ABSTRACT
Background
To investigate the anti-spike antibody response to vaccination in kidney transplant recipients (KTRs) previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as compared with KTRs with no history of coronavirus disease 2019 (COVID-19) from India.
Methods
SARS-CoV-2 spike immunoglobulin (Ig) G antibody response was measured in 105 post-COVID-19 KTRs with PCR-confirmed SARS-CoV-2 infection who received either no vaccination (cohort 1), a single dose (cohort 2) or two doses (cohort 3) of vaccine and compared with 103 two-dose vaccinated COVID-19-naïve KTRs with no history of COVID-19 (cohort 4).
Results
Out of 103 COVID-19-naïve two-dose vaccinated KTRs, <50% became seropositive with anti-spike antibody titres >50 arbitrary unit/mL subsequent to complete vaccination, the seroconversion rate being comparable in subjects receiving CovishieldTM versus CovaxinTM vaccines. However, the seropositive KTRs vaccinated with CovishieldTM had higher anti-spike antibody titres as compared with those who received CovaxinTM. We observed higher anti-SARS-CoV-2 spike antibody levels in post-COVID-19 KTRs after one dose of vaccine as compared with COVID-19-naïve two-dose vaccinated KTRs. Importantly, the second dose in post-COVID-19 KTRs did not significantly increase anti-spike antibody levels compared with the single-dose recipients.
Conclusions
Our data present that in KTRs with previous SARS-CoV-2 infection, a single dose of vaccine (CovishieldTM) may be effective in mounting an optimal immune response. In contrast, COVID-19-naïve two-dose vaccinated KTRs respond poorly (<50%) to the current recommendation of a two-dose regimen in India.
Keywords: anti-spike antibody, COVID-19, kidney transplant recipients, previously infected, SARS-CoV-2
INTRODUCTION
Kidney transplant recipients (KTRs) are at an elevated risk of developing severe coronavirus disease 2019 (COVID-19) [1]. Studies have demonstrated increased morbidity and mortality in transplant patients [1–17]. In the absence of a definitive cure for COVID-19, vaccines are perhaps the most promising option available to control the pandemic. There are several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines currently available whose immunogenicity and safety have been assessed in various clinical trials [18]. However, no vaccine trial included transplant recipients. Recent investigations demonstrate that even though mRNA vaccines induce robust immune response in non-transplant individuals protecting against severe COVID-19, KTRs develop significantly lower antibody response post-vaccination [19–30]. In contrast, studies evaluating the serologic response of transplant recipients to COVID-19 infection provide conflicting results reporting normal levels of anti-SARS-CoV-2 antibodies in KTRs subsequent to past COVID-19 infection [31–33]. However, the majority of these studies explored the immune response to mRNA vaccines, currently not available in India; similar data following immunization with vaccines approved in India are not available. Importantly, the dynamics of vaccination after natural infection in transplant recipients remain unexplored. In this study, we investigated the spectrum of antibody responses to SARS-CoV-2 in a cohort of KTRs with different vaccination status.
MATERIALS AND METHODS
Study design and population
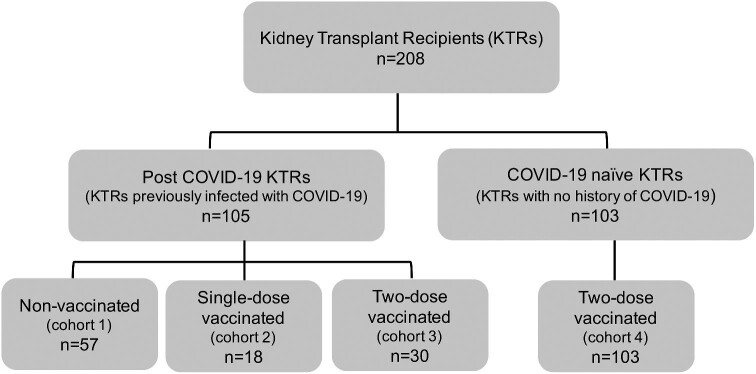
SARS-CoV-2 anti-spike IgG antibody titres were assessed in 208 KTRs, treated at a tertiary care hospital in New Delhi, India between 1 April 2020 and 30 November 2021. Out of the 208 KTRs, 105 KTRs were previously infected with COVID-19 (confirmed with SARS-CoV-2 real-time reverse transcription polymerase chain reaction) and had not received convalescent plasma during treatment. The 105 KTRs were either not vaccinated (referred to as ‘post-COVID-19 non-vaccinated’) or received a single dose (referred to as ‘post-COVID-19 single-dose vaccinated’) or both doses (referred to as ‘post-COVID-19 two-dose vaccinated’) of the approved vaccines, CovishieldTM (ChAdOx1-nCOV or AZD1222, Oxford-AstraZeneca, manufactured by Serum Institute of India, Pune, India) and CovaxinTM [BBV-152, manufactured by Bharat Biotech, Hyderabad, in collaboration with Indian Council of Medical Research (ICMR), India] subsequent to their recovery from COVID-19. The remaining 103 KTRs with no history of COVID-19 were fully vaccinated with two doses of either of the approved vaccines (referred to as ‘COVID-19-naïve two-dose vaccinated’). The distribution of study cohorts is summarized in Figure 1. Necessary institutional approvals were secured for carrying out the data analysis and manuscript development.
Figure 1:
Details of the four study cohorts of KTRs based on COVID-19 infection and vaccinations.
Data collection
Data were collected retrospectively from the medical records of the hospitals’ or patients’ follow-up submissions. Clinical data collected included demographics (age, height, weight, sex, duration) from transplant to COVID-19, comorbidities, baseline immunosuppression regimen and details of vaccination.
Outcomes
The primary objective of this study was to quantitatively evaluate the SARS-CoV-2 anti-spike IgG antibody response in previously infected KTRs with respect to their vaccination status, comparing with fully vaccinated uninfected KTRs. The secondary outcomes included evaluating the association and correlation of anti-spike antibody levels with comorbidities and other baseline transplant characteristics.
Anti-spike IgG antibody evaluation
Anti-spike IgG antibodies to SARS-CoV-2 were assayed with the AdviseDx SARS-CoV-2 IgG II assay (Abbott Diagnostics, Chicago, IL, USA) using a chemiluminescent microparticle immunoassay intended for the qualitative and semi-quantitative detection of IgG antibodies to SARS-CoV-2 in human serum and plasma on the Alinity i system (Abbott Diagnostics, Chicago, IL, USA). The analytical measurement interval is stated as 22–40 000 arbitrary unit (AU)/mL, and the positivity cut-off is ≥50 AU/mL (manufacturer defined). According to the manufacturer, the observed limit of quantification on the Alinity i system was 7.2 AU/mL, representing the lowest concentration at which a maximum allowable precision was met. The observed limit of detection (LoD) on the Alinity i system was 4.8 AU/mL and represents the lowest concentration at which the analytes can be detected [34]. However, in real-world reports generated in the laboratory, low values are reported as undetectable or numeric values <7.2 AU/mL. Undetectable reports are considered zero (0.0) for calculation purposes.
These tests were conducted 10–45 weeks post onset of COVID-19 infection or 10–120 days since the last dose of vaccination. Samples were collected as either serum or plasma using EDTA vials from each participant and analysed at Dr Lal's Path Lab, New Delhi, India.
Statistical analysis
Data were tabulated using Microsoft Excel, imported to SPSS statistical software version 16.0 (SPSS Inc., Chicago, IL, USA) for analysis, and R-software version 3.6.1 was applied for determining the median difference 95% confidence interval. The continuous variables were summarized as mean ± standard deviation (SD) and median [inter-quartile range (IQR)]. The qualitative variables were reported with number and percentage.
To compare normally distributed continuous variables among the cohorts, a one-way ANOVA followed by a Tukey's test was performed. Skewed distributed variables were tested using the non-parametric Kurskal–Wallis followed by the Mann–Whintney U-test, and P-values were adjusted as per the Bonferroni correction. For comparing qualitative variables among the cohorts, the chi-squared test was applied. The unpaired Student's t-test, the Mann–Whitney U-test and the simple logistic regression were performed to find the association between responders and non-responders with regard to demographic and other clinical variables. We applied Spearman’s correlation to find the strength of association between anti-spike antibody titres and other continuous variables. The Bonferroni correction was applied, keeping the small sample size in consideration and multiple variable testing. P-value <0.05 was considered as significant.
RESULTS
Demographics, comorbidities and baseline transplant characteristics of study cohorts
The study subjects were categorized into four cohorts based on their COVID-19 infection and vaccination status (Figure 1). A total of 208 KTRs were included in the study, out of which 105 KTRs were infected in the past with COVID-19 and 103 KTRs remained uninfected. Amongst the previously infected 105 KTRs, 57 patients were non-vaccinated (cohort 1: post-COVID-19 non-vaccinated), whereas 18 patients received only one dose (cohort 2: post-COVID-19 single-dose vaccinated) and 30 KTRs received both vaccination doses (cohort 3: post-COVID-19 two-dose vaccinated) of either of the approved vaccines. The 103 uninfected KTRs were fully vaccinated with the recommended two-dose regimen of the approved vaccines in India (cohort 4: COVID-19-naïve two-dose vaccinated). Table 1 summarizes the demographics, baseline characteristics, comorbidities and vaccination details of the four study cohorts. No significant difference was observed between the cohorts with respect to mean weight, height, gender and median time interval from transplant to COVID-19 and laboratory investigations. There was a significant difference observed in the average age of the KTRs between the four cohorts. The average age of the post-COVID-19 two-dose vaccinated KTRs (54.70 ± 11.35 years) was significantly higher than the post-COVID-19 non-vaccinated (44.11 ± 12.63 years; P = 0.001) and COVID-19-naïve two-dose vaccinated KTRs (45.91 ± 12.21 years; P = 0.004).
Table 1.
Demographics and baseline characteristics of COVID-19-naïve and post-COVID-19 KTRs based on their vaccination status
| Post-COVID-19 KTRs | COVID-19-naïve | |||||
|---|---|---|---|---|---|---|
| Non-vaccinated N = 57 | Single-dose vaccinated n = 18 | Two-dose vaccinated n = 30 | Two-dose vaccinated N = 103 | P-value (F-test/chi-squared test) | Multiple group comparison (Tukey's test)/Bonferroni adjustment | |
| Demographics | ||||||
| Age (years), mean (SD) | 44.11 (12.63) | 47.83 (14.57) | 54.70 (11.35) | 45.91 (12.21) | 0.002a | ● Post-COVID-19 two-dose vaccinated versus post-COVID-19 non-vaccinated: P = 0.001 |
| ● Post-COVID-19 two-dose vaccinated versus COVID-19-naïve two-dose vaccinated: P = 0.004 | ||||||
| Height (m), mean (SD) | 1.68 (0.10) | 1.67 (0.09) | 1.68 (0.09) | 1.66 (0.09) | 0.511a | NA |
| Weight (kg), mean (SD) | 66.51 (13.7) | 74.6 (16.11) | 30 (70.1) | 65.86 (12.82) | 0.056a | NA |
| Gender, n (%) | ||||||
| Male | 40 (70.2) | 13 (72.2) | 22 (73.3) | 70 (68.0) | 0.941b | NA |
| Comorbidities, n (%) | ||||||
| Any | 51 (89.5) | 18 (100.0) | 29 (96.7) | 91 (88.3) | 0.276 | NA |
| DM | 30 (52.6) | 9 (50.0) | 16 (53.3) | 45 (43.7) | 0.663b | NA |
| HTN | 48 (84.2) | 17 (94.4) | 28 (93.3) | 86 (83.5) | 0.376b | NA |
| CLD | 5 (8.8) | 0 (0.0) | 2 (6.7) | 6 (5.8) | 0.647b | NA |
| COAD | 2 (3.5) | 1 (5.6) | 6 (20.0) | 1 (1.0) | 0.001b | Post-COVID-19 two-dose vaccinated versus COVID-19-naïve two-dose vaccinated: P = 0.006 |
| Vascular disease (CAD/PVD) | 5 (8.8) | 4 (22.2) | 10 (33.3) | 2 (1.9) | <0.001b | ● Post-COVID-19 two-dose vaccinated versus COVID-19-naïve two-dose vaccinated: P < 0.001 |
| ● Post-COVID-19 single-dose vaccinated versus COVID-19-naïve two-dose vaccinated: P = 0.012 | ||||||
| Chronic allograft dysfunction | 11 (19.3) | 5 (27.8) | 6 (20.0) | 35 (34.0) | 0.182b | NA |
| Baseline transplant characteristics, n (%) | ||||||
| Duration from transplant to COVID-19 onset (weeks), median (IQR) | 301 (116.5–477) | 243 (112–411) | 199 (112–329) | NA | 0.451c | NA |
| Baseline immunosuppression | ||||||
| Steroid | 57 (100.0) | 18 (100.0) | 30 (100.0) | 103 (100) | e | e |
| CNI | 56 (98.2) | 18 (100.0) | 30 (100.0) | 101 (98.1) | 0.560b | NA |
| MMF/MPA | 57 (100.0) | 15 (83.3) | 29 (96.7) | 88 (85.4) | <0.008b | Post-COVID-19 non-vaccinated versus COVID-19-naïve two-dose vaccinated P = 0.018 |
| Vaccination details | ||||||
| Type of vaccine | ||||||
| Covishield | NA | 17 (94.4) | 20 (66.7) | 75 (72.8)d | 0.096 | NA |
| Covaxin | NA | 1 (5.6) | 10 (33.3) | 25 (24.3)d | ||
| Anti-spike antibody (AU/mL), median (IQR) | 745 (239–3022) | 3436 (661–10 450) | 3706 (867–10 660) | 17.1 (1.6–2125) | P < 0.001c | ● COVID-19-naïve two-dose vaccinated versus post-COVID-19 non-vaccinated, single-dose and two-dose vaccinated groups: P < 0.001c |
| ● Post-COVID-19 non-vaccinated versus post-COVID-19 two-dose vaccinated: P = 0.066, | ||||||
| ●Post-COVID-19 non-vaccinated versus post-COVID-19 two-dose vaccinated: P = 0.006 | ||||||
| Seropositivity | ||||||
| Anti-spike antibody titre >50 AU/mL | 54 (94.7) | 18 (100.0) | 29 (96.7) | 50 (48.5) | P < 0.001 | COVID-19-naïve two-dose vaccinated versus post-COVID-19 non-vaccinated, single-dose and two-dose vaccinated groups: P < 0.001 |
| Duration from COVID-19 onset to anti-spike antibody test (weeks) | 12.0 (10.0–31.5) | 18.0 (10.8–44.3) | 35.0 (25.8–43.0) | NA | P < 0.001c | Post-COVID-19 non- vaccinated versus post-COVID-19 two-dose vaccinated: P < 0.001 |
| Duration from last vaccine dose to anti-spike antibody test (days) | NA | 47.0 (28.0–84.8) | 65.5 (24.0–95.5) | 54.0 (29.0–120.0) | 0.584c | NA |
CAD/PVD, coronary artery disease/peripheral vascular disease; TLC, total leucocyte count; and Hb, haemoglobin. NA, not applicable because not significant in overall comparison (F-test/chi-squared test).
aOne-way ANOVA followed by Tukey's test (The Tukey's/Bonferroni is applicable only when the overall effect was significant).
bChi-squared/exact chi-squared test.
cMann–Whitney U-test with Bonferroni corrected P-values.
dIn group ‘COVID-19-naïve two-dose vaccinated’, three patients received vaccine other than Covishied and Covaxin.
eData/P-value could not be computed.
Nearly all the individuals in the study documented the presence of pre-existing comorbidities. Comorbidities such as diabetes mellitus (DM), hypertension (HTN), chronic liver disease (CLD) and chronic allograft dysfunction were comparable between the cohorts. Interestingly, significantly fewer COVID-19-naïve two-dose vaccinated KTRs reported a history of chronic obstructive airway disease (COAD) (1.9% P = 0.001) and vascular disease (1%, P < 0.001).
Treatment with mycophenolate mofetil/mycophenolic acid (MMF/MPA) was significantly higher in the post-COVID-19 non-vaccinated cohort (100%) as compared with the COVID-19-naïve two-dose vaccinated cohort (85.4%, P = 0.018), whereas treatment with steroids and calcineurin inhibitors (CNIs) was comparable.
Vaccination details of the study cohorts
Details about vaccination are summarized in Table 1. Amongst the 18 post-COVID-19 single-dose vaccinated KTRs, 17 received CovishieldTM and only 1 received CovaxinTM, whereas out of the 30 post-COVID-19 two-dose vaccinated KTRs, 20 individuals received CovishieldTM and 10 received CovaxinTM. Amongst the COVID-19-naïve two-dose vaccinated cohort, 75 KTRs were vaccinated with CovishieldTM and 25 with CovaxinTM; 3 KTRs were vaccinated with other anti-SARS-CoV-2 vaccines approved in India.
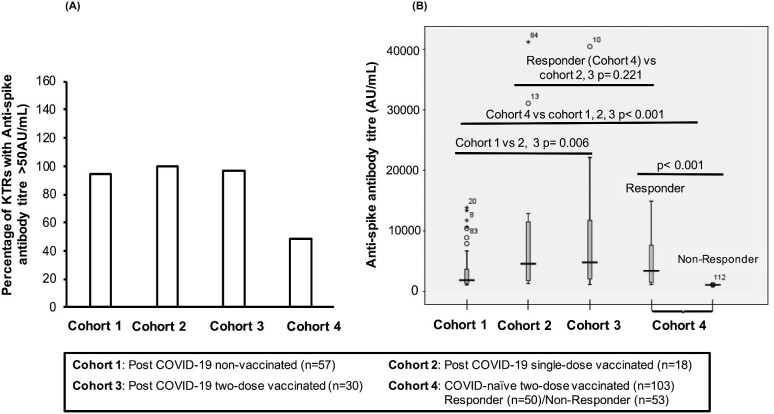
To assess the immune response elicited upon vaccination against SARS-CoV-2, anti–SARS-CoV-2 spike protein IgG (‘anti-spike antibody’) levels were measured. Individuals with antibody titres >50 AUs/mL were considered seropositive. Despite being fully vaccinated, only 50 out the 103 COVID-19-naïve two-dose vaccinated KTRs became seropositive (Figure 2A); more than half of the cohort (53/103; 51.5%) had anti-spike antibody titres < 50 AUs/mL (P < 0.001). Amongst KTRs with past COVID-19 infection, 96.7% of non-vaccinated patients, 100% of vaccines receiving single-dose vaccination and 94.7% of two-dose vaccinated KTRs were seropositive (Figure 2A). Out of the four KTRs that were non-responsive, three KTRs were non-vaccinated and one subject, despite receiving both doses, did not express antibody >60 AUs/mL.
Figure 2:
(A) Percentage of seropositive KTRs (anti-spike antibody titre >50 AU/mL) in each patient cohort. (B) Box plot for anti spike SARS-CoV-2 antibodies for different cohorts. Anti-spike antibody levels (AU/mL) were measured for cohort 1 (post-COVID-19 non-vaccinated), cohort 2 (post-COVID-19 single-dose vaccinated), cohort 3 (post-COVID-19 two-dose vaccinated) and cohort 4 (COVID-19-naïve two-dose vaccinated). For cohort 4, seropositive responders (anti-spike antibody titre > 50 AU/mL) and non-responders (anti-spike antibody titre <50 AU/mL) were analysed separately. *P-value < 0.05 is significant.
The anti-spike antibody levels were significantly different between the cohorts (P < 0.001). Notably, the median antibody titres of the COVID-19 two-dose vaccinated KTRs, inclusive of seropositive and seronegative individuals, [17.1 (IQR 1.6–2125) AU/mL] were significantly lower than KTRs with past COVID-19 infection, irrespective of their vaccination status (P < 0.001) (Table 1 and Figure 2B).
Amongst the KTRs with past COVID-19 infection, non-vaccinated patients had lower median antibody titres [745 (IQR 239–3022) AU/mL] as compared with post-COVID-19 single-dose vaccinees [3436 (IQR 661–10 450) AU/mL; P = 0.066] or post-COVID-19 two-dose vaccinated KTRs [3706(IQR 867–10 700) AU/mL; P = 0.006]. Interestingly, median antibody titres of post-COVID-19 KTRs vaccinated with a single dose were comparable to those who received two doses (P = 1.00, Bonferroni adjusted) (Table 1 and Figure 2B).
Amongst the vaccinated cohort, the median time interval for assessment of the serological response past vaccination was comparable. Anti-spike antibody tests were conducted for post-COVID-19 single-dose vaccinated cohort at median 47 days (IQR 28–84.8), post-COVID-19 two-dose vaccinated cohort at median 65.5 days (IQR 24.0–95.5) and COVID-19-naïve two-dose vaccinated cohort at median 54 days (IQR 29–120) past last vaccine dose. For post-COVID-19 non-vaccinated cohort, the anti-spike antibody was assessed at median 12 weeks (IQR 10–31.5) from onset of COVID-19.
Demographics, comorbidities and baseline transplant characteristics of responders versus non-responders in fully vaccinated uninfected KTRs
Out of the 103 COVID-19-naïve two-dose vaccinated subjects, 50 seropositive individuals with anti-spike antibody titre >50 AU/mL were considered as ‘responders’ and the remaining subjects with anti-spike antibody titre <50 AU/mL were identified as ‘non-responders’. The demographics, baseline transplant characteristics, comorbidities and laboratory investigations, along with vaccination details are documented in Table 2. The average age (48.28 ± 12.34 years) of non-responder was significantly higher, whereas average weight (63.22 ± 10.63 kg) was lower than the responders (age: 43.40 ± 11.67 years, P = 0.042; weight: 68.55 ± 14.41 kg; P = 0.038). No significant difference was observed regards to height, gender, comorbidities and immunosuppressive treatment. Median anti-spike antibody titre for non-responders was 1.9 (IQR 0.33–5.45) AU/mL and that of responders were 2313.0 (IQR 389.1–6518.0) AU/mL (P < 0.001) (Table 2 and Figure 2B).
Table 2.
Comparison between responder and non-responders in COVID-19-naïve two-dose vaccinated KTRs (no history of COVID-19)
| Parameters | Total (n = 103) | Responders (anti-spike antibody titre >50 AU/mL) (n = 50) | Non-responders (anti-spike antibody titre <50 AU/mL) (n = 53)a | Mean or median difference/odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 45.91 (12.21) | 43.40 (11.67) | 48.28 (12.34) | 4.88 (0.18 to 9.59) | 0.042 |
| Height (m), mean (SD) | 1.66 (0.09) | 1.67 (0.10) | 1.65 (0.09) | –0.017 (–0.05 to 0.019) | 0.350 |
| Weight (kg), mean (SD) | 65.86 (12.82) | 68.55 (14.41) | 63.22 (10.63) | –5.23 (–10.16 to –0.30) | 0.038 |
| Gender, n (%) | |||||
| Male | 70 (68.0) | 37 (74.0) | 33 (62.3) | 1.73 (0.74–4.0) | 0.202 |
| Comorbidities, n (%) | |||||
| DM | 45 (43.7) | 25 (50.0) | 20 (37.7) | 0.61 (0.28–1.33) | 0.210 |
| HTN | 86 (83.5) | 42 (84.0) | 44 (83.0) | 0.93 (0.33–2.64) | 1.00 |
| CLD | 6 (5.8) | 1 (2.0) | 5 (9.4) | 5.10 (0.58–45.32) | 0.206 |
| COAD | 1 (1.0) | 1 (2.0) | 0 (0.0) | b | 0.485 |
| Vascular disease (CAD/PVD) | 2 (1.9) | 2 (4.0) | 0 (0.0) | b | 0.233 |
| Chronic allograft dysfunction | 35 (34.0) | 18 (36.0) | 17 (32.1) | 0.84 [0.37–1.90] | 0.684 |
| Diabetic neuropathy | 25 (24.3) | 14 (28.0) | 11 (20.8) | 0.67 [0.27–1.67] | 0.391 |
| Baseline immunosuppressant, n (%) | |||||
| MMF | 88 (85.4) | 40 (80.0) | 48 (90.6) | 2.40 (0.76–7.60) | 0.129 |
| CNI | 101 (98.1) | 49 (98.0) | 52 (98.1) | 1.06 (0.07–17.44) | 1.00 |
| Vaccination details | |||||
| Vaccine type | |||||
| Covishield | 75 (72.8) | 36 (72.0) | 39 (78.0) | P = 0.488 (responders versus non-responders) | |
| Covaxin | 25 (24.2) | 14 (28.0) | 11 (22.0) | ||
| Anti-spike antibody (AU/mL), median (IQR) | 17.1 (1.6–212.5) | 2313.0 (389.1–6518.0) | 1.9 (0.33–5.45) | 2173 (1012.8–3447.6) | <0.001 |
| Covishield | 2943 (965–7055) | 0.042 | |||
| Covaxin | 378.05 (161.5–4272) | ||||
| Duration from last vaccine dose (days), median (IQR) | 54.0 (29.0–120.0) | 41.50 (25.0–90.50) | 74.0 (34.0–137.0) | –20.0 (–42.0 to –1.0) | 0.036 |
| Duration of transplant to vaccination (weeks), median (IQR) | 281 (132 to 378) | 233.0 (142 to 423) | 295 (123 to 360) | –3.0 (–96 to 63.0) | 0.911 |
aThree patients received vaccine other than Covishied and Covaxin.
bCannot be computed due to zero count.
Out of 103 COVID-19-naïve two-dose vaccinated KTRs, 75 subjects received CovishieldTM and 25 were vaccinated by CovaxinTM (Table 1). The seroconversion rate in KTRs receiving CovishieldTM (36 out of 75, 48%) versus CovaxinTM (14 out of 25, 56%) was comparable (P = 0.488) (Table 2). The distribution of subjects receiving CovishieldTM and CovaxinTM between the responders (CovishieldTM: 36/50 and CovaxinTM: 14/50) and non-responders (CovishieldTM: 39/50 and CovaxinTM: 11/50) was also comparable (P = 0.488). However, amongst the responders, subjects receiving CovishieldTM had a significantly higher anti-spike antibody titre [median (IQR): 378.05 (161.5–4272); P = 0.042]. Interestingly, the median time interval for anti-spike antibody assessment past vaccination was higher for non-responders [74 days (IQR 34–137)] as compared with responders [41.5 days (IQR 25–90.5); P = 0.036] (Table 2).
Association and correlation of anti-spike antibody levels with demographics, comorbidities and other baseline characteristics of the KTRs
No significant association of anti-spike antibody levels assessed in KTRs from all cohorts with demographics, comorbidities, vaccination and anti-spike antibody assessment details was observed (Table 3). Similar results were obtained when we studied the association of anti-spike antibody titres of only seropositive KTRs (anti-spike antibody levels >50 AU/mL) with the baseline characteristics (Supplementary data, Table S1). Further, the correlation between anti-spike antibody levels in post-COVID-19 (non-vaccinated, single-dose and two-dose vaccinated) and COVID-19-naïve two-dose vaccinated KTRs with clinical variables such as age, transplant duration up to vaccination, duration from onset of COVID-19 to vaccination and days from last dose of vaccine to serological assessment was studied (Table 4). No significant correlation was observed except in cohort 3, where anti-spike antibody levels from previously infected KTRs who received both doses of vaccine showed a negative correlation between age and anti-spike antibody levels (Spearman’s correlation coefficient: –0.380; P = 0.038).
Table 3.
Association of anti-spike antibody levels with baseline characteristics and comorbidities in post-COVID-19 non-vaccinated, vaccinated (both single and two-dose vaccinated) and COVID-19-naïve two-dose vaccinated KTRs. The data shown corresponds to anti-spike antibody titres (AU/mL)
| Post-COVID-19 KTRs | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Non-vaccinated n = 57 | P-value | Vaccinated (single-dose and two-dose vaccinated) n = 48 | P-value | COVID-19-naïve two-dose vaccinated n = 103 | P-value |
| Demographics | ||||||
| Age | ||||||
| <60 years | 768 (250–2228) n = 47 | 0.973 | 3697 (716–10 450) n = 37 | 0.873 | 57.90 (2.23–2551) n = 92 | 0.184 |
| >60 years | 499 (153–6348) n = 10 | 3540 (371–10 626) n = 11 | 3.90 (0.80–220.0) n = 11 | |||
| Gender | ||||||
| Male | 768 (250–1941) n = 39 | 0.993 | 3697 (974–10 360) n = 35 | 0.719 | 87.2 (1.35–2756) n = 70 | 0.522 |
| Female | 694 (184.8–4294) n = 17 | 2298 (426–12 220) n = 13 | 6.80 (2.0–1339) n = 33 | |||
| Transplant duration | ||||||
| ≤1 year | 1710 (523–10 760) n = 5 | 0.284 | Three cases | 0.841 | NA | NA |
| >1 year | 745 (249–2228) n = 51 | 3540 (716–10 450) n = 45 | NA | |||
| Vaccination detailsAnti-spike antibody levels (AU/mL) from onset of COVID (weeks) | ||||||
| <12 weeks | 768 (205–3168) n = 31 | 0.921 | 3114 (633–6188) n = 10 | 0.339 | NA | NA |
| >12–24 weeks | 1132 (249–1941) n = 11 | 750 (519–4835) n = 7 | NA | |||
| >24 weeks | 750 (382–9774) n = 15 | 5883 (1233–10 780) n = 31 | NA | |||
| Anti-spike antibody levels from last vaccine dose (days) | ||||||
| <21 days | NA | NA | 3619 (510–7960) n = 10 | 0.910 | 31.1 (0.08–1191) n = 10 | 0.280 |
| >21 days | NA | 3741 (733–10 560) n = 38 | 17.1 (1.9–2534) n = 93 | |||
| Comorbidities | ||||||
| Any | ||||||
| Present | 76 (250–2390) n = 50 | 0.969 | 3697 (682–10 550) n = 47 | NA | 17.10 (1.90–1801) N = 91 | 0.930 |
| Absent | 1228 (66.8–4508) n = 6 | One-case only | 390.9 (0.70–3416) n = 12 | |||
| DM | ||||||
| Present | 7680 (238–1772) n = 29 | 0.825 | 3481 (532–5403) n = 25 | 0.197 | 119 (4.0–4710) n = 45 | 0.022 |
| Absent | 745 (250–4150) n = 29 | 7022 (1233–12 120) n = 23 | 8.0 (0.40–839.5) n = 58 | |||
| HTN | ||||||
| Present | 754 (249–2228) n = 47 | 0.713 | 3697 (614–10 580) n = 45 | 0.873 | 23.5 (1.80–2219) n = 86 | 0.657 |
| Absent | 1710 (181–4294) n = 9 | 3436 n = 3a | 4.40 (1.0–2214) n = 17 | |||
| CLD | ||||||
| Present | 1196 (645–4680) n = 5 | 0.339 | Only two cases | 0.255 | 5.0 (0.8–52.10) n = 6 | 0.084 |
| Absent | 745 (228–2228) n = 51 | 3706 (733–10 560) n = 46 | 56.10 (2.05–2534) n = 97 | |||
| COAD | ||||||
| Present | Two cases | 0.026 | 4234 (371–10 620) n = 7 | 0909 | One case | 0.719 |
| Absent | 877 (273–2950) n = 54 | 3540 (862–10 450) n = 41 | 17.0 (1.6–2219) n = 102 | |||
| Vascular disease (CAD/PVD) | ||||||
| Present | 164 (128– 5321) n = 5 | 0.449 | 2997 (480–6472) n = 14 | 0.297 | Two cases | 0.038 |
| Absent | 768 [280–2877] n = 51 | 3628 (918–11 110) n = 34 | 16.90 (1.6–1759) n = 101 | |||
| Chronic allograft dysfunction | ||||||
| Present | 1196 (515–6791) n = 11 | 0.348 | 4000 (2700–10 780) n = 11 | 0.244 | 100.5 (0.40–978.9) n = 35 | 0.473 |
| Absent | 754 (216–2552) n = 45 | 3481 (583–9172) n = 37 | 16.45 (2.0–2595) n = 68 | |||
aIQR could not be calculated due to insufficient n values.
Table 4.
Correlation of clinical variables with anti-spike antibody levels in post-COVID-19 (non-vaccinated and single-dose and two-dose vaccinated) and COVID-19-naïve two-dose vaccinated KTRs
| Post-COVID-19 KTRs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-vaccinated | Single-dose vaccinated | Two-dose vaccinated | COVID-19-naïve two-dose vaccinated | |||||
| Variables | Spearman's correlation | P-value | Spearman's correlation | P-value | Spearman's correlation | P-value | Spearman's correlation | P-value |
| Age (years) | 0.037 | .788 | 0.445 | 0.064 | –0.380 | 0.038 | –0.107 | 0.281 |
| Transplant duration up to vaccination date (weeks) | NA | NA | 0.303 | 0.222 | –0.072 | 0.706 | 0.158 | 0.11 |
| Duration from onset of COVID-19 to vaccination date (weeks) | NA | NA | 0.395 | 0.104 | 0.015 | 0.938 | NA | NA |
| Days from last dose of vaccine | NA | NA | 0.061 | 0.810 | –0.068 | 0.720 | –0.160 | 0.107 |
Bold value represent the statistically significant value. The italic has no significance.
DISCUSSION
The current study, to the best of our knowledge, is the first to investigate the longitudinal serological response to SARS-CoV-2 natural infection and subsequent vaccination in KTRs in India. We have assessed the anti-spike antibody levels in 105 COVID-19-infected KTRs who have not been vaccinated (cohort 1) or vaccinated with either a single (cohort 2) or two doses (cohort 3) with 103 COVID-19-naïve two-dose vaccinated KTRs (cohort 4).
Recent studies have demonstrated that KTRs elicit an impaired immune response to the SARS-CoV-2 vaccine, with only 4–48% of KTRs showing detectable anti-spike IgG after complete vaccination [19–24, 30]. However, all these investigations were done in response to mRNA vaccines, which are currently unavailable in India. In our study cohorts, the majority of the subjects were vaccinated by CovishieldTM, an adenovirus-vectored vaccine expressing the SARS-CoV-2 spike protein (ChAdOx1-nCOV or AZD1222, acquired from Oxford University and AstraZeneca, manufactured by Serum Institute of India, Pune, India) and rest with inactivated whole virus–based vaccine CovaxinTM [BBV-152, manufactured by Bharat Biotech, Hyderabad, in collaboration with the Indian Council of Medical Research (ICMR), India]. The data available on the serological response to CovishieldTM and CovaxinTM is based on immune responses from immune-competent individuals [35–37], and similar information from KTRs is scarce. Recently, Prendecki et al. [38] reported immunological responses to twodose vaccination with ChAdOx1 (Oxford University–AstraZeneca) in KTRs; only 44% of infection-naïve KTRs receiving ChAdOx1 seroconverted. In agreement with the available literature, we also observed that only 48.5% KTRs (50/103) with no history of COVID-19 (COVID-19-naïve two-dose vaccinated cohort) responded positively (anti-spike antibody titre of >50 AU/mL) despite receiving a complete two doses of the vaccination regimen. This is lower in comparison with a recent report from India by Kute et al. [39], where 19 out of 31 uninfected KTRs (61.2%) seroconverted, probably because they considered a lower cut-off of ≥15 AU/mL (as opposed to 50 AU/mL in our study) as an indication of an antibody response.
In our study, proportions of responders and non-responders receiving CovishieldTM versus CovaxinTM were comparable. No significant difference was observed in the seroconversion rate of CovishieldTM and CovaxinTM. However, the serological response to vaccination was significantly lower in responders who received CovaxinTM as compared with those who were administered CovishieldTM. Similar observations have been reported by two independent studies, including one on rheumatology patients [36, 40]. Interestingly, the non-responders were significantly older than the responders, with older age being associated with poorer vaccination responses [20].
Notably, amongst KTRs who were previously infected with COVID-19, only four subjects reported anti-spike antibody <50 AU/mL, out of which three were non-vaccinated and one subject had received both vaccine doses. This is in agreement with a recent study, where only 5% of previously infected KTRs were seronegative after vaccination [38]. Similarly, in a study in an immunocompetent population, investigators reported that 100% of healthcare workers with a history of COVID-19 became seropositive after the vaccination [36].
Interestingly, the SARS-CoV-2 anti-spike IgG antibody response [with a median antibody titre of 768 (249.0–3022)] in post-COVID-19 non-vaccinated KTRs was significantly higher than the COVID-19-naïve two-dose vaccinated KTRs. This observation is supported by studies that have investigated the development of SARS-CoV-2 antibodies in transplant recipients, including KTRs following natural COVID-19 infection [31–33]. It is to be noted that the low antibody levels in vaccinated uninfected KTRs are likely attributable to non-responders in the cohort. Further analysis of the median anti-spike antibody titres of the responders from the COVID-19-naïve two-dose vaccinated cohort revealed that the immune response elicited by vaccination in uninfected KTRs was comparable to KTRs with COVID-19 history (P = 0.221).
The antibody responses to CovishieldTM and CovaxinTM vaccines in KTRs who have been previously infected with SARS-CoV-2 are largely unknown. In our study, we observed that previously infected KTRs showed a significant 4-fold increase in anti-spike antibody response to a single dose of vaccine. However, the median antibody titres between KTRs who received only one dose as compared with those who received both doses were comparable. This is in agreement to multiple studies conducted in non-transplant individuals [35–37, 41, 42] and a study by Benotmane et al. [43] in KTRs, where one dose of vaccine yielded significantly higher anti-spike antibody titre in COVID-19 recovered subjects. The robust post-vaccination immune response in individuals with past COVID-19 infection could be explained by immune memory that may persist for months [44–46], possibly resulting in a quicker and sustained response to COVID-19 vaccines.
We also analysed association of anti-spike antibody levels with demographics, vaccination details and comorbidities, and no significant association was observed. Similar results were reported by Singh et al. [36], with no significant difference in seropositivity rate with regard to age, sex, BMI, blood group, and any comorbidities, including its duration and treatment. However, when we calculated the correlation of anti-spike antibody with clinical variables, we observed a negative correlation of antibody levels with age in post-COVID-19 two-dose vaccinated KTRs; older age has been associated with a poorer immune response to vaccination.
There are several limitations to this study, including the relatively small sample size, especially in KTRs vaccinated with Covaxin. We also acknowledge the variation in anti-spike antibody levels of individuals, which could be due to variation in the interval between transplantation, COVID-19 diagnosis and vaccination days. It has been reported that patients who contracted COVID-19 within the first year of transplant may have a poorer immune response due to immunosuppression therapy [20]. Another important consideration is the variation in timing of antibody testing past infection or vaccination within our study cohorts. It is possible that anti-SARS-CoV-2 antibody levels have started to decline over time in some individuals, especially in KTRs who experience mild COVID-19 symptoms or with compromised immune response [46]. This could be applicable in our study, where we observed that the median time interval for assessment of anti-spike antibody after the last vaccine dose in non-responders of the COVID-19-naïve two-dose vaccinated cohort was longer [74 days (IQR 34–137)] as compared with responders [41.5 days (IQR 25–90.5)]. This could be one of the reasons for low antibody levels in some of the non-responders.
Our findings provide evidence that KTRs infected with COVID-19 develop anti-spike antibody following natural infection, albeit lower than the KTRs that received vaccination subsequent to their recovery from COVID-19. The antibody response significantly increases after the administration of a single dose of vaccine (CovishieldTM), suggesting that a previous infection with SARS-CoV-2 primed the immune system of the KTRs against COVID-19. Although no increase in the antibody level was observed following the second dose of vaccination, it is possible that the second dose could improve the longevity and durability of the response. However, due to inadequate number, the inference could not be extrapolated to patients receiving CovaxinTM. Further studies with larger sample sizes, sequential anti-spike antibody monitoring over time and comparing COVID-19-infected KTRs with COVID-19-naïve KTRs are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the support extended by Dr A.K. Hooda, Director, Medical Services, Indraprastha Apollo Hospital, for granting us the permission to carry out the entire exercise; Dr Rajeev Kumar Malhotra for the statistical analysis; and Dr Anupriya Khare Roy for editing the manuscript. We acknowledge the assistance provided by Mr Desmond Dyslva, Mr Nirmal Maseeh, Mr Santosh Singh, Mr Mukul, Mr Ravi Kumar and Mr Ashwani Gupta for the graphical inputs and data compilation. We also thank the AVATAR Foundation and Dr Lal PathLabs for logistic support.
Contributor Information
Sanjiv Jasuja, Department of Nephrology, Indraprastha Apollo Hospital, New Delhi, India.
Vivekanand Jha, George Institute for Global Health, UNSW, New Delhi, India; School of Public Health, Imperial College, London, UK; Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, India.
Gaurav Sagar, Department of Nephrology, Indraprastha Apollo Hospital, New Delhi, India.
Anupam Bahl, Department of Nephrology, Indraprastha Apollo Hospital, New Delhi, India.
Shalini Verma, Department of Clinical Research, AVATAR Foundation, New Delhi, India.
Neharita Jasuja, Department of Clinical Research, AVATAR Foundation, New Delhi, India.
Jasmeet Kaur, Department of Histocompatibility and Transplant Immunology, Dr Lal PathLabs Ltd, National Reference Laboratory, New Delhi, India.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONS
All authors contributed equally in research design and execution, data collection, analysis and interpretation of data and writing of the manuscript. All authors provided intellectual content of critical importance to the work described, approved the version for publication and agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST STATEMENT
V.J. has research grants from Baxter, GSK and NephroPlus, and reports honoraria from speaking engagements (lectures, presentations, speakers’ bureaus, manuscript writing or educational events) from AstraZeneca, Boehringer Ingelheim, Baxter and Zydus Cadila, and participation in the Advisory Board of Zydus Cadila and GSK, outside the published work. All the other authors reported no conflict. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Kronbichler A, Gauckler P, Windpessl Met al. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol 2020; 16: 365–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banerjee D, Popoola J, Shah Set al. COVID-19 infection in kidney transplant recipients. Kidney Int 2020; 97: 1076–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jha PK, Yadav DK, Siddini Vet al. A retrospective multi-center experience of renal transplants from India during COVID-19 pandemic. Clin Transplant 2021; 35: e14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kute VB, Bhalla AK, Guleria Set al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation 2021; 105: 851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elias M, Pievani D, Randoux Cet al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol 2020; 31: 2413–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Requião-Moura LR, de Sandes-Freitas TV, Viana LAet al. High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: results from the Brazilian multicenter cohort study. PLoS One 2021; 16: e0254822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jasuja S, Sagar G, Bahl Aet al. COVID-19 infection clinical profile, management, outcome, and antibody response in kidney transplant recipients: a single centre experience. Int J Nephrol 2021; 2021: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aubert O, Yoo D, Zielinski Det al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health 2021; 6: e709–e719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Azzi Y, Bartash R, Scalea Jet al. COVID-19 and solid organ transplantation: a review article. Transplantation 2021; 105: 37–55 [DOI] [PubMed] [Google Scholar]
- 10. Elec FI, Bolboacă SD, Muntean Aet al. Comparing the first and second wave of COVID-19 in kidney transplant recipients: an East-European perspective. Eur Surg Res 2022; 63: 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kremer D, Pieters TT, Verhaar MCet al. A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: lessons to be learned. Am J Transplant 2021; 21: 3936–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kute VB, Meshram HS, Navadiya VVet al. Consequences of the first and second COVID-19 wave on kidney transplant recipients at a large Indian transplant centre. Nephrology 2022; 27: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elhadedy MA, Marie Y, Halawa A. COVID-19 in renal transplant recipients: case series and a brief review of current evidence. Nephron 2021; 145: 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miarons M, Larrosa-García M, García-García Set al. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation 2021; 105: 138–150 [DOI] [PubMed] [Google Scholar]
- 15. Kakkanattu TJ, Sankarasubbaiyan S, Yadav AKet al. Outcome and determinants of outcome of COVID-19 infection among hemodialysis patients: findings from a national dialysis network program in India. Kidney Int Rep 2021; 6: 1429–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tau N, Yahav D, Schneider Set al. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am J Transplant 2021; 21: 2910–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yadav AK, Sankarasubbaiyan S, Gowda BGMet al. The high mortality and impact of vaccination on COVID-19 in hemodialysis population in India during the second wave. Kidney Int Rep 2021; 6: 2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. npj Vaccines 2021; 6: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebinger JE, Fert-Bober J, Printsev Iet al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021; 27: 981–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caillard S, Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol 2021; 17: 785–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naaber P, Tserel L, Kangro Ket al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Regional Health Eur 2021; 10: 100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rincon-Arevalo H, Choi M, Stefanski A-Let al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol 2021; 6: eabj1031. [DOI] [PubMed] [Google Scholar]
- 23. Benotmane I, Gautier-Vargas G, Cognard Net al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int 2021; 99: 1498–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benotmane I, Gautier-Vargas G, Cognard Net al. Weak anti–SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int 2021; 99: 1487–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cucchiari D, Egri N, Bodro Met al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant 2021; 21: 2727–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korth J, Jahn M, Dorsch Oet al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021; 13: 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grupper A, Rabinowich L, Schwartz Det al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21: 2719–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyarsky BJ, Werbel WA, Avery RKet al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021; 325: 2204–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rozen-Zvi B, Yahav D, Agur Tet al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect 2021; 27: 1173.e1–1173.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seija M, Rammauro F, Santiago Jet al. Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant. Clin Kidney J 2021; 15: 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fung M, Chiu CY, DeVoe Cet al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: a case series from the United States. Am J Transplant 2020; 20: 3225–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benotmane I, Gautier Vargas G, Wendling Met al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant 2020; 20; 3162–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prendecki M, Clarke C, Gleeson Set al. Detection of SARS-CoV-2 antibodies in kidney transplant recipients. J Am Soc Nephrol 2020; 31: 2753–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. English E, Cook LE, Piec Iet al. Performance of the Abbott SARS-CoV-2 IgG II quantitative antibody assay including the new variants of concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and first steps towards global harmonization of COVID-19 antibody methods. J Clin Microbiol 2021; 59: e0028821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Havervall S, Marking U, Greilert-Norin Net al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. EBioMedicine 2021; 70: 103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh AK, Phatak SR, Singh Ret al. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: the final results of Cross-Sectional Coronavirus Vaccine-Induced Antibody Titre (COVAT) study. Vaccine 2021; 39: 6492–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jeewandara C, Kamaladasa A, Pushpakumara PDet al. Immune responses to a single dose of the AZD1222/Covishield vaccine in health care workers. Nat Commun 2021; 12: 4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prendecki M, Thomson T, Clarke CLet al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet North Am Ed 2021; 398: 1482–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kute VB, Shah N, Meshram HSet al. Safety and efficacy of Oxford vaccine in kidney transplant recipients: a single-center prospective analysis from India. Nephrology (Carlton) 2022; 27: 292–293 [DOI] [PubMed] [Google Scholar]
- 40. Shenoy P, Ahmed S, Paul Aet al. Inactivated vaccines may not provide adequate protection in immunosuppressed patients with rheumatic diseases. Ann Rheum Dis 2022; 81: 295–296 [DOI] [PubMed] [Google Scholar]
- 41. Lombardi A, Consonni D, Oggioni Met al. SARS-CoV-2 anti-spike antibody titres after vaccination with BNT162b2 in naïve and previously infected individuals. J Infect Public Health 2021; 14: 1120–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderson M, Stec M, Rewane Aet al. SARS-CoV-2 antibody responses in infection-naive or previously infected individuals after 1 and 2 doses of the BNT162b2 vaccine. JAMA Netw Open 2021; 4: e2119741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Benotmane I, Gautier -Vargas G, Gallais Fet al. Strong antibody response after a first dose of a SARS-CoV-2 mRNA-based vaccine in kidney transplant recipients with a previous history of COVID-19. Am J Transplant 2021; 21: 3808–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chavarot N, Leruez-Ville M, Scemla Aet al. Decline and loss of anti–SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int 2021; 99: 486–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bajpai D, Shah D, Bose Set al. Development and longevity of antibodies against SARS-CoV-2 in kidney transplant recipients after symptomatic COVID-19. Transpl Infect Dis 2021; 23: e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pradenas E, Trinité B, Urrea Vet al. Stable neutralizing antibody levels 6 months after mild and severe COVID-19 episodes. Med (N Y) 2021; 2: 313–320.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.