Abstract
Predictors of early diagnosis and severe infection in children with coronavirus disease 2019 (COVID-19), which has killed more than 4 million people worldwide, have not been identified. However, some biomarkers, including cytokines and chemokines, are associated with the diagnosis, pathogenesis and severity of COVID-19 in adults. We examined whether such biomarkers can be used to predict the diagnosis and prognosis of COVID-19 in pediatric patients. Eighty-nine children were included in the study, comprising three patient groups of 69 patients (6 severe, 36 moderate and 27 mild) diagnosed with COVID-19 by real-time polymerase chain reaction observed for 2–216 months and clinical findings and 20 healthy children in the same age group. Hemogram, coagulation, inflammatory parameters and serum levels of 16 cytokines and chemokines were measured in blood samples and were analyzed and compared with clinical data. Interleukin 1-beta (IL-1β), interleukin-12 (IL-12) and interferon gamma-induced protein 10 (IP-10) levels were significantly higher in the COVID-19 patients (p = 0.035, p = 0.006 and p < 0.001). Additionally, D-dimer and IP-10 levels were higher in the severe group (p = 0.043 for D-dimer, area under the curve = 0.743, p = 0.027 for IP-10). Lymphocytes, C-reactive protein and procalcitonin levels were not diagnostic or prognostic factors in pediatric patients (p = 0.304, p = 0.144 and p = 0.67). Increased IL-1β, IL-12 and IP-10 levels in children with COVID-19 are indicators for early diagnosis, and D-dimer and IP-10 levels are predictive of disease severity. In children with COVID-19, these biomarkers can provide information on prognosis and enable early treatment.
Keywords: COVID-19, pediatrics and cytokine
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has spread worldwide since being identified in December 2019 and has caused the death of millions of people [1]. Despite protection measures and vaccination programs, the ongoing COVID-19 pandemic poses various challenges for clinicians. Timely diagnosis and hospitalization, risk stratification, effective use of intensive care services and selection of appropriate treatments are essential for resolving COVID-19 [2]. Clinical evaluation is indispensable, but laboratory parameters and biomarkers are helpful in diagnosis and determining disease severity [1, 2]. COVID-19 is not a localized respiratory tract infection, but a multisystemic disease caused by a widespread systemic process involving the interaction of immunological, inflammatory and coagulation cascades [3, 4].
The clinical course of COVID-19 is milder in children than in adults, possibly because of trained immunity, vaccines, frequent viral infections, better lung regeneration or lack of comorbities [5]. The answers to the unknowns in the pathogenesis of COVID-19 could be related to cytokines and immunological mechanisms [5, 6].
Some cytokines increase in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [6]. The severity of COVID-19 may be related to the cytokine storm. Interleukin-2 (IL-2), interleukin-7 (IL-7), interleukin-10 (IL-10), granulocyte colony-stimulating factor, IFN-γ-inducible protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP-1α) and tumor necrosis factor alpha (TNF-α) levels were higher in critical than non-critical COVID-19 adult patients [1]. Although the milder course of COVID-19 infection in children may be associated with lower cytokine levels, the data are limited [5]. In addition, a severe clinical condition, called pediatric inflammatory multisystem syndrome, caused by a cytokine storm, develops in some patients [7].
Because there is a relationship between cytokine levels and disease severity, cytokine antagonists have been used for treatment, especially in adults with severe disease [8].
We evaluated whether cytokines, chemokines and some other biomarkers could be used for diagnosis in pediatric COVID-19 patients. In addition, we evaluated biomarkers predictive of prognosis by examining the relationship between disease severity and cytokine levels.
MATERIALS AND METHODS
Sixty-nine children with COVID-19 who applied to three reference hospitals in Istanbul province between 1 April and 30 August 2020 were enrolled to our study. There were 20 healthy controls, randomly selected from outpatient clinics, consist of 11 girls and 9 boys aged between 9 and 216 months. Approval for the study was obtained from the Ethics Committee of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty (number: 59562). Written informed consent was obtained from the families of all subjects.
The COVID-19 patients were divided into three groups based on disease severity (mild, moderate and severe/critically ill) according to Dong’s classification [9]. There were 27 patients with mild disease, 36 with moderate disease and 6 patients with severe disease.
Mild: Symptoms of acute upper respiratory tract infection, including fever, fatigue, myalgia, cough, sore throat, runny nose and sneezing. Physical examination shows congestion of the pharynx and no auscultatory abnormalities. Some cases may have no fever or have only digestive symptoms, such as nausea, vomiting, abdominal pain and diarrhea.
Moderate: Pneumonia, frequent fever and cough (mostly dry cough, followed by productive cough); some may have wheezing, but no obvious hypoxemia such as shortness of breath, and sputum or dry and/or wet snoring can be detected in the lungs. Some cases may have no clinical signs and symptoms, but chest computed tomography shows lung lesions, which are subclinical.
Severe/critical: Early respiratory symptoms, such as fever and cough, may be accompanied by gastrointestinal symptoms, such as diarrhea. The disease usually progresses at ∼1 week, and dyspnea occurs with central cyanosis. Oxygen saturation is <92% with other hypoxia manifestations or children can quickly progress to acute respiratory distress syndrome or respiratory failure and may also have shock, encephalopathy, myocardial injury or heart failure, coagulation dysfunction and acute kidney injury. Organ dysfunction can be life-threatening.
The following demographic and clinical variables were evaluated: age, sex, COVID-19 PCR result, comorbid diseases, signs and symptoms, laboratory and radiologic findings and treatment modalities. Children meeting the following criteria were enrolled in the study:
Healthy children with no symptoms or signs of COVID 19, negative COVID PCR test results and no underlying diseases.
Patients presenting with complaints suggesting COVID-19 with a positive RT-PCR test.
Patients presenting with complaints suggesting COVID-19 with a negative RT-PCR test plus radiological findings compatible with COVID-19 plus exposure to a microbiologically confirmed COVID-19 patient, plus positive serology for COVID-19 immunoglobulin G.
Serum samples were taken at hospital admission and 3 to 5 days after symptom onset. The plasma concentrations of IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, IL-13, IL-37, IL-38, IFN-γ, IP-10, TNF-α, MCP-1 and monocyte chemoattractant protein-3 (MCP-3) were measured using enzyme-linked immunosorbent assay kits, according to the manufacturer’s instructions.
Statistical analysis
Medians and 95% confidence intervals for medians, frequencies and percentages were given as descriptive statistics. The distribution of variables was examined by Kolmogorov–Smirnov test. The Kruskal–Wallis and Mann–Whitney U tests were used for the analysis of quantitative independent data, the χ2 test for qualitative independent data and Fisher’s test was used when the assumptions for the χ2 test were not met. Spearman’s correlation analysis was performed to examine the associations among the variables. After Kruskal–Wallis test, Dunn’s test was used to assess differences between groups. A receiver operating characteristics (ROC) curve and the area under the ROC curve (AUC) of serum cytokine and chemokine levels were estimated for patients with severe disease. Statistical analysis was performed using SPSS software (ver. 26.0; SPSS Inc., Chicago, IL). A p value of <0.05 was taken to indicate statistical significance.
RESULTS
Demographic and clinical characteristics
Sixty-nine pediatric cases (37 females and 32 males) of median age 150 months (range 2–216) with COVID-19 and 20 healthy children (11 females and 9 males) of median age 151.5 months (range 9–216) were enrolled in the study. A total of 14 (20.2%) patients had comorbid conditions. The most common comorbidities were neurological diseases, followed by chronic respiratory diseases, malignancies and rheumatological diseases (neurological disorders, asthma, bronchopulmonary dysplasia, juvenile idiopathic arthritis, obesity-1, metabolic disorder and malignancy). A history of close contact with a family member with SARS-CoV-2 was reported by 59 (85.5%) patients. There were 27 patients with mild disease, 36 with moderate disease and 6 patients with severe disease. The most commonly reported initial symptoms were fever (78.2%), cough (66.6%), difficulty breathing (40.5%) and headache (18.8%). Gastrointestinal complaints were the least common symptoms. Abnormal radiological findings suggestive of COVID-19 were detected in 60.8% of the patients.
Three (4.3%) of the patients were on favipiravir and 34 (49%) patients were on an antibacterial for suspicion of secondary bacterial infection. Invasive mechanical ventilation was required for six (8.6%) patients and two (2.8%) underwent plasmapheresis. The demographic and clinical characteristics of children with COVID-19 are shown in Table 1.
Table 1.
Chemokine and cytokine levels in children with COVID-19
| Mild (n = 27) | Moderate (n = 36) | Severe/critical (n = 6) | Total (n = 69) | |
|---|---|---|---|---|
| Age months; median (min–max) | 129 (2–204) | 168 (4–216) | 144 (55–216) | 150 (2–216) |
| Comorbidity (n, %) | 4 (14.8%) | 7 (19.4%) | 3 (50%) | 14 (20.2%) |
| Symptoms and signs | ||||
| Fever | 19 (70.3%) | 30 (45.4%) | 5 (83.3%) | 54 (78.2%) |
| Cough | 10 (37%) | 31 (86.1%) | 5 (83.3%) | 46 (66.6%) |
| Dyspnea | 1 (3.7%) | 21 (58.3%) | 6 (100%) | 28 (40.5%) |
| Headache | 9 (33.3%) | 2 (55.5%) | 2 (33.3%) | 13 (18.8%) |
| Myalgia | 6 (22.2%) | 5 (13.8%) | 1 (16.6%) | 12 (17.3%) |
| Rinorrhea | 7 (25.9%) | 3 (8.3%) | 0 | 10 (14.4%) |
| Chest pain | 2 (7.4%) | 4 (11.1%) | 1 (16.6%) | 7 (10.1%) |
| Vomiting | 0 | 1 (2.7%) | 0 | 1 (1.4%) |
| Diarrhea | 2 (7.4%) | 2 (5.5%) | 0 | 4 (5.7%) |
| Laboratory parameters (median (95% CI)) | ||||
| Lymphocytes (/ml) | 1800 (1440–2400) | 2100 (1700–2300) | 970 (800–1200) | 1900 (1600–2230) |
| D-dimer (mg/l) | 0.310 (0.27–0.39) | 0.625 (0.42–0.90) | 1.555 (0.31–1.87) | 0.445 (0.340–0.690) |
| C-reactive protein (mg/dl) | 1.87 (1.07–2.90) | 5.25 (2.36–16.70) | 54.35 (0.56–188) | 2.80 (1.66–6.10) |
| Procalcitonin (ng/ml) | 0.04 (0.04–0.07) | 0.05 (0.04–0.10) | 0.238 (0.047–0.880) | 0.050 (0.040–0.070) |
| Abnormal radiologic findings (n, %) | 0 | 36 (100%) | 6 (100%) | 42 (60.8%) |
| Treatment (n, %) | ||||
| Favipiravir | 0 | 0 | 3 (50%) | 3 (4.3%) |
| Antibacterial | 0 | 28 (78%) | 6 (100%) | 34 (49%) |
| Mechanical ventilation | 0 | 0 | 5 (83.3%) | 5 (7.2%) |
| Plasmapheresis | 0 | 0 | 2 (33.3%) | 2 (2.8%) |
Comparison of chemokine and cytokine levels in children with COVID-19 and healthy groups
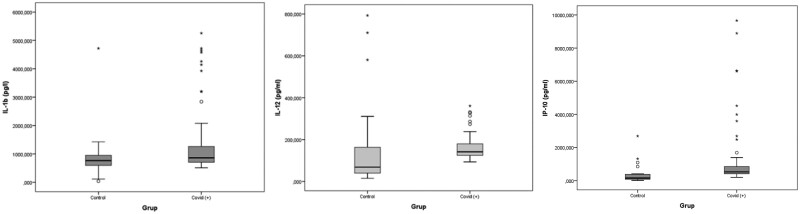
The IL-1β, IL-12 and IP-10 levels increased significantly in the patients with COVID-19 compared to the healthy controls (p = 0.035, p = 0.006 and p < 0.001) (Table 2, Fig. 1).
Table 2.
Comparison of COVID (+) and healthy groups
| Control (n = 20) Median (95% CI) | COVID (+) (n = 69) Median (95% CI) | p | |
|---|---|---|---|
| Age (month) | 151.5 (9–216) | 150 (2–216) | 0.753 |
| Cytokine levels | |||
| IFN-γ (ng/ml) | 22.85 (18.11–278.91) | 25.4 (15.55–291.6) | 0.443 |
| IL-1a (pg/ml) | 27.755 (16.11–291.51) | 26.79 (11.74–336.2) | 0.623 |
| IL-1b (pg/l) | 762.5 (36.99–4720.24) | 861.92 (509.21–5255.39) | 0.035* |
| IL-12 (pg/ml) | 68.515 (14.66–792.88) | 141.19 (93.2–360.83) | 0.006* |
| IL-4 (ng/l) | 57.605 (14.66–792.88) | 64.35 (-10–792.88) | 0.844 |
| IL-6 (ng/l) | 38.44 (22.33–322.68) | 41.58 (21.86–451.29) | 0.683 |
| IL-7 (ng/l) | 79.96 (9.57–312.77) | 76.65 (9.57–845.22) | 0.687 |
| TNF-α (ng/l) | 63.25 (39.43–615.01) | 64.65 (31.25–736.99) | 0.746 |
| IL-13 (ng/l) | 7.33 (4.34–47.05) | 6.8 (3.72–83.77) | 0.644 |
| IL-37 (ng/l) | 26.675 (11.96–238.36) | 31.61 (6.34–257.03) | 0.196 |
| IL-38 (ng/l) | 16.615 (10.64–170.31) | 15.26 (7.15–170.31) | 0.215 |
| MCP-1 (ng/l) | 153.66 (55.36–730.97) | 122.94 (12.31–1266.3) | 0.494 |
| MCP-3 (ng/l) | 55.49 (32.84–452.89) | 58.77 (39.7–475.15) | 0.969 |
| IP-10 (pg/ml) | 154.265 (12.1–2689.2) | 529.05 (199.98–9661.29) | <0.001* |
| IL-10 (pg/ml) | 70.71 (12.2–633.1) | 79.69 (23.49–997.27) | 0.189 |
| IL-2R (ng/ml) | 4.28 (0.8–54.13) | 3.46 (1.49–178.3) | 0.606 |
IFN-γ: interferon gamma; IL-1a: interleukin-1a; IL-1b: interleukin-1b; IL-12: interleukin-12; IL-4: interleukin-4; IL-6: interleukin-6; IL-7: interleukin-7; IL-10: interleukin-10; IL-13: interleukin-13; IL-37: interleukin-37; IL-38: interleukin-38; IL-2R: interleukin-2 receptor; IP-10: IFN-γ-inducible protein-10; MCP-1: monocyte chemoattractant protein-1; MCP-3: monocyte chemoattractant protein-3; TNF-α: tumor necrosis factor alpha.
*P < .05 indicates statistical significance were represented in Bold.
Fig. 1.
Box plot graphics for variables considered to be significant.
Lymphocytes, CRP, procalcitonin, D-dimer, chemokine and cytokine levels were compared between three patient groups with COVID 19 disease and the healthy group.
The median D-dimer value in the severe group (1.555; min–max, 0.21–3.2) was significantly higher than in the mild disease and healthy groups (0.625; min–max, 0.19–4.93 and 0.310; min–max, 0.19–14.3). The median IP-10 level was 896.7 in the healthy group (min–max, 409.14–8892.8) and 54.67 (min–max, 39.73–333.35), 526.525 (min–max, 200.8–9661.2) and 154.265 (min–max, 12.1–2689.2) in the mild, moderate and healthy groups, respectively, and was significantly higher in the severe group (p = 0.043) (Table 3, Figs. 2 and 3). According to the results of the process characteristic curve (ROC) analysis, the AUC was significant for IP-10. Accordingly, the IP-10 level is associated with disease severity in children. According to the Youden index, an IP-10 level of >580.97 as a cutoff had 83.33% sensitivity and 66.67% specificity (Fig. 4).
Table 3.
Comparisons of the laboratory, cytokine and chemokine levels of children with COVID 19 according to disease severity
| Median (95% CI) | Healthy | Severe | Moderate | Mild | p |
|---|---|---|---|---|---|
| Age (month) | 151.5 (9–216) | 144 (55–216) | 168 (4–216) | 129 (2–204) | 0.423 |
| Lymphocyte (/ml) | 2290 (1560–3400) | 970 (400–2800) | 2100 (300–5600) | 1800 (100–9200) | 0.304 |
| CRP | 54.35 (0.18–242) | 5.25 (0.5–240) | 1.87 (0.2–131) | 0.144 | |
| PCT | 0.238 (0.03–3.6) | 0.05 (0.01–2.6) | 0.04 (0.01–3.03) | 0.670 | |
| D-dimer | 1.555 (0.21–3.2) | 0.625 (0.19–4.93) | 0.310 (0.19–14.3) | 0.043* | |
| IFN-γ (ng/ml) | 22.85 (18.11–278.91) | 40.27 (19.66–291.6) | 25.92 (15.55–278.91) | 23.39 (17.24–125.05) | 0.272 |
| IL-1a (pg/ml) | 27.755 (16.11–291.51) | 32.365 (17.61–336.2) | 28.755 (12.51–295.22) | 25.17 (11.74–284.15) | 0.639 |
| IL-1b (pg/l) | 762.5 (36.9–4720.2) | 1238.53 (509.2–5255.3) | 814.73 (545.85–4720.2) | 861.92 (603.2–4143.1) | 0.161 |
| IL-12 (pg/ml) | 68.515 (14.66–792.88) | 152.865 (129.04–180.2) | 137.385 (93.2–331.4) | 146.74 (94.65–360.8) | 0.058 |
| IL-4 (ng/l) | 57.605 (14.66–792.88) | 110.1 (24.52–705.06) | 61.955 (10–792.88) | 50.05 (14.66–710.22) | 0.322 |
| IL-6 (ng/l) | 38.44 (22.33–322.68) | 78.35 (31.42–407.5) | 40.18 (21.86–451.29) | 41.58 (25.33–322.68) | 0.307 |
| IL-7 (ng/l) | 79.96 (9.57–312.77) | 128.47 (68.87–845.22) | 67.385 (9.57–769.97) | 79.66 (41.98–353.89) | 0.460 |
| TNF-α (ng/l) | 63.25 (39.43–615.01) | 85.475 (50.69–661.58) | 67.26 (32.83–736.99) | 62.55 (31.25–272.33) | 0.273 |
| IL-13 (ng/l) | 7.33 (4.34–47.05) | 13.97 (4.01–83.77) | 6.455 (3.72–82.27) | 6.71 (4.25–49.08) | 0.371 |
| IL-37 (ng/l) | 26.675 (11.96–238.36) | 45.195 (17.48–254.97) | 31.525 (7.98–257.03) | 30.29 (6.34–178.33) | 0.472 |
| IL-38 (ng/l) | 16.615 (10.64–170.31) | 31.02 (12.28–170.31) | 14.095 (8.6–165.5) | 15.26 (7.15–166.23) | 0.304 |
| MCP-1 (ng/l) | 153.66 (55.36–730.97) | 161.62 (39.73–1103.87) | 118 (12.31–1266.3) | 122.94 (55.36–730.97) | 0.477 |
| MCP-3 (ng/l) | 55.49 (32.84–452.89) | 160.18 (39.84–442.75) | 58.065 (39.7–475.15) | 54.67 (39.73–333.35) | 0.376 |
| IP-10 (pg/ml) | 154.265 (12.1–2689.2) | 896.7 (409.14–8892.8) | 526.525 (200.8–9661.2) | 524 (199.98–2689.2) | 0.012* |
| IL-10 (pg/ml) | 70.71 (12.2–633.1) | 138.88 (49.07–997.27) | 77.43 (24.68–861.53) | 84.52 (23.49–718.22) | 0.593 |
| IL-2R (ng/ml) | 4.28 (0.8–54.13) | 6.19 (1.91–178.3) | 3.65 (1.49–101.62) | 3.23 (1.8–30.73) | 0.198 |
CRP: C-reactive protein; IFN-γ: interferon gamma; IL-1a: interleukin-1a; IL-1b: interleukin-1b; IL-2R: interleukin-2 receptor; IL-4: interleukin-4; IL-6: interleukin-6; IL-7: interleukin-7; IL-10: interleukin-10; IL-12: interleukin-12; IL-13: interleukin-13; IL-37: interleukin-37; IL-38: interleukin-38; IP-10: IFN-γ-inducible protein-10; MCP-1: monocyte chemoattractant protein-1; MCP-3: monocyte chemoattractant protein-3; PCT: procalcitonin; TNF-α: tumor necrosis factor alpha.
*P < .05 indicates statistical significance were represented in Bold.
Fig. 2.
Box plot graphics for D-dimer levels.
Fig. 3.
Box plot graphics for IP-10 levels.
Fig. 4.
The ROC curve of plasma IP-10 levels. AUC: area under the ROC curve; IP-10: IFN-γ-inducible protein-10; SE: standard error.
DISCUSSION
The IP-10 level was an independent predictive factor for disease severity in pediatric patients. In addition, IL-1β, IL-12 and IP-10 levels were higher in COVID-19 pediatric patients than in healthy children. These biomarkers are implicated in COVID-19 pathogenesis.
Definitive diagnosis of COVID-19 is made by RT-PCR [1]. However, because RT-PCR is not always rapid and accurate, radiological and laboratory parameters are also used for diagnostic purposes [1, 2]. High levels of inflammatory markers such as leukocytosis, lymphopenia, CRP, procalcitonin and ferritin are common findings in infectious diseases, and not specific for COVID-19 [1, 3].
In addition to the increase in these markers in adult COVID-19 patients, studies stating that these markers vary with the severity of the disease have begun to increase [10–12]. In a study, cutoff values of >10 mg/l and >0.5 ng/ml, for CRP and PCT, respectively, were shown to be poor prognostic factors [10]. In another study, it was found that a cutoff value of 26 mg/l for CRP could be used to predict progression to serious disease [11]. In a meta-analysis, a high PCT value was associated with an approximately 5-fold higher risk of severe infection [12]. In this study, although the lymphopenia, CRP and procalcitonin elevations were more prominent in severe disease, no significant difference was found according to disease severity. Similarly, Qian, et al. [13] reported that lymphopenia is a rare finding in children, unlike adults, and there was no difference according to disease severity.
COVID-19 infection alters coagulation parameters [3]. High fibrinogen, a normal or slightly prolonged prothrombin time and activated partial thromboplastin time, and a normal platelet count are common findings [14]. COVID-19 differs from typical disseminated intravascular coagulation in the patients who do not have significant bleeding [14, 15].
The most important change in coagulation parameters in COVID-19 patients is a high D-dimer level. In adults, the D-dimer level has prognostic value and is correlated with disease severity and in-hospital mortality [15–17]. Therefore, D-dimer may be an early marker of COVID-19 [16, 17]. In this study, the D-dimer level was associated with disease severity.
The role of cytokines and chemokines in disease pathogenesis and severity has been investigated [3]. These factors are implicated in COVID-19 pathogenesis, as evidenced by the MIS-C cases that emerged after COVID-19 infection due to cytokine storm [7]. Although in adults the cytokine response is associated with disease severity and a poor prognosis, data on the inflammatory response to COVID-19 in children are sparse [4, 18].
The IL-6 and IL-1α levels are markedly increased in adult COVID-19 patients. A high baseline IL-6 level is correlated with lung involvement and levels of acute inflammatory markers [19]. IL-6 is a marker of disease severity and is predictive of mortality. Also, IL-6 can be used to monitor the treatment response [3, 19]. IL-1 and IL-6 blockade in severe COVID-19 patients reduces fever, systemic inflammation and the risk of intubation [20]. In a study, the risk of death and the need for mechanical ventilation were significantly lower in COVID-19 patients taking anakinra, an anti-IL-1 antibody [21]. These studies were conducted in adults with severe disease; few studies have involved children. Ozsurekci, et al. [22] reported that the IL-6 level was significantly increased in adults; there was no difference in IL-1 and IL-6 levels in pediatric patients compared to the controls. In this study, although the IL-1β and IL-12 levels were higher in the COVID-19 patients, there was no significant difference in IL-6 levels between the two groups. Also, there was no significant difference in IL-1β, IL-12 and IL-6 levels according to disease severity. The milder course of COVID-19 in children compared to adults can be explained by the absence of a significant increase in the levels of these cytokines. Because IL-1 and IL-6 are not predictive of prognosis, other cytokines and chemokines implicated in pathogenesis are under investigation [3].
In adults, the fibroblast growth factor, IFN-γ, IP-10, TNF-α, MCP-1, MIP-1A, MIP-1B, IL-1β, IL-1, IL-7, IL-8, IL-9 and IL-10 levels were higher than in healthy subjects. In addition, the IL-2, IL-7, IL-10, IP-10, MCP-1, MIP-1A and TNF-α levels were moderately significantly higher in patients hospitalized in the intensive care unit [1]. In a study of pediatric and adult patients, no significant differences were found in the levels of MIP-1, TNF-α, MCP-1, IL-4, IL-10, IL-13, IL-17, IFN-γ and IL-27. The IP-10 level was significantly higher in pediatric and adult patients with severe disease, and that of MIP-3β was significantly lower in healthy controls [22]. In a study involving 127 children with mild, moderate or severe COVID-19, the IL-2, IL-4, IL-6, TNF-α and INF-γ levels were similar [13].
In this study, although there were no significant differences in the IL-2, IL-7, IL-10, IL-13, IL-37, IL-38, MCP-1, MCP-3, TNF-α and IFN-γ levels, the IP-10 level was high in COVID-19 pediatric patients. In addition, IP-10 was an independent predictive biomarker for disease severity in children.
There were several limitations to this study. First, the small numbers of patients and controls. Because the disease course is milder in children compared to adults, there were few patients with severe disease. More patients are needed to identify a diagnostic and prognostic biomarker. Second, serum samples were collected within 3–5 days of symptom onset. Because the disease worsens after 7 days, an increase in these biomarkers may not be detected in early serum samples [3]. Further studies should obtain serum samples in both the early and late stages of COVID-19.
In conclusion, the IP-10, IL-1 and IL-12 levels were identified as diagnostic factors for COVID-19. Also, the D-dimer and IP-10 levels were predictive of disease prognosis. Our findings will guide rational use of anti-cytokine therapies in pediatric patients with COVID-19.
FUNDING
This work was supported by Istanbul University- Cerrahpaşa Scientific Research Projects (TSG-2020-34877).
ETHICS STATEMENT
Ethical permission for patients and healthy controls were obtained from the Ethics Committee of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty (permit number: 59562) and all patients gave informed consent.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 3. Madhusudan S, Muralidharan J.. Biomarkers in COVID-19: an up-to-date. Front Pediatr 2021;30:607–47. [Google Scholar]
- 4. Pierce CA, Preston‐Hurlburt P, Dai Y, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med 2020;12:eabd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuan Y, Wang Q, Sun D, et al. Differences in immune responses between children and adults with COVID-19. Curr Med Sci 2021;41:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu M, Martins T, Peterson L, et al. Clinical significance of measuring serum cytokine levels as inflammatory biomarkers in adult and pediatric COVID-19 cases: a review. Cytokine 2021;142:155478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020;130:5942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen W, Su W, Tang H, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov 2020;6:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020;145:e20200702. [DOI] [PubMed] [Google Scholar]
- 10. Huang I, Pranata R, Lim MA, et al. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease 2019: a meta-analysis. Ther Adv Respir Dis 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang G, Wu C, Zhang Q, et al. C-Reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis 2020;29:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lippi G, Plebani M.. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020;505:190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qian G, Zhang Y, Xu Y, et al. Reduced inflammatory responses to SARS-CoV-2 infection in children presenting to hospital with COVID-19 in China. E Clin Med 2021;34:100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell WB. Thromboinflammation in COVID-19 acute lung injury. Paediatr Respir Rev 2020;35:20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin J, Yan H, Chen H, et al. COVID-19 and coagulation dysfunction in adults: a systematic review and meta-analysis. J Med Virol 2021;93:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu J, Pang J, Ji P, et al. Coagulation dysfunction is associated with severity of COVID-19: a meta-analysis. J Med Virol 2021;93:962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alnor A, Sandberg MB, Gils C, et al. Laboratory tests and outcome for patients with coronavirus disease 2019: a systematic review and meta-analysis. J Appl Lab Med 2020;5:1038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Zhang C, Huang F, et al. 2019-novel coronavirus (2019-nCoV) infections trigger and exaggerated cytokine response aggravating lung injury. ChinaXiv. 2020. ChinaXiv:202002.00018v1.
- 19. Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med 2020;12:e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buckley LF, Wohlford GF, Ting C, et al. Role for anti-cytokine therapies in severe coronavirus disease. Crit Care Expl 2020;10:e017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020;2:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozsurekci Y, Aykac K, Er AG, et al. Predictive value of cytokine/chemokine responses for the disease severity and management in children and adult cases with COVID-19. J Med Virol 2021;93:2828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]