Abstract
Background
Detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens in blood has high sensitivity in adults with acute coronavirus disease 2019 (COVID-19), but sensitivity in pediatric patients is unclear. Recent data suggest that persistent SARS-CoV-2 spike antigenemia may contribute to multisystem inflammatory syndrome in children (MIS-C). We quantified SARS-CoV-2 nucleocapsid (N) and spike (S) antigens in blood of pediatric patients with either acute COVID-19 or MIS-C using ultrasensitive immunoassays (Meso Scale Discovery).
Methods
Plasma was collected from inpatients (<21 years) enrolled across 15 hospitals in 15 US states. Acute COVID-19 patients (n = 36) had a range of disease severity and positive nasopharyngeal SARS-CoV-2 RT-PCR within 24 hours of blood collection. Patients with MIS-C (n = 53) met CDC criteria and tested positive for SARS-CoV-2 (RT-PCR or serology). Controls were patients pre–COVID-19 (n = 67) or within 24 hours of negative RT-PCR (n = 43).
Results
Specificities of N and S assays were 95–97% and 100%, respectively. In acute COVID-19 patients, N/S plasma assays had 89%/64% sensitivity; sensitivities in patients with concurrent nasopharyngeal swab cycle threshold (Ct) ≤35 were 93%/63%. Antigen concentrations ranged from 1.28–3844 pg/mL (N) and 1.65–1071 pg/mL (S) and correlated with disease severity. In MIS-C, antigens were detected in 3/53 (5.7%) samples (3 N-positive: 1.7, 1.9, 121.1 pg/mL; 1 S-positive: 2.3 pg/mL); the patient with highest N had positive nasopharyngeal RT-PCR (Ct 22.3) concurrent with blood draw.
Conclusions
Ultrasensitive blood SARS-CoV-2 antigen measurement has high diagnostic yield in children with acute COVID-19. Antigens were undetectable in most MIS-C patients, suggesting that persistent antigenemia is not a common contributor to MIS-C pathogenesis.
Keywords: SARS-CoV-2, COVID-19, antigen, ultrasensitive immunoassay, antigenemia
In a U.S. pediatric cohort tested with ultrasensitive immunoassays, severe acute respiratory syndrome coronavirus 2 nucleocapsid antigens were detectable in most patients with acute coronavirus disease 2019, and spike antigens were commonly detectable. Both antigens were undetectable in almost all multisystem inflammatory syndrome in children patients.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pediatric populations can present with a range of disease severity, from asymptomatic infection and mild illness to severe systemic disease with involvement of multiple organ systems [1]. Multisystem inflammatory syndrome in children (MIS-C) is a relatively rare sequela of SARS-CoV-2 infection initially recognized in early 2020, when otherwise healthy children were hospitalized with severe systemic inflammation, with timing consistent with a postinfectious syndrome [2].
Diagnosis of acute SARS-CoV-2 infection in children is similar to diagnosis in adults, including detection of viral RNA by nucleic acid amplification testing (NAAT) or viral antigens by immunoassay testing of upper respiratory specimens. Both types of measurements are likely to act as relative indicators of viral load, and nucleocapsid (N) antigen concentrations in nasopharyngeal (NP) swab samples have been shown to correlate closely with NAAT cycle threshold (Ct) values [3, 4]. In contrast, MIS-C is a syndrome defined by clinical criteria (including fever, inflammation, severity requiring hospitalization, and multisystem involvement) plus evidence of current or recent SARS-CoV-2 infection (by NAAT, serology or antigen test, or exposure in the past 4 weeks), with no alternative diagnosis [5]. Some patients with multisystem involvement meet clinical criteria for both severe acute coronavirus disease 2019 (COVID-19) and MIS-C [1].
Recent advances in SARS-CoV-2 diagnostic approaches include assays developed using two ultrasensitive and quantitative antigen detection technologies (Single Molecule Array [Simoa]; Quanterix, Billerica, MA; and S-PLEX electrochemiluminescence immunoassay; MesoScale Discovery, Rockville, MD) for use in both respiratory and nonrespiratory specimens. Simoa and S-PLEX assays have detected N antigens with high sensitivity in the blood of adults with acute COVID-19 [6–8]. Data on the clinical performance of these assays for diagnosing children with either acute COVID-19 or MIS-C are limited. A recent study using Simoa-based assays concluded that SARS-CoV-2 spike (S) antigens were detectable in the blood of children with MIS-C. This finding raised the question of whether antigen detection could provide diagnostic utility in MIS-C and generated hypotheses about possible disease mechanism and therapeutic approaches based on a potential intestinal source of antigen leakage [9].
In this study, we applied ultrasensitive and quantitative S-PLEX assays for SARS-CoV-2 N [3, 4, 8, 10] and S antigens to plasma of hospitalized pediatric patients with either acute COVID-19 or MIS-C enrolled in a large multisite study comparing the 2 presentations. We sought to characterize the range of SARS-CoV-2 antigen concentrations in blood of children with acute COVID-19 or MIS-C, further clarifying diagnostic options for two important presentations of pediatric COVID-19.
METHODS
Clinical Cohorts and Sample Collection
COVID-19 acute and COVID-19 MIS-C samples were collected in the Overcoming COVID-19 Immunobiology Study that investigates severe pediatric complications related to COVID-19 [2]. Samples were collected between 17 June 2020 and 17 June 2021 across 15 pediatric hospital sites in 15 US states. Sites relied on the Boston Children’s Hospital Institutional Review Board (IRB); informed consent was obtained from at least 1 parent or legal guardian. Patients were approached for enrollment and research sample collection as soon as possible after admission. Patients were classified as having acute COVID-19 if they had a positive SARS-CoV-2 reverse transcription–polymerase chain reaction (RT-PCR) test and symptoms consistent with COVID-19 (Supplementary Methods). Only patients with acute COVID-19 who had a research NP swab sample collected within 24 hours of the research blood sample were included. The research NP sample was frozen and tested by RT-PCR at Vanderbilt University Medical Center (Halasa Lab) using the Centers for Disease Control and Prevention (CDC) Emergency Use Authorization (EUA) protocol (https://www.fda.gov/media/134922/download). N1 and N2 target Ct values were averaged for analysis. All patients with MIS-C met CDC diagnostic criteria and were required to have a positive SARS-CoV-2 test (Supplementary Methods) [2, 5]. For all patients with MIS-C, the RT-PCR result associated with each research blood sample was the most recent (preceding) clinical RT-PCR result reported by the hospital site. Clinical Ct values were not available. Some patients with MIS-C also had a research NP swab collected within 24 hours of the research blood sample, which was tested by RT-PCR at Vanderbilt. Additional details are provided in the Supplementary Methods.
Pre–COVID-19 control samples were discarded heparin plasma samples from pediatric patients (aged ≤18 years) with suspected Clostridioides difficile infection or discarded EDTA (ethylenediaminetetraacetic acid) plasma samples from pediatric patients with suspected sepsis, captured prior to December 2019 under separate IRB protocols.
COVID-19–negative control samples were discarded heparin plasma samples from symptomatic and asymptomatic pediatric patients (aged ≤18 years) who had tested negative on SARS-CoV-2 RT-PCR testing of a respiratory sample collected on the same date (25 April–3 May 2021). Samples were frozen within 24 hours of initial collection.
SARS-CoV-2 Antigen and Serologic Assays
Detection of SARS-CoV-2 N and S proteins was performed using MSD S-PLEX CoV-2 N and MSD S-PLEX CoV-2 S assay kits (Meso Scale Discovery, Rockville, MD). The assays were run according to protocols in the kit package inserts [11, 12]. Plasma samples were diluted 4-fold in assay buffer prior to analysis. Sample quantitation was achieved using a calibration curve generated using a recombinant antigen standard. For graphing and analysis, any concentrations below the limit of detection (LOD) were assigned the LOD value, and any concentrations above the highest calibration standard were assigned its value. The LOD and assay cutoff for the N assay were 0.64 and 1.28 pg/mL, respectively, and for the S assay were 1.12 and 1.65 pg/mL, respectively (assay details in the Supplementary Methods).
All samples were also tested using an MSD multiplexed serologic assay that measured immunoglobulin G (IgG) antibodies against SARS-CoV-2 N, S, and the spike receptor binding domain (RBD) and N-terminal domain (NTD), as well as antibodies against S from SARS-CoV-1 and common circulating coronaviruses (229E, HKU1, NL63, and OC43). Details of this MSD antibody panel are in the Supplementary Methods. The assays were run according to the protocol provided with the assay kits [13]. Statistical methods are detailed in the Supplementary Methods.
RESULTS
Measurement of SARS-CoV-2 Antigen Levels in Pediatric Plasma
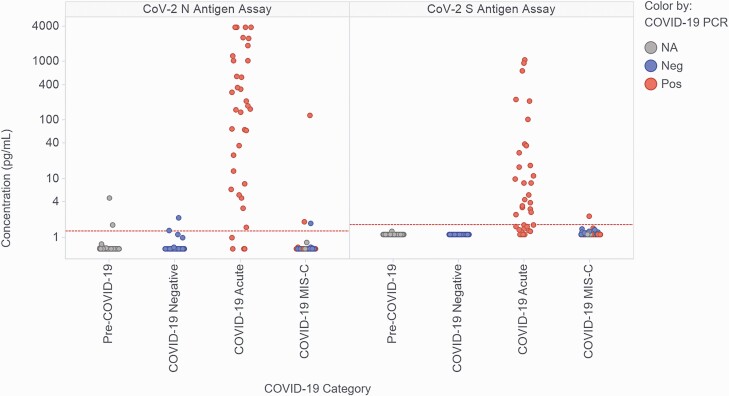
Table 1 summarizes patient demographics and clinical data for patients with acute COVID-19 (n = 36; age range: 0.1–20.8 years; 22% previously healthy) and patients with MIS-C (n = 53; age range: 1.0–19.1 years; 79% previously healthy). Supplementary Table 1 summarizes key laboratory and blood sample handling data. Figure 1 shows measured concentrations of N and S antigens in plasma for the 4 categories of study patients: pre–COVID-19 controls (n = 67), RT-PCR–negative (ruled out) controls (COVID-19 negative; n = 43), acute COVID-19 cases (COVID-19 acute; n = 36), and MIS-C cases (COVID-19 MIS-C; n = 53). As expected, antigen concentration measurements for the 2 negative control categories were low; only 4 (3.6%) samples (all N measurements) were slightly above the assay cutoffs. The N and S antigen concentrations in acute COVID-19 cases (all with positive RT-PCR results on admission) spanned a wide range: less than 1.28 pg/mL (assay cutoff value) to greater than 3844 pg/mL (top of the calibration curve) for N, and less than 1.65 pg/mL (assay cutoff value) to 1071 pg/mL for S. Two of the 36 patients with acute COVID-19 had received intravenous immunoglobulin (IVIG) prior to blood collection (9.1 and 1.2 days, respectively); their N/S concentrations were 1024.8/8.65 pg/mL and 3844.0/1071.2 pg/mL, respectively, suggesting that IVIG did not inhibit antigen detection (the first patient also received monoclonal antibody treatment pre-sampling [timing unknown]).
Table 1.
Patient Demographics and Clinical Data
| Patient Demographics and Clinical Data | Acute COVID-19 (n = 36) | MIS-C (n = 53) | P |
|---|---|---|---|
| Male, n (%) | 20 (56) | 33 (62) | .5 |
| Age, median (IQR), y | 12.9 (5.2, 18.1) |
12.3 (8.7, 14.8) |
.8 |
| Race/ethnicity, n (%) | |||
| Hispanic, any race | 10 (28) | 16 (30) | .8 |
| White, non-Hispanic | 12 (33) | 18 (34) | 1.0 |
| Black, non-Hispanic | 7 (19) | 17 (32) | .2 |
| Other, non-Hispanica | 6 (17) | 1 (2) | .02 |
| Other/unknown/refused | 11 (31) | 18 (34) | .7 |
| Previously healthy, n (%) | 8 (22) | 42 (79) | <.001 |
| Underlying conditions, n (%) | |||
| Obesity | 7 (19) | 4 (8) | .1 |
| Respiratory system disorders | 17 (47) | 7 (13) | <.001 |
| Cardiovascular system disorders | 3 (8) | 0 | .06 |
| Neurologic or neuromuscular disorders | 14 (39) | 0 | <.001 |
| Hematologic disorder | 6 (17) | 1 (2) | .02 |
| GI and hepatic dysfunction | 13 (36) | 0 | <.001 |
| Endocrine, metabolic, or genetic disorder | 10 (28) | 2 (4) | <.01 |
| Otherb | 6 (17) | 2 (4) | .06 |
| Hospital course and treatment | |||
| Received fresh frozen or convalescent plasma, n (%) | 4 (11) | 2 (4) | .2 |
| Received IVIG prior to blood collection, n (%) | 2 (6) | 39 (74) | <.001 |
| Received steroids prior to blood collection, n (%) | 18 (50) | 44 (83) | .001 |
| ICU admission, n (%) | 20 (56) | 50 (94) | <.001 |
| Received any respiratory support,c n (%) | 23 (64) | 37 (70) | .6 |
| Invasive mechanical ventilation, n (%) | 10 (28) | 13 (25) | .3 |
| Total hours intubated, median (IQR) | 202.8 (141, 333.8) |
120.2 (46.2, 181.4) |
.04 |
| Shock requiring vasopressors, n (%) | 6 (17) | 31 (58) | <.0001 |
| Days in study hospital, median (IQR) | 6.7 (1.9, 19.7) |
6.9 (5.7, 8.9) |
.9 |
| Died before discharge, n (%) | 1 (3) | 0 | .4 |
Pearson chi-square or Fisher’s exact test (2-sided) applied if <5 counts used for categorical data. Mann-Whitney U with Tukey’s median IQR was used for numerical data.
Abbreviations: COVID-19, coronavirus disease 2019; GI, gastrointestinal; IQR, interquartile range; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MIS-C, multisystem inflammatory syndrome in children.
For Acute COVID-19, n = 5 patients identified as Asian and n = 1 did not specify their race. For MIS-C, n = 1 did not specify their race.
Other underlying conditions include active or prior oncologic issues, nononcologic immunosuppressive disorder, rheumatologic/autoimmune disorder, or renal or urologic dysfunction.
Respiratory support included noninvasive supplementary oxygen or mechanical ventilation.
Figure 1.
Measured levels of SARS-CoV-2 nucleocapsid (N) and spike (S) antigen in the plasma of children and young-adult study participants. Participants were classified as controls with samples collected prior to 2020 (Pre-COVID-19; n = 67), controls ruled out for acute COVID-19 by negative nasopharyngeal swab RT-PCR (COVID-19 Negative; n = 43), patients with RT-PCR–confirmed acute COVID-19 infections (COVID-19 Acute; n = 36), and patients diagnosed as MIS-C (COVID-19 MIS-C; n = 53). Data points are colored based on the results of the most recent clinical COVID-19 RT-PCR test prior to sample collection. Of the patients with MIS-C, the most recent clinical RT-PCR results prior to research blood sample collection were as follows: 20 RT-PCR positive, 29 RT-PCR negative, 4 NA (3 not performed, 1 inconclusive). The horizontal dashed red lines represent the assay thresholds for classifying samples as antigen positive. Abbreviations: COVID-19, coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children; NA, not available; Neg, negative; Pos, positive; RT-PCR, reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In contrast, antigen concentrations in patients with MIS-C (of whom 20 of 53 [38%] had a positive RT-PCR result at admission) were almost all undetectable. Concentrations of N ranged from less than 1.28 pg/mL to 121 pg/mL, with only 3 (5.7%) samples above the cutoff value, 2 of which were from patients who were RT-PCR positive on admission. Concentrations of S ranged from less than 1.65 pg/mL to 2.3 pg/mL, with only 1 sample above the cutoff value. The only patient with MIS-C that had an N concentration more than 5-fold above the assay cutoff (patient A, 121 pg/mL) was also the patient positive for S antigen, and had positive clinical RT-PCR results 25 days and 3 days prior to the research antigen testing; however, she was SARS-CoV-2 antibody negative on clinical testing (3 days prior to antigen testing) and follow-up research testing (of the same sample tested for antigens). Of the 53 patients with MIS-C, 35 had a research NP swab collected within 0–2 days of the blood sample (31/35 on day 0). Of those 35 swabs, 7 (20%) tested positive by RT-PCR. The patient with the lowest Ct value (22.8) was patient A; the other 6 RT-PCR–positive patients all had Ct values greater than 37. Of the 53 patients with MIS-C, 40 had received IVIG prior to blood collection, including patient A. Of the 13 who had not received IVIG, none had detectable antigenemia.
The sensitivity and specificity of the N and S antigen assays in the study cohorts are shown in Table 2. The assays demonstrated high specificity in control patients. The specificity of the N assay was 97% in pre–COVID-19 control samples and 95% in COVID-19–negative control samples; S assay specificity was 100% in pre–COVID-19 samples and 100% in COVID-19–negative samples. The N assay sensitivity was 89% in all acute COVID-19 cases and 93% in cases with a Ct value of 35 or less on research NP swab RT-PCR. The S assay was less sensitive in acute COVID-19, with 64% sensitivity in all cases and 63% sensitivity in those with RT-PCR Ct values of 35 or less. Both assays were considerably less sensitive for identifying MIS-C, with sensitivities of 5.7% (N) and 1.9% (S), respectively.
Table 2.
Sensitivity and Specificity of Assays for SARS-CoV-2 Nucleocapsid (N) and Spike (S) Antigens in Plasma of Pediatric Patients With and Without COVID-19
| Patient Category | MSD S-PLEX CoV-2 N Antigen Assay | MSD S-PLEX CoV-2 S Antigen Assay | ||
|---|---|---|---|---|
| Count | % (95% CI) | Count | % (95% CI) | |
| Specificity | ||||
| Pre–COVID-19 controls | 65/67 | 97 (90, 99) | 67/67 | 100 (95, 100) |
| COVID-19 Negative controls | 41/43 | 95 (85, 99) | 43/43 | 100 (92, 100) |
| Sensitivity | ||||
| COVID-19 Acute | 32/36 | 89 (75, 96) | 23/36 | 64 (48, 78) |
| COVID-19 MIS-C | 3/53 | 5.7 (1.9, 15) | 1/53 | 1.9 (.3, 9.9) |
| COVID-19 Acute (Ct ≤ 35)a | 28/30 | 93 (79, 98) | 19/30 | 63 (46, 78) |
| COVID-19 MIS-C (RT-PCR positive)b | 2/20 | 10 (2.8, 30) | 1/20 | 5.0 (.9, 24) |
Specificity was calculated as % with negative antigen assay results in each control cohort. Sensitivity was calculated as % with positive antigen assay results in each case cohort. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; Ct, cycle threshold; MIS-C, multisystem inflammatory syndrome in children; RT-PCR, reverse transcription– polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sensitivity calculated after limiting analysis to COVID-19 Acute patients with Ct values ≤35 on research RT-PCR testing.
Sensitivity calculated after limiting analysis to COVID-19 MIS-C patients with positive clinical RT-PCR results.
Correlation of SARS-CoV-2 S and N Antigens with RT-PCR Ct values and Disease Severity
Supplementary Figure 1 shows the correlation of the measured S and N antigen concentrations in plasma with RT-PCR Ct values from the corresponding research NP swabs collected from patients with acute COVID-19. The correlations are relatively weak, with R2 values of 0.17 and 0.083 for N and S, respectively. The slopes are also much lower than would be expected for a linear dependence of plasma antigen concentration on respiratory RNA levels.
Supplementary Figure 2 examines the association of plasma N and S antigen concentrations with indicators of disease severity in patients with acute COVID-19. Admission to the intensive care unit (ICU) was associated with increased median (interquartile range [IQR]) concentrations of both N (547.5 [53.0–2590.2] vs 51.6 [6.3–166.0] pg/mL; P = .0009) and S (8.8 [2.3–130.6] vs 2.1 [1.4–4.0] pg/mL; P = .002) antigens in plasma. Similarly, requiring any respiratory support (vs no respiratory support) was also associated with increased concentrations of N (320.0 [27.0–2130.3] vs 36.8 [4.5–160.2] pg/mL; P = .003) and S (8.4 [1.90–86.0] vs 1.8 [<1.65–3.9] pg/mL; P = .004), although the specific level of respiratory support (mechanical support vs noninvasive supplemental oxygen) did not generate statistically significant differences for either antigen.
Measured concentrations of N and S within each sample are compared in Supplementary Figure 3. Log-transformed concentration values (for samples that provided concentrations above the cutoff values for both assays) are moderately correlated with an R2 value of 0.52. The median ratio (22; IQR: 5.2–51) of N to S indicates consistently higher N than S concentrations but high variability in the relative amounts of the 2 antigens. There were no samples in which S but not N was detected.
Correlation of SARS-CoV-2 Antigen and Antibody Measurements
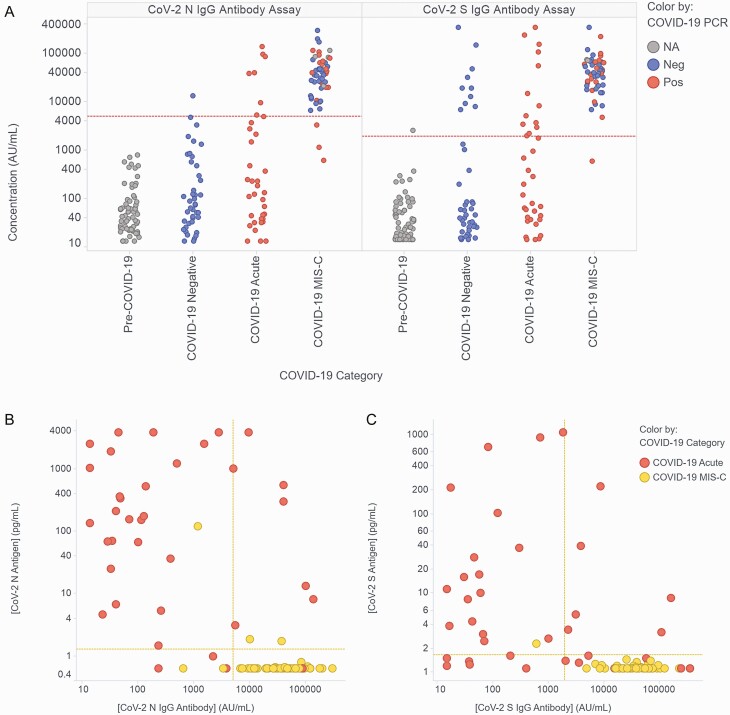
In addition to antigen testing, all samples were also tested using a multiplexed serologic assay (see Methods, Supplementary Methods, Supplementary Figure 4). Figure 2a shows measured concentrations of anti-N and anti-S antibodies in the 4 patient cohorts from Figure 1. As expected, antibody levels in control samples were low: levels were below assay cutoffs for N and S antibodies in 100% (67/67) and 99% (66/67) of pre–COVID-19 samples and 99% (42/43) and 77% (33/43) of COVID-19–negative samples. All 10 COVID-19–negative control samples classified as positive by serology for S were also positive for anti-RBD activity (Supplementary Figure 4), and likely represent prior vaccination or infection (data were not available for most COVID-19–negative patients, but the sample with the highest anti-S level was from a vaccinated patient). Of the patients with acute COVID-19 (all of whom were unvaccinated), 22% (8/36) and 36% (13/36) of samples were above the cutoff values for N and S antibodies. Nearly all patients with MIS-C had seroconverted, with 94% (50/53) and 98% (52/53) above the N and S cutoff values (8/53 were eligible for vaccination at the time of study participation based on date, but vaccination status is unknown).
Figure 2.
Measured levels of plasma IgG antibodies against SARS-CoV-2 nucleocapsid (N) and spike (S) antigens and correlation with concentrations of N and S antigens. (A) Measurement of IgG antibodies against SARS-CoV-2 N and S antigen in the plasma of children and young-adult study participants. Participants were classified as described in Figure 1. Data points are colored based on the results of the most recent clinical COVID-19 RT-PCR test prior to sample collection. The horizontal dashed red lines represent the assay thresholds for classifying samples as antibody positive (Supplementary Methods). (B, C) Correlation of the levels of antigen and anti-antigen antibodies for SARS-CoV-2 N (B) and S (C) for the data points categorized as COVID-19 Acute or COVID-19 MIS-C in Figures 1 and 2a. Horizontal and vertical dashed yellow lines represent the assay thresholds for classifying samples as antigen or antibody positive, respectively. Abbreviations: COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; MIS-C, multisystem inflammatory syndrome in children; NA, not available; Neg, negative; Pos, positive; RT-PCR, reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2 b and 2c compare N and S antigen concentrations with antibody concentrations in each sample. For the acute COVID-19 samples, seroconversion had no apparent effect on the detection of N antigen, with N detected in 89% (25/28) of seronegative and 88% (7/8) of seropositive samples, and led to a small decrease in the detection of S antigen, with S detected in 74% (17/23) of seronegative samples and 46% (6/13) of seropositive samples.
DISCUSSION
Our results using the MSD S-PLEX CoV-2 N and S assays demonstrate that, early in the hospital course, SARS-CoV-2 N and S antigens are detectable in the blood of most pediatric patients with acute COVID-19 but in few patients with MIS-C. The specificity of both N and S assays was near 100% in samples from pre–COVID-19 and COVID-19–negative controls. Blood concentrations of N and S antigens in acute COVID-19 correlated with disease severity, as indicated by ICU admission and need for respiratory support, but did not strongly correlate with RT-PCR Ct values in temporally matched NP samples. In contrast, antigen levels measured in NP swab samples have been shown to correlate closely with Ct values in NP samples [3, 4], suggesting that antigen levels in blood may be influenced by additional factors, such as infection in other tissues or variable antigen clearance from blood.
In patients with acute COVID-19, the sensitivity of the S-PLEX blood N antigen assay was 89% overall and 93% if the corresponding NP sample Ct was 35 or less, consistent with prior findings in adults with acute COVID-19. Shan et al [7] found that a Simoa assay for N antigen in blood had 97.5% positive and 100% negative agreement with NP RT-PCR. Wang et al [8], applying the S-PLEX N antigen assay to plasma of adults with acute COVID-19, demonstrated 91.9% clinical sensitivity (antigen-negative samples belonged to patients with high respiratory sample Ct values) and 94.2% clinical specificity. Notably, they observed plasma N concentration ranges and association with disease severity similar to what we observed in children. The S-PLEX S assay targets the RBD within the S1 subunit; it can capture either the S1 domain (created by proteolytic cleavage at the S1–S2 junction [14]) or the full-length extracellular S1–S2 domain. Our S assay had lower clinical sensitivity than our N assay in pediatric patients with acute COVID-19 (64% vs 89%), likely due to the consistently lower concentrations of S relative to N (~22-fold).
The clinical overlap between MIS-C and toxic shock syndrome led to the hypothesis that SARS-CoV-2 spike peptides might function as a superantigen, contributing to T-cell activation and MIS-C [15, 16]. Yonker et al [9] tested the hypothesis that persistent SARS-CoV-2 infection in the gastrointestinal trat leads to antigenemia, potentially underlying MIS-C. Using Simoa-based assays previously developed and applied to the plasma of adults with acute COVID-19 by Ogata et al [6], they found higher signals from S1–S2, S1, and N assays in blood of patients with MIS-C (medians of ~70, 50, and 5 pg/mL, respectively) relative to children with acute COVID-19 and pre-pandemic controls, and identified SARS-CoV-2 RNA in stool in 7 of 12 patients. They reported no difference in blood antigen concentrations between healthy pre–COVID-19 controls and patients with acute COVID-19.
We were unable to confirm the findings of the single-center study by Yonker et al [9]. One possible explanation may be differences in the clinical cohorts studied. Our study is larger and includes patients enrolled across the United States with MIS-C and with a range of acute COVID-19 disease severity, including those requiring mechanical ventilation and one that died (Table 1). Blood collection in our patients was performed at a single time point soon after admission; we did not perform serial sampling or attempt to associate antigen levels with detection of virus in stool samples. The assays deployed in the 2 studies utilize different antibodies, so it is possible that S-PLEX assays were less efficient in detecting antigens in the blood of patients with MIS-C. However, we reliably detected both N and S in children with acute COVID-19, and the MSD N antigen assay has demonstrated strong performance in pediatric and adult NP samples [3, 4], adult saliva [10], and adult blood [8]. We note that in the single patient in our MIS-C cohort with elevated antigen levels, no SARS-CoV-2 antibodies were detected. Given the overlap between diagnoses of acute severe COVID-19 with cardiovascular involvement and MIS-C [1], it is possible that this patient was in fact misclassified. Loss of antigens in complexes with host antibodies is possible but is also unlikely to explain fully the disparate findings, as we still detected antigen after seroconversion in some patients with acute COVID-19. Differences in assay specificity may also explain differences in detection.
Our study has several strengths. We utilized rigorously validated and commercially available ultrasensitive and quantitative assays for N and S antigens, with the N antigen assay having already demonstrated high sensitivity and specificity in multiple sample types, including blood from adults [3, 4, 8, 10]. Samples were drawn from a diverse and nationally representative multisite CDC study with carefully adjudicated cohorts of children and young adults with acute COVID-19 (with a range of severity) and MIS-C. These samples had clearly documented timing for both RT-PCR testing and blood sample collection, allowing analysis of MIS-C blood samples drawn close to a validated clinical RT-PCR test result for comparison. The samples were collected with careful attention to handling, with minimal opportunity for antigen degradation.
Our study also had several limitations. First, all acute COVID-19 patient samples were from hospitalized patients, and thus our results may or may not overestimate the sensitivity of the assay in children with mild COVID-19. Second, 75% of patients with MIS-C received IVIG prior to collection of the blood sample used in this analysis, raising the question of whether IVIG may have interfered with antigen detection. However, we did not detect N or S antigens in any of the 13 patients with MIS-C who had pre-IVIG sampling, and the 2 patients with acute COVID-19 who received IVIG prior to blood collection both had high blood antigen concentrations. Moreover, the presence of SARS-CoV-2–specific antibodies did not appear to inhibit antigen detection in patients with acute COVID-19. The Yonker et al study similarly included many post-IVIG samples, and concluded that IVIG initiation did not seem to have an impact on S antigen levels measured on serial samples in the few patients who had pre-IVIG measurements [9].
In conclusion, in this multicenter representative cohort of US children with acute COVID-19 or MIS-C, we demonstrate that blood SARS-CoV-2 antigen measurement may be useful for diagnosing hospitalized children with acute COVID-19. Our findings do not support the hypothesis that ongoing SARS-CoV-2 spike antigenemia is a major contributor to MIS-C pathogenesis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Authors’ Contributions : G. B. S., A. M., and N. R. P. conceived and designed the assay evaluation and sample analysis, and A. G. R., T. N., and N. B. H. designed the Overcoming COVID-19 Study. All members of the study group acquired the data. G. B. S., N. M., P. B, J. J., N. P., and D. R. acquired the S-PLEX data. A. G. R., T. N., L. L. L., S. P. S., T. C. W., J. C. F., K. M. T., M. S. Z., J. E. S., N. B. H., M. L. C., A. B. M., M. A. S., K. I., H. R. F., B. M. C., H. C., and S. J. G. provided samples and clinical data from the Overcoming COVID-19 Immunobiology Study. G. A.-M., J. J., and Y. Z. acquired control samples. G. B. S. and N. R. P. analyzed and interpreted the data and drafted the manuscript. Critical revisions to the manuscript were made by all members of the study group. N. R.. P. and A. G. R. obtained the funds for the study. G. B. S., T. N., Y. Z, A. G. R., and N. R. P. verified all data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments . The authors thank Dr. Mark Kellogg, Rebecca Sprague, Caitlin Barrett, and Kaitlyn Daugherty for assistance with control samples.
Disclaimer. This work represents the findings and conclusions of the authors and not the US Centers for Disease Control and Prevention.
Financial support . This work was supported in part by a grant to N.R.P. from the Boston Children’s Hospital Emerging Pathogens and Epidemic Response Cluster of Clinical Research Excellence. S-PLEX assays were provided as an in-kind service by Meso Scale Diagnostics. The US Centers for Disease Control and Prevention funded the referenced Overcoming COVID-19 Immunobiology Study (contract numbers 75D30119C05584 and 75D30120C07725; to A. G. R.). J. C. F is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K23DK119463. A. B. M. is supported by NICHD K23HD096018. M. S. Z. is supported by NHLBI K23HL146936.
Contributor Information
George B Sigal, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Tanya Novak, Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children’s Hospital, and Department of Anesthesia, Harvard Medical School, Boston, Massachusetts, USA.
Anu Mathew, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Janet Chou, Department of Pediatrics, Boston Children’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
Yubo Zhang, Institutional Centers for Clinical and Translational Research, Boston Children’s Hospital, Boston, Massachusetts, USA.
Navaratnam Manjula, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Pradeepthi Bathala, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Jessica Joe, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Nikhil Padmanabhan, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Daniel Romero, Meso Scale Diagnostics, LLC, Rockville, Maryland, USA.
Gabriella Allegri-Machado, Department of Laboratory Medicine, Boston Children’s Hospital, Boston, Massachusetts, USA.
Jill Joerger, Department of Laboratory Medicine, Boston Children’s Hospital, Boston, Massachusetts, USA.
Laura L Loftis, Division of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA.
Stephanie P Schwartz, Department of Pediatrics, University of North Carolina at Chapel Hill Children’s Hospital, Chapel Hill, North Carolina, USA.
Tracie C Walker, Department of Pediatrics, University of North Carolina at Chapel Hill Children’s Hospital, Chapel Hill, North Carolina, USA.
Julie C Fitzgerald, Division of Critical Care, Department of Anesthesiology and Critical Care, The University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania, USA.
Keiko M Tarquinio, Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA.
Matt S Zinter, Divisions of Critical Care and Bone Marrow Transplantation, Department of Pediatrics, University of California, San Francisco, San Francisco, California, USA.
Jennifer E Schuster, Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, Missouri, USA.
Natasha B Halasa, Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Melissa L Cullimore, Department of Pediatrics, College of Medicine, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Aline B Maddux, Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, Colorado, USA.
Mary A Staat, Department of Pediatrics, University of Cincinnati, Division of Infectious Diseases, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Katherine Irby, Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock, Arkansas, USA.
Heidi R Flori, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Mott Children’s Hospital and University of Michigan, Ann Arbor, Michigan, USA.
Bria M Coates, Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois, USA.
Hillary Crandall, Division of Pediatric Critical Care, Department of Pediatrics, University of Utah, Salt Lake City, Utah, USA.
Shira J Gertz, Division of Pediatric Critical Care, Department of Pediatrics, Saint Barnabas Medical Center, Livingston, New Jersey, USA.
Adrienne G Randolph, Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children’s Hospital, and Department of Anesthesia, Harvard Medical School, Boston, Massachusetts, USA.
Nira R Pollock, Department of Laboratory Medicine, Boston Children’s Hospital and Harvard Medical School, Boston, Massachusetts, USA.
References
- 1. Feldstein LR, Tenforde MW, Friedman KG, et al. . Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldstein LR, Rose EB, Horwitz SM, et al. . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pollock NR, Savage TJ, Wardell H, et al. . Correlation of SARS-CoV-2 nucleocapsid antigen and RNA concentrations in nasopharyngeal samples from children and adults using an ultrasensitive and quantitative antigen assay. J Clin Microbiol 2021; 59:e03077-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H, Hogan CA, Verghese M, et al. . Ultra-sensitive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen detection for the diagnosis of coronavirus disease 2019 (COVID-19) in upper respiratory samples. Clin Infect Dis 2021; 73:2326–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Health Advisory. CDC Health Alert Network; May 14, 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 12 January 2021.
- 6. Ogata AF, Maley AM, Wu C, et al. . Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin Chem 2020; 66:1562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shan D, Johnson JM, Fernandes SC, et al. . N-protein presents early in blood, dried blood and saliva during asymptomatic and symptomatic SARS-CoV-2 infection. Nat Commun 2021; 12:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H, Hogan CA, Verghese M, et al. . SARS-CoV-2 nucleocapsid plasma antigen for diagnosis and monitoring of COVID-19. Clin Chem 2021; 68:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yonker LM, Gilboa T, Ogata AF, et al. . Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021; 131:e149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ren A, Sohaei D, Zacharioudakis I, et al. . Ultrasensitive assay for saliva-based SARS-CoV-2 antigen detection. Clin Chem Lab Med 2022. Available at: 10.1515/cclm-2021-1142. [DOI] [PubMed] [Google Scholar]
- 11. Meso Scale Discovery. S-PLEX SARS-CoV-2 N kit [package insert]. Available at: https://www.mesoscale.com/~/media/files/product%20inserts/s-plex-sars-cov-2%20n-kit-product-insert.pdf. Accessed 30 October 2021.
- 12. Meso Scale Discovery. S-PLEX SARS-CoV-2 Spike kit [package insert]. Available at: https://www.mesoscale.com/~/media/files/product%20inserts/s-plex%20sars-cov-2%20spike%20kit%20product%20insert.pdf. Accessed 30 October 2021.
- 13. Meso Scale Discovery. V-PLEX COVID-19 Serologic Assay kit [package insert]. Available at: Available at: https://www.mesoscale.com/~/media/files/product%20inserts/v-plex%20covid-19%20serology%20assays%20insert.pdf. Accessed 30 October 2021.
- 14. Zhu C, He G, Yin Q, et al. . Molecular biology of the SARs-CoV-2 spike protein: a review of current knowledge. J Med Virol 2021; 93:5729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng MH, Zhang S, Porritt RA, et al. . Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci USA 2020; 117:25254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porritt RA, Paschold L, Rivas MN, et al. . HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J Clin Invest 2021; 131:e146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.