Abstract
Vaccination against viruses has rarely been associated with Guillain-Barré syndrome (GBS), and an association with the COVID-19 vaccine is unknown. We performed a population-based study of National Health Service data in England and a multicentre surveillance study from UK hospitals to investigate the relationship between COVID-19 vaccination and GBS.
Firstly, case dates of GBS identified retrospectively in the National Immunoglobulin Database from 8 December 2021 to 8 July 2021 were linked to receipt dates of COVID-19 vaccines using data from the National Immunisation Management System in England. For the linked dataset, GBS cases temporally associated with vaccination within a 6-week risk window of any COVID-19 vaccine were identified. Secondly, we prospectively collected incident UK-wide (four nations) GBS cases from 1 January 2021 to 7 November 2021 in a separate UK multicentre surveillance database. For this multicentre UK-wide surveillance dataset, we explored phenotypes of reported GBS cases to identify features of COVID-19 vaccine-associated GBS.
Nine hundred and ninety-six GBS cases were recorded in the National Immunoglobulin Database from January to October 2021. A spike of GBS cases above the 2016–2020 average occurred in March–April 2021. One hundred and ninety-eight GBS cases occurred within 6 weeks of the first-dose COVID-19 vaccination in England [0.618 cases per 100,000 vaccinations; 176 ChAdOx1 nCoV-19 (AstraZeneca), 21 tozinameran (Pfizer) and one mRNA-1273 (Moderna)]. The 6-week excess of GBS (compared to the baseline rate of GBS cases 6–12 weeks after vaccination) occurred with a peak at 24 days post-vaccination; first-doses of ChAdOx1 nCoV-19 accounted for the excess. No excess was seen for second-dose vaccination. The absolute number of excess GBS cases from January–July 2021 was between 98–140 cases for first-dose ChAdOx1 nCoV-19 vaccination. First-dose tozinameran and second-dose of any vaccination showed no excess GBS risk. Detailed clinical data from 121 GBS patients were reported in the separate multicentre surveillance dataset during this timeframe. No phenotypic or demographic differences identified between vaccine-associated and non-vaccinated GBS cases occurring in the same timeframe.
Analysis of the linked NID/NIMS dataset suggested that first-dose ChAdOx1 nCoV-19 vaccination is associated with an excess GBS risk of 0.576 (95% confidence interval 0.481–0.691) cases per 100 000 doses. However, examination of a multicentre surveillance dataset suggested that no specific clinical features, including facial weakness, are associated with vaccination-related GBS compared to non-vaccinated cases. The pathogenic cause of the ChAdOx1 nCoV-19 specific first dose link warrants further study.
Keywords: COVID-19 vaccination, Guillain-Barré syndrome
Based on a combined analysis of COVID-19 vaccination and Guillain-Barre syndrome (GBS) in England, and a multicentre surveillance cohort, Keh et al. report that GBS is associated with first-dose ChAdOx1 nCoV-19 vaccine at 5.8 cases per million vaccinations. No specific phenotype differentiates vaccine-associated GBS.
Introduction
The first year of the COVID-19 pandemic produced robust information on the neurological and neuropsychiatric sequelae of SARS-CoV-2 infection.1 In the peripheral nervous system, brachial neuritis, facial palsy and Guillain-Barré syndrome (GBS) were subjects of particular interest.
From January 2020, case reports and case series of patients with GBS occurring around the time of SARS-CoV-2 infection raised the possibility of a link between GBS and COVID-19.2,3 However, a large national study of the UK population as well as one in Singapore showed a decreased incidence of GBS during the pandemic and failed to find a definitive link between GBS and COVID-19 infection.4,5
GBS became an adverse event of special interest (AESI) related to vaccination in the 1970s when an excess of GBS cases was detected in the United States during the 1976/1977 A/New Jersey/76 influenza (‘swine flu’) vaccination campaign within 6 weeks of vaccination.6 Serial epidemiological analyses established that the rate of GBS attributable to the ‘swine flu’ vaccine was approximately 4.9–5.9 per million vaccines, mostly from 14–28 days post-vaccination.6 Although GBS has been identified in subsequent annual surveillance of influenza vaccination programmes at a rate of 1–1.6 per million doses,7,8 a pathogenic explanation has not been found. Expert consensus largely derived from the vaccination surveillance is that GBS risk attributable to vaccination exists for up to 6 weeks (42 days).7
The global rollout of COVID-19 vaccines triggered extensive monitoring, with GBS as an AESI. Very rare adverse events, not visible even in large clinical trials, can be identified when mass vaccination monitoring systems are in place, particularly when a unique disease phenotype emerges. Vaccine-induced immune thrombotic thrombocytopenia (VIIT)9 and more recently myocarditis10 were identified this way and are unique diseases. VIIT occurs most commonly in association with ChAdOx1 nCoV-19 (AstraZeneca) recombinant adenoviral vector vaccine. VIIT manifests as thromboses, including cerebral venous sinus thrombosis, with an estimated incidence of 1 case per 100 000 exposures.9 Myocarditis seems specific to the tozinameran (Pfizer) and mRNA-1273 (Moderna) vaccines.10
Non-replicating viral vectors can deliver vaccination antigen or other pharmaceuticals to the host. Adenoviruses are commonly used, and four current adenoviral vector COVID-19 vaccines are authorized in at least one country (AstraZeneca, Sputnik V, Janssen and Convidecia). Despite the single observational study by McNeil et al.11 suggesting a link between GBS and vaccination against adenovirus infection, adenovirus vectors are thought to be benign. ChAdOx1 nCoV-19 utilizes a replication-deficient simian adenovirus vector designed to evade anti-human adenovirus neutralizing antibodies to stimulate a robust immune response.
The UK COVID-19 vaccination programme began on 8th December 2020 with tozinameran, then ChAdOx1 nCoV-19 in January and subsequently mRNA-1273 vaccinations. Vaccination was delivered sequentially to cohorts of the most vulnerable and elderly followed by deciles of age. Fifty percent of adults over 50 years of age had had their first vaccination by mid-February 2021.
We aimed to combine multiple national data sources and systematically investigate any temporal relationship between COVID-19 vaccination and excess cases of GBS during the UK COVID-19 vaccination programme. We retrospectively interrogated a large database of patients hospitalized with GBS in England, Scotland and Northern Ireland treated with immunoglobulin from the National Immunoglobulin Database (NID). Using the common NHS identifier, we combined the English data with data from the National Immunisation Management System (NIMS) on all COVID-19 vaccinations data in England. Separately, we characterized a large surveillance dataset of the incident UK GBS cases, presenting both after COVID-19 vaccination, and also without vaccination during the same period, recording the timing of onset after COVID-19 vaccination.
Materials and methods
Retrospective analyses of NID/NIMS datasets
All cases of GBS admitted to hospital in England, Scotland and Northern Ireland and considered for immunoglobulin treatment are recorded in the NID. Because NHS England (NHSE) procures the total immunoglobulin supply for the UK except Wales, and mandates that all immunoglobulin prescriptions are approved by the local clinical panel and reported onto the Immunoglobulin Database within 90 days of administration to facilitate repayment of immunoglobulin costs to the trusts, there is nearly 100% compliance with detailed recording of immunoglobulin use across the three UK regions.12 All cases are confirmed as GBS by the admitting clinician and reviewed and authorized by an independent Immunoglobulin Assessment Panel, although Brighton Criteria are not recorded.
We extracted NID GBS cases from 1 January to 31 October 2021 and recorded diagnosis, their unique identifier and date of first immunoglobulin prescription. These numbers were compared to the historical GBS cases recorded in the NID from 2016 to 2020.
UK Department of Health and Social Care (DHSC) guidance for GBS treatment recommends intravenous immunoglobulin (IVIg) as first line therapy for patients with Hughes Grade 4 or more (significant disability) disease progressing towards intubation or ventilation or with high probability of respiratory insufficiency (mEGRIS score ≥3) or predicted poor prognosis (mEGOS ≥4).13 Although plasma exchange (PLEX) is also considered a first line option, in practice, this is not readily available and is very seldom used. Utilizing IVIg use as a proxy for GBS incidence under-estimates the true incidence of GBS, as milder cases are not treated. However, IVIg is estimated to be given to 86% of European and UK GBS cases,14 and the 2021 data can be compared to previous years with similar clinical behaviours for admitted patients.
The NIMS database is a national point of care system for capturing vaccination data from England. Patients in the NIMS database are also registered with their NHS number. The COVID-19 vaccination data were linked to the English cases of GBS identified from the NID from 8 December 2020 to 8 July 2021 using the unique NHS identifier issued by NHS England. The start date was the commencement of the COVID-19 vaccination programme in the UK. Of note, the NIMS database captures information on vaccinations for England only (population: 55 980 000 prevalent persons in 2021); Scottish (5 470 000 prevalent persons) and Northern Irish (1 885 000 prevalent persons). GBS data were not included in this section of the analysis. At the time of analysis, data on immunoglobulin administration for GBS were available to mid-July 2021. To accommodate the need for a 6-week post-vaccination follow-up, information on vaccinations up to and including 27 May 2021 was included. The exposed population, which was used to calculate GBS rates post-vaccination, was taken from weekly published cumulative counts of vaccine usage in England up to this date.
Prospective surveillance study
We conducted a prospective surveillance study to compare the demographic and phenotypic characteristics of GBS cases reported from 1 January 2021 to 7 November 2021, comparing GBS cases reported as having received COVID-19 vaccination and cases without vaccination. We recognize the included cases are heavily influenced by reporting bias, as there was significant interest in the possibility of vaccination-related GBS at the time. The surveillance study should not be used in direct comparison to the retrospective analyses of linked data described above.
Reports of GBS were submitted by members of the British Peripheral Nerve Society (BPNS) and the Association of British Neurologists (ABN), with regular reminders to collect information on hospital presentations of GBS during the study period. Data were entered into the International Neuromuscular COVID-19 database (https://www.ucl.ac.uk/centre-for-neuromuscular-diseases/news/2020/may/international-neuromuscular-covid-19-database), at the Centre for Neuromuscular Disease, where database questions had been adapted to allow for reporting of COVID-19 vaccine-related neuromuscular cases. Surveillance study data collection ended on 7 November 2021 to allow time for retrospective case reporting. Anonymized clinical data on demographics, GBS diagnostic criteria, vaccine details and prior COVID-19 infection, symptoms and management were collected. Cases were classified according to Brighton Collaboration GBS Working Group criteria15 by the study team to describe the level of diagnostic certainty as recorded previously.4
Two cases were excluded from the analysis, as the reporting clinicians later informed us of a change in diagnosis.
Statistical analysis
Statistical analysis was performed using STATA 1616 and R v4.0.2/v4.0.3 (R Core Team).17
An ecological analysis presenting the number of GBS cases identified in the NID by calendar month was used to compare the incidence of GBS in the UK across the years 2016–2020 with that seen in January–July 2021.
The Spearman’s rank correlation test was used to test for correlation between the time after the first vaccine dose and the incidence of GBS. One-way ANOVA was used to compare yearly GBS rates within age deciles of the linked NID/NIMS data in 2021 compared to 2019 and 2020 to determine if age distribution of GBS differed compared to previous years, using ONS population estimates as the denominators for the calculation of rates. As GBS numbers were only available until July 2021, an estimate of annual numbers was produced on the assumption of a stable GBS incidence through the year to enable comparison to prior years. ONS population estimates for 2021 were assumed to be the same as for 2020.
For the prospective surveillance study, Chi-square and Kruskal–Wallis tests were used to test for correlations between patient demographics, GBS characteristics or treatment details and exposure to COVID-19 vaccination within 6 weeks of GBS onset.
A significance value of P < 0.05 was used throughout.
Ethics
The UK Health Research Authority was consulted and advised that the study did not require review by an NHS Research Ethics Committee, as this was an analysis of previously collected, non-identifiable anonymized data.
Data availability
Data are available on request to the corresponding author.
Results
Retrospective analyses of NID/NIMS datasets
Ecological analysis—GBS cases recorded in the NID from 2016 to 2020
Between 2016 and 2020, the NID recorded a mean of 1283 immunoglobulin-treated cases of GBS per year [95% confidence interval (CI) 1159–1408, mean 107 cases per month in the three participating UK nations]. In England, a mean of 1148 GBS cases occurred annually between 2016 and 2020 (95%CI 1022–1274, or 3.1 GBS cases per day). These annualized case counts represent the vast majority of hospitalized GBS cases in England, Scotland and Northern Ireland, resulting in an estimated GBS incidence rate of 1.99 per 100 000 individuals per year (95%CI 1.79–2.18). This was comparable to previous European and North American studies with incidence rates of between 0.84–1.91 per 100 000 individuals per year.18 As previously described, the UK experienced an overall reduction in cases in 2020 during the height of the COVID-19 pandemic, resulting in the lowest case number (1053 cases in 2020) and estimated incidence (1.57 cases per 100 000 individuals per year) in the five-year period.4
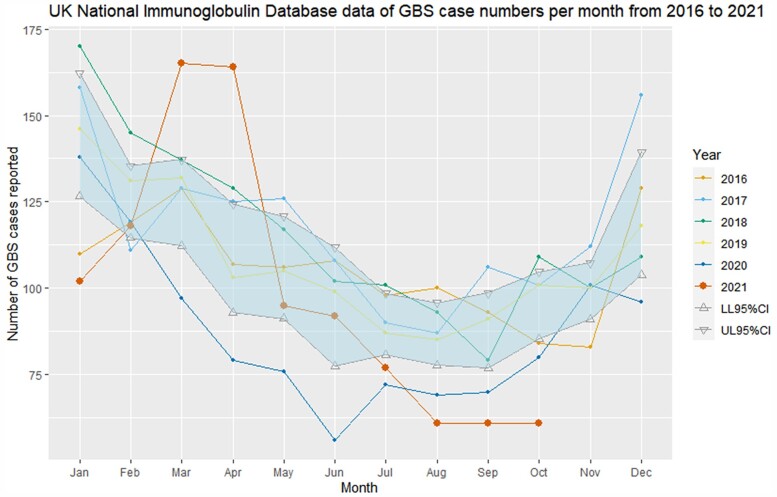
Fig. 1 summarizes the monthly frequency of incident GBS cases in the England, Scotland and Northern Ireland from 2016 to 2020 and compares these to the monthly incident case numbers from January to October 2021 during the UK vaccination programme. A total of 996 GBS cases were recorded from January to October 2021, fewer than observed from January–October 2016 to 2019 (pre-pandemic range: 1054–1182 cases) but higher than for January–October 2020 (pandemic range: 856 cases).
Figure 1.
NHSE Immunoglobulin Database GBS cases 2016–2021. NHSE Immunoglobulin Database-derived numbers of incident GBS cases reported per month between 2016 and 2021 (year to date). The shaded area represents 95% CI of mean monthly case numbers from 2016–2020 and compares them with the absolute monthly case count for January–October 2021.
The number of GBS cases in January 2021 was significantly lower than the mean number seen in the same month 2016–2020, continuing the trend of lower GBS rates from 2020. However, England, Scotland and Northern Ireland experienced a spike of GBS cases in March and April 2021, before rates fell again below the lower range of the 95% CI for the 2016–2020 mean incident GBS case number in July to October 2021.
Analyses of linked NID/NIMS data
Annual GBS cases in England incident from 2019, 2020 and January to July 2021 were stratified by age decile, enabling age-specific incidence rate to be compared to recent years ( Table 1). To enable a comparison across years, an estimate of annual case numbers for 2021 was produced based on assumption of minimal seasonal variation. An excess of cases in the 50–59 and 60–69 age groups was identified compared to 2019 and 2020, with statistically significant differences between age groups (P = 0.00033).
Table 1.
GBS rates in England between 2019 and July 2021, separated by age group
| Age group, years | Number of cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 January –July (Estimated annual 2021 total) | |||||||
| GBS cases | ONS (million) | GBS rate per 100 000 patient years | GBS cases | ONS (million) | GBS rate per 100 000 patient years | GBS cases | ONSa (million) | GBS rate per 100 000 patient years | |
| 18–29 | 109 | 8.55 | 1.28 | 90 | 8.48 | 1.06 | 38 (65) | 8.48 | 0.77 |
| 30–39 | 117 | 7.54 | 1.55 | 99 | 7.56 | 1.31 | 48 (82) | 7.56 | 1.09 |
| 40–49 | 120 | 7.13 | 1.68 | 110 | 7.11 | 1.55 | 84 (144) | 7.11 | 2.04 |
| 50–59 | 191 | 7.58 | 2.52 | 138 | 7.64 | 1.81 | 161 (276) | 7.64 | 3.63 |
| 60–69 | 213 | 5.91 | 3.60 | 200 | 5.98 | 3.34 | 145 (248) | 5.98 | 4.18 |
| 70–79 | 202 | 4.72 | 4.28 | 163 | 4.82 | 3.38 | 101 (173) | 4.82 | 3.61 |
| 80+ | 98 | 2.84 | 3.45 | 60 | 2.86 | 2.10 | 27 (46) | 2.86 | 1.63 |
Estimated annual total for 2021 (italics) was extrapolated assuming stable GBS incidence across the year based on numbers from January to July 2021. GBS rate = incidence rate of GBS cases (per 100 000 patient years, assuming that each individual in the ONS population estimate was followed for a full year, or in the case of 2021 estimates, for 7 months); ONS = Office of National Statistics population estimates.
For 2021, ONS population estimates were assumed to be the same as for 2020.
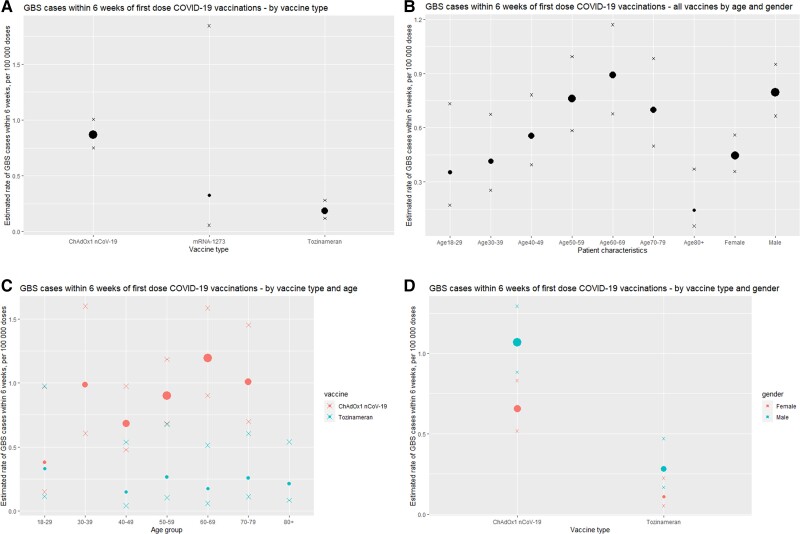
Using the linked NID/NIMS data, the first record of GBS occurring within 6 weeks after a COVID-19 vaccination was in January 2021. Of note, not all GBS patients in NID had a vaccination record. One hundred and ninety eight cases of GBS identified in the linked NID/NIMS data study period occurred within 6 weeks of the first dose of any COVID-19 vaccine (0.618 cases per 100 000 vaccinations in 6 weeks, all ages). A total of 32.1 million first dose vaccinations were recorded during the reporting period (20.3 million ChAdOx1 nCoV-19, 11.5 million tozinameran and 0.3 million mRNA-1273). Of the 198 linked GBS cases, 176 followed a first dose ChAdOx1 nCoV-19 vaccine (rate 0.868 per 100 000) and 21 followed a first dose tozinameran vaccine (rate 0.183 per 100 000). Only one case was reported within 6 weeks of mRNA-1273vaccination. Only 23 GBS cases were reported within 6 weeks of any second vaccine dose.
Table 2 and Fig. 2 summarize patient characteristics of GBS cases in England occurring within 6 weeks of first COVID-19 vaccination. The GBS incidence after first vaccination was highest in males receiving the ChAdOx1 nCoV-19vaccine (1.069 per 100 000 doses).
Table 2.
Patient characteristics of GBS cases documented in England occurring within 6 weeks of first dose COVID-19 vaccination with specific breakdown of gender and age for ChAdOx1 nCoV-19 and tozinameran recipients
| Patient characteristics | Cases | Estimated first dose vaccinations (million) | Estimated 6-week GBS case rate (/100 000 first doses) | 95% CI | |
|---|---|---|---|---|---|
| Gender | Female | 76 | 17.0 | 0.448 | 0.358–0.560 |
| Male | 120 | 15.1 | 0.795 | 0.665–0.951 | |
| Age group, years | 18–29 | 7 | 2.0 | 0.355 | 0.172–0.732 |
| 30–39 | 16 | 3.9 | 0.415 | 0.255–0.674 | |
| 40–49 | 33 | 5.9 | 0.557 | 0.396–0.782 | |
| 50–59 | 54 | 7.1 | 0.762 | 0.584–0.994 | |
| 60–69 | 51 | 5.7 | 0.890 | 0.677–1.170 | |
| 70–79 | 33 | 4.7 | 0.699 | 0.498–0.982 | |
| 80+ | 4 | 2.8 | 0.145 | 0.056–0.372 | |
| Vaccine | ChAdOx1 nCoV-19 | 176 | 20.3 | 0.868 | 0.749–1.006 |
| Tozinameran | 21 | 11.5 | 0.183 | 0.120–0.280 | |
| mRNA-1273 | 1 | 0.3 | 0.325 | 0.057–1.844 | |
| ChAdOx1 nCoV-19 only | |||||
| Gender | Female | 68 | 10.4 | 0.656 | 0.517–0.831 |
| Male | 106 | 9.9 | 1.069 | 0.884–1.293 | |
| Age, years | 18–29 | 4 | 1.1 | 0.380 | 0.148–0.977 |
| 30–39 | 16 | 1.6 | 0.986 | 0.607–1.601 | |
| 40–49 | 30 | 4.4 | 0.683 | 0.478–0.975 | |
| 50–59 | 50 | 5.6 | 0.899 | 0.682–1.185 | |
| 60–69 | 48 | 4.0 | 1.196 | 0.902–1.586 | |
| 70–79 | 28 | 2.8 | 1.007 | 0.697–1.455 | |
| 80+ | 0 | 0.9 | – | – | |
| Tozinameran only | |||||
| Gender | Female | 7 | 6.5 | 0.108 | 0.052–0.224 |
| Male | 14 | 5.0 | 0.280 | 0.167–0.470 | |
| Age | 18–29 | 3 | 0.9 | 0.332 | 0.113–0.976 |
| 30–39 | 0 | 2.1 | – | – | |
| 40–49 | 2 | 1.4 | 0.147 | 0.040–0.538 | |
| 50–59 | 4 | 1.5 | 0.264 | 0.103–0.678 | |
| 60–69 | 3 | 1.7 | 0.175 | 0.059–0.514 | |
| 70–79 | 5 | 1.9 | 0.258 | 0.110–0.604 | |
| 80+ | 4 | 1.9 | 0.210 | 0.082–0.539 | |
Note mRNA-1273 recipients were not separately analysed due to only a single case being reported.
Figure 2.
GBS case rate within 6 weeks of first-dose of COVID-19 vaccination in England. Estimated rate of GBS cases within 6 weeks of first COVID-19 vaccination (per 100 000 doses) separated by (A) vaccine type, (B) age/gender, (C) age for ChAdOx1 nCoV-19 and tozinameran vaccines and (D) gender for ChAdOx1 nCoV-19 and tozinameran vaccines. Size of dots are weighted based on GBS case numbers. Crosses represent upper and lower limits of 95% CIs.
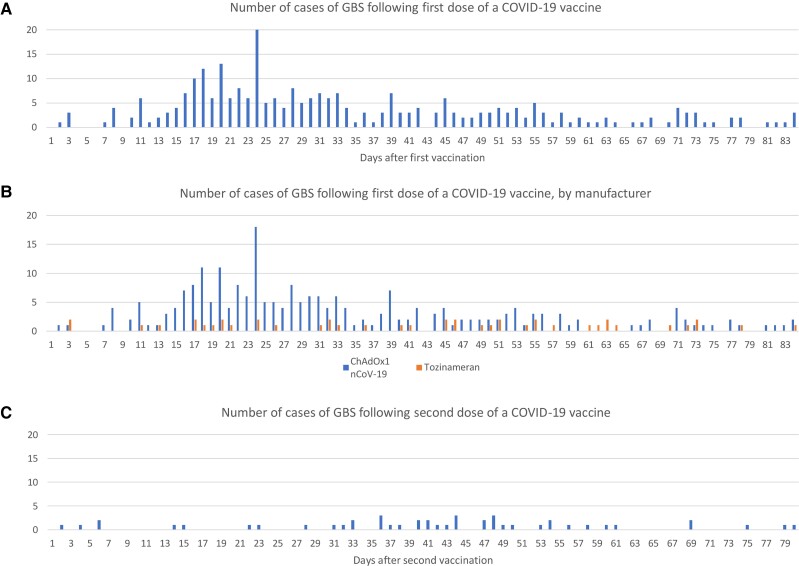
The daily number of incident GBS cases, with an 84-day post-vaccine follow-up from dose 1 and 2 of COVID-19 vaccination was plotted (Fig. 3). A peak of GBS cases was observed around 24 days following a first dose, with higher numbers of cases seen in the period of 2 to 4 weeks after vaccination than in other periods. First doses of ChAdOx1 nCoV-19 vaccine accounted for the majority of this increase. A similar pattern was not seen following a second dose of any vaccine. Using the Spearman’s rank correlation test of randomness, the occurrence of GBS was random for all times after the first-dose tozinameran vaccine (P = 0.84) (no peak associated with tozinameran vaccine) and after the second-dose vaccination of all vaccines (P = 0.85). However, it was non-random for ‘all first-dose’ vaccinations (P = 0.009) and the first dose of the ChAdOx1 nCoV-19 vaccination (P = 0.004).
Figure 3.
GBS case numbers in England by day following vaccination. Number of GBS cases 0 to 84 days following (A) any first dose vaccination, (B) first dose stratified by vaccine brand and (C) second dose COVID-19 vaccination.
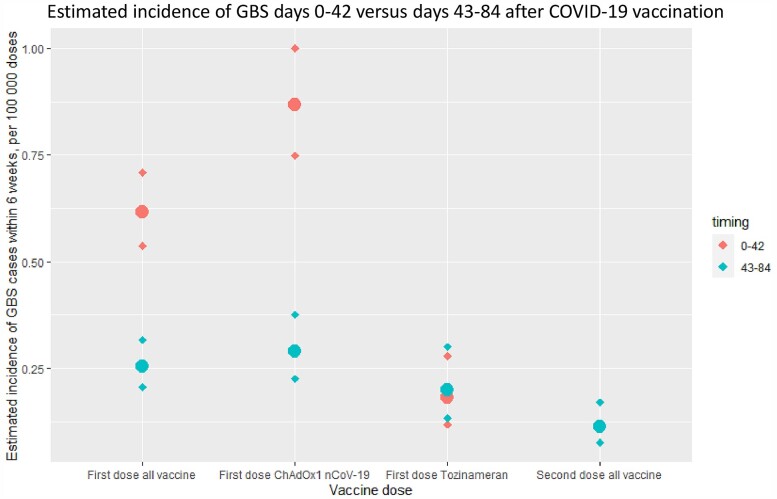
Using case numbers from Days 43–84 after first-dose vaccination as a comparison group (assuming this group represents a baseline random GBS rate), the excess risk in the first 42 days post-ChAdOx1 nCoV-19 vaccine was 0.576 GBS cases per 100 000 doses (95% CI 0.481–0.691). With an estimated 20.3 million first ChAdOx1 nCoV-19 doses given at the time of analysis, this suggested an absolute number of 98–140 excess GBS cases. There was no significant difference between the GBS cases associated with the first dose of tozinameran and second-dose vaccination numbers in the Day 0–42 or Day 43–84 case numbers. Fig. 4 summarizes the estimated excess risk for different vaccinated groups.
Figure 4.
Excess risk in first 42 days following vaccination in England. Estimated incidence of GBS cases within 6 weeks (per 100 000 vaccine doses), comparing reports of GBS cases 0–42 and 43–84 days for first-dose vaccines (all vaccines, ChAdOx1 nCoV-19, tozinameran) and second-dose vaccines. Diamonds represent upper and lower limits of 95% CIs. An excess of GBS cases was noted in the first 42 days following first-dose vaccination, accounted for by the ChAdOx1 nCoV-19 vaccine.
Prospective surveillance study
Between 1 January and 7 November 2021, 121 cases of GBS were reported by the BPNS/ABN network. Since clinicians and the public were highly sensitized to vaccination and concerns about risk, the reporting of this dataset is biased in favour of vaccine-related cases. The median age of reported cases was 59 years, with 59% being male. A total of 106 patients (87.3%) had received a COVID-19 vaccination prior to GBS onset, with 80 (66.1% of the total dataset) having received a first-dose vaccination within 42 days of GBS onset. In comparison, from the linked NID/NIMS dataset, 198 of the 659 GBS cases in England during this timeframe (30%) were reported to be within 42 days of vaccination. This highlights the reporting bias of the dataset towards vaccine-associated GBS. As expected with the timing of the vaccine rollout, 90% of GBS cases reported from January to April 2021 were within 6 weeks of vaccination, compared to only 35% of cases from May 2021 onwards.
Further demographic and clinical characteristics of the patients described within the dataset are shown in Table 3.
Table 3.
Characteristics of patients from clinician-reported surveillance study
| Patient characteristic (n = patients with available data) | Number (% or range) | |
|---|---|---|
| Total number of cases | 121 | |
| Median age (n = 121) | 59 years (17–85) | |
| Gender (n = 121) | Female | 50 (41.3%) |
| Male | 71 (58.7%) | |
| Race (n = 113) | White British | 102 (90.3%) |
| Latin American | 1 (0.9%) | |
| South Asian | 5 (4.4%) | |
| Middle Eastern | 2 (1.8%) | |
| Black | 3 (2.7%) | |
| COVID-19 Vaccination received (n = 121) | Yes | 106 (87.6%) |
| No | 15 (12.4%) | |
| Vaccine manufacturer (n = 102) | ChAdOx1 nCoV-19 | 89 (87.3%) |
| Tozinameran | 13 (12.7%) | |
| Number of vaccine doses (n = 104) | One dose | 88 (84.6%) |
| Two doses | 16 (15.4%) | |
| Median time from vaccination to GBS onset (n = 97) | 17 days (0–297) | |
| Cases within 42 days of first dose vaccination (n = 97) | 80 (82.3%) | |
| Recent or previous COVID-19 infection (n = 15) | COVID-19 diagnosed after GBS onset | 5 |
| COVID-19 prior to GBS | 10 | |
| Median time from COVID-19 infection to GBS (n = 10) | 24 (3–266) | |
| Highest level of care (n = 118) | Home | 11 (9.3%) |
| Ward | 86 (72.9%) | |
| High Dependency Unit | 8 (6.8%) | |
| Intensive Care Unit | 13 (11.0%) | |
| Hughes GBS score on first assessment (n = 111) | 0—Normal | 6 (5.4%) |
| 1—Slight clinical symptoms/signs | 8 (7.42%) | |
| 2—Able to walk 5 m unaided, unable to run | 32 (28.8%) | |
| 3—Able to walk 5 m with help | 24 (21.6%) | |
| 4—Bedridden/chairbound | 38 (34.2%) | |
| 5—Ventilator-assisted breathing | 3 (2.7%) | |
| Brighton Criteria classification15 (n = 121) | Level 1 (highest certainty) | 50 (41.3%) |
| Level 2 | 36 (29.8%) | |
| Level 3 | 9 (7.4%) | |
| Level 4 (lowest certainty) | 26 (21.5%) | |
| Presence of bilateral flaccid limb weakness (n = 121) | 105 (86.8%) | |
| Decreased or absent deep tendon reflexes in the weak limbs (n = 121) | 114 (94.2%) | |
| Monophasic pattern with nadir 12 h to 28 days from onset (n = 121) | 112 (92.6%) | |
| Lumbar puncture showing albumino-cytological dissociation (n = 103) | 94 (91.3%) | |
| Nerve conduction studies in keeping with GBS (n = 84) | 82 (97.6%) | |
| Nerve conduction studies subtype (n = 79) | Axonal | 7 (8.9%) |
| Conduction block | 2 (2.5%) | |
| Demyelinating | 63 (79.7%) | |
| Equivocal | 2 (2.5%) | |
| Mixed | 5 (6.3%) | |
| Other diagnoses still being considered at latest time of reporting (n = 118) | 3 (2.5%) | |
| Treatment (n = 121) | No treatment | 18 (14.9%) |
| Intravenous immunoglobulin | 90 (74.4%) | |
| Steroids | 5 (4.1%) | |
| Plasma exchange | 5 (4.1%) | |
| Combination of above | 3 (2.5%) | |
Data are presented either as n (%) or median (range).
Forty-two patients (34.7%) were reported to have facial weakness in association with other GBS findings. Facial weakness was bilateral in 37 of these patients. Only seven patients (5.8%) had pure bilateral facial paralysis with paraesthesia.
Only four patients were reported with positive ganglioside antibodies (GM1, GM2, GM1/GD1b, GD1a/GD1b), but this was likely to have been under-reported because of the time delays in anti-ganglioside results being available and inconsistent testing. Equally, limited COVID access to neurophysiology services may explain why only 84 of the 121 patients underwent nerve conduction studies; these data deficiencies limit the Brighton Collaboration diagnostic categorization.
Comparing surveillance study patients who had a first-dose vaccination within 42 days of GBS onset to those who were unvaccinated and patients who had GBS more than 42 days after first-dose vaccination, no significant differences were found in terms of gender (P = 0.54), age (P = 0.25), highest level of care (P = 0.36), Hughes GBS disability score on admission (P = 0.75), Brighton level of diagnostic certainty (P = 0.53), nerve conduction study changes (P = 0.44), treatment (P = 0.52) or presence of unilateral or bilateral facial weakness (P = 0.77).
Discussion
We have presented parallel studies designed to investigate a possible relationship between COVID-19 vaccination and GBS. The first study linked nationalized databases and the second was based on prospective case reporting.
These data suggested a clear and plausible excess of GBS cases occurring within 42 days after the first dose of ChAdOx1 nCoV-19 COVID-19 vaccination. The complexities of timing and delivery of multiple vaccines to age and at-risk cohorts of patients in the UK, on a naturally unstable GBS baseline, make accurate identification and quantification of the risks difficult in real time. The data provided an estimate of 5.8 cases of GBS (4.8–6.9) per million first doses of ChAdOx1 nCoV-19 vaccine and no measurable excess of GBS associated with first doses of tozinameran. This equated to an absolute excess of between 98 and 140 cases of GBS attributable to ChAdOx1 nCoV-19 vaccination up to 8 July 2021 in England. This should be compared to estimates that the vaccination programme directly averted over 52 600 hospitalizations, between 21.3 and 22.9 million infections and between 57 500 and 62 700 deaths over the same time period.19
We decided to use GBS case numbers from Days 43–84 after vaccination as a comparator group, rather than an externally-derived control based on historical GBS numbers, as GBS case numbers during the COVID-19 pandemic may be different from historical baselines.4 GBS incidence in this group was estimated to be 1.9 cases per day, lower than the historical 2016–2020 average of 3.1 cases per day based on NID data in England, supporting the hypothesis that the baseline GBS case numbers in 2021 continued to be lower than pre-pandemic levels.
The total number of cases of GBS in the NID from January to October 2021 was lower than the 10-month total for January to October 2016 to 2019 but was higher than total case numbers during 2020. A monthly increase in GBS cases in March and April 2021 was notable, but total numbers fell back into the normal range thereafter, and thus this ‘spike’ was the only hint of a causative link in simple occurrence data.
The spike in case numbers and the subsequent return to normal levels might be explained in several ways. The baseline onto which any vaccination-related cases are built varies because of the normal seasonal reduction in GBS and the unknown effects of ongoing social distancing measures, which reduced the 2020 cases of GBS by about one-third. Social distancing and other lockdown measures in the UK were slowly relaxed in the first half of 2021 and some GBS increases might have been expected through greater pathogen exposure. In addition, vaccinated individuals may have rapidly changed their social behaviour after vaccinations, increasing exposure to other infections known to increase GBS risk, but this behaviour would have been expected to continue and grow as more were vaccinated, which was not the pattern demonstrated here. The remaining nationally-mandated social distancing measures may have continued to account for the lower than usual numbers of GBS cases in July–October 2021.
If all COVID-19 vaccines were associated with an increased risk of GBS across all age groups, a more sustained rise in GBS incidence would be expected. A total of 9 646 715 mostly older and more vulnerable people were vaccinated from early December 2020 to 1 February 2021, but the increase in GBS cases was not seen until mid-February.
ChAdOx1 nCoV-19 vaccinations commenced in early January, quickly overtaking tozinameran as the predominant vaccine administered, to a significant proportion of the apparently more susceptible 50- to 70-year-old age group, with 24 858 665 adults receiving the first dose of COVID-19 vaccine from 1 Feb 2021 to 1 May 2021 (representing 50.5% of the UK population).20
Interestingly, there was no recognizable increase in GBS after the second dose, which may have been due to a pathophysiological phenomenon, individual susceptibility, or because patients experiencing adverse effects from the first dose did not take-up the second dose of vaccine. A single patient was reported to have GBS-like illness following both first- and second-doses of the ChAdOx1 nCov-19 vaccine. The patient initially developed a facial diplegia and paraesthesia phenotype with subsequent gait disturbance and elevated CSF protein but improved with IVIg. Two months later, 2 weeks after their second dose of ChAdOx1 nCoV-19 vaccination, they developed increasing weakness with neuropathic pain, elevated CSF protein, demyelinating changes on nerve conduction studies and MRI enhancement of the cauda equina, with only partial response to IVIg treatment.
The single patient in our surveillance study who had recurring neuropathic symptoms after the second-dose vaccination appeared to be a unique report in the UK at the time and may have represented an acute-onset chronic inflammatory demyelinating polyradiculoneuropathy (A-CIDP) rather than true GBS. A recent study from Israel on COVID-19 vaccinations in patients with previous GBS described a single patient with recurrent GBS-like illness time-linked to both doses of their tozinameran vaccination, but limited information was available regarding the robustness of the GBS diagnosis.21
A small excess of GBS in males was seen in the vaccine-associated GBS cohort as documented in the GBS literature,15 but the reason for this remains unclear. Furthermore, the phenotype of the reported cases of GBS in the surveillance study gave no recognizable vaccine-specific GBS features, unlike vaccine-induced immune thrombotic thrombocytopenia (VITT), with distinguishable clinical features and an available biomarker in the anti-PF4 antibodies.11 Cases of severe (bi)-facial palsy have been discussed widely,22–25 although this feature did not occur more often than in the non-vaccine associated cases in our dataset.
The reason for the association between ChAdOx1 nCoV-19-only vaccination and GBS is unclear. COVID-19 infection does not lead to a strong, or possibly any, increased risk of GBS,4,5 and the lack of increased risk associated with tozinameran vaccination implies that it is unlikely that the COVID-19 spike protein is the causative factor for the increased risk. The excess incidence is estimated to be 5.8 cases per million doses, similar to the estimates for the 1976 ‘swine flu’ vaccine and higher (but within the same order of magnitude) as the reported excess cases for the modern influenza and yellow fever vaccines. It is far below the 1:1000 cases of GBS in C. jejuni gastroenteritis or Zika-virus. A non-specific immune activation in susceptible individuals might therefore be implicated, but if that were the case, similar risks might apply to all vaccine types. It is therefore logical to suggest the simian adenovirus vector may account for the increased risk. Adenoviruses have not been strongly associated with GBS in previous studies,26 and any association between adenoviral vaccination and GBS11 has only been reported once. Nevertheless, adenovirus testing is not routinely performed in cases of GBS in the UK, and whether adenoviruses account for a proportion of ‘idiopathic’ or ‘serology negative’ GBS requires further investigation.
Although the first retrospective analysis presented here employed two cross-referenced national and mandated datasets, there were still confounders, bias and criticisms as applicable to many epidemiological studies. We were unable to individually validate cases of immunoglobulin-treated GBS. However, these patients would need to be unwell enough to be admitted to hospital, and the diagnosis was assessed not only by the admitting physicians but also by an Immunoglobulin Assessment Panel who authorize the treatment. Furthermore, the data were controlled against data from earlier years in which GBS rates were consistent with other international, methodologically robust epidemiological studies with high levels of ascertainment and clinical validity. We recognized that patients with ‘mild GBS’ may not attend hospital or be treated, but this has been the same in past years. We also recognized that patients may have been more reluctant to attend hospital, although this was not obviously observed in 2021, and paralyzed patients were likely to have attended more than those with lesser disabilities.
We explored detailed phenotypes by collecting data on GBS presentations to UK neurologists with a continuation of the BPNS/ABN surveillance study. In total, 121 cases were reported, representing only 13% of GBS cases during that period of 2021. Clinicians were much more likely to report cases with recent vaccination compared to those who were unvaccinated. Nonetheless, this dataset allowed for deeper confirmation of GBS diagnoses, with 79% of the cohort meeting Brighton Collaboration diagnostic criteria level 1, 2 or 3 [compared to 15% of the cases reported to the MHRA ‘Yellow Card’ system (personal communication)]. Within our dataset, there were no differences identified between patients who had recent vaccination and those who had not in terms of baseline demographics, disease course or treatment. Although BPNS and ABN members reported cases of facial weakness associated with GBS, there was no increase linked to vaccination status. The description of some of these cases best fit the ‘atypical’ GBS presentation of ‘bilateral facial weakness with paraesthesia’, which is felt to represent <5% of total GBS cases.27
Another recently published UK-based study analysed neurological complications in the context of recent COVID-19 vaccination and infection and reported comparable excess in GBS cases of 3.8 per million doses of ChAdOx1 nCoV-19 vaccine. The incidence rate ratio (IRR) of 2.90 at 15–21 days post-vaccination suggested a similarly plausible time-locked association to that which we observed, in keeping with the pathological mechanism of GBS.28 However, they also reported a possible increase in GBS cases in relation to COVID-19 infection of 14.5 per million COVID-19 infections, with an IRR of 5.25.28 We note that neurological manifestations were identified using hospital coding data, which may be less accurate than NID identification of Immunoglobulin Panel-scrutinized GBS cases. Moreover, mortality rate was 1.8% in that cohort, lower than the 3–10% mortality generally associated with GBS.27 In addition, 16% of COVID-19-associated GBS cases in their cohort (7/43 patients) were diagnosed on the same day as a COVID-19 diagnosis, which makes it less likely that GBS was caused by a post-infectious immune process triggered by COVID-19 infection. For these and other reasons, we believe that our study’s findings on excess risk may provide a more accurate estimate of risk.
Our study reports an association between first-dose ChAdOx1 nCoV-19COVID-19 vaccination and GBS, accounting for an estimated excess incidence of 5.8 GBS cases per million first doses. The cause for this association remains unclear, and excess risk remains comparable to previous vaccine-associated GBS. The risk in proportion to the benefits of vaccination is very small. Further studies are required to confirm these observations, determine causality, explore the pathogenic mechanisms and investigate the effects of other COVID-19 vaccine preparations in use elsewhere in the world.
Supplementary Material
Appendix 1
BPNS/ABN COVID-19 Vaccine GBS Study Group
Further details are available in the Supplementary material.
Hadi Manji, Tim Lavin, James B. Lilleker, David Gosal, Robert D.M. Hadden, Taylor Watson-Fargie, Kathryn Brennan, Andreas Themistocleous, Jacquie Deeb, Ana Romeiro, Puja R. Mehta, Dimitri Kullmann, James Miller, Amar Elsaddig, Adam Molyneux, Plamen Georgiev, Aaron Ben-Joseph, James Holt, Jacob Roelofs, Fadi Alkufri, David Allen, Simon Shields, Stephen Murphy, Harri Sivasathiaseelan, Richard Sylvester, Abdul Al-Saleh, Rhys Roberts, Kannan Nithi, Lahiru Handdunnethi, Kate Wannop, Amit Batla, Anna Sadnicka, Jananee Sivaganasundaram, Tatyana Yermakova, Ravi Dasari, Graziella Quattrocchi, Harriet Ball, Rebecca Cooper, Daniel Whittam, Mohanned Mustafa, Gabriel Yiin, Shayan Ashjaei, Andrew J. Westwood, Michelle Dsouza, Eng Chuan Foo, Shwe Zin Tun, Khine Khine Lwin, Gorande Kanabar.
Contributor Information
Ryan Y S Keh, MRC Centre for Neuromuscular Diseases, National Hospital of Neurology and Neurosurgery, Queen Square, University College London Hospitals NHS Foundation Trust, London, UK; Manchester Centre for Clinical Neurosciences, Salford Royal NHS Foundation Trust, Manchester, UK.
Sophie Scanlon, Medicines and Healthcare products Regulatory Agency, UK.
Preeti Datta-Nemdharry, Medicines and Healthcare products Regulatory Agency, UK.
Katherine Donegan, Medicines and Healthcare products Regulatory Agency, UK.
Sally Cavanagh, NHS England & Improvement (NHSEI), National Health Service, London, UK.
Mark Foster, Medical Data Solutions and Services, Ardwick, Manchester, UK.
David Skelland, NHS Arden and Greater East Midlands (GEM) Commissioning Support Unit (CSU), Warwick, UK.
James Palmer, NHS England & Improvement (NHSEI), National Health Service, London, UK.
Pedro M Machado, MRC Centre for Neuromuscular Diseases, National Hospital of Neurology and Neurosurgery, Queen Square, University College London Hospitals NHS Foundation Trust, London, UK; Institute of Neurology, University College London, London, UK.
Stephen Keddie, MRC Centre for Neuromuscular Diseases, National Hospital of Neurology and Neurosurgery, Queen Square, University College London Hospitals NHS Foundation Trust, London, UK; Institute of Neurology, University College London, London, UK; Department of Neurology, The Royal London Hospital, Barts Health NHS Trust, London, UK.
Aisling S Carr, MRC Centre for Neuromuscular Diseases, National Hospital of Neurology and Neurosurgery, Queen Square, University College London Hospitals NHS Foundation Trust, London, UK; Institute of Neurology, University College London, London, UK.
Michael P Lunn, MRC Centre for Neuromuscular Diseases, National Hospital of Neurology and Neurosurgery, Queen Square, University College London Hospitals NHS Foundation Trust, London, UK; Institute of Neurology, University College London, London, UK.
BPNS/ABN COVID-19 Vaccine GBS Study Group:
Hadi Manji, Tim Lavin, James B Lilleker, David Gosal, Robert DM Hadden, Taylor Watson-Fargie, Kathryn Brennan, Andreas Themistocleous, Jacquie Deeb, Ana Romeiro, Puja R Mehta, Dimitri Kullmann, James Miller, Amar Elsaddig, Adam Molyneux, Plamen Georgiev, Aaron Ben-Joseph, James Holt, Jacob Roelofs, Fadi Alkufri, David Allen, Simon Shields, Stephen Murphy, Harri Sivasathiaseelan, Richard Sylvester, Abdul Al-Saleh, Rhys Roberts, Kannan Nithi, Lahiru Handdunnethi, Kate Wannop, Amit Batla, Anna Sadnicka, Jananee Sivaganasundaram, Tatyana Yermakova, Ravi Dasari, Graziella Quattrocchi, Harriet Ball, Rebecca Cooper, Daniel Whittam, Mohanned Mustafa, Gabriel Yiin, Shayan Ashjaei, Andrew J Westwood, Michelle Dsouza, Eng Chuan Foo, Shwe Zin Tun, Khine Khine Lwin, and Gorande Kanabar
Funding
No specific funding was received for this work. P.M.M., M.P.L. and A.S.C. are supported by the National Institute for Health Research (NIH) UCLH Biomedical Research Centre (BRC). R.Y.S.K. is funded by GBS-CIDP Foundation International.
Competing interests
P.M.M. has received consulting/speaker’s fees from Abbvie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript. The remaining authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Rogers JP, Watson CJ, Badenoch J, et al. Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry. 2021;92(9):932–941. [DOI] [PubMed] [Google Scholar]
- 2. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Umapathi T, Er B, Koh JS, Goh YH, Chua L. Guillain-Barré syndrome decreases in Singapore during the COVID-19 pandemic. J Peripher Nerv Syst. 2021;26(2):235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langmuir AD, Bregman DJ, Kurland LT, Nathanson N, Victor M. An epidemiologic and clinical evaluation of Guillain-Barré syndrome reported in association with the administration of swine influenza vaccines. Am J Epidemiol. 1984;119(6):841–879. [DOI] [PubMed] [Google Scholar]
- 7. Salmon DA, Proschan M, Forshee R, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381(9876):1461–1468. [DOI] [PubMed] [Google Scholar]
- 8. Arya DP, Said MA, Izurieta HS, et al. Surveillance for Guillain-Barré syndrome after 2015-2016 and 2016-2017 influenza vaccination of Medicare beneficiaries. Vaccine. 2019;37(43):6543–6549. [DOI] [PubMed] [Google Scholar]
- 9. Cines DB, Bussel JB. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326(12):1210–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McNeil MM, Paradowska-Stankiewicz I, Miller ER, et al. Adverse events following adenovirus type 4 and type 7 vaccine, live, oral in the Vaccine Adverse Event Reporting System (VAERS), United States, October 2011-July 2018. Vaccine. 2019;37(44):6760–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foster M. Immunoglobulin database annual report 2019/20. Accessed 11 November 2021. https://igd.mdsas.com/wpcontent/uploads/ImmunoglobulinDatabaseAnnualReport201920.pdf.
- 13.NHS England. Updated Commissioning Guidance for the use of therapeutic immunoglobulin (Ig) in immunology, haematology, neurology and infectious diseases in England December 2018. Accessed 11 November 2021. https://www.england.nhs.uk/wpcontent/uploads/2019/03/PSS9-Immunoglobulin-Commissioning-Guidance-CQUIN-1920.pdf.
- 14. Doets AY, Verboon C, van den Berg B, et al. Regional variation of Guillain-Barre´ syndrome. Brain. 2018;141:2866–2877. [DOI] [PubMed] [Google Scholar]
- 15. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29(3):599–612. [DOI] [PubMed] [Google Scholar]
- 16. StataCorp . Stata Statistical Software: Release 16.StataCorp LLC; 2019. [Google Scholar]
- 17. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 18. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150–163. [DOI] [PubMed] [Google Scholar]
- 19.Public Health England. COVID-19 vaccine surveillance report: Week 30. Published 29 July 2021. Accessed 11 November 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1007376/Vaccine_surveillance_report_-_week_30.pdf.
- 20.Public Health England. Vaccinations in the United Kingdom. Accessed 20 September 2021. https://coronavirus.data.gov.uk/details/vaccinations.
- 21. David SSB, Potasman I, Rahamim-Cohen D. Rate of recurrent guillain-barré syndrome after mRNA COVID-19 vaccine BNT162b2. JAMA Neurol. 2021;78(11):1409–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mason MC, Liaqat A, Morrow J, Basso R, Gujrati Y. Bilateral facial nerve palsy and COVID-19 vaccination: causation or coincidence?. Cureus. 2021;13(8):e17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prasad A, Hurlburt G, Podury S, Tandon M, Kingree S, Sriwastava S. A novel case of bifacial diplegia variant of guillain-barré syndrome following janssen COVID-19 vaccination. Neurol Int. 2021;13(3):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Repajic M, Lai XL, Xu P, Liu A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021;13:100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. James J, Jose J, Gafoor VA, Smita B, Balaram N. Guillain-Barré syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: a case series. Neurol Clin Neurosci. 2021;9:402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobs BC, Rothbarth PH, van der Meché FG, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51(4):1110–1115. [DOI] [PubMed] [Google Scholar]
- 27. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol. 2019;15:671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patone M, Handunnnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request to the corresponding author.