Abstract
Background
We studied humoral responses after coronavirus disease 2019 (COVID-19) vaccination across varying causes of immunodeficiency.
Methods
Prospective study of fully vaccinated immunocompromised adults (solid organ transplant [SOT], hematologic malignancy, solid cancers, autoimmune conditions, human immunodeficiency virus [HIV]) versus nonimmunocompromised healthcare workers (HCWs). The primary outcome was the proportion with a reactive test (seropositive) for immunoglobulin G to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain. Secondary outcomes were comparisons of antibody levels and their correlation with pseudovirus neutralization titers. Stepwise logistic regression was used to identify factors associated with seropositivity.
Results
A total of 1271 participants enrolled: 1099 immunocompromised and 172 HCW. Compared with HCW (92.4% seropositive), seropositivity was lower among participants with SOT (30.7%), hematological malignancies (50.0%), autoimmune conditions (79.1%), solid tumors (78.7%), and HIV (79.8%) (P < .01). Factors associated with poor seropositivity included age, greater immunosuppression, time since vaccination, anti-CD20 monoclonal antibodies, and vaccination with BNT162b2 (Pfizer) or adenovirus vector vaccines versus messenger RNA (mRNA)-1273 (Moderna). mRNA-1273 was associated with higher antibody levels than BNT162b2 or adenovirus vector vaccines after adjusting for time since vaccination, age, and underlying condition. Antibody levels were strongly correlated with pseudovirus neutralization titers (Spearman r = 0.89, P < .0001), but in seropositive participants with intermediate antibody levels, neutralization titers were significantly lower in immunocompromised individuals versus HCW.
Conclusions
Antibody responses to COVID-19 vaccines were lowest among SOT and anti-CD20 monoclonal recipients, and recipients of vaccines other than mRNA-1273. Among those with intermediate antibody levels, pseudovirus neutralization titers were lower in immunocompromised patients than HCWs. Additional SARS-CoV-2 preventive approaches are needed for immunocompromised persons, which may need to be tailored to the cause of immunodeficiency.
Keywords: COVID-19 vaccines, SARS-COV-2 antibody, immunocompromised, SARS-CoV-2 neutralization
In this prospective study of 1271 participants, seropositivity after COVID-19 vaccination varied by condition: HCW (92.4%), HIV (79.8%), autoimmune conditions (79.1%), solid tumors (78.7%), hematological malignancies (50.0%), and SOT (30.7%). Additional strategies are needed to protect immunocompromised patients from COVID-19.
Recent studies in immunocompromised individuals have shown that coronavirus disease 2019 (COVID-19) vaccines elicit poor antibody responses [1–4]. Several unknowns persist, however, including factors associated with inadequate humoral responses across varied causes of immunocompromising conditions, and whether antibodies from immunocompromised individuals have similar neutralizing ability as those of nonimmunocompromised individuals. To address these knowledge gaps, we performed the COVID-19 Vaccination in the Immunocompromised Study (COVICS). Our objectives were to measure antibody responses and neutralization titers after COVID-19 vaccination in individuals with a broad range of immunocompromising conditions compared to nonimmunocompromised healthcare workers (HCWs).
METHODS
COVICS is a prospective observational electronic medical record (EMR)-embedded study of adults who had completed their COVID-19 vaccine series. The study was approved by the University of Pittsburgh institutional review board (IRB; study 21030056). Enrollment began on April 14, 2021, and occurred online. To obtain serum across the University of Pittsburgh Medical Center (UPMC) Health System, a study-specific severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) order was built in the EMR without billing of the patient. Serum could be drawn at any of 16 UPMC hospital-based laboratories across Western Pennsylvania. Test results were shared via the EMR.
Enrollment
Detailed information on recruitment materials (all of which were IRB approved), the consent process, and enrollment metrics can be found in the Supplement (including Supplementary Figures S8 and S9). Briefly, the study primarily used online infrastructure for advertisement and enrollment. HCWs expressed interest about participating in the study after receiving information through word of mouth or department-wide e-mails. Immunocompromised individuals primarily self-referred to the study after learning about it through direct messages sent to them via our patient portal MyUPMC (25%), a UPMC newsletter e-mail (24%), a disease specialist (21%), or another member of the healthcare team (11%); additional recruitment materials are described in Supplementary Figure S8. A link to the study website and a phone number for the study team were provided in all enrollment materials. Once an individual expressed interest in participating, his or her medical record was reviewed to confirm eligibility. Eligible participants were contacted by a clinical research coordinator (CRC) or the principal investigator (PI, G.H.) and given the option to either enroll on the phone with the CRC or PI, or to self-enroll by watching an 8-minute, IRB-approved, Research Electronic Data Capture (REDCap)-embedded video of the PI describing the study. Participants who opted to self-enroll were also given the option to ask questions, after which they were contacted by a CRC or the PI before signing the consent form. Participants who preferred to enroll in person in a clinic were scheduled for a research visit. All participants were required to sign an electronic or paper consent form; once signed, an antibody order was placed by a CRC (or PI) in the EMR, cosigned by the PI, and then sent via e-mail to the participant.
Participants
Immunocompromised individuals were eligible if they had any of the following: solid organ transplantation (SOT), hematological malignancy, solid cancer undergoing systemic or radiation therapy within the prior 12 months, autoimmune or chronic inflammatory disease undergoing therapy within the prior 12 months, and human immunodeficiency virus (HIV). For the control arm, we enrolled nonimmunocompromised HCWs. Participants with a known history of COVID-19 were excluded. Prior COVID-19 was ascertained by participant (immunocompromised patient or HCW) self-report, then confirmed by manual chart review for positive SARS-CoV-2 polymerase chain reaction (PCR) results within the UPMC EMR, conducted by either the PI (G.H.) or the study coordinators. All participants were required to have completed vaccination with 2 doses of a messenger RNA (mRNA) vaccine (mRNA-1273 [Moderna] or BNT162b2 [Pfizer]) or the adenovirus vaccine ChAdOx1 nCoV-19 (AstraZeneca), or a single dose of the adenovirus vaccine Ad26.COV2.S (Johnson & Johnson) at least 14 days before testing.
Data Collection and Outcomes
Data were collected using the REDCap hosted at the University of Pittsburgh [5]. For medical history adjudication, a 2-step process was used: participants self-reported their own underlying immunocompromising conditions, which were then confirmed by the PI (G.H.) or a CRC by manual chart review. Our online enrollment system was designed to not allow HCWs to enroll should they answer “yes” to having an immunocompromising condition, which was also confirmed by manual chart review. The REDCap database asked participants to record (by multiple choice, with a free-text option) specific categories of medications they were taking, primarily immunosuppressive drugs for SOT or autoimmune conditions, whether they were receiving antiretroviral therapy for HIV, and whether they were receiving systemic therapy or radiation therapy for cancer. Medical records for all participants with missing or incomplete medication data were manually reviewed by the PI (G.H.) or a CRC (L.C.), who then updated the REDCap records. G.H. and L.C. also confirmed all plasma HIV RNA levels and CD4+ T-cell counts. Specific cancer chemotherapy drugs and other nonimmunocompromising comorbidities were extracted from the EMR with the assistance of UPMC’s Clinical Analytics Group (K.C.).
Serum was processed at UPMC’s Clinical Laboratory Improvement Amendments 88-accredited central laboratory, then aliquoted for additional testing at the University of Pittsburgh's Division of Infectious Diseases laboratories. The primary outcome was the proportion of immunocompromised individuals versus HCW with a reactive (seropositive) Beckman Coulter assay (see the following section) for IgG to SARS-CoV-2 spike receptor-binding domain (RBD). We also compared the distribution of antibody levels across subgroups, compared IgG levels with RBD with those of another assay (Bio-Rad Bio-Plex; see the following section), and performed pseudovirus neutralization assays (described later) in a subset of participants.
Antibody Assays
Serum was tested using the Beckman Coulter SARS-CoV-2 platform (IgG against the spike protein RBD) per the manufacturer’s instructions [6–8]. Serum IgG results are expressed as extinction coefficient signal/cutoff (S/CO) ratios or “levels” and interpreted as reactive (≥ 1.00), equivocal (0.80–1.00), or nonreactive (≤ 0.80) [8]. For data analysis, reactive results were defined as seropositive, and equivocal or nonreactive results were defined as seronegative. Sera from a subset of 245 participants (197 immunocompromised and 48 HCWs), stratified by S/CO antibody level (97 with levels < 1, 79 with levels 1–10, and 69 with levels > 10), also underwent testing for IgG to RBD using the Bio-Rad Bio-Plex Pro Human SARS-CoV-2 Serology Assay, as previously described [9] (characteristics in Supplementary Table S1).
Pseudovirus Neutralization Assays
To determine the ability of serum from vaccinated individuals to neutralize SARS-CoV-2, sera from 100 study participants (50 immunocompromised, 50 HCWs) underwent testing using a previously reported pseudovirus neutralization assay [10] (characteristics in Supplementary Table S2). Sera were selected based on S/CO antibody levels (17 with levels < 1 [all immunocompromised], 42 with levels 1–10 [17 immunocompromised, 25 HCWs], and 41 with levels > 10 [16 immunocompromised, 25 HCWs]). Serially diluted sera were incubated in the presence of SARS-CoV-2 pseudovirus (S gene from Wuhan-hu-1/lineage B with D614G mutation) and used to infect 293T-hACE2 cells with luminescence measured after 48 hours (Supplement). The proportion of cells infected by SARS-CoV-2 in the presence of sera from vaccinated individuals was calculated. Results are reported as the highest serum dilution that neutralizes > 50% of the pseudovirus (NT50) [10].
Statistical Methods
Baseline characteristics, seropositivity with 95% Clopper-Pearson exact confidence intervals (CIs), antibody levels, and NT50 were compared between immunocompromised participants and HCW using 2-sample Student t tests, Wilcoxon rank-sum tests, or χ2 tests as appropriate. Within each group, these same variables were presented descriptively by underlying condition. For the binary outcome variable of seropositive versus seronegative, we computed the unadjusted odds ratio (ORs) and 95% CI for the individual risk factors. Stepwise multivariable logistic regression analysis was then used to calculate adjusted ORs for seropositivity, which included factors found to be associated with seropositivity at the P < .10 level. Throughout the text, only adjusted ORs are provided, which represent the ORs of seropositivity (or of being seropositive); the tables show both adjusted and unadjusted ORs for seropositivity. We performed an additional exploratory analysis using antibody levels as a continuous outcome measure, with the same independent variables used to calculate the ORs for seropositivity. These results are presented as incidence rate ratios in the supplement (Supplementary Tables S11–S16). The Spearman correlation coefficient was estimated between antibody levels (Beckman assay) and pseudovirus NT50, and between antibody levels by the Beckman and Bio-Rad assays. Analyses were performed using Stata SE, version 16.1 (College Station, TX). Two-sided tests with an α = .05 was used to denote statistical significance.
RESULTS
Participants
Between April 14 and July 19, 2021, 1271 participants were enrolled: 1099 immunocompromised participants (86.5%) and 172 HCWs (13.5%) (Table 1). All HCWs (100%) self-enrolled by watching a REDCap video; immunocompromised individuals primarily either self-enrolled (46.7%) or enrolled virtually with a CRC or the PI (53.2%), with only 0.1% being enrolled in clinics (Supplementary Figure S9). The immunocompromised group included 450 participants with SOT (41.0%), 263 with autoimmune conditions (23.9%), 156 with hematological malignancies (14.2%), 136 with solid tumors (12.4%), and 94 with HIV (8.6%). Most participants received the mRNA-1273 (48.3%, 614/1271) or BNT162b2 vaccines (50.7%, 644/1271). Only 1.0% (13/1271) received an adenovirus vector vaccine (Ad26.COV2.S [85%, 11/13]; ChAdOx1 nCoV-19, 15% [2/13]). Compared with HCWs, immunocompromised participants were older (median age for HCW versus immunocompromised 42.6; interquartile range [IQR], 34.2, 57.4 vs 63.1 [52.5, 69.7], respectively, P < .001) and less likely to be female (75.0% vs 50.0% respectively, P < .001). Additionally, immunocompromised participants were much more likely to have underlying comorbidities (cardiac, cerebral, or peripheral vascular disease, pulmonary disease, diabetes, chronic kidney disease, dyslipidemia, and liver disease), with the exception of obesity, which was equally prevalent between HCWs and immunocompromised participants (Table 1). Days from vaccination to antibody level drawn was longer for HCWs compared with immunocompromised participants (median [IQR] 132.5 [116.5, 148.5] vs 94 [69, 119] days, respectively, P < .001), reflecting earlier vaccine rollout for HCWs.
Table 1.
Descriptive Characteristics in HCWs and Immunocompromised Participants
| Characteristic (N = 1271) |
HCWs (N = 172) (13.5%) |
Immunocompromised (N = 1099) (86.5%) | P a | Participants by Immunocompromising Condition | ||||
|---|---|---|---|---|---|---|---|---|
| SOT (N = 450) (41.0%) | Autoimmune (N = 263) (23.9%) | Hematologic Malignancy (N = 156) (14.2%) | HIV (N = 94) (8.6%) | Solid Tumor (N = 136) (12.4%) | ||||
| Age in y, mean (SD) | 44.2 (13.3) | 60.1 (13.3) | <.001 | 60.3 (13.0) | 55.3 (14.8) | 67.7 (10.3) | 57.4 (10.0) | 61.7 (12.2) |
| Age in y, n (%) | ||||||||
| 19–44 | 99 (57.6%) | 172 (15.7%) | 66 (14.7%) | 72 (27.4%) | 5 (3.2%) | 10 (10.6%) | 19 (14.0%) | |
| 45–60 | 50 (29.1%) | 300 (27.3%) | 118 (26.2%) | 76 (28.9%) | 21 (13.5%) | 49 (52.1%) | 36 (26.5%) | |
| >60 | 23 (13.4%) | 627 (57.1%) | 266 (59.1%) | 115 (43.7%) | 130 (83.3%) | 35 (37.2%) | 81 (59.6%) | |
| Sex, n (%) | <.001 | |||||||
| Female | 129 (75.0%) | 549 (50.0%) | 170 (37.8%) | 189 (71.9%) | 72 (46.2%) | 10 (10.6%) | 108 (79.4%) | |
| Male | 43 (25.0%) | 550 (50.0%) | 280 (62.2%) | 74 (28.1%) | 84 (53.8%) | 84 (89.4%) | 28 (20.6%) | |
| Race, n (%) | 0.49 | |||||||
| Non-White | 16 (9.3%) | 85 (7.7%) | 42 (9.3%) | 16 (6.1%) | 9 (5.8%) | 14 (14.9%) | 4 (2.9%) | |
| White | 156 (90.7%) | 1014 (92.3%) | 408 (90.7%) | 247 (93.9%) | 147 (94.2%) | 80 (85.1%) | 132 (97.1%) | |
| Comorbidities, n (%)b | ||||||||
| Cardiac disease | 42 (25.1%) | 694 (66.2%) | <.001 | 390 (89.2%) | 111 (44.9%) | 74 (51.4%) | 61 (66.3%) | 58 (45.0%) |
| CVD | 2 (1.2%) | 91 (8.7%) | <.001 | 58 (13.3%) | 10 (4.0%) | 12 (8.3%) | 6 (6.5%) | 5 (3.9%) |
| PVD | 0 (0.0%) | 2 (0.2%) | <.001 | 2 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Pulmonary disease | 38 (22.8%) | 356 (33.9%) | 0.003 | 142 (32.5%) | 93 (37.7%) | 41 (28.5%) | 37 (40.2%) | 43 (33.3%) |
| Diabetes | 7 (4.2%) | 271 (25.8%) | <.001 | 196 (44.9%) | 24 (9.7%) | 18 (12.5%) | 24 (26.1%) | 9 (7.0%) |
| CKD | 0 (0.0%) | 338 (32.2%) | <.001 | 296 (67.7%) | 8 (3.2%) | 12 (8.3%) | 16 (17.4%) | 6 (4.7%) |
| Obesity | 89 (53.3%) | 543 (51.8%) | 0.713 | 247 (56.5%) | 120 (48.6%) | 66 (45.8%) | 43 (46.7%) | 67 (51.9%) |
| DLD | 47 (28.1%) | 598 (57.0%) | <.001 | 305 (69.8%) | 100 (40.5%) | 83 (57.6%) | 54 (58.7%) | 56 (43.4%) |
| Liver disease | 8 (4.8%) | 183 (17.4%) | <.001 | 120 (27.5%) | 28 (11.3%) | 15 (10.4%) | 13 (14.1%) | 7 (5.4%) |
| Vaccine type, n (%) | 0.19 | |||||||
| mRNA-1273 (Moderna) | 73 (42.4%) | 541 (49.2%) | 217 (48.2%) | 133 (50.6%) | 86 (55.1%) | 29 (30.9%) | 76 (55.9%) | |
| BNT162b2 (Pfizer) | 96 (55.8%) | 548 (49.9%) | 228 (50.7%) | 127 (48.3%) | 70 (44.9%) | 63 (67.0%) | 60 (44.1%) | |
| Adenovirus | 3 (1.7%) | 10 (0.9%) | 5 (1.1%) | 3 (1.1%) | 0 (0.0%) | 2 (2.1%) | 0 (0.0%) | |
| Days from second vaccine to antibody sample, median (IQR) | 132.5 (118.0,150.0) | 93.0 (69.0,118.0) | <.001 | 92.0 (68.0,118.0) | 91.0 (68.0,114.0) | 94.5 (73.0,113.0) | 85.5 (62.0,105.0) | 111.0 (84.0,133.0) |
Abbreviations: CKD, chronic kidney disease; CVD, cerebrovascular disease; DLD, dyslipidemia; HCWs, healthcare workers; HIV, human immunodeficiency virus; IQR, interquartile range; mRNA, messenger RNA; PVD, peripheral vascular disease; SOT, solid organ transplant.
P values are the comparisons between HCWs and all immunocompromised patients calculated by likelihood ratio χ2 test or Wilcoxon rank-sum test.
Comorbidities extracted from the University of Pittsburgh Medical Center electronic medical record; data available for 167 HCWs and 1049 immunocompromised participants.
Outcomes
Seropositivity
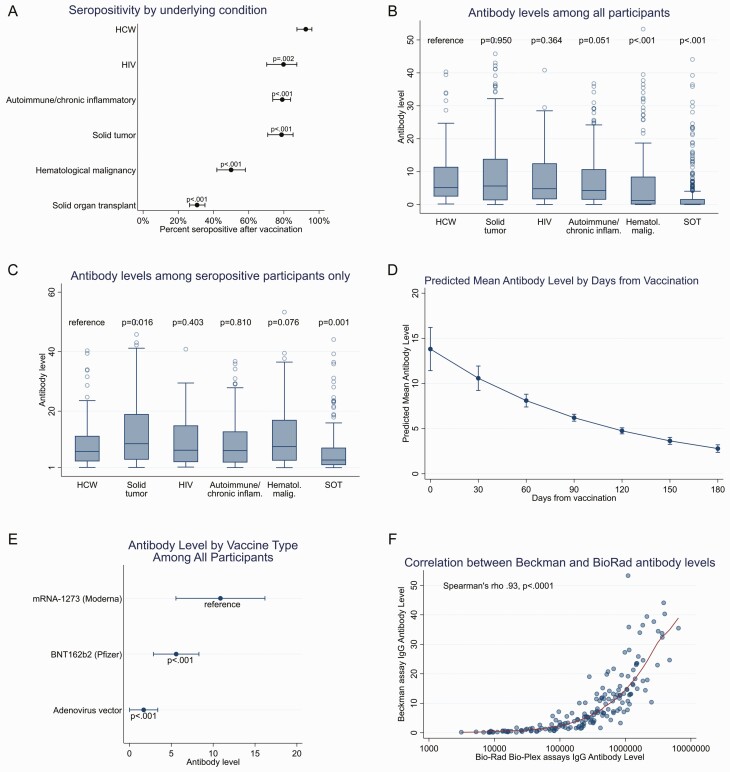
Compared with HCWs of whom 92.4% were seropositive (95% CI, 87.4–95.9), seropositivity was significantly lower among all groups of immunocompromised individuals: SOT (30.7% [26.4–35.2]), hematological malignancies (50.0% [41.9–58.1]), solid tumors (78.7% [70.8–85.2%]), autoimmune conditions (79.1% [73.7–83.8%], and HIV (79.8% [70.2–87.4]), P < .01 for all (Table 2, Figure 1A). Next, we examined risk factors for a negative antibody response by underlying condition.
Table 2.
Seropositivity and Antibody Levels in HCWs and Immunocompromised Participants
| Outcome | HCWs (N = 172) | Immuno-compromised Patients (N = 1099) | P * | Patients by Immunocompromised Condition | P ** | ||||
|---|---|---|---|---|---|---|---|---|---|
| SOT (N = 450) | Autoimmune (N = 263) | Hematologic Malignancy (N = 156) | HIV (N = 94) | Solid Tumor (N = 136) | |||||
| Seropositivity, n (%) | 159 (92.4%) | 606 (55.1%) | <.001 | 138 (30.7%) | 208 (79.1%) | 78 (50.0%) | 75 (79.8%) | 107 (78.7%) | <.001 |
| Median antibody level (IQR) | 5.2 (2.5,11.5) | 1.6 (0.1,7.2) | <.001 | 0.1 (0.0,1.7) | 4.3 (1.5,10.8) | 1.3 (0.1,8.5) | 4.8 (1.6,12.5) | 5.7 (1.3,13.9) | <.001 |
| Categorical antibody level, n (%)a | <.001 | <.001 | |||||||
| 0 to < 5 | 84 (49.1%) | 736 (68.4%) | 386 (87.5%) | 138 (53.7%) | 99 (65.6%) | 49 (52.7%) | 64 (47.8%) | ||
| 5–10 | 36 (21.1%) | 131 (12.2%) | 27 (6.1%) | 49 (19.1%) | 16 (10.6%) | 18 (19.4%) | 21 (15.7%) | ||
| > 10 | 51 (29.8%) | 209 (19.4%) | 28 (6.3%) | 70 (27.2%) | 36 (23.8%) | 26 (28.0%) | 49 (36.6% | ||
Abbreviations: HCWs, healthcare workers; HIV, human immunodeficiency virus; IQR, interquartile range; SOT, solid organ transplant.
P value for comparison between HCWs and all immunocompromised patients (likelihood ratio χ2 test).
P value for comparison between patients by immunocompromised condition (likelihood ratio χ2 test).
Antibody levels and categories are defined by signal to cut-off ratio from the Beckman anti-receptor-binding domain assay.
Figure 1.
Seropositivity and antibody levels. Results reflect anti-RBD antibody levels (signal to cut-off [S/CO] ratio) measured by the Beckman assay, unless otherwise indicated. A, Seropositivity in healthcare workers (HCWs) and immunocompromised participants. P values refer to comparisons between HCWs and immunocompromised participants (χ2 test). Whiskers denote 95% confidence intervals. B, All antibody levels (seropositive and seronegative) in nonimmunocompromised HCWs and immunocompromised participants. C, Comparisons of antibody levels among only participants with positive results. P value determined by Wilcoxon rank-sum test. D, Decline in antibody levels per month following vaccination; whiskers denote 95% confidence intervals. E, Antibody levels stratified by vaccine type among all participants, after adjustment of age, time from vaccination, and underlying immunocompromising condition; whiskers denote 95% confidence intervals. F, Correlation of antibody levels measured by the Beckman (anti-RBD) and Bio-Rad Bio-Plex (anti-RBD) assays. Abbreviations: HIV, human immunodeficiency virus; RBD, receptor-binding domain; SOT, solid organ transplant.
Healthcare workers
By multivariate analysis, only time from vaccination (but not type of vaccine) was significantly associated with a lower odds of seropositivity (adjusted OR 0.97 [95% CI, 0.94–0.99], P = .004) (Table 3). The probability of developing a reactive antibody level decreased with each month after vaccination, with 30-, 60-, 90-, 120-, and 150-day seropositivity of 99.8%, 99.5%, 98.6%, 96.5%, and 91.8%, respectively. There was no association between age and seropositivity.
Table 3.
Association Between Demographics, Vaccine Type, and Time Since Vaccination With Antibody Responses, Stratified by Underlying Condition
| Characteristic | Healthcare Workers | SOT recipients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | |||
| Reactive (N=159) | Nonreactive (N=13) | Reactive (N=138) | Nonreactive (N=312) | |||||||||
| Age group, y, n (%) | ||||||||||||
| 19-44 | ||||||||||||
| 45-60 | 45 (90.0%) | 5 (10.0%) | 0.78 (0.24,2.53) | 0.682 | 31 (26.3%) | 87 (73.7%) | 0.40 (0.21,0.76) | 0.005 | 0.32 (0.16-0.65) | 0.002 | ||
| 60+ | 23 (100.0%) | 0 (0.0%) | ... | ... | 76 (28.6%) | 190 (71.4%) | 0.45 (0.26,0.78) | 0.005 | 0.29 (0.15-0.55) | <.001 | ||
| Sex, n (%) | ||||||||||||
| Male | 40 (93.0%) | 3 (7.0%) | Reference | 77 (27.5%) | 203 (72.5%) | Reference | Reference | ... | ||||
| Female | 119 (92.2%) | 10 (7.8%) | 0.86 (0.23,3.29) | 0.83 | 61 (35.9%) | 109 (64.1%) | 1.48 (0.98,2.22) | 0.062 | 1.63 (1.02-2.61) | 0.043 | ||
| Race, n (%) | ||||||||||||
| White | 146 (93.6%) | 10 (6.4%) | Reference | Reference | 131 (32.1%) | 277 (67.9%) | Reference | Reference | ... | |||
| Non-White | 13 (81.3%) | 3 (18.8%) | 0.30 (0.07,1.21) | 0.091 | 0.50 (0.05-4.72) | 0.54 | 7 (16.7%) | 35 (83.3%) | 0.42 (0.18,0.98) | 0.044 | 0.38 (0.16-0.94) | 0.04 |
| Vaccine received, n (%) | ||||||||||||
| mRNA-1273 (Moderna) | 72 (98.6%) | 1 (1.4%) | Reference | Reference | 81 (37.3%) | 136 (62.7%) | Reference | Reference | ... | |||
| BNT162b2 (Pfizer) | 86 (89.6%) | 10 (10.4%) | 0.12 (0.01,0.96) | 0.045 | 0.21 (0.02-1.75) | 0.148 | 56 (24.6%) | 172 (75.4%) | 0.55 (0.36,0.82) | 0.004 | 0.51 (0.32-0.80) | 0.004 |
| Adenovirus vector | 1 (33.3%) | 2 (66.7%) | 0.01 (0.00,0.16) | 0.002 | 0.01 (0.00-2.82) | 0.113 | 1 (20.0%) | 4 (80.0%) | 0.42 (0.05,3.82) | 0.441 | 0.24 (0.02-2.90) | 0.26 |
| Median days from vaccine (IQR) | 131 (118,148) | 181 (148,195) | 0.95 (0.93,0.98) | <.001 | 0.97 (0.94 – 0.99) | 0.004 | 87.5 (63,112) | 92.5 (69.5,119.5) | 1.00 (0.99,1.00) | 0.23 | ||
| Characteristic | Autoimmune Conditions | Hematologic Malignancy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for reactive antibody result (95% CI) | P Value | Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | |||
| Reactive (N=208) | Nonreactive (N=55) | Reactive (N=78) | Nonreactive (N=78) | |||||||||
| Age group, y, n (%) | ||||||||||||
| 19-44 | 54 (75.0%) | 18 (25.0%) | Reference | |||||||||
| 45-60 | 61 (80.3%) | 15 (19.7%) | 1.36 (0.62,2.95) | 0.44 | 17 (65.4%) | 9 (34.6%) | Reference | |||||
| 60+ | 93 (80.9%) | 22 (19.1%) | 1.41 (0.69,2.86) | 0.34 | 61 (46.9%) | 69 (53.1%) | 0.47 (0.19,1.13) | 0.09 | 0.33 (0.11-0.99) | 0.05 | ||
| Sex, n (%) | ||||||||||||
| Male | 57 (77.0%) | 17 (23.0%) | Reference | 40 (47.6%) | 44 (52.4%) | Reference | ||||||
| Female | 151 (79.9%) | 38 (20.1%) | 1.19 (0.62,2.27) | 0.61 | 38 (52.8%) | 34 (47.2%) | 1.23 (0.65,2.31) | 0.52 | 0.52 | |||
| Race, n (%) | ||||||||||||
| White | 197 (79.8%) | 50 (20.2%) | Reference | 72 (49.0%) | 75 (51.0%) | Reference | ||||||
| Non-White | 11 (68.8%) | 5 (31.3%) | 0.56 (0.19,1.68) | 0.3 | 6 (66.7%) | 3 (33.3%) | 2.08 (0.50,8.65) | 0.31 | 0.31 | |||
| Vaccine received, n (%) | ||||||||||||
| mRNA-1273 (Moderna) | 114 (85.7%) | 19 (14.3%) | Reference | 46 (53.5%) | 40 (46.5%) | Reference | ||||||
| BNT162b2 (Pfizer) | 92 (72.4%) | 35 (27.6%) | 0.44 (0.24,0.82) | 0.009 | 0.46 (0.24-0.89) | 0.021 | 32 (45.7%) | 38 (54.3%) | 0.73 (0.39,1.38) | 0.34 | 0.34 | |
| Adenovirus vector | 2 (66.7%) | 1 (33.3%) | 0.33 (0.03,3.86) | 0.38 | ... | ... | ... | ... | ... | |||
| Median days from vaccine (IQR) | 86.5 (66.5,112) | 108 (77,128) | 0.99 (0.98,1.00) | 0.004 | 0.99 (0.98-0.99) | 0.003 | 93.5 (75,114) | 95 (69,112) | 1.00 (0.99,1.01 | 0.98 | 0.98 | |
| Characteristic | Solid Tumors | Persons with HIV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | Antibody result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | |||
| Reactive (N=107) | Nonreactive (N=29) | Reactive (N=75) | Non-reactive (N=19) | |||||||||
| Age group, y, n (%) | ||||||||||||
| 19-44 | 14 (73.7%) | 5 (26.3%) | Reference | 9 (90.0%) | 1 (10.0%) | Reference | ||||||
| 45-60 | 32 (88.9%) | 4 (11.1%) | 2.86 (0.67,12.27) | 0.16 | 39 (79.6%) | 10 (20.4%) | 0.43 (0.05,3.83) | 0.45 | ||||
| 60+ | 61 (75.3%) | 20 (24.7%) | 1.09 (0.35,3.40) | 0.88 | 27 (77.1%) | 8 (22.9%) | 0.38 (0.04,3.42) | 0.39 | ||||
| Sex, n (%) | ||||||||||||
| Male | 21 (75.0%) | 7 (25.0%) | Reference | 66 (78.6%) | 18 (21.4%) | Reference | ||||||
| Female | 86 (79.6%) | 22 (20.4%) | 1.30 (0.49,3.46) | 0.6 | 9 (90.0%) | 1 (10.0%) | 2.45 (0.29,20.67) | 0.41 | ||||
| Race, n (%) | ||||||||||||
| White | 103 (78.0%) | 29 (22.0%) | Reference | 62 (77.5%) | 18 (22.5%) | Reference | ||||||
| Non-White | 4 (100.0%) | 0 (0.0%) | ... | ... | 13 (92.9%) | 1 (7.1%) | 3.43 (0.42,28.17) | 0.25 | ||||
| Vaccine received, n (%) | ||||||||||||
| mRNA-1273 (Moderna) | 68 (89.5%) | 8 (10.5%) | Reference | 25 (86.2%) | 4 (13.8%) | Reference | ||||||
| BNT162b2 (Pfizer) | 39 (65.0%) | 21 (35.0%) | 0.22 (0.09,0.54) | 0.001 | 0.23 (0.09-0.57) | 0.002 | 50 (79.4%) | 13 (20.6%) | 0.62 (0.18,2.08) | 0.44 | ||
| Adenovirus vector | ... | ... | ... | ... | 0 (0.0%) | 2 (100.0%) | ... | ... | ||||
| Median days from vaccine (IQR) | 105.5 (82,133) | 115 (97,143) | 0.99 (0.98,1.00) | 0.13 | 78 (61,105) | 96 (71,112) | 0.99 (0.97,1.00) | 0.12 | 0.99 (0.97-1.00) | 0.155 | ||
Statistically significant associations highlighted in bold. Variables with a P value <.1 were entered in the multivariate model from which adjusted odds ratios were calculated.
Abbreviations: CI, confidence interval; HCWs, healthcare workers; HIV, human immunodeficiency virus; IQR, interquartile range; SOT, solid organ transplant.
Solid organ transplant recipients
By multivariate analysis, we identified age > 45 years (OR for 0.44 [0.26–0.74], P = .002), non-White race (OR 0.38 [0.16–0.94], P = .036), vaccination with BNT162b2 as opposed to mRNA-1273 (OR 0.50 [0.32–0.80]), P = .004), vaccination within 1 year of SOT (OR vs > 1 year 0.45 [0.24–0.87], P = .017), and administration of 2 or greater immunosuppressive medications (OR vs 1 drug 0.28 [0.18–0.44], P < .001) as factors independently associated with a lower odds of seropositivity (Tables 3 and 4). Antimetabolite use was collinear with the number of immunosuppressive drugs prescribed, and use of 2 or more drugs was associated with similar vaccine responses regardless of antimetabolite use (Supplementary Table 3). Compared with liver transplant recipients, nonliver recipients were significantly less likely to be seropositive (adjusted OR for kidney, lung, or heart vs liver transplant 0.53 [0.29–0.98], P = .041; 0.21 [0.08–0.54], P = .001; and 0.26 [0.13–0.51], P < .001, respectively). These differences in seropositivity by organ type persisted even after adjusting for the number of immunosuppressive medications, although there was a nonstatistically significant but potentially meaningful difference in the number of immunosuppressive drugs by organ type (Supplementary Table 4). Neither time since vaccination nor a recent rejection episode impacted vaccine responses, though only 9 SOT recipients had been treated for rejection within 3 months before vaccination.
Table 4.
Association Between Antibody Responses and Variables Unique to Each Underlying Condition
| SOT Recipients | ||||||
|---|---|---|---|---|---|---|
| Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | ||
| Characteristic | Reactive (N = 138) | (95% CI) | ||||
| SOT, n (%) | ||||||
| Liver | 42 (50.0%) | 42 (50.0%) | Reference | Reference | … | |
| Lung | 10 (14.7%) | 58 (85.3%) | 0.17 (0.08–0.38) | 0.004 | 0.21 (0.08–0.54) | 0.001 |
| Heart | 27 (24.3%) | 84 (75.5%) | 0.32 (0.17–0.59) | < .001 | 0.26 (0.13–0.51) | < .001 |
| Kidney | 59 (31.7%) | 127 (68.3%) | 0.46 (0.27–0.79) | < .001 | 0.53 (0.29–0.98) | 0.04 |
| Pancreas | 0 (0.0%) | 1 (100%) | … | … | ||
| Treated for rejection within 3 mo, n (%) | ||||||
| No | 134 (30.4%) | 307 (69.6%) | Reference | |||
| Yes | 4 (44.4%) | 5 (55.6%) | 1.83 (0.48–6.93) | 0.372 | ||
| Time from SOT, n (%) | ||||||
| 2+ y | 121 (33.3%) | 242 (66.7%) | Reference | |||
| 0–1 y | 17 (19.5%) | 70 (80.5%) | 0.49 (0.27–0.86) | 0.014 | 0.45 (0.24–0.87) | 0.02 |
| Calcineurin inhibitors, n (%) | ||||||
| No | 16 (39.0%) | 25 (61.0%) | Reference | |||
| Yes | 122 (29.8%) | 287 (70.2%) | 0.66 (0.34–1.29) | 0.226 | ||
| Antimetabolites, n (%) | ||||||
| No | 70 (47.6%) | 77 (52.4%) | Reference | |||
| Yes | 68 (22.4%) | 235 (77.6%) | 0.32 (0.21–0.49) | < .001 | ||
| mTOR inhibitors, n (%) | ||||||
| No | 121 (30.5%) | 276 (69.5%) | Reference | |||
| Yes | 17 (32.1%) | 36 (67.9%) | 1.08 (0.58–1.99) | 0.813 | ||
| No. of immunosuppressive drugs, n (%) | ||||||
| 1 | 58 (53.2%) | 51 (46.8%) | Reference | Reference | ... | |
| 2 | 57 (25.0%) | 171 (75.0%) | 0.29 (0.18–0.47) | < .001 | 0.31 (0.18–0.53) | < .001 |
| 3+ | 23 (20.4%) | 90 (79.6%) | 0.22 (0.12–0.41) | <.001 | 0.24 (0.12–0.50) | <.001 |
| –Autoimmune Conditions | ||||||
|---|---|---|---|---|---|---|
| Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | ||
| Characteristic | (95% CI) (N = 208) | Nonreactive | ||||
| TNF-alpha inhibitor, n (%) | ||||||
| No | 124 (81.6%) | 28 (18.4%) | Reference | |||
| Yes | 84 (75.7%) | 27 (24.3%) | 0.70 (0.39,1.28) | 0.25 | ||
| Mercaptopurine, n (%) | ||||||
| No | 201 (79.1%) | 53 (20.9%) | Reference | |||
| Yes | 7 (77.8%) | 2 (22.2%) | 0.92 (0.19,4.57) | 0.92 | ||
| JAK inhibitor, n (%) | ||||||
| No | 200 (80.0%) | 50 (20.0%) | Reference | |||
| Yes | 8 (61.5%) | 5 (38.5%) | 0.40 (0.13,1.28) | 0.12 | ||
| IL-inhibitor, n (%) | ||||||
| No | 200 (78.7%) | 54 (21.3%) | Reference | |||
| Yes | 8 (88.9%) | 1 (11.1%) | 2.16 (0.26,17.65) | 0.47 | ||
| Calcineurin inhibitor, n (%) | ||||||
| No | 205 (79.2%) | 54 (20.8%) | Reference | |||
| Yes | 3 (75.0%) | 1 (25.0%) | 0.79 (0.08,7.75) | 0.84 | ||
| Antimetabolites, n (%) | ||||||
| No | 157 (77.7%) | 45 (22.3%) | Reference | |||
| Yes | 51 (83.6%) | 10 (16.4%) | 1.46 (0.69,3.11) | 0.32 | ||
| Methotrexate , n (%) | ||||||
| No | 165 (79.3%) | 43 (20.7%) | Reference | |||
| Yes | 43 (78.2%) | 12 (21.8%) | 0.93 (0.45,1.92) | 0.85 | ||
| Receiving anti-CD20 monoclonal antibody, n (%) | ||||||
| No | 206 (81.4%) | 47 (18.6%) | Reference | |||
| Yes | 2 (20.0%) | 8 (80.0%) | 0.06 (0.01,0.28) | <.001 | 0.05 (0.01-0.23) | <.001 |
| Hematological Malignancy | ||||||
|---|---|---|---|---|---|---|
| Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | ||
| Characteristic | Reactive (N = 78) | Nonreactive (N = 78) | ||||
| Systemic therapy over the past 12 mo, n (%) | ||||||
| No | 59 (59.0%) | 41 (41.0%) | Reference | Reference | ||
| Yes | 19 (33.9%) | 37 (66.1%) | 0.36 (0.18,0.71) | 0.003 | 0.29 (0.12–0.69) | 0.005 |
| Radiotherapy over the past 12 mo, n (%) | ||||||
| No | 76 (50.7%) | 74 (49.3%) | Reference | |||
| Yes | 2 (33.3%) | 4 (66.7%) | 0.49 (0.09,2.74) | 0.41 | ||
| Anti-CD20 therapy, n (%) | ||||||
| No | 74 (57.8%) | 54 (42.2%) | Reference | Reference | ||
| Yes | 4 (14.3%) | 24 (85.7%) | 0.12 (0.04,0.37) | < .001 | 0.16 (0.04–0.58) | 0.005 |
| Solid Tumors | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Antibody Result | Unadjusted OR for Reactive Antibody Fesult (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | |
| Reactive (N = 107) | Nonreactive (N = 29) | |||||
| Systemic therapy over the past 12 mo | ||||||
| No | 41 (89.1%) | 5 (10.9%) | Reference | |||
| Yes | 66 (73.3%) | 24 (26.7%) | 0.34 (0.12,0.95) | 0.039 | 0.44 (0.15-1.30) | 0.135 |
| Radiotherapy over the past 12 mo, n (%) | ||||||
| No | 54 (72.0%) | 21 (28.0%) | Reference | |||
| Yes | 53 (86.9%) | 8 (13.1%) | 2.58 (1.05,6.33) | 0.039 | 2.45 (0.95-6.34) | 0.065 |
| Anti-CD20 therapy, n (%) | ||||||
| No | 103 (80.5%) | 25 (19.5%) | Reference | |||
| Yes | 2 (100.0%) | 0 (0.0%) | … | … | ||
| HIV | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Antibody Result | Unadjusted OR for Reactive Antibody Result (95% CI) | P Value | Adjusted OR for Reactive Antibody Result (95% CI) | P Value | |
| Reactive (N = 75) | Nonreactive (N = 19) | |||||
| Receiving antiretroviral therapy, n (%) | ||||||
| No | 0 | 0 | Reference | |||
| Yes | 75 (79.8%) | 19 (20.2%) | … | … | ||
| HIV viral load, n (%) | ||||||
| Undetectable | 73 (79.3%) | 19 (20.7%) | Reference | |||
| Detectable | 2 (100.0%) | 0 (0.0%) | … | … | ||
| CD4 count (cells/µL), n (%) | ||||||
| >200 | 71 (86.6%) | 11 (13.4%) | Reference | |||
| <200 | 4 (33.3%) | 8 (66.7%) | 0.08 (0.02,0.30) | < .001 | 0.08 (0.02-0.31) | < .002 |
Statistically significant associations highlighted in bold. Variables with a P value < .1 were entered in the multivariate model from which adjusted odds ratios were calculated. Specific cancer therapies found in Supplementary Table S6. Specific hematological cancers found in Supplementary Table S5. Specific autoimmune conditions found in Supplementary Table S7.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IL, interleukin; IQR, interquartile range; SOT, solid organ transplant; TNF, tumor necrosis factor.
Participants with Autoimmune Conditions
Vaccination with BNT162b2 or use of anti-CD20 monoclonal antibodies were associated with a lower odds of seropositivity compared with participants who received mRNA-1273 or those who had not received an anti-CD20 monoclonal antibody (adjusted OR 0.46 [0.24–0.89], P = .02, and 0.05 [0.01–0.23], P < .001, respectively) (Tables 3 and 4, Supplementary Table 7). No other immunosuppressive therapy, including use of tumor necrosis alpha inhibitors or antimetabolites impacted antibody responses. Although individuals with multiple sclerosis were less likely to be seropositive compared with those with other autoimmune conditions (Supplementary Table 7) (unadjusted OR 0.12 [0.02–0.69], P = .018), 83.3% (5/6) of individuals with multiple sclerosis had received an anti-CD20 monoclonal antibody. Longer time from vaccination predicted a lower odds of seropositivity (adjusted OR 0.99 [0.98–0.99], P = .002). The probability of developing a reactive antibody level decreased with each month after vaccination, with 30-, 60-, 90-, 120-, and 150-day seropositivity of 89.9%, 85.9%, 80.5%, 73.7%, and 65.3%, respectively.
Participants with Cancer
Administration of cancer-specific therapy within 12 months before vaccination was associated with a lower odds of seropositivity for both patients with hematological malignancies and those with solid tumors (Tables 3 and 4, Supplementary Tables 5 and 6). This observation was driven by individuals who had received an anti-CD20 monoclonal antibody within the prior 12 months (OR for seropositivity after adjusting for age, vaccine type, and days since vaccination 0.1 [0.04–0.23], P < .001). No other therapies, including radiation therapy, cytotoxic chemotherapy, checkpoint inhibitors, or other cancer therapies were statistically significantly associated with vaccine responses (Supplementary Table 6). Underlying hematological malignancy also did not appear to influence seropositivity (Supplementary Table 5). Only 9 participants had undergone hematopoietic stem cell transplant, whereas 3 had undergone CAR-T-cell therapy; seropositivity was 66.7% (6/9) and 33.3% (1/3), respectively. Among patients with solid tumors (but not hematological malignancies), BNT162b2 was associated with a 78% lower odds of seropositivity compared with mRNA-1273 (adjusted OR 0.22 [0.09–0.57], P = .002). Time from vaccination did not impact seropositivity.
Participants with HIV
All persons with HIV (100%) were receiving antiretroviral therapy; 97.9% (92/94) were virally suppressed, and 87.2% (82/94) had CD4 counts > 200 cells/µL (Tables 3 and 4). The only variable associated with a lower odds of seropositivity was having a CD4 count < 200 cells/µL (adjusted OR 0.08 [0.02–0.31], P < .001). Among participants with CD4 counts > 200 cells/µL, 86.6% (71/82) were seropositive, with no statistically significant difference compared with HCWs (P = .15).
Impact of Anti-CD20 Monoclonal Antibodies
Overall, 4.6% (51/1099) of immunocompromised participants had received an anti-CD20 monoclonal antibody within 12 months before vaccination (Supplementary Table 8). Only 17.7% (9/51) of these participants were seropositive compared with 57.0% (597/1099) of immunocompromised participants who had not received an anti-CD20 monoclonal antibody. After adjusting for age, vaccine type, and time from vaccination, the odds of seropositivity in the setting of anti-CD20 monoclonal antibody use was 84% lower than among participants who had not received an anti-C20 monoclonal antibody (OR 0.16 [0.08–0.34], P < .001).
Antibody Levels
Antibody S/CO levels were significantly lower in immunocompromised participants compared with HCWs (Figure 1B). This finding was driven by the higher proportion of participants with negative antibody results in the immunocompromised group. When we analyzed seropositive study participants only (Figure 1C), antibody levels of seropositive HCW (median 6.47 [IQR 3.12–11.69]) were not significantly higher than those of seropositive persons with autoimmune conditions (6.47 [2.74–13.18), hematological malignancies (8.11 [3.37–17.06]), solid tumors (9.05 [3.69–19.02]), or HIV (6.87 [2.88–15.20]). By contrast, antibody levels were significantly lower among SOT recipients compared with HCWs (3.57 [1.87–7.27], P < .01). Overall, we found that antibody levels declined by 1.55 per month from vaccination (Figure 1D). When antibody levels were analyzed as the continuous outcome measure (instead of seropositivity as a categorical variable) with the same predictor variables used to calculate ORs for seropositivity, we found that overall, variables associated with higher/lower antibody levels were generally similar to those associated with seropositivity (vs seronegativity) (Supplementary Tables S11–S16). The main differences found, compared with seropositivity as the outcome measure, were that time since vaccination was significantly associated with lower antibody levels across all subgroups (indicating that antibody titers decline over time), as was the use of non-mRNA-1273 SARS-CoV-2 vaccines (as described in detail later).
Impact of Type of Vaccine
After adjusting for time since vaccination, age, and underlying condition, vaccination with mRNA-1273 was associated with significantly higher antibody levels compared with vaccination with either BNT162b2 or an adenovirus vector vaccine for all subgroups of participants (mean levels [95% CI] 10.24 [4.70–15.78] vs 5.25 (2.42–8.08), P < .001; and 1.82 (0.00–3.91), P = .001, respectively) (Figure 1E). Antibody levels for all patient subgroups stratified by vaccine type are shown in Supplementary Figures S1–S7.
Comparison of Levels With 2 Different Assays
Antibody levels using the Beckman assay strongly correlated with anti-RBD titers using the Bio-Rad assay (Spearman r = 0.93, P < .0001, Figure 1F), although 13% of the results were discordant (Supplementary Table 9). Concordance varied by type of underlying condition (Supplementary Table 10); there was 100% concordance between the 2 assays for the 48 HCW samples.
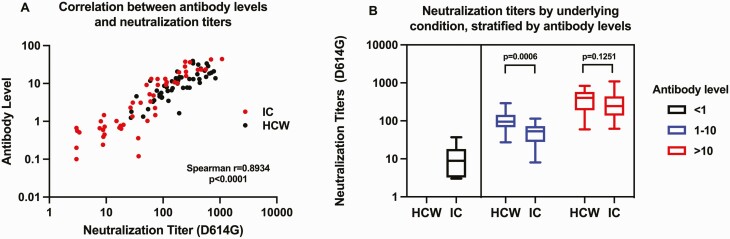
Pseudovirus Neutralization Assays
As mentioned in the methods, pseudovirus neutralization assays were performed on a subset of 100 study participants (50 HCWs, 50 immunocompromised). We observed a strong, positive correlation between antibody levels and neutralization titers (Spearman r = 0.89, P < .0001) (Figure 2A), suggesting that higher antibody levels could confer greater protection. Next, we performed four neutralization titer (NT50) comparisons: first, we compared neutralization titers across the 50 immunocompromised participants and 50 HCWs (N=100); second, we compared neutralization titers across 33 immunocompromised participants and 50 HCWs with antibody levels >1 (N=83); third, we compared neutralization titers across 17 immunocompromised participants and 25 HCWs with antibody levels of 1 to 10 (N=42); and fourth, we we compared neutralization titers across 16 immunocompromised participants and 25 HCWs with antibody levels > 10 (N=41).
Figure 2.
A, Scatter plot of 50% neutralization titer (NT50) for D614G pseudovirus (x-axis) by anti-RBD antibody levels (signal to cut-off [S/CO] ratio) measured by Beckman assay (y-axis). NT50 was defined as the highest serum dilution that neutralizes >50% of the D614G pseudovirus. Black filled circles are data from nonimmunocompromised healthcare workers; red filled circles are data from immunocompromised participants. B, Comparisons of antibody levels and NT50 across 100 study participants. Black, blue, and red boxes represent participants with antibody levels <1, 1–10, and >10, respectively. Among participants with antibody S/CO levels 1–10, NT50 were significantly lower among IC participants compared with HCWs. Abbreviations: HCWs, healthcare workers; IC, immunocompromised; RBD, receptor-binding domain.
In all 100 participants, neutralization titers were lower among immunocompromised individuals compared with HCWs (median NT50 [IQR], 52.2 [9.4, 159.5] vs 181.5 [92.7, 401.2], respectively, P < .01), mirroring the overall lower antibody levels from this sample of immunocompromised participants (median level [IQR] = 2.7 [.7, 13.2] vs 10.2 [4.6, 14.9] P = .002, respectively). In participants with levels > 1, neutralization titers were also lower among immunocompromised patients compared with HCWs (median NT50 [IQR] of 95.075 [53.15, 245.2] vs 181.45 [92.74, 401.2], respectively, P = .02) although antibody levels were similar (median level [IQR] 10 [3.1–20.3] vs 10 [4.59–14.93], respectively, P = .83). Importantly, for participants with antibody levels between 1 and 10, neutralization titers were significantly lower among immunocompromised participants compared with HCWs (median NT50 [IQR] 55.5 [29.1, 72.7] vs 93.9 [67.9, 132.9], respectively, P = .002) (Figure 2B), despite there being no difference in antibody levels between the groups (median level [IQR] 3.1 [1.5, 7.2] vs 4.6 [3.4, 7.1], respectively, P = .31). By contrast, there was no difference in neutralization titers between the groups of immunocompromised participants versus HCWs with antibody levels > 10 (median NT50 [IQR] 249.2 [170.25, 440.4] vs 370.9 [184.9, 571.7], respectively, P = .252), nor was there a difference in antibody levels in this group (median level [IQR] 21.7 [13.2, 32.3] vs 14.8 [13.1, 21.8]), respectively, P = .18).
Breakthrough Infection
With a median of 67 days (IQR 45–85 days) of follow-up, 2 participants developed COVID-19, both of whom were seronegative. The first patient had advanced HIV infection (CD4 < 200 cells/µL) and was hospitalized with COVID-19 pneumonia. The second patient had undergone kidney transplantation and died of COVID-19–related respiratory failure. An additional 378 patients had undergone clinical SARS-CoV-2 PCR testing, all of whom were negative.
DISCUSSION
We prospectively characterized humoral immune responses to COVID-19 vaccines in 1099 immunocompromised participants compared with 172 HCWs and identified 5 major findings. First, compared with HCWs (92.4% seropositive), seropositivity was lowest among SOT recipients (30.7%), followed by participants with hematological malignancies (50.0%), solid tumors (78.7%), autoimmune conditions (79.1%), and HIV (79.8%). Second, the lower seropositivity across immunocompromised individuals was generally associated with more profound immunosuppression or with the use of anti-CD20 monoclonal antibodies. Third, antibody levels exhibited a decline over time, and levels in seropositive individuals were similarly distributed across all patient groups, with the exception of SOT recipients who exhibited lower antibody levels than others. Fourth, vaccination with mRNA-1273 was associated with higher antibody levels compared with BTN162b2 or adenovirus vector vaccines, even after adjusting for time since vaccination, age, and underlying condition. Finally, although antibody levels strongly correlated with neutralization titers, we found that compared with HCWs with antibody levels between 1 and 10, sera from immunocompromised participants with the same antibody levels exhibited significantly lower neutralization titers, suggesting that lower levels of neutralizing antibodies are produced by certain immunocompromised participants compared with nonimmunocompromised HCWs. Taken together, our findings highlight the heterogeneity of the humoral immune response to COVID-19 vaccines based on underlying immunosuppression and vaccine type and suggest that the presence of detectable antibodies in immunocompromised individuals does not equate to the same levels of virus neutralization as HCW.
Three factors that were variably associated a lower probability of having a detectable antibody level after vaccination were type of vaccine, time since vaccination, and age. First, the odds of seropositivity after vaccination with BNT162b2 (compared with mRNA-1273) was 50%, 77%, and 54% lower among patients with SOT, solid tumors, and autoimmune conditions, respectively. Additionally, across all groups of participants, absolute antibody levels were significantly higher with mRNA-1273 compared with other vaccine types, despite adjusting for time elapsed since vaccination, age, and immunocompromising condition. The underlying cause of these observations remains unknown but may be a result of vaccination schedules, vaccine doses, or other reasons. In the literature, the impact of BNT162b2 versus mRNA-1273 vaccination on seroconversion and antibody titers has been contradictory [1, 11], though 2 recent studies of healthy volunteers showed greater immunogenicity with mRNA-1273 compared with BNT162b2 [12, 13]. Whether using higher mRNA vaccine doses or prolonging the interval between vaccine doses (as has been done with BNT162b2) [14] will result in superior immune responses than current strategies requires further study. Additionally, whether immunocompromised individuals should be prioritized for vaccination with mRNA-1273 remains to be determined.
Second, a greater interval between vaccination and testing was associated with a lower odds of a positive antibody response in HCW and individuals with autoimmune conditions. HCWs in our study underwent testing at a median of 4.4 months after vaccination, which may explain why their seropositivity (92.4%) was surprisingly lower than the 100% seroconversion described in the phase 1/2 mRNA vaccine trials, in which antibody levels were measured 1 to 2 months after vaccination [15, 16]. Although we found no association between time since vaccination and the odds of seropositivity among the other groups, we did find that longer time since vaccination predicted lower antibody levels across all patient groups. It is therefore plausible that we may have observed a difference in seropositivity with longer follow-up. Furthermore, the longer interval between vaccination and antibody testing for HCWs (median 132.5 days) versus immunocompromised participants (median 93 days) may have resulted in detecting lower seropositivity in HCWs than what would have been expected had HCWs and immunocompromised participants undergone testing at the same time interval after vaccination. Similarly, because antibody levels are expected to wane faster in immunocompromised individuals (as demonstrated by our cohort with autoimmune conditions), we anticipate that both seropositivity and antibody levels in our immunocompromised participants would be lower than reported had these patients been tested at the same intervals after vaccination as were HCWs. Waning levels of circulating antibodies may explain the observation of declining vaccine efficacy over time [17] and support recommendations for vaccine boosters in certain individuals [18]. However, additional work is needed to define the extent of the long-lived plasma cell and memory B-cell pools that are produced after vaccination [19]. Finally, we observed an impact of advanced age on seropositivity in patients with SOT and hematological malignancies. Although this observation has been inconsistently reported in the literature [1, 11], it may be related to the impact of immunosenescence among individuals with blunted immune responses and may suggest a need for higher vaccine doses in older individuals, as is the case for influenza vaccination [20].
We identified unique risk factors for poor humoral responses associated with the underlying condition. In SOT recipients, the odds of seropositivity in participants who were vaccinated within 1 year after transplantation was 55% lower than those who received the vaccines after the first year of transplantation. This observation is likely the result of more profound immunosuppression in the first year after SOT. Some studies have demonstrated that antimetabolites (mycophenolate and azathioprine) are associated with poor humoral responses to COVID-19 vaccines after SOT [1, 2, 21, 22]. In contrast, we found that the odds of seropositivity among SOT recipients receiving 2 or more immunosuppressive drugs was 72% lower compared with those receiving only 1 drug, irrespective of whether the participant was receiving an antimetabolite, suggesting that the overall degree of immunosuppression, not specific drugs, is the main predictor of poor humoral responses after SOT. This finding is corroborated by our observation that antimetabolites did not impact vaccine responses in individuals with autoimmune conditions. We also found that seropositivity in lung, heart, or kidney transplant recipients was significantly lower than that of liver transplant recipients. This risk was most pronounced in lung or heart transplant recipients, in whom the odds of seropositivity was 79% and 74% lower than that of liver transplant recipients, respectively. Whether recipients of thoracic organ transplants may benefit from modified vaccine schedules and doses is not currently known.
Among participants with cancer, receipt of anticancer therapy over the preceding 12 months conferred a lower odds of seropositivity. However, this observation was driven primarily by using anti-CD20 monoclonal antibodies but not cytotoxic chemotherapy, checkpoint inhibitors, radiation therapy, or others. The existing literature evaluating the specific risk of poor humoral responses in the oncology population has been conflicting, with some [3, 11] but not all [4] studies demonstrating an association between vaccine responses and cytotoxic chemotherapy and/or immunotherapy. Our findings are nonetheless similar to those of a prior report of influenza vaccination in patients with solid tumors undergoing chemotherapy, in whom seroconversion occurred in > 70% of participants regardless of timing of vaccination [23]. Similarly, among participants with autoimmune conditions, only anti-CD20 monoclonal antibodies, but not the underlying condition nor other immunosuppressive medications, predicted a poor humoral response. Indeed, the odds of seropositivity among all individuals who had received an anti-CD20 monoclonal antibody within 12 months before administration of a COVID-19 vaccine was 84% lower than those who had not received these drugs. Although this observation is consistent with the findings of a recent meta-analysis of the impact of anti-CD20 monoclonal antibodies on other vaccines [24], several unknowns persist, including the effect of anti-CD20 monoclonal antibodies on memory T-cell and memory B-cell reservoirs, particularly among individuals who have been administered a COVID-19 vaccine before receiving an anti-CD20 monoclonal antibody. Additional studies are needed to determine the influence of specific cancer therapies and immunomodulators on vaccine responses, and whether adjusting the vaccine schedule around these therapies, in particular anti-CD20 monoclonal antibodies, may result in superior humoral responses. Finally, we found that most persons with HIV were seropositive after vaccination, although having a CD4 count <200 cells/µL was not surprisingly associated with a lower odds of seropositivity. Because the prevalence of underlying comorbidities in our HIV-infected cohort appears to mirror that of HIV-infected individuals in the United States [25, 26], our results may be generalizable to people with HIV at large.
A significant deficit in our understanding of COVID-19 immune responses is the lack of immune correlates of protection. Although antibody levels strongly correlated with neutralization titers (Figure 2B), seropositive immunocompromised participants with antibody levels between 1 and 10 exhibited significantly lower neutralization titers compared with HCWs with the same antibody levels. Despite the heterogeneity of the immunocompromised group, whether this observation means that antibodies elicited by vaccines may be less protective in certain subgroups of seropositive immunocompromised individuals compared to nonimmunocompromised individuals is not currently known. However, these results suggest that clinicians should be cautious when counseling patients who are found to be seropositive after vaccination, as the presence of antibodies need not imply protection. Moreover, although there was a strong correlation between antibody levels using the Beckman and Bio-Rad platforms, discordances in negative/positive samples were observed in 13% of sera. These discrepancies in testing platforms, although uncommon, underscore the difficulties clinicians may face when advising patients about the results of postvaccination serological testing, as the antibody results may differ based on the assay used. Indeed, because of the absence of a reference standard, the US Food and Drug Administration does not currently recommend routine antibody testing after vaccination [27] until additional research is performed to correlate antibody levels with specific degrees of protection.
Limitations of this study include lack of assessment of cellular immunity and timing of chemotherapy in relation to vaccination, low number of hematopoietic stem cell transplant recipients, and heterogeneity of immunosuppressive therapies for cancer and autoimmune conditions. In addition, that immunocompromised participants were significantly older than HCWs, and that HCWs were predominantly female, may have influenced our results, although vaccine efficacy in the phase 3 mRNA vaccine trials did not appear to be affected by either age or sex [28, 29]. Despite using a 2-tiered approach (self-report and chart review) to exclude participants with a history of COVID-19, a few participants with a prior positive SARS-CoV-2 PCR result outside of our system may have been missed. Similarly, although every attempt was made to ensure the accuracy of underlying medical conditions and medications, potential errors may have been introduced through either patient self-reports or EMR extraction of data. We also did not assess neutralizing antibody titers against the Delta or newly described Omicron SARS-CoV-2 variants. Given that vaccine efficacy against these 2 variants is likely reduced [30, 31], our findings underscore the need for continued vigilance and safe living practices among immunocompromised individuals, who remain at risk because of suboptimal vaccine responses. The small number of participants who received an adenovirus vector vaccine also limits our ability to draw conclusions about antibody responses to these vaccines. As noted previously, because immune correlates of protection are not yet defined, our finding of lower neutralization ability in a subset of immunocompromised participants should be validated in future studies. Additionally, although Centers for Disease Control and Prevention guidance advocates for up to 4 vaccine doses in certain groups of immunocompromised individuals [32], we only describe immune responses after the initial vaccination series, which was the standard of care at the time the study was conducted (April–July 2021). Nevertheless, it remains important to define the immune response to the initial vaccination series in order to test additional strategies to improve vaccine response such as other vaccine doses, schedules, or types. Although our study was not designed to evaluate the clinical effectiveness of COVID-19 vaccines, it is reassuring that only 2 study participants (both of whom were seronegative) developed COVID-19 after vaccination, though others may have been diagnosed with COVID-19 outside our healthcare system. Finally, the online design of the study and the requirement for a signed consent form may have resulted in selection bias by recruiting primarily health-literate and computer-literate individuals, who may be extremely engaged in their own healthcare and thus participated to learn about their response to vaccination.
In summary, our data highlight the complexities in understanding the immune response to vaccines and the need to correlate immune responses with clinical efficacy of COVID-19 vaccines. Because not all immunocompromised patients appear to benefit from additional mRNA vaccine doses [33–35], further studies are needed to determine the effectiveness of other strategies such as monoclonal antibody prophylaxis [36] or additional vaccine doses in combination with modification of immunosuppression to protect seronegative immunocompromised individuals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank all COVICS study participants, without whom this work would not have been possible. We also thank the following individuals for providing multidisciplinary commitment to advance scientific discovery and quality of patient care in response to the COVID-19 pandemic: Jordan Bartlow, Logan Baylor, Sarah Behr, Shannon Buono, Lori Caruso, Vibha Chauhan, Charles Chilleo, Maddie Chrisman, Shawna Chylinski, Cathy Cochran, Kate Codd-Palmer, Nicole Czolba, Celeste Duprey, Kelly Friday, Megan Fritz, Dr. Mark Gladwin MD, Dr. Ken Ho, Kailey Hughes, Dr. Naudia Jonnasaint, David Jordan, Trevor Katich, Jenna Keeling, Kristin Kerfoot, Joshua Kohl, Dr. Fadi Lakkis, Elaine Lander, Michelle Lucas, Aimee Majeski, Dr. Rachel Marini, Dr. Oscar Marroquin, Susan Martin, Traci McGaha, Rachel McGargle, Stephanie Montgomery, Dr. Alison Morris, Ben Morris, Audrey Paul, Dr. Barbara Postol, Dr. Sharon Riddler, Nicole Radulovich, Jennifer Roscher, Margherita Sciullo, Amber Shaffer, Jordan Shayer, Lisa Sheehan, Kristin Shoemaker, Lori Snyder, Courtney Starrett, Dr. Mindi Styn, Colleen Sullivan, Abbey Sung, Christina Tedesco, Jeffrey Tischler, Dr Peter Veldkamp, Jamie Voyten, Mary K. Wisniewski, and the entire Optimizing Treatment and Impact of Monoclonal Antibodies through Evaluation for COVID-19 team.
Financial support. This work was supported by UPMC, the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI154546 (G. H.) and the University of Pittsburgh Clinical and Translational Science Institute and the DSF Charitable Foundation (J. L. J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. G. H. receives research funds from Karius, Inc. J. W. M. is a consultant to Gilead Sciences, and owns shares in Co-Crystal Pharmaceuticals, Infectious Disease Connect, and Abound Bio. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Ghady Haidar, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Mounzer Agha, Hillman Cancer Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Andrew Bilderback, Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Amy Lukanski, Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Kelsey Linstrum, Health Care Innovation, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Rachel Troyan, Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Scott Rothenberger, Division of General Internal Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Deborah K McMahon, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Melissa D Crandall, Clinical Laboratory, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Michele D Sobolewksi, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
P Nathan Enick, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Jana L Jacobs, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Kevin Collins, Clinical Analytics, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Cynthia Klamar-Blain, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Bernard J C Macatangay, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Urvi M Parikh, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Amy Heaps, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Lindsay Coughenour, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Marc B Schwartz, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Jeffrey M Dueker, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Fernanda P Silveira, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Mary E Keebler, Department of Cardiology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Abhinav Humar, Division of Transplantation, Department of Surgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
James D Luketich, Department of Cardiothoracic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Matthew R Morrell, Division of Pulmonary and Critical Care, School of Medicine, University of Utah, Salt Lake City, Utah, USA.
Joseph M Pilewski, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
John F McDyer, Division of Pulmonary, Allergy and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Bhanu Pappu, Hillman Cancer Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Robert L Ferris, Hillman Cancer Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Stanley M Marks, Hillman Cancer Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
John Mahon, Clinical Laboratory, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Katie Mulvey, Clinical Laboratory, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Sundaram Hariharan, Division of Transplantation, Department of Surgery, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Transplant Nephrology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Glenn M Updike, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; UPMC Magee-Womens Hospital, Pittsburgh, Pennsylvania, USAand.
Lorraine Brock, Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Robert Edwards, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; UPMC Magee-Womens Hospital, Pittsburgh, Pennsylvania, USAand.
Richard H Beigi, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; UPMC Magee-Womens Hospital, Pittsburgh, Pennsylvania, USAand.
Paula L Kip, Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Alan Wells, Clinical Laboratory, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA; Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Tami Minnier, Wolff Center, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Derek C Angus, Health Care Innovation, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
John W Mellors, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
References
- 1. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. Jama 2021; 325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant 2021; 21:2719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021; 7:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021; 39:1081–1090.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration. Emergency Use Authorization Beckman Coulter access immunoassay systems instructions for use. 2020. Available at: https://www.fda.gov/media/139627/download. Accessed 30 March 2022.
- 7. Zilla MW, Keetch C, et al. Variable performance of commercially available EUA SARS-CoV-2 antibody assays in critically ill and mildly symptomatic patients assessed at a large academic medical center. Am J Clin Pathol 2021; 155:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zilla ML, Keetch C, Mitchell G, McBreen J, Shurin MR, Wheeler SE.. SARS-CoV-2 serologic immune response in exogenously immunosuppressed patients. J Appl Lab Med 2021; 6:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cox CMV, Tan W, Gao Q.. Quantitative Bio-Plex Pro Human IgG, IgA, and IgM SARS-CoV-2 Serology Assays Using a Standard Curve. https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_3208.pdf. 2021. Published 2021. Accessed 28 September 2021.
- 10. Nace DA, Kip KE, Mellors JW, et al. Antibody responses after mRNA-based COVID-19 vaccination in residential older adults: implications for reopening. J Am Med Dir Assoc 2021; 22:1593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021; 39:1091–1098.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions. MMWR Morb Mortal Wkly Rep 2021; 70:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L.. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA 2021; 326:1533–5. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parry H, Bruton R, Stephens C, et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. medRxiv 2021. doi: 10.1101/2021.05.15.21257017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020; 586:589–93. [DOI] [PubMed] [Google Scholar]
- 17. Thomas SJ, Moreira ED, Kitchin N, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. medRxiv 2021: doi: 10.1101/2021.07.28.21261159. Posted July 28, 2021. [DOI] [Google Scholar]
- 18. U. S. Food and Drug Administration. FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations. 2021. Published September 22, 2021. Accessed 28 September 2021.
- 19. Parham P. The Immune System. New York, NY: Garland Science, Taylor & Francis Group; 2015. [Google Scholar]
- 20. Centers for Disease Control and Prevention. Fluzone High-Dose Seasonal Influenza Vaccine. https://www.cdc.gov/flu/prevent/qa_fluzone.htm2021. Published August 27, 2021. Accessed 28 September 2021.
- 21. Itzhaki Ben Zadok O, Shaul AA, Ben-Avraham B, et al. Immunogenicity of the BNT162b2 mRNA vaccine in heart transplant recipients - a prospective cohort study. Eur J Heart Fail 2021; 23:1555–9. [DOI] [PubMed] [Google Scholar]
- 22. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021; 75:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waqar SN, Boehmer L, Morgensztern D, et al. Immunogenicity of influenza vaccination in patients with cancer. Am J Clin Oncol 2018; 41:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK.. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv 2021; 5:2624–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallant J, Hsue PY, Shreay S, Meyer N.. Comorbidities among US patients with prevalent HIV infection-a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 26. Lerner AM, Eisinger RW, Fauci AS.. Comorbidities in persons with HIV: the lingering challenge. JAMA 2020; 323:19–20. [DOI] [PubMed] [Google Scholar]
- 27. U.S. Food and Drug Administration. Antibody Testing Is Not Currently Recommended to Assess Immunity After COVID-19 Vaccination: FDA Safety Communication. https://www.fda.gov/medical-devices/safety-communications/antibody-testing-not-currently-recommended-assess-immunity-after-covid-19-vaccination-fda-safety. 2021. Published May 19, 2021. Accessed 25 June 2021.
- 28. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cele S, Jackson L, Khan K, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv [Preprint]. December 21, 2021. [cited 2022 Feb 14]. Available from 10.1101/2021.12.08.21267417. [DOI] [Google Scholar]
- 31. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed 20 December 2021.
- 33. Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385:1244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A.. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021; 385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med 2021; 174:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. AZD7442 PROVENT Phase III prophylaxis trial met primary endpoint in preventing COVID-19. August 20, 2021. https://www.astrazeneca.com/media-centre/press-releases/2021/azd7442-prophylaxis-trial-met-primary-endpoint.html. Accessed 31 October 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.