, Cohen C. et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dolgin E. COVID vaccine immunity is waning - how much does that matter? Nature 2021;597:606–7. [DOI] [PubMed] [Google Scholar]
- 30. Shrotri M, Navaratnam AMD, Nguyen V. et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021;398:385–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O. et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med 2021;385:e85 doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Standing Committee on Vaccination (STIKO). Beschluss der STIKO zur 12. Aktualisierung der COVID-19-Impfempfehlung. Epid Bull 2021;43:3–11. [Google Scholar]
- 33. Paschall AV, Ozdilek A, Briner SL, Brindley MA, Avci FY.. Modulation of immunosuppressant drug treatment to improve SARS-CoV-2 vaccine efficacy in mice. Vaccine 2022;40:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ACR. COVID-19 Vaccine Clinical Guidance Summary for Patients with Rheumatic and Musculoskeletal Diseases, Version 4, 2021. https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf (13 January 2022, date last accessed).
- 35. Izmirly PM, Kim MY, Samanovic M. et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2022;74:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moyon Q, Sterlin D, Miyara M. et al. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann Rheum Dis 2021; doi: 10.1136/annrheumdis-2021-221097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kearns P, Siebert S, Willicombe M. et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – The OCTAVE Trial, 2021. https://ssrn.com/abstract=3910058 (13 January 2022, date last accessed).
- 38. Wang Z, Yang X, Zhong J. et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat Commun 2021;12:1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bertoletti A, Le Bert N, Qui M, Tan AT.. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol Immunol 2021;18:2307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McMahan K, Yu J, Mercado NB. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021;590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.