Abstract
Emerging as a new epidemic, long COVID or post-acute sequelae of coronavirus disease 2019 (COVID-19), a condition characterized by the persistence of COVID-19 symptoms beyond 3 months, is anticipated to substantially alter the lives of millions of people globally. Cardiopulmonary symptoms including chest pain, shortness of breath, fatigue, and autonomic manifestations such as postural orthostatic tachycardia are common and associated with significant disability, heightened anxiety, and public awareness. A range of cardiovascular (CV) abnormalities has been reported among patients beyond the acute phase and include myocardial inflammation, myocardial infarction, right ventricular dysfunction, and arrhythmias. Pathophysiological mechanisms for delayed complications are still poorly understood, with a dissociation seen between ongoing symptoms and objective measures of cardiopulmonary health. COVID-19 is anticipated to alter the long-term trajectory of many chronic cardiac diseases which are abundant in those at risk of severe disease. In this review, we discuss the definition of long COVID and its epidemiology, with an emphasis on cardiopulmonary symptoms. We further review the pathophysiological mechanisms underlying acute and chronic CV injury, the range of post-acute CV sequelae, and impact of COVID-19 on multiorgan health. We propose a possible model for referral of post-COVID-19 patients to cardiac services and discuss future directions including research priorities and clinical trials that are currently underway to evaluate the efficacy of treatment strategies for long COVID and associated CV sequelae.
Keywords: COVID-19, Long COVID, Post-acute sequelae of COVID-19, Cardiovascular disease, Coronavirus, Long term
Graphical Abstract
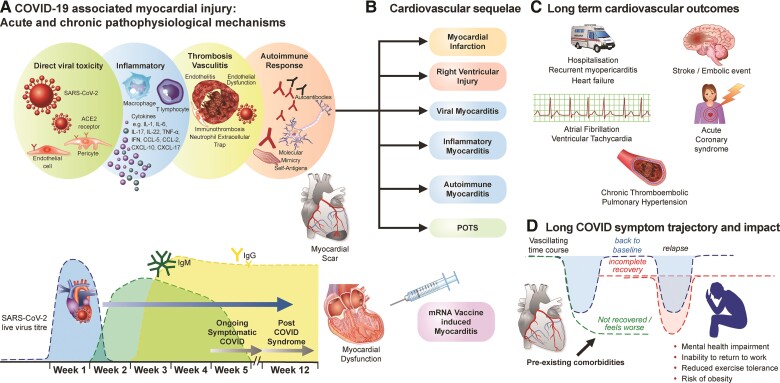
Graphical Abstract.
Central illustration depicting (A) Pathophysiological mechanisms underlying acute and chronic severe acute respiratory syndrome coronavirus 2-induced myocardial injury (including effects of vaccine) and its relation with timing of infection and onset of long COVID symptoms, (B) post-COVID-19 cardiovascular sequelae, (C) anticipated long-term cardiovascular complications and outcomes, and (D) the unpredictable trajectory of long COVID and its impact on mental health, ability to work, exercise tolerance, and potential to exacerbate the obesity epidemic. ACE2, angiotensin-converting enzyme 2; CCL, chemokine ligand; COVID, coronavirus disease; IL, interleukin; IFN, interferon; Ig, immunoglobulin; PCR, polymerase chain reaction; POTS, postural orthostatic tachycardia syndrome; RNA, ribonucleic acid; TNF, tumour necrosis factor.
Background
The crippling effect of coronavirus disease 2019 (COVID-19) on healthcare and economies globally has undoubtedly been one of the worst disasters experienced by humans in the last decades. Worldwide, survivors of COVID-19 now exceed hundreds of millions,1 with some reporting incomplete recovery months beyond the acute illness, a condition commonly referred to as long COVID. Persistent symptoms of breathlessness, chest pain, fatigue, headaches, brain fog, and palpitations are a constant reminder of the devastation caused by this virus and the need to remain vigilant for any long-term damage. Now, more than ever before, management of cardiometabolic risk factors should become a priority for physicians, as their formidable power in intensifying COVID-19 illness severity has been convincingly documented. The long-term impact of COVID-19 on cardiovascular (CV) health and mortality is also emerging as a major global concern.
In this review, we discuss the definition of long COVID, epidemiology of cardiopulmonary manifestations in the context of long COVID, pathophysiological mechanisms for acute and chronic cardiac injury secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, its management, and future directions.
Long COVID and epidemiology of cardiac symptoms
The term ‘long COVID’ was originally coined by a patient2,3 and asserts the notion that suffering does not stop with resolution of acute infection. While there is no universally accepted definition, in December 2020 the United Kingdom (UK) National Institute for Health and Care Excellence guidelines4 defined long COVID as persistence of symptoms beyond 4 weeks of SARS-CoV-2 infection. This term comprises two phases: ongoing symptomatic phase (4–12 weeks) and post-COVID-19 syndrome (>12 weeks) based on the duration of symptoms. More recently, the World Health Organization provided a case definition for post-COVID-19 condition,5 a term used to refer to persistence of symptoms beyond 3 months of SARS-CoV-2 infection, lasting for at least 2 months and not explained by any other illness. Other terms used to describe long COVID include post-acute COVID-19 syndrome,6 post-acute sequelae of COVID-19,7 and long-haul COVID.8
Long COVID is a vacillating disease,9 characterized by a diverse range of symptoms spanning multiple organ systems, as depicted in Figure 1 and Graphical Abstract, and commonly includes fatigue, breathlessness, post-exertional malaise (PEM), brain fog, headaches, nausea, vomiting, anxiety, depression, skin rash, joint pain, and palpitations. Patient advocacy groups6 (e.g. long COVID SOS, COVID Advocacy Exchange, the National Patient Advocate Foundation COVID Care Resource Center, long-haul COVID fighters, Body Politic COVID-19 Support Group) have enhanced our understanding of this disease by drawing our attention to its multifaceted nature. Several experts10,11 have noted its marked similarities with other post-viral symptoms (e.g. Epstein-Barr,12 human herpesvirus,13 influenza, SARS,14 and Ebola viruses15), although few options exist for the management of such syndromes.
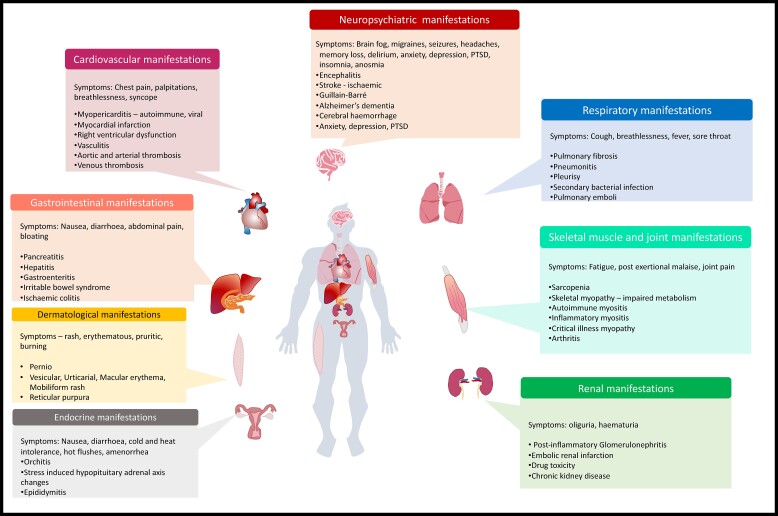
Figure 1.
Long COVID is characterized by a diverse range of symptoms and signs spanning multiple organ systems including the respiratory system, neurological system, cardiovascular system, gastrointestinal system, dermatological system, endocrine/genitourinary systems, and skeletal muscle/joints as illustrated here. PTSD, post-traumatic stress disorder.
The reported prevalence of long COVID has varied across and within many countries: UK 1.6–71%,16–19 Germany 35–77%,20,21 China 49–76%,22,23 Africa 68%,24 India 22%,25,26 Bangladesh 16–46%,27,28 Denmark 1%,29 Italy 5–51%,30,31 USA 16–53%,32,33 Norway 61%.34 Studies assessing hospitalized patients have typically reported higher prevalence estimates (e.g. 76% in Huang et al.,22 71% in Evans et al.19) when compared with community studies (e.g. Sudre et al.16), reflecting the complex relationship between severity of acute illness, higher burden of co-morbidities, and persistent symptoms. Differences in the study population may explain the vast disparity in prevalence estimates across the various studies. The timing of assessment also appears to be important as symptom frequency can diminish over time from the infection. Hossain et al. 28 reported a reduction in the burden of long COVID symptoms which affected 21.2% of their cohort at 4 weeks and 16.5% by 12 weeks post-COVID diagnosis. A similar temporal improvement in symptom burden was also observed by Wu et al.35 and Cassar et al.36 Varying definitions of long COVID may also affect the relative frequencies. Mahmud et al.27 defined long COVID as the persistence of symptoms beyond 2 weeks (i.e. time taken for viral clearance) and reported a symptom prevalence of 46%. In contrast, more conservative definitions such as the one used by the UK Office of National Statistics (requiring the presence of functional limitation and exclusion of symptoms explained by co-morbidities) have resulted in lower prevalence estimates. The study design is also likely to be relevant as retrospective reporting from electronic healthcare records suffers from ascertainment bias, while prospective studies with comprehensive assessments37 are likely to attract patients with a high burden of symptoms seeking an explanation. In addition to these, disparities in vaccinations, SARS-CoV-2 variants, co-morbidities, study sample size, and use of varying non-COVID control groups appear to drive heterogeneity in prevalence estimates, as illustrated in Figure 2.
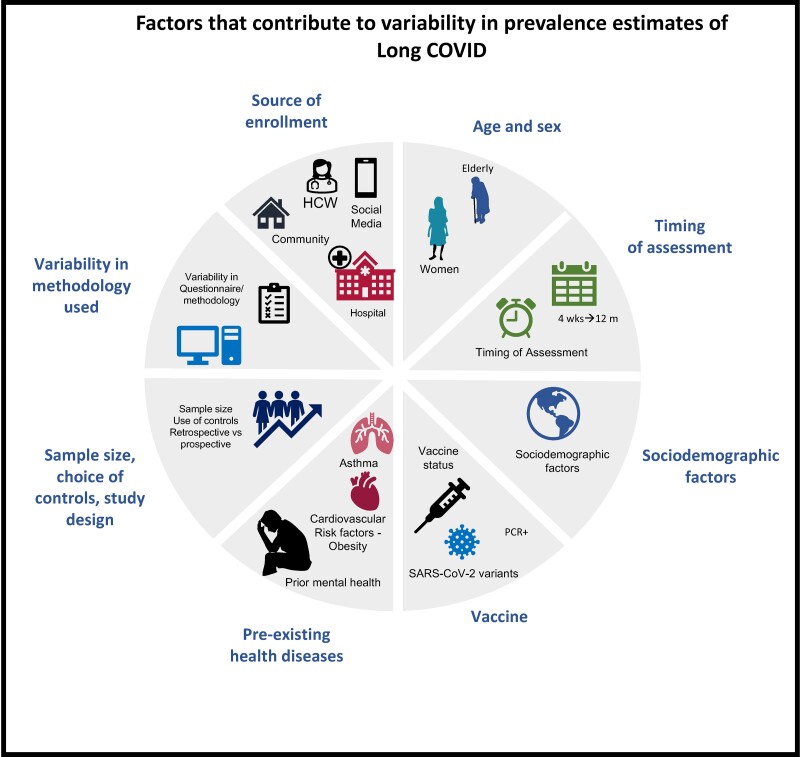
Figure 2.
Factors that contribute to variability in prevalence estimates of long COVID. Prevalence estimates of long COVID are highly variable across studies due to a number of factors that introduce bias. These include differences in cohort characteristics, age, and sex of subjects enrolled, timing of assessment, sociodemographic factors, vaccines and variants, pre-existing health problems, sample size, study design, and variability in questionnaires or tools used. HCW, healthcare workers; m, months; PCR, polymerase chain reaction; wks, weeks.
Contrary to the variability seen in reported disease prevalence, risk factors for long COVID tend to be fairly consistent, with female sex, escalating age, obesity, asthma, poor general health, poor pre-pandemic mental health, poor sociodemographic factors emerging as important determinants across several studies.16–18,38–40 In particular, the impact of nationwide lockdowns, remote working, and limited physical activity on pre-existing trends of an increasingly obese population with poor dietary intake and physical activity patterns is noteworthy.41–43 According to national statistics data from the UK (from 2019),44 among adults 16 and over, a staggering 68% of men and 60% of women were either overweight or obese. Obesity increased across all age groups up to 75 years old. In a separate report by the national child measurement programme from the UK, one in three children leaving primary school were noted to be overweight or obese and one in five obese in 2019.45 A recent update from the American Heart Association (AHA)46 on stroke and CV disease has also highlighted the high prevalence of obesity, metabolic syndrome, poor dietary habits, and physical inactivity among children and adults from the US. It is now well established that obesity and other cardiometabolic risk factors commonly promote inflammation and endothelial dysfunction,47,48 which may lower the cardiometabolic reserve and threshold for exertional symptoms. Consistent with this, numerous population and prospective cohort studies have documented an independent link between obesity and long COVID.16,19,38 Thompson et al.,38 in a prospective study of 6907 patients (mean age 19–63 years), reported that being overweight or obese was associated with a 25% higher likelihood of long COVID than not belonging to this category. Similarly, Sudre et al. 16 reported that patients with prolonged symptoms were more likely to be obese than those without.
Cardiopulmonary symptoms including chest pain, dyspnoea, fatigue, palpitations, and cough are common among long haulers. In one UK study,16 13.3% of 4182 symptom app users (predominantly community patients) experienced at least one persistent symptom beyond 4 weeks of infection, of which half were thought to be cardiac in origin. In December 2020, the UK ONS49 provided similar estimates of long COVID prevalence, though a recent analysis with a stricter case definition (symptoms explained by co-morbidities were not counted) by the ONS18,50 and other groups29 suggests lower prevalence estimates (1.2–1.5%). Patient-led or survey-based research from long COVID support groups37,51 has also provided insights into the longitudinal trajectory of persistent symptoms. In an international online survey study undertaken by Davis et al. 37 of 3762 patients, cardiac symptoms including chest pain (∼53%), palpitations (∼68%), fainting (∼13%) were observed in up to ∼86% of patients by 7 months from infection. Postural orthostatic tachycardia syndrome (POTS), characterized by an increase in heart rate of at least 30 b.p.m. from supine to standing position, was noted in 31% of patients.52 Ziauddeen et al. 51 similarly studied the prevalence of long COVID among 2550 patients using a social media survey. Cardiopulmonary symptoms were reported by 89% of participants in their study.
There are now several prospective follow-up studies of hospitalized patients. One of the earliest published reports of long COVID came from an Italian study of hospitalized patients by Carfi et al.,53 demonstrating a particularly high burden of cardiopulmonary symptoms (>43%). In a subsequent follow-up study of 1733 hospitalized patients from Wuhan, China, Huang et al. 22 observed that at 6 months post-infection, 63% of patients reported fatigue, 26% breathlessness, and 5–9% experienced chest pain and palpitations. By 12 months,23 investigators of the same study showed that symptoms of breathlessness (30%) and chest pain (7%) were slightly more common, while fatigue had improved (20%). Evans et al. 19 from the UK also undertook a follow-up study of 1077 hospitalized patients. At a median of 5 months post-discharge, 48% reported persistent fatigue, 41% dyspnoea, and 21–28% chest pain and palpitations.
Long COVID has been proposed to be a form of chronic fatigue syndrome (CFS)/myalgic encephalitis.52 While there are marked similarities between the two, subtle yet important differences exist. Central to the diagnosis of CFS is the observation that patients experience PEM,54,55 defined as fatigue following even minor physical or mental exertion. For a diagnosis of CFS, symptoms should last for a minimum of 6 months and occur at least 50% of the time. Current definitions of long COVID do not obligate the presence of PEM, with symptom duration yet to be defined. A further point to note is that, while the evidence for exercise rehabilitation in CFS patients is mixed, early positive data supporting tailored rehabilitation in previously hospitalized patients with long COVID are emerging with some improvement seen in exercise capacity and cognition at 4 months from discharge.56 Finally, breathlessness, one of the most common long COVID symptom, is not essential for the diagnosis of CFS and may be due to distinct mechanisms. Nonetheless, the similarities between CFS and long COVID are striking, with both syndromes having links to psychological and neurostructural/metabolic alterations57–59 (e.g. grey matter volume reduction in the limbic cortex in post-COVID patients60), highlighting the potential benefits of neuroprotective interventions in such patients.
Possible mechanisms underlying cardiac manifestations
Given the abundance of cardiac symptoms among patients with long COVID, a deeper discussion of potential mechanisms underlying cardiac injury is warranted.
Acute phase
The role of angiotensin-converting enzyme 2 receptors in SARS-CoV-2 involvement of the heart is now well established. Several mechanisms have been proposed to contribute to myocardial injury including direct cytotoxic injury,1 dysregulation of renin–angiotensin–aldosterone system,3 endotheliitis and thromboinflammation, and4 dysregulated immune response with cytokine release.6
Autopsy studies
The pattern of myocardial injury following SARS-CoV-2 infection derived from autopsy studies suffers from referral bias but has provided initial pathophysiological clues. In an early autopsy series of 80 consecutive SARS-CoV-2 PCR positive cases,61 only four patients (5%) had suspected cardiac injury. Two patients had co-morbid conditions and died of sudden cardiac death. One had acute myocardial infarction and another exhibited right ventricular lymphocytic infiltrates. These early results suggested that extensive myocardial injury as a primary cause of death may be infrequent.
In a subsequent multicentre autopsy study, Basso et al. 62 characterized the hearts in 21 selected autopsies. Myocarditis (defined as lymphocytic infiltration and myocyte necrosis) was evident in 14% of cases, interstitial macrophage infiltration in 86%, and pericarditis and right ventricular injury in 19%, respectively. Halushka and Vander Heide63 performed a review of 22 publications describing the autopsy results of 277 patients. Lymphocytic myocarditis was reported in 7.2%; however, only 1.4% met the well-established histological criteria64 for myocarditis, suggesting that true myocarditis was rare.65–67 In another study, Lindner et al.68 demonstrated the presence of SARS-CoV-2 viral particles in the hearts of 24/39 (59%) consecutive autopsies; the viral load was clinically relevant in 16/39 (41%). Of note, viral particles were not isolated within cardiomyocytes, but rather in interstitial cells including pericytes and macrophages. The high viral load in some cases was also not associated with inflammation, consistent with the low prevalence of myocarditis on autopsy studies.67
Microvascular injury due to SARS-CoV-2
Cardiac troponin levels are frequently elevated in COVID-19 patients,69 indicating myocardial injury and/or ischaemia. The work of Bois et al.70 appears to support the concept of microthrombi occurring in association with COVID-19. In a small series of 15 individuals, the authors observed that post-mortem fibrin microthrombi were more common (80%) than acute ischaemic injury (13%) and myocarditis (33%) suggesting a role for thrombosis in accentuating myocardial injury.
Fox and Vander Heide65 have conceptualized the array of pathophysiological mechanisms underlying myocardial injury. The authors proposed that hypoxia and pulmonary microvascular damage may lead to right heart stress and myocyte necrosis. The latter may be further augmented through localized microvascular effects, endotheliitis,71 associated microthrombi, and altered renin–angiotensin homeostasis.72 Elevated cytokines73,74 [e.g. interleukins (IL)-1, IL-16, IL-17, IL-22, interferon (IFN)-γ, tumour necrosis factor (TNF)-α] could also contribute to myocardial injury by inducing endothelial dysfunction, activation of platelets, recruitment of neutrophils, and eventually triggering a hypercoagulable state. In this framework, viral myocarditis would play an infrequent role in SARS-CoV-2 infection.
Post-acute and chronic phase
Mechanisms for enduring cardiac damage post-acute illness are still poorly understood. One possible explanation is a chronic inflammatory response evoked by persistent viral reservoirs in the heart following the acute infection,75 which may, in turn, be exacerbated by obesity-related inflammatory signalling driven in part by perivascular adipose tissue via the release of adipokines such as monocyte chemoattractant protein-1 and Regulated upon Activation, Normal T Cell Expressed, and Presumably Secreted, chemokines that aggravate endothelial dysfunction via endothelial nitric oxide synthetase uncoupling and reactive oxygen species production.76 An unintended consequence of such processes would be insidious tissue damage, followed by chronic myocardial fibrosis leading to impaired ventricular compliance, impaired myocardial perfusion, increased myocardial stiffness, reduced contractility and potential arrhythmias.
A second mechanism for delayed damage is an autoimmune response to cardiac antigens through molecular mimicry.77 High-throughput proteome analysis by Wang and others78–80 has identified a range of autoantibodies to humoral and tissue antigens in patients with severe COVID-19. Autoantibodies to cholinergic and adrenergic receptors have also been detected in individuals with CFS.81,82 Recently, a number of longitudinal cytokine profiling and proteomic studies83,84 have revealed an increased expression of prothrombotic factors (e.g. factor VIII, prothrombin, plasminogen activator inhibitor-1) beyond the acute infection. This is in keeping with the burgeoning reports of delayed embolic complications.85–87 The high prevalence of pulmonary vascular thrombosis (5–30%),87,88 particularly in hospitalized patients, is also expected to heighten the future risk of chronic thrombo-embolic pulmonary hypertension.89 Endothelial dysfunction90 and its complications may also develop in patients, with evidence of persistent impairment detected in younger individuals 3–4 weeks after SARS-CoV-2 infection.91 The Graphical Abstract summarizes the relevant pathophysiological mechanisms for acute and chronic cardiac injury secondary to SARS-CoV-2 infection and potential long-term consequences. It is worth noting that many of these complications mirror those encountered by survivors of other epidemics caused by SARS, Middle East respiratory syndrome (MERS), H1N1A, underscoring the need to recognize the impact of respiratory viral infections on CV health as previously outlined by Xiong et al. 92 in an earlier review.
Post-acute COVID-19 cardiovascular sequelae
The high mortality and poor outcomes associated with myocardial injury93–97 during acute COVID-19 infection have galvanized an interest among research communities to characterize the long-term CV effects of SARS-CoV-2 infection. Insights from both prospective and retrospective studies continue to shape our understanding of its long-term effects. While retrospective studies rely on electronic medical health records and labelled datasets,7,34,98 prospective studies19,22 have innovatively turned to remote (e.g. telemedicine, symptom apps) assessments and face–face reviews.
Retrospective cohort studies
There is now compelling evidence from large retrospective cohort studies that speak to the rising cases of new cardiac diagnoses. In a study of 73 435 (median age 61 years, 88% men) non-hospitalized patients using the US Department of Veterans Affairs health services, Al-Aly et al. 7 demonstrated a high risk of death and incident CV and metabolic diseases associated with COVID-19 beyond 30 days of infection. A UK-based study of 47 780 hospitalized COVID-19 patients (mean age 65 years, 55% men) demonstrated that a diagnosis of COVID-19 was linked to a three-fold increased risk of major adverse CV events up to 4 months from diagnosis (vs. non-hospitalized controls). In this study, Ayoubkhani et al. 98 further noted that the increased risk was not confined to the older age group and was more pronounced in non-White patients. Daugherty et al.,99 in a related paper, compared the incidence of new cardiometabolic diagnoses in post-COVID-19 patients with two important controls groups—non-COVID-19 controls (from 2019 to 2020) and those recovering from lower respiratory tract infection (LRTI). In their study, COVID-19 was associated with a nearly two-fold increased risk of incident CV diagnoses. However, when comparisons were made with LRTI controls, the excess risk of cardiomyopathy was no longer significant. These findings are in line with another study that used primary healthcare data in the UK (OpenSAFELY platform). Tazare et al.100 revealed that the excess risk of major adverse CV events among previously hospitalized COVID-19 patients was similar to patients admitted with a diagnosis of pneumonia, although the risk of developing type 2 diabetes was higher after COVID-19.
Prospective studies
In the post-acute period, cardiac abnormalities have been reported in several prospective observational studies.101–134 A summary of selected studies (n > 50) employing three widely used investigative tools—echocardiography, cardiac magnetic resonance (CMR), and cardiopulmonary exercise test—is provided in Table 1 101,102,109,112,114–116,120–122,124,125,128,130,131,133,135 and highlights the vast heterogeneity in the prevalence of abnormalities.
Table 1.
Prevalence of cardiac abnormalities in studies (n > 50) that utilized echocardiography, cardiac magnetic resonance, and cardiopulmonary exercise test during follow-up of COVID-19 patients
| First author | No. of patients | Age | Patient characteristics | Follow-up time | Controls | Cardiopulmonary symptoms | Echo findings |
|---|---|---|---|---|---|---|---|
| Echocardiography | |||||||
| Hall et al.135 | 200 | 55 ± 15 years; 62% male | Hospitalized patients; 27.5% mechanical ventilation | 4–6 weeks post-discharge | – | 18% new-onset/worsening of dypsnoea | 14% had either newly diagnosed or previously present abnormalities |
| Sechi et al.130 | 105 | 57 ± 14 years; 53% male | Hospitalized; 26% mechanical ventilation | Median 41 days post-symptom onset | 105 matched controls | 5% chest pain, 5% dyspnoea, 7% fatigue | No cardiac abnormalities |
| Catena et al.116 | 105 | 57 ± 14 years; 53% male | Hospitalized patients; 26% mechanical ventilation | Median 41 days post-symptom onset | – | 5% chest pain, 5% dyspnoea, 7% fatigue | No differences in cardiac function between troponin+ and troponin− COVID-19 patients |
| de Graaf et al.131 | 81 | 61 ± 13 years; 63% male | Hospitalized patient; 41% mechanical ventilation | 6 weeks post-discharge | – | 62% dyspnoea, 14% chest pain, 32% limited functional status | 18% LV dysfunction, 10% RV dysfunction |
| Moody et al.125 | 79 | 57 ± 11 years; 74% males | Hospitalized patients; 80% mechanically ventilated | 3 months post-discharge | – | – | 9% LV dysfunction, 14% RV dysfunction, 3% dilated LV, 9% dilated RV, 4% pericardial effusion |
| Sonnweber et al.109 | 145 | 57 ± 14 years; 57% males | 75% hospitalized; 22% ICU admission | 60 days and 100 days post-symptom onset | – | 36% dyspnoea | 3% LV systolic dysfunction—60 and 100 days, 55% diastolic dysfunction—60 days, 60% diastolic dysfunction—100 days, 10% pulmonary hypertension—60 and 100 days. Pericardial effusion 6% at 60 days and 1% at 100 days |
| CMR | |||||||
| Kotecha et al.101 | 148 | 64 ± 12 years; 70% male | Severe COVID-19 and elevated troponin; 32% mechanically ventilated | Median 68 days post-discharge or confirmed diagnosis | 40 co-morbidity matched and 40 healthy | No symptoms | 11% LV dysfunction, 26% myocarditis, 23% ischaemia/infarction, 6% had dual pathology |
| Puntmann et al.122 | 100 | 49 ± 14 years; 53% male | 67% non-hospitalized | Median 71 days post-positive COVID-19 test | 50 healthy and 57 co-morbidity matched controls | 36% breathlessness, 17% chest pain, 20% palpitations | 60% myocardial inflammation, 78% any abnormality including LV, RV dysfunction, late gadolinium enhancement, and pericardial enhancement |
| Raman et al.120 | 58 | 55 ± 13 years; 59% male | Hospitalized patients; 21% mechanically ventilated | 2–3 months post-symptom onset | 30 co-morbidity matched controls | 89% cardiopulmonary symptoms | No evidence of active myocardial oedema, no significant difference in scar burden with controls. Native T1 was elevated in 26% |
| Dennis et al.121 | 201 | 45 (21–71 years); 29% male | 19% hospitalized | Median 141 day post-symptom onset | 36 healthy controls | 98% fatigue, 88% breathlessness, 76% chest pain | 9% systolic dysfunction, 19% myocarditis |
| Zhou et al.112 | 97 | 47 ± 19 years; 54% male | Hospitalized patients (non-ventilated) | 2–4 weeks after discharge | – | – | All patients had echo. 1% LV dysfunction. CMR in four patients. One had subepicardial hyper-enhancement with no elevated T2 |
| Joy et al.128 | 74 | 39 (30–48 years); 38% male | Healthcare workers with predominantly mild infection; 3% hospitalized | 6 months post-infection | 75 SARS-CoV-2 antibody negative healthcare workers | 11% symptomatic, 3% sore throat, 3% fatigue, 2% breathlessness | 4% myocarditis like scar |
| Knight et al.114 | 29 | 64 ± 9 years; 83% male | Hospitalized with elevated troponin, 34% mechanically ventilated | Mean 46 days post-symptom onset | – | – | 69% had pathology, 3% mild LV dysfunction, 3% severe biventricular dysfunction, 38% non-ischaemic injury, 17% ischaemic injury, 14% dual pathology, 7% pericardial effusion |
| Eiros et al.124 | 139 | 52 (41–57 years); 28% male | Healthcare workers; 16% hospitalized | Median 10.4 weeks post-symptom onset | – | 27% fatigue,19% chest pain, 14% palpitations | 75% had CMR abnormalities, 4% oedema on T2, 42% T1, 37% extracellular volume, 30% pericardial effusion, 5% LV dysfunction, 14% had pericarditis, 37% had myocarditis, 11% fulfilled criteria for both pericarditis and myocarditis |
| Myhre et al.133 | 58 | 56 (50–70 years); 56% male | Hospitalized; 19% mechanically ventilated | Median 175 days | 32 healthy controls | 64% fatigue, 55% dyspnoea, 4% chest pain | 21% had pathology on CMR, 5% LV dysfunction, 17% late gadolinium enhancement |
| CPET | |||||||
| Clavario et al.102 | 110 | 62 (54–69 years); 59% male | Hospitalized (excluded pts requiring mechanical ventilation/ICU) | 3 months post-hospital discharge | – | 74% at least one symptom. 50% dyspnoea, 26% chest pain, 49% fatigue, 23% palpitations | Median predicted pVO2 90.9 (79.2–109). 35% had pVO2 < 80% predicted. DLE maximal strength independently associated with pVO2. 24% had cardiac limitation to exercise, 8% respiratory and cardiac, 47% non-cardiopulmonary limitation |
| Rinaldo et al.115 | 75 | Mean 57 years; 57% males | Hospitalized (39 critical, 18 severe, 18 mild–moderate disease) | Mean 97 days from discharge | – | 52% had dyspnoea with normal activity | Mean pVO2 83% of predicted. 55% pVO2 < 85% of predicted. VE/VCO2 slope 28 ± 13. Patients with reduced exercise capacity had normal breathing reserve, 17% had circulatory limitation (heart rate reserve <15%), 20% reduced AT (<45%). Patients with a reduced exercise capacity showed an early AT, indicating a higher degree of deconditioning, lower peak oxygen pulse, reduced VO2/WR slope |
| Raman et al.120 | 58 | 55 ± 13 years; 59% male | Hospitalized patients at 3 months from symptom onset | 3 months from symptom onset | 30 co-morbidity matched controls | 83% had at least one cardiopulmonary symptom | 55% had pVO2 < 80% predicted, VE/VCO2 slope 33.29–40 HRR in first minute was slower in patients compared with controls |
Data are presented as mean ± standard deviation or median (interquartile range).
AT, anaerobic threshold; BMI, body mass index; COVID, coronavirus disease; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise test; DLco, carbon monoxide gas transfer; GLS, global longitudinal strain; HRR, heart rate recovery; ICU, intensive care unit; LV, left ventricle; PCR, polymerase chain reaction; pVO2, peak oxygen consumption; RV, right ventricle; PAP, pulmonary artery pressure; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VE/VCO2, slope ventilatory equivalent for carbon dioxide; WR, work rate.
Numerous studies have evaluated the role of 12-lead electrocardiogram (ECG) in screening patients for post-acute cardiac manifestations,36,103,117,124,136 although commonly pre-COVID-19 control ECGs are missing. Dynamic ECG changes (e.g. depolarization, repolarization abnormalities, arrhythmias)137,138 while frequent during acute illness, tend to resolve in the majority of hospitalized patient by 6 months post-acute COVID-19 and are often comparable to risk-factor matched controls.36,103,117,136 Nevertheless, sinus arrhythmia is frequent in the post-acute phase and manifests as transient or sustained periods of sinus tachycardia or bradycardia.103–105 In a study of 234 patients, Radin et al. observed that 13.7% of patients wearing a Fitbit displayed persistently elevated heart rate (>5 beats above the pre-COVID baseline resting heart rate) up to 133 days post-infection.139 Currently, there are no published studies on the role of prolonged ECG monitoring (Holter) in post-COVID-19 management. Prior studies of post-influenza patients have demonstrated a high burden of atrial140 and ventricular arrhythmias, which are known to correlate with inflammatory markers.141–145 These findings imply that a subset of COVID-19 patients (e.g. with ongoing inflammation) may potentially benefit from ECG monitoring in the long term.
Both transthoracic echocardiography and CMR are cornerstones in the diagnosis of acute and chronic cardiac pathology.146,147 Cardiac abnormalities commonly reported on follow-up imaging include myopericarditis, right ventricular dysfunction, and ischaemia/infarction.
Myopericarditis may be suspected clinically based on the presence of one clinical (pericarditic chest pain, heart failure or progression of heart failure symptoms, palpitations, syncope, new-onset arrhythmia) and one diagnostic criteria (ECG abnormalities, troponin elevation, wall motion abnormalities on echocardiogram, CMR abnormalities) as per the 2013 European Society of Cardiology (ESC) Position statement148 and 2020 AHA scientific statement.149,150 While endomyocardial biopsy (EMB) is the gold standard investigation for histological evaluation of suspected fulminant cases,148–150 CMR remains the best alternative for non-invasive evaluation of stable cases149,151 and provides information on several pathological processes including myocardial oedema, hyperaemia, necrosis, and fibrosis by exploiting alterations in fundamental magnetic properties of the tissue (T1 and T2 relaxation). In 2018, the Journal of American College of Cardiology scientific expert panel provided recommendations for updated CMR criteria to improve detection of active myocarditis.151 This required an increase in at least one T1-based method including T1 mapping (sensitive to hyperaemia, fibrosis, necrosis, and oedema) and one T2-based method including T2 mapping (sensitive to oedema).151 Although the incorporation of quantitative tissue mapping has augmented the sensitivity of CMR for myocarditis, two limitations exist with such an approach. The first is that CMR diagnostic performance may be impacted by co-morbidities.152,153 The second is that readouts of T1 and T2 values of the myocardium are poorly standardized.154 As a result, combining quantitative CMR data from multiple centres is problematic.
In June 2020, a follow-up CMR study of 100 patients (67% non-hospitalized) reported an alarmingly high rate (60%) of persistent myocardial inflammation at 71 days post-infection.122 At least 22% of patients in this study were found to be co-morbid. Other studies have also reported a high prevalence of CMR abnormalities, though comparator groups were typically healthy making it challenging to rule out confounding effects of co-morbidities such as hypertension and diabetes. In contrast to these early studies, a subsequent study128 of healthcare workers, which enrolled co-morbidity matched controls with mild infection, reported a lower prevalence of CMR abnormalities, with no significant difference in 6-month CMR tissue abnormalities between seropositive and seronegative healthcare workers. Similarly, a study of 1285 UK Biobank participants155 with pre- and post-SARS-CoV-2 infection imaging revealed no link between prior infection and longitudinal changes in cardiac or aortic phenotypes before and after adjusting for potential confounders including co-morbidities.
There are now abundant studies evaluating the burden of myocarditis among athletes in view of associated risks of sudden cardiac death. The majority was undertaken within 1–2 months of infection105,126,127,129 and the prevalence of myocarditis on objective testing was found to be generally low (0–3%). Studies evaluating persistent symptom burden among athletes beyond 8 weeks of infection are lacking, though it is likely, given the lower risk profile of athletes (e.g. less likely to be obese and co-morbid), that ongoing symptoms are infrequent in this population.
The role of CMR in elucidating a cause for elevated troponin following acute COVID-19 is less contentious. Several prior studies156–158 have confirmed its incremental value in clarifying a diagnosis when the aetiology of troponin elevation is unclear. In a study of 148 troponin-positive patients, Kotecha et al. 101 reported that 26% had myocarditis, 22% had inducible ischaemia/infarction, and 6% had evidence of both.
Echocardiography is vital in the early diagnosis of cardiac pathology in COVID-19 infection (suspected myocarditis, Takotsubo syndrome, myocardial infarction, pericardial effusion, etc.), particularly where haemodynamic stability is uncertain.69,159 Right ventricular dilatation and dysfunction are the most common echocardiographic abnormalities with prognostic significance.125,160,161 When the acute infection abates,161 right ventricular abnormalities improve in most patients.36,125 Left ventricular systolic dysfunction is comparatively less frequent.101,125 Follow-up echo and CMR studies have confirmed that even among those with severe acute infection125 or elevated troponin,101 systolic impairment is rare, affecting up to 9–11% of patients. Concordant with this, in the UK-wide national follow-up study (PHOSP-COVID), levels of N-terminal pro B-type natriuretic peptide (NT-proBNP) were abnormal in only 7% of patients at 5 months post-hospitalization.19 Unlike systolic dysfunction, abnormalities in diastology may be common (up to 60% of hospitalized patients).109 The lack of pre-COVID imaging, however, makes it challenging to disaggregate what is cause and effect in patients.
Computed tomography (CT) angiography (CTA) has garnered considerable attention for its ability to detect pulmonary emboli,162–164 epicardial coronary stenoses, or vascular pathology (e.g. mural thrombus or vasculitis) related to acute SARS-CoV-2 infection. Perivascular fat attenuation index,165,166 a biomarker of vascular inflammation on CTA, has shown promise for prognostic risk stratification. In an early study by Kotanidis et al.,167 a new radiotranscriptomic signature of vascular inflammation demonstrated an association between SARS-CoV-2 variant B1.1.7, vascular inflammation, and increased mortality risk. Delayed arterial and venous thrombo-embolic complications168–170 have also been reported in the post-acute period. Dual-energy CTA, in a study of 55 patients at 3 months post-infection, detected both proximal arterial thrombosis (5.4%) and distal microangiopathy (65.5%) in a significant proportion of symptomatic patients.171
Cardiopulmonary exercise testing has shed light on the relevant pathophysiological brakes applied by COVID-19 on exercise capacity.36,102,106–108,115,118–120 Several studies have demonstrated a reduction in peak oxygen consumption post-acute COVID-19.36,102,106,115,120,172 The predominant mechanism for this finding seems to be muscular deficiencies (impairment in oxygen extraction173), manifesting primarily as submaximal exercise tests or an early anaerobic threshold. Generalized muscle wasting or sarcopenia is also common.174,175 Physical inactivity, cytokine storm, poor nutrition, intensive care therapy, mechanical ventilation, and drugs (e.g. dexamethasone) have all been implicated. The ratio of exercise minute ventilation coupled with carbon dioxide output (VE/VCO2), a marker of ventilatory efficiency, may also be abnormal.36,102,120 However, breathing reserve is relatively preserved, arguing against pulmonary factors and supporting hyperventilation or dysfunctional breathing as a potential cause.172 Heart rate recovery provides a surrogate measure of autonomic health in patients. Following COVID-19, delayed heart rate recovery has been noted in some patients,36,176 though the majority recovers spontaneously over time.36
There are now numerous reports177–181 and cohort studies38 where POTS has been suspected among patients. In a retrospective study of 20 patients182 referred to a dysautonomia clinic, orthostatic instability on tilt table test or 10 min stand test was observed in 75% of patients. Postural orthostatic tachycardia syndrome was the most common diagnosis in this study, followed by neurocardiogenic syncope (15%) and orthostatic hypotension (10%).
A table summarizing all the relevant cardiac investigations, their advantages, and role in post-COVID management can be found in the Supplementary material online, Table S1.
Cardiovascular injury in the context of multiorgan involvement
Understanding cardiac involvement in the context of multisystem health can provide clues into mechanisms of ongoing injury through recognition of unique patterns of tissue damage (e.g. inflammatory changes or embolic manifestations). In an early study of 58 post-hospitalized COVID-19 patients and 30 matched controls, Raman et al.120 undertook multiorgan magnetic resonance imaging (MRI) and reported tissue abnormalities involving the lungs (60%), heart (26%), liver (10%), kidneys (29%), and brain (11%) in patients. Magnetic resonance imaging abnormalities in almost every organ correlated with inflammatory markers, suggesting that chronic inflammation could impede recovery. Following on from this work, the PHOSP-COVID study also demonstrated that failure to recover from multiorgan symptoms was associated with markers of persistent inflammation.19 In another study of 201 patients, Dennis et al. 121 evaluated the prevalence of multiorgan damage among predominantly non-hospitalized patients and noted that symptoms of long COVID clustered among those with multiorgan injury on MRI.
Persistent endothelial dysfunction,47,183,184 microvascular dysfunction,101 and prothrombotic tendencies185 may also contribute to multiorgan dysfunction.186 Selected studies of advanced imaging modalities including positron emission tomography, CT, and MRI have noted perfusion deficits in the heart and lungs101,187,188 of COVID-19 survivors at ∼40–60 days from infection. In one study of hospitalized patients, multiorgan MRI demonstrated evidence of small vessel disease (9.3%) and ischaemic changes (3.7%) in the brain (9.3%) and 1.9% had myocardial infarction120 2–3 months post-infection. Another study of 104 hospitalized189 patients observed that inducible myocardial perfusion defects were common among patients with moderate to severe disease but did not differ in burden compared with co-morbidity and risk-factor matched controls. Several studies are currently underway to characterize the burden of vascular and thrombotic complications190,191 and to examine the potential benefits of prolonged antithrombotic (extended thromboprophylaxis) and vascular protective therapies (e.g. statins, risk-factor management) in post-acute COVID-19 patients as indicated in Table 2 and Supplementary material online, Table S2.
Table 2.
Examples of prospective clinical trials evaluating cardiovascular outcomes beyond 4 weeks of COVID-19
| Category | Title | Interventions | Outcome measures | Sponsor/collaborators | Enrolment | Funded by | |
|---|---|---|---|---|---|---|---|
| NCT04324463 | Cardiovascular | Anti-Coronavirus Therapies to Prevent Progression of Coronavirus Disease 2019 (COVID-19) Trial (ACTCOVID-19) |
Colchicine Aspirin Rivaroxaban |
Colchicine v control: 45 days composite of hospitalization and death, disease progression, composite of MACE Aspirin vs. control: 45 days composite of hospitalization and death, disease progression, composite of MACE Aspirin and rivaroxaban vs. control: 45 days composite of hospitalization and death, disease progression, composite of MACE |
Population Health Research Institute, Bayer | 4000 | |
| NCT04381936 | Cardiovascular | Randomized Evaluation of COVID-19 Therapy (RECOVERY) |
Aspirin Colchicine Empagliflozin Anakinra Steroids |
Primary and secondary outcome measures are 28-day mortality, need for ventilation, and hospital stay. Other outcome measures including risk of thrombotic event up to 6 months after randomization. |
University of Oxford UK Research and Innovation, National Institute for Health Research, UK Wellcome, Bill and Melinda Gates Foundation, Department for International Development, UK, Health Data Research, UK Medical Research Council, Population Health Research Unit NIHR Clinical Trials Unit Support Funding, NIHR Health Protection Research Unit in Emerging and Zoonotic Infections |
45 000 | UK |
| NCT04662684 | Cardiovascular | Medically Ill Hospitalized Patients for COVID-19 THrombosis Extended ProphyLaxis with Rivaroxaban ThErapy: The MICHELLE Trial (MICHELLE) | Rivaroxaban |
Primary outcome measures are 35 days post-hospital discharge VTE and VTE-related death. Secondary outcome measures are 35 days post-hospital discharge bleeding and other outcome measures which are composite of myocardial infarction, stroke, arrhythmia, heart failure and all-cause death. |
Science Valley Research Institute Bayer |
320 | Brazil |
| NCT04406389 | Cardiovascular | Anticoagulation in Critically Ill Patients With COVID-19 (The IMPACT Trial) (IMPACT) |
Enoxaparin Unfractionated heparin Fondaparinux Argatroban |
Primary outcome measure 30-day mortality, secondary outcome measures include 6-month incidence of VTE, length of ICU stay, number of major bleeding events | Weill Medical College of Cornell University | 186 | USA |
| NCT04486508 | Cardiovascular | Intermediate-dose vs. Standard Prophylactic Anticoagulation and Statin vs. Placebo in ICU Patients With COVID-19 (INSPIRATION) |
Enoxaparin Unfractionated heparin (intermediate-dose) Atorvastatin Matched placebo |
Primary outcome measure is 30 days composite of acute VTE, arterial thrombosis, mortality, treatment with ECMO Secondary outcome measures include 30-day MACE, arrhythmia, mortality, major bleeding, thrombocytopenia, raised liver enzymes, atrial fibrillation; 60, 90 days post-COVID-19 functional status |
Rajaie Cardiovascular Medical and Research Center, Brigham and Women's Hospital, Tehran Heart Center, Masih Daneshvari Hospital Hazrat Rasool Hospital,Modarres Hospital, Firuzgar hospital affiliated to Iran University of Medical Sciences, Imam Khomeini Hospital Sina Hospital, Iran, Tabriz University of Medical Sciences Shariati Hospital, Imam Ali Hospital Labbafinejhad Hospital |
600 | Iran |
| NCT04900155 | Cardiovascular | Evaluation of the effect of long-term lipid-lowering therapy in STEMI patients with Coronavirus Infection COVID-19 (CONTRAST-3) |
Atorvastatin Atorvastatin and ezetimibe |
Primary outcome measure is 96-week lipid profile, ventricular rhythm disturbance, electrical instability and autonomic regulation, left ventricular systolic function, myocardial deformation, MACE | Penza State University | 200 | Russia |
| NCT04460651 | Cardiovascular | Prevention and Treatment of COVID-19 With EPA in Subjects at Risk-Intervention Trial (PREPARE-IT) |
Icosapent ethyl Placebo |
Primary outcome measure 60-day SARS-CoV-2 positivity, COVID-19 hospitalization; secondary outcomes measures include 60-day CRP, triglycerides, COVID-19-related hospitalization, 28-day non-fatal myocardial infarction and stroke |
Estudios Clínicos Latino América Amarin Pharma Inc. |
4093 | Argentina |
| NCT04505098 | Cardiovascular | A Pragmatic Randomized Trial of Icosapent Ethyl for High-Cardiovascular Risk Adults (MITIGATE) | Icosapent ethyl | Primary outcome measure is percentage of people with moderate to severe viral upper respiratory tract infection, worse clinical status. Secondary outcome measures included 12-month mortality, MACE, heart failure |
Kaiser Permanente Amarin Corporation |
16 500 | California |
| NCT04350593 | Cardiovascular | Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) |
Dapagliflozin Placebo |
Primary outcome measure 30-day organ dysfunction, heart failure, respiratory decompensation, ventricular tachycardia, vasopressor therapy, renal replacement therapy. Secondary outcome measure 30 days time to hospital discharge, days alive and free from respiratory decompensation |
Saint Luke's Health System Saint Luke's Hospital of Kansas City AstraZeneca George Clinical Pty Ltd |
1250 | USA |
| ISRCTN11721294 | Cardiac | Rehabilitation for cardiac arrhythmia | Rehabilitation | Primary outcome measure: autonomic function measured using 12-lead ECG Holter device to record the heart activity for 10 min and 24 h at baseline and after rehabilitation programme (6 weeks) | Saudi Arabian | 110 | Saudi Arabian Cultural Bureau (SACB) |
COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ECG, electrocardiogram; ECMO, extracorporeal membrane oxygenation; MACE, major adverse cardiovascular events; NYHA, New York Heart Association; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; VTE, venous thrombo-embolism; STEMI, ST elevation myocardial infarction.
Chronic cardiovascular disease and impact of COVID-19
Up to a third of patients hospitalized with COVID-19 have a history of chronic CV diseases.192,193 Presence of concurrent cardiac disease is typically associated with higher in-hospital mortality, thrombo-embolic risk, and septic shock rates.97,194 Even in the post-acute period, patients with a history of heart failure are at a two- to four-fold risk of decompensation and mortality.195,196 Increased rates of heart failure exacerbation may present even beyond 30 days after SARS-CoV2 infection.98
One reason for the rising epidemic of post-discharge heart failure events is the withdrawal of guideline-directed medical therapy during acute illness.197 Investigators of the TRED-HF study198 have previously demonstrated the negative impact of heart failure therapy withdrawal in recovered dilated cardiomyopathy patients, resulting in relapse and poor outcomes. The successful resumption and optimization of heart failure therapies199 may therefore be important in halting heart failure readmissions post-acute COVID-19.
The shared cardiometabolic profile of COVID-19 and cardiac diseases implies that COVID-19 may play a role in destabilizing subclinical diseases (e.g. coronary artery disease and heart failure). This would explain the high prevalence of type 2 myocardial infarction200 in those with severe COVID-19 and also the rising incidence of ‘new’ CV diagnoses.7,98,99 Other mechanisms that may contribute include dysregulation of the renin–angiotensin–aldosterone system,6 endothelial dysfunction,186,201,202 renal injury,203 and steroid use.204,205
Proposed model for investigation and management of cardiovascular sequelae and long COVID
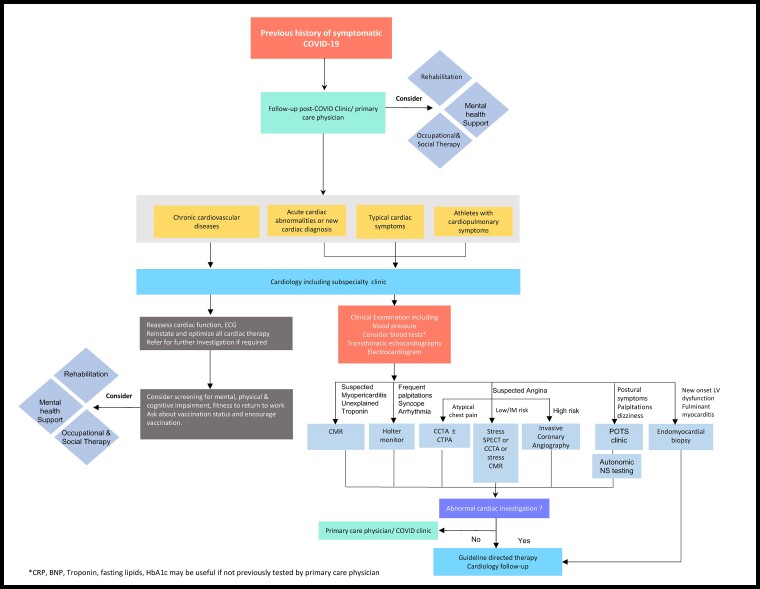
Although the true burden of CV pathology post-acute COVID-19 remains elusive, the prevalence of cardiac symptoms in this phase appears high. There is a strong need for evidence in support of cost-effective strategies to exclude significant CV pathology. An approach considered reasonable by some experts206–208 involves screening of high-risk individuals for ongoing cardiac involvement including those with abnormal cardiac investigations during the acute phase, new CV diagnosis post-COVID-19, and athletes. Figure 3 shows a possible pragmatic algorithm to guide physicians on the indication for cardiology follow-up and management approaches. Screening of high-risk individuals could comprise of a thorough history, clinical examination, blood test panel (C-reactive protein, troponin, B-type natriuretic peptide/NT-proBNP, glycated haemoglobin, lipids), ECG, and transthoracic echocardiography at least 8–12 weeks from infection. For patients with clinically significant abnormalities after the screening, additional testing is recommended. Non-invasive tests such as CMR, stress single positron emission computed tomography, Holter, coronary CTA can be considered following screening investigations; invasive coronary angiography or EMB may be indicated for high-risk individuals. Referral to specialist clinics (e.g. POTS, arrhythmia clinic, psychology support) should be considered where relevant. Patients with chronic CV diseases presenting for routine follow-up should be asked about their history of COVID-19 infection and vaccination status. A brief assessment of mental, physical, and cognitive health may be required for selected patients who report ongoing symptoms as this could facilitate early referral to appropriate services (rehabilitation,209 physiotherapy,210 psychology,211 occupational therapy, and social and welfare support) (Figure 3) and alleviate patient burden.
Figure 3.
Suggested possible algorithm for follow-up care and management of post-acute cardiovascular sequelae of COVID-19. Given the pressures imposed by a backlog of referrals to cardiology services during the COVID-19 pandemic, appropriate referral of patients post-acute COVID-19 is of utmost importance. This figure provides a suggested framework for referral of long COVID patients to cardiology services and the potential role of additional investigations in specific cases where appropriate. BNP, B-type natriuretic peptide; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance; CRP, C-reactive protein; CTPA, computed tomography pulmonary angiography; ECG, electrocardiogram; HbA1c, glycated haemoglobin; NS, nervous system; POTS, postural orthostatic tachycardia syndrome; Trop, troponin.
With regard to return-to-play guidance for athletes, numerous recommendations have been put forward by consensus societies.212–214 Although earlier guidelines adopted a conservative approach, recent studies of college and professional athletes have led to a revision126,129 in recommendations. Graded resumption to exercise and sports is now considered reasonable for mild infections, whereas exercise restriction for 3 months is still recommended for individuals with suspected myocarditis as per the 2019 position statement from the Sport Cardiology Section of the European Association of Preventive Cardiology.215
The management of COVID-19-related chronic myocarditis is a subject of considerable debate. In individuals with complicated (non-COVID) myocarditis (i.e. unexplained dilated left ventricle and severe dysfunction, ongoing troponin leak, advanced brady- or tachyarrhythmias), the ESC148 and AHA149 recommend EMB for clarification of myocarditis subtype to guide specific treatment options (e.g. immunomodulatory therapy vs. antivirals).150 Currently, there is no COVID-19 specific guidance on this, though several studies are underway to evaluate the most effective management strategy. The efficacy of oral non-steroidal anti-inflammatory drugs and/or colchicine is also being evaluated for COVID-19-associated pericarditis.216
For the management of post-COVID-19 acute coronary syndromes, patients are typically treated in accordance with the ESC217 and AHA218 guidelines released in 2020 and 2014, respectively. Similarly, heart failure management revolves around optimal utilization of contemporaneous therapies as per guidelines.219,220 There are currently no published trials on the efficacy of prolonged thromboprophylaxis post-acute COVID-19; however, numerous intervention trials (e.g. HEAL-COVID,221 STIMULATE ICP)216 are currently ongoing to address this gap.
After exclusion of significant CV and other organ pathology, the management of long COVID tends to be largely supportive.222 Given the strong association between obesity and long COVID, measures to reduce weight through caloric restriction, diet, tailored graded exercise, stress reduction, and good sleep hygiene could be beneficial in the long run,223 with growing evidence indicating its favourable effects on systemic inflammation,224 vascular dysfunction,225 and metabolic syndrome.226 Additionally, a pragmatic approach that is holistic and targeted at alleviating symptoms may also be required. Non-pharmacological approaches including pulmonary rehabilitation,227 breathing exercise,228,229 and alternative therapies230 (e.g. singing therapy,231 acupuncture, body rotation, and stretching) have also been suggested to help breathlessness symptoms. Those returning to work may benefit from phased return, allowing individuals with incomplete mental and physical recovery to gradually resume employment.232 Given that psychosocial factors are a major determinant of incomplete recovery,38,233 early referral for mental health assessment/cognitive behavioural therapy may benefit some patients.
Postural orthostatic tachycardia syndrome and symptoms of dysautonomia can be debilitating for patients.178–181 The management of POTS centres around accurate diagnosis following specialist assessment, correction of reversible causes (dehydration, heat), optimization of chronic disease management, and patient education. In some patients with ongoing palpitations, beta-blockers can be helpful in treating symptoms.234 Graded exercise programmes235 encouraging patients to adopt an upright posture may attenuate postural symptoms after prolonged bed rest. Compression panty-hose style stockings with 30–40 mmHg counter pressure may help symptoms of orthostatic hypotension through reduced peripheral venous pooling.236 In the event that symptoms persist despite compliance with the aforementioned approaches, pharmacological therapies (e.g. ivabradine, fludrocortisone, midodrine, clonidine, and methyldopa237) may be considered.
Special considerations
Impact of acute therapies
The vast majority of persistent CV abnormalities following COVID-19 is due to tissue injury sustained during the acute illness. The impact of acute therapies on long-term CV health deserves further investigation. Currently, anti-inflammatory drugs such as dexamethasone238 and tocilizumab239 or antivirals such as remdesivir240 have been identified as key weapons in the therapeutic armamentarium against severe COVID-19. However, the extent to which they affect cardiopulmonary recovery in the long term is still unclear and data regarding cardiac injury rates are not yet widely available. Whether or not ongoing inflammation in long COVID may reflect a rebound phenomenon in dexamethasone- or tocilizumab-treated patients also warrants further study. The complicated role of anticoagulation in patients deserves some consideration. In the acute phase, mounting evidence confirms a lack of benefit of aspirin in reducing mortality among hospitalized241 and non-hospitalized outpatients.242 Data in support of therapeutic dose anticoagulation are mixed with the severity of illness (non-critical hospitalized patients benefitting most) being a critical determinant of treatment success.243 The multiplatform adaptive randomized controlled clinical trial,244 which combined data from ACTIV-4a, REMAP-CAP, and ATTACC studies, reported an improved survival until hospital discharge and organ support-free days with therapeutic dose heparin in moderately ill patients but not during critical illness. In contrast, other studies (ACTION,245 INPIRATION,246 and RAPID trials)247 reported no difference in primary outcome measures among patients receiving therapeutic vs. prophylactic dose anticoagulation. Investigators of the ACTIV-4B242 study also reported no improvement in 45-day survival among non-hospitalized patients receiving aspirin, low dose, and high dose apixaban vs. placebo. Further research is therefore needed to better understand the long-term benefits of anticoagulation in patients.
Role of vaccination—risks vs. benefits
The most effective way of preventing serious complications from SARS-CoV-2 infection is through vaccination.248–254 Previous experience with influenza vaccine has taught us to expect a favourable relationship between vaccination and CV outcomes.255,256 There are at least eight major SARS-CoV-2 vaccines available globally, with excellent efficacy. Early data from a patient-led observational study257 has hinted at the possibility of long COVID symptoms being alleviated through vaccination. Of 900 people with long COVID, 56.7% of those vaccinated saw an overall improvement, 18.7% a deterioration, and 24.6% were unchanged post-vaccination. In another survey study (COVID symptom app study),258,259 the odds of experiencing symptoms more than 28 days post-vaccination was halved by two vaccinations (n = 906). Some experts posit that an accelerated viral clearance and a muted chronic inflammatory response could explain symptom reduction following vaccination.260
While there is little doubt that early inoculation confers the greatest protection against severe COVID-19, rare cases of vaccine-induced adverse effects have led to a rise in vaccine hesitancy.261 In particular, two widely available vaccine strategies including mRNA vaccines250,262 and vector-based (ChAdOx1 nCov-19263 and Ad26.COV2.S/Janssen)251 have been linked to cases of myocarditis264–266 and vaccine-induced prothrombotic immune thrombocytopenia (VITT),267 respectively. Although rare (∼5 per million), VITT due to antibodies to platelet factor 4 typically occurs following a single vaccine dose and may be fatal in some (pulmonary embolus, cerebral venous thrombosis). In contrast, myocarditis, potentially due to an autoimmune response (triggered by molecular mimicry between spike protein and self-antigen), tends to be more common after the second mRNA vaccine dose but is comparatively less life-threatening as the majority of cases spontaneously resolves.264–266
Future direction
Current evidence for the treatment of long COVID is lacking, though many clinical trials for long COVID and CV sequelae (Table 2) are currently underway. A list of selected clinical trials in long COVID are presented in Supplementary material online, Table S2 to demonstrate the diversity of treatments under investigation. Studies include a variety of rehabilitation programmes (telemedicine and face–face) for treatment of fatigue, cognitive decline and breathlessness, therapies targeted at cognition (e.g. transcranial stimulation), metabolic modulators (e.g. niagen), immunomodulatory therapies (e.g. steroids, laranilubmab, tocilizumab, atorvastatin, colchicine), antifibrotic treatments (e.g. pirfenidone, LYT-100), and anticoagulation (e.g. apixaban). The World Health Organization (see https://clinicaltrials.gov/ct2/who_table or https://www.who.int/clinical-trials-registry-platform) and ClinicalTrials.gov (see https://clinicaltrials.gov/ct2/results?cond=COVID-19) list more than 730 studies related to COVID-19; >80 have a major emphasis on long-term CV outcomes. As examples, selected studies with 400 or more participants are listed in Table 3.
Table 3.
Examples of large (n ≥ 400) prospective observational studies evaluating short- and long-term cardiovascular outcomes associated with COVID-19
| National clinical trials number | Title | Cardiovascular outcome measures | Age | Enrolment | Study type |
|---|---|---|---|---|---|
| NCT04358029 | Cardiac arrhythmias in patients with coronavirus disease (COVID-19) | Cardiac arrhythmias, mode of death, number of recurrence of atrial arrhythmias | Child, adult, older adult | 10 000 | Observational |
| NCT04465552 | Arrhythmic manifestations and management in hospitalized COVID-19 patients | Arrhythmic manifestations employed treatment strategies and long-term outcomes in hospitalized COVID-19 patients | 18 years and older (adult, older adult) | 666 | Observational |
| NCT04508712 | Long-term outcomes in patients with COVID-19 | Cardiac function | 18 years and older (adult, older adult) | 900 | Observational |
| NCT04624503 | Prognostic and clinical impact of cardiovascular involvement in patients With COVID-19 (CARDIO-COVID) | Cardiovascular mortality, all-cause mortality, major adverse cardiovascular events, NYHA class, left ventricular systolic function (cardiac magnetic resonance, echocardiography) | 18–85 years (adult, older adult) | 500 | Observational |
| NCT04359927 | Long-term effects of coronavirus disease 2019 on the cardiovascular system: CV COVID-19 Registry (CV-COVID-19) | Cardiovascular mortality, acute myocardial infarction, stroke | 18 years and older (adult, older adult) | 10 000 | Observational |
| NCT04724707 | Russian Cardiovascular Registry of COVID-19 | Death, hospitalization for cardiovascular reasons, mechanical support or heart transplant, ICD or CRT, Arrythmias | 18 years and older (adult, older adult) | 900 | Observational |
| NCT04384029 | The Geneva COVID-19 CVD Study | Clinical outcomes related to pre-existing cardiovascular risk factors at admission, new onset of CVD induced by COVID-19 | 18 years and older (adult, older adult) | 1927 | Observational |
| NCT04375748 | Hospital registry of acute myocarditis: evolution of the proportion of positive SARS-CoV-2 (COVID-19) cases (MYOCOVID) | Prognosis of the acute myocarditis, cardiac MRI parameters | Child, adult, older adult | 400 | Observational |
COVID-19, coronavirus disease 2019; CRT, cardiac resynchronization therapy; CVD, cardiovascular disease; ICD, implantable cardioverter-defibrillator; MRI, magnetic resonance imaging; NYHA, New York Heart Association; ICD, implantable cardioverter defibrillator; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VTE, venous thrombo-embolism.
Research priorities
Current priorities for research include (i) establishing the prevalence of persistent or chronic SARS-CoV-2 induced CV injury; (ii) elucidating causal mechanisms including the role of immune system, obesity, endotheliopathy, and genetic predispositions; (iii) developing an understanding of CV symptom burden (as part of the long COVID spectrum) and its association with pathology; (iv) developing and refining scalable diagnostic methods with high specificity for COVID-19-associated CV complications (including POTS); (v) identifying novel therapeutic solutions or repurposing old drugs that can protect or reverse COVID-19-associated long-term CV injury; (vi) evaluating the role of vaccination and SARS-CoV-2 variants on cardiac injury; (vii) evaluating the long-term impact of SARS-CoV-2 infection on those with pre-existing cardiac diseases and future risk of heart failure, ischaemic events, and arrhythmias; (viii) evaluating the effects of COVID-19-related autonomic dysfunction on CV homeostasis; and (ix) understanding the impact of long COVID on healthcare costs and on working population.4,230,233 A concerning trend observed in recent studies36,19,121,268 is the marked dissociation seen between symptoms and objective measures of health highlighting the limitations of routine clinical investigations. In this regard, deeper phenotyping efforts including advanced cardiopulmonary imaging (e.g. hyperpolarized xenon269), mass spectrometry,270 metabolomics,271 proteomics,272 whole-genome sequencing, and gut microbiome273 studies promise to unscramble the Rubix cube of pathophysiological processes that underpin long COVID. It is anticipated that multiple endophenotypes230,233 (inflammatory, metabolic, autoimmune, neurocognitive, psychological233) will surface, through artificial intelligence-driven data analysis, enabling precision diagnostics, prognostic risk prediction, and therapeutics for patients.
Conclusion
Long COVID is emerging as a major public health issue. Our current understanding of pathophysiological mechanisms and treatment options remains limited; however, there is great optimism as several national and international research initiatives promise to disentangle the complexities of this disease. The high burden of cardiopulmonary symptoms along with other organ manifestations underscores the need for multispecialty input,274,275 a model that is likely to also profit other chronic diseases. Proactive screening and investigation, where appropriate, could allay fears and anxiety among patients. Considerable efforts to find the right balance between cost-effective investigations and benefit to patients are needed to ensure sustainable service provision in these challenging economic times. Finally, the vast inequalities43,276 in healthcare provision exposed by COVID-19 will continue to be magnified by long COVID, a problem that calls for global humanitarian efforts to promote and fund equitable access to healthcare, social and welfare support, and vaccines across the world.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
B.R., D.A.B., T.F.L., and S.N. drafted and revised the manuscript.
Contributor Information
Betty Raman, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
David A. Bluemke, Department of Radiology, University of Wisconsin School of Medicine and Public Health, 3252 Clinical Science Center, 600 Highland Ave, Madison, WI 53792, USA Department of Medical Physics, University of Wisconsin School of Medicine and Public Health, 3252 Clinical Science Center, 600 Highland Ave, Madison, WI 53792, USA.
Thomas F. Lüscher, Royal Brompton & Harefield Hospitals and National Heart and Lung Institute, Imperial College, London, UK Center for Molecular Cardiology, University of Zurich, Zurich, Switzerland.
Stefan Neubauer, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK.
Funding
This work was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford British Heart Foundation (BHF) Centre of Research Excellence (grant number RE/18/3/34214).
Conflict of interest
B.R. has no conflict of interest related to the manuscript but has received grants from Oxford BHF CRE, NIHR Oxford Biomedical Research Centre, and UK Research and Innovation. B.R. holds US patent 16/674104. B.R. has consulted for Axcella therapeutics. S.N. reports grants from the NIHR Oxford Biomedical Research Centre and UK Research and Innovation and is a shareholder in Perspectum. S.N. was a non-executive director and consultant to Perspectum until 2019. S.N. holds US patents 61/630508 and 61-630510 licensed to Perspectum. S.N. is also a non-executive director and consultant to Caristo Diagnostics and holds two patents (one granted and one in review) on pericoronary fat assessment for risk prediction. T.F.L. has no conflicts of interest related to this manuscript but received educational and research grants from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daichi-Sankyo, Novartis, Sanofi, Servier, and Vifor outside this field. D.A.B. has no conflicts of interest related to this manuscript but has received consultant fees from Bayer.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long COVID: let patients help define long-lasting COVID symptoms. Nature 2020;586:170. [DOI] [PubMed] [Google Scholar]
- 3. Perego E, Callard F, Stras L, Melville-Johannesson B, Pope R, Alwan N. Why we need to keep using the patient made term “Long Covid”. https://blogs.bmj.com/bmj/2020/10/01/why-we-need-to-keep-using-the-patient-made-term-long-covid/ (1 October 2020).
- 4. National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE guideline [NG188]. https://www.nice.org.uk/guidance/ng188 (18 December 2020). [PubMed]
- 5. World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (6 October 2021).
- 6. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–264. [DOI] [PubMed] [Google Scholar]
- 8. Phillips S, Williams MA. Confronting our next national health disaster—long-haul Covid. N Engl J Med 2021;385:577–579. [DOI] [PubMed] [Google Scholar]
- 9. Nabavi N. Long covid: how to define it and how to manage it. BMJ 2020;370:m3489. [DOI] [PubMed] [Google Scholar]
- 10. Islam MF, Cotler J, Jason LA. Post-viral fatigue and COVID-19: lessons from past epidemics. Fatigue Biomed Health Behav 2020;8:61–69. [Google Scholar]
- 11. Lyons D, Frampton M, Naqvi S, Donohoe D, Adams G, Glynn K. Fallout from the COVID-19 pandemic—should we prepare for a tsunami of post viral depression? Ir J Psychol Med 2020;37:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotchin N, Read R, Smith D, Crawford D. Active Epstein-Barr virus infection in post-viral fatigue syndrome. J Infect 1989;18:143–150. [DOI] [PubMed] [Google Scholar]
- 13. Bond P. A role for herpes simplex virus in the aetiology of chronic fatigue syndrome and related disorders. Med Hypotheses 1993;40:301–308. [DOI] [PubMed] [Google Scholar]
- 14. Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol 2011;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carod-Artal FJ. Post-Ebolavirus disease syndrome: what do we know? Expert Rev Anti Infect Ther 2015;13:1185–1187. [DOI] [PubMed] [Google Scholar]
- 16. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whitaker M, Elliott J, Chadeau-Hyam M, Riley S, Darzi A, Cooke G, et al. Persistent symptoms following SARS-CoV-2 infection in a random community sample of 508,707 people. medRxiv 2021. doi: 10.1101/2021.06.28.21259452 [DOI] [Google Scholar]
- 18. Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 (1 April 2021).
- 19. Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre prospective cohort study. Lancet Respir Med 2021;9:1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021;6:100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, et al. Persistent symptoms in adult patients one year after COVID-19: a prospective cohort study. Clin Infect Dis 2021. doi: 10.1093/cid/ciab611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dryden M, Vika C. Post acute sequelae of SARS-CoV-2 infection (PASC)—formally long COVID. https://www.nioh.ac.za/wp-content/uploads/2021/04/NIOH-Webinar-Invitation_-COVID-19-_Long-Covid-and-the-workplace_22-April-2021-Dr-Dryden.pdf (21 April 2021).
- 25. Naik S, Soneja M, Haldar S, Soneja M, Mundadan NG, Garg P, et al. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov Ther 2021;15:254–260. [DOI] [PubMed] [Google Scholar]
- 26. Chopra N, Chowdhury M, Singh AK, Ma K, Kumar A, Ranjan P, et al. Clinical predictors of long COVID-19 and phenotypes of mild COVID-19 at a tertiary care centre in India. Drug Discov Ther 2021;15:156–161. [DOI] [PubMed] [Google Scholar]
- 27. Mahmud R, Rahman MM, Rassel MA, Monayem FB, Sayeed SJB, Islam MS, et al. Post-COVID-19 syndrome among symptomatic COVID-19 patients: a prospective cohort study in a tertiary care center of Bangladesh. PLoS One 2021;16:e0249644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hossain MA, Hossain KMA, Saunders K, Uddin Z, Walton LM, Raigangar V, et al. Prevalence of long COVID symptoms in Bangladesh: a prospective inception cohort study of COVID-19 survivors. BMJ Glob Health 2021;6:e006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis 2021;21:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Venturelli S, Benatti SV, Casati M, Binda F, Zuglian G, Imeri G, et al. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect 2021;149:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open 2021;4:e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 2021;4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirschtick JL, Titus AR, Slocum E, Power LE, Hirschtick RE, Elliott MR, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis 2021;73:2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blomberg B, Mohn KG-I, Brokstad KA, Zhou F, Linchausen DW, Hansen B-A, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021;27:1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Resp Med 2021;9:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cassar MP, Tunnicliffe EM, Petousi N, Lewandowski AJ, Xie C, Mahmod M, et al. Symptom persistence despite improvement in cardiopulmonary health—insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine 2021;41:101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Risk factors for long COVID: analyses of 10 longitudinal studies and electronic health records in the UK. medRxiv. doi: 10.1101/2021.06.24.21259277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/4june2021 (4 June 2021).
- 40. Office for National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. 11 July 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1july2021 (15 August 202).
- 41. Murray CJ, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grosso G. Obesity during COVID-19: an underrated pandemic? EClinicalMedicine 2021;39:101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horton R. Offline: COVID-19 is not a pandemic. Lancet 2020;396:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. NHS Digital England. Health Survey for England 2019. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019 (October 2021).
- 45. NHS Digital England. National child measurement programme 2019. https://digital.nhs.uk/data-and-information/publications/statistical/national-child-measurement-programme/2019-20-school-year (October 2021).
- 46. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 47. Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost 2021;19:2546–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ritchie S, Connell J. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis 2007;17:319–326. [DOI] [PubMed] [Google Scholar]
- 49. Office of National Statistics. The prevalence of long COVID symptoms and COVID-19 complications. https://www.ons.gov.uk/news/statementsandletters/theprevalenceoflongcovidsymptomsandcovid19complications (16 December 2020).
- 50. Office of National Statistics. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2september2021 (2 September 2021).
- 51. Ziauddeen N, Gurdasani D, O’Hara ME, Hastie C, Roderick P, Yao G, et al. Characteristics of long covid: findings from a social media survey. medRxiv 2021. doi: 10.1101/2021.03.21.21253968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Newman M. Chronic fatigue syndrome and long covid: moving beyond the controversy. BMJ 2021;373:n1559. [DOI] [PubMed] [Google Scholar]
- 53. Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med 2011;270:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr 2003;11:7–115. [Google Scholar]
- 56. Singh SJ, Barradell AC, Greening NJ, Bolton C, Jenkins G, Preston L, et al. British Thoracic Society survey of rehabilitation to support recovery of the post-COVID-19 population. BMJ Open 2020;10:e040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. VanElzakker MB, Brumfield SA, Lara Mejia PS. Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a critical review of research methods. Front Neurol 2019;9:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cader S, O’Donovan D, Shepherd C, Chaudhuri A. FP03-MO-02 neuropathology of post-infectious chronic fatigue syndrome. J Neurol Sci 2009;285:S60–S61. [Google Scholar]
- 59. VanElzakker MB. Chronic fatigue syndrome from vagus nerve infection: a psychoneuroimmunological hypothesis. Med Hypotheses 2013;81:414–423. [DOI] [PubMed] [Google Scholar]
- 60. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. Brain imaging before and after COVID-19 in UK Biobank. medRxiv 2021. doi: 10.1101/2021.06.11.21258690 [DOI] [Google Scholar]
- 61. Edler C, Schroder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 2020;134:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J 2020;41:3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol 2021;50:107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aretz H T. Myocarditis: the Dallas criteria. Hum Pathol 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 65. Fox SE, Vander Heide RS. COVID-19: the heart of the matter—pathological changes and a proposed mechanism. J Cardiovasc Pharmacol Ther 2021;26:217–224. [DOI] [PubMed] [Google Scholar]
- 66. Hooper JE, Padera RF, Dolhnikoff M, da Silva LFF, Duarte-Neto AN, Kapp ME, et al. A postmortem portrait of the coronavirus disease 2019 (COVID-19) pandemic: a large multi-institutional autopsy survey study. Arch Pathol Lab Med 2021;145:529–535. [DOI] [PubMed] [Google Scholar]
- 67. Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol 2021;77:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol 2020;5:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020;76:2043–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden AC, et al. COVID-19-associated nonocclusive fibrin microthrombi in the heart. Circulation 2021;143:230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vrints CJM, Krychtiuk KA, Van Craenenbroeck EM, Segers VF, Price S, Heidbuchel H. Endothelialitis plays a central role in the pathophysiology of severe COVID-19 and its cardiovascular complications. Acta Cardiol 2021;76:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Robinson FA, Mihealsick RP, Wagener BM, Hanna P, Poston MD, Efimov IR, et al. Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection. Am J Physiol Heart Circ Physiol 2020;319:H1059–H1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther 2021;6:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hu Z, Li S, Song X. Cytokine storm with rapidly elevated interleukin-6 indicates sudden death in patients with critical COVID-19. Cytokine Growth Factor Rev 2021;58:30–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis—diagnosis, treatment options, and current controversies. Nat Rev Cardiol 2015;12:670–680. [DOI] [PubMed] [Google Scholar]
- 76. Kim HW, de Chantemèle EJ B, Weintraub NL. Perivascular adipocytes in vascular disease. Arterioscler Thromb Vasc Biol 2019;39:2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Blagova O, Varionchik N, Zaidenov V, Savina P, Sarkisova N. Anti-heart antibodies levels and their correlation with clinical symptoms and outcomes in patients with confirmed or suspected diagnosis COVID-19. Eur J Immunol 2021;51:893–902. [DOI] [PubMed] [Google Scholar]
- 78. Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021;595:283–288. [DOI] [PubMed] [Google Scholar]
- 79. Franke C, Ferse C, Kreye J, Reincke SM, Sanchez-Sendin E, Rocco A, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun 2021;93:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Richter AG, Shields AM, Karim A, Birch D, Faustini SE, Steadman L, et al. Establishing the prevalence of common tissue-specific autoantibodies following severe acute respiratory syndrome coronavirus 2 infection. Clin Exp Immunol 2021;205:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Konstantinov K, Von Mikecz A, Buchwald D, Jones J, Gerace L, Tan E. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. J Clin Investig 1996;98:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Loebel M, Grabowski P, Heidecke H, Bauer S, Hanitsch LG, Wittke K, et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behav Immun 2016;52:32–39. [DOI] [PubMed] [Google Scholar]
- 83. Talla A, Vasaikar SV, Lemos MP, Moodie Z, Pebworth M-PL, Henderson KE, et al. Longitudinal immune dynamics of mild COVID-19 define signatures of recovery and persistence. bioRxiv 2021. doi: 10.1101/2021.05.26.442666 [DOI] [Google Scholar]
- 84. von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundström A, Magnusson M, Mackman N, et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv 2021;5:756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Garg A, Goyal S, Patel P. A case of COVID-19 infection with delayed thromboembolic complication on warfarin. Cureus 2020;12:e8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schweblin C, Hachulla AL, Roffi M, Glauser F. Delayed manifestation of COVID-19 presenting as lower extremity multilevel arterial thrombosis: a case report. Eur Heart J Case Rep 2020;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vechi HT, Maia LR, MdM A. Late acute pulmonary embolism after mild Coronavirus Disease 2019 (COVID-19): a case series. Rev Inst Med Trop Sao Paulo 2020;62:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Riyahi S, Dev H, Behzadi A, Kim J, Attari H, Raza SI, et al. Pulmonary embolism in hospitalized patients with COVID-19: a multicenter study. Radiology 2021;301:E426–E433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Suzuki YJ, Nikolaienko SI, Shults NV, Gychka SG. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med Hypotheses 2021;147:110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ambrosino P, Calcaterra I, Molino A, Moretta P, Lupoli R, Spedicato GA, et al. Persistent endothelial dysfunction in post-acute COVID-19 syndrome: a case-control study. Biomedicines 2021;9:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, et al. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 2021;320:H404–H410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xiong T-Y, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 2020;41:1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol 2020;5:1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ 2021;372:n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021;373:n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tazare J, Walker AJ, Tomlinson L, Hickman G, Rentsch CT, Williamson EJ, et al. Rates of serious clinical outcomes in survivors of hospitalisation with COVID-19: a descriptive cohort study within the OpenSAFELY platform. MedRxiv 2021. doi: 10.1101/2021.01.22.21250304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J 2021;42:1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Clavario P, De Marzo V, Lotti R, Barbara C, Porcile A, Russo C, et al. Assessment of functional capacity with cardiopulmonary exercise testing in non-severe COVID-19 patients at three months follow-up. MedRxiv 2020. doi: 10.1101/2020.11.15.20231985 [DOI] [Google Scholar]
- 103. Małek ŁA, Marczak M, Miłosz-Wieczorek B, Konopka M, Braksator W, Drygas W, et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging 2021;53:1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang H, Li R, Zhou Z, Jiang H, Yan Z, Tao X, et al. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2021;23:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vago H, Szabo L, Dohy Z, Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. Cardiovasc Imaging 2021;14:1279–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mohr A, Dannerbeck L, Lange TJ, Pfeifer M, Blaas S, Salzberger B, et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med 2021;16:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gao Y, Chen R, Geng Q, Mo X, Zhan C, Jian W, et al. Cardiopulmonary exercise testing might be helpful for interpretation of impaired pulmonary function in recovered COVID-19 patients. Eur Respir J 2021;57:2004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Debeaumont D, Boujibar F, Ferrand-Devouge E, Artaud-Macari E, Tamion F, Gravier FE, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID-19 who survived hospitalization: a pilot study. Phys Ther 2021;101:pzab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J 2021;57:2003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hendrickson BS, Stephens RE, Chang JV, Amburn JM, Pierotti LL, Johnson JL, et al. Cardiovascular evaluation after COVID-19 in 137 collegiate athletes: results of an algorithm-guided screening. Circulation 2021;143:1926–1928. [DOI] [PubMed] [Google Scholar]
- 111. Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol 2021;6:116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhou M, Wong C-K, Un K-C, Lau Y-M, Lee JC-Y, Tam FC-C, et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS One 2021;16:e0246732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation 2021;143:609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, et al. COVID-19: myocardial injury in survivors. Circulation 2020;142:1120–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rinaldo RF, Mondoni M, Parazzini EM, Pitari F, Brambilla E, Luraschi S, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J 2021;58:2100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Catena C, Colussi G, Bulfone L, Da Porto A, Tascini C, Sechi LA. Echocardiographic comparison of COVID-19 patients with or without prior biochemical evidence of cardiac injury after recovery. J Am Soc Echocardiogr 2021;34:193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol 2021;6:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Motiejunaite J, Balagny P, Arnoult F, Mangin L, Bancal C, d’Ortho M-P, et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild Covid-19 survivors? Front Physiol 2021;11:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, et al. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol 2021;130:1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021;31:100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021;11:e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ng MY, Ferreira VM, Leung ST, Lee JCY, Fong AHT, Liu RWT, et al. Patients recovered from COVID-19 show ongoing subclinical myocarditis as revealed by cardiac magnetic resonance imaging. JACC Cardiovasc Imaging 2020;13:2476–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Eiros R, Barreiro-Perez M, Martin-Garcia A, Almeida J, Villacorta E, Perez-Pons A, et al. Pericarditis and myocarditis long after SARS-CoV-2 infection: a cross-sectional descriptive study in health-care workers. MedRxiv 2020. doi: 10.1101/2020.07.12.20151316 [DOI] [Google Scholar]
- 125. Moody WE, Liu B, Mahmoud-Elsayed HM, Senior J, Lalla SS, Khan-Kheil AM, et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr 2021;34:562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol 2021;6:1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol 2021;6:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, et al. Prospective case–control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging 2021;14:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR, et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 2021;144:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sechi LA, Colussi G, Bulfone L, Brosolo G, Da Porto A, Peghin M, et al. Short-term cardiac outcome in survivors of COVID-19: a systematic study after hospital discharge. Clin Res Cardiol 2021;110:1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. de Graaf M, Antoni M, Ter Kuile M, Arbous M, Duinisveld A, Feltkamp M, et al. Short-term outpatient follow-up of COVID-19 patients: a multidisciplinary approach. EClinicalMedicine 2021;32:100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Daher A, Cornelissen C, Hartmann N-U, Balfanz P, Müller A, Bergs I, et al. Six months follow-up of patients with invasive mechanical ventilation due to COVID-19 related ARDS. Int J Environ Res Public Health 2021;18:5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Myhre PL, Heck SL, Skranes JB, Prebensen C, Jonassen CM, Berge T, et al. Cardiac pathology 6 months after hospitalization for COVID-19 and association with the acute disease severity: cardiac MRI 6 months after COVID-19. Am Heart J 2021;242:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging 2020;13:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hall J, Myall K, Lam JL, Mason T, Mukherjee B, West A, et al. Identifying patients at risk of post-discharge complications related to COVID-19 infection. Thorax 2021;76:408–411. [DOI] [PubMed] [Google Scholar]
- 136. Li X, Wang H, Zhao R, Wang T, Zhu Y, Qian Y, et al. Elevated extracellular volume fraction and reduced global longitudinal strains in patients recovered from COVID-19 without clinical cardiac findings. Radiology 2021;299:E230–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Long B, Brady WJ, Bridwell RE, Ramzy M, Montrief T, Singh M, et al. Electrocardiographic manifestations of COVID-19. Am J Emerg Med 2021;41:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Desai AD, Boursiquot BC, Melki L, Wan EY. Management of arrhythmias associated with COVID-19. Curr Cardiol Rep 2021;23:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Radin JM, Quer G, Ramos E, Baca-Motes K, Gadaleta M, Topol EJ, et al. Assessment of prolonged physiological and behavioral changes associated with COVID-19 infection. JAMA Network Open 2021;4:e2115959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chang TY, Chao TF, Liu CJ, Chen SJ, Chung FP, Liao JN, et al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm 2016;13:1189–1194. [DOI] [PubMed] [Google Scholar]
- 141. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 142. Lakin R, Polidovitch N, Yang S, Guzman C, Gao X, Wauchop M, et al. Inhibition of soluble TNFα prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J Mol Cell Cardiol 2019;129:165–173. [DOI] [PubMed] [Google Scholar]
- 143. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 2015;12:230–243. [DOI] [PubMed] [Google Scholar]
- 144. Liuba I, Ahlmroth H, Jonasson L, Englund A, Jönsson A, Säfström K, et al. Source of inflammatory markers in patients with atrial fibrillation. Europace 2008;10:848–853. [DOI] [PubMed] [Google Scholar]
- 145. Chen Y, Wu S, Li W, Wang B, Han X, Yang Y, et al. Higher high-sensitivity c reactive protein is associated with future premature ventricular contraction: a community based prospective cohort study. Sci Rep 2018;8:5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Flachskampf FA, Biering-Sørensen T, Solomon SD, Duvernoy O, Bjerner T, Smiseth OA. Cardiac imaging to evaluate left ventricular diastolic function. JACC Cardiovasc Imaging 2015;8:1071–1093. [DOI] [PubMed] [Google Scholar]
- 147. Pennell DJ. Cardiovascular magnetic resonance. Circulation 2010;121:692–705. [DOI] [PubMed] [Google Scholar]
- 148. Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 2016;134:e579–e646. [DOI] [PubMed] [Google Scholar]
- 149. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail 2020;13:e007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 151. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–3176. [DOI] [PubMed] [Google Scholar]
- 152. Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, et al. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the international T1 multicenter cardiovascular magnetic resonance study. Circ Cardiovasc Imaging 2015;8:e003285. [DOI] [PubMed] [Google Scholar]
- 153. Ridouani F, Damy T, Tacher V, Derbel H, Legou F, Sifaoui I, et al. Myocardial native T2 measurement to differentiate light-chain and transthyretin cardiac amyloidosis and assess prognosis. J Cardiovasc Magn Reson 2018;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bai W, Raman B, Petersen SE, Neubauer S, Raisi-Estabragh Z, Aung N, et al. Longitudinal changes of cardiac and aortic imaging phenotypes following COVID-19 in the UK biobank cohort. medRxiv 2021. doi: 10.1101/2021.11.04.21265918 [DOI] [Google Scholar]
- 156. Pathik B, Raman B, Mohd Amin NH, Mahadavan D, Rajendran S, McGavigan AD, et al. Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2016;17:1146–1152. [DOI] [PubMed] [Google Scholar]
- 157. Dastidar AG, Baritussio A, De Garate E, Drobni Z, Biglino G, Singhal P, et al. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging 2019;12:1973–1982. [DOI] [PubMed] [Google Scholar]
- 158. Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 2007;28:1242–1249. [DOI] [PubMed] [Google Scholar]
- 159. Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 2020;21:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging 2020;13:2459–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kim J, Volodarskiy A, Sultana R, Pollie MP, Yum B, Nambiar L, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol 2020;76:1965–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Buikema JW, Asselbergs FW, Tekstra J. COVID-19 related thrombi in ascending and descending thoracic aorta with peripheral embolization: a case report. Eur Heart J Case Rep 2021;5:ytaa525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Çora AR, Çelik E, Karadem KB. Aortic thrombosis in the course of Covid-19 disease; two rare cases. Ann Vasc Surg 2021;73:119–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. de Carranza M, Salazar DE, Troya J, Alcázar R, Peña C, Aragón E, et al. Aortic thrombus in patients with severe COVID-19: review of three cases. J Thromb Thrombolysis 2021;51:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol 2019;4:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Kotanidis CP, Xie C, Adlam D, Rodrigues JC, Siddique M, Lockstone H, et al. Radiotranscriptomic analysis of perivascular adipose tissue quantifies vascular inflammation in Covid-19 from routine CT angiograms: stratification of “new UK variant” infection and prediction of in-hospital outcomes [abstract]. Heart 2021;107:A177. [Google Scholar]
- 168. Fan BE, Umapathi T, Chua K, Chia YW, Wong SW, Tan GWL, et al. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J Thromb Thrombolysis 2021;51:971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Patell R, Bogue T, Koshy A, Bindal P, Merrill M, Aird WC, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood 2020;136:1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Engelen M, Vanassche T, Balthazar T, Claeys E, Gunst J, Jacquemin M, et al. Incidence of venous thromboembolism in patients discharged after COVID-19 hospitalisation [abstract]. Res Pract Thromb Haemost 2020;4:CO01.3. [Google Scholar]
- 171. Remy-Jardin M, Duthoit L, Perez T, Felloni P, Faivre JB, Fry S, et al. Assessment of pulmonary arterial circulation 3 months after hospitalization for SARS-CoV-2 pneumonia: dual-energy CT (DECT) angiographic study in 55 patients. EClinicalMedicine 2021;34:100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Donna M M, Danielle L B, Lala A, Trivieri Maria G, Contreras Johanna P, Natelson Benjamin H. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail 2021;9:927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Singh I, Joseph P, Heerdt PM, Cullinan M, Lutchmansingh DD, Gulati M, et al. Persistent exertional intolerance after COVID-19: insights from invasive cardiopulmonary exercise testing. Chest 2021. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Di Filippo L, De Lorenzo R, D’Amico M, Sofia V, Roveri L, Mele R, et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr 2021;40:2420–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Morley JE, Kalantar-Zadeh K, Anker SD. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle 2020;11:863–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Dorelli G, Braggio M, Gabbiani D, Busti F, Caminati M, Senna G, et al. Importance of cardiopulmonary exercise testing amongst subjects recovering from COVID-19. Diagnostics 2021;11:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Johansson M, Ståhlberg M, Runold M, Nygren-Bonnier M, Nilsson J, Olshansky B, et al. Long-Haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep 2021;3:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med 2021;21:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kanjwal K, Jamal S, Kichloo A, Grubb BP. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag 2020;11:4302–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn DI, Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res 2020;30:449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Umapathi T, Poh MQW, Fan BE, Li KFC, George J, Tan JYL. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin Auton Res 2020;30:571–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res 2021;69:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chioh FW, Fong SW, Young BE, Wu KX, Siau A, Krishnan S, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife 2021;10:e64909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Gavriilaki E, Eftychidis I, Papassotiriou I. Update on endothelial dysfunction in COVID-19: severe disease, long COVID-19 and pediatric characteristics. J Lab Med 2021;45:293–302. [Google Scholar]
- 185. Ho FK, Man KK, Toshner M, Church C, Celis-Morales C, Wong IC, et al. Thromboembolic risk in hospitalised and non-hospitalised COVID-19 patients: a self-controlled case series analysis of a nation-wide cohort. Mayo Clin Proc 2021;96:2587–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lukose T, De S, Ganga R, Ravina M, Sahu D, Moideen A, et al. Lung perfusion SPECT/CT imaging in post COVID-19 pneumonia: the spectrum of imaging findings. J Nucl Med 2021;62:2002. [Google Scholar]
- 188. Patelli G, Paganoni S, Besana F, Codazzi F, Ronzoni M, Manini S, et al. Preliminary detection of lung hypoperfusion in discharged Covid-19 patients during recovery. Eur J Radiol 2020;129:109121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Thornton G, Shetye A, Knott K, Razvi Y, Vimalesvaran K, Kurdi H, et al. Myocardial perfusion after COVID-19 infection: no persisting impaired myocardial blood flow in surviving patients. Eur Heart J Cardiovasc Imaging 2021;22:jeab090. [Google Scholar]
- 190. Mangion K, Morrow A, Bagot C, Bayes H, Blyth KG, Church C, et al. The chief scientist office cardiovascular and pulmonary imaging in SARS coronavirus disease-19 (CISCO-19) study. Cardiovasc Res 2020;116:2185–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Tholin B, Ghanima W, Einvik G, Aarli B, Brønstad E, Skjønsberg OH, et al. Incidence of thrombotic complications in hospitalised and non-hospitalised patients after COVID-19 diagnosis. Br J Haematol 2021;194:542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Drake TM, Riad AM, Fairfield CJ, Egan C, Knight SR, Pius R, et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet 2021;398:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol 2020;76:2334–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Chatrath N, Kaza N, Pabari PA, Fox K, Mayet J, Barton C, et al. The effect of concomitant COVID-19 infection on outcomes in patients hospitalized with heart failure. ESC Heart Fail 2020;7:4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Rey JR, Caro-Codón J, Rosillo SO, Iniesta ÁM, Castrejón-Castrejón S, Marco-Clement I, et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail 2020;22:2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Arrigo M, Nijst P, Rudiger A. Optimising heart failure therapies in the acute setting. Card Fail Rev 2018;4:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sandoval Y, Januzzi Jr JL, Jaffe AS. Cardiac troponin for the diagnosis and risk-stratification of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol 2020;76:1244–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC working group for atherosclerosis and vascular biology, and the ESC council of basic cardiovascular science. Cardiovasc Res 2020;116:2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep 2020;5:1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. New MI, Peterson RE, Saenger P, Levine LS. Evidence for an unidentified ACTH-induced steroid hormone causing hypertension. J Clin Endocrinol Metab 1976;43:1283–1293. [DOI] [PubMed] [Google Scholar]
- 205. Dustan HP, Bravo EL, Tarazi RC. Volume-dependent essential and steroid hypertension. Am J Cardiol 1973;31:606–615. [DOI] [PubMed] [Google Scholar]
- 206. Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19. JACC Cardiovasc Imaging 2020;13:2635–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dixit NM, Churchill A, Nsair A, Hsu JJ. Post-acute COVID-19 syndrome and the cardiovascular system: what is known? Am Heart J Plus 2021;5:100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020;17:1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition—a cohort study. Chron Respir Dis 2021;18:147997312110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Thomas P, Baldwin C, Bissett B, Boden I, Gosselink R, Granger CL, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother 2020;66:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Naidu SB, Shah AJ, Saigal A, Smith C, Brill SE, Goldring J, et al. The high mental health burden of “Long COVID” and its association with on-going physical and respiratory symptoms in all adults discharged from hospital. Eur Respir J 2021;57:2004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Bhatia RT, Marwaha S, Malhotra A, Iqbal Z, Hughes C, Börjesson M, et al. Exercise in the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of sports cardiology & exercise of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol 2020;27:1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Santos-Ferreira D, Tomás R, Dores H. Return-to-play guidelines for athletes after COVID-19 infection. JAMA Cardiol 2021;6:478–479. [DOI] [PubMed] [Google Scholar]
- 214. Elliott N, Martin R, Heron N, Elliott J, Grimstead D, Biswas A. Infographic. Graduated return to play guidance following COVID-19 infection. Br J Sports Med 2020;54:1174–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Pelliccia A, Solberg EE, Papadakis M, Adami PE, Biffi A, Caselli S, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J 2019;40:19–33. [DOI] [PubMed] [Google Scholar]
- 216. University College London. Long Covid: UCL leads £8m studies into treatments and diagnosis. https://www.ucl.ac.uk/news/headlines/2021/jul/ucl-leads-ps8m-studies-long-covid-treatments-and-diagnosis (19 July 2021).
- 217. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 218. Ezra A A, Nanette K W, Ralph G B, Donald E C, Theodore G G, David R H, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 219. Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, et al. Advanced heart failure: a position statement of the heart failure association of the European Society of Cardiology. Eur J Heart Fail 2018;20:1505–1535. [DOI] [PubMed] [Google Scholar]
- 220. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 221. ClinicalTrials.gov. HElping Alleviate the Longer-term Consequences of COVID-19 (HEAL-COVID) (HEAL-COVID) 2021. https://clinicaltrials.gov/ct2/show/NCT04801940 (8 August 2021).
- 222. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute Covid-19 in primary care. BMJ 2020;370:m3026. [DOI] [PubMed] [Google Scholar]
- 223. Richette P, Poitou C, Garnero P, Vicaut E, Bouillot JL, Lacorte JM, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis 2011;70:139–144. [DOI] [PubMed] [Google Scholar]
- 224. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010;2010:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Koenen M, Hill MA, Cohen P, Obesity SJ. Adipose tissue and vascular dysfunction. Circ Res 2021;128:951–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Gloeckl R, Leitl D, Jarosch I, Schneeberger T, Nell C, Stenzel N, et al. Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ Open Res 2021;7:00108–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Asly M, Hazim A. Rehabilitation of post-COVID-19 patients. Pan Afr Med J 2020;36:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Jones M, Harvey A, Marston L, O’Connell NE. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in adults. Cochrane Database Syst Rev 2013;5:CD009041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 231. Lord VM, Cave P, Hume VJ, Flude EJ, Evans A, Kelly JL, et al. Singing teaching as a therapy for chronic respiratory disease—a randomised controlled trial and qualitative evaluation. BMC Pulm Med 2010;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Habersaat KB, Betsch C, Danchin M, Sunstein CR, Böhm R, Falk A, et al. Ten considerations for effectively managing the COVID-19 transition. Nat Hum Behav 2020;4:677–687. [DOI] [PubMed] [Google Scholar]
- 233. Park CC, Chaturvedi N, Sterne J, Steves C, Williams D. Short report on long COVID. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1007511/S1327_Short_Long_COVID_report.pdf (22 July 2021).
- 234. Arnold AC, Okamoto LE, Diedrich A, Paranjape SY, Raj SR, Biaggioni I, et al. Low-dose propranolol and exercise capacity in postural tachycardia syndrome. A randomized study. Neurology 2013;80:1927–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Parsaik A, Allison TG, Singer W, Sletten DM, Joyner MJ, Benarroch EE, et al. Deconditioning in patients with orthostatic intolerance. Neurology 2012;79:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Podoleanu C, Maggi R, Brignole M, Croci F, Incze A, Solano A, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled study. J Am Coll Cardiol 2006;48:1425–1432. [DOI] [PubMed] [Google Scholar]
- 237. Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic hypotension. J Am Coll Cardiol 2018;72:1294–1309. [DOI] [PubMed] [Google Scholar]
- 238. Collaborative Group RECOVERY, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020;383:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2022;399:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Connors JM, Brooks MM, Sciurba FC, Krishnan JA, Bledsoe JR, Kindzelski A, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA 2021;326:1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators; Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med 2021;385:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, et al. Therapeutic anticoagulation with heparin in noncritically Ill patients with Covid-19. N Engl J Med 2021;385:790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Lopes RD, Furtado RH, Macedo AVS, Bronhara B, Damiani LP, Barbosa LM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 2021;397:2253–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Sadeghipour P, Talasaz A, Rashidi F, Sharif-Kashani B, Beigmohammadi M, Farrokhpour M, et al. Effect of inter-mediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA 2021;325:1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Ní Áinle F, et al. Heparin for moderately Ill patients with Covid-19. medRxiv 2021. doi: 10.1101/2021.07.08.21259351. [DOI] [Google Scholar]
- 248. McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021;397:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 2013;310:1711–1720. [DOI] [PubMed] [Google Scholar]
- 256. Johnstone J, Loeb M, Teo KK, Gao P, Dyal L, Liu L, et al. Influenza vaccination and major adverse vascular events in high-risk patients. Circulation 2012;126:278–286. [DOI] [PubMed] [Google Scholar]
- 257. Strain WD, Sherwood O, Banerjee A, Vvd T, Hishmeh L, Rossman J. The impact of COVID vaccination on symptoms of long COVID. An international survey of people with lived experience of long COVID. SSRN 2021. doi: 10.2139/ssrn.3868856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Post-vaccination SARS-CoV-2 infection: risk factors and illness profile in a prospective, observational community-based case–control study. medRxiv 2021. doi: 10.1101/2021.05.24.21257738. [DOI] [Google Scholar]
- 259. Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case–control study. Lancet Infect Dis 2022;22:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 2021;27:790–792. [DOI] [PubMed] [Google Scholar]
- 261. Murphy J, Vallières F, Bentall RP, Shevlin M, McBride O, Hartman TK, et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat Commun 2021;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Larson KF, Ammirati E, Adler ED, Cooper LT, Hong KN, Saponara G, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation 2021;144:506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Munker D, Veit T, Barton J, Mertsch P, Mümmler C, Osterman A, et al. Pulmonary function impairment of asymptomatic and persistently symptomatic patients 4 months after COVID-19 according to disease severity. Infection 2021. doi: 10.1007/s15010-021-01669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Grist JT, Chen M, Collier GJ, Raman B, AbuEid G, McIntyre A, et al. Hyperpolarized 129Xe MRI abnormalities in dyspneic participants 3 months after COVID-19 pneumonia: preliminary results. Radiology 2021;301:E353–E360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 2021;20:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Pasini E, Corsetti G, Romano C, Scarabelli TM, Chen-Scarabelli C, Saravolatz L, et al. Serum metabolic profile in patients with long-Covid (PASC) syndrome: clinical implications. Front Med 2021;8:714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Filbin MR, Mehta A, Schneider AM, Kays KR, Guess JR, Gentili M, et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep Med 2021;2:100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Giloteaux L, Goodrich JK, Walters WA, Levine SM, Ley RE, Hanson MR. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Sivan M, Rayner C, Delaney B. Fresh evidence of the scale and scope of long covid. BMJ 2021;373:n853. [DOI] [PubMed] [Google Scholar]
- 275. Norton A, Olliaro P, Sigfrid L, Carson G, Paparella G, Hastie C, et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis 2021;21:601–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Abecassis A. Five priorities for universal COVID-19 vaccination. Lancet 2021;398:285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.