Abstract
Context
The type 2 deiodinase and its Thr92Ala-DIO2 polymorphism have been linked to clinical outcomes in acute lung injury and pulmonary fibrosis.
Objective
Our objectives were to evaluate were cumulative mortality during admission according to Thr92Ala-DIO2 polymorphism.
Methods
Here we conducted an observational, longitudinal, and prospective cohort study to investigate a possible association between the Thr92Ala-DIO2 polymorphism and intrahospital mortality from COVID-19 in adult patients admitted between June and August 2020. Blood biochemistry, thyroid function tests, length of stay, comorbidities, complications, and severity scores were also studied according to Thr92Ala-DIO2 polymorphism.
Results
In total, 220 consecutive patients (median age 62; 48-74 years) were stratified into 3 subgroups: Thr/Thr (n = 79), Thr/Ala (n = 119), and Ala/Ala (n = 23). While the overall mortality was 17.3%, the lethality was lower in Ala/Thr patients (12.6%) than in Thr/Thr patients (21.7%) or Ala/Ala patients (23%). The heterozygous genotype (Thr/Ala) was associated with a 47% reduced risk of intrahospital mortality whereas univariate and multivariate logistic regression adjusted for multiple covariates revealed a reduction that ranged from 51% to 66%. The association of the Thr/Ala genotype with better clinical outcomes was confirmed in a metanalysis of 5 studies, including the present one.
Conclusion
Here we provide evidence for a protective role played by Thr92Ala-DIO2 heterozygosity in patients with COVID-19. This protective effect follows an inheritance model known as overdominance, in which the phenotype of the heterozygote lies outside the phenotypical range of both homozygous.
Keywords: thyroid, type II deiodinase, polymorphism and COVID-19
The coronavirus 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) virus, has created a major global health crisis. Despite having infected hundreds of millions and killed more than 5 million, gaps remain in our understanding of the pathophysiology of the disease (1). SARS-Cov-2 infection can cause pulmonary and systemic inflammation, leading to multiple organ dysfunction in high-risk populations such as elderly people, pregnant women, patients with obesity, hypertension, and diabetes, and those on long-term immunosuppressive therapy (2-5). The lethality among hospitalized patients ranges from 11% and 15% (2, 6, 7).
The most feared complication of COVID-19 is severe acute respiratory syndrome (SARS) which has an in-hospital prevalence of 17% to 29% (2, 7). Acute lung injury (ALI) is 1 of the main causes of respiratory failure that usually develops in response to major insults, such as sepsis, trauma, viral or bacterial pneumonia, and multiple blood transfusions during hospitalization (8). In this regard, it is well accepted that low triiodothyronine syndrome is a strong predictor of poor prognosis in critically ill hospitalized patients, including patients admitted to the intensive care unit (ICU), pneumonia, severe burns, stroke, septic shock, respiratory failure, cardiovascular diseases, and multiple trauma (9-16). This has also been found in patients with COVID-19, with strong association between reduced levels of free T3 (fT3) at admission of hospitalized patients with intrahospital severity and mortality (17-21).
While different strategies have been employed to minimize ALI and improve lung function, we note a growing interest in treating lung disease with thyroid hormone (TH) in pulmonary edema and acute respiratory distress syndrome (ARDS). Instillation of the TH T3 increased alveolar fluid clearance in rat lungs with hypoxia-induced lung injury (22). In addition, TH exhibits antifibrotic properties that are associated with the protection of alveolar epithelial cells and restoration of mitochondrial function. TH can reach the lungs via systemic circulation but can also be activated locally, within type II pulmonary alveolar epithelial cells, through the activity of the iodothyronine deiodinase 2 (DIO2). Remarkably, DIO2 expression and activity were elevated in lungs from patients with idiopathic pulmonary fibrosis, and its knockout worsened bleomycin-induced lung fibrosis in mice (23).
Accordingly, Ma et al reported increased severity of ALI in mice with reduced DIO2 expression (through RNA silencing), suggesting a protective role of DIO2 in ALI. They also reported that carrying a polymorphism in DIO2 (Thr92Ala) in humans confers protection against ALI. Increased DIO2 expression may dampen the ALI inflammatory response, thereby strengthening the premise that TH metabolism is intimately linked to the integrated response to inflammatory injury in critically ill patients (24).
DIO2 is located on the long arm of chromosome 14, at position 14q0.24.3. To date, 6 single nucleotide polymorphisms (SNPs) of these genes have been described in the literature, including rs225014, ORFa-Gly3Asp, rs225010, rs225012, rs2267872, and rs1388378 (25-27). The rs225014 SNP, also known as the Thr92Ala-DIO2 polymorphism, is located in exon 2 of DIO2. It causes the substitution of threonine for an alanine at position 92, which has reduced activity (28, 29). Nonetheless, carrying the Thr92Ala-DIO2 polymorphism does not affect thyroid function tests in individuals who have a normal thyroid gland, but this is controversial in thyroidectomized patients kept on levothyroxine (LT4) (30). In a large report that included approximately 550 LT4-treated patients, the Thr92Ala-DIO2 polymorphism had no effect on the serum T4:T3 ratio (31), but in another series of 140 patients it was associated with a higher T4:T3 ratio (32).
The minor allele Ala92-Dio2 has a high prevalence in the population (38.8-47.6%). Several studies have analyzed its association with multiple chronic diseases, such as type 2 diabetes mellitus (28, 33-35), obesity (36), arterial hypertension (37, 38), osteoarthritis (39), osteoporosis (40), and dementias (41), many of which are risk factors for a worse prognosis for COVID-19 (42-44), but controversy has prevented the formulation of a unifying hypothesis as to its role (29). Here we tested whether the Thr92Ala-DIO2 polymorphism is associated with intrahospital mortality from COVID-19.
Material and Methods
Subjects
This study was performed at the Dom José Maria Pires Metropolitan Hospital (João Pessoa, Paraíba, Brazil). Adults (≥18 years) who presented to the emergency department between June and August 2020 with COVID-19 symptoms and were assessed through blood biochemistry were preliminarily recruited into this observational, longitudinal, and prospective cohort study. The study was approved by the Human Research Ethics Committee of the Lauro Wanderley University Hospital (CAAE:31562720.9.0000.5183). This study was performed in agreement with the Declaration of Helsinki and in compliance with local and national regulations.
Inclusion and Exclusion Criteria
A total of 220 consecutive patients with a positive result on standard of care reverse transcription polymerase chain reaction test (RT-qPCR; Biomol OneStep/COVID-19, IBMP, Paraná, Brazil) for SARS-CoV-2 in nasopharyngeal swabs, or, in cases of negative RT-qPCR, having clinical, radiological, and serological (IgG positive for SARS-CoV-2) criteria. Samples were centrifuged at 2000g for 15 minutes at 4°C and subsequently frozen at −80°C until measurement. Exclusion criteria were (1) patients younger than 18 years, (2) patients with a history of thyroid disease and diagnosis of pregnancy, and (3) patients who had used iodinated contrast in the last 6 months or drugs that interfere with thyroid metabolism. Race or ethnic background were not considered in the study. Although historically the Brazilian population was formed by natives of that country, Europeans, and Africans, the degree to which these races are now intermixed minimizes the relevance of such distinctions in Brazilian populational studies.
Severity Scoring
Within the first 48 hours of admission, patient severity was first defined using 3 scoring systems: (1) the quick Sepsis-related Organ Failure Assessment (qSOFA), the National Early Warning Score 2 (NEW2), and chest computed tomography severity score proposed by Pan et al (45).
Blood Biochemistry
Blood samples (50 mL) were collected within first 48 hours of hospital admission (before interventions or therapy that could potentially interfere or alter TH or cytokine serum levels, including steroids and heparin). Plasma concentrations of interleukin 6 (IL-6), high-sensitive C-reactive protein (CRP), D-dimer, creatinine, alanine transaminase (ALT), aspartate transaminase (AST), and lactate dehydrogenase (LDH), thyroid function (fT3, free thyroxine [fT4], reverse triiodothyronine [rT3], thyrotropin [TSH]), thyroglobulin, antithyroid peroxidase antibodies (anti-TPO), and albumin were assessed using measured by chemiluminescence immunoassay (MAGLUMI-2000-PLUS, Shenzhen New Industries Biomedical Engineering Co., Shenzhen, China), according to the manufacturer’s protocol. The complete blood cells count with differential was performed on a MEK-7300 hematological analyzer (Nihon Kohden®, Tokyo, Japan). The neutrophil to lymphocyte ratio was calculated by the absolute neutrophil count divided by the absolute lymphocyte count.
Genotyping Analysis
Genomic DNA was extracted from whole blood using standard techniques. In this study the polymorphism was determined by the TaqMan® SNP Genotyping method (7500 Real Time PCR Systems, Applied Biosystems, CA), using the assay for genotyping with TaqMan® probes and primers; in a combination of hybridization and DNA polymerase activity, associated with fluorescence detection (46). For these analyses, a 5-μL solution was be used, containing 20 ng of sample DNA, 2.5 μL of universal Taqman PCR Master Mix (with final concentration 1×), 0.25 μL of the assay (probe and primers) for a concentration of 20×, and 0.125 μL for a concentration of 40× (maintaining a final concentration of 1×), in addition to 2.25 μL of Milli-q® water. For all reactions, a negative and a positive control were performed. For the polymerase chain reaction (PCR), cycles of 10 minutes at 95°C were used for the initial denaturation phase, followed by 50 cycles at 92°C for 15 seconds and 60°C for 90 seconds. We used the software Sequence Detection, version 1.3 (Applied Biosystems, CA) to analyze the data.
Outcomes
Primary
Primary outcomes were cumulative mortality during admission according to Thr92Ala-DIO2 polymorphism.
Secondary
Secondary outcomes were blood biochemistry, thyroid function tests, length of stay, comorbidities, complications, and severity scores according to Thr92Ala-DIO2 polymorphism.
Statistical Analysis
To study mortality, we performed power calculations based on Cohen’s method using GPower 3.1.9.7 software. The current sample size showed more than 90% power to detect significance (α < .05) in association with allele and haplotype under study conditions, and an effect size index of 0.3 was used. Chi-squared tests were used to determine whether samples were in Hardy–Weinberg equilibrium.
The data were expressed as median ± interquartile range (IQR). We used Kruskal–Wallis test analysis followed by Dunn’s post hoc test with Benjamini–Hochberg multiple comparison corrections. Mann–Whitney, chi-squared, or Cochran–Armitage test were used for nonparametric variables. The Kaplan–Meier method and log-rank test were used in our study to investigate the relationship between variables and COVID-19 prognosis.
To assess the relative risk of mortality (odds ratio [OR]), we used univariate and multivariate logistic regression. The significance level of P < .05 was accepted as statistically significant. The statistical program GraphPad Prism, v0.7.00 (2016), was used to perform statistical tests.
Next, we used uni- and multivariate logistic regression analysis on the total group (220 patients) to investigate the potential association between the heterozygous allele (Thr/Ala) vs the homozygous alleles (Thr/Thr and Ala/Ala) with mortality. Five multivariate logistic regression models were estimated to individualize mortality by prognostic factors. In the first model (Model 1), the following sociodemographic variables and clinical characteristics were included: male gender, age >60 years, diabetes, arterial hypertension, and length of hospital stay. The second and third models (Model 2 and 3) aimed to evaluate laboratory tests; Model 2 (where the thyroid function was assessed—TSH, fT4, fT3, and rT3) and Model 3 (where markers of inflammation, tissue damage or hemochromocytometric parameters were analyzed—IL-6, CRP, D-dimer, neutrophils, and DHL). Finally, Model 4 was adjusted for Models 1 and 3, and Model 5, for all variables of the analyzed models.
Meta-analysis
A systematic review was conducted according to the recommendations outlined in the PRISMA (47). The electronic databases Pubmed, Cochrane, and SciELO were combed for studies of genetic association between the Thr92Ala-DIO2 polymorphism and diseases. We limited the search to humans and used the following strategy: “rs225014” or rs225014-T/C or “thr92ala” or “dio2 a/g” or “T92A.” Only studies observing these 3 inclusion criteria were selected: (1) observational studies (cohort, case–control, and cross-sectional studies) on the Thr92Ala-DIO2 polymorphism, (2) studies that included patients with diseases nonrelated to thyroid dysfunction, and (3) presence of control group. Data were independently extracted by 2 authors (F.E.L.B., G.C.) using a predetermined structured form. Disagreements were solved through discussion or when needed by consulting a third author (H.E.R.).
Statistics
The strength of the association between Thr92Ala-DIO2 polymorphism and diseases was measured by OR with 95% CI for dominant (Thr/Thr vs Thr/Ala + Ala/Ala), recessive (Ala/Ala vs Thr/Ala + Thr/Thr), and overdominant (Thr/Ala vs Thr/Thr + Ala/Ala). The chi-squared–based Q-test was used to assess the between-study heterogeneity and P = .1 was considered to indicate significant heterogeneity among studies. I2 statistic and Cochran Q test were applied to examine the heterogeneity and the pooled OR estimation of each study was calculated by the fixed and random-effects model (the Mantel–Haenszel method). The I2 statistic was specifically documented for the percentage of study variability observed due to heterogeneity rather than chance (I2 = 0-25%, no heterogeneity; I2 = 25-50%, moderate heterogeneity; I2 = 50-75%, high heterogeneity; I2 = 75-100%, extreme heterogeneity) (48).
All statistical tests were performed using Review Manager 5.4 (The Cochrane Collaboration, UK). P < .05 was considered statistically significant.
Results
Patient Demographics and Clinical Characteristics
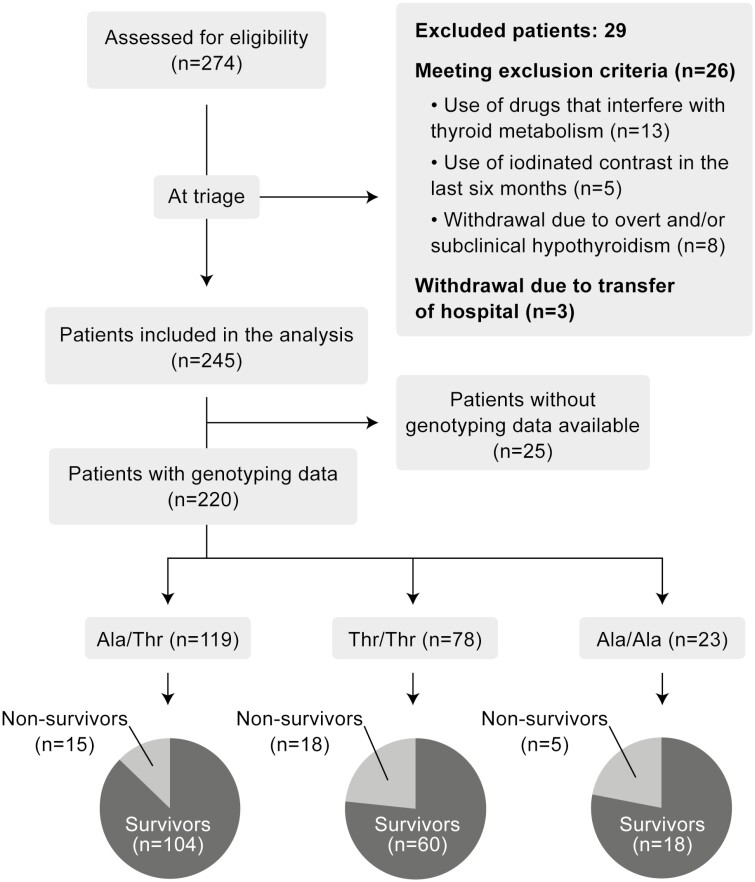
A total of 274 consecutive patients admitted with COVID-19 were evaluated for potential enrollment in the study. In total, 245 patients met the inclusion criteria and were considered as potential subjects for the study. An additional 25 patients were excluded for lack of genotype determination. The remaining 220 patients completed the study (Fig. 1). The median age was 62 (48-74) years, and 135 patients (61.3%) were male. Baseline sociodemographic and clinical characteristics are summarized in Table 1. Most patients had underlying diseases, including hypertension (65.4%), diabetes (42.3%), and cardiopathy (12.7%), neoplasia (0.9%), and chronic pneumopathy (4.5%). The group of 220 patients was stratified into 3 subgroups: Thr/Thr (n = 79), Thr/Ala (n = 119) and Ala/Ala (n = 23) (Fig. 1). The Thr allele frequency was 0.62 and the Ala allele frequency was 0.37, with a distribution that was in Hardy–Weinberg equilibrium (P = .07; chi-squared test).
Figure 1.
Flowchart of the study.
Table 1.
Demographic and clinical characteristics of the patient cohort and their association with Thr92Ala-DIO2 polymorphism and mortality (n = 220)
| Variables | Mann–Whitney test and Cochran–Armitage test | Univariate logistic regression Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Thr/Thr | Thr/Ala | Ala/Ala | P | OR | IC 95% | P | |
| (n = 220) | (n = 78) | (n = 119) | (n = 23) | |||||
| Age (y), median (IQR) | 62 (48-74) | 63 (51-75) | 60 (47-74) | 63 (47-77) | .539 | 1.016 | 0.993-1.040 | .176 |
| BMI(kg/m2) | 29.8 (26-34) | 29.8 (27-33) | 29.8 (25-34) | 29.4 (26-34) | .923 | 0.993 | 0.938-1.050 | .859 |
| Age > 60 year, n (%) | 116 (52.7) | 43 (56.5) | 60 (50.4) | 13 (56.5) | .501 | 1.467 | 0.725-3.044 | .291 |
| Gender male, n (%) | 135 (61.3) | 41 (52.5) | 79 (66.4) | 15 (65.2) | .16 | 0.647 | 0.319-1.318 | .226 |
| Length of hospital stay (d), median (IQR) | 6 (4-10) | 7 (4-11) | 6 (4-10) | 6 (4-12) | .611 | 1.099 | 1.052-1.154 | <.0001 |
| Comorbidities | ||||||||
| Hypertension, n (%) | 144 (65.4) | 58 (74.3) | 72 (60.5) | 14 (60.9) | .049 | 1.175 | 0.565-2.559 | .672 |
| Diabetes mellitus, n (%) | 93 (42.3) | 33 (42.3) | 51 (42.8) | 9 (39.1) | .881 | 1.352 | 0.661 -2.755 | .405 |
| Cardiopathy, n (%) | 28 (12.7) | 12 (15.4) | 15 (12.6) | 1 (4.3) | .628 | 0.333 | 0.052-1.187 | .146 |
| Chronic pneumopathy, n(%) | 10 (4.5) | 5 (6.4) | 4 (3.3) | 1 (4.3) | .317 | 0.51 | 0.027-2.891 | .540 |
| Neoplasia, n (%) | 2 (0.9) | 0 (0) | 1 (0.8) | 1 (4.3) | .631 | 4.89 | 0.190-125.5 | .265 |
| Obesity, n (%)a | 103 (49) | 37 (49) | 55 (49.1) | 11 (47.8) | .982 | 0.39 | 0.169-0.852 | .021 |
| Complications | ||||||||
| NTIS, n (%) | 14 (6.4) | 4 (5.1) | 7 (5.9) | 3 (13) | .055 | 4.1 | 1.268-12.5 | .0142 |
| Cardiovascular shock, n (%) | 28 (12.7) | 12 (15.4) | 13 (10.9) | 3 (13) | .357 | 54.2 | 19.3-181.4 | <.0001 |
| Endotracheal intubation, n (%) | 29 (13.2) | 14 (17.9) | 12 (10) | 3 (13) | .111 | 129.3 | 39-600 | <.0001 |
| ICU admission, n (%) | 55 (25) | 24 (30.7) | 26 (21.8) | 5 (21.7) | .37 | 36.8 | 14.8-106.9 | <.0001 |
| Scores systems | ||||||||
| NEWS2 score, median (IQR) | 6 (5 -7) | 5 (4-6) | 6 (5 -7) | 6 (5 -7) | .346 | 1.162 | 0.960-1.348 | .134 |
| q-SOFA score, median (IQR) | 1 (1-1) | 1 (1-1) | 1 (0-1) | 1 (1-1) | .435 | 1.897 | 0.892-4.256 | .105 |
| CT COVID score, median (IQR) | 20 (15-20) | 20 (15-20) | 20 (15-20) | 20 (15-20) | .786 | 1.043 | 0.972-1.133 | .277 |
Mann–Whitney test was performed for continuous variables (age, NEWS2, qSOFA and CT COVID score) while Cochran–Armitage test was performed for all other variables.
Abbreviations: BMI, body mass index; CT, computed tomography; ICU, intensive care unit; IQR, interquartile range; NEWS2, National Early Warning Score 2; NTIS, nonthyroid illness syndrome; qSOFA, quick Sepsis-related Organ Failure Assessment.
a (n = 210 patients).
Blood biochemistry is shown in Table 2. Several thyroid function tests and markers of inflammation, tissue damage, or hemochromocytometric parameters were found to predict mortality using the univariate logistic regression (Table 2). Most parameters were not affected by the patient’s genotype except for fT3, ALT, and hemoglobin levels (Table 2).
Table 2.
Blood biochemistry in COVID-19 patients and their association with Thr92Ala-DIO2 polymorphism and mortality
| Kruskal–Wallis test median (IQR) | Univariate logistic regression mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters (normal range) | Total | A (Thr/Thr) | B (Thr/Ala) | C (Ala/Ala) | P | Benjamini–Hochberg’s | OR | IC 95% | P |
| (n = 221) | (n = 79) | (n = 119) | (n = 23) | test | |||||
| TSH (0.4-5.8 µIU/mL) | 1.66 (0.9-3) | 1.55 (0.9-3) | 1.62 (0.9-2.9) | 2.15 (1.0-3.8) | .322 | 0.986 | 0.796-1.193 | .895 | |
| fT4 (0.89-1.72 ng/dL) | 1.33 (1-1.6) | 1.3 (1-1.5) | 1.35 (1.0-1.7) | 1.28 (0.8-1.5) | .465 | 1.011 | 0.497-1.989 | .975 | |
| fT3 (2.0-4.2 pg/mL) | 2.96 (2.6-3.4) | 2.92 (2.6-3.3) | 3.14 (2.6-3.5) | 2.77 (2.1-3.2) | .029 | A-B (P = .054) B-C (P = .023) |
0.460 | 0.276-0.739 | .002 |
| rT3 (0.1-0.35 ng/mL) | 0.48 (0.3-0.6) | 0.38 (0.2-0.6) | 0.52 (0.3-0.6) | 0.53 (0.3-0.6) | .249 | 0.086 | 0.013-0.461 | .006 | |
| Thyroglobulin (1.59- 59.9 ng/mL) | 15.1 (6-26) | 15.6 (6-28.7) | 14.5 (6-23) | 15.8 (6-45) | .725 | 0.994 | 0.976-1.005 | .414 | |
| FT3/RT3 | 6.60 (4.6-9.9) | 7 (4.7-10.6) | 6.61 (4.7-9.8) | 4.83 (3.7-6.7) | .056 | B-C(P = .045) A-C (P = .016) | 1.017 | 0.963-1.067 | .496 |
| FT3●RT3 | 1.28 (0.8-2.1) | 1.1 (0.7-1.9) | 1.46 (0.8-2.3) | 1.24 (0.7-1.9) | .075 | A-B (P = .025) | 0.441 | 0.249-0.717 | .002 |
| FT4/FT3 | 0.43 (0.3-0.5) | 0.45 (0.3-0.5) | 0.43 (0.3-0.5) | 0.48 (0.3-0.6) | .691 | 9.697 | 1.737-57.4 | .01 | |
| IL-6 (<3.4 pg/mL) | 49.8 (21-87) | 43 (23-83) | 55.5 (23-96) | 32.8 (19-84) | .533 | 1.000 | 0.999-1.001 | .739 | |
| D-dimer (<500 ng/mL) | 759 (487-1628) | 924 (546-1579) | 696 (458-1496) | 706 (488-3629) | .447 | 1.000 | 1.000-1.000 | .047 | |
| LDH (207-414 U/L) | 742 (538-1014) | 723 (522-1006) | 772(564-1051) | 718 (488-992) | .529 | 1.001 | 1.000-1.002 | .008 | |
| Creatinine (mg/dL) | 1.1 (0.9-1.3) | 1.13 (0.9-1.4) | 1.1 (0.8-1.3) | 1.0 (0.9-1.3) | .342 | 1.017 | 0.754-1.197 | .851 | |
| CRP (<5.0 mg/dL) | 83 (36-151) | 68 (30-145) | 100 (42-162) | 75.3 (33-142) | .337 | 1.009 | 1.004-1.015 | .002 | |
| ALT (8-42 U/L) | 62 (39-102) | 53 (38-97) | 68 (46-111) | 48 (23-114) | .06 | A-B(P = .046) | 0.992 | 0.984-0.999 | .067 |
| AST (8-42 U/L) | 54 (38-80) | 49 (36-77) | 56 (40-84) | 52 (33-75) | .206 | 0.996 | 0.988-1.003 | .382 | |
| Neutrophils (1935-6700 103 cells/µL) | 7412 (5348-10130) | 7701(5237-9290) | 7303 (5340-10660) | 7560 (5642-9945) | .978 | 1.000 | 1.000-1.000 | .053 | |
| Hemoglobin (13-18 g/dL) | 13.4 (12-14.4) | 13 (11-14) | 13.6 (12-14) | 13.3 (12-14) | .032 | A-B(P = .009) | 0.816 | 0.680-0.978 | .027 |
| N/L ratio (1-3) | 9.27 (5.8-14) | 9.5 (5.8-14) | 9.11 (6-14) | 10.5 (5.2-14) | .954 | 1.077 | 1.025-1.135 | .004 | |
| Albumin (3.5-5.5 g/dL) | 3.3 (2.9-3.7) | 3.3 (2.9-3.7) | 3.3 (2.9-3.7) | 3.2 (2.7-3.4) | .609 | 0.328 | 0.158-0.653 | .002 | |
Kruskal–Wallis test and univariate logistic regression (mortality) were performed for all variables.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; CRP, C-reactive protein; fT3, free triiodothyronine; fT4, free thyroxine; IL-6, interleukin 6; IQR, interquartile range; LDH, lactate dehydrogenase; N/L ratio, neutrophil–lymphocyte ratio; OR, odds ratio; rT3, reverse triiodothyronine; TSH, thyrotropin.
Clinical Outcomes
Primary
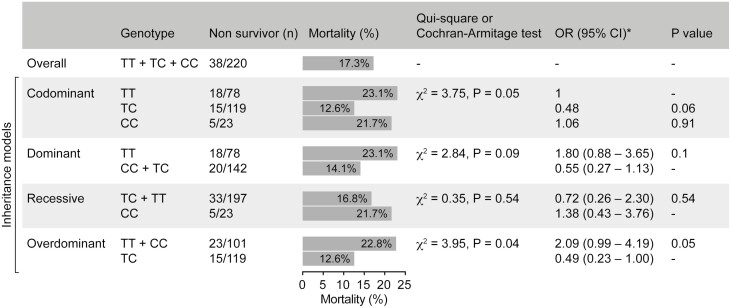
The overall mortality, regardless of genotype, was 38 (17.3%) (Fig. 2). Mortality was lower in Ala/Thr patients (12.6%) than in Thr/Thr patients (21.7%) or Ala/Ala patients (23%) (Fig. 2). Logistic regression analysis confirmed that the presence of the Thr/Ala allele was associated with reduced mortality when compared with Thr/Thr, even after correcting for 14 comorbidities and other covariates (Table 3).
Figure 2.
DIO2 Thr92Ala polymorphism and mortality (Cochran–Armitage test and chi-squared test); CI, confidence interval; OR, odds ratio; X2, chi squared.
Table 3.
Multivariable regression analyses of mortality, considering multiple covariates, Thr92Ala-DIO2 polymorphism, and overdominant inheritance model
| Ala/Thr vs Ala/Ala | Ala/Thr vs Thr/Thr | Thr/Thr vs Ala/Ala | Ala/Thr vs Ala/Ala + Thr/Thr (Overdominant model) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR* | CI 95% | P | OR* | CI 95% | P | OR* | CI 95% | P | OR* | CI 95% | P | ||||
| Mortality | 0.52 | 0.18-1.75 | .26 | 0.49 | 0.23-1.04 | 0.06 | 1.06 | 0.36-3.57 | .9 | 0.52 | 0.26-1.04 | 0.07 | |||
| Model 5 | Model 4 | Model 1 | Male gender | 0.52 | 0.18-1.76 | .26 | 0.49 | 0.23-1.04 | 0.06 | 1.06 | 0.36-3.57 | .9 | 0.52 | 0.26–1.04 | 0.07 |
| Age > 60 year | 0.53 | 0.18-1.79 | .27 | 0.50 | 0.23-1.06 | 0.07 | 1.07 | 0.36-3.59 | .9 | 0.53 | 0.26–1.06 | 0.07 | |||
| Diabetes | 0.48 | 0.16-1.62 | .20 | 0.45 | 0.21-0.98 | 0.04 | 1.05 | 0.36-3.53 | .9 | 0.49 | 0.23–0.98 | 0.047 | |||
| Hypertension | 0.52 | 0.18-1.75 | .26 | 0.49 | 0.23-1.06 | 0.07 | 1.05 | 0.36-3.55 | .9 | 0.53 | 0.26–1.06 | 0.08 | |||
| Hospital stay | 0.47 | 0.15-1.65 | .21 | 0.48 | 0.21-1.07 | 0.07 | 0.97 | 0.32-3.39 | 1.0 | 0.51 | 0.24–1.05 | 0.07 | |||
| Model 1 | 0.43 | 0.14-1.54 | .17 | 0.43 | 0.19-0.99 | 0.048 | 0.99 | 0.32-3.51 | 1.0 | 0.46 | 0.21–0.98 | 0.046 | |||
| Model 2 | TSH | 0.51 | 0.17-1.72 | .24 | 0.49 | 0.23-1.03 | 0.06 | 1.04 | 0.35-3.51 | .9 | 0.52 | 0.26–1.05 | 0.07 | ||
| Free T3 | 0.74 | 0.24-2.65 | .62 | 0.54 | 0.25-1.16 | 0.12 | 1.37 | 0.44-4.87 | .6 | 0.62 | 0.3–1.28 | 0.20 | |||
| Free T4 | 0.51 | 0.17-1.74 | .25 | 0.49 | 0.23-1.03 | 0.06 | 1.06 | 0.36-3.55 | .9 | 0.52 | 0.25–1.04 | 0.07 | |||
| Reverse T3 | 0.48 | 0.16-1.66 | .22 | 0.54 | 0.25-1.17 | 0.12 | 0.89 | 0.29-3.07 | .8 | 0.56 | 0.27–1.13 | 0.11 | |||
| Model 2 | 0.61 | 0.19-2.24 | .42 | 0.56 | 0.25-1.22 | 0.14 | 0.92 | 0.25-1.22 | .9 | 0.64 | 0.3–1.32 | 0.23 | |||
| Model 3 | IL-6 | 0.51 | 0.17-1.73 | .25 | 0.49 | 0.23-1.04 | 0.06 | 1.05 | 0.26-3.52 | .9 | 0.52 | 0.25–1.04 | 0.07 | ||
| CRP | 0.55 | 0.16-2.19 | .36 | 0.41 | 0.18-0.9 | 0.03 | 1.34 | 0.4-5.36 | .6 | 0.46 | 0.21–0.96 | 0.04 | |||
| D-DIMER | 0.57 | 0.19-1.97 | .34 | 0.51 | 0.23-1.11 | 0.09 | 1.12 | 0.37-3.85 | .9 | 0.55 | 0.27–1.13 | 0.11 | |||
| Neutrophil | 0.47 | 0.16-1.61 | .20 | 0.44 | 0.2-0.96 | 0.04 | 1.06 | 0.36-3.59 | .9 | 0.48 | 0.23–0.97 | 0.04 | |||
| LDH | 0.46 | 0.15-1.58 | .19 | 0.47 | 0.21-1.05 | 0.07 | 0.97 | 0.32-3.33 | 1.0 | 0.49 | 0.23–1.03 | 0.06 | |||
| Model 3 | 0.52 | 0.14-2.2 | .34 | 0.36 | 0.15-0.85 | 0.02 | 1.44 | 0.41-6.08 | .6 | 0.42 | 0.18–0.92 | 0.03 | |||
| Model 4 | 0.46 | 0.12-2.04 | .27 | 0.32 | 0.12-0.8 | 0.02 | 1.43 | 0.38-6.41 | .6 | 0.38 | 0.16–0.87 | 0.03 | |||
| Model 5 | 0.56 | 0.13-2.85 | .46 | 0.32 | 0.12-0.85 | 0.03 | 1.74 | 0.4-9.02 | .5 | 0.40 | 0.16–0.98 | 0.049 | |||
Multivariable regression analyses: Model 1, adjusted for male gender, age >60 years, diabetes, arterial hypertension, and length of hospital stay. Model 2, adjusted for thyrotropin, free triiodothyronine, free thyroxine, and reverse triiodothyronine. Model 3, adjusted for interleukin-6, neutrophils, lactate dehydrogenase, C-reactive protein, and D-dimer. Model 4, adjusted for Model 1 and 3. Model 5, adjusted for all of the above variables.
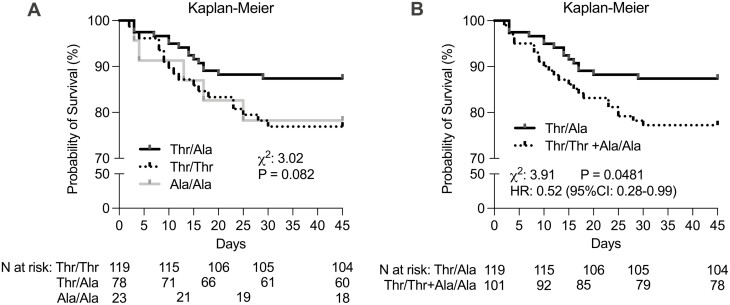
The Cochran–Armitage test was used to assess the relationship between genotype and mortality according to 4 inheritance models. The dominant, recessive, and codominant models did not predict a relationship, although the last one revealed a borderline statistical significance for the heterozygous group (Fig. 2). At the same time, an association between lower mortality and Thr92Ala-DIO2 heterozygosity was statistically significant when the overdominant model was utilized. This is illustrated in the Kaplan–Meier survival analysis (Fig. 3). Logistic regression analysis confirmed that the presence of the Thr/Ala allele was associated with reduced mortality when compared with Thr/Thr and Ala/Ala, even after accounting for comorbidities (Table 3).
Figure 3.
Kaplan–Meier survival curve and their association with mortality. Kaplan–Meier survival curves of DIO2 Thr92Ala polymorphism for the overall survival in patients with COVID-19. (Thr/Thr vs Thr/Ala vs Ala/Ala and Thr/Ala vs Thr/Thr + Ala/Ala). IQR, interquartile range; HR, hazard ratio.
Secondary
As a group, the median hospital stay was 6 (4-10) days. The severity score systems indicated that patients were moderately/gravely affected by the disease, with developments such as admission to ICU (25%), cardiovascular shock (12.7%), and/or endotracheal intubation (13.2%). As expected, length of stay, ICU admission, cardiovascular shock, and endotracheal intubation correlated with mortality as assessed through univariate logistic regression analysis (Table 1). The analyses of these parameters in the 3 genotype subgroups, namely Thr/Thr, Ala/Thr, Ala/Ala, did not reveal significant differences except for hypertension as an underlying condition, which was higher in the Thr/Thr group (Table 1).
Meta-analysis
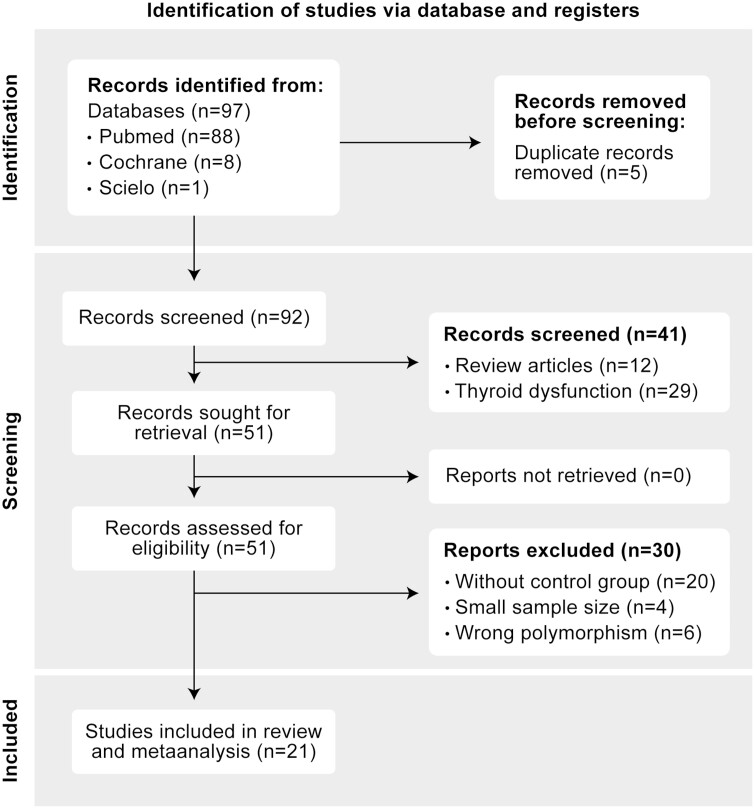
The results of the systematic search and selection led us to 97 studies, which after application of the inclusion and exclusion criteria, were reduced to 21 studies totaling 8400 cases and 20 165 controls (Fig. 4). The analysis focused on 2 major types of studies: (1) those that reported the frequency of the 2 different alleles (Ala and Thr) for each disease and (2) those that reported clinical outcomes for each disease as a function of these 2 different alleles.
Figure 4.
Flow diagram utilized in metanalysis (PRISMA 2020).
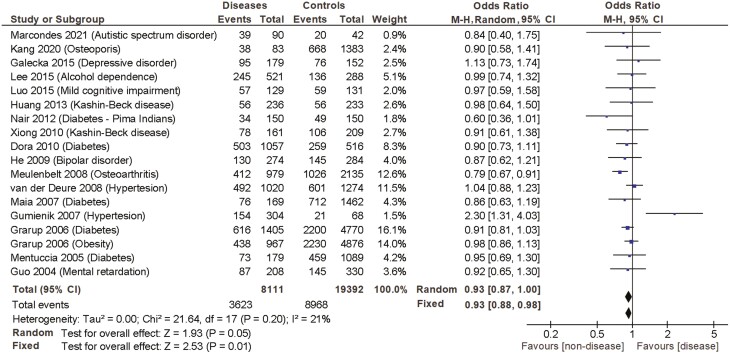
In the frequency analysis, the reported allele frequency was analyzed according to the 3 inheritance models, dominant, overdominant, and recessive. The summary of the resulting fixed and random-effects meta-analysis for the haplotypic association of DIO2 SNPs (Thr/Thr, Thr/Ala, and Ala/Ala) with the different diseases, as well as heterogeneity test, are shown in Table 4 and Fig. 5. The application of the recessive model revealed that the Ala/Ala genotype was associated with a greater risk of having any of the diseases (random OR 1.19 [1.01-1.41], P = .04). At the same time, the application of the overdominant model revealed that carrying the Thr/Ala genotype was associated with a smaller risk of having the diseases (random OR 0.93 [0.87-1.0], P = .05). No significant associations were observed when the dominant model was applied. Notably, both the Q statistical test (chi squared = 53.7, P < .0001) and the I2 statistic (I2 = 68%) showed high heterogeneity for the recessive model, whereas the dominant (chi squared = 22.4, P = .17, I2 = 24%) and overdominant models exhibited no heterogeneity (chi squared = 21.6, P = .20, I2 = 21%) (Table 4 and Fig. 5).
Table 4.
Fixed and random-effects meta-analysis of the haplotypic association between the occurrence of a series of diseases and the Thr92Ala-DIO2 polymorphism according to 3 inheritance models: dominant, overdominant and recessive
| Dominant model | Overdominant | Recessive | ||||||
|---|---|---|---|---|---|---|---|---|
| (Thr/Thr vs Thr/Ala + Ala/Ala) | (Thr/Ala vs Thr/Thr + Ala/Ala) | (Ala/Ala vs Thr/Ala + Thr/Thr) | ||||||
| Author, year | N | Major endpoint | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) |
| 1. Marcondes, 2021 | 132 | Autism | .4 | 1.41 (0.62-3.19) | .6 | 0.84 (0.40-1.75) | .7 | 0.86 (0.37-1.99) |
| 2. Kang, 2020 | 6022 | Osteoporosis | .4 | 0.82 (0.50-1.34) | .7 | 0.90 (0.58-1.41) | .1 | 1.48 (0.89-2.48) |
| 3. Galecka, 2015 | 331 | Recurrent depressive disorder | .5 | 1.16 (0.75-1.79) | .6 | 1.13 (0.73-1.74) | .01 | 0.07 (0.01-0.56) |
| 4. Lee, 2015 | 809 | Alcohol dependence | .7 | 0.94 (0.70-1.27) | 1.0 | 0.99 (0.74-1.32) | .6 | 1.12 (0.76-1.63) |
| 5. Luo, 2015 | 260 | Mild cognitive impairment | .8 | 0.92 (0.55-1.55) | .9 | 0.97(0.59-1.58) | .6 | 1.16 (0.65-2.06) |
| 6. Huang, 2013 | 469 | Kashin–Beck disease | .8 | 1.06 (0.71-1.60) | .9 | 0.98 (0.64-1.50) | .6 | 0.70 (0.22-2.23) |
| 7. Nair, 2012 | 300 | Diabetes (Pima Indians) | .2 | 0.43 (0.13-1.43) | .05 | 0.60 (0.36-1.01) | .01 | 1.86 (1.13-3.04) |
| 8. Xiong, 2010 | 370 | Kashin-Beck disease | .9 | 0.96 (0.61-1.51) | .7 | 0.91 (0.61-1.38) | .5 | 1.21 (0.73-2.02) |
| 9. Dora, 2010 | 1573 | Diabetes | .5 | 0.93 (0.75-1.15) | .3 | 0.90 (0.73-1.11) | .02 | 1.43 (1.05-1.96) |
| 10. He, 2009 | 558 | Bipolar disorder | .009 | 0.61 (0.42-0.88) | .4 | 0.87 (0.62-1.21) | .0001 | 2.27 (1.50-3.44) |
| 11. Meulenbelt, 2008 | 3114 | Osteoarthritis | .9 | 1.01 (0.86-1.18) | .002 | 0.79 (0.67-0.91) | <.0001 | 1.64 (1.32-2.04) |
| 12. Van der Deure, 2008 | 2294 | Hypertension | .7 | 1.04 (0.88 -1.23) | .6 | 1.04 (0.88-1.23) | .2 | 0.84 (0.66-1.07) |
| 13. Maia, 2007 | 1631 | Diabetes | .7 | 1.06 (0.77-1.47) | .4 | 0.86 (0.63-1.19) | .4 | 1.22 (0.78-1.92) |
| 14. Gumieniak, 2007 | 372 | Hypertension | .007 | 0.48 (0.28-0.82) | .004 | 2.30 (1.31-4.03) | .7 | 0.85(0.41-1.76) |
| 15. Grarup, 2006 | 6175 | Diabetes | .3 | 1.06 (0.94-1.20) | .1 | 0.91 (0.81-1.03) | .4 | 1.08 (0.90-1.28) |
| 16. Grarup, 2006 | 5843 | Obesity | .1 | 1.11 (0.97-1.28) | .8 | 0.98 (0.86-1.13) | .1 | 0.82 (0.66-1.02) |
| 17. Mentuccia, 2005 | 1268 | Diabetes | .5 | 0.91 (0.66-1.24) | .7 | 0.95 (0.65-1.30) | .1 | 1.52 (0.93-2.49) |
| 18. Guo, 2004 | 538 | Mental retardation | .7 | 1.07 (0.75-1.52) | .6 | 0.69 (0.48-0.99) | .9 | 1.03 (0.65-1.65) |
| Metanalysis total (fixed) | 27 503 | .7 | 1.01 (0.95-1.07) | .01 | 0.93 (0.88-0.98) | .001 | 1.15 (1.06-1.24) | |
| Metanalysis total (random) | 27 503 | .7 | 0.99 (0.92-1.07) | .05 | 0.93 (0.87-1.00) | .04 | 1.19 (1.01-1.41) | |
| Heterogeneity analysis | Tau2 = .01; chi2 = 22.43, | Tau2 = 0.00; chi2 = 21.64, | Tau2 = .07; chi2 = 53.73, | |||||
| P = .17; I2 = 24% | P = 0.20; I2 = 21% | P < .0001; I2 = 68% | ||||||
| Number of studies with P ≤ .05 | 3 studies | 3 studies | 5 studies | |||||
| Advantage (n)/disadvantage (n) | 2/1 | 2/1 | 1/4 | |||||
Figure 5.
Fixed and random-effects meta-analysis of the haplotypic association between the occurrence of a series of diseases and the Thr92Ala-DIO2 polymorphism according to overdominant inheritance models.
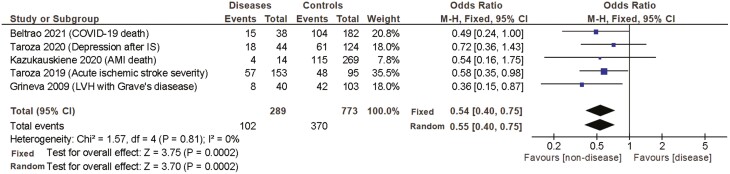
A similar analysis was performed while considering the clinical outcomes. The reported outcome was also analyzed according to the 3 inheritance models. The summary of the resulting fixed and random-effects meta-analysis for the haplotypic association of DIO2 SNPs with the different outcomes, as well as heterogeneity test, are shown in Table 5 and Fig. 6. The dominant inheritance model revealed that carrying the Thr/Thr genotype was associated with a worse outcome (fixed OR 2.18 [1.43-3.3], P = .0003). At the same time, the overdominant model revealed an inverse association between the Thr/Ala genotype and the severity of the clinical outcome (fixed OR 0.54 [0.40-0.75], P = .0002). No significant associations between genotype and clinical outcomes were observed after using the recessive inheritance model (fixed OR 1.27 [0.88-1.83], P = .20) (Table 5 and Fig. 6).
Table 5.
Fixed and random-effects meta-analysis of the haplotypic association between disease outcome and the Thr92Ala-DIO2 polymorphism according to 3 inheritance models: dominant, overdominant and recessive
| Dominant model | Overdominant | Recessive | ||||||
|---|---|---|---|---|---|---|---|---|
| (Thr/Thr vs Thr/Ala + Ala/Ala) | (Thr/Ala vs Thr/Thr + Ala/Ala) | (Ala/Ala vs Thr/Ala + Thr/Thr) | ||||||
| Author, year | N | Major endpoint | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) |
| 1. Beltrao, 2021 | 220 | COVID-19 death | .1 | 1.83 (0.90-3.71) | .05 | 0.49 (0.24-1.00) | .6 | 1.38 (0.48-3.98) |
| 2. Taroza, 2020 | 168 | Depression after AIS | .3 | 2.20 (0.47-10.2) | .4 | 0.72 (0.36-1.43) | .6 | 1.21 (0.61-2.40) |
| 3. Kazukauskiene, 2020 | 283 | Cardiac-related death in ICU | .1 | 2.52 (0.77-8.23) | .3 | 0.54 (0.16-1.75) | .6 | 0.42 (0.02-7.29) |
| 4. Taroza, 2019 | 248 | AIS severity | .4 | 1.53 (0.52-4.49) | .04 | 0.58 (0.35-0.98) | .1 | 1.54 (0.92-2.57) |
| 5. Grineva, 2009 | 143 | LVH with Graves’s disease | .007 | 3.06 (1.36-6.90) | .02 | 0.36 (0.15-0.87) | .4 | 0.49 (0.10-2.34) |
| Metanalysis total (Fixed) | 1062 | .0003 | 2.18 (1.43-3.3) | .0002 | 0.54 (0.40-0.75) | .20 | 1.27 (0.88-1.83) | |
| Metanalysis total (Random) | 1062 | .0004 | 2.17 (1.41-3.3) | .0002 | 0.55 (0.40-0.75) | .17 | 1.30 (0.90-1.88) | |
| Heterogeneity analysis | Tau2 = 0.00; chi2 = 1.37, | Tau2 = 0.00; chi2 = 1.57, | Tau2 =0 .00; chi2 = 2.57, | |||||
| P = .85; I2 = 0% | P = 0.81; I2 = 0% | P = .63; I2 = 0% | ||||||
| Number of studies with P ≤ .05 | 1 studies | 3 studies | 0 studies | |||||
| Advantage (n)/Disadvantage (n) | 0/1 | 3/0 | 0/0 | |||||
Abbreviations: AIS, acute ischemic stroke; ICU, intensive care unit; LVH, left ventricular hypertrophy.
Figure 6.
Fixed and random-effects meta-analysis of the haplotypic association between disease outcome and the Thr92Ala-DIO2 polymorphism according to overdominant inheritance models. AMI, acute myocardial infarction; IS, ischemic stroke; CI, confidence interval; ICU, intensive care unit; LVH, left ventricular hypertrophy; M-H, Mantel–Haenszel.
Discussion
To our knowledge, this is the first prospective study of patients hospitalized with COVID-19 in which the primary clinical outcome (mortality) was analyzed according to the Thr92Ala-DIO2 polymorphism. Our findings revealed that carrying the Thr/Ala genotype was associated with lower mortality rates. The Kaplan–Meier curve shows that the heterozygous genotype (Thr/Ala) was associated with a 47% reduced risk of intrahospital mortality (Fig. 3). The univariate and multivariate logistic regression, adjusted for multiple covariates, indicated a reduction in mortality that ranged from 51% to 66% (Table 3).
These results were unexpected and prompted us to examine other studies that correlated the Thr92Ala-DIO2 polymorphism with other clinical outcomes. The metanalysis of 5 studies (including the present study) involving 1062 patients revealed that carriers of the Thr/Ala genotype exhibited significantly better clinical outcomes. In addition, the metanalysis of 16 studies involving 27 503 patients revealed that carriers of the Thr/Ala genotype were less likely to be found among patients with 12 medical conditions. These results shed light on a previously unappreciated aspect of the Thr92Ala-DIO2 polymorphism, which is a likely advantage for carriers of the heterozygous genotype. Such a condition, in which the phenotype of the heterozygote lies outside the phenotypical range of both homozygous, is known as overdominance.
Overdominance has been described for other conditions as well. For example, there is evidence that genetic heterozygosity in humans provides greater resistance to certain viral infections. A study that evaluated human leukocyte antigen (HLA) polymorphisms in patients with HIV revealed that heterozygosity of 1 or more loci was associated with a slower progression to AIDS and reduced mortality (49). Heterozygosity advantage of human leukocyte antigen polymorphisms has also been reported for hepatitis B virus and hepatitis C infections (50, 51).
Sequencing DIO2 from archaic human subspecies led some to conclude that Neanderthals and Denisovans displayed only the Ala92-DIO2 allele, suggesting that those hominines were homozygous for Ala92-DIO2 (52). The fact that Ala92-D2 is about 20% less catalytically active (29) suggests that the minor allele could have protected against iodine deficiency because it metabolizes less T4. The Thr92-DIO2 appeared for the first time during the Upper Pleistocene and has been conserved during the Neolithic age. The fact that the Thr92-DIO2 allele became the major one observed in the modern human suggests that its existence confers an evolutionary advantage.
The present studies suggest that such an evolutionary advantage is identified by the increased survival of Thr/Ala patients during admission for COVID-19. In fact, the metanalyses suggest that this protective effect might be broader and include multiple other chronic conditions and severe diseases, such as ischemic stroke, myocardial infarction, and left ventricular hypertrophy. While the strength of these associations needs to be confirmed in other settings and with much larger populations, one can only speculate as to the mechanistic explanation involved. COVID-19-induced respiratory failure promotes angiocentric inflammation, characterized by generalized thrombosis with microangiopathy, vasoconstriction, and distinct intussusceptive angiogenesis (53). A series of genes involved in these pathogenetic mechanisms of COVID-19 have been recently identified, including CXCR4 (53, 54) and SLC44A2 (55-57). Genes involved in the activation of the immune response and secretion of cytokines, such as CDK2 (58), BST2 (59), and CCL4 (60-62), have also been identified. In the specific case of lung inflammatory diseases, a study in mice found that endoplasmic reticulum stress may play a central role. Moreover, inhibition of endoplasmic reticulum stress alleviated endotoxin-induced ALI both in vivo and in vitro (63, 64). This is relevant given that expressing the Thr92Ala-DIO2 gene has been linked to endoplasmic reticulum stress in cell and animal models (29).
While studying post-mortem samples of the human temporal lobe and HEK-293 cells stably expressing Ala92-DIO2, it was observed in both models that the expression of different DIO2 alleles correlates with the expression of 81 genes related to inflammation, oxidative stress, apoptosis, mitochondrial dysfunction, DNA repair, growth factor signaling, and neurodegenerative diseases (65). Remarkably, the Thr/Ala genotype exhibited a strong positive correlation with the expression of CXCR4, SLC44a2 in both cells and human brain samples; the Thr/Thr genotype correlated positively with the expression of CDK2, BST2; and Ala/Ala genotype correlated positively with CXCR4, SLC44a2, and BST2.
The present study is limited by the lack of a conclusive mechanistic explanation for the protective role played by the Thr92Ala-DIO2 heterozygosity. The study is also limited by the relatively small number of patients, which has an effect size index of 0.3 (best if below 0.2), and by the fact that we only considered patients hospitalized with COVID-19 with moderate to severe conditions. Individuals with mild disease were not evaluated.
Conclusion
Here we provide evidence for a protective role played by the Thr92Ala-DIO2 heterozygosity in patients with COVID-19. An accompanying metanalysis suggests that this advantage is extended to other conditions.
Acknowledgments
We gratefully acknowledge the contributions and efforts of all patients who participated in this study and the physicians, residents, students, nutritionists, pharmacists, and healthcare professionals involved in data collection and patient care. We thank the hospital management of Dom José Maria Pires Metropolitan Hospital, the Teaching and Research Management of Lauro Wanderley University Hospital, and University Center of João Pessoa—UNIPE. We thank Dr Laura Ward and Natassia Bufalo for initial discussions about D2-Polymorphism genotyping.
Glossary
Abbreviations
- AIDS
acquired immune deficiency syndrome
- AIS
acute ischemic strokes
- ALI
acute lung injury
- ALT
Alanine transaminase
- AMI
acute myocardial infarction
- anti-TPO
antithyroid peroxidase antibodies
- ARDS
acute respiratory distress syndrome
- AST
aspartate aminotransferase
- BMI
body mass index
- BST2
bone marrow stromal antigen 2
- CCL
C-C motif chemokine ligand
- CDK2
cyclin-dependent kinase 2
- CI
confidence interval
- COVID-19
coronavirus disease 19
- CRP
C-reactive protein
- CT
computed tomography
- CXCR4
C-X-C chemokine receptor type 4
- DIO2
type II deiodinase
- DIO3
type III deiodinase
- fT3
free triiodothyronine
- fT4
free thyroxine
- HLA
human leukocyte antigen
- HIV
human immunodeficiency virus
- HR
hazard ratio
- ICU
intensive care unit
- IL-6
interleukin 6
- IR
interquartile range
- IS
ischemic stroke
- LDH
lactate dehydrogenase
- LVH
left ventricular hypertrophy
- M-H
Mantel–Haenszel
- NEWS2
National Early Warning Score 2
- NTIS
nonthyroidal illness syndrome
- OR
odds ratio
- qSOFA
Quick Sepsis-related Organ Failure Assessment
- RT-qPCR
real-time reverse transcription polymerase chain reaction
- SARS
severe acute respiratory syndrome
- rT3
reverse triiodothyronine
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SNP
single nucleotide polymorphisms
- SLC44a2
solute carrier family 44 member 2
- TH
thyroid hormone
- TSH
thyrotropin
Contributor Information
Fabyan Esberard de Lima Beltrão, Lauro Wanderley University Hospital, Federal University of Paraíba, João Pessoa, Paraíba, Brazil; Post-Graduation Program in Nutritional Sciences, Department of Nutrition, Center for Health Sciences, Federal University of Paraíba, João Pessoa, Paraíba, Brazil; University Center of João Pessoa – UNIPE, João Pessoa, PB, Brazil.
Daniele Carvalhal de Almeida Beltrão, University Center of João Pessoa – UNIPE, João Pessoa, PB, Brazil.
Giulia Carvalhal, Center for Biological and Health Sciences, Federal University of Campina Grande, Campina Grande, Paraíba, Brazil.
Fabricia Elizabeth de Lima Beltrão, Lauro Wanderley University Hospital, Federal University of Paraíba, João Pessoa, Paraíba, Brazil.
Jair de Souza Braga Filho, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil.
Jocyel de Brito Oliveira, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil.
Joice dos Santos de Jesus, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil.
Gabriel Jeferson Rodríguez Machado, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil.
Hatilla dos Santos Silva, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil.
Helena Mariana Pitangueira Teixeira, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil.
Juliana Lopes Rodrigues, Laboratory of Immunopharmacology and Molecular Biology, Health Sciences Institute, Federal University of Bahia, Brazil.
Camila Alexandrina Viana de Figueiredo, Laboratory of Immunopharmacology and Molecular Biology, Health Sciences Institute, Federal University of Bahia, Brazil.
Ryan dos Santos Costa, Laboratory of Immunopharmacology and Molecular Biology, Health Sciences Institute, Federal University of Bahia, Brazil.
Fabio Hecht, The Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
Antonio C Bianco, Section of Endocrinology and Metabolism, Division of the Biological Sciences, University of Chicago, Chicago, IL, USA.
Maria da Conceição Rodrigues Gonçalves, Post-Graduation Program in Nutritional Sciences, Department of Nutrition, Center for Health Sciences, Federal University of Paraíba, João Pessoa, Paraíba, Brazil.
Helton Estrela Ramos, Bioregulation Department, Health and Science Institut, Federal University of Bahia, Salvador, Bahia, Brazil; Postgraduate Program in Medicine and Health, Medical School of Medicine, Federal University of Bahia, Salvador, Brazil; Postgraduate Program in Interactive Processes of Organs and Systems, Health & Science Institute, Federal University of Bahia, Salvador, BA, Brazil.
Financial Support
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure Summary
A.B. is a consultant for AbbVie, Synthonics, and Allergan. The other authors declare no relevant disclosures.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256:117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giovanetti M, Angeletti S, Benvenuto D, Ciccozzi MA. doubt of multiple introduction of SARS-CoV-2 in Italy: a preliminary overview. J Med Virol. 2020;92(9):1634-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome Coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35(13):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9(4):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293-301. [DOI] [PubMed] [Google Scholar]
- 9. Guo J, Hong Y, Wang Z, Li Y. Prognostic value of thyroid hormone FT3 in general patients admitted to the intensive care unit. Biomed Res Int. 2020;2020:6329548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu MQ, Chen Z, Chen LX. Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol Sin. 2016;37(4):425-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gangemi EN, Garino F, Berchialla P, et al. Low triiodothyronine serum levels as a predictor of poor prognosis in burn patients. Burns. 2008;34(6):817-824. [DOI] [PubMed] [Google Scholar]
- 12. Alevizaki M, Synetou M, Xynos K, Pappa T, Vemmos KN. Low triiodothyronine: a strong predictor of outcome in acute stroke patients. Eur J Clin Invest. 2007;37(8):651-657. [DOI] [PubMed] [Google Scholar]
- 13. Borkowski J, Siemiatkowski A, Wołczyński S, Czaban SL, Jedynak M. Assessment of the release of thyroid hormones in septic shock – prognostic significance. Pol Merkur Lekarski. 2005;18(103):45-48. [PubMed] [Google Scholar]
- 14. Scoscia E, Baglioni S, Eslami A, Iervasi G, Monti S, Todisco T. Low triiodothyronine (T3) state: a predictor of outcome in respiratory failure? Results of a clinical pilot study. Eur J Endocrinol. 2004;151(5):557-560. [DOI] [PubMed] [Google Scholar]
- 15. Iervasi G, Pingitore A, Landi P, et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107(5):708-713. [DOI] [PubMed] [Google Scholar]
- 16. Schilling JU, Zimmermann T, Albrecht S, Zwipp H, Saeger HD. Low T3 syndrome in multiple trauma patients—a phenomenon or important pathogenetic factor? Med Klin. 1999;94(Suppl 3):66-69. [DOI] [PubMed] [Google Scholar]
- 17. Gao W, Guo W, Guo Y, et al. Thyroid hormone concentrations in severely or critically ill patients with COVID-19. J. Endocrinol. Invest. 2020;44(5):1031-1040.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen M, Zhou W, Xu W. Thyroid function analysis in 50 patients with COVID-19: a retrospective study. Thyroid 2021;31(1):8-11. [DOI] [PubMed] [Google Scholar]
- 19. Zou R, Wu C, Zhang S, et al. Euthyroid sick syndrome in patients with COVID-19. Front Endocrinol (Lausanne). 2020;11:566439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beltrão FEL, Beltrãão DCA, Carvalhal G, et al. Thyroid hormone levels during hospital admission inform disease severity and mortality in COVID-19 patients. Thyroid 2021;31(11):1639-1649. [DOI] [PubMed] [Google Scholar]
- 21. Campi I, Bulgarelli I, Dubini A, et al. The spectrum of thyroid function tests during hospitalization for SARS COV-2 infection. Eur J Endocrinol. 2021;184(5):699-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhargava M, Runyon MR, Smirnov D, et al. Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs. Am J Respir Crit Care Med. 2008;178(5):506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu G, Tzouvelekis A, Wang R, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med. 2018;24(1):39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma SF, Xie L, Pino-Yanes M, et al. Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol. 2011;45(6):1203-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo TW, Zhang FC, Yang MS, et al. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J Med Genet. 2004;41(8):585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang K, Xi H, Wang X, et al. A family-based association study of DIO2 and children mental retardation in the Qinba region of China. J Hum Genet. 2012;57(1):14-17. [DOI] [PubMed] [Google Scholar]
- 28. Mentuccia D, Proietti-Pannunzi L, Tanner K, et al. Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor. Diabetes. 2002;51(3):880-883. [DOI] [PubMed] [Google Scholar]
- 29. Jo S, Fonseca TL, Bocco BMLC, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Investig. 2019;129(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wouters HJCM, van Loon HCM, van der Klauw MM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017;27(2):147-155. [DOI] [PubMed] [Google Scholar]
- 31. Panicker V, Saravanan P, Vaidya B, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94(5):1623-1629. [DOI] [PubMed] [Google Scholar]
- 32. Castagna MG, Dentice M, Cantara S, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017;102(5):1623-1630. [DOI] [PubMed] [Google Scholar]
- 33. Dora JM, Machado WE, Rheinheimer J, Crispim D, Maia AL. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur J Endocrinol. 2010;163(3):427-434. [DOI] [PubMed] [Google Scholar]
- 34. Nair S, Muller YL, Ortega E, Kobes S, Bogardus C, Baier LJ. Association analyses of variants in the DIO2 gene with early-onset type 2 diabetes mellitus in Pima Indians. Thyroid. 2012;22(1):80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Canani LH, Capp C, Dora JM, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90(6):3472-3478. [DOI] [PubMed] [Google Scholar]
- 36. Grarup N, Andersen MK, Andreasen CH, et al. Studies of the common DIO2 Thr92Ala polymorphism and metabolic phenotypes in 7342 Danish white subjects. J Clin Endocrinol Metab. 2007;92(1):363-366. [DOI] [PubMed] [Google Scholar]
- 37. Gumieniak O, Perlstein TS, Williams JS, et al. Ala92 type 2 deiodinase allele increases risk for the development of hypertension. Hypertension. 2007;49(3):461-466. [DOI] [PubMed] [Google Scholar]
- 38. van der Deure WM, Peeters RP, Uitterlinden AG, et al. Impact of thyroid function and polymorphisms in the type 2 deiodinase on blood pressure: the Rotterdam Study and the Rotterdam Scan Study. Clin Endocrinol (Oxf). 2009;71(1):137-144. [DOI] [PubMed] [Google Scholar]
- 39. Meulenbelt I, Min JL, Bos S, et al. Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis. Hum Mol Genet. 2008;17(12):1867-1875. [DOI] [PubMed] [Google Scholar]
- 40. Kang YE, Kang YM, Park B, Shong M, Yi HS. Type 2 deiodinase Thr92Ala polymorphism is associated with a reduction in bone mineral density: a community-based Korean genome and epidemiology study. Clin Endocrinol (Oxf). 2020;93(3):238-247. [DOI] [PubMed] [Google Scholar]
- 41. Luo M, Zhou XH, Zou T, Keyim K, Dong LM. Type II deiodinase polymorphisms and serum thyroid hormone levels in patients with mild cognitive impairment. Genet Mol Res. 2015;14(2):5407-5416. [DOI] [PubMed] [Google Scholar]
- 42. Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friedman AN, Guirguis J, Kapoor R, et al. Obesity, inflammatory and thrombotic markers, and major clinical outcomes in critically ill patients with COVID-19 in the US. Obesity (Silver Spring). 2021;29(10):1719-1730. [DOI] [PubMed] [Google Scholar]
- 44. Ye Q, Wang B, Mao J, et al. Epidemiological analysis of COVID-19 and practical experience from China. J Med Virol. 2020;92(7):755-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De la Vega FM, Lazaruk KD, Rhodes MD, Wenz MH. Assessment of two flexible and compatible SNP genotyping platforms: TaqMan SNP Genotyping Assays and the SNPlex genotyping system. Mutat Res. 2005;573(1-2):111-135. [DOI] [PubMed] [Google Scholar]
- 47. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carrington M, Nelson GW, Martin MP, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283(5408):1748-1752. [DOI] [PubMed] [Google Scholar]
- 50. Thursz MR, Thomas HC, Greenwood BM, Hill AV. Heterozygote advantage for HLA class-II type in hepatitis B virus infection. Nat Genet. 1997;17(1):11-12. [DOI] [PubMed] [Google Scholar]
- 51. Hraber P, Kuiken C, Yusim K. Evidence for human leukocyte antigen heterozygote advantage against hepatitis C virus infection. Hepatology. 2007;46(6):1713-1721. [DOI] [PubMed] [Google Scholar]
- 52. Ricci C, Kakularam KR, Marzocchi C, et al. Thr92Ala polymorphism in the type 2 deiodinase gene: an evolutionary perspective. J Endocrinol Invest. 2020;43(12):1749-1757. [DOI] [PubMed] [Google Scholar]
- 53. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ackermann M, Mentzer SJ, Kolb M, Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur Respir J. 2020;56(5):2003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nair TS, Kakaraparthi BN, Yang L, et al. Slc44a2 deletion alters tetraspanin and N-cadherin expression: reduced adhesion and enhanced proliferation in cultured mesenchymal lung cells. Tissue Cell. 2021;73:101599. [DOI] [PubMed] [Google Scholar]
- 57. Bennett JA, Mastrangelo MA, Ture SK, et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat Commun. 2020;11(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bouhaddou M, Memon D, Meyer B, et al. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182(3):685-712.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dolskiy AA, Bodnev SA, Nazarenko AA, et al. Deletion of BST2 cytoplasmic and transmembrane N-terminal domains results in SARS-CoV, SARS-CoV-2, and influenza virus production suppression in a vero cell line. Front Mol Biosci. 2020;7:616798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerging Microbes Infect. 2020;9(1):761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842-844. [DOI] [PubMed] [Google Scholar]
- 62. Coperchini F, Chiovato L, Ricci G, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: further advances in our understanding the role of specific chemokines involved. Cytokine Growth Factor Rev. 2021;58:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ, Lee YC. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-κB/HIF-1α signaling pathway. Sci Rep. 2013;3(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zeng M, Sang W, Chen S, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26-37. [DOI] [PubMed] [Google Scholar]
- 65. McAninch EA, Jo S, Preite NZ, et al. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015;100(3):920-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.