Abstract
Background
Coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) has been reported in ~5%–10% of critically ill COVID-19 patients. However, incidence varies widely (0%–33%) across hospitals, most cases are unproven, and CAPA definitions and clinical relevance are debated.
Methods
We reframed the debate by asking, what is the likelihood that patients with CAPA have invasive aspergillosis? We use diagnostic test performance in other clinical settings to estimate positive predictive values (PPVs) and negative predictive values (NPVs) of CAPA criteria for invasive aspergillosis in populations with varying CAPA incidence.
Results
In a population with CAPA incidence of 10%, anticipated PPV/NPV of diagnostic criteria are ~30%–60%/≥97%; ~3%–5% of tested cohort would be anticipated to have true invasive aspergillosis. If CAPA incidence is 2%–3%, anticipated PPV and NPV are ~8%–30%/>99%.
Conclusions
Depending on local epidemiology and clinical details of a given case, PPVs and NPVs may be useful in guiding antifungal therapy. We incorporate this model into a stepwise strategy for diagnosing and managing CAPA.
Keywords: Aspergillus, CAPA, coronavirus disease 2019-associated pulmonary aspergillosis, COVID-19, galactomannan
We present a model by which clinicians can estimate the likelihood that critically ill patients with COVID-19 have invasive aspergillosis, using knowledge of local epidemiology and diagnostic test performance. We propose a stepwise approach to diagnostic and treatment decision making.
Pulmonary aspergillosis is well recognized among patients with severe coronavirus disease 2019 (COVID-19) [1, 2]. Nevertheless, the incidence of COVID-19-associated pulmonary aspergillosis (CAPA) remains unclear [3–5]. Rates ranging from 0% to 33% have been reported in critically ill patients with COVID-19 at some hospitals [2, 6]. In general, CAPA incidence has been higher in reports from Europe than in those from North America. Discrepancies between studies may reflect differences in local epidemiology, environmental factors, treatment of COVID-19, thresholds for testing, disease definitions, diagnostic criteria, and patient populations. In studies that have retrospectively applied standardized CAPA definitions, pooled incidence of CAPA in intensive care units (ICUs) was 2% to 11% [3, 7–13]. Only 2% of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-infected decedents in autopsy studies published through September 2020 had histopathologic evidence of aspergillosis or other invasive mould infections; incidence was also 2% among mechanically ventilated decedents [4]. Therefore, robust debate has arisen over the diagnosis of CAPA, its incidence, and clinical relevance [3, 5]. In this article, we reframe the debate over CAPA around the clinical question, what is the likelihood that patients fulfilling diagnostic criteria for CAPA have invasive aspergillosis?
Diagnosing Coronavirus Disease 2019-Associated Pulmonary Aspergillosis
In general, CAPA definitions are based on a combination of clinical and host factors, imaging findings, and mycologic test results in critically ill patients with COVID-19. Advanced age, invasive respiratory support, and receipt of tocilizumab were CAPA risk factors among SARS-CoV-2-infected ICU patients in a multinational study [9]. Chest radiographic or computerized tomography scan findings in CAPA are difficult to distinguish from those of severe COVID-19. Although there is overlap in CAPA criteria proposed by expert panels, correlation between any 2 standardized definitions is modest [13]. Mortality is increased among SARS-CoV-2-infected ICU patients who fulfill CAPA definitions, but it is uncertain how often deaths are attributable to fungal infection [9]. It is clear that CAPA definitions overstate incidence of invasive aspergillosis. Several patients diagnosed with CAPA have survived despite not receiving antifungal therapy; others diagnosed antemortem did not have evidence of fungal disease upon autopsy [4, 19, 20].
Across guidelines, the major driver of diagnosis is detection of Aspergillus in respiratory tract samples by methods such as culture, galactomannan (GM) detection, or polymerase chain reaction (PCR) [14]. These are not definitive diagnostic tests, because they do not distinguish between colonization and disease [15]. The preferred sample for testing, after respiratory tract tissue, is bronchoalveolar lavage (BAL) collected by bronchoscopy [2]. Bronchoscopies of patients with COVID-19 are safe using risk-minimizing protocols, and they are now endorsed for diagnosing coinfections [2, 16, 17]. In general, detection of Aspergillus in BAL in absence of proven tissue invasion constitutes probable disease by consensus definitions, provided other criteria are also fulfilled [2, 7, 10, 11].
Respiratory samples such as sputa, bronchial and tracheal aspirates, and non-bronchoscopic BAL (NBL) are used in many CAPA studies, despite increased potential for upper airway microbial contamination and lack of validation for GM testing or Aspergillus PCR [2, 11, 18]. Limited data suggest that performance of GM detection in NBL may be comparable to that of GM or PCR in BAL [11, 18]. In the absence of other positive diagnostic markers, Aspergillus detection in non-BAL respiratory samples is supportive of possible disease [2, 7, 10, 11]. Blood cultures and serum GM are insensitive for diagnosing pulmonary aspergillosis, but detection in at-risk hosts may reflect disseminated disease [9]. Beta-d-glucan detection in respiratory samples or serum cannot distinguish between Aspergillus, Candida, or other fungi, and it is prone to false positivity.
There are no conclusive data on diagnostic test performance for CAPA, due largely to the paucity of proven cases. Performance of cultures, GM detection, and PCR on BAL for diagnosing invasive aspergillosis is more firmly established in other populations, including in critically ill patients who do not have COVID-19 [21]. Bronchoalveolar lavage galactomannan testing is performed widely in Europe and the United States. Aspergillus PCR is commonly used in the former but not the latter [22]. For illustrative purposes, we will use BAL galactomannan as the representative CAPA diagnostic test, based on its global utilization, validation in other populations, and widespread availability of a commercial, US Food and Drug Administration-cleared sandwich enzyme-linked immunosorbent assay (Platelia Aspergillus EIA). Our approach can be applied to PCR and other tests, and to testing of samples other than BAL, using sensitivities and specificities that are reported or estimated for invasive aspergillosis.
Do Patients Diagnosed With Coronavirus Disease 2019-Associated Pulmonary Aspergillosis Have Invasive Aspergillosis?
Positive predictive values (PPVs) and negative predictive values (NPV) of a test (or diagnostic criteria) are determined by sensitivity, specificity, and pretest disease likelihood. In a Cochrane review of published data, BAL galactomannan sensitivity/specificity for invasive aspergillosis in immunocompromised hosts were 88%/81% and 78%/93% at positive cutoff galactomannan indices ≥0.5 and ≥1.0, respectively [23]. At our center, sensitivity/specificity at the respective cutoffs were 93%/89% and 67%/97% in lung transplant recipients [15]. In a study of a diverse population that included critically ill patients not receiving immunosuppressive drugs, respective values were 93%/87% and 80%/94% [24]. These data can be used to estimate the likelihood that patients diagnosed with CAPA have invasive aspergillosis, assuming that BAL galactomannan test performance for patients with severe COVID-19 is comparable to that reported in other populations (Table 1).
Table 1.
Anticipated Positive and Negative Predictive Values of CAPA Diagnostic Criteria for Invasive Aspergillosis
| CAPA Likelihood | BAL GM Cutoff 0.5 (Sens/Spec: 85%/80%)a |
BAL GM Cutoff 1.0 (Sens/Spec: 75%/90%)a |
BAL GM Cutoff 1.0 (Sens/Spec: 80%/94%)a |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PPV for IPA | NPV for IPA | IPA Incidence | PPV for IPA | NPV for IPA | IPA Incidence | PPV for IPA | NPV for IPA | IPA Incidence | |
| 1% | 4% | >99% | <0.1% | 7% | >99% | <0.1% | 12% | >99% | <0.1% |
| 2% | 8% | >99% | 0.1% | 13% | >99% | 0.2% | 21% | >99% | 0.3% |
| 3% | 12% | >99% | 0.5% | 19% | >99% | 0.5% | 32% | >99% | 1% |
| 5% | 22% | 99% | 1% | 28% | 99% | 1% | 40% | 99% | 2% |
| 10% | 32% | 98% | 3% | 45% | 97% | 3% | 60% | 98% | 5% |
| 15% | 43% | 97% | 6% | 57% | 95% | 6% | 71% | 96% | 9% |
| 20% | 52% | 96% | 9% | 65% | 94% | 10% | 76% | 95% | 12% |
Abbreviations: BAL, bronchoalveolar lavage; CAPA, coronavirus disease 2019-associated pulmonary aspergillosis; GM, galactomannan; PPV, positive predictive value; NPV, negative predictive value; Sens, sensitivity; Spec, specificity.
CAPA has been diagnosed in 0% to 33% of critically ill COVID-19 patients in intensive care units (ICUs) at different hospitals. Optimal BAL galactomannan cutoffs for diagnosing invasive aspergillosis in patients with COVID-19 are not defined [7, 11]. Cutoffs and test performance in non-COVID-19 populations can be used to estimate positive predictive values (PPVs) and negative predictive values (NPVs) for invasive aspergillosis in ICUs with various underlying burdens of CAPA (column 1). Bolded text shows PPVs > 15% and NPVs ≥94%, representing settings in which CAPA criteria might be useful in guiding treatment decisions. PPVs ≥15%–30% may be sufficiently high to justify empiric antifungal treatment, depending on constellation of clinical findings and other data in individual patients (Table 3). NPVs are likely high enough to justify withholding antifungal treatment. Clinicians can modify calculations based on local epidemiology and knowledge of test performance.
If a critically ill patient with COVID-19 meets CAPA definitions in a population in which 10% of patients have CAPA (ie, median incidence in the recent multinational study, and upper range of pooled incidence in studies that retrospectively applied standardized criteria) [9, 13], then the estimated probability of invasive aspergillosis based on BAL galactomannan index ≥0.5 would be 32% (ie, PPV, assuming sensitivity/specificity of 85%/80%). At cutoff BAL galactomannan index ≥1, the estimated likelihood of invasive aspergillosis would be 45% to 60%, over the range of sensitivities/specificities cited in the preceding paragraph. In contrast, if pretest likelihood of meeting CAPA definitions is only 2% (ie, low range of pooled incidence with standardized diagnostic criteria), expected likelihoods of invasive aspergillosis given BAL galactomannan indices ≥0.5 and ≥1 would be reduced to 8% and 13% to 21%, respectively. At 5% pretest likelihood, corresponding expected probabilities of invasive aspergillosis would be 22% and 28% to 40%.
Patients in groups above would be extremely unlikely to have invasive aspergillosis if BAL galactomannan was negative (expected NPVs 97% to >99%). For populations in which 2%, 5%, and 10% of patients fulfill criteria for CAPA, the anticipated incidence of true invasive aspergillosis is approximately 0.5% to 1%, 1% to 2%, and 3% to 5%, respectively (across the range of BAL galactomannan performance in Table 1). These values are broadly in keeping with the 2% incidence of invasive mould infections from autopsy studies of mechanically ventilated patients dying with COVID-19 [4]. Therefore, aggregate clinical and autopsy data on CAPA are largely in agreement; seeming discrepancies between results of antemortem and postmortem studies reflect that the latter, for the most part, have described a subset of the former with invasive disease (Figure 1). Based on these considerations, we propose definitions that may be useful in thinking about, and distinguishing between, CAPA and invasive aspergillosis (Table 2).
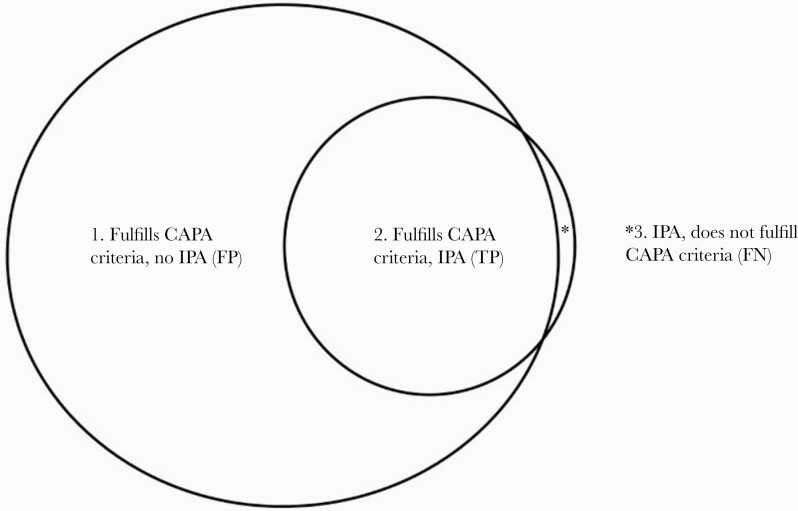
Figure 1.
Coronavirus disease 2019-associated pulmonary aspergillosis (CAPA) and invasive pulmonary aspergillosis (IPA). The relationship between CAPA and IPA in critically ill patients with coronavirus disease 2019 (COVID-19) is represented by a Venn diagram. Coronavirus disease 2019-associated pulmonary aspergillosis criteria (large circle on left) signify the likely presence of Aspergillus in the respiratory tract. Invasive pulmonary aspergillosis (small circle on right) is defined by Aspergillus invasion and attendant damage of respiratory tract tissue. Some patients who fulfill CAPA diagnostic criteria have IPA (group 2), but others do not (group 1). In groups 1 and 2, a diagnosis of CAPA can be considered false positive (FP) or true positive (TP) for IPA, respectively. Several critically ill patients with COVID-19 may have IPA without fulfilling criteria for CAPA (group 3, represented by the asterisk in the Venn diagram). In this group, CAPA is false negative (FN) for IPA. It is plausible, but as yet unproven, that IPA in some patients is preceded by CAPA that represents Aspergillus colonization of the respiratory tract.
Table 2.
Definitions of CAPA and Invasive Aspergillosis
| Entity | Definition |
|---|---|
| CAPA | The likely presence of Aspergillus in the respiratory tract of patients with COVID-19, which may or may not be associated with tissue invasion and damage. It is plausible that CAPA representing respiratory tract colonization is a risk factor for development of invasive aspergillosis. |
| Invasive aspergillosis | Invasive Aspergillus infection of organs with attendant tissue damage, most commonly in the lungs, bronchi, trachea, or sinuses |
Abbreviations: CAPA, coronavirus disease 2019-associated pulmonary aspergillosis; COVID-19, coronavirus. disease 2019.
Combination testing has been proposed to improve diagnosis of CAPA [11], but its value is unproven. Multiple tests are powerful if results are concordant, because the likelihood ratio for combined tests is the product of likelihood ratios for individual tests. If results are discordant, however, a positive test is at least partially offset by a negative result with another test. It is fair to assume that positive or negative results by multiple tests make invasive aspergillosis more and less likely, respectively, while recognizing that discordant test results are difficult to interpret. For any test, more strongly positive and repeatedly positive results increase the probability of true disease.
Calculations above likely represent a best-case scenario for PPV of CAPA. The PPVs and NPVs in Table 1 are most relevant to patients who meet criteria for probable, rather than possible disease. In clinical practice, false positivity is likely to be greater for those with possible CAPA, because definitions are typically based on assays of non-BAL respiratory samples that have increased propensity to microbial contamination. The assumption that diagnostic test performance in critically ill patients with COVID-19 is comparable to that in other populations is unproven.
How Should Clinicians Approach the Diagnosis and Treatment of Coronavirus Disease 2019-Associated Pulmonary Aspergillosis?
Antifungal treatment of probable and possible CAPA is endorsed by at least some consensus guidelines [2], although it has not been associated with lower mortality in retrospective studies. In absence of clinical trial data, we recommend a 6-step approach to diagnosing and managing CAPA (Table 3).
Table 3.
Stepwise Approach to Diagnosis and Management of CAPA
| Step | Objectives | Comments |
|---|---|---|
| Understand local epidemiology of CAPA and aspergillosis | Use retrospective reviews and pathology/autopsy data to get rough estimate of burdens at your hospital | Pilot data for CAPA incidence locally may be useful. Historic incidence of aspergillosis in vulnerable populations (eg, transplant) and ICUs may give sense of relative local burdens |
| 2.Define at-risk patient populations for CAPA | Use local data and review of published literature to define risk factors relevant at your hospital | Test performance, PPVs and NPVs will be most useful if testing is directed toward populations with reasonable pretest likelihoods of aspergillosis, rather than including all patients with COVID-19 |
| 3.Estimate PPVs and NPVs given approximate pretest likelihoods | Use data from steps 1 and 2 to calculate estimated PPVs and NPVs (Table 1) | Even if exact numbers are not available, it may be possible to approximate PPVs and NPVs for aspergillosis within ranges, and classify these as relatively low, medium, or high |
| 4.Develop strategies to direct testing to at-risk populations | Engage clinical services relevant to at-risk patients to develop testing, interpretive and management protocols | Many services are involved in care of critically ill patients with COVID-19. Engagement with and buy-in from services will improve compliance with protocols and treatment recommendations. Directed testing rather than routine surveillance testing will decrease false positives for aspergillosis |
| 5.Determine thresholds to justify antifungal treatment | Develop treatment protocols based on estimated PPVs and NPVs, using team approach | Agree among clinical and stewardship services on likelihoods of aspergillosis that justify treatment, and how much potential antifungal overtreatment you are willing to tolerate |
| 6.Individualize decisions in each patient | Make treatment decisions for each patient by considering clinical data and case details | In each patient, clinical parameters (eg, new findings, lack of alternative diagnoses, length of stay, etc), radiography (eg, new lesions), and laboratory data (eg, higher values, repeat or multiple positive results, etc) may refine assessments of disease likelihood and need for treatment |
Abbreviations: CAPA, coronavirus disease 2019-associated pulmonary aspergillosis; COVID-19, coronavirus; ICU, intensive care unit; NPV, negative predictive value; PPV, positive predictive value.
In determining whether to treat a patient with CAPA, clinicians must decide upon the threshold likelihood of invasive aspergillosis that would trigger empiric antifungal therapy. Threshold PPVs or NPVs that justify treatment decisions for aspergillosis or other fungal infections are not firmly established. Data in immunosuppressed or critically ill patients suggest that antifungal prophylaxis is beneficial in preventing invasive fungal infections when baseline disease rates are ≥15% to 30% [25]. Hypothesizing that this target range encompasses a threshold for empiric treatment, we can identify settings in which likelihood of invasive aspergillosis (ie, PPV) is expected to be above these values for patients with CAPA (Table 1, shaded boxes). In populations in which ≥10% of patients with COVID-19 have CAPA, anticipated PPVs of diagnostic criteria are>30% for invasive aspergillosis over a range of BAL galactomannan sensitivities/specificities and cutoffs. In a critically ill population with 5% CAPA incidence, anticipated PPVs for invasive aspergillosis are >20% given the same BAL GM performance. If CAPA incidence is 2%–3%, anticipated PPVs are >15% if BAL GM sensitivity and specificity are ≥75% and ≥90%, respectively. Therefore, depending on one’s threshold to treat, reasonable cases can be made for empiric antifungal therapy, other than in ICUs in which very low percentages (approximately ≤2% to 5%) of patients meet CAPA diagnostic criteria. Furthermore, excellent NPVs support withholding prophylactic antifungals for ICU residents who do not have CAPA. In adapting this model, clinicians can revise PPV and NPV calculations to reflect their ICU populations and new or local data on diagnostic test performance.
Discussion here highlights the importance of understanding local epidemiology and of applying these insights to decisions about CAPA testing and treatment. If epidemiology at a hospital is unknown, perspective may be provided by a quality improvement review of invasive fungal infections in traditional high-risk populations. Indiscriminate testing of COVID-19 patients for CAPA is likely to be counterproductive or deleterious. Centers should identify, as best as possible, (1) their critically ill COVID-19 subpopulations at increased risk for CAPA and (2) direct diagnostic testing and management algorithms toward them. Priority subpopulations can be defined by demographic and clinical factors identified using local data and/or published studies. Examples of such factors might be ICU stays ≥3 days (particularly prolonged stays), receipt of tocilizumab, or other anti-interleukin-6 agents, invasive respiratory support, worsening respiratory status in absence of established etiology despite optimized COVID-19 and antibacterial treatment, new or evolving imaging findings, and tracheobronchial lesions. Successful strategies are best developed and implemented as collaborations between clinical services caring for critically ill COVID-19 patients. Treatment decisions must be individualized. Calculations such as those in Table 1 are starting points for interpreting diagnostic testing for aspergillosis, but they are useful only in context of all clinical data for a patient. In the end, the likelihood of disease and need for treatment is determined by the clinician, not by any single test or criterion. Indeed, each piece of clinical information and data should be considered as its own “test”, results of which increase or decrease the likelihood of invasive aspergillosis. Management of any patient, then, is shaped by combination of these results.
In most cases, it is infeasible to calculate a precise running tally of disease likelihood. However, clinicians can make qualitative assessments to guide decision making. An example is as follows: “This mechanically ventilated patient with COVID-19 who was treated with tocilizumab has worsening respiratory status and imaging. The work-up thus far is negative. Positive respiratory tract galactomannan increases the likelihood of invasive aspergillosis such that I am going to treat empirically. Negative respiratory tract galactomannan and fungal culture make invasive aspergillosis unlikely, so I am comfortable holding antifungal treatment even though the patient has some risk factors.”
CONCLUSIONS
This article proposes a conceptual framework for approaching CAPA, which can also serve as a model for aspergillosis in other critically ill populations, including those with severe influenza or other respiratory viral infections [26, 27]. Ideally, diagnostic criteria and management recommendations for CAPA would be investigated in clinical trials. However, given poor outcomes of patients diagnosed with CAPA, randomized placebo-controlled antifungal treatment trials are unlikely. A randomized, multicenter trial of isavuconazole versus placebo for prevention of CAPA was canceled due to insufficient enrollment (Isavu-CAPA Trial; ClinicalTrials.gov ID NCT04707703). Therefore, clinicians should weigh concepts here in managing patients, while understanding that they are not validated. For ICUs in which the incidence of CAPA is 5%–10%, our analysis suggests that PPV of CAPA diagnostic criteria is ≥30% for invasive aspergillosis (Table 1). Clinicians may decide that this PPV is sufficiently high to justify empiric antifungal therapy in at-risk patients meeting definitions of CAPA, depending on the constellation of clinical findings and other data (Table 3).
Clinicians also must remember that Candida and, in certain locations, Mucorales can be important causes of COVID-19 superinfections [28, 29]. These fungi are not detected by galactomannan or Aspergillus-specific PCR. Mucorales typically cause rhinosinusitis, central nervous system, and multifocal disease in patients with COVID-19, which may be mixed with Aspergillus and other moulds [29]. Unlike aspergillosis, candidiasis rarely involves sinuses, upper airways, or lung parenchyma [30]. In the end, understanding of local epidemiology is crucial to developing optimal strategies for diagnosing and treating COVID-19-associated fungal infections.
Acknowledgments
Potential conflicts of interest. C. J. C. has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for studies unrelated to this project, served on advisory boards or consulted for Astellas, Merck, the Medicines Company, Cidara, Scynexis, Shionogi, Qpex, and Needham & Company, and spoken at symposia sponsored by Merck and T2Biosystems. M. H. N. has been awarded investigator-initiated research grants from Astellas, Merck, Pulmocide, and Scynexis for studies unrelated to this project, and served on advisory boards or consulted for Astellas, Pulmocide, and Scynexis. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Thompson GR III, Cornely OA, Pappas PG, et al. Invasive aspergillosis as an under-recognized superinfection in COVID-19. Open Forum Infect Dis 2020; 7:ofaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verweij PE, Bruggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med 2021; 47:819–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fekkar A, Neofytos D, Nguyen MH, Clancy CJ, Kontoyiannis DP, Lamoth F.. COVID-19-associated pulmonary aspergillosis (CAPA): how big a problem is it? Clin Microbiol Infect 2021; 27:1376–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kula BE, Clancy CJ, Hong Nguyen M, Schwartz IS.. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe 2021; 2:e405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP.. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): a comparison with influenza associated aspergillosis (IAPA). J Infect Dis 2021. doi: 10.1093/infdis/jiab163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clancy CJ, Nguyen MH.. Coronavirus disease 2019, superinfections, and antimicrobial development: what can we expect? Clin Infect Dis 2020; 71:2736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 2021; 21:e149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chong WH, Neu KP.. Incidence, diagnosis and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect 2021; 113:115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prattes J, Wauters J, Giacobbe DR, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients-a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect 2021. doi: 10.1016/j.cmi.2021.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verweij PE, Gangneux JP, Bassetti M, et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020; 1:e53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis 2021; 73:e1634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bassetti M, Azoulay E, Kullberg BJ, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis 2021; 72:S121–7. [DOI] [PubMed] [Google Scholar]
- 13. Kariyawasam RM, Dingle TC, Kula BE, et al. COVID-19 associated pulmonary aspergillosis: systematic review and patient-level meta-analysis [preprint]. medRxiv 2022. doi: 10.1016/j.cmi.2022.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yusuf E, Seghers L, Hoek RAS, van den Akker JPC, Bode LGM, Rijnders BJA.. Aspergillus in critically ill COVID-19 patients: a scoping review. J Clin Med 2021; 10:2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luong ML, Clancy CJ, Vadnerkar A, et al. Comparison of an Aspergillus real-time polymerase chain reaction assay with galactomannan testing of bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in lung transplant recipients. Clin Infect Dis 2011; 52:1218–26. [DOI] [PubMed] [Google Scholar]
- 16. Pritchett MA, Oberg CL, Belanger A, et al. Society for Advanced Bronchoscopy Consensus Statement and Guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis 2020; 12:1781–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lormans P, Blot S, Amerlinck S, Devriendt Y, Dumoulin A.. COVID-19 acquisition risk among ICU nursing staff with patient-driven use of aerosol-generating respiratory procedures and optimal use of personal protective equipment. Intensive Crit Care Nurs 2021; 63:102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Biesen S, Kwa D, Bosman RJ, Juffermans NP.. Detection of invasive pulmonary aspergillosis in COVID-19 with non-directed bronchoalveolar lavage. Am J Respir Crit Care Med 2020; 202:1171–3. doi: 10.1164/rccm.202005-2018LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alanio A, Delliere S, Fodil S, Bretagne S, Megarbane B.. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med 2020; 8:e48–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flikweert AW, Grootenboers M, Yick DCY, et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care 2020; 59:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blot SI, Taccone FS, Van den Abeele AM, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 2012; 186:56–64. [DOI] [PubMed] [Google Scholar]
- 22. Permpalung N, Chiang TP, Massie AB, et al. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis 2021; 74:83–91. doi: 10.1093/cid/ciab223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Heer K, Gerritsen MG, Visser CE, Leeflang MM.. Galactomannan detection in broncho-alveolar lavage fluid for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev 2019; 5:CD012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. D’Haese J, Theunissen K, Vermeulen E, et al. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol 2012; 50:1258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clancy CJ, Nguyen MH.. Rapid diagnosis of invasive candidiasis: ready for prime-time? Curr Opin Infect Dis 2019; 32:546–52. [DOI] [PubMed] [Google Scholar]
- 26. Apostolopoulou A, Clancy CJ, Skeel A, Nguyen MH.. Invasive pulmonary aspergillosis complicating non-influenza respiratory viral infections in solid organ transplant recipients. Open Forum Infect Dis 2021; 8:ofab478. doi: 10.1093/ofid/ofab478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verweij PE, Rijnders BJA, Bruggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 2020; 46:1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White PL, Dhillon R, Healy B, Wise MP, Backs M.. Candidaemia in COVID -19, a link to disease pathology or increased clinical pressures? Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1597 [DOI] [Google Scholar]
- 29. Joshi S, Telang R, Tambe M, et al. Outbreak of mucormycosis in coronavirus disease patients, Pune, India. Emerg Infect Dis 2021; 28:1–8. doi: 10.3201/eid2801.211636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Senger SS, Thompson GR 3rd, Samanta P, Ahrens J, Clancy CJ, Nguyen MH.. Candida empyema thoracis at two academic medical centers: new insights into treatment and outcomes. Open Forum Infect Dis 2021; 8:ofaa656. [DOI] [PMC free article] [PubMed] [Google Scholar]