Abstract
Background
Open-label platform trials and a prospective meta-analysis suggest efficacy of anti–interleukin (IL)-6R therapies in hospitalized patients with coronavirus disease 2019 (COVID-19) receiving corticosteroids. This study evaluated the efficacy and safety of sarilumab, an anti–IL-6R monoclonal antibody, in the treatment of hospitalized patients with COVID-19.
Methods
In this adaptive, phase 2/3, randomized, double-blind, placebo-controlled trial, adults hospitalized with COVID-19 received intravenous sarilumab 400 mg or placebo. The phase 3 primary analysis population included patients with critical COVID-19 receiving mechanical ventilation (MV). The primary outcome was proportion of patients with ≥1-point improvement in clinical status from baseline to day 22.
Results
There were 457 and 1365 patients randomized and treated in phases 2 and 3, respectively. In phase 3, patients with critical COVID-19 receiving MV (n = 298; 28.2% on corticosteroids), the proportion with ≥1-point improvement in clinical status (alive, not receiving MV) at day 22 was 43.2% for sarilumab and 35.5% for placebo (risk difference, +7.5%; 95% confidence interval [CI], –7.4 to 21.3; P =.3261), a relative risk improvement of 21.7%. In post hoc analyses pooling phase 2 and 3 critical patients receiving MV, the hazard ratio for death for sarilumab vs placebo was 0.76 (95% CI, .51 to 1.13) overall and 0.49 (95% CI, .25 to .94) in patients receiving corticosteroids at baseline.
Conclusions
This study did not establish the efficacy of sarilumab in hospitalized patients with severe/critical COVID-19. Post hoc analyses were consistent with other studies that found a benefit of sarilumab in patients receiving corticosteroids.
Clinical Trials Registration
Keywords: monoclonal antibodies, interleukin-6 receptor, hospitalized, COVID-19
Sarilumab did not lead to significant improvements in clinical status or mortality in hospitalized patients with coronavirus disease 2019, most of whom were not receiving corticosteroids. However, there may be benefit of anti–interleukin-6R therapy in patients receiving corticosteroids.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus first identified in December 2019, is the causative agent of coronavirus disease 2019 (COVID-19). While most patients with COVID-19 have mild disease, the leading cause of hospitalization and death is respiratory failure, including acute respiratory distress syndrome [1, 2]. A key driver of this deterioration may be a dysregulated inflammatory response [3] based on the observation of elevated circulating levels of inflammatory mediators such as C-reactive protein (CRP) and interleukin-6 (IL-6) [4, 5]. Early in the pandemic, small, uncontrolled studies reported that treating hospitalized patients with COVID-19 with 2 different IL-6 receptor (IL-6R)-blocking antibodies, tocilizumab and sarilumab, resulted in potentially dramatic clinical improvement [6–11]. More recently, 2 platform trials found that tocilizumab or sarilumab may improve clinical outcomes in patients with COVID-19, the majority of whom were also receiving corticosteroids [12, 13].
On 13 March 2020, COVID-19 was declared a national emergency in the United States. On 18 March 2020, we initiated a clinical trial to evaluate the efficacy and safety of intravenous (IV) sarilumab, an anti–IL-6R monoclonal antibody, for the treatment of hospitalized patients with COVID-19 in the United States. Here, we report the final results of this trial.
METHODS
Study Design
We conducted an adaptive, phase 2/3, randomized, double-blind, placebo-controlled, multicenter trial (Supplementary Figure 1); 62 of 65 sites enrolled patients (see list of sites in the Supplementary Material). Since the trial was being conducted during a pandemic with a novel coronavirus, the protocol allowed adaptations, including closing treatment arms or randomization strata, modification of the provisional phase 3 end points, and sample size re-estimation before phase 3 database lock and study readout.
Participants
Eligible patients were aged ≥18 years and hospitalized with laboratory-confirmed SARS-CoV-2 infection that required supplemental oxygen and/or assisted ventilation (see Supplementary Material, Inclusion and Exclusion Criteria). All patients received the local standard of care (SOC), including corticosteroids and open-label use of putative treatments for COVID-19 (as described in the protocol).
Randomization and Masking
In phase 2, patients were randomized 2:2:1 to IV sarilumab 400 mg, sarilumab 200 mg, or placebo (for flow diagram of phase 2, see Supplementary Material, Phase 2 Study Results). Randomization was stratified by corticosteroid use and disease severity (severe, critical, multisystem organ dysfunction [MSOD], and immunocompromised state). “Severe” COVID-19 was defined as patients receiving low-flow supplemental oxygen. “Critical” COVID-19 was defined as requiring supplemental oxygen by nonrebreather mask or high-flow nasal device, noninvasive ventilation, invasive mechanical ventilation (MV), or management in an intensive care unit. “MSOD” was defined as use of vasopressors, extracorporeal life support, or renal replacement therapy.
Trial Adaptations
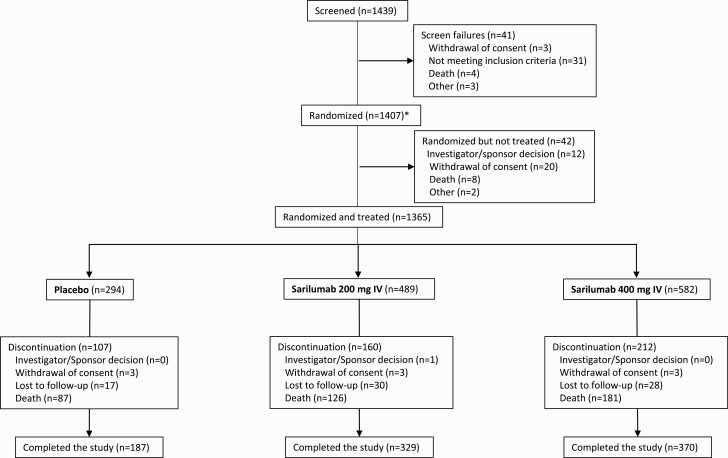
A summary of protocol amendments and study adaptations can be found in the Supplementary Material. A prespecified phase 2 interim analysis of 457 patients suggested potential benefit of sarilumab 400 mg in patients only in the critical stratum [14] (for final phase 2 results, please see Supplementary Material, Phase 2 Study Results). Subsequently, the Independent Data Monitoring Committee recommended discontinuation of enrollment into the severe and MSOD strata and elimination of the 200-mg dose. Thereafter, the phase 3 protocol was amended (Supplementary Figure 1) to restrict enrollment to critical patients receiving MV, critical patients not on MV, severe patients, patients on MSOD, and immunocompromised patients with further randomization (2:1) to sarilumab 400 mg and placebo (cohort 1, n = 1407; Figure 1) and to add 2 new cohorts of critical patients receiving MV randomized to sarilumab 800 mg or placebo (cohort 2, n = 33; Supplementary Material, phase 3 (cohort 2) Study Results); critical patients not receiving MV, but requiring high-flow oxygen or noninvasive ventilation were randomized to sarilumab 800 mg or placebo (cohort 3, n = 9; Supplementary Material, phase 3 (cohort 3) Study Results). In addition, the following adaptations were implemented prior to database lock: the phase 3 primary analysis population was changed to patients randomized to the critical stratum receiving MV (without extracorporeal membrane oxygenation [ECMO]) who were randomized to the sarilumab 400-mg group or placebo, the primary end point was changed from the time to ≥2-point improvement in clinical status using a 7-point ordinal scale to the proportion with ≥1-point improvement from baseline to day 22, and the sample size was recalculated.
Figure 1.
Phase 3, cohort 1: flow diagram. * Includes 9 screen failures that were randomized.
Results from the phase 3 immunocompromised stratum (n = 35 patients randomized and treated) are not shown; results for cohorts 2 and 3 (sarilumab 800 mg) are reported in the Supplementary Material.
Outcomes
Following the interim analysis from the phase 2 part of the study, the prespecified primary end point for phase 3 cohort 1 was the proportion of critical patients receiving MV at baseline with ≥1-point improvement in clinical status on a 7-point ordinal scale from baseline to day 22 (Clinical Status Scale in the Supplementary Material) [15]. In this population, a 1-point improvement indicates that the patient was alive and no longer receiving MV. Other prespecified end points included the proportion of patients who died by day 60 and the proportion of patients who recovered (discharged or alive without supplemental oxygen use) by day 22. All outcomes were assessed by the site investigators who were blinded to treatment assignment and serum IL-6 levels conducted at a central laboratory. Safety end points are described in the Supplementary Material.
Study Oversight
See the Supplementary Material regarding study oversight.
Statistical Analyses
Statistical details are provided in the Supplementary Material and the statistical analysis plan.
The phase 3 primary analysis population included all patients with critical COVID-19 receiving MV without ECMO at baseline who were randomized to the sarilumab 400-mg or placebo groups.
Post hoc analyses were conducted using the pooled data from phase 2 and phase 3 (cohort 1) to descriptively analyze the end points of the proportion of patients with ≥1-point improvement in clinical status to day 22, proportion alive and not receiving MV, and time to death. All post hoc analyses were unstratified.
RESULTS
From 18 March 2020 to 2 July 2020, 457 patients were randomized and treated in phase 2 (placebo, n = 90; sarilumab 200 mg, n = 187; sarilumab 400 mg, n = 180), and 1365 patients were randomized and treated in phase 3 cohort 1 (placebo, n = 294; sarilumab 200 mg, n = 489; sarilumab 400 mg, n = 582; Figure 1). A summary of phase 2 and phase 3 analysis populations by disease severity strata is presented in Supplementary Table 1. Results of the phase 2 portion of the study are presented in Supplementary Material.
Baseline Demographics and Characteristics
In phase 3, 750 (54.9%) patients were randomized to the critical stratum (298 [21.8%] receiving MV at baseline, 450 [33.0%] not receiving MV, 2 [.3%] receiving ECMO), 347 (25.4%) were randomized to the severe stratum, and 233 (17.1%) were randomized to the MSOD stratum. The median age of patients in the critical stratum was 61 years, 68% were male, and the median duration of illness was 9.0 days. The median serum CRP was 168.80 mg/L, serum IL-6 concentration was 110.91 pg/mL, and viral load in nasopharyngeal swabs was 3.95 log10 copies/mL. At randomization, 34.3% of critical patients were receiving systemic corticosteroids, and this was balanced across treatment groups (Supplementary Table 2).
Demographics and baseline characteristics for phase 3 severe and MSOD strata and for the pooled phase 2/3 dataset are presented in Supplementary Tables 3 and 4, respectively.
Phase 3 Efficacy Outcomes
Primary Outcome
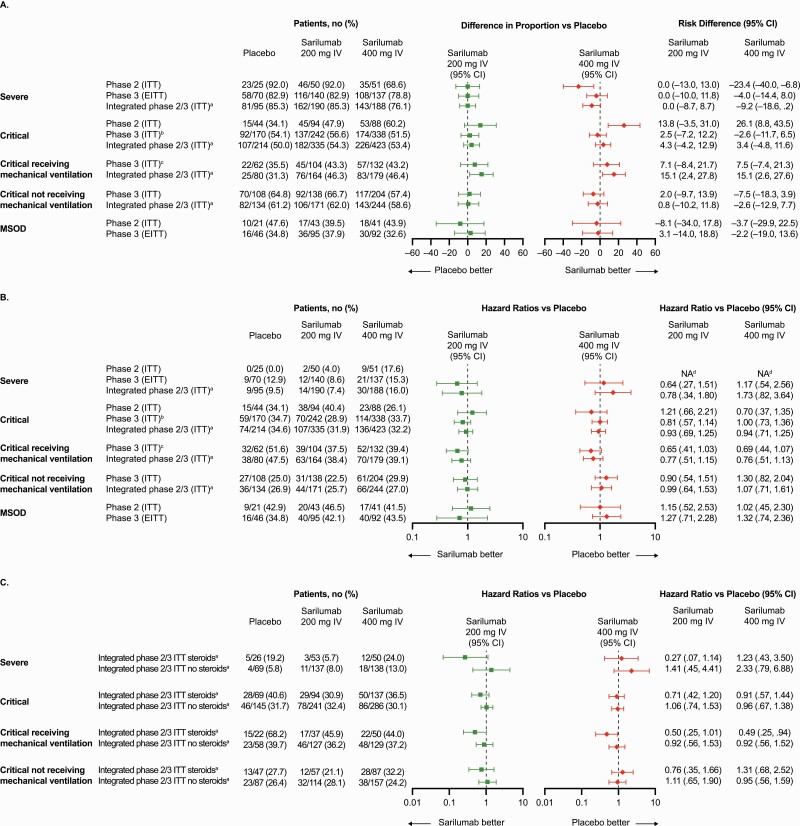
In the prespecified primary analysis among critical patients receiving MV, 43.2% of patients in the 400-mg group and 35.5% patients in the placebo group had ≥1-point improvement in clinical status (alive not receiving MV) at day 22 (risk difference [RD], +7.5%; 95% confidence interval [CI], –7.4 to 21.3; P = .3261; Table 1, Figure 2A), representing a relative risk improvement of 21.7%.
Table 1.
Phase 3, Cohort 1: Primary Efficacy End Point
| Proportion of Patients With ≥1-Point Improvement in Clinical Status from Baseline to Day 22 (Critical Intention-to-Treat Patients Receiving Mechanical Ventilation at Baseline) | |||
|---|---|---|---|
| Primary Efficacy Endpoint | Placebo Group (n = 62) |
Sarilumab 200 mg IV Group (n = 104) |
Sarilumab 400 mg IV Group (n = 132) |
| Patients, no.(%) | 22 (35.5) | 45 (43.3) | 57 (43.2) |
| 95% CI | 23.6–47.4 | 33.7–52.8 | 34.7–51.6 |
| Risk difference vs placebo, % | ... | 7.1 | 7.5 |
| 95% CI | ... | -8.4 to 21.7 | -7.4 to 21.3 |
| P value | ... | .3707 | .3261 |
Abbreviations: CI, confidence interval; IV, intravenous.
Figure 2.
Improvement in clinical status and risk of death in phase 3, cohort 1. A, Risk difference for various population strata achieving ≥1-point improvement in clinical status from baseline to day 22 using the 7-point ordinal scale. B, Hazard ratios for the risk of death in various population strata. C, Hazard ratios for the risk of death in various population strata by steroid use. aUnstratified analysis. bKey secondary end point for phase 3, cohort 1. cPrimary end point for phase 3, cohort 1. dHazard ratio could not be calculated, as the number of events was too small. Abbreviations: CI, confidence interval; EITT, exploratory intention to treat; ITT, intention to treat; IV, intravenous; MSOD, multisystemic organ dysfunction.
Secondary Outcomes
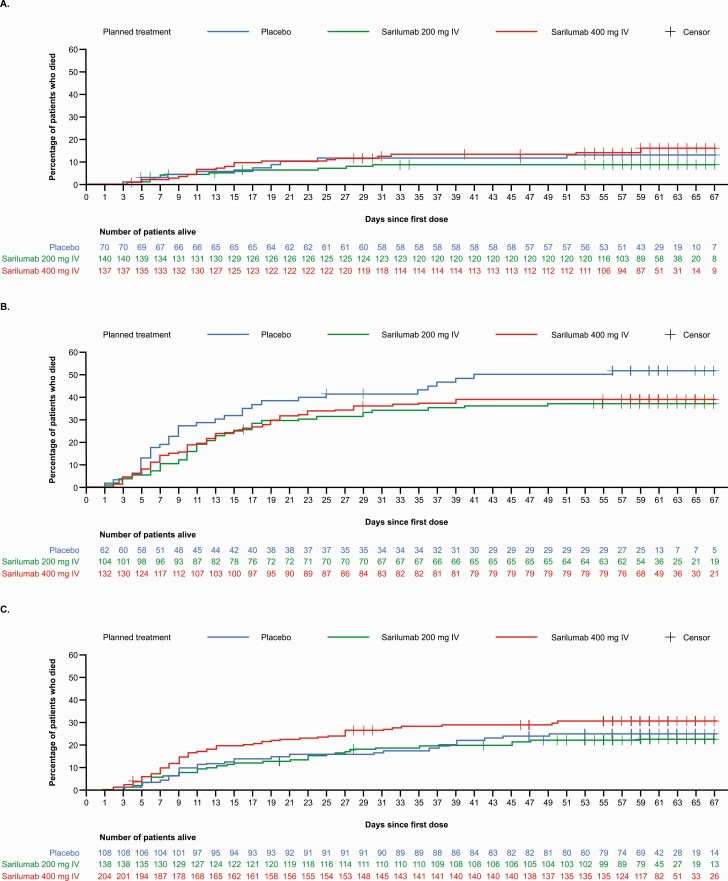
Among critical patients receiving MV at baseline, mortality by day 29 was 36.4% in the 400-mg group and 41.9% in the placebo group (RD, –5.5%; 95% CI, –20.2 to 8.7; relative risk reduction, 13.3%; key secondary end point) and mortality by day 60 was 39.4% in the sarilumab 400-mg group and 51.6% in the placebo group (RD, –11.9%; 95% CI, –26.4 to 2.9; relative risk reduction, 23.7%; Supplementary Table 5). Time to death in critical patients receiving MV at baseline is shown in Figure 3. Recovery by day 22 occurred in 31.8% of critical patients receiving MV at baseline in the 400-mg group and 25.8% in the placebo group (RD, +5.7%; 95% CI, –8.4 to 18.2; relative risk reduction, 23.3%; key secondary end point; Supplementary Table 5).
Figure 3.
Kaplan-Meier curve of time to death in phase 3, cohort 1. A, Severe patients (exploratory intention-to-treat [ITT] population). B, Critical patients on mechanical ventilation at baseline (ITT population). C, Critical patients not on mechanical ventilation at baseline (ITT population). Abbreviation: IV, intravenous.
Among critical patients not receiving MV at baseline, 57.4% of patients in the 400-mg group and 64.8% of patients in the placebo group had ≥1-point improvement in clinical status at day 22 (RD –7.5%; 95% CI, –18.3 to 3.9; Supplementary Table 5). Mortality by day 29 was 26.5% in the 400-mg group and 15.7% in the placebo group (RD, +10.7%; 95% CI, .9 to 19.3; Supplementary Table 5), and mortality by day 60 was 29.9% in the 400-mg group and 25% in the placebo group (RD, +4.8%; 95% CI, 5.8 to 14.6; Supplementary Table 5). Time to death in critical patients not receiving MV at baseline is shown in Figure 3. Recovery by day 22 occurred in 54.4% of patients in the 400-mg group and 63.9% in the placebo group (RD, –9.5%; 95% CI, –20.4 to 2.0; Supplementary Table 5).
Prespecified Exploratory Analyses
There was no evidence of efficacy of sarilumab compared with placebo in severe or MSOD patients (Supplementary Table 6, Figures 2 and 3A).
Post Hoc Analyses
In post hoc analyses of pooled phase 2 and phase 3 critical patients receiving MV, 46.4% of patients receiving 400 mg and 31.3% of patients receiving placebo had ≥1-point improvement in clinical status at day 22 (RD, 15.1%; 95% CI, 2.6 to 27.6; Figure 2A). Similar findings were observed for mortality (Figure 2B). No benefit in clinical status was seen in critical patients not receiving MV at baseline (RD, –2.6%; 95% CI, –12.9 to 7.7; Figure 2A).
Among critical patients receiving MV and corticosteroids at baseline, 44.0% (22 of 50) of patients died in the 400-mg group compared with 68.2% (15 of 22) in the placebo group (hazard ratio [HR], 0.49; 95% CI, .25 to .94; Figure 2C). Among critical patients receiving MV without corticosteroids at baseline, 37.2% (48 of 129) died in the 400-mg group compared with 39.7% (23 of 58) in the placebo group (HR, 0.92; 95% CI, .56 to 1.52; Figure 2C).
Safety
In the phase 3 portion of the study in critical patients, the safety profiles of sarilumab 200 mg and 400 mg were comparable and were similar to placebo (Supplementary Table 7). Adverse events were consistent with advanced COVID-19 and its complications. Serious adverse events (SAEs) of cardiac arrest, COVID-19, respiratory failure, acute respiratory failure, septic shock, and acute kidney injury were reported in a similar proportion of patients in the sarilumab dose groups in comparison with the placebo group, suggesting that these events were related to COVID-19 (Table 2).
Table 2.
Phase 3, Cohort 1 Critical Stratum: Treatment-Emergent Serious Adverse Events and Adverse Events of Special Interest Occurring in ≥5% of Patients in Any Group for the Safety Population
| Critical Patients Receiving Mechanical Ventilation at Baseline | Critical Patients Not Receiving Mechanical Ventilation at Baseline | All Critical Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| System Organ Class Preferred Term |
Placebo (n = 62) |
Sarilumab 200 mg IV Group (n = 104) |
Sarilumab 400 mg IV Group (n = 132) |
Placebo (n = 108) |
Sarilumab 200 mg IV Group (n = 138) |
Sarilumab 400 mg IV Group (n = 204) |
Placebo (n = 170) |
Sarilumab 200 mg IV Group (n = 242) |
Sarilumab 400 mg IV Group (n = 338) |
| SAEs | |||||||||
| SAEs | 103 | 143 | 174 | 96 | 114 | 183 | 199 | 257 | 359 |
| Patients with ≥1 SAE | 44 (71.0) | 65 (62.5) | 88 (66.7) | 45 (41.7) | 54 (39.1) | 93 (45.6) | 89 (52.4) | 119 (49.2) | 183 (54.1) |
| Infections and infestations | |||||||||
| COVID-19 | 6 (9.7) | 9 (8.7) | 9 (6.8) | 9 (8.3) | 10 (7.2) | 14 (6.9) | 15 (8.8) | 19 (7.9) | 23 (6.8) |
| Septic shock | 6 (9.7) | 5 (4.8) | 6 (4.5) | 3 (2.8) | 1 (.7) | 6 (2.9) | 9 (5.3) | 6 (2.5) | 12 (3.6) |
| Respiratory, thoracic, and mediastinal disorders | |||||||||
| Respiratory failure | 5 (8.1) | 9 (8.7) | 7 (5.3) | 10 (9.3) | 14 (10.1) | 14 (6.9) | 15 (8.8) | 23 (9.5) | 22 (6.5) |
| Acute respiratory failure | 3 (4.8) | 4 (3.8) | 5 (3.8) | 7 (6.5) | 2 (1.4) | 12 (5.9) | 10 (5.9) | 6 (2.5) | 17 (5.0) |
| Pneumothorax | 2 (3.2) | 6 (5.8) | 1 (0.8) | 3 (2.8) | 2 (1.4) | 8 (3.9) | 5 (2.9) | 8 (3.3) | 9 (2.7) |
| Cardiac disorders | |||||||||
| Cardiac arrest | 5 (8.1) | 6 (5.8) | 11 (8.3) | 2 (1.9) | 1 (.7) | 14 (6.9) | 7 (4.1) | 7 (2.9) | 25 (7.4) |
| Renal and urinary disorders | |||||||||
| Acute kidney injury | 6 (9.7) | 9 (8.7) | 3 (2.3) | 4 (3.7) | 3 (2.2) | 7 (3.4) | 10 (5.9) | 12 (5.0) | 10 (3.0) |
| General disorders and administration site conditions | |||||||||
| Multiple organ dysfunction syndrome | 3 (4.8) | 1 (1.0) | 8 (6.1) | 1 (.9) | 3 (2.2) | 2 (1.0) | 4 (2.4) | 4 (1.7) | 10 (3.0) |
| AESIs | |||||||||
| AESIs | 61 | 102 | 128 | 49 | 89 | 124 | 110 | 191 | 255 |
| Patients with ≥1 AESI | 31 (50.0) | 56 (53.8) | 71 (53.8) | 30 (27.8) | 51 (37.0) | 77 (37.7) | 61 (35.9) | 107 (44.2) | 150 (44.4) |
| Investigations | |||||||||
| Alanine aminotransferase increased | 8 (12.9) | 23 (22.1) | 16 (12.1) | 13 (12.0) | 23 (16.7) | 37 (18.1) | 21 (12.4) | 46 (19.0) | 55 (16.3) |
| Aspartate aminotransferase increased | 8 (12.9) | 21 (20.2) | 18 (13.6) | 12 (11.1) | 19 (13.8) | 19 (9.3) | 20 (11.8) | 40 (16.5) | 38 (11.2) |
| Transaminases increased | 3 (4.8) | 4 (3.8) | 12 (9.1) | 3 (2.8) | 3 (2.2) | 16 (7.8) | 6 (3.5) | 7 (2.9) | 28 (8.3) |
| Liver function test increased | 2 (3.2) | 2 (1.9) | 8 (6.1) | 0 (0.0) | 1 (.7) | 0 (0.0) | 3 (1.8) | 2 (.8) | 10 (3.0) |
| Infections and infestations | |||||||||
| Pneumonia | 4 (6.5) | 3 (2.9) | 8 (6.1) | 2 (1.9) | 1 (.7) | 3 (1.5) | 6 (3.5) | 4 (1.7) | 11 (3.3) |
| Staphylococcal infection | 4 (6.5) | 2 (1.9) | 5 (3.8) | 2 (1.9) | 2 (1.4) | 3 (1.5) | 6 (3.5) | 4 (1.7) | 8 (2.4) |
Data are shown as no. (%) unless noted otherwise.
Abbreviations: AESI, adverse event of special interest; IV, intravenous; SAE, serious adverse event.
Adverse events of special interest (AESIs) reported in more than 5% of the patients in any treatment group were elevations of liver function tests, reported numerically more often in patients receiving sarilumab than placebo (Table 2). None of the liver transaminase elevation cases met Hy’s law criteria.
In the phase 3 portion of the study, patients with severe disease or MSOD exhibited a safety profile similar to that of critical patients, and no new safety findings were observed (Supplementary Table 8). Numerically, more patients reported elevation of liver function tests and SAEs in the sarilumab 200-mg and 400-mg dose groups in comparison with placebo (Supplementary Table 9), which is expected based on the known safety profile of sarilumab [16].
DISCUSSION
In the phase 3 cohort 1 of this phase 2/3 clinical trial conducted early during the COVID-19 pandemic, a numerically lower proportion of critical patients receiving MV in the sarilumab 400-mg group had died by day 29 compared with placebo (36.4% vs 41.9%, respectively). This was a smaller effect than that observed with dexamethasone in the RECOVERY trial [17], and sarilumab did not lead to significant improvement in clinical status or mortality in hospitalized patients with COVID-19, the majority of whom were not receiving corticosteroids as SOC. There was also no benefit of sarilumab seen in patients receiving either low- or high-flow supplemental oxygen or noninvasive ventilation. It is important to note that since the majority of our study enrollment was prior to demonstration of the clinical utility of corticosteroids in COVID-19, only a minority of patients in our trial (approximately 30%) were receiving concomitant corticosteroids. Therefore, these overall outcomes may not be generalizable to the hospitalized population receiving sarilumab in addition to corticosteroids as part of SOC.
In our post hoc analyses where we pooled phase 2 and 3 datasets, critical patients receiving MV at baseline had a higher mortality rate of 68% with steroids alone (placebo group) compared with 44% with steroids and sarilumab 400 mg. An additive benefit of anti–IL-6R therapy to corticosteroids in patients with COVID-19 has previously been reported in 2 platform trials. The REMAP-CAP trial, in which >90% of patients were receiving corticosteroids as part of SOC, reported a mortality benefit of tocilizumab and sarilumab compared with SOC alone in hospitalized patients requiring organ support [12, 18]. The RECOVERY trial in hospitalized patients with COVID-19 with hypoxemia and CRP >75 mg/L, in which 82% of patients were receiving corticosteroids as SOC, demonstrated that tocilizumab led to a statistically significant reduction in mortality (29% in the tocilizumab group vs 33% in SOC only group). Tocilizumab treatment of patients not receiving MV reduced the risk of progressing to MV or death (33% vs 38% SOC). Similar to the REMAP-CAP trial, the majority (82%) of patients were receiving corticosteroids at baseline, and the mortality benefit of tocilizumab was not seen in patients not receiving corticosteroids [13].
Strengths of this trial include the randomized, double-blind, placebo-controlled design and stratification based on disease severity and corticosteroid use. In addition, unlike the RECOVERY trial, we systematically collected all SAEs and AESIs, providing important safety information on the use of sarilumab at doses up to 800 mg in the hospitalized COVID-19 population.
Our trial had several limitations. The phase 3 sample size estimation based on the phase 2 interim data may have led to an underpowered study, which was further impacted by the 2:1 randomization ratio, leading to a small placebo treatment group. The heterogeneity in the critically ill population, as seen in clinical trials in acute respiratory distress syndrome not related to COVID-19, highlights the need for large real-world trials such as RECOVERY and SOLIDARITY to adequately assess efficacy of therapeutics in the hospitalized COVID-19 population [19, 20]. Another limitation is that adequate dose response and multiple dosing were not evaluated as part of this study. Although an 800-mg treatment arm was included late in the trial, the small sample size makes interpretation challenging.
Although this study alone does not provide sufficient evidence of the clinical utility of sarilumab to treat COVID-19, our post hoc data are consistent with a mortality benefit observed with IL-6R inhibitors in other large studies, when used in conjunction with corticosteroids for patients receiving MV [18, 21]. Furthermore, based on results from the prospective meta-analysis that included data from RECOVERY, REMAP-CAP, and the current study, IL-6R inhibitors are currently recommended in combination with corticosteroids in certain hospitalized patients [22]. The safety of sarilumab in COVID-19 was similar to that observed in previous clinical studies with sarilumab, with no new safety signals identified in this setting. While further studies would be required to definitively demonstrate a mortality benefit for use in COVID-19, the recent addition of tocilizumab and sarilumab into treatment guidelines will make conducting large, controlled trials with sarilumab challenging [23–25].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. S. S., D. J. L., R. B., A. G., A. Boyapati, J. P., B. J. M., L. A. P., S. M. B., A. T. D., B. A., M. C. N., N. B., G. H., G. D. Y., and D. M. W. contributed to study concept and design. N. H., G. J. C., A. Boyapati, E. L.-T., D. G., M. N. G., S. S., S. J. S., V. M., D. K. S., M. E. S., W. P., J. A. A., S. M. B., and B. K. were involved in data collection. S. S., D. J. L., R. H., A. M., A. G., S. S.-K., M. P. O., J. P., E. L.-T., D. R., L. A. P., D. G., B. K., and B. A. provided administrative, technical, or material support. R. B., B. J. M., A. W., and B. A. provided statistical analysis. S. S., D. J. L., R. B., R. H., A. M., A. G., S. S.-K., M. P. O., A. Boyapati, J. P., B. J. M., L. A. P., W. K., A. W., J. A. K., J. E. H., M. A. F., A. Baris, A. T. D., B. A., M. C. N., N. B., G. H., G. D. Y., and D. M. W. provided analysis and interpretation of the data. S. S., D. J. L., R. H., A. M., W. K., and M. C. N. drafted the manuscript. S. S., D. J. L., R. B., N. H., G. J. C., A. M., A. G., A. Boyapati, J. P., B. J. M., L. A. P., W. K., A. W., M. N. G., S. S., S. J. S., V. M., D. K. S., M. E. S., W. P., J. A. A., S. M. B., J. E. H., B. K., A. T. D., B. A., M. C. N., N. B., G. A. H., G. D. Y., and D. M. W. provided critical revision of the manuscript for important intellectual content. All authors provided approval to submit.
Acknowledgments. The authors thank the patients, their families, and investigational site members involved in this study (principal investigators and subprincipal investigators are listed in the Supplementary Material, Study Sites and Investigators); the members of the Independent Data Monitoring Committee (Steve Dahlberg, MS, Mitchell Levy, MD, Victor Ortega, MD, PhD, Kevin Winthrop, MD, Thomas Cook, PhD [nonvoting member]); Caryn Trbovic, PhD, S. Balachandra Dass, PhD, and Brian Head, PhD, from Regeneron Pharmaceuticals for assistance with development of the manuscript; and Prime, Knutsford, United Kingdom, for manuscript formatting and copy editing suggestions. We dedicate this article to the memory of Colby Burk, MS, for his contributions and commitment to patients with coronavirus disease 2019 (COVID-19).
Disclaimer. S. S., R. H., J. P., D. R., and L. A. P. were all employees of Regeneron Pharmaceuticals, Inc, at the time of the study; S. S. is currently at Excision BioTherapeutics, New York, New York; J. P. is currently at Bill & Melinda Gates Medical Research Institute, Cambridge, Massachusetts; D. R. is currently at Apellis Pharmaceuticals, Inc, Waltham, Massachusetts; and L. A. P. is currently at Vir Biotechnology, St. Louis, Missouri.
Financial support. This work was supported by Regeneron Pharmaceuticals, Inc, Sanofi, and the Biomedical Advanced Research and Development Authority. Certain aspects of this project were funded in whole or in part with federal funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA), under Other Transaction number HHSO100201700020C.
Potential conflicts of interest . S. Sivapalasingam is an Excision BioTherapeutics employee/stockholder and former Regeneron Pharmaceuticals employee and current stockholder and reports grants from BARDA. D. J. L., R. B., A. M., A. G., S. S. K., M. P. O., A. Boyapati, B. J. M., E. L.-T., D. G., W. K., A. W., J. A. K., J. E. H., M. A. F., A. Baras, B. K., A. T. D., B. A., M. C. N., N. B., G. A. H., G. D. Y., and D. M. W. are Regeneron Pharmaceuticals employees/stockholders and report grants from BARDA and personal fees and other from Regeneron Pharmaceuticals. D. J. L., M. C. N., N. B., and D. M. W. have a patent pending. G. C. reports grants from BARDA and personal fees and other from Regeneron Pharmaceuticals. J. P. is a Bill & Melinda Gates Medical Research Institute employee, a former Regeneron Pharmaceuticals employee and current stockholder, and reports grants from BARDA. D. R. is an Apellis Pharmaceuticals employee/stockholder and a former Regeneron Pharmaceuticals employee and current stockholder. R. H. is a former Regeneron Pharmaceuticals employee and current stockholder and reports grants from BARDA and personal fees and other from Regeneron Pharmaceuticals. L. A. P. is a Vir Biotechnology employee/stockholder and former Regeneron Pharmaceuticals employee and current stockholder and has received grants from BARDA. M. N. G. has received support to conduct the study from Regeneron Pharmaceuticals and reports grants from BARDA, the National Institutes of Health (NIH), the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention for COVID and non-COVID research that is not related to this article. S. Saggar reports grants from BARDA and fees from Allergan/AbbVie. S. J. S. reports grants from BARDA. V. M., M. E. S., and W. P. report grants from BARDA. D. K. S. has received support to conduct the study from Regeneron Pharmaceuticals and reports grants from BARDA. J. A. A. has received support to conduct the study from Regeneron Pharmaceuticals; reports grants from BARDA, Atea, Emergent Biosolutions, Frontier Technologies, Gilead, Janssen, Pfizer, and ViiV; and reports grants and personal fees from GlaxoSmithKline and Merck. S. M. B. reports grants from BARDA, Sedana, Janssen, NIH, and the Department of Defense; personal fees from Hamilton and New York University and other from Faron; and book fees from Oxford University and Brigham Young University. The remaining author: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data sharing. Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported here. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (eg, US Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency) if there is legal authority to share the data and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.
Contributor Information
Sumathi Sivapalasingam, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
David J Lederer, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Rafia Bhore, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Negin Hajizadeh, Institute for Clinical Outcomes Research, Feinstein Institutes for Medical Research, Northwell Health, Manhasset, New York, New York, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Northwell Health, Hempstead, New York, New York, USA.
Gerard Criner, Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania, USA.
Romana Hosain, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Adnan Mahmood, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Angeliki Giannelou, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Selin Somersan-Karakaya, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Meagan P O’Brien, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Anita Boyapati, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Janie Parrino, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Bret J Musser, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Emily Labriola-Tompkins, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Divya Ramesh, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Lisa A Purcell, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Daya Gulabani, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Wendy Kampman, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Alpana Waldron, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Michelle Ng Gong, Department of Medicine, Montefiore–Moses, Bronx, New York, USA.
Suraj Saggar, Department of Infectious Disease, Holy Name Medical Center, Teaneck, New Jersey, USA.
Steven J Sperber, Department of Infectious Disease, Hackensack Meridian School of Medicine and Hackensack University Medical Center, Hackensack, New Jersey, USA.
Vidya Menon, Department of Medicine, NYC Health + Hospitals/Lincoln, Bronx, New York, USA.
David K Stein, Department of Medicine, Jacobi Medical Center, Bronx, New York, USA.
Magdalena E Sobieszczyk, Department of Medicine, Columbia University, New York, New York, USA.
William Park, Pulmonary and Sleep Disorder Clinic, Valley Medical Center, Renton, Washington, USA.
Judith A Aberg, Division of Infectious Diseases, Icahn School of Medicine at Mount Sinai, New York, New York, USAand.
Samuel M Brown, Department of Internal Medicine, Intermountain Medical Center and University of Utah, Salt Lake City, Utah, USA.
Jack A Kosmicki, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Julie E Horowitz, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Manuel A Ferreira, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Aris Baras, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Bari Kowal, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
A Thomas DiCioccio, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Bolanle Akinlade, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Michael C Nivens, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Ned Braunstein, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
Gary A Herman, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
George D Yancopoulos, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
David M Weinreich, Regeneron Pharmaceuticals, Inc, Tarrytown, New York, USA.
References
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; 180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 2020; 214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Del Valle DM, Kim-Schulze S, Hsin-Hui H, et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. Nat Med 2020; 26:1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020; 117:10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J.. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020; 92:814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morena V, Milazzo L, Oreni L, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med 2020; 76:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benucci M, Giannasi G, Cecchini P, et al. COVID-19 pneumonia treated with sarilumab: a clinical series of eight patients. J Med Virol 2020; 92:2368–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montesarchio V, Parrela R, Iommelli C, et al. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J Immuno Ther Cancer 2020; 8:e001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest 2020; 158:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19—preliminary report. N Engl J Med 2021; 2021.2001.2007.21249390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Regeneron Pharmaceuticals Inc. Regeneron and Sanofi provide update on US phase 2/3 adaptive-designed trial of KEVZARA® (sarilumab) in hospitalized COVID-19 patients. Available at: https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive. Accessed 10 February 2022.
- 15. Peterson RL, Vock DM, Powers JH, et al. Analysis of an ordinal endpoint for use in evaluating treatments for severe influenza requiring hospitalization. Clin Trials 2017; 14:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleischmann R, Genovese MC, Lin Y, et al. Long-term safety of sarilumab in rheumatoid arthritis: an integrated analysis with up to 7 years’ follow-up. Rheumatology (Oxford) 2020; 59:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Recovery Collaborative Group.; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Remap-Cap I, Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384:1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nuffield Department of Population Health. Randomised evaluation of COVID-19 therapy (RECOVERY). Available at: https://www.recoverytrial.net/files/recovery-protocol-v7-0-2020-06-18.pdf. Accessed 18 February 2021.
- 20. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med 2021; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. Therapeutics and COVID-19: living guideline. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2. Accessed 13 July 2021. [PubMed]
- 23. National Institutes of Health. Coronavirus disease 2019 (COVID-19) Treatment Guidelines. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf. Accessed 3 November 2021. [PubMed]
- 24. World Health Organization. Therapeutics and COVID-19: living guideline. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.1. Accessed 10 February 2022. [PubMed]
- 25. WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 2021; 326:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.