Abstract
The aim of this retrospective analysis was to provide information on how infections with respiratory syncytial virus (RSV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) differ in symptoms, clinical course, outcome, and utilization of hospital care. We investigated 748 polymerase chain reaction results from symptomatic children aged 0–4 years in Cologne, Germany. One hundred sixty-nine patients tested positive for RSV (22.6%) and 24 children for SARS-CoV-2 (3.2%). Symptomatic patients with RSV infection were hospitalized significantly longer. RSV-positive patients needed oxygen supplementation significantly more often as well as high-flow therapy. With regard to care efforts, RSV-infected patients put higher pressure on the hospital and utilized more hospital resources.
Keywords: Germany, COVID-19, RSV infections, hospitalization, children
Symptomatic children (aged 0–4 years) with respiratory syncytial virus (RSV) infection were hospitalized significantly longer than children infected with SARS-CoV-2. With regard to care efforts, RSV-infected patients put higher pressure on the hospital and utilized more hospital resources.
Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections in infants and young children [1]. RSV typically affects children in the first 2 years of life and/or those with limited respiratory or cardiovascular function [2]. During the global coronavirus disease 2019 (COVID-19) pandemic, RSV and other respiratory viruses such as influenza changed dramatically in their incidence. Data from Australia and New Zealand showed a decrease of RSV infections throughout the winter season 2020 (April–June 2020) in the southern hemisphere [3, 4]. Data from the European Centre for Disease Prevention and Control confirmed these findings for the winter season 2020–2021. Before the COVID-19 pandemic, the highest RSV incidences were usually detected from December to March with first cases occurring in autumn (eg, September–October) [5]. In 2021 we observed an enormous increase of RSV infections worldwide, characterized by an obvious shift of incidence toward late summer with the first cases by the end of July [6–8].
Children with RSV infection usually present with upper respiratory symptoms (eg, fever, rhinitis, and nasal congestion) as well as with lower respiratory symptoms (eg, cough and tachydyspnea) [1]. Therapy of choice is supportive care such as maintaining hydration and nutrition as well as keeping the oxygen saturation >90% [9].
In parallel, the COVID-19 pandemic is still ongoing with increasing numbers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, especially in the pediatric cohort [10]. The course of disease is often mild [11] and fatal outcomes are rare [12]. However, the symptoms of the respiratory tract reflect the main clinical appearance in SARS-CoV-2 in children as well.
The absolute number of SARS-CoV-2–positive tested patients, but especially RSV-positive tested patients, requiring inpatient treatment and inevitable hygiene measures such as the cohorting of infected patients currently exerts high pressure on hospitals in Germany [13]. This work presents data of 193 children aged 0–4 years collected over a period of 21 months from March 2020 to November 2021 who tested positive for RSV or SARS-CoV-2. The aim of this retrospective analysis was to provide information on how infections with RSV and SARS-CoV-2 differed in terms of symptoms, clinical course, outcome, and incidence over the observation period.
METHODS
We included symptomatic children between 0 and 4 years of age who had shown a positive reverse-transcription polymerase chain reaction (RT-PCR) result for RSV or SARS-CoV-2 and were admitted to our hospital between 13 March 2020 and 30 November 2021. During this period, the wild type of SARS-CoV-2 (the Alpha variant; first cases in January 2021), and the Delta variant (first cases at the end of July 2021) were circulating in Germany. We excluded asymptomatic patients as well as patients aged >4 years. The patients received a nasopharyngeal and/or oropharyngeal swab. All swabs were conducted by trained staff and were tested for RSV, SARS-CoV-2, and influenza A/B. High-flow nasal cannula (HFNC) was defined as a flow >4 L/minute. Former preterm infants as well as multimorbid children and those suffering from chronic renal diseases were regarded as patients at risk.
In the case of numerical variables, the descriptive presentation of the results was performed by specifying the mean value and the standard deviation, or the median indicating the minimum and maximum values of nonnormally distributed variables. We checked for normal distribution by using the Kolmogorov–Smirnov test. Categorical variables were described as a percentage of the underlying collective. Statistical analysis was performed using the Student t test for normally distributed values, the Mann–Whitney U test for nonnormally distributed values, or the χ2 test for categorical values. Results were regarded as differing significantly when the P value was < .05.
RESULTS
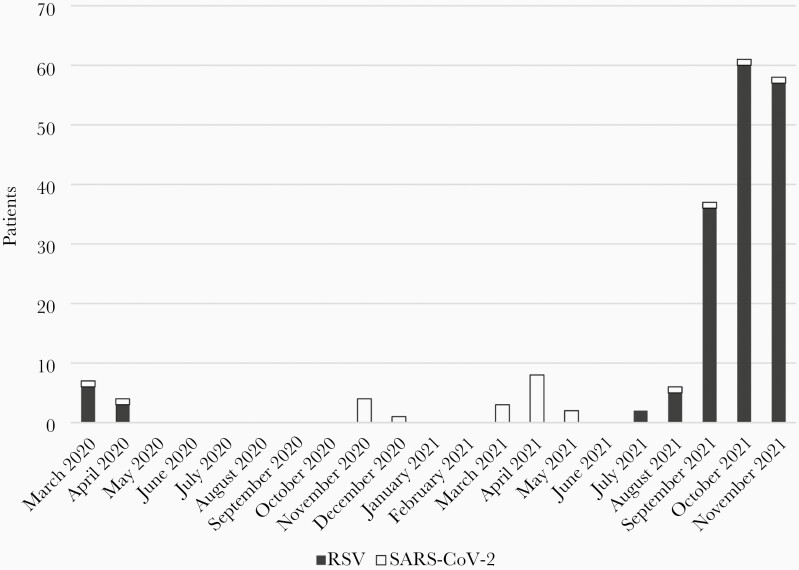
Between March 2020 and November 2021, 748 children between 0 and 4 years of age admitted to our hospital with respiratory symptoms received a naso- and/or oropharyngeal swab. One hundred sixty-nine (22.6%) patients tested positive for RSV, 24 (3.2%) tested positive for SARS-CoV-2, and 2 (0.3%) tested positive for influenza A. We observed the first cases of RSV infections in July 2021 with an enormous increase of cases in October 2021. For more data, see Figure 1.
Figure 1.
Respiratory syncytial virus (RSV)– and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–positive patients throughout the observation time (n = 193).
Only 17 of 748 infants received a more comprehensive multiplex PCR analysis to detect other viruses that may cause respiratory symptoms in infants. These were, in particular, patients who were critically ill at presentation. Multiplex PCR is not performed routinely at our hospital due to high costs. In these 17 patients, infection with rhinovirus was detected in 10 cases, bocavirus in 7 cases, adenovirus in 2 cases, metapneumovirus in 2 cases, coronavirus NL63 in 2 cases, and parainfluenza type 3 in 1 case (coinfection with >1 virus occurred in 8 cases; 4 of 8 [50%] had a coinfection of bocavirus and rhinovirus). The median age of our patients was 14 months (min, 0 months; max, 59 months).
Statistically, there was no significant difference in age between the groups, even though patients infected with RSV were slightly younger than those infected with SARS-CoV-2. RSV patients presented significantly more often with a cough (88.8% vs 45.8%; P < .001), whereas SARS-CoV-2 patients suffered significantly more often from fever (87.5% vs 42.0%; P < .001) and gastrointestinal symptoms (25.0% vs 8.3%; P < .05). Patients with an RSV infection stayed in hospital significantly longer than patients with a SARS-CoV-2 infection (4 days [min, 0; max, 21] vs 2 days [min, 1; max, 8]; P < .05). Moreover, RSV-positive patients needed oxygen supplementation significantly more often (39.6% vs 8.3%; P < .05) as well as HFNC (24.3% vs 0.0%; P < .05), even though there was no difference with respect to intubation. It is of note that no infant with a positive SARS-CoV-2 test had to be admitted to the pediatric intensive care unit (PICU), whereas 17 infants with RSV required intensive care. By calculating the relative risk (RR), we could show that the risk of PICU admission (RR, 1.16 [95% confidence interval {CI}, 1.10–1.23]), intubation (1.14 [95% CI, 1.08–1.21]), and nasal continuous positive airway pressure (nCPAP) (RR, 1.15 [95% CI, 1.08–1.21]) for RSV patients was almost 20% higher than for SARS-CoV-2 patients. For more data, see Table 1.
Table 1.
Comparison of Respiratory Syncytial Virus (RSV)– Versus Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Infected Patients (n = 193) and Characteristics of Patients With Neither RSV Nor SARS-CoV-2 (n = 555)
| Characteristic | RSV (n = 169) |
SARS-CoV-2 (n = 24) |
P Value | RR (95% CI) |
|---|---|---|---|---|
| Median age, mo (min; max) | 3 (0; 57) | 7 (0; 44) | .601 | … |
| Male sex | 57.4% (n = 97) | 54.2% (n = 13) | .765 | 1.02 (.91–1.13) |
| Patients at risk | 13.6% (n = 23) | 12.5% (n = 3) | .882 | 1.01 (.87–1.18) |
| Signs and symptoms | ||||
| Dyspnea | 20.7% (n = 35) | 12.5% (n = 3) | .344 | 1.07 (.95–1.19) |
| Fever | 42.0% (n = 71) | 87.5% (n = 21) | <.001 | 0.80 (.71–.89) |
| Cough | 88.8% (n = 150) | 45.8% (n = 11) | <.001 | 1.57 (1.18–2.10) |
| Rhinitis | 50.9% (n = 86) | 33.3% (n = 8) | .107 | 1.09 (.98–1.21) |
| GI symptoms | 8.3% (n = 14) | 25.0% (n = 6) | .012 | 0.78 (.58–1.05) |
| Median time of hospitalization, d (min; max) |
4 (0; 21) | 2 (1; 8) | .049 | … |
| Intensive care treatment | 10.1% (n = 17) | 0.0% (n = 0) | .104 | 1.16 (1.10–1.23) |
| O2 supplementation | 39.6% (n = 67) | 8.3% (n = 2) | .003 | 1.18 (1.08–1.29) |
| HFNC | 24.3% (n = 41) | 0.0% (n = 0) | .007 | 1.19 (1.11–1.27) |
| nCPAP | 1.8% (n = 3) | 0.0% (n = 0) | .511 | 1.15 (1.08–1.21) |
| IMV | 1.2% (n = 2) | 0.0% (n = 0) | .592 | 1.14 (1.08–1.21) |
| Mortality | 0% (n = 0) | 0% (n = 0) | >.999 | … |
| Neither RSV Nor SARS-CoV-2 (n = 555) |
||||
| Median age, mo (min; max) | 16.0 (0; 59) | |||
| Male sex | 58.9% (n = 327) | |||
| Patients at risk | 15.3% (n = 85) | |||
| Signs and symptoms | ||||
| Dyspnea | 14.2% (n = 79) | |||
| Fever | 60.9% (n = 338) | |||
| Cough | 39.5% (n = 219) | |||
| Rhinitis | 26.1% (n = 145) | |||
| GI symptoms | 24.7% (n = 137) | |||
| Median time of hospitalization, d (min; max) | 3 (0; 72) | |||
| Intensive care treatment | 4.3% (n = 24) | |||
| O2 supplementation | 10.8% (n = 60) | |||
| HFNC | 7.4% (n = 41) | |||
| nCPAP | 0.7 (n = 4) | |||
| IMV | 2.2% (n = 12) | |||
| Mortality | 0.7% (n = 4) | |||
Abbreviations: CI, confidence interval; GI, gastrointestinal; HFNC, high-flow nasal cannula; IMV, invasive mechanical ventilation; nCPAP, nasal continuous positive airway pressure; O2, oxygen; RR, relative risk; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Values in bold denote significant P Value (<.05).
DISCUSSION
In our cohort, symptomatic patients with RSV were sicker than patients with SARS-CoV-2 and were hospitalized significantly longer. Moreover, patients with RSV infection required a higher level of medical care and more intensive nursing than patients infected with SARS-CoV-2 due to a higher need of respiratory support with HFNC and oxygen supplementation. It is noteworthy that 11 of 41 patients (26.8%) required >15 L/minute of flow. Therefore, the incidence of nCPAP support might be higher in other centers that limit flow to 10–12 L/minute in young infants, and the need for nCPAP support in RSV infections might be underestimated in our survey. Moreover, we were able to underline the higher need for medical care and more intensive nursing by showing that RSV patients have a higher risk of intensive care treatment at the PICU, a higher risk of nCPAP, and a higher risk of intubation.
This analysis was limited by its retrospective nature and its local cohort. Despite the shown differences in clinical appearance, in times of high prevalence of both infections, clinical symptoms cannot be used to reasonably differentiate between these 2 infections. Thus, RT-PCR testing is necessary for the diagnosis and subsequent cohorting of infected patients. Although the outcome of both infections is good, one should not forget long-term outcome after RSV infections such as preschool wheeze, asthma, and repeated respiratory infections. Long-term outcomes of SARS-CoV-2-infections are unknown or suspected to play a small role with regard to children at present.
In summary, RSV-infected patients require a higher level of medical care and had to stay in hospital for longer than SARS-CoV-2–infected children, thereby placing higher stress on hospital resources and the healthcare system in general.
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Meissner HC. Viral bronchiolitis in children. N Engl J Med 2016; 374:62–72. [DOI] [PubMed] [Google Scholar]
- 2. Hall CB, Weinberg GA, Blumkin AK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeoh DK, Foley DA, Minney-SmithCA, et al. The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis 2021; 72:2199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trenholme A, Webb R, Lawrence S, et al. COVID-19 and infant hospitalizations for seasonal respiratory virus infections, New Zealand, 2020. Emerg Infect Dis 2020; 27:641–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. European Centre for Disease Control and Prevention. Surveillance atlas of infectious diseases. https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases. Accessed 30 November 2021.
- 6. Hernandez-Rivas L, Pedraz T, Calvo C, et al. Respiratory syncytial virus outbreak during the COVID-19 pandemic. How has it changed? [in Spanish] [manuscript published online ahead of print 22 December 2021]. Enferm Infecc Microbiol Clin 2021. doi: 10.1016/j.eimc.2021.12.003. [DOI] [Google Scholar]
- 7. Lumley SF, Richens N, Lees E, et al. Changes in paediatric respiratory infections at a UK teaching hospital 2016-2021; impact of the SARS-CoV-2 pandemic. J Infect 2022; 84:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic—United States, 2020-2021. MMWR Morb Mortal Wkly Rep 2021; 70:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ralston SL, Lieberthal AS, Meissner AC, et al. American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management and prevention of bronchiolitis. Pediatrics 2015; 136:782. [DOI] [PubMed] [Google Scholar]
- 10. Robert Koch Institut. Weekly situation report on the novel Coronavirus [in German]. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2021-11-25.pdf?__blob=publicationFile. Accessed 1 December 2021. [Google Scholar]
- 11. Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults. a systematic review and meta-analysis. JAMA Pediatr 2020; 175:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. German Society for Pediatric Infectious Diseases; German Society for Hospital Hygiene. Hospitalization and mortality from COVID-19 among children in Germany [in German]. https://dgpi.de/stellungnahme-dgpi-dgkh-hospitalisierung-und-sterblichkeit-von-covid-19-bei-kindern-in-deutschland-18-04-2021/. Accessed 30 November 2021.
- 13. Sticht C via Deutsche Presse Agentur (dpa). Many more children than usual with respiratory infections [in German]. https://www.aerzteblatt.de/nachrichten/127834/Viel-mehr-Kinder-als-ueblich-mit-Atemwegsinfekten. Accessed 6 December 2021.