Abstract
Background
A longitudinal study was performed to determine the breadth, kinetics, and correlations of systemic and mucosal antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Methods
Twenty-six unvaccinated adults with confirmed coronavirus disease 2019 (COVID-19) were followed for 6 months with 3 collections of blood, nasal secretions, and stool. Control samples were obtained from 16 unvaccinated uninfected individuals. SARS-CoV-2 neutralizing and binding antibody responses were respectively evaluated by pseudovirus assays and multiplex bead arrays.
Results
Neutralizing antibody responses to SARS-CoV-2 were detected in serum and respiratory samples for 96% (25/26) and 54% (14/26), respectively, of infected participants. Robust binding antibody responses against SARS-CoV-2 spike protein and S1, S2, and receptor binding (RBD) domains occurred in serum and respiratory nasal secretions, but not in stool samples. Serum neutralization correlated with RBD-specific immunoglobulin (Ig)G, IgM, and IgA in serum (Spearman ρ = 0.74, 0.66, and 0.57, respectively), RBD-specific IgG in respiratory secretions (ρ = 0.52), disease severity (ρ = 0.59), and age (ρ = 0.40). Respiratory mucosal neutralization correlated with RBD-specific IgM (ρ = 0.42) and IgA (ρ = 0.63).
Conclusions
Sustained antibody responses occurred after SARS-CoV-2 infection. Notably, there was independent induction of IgM and IgA binding antibody and neutralizing responses in systemic and respiratory compartments. These observations have implications for current vaccine strategies and understanding SARS-CoV-2 reinfection and transmission.
Keywords: SARS-CoV-2, mucosal immunity
Respiratory/mucosal and serum/systemic antibody responses to SARS-CoV-2 infection are distinct host defense mechanisms independently influenced by age, severity of illness, and immunoglobulin class. Stimulation of respiratory immunity will be important for SARS-CoV-2 vaccines designed to limit transmission.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a betacoronavirus that was first detected in Wuhan, China, presumably following introduction from an animal reservoir [1]. The ability of SARS-CoV-2 to cause a severe respiratory illness, coronavirus disease 2019 (COVID-19), and rapidly spread through human populations has led to the most devastating pandemic in a century, resulting in an estimated 293 million infections and 5.4 million deaths globally [2]. A rapid scale-up of nonpharmaceutical public health interventions, including policies with social and economic impacts (eg, lockdowns and school and border closures), coupled with the introduction of highly effective vaccines, have limited transmission of the virus within communities [3]. However, SARS-CoV-2 infection risk persists owing to stark inequalities of global vaccine coverage, shortfalls in vaccine uptake, and the ongoing emergence of new variants of concern, such as delta, B.1.617.2 [4], and omicron, B.1.1.529 [5]. Ultimately, mitigating the public health impact will require globally coordinated mass immunization campaigns with highly effective vaccines that prevent both illness and virus transmission.
An increasingly nuanced picture of the development of systemic immunity to SARS-CoV-2 has emerged for the acute and early convalescent phases of COVID-19 [6]. Serum antibody to the SARS-CoV-2 spike (S) protein, and specifically to the receptor binding domain (RBD), is both critical to neutralization of the virus [7, 8], and the strongest correlate of protection from illness [9, 10]. The role of mucosal immunity is less well articulated, although the potent nature of dimeric IgA in neutralizing SARS-CoV-2 has been described [11]. The duration of immunity to SARS-CoV-2 and correlates of protection against subsequent reinfection are not fully defined. Finally, the propensity towards serious illness in older adults despite their mounting vigorous antibody responses remains unexplained [12].
A comprehensive approach to investigating antibody responses to SARS-CoV-2 provides insights into these critical questions. Here, we examined samples of blood, nasal wash, and stool collected serially from a cohort of SARS-CoV-2–infected individuals for 6 months from the onset of their infection. Detailed characterization of the specificity, functionality, and kinetics of the antibody responses to SARS-CoV-2 demonstrates clear distinctions in systemic and mucosal immune responses.
METHODS
Study Participants and Design
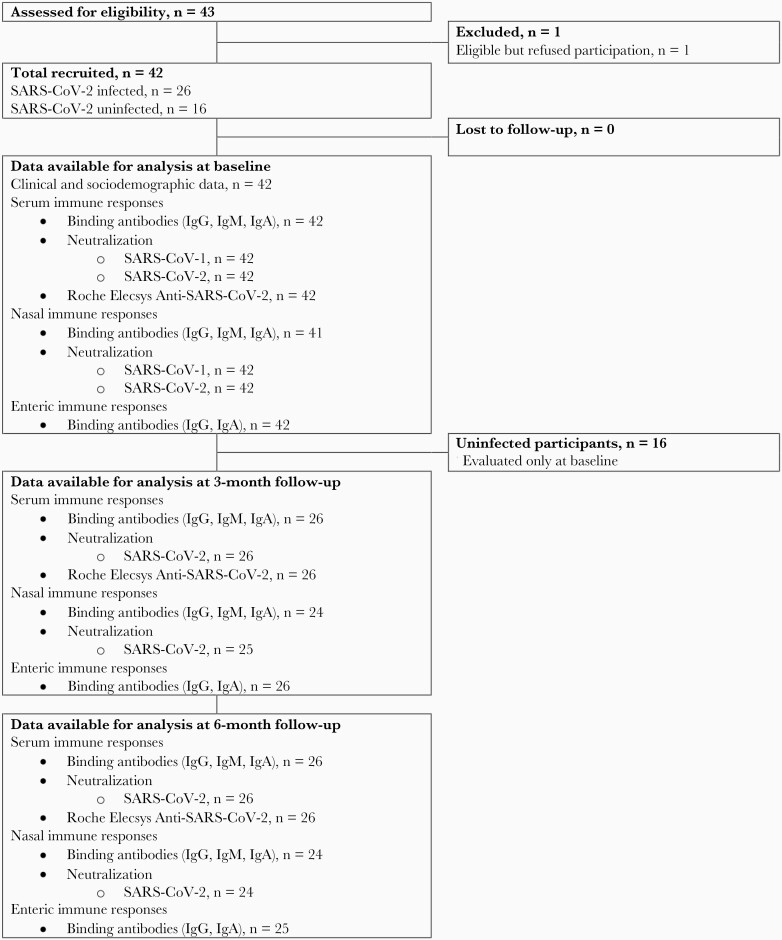
Twenty-six unvaccinated SARS-CoV-2–infected adults, aged 18–77 years, were enrolled at 1 month or more after COVID-19 diagnosis, which was confirmed by reverse transcription polymerase chain reaction (RT-PCR). All were followed up in the Clinical Research Unit of Dartmouth Hitchcock Medical Center (Lebanon, New Hampshire, USA) for at least 6 months with 2 additional specimen collection points at 2 and 5 months after the initial visit (Figure 1). As controls, 16 unvaccinated uninfected adults, aged 22–66 years, were sampled at a single point early in the epidemic. During the period of follow-up, no study participants received a COVID-19 vaccine, and none of the infected participants had clinical evidence of a second infection with SARS-CoV-2.
Figure 1.
Follow-up of study population at Dartmouth Hitchcock Medical Center (Lebanon, NH) between 8 April 2020 and 4 February 2021. Abbreviations: Ig, immunoglobulin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Blood, nasal secretions, and stool samples were collected at each study visit. Nasal secretions were obtained by forceful exhalation after instillation of 5 mL of normal saline into each nares in a recumbent position. Stool was self-collected either at the visit or the following day. At enrollment, a brief questionnaire evaluated the participants’ severity of symptoms. Five with mild illness reported no interference with tasks of daily living. Fifteen with moderate symptoms reported having a debilitating illness that prevented the activities of daily living with varying chief complaints of fatigue, shortness of breath, diarrheal illness, and/or fever. Six had severe enough illness to require hospitalization, primarily for respiratory support. Using this information, a rank order of severity of illness was established and used to investigate correlations of immune responses and clinical symptoms. The study was approved by the Dartmouth-Hitchcock institutional review board and written informed consent was obtained from all participants using an approved template.
Laboratory Procedures
Neutralization was evaluated using a vesicular stomatitis virus pseudovirus expressing the SARS-CoV-1 or SARS-CoV-2 S proteins, which were developed using plasmids supplied by the National Institute of Allergy and Infectious Diseases Rocky Mountain Laboratory [13]. Neutralizing activity was determined as a measure of functional antibody responses in serum and nasal secretions. Assay validation was performed on a subset of 40 serum samples (36 paired serum samples from 18 SARS-CoV-2–infected participants from their first and second visits and 4 samples from uninfected participants). These samples were run in parallel by the Duke Surgical Research Facility using a SARS-CoV-2-S lentivirus system that is the reference for measuring neutralization in COVID-19 vaccine trials [14]. The assays were found to be precisely correlated at both the first (Spearman ρ = 0.93, P < .0001) and second study visits (Spearman ρ = 0.91, P < .0001) (Supplementary Figure 1).
Binding antibodies were evaluated using custom multiplex bead arrays [15]. For the purposes of this study, data analysis was confined to the IgG, IgM, and IgA responses to components of the S protein of SARS-CoV-2. At the time of investigation, the original Wuhan SARS-CoV-2 strain was the only locally circulating virus, and homologous antigens were used in the antibody assays. The Roche Elecsys Anti-SARS-CoV-2 immunoassay, which detects total antibody binding responses to the nucleocapsid (N) protein in serum, was performed by the Dartmouth-Hitchcock Clinical Laboratory Services.
Statistical Analysis
Statistical analyses were performed using Stata version 13 and R version 3.6.1. Heatmaps were made using the R package “pheatmap.” Features were filtered to eliminate any end point for which >25% of subject were within 10 standard deviations of the blank used in the assay. The data was log transformed, scaled, and centered, and the z-score was plotted [16]. To accommodate variation in the time of initial sampling, longitudinal data displays were stratified into 50-day blocks to 200 days after symptom onset. Linear regressions with adjustment for participant age and sex were used to evaluate mean percent differences in immune markers between infected and uninfected participants. The mean percent differences were estimated with the formula (eβ − 1) × 100%, where the β coefficient represents the mean difference in loge-transformed marker level (markers with a level of 0 were recoded to 2 prior to the loge-transformation). Longitudinal patterns in log2 serum and nasal wash neutralization and RBD-specific binding antibodies were investigated using linear multilevel mixed-effects models with participant-specific random effects, linear time trends (ie, per 50-day units), and the variables of participant age, sex, and COVID-19 severity category. Multiplicative interaction was investigated using likelihood ratio tests. Pairwise correlations between antibody concentrations and neutralization titers in serum, nasal secretions, and stool samples at the time of the initial visit as well as participant age and severity rank order (ie, from 1 [least symptomatic] to 26 [most severe]) were estimated with Spearman rank correlation coefficients and visualized in matrices using the “corrplot” R package version 0.88 [17]. Spearman rank correlation coefficients and scatterplots were also used to compare SARS-CoV-2 pseudovirus neutralization between the vesicular stomatitis virus and lentivirus assays.
RESULTS
Sera, nasal secretions, and stools were collected between 8 April 2020 and 4 February 2021 from 26 SARS-CoV-2–infected adult participants (50.0% female; median age, 57.5 years [range, 18–77 years]; Figure 1). Initial samples were collected from infected participants at a median of 42.5 days (range, 17–154 days) after the onset of symptoms compatible with COVID-19 or a positive diagnostic test (whichever came first) for the study baseline. Subsequent visits for the infected participants were at a median of 103.5 days (range, 77–217 days) after symptom onset for the second visit and 200 days (range, 163–309 days) after symptom onset for the third visit. Serum, nasal secretions and stool were collected once between 17 April 2020 and 15 June 2020 in a control group of 16 uninfected adults (56.3% female; median age, 34 years [range, 22–76 years]).
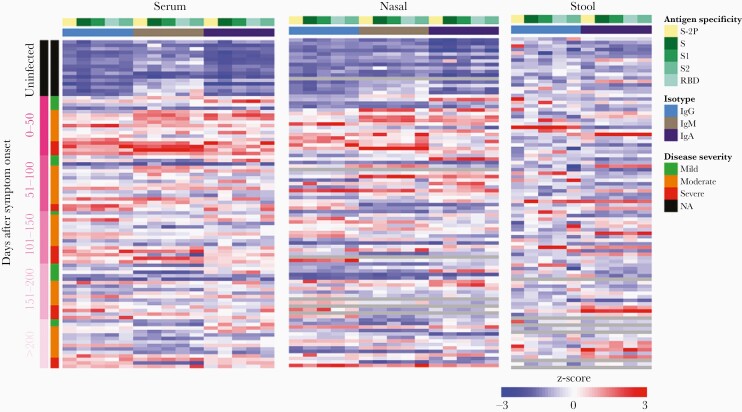
Neutralizing and binding antibody responses over time to specific viral proteins in serum, nasal wash, and stool in SARS-CoV-2–infected and uninfected participants were compared using a heat map (Figure 2). This overview demonstrates detection of both systemic and mucosal responses to all components of SARS-CoV-2 S evaluated in infected individuals. Responses above the mean (ie, with a Z-score > 0) among the 42 participants are shown in red and are typical of convalescent subjects, whereas responses below the mean (ie, with a Z-score < 0) are indicated in blue and observed particularly among naive subjects plotted in the top one-sixth of the map. Robust antibody responses were present in nasal secretions and serum but not in stool. Serum immune responses appear to diminish through longitudinal time points.
Figure 2.
Heat-map overview of binding antibody responses to specific SARS-CoV-2 proteins in serum, nasal wash, and stool in infected and uninfected participants at Dartmouth Hitchcock Medical Center (Lebanon, NH) between 17 April 2020 and 4 February 2021. Abbreviations: Ig, immunoglobulin; NA, not applicable; RBD, receptor-binding domain; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Serum Antibody Responses
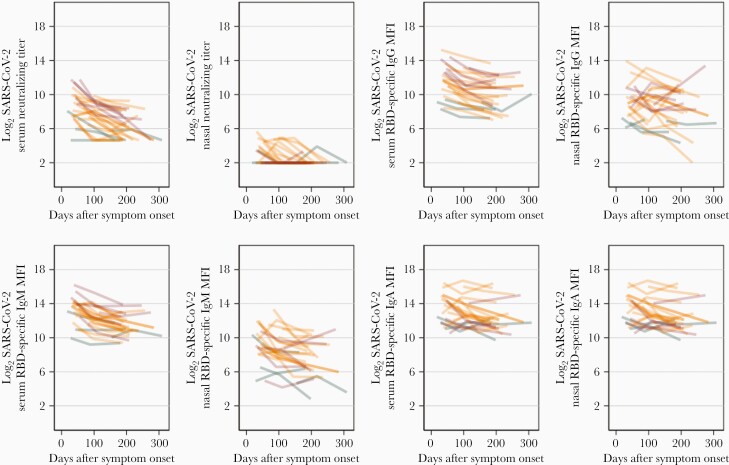
Neutralizing antibodies to SARS-CoV-2 were detected at the first visit in 25/26 (96.2%) infected individuals (Figure 3). During the 5-month period of follow-up, serum neutralization titers to SARS-CoV-2 declined in 23/26 (88.5%) of infected participants with a median half-life of 60.3 days (interquartile range, 42.6–105.4 days). The declines in serum neutralization over time were approximately log-linear, and there was no evidence of effect modification in the rate of decline by disease severity (P = .08, likelihood ratio test). In a multilevel linear regression adjusted for severity category, age, sex, and within-individual correlations, log2 serum neutralization declined on average by 0.70 (95% confidence interval [CI], .55–.85) per 50-day block. Relative to participants with mild COVID-19, participants with severe COVID-19 had on average higher (2.22; 95% CI, .69–3.75) log2 serum neutralization titers over the duration of follow-up; levels did not differ significantly between moderate and mild cases (1.00; 95% CI, −.29 to 2.30). There was not an obvious biphasic decline, as suggested by others (Figure 3) [18].
Figure 3.
Longitudinal profile of serum and respiratory secretion neutralization after SARS-CoV-2 infection among 26 participants presenting with mild (n = 5, green), moderate (n = 15, orange), and severe (n = 6, red) COVID-19. Abbreviations: COVID-19, coronavirus disease 2019; Ig, immunoglobulin; MFI, mean fluorescence intensity; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Neutralizing titers to SARS-CoV-1 were low but measurable in serum from 9/26 (34.6%) of infected individuals at the time of the initial sampling. For those 9, SARS-CoV-1 titers were not sustained over the follow-up. No measurable titers to SARS-CoV-1 or SARS-CoV-2 were seen in the uninfected individuals. These findings suggest the observed antibody response to SARS-CoV-1 in infected individuals most likely reflects de novo recognition of shared epitopes with SARS-CoV-2.
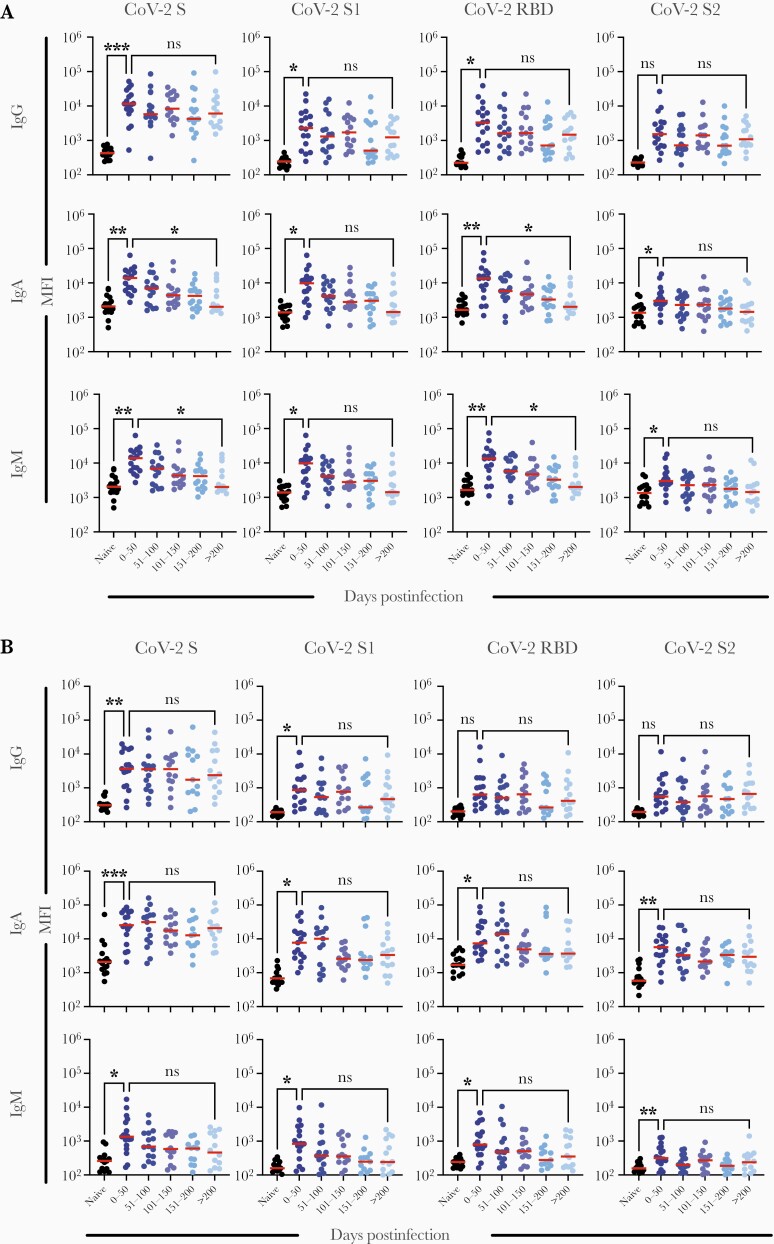
Multiplex arrays were used to quantify serum binding antibodies to SARS-CoV-2 S proteins and compared to serum neutralization titers. Significant differences in the levels of serum IgG, IgM, and IgA antibody to SARS-CoV-2 S were observed between uninfected and infected individuals at the time of the initial visit (Figure 4A and Supplementary Table 1). Analysis of results from subsequent follow-up visits suggested a decay in serum binding antibodies of each isotype against a panel of SARS-CoV-2 S antigens (Figure 4A). In multilevel linear regressions adjusted for severity category, age, sex, and within-individual correlations, log2 serum RBD-specific IgG, IgM, and IgA declined on average by 0.43 (95% CI, .32–.54), 0.48 (95% CI, .37–.60), and 0.27 (95% CI, .17–.37) per 50-day block over the duration of follow-up. The closest competitor to neutralization in terms of decay was antibody to SARS-CoV-2 RBD IgM with a median half-life of 87.4 days (interquartile range, 64.6–160.7). When serum isotype antibody responses are plotted in individual subjects the uniformity of decay is evident (Figure 3).
Figure 4.
Changes over time in binding antibodies to portions of the coronavirus spike protein by Ig class in (A) serum and (B) respiratory secretions (n = 26). Abbreviations: CoV-2, coronavirus 2; Ig, immunoglobulin; MFI, mean fluorescence intensity; RBD, receptor-binding domain; S, spike.
In contrast, the serum response to the SARS-CoV-2 N protein measured by the Roche Elecsys Anti-SARS-CoV-2 immunoassay rose significantly over the same period of follow-up in infected individuals (Supplementary Figure 2).
Nasal and Enteric Antibody Responses
At the time of the first visit, 14/26 (54%) of infected individuals also showed measurable neutralization of SARS-CoV-2 in nasal secretions. Neutralization was lower than to serum, with a maximum 60% neutralizing titer of 1:51 at the first visit (Figure 3). By the time of the second sampling, neutralization was undetectable in nasal secretions from 15/23 infected participants. At the final follow-up, nasal neutralizing antibody could not be detected. In a multilevel linear regression adjusted for disease severity category, age, sex, and within-individual correlations, log2 nasal wash neutralization declined on average by 0.29 (95% CI, .15–.42) per 50-day block. No association was observed between nasal wash neutralization titers and disease severity. Nasal neutralization to SARS-CoV-1 was seen in 3 individuals at the first visit.
Elevated IgA and IgG responses to SARS-CoV-2 S protein components were detected in nasal secretions of infected participants and were significantly higher than in the uninfected cohort at the first visit (Figure 4B). Log2 nasal RBD-specific IgG, IgM, and IgA declined on average by 0.30 (95% CI, .07–.54), 0.53 (95% CI, .35–.71), and 0.32 (95% CI, .18–.47), respectively, per 50-day block over the duration of follow-up (Figure 4B).
There were minimal differences in stool IgA or IgG antibodies to SARS-CoV-2 S proteins between the infected and uninfected participants (Figure 1, Supplementary Table 1, and Supplementary Figure 3). This is in contrast to other reports [19] and the demonstration of SARS-CoV-2 RNA in stool [20]. Assays for IgM binding antibodies were not performed on stool samples. Neutralizing antibodies in stool could not be measured because of nonspecific interference in the assay.
Correlation of Antibodies With Severity of Illness and Age
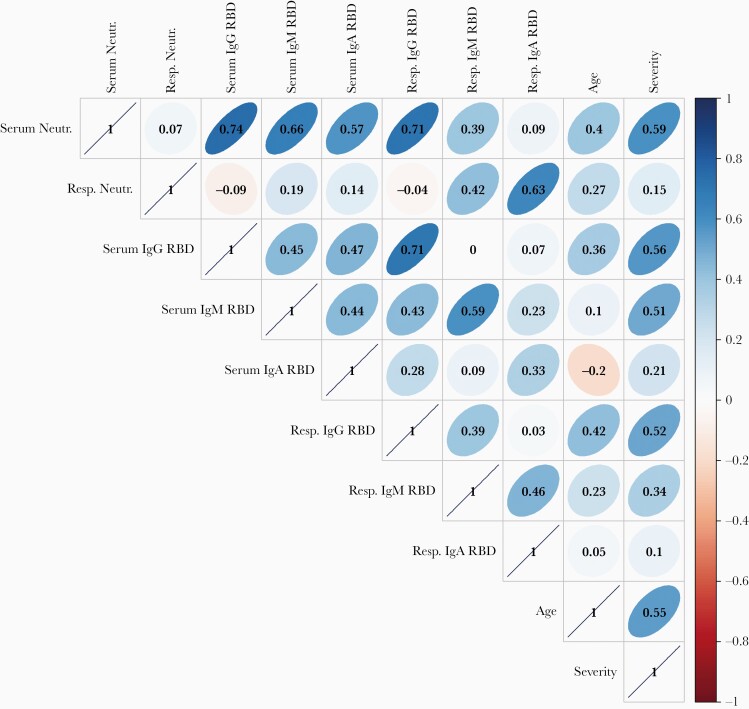
We performed a correlation analysis to define relationships between sample types, assays, disease severity, and age at the first study visit (the complete data set is shown in Supplementary Figure 3). In the serum, strong positive correlations were found between measurements of virtually all binding antibodies to SARS-CoV-2 S proteins in all Ig classes and neutralization (Spearman ρ > 0.55 for all). We focused on RBD responses in Figure 5. The level of RBD-specific IgG antibodies in serum was moderately positively correlated with age (Spearman ρ = 0.36) but more strongly correlated with the ranking of disease severity (Spearman ρ = 0.56). Serum RBD IgM antibodies showed no correlation with age (Spearman ρ = 0.10) but a strong correlation with severity of disease (Spearman ρ = 0.51). Serum IgA was not associated with age or severity (Spearman ρ < 0.25 for both).
Figure 5.
Pairwise spearman rank correlation coefficients of serum and respiratory SARS-CoV-2–specific neutralization, RBD binding antibody levels, participant age and severity (ie, in increasing rank order), and binding antibody levels in serum and nasal wash collected at the first study visit in SARS-CoV-2–infected participants (n = 25). The narrowness of the ellipse and intensity of the color indicate the strength of a given correlation coefficient, and the corresponding numerical values are defined by the vertical bar on the right. Abbreviations: Ig, immunoglobulin; Neutr., neutralization; RBD, receptor-binding domain; Resp., respiratory; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
There was a strong positive correlation of increasing age with increasing severity of disease (Spearman ρ = 0.59). Strikingly, there was no correlation of serum antibody titers in any Ig class with nasal wash neutralizing titers (Spearman ρ < 0.20 for all). Sex was not strongly associated with any measure of antibody responses.
In the nasal secretions, IgA was most strongly correlated with neutralization (Spearman ρ = 0.63; Figure 5 and Supplementary Figure 3). As with serum, the height of RBD-specific IgG antibodies in nasal secretions was moderately correlated with age (Spearman ρ = 0.42) and more strongly correlated with ranking of severity of disease (Spearman ρ = 0.52). In contrast, the height of nasal wash neutralization, IgM, and IgA had little relationship to age or severity of disease (Spearman ρ < 0.35 for all). Nasal IgG responses had no relationship to IgA responses (Spearman ρ = 0.03) but strongly correlated with the serum IgG values (Spearman ρ = 0.71). IgM responses in nasal wash were moderately correlated with IgA responses (Spearman ρ = 0.46).
DISCUSSION
We followed the evolution of systemic and mucosal upper respiratory tract immunity over a 6-month period following infection with SARS-CoV-2. Despite the relatively small number of SARS-CoV-2–infected volunteers studied, we were able to demonstrate correlations of SARS-CoV-2–specific antibody responses with each other, and with sample type, age, and severity of illness. Naive individuals provided a comparator for interpreting responses to SARS-CoV-2 infection. An earlier report by our group used a systems biology approach to define antibody functionality by coronavirus type, epitope, and Ig class at the initial time point [15]. In the current study, we incorporated data from sequential sampling to provide information on persistence of antibody responses with a focus on interrelationships of the mucosal and systemic immune compartments.
Our results demonstrate that induction of a SARS-CoV-2–specific antibody response occurs both systemically and in the respiratory tract. Enteric antibody was not seen. This is consistent with poliovirus findings—enteric antibody response to oral attenuated vaccine, while vigorous in infants and young children, was absent in adults [21]. The SARS-CoV-1 responses were not suggestive of an anamnestic response as they were low titer, not IgM, not sustained, and had no relationship to height of SARS-CoV-2 neutralizing antibody or to age.
Serum and mucosal neutralizing antibody titers showed a marked and uniform decline during follow-up. In contrast, other studies have shown more variable declines in titer [22]. This decline correlated with decline in serum IgM and IgA binding antibodies to RBD, the key recognition site for neutralizing antibodies [6], and suggests that IgM and/or IgA may be a major component of serum neutralization in the early convalescent period as indicated by other recent work [23, 24]. Supporting this is the observation that IgM depletion, in contrast to depletion of IgA or IgG, markedly lowers serum neutralizing titers [25]. The decline in neutralizing titer contrasts with our ability to isolate greater numbers of neutralizing monoclonal antibodies with increasing avidity over time from 8 members of this same cohort [26]. However, neutralizing antibodies are not the majority of circulating clonotypes ([27] and J.-W. Lee unpublished observation).
The sustainability of serum neutralizing antibody responses is a shared concern between SARS-CoV-2 natural infection and vaccination [28–30]. The half-life of serum neutralizing antibody after mRNA vaccination was 55 days [30], very similar to our observations of a half-life of 70 days after infection. Mucosal IgG antibody levels to all S components measured did not significantly decline in the 200 days after infection, suggesting that some components of the mucosal antibody response are longer lived. This is in contrast to the limited number of publications on the short duration of IgA antibody after other viral infections initiated at the respiratory mucosa, for example measles in which the half-life was in the range of 10 days [31].
Relatively few articles have addressed mucosal response to SARS-CoV-2 infection [32, 33]. When done, most have reported using techniques that do not optimally address an IgA-mediated response [34]. In spite of the differences in dilutions introduced by the collection methods for serum and nasal wash, our ability to measure IgA in the upper respiratory tract through nasal secretions revealed several important relationships between the respiratory and systemic antibody responses to SARS-CoV-2 infection.
Nasal and serum neutralization are not correlated. However, nasal and serum IgG are correlated. This presumably reflects serum antibody that has leaked across the epithelial barrier or utilized the FcRN receptor.
Nasal IgA although not correlated with nasal IgG nor with serum IgG or IgM, is correlated with nasal neutralization and nasal IgM.
Serum and nasal IgA and IgM titers are not correlated with the age of the subject, but serum and nasal IgG are correlated with age.
These observations have implications for route of administration, induction of immunity, and replication capacity of SARS-CoV-2 vaccines. Notably, prior studies by our group have emphasized striking differences in the induction of systemic and mucosal antibody to poliovirus that are dependent on the type and route of vaccine delivery, whether by systemic presentation of inactivated polio vaccine (Salk) or by enteric presentation of live replicating virus delivered orally (Sabin) [35]. SARS-CoV-2 proved highly effective at inducing mucosal IgA in the respiratory tract. Although high levels of protection from illness are seen with systemically administered SARS-CoV-2 vaccines, concerns remain about the extent to which these vaccines limit virus replication in the upper respiratory tract [36]. This concern has been heightened by the recent demonstrations of extensive replication [37] and transmission of the SARS-CoV-2 delta variant [38]. There is evidence that mRNA-based vaccines can confer a substantial degree of protection against infection [39]. However, animal models consistently demonstrate that vaccination targeted to the induction of mucosal antibody may be critical in limiting SARS-CoV-2 replication on challenge and, by implication, interrupting transmission [40, 41].
Severity of COVID-19 disease has an important positive correlation with the height of neutralizing responses in the serum [42]. From our data, the following conclusions can be drawn.
Nasal neutralizing and IgA spike antibodies have no correlation with severity of illness.
Severity of illness is the strongest correlate with increasing serum neutralizing and IgG spike immune responses.
We highlight the influence of age and severity of illness on the immune response following SARS-CoV-2 infection. Older individuals have markedly higher mortality from COVID-19 [43], yet have higher serum neutralizing antibody responses as compared to younger individuals [32]. The converse—that immune responses wane with age—is generally accepted [44], as is the case with SARS-CoV-2 mRNA vaccines [30]. Vaccines are given in higher doses (eg, for influenza) or with novel adjuvants in older adults to induce comparable immune responses to those seen in younger adults [45]. If heightened virus replication stimulates an enhanced antibody response, it would be reasonable to expect mucosal responses in the upper respiratory tract to also be higher in the sicker individuals. This was not the case in our study. Our results broadly align with previous work, which does not show a correlation between the height of virus shedding and severity of symptoms, although the more symptomatic individuals appeared to be infected for longer periods of time [46, 47].
As attention is paid to the diminishing neutralizing antibody responses over time postvaccination, it is important to recognize that this is also seen after natural infection and that other components of the immune response to infection or vaccination may be equally important in providing protection against more serious illness. In particular, the induction of mucosal IgA, which is locally produced and can uniquely transcytose across the epithelial cell barrier, can be postulated to be critical in the inactivation of viruses like SARS-CoV-2 that replicate primarily in the respiratory tract and must be a target for vaccine-induced immunity [48, 49].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Chengzi Kaku, David De Gijsel, Anais Ovide, Mrunal Sakharkar, Jiwon Lee, Edward Usherwood, Jacqueline Smith, Paul Guyre, Laura M. Walker, David Montefiore, and the Norris Cotton Cancer Center Immune Monitoring and Flow Cytometry Resource all contributed immensely to various parts of the effort.
Author contributions. P. W. contributed to the concept, organized the clinical trial, and wrote the primary drafts of the paper. A. P.-R. helped with data analysis, preparation of graphics for the paper, and writing of the manuscript. H. N. performed laboratory experiments and contributed to graphics. E. B. performed statistics, data analysis, and assisted with drafting the manuscript. R. C. performed key laboratory assays and contributed to the manuscript. W. W.-A. prepared specimens, kept the data base, and performed laboratory analysis. A. M. contributed to manuscript preparation and data presentation. J. W. performed laboratory analysis and contributed to the concept. R. N. analyzed responses to the N protein. M. E. contributed to the concept, writing of the manuscript, and analysis of data.
Financial support. This work was supported by the Bill and Melinda Gates Foundation (grant number DGR5180); and the Dartmouth-Hitchcock Department of Medicine and Division of Infectious Disease and International Medicine (SEAM Award AY21).
Contributor Information
Peter F Wright, Division of Infectious Disease and International Health, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA.
Alejandra C Prevost-Reilly, Dartmouth College, Hanover, New Hampshire, USA.
Harini Natarajan, Dartmouth College, Hanover, New Hampshire, USA.
Elizabeth B Brickley, London School of Hygiene and Tropical Medicine, London, United Kingdom.
Ruth I Connor, Division of Infectious Disease and International Health, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA.
Wendy F Wieland-Alter, Division of Infectious Disease and International Health, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA.
Anna S Miele, Dartmouth College, Hanover, New Hampshire, USA.
Joshua A Weiner, Thayer School of Engineering, Dartmouth College, Hanover, New Hampshire, USA.
Robert D Nerenz, Department of Pathology, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, USA.
Margaret E Ackerman, Thayer School of Engineering, Dartmouth College, Hanover, New Hampshire, USA.
References
- 1. Wu F, Zhao S, Yu B, et al. . A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. John Hopkins University of Medicine. Coronavirus resource center. https://coronavirus.jhu.edu. Accessed 4 January 2022.
- 3. Pearce N, Lawlor DA, Brickley EB.. Comparisons between countries are essential for the control of COVID-19. Int J Epidemiol 2020; 49:1059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mlcochova P, Kemp S, Dhar MS, et al. . SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021; 599:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng B, Abdullahi A, Ferreira IATM, et al. . Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022; 603:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sette A, Crotty S.. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184:861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robbiani DF, Gaebler C, Muecksch F, et al. . Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020; 584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ju B, Zhang Q, Ge J, et al. . Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020; 584:115–9. [DOI] [PubMed] [Google Scholar]
- 9. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. . Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Röltgen K, Boyd SD.. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe 2021; 29:1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Z, Lorenzi JCC, Muecksch F, et al. . Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med 2021;13:eabf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. . Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020; 183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Letko M, Marzi A, Munster V.. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 2020; 5:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sholukh AM, Fiore-Gartland A, Ford ES, et al. . Evaluation of cell-based and surrogate SARS-CoV-2 neutralization assays. J Clin Microbiol 2021; 59:e0052721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butler SE, Crowley AR, Natarajan H, et al. . Distinct features and functions of systemic and mucosal humoral immunity among SARS-CoV-2 convalescent individuals. Front Immunol 2021; 11:618685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolde R. pheatmap: Pretty heatmaps (2). https://rdrr.io/cran/pheatmap/. Accessed 6 April 2022. [Google Scholar]
- 17. Wei T, Simko V.. R package 'corrplot': Visualization of a Correlation Matrix. (Version 0.92). https://github.com/taiyun/corrplot. Accessed 6 April 2022. [Google Scholar]
- 18. Goel RR, Painter MM, Apostolidis SA, et al. . mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021; 374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Britton GJ, Chen-Liaw A, Cossarini F, et al. . Limited intestinal inflammation despite diarrhea, fecal viral RNA and SARS-CoV-2-specific IgA in patients with acute COVID-19. Sci Rep 2021; 11:13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Guo C, Tang L, et al. . Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Connor RI, Brickley EB, Wieland-Alter WF, et al. . Mucosal immunity to poliovirus. Mucosal Immunol 2022; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Zuiani A, Fischinger S, et al. . Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell 2020; 183:1496–507.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterlin D, Mathian A, Miyara M, et al. . IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med 2021; 13:eabd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gasser R, Cloutier M, Prévost J, et al. . Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep 2021; 34:108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Natarajan H, Xu S, Crowley AR, et al. . Antibody attributes that predict the neutralization and effector function of polyclonal responses to SARS-CoV-2. BMC Immunol 2022; 23:7. doi: 10.1186/s12865-022-00480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakharkar M, Rappazzo CG, Wieland-Alter WF, et al. . Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol 2021; 6:eabg6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voss WN, Hou YJ, Johnson NV, et al. . Prevalent, protective, and convergent IgG recognition of SARS-CoV-2 non-RBD spike epitope. Science 2021; 6546:1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pegu A, O’Connell S, Schmidt SD, et al. . Durability of mRNA-1273-induced antibodies against SARS-CoV-2 variants. Science 2021; 373:1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beaudoin-Bussières G, Laumaea A, Anand SP, et al. . Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. MBio 2020; 11:e02590–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doria-Rose N, Suthar MS, Makowski M, et al. . Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med 2021; 384:2259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friedman MG, Phillip M, Dagan R.. Virus-specific IgA in serum, saliva, and tears of children with measles. Clin Exp Immunol 1989; 75:58–63. [PMC free article] [PubMed] [Google Scholar]
- 32. Cervia C, Nilsson J, Zurbuchen Y.. Systemic and mucosal antibody responses specific to SARS-CoV-2 during mild versus severe COVID-19. J Allergy Clin Immunol 2021; 147:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isho B, Abe KT, Zuo M, et al. . Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol 2020; 5:eabe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson J, Pham DM, Werkhaven JA, et al. . Optimal collection and assay of upper respiratory specimens for determination of mucosal immune responses to influenza. In: Brown LE, Hampson AW and Webster RG, eds. Options for the control of influenza III. Amsterdam, The Netherlands: Elsevier Science, 1996: 263–70. [Google Scholar]
- 35. Wright PF, Connor RI, Wieland-Alter WF, et al. . Vaccine-induced mucosal immunity to poliovirus: analysis of cohorts from an open-label, randomised controlled trial in Latin American infants. Lancet Infect Dis 2016; 16:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baden LR, El Sahly HM, Essink B, et al. . COVE study group efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riemersma KK, Grogan BE, Kita-Yarbro A.. Shedding of infectious SARS-CoV-2 despite vaccination when the delta variant is prevalent—Wisconsin, July 2021. medRxiv, doi: 10.1101/2021.07.31.21261387, 6. November 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 38. Brown CM, Vostok J, Johnson H.. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1059–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson MG, Burgess JL, Naleway AL, et al. . Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers — eight U.S. Locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021; 70:495– 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hassan AO, Kafai NM, Dmitriev IP, et al. . A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 2020; 183:169–84.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mudrick HJ, McGlinch EB, Parret BJ.. Comparison of mucosal and intramuscular immunization against SARS-CoV-2 with replication-defective and replicating single-cycle adenovirus vaccines. bioRxiv, doi: 10.1101/2021.04.20.440651, 20. April 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- 42. Vanshylla K, Di Cristanziano V, Kleipass F, et al. . Kinetics and correlates of the neutralizing antibody response to SARS-CoV-2 infection in humans. Cell Host Microbe 2021; 29:917–29.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guan W, Ni Z, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Eng J of Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB.. Immunosenescence and human vaccine immune responses. Immun Ageing 2019; 13:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cowling BJ, Perera RAPM, Valkenburg SA, et al. . Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a randomized, controlled trial. Clin Infect Dis 2020; 71:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kissler SM, Fauver JR, Mack C, et al. . Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health strategies. PLoS Biol 2021; 19:e3001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bustos P, Tambley C, Acevedo A, et al. . Quantitative detection of SARS-CoV-2 RNA in nasopharyngeal samples from infected patients with mild disease. J Med Virol 2021; 93:2439–45. [DOI] [PubMed] [Google Scholar]
- 48. Ku Z, Xie X, Hinton PR, et al. . Nasal delivery of an IgM offers broad protection from SARS-CoV-2 variants. Nature 2021; 595:718–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lund FE, Randall TD.. The scent of a vaccine. Science 2021; 373:397–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.