Abstract
Objectives
Immunogenicity to the SARS-CoV-2 mRNA vaccines in adolescents and young adults (AYA) with childhood-onset rheumatic diseases (cRD) is unknown. We aimed to evaluate the humoral immunogenicity and safety of the vaccines in our AYA with cRD.
Methods
A monocentric observational study with 159 AYA (50.3% female and 70.4% Chinese). Humoral immunogenicity was assessed at 2–3 and 4–6 weeks following first and second vaccination by cPass™ SARS-CoV-2 Neutralization Antibody Assay. Inhibition signal of ≥30% defined the cut-off for positive detection of the SARS-CoV-2 neutralizing antibodies. Vaccine safety and disease activity were assessed within 6 weeks after second vaccination.
Results
A total of 64.9% and 99.1% of 159 patients (median age: 16.9, IQR: 14.7–19.5) mounted positive SARS-CoV-2 neutralizing responses after first and second vaccination, respectively. Most patients (89.8%) had ≥90% inhibition signal after second vaccination. Methotrexate and mycophenolate mofetil increased the risk associated with negative cPass neutralization responses following the first vaccination. Holding both medications after each vaccination did not affect immunogenicity. There was no symptomatic COVID-19 infection. Local reaction remained the most common (23.3–25.2%) adverse event, without serious complication. Two and seven patients flared following the first and second vaccination, respectively. Subgroup analyses of the 12–18-year-old cohort did not show any differences in vaccine efficacy, predictors of poor response and general safety, but higher proportion of disease flares.
Conclusions
SARS-CoV-2 mRNA vaccines were efficacious after the two-dose regimen in almost all AYA with cRD without serious adverse event. The rate of disease flare observed is 4.4% after the second mRNA vaccine dose.
Keywords: SARS-CoV-2 vaccine, COVID-19, childhood-onset rheumatic disease, vaccine efficacy, vaccine safety, paediatric age, paediatric rheumatology
Rheumatology key messages.
Almost all patients mounted adequate SARS-CoV-2 neutralizing antibodies following first and second SARS-CoV-2 mRNA vaccination.
Methotrexate and mycophenolate-mofetil significantly dampened first vaccine immunogenicity but not at all after the second.
No major adverse events were noted following vaccination, with 4% rate of disease flare.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], has led to significant mortality and disrupted the global socio-economic ecosystem [2]. The clinical spectrum of COVID-19 ranges from severe respiratory and multi-organ failure leading to death, to an asymptomatic or mild disease course [3–5]. Meta-analysis showed that individuals with rheumatic diseases (RD) do not appear to have more severe SARS-CoV-2 infection in terms of hospitalization, intensive care admission and mechanical ventilation [6]. Any increased COVID-19 severity in these patients found across studies is associated with the currently known risk factors of older age, male gender, high body mass index and the presence of comorbidities [7]. Although an increased COVID-19 mortality in individuals with RD is not consistently observed, an increased un-adjusted odds of mortality of 1.74 (95% CI of 1.08, 2.80) was found in a meta-analysis with 16 included studies [6]. Current available paediatric studies have reported hospitalization rates of 18–51.3% [8–11], but limited data exists regarding the clinical course of COVID-19 in children with RD. Although children have a lower COVID-19 mortality rate compared with adults [12, 13], the paediatric-specific occurrence of multisystem inflammatory syndrome in children [9] and known vulnerability to other severe infections from therapeutic immunomodulation [14] are concerns for a potentially more severe clinical course of COVID-19 in children with RD.
Henceforth, it is clinically prudent to protect this vulnerable population from the imminent and eventual exposure to SARS-CoV-2 as the virus becomes endemic [15]. Both the Pfizer-BioNTech and Moderna SARS-CoV-2 mRNA vaccines have demonstrated a noninferior immune response in non-diseased, healthy adolescents in comparison to young adults and should constitute part of the protective strategy [16, 17]. Notably, the Pfizer-BioNTech vaccine produced a higher 50% neutralizing titre in 12–15-year-olds when compared with 16–25-year-olds, which illustrates a robust immune response in the former and provides evidence for immunization in this age group [16]. However, there is no clear vaccination recommendation or guideline for children with RD due to the paucity of data on the impact of SARS-CoV-2 mRNA vaccines on childhood onset RD (cRD). Herein, we aim to comprehensively characterize the protective humoral neutralization response in our cohort of patients with cRD after inoculation with the COVID-19 mRNA vaccine, evaluate the impact of different immunomodulatory drugs as well as examine the safety profile of this two-dose immunization strategy.
Methods
Participants and study approval
Patients with cRD who are currently evaluated at the rheumatology clinics at KK Women’s and Children’s Hospital, Singapore and had received Pfizer-BioNTech or Moderna COVID-19 mRNA vaccines were recruited from 31 May 2021 to 20 September 2021. The vaccines were given to patients with inactive or low disease activity on stable immunosuppressive therapies. Written informed consent was obtained prior to the study initiation. The study was approved by SingHealth Centralized Institutional Review Board (CIRB: 2019–2961, 2019–2239). Blood samples were collected at 2–3 weeks after first vaccination and 4–6 weeks after the second. Blood samples were collected in serum separation blood collection tubes and centrifuged at 1300 g for 10 min before being aliquoted into cryovials and stored at –80°C until analysis.
Humoral immune response to mRNA vaccines
The frozen serum was thawed in batches for the experiment to assess the humoral immune response by the commercial blocking ELISA, cPass SARS-CoV-2 neutralization antibody detection kit (GenScript USA, Inc., NJ, USA) [18], according to the manufacturer’s protocol. This validated kit, which has received the US Food and Drug Administration authorization [19], assays the magnitude of antibody-mediated blockage of angiotensin-converting enzyme 2 receptor protein and its interaction with the SARS-CoV-2 receptor binding domain (RBD) required for viral entry into susceptible cells [20]. In SARS-CoV-2 infection, the RBD-targeting neutralizing antibodies are immunodominant [21]. The percent signal inhibition was calculated as [1-(Optical Density of sample/Optical Density of negative control)]×100%. An inhibition signal of ≥30% was used as the cut-off for positive detection of SARS-CoV-2 neutralizing antibodies [18]. At this recommended cut-off by the manufacturer, the cPass assay exhibits a 100% specificity and 96.1% sensitivity in COVID-19 patients at >14 days after SARS-CoV-2 PCR positive [22]. Additionally, this level also correlates strongly with the current gold standard for SARS-CoV-2 neutralizing antibody characterization, the plaque reduction neutralization test of 90% (PRNT90) [23].
Safety monitoring
Patients were contacted by phone on the day of first vaccination to 6 weeks after the second to complete a questionnaire regarding adverse events.
Statistical analysis
Non-parametric analyses were used to describe data and were shown as median (interquartile range, IQR) for continuous variables and percentages for categorical variables. Chi squared/Fisher’s exact, Mann–Whitney U, and Kruskal–Wallis tests were applied to compare differences between groups where appropriate. Predictors of vaccine response were examined using logistic regression. Variables that gave P < 0.1 in the univariate logistic regression were entered into the multivariate logistic regression by a backward conditional method and confirmed with a forward conditional method yielding odd ratios (ORs) and 95% CI for predictor variables. All analyses were performed using SPSS, version 23.0 (IBM Corp., NY, USA) and GraphPad Prism V.7 (GraphPad Software, Inc., CA, USA) with statistical significance set at P < 0.05.
Results
The study cohort comprised 159 consecutive patients with cRD (50.3% female, 70.4% Chinese) with the median age at first vaccination of 16.9 years (IQR: 14.7–19.5) and disease duration of 5.0 years (IQR: 2.0–7.0), Table 1. Overall, 147 (92.5%) and 12 (7.5%) patients had Pfizer-BioNTech and Moderna SARS-CoV-2 mRNA vaccines, respectively. JIA composed the majority (56.6%) followed by SLE (18.2%), and Undifferentiated CTD (UCTD, 6.3%), Table 1. Other diagnoses that had four or less patients each are shown in Supplementary Table S1, available at Rheumatology online. Anti-tumour necrosis factor (aTNF) was the most common biological DMARD used. As for conventional DMARDs, HCQ, SSZ and MTX were among the most prescribed. All patients taking prednisolone had concomitant DMARDs. Of 43 patients taking HCQ, 11 of them were on monotherapy.
Table 1.
Clinical characteristics and medications of patients in our cohort (n = 159)
| Clinical characteristics | n (%) |
|---|---|
| Female | 80 (50.3) |
| Race | |
| Chinese | 112 (70.4) |
| Malay | 18 (11.3) |
| Indian | 13 (8.2) |
| Others | 16 (10.1) |
| Age at first vaccination (years)a | 16.9 (14.7–19.5) |
| Disease duration at first vaccination (years)a | 5.0 (2.0–7.0) |
| Duration from first vaccination to sample collection (weeks)a | 2.4 (2.1–2.7) |
| Duration from second vaccination to sample collection (weeks)a | 4.6 (3.9–5.1) |
| Diagnosis | |
| JIA | 90 (56.6) |
| SLE | 29 (18.2) |
| Overlap syndrome | 2 (1.3) |
| UCTD | 10 (6.3) |
| JDM | 8 (5.0) |
| Others | 20 (12.6) |
| Medications | |
| Prednisolone | 29 (18.2) |
| Methotrexate | 41 (25.8) |
| Azathioprine | 7 (4.4) |
| Mycophenolate mofetil | 23 (14.5) |
| Sulfasalazine | 42 (26.4) |
| Hydroxychloroquine | 43 (27.0) |
| Anti-TNFα | 49 (30.8) |
| Tocilizumab | 4 (2.5) |
| Rituximab | 1 (0.6) |
Median (IQR); UCTD: undifferentiated CTD; Others (details in Supplementary Table S1, available at Rheumatology online).
Humoral neutralization response to mRNA COVID-19 vaccine in patients with cRD
All patients received two doses of mRNA COVID-19 vaccine, 131 (82.4%) and 108 (67.9%) patients had serum samples available for the neutralization assays after their first and second vaccination, respectively, Tables 2 and 3. A total of 65% of our patients mounted an adequate neutralization response (signal inhibition ≥30%) after the first vaccination (Table 2). This figure increased to 99.1% after the second dose, Table 3. There is no difference between the patient group with no (n = 51) and those with post second vaccination antibody response assayed (n = 108) in their demographics, diagnosis, gender, age at vaccination, disease duration and medication used (Supplementary Table S2, available at Rheumatology online). The antibody response after the second dose in the patients with cRD is comparable to our age-matched healthy cohort (n = 17) with all having an adequate post second dose response (Supplementary Table S3, available at Rheumatology online). Moreover, 89.8% who had completed two doses developed over a 90% inhibition level, but only 3.8% of the singly vaccinated patients achieved the same, Table 2 and 3.
Table 2.
cPass % inhibition data after first vaccination for our whole COVID-19 vaccine cohort
| cPass | % inhibition |
|---|---|
| First vaccination with sample, n = 131 (82.4%)a | 43.7 (17.1–71.8) |
| cPass1 | n (%) |
| ≥30% | 85 (64.9) |
| ≥50% | 57 (43.5) |
| ≥70% | 34 (26.0) |
| ≥90% | 6 (3.8) |
Median (IQR), cPass1, percent signal inhibition after first COVID-19 mRNA vaccine dose.
Table 3.
cPass % inhibition data after second vaccination for our whole COVID-19 vaccine cohort
| cPass | % inhibition |
|---|---|
| Second vaccination with sample, n = 108 (67.9%)a | 96.9 (95.4–97.3) |
| cPass2 | |
| ≥ 30% | 107 (99.1) |
| ≥ 50% | 105 (97.2) |
| ≥ 70% | 104 (96.3) |
| ≥ 90% | 97 (89.8) |
Median (IQR), cPass2, percent signal inhibition after second COVID-19 mRNA vaccine dose.
Forty-six patients did not have an adequate neutralization response after the first vaccination, Table 4. Most JIA patients (63.5%) had an adequate response (P = 0.004) but around one-third of cSLE patients did not (P = 0.020). Regarding medication, a significant proportion of patients taking MMF could not mount an adequate response relative to other medications. Patients taking MTX seem to have an inadequate response as well despite non-significance. In those with the neutralization responses characterized after the first COVID-19 vaccination, 16/23 (69.6%) of MMF and 34/36 (94.4%) of MTX taking patients held their respective medication after vaccination for a week. Interestingly, there were no significant differences in the proportion of patients whether they held their MTX or MMF as determined by their neutralization responses [cPass < 30% vs cPass ≥ 30%: MTX 17/17 vs 17/19 (P = 0.487) and MMF 12/17 vs 4/6 (P = 1.000)].
Table 4.
Clinical characteristics and medications of patients by the adequacy of neutralization response after the first COVID-19 vaccination (n = 131)
| Parameters | Total | cPass1 < 30% | cPass1 > 30% | P-value |
|---|---|---|---|---|
| n = 131 | n = 46 | n = 85 | ||
| Female | 68 | 27 (58.7) | 41 (48.2) | 0.253 |
| Race | 0.732 | |||
| Chinese | 91 | 34 (73.9) | 57 (67.1) | |
| Malay | 16 | 4 (8.7) | 12 (14.1) | |
| Indian | 10 | 4 (8.7) | 6 (7.1) | |
| Others | 14 | 4 (8.7) | 10 (11.7) | |
| Age at first vaccination (years)a | 16.8 (14.4-19.0) | 16.6 (14.7-18.9) | 0.589 | |
| Duration of disease (years)a | 4.0 (1.8-6.0) | 4.0 (2.0-7.0) | 0.369 | |
| Duration of sampling after first vaccination (weeks)a | 2.4 (2.1-2.7) | 2.6 (2.3-2.9) | 0.046 | |
| Diagnosis | 0.026 | |||
| SLE | 25 | 14 (30.4) | 11 (12.9) | 0.020 |
| JIA | 71 | 17 (37.0) | 54 (63.5) | 0.004 |
| JDM | 6 | 2 (4.3) | 4 (4.7) | 0.925 |
| UCTD | 10 | 2 (4.3) | 8 (9.4) | 0.493 |
| Medication | ||||
| Prednisolone | 26 | 12 (26.1) | 14 (16.5) | 0.188 |
| MTX | 36 | 17 (37.0) | 19 (22.4) | 0.074 |
| HCQ | 38 | 17 (37.0) | 21 (24.7) | 0.140 |
| AZA | 6 | 0 | 6 (7.1) | 0.090 |
| SSZ | 33 | 5 (10.9) | 28 (32.9) | 0.005 |
| MMF | 23 | 17 (37.0) | 6 (7.1) | <0.001 |
| Anti-TNF α | 40 | 13 (28.3) | 27 (31.8) | 0.678 |
Median (IQR); UCTD, Undifferentiated CTD; cPass1, percent signal inhibition after first COVID-19 mRNA vaccine dose.
Effect of immunosuppressive treatments on the immunogenicity of the COVID-19 mRNA vaccine
We then performed univariate and multivariate logistic regression analyses to determine the relationship of a negative cPass result (signal inhibition <30%) after the first vaccine dose with gender, age, disease duration, diagnosis and medications (Supplementary Table S4, available at Rheumatology online). In the univariate analyses, SLE and treatment with MMF were associated with a lack of response while JIA and treatment with SSZ were associated with a positive neutralization response. Following multivariate logistic regression analysis to adjust for confounders, an increased risk of a negative cPass inhibition was found only with MTX (OR: 7.616, 95% CI: 2.668, 21.738, P < 0.001) and MMF (OR: 10.643, 95% CI: 3.043, 37.227, P < 0.001) treatments.
When the post first-dose cPass inhibition level was compared across different diseases, those with SLE (median: 22.4%, IQR: 4.4–48.8) had a significantly lower inhibition level compared with those with Enthesis-Related Arthritis (ERA) (median: 50.0%, IQR: 33.3–73.7), P = 0.039 (Supplementary Fig. S1A, available at Rheumatology online). However, no difference in the cPass inhibition level was observed across different diseases after the second vaccine dose (Supplementary Fig. S1B, available at Rheumatology online).
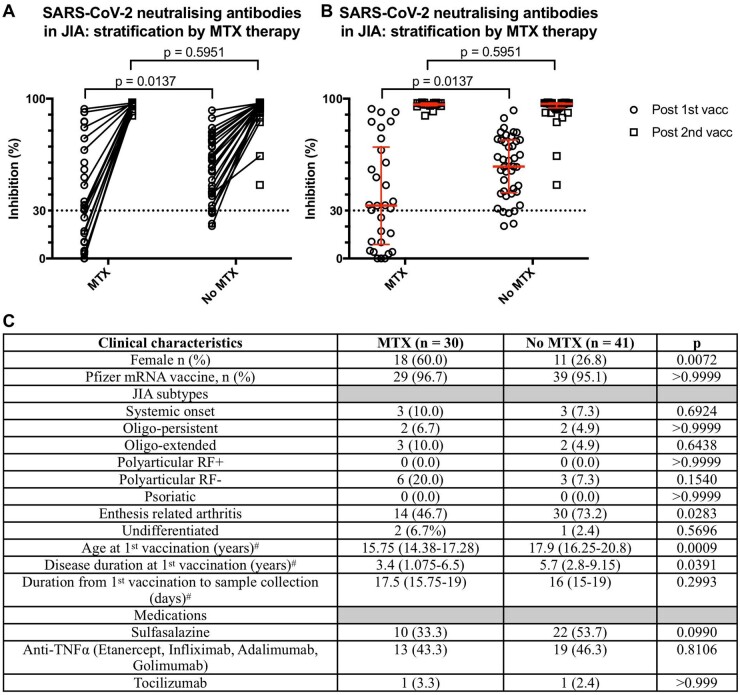
MTX decreased the first dose neutralization response in patients with JIA
In our JIA cohort, dichotomized by treatment with MTX, a significant reduction in the first dose inhibition level was observed in those taking MTX (median: 33.2%, IQR: 8.8–69.7) vs those not (median: 57.5%, IQR: 41.2–74.1), P = 0.014 (Fig. 1A and B). However, the JIA patients treated with MTX differed significantly from the non-treated group in their clinical characteristics: female and ERA predominance, age at first vaccination and disease duration (Fig. 1C). Hence, we proceeded to analyse the ERA subtype separately, the most common JIA subtype in our cohort (44 of 77, 62.0%), to control for these potential confounders (Supplementary Fig. S2A and B, available at Rheumatology online). Although a reduced inhibition level was still observed in the MTX (median: 34.5%, IQR: 23.5–77.7) vs non-MTX treated in the ERA subgroup (median: 56.1%, IQR: 40.6–73.0), it was not significant (P = 0.182). Additionally, no difference in the neutralization response was observed with age in the ERA group with no MTX treatment (Supplementary Fig. S2C, available at Rheumatology online).
Fig. 1.
Neutralization response after COVID-19 mRNA vaccination in patients with JIA
(A) Symbols and lines plot of percent signal inhibition in patients with JIA stratified by MTX treatment. (B) Scatter plot with median and interquartile ranges of percent signal inhibition in patients with JIA stratified by MTX treatment. (C) Clinical characteristics of JIA patients with cPass inhibition results after first vaccine dose. vacc: COVID-19 mRNA vaccine dose. Statistical analysis by Fisher’s exact or Mann–Whitney U tests. #Data presented as median (interquartile range).
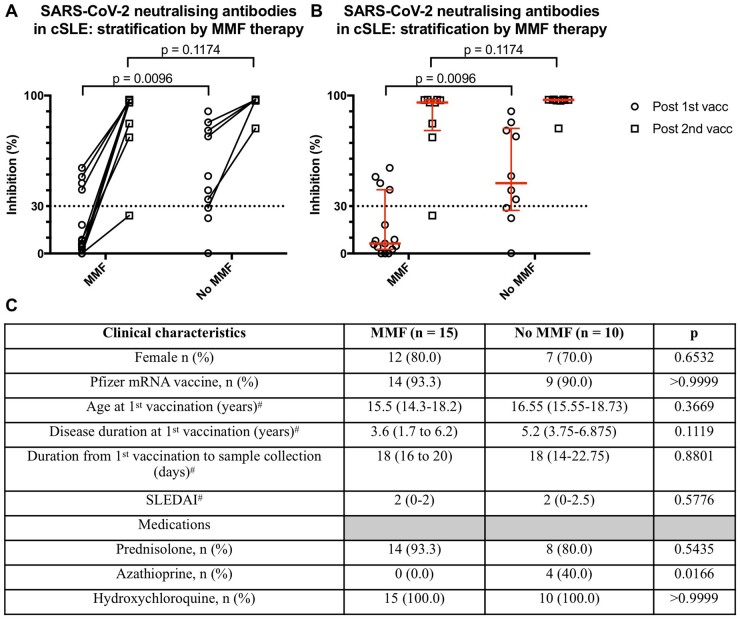
MMF decreased the first dose neutralization response in patients with SLE
We carried out a subgroup analysis on all patients with cSLE stratified by MMF treatment comparing the percent signal inhibition (Fig. 2A and B). There was a significant reduction in the inhibition level after the first vaccination in those taking MMF (median: 6.3%, IQR: 2.6–40.4) vs those without (median: 44.5%, IQR: 27.3–79.2), P = 0.010. For those with first dose cPass result, the two groups stratified by MMF treatment were comparable in terms of age, gender, type of COVID-19 mRNA vaccine, disease duration and activity, and medications except for a higher number of patients on azathioprine therapy in those without MMF treatment (P = 0.017). The immunomodulatory effect of MMF on the neutralization was absent after the second dose. Only one 18-year-old clinically inactive lupus patient (on stable doses of MMF, HCQ, tacrolimus and not on steroids) failed to achieve the cPass assay cut-off (≥30%) assessed on day 39 after receiving the second Pfizer COVID-19 mRNA vaccine.
Fig. 2.
Neutralization response after COVID-19 mRNA vaccination in patients with SLE
(A) Symbols and lines plot of percent signal inhibition in patients with SLE stratified by MMF treatment. (B) Scatter plot with median and interquartile ranges of percent signal inhibition in patients with SLE stratified by MMF treatment. (C) Clinical characteristics of lupus patients with cPass inhibition results after first vaccine dose. cSLE: childhood onset SLE; vacc: COVID-19 mRNA vaccine dose. Statistical analysis by Fisher’s exact or Mann–Whitney U tests. #Data presented as median (interquartile range).
Safety of COVID-19 mRNA vaccine in patients with rheumatic diseases
As shown in Table 5, pain at injection site remained the most common adverse reaction, up to one-quarter of our 159 patients. There was no serious adverse reaction noted up to 6 weeks post-vaccination. Fever and arthralgia were more frequent after second vaccination as compared with headache, myalgia and local swelling for the first. Moreover, there was no symptomatic COVID-19 infection case observed.
Table 5.
Safety profiles of SARS-CoV-2 mRNA vaccine in our mRNA vaccine cohort
| Clinical manifestations | After first vaccination, n (%) | After second vaccination, n (%) |
|---|---|---|
| Fever | 2 (1.3) | 8 (5.0) |
| Injection site reaction | 40 (25.2) | 37 (23.3) |
| Pain | 38 (23.9) | 37 (23.3) |
| Swelling | 2 (1.3) | 0 (0.0) |
| Erythema | 0 (0.0) | 0 (0.0) |
| Malaise | 17 (10.7) | 17 (10.7) |
| Arthralgia | 1 (0.6) | 2 (1.3) |
| Myalgia | 4 (2.5) | 2 (1.3) |
| Headache | 9 (5.7) | 3 (1.9) |
| Rash | 1 (0.6) | 1 (0.6) |
| Swollen lymph node | 0 (0.0) | 1 (0.6) |
Two patients (1.3%) experienced disease flare after first vaccination: a 20-year-old female with extended oligo-JIA, on infliximab for her arthritis and uveitis had both joint and eye disease flares, after 6 days, and a 16-year-old girl with UCTD had a flare of autoimmune haemolytic anaemia after 23 days. Seven patients (4.4%) reported disease flares after second vaccination with a median of 4.7 weeks (IQR: 2.1–6.0): five-JIA patients of which four were on MTX and two on SSZ with a median 4.7 weeks, IQR: 2.2–7.5, a 14-year-old Caucasian girl with juvenile dermatomyositis on weaning MTX 6 weeks after, and a 15-year-old Indian girl with overlap syndrome off medication developing myositis 2.14 weeks after.
Pfizer-BioNTech mRNA response in 12–18 years old patients with cRD
In our cohort, 95 (59.7%) were adolescents aged 12–18 [median age 15.2 (IQR: 14.1–16.6)]. This subgroup had similar disease distribution and medications used, proportion of patients with cPass neutralization response <30% inhibition (35.6%) and predictors for a negative cPass results including MTX (OR 4.800, 95% CI: 1.615, 14.263, P = 0.005) and MMF (OR 10.286, 95% CI: 2.681, 39.462, P = 0.001) (Supplementary Tables S5 and S6, available at Rheumatology online). The vaccine safety profile was comparable to the whole cohort report (Supplementary Table S7, available at Rheumatology online). Six out of seven patients who had flares of their underlying RDs after the second vaccination belonged to the adolescent subgroup, which constituted more than half of our study cohort (59.7%).
Discussion
In this study, we have demonstrated a robust humoral neutralization response against the SARS-CoV-2 spike RBD domain in 99.1% of adolescents and young adults (AYA) with cRD after two doses of the COVID-19 mRNA vaccine. Importantly, this positive cPass assay correlates strongly with a plaque reduction neutralization test of 90% (PRNT90) [23], a current gold standard for SARS-CoV-2 neutralizing antibody characterization. Notably, we observe an attenuation of this neutralization response with MMF and MTX after the first dose, which is lost after completing the two-dose vaccine regime. Thus, we strongly recommend that any unnecessary delay in administering the second mRNA vaccine dose must be avoided in patients treated with either one of these two DMARDs.
While there are studies on the immune response of adult patients with RD after COVID-19 vaccination [24–33], data heterogeneity on the humoral response to COVID-19 vaccination in patients with autoimmune diseases exists and hinders the harmonization of these information for clinical applications. Differences in vaccine types, clinical cohorts, duration of observation for adverse effects, methods used and the timing of assessing the vaccination response (after first and/or second vaccination) contribute to this quandary. Additionally, the differences in analysing this humoral response either as a categorical (with seropositivity rate) or continuous (with measure of central tendency and spread) variable further compounds the difficulty for cross-study comparison and generalization. Bearing these issues in mind, we will highlight how our study builds upon this information with new insights.
Our study is the first to demonstrate a robust humoral neutralization response in a distinctively AYA with cRD who have completed COVID-19 mRNA vaccination. Notably, our cohort has a higher proportion (97 out of 108, 89.8%) with an inhibition level of ≥90% compared with an adult cohort (16 out of 22, 72.7%) that was similarly evaluated with the cPass assay at an earlier blood sampling timepoint of 7 days after the second vaccine dose [28]. Significantly, this is in spite of the lack of MMF and MTX use in the adult study [28], which would depress vaccine immune responses [34].
Second, we illustrated the suppressive effect of two DMARDs, MTX and MMF, on the neutralization response after the first vaccine dose. The immunomodulatory effect of MTX was exhibited via multivariate logistic regression analysis and stratified bivariate analysis in the JIA cohort, whereby the humoral neutralization response was evaluated as a dichotomous categorical and continuous variable, respectively. Although a lower inhibition was still observed in the MTX-treated group (median: 34.5%, IQR: 23.5–77.7 vs median: 56.1%, IQR: 40.6–73.0, P = 0.182) when ERA was considered separately, this effect is not statistically significant. This immunosuppressive effect of MTX after the first but not the second vaccine dose was similarly shown in adult patients with RD (n = 574) who had been inoculated mainly with the ChAdOx1 (AstraZeneca) adenoviral vaccine [29]. However, other studies involving adults with RD inoculated with the BNT162b2 (BioNTech) mRNA vaccine demonstrated a persistent attenuation of the humoral response by MTX even after completing the two-dose regime [25, 26].
For MMF, a 10-fold higher risk for a negative neutralization response (<30% inhibition) was also observed. Interestingly, this attenuation was only observed after the first dose but not with completion of COVID-19 vaccination. Contrastingly, in adults with RD, the suppressive effect of MMF persists even after the second vaccine dose with a lower seroconversion rate [25, 27, 32]. The persistence of MMF’s suppressive effect may be attributed to variability in assays used and more importantly age, as a stronger immune response to COVID-19 mRNA vaccination has been reported in younger age groups [16]. This suggests that the American College of Rheumatology’s recommendation of withholding DMARDs following each vaccine dose may not be relevant for youths with cRD given their robust humoral immune responses in spite of concurrent DMARD therapy [35]. Nevertheless, combinatorial DMARD therapy may dampen vaccine responses in AYA as evidenced by the young adult with cSLE in our cohort who was simultaneously receiving MMF, HCQ and tacrolimus and failed to achieve the cPass assay cut-off. Further validation of our observations in a larger cohort is required to elucidate the influence of DMARDs on vaccine responses.
The safety profiles of these mRNA vaccine in our youth cohort are comparable to those reported for healthy adolescents, for which pain at the injection site remains the commonest local reaction with few serious adverse reactions reported [16]. However, 1.3% of our patients flared after their first and 4.4% flared after their second vaccination. Younger patients (12–18 years old) may confer a higher risk of flare as six out of seven of patients who flared after second vaccination belonged to this subgroup.
The limitations of our study include a non-randomised design and the use of a surrogate virus neutralization test with a readout that measures the degree of antibody-mediated blockage of ACE2-spike protein–protein interaction instead of a quantifiable antibody titre [18]. However, unlike the time-consuming PRNT which requires a specialized biosafety level (BSL) 3 facility due to the use of live viruses, the quicker ELISA-based cPass assay can be done in a BSL2 laboratory and this greatly increases its accessibility [18]. The cPass surrogate viral neutralization test also gives results that are either similar or superior to the commercial kits that were utilized in the studies with rheumatic disease cohorts [23], specifically the SARS-CoV-2 ELISA kits from Abbott (Chicago, IL, US) [27], DiaSorin (Saluggia, Italy) [25], Euroimmun (Lübeck, Germany) [28, 32] and Roche Diagnostics (Basel, Switzerland) [24, 32]. It is crucial to emphasize that these assays, including cPass, provide a crude correlate for protection, an absolute protective threshold that confers definite protection against SARS-CoV-2 infection is still not known [36, 37]. Without a known protective threshold, there is no advantage of an antibody quantification over a viral neutralization assay like cPass, which measures the ability of the serum/plasma to inhibit a mechanistically important ACE2-SARS-CoV-2-spike protein–protein interaction.
In conclusion, we have shown that in AYA with cRD, a robust humoral neutralization response is elicited after two-dose COVID-19 mRNA vaccination with the main adverse effects being self-resolving localized injection site reaction with pain and swelling. By building upon current evidence from adult studies that support the efficacy and safety of the mRNA vaccine with insights from a well-characterised AYA cohort with cRD, our findings can further allay the fears due to lack of information that contributes to vaccine hesitancy [38, 39]. In addition, we have shown that the two-dose mRNA vaccine regimen is efficacious and adequate for AYA with cRD. Further studies looking at the humoral immune response decay rate is underway in this cohort. The results obtained will eventually assist in guiding the booster intervals after completing the two-dose vaccination regime.
Supplementary Material
Acknowledgements
We thank the patients and families who contributed their time and efforts in this urgent but crucial study and special thanks to our phlebotomists Ms Yu CH, Chia MF, Ang JYJ, Norhani Bte Tenan, and Mr Ganesan P, Prakash C for their tirelessly assisting in collect blood samples for the study.
Funding: This study was conducted with the provision of grant funding from the SingHealth Duke-NUS Academic Medicine COVID-19 Rapid Response Research AM/COV004/2020 (S.A.). This research was also supported by the National Research Foundation Singapore under its National Medical Research Council (NMRC) Centre Grant Programme (NMRC/CG/M003/2017) (S.A., T.A.) and is administered by the Ministry of Health, Singapore’s NMRC. Other NMRC grant support, NMRC/TA/0059/2017 (J.G.Y.), MNRC/MOHIAFCAT2/005/2015 (S.A.), NMRC/TCR/0015-NCC/2016 (S.A.), NMRC/OFLCG/002/2018 (S.A.), CIRG19may0052 (S.A.), NMRC/STPRG-FY19-001 (L.-F.W.) and NMRC/COVID19RF-003 (L.-F.W.) is gratefully acknowledged.
Disclosure statement: The authors have declared no conflicts of interest
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information, available at Rheumatology online. The data used to support the findings of this study are included within the article.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Zhang YZ, Holmes EC.. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 2020;181:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakraborty I, Maity P.. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ 2020;728:138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 5. Yang R, Gui X, Xiong Y.. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw Open 2020;3:e2010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway R, Grimshaw AA, Konig MF. et al. SARS-CoV-2 infection and COVID-19 outcomes in rheumatic disease: a systematic literature review and meta-analysis. Arthritis Rheumatol 2022;https://doi.org/10.1002/art.42030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kroon FPB, Najm A, Alunno A. et al. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis 2022;81:422–32. [DOI] [PubMed] [Google Scholar]
- 8. Demir F, Ulu K, Çağlayan Ş. et al. Clinical course of COVID-19 in children with rheumatic disease under biologic therapy. Clin Exp Rheumatol 2021;39(Suppl 128):36–7. [PubMed] [Google Scholar]
- 9. Sozeri B, Ulu K, Kaya-Akça U. et al. The clinical course of SARS-CoV-2 infection among children with rheumatic disease under biologic therapy: a retrospective and multicenter study. Rheumatol Int 2022;42:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villacis-Nunez DS, Rostad CA, Rouster-Stevens K. et al. Outcomes of COVID-19 in a cohort of pediatric patients with rheumatic diseases. Pediatr Rheumatol Online J 2021;19:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aydın F, Kurt T, Sezer M. et al. Biological therapies in children with rheumatic diseases during the COVID-19 pandemic: a single-centre experience. Int J Clin Pract 2021;75:e14030. [DOI] [PubMed] [Google Scholar]
- 12. Bhopal SS, Bagaria J, Olabi B. et al. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolescent Health 2021;5:e12–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCormick DW, Richardson LC, Young PR. et al. Deaths in children and adolescents associated with COVID-19 and MIS-C in the United States. Pediatrics 2021;148:e2021052273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swart J, Giancane G, Horneff G. et al. Pharmacovigilance in juvenile idiopathic arthritis patients treated with biologic or synthetic drugs: combined data of more than 15,000 patients from Pharmachild and national registries. Arthritis Res Ther 2018;20:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillips N. The coronavirus is here to stay – here’s what that means. Nature 2021;590:382–4. [DOI] [PubMed] [Google Scholar]
- 16. Frenck RW Jr, Klein NP, Kitchin N. et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. New Engl J Med 2021;385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali K, Berman G, Zhou H. et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. New Engl J Med 2021;385:2241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan CW, Chia WN, Qin X. et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol 2020;38:1073–8. [DOI] [PubMed] [Google Scholar]
- 19. Coronavirus (COVID-19) Update: FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection. 2020. [updated 6 November 2020]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-test-detects-neutralizing-antibodies-recent-or (14 October 2021, date last accessed).
- 20. Shang J, Ye G, Shi K. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020;581:221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Premkumar L, Segovia-Chumbez B, Jadi R. et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020;5:eabc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nandakumar V, Profaizer T, Lozier BK. et al. Evaluation of a surrogate enzyme-linked immunosorbent assay-based Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) cPass neutralization antibody detection assay and correlation with immunoglobulin G commercial serology assays. Archi Pathol Lab Med 2021;145:1212–20. [DOI] [PubMed] [Google Scholar]
- 23. Taylor SC, Hurst B, Charlton CL. et al. A New SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol 2021;59:e02438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spiera R, Jinich S, Jannat-Khah D.. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis 2021;80:1357–9. [DOI] [PubMed] [Google Scholar]
- 25. Furer V, Eviatar T, Zisman D. et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. [DOI] [PubMed] [Google Scholar]
- 26. Haberman RH, Herati R, Simon D. et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Braun-Moscovici Y, Kaplan M, Braun M. et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the Pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. [DOI] [PubMed] [Google Scholar]
- 28. Geisen UM, Berner DK, Tran F. et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boekel L, Steenhuis M, Hooijberg F. et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021;3:e778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Picchianti-Diamanti A, Aiello A, Laganà B. et al. Immunosuppressive therapies differently modulate humoral- and T-cell-specific responses to COVID-19 mRNA vaccine in rheumatoid arthritis patients. Front Immunol 2021;12:740249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubbert-Roth A, Vuilleumier N, Ludewig B. et al. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol 2021;3:e470–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruddy JA, Connolly CM, Boyarsky BJ. et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simon D, Tascilar K, Fagni F. et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2021;80:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedman MA, Curtis JR, Winthrop KL.. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Curtis JR, Johnson SR, Anthony DD. et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nature Med 2021;27:1147–8. [DOI] [PubMed] [Google Scholar]
- 37. Earle KA, Ambrosino DM, Fiore-Gartland A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021;39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaur P, Agrawat H, Shukla A.. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int 2021;41:1601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beesley RP, Costello W, Angevare SP. et al. Survey of adult and paediatric rheumatology patients suggests information about COVID-19 vaccination will aid uptake. Rheumatol 2021;60:3474–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information, available at Rheumatology online. The data used to support the findings of this study are included within the article.