Abstract
Background
BNT162b2 by Pfizer-BioNTech and mRNA-1273 by Moderna are the most commonly used vaccines to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. Head-to-head comparison of the efficacy of these vaccines in immunocompromised patients is lacking.
Methods
Parallel, 2-arm (allocation 1:1), open-label, noninferiority randomized clinical trial nested into the Swiss HIV Cohort Study and the Swiss Transplant Cohort Study. People living with human immunodeficiency virus (PLWH) or solid organ transplant recipients (SOTR; ie, lung and kidney) from these cohorts were randomized to mRNA-1273 or BNT162b2. The primary endpoint was antibody response to SARS-CoV-2 spike (S1) protein receptor binding domain (Elecsys Anti-SARS-CoV-2 immunoassay, Roche; cutoff ≥0.8 units/mL) 12 weeks after first vaccination (ie, 8 weeks after second vaccination). In addition, antibody response was measured with the Antibody Coronavirus Assay 2 (ABCORA 2).
Results
A total of 430 patients were randomized and 412 were included in the intention-to-treat analysis (341 PLWH and 71 SOTR). The percentage of patients showing an immune response was 92.1% (95% confidence interval [CI]: 88.4–95.8; 186/202) for mRNA-1273 and 94.3% (95% CI: 91.2–97.4; 198/210) for BNT162b2 (difference: -2.2%; 95% CI: -7.1 to 2.7), fulfilling noninferiority of mRNA-1273. With the ABCORA 2 test, 89.1% had an immune response to mRNA-1273 (95% CI: 84.8–93.4; 180/202) and 89.5% to BNT162b2 (95% CI: 85.4–93.7; 188/210). Based on the Elecsys test, all PLWH had an antibody response (100.0%; 341/341), whereas for SOTR, only 60.6% (95% CI: 49.2–71.9; 43/71) had titers above the cutoff level.
Conclusions
In immunocompromised patients, the antibody response of mRNA-1273 was noninferior to BNT162b2. PLWH had in general an antibody response, whereas a high proportion of SOTR had no antibody response.
Keywords: SARS-CoV-2, randomized controlled trial, HIV, organ transplant, platform trial, vaccine
This randomized trial confirmed the noninferiority of the severe acute respiratory syndrome coronavirus 2 vaccine mRNA-1273 compared with BNT162b2 in terms of antibody response in immunocompromised patients. Although people with human immunodeficiency virus had a sufficient antibody response, a high proportion of transplant recipients had no antibody response.
Coronavirus disease 2019 (COVID-19) emerged in late 2019 in Wuhan, China, and was declared a pandemic by the World Health Organization on March 11, 2020 [1–3]. Since January 2021, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines by Pfizer-BioNTech (BNT162b2; Comirnaty) and Moderna (mRNA-1273; Spikevax) have been approved in Switzerland and are used to vaccinate the Swiss population [4]. Both vaccines were tested in largescale placebo-controlled approval studies including tens of thousands of individuals [5, 6]. Vaccines were found to be safe with excellent efficacy of 95% and 94%, respectively, in terms of preventing COVID-19 illness 14 days after the second vaccination. However, data for immunocompromised patients who have a high risk of COVID-19 infection with adverse outcome are still limited. The approval studies included only a few patients living with human immunodeficiency (PLWH), with no information on CD4 cell counts and no solid organ transplant recipients [5, 6]. To date, there is no randomized evidence on the comparative effectiveness of BNT162b2 and mRNA-1273 in immunocompromised patients.
Having 2 Swiss national cohort studies with immunocompromised patients in Switzerland (ie, the Swiss HIV Cohort Study [SHCS] [7] and the Swiss Transplant Cohort Study [STCS] [8, 9]) a Corona Vaccine Trial Platform nested in these cohorts was established. We aimed to assess the noninferiority of mRNA-1273 to BNT162b (the first in Switzerland licensed SARS-CoV-2 vaccines) in a randomized trial with respect to antibody response and safety in immunocompromised patients 12 weeks after the first vaccination (ie, 8 weeks after second vaccination).
METHODS
Trial Oversight
The full version of the study protocol as approved by the ethical committee Nordwest- and Zentralschweiz, Switzerland (BASEC Nr. 2021-000593), is available on the trial registration site (https://clinicaltrials.gov/ct2/show/NCT04805125); a condensed version has been published [9]. In brief, we conducted a parallel 2-arm (allocation 1:1), open-label, noninferiority randomized clinical trial comparing the 2 in Switzerland-approved SARS-CoV-2 mRNA vaccines that are used to vaccinate the Swiss population. Treating cohort physicians or delegated staff contacted potentially eligible cohort participants from the SHCS and the STCS and obtained written informed consent.
Participants, Randomization, and Blinding
Individuals who were enrolled in the SHCS or the STCS (ie, lung transplant and kidney transplant recipients; heart and liver transplant centers could not join the trial for organizational reasons) were eligible for trial participation if they were aged 18 years or older, and if the COVID-19 vaccination was recommended by the treating physician. We excluded pregnant women, patients with any acute respiratory tract infection, SARS-CoV-2-positive patients with an infection occurring in the past 3 months, and persons with any emergency condition requiring immediate hospitalization. In addition, we excluded organ transplant recipients who received the new organ within the past month, had received T-cell-depleting agents within the past 3 months, pulse corticosteroids (within the past few months), rituximab (within the past 6 months), or if they were in need of chemotherapy treatment. The 3 cohort centers—University Hospital Basel (SHCS + STCS), University Hospital Zürich (SHCS + STCS), and University Hospital Bern (SHCS)—recruited all participants.
Randomization was performed in the Research Electronic Data Entry system [10] separately for the 2 cohorts stratified by study center, age group, sex, and presence of comorbidities. We used minimization with a random element across stratification factors to control for imbalances in treatment arms.
Participants, treating physicians, and outcome assessors for clinical outcomes were not blinded. Laboratory staff who assessed immunological parameters was blinded to treatment allocation. Serious adverse events were adjudicated by the data safety monitoring board that was blinded to intervention allocation.
Interventions
BNT162b2 licensed by Pfizer-BioNTech (Comirnaty) and mRNA-1273 licensed by Moderna (Spikevax) were stored and applied according to the recommendation of the manufacturers [9, 11–13]. Both vaccines were administered on days 0 and 28 into the deltoid muscle (30 μg of BNT162b2 in 0.3 mL or 100 μg of mRNA-1273 in 0.5 mL).
Outcomes
All outcomes were assessed 12 weeks (±7 days) after the first vaccination. In cases in which patients were not available within this time window (eg, because of vacations), outcome data were collected on the closest possible date.
The primary outcome was a positive antibody response to SARS-CoV-2 spike (S1) protein receptor-binding domain in human serum or plasma assessed by the commercial immunoassay Elecsys Anti-SARS-CoV-2 S (Elecsys S) from Roche Diagnostics [14]. The outcome is binary using a threshold of ≥0.8 units/mL as defined by the manufacturer. Further immunological outcomes were positive antibody response using the Antibody CORonavirus Assay (ABCORA) 2 that assesses seropositivity by measuring specific immunoglobulin G (IgG), IgA, and IgM responses to SARS-CoV-2 receptor binding domains, S1, S2, and N [15]. The following clinical outcomes were chosen: (1) newly polymerase chain reaction–confirmed asymptomatic COVID-19 infections; (2) newly confirmed symptomatic COVID-19 infections; (3) severe COVID-19 infections (see study protocol for more details [9]); (4) COVID-19 burden of diseases (0 for no COVID-19; 1 for nonsevere COVID-19; 2 for severe COVID-19); and (5) COVID-19 infection of a household member. Safety outcomes were assessed during the 12-week study visit and were reduced for feasibility and relevance reasons to the following (asked separately after the first and second vaccine): (1) any local symptom (redness or swelling or prolonged pain at injection site) limiting continuation of normal daily activities during the first 7 days after vaccination; (2) any systemic symptom (fever, generalized muscle or joint pain) limiting continuation of normal daily activities during the first 7 days after vaccination; and (3) any vaccine-related symptom leading to contacting a physician during the first 7 days after vaccination. Serious adverse events (SAEs; see study protocol for more details [9]) were documented throughout the trial and routinely assessed during the 12-week study visit.
Sample Size
This study is powered to assess the noninferiority in terms of immune response (antibodies to SARS-CoV-2 spike (S1) protein receptor binding domain) between the 2 SARS-CoV-2 vaccines BNT162b2 or mRNA-1273. Data in the general population showed that titers were high in nearly 100% after the second vaccination; however, no data were available for immunocompromised patients when this study was planned [16, 17]. We assumed an immune response in 90% of patients in both groups and powered our noninferiority trial so that a 95% 2-sided confidence interval (CI) excludes a difference in favor of the reference group of more than 10%. In total, 380 patients (190 in each treatment arm) were required for a statistical power of 90% and a type I error of 0.025. The sample size was increased to 430 patients to account for losses to follow-up. Sample size was calculated using the “ssc_propcomp” function of the R statistical software package SampleSize4ClinicalTrials [18].
Analysis
Trial participants’ baseline characteristics, secondary outcomes regarding antibody response, clinical outcomes, SAEs, and safety outcomes are described as frequencies and percentages with 95% CIs or medians and interquartile range (IQR). Noninferiority of the primary outcome is established if the lower limit of a 95% 2-sided Wald CI for the difference in antibody response proportion between participants receiving mRNA-1273 and BNT162bs vaccines is above –10%, where 10% is the predefined noninferiority margin. Trial participants were primarily analyzed according to their allocated randomization group (intention to treat) but also according to a per-protocol principle that was defined as restricting the analysis to participants who received both vaccine doses they were allocated to. An additional “strict” per-protocol analysis was conducted restricted to individuals who received both dosages of their allocated vaccine (within the interval of 4 weeks ± 1 week) and had available outcome data at week 12 week (within the prespecified interval of ± 1 week; results presented in the appendix only). We performed sensitivity analyses by excluding participants with positive antibody response to the nucleocapsid protein at baseline as indicated by the Elecsys Anti-SARS-CoV-2 test Elecsys N test (sign of previous infection).
Quantitative SARS-CoV-2 S protein receptor binding domain with values ≥0.80 U/mL were considered “positive” for anti-SARS-CoV-2 S antibodies. In a first post hoc analysis setting the threshold for predicting a protective immune response was changed to 100 U/mL as indicated by Hall et al [19] and Khoury and colleagues [20].
In a second post hoc analyses, we chose for the ABCORA 2 sum S1 (sum of S1 signal over cutoff values of IgG, IgA, IgM) a threshold of 17 to predict neutralization activity against the vaccine strain Wuhan-Hu-1 in sera. The prediction is based on a head-to-head measurement of pseudovirus neutralization and ABCORA 2 binding. It was shown that 100% of the sera with a threshold above 17 had measurable neutralization activity (above a titer of 1:100) in our pseudovirus neutralization tests [15]. Subgroup analyses were conducted by cohort, as well as for specific subpopulations such as PLWH with less and more than 200 CD4 cells/μL, with a suppressed and unsuppressed HIV viral load (ie, >50 copies/mL), for transplanted patients under intense (triple or quadruple immunosuppressive regimen) or less intense immunosuppressive therapy (dual immunosuppressive regimen) and for study participants according to sex (male/female), age group (<60, 60 to 69, ≥70 years) and history of cardiovascular diseases or metabolic syndrome (see appendix for definition). No interim analysis was conducted. All analysis were done in R Project for Statistical Computing (version 4.0.3) software [21].
RESULTS
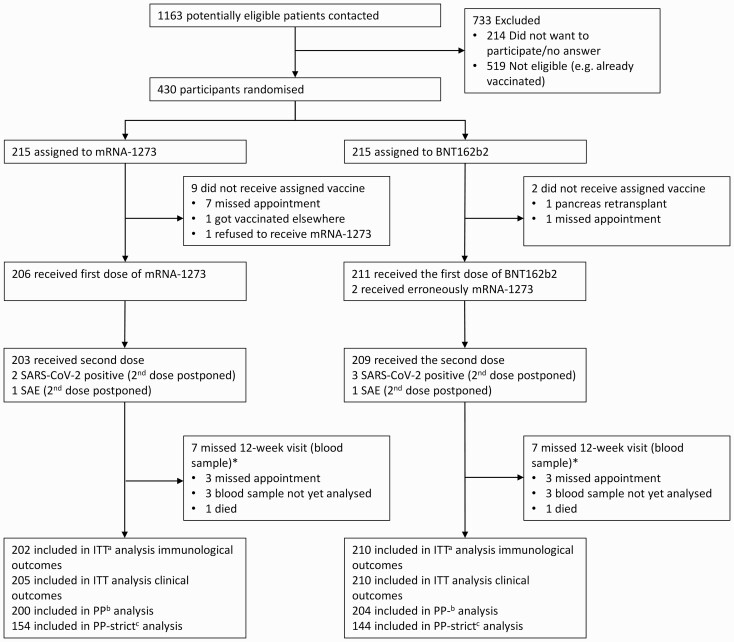
A total of 430 patients were randomized; of those, 419 received a first vaccination dose in the frame of this study between April 19 and June 9, 2021; 412 patients received a second dose. We included 412 patients in the intention-to-treat dataset for immunological outcomes, 415 in the intention-to-treat dataset for clinical outcomes, and 404 in the per-protocol dataset (Figure 1).
Figure 1.
Flow chart.
*3 patients who missed the study visits could be contacted by phone to assess clinical outcomes.
aIncluding all patients because they were randomized and have available outcome data.
bIncluding patients who received the intervention they were allocated to and have available outcome data.
cIncluding patients who received the intervention they were allocated to, with an interval of 4 weeks (± 1 week) between first and second vaccination dose and provided outcome data at 12 weeks (± 1 week). Results only presented within appendix.
Abbreviations: ITT, intention to treat; pp, per-protocol; SAE, serious adverse event.
Trial participants had a median age of 53 years (IQR: 43–61), the majority was male (75.8%; 326/430) and from the SHCS (81.9%; 352/430; Table 1). Of the 352 included PLWH, 2.0% (7/352) had CD4 cell counts below 200/µL and 5.7% (20/352) had an unsuppressed viral load (>50 copies/mL). Of the 78 organ transplant recipients, 79.5% (62/78) were on an intensive immunosuppressive therapy; 41 (52.6%) had received a lung and 37 (47.4%) a kidney transplantation. A total of 39 (9.1%) patients had a reactive antibody test to the nucleocapsid protein at baseline as determined by the Elecsys N test (mRNA-1273: 4.2%; 9/215; BNT162b2: 14.0%; 30/215) indicating a prior SARS-CoV-2 infection. Baseline data stratified by cohort are presented in the supplementary appendix (Table S1 and Table S2). The duration between the first and the second vaccine was a median of 28 days (IQR: 28–28) and the 12-week follow-up was conducted after a median of 84 days after the first vaccination (IQR: 84–86; Table S3).
Table 1.
Demographics and Clinical Characteristics of the Study Population at Baseline
| Characteristic | mRNA-1273 (Moderna) N = 215 | BNT162b2 (Pfizer-BioNTech) N = 215 | Total N = 430 |
|---|---|---|---|
| Median age (IQR) | 53 (43, 60) | 53 (43, 61) | 53 (43, 61) |
| Sex | |||
| Male | 161 (74.9%) | 165 (76.7%) | 326 (75.8%) |
| Female | 54 (25.1%) | 50 (23.3%) | 104 (24.2%) |
| Cohort | |||
| SHCS | 177 (82.3%) | 175 (81.4%) | 352 (81.9%) |
| STCS | 38 (17.7%) | 40 (18.6%) | 78 (18.1%) |
| Centers | |||
| University Hospital Basel | 77 (35.8%) | 81 (37.7%) | 158 (36.7%) |
| University Hospital Bern | 53 (24.7%) | 49 (22.8%) | 102 (23.7%) |
| University Hospital Zurich | 85 (39.5%) | 85 (39.5%) | 170 (39.5%) |
| History of cardiovascular disease or metabolic syndrome | |||
| Yes | 75/215 (34.9%) | 77/215 (35.8%) | 152/430 (35.3%) |
| No | 140/215 (65.1%) | 138/215 (64.2%) | 278/430 (64.7%) |
| CD4 cell count (cells/µL)a | |||
| <200 | 3/177 (1.7%) | 4/175 (2.3%) | 7/352 (2.0%) |
| 200–350 | 13/177 (7.3%) | 10/175 (5.7%) | 23/352 (6.5%) |
| 350–500 | 18/177 (10.2%) | 26/175 (14.9%) | 44/352 (12.5%) |
| >500 | 143/177 (80.8%) | 135/175 (77.1%) | 278/352 (79.0%) |
| Unsuppressed viral load (≥200 copies/mL)a | |||
| Yes | 7/177 (4.0%) | 13/175 (7.4%) | 20/352 (5.7%) |
| No | 170/177 (96.0%) | 162/175 (92.6%) | 332/352 (94.3%) |
| Transplanted organb | |||
| Lung transplant | 20/38 (52.6%) | 21/40 (52.5%) | 41/78 (52.6%) |
| Kidney transplant | 18/38 (47.4%) | 19/40 (47.5%) | 37/78 (47.4%) |
| Immunosuppressive therapyb | |||
| Less intense (<2 regimen) | 5/38 (13.2%) | 11/40 (27.5%) | 16/78 (20.5%) |
| Intense (3 or 4 regimen) | 33/38 (86.8%) | 29/40 (72.5%) | 62/78 (79.5%) |
| Antibody test to the nucleocapsid proteinc | |||
| Nonreactive | 197 (91.6%) | 181 (84.2%) | 378 (87.9%) |
| Reactive | 9 (4.2%) | 30 (14.0%) | 39 (9.1%) |
| Missing | 9 (4.2%) | 4 (1.9%) | 13 (3.0%) |
Abbreviations: IQR, interquartile range; SHCS, Swiss HIV Cohort Study; STCT, Swiss Transplant Cohort Study.
Only considering patients from the Swiss HIV Cohort Study.
Only considering patients from the Swiss Transplant Cohort Study.
Elecsys N test [14] reactive to nucleocapsid protein indicates previous contact to severe acute respiratory syndrome coronavirus 2.
Overall, 92.1% of participants randomized to mRNA-1273 (95% CI: 88.4–95.8; 186/202) had an antibody response (Elecsys S test) compared with 94.3% (95% CI: 91.2–97.4; 198/210) randomized to BNT162b2. With a difference of -2.2% (95% CI: -7.1 to 2.7), the vaccine mRNA-1273 from Moderna was noninferior to BNT162b2 from Pfizer-BioNTech (Table 2). This result was confirmed by the ABCORA 2 test for which a total of 89.3% (95% CI: 86.3–92.3; 368/412) had an antibody response (mRNA-1273: 89.1%; 95% CI: 84.8–93.4; 180/202 vs BNT162b2: 89.5%; 95% CI: 85.4–93.7; 188/210). When assessing the ABCORA 2 sum S1 threshold of 17, 83.5% (95% CI: 79.9–87.1; 344/412) had neutralizing antibodies (mRNA-1273: 84.7%; 95% CI: 79.7–89.6; 171/202 vs BNT162b2: 82.4%; 95% CI: 77.2–87.5; 173/210; Table S4). The analyses conducted on the per-protocol dataset were in line with the findings from the intention-to-treat dataset (Table 2 and Table S4). Although all PLWH (341/341) showed an immune response, only 60.6% (95% CI: 49.2–71.9; 43/71) of solid organ transplant recipients had an immune response (Elecsys S test). This number decreased to 39.4% (95% CI: 28.1–50.8; 28/71) among organ transplant recipients when using the more stringent ABCORA 2 test and to 21.1% (95% CI: 11.6–30.6; 15/71) when using the ABCORA 2 sum S1 threshold. Results from prespecified subgroup analyses (Tables S4–6) suggest that fewer patients with a lung transplant had an immune response (48.7%; 95% CI: 33.0–64.1; 19/39) compared with kidney transplant recipients (75.0%; 95% CI: 60.0–90.0; 24/32). Furthermore, 85.7% (95% CI: 67.4–100.0; 12/14) of transplant recipients with less intensive immunosuppressive therapy had an immune response, whereas this was only the case for 54.4% (95% CI: 41.5–67.3; 31/57) of transplant patients with an intensive immunosuppressive therapy. When using a cutoff of 100 U/mL for Elecsys S, the proportion of patients with an immune response decreased to 86.4% (95% CI: 83.1–89.7; 356/412) for all patients, 99.4% (95% CI: 98.6–100.0; 339/341) for PLWH, and 23.9% (95% CI: 14.0–33.9; 17/71) for transplant recipients (Tables S4–6). Sensitivity analyses excluding patients with a reactive antibody test to the protein at baseline were in line with the previously mentioned results (Tables S7–9).
Table 2.
Proportion of Patients With an Immune Response 12 Weeks After the First SARS-CoV-2 Vaccination
| SHCS and STCS | SHCS | STCS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mRNA-1273 (Moderna) |
BNT162b2 (Pfizer-BioNTech) | Total | Difference | mRNA-1273 (Moderna) |
BNT162b2 (Pfizer-BioNTech) | Total | mRNA-1273 (Moderna) |
BNT162b2 (Pfizer-BioNTech) | Total | |
| Intention to treat | ||||||||||
| Immune response (Elecsys S [14])a | 92.1% (88.4; 95.8%) 186/202 |
94.3% (91.2; 97.4%) 198/210 |
93.2% (90.8; 95.6%) 384/412 |
–2.2% (–7.1; 2.7%) | 100.0% (-) 169/169 |
100.0% (-) 172/172 |
100.0% (-) 341/341 |
51.5% (34.5; 68.6%) 17/33 |
68.4% (53.6; 83.2%) 26/38 |
60.6% (49.2; 71.9%) 43/71 |
| Immune response (ABCORA2 [15]) | 89.1% (84.8; 93.4%) 180/202 |
89.5% (85.4; 93.7%) 188/210 |
89.3% (86.3; 92.3%) 368/412 |
–0.4% (–6.4;5.6%) |
100.0% (-)169/169 |
99.4% (98.3; 100.0%) 171/172 |
99.7% (99.1–100.0) 340/341 |
33.3% (17.3; 49.4%) 11/33 |
44.7% (28.9; 60.6%) 17/38 |
39.4% (28.1; 50.8%) 28/71 |
| Per-protocolb | ||||||||||
| Immune response (Elecsys S [14])a | 92.0% (88.2; 95.8%) 184/200 |
94.6% (91.5; 97.7%) 193/204 |
93.3% (90.9; 95.8%) 377/404 |
–2.6% (–7.5;2.3%) | 100.0% (-) 168/168 |
100.0% (-) 170/170 |
100.0% (-) 338/338 |
50.0% (32.7; 67.3%) 16/32 |
67.7% (51.9; 83.4%) 23/34 |
59.1% (47.2; 71.0%) 39/66 |
| Immune response (ABCORA 2 [15]) | 89.0% (84.7; 93.3%) 178/200 |
89.7% (85.5; 93.9%) 183/204 |
89.4% (86.4; 92.4%) 361/404 |
–0.7% (–6.7; 5.3%) |
100.0% (-) 168/168 |
99.4% (98.3; 100.0%) 169/170 |
99.7% (99.1; 100.0%) 337/338 |
31.3% (15.2; 47.3%) 10/32 |
41.2% (24.6; 57.7%) 14/34 |
36.4% (24.8; 48.0%) 24/66 |
Sensitivity analysis for the per-protocol estimate, including only patients who received the intervention they were allocated to, with an interval of 4 weeks (± 1 week) between first and second vaccination dose and provided outcome data at 12 weeks (± 1 week) is available in the Supplementary Appendix.
Abbreviations: ABCORA 2, Antibody Coronavirus Assay 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SHCS, Swiss HIV Cohort Study; STCT, Swiss Transplant Cohort Study.
Using the threshold of at 0.8 U/mL.
Including patients who received the intervention they were allocated to and have available outcome data.
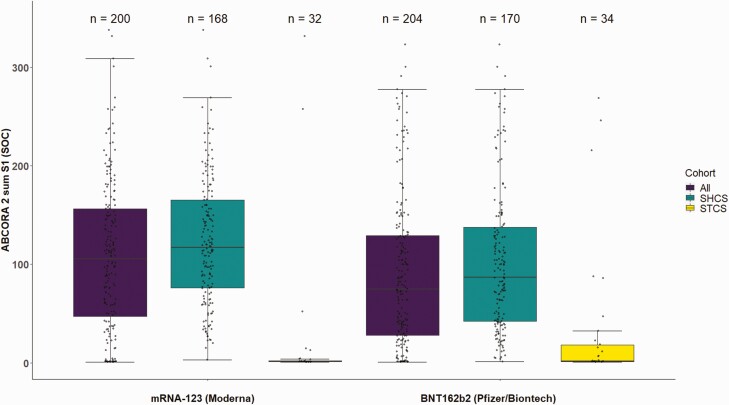
Based on per-protocol data, mean ABCORA 2 sum S1 levels were 107.2 (95% CI: 96.7–117.9) for mRNA-1273 and 90.6 (95% CI: 80.0–101.3) for BNT162b2 (Figure 2, Table S10; per-protocol strict in Table S11). Results for Elecsys S titer levels are presented in the appendix (Figure S1; Table S12). For PLWH, the ABCORA 2 sum S1 titers were 123.5 (95% CI: 113.5–133.4) and 102.3 (95% CI: 91.2–113.4) for mRNA-1273 and BNT162b2, respectively, and the proportion of patients with a neutralization activity defined by the ABCORA 2 sum S1 threshold was higher with mRNA-1273 (98.8%; 95% CI: 97.2–100.0; 176/169) compared with BNT162b2 (94.2%; 95% CI: 90.7–97.7; 176/169; Table S5). Organ transplant recipients mean ABCORA 2 sum S1 levels were 22.1 (95% CI: 0.0–48.4) after receiving mRNA-1273 and 32.1 (95% CI: 7.5–56.7) following vaccination with BNT162b2 (Figure 2, Table S10), and the proportion of patients with a neutralization activity (ABCORA 2 sum S1) was 12.1% (95% CI: 1.0–23.4; 4/33) for mRNA-1273 and 29.0% (95% CI: 14.0–43.4; 11/38) for BNT162b2; Table S5)
Figure 2.
Antibody response in immunocompromised patients after receiving 2 doses of SARS-CoV-2 vaccines (per-protocol data set) using ABCORA 2 [15].
Figure shows combined reactivity of IgM, IgA, and IgG to the subunit S1 in patients who received the allocation they were randomized to and provided a blood sample at follow-up (per-protocol). Depicted are sum S1 (sum of S1 signal over cutoff values IgG, IgA, IgM) off all patients, patients from Swiss HIV Cohort Study (SHCS), Swiss Transplant Cohort Study (STCS). Box plots indicate the interquartile ranges with vertical lines representing the minimum and maximum values. ABCORA 2, Antibody Coronavirus Assay 2; Ig, immunoglobulin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
At 12-week follow-up, a total of 5 patients reported that they tested SARS-CoV-2 positive (all before receiving the second dose of vaccine). No severe COVID-19 infections occurred, and no household members were reported as SARS-CoV-2 positive (Table 3). Symptoms limiting the normal daily activities occurred frequently after the second vaccination and systemic symptoms appeared more frequently after the second dose of mRNA-1273 (21.8%; 95% CI: 16.3–28.1; 44/202) compared with vaccination with BNT162b2 (10.7%; 6.8–15.8; 22/205). A total of 18 patients had at least 1 SAE requiring hospitalization and 2 of these patients died. None of the SAEs were classified as clearly related to study medication (see judgment from treating physicians and data safety monitoring board in Table S13). The clinical outcomes reported separately for the SHCS and the STCS are listed in Tables S14 and S15.
Table 3.
Clinical Outcomes and Adverse Events
| Outcomes | mRNA-1273 (Moderna) | BNT162b2 (Pfizer-BioNTech) | Total |
|---|---|---|---|
| Confirmed SARS-CoV-2 infection | 2/205c (1.0%; 0.0–2.3%) | 3/210c (1.4%; 0.0–3.0%) | 5/415c (1.2%; 0.2–2.3%) |
| Symptomatic | 2/205 (1.0%; 0.0–2.3%) | 1/210 (0.5%; 0.0–1.4%) | 3/415 (0.7%; 0.0–1.5%) |
| Asymptomatic | 0/205 (0.0%) | 2/210 (1.0%; 0.1–3.4%) | 2/415 (0.5%; 0.0–1.1%) |
| Severe COVID-19 infection | 0/205 (0.0%) | 0/210 (0.0%) | 0/415 (0.0%) |
| COVID-19 burden of diseasea (mean, SD) | 0.010 (0.099 SD) | 0.014 (0.118 SD) | 0.012 (0.109 SD) |
| Confirmed SARS-COV-2 infection of household members | 0/205 (0.0%) | 0/210 (0.0%) | 0/415 (0.0%) |
| Safety outcomes after first vaccine | |||
| Any local symptoms limiting continuation of normal daily activities during the first 7 days | 13/205 (6.3%; 3.0–9.7%) | 14/210 (6.7%; 3.3–10.0%) | 27/415 (6.5%; 4.1–8.9%) |
| Any systemic symptoms limiting continuation of normal daily activities during the first 7 days | 14/205 (6.8%; 3.4–10.3%) | 12/210 (5.7%; 2.6–8.9%) | 26/415 (6.3%; 3.9–8.6%) |
| Any vaccine related symptom leading to contacting a physician during the first 7 days | 2/205 (1.0%; 0.0–2.3%) | 1/210 (0.5%; 0.0–1.4%) | 3/415 (0.7%; 0.0–1.5%) |
| Safety outcomes after second vaccine | |||
| Any local symptoms limiting continuation of normal daily activities during the first 7 days | 18/202 (8.9%; 5.0–12.8%) | 13/205 (6.3%; 3.0–9.7%) | 31/407 (7.6%; 5.0–10.2%) |
| Any systemic symptoms limiting continuation of normal daily activities during the first 7 days | 44/202 (21.8%; 16.1–27.5%) | 22/205 (10.7%; 6.5–15.0%) | 66/407 (16.2%; 12.6–19.8%) |
| Any vaccine related symptom leading to contacting a physician during the first 7 days | 3/202 (1.5%; 0.0–3.2%) | 2/205 (1.0%; 0.0–2.3%) | 5/407 (1.2%; 0.2–2.3%) |
| Serious adverse eventsb | 9/205 (4.4%; 1.6–7.2%) | 9/210 (4.3%; 1.5–7.0%) | 18/415 (4.3%; 2.4–6.3%) |
| Patient died | 1/205 (0.5%; 0.0–1.4%) | 1/210 (0.5%; 0.0–1.4%) | 2/415 (0.5%; 0.0–1.1%) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
The burden of disease was judged as 0 for no SARS-CoV-2 infection, 1 for nonsevere SARS-CoV-2 infections and 2 for severe SARS-CoV-2 infections.
All infections occurred after the first vaccination, but before the second vaccine was administered.
All infections occurred after the first vaccination, but before the second vaccine was administered.
DISCUSSION
This randomized head-to-head comparison showed noninferiority of the mRNA-1273 (Moderna) compared with the BNT162b2 (Pfizer-BioNTech) vaccine in terms of antibody response at 12 weeks. An antibody response was seen in the majority of the included patients independently of the antibody test (Elecsys S [14] or ABCORA 2 [15]). Although all patients from the HIV cohort (with 1 exception using the ABCORA 2 test) had an immune response after vaccination, only 61% of the patients from the transplant cohort had an immune response using the Elecsys S test. An ABCORA 2 sum S1 value >17 allowed the prediction of whether sera harbor neutralization activity was based on a previous established algorithm [15]. Based on this analysis, we found that nearly 80% of organ transplant recipients had with high certainty not developed neutralization activity.
Our results confirmed the findings from a published observational study reporting that immune response in solid organ transplant recipients was detectable in 54% of patients (357/658) [22]. A randomized clinical trial conducted by Hall et al has shown that solid organ transplant recipients have a higher immune response after a third SARS-CoV-2 vaccination; hence, a third vaccine should be considered in this population [19]. Two recently published case reports in 14 and 12 virologically suppressed PLWHs found high antibody titers after the second vaccination with mRNA-1273 [23, 24]. These results are in line with our findings, which provide now more robust evidence for mRNA-1273 and for BNT162b2 in PLWH with a suppressed viral load.
Current research indicates that the immune response is stronger in immunocompetent individuals when applying the mRNA-1273 vaccine (Moderna) compared with BNT162b2 (Pfizer-BioNTech) because of its higher mRNA content and the longer interval (4 vs 3 weeks) [25]. Our conducted post hoc analysis found more PLWHs with neutralization activity (according to ABCORA 2 sum S1) after receiving mRNA-1273 compared with BNT162b2. In addition, the assessed titer levels were somewhat higher with mRNA-1273 by Moderna (Figure 2), but because of the large variance in the data (ie, 95% CI), we cannot conclusively confirm a difference between the 2 vaccines. A retrospective study in more than 50 000 vaccinated individuals found fewer breakthrough infections when the mRNA-1273 vaccine was used [26]. Further high-quality evidence is needed to assess if the mRNA-1273 might be superior for specific clinical endpoints (eg, severe COVID-19, mortality).
The study has the following limitations. First, sample size for transplant patients and for subgroup analyses was small. Therefore, suggested differences (eg, between kidney and lung transplant patients) are of exploratory nature and have to be interpreted carefully. Second, the prespecified neutralization cutoff for the primary outcome (ie, of ≥0.8 U/mL) was chosen when little information was available. Now, it is unclear how useful this cutoff from the Elecsys S test is in terms of predictive resistance to infection. Although other studies took the same cutoff (ie, of ≥0.8 U/mL) [23, 27], Hall et al chose a cutoff of 100 U/mL. We believe that by including a second high-quality test (ABCORA 2 antibody response and sum S1) as well as assessing the results with a cutoff of 100 U/mL allows us to make a sensible interpretation of study results. However, these cutoffs will have to be further adjusted in the future (ie, to account for SARS-CoV-2 variants of concern).
In conclusion, the proportion of patients with an immune response was comparable between mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech). In general, PLWH had a good immune response, whereas solid organ transplant patients had a low immune response. These patients should be prioritized when third (“booster”) vaccines are administered.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Members of the Swiss HIV Cohort Study. Abela IA, Aebi-Popp K, Anagnostopoulos A, Battegay M, Bernasconi E, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H, Fux CA, Günthard HF (President of the SHCS), Hachfeld A, Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Huber M, Kahlert CR (chair of the Mother & Child Substudy), Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Kusejko K (head of the data center), Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nemeth J, Nicca D, Paioni P, Pantaleo G, Perreau M, Rauch A (chair of the Scientific Board), Schmid P, Speck R, Stöckle M (chair of the Clinical and Laboratory Committee), Tarr P, Trkola A, Wandeler G, Yerly S.
Members of the Swiss Transplant Cohort Study. Patrizia Amico, Andres Axel, John-David Aubert, Vanessa Banz, Beckmann Sonja, Guido Beldi, Christoph Berger, Ekaterine Berishvili, Isabelle Binet, Pierre-Yves Bochud, Sanda Branca, Heiner C. Bucher, Thierry Carrel, Emmanuelle Catana, Yves Chalandon, Sabina De Geest, Olivier De Rougemont, Michael Dickenmann, Joëlle Lynn Dreifuss, Michel Duchosal, Thomas Fehr, Sylvie Ferrari-Lacraz, Nicola Franscini, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Déla Golshayan, Nicolas Goossens, Karine Hadaya, Jörg Halter, Dominik Heim, Christoph Hess, Sven Hillinger, Hans Hirsch, Patricia Hirt, Günther Hofbauer, Uyen Huynh-Do, Franz Immer, Michael Koller (head of the data center), Mirjam Laager, Bettina Laesser, Roger Lehmann, Alexander Leichtle, Christian Lovis, Oriol Manuel, Hans-Peter Marti, Pierre Yves Martin, Michele Martinelli, Valérie McLin, Katell Mellac, Aurélia Merçay, Karin Mettler, Nicolas Mueller (chair Scientific Committee), Antonia Müller, Ulrike Müller-Arndt, Beat Müllhaupt, Mirjam Nägeli, Graziano Oldani, Manuel Pascual (executive office), Klara Posfay-Barbe, Juliane Rick, Anne Rosselet, Simona Rossi, Silvia Rothlin, Frank Ruschitzka, Thomas Schachtner, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Macé Schuurmans, Thierry Sengstag, Federico Simonetta, Susanne Stampf, Jürg Steiger (head, executive office), Guido Stirniman, Ueli Stürzinger, Christian Van Delden (executive office), Jean-Pierre Venetz, Jean Villard, Julien Vionnet, Madeleine Wick (STCS coordinator), Markus Wilhlem, Patrick Yerly
Notes
Author contributions. H. C. B., B. S., F. C., N. J. M., H. F. G., M. P. S., A. R., M. T., M. T. K., M. B., and K. K. designed the study. K. K. was responsible for preparing the data platform; F. C. conducted sample size calculation and all analyses. B. S., H. C. B., P. A., M. P. S., A. L. E., B. H., D. L. B., M. M. S., T. F. M., M. T., A. R., and H. F. G. coordinated patient recruitment and follow-up at local centers. I. A. A., A. T., and A. A. conducted all laboratory analyses; B. S. wrote the first draft of the manuscript, and all authors read and approved the final version of the manuscript.
Acknowledgments. The authors thank all cohort patients who voluntarily participated in this study. They also thank the members of the data safety monitoring board (Pietro Vernazza, Andreas F. Widmer, and Tracy Glass). They are grateful for all work conducted at the local centers in Basel (Erol Cetinkaya, Patricia Francois, Diana Grauwiler, Alexandra Griessbach, Lars Hemkens, Hans H. Hirsch, Tobias Kunz, Maria Pascarella, Rebekka Platter, Louise Seiler, Ala Taji Heravi), Zürich (Daniela Gsell, Nino Scherrer, Herbert Kuster, Laura Tschuor, Rosita Nujic, Christina Grube, Flurina Brunschweiler, Rheliana Katzensteiner, Christine Schneider, Claudia Schmidt, Dominik Damm, Fabian Knörr), Bern (Daniela Hirter, Manuela Correia da Silva Saúde, Pia Scherler, Anna Rehbock), and the Institute of Medical Virology, University of Zürich (Selina Epp, Lorena Mancini). They thank Markus Born Tiago Stämpfli from the Impfzentrum Basel-Stadt and Esther Ammann, Kantonsapotheke Basel-Stadt for their support for setting up the vaccination center at the University Hospital Basel. Furthermore, the authors are grateful to Daniel Smith from the coordination and data center of the Swiss HIV Cohort Study for his support in setting up the trial platform. In addition, they thank the ethical committee Nordwest- and Zentralschweiz, Switzerland, for its fast feedback, allowing us to start this trial in a timely manner.
Financial support. This study received financial support from the Swiss National Science Foundation (grant 31CA30_196245). The Swiss HIV Cohort Study (SHCS) and the Swiss Transplant Cohort Study (STCS) are funded by the Swiss National Science Foundation (SHCS: grants 177499 and 201369, STCS: grant 33CS30_177522). The diagnostic work was supported by a grant of the Pandemiefonds of the University of Zurich Foundation to A. T. The Promedica Foundation (grant 14851M) is supporting I.A.A. with a research grant. B. S. is supported by an Advanced Postdoc.Mobility grant (grant P300PB_177933) and a return grant (grant P4P4PM_194496) from the Swiss National Science Foundation. Roche Switzerland provided the antibody tests (Elecsys Anti-SARS-CoV-2) free of charge. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. H. C. B. has received in the 36 months before the submission of this manuscript grants, support for traveling, consultancy fees, and honorarium from Gilead, BMS, Viiv Healthcare, Roche, and Pfizer that were not related to this project. He serves as the president of the Association contre le HIV et autres infections transmissibles. In this function, he has received support for the Swiss HIV Cohort Study from ViiV Healthcare, Gilead, BMS, and MSD. He also reports participation on a Data Safety Monitory Board or Advisory Board for Gilead Viiv Health Care and receipt of funding for consultancy services for his institution from Roche. A. T. received a consultant fee from Roche and Neuroimmune; has received unrestricted research funding from Gilead and Roche not related to this study; reports grants or contract unrelated to this study from the Swiss National Science Foundation, Swiss Federal Office of Public Health, Gilead COVID grant initiative, Pandemiefonds of the UZH Foundation, Swiss Red Cross “Glückskette” Corona Funding, and the University Hospital Zurich, Innovation Pool Project; has received payment or honoraria from Schweizer Lungen Liga for a COVID lecture unrelated to the study; participated on a Data Safety Monitory Board or Advisory Board for Neuroimmune; and received material for COVID-19 diagnostics evaluation unrelated to the study from Roche. D. L. B. received honoraria for advisory boards from the companies Gilead, MSD, and ViiV; consulting fees for participation on advisory boards for Gilead, ViiV, and Merck; and payment or honoraria for lectures from Gilead, Merck, ViiV, and Abbvie outside of the study. H. F. G., outside of this study, reports grants from the Swiss National Science Foundation, National Institutes of Health (NIH), and the Swiss HIV Cohort Study; unrestricted research grants from Gilead Sciences, Roche, and Yvonne Jacob Foundation; personal fees from consulting or advisory boards or data safety monitoring boards for Merck, Gilead Sciences, ViiV Healthcare, Mepha, Janssen, Novartis, and Sandoz; and payment for lectures, presentations, speakers bureaus, manuscript writing, or education events from Medscape. H. F. G.’s institution received money for participation in the following clinical COVID-19 studies: 540-7773/5774 (Gilead), TICO (ACTIV-3, INSIGHT/NIH), and the Morningsky study (Roche). A. R. reports support for attending meetings and/or travel paid to his institution by Gilead Sciences and Pfizer and participation on a Data Safety Monitory Board or Advisory Board for MSD and Gilead Sciences. N. J. M. reports grants or contracts unrelated to the study from the Swiss Transplant Cohort Study, payment for expert testimony from the Federal Office of Public Health, Switzerland, support for attending meetings and/or travel from Biotest, participation on a Data Safety Monitoring Board or Advisory Board for Takeda and MSD, and is a board member of the Swiss Society for Infectious Diseases. A. A. reports receipt of Roche reagents for the analyses on cobas e 411. M. S. reports conference grants from Gilead and MSD; participation on an Advisory Board for Moderna, Pfizer, MSD, ViiV, Gilead, and Mepha; and is chair of the clinic and laboratory committee of the Swiss HIV cohort study. All other authors have declared that no competing interests exist. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Benjamin Speich, Basel Institute for Clinical Epidemiology and Biostatistics, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland; Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom.
Frédérique Chammartin, Basel Institute for Clinical Epidemiology and Biostatistics, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Irene A Abela, University of Zurich, Institute of Medical Virology, Zurich, Switzerland; Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Patrizia Amico, Clinic for Transplantation Immunology and Nephrology, University Hospital Basel, University of Basel, Basel, Switzerland.
Marcel P Stoeckle, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, University of Basel, Switzerland.
Anna L Eichenberger, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Barbara Hasse, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Dominique L Braun, University of Zurich, Institute of Medical Virology, Zurich, Switzerland; Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Macé M Schuurmans, Division of Pulmonology, University Hospital Zurich, Zurich, Switzerland.
Thomas F Müller, Nephrology Clinic, University Hospital Zurich, Zürich, Switzerland.
Michael Tamm, Clinic of Respiratory Medicine and Pulmonary Cell Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Annette Audigé, University of Zurich, Institute of Medical Virology, Zurich, Switzerland.
Nicolas J Mueller, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Andri Rauch, Department of Infectious Diseases, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Huldrych F Günthard, University of Zurich, Institute of Medical Virology, Zurich, Switzerland; Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Michael T Koller, Swiss Transplant Cohorts Study, University Hospital Basel, University of Basel, Basel, Switzerlandand.
Alexandra Trkola, University of Zurich, Institute of Medical Virology, Zurich, Switzerland.
Matthias Briel, Basel Institute for Clinical Epidemiology and Biostatistics, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland; Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Canada.
Katharina Kusejko, University of Zurich, Institute of Medical Virology, Zurich, Switzerland; Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland.
Heiner C Bucher, Basel Institute for Clinical Epidemiology and Biostatistics, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Swiss HIV Cohort Study and the Swiss Transplant Cohort Study:
I A Aebi-Popp, K Anagnostopoulos, A Battegay, M Bernasconi, E Braun, D L Bucher, H C Calmy, A Cavassini, M Ciuffi, A Dollenmaier, G Egger, M Elzi, L Fehr, J Fellay, J Furrer, H Fux, C A Günthard, A Haerry, B Hirsch, H H Hoffmann, M Hösli, I Huber, M Kahlert, L Keiser, O Klimkait, T Kouyos, R D Kovari, H Kusejko, G Martinez de Tejada, B Marzolini, C Metzner, K J Müller, N Nemeth, J Nicca, D Paioni, P Pantaleo, G Perreau, M Rauch, P Speck, R Stöckle, P Trkola, A Wandeler, G Yerly, Patrizia Amico, Andres Axel, John David Aubert, Vanessa Banz, Beckmann Sonja, Guido Beldi, Christoph Berger, Ekaterine Berishvili, Isabelle Binet, Pierre Yves Bochud, Sanda Branca, Heiner C Bucher, Thierry Carrel, Emmanuelle Catana, Yves Chalandon, Sabina De Geest, Olivier De Rougemont, Michael Dickenmann, Joëlle Lynn Dreifuss, Michel Duchosal, Thomas Fehr, Sylvie Ferrari-Lacraz, Nicola Franscini, Christian Garzoni, Paola Gasche Soccal, Christophe Gaudet, Déla Golshayan, Nicolas Goossens, Karine Hadaya, Jörg Halter, Dominik Heim, Christoph Hess, Sven Hillinger, Hans Hirsch, Patricia Hirt, Günther Hofbauer, Uyen Huynh-Do, Franz Immer, Michael Koller, Mirjam Laager, Bettina Laesser, Roger Lehmann, Alexander Leichtle, Christian Lovis, Oriol Manuel, Hans Peter Marti, Pierre Yves Martin, Michele Martinelli, Valérie McLin, Katell Mellac, Aurélia Merçay, Karin Mettler, Nicolas Mueller, Antonia Müller, Ulrike Müller-Arndt, Beat Müllhaupt, Mirjam Nägeli, Graziano Oldani, Manuel Pascual, Klara Posfay-Barbe, Juliane Rick, Anne Rosselet, Simona Rossi, Silvia Rothlin, Frank Ruschitzka, Thomas Schachtner, Urs Schanz, Stefan Schaub, Aurelia Schnyder, Macé Schuurmans, Thierry Sengstag, Federico Simonetta, Susanne Stampf, Jürg Steiger, Guido Stirniman, Ueli Stürzinger, Christian Van Delden, Jean Pierre Venetz, Jean Villard, Julien Vionnet, Madeleine Wick, Markus Wilhlem, and Patrick Yerly
References
- 1. WHO. Pneumonia of unknown cause – China. WHO. https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229. Accessed: 16 March 2022.
- 2. Wang C, Horby PW, Hayden FG, Gao GF.. A novel coronavirus outbreak of global health concern. Lancet 2020; 395:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed: 14 February 2022.
- 4. Federal Office of Public Health. Coronavirus: vaccination. https://www.bag.admin.ch/bag/en/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/impfen.html#288501448 Accessed: 14 Feburary 2022.
- 5. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scherrer AU, Traytel A, Braun DL, et al. Cohort profile update: the Swiss HIV Cohort Study (SHCS). Int J Epidemiol 2022; 51(1):33–4j. [DOI] [PubMed] [Google Scholar]
- 8. Koller MT, van Delden C, Muller NJ, et al. Design and methodology of the Swiss Transplant Cohort Study (STCS): a comprehensive prospective nationwide long-term follow-up cohort. Eur J Epidemiol 2013; 28:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Speich B, Chammartin F, Smith D, et al. A trial platform to assess approved SARS-CoV-2 vaccines in immunocompromised patients: first sub-protocol for a pilot trial comparing the mRNA vaccines Comirnaty(R) and COVID-19 mRNA Vaccine Moderna(R). Trials 2021; 22:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: BUILDING an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA briefing document: Moderna COVID-19 vaccine. vaccines and related biological products advisory committee meeting 17 December 2020. https://www.fda.gov/media/144434/download Accessed: 14 February 2022.
- 12. Centers for Disease Control and Prevention (CDC). Pfizer-BioNTech COVID-19 vaccine. Storage and handling summary. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/downloads/storage-summary.pdf Accessed: 14 February 2022.
- 13. European Medicines Agency (EMA). COVID-19 vaccine Moderna. Annex 1. https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf Accessed: 14 February 2022.
- 14. Roche. Elecsys®Covid-19 solutions. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html Accessed: 14 February 2022.
- 15. Abela IA, Pasin C, Schwarzmuller M, et al. Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses responses to SARS-CoV-2 immunity. Nat Commun 2021; 12:6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020; 586:589–93. [DOI] [PubMed] [Google Scholar]
- 17. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021; 384:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chow S, Shao J, Wang H.. Sample size calculations in clinical research. 2nd ed. Chapman & Hall/CRC Biostatistics Series; 2008. [Google Scholar]
- 19. Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 21. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. Available at: https://www.R-project.org/. Accessed: 16 March 2022. [Google Scholar]
- 22. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325(21):2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021; 35(14):2399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with HIV. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L.. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021; 326(15):1533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv 2021. [Google Scholar]
- 27. Strauss AT, Hallett AM, Boyarsky BJ, et al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl 2021; 27(12):1852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.