Abstract
Penetration of fluconazole into female genital tissues was examined. Fluconazole was administered orally at a dose of 150 mg to patients undergoing total abdominal hysterectomy 1 to 151 h prior to surgery. During surgery, blood, uterus, ovary, and oviduct were sampled. Fluconazole concentrations in each tissue were determined by high-performance liquid chromatography. The peak concentrations in serum reached approximately 6.1 μg/ml 1.0 h after a drip infusion was begun. At each time after the infusion, the concentrations in portio vaginalis, cervix uteri, myometrium, endometrium, ovary, and oviduct were higher than those in the serum: the peaks in the tissues ranged from 6.4 to 9.5 μg/g around 1.0 h after the drip infusion was begun. Thus, the levels of penentration of fluconazole into gynecological tissues appeared to be similar to or slightly above those in serum samples. Fluconazole can rapidly penetrate from plasma into the female genital organs, supporting high efficacy of fluconazole against fungal infections in the field of gynecology.
Vaginal candidiasis is an infection caused by Candida albicans or related fungi. The fungi isolated from the vaginas of patients with candidiasis include C. albicans (80 to 90% of patients), Candida glabrata, and Candida tropicalis.
The usual transvaginal treatment of candidiasis can be messy and displeasing and can include local irritation, burning, and frequency of micturition. Consequently, complicance with this form of treatment is poor, as women often stop the treatment as soon as their symptoms disappear but before eradication of the fungi. The rectum is a carrier of the fungi, and relapses are common.
Fluconazole is one of the antifungal agents which are effective against not only systemic fungal infection but vaginal candidiasis; however, there have been no published data on penetration of oral fluconazole into gynecological tissues. The concentrations of fluconazole in serum and gynecological tissues (uterus, ovaries, and oviducts) were determined to reveal the drug’s effective distribution.
MATERIALS AND METHODS
Drug.
Fluconazole [α-(2,4-difluorophenyl)-α-(1H-1,2,4-triazol-1-ylmethyl)-1H-1,2,4-triazole-1-ethanol] (150 milligrams) was used in this study.
Subjects.
Fifty-seven patients (mean age ± standard deviation, 54.7 ± 7.5 years; mean weight ± standard deviation, 54.8 ± 6.4 kg) were chosen for the study. Patients underwent total abdominal hysterectomy for the treatment of myoma uteri and other conditions at the Department of Obstetrics and Gynecology, School of Medicine, Gifu University; Gifu Municipal Hospital; Gihoku General Hospital; and Gifu Prefectural Gifu Hospital from June 1996 to March 1997. They agreed to participate in this study. These patients showed no abnormalities in liver function, having normal levels of transaminase in serum, or in renal function, having normal levels of urea nitrogen and creatinine in serum.
Experimental methods. (i) Drug administration.
Before the operation, 150 mg of fluconazole was orally administered to each patient.
(ii) Sampling method.
After ligation of the uterine artery on one side in the hysterectomy, blood from the uterine artery on the other side and cubital venous blood were taken. Also, approximately 1 g each of tissue from the portio vaginalis, cervix uteri, myometrium, ovary, and oviduct was taken from the normal portions of the samples immediately after hysterectomy. After centrifugal separation (1,000 × g, 15 min) of blood samples, the supernatant was immediately frozen and stored at −80°C. Each tissue sample was homogenized, immediately frozen, and stored at −80°C.
(iii) Measurement of drug concentration.
The concentrations of fluoconazole were measured by a modification of the validated high-performance liquid chromatography method of John et al. (13). Each tissue sample was homogenized with a solution containing 2 ml of 5 M NaOH and 3 ml of ethyl acetate. In accordance with a cross-matrix compatibility study, serum was used as a control matrix to prepare a standard curve for tissue samples. Samples prepared from a rat control liver were assayed against the standard curve for serum. Levels of accuracy for rat liver were less than 13%. The percent recovery was 80% for serum.
Chromatographic analysis was performed on an octyldecyl silane-M column (25 cm [length] by 4.6 mm [inner diameter], 5-μm particles) by isocratic elution, with a mobile phase of 0.5% ammonium acetate (pH 3.8)–acetonitrile (3:1 [vol/vol]) and UV detection of 260 nm.
Standard curves showed a good linear relationship between peak-height ratios and fluconazole concentration, with γ2 consistently being >0.999. The lower limits of quantitation were 0.4 μg/ml in serum (with 0.5-ml samples) and 0.2 μg/sample in tissues (with 0.03- to 0.86-g samples). The between-day (6 days) precision values (as percents coefficient of variation) were less than 3.2%.
(iv) Method of analysis.
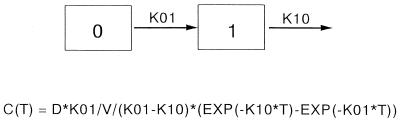
Results were analyzed with a one-compartment first-order-lag-time–first-order-elimination model (Fig. 1) as follows: C(T) = D* K01/V/(K01−K10)* [EXP(−K10* T) − EXP (−K01* T)], where C is concentration, T is time (in hours), D is dose, K01 is absorption rate constant, K10 is elimination rate constant, and EXP is exponent. The area under the concentration-time curve from 0 h to T (AUC0–T) was calculated by the trapezoidal method. Thus, AUC0–∞ = AUC0–T + CT/kel, where AUC0–∞ is the area under the concentration-time curve from 0 h to infinity, CT is the concentration detected in the plasma at the final time point, and kel is the elimination rate constant).
FIG. 1.
Diagram of the one-compartment first-order-lag-time–first-order-elimination pharmacokinetic model. See Materials and Methods for an explanation of the abbreviations.
RESULTS
The drug concentrations in serum and each genital tissue after intravenous administration of 150 mg of fluconazole are shown in Table 1 and Fig. 1 and 2.
TABLE 1.
Drug concentrations in serum and each genital tissue after oral administration of 150 mg of fluconazole
| Patient | Time after administration (h) | Concn (μg/ml) in serum from:
|
Concn (μg/g) in tissue from:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Uterine artery | Antecubital vein | Portio vaginalis | Cervix uteri | Endometrium | Myometrium | Oviduct | Ovary | ||
| 1 | 1.83 | 3.6 | 3.7 | 3.0 | 3.1 | 3.2 | 3.4 | 3.4 | NTa |
| 2 | 2.08 | 3.0 | 2.7 | 2.6 | 2.7 | 2.6 | 2.5 | 2.6 | NT |
| 3 | 2.50 | 4.1 | 4.1 | 4.1 | 4.6 | 4.2 | 4.3 | 3.9 | 4.0 |
| 4 | 2.57 | 3.7 | 3.8 | 3.6 | 3.6 | 3.8 | 4.3 | 4.4 | 4.3 |
| 5 | 2.57 | 2.1 | 1.9 | 2.1 | 1.7 | 1.8 | 2.2 | 1.9 | NT |
| 6 | 2.58 | 3.9 | 3.8 | 3.2 | 3.2 | NT | 2.8 | 4.5 | 4.3 |
| 7 | 2.67 | 3.5 | 3.6 | 4.1 | 3.2 | 4.5 | 3.8 | 4.4 | NT |
| 8 | 2.67 | 2.3 | 2.5 | 2.3 | 2.3 | NT | 2.3 | 4.2 | 2.6 |
| 9 | 2.75 | 4.3 | 4.3 | 3.8 | 4.5 | NT | 4.6 | 4.2 | 4.7 |
| 10 | 2.83 | 3.3 | 3.2 | 2.3 | 2.8 | 3.5 | 3.0 | 3.4 | 3.2 |
| 11 | 3.08 | 1.5 | 1.5 | 1.2 | 1.4 | 1.3 | 1.5 | 1.2 | 1.3 |
| 12 | 3.17 | 4.0 | 4.1 | 4.2 | 3.8 | 5.3 | 4.7 | 5.2 | 4.6 |
| 13 | 4.25 | 3.1 | 3.5 | 3.3 | 3.7 | 4.2 | 3.2 | 4.3 | 3.4 |
| 14 | 6.00 | 3.7 | 3.8 | 3.9 | 3.3 | 3.6 | 3.8 | 4.1 | NT |
| 15 | 9.33 | 4.1 | 4.0 | 4.0 | 3.6 | NT | 4.3 | NT | 3.2 |
| 16 | 10.42 | 3.4 | 3.5 | 3.2 | 3.2 | 3.3 | 3.4 | 3.6 | NT |
| 17 | 11.50 | 3.0 | 2.9 | 2.4 | 2.6 | 2.8 | 2.8 | NT | NT |
| 18 | 13.00 | 4.5 | 4.5 | 4.5 | 3.1 | 5.7 | 5.4 | 4.9 | NT |
| 19 | 13.00 | 3.4 | 3.2 | 3.4 | 2.8 | 3.6 | 3.2 | 4.4 | 3.4 |
| 20 | 13.50 | 3.2 | 3.4 | 3.4 | 3.1 | NT | 3.4 | 3.6 | 3.3 |
| 21 | 14.00 | 3.7 | 3.7 | 4.0 | 3.5 | NT | 3.7 | 4.4 | 3.6 |
| 22 | 14.50 | 3.4 | 3.3 | NT | 3.2 | 3.8 | 3.5 | 4.1 | 3.7 |
| 23 | 17.25 | 3.7 | 3.7 | 3.5 | 3.3 | 3.4 | 3.5 | 3.8 | NT |
| 24 | 19.25 | NT | 3.6 | NT | NT | NT | NT | NT | NT |
| 25 | 21.50 | 3.6 | 3.4 | 3.3 | NT | NT | 3.2 | 3.7 | 3.4 |
| 26 | 21.50 | 3.5 | NT | 2.9 | NT | NT | 3.4 | 2.8 | 3.8 |
| 27 | 22.67 | 3.2 | 3.2 | 3.0 | NT | NT | 2.9 | 3.7 | NT |
| 28 | 23.17 | 2.0 | 2.2 | 1.9 | 2.0 | 2.3 | 2.2 | 2.3 | 2.1 |
| 29 | 24.25 | 2.6 | 2.7 | 2.7 | 2.6 | 2.9 | 2.9 | NT | NT |
| 30 | 24.57 | 2.3 | 2.3 | 2.4 | 2.4 | 2.6 | 2.7 | 2.4 | NT |
| 31 | 24.83 | 2.5 | 2.5 | 2.0 | 2.4 | 2.4 | 2.7 | NT | NT |
| 32 | 26.00 | 2.8 | 2.8 | 3.2 | 2.4 | 2.6 | 2.9 | 3.3 | NT |
| 33 | 26.33 | 2.8 | 2.6 | 2.4 | 2.4 | 2.6 | 2.5 | 2.8 | NT |
| 34 | 27.50 | NT | 3.5 | 4.3 | NT | NT | 5.1 | 4.1 | 3.4 |
| 35 | 31.25 | 2.2 | 2.2 | 2.1 | 2.2 | NT | 2.2 | 2.1 | 1.9 |
| 36 | 37.00 | 1.9 | 1.8 | 2.1 | 2.0 | NT | 2.4 | 2.2 | 2.1 |
| 37 | 37.50 | 2.3 | 2.2 | NT | 2.1 | NT | 2.3 | 2.9 | 2.4 |
| 38 | 37.50 | 1.6 | 1.5 | 1.5 | 1.8 | 1.3 | 1.5 | 1.4 | 1.5 |
| 39 | 39.50 | NT | 1.6 | 1.6 | 1.4 | 1.7 | 1.5 | 1.5 | 1.5 |
| 40 | 41.50 | 1.4 | 1.3 | 1.3 | 1.5 | 1.5 | 1.4 | 1.7 | 1.6 |
| 41 | 41.50 | 2.3 | 2.1 | NT | NT | NT | 1.9 | 2.1 | 2.0 |
| 42 | 41.67 | 1.5 | 1.5 | 1.5 | 1.4 | NT | 1.7 | 1.6 | 1.5 |
| 43 | 41.83 | 2.2 | 2.2 | 1.6 | 2.2 | 1.9 | 2.1 | 2.4 | 2.0 |
| 44 | 42.00 | 3.8 | 3.5 | 3.2 | NT | NT | 3.5 | 4.1 | 3.9 |
| 45 | 43.50 | 1.5 | 1.4 | 1.6 | 1.6 | NT | 1.8 | 1.8 | 1.6 |
| 46 | 45.50 | NT | NT | 1.2 | 1.3 | 1.3 | 1.5 | NT | NT |
| 47 | 46.17 | NT | 2.0 | NT | NT | NT | NT | NT | NT |
| 48 | 49.50 | NT | 1.2 | 1.2 | 1.2 | 1.5 | 1.3 | 1.3 | 1.4 |
| 49 | 50.00 | 1.4 | 1.5 | 1.2 | 1.1 | 1.3 | 1.3 | 1.3 | 1.5 |
| 50 | 52.25 | 1.5 | 1.5 | 1.0 | 1.1 | 1.1 | 1.7 | 1.3 | 1.1 |
| 51 | 64.50 | NT | 1.8 | 2.1 | 1.8 | 1.5 | 1.6 | 1.6 | 1.4 |
| 52 | 68.50 | 1.1 | 1.0 | 0.8 | 0.8 | NT | 1.3 | 1.1 | 1.1 |
NT, not tested.
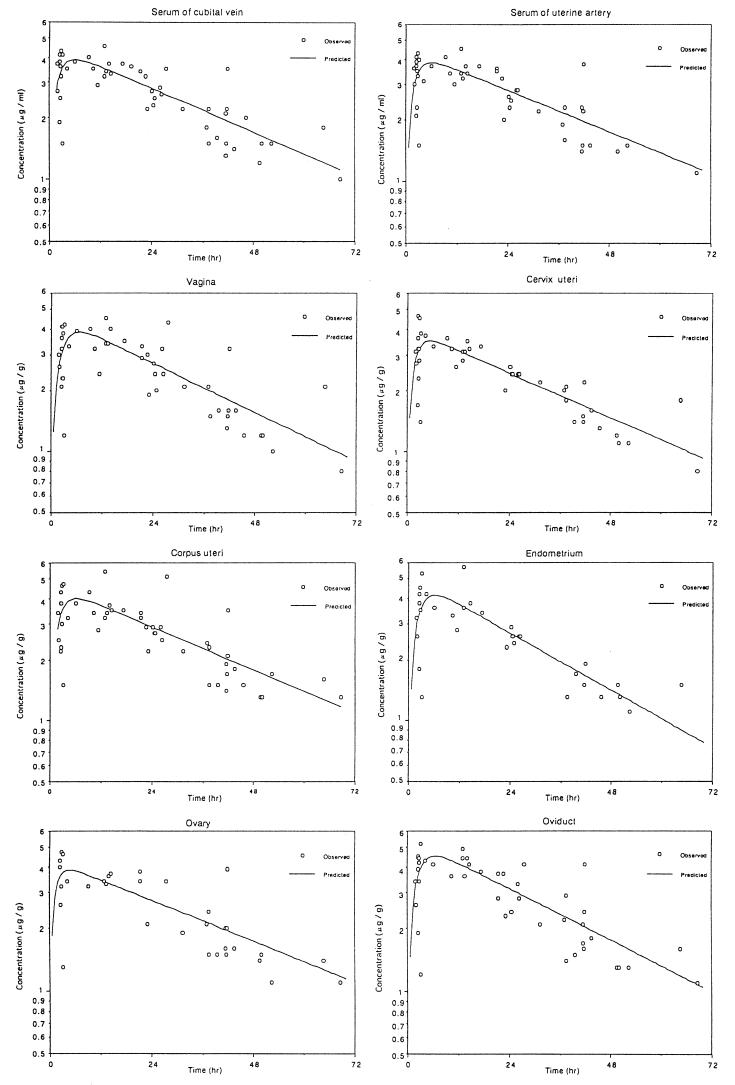
FIG. 2.
Drug concentrations in serum and each genital tissue after oral administration of fluconazole (150 mg).
The maximum concentration of fluconazole in serum was observed to be 6.1 μg/ml at 1.0 h after the drip infusion was begun (Table 2). The peak concentrations in tissues from the portio vaginalis, cervix uteri, myometrium, endometrium, ovary, and oviduct ranged from 6.4 to 9.5 μg/g around 1.0 h after the drip infusion was begun. The levels of penetration of fluconazole into gynecological tissues appeared to be similar to or slightly above those in serum samples.
TABLE 2.
Pharmacokinetic parameters of 150 mg of orally administered fluconazole
| Tissue | Parmacokinetic parametera
|
|||
|---|---|---|---|---|
| Cmax (h) | Tmax (h) | t1/2 (h) | AUC0–∞ (μg/ · h/ml or g) | |
| Serum from cubital vein | 3.9 | 6.1 | 33.5 | 214 |
| Serum from uterine artery | 3.8 | 6.0 | 35.4 | 221 |
| Vagina | 3.8 | 6.8 | 30.0 | 194 |
| Cervix uteri | 3.5 | 5.3 | 33.0 | 186 |
| Corpus uteri | 4.0 | 6.2 | 33.9 | 223 |
| Endometrium | 4.1 | 6.2 | 25.5 | 180 |
| Oviduct | 4.5 | 6.6 | 29.1 | 222 |
| Ovary | 3.9 | 4.7 | 36.5 | 221 |
Cmax, maximum concentration of drug in serum; Tmax, time to maximum concentration of drug in serum; t1/2, half-life; AUC0–∞, area under the concentration-time curve from 0 h to infinity.
No abnormal symptoms or laboratory findings attributable to fluconazole were observed in the study.
DISCUSSION
Systemic fungal infections and superficial fungal infections have been increasing in recent years, because of the increasing population of compromised hosts: patients with immune deficiencies due to diseases, immunosuppression as a result of host reactions to organ transplants, and neutropenia from cancer or cancer chemotherapy (1–3, 17, 18).
In humans, the volume of distribution approximates that of total body water (0.8 liter/g). In contrast to the protein binding capacities of other azole antifungals, that of fluconazole is low (approximately 11%), and therefore most fluconazole circulates as a free form (4, 5, 10, 20, 22). Alternatively, fluconazole can penetrate into gynecological tissues in concentrations equivalent to those in serum, as shown in this study. The peak concentrations in each gynecological tissue, such as the uterus, ovaries, and oviducts, were 6.4 to 9.5 μg/g around 1.0 h after the drip infusion was begun, levels which were similar to or slightly above those in serum. The penetration of fluconazole appears to occur rapidly from serum to each genital tissue.
Fluconazole inhibited hyphal branching almost totally at concentrations of 0.2 mg/liter in a morphology study of C. albicans (23). The MICs of fluconazole for pathogenic yeasts fell within achievable concentrations in serum and gynecological tissues even around 50 h after drug administration.
The presented penetration of oral fluconazole into gynecological tissues suggests that fluconazole has a considerable potential for treatment of not only human systemic fungal infections but also vaginal candidiasis with excellent activity.
REFERENCES
- 1.Bodey, G. P. 1986. Fungal infection and fever of unknown origin in neutropenic patients. Am. J. Med. 80(Suppl. 5C):112–119. [PubMed]
- 2.Bodey, G. P. 1986. Infection in cancer patients. Am. J. Med. 81(Suppl. 1A):11–26. [DOI] [PubMed]
- 3.Bodey G P, Fainstein V. Systemic candidiasis. In: Bodey G P, Fainstein V, editors. Candidiasis. New York, N.Y: Raven Press; 1985. pp. 135–168. [Google Scholar]
- 4.Brammer K W. Management of fungal infection in neutropenic patients with fluconazole. Hematol Transfusiol. 1990;33:546–550. doi: 10.1007/978-3-642-74643-7_97. [DOI] [PubMed] [Google Scholar]
- 5.Brammer, K. W., P. R. Farrow, and J. K. Faulkner. 1990. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev. Infect. Dis. 12 (Suppl. 3):318–326. [DOI] [PubMed]
- 6.Cohen J. Treatment of systemic yeast infection with fluconazole. J Antimicrob Chemother. 1989;23:294. doi: 10.1093/jac/23.2.294. [DOI] [PubMed] [Google Scholar]
- 7.Conti D J, Tolkoff-Rubin N E, Baker G E, Jr, Doran M, Cosimi A B, Delmonico F, Auchincloss H, Jr, Russell P S, Rubin R H. Successful treatment of invasive fungal infection with fluconazole in organ transplant recipients. Transplantation. 1989;48:692–694. [PubMed] [Google Scholar]
- 8.El-Yazigi A, Ellis M, Ernst P, Spence D, Hussain R, Baillie F J. Pharmacokinetics of oral fluconazole when used for prophylaxis in bone marrow transplant recipients. Antimicrob Agents Chemother. 1997;41:914–917. doi: 10.1128/aac.41.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiruma K, Oh H, Mirio S, Hirasawa A, Aotsuka N, Wakita H, Endo N, Asai T, Yoshida S, Igarashi T, Ito K, Ishige K, Kashimura M, Itaya T. The prophylactic effect of oral high-dose amphotericin B for fungal infection in the patients with hematological disorders—a comparison with the efficacy of usual-dose amphotericin B. Prog Med. 1990;10:494–500. [Google Scholar]
- 10.Humphrey M J, Jevons S, Tarbit M H. Pharmacokinetic evaluation of UK-49858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother. 1985;28:648–653. doi: 10.1128/aac.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikemoto H, Watanabe K, Mori T, Taniuchi A, Akahonai Y, Mikuni C, Yoshida K, Kasai M, Kawamura K, Yoshida T, Konno K, Oizumi K, Aonuma S, Hayashi I, Itoh A, Shimada K, Oka S, Nakata H, Miyahara T, Shimada J, Hori S, Aoki N, Hirosawa S, Nakamura Y, Ebatake K, Sakurai M, Tanimoto H, Nakatani T, Onozawa Y, Odagiri S, Murohashi K, Suzuki K, Ishii S, Saito H, Shimokata K, Oono R, Hotta T, Yamamoto M, Ina Y, Arakawa H, Nakashima M, Hosoda S, Fujiyama Y, Masaoka T, Shibata H, Tsubura E, Nakagawa M, Nakayama S, Nagai K, Soejima R, Yagi S, Matsushima T, Adachi M, Hitomi Y, Yasuda H, Irino S, Kubota Y, Sawae Y, Ishimaru T, Takagi K, Hara K, Hirota M, Yamaguchi K, Kono S, Hayashi T, Sasayama K, Yasuoka A, Ito N, Okuno K, Oe T, Matsukura S, Tsuruta, Nasu M, Shigeno H, Goto Y, Yamaguchi H, Uchida K. Clinical study of fluconazole on deep-seated fungal infections. Jpn J Antibiot. 1989;42:63–116. [PubMed] [Google Scholar]
- 12.Itoh A. Statistical evaluation of the cases with deep mycosis in Japan. Jpn J Med Mycol. 1980;21:239–248. [Google Scholar]
- 13.John H R, Linda H H, Michael A A, David A S. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1991;35:846–850. doi: 10.1128/aac.35.5.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura I, Kuramoto J, Yahata Y, Irino S, Takahashi I, Sezaki T, Tsubota T, Watanabe Y, Hara M, Hino N, Sugiyama M, Yorimitsu S. A co-operative study on prophylactic effect of oral administration of high-dose amphotericin B syrup for systemic fungal infection in patients with hematological neoplasms. Cancer Chemother. 1990;17:1027–1032. [PubMed] [Google Scholar]
- 15.Mysrowitz R L, Pazin G J, Allen C M. Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology. Am J Clin Pathol. 1977;68:29–38. doi: 10.1093/ajcp/68.1.29. [DOI] [PubMed] [Google Scholar]
- 16.Pfizer Central Research. Unpublished data. Pfizer Central Research, Sandwich, Kent, United Kingdom.
- 17.Pizzo P A. Granulocytopenia and cancer therapy. Cancer. 1984;54:2649–2661. doi: 10.1002/1097-0142(19841201)54:2+<2649::aid-cncr2820541409>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Samonis G, Rolston K, Karl C, Miller P, Bodey G P. Prophylaxis of oropharyngeal candidiasis with fluconazole. Rev Infect Dis. 1990;12:369–373. doi: 10.1093/clinids/12.supplement_3.s369. [DOI] [PubMed] [Google Scholar]
- 19.Shiba K, Saito A, Miyahara T. Pharmacokinetic evaluation of fluconazole in healthy volunteers. Jpn J Antibiot. 1989;42:17–30. [PubMed] [Google Scholar]
- 20.Shiba K, Saito A, Miyahara T. Safety and pharmacokinetics of single oral and intravenous doses of fluconazole in healthy subjects. Clin Ther. 1990;12:206–215. [PubMed] [Google Scholar]
- 21.Urabe A, Takau F, Mizoguchi H, Nomura T, Ogawa T, Maekawa T, Omine M, Miura Y, Hirashima K, Takatani O, Sato N, Wakabayashi Y, Shimada K, Asano S, Fujioka S, Saito T, Togawa A, Yamaguchi H, Mutoh Y, Mori M, Kinugasa K, Shirai T, Murase T, Aoki N, Ohshima T, Toyama K, Tsuruoka N, Yamaguchi H. Prophylactic and therapeutic effects of oral administration of amphotericin B in mycosis associated with hematologic diseases. Jpn J Antibiot. 1990;43:116–130. [PubMed] [Google Scholar]
- 22.Walsh T J, Foulds G, Pizzo P A. Pharmacokinetics and tissue penetration of fluconazole in rabbits. Antimicrob Agents Chemother. 1989;33:467–469. doi: 10.1128/aac.33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi H, Uchida K, Kawasaki K, Matsunaga T. In vitro activity of fluconazole, a novel bistriazole antifungal agent. Jpn J Antibiot. 1989;42:1–16. [PubMed] [Google Scholar]