Abstract
Background
LUNG INJURY COVID-19 (clinicaltrials.gov NCT 21/399-E) is a registry-based prospective observational cohort study to evaluate long-term outcomes and recovery 12 months after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection according to severity.
Methods
Three hundred five coronavirus disease 2019 (COVID-19) survivors were included (moderate, 162; severe, 143). Twelve months after SARS-CoV-2 infection, there was resolution of respiratory symptoms (37.9% in severe vs 27.3% in moderate pneumonia; P = .089).
Results
Exertional dyspnea was present (20% in severe vs 18.4% in moderate; P = .810). Abnormalities on chest radiology imaging were detected more often in severe COVID-19 infection vs moderate infection (29% vs 8.8%; P < .001). Pulmonary function testing (forced spirometry or diffusion) performed at 12 months of mean follow-up according to protocol detected anomalies in 31.4% of patients with severe COVID-19 courses and in 27.7% of moderate patients. Risk factors associated with diffusion impairment at 12 months were age (odds ratio [OR], 1.05; 95% CI, 1.01–1.10; P = .008), forced expiratory volume in 1 second predicted at follow-up (OR, 0.96; 95% CI, 0.93–0.99; P = .017), and dyspnea score at follow-up (OR, 3.16; 95% CI, 1.43–6.97; P = .004). Computed tomography (CT) scans performed at 12 months of mean follow-up showed evidence of fibrosis in almost half of patients with severe COVID-19 courses, who underwent CT according to protocol.
Conclusions
At 12 months from infection onset, most patients refer to symptoms, particularly muscle weakness and dyspnea, and almost one-third of patients with severe COVID-19 pneumonia had impaired pulmonary diffusion and abnormalities on chest radiology imaging. These results emphasize the importance of systematic follow-up after severe COVID-19, with appropriate management of pulmonary sequelae.
Keywords: long-term effects, SARS-CoV-2 infection, sequelae
Physicians are observing persisting symptoms and unexpected substantial organ dysfunction after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1], as previously observed in the severe acute respiratory syndrome (SARS) outbreak [2].
In previous coronavirus infections, studies concluded that between 20% and 60% of survivors of the global SARS outbreak caused by SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) experienced persistent physiological impairment and abnormal radiology consistent with pulmonary fibrosis [3–6]. The appearance of pulmonary fibrosis correlated with the severity and duration of acute illness [6, 7]. Drawing on these experiences, respiratory complications are predicted to be an important sequela of coronavirus disease 2019 (COVID-19).
In SARS-CoV-2 infection, several studies have reported lingering radiological and pulmonary diffusion abnormalities in a sizeable proportion of patients up to 3–6 months after hospital discharge. More than 70% of patients reported at least 1 symptom that persisted, and >50% of patients presented with residual chest imaging abnormalities 6 months after illness onset [8]. Some reports had data up to 3 months after onset that indicated that one-fifth of the patients displayed a reduced lung diffusing capacity and one-fourth of discharged patients had chest computed tomography (CT) scan abnormalities [9, 10]. This is particularly relevant for patients who required mechanical ventilation during their hospital stay [10].
SARS-CoV-2 infection is a new disease, and uncertainty remains regarding the possible long-term health sequelae, particularly in survivors of severe disease courses, for whom long-term complications and incomplete recovery after discharge would be expected. The clinical evolution and recovery are heterogeneous. At short-term follow-up, lung parenchymal abnormalities seem to have improved in most cases, while some patients persistently show abnormalities [8–10]. Potential long-term consequences specifically after severe COVID-19 still need to be investigated. Greater knowledge about the evolution of the disease and its possible complications is necessary for proper planning and optimization of resources.
METHODS
Study Design and Population
We initiated LUNG INJURY COVID-19, a registry to assess pulmonary sequelae following moderate or severe COVID-19 infection. This study was designed as a prospective clinical cohort to evaluate long-term outcomes and recovery 12 months after SARS-CoV-2 infection onset according to severity. This study includes adults who survived acute COVID-19 pneumonia and appeared for clinical follow-up after either moderate or severe COVID-19 infection.
This observational cohort study includes consecutive adults aged 18–84 years who survived laboratory-confirmed SARS-CoV-2 pneumonia and were referred to the follow-up clinic in the Pneumology Department at Hospital Clínico San Carlos in Madrid (Spain) from March 23 to August 20, 2020. Patients were referred from primary or specialized care within clinical practice after suffering a moderate or severe SARS-CoV-2 infection for follow-up. There were no exclusion criteria, except for patients’ or families’ explicit refusal to participate.
In this first analysis of our cohort, we report on chronic pulmonary sequelae in patients who had experienced moderate or severe COVID-19 pneumonia, with the goal of improving the current understanding of the heterogeneous COVID-19 trajectories.
Data Source
Baseline information (demographic data, comorbidities measured by the Charlson index [11], oxygen saturation in emergency room assessment) and clinical assessments during hospitalization (radiology, laboratory findings, clinical signs and symptoms, severity according to the use of ventilatory support and/or admission to the intensive care unit [ICU]) were retrieved from medical records.
Following the British Thoracic Society Guidance on Respiratory Follow Up of Patients with Diagnosis of COVID-19 Pneumonia [12], the following procedures were performed: assessment of symptoms and breathlessness, British modified Medical Research Council (mMRC) [13] dyspnea score determination, assessment of oxygen saturation, routine blood examinations, and chest x-ray. Pulmonary damage was quantified by adapting the Radiographic Assessment of Lung Edema (RALE) score to COVID-19 [14]. If chest x-ray changes had fully resolved and the patient had made a good recovery, we considered discharge. If chest x-ray had not cleared satisfactorily and/or the patient had ongoing respiratory symptoms, we considered pulmonary function testing including spirometry and measurement of carbon monoxide diffusing capacity (DLCO; Masterscreen, Jaeger, Germany). All procedures were carried out according to American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines [15, 16]. Arterial blood gases were obtained when hemoglobin oxygen saturation was below 90% or at the physician’s discretion. When there were persistent alterations in the radiography and/or impairment in pulmonary function tests (forced vital capacity [FVC] <80% without forced expiratory volume in 1 second/FVC <70% and/or DLCO <80%), a high-resolution CT scan was performed. HRCT scans (Optima CT660, General Electric, Boston, Massachusetts, USA) were obtained in the supine position during end-inspiration breath-holding. If there was clinical suspicion for pulmonary embolism (PE), additional contrast CTs were performed. The images were classified according to guidance by the Fleischner Society [17] based on the presence of ground-glass opacities, parenchymal bands, bronchiectasis, irregular lines, and reticular patterns. Images were interpreted by 2 senior radiologists experienced in chest radiology. All imaging data were analyzed in the absence of clinical or laboratory results or pulmonary function tests. After independent evaluation, any discrepancies were resolved through discussion and negotiation. A normal radiological pattern was defined as the absence of abnormalities.
Patients were stratified into 2 groups following the World Health Organization Clinical Progression Scale [18] according to severity: (1) moderate disease: patients with clinical signs of pneumonia and oxygen saturation (SpO2) ≥90% requiring supplemental oxygen by mask or nasal prongs; (2) severe disease: hospitalized patients who require respiratory support through noninvasive ventilation (NIV), high-flow nasal cannula oxygen (HFNC), or intubation and mechanical ventilation (IMV).
Statistical Analysis
Descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]). Differences between the moderate and severe COVID-19 groups were analyzed for statistical significance using the chi-square or Fisher exact test for categorical variables and the 2-sample t test or Wilcoxon rank-sum test for continuous variables, as applicable. Demographic factors, pulmonary and pulmonary function tests, and radiological sign associations with the COVID-19 severity groups were estimated using linear or logistic regression models where applicable. The conventional level of statistical significance of .05 was used for all analyses.
RESULTS
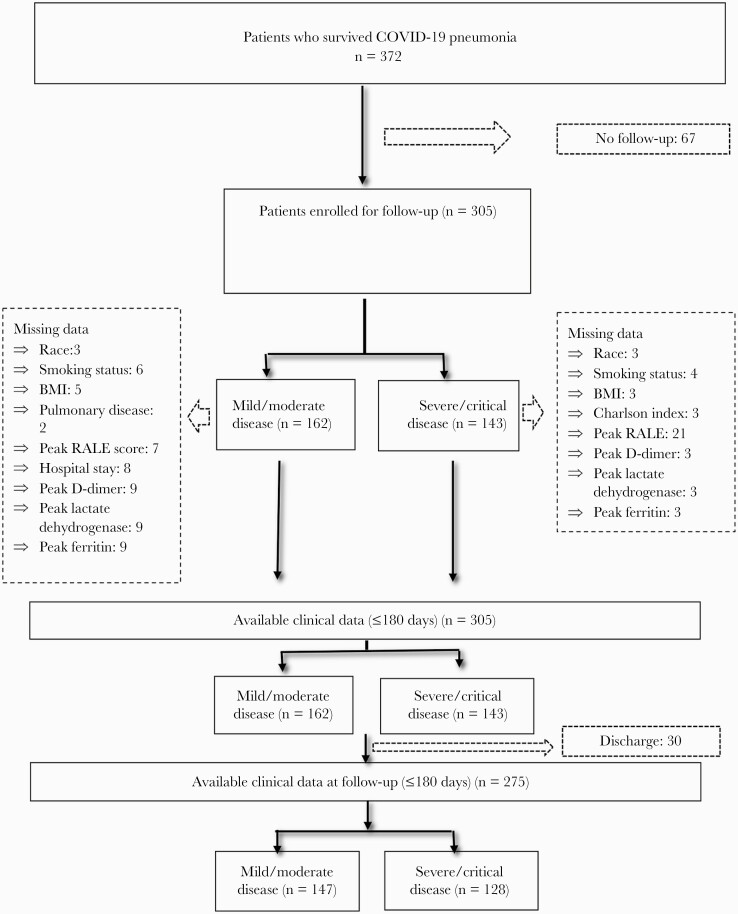
Between March 23 and August 20, 2020, this registry evaluated 305 patients with pneumonia from SARS-CoV-2 who were treated at Hospital Clínico San Carlos and referred to the follow-up clinic in the Pneumology Department. Sixty-seven were not considered for follow-up because they were living outside the hospital care area (Figure 1).
Figure 1.
Flowchart of the sampling process. Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; RALE, Radiographic Assessment of Lung Edema.
Baseline Characteristics and Symptoms
Three hundred five patients were included in the analysis (143 with severe COVID-19 infection and 162 with moderate infection); 75.5% of patients with severe infection required ICU admission, and 63.5% of patients with moderate pneumonia were hospitalized. Patients with severe COVID-19 were older than patients with moderate disease (mean [SD] age, 64.7 [15] vs 60.4 [14.2] years; P = .010), were mostly male (63.6% vs 41.3%; P < .001), and, as expected, they had more comorbidities (mean [SD] Charlson, 2.9 [2.2] vs 2.3 [2.2]; P = .024). Latin race (26.6% vs 7.9%; P < .001) and obesity (55.9% vs 33.3%; P < .001) were more prevalent in the severe group (P < .001). In addition, body mass index (BMI) was significantly higher in patients with severe disease (31.3 [5.3] vs 28.5 [5.3]; P < .001). However, in severe and moderate COVID-19 patients, history of lung disease (27.9% vs. 37.3%; P = .062) and smoking history were not significantly different. A logistic regression analysis showed that male sex, Latin race, and BMI were factors significantly associated with severe COVID-19 infection (Table 1).
Table 1.
Baseline Characteristics of Enrolled Patients With Severe and Moderate Infection
| Enrolled Patients | Severe Disease (n = 143) | Moderate Disease (n = 162) | P Valuea |
|---|---|---|---|
| Characteristics at baseline | |||
| Gender (male), % | 63.64 | 41.36 | <.001 |
| Age, y (%) | 64.79 (15.05) | 60.46 (14.20) | .010 |
| Race, % | <.001 | ||
| -Caucasian | 73.64 | 92.06 | |
| -Latin | 26.36 | 7.94 | |
| -Asian-Indian | 0.77 | 0 | |
| Smoking status, % | .728 | ||
| -Never-smoker | 60.16 | 57.14 | |
| -Current smoker | 9.38 | 7.94 | |
| -Former smoker | 30.47 | 34.92 | |
| Body mass index, mean (SD), kg/m2 | 31.36 (5.38) | 28.52 (5.38) | <.001 |
| Charlson index, mean (SD) | 2.98 (2.20) | 2.31 (2.26) | .024 |
| Obesity, % | 55.91 | 33.33 | <.001 |
| Pulmonary disease, % | 27.94 | 37.32 | .062 |
| COPD, % | 9.6 | 12 | .342 |
| Asthma, % | 11.81 | 15.57 | .248 |
| SAHS, % | 8 | 11.76 | .220 |
| ILD, % | 0 | 2.54 | |
| Neoplasia, % | 14.69 | 13.58 | .445 |
| AATD, % | 0.81 | 3.3 | .145 |
| PTE, % | 1.65 | 0 | .257 |
| Home oxygen therapy, % | 2.43 | 2.36 | .976 |
| Characteristics of acute COVID-19 | |||
| Hospitalization, % | 100 | 63.58 | <.001 |
| Length of hospital stay, median (IQR), d | 28 (20–39) | 5 (0–11) | <.001 |
| ICU/UCIR, % | 75.52 | 0 | <.001 |
| Ventilatory support therapy, % | 98.60 | 0 | <.001 |
| Tracheostomy, % | 13.77 | 0 | <.001 |
| PTE, % | 3.45 | 0 | .158 |
| RALE | |||
| -Peak RALE score, mean (SD) | 4.66 (2.29) |
2.51 (2.01) |
<.001 |
| -RALE score at discharge, mean (SD) | 4.53 (2.29) | 3.0 (2.25) | <.001 |
| Bilateral involvement, % | 91.06 | 68.09 | <.001 |
| Laboratory findingsb | |||
| -Lactate dehydrogenase, median (IQR), U/L | 474 (389.5–561) | 460 (378–545) | .850 |
| -Ferritin, median (IQR), ng/mL | 446 (237–749) | 420 (225–675) | .813 |
| -D-dimer, median (IQR), ng/mL | 941 (474–1548) | 566.5 (379–1207) | .004 |
| -D-dimer >500 ng/mL, % | 71.56 | 63.64 | .177 |
| Clinical associations with a severe COVID-19 disease course | OR (95% CI) | P-value | |
| Age | .997 (.956-1.040) | .920 | |
| Sex (male) | 3.102 (1.520-6.331) | .002 | |
| Race (Latin) | 8.444 (2.527-28.212) | .001 | |
| Body mass index | 1.106 (1.036-1.180) | .002 | |
| Never smoker | .905 (.429-1.906) | .793 | |
| Charlson | 1.230 (.915-1.655) | .169 | |
| Respiratory comorbidity | .498 (.232-1.069) | .074 | |
Data are expressed as No. (%), mean (SD), or median (IQR).
Abbreviations: AATD, alpha 1 antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; ILD, diffuse interstitial lung disease; IQR, interquartile range; PTE, pulmonary thromboembolism; RALE, Radiographic Assessment of Lung Edema; SAHS, sleep apnea hypopnea syndrome; UCRI, intermediate respiratory care unit.
P values from 2-sample t tests, Wilcoxon rank-sum test, chi-square test, or Fisher exact.
All laboratory findings are values at discharge.
Outcomes at Medium-term Follow-up
At follow-up, patients in both COVID-19 severity groups indicated relevant symptoms, with persistent exertional dyspnea in both groups. Dyspnea ≥2 mMRC among severe cases was significantly more prevalent than in moderate cases (55.5% vs 44%; P = .042). More than half of the patients with severe infection (58.1%) had unresolved x-ray abnormalities affected by either pulmonary opacities, parenchymal bands, or reticulations. Only 23.7% of patients in the moderate COVID-19 group had imaging abnormalities. In 161 (52.7%) patients, pulmonary function was tested due to the chest x-ray not having cleared satisfactorily and/or the patient having ongoing respiratory symptoms. Of these, impaired functional data were present in more patients with severe COVID-19 infection vs the group of moderate patients (59.4% vs 40.2%; P < .001). The median (SD) DLCO in severe vs moderate patients was 70.8 (21.3) vs 82.6 (25.7; P < .001). More than a third of patients in both groups had elevated D-dimer >500 ng/mL, although levels were higher in patients with severe COVID-19 infection vs in the group of moderate patients (median [IQR], 432 [278–742] vs 350.5 [219–597]; P = .023). Only 30 (10%) patients were considered for discharge and did not continue follow-up. Findings in the medium-term follow-up are provided in Table 2.
Table 2.
Features at Medium-term Follow-up (≤180 Days From Initial Symptoms)
| Clinical Characteristics | Severe Disease (n = 143) | Moderate Disease (n = 162) | P Valuea |
|---|---|---|---|
| Symptom resolution, % | 13.08 | 14.06 | .817 |
| Symptoms, % | |||
| -Cough | 14.41 | 26.32 | .033 |
| -Chest pain | 24.11 | 31.37 | .235 |
| -Asthenia | 29.46 | 37.00 | .244 |
| -Memory loss | 10.48 | 4.55 | .125 |
| -Hair loss | 16.51 | 6.59 | .032 |
| Dyspnea mMRC, mean (SD), No. | 1.45 (0.07) | 1.33 (0.06) | .143 |
| Dyspnea mMRC ≥2, % | 55.55 | 44.09 | .042 |
| Crackly, % | 16.52 | 8.33 | .090 |
| Average time,b median (IQR), d | 135 (116–153) | 133 (116–158) | .777 |
| Radiological Features | Severe Disease (n = 139) | Moderate Disease (n = 157) | |
|---|---|---|---|
| Normal radiological pattern, % | 41.86 | 76.30 | .001 |
| RALE score, mean (SD) | 0.62 (0.14) | 0.12 (0.06) | .002 |
| RALE score 0, % | 85.27 | 95.56 | .033 |
| Pulmonary opacities, % | 42.15 | 13.11 | .001 |
| Bands or reticulations, % | 33.06 | 14.75 | .001 |
| Hypoattenuation, % | 5.79 | 5.00 | .506 |
| Average time,b median (IQR), d | 119 (91–144) | 93 (61–128) | .001 |
| Pulmonary Functional Data | Severe Disease (n = 79) | Moderate Disease (n = 82) | |
|---|---|---|---|
| Functional data normalization, % | 40.51 | 59.76 | <.001 |
| FEV1, mean (SD), % predicted | 92.58 (20.51) | 99.83 (20.88) | .048 |
| FVC, mean (SD), % predicted | 94.90 (25.71) | 102.89 (20.93) | .036 |
| FEV/FVC, mean (SD) | 76.83 (12.67) | 75.98 (1.40) | .681 |
| SpO2, mean (SD), % | 96.02 (1.86) | 96.81 (11.65) | .002 |
| DLCO, mean (SD), % predicted | 70.84 (21.35) | 82.60 (25.73) | <.001 |
| Average time,b median (IQR), d | 138 (117–156) | 131 (109–147) | .271 |
| Serum Biomarkers | Severe Disease (n = 86) | Moderate Disease (n = 97) | |
|---|---|---|---|
| Lactate dehydrogenase, median (IQR), U/L | 387 (347–442) | 387 (327–415) | .180 |
| Ferritin, median (IQR), ng/mL | 88 (39–173) | 65 (34.5– 110) | .287 |
| D-dimer, median (IQR), ng/mL | 432 (278–742) | 350.5 (219–597) | .023 |
| D-dimer >500 ng/mL, % | 37.35 | 33.33 | .347 |
| Average time,b median (IQR), d | 120 (109–130) | 115 (107–122) | .445 |
Abbreviations: DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; mMRC, modified Medical Research Council; RALE, Radiographic Assessment of Lung Edema; SpO2, oxygen saturation.
P values from 2-sample t test, Wilcoxon rank-sum test, chi-square test, or Fisher exact test. Average time from initial symptoms to evaluation.
time from initial symptoms to the follow-up data.
Outcomes at Long-term Follow-up
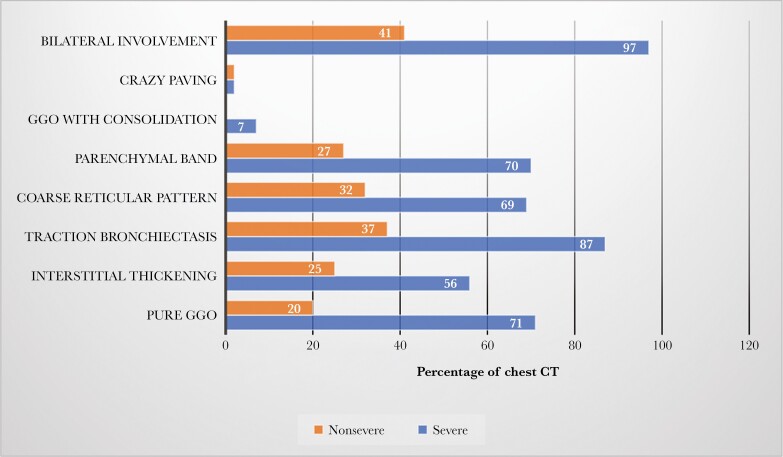
The assessment of symptoms after more than 10 months since SARS-CoV-2 infection showed resolution in a third of patients in both groups (37.9% in severe vs 27.3% in moderate; P = .089). Long-term abnormalities in chest x-ray imaging after SARS-CoV-2 infection were more common in patients with severe COVID-19 infection vs in the group of moderate patients (29% vs 8.8%; P < .001). In 153 (55.6%) patients, pulmonary function was tested due the chest x-ray not having cleared satisfactorily and/or the patient having ongoing respiratory symptoms. Of these, functional normalization dates were more common in patients with moderate COVID-19 infection vs the group of severe patients (64.1% vs 50.6%; P < .001). There were no differences between severity groups in spirometry parameters. The mean (SD) DLCO in severe vs moderate patients was 75.7 (24.6) vs 83.7 (21.1; P = .032). Seventy-nine patients underwent a CT scan according to protocol, with a greater median time between infection onset and CT in patients with severe COVID-19 infection vs those in the moderate group (median [IQR], 332 [250–381] days vs 306 [239–354] days; P = .451). Radiological features were significantly more prevalent after severe infection than after moderate COVID-19, as shown in Figure 2. Serum laboratory testing was carried out in 116 patients (42.1%) because they maintained abnormal results from previous visits. More than a third of patients in both groups had elevated D-dimer >500 ng/mL; no significant differences were found between severity groups. Findings in the long-term follow-up are provided in Table 3.
Figure 2.
Proportion of chest CT with imaging abnormalities after 1 year in severe vs nonsevere COVID infection. Abbreviations: COVID-19, coronavirus disease 2019; CT, computed tomography; GGO, ground-glass opacity.
Table 3.
Features at Long-term Follow-up (>180 Days From Initial Symptoms)
| Clinical Characteristics | Severe Disease (n = 128) | Moderate Disease (n = 147) | P Valuea |
|---|---|---|---|
| Symptom resolution, % | 37.96 | 27.35 | .089 |
| Symptoms, % | |||
| -Cough | 3.30 | 11.90 | .030 |
| -Chest pain | 4.35 | 13.95 | .025 |
| -Asthenia | 7.69 | 20.00 | .017 |
| -Memory loss | 4.55 | 3.95 | .850 |
| -Hair loss | 0.00 | 1.27 | .287 |
| Dyspnea mMRC, mean (SD), No. | 0.82 (0.07) | 0.89 (0.06) | .392 |
| Dyspnea mMRC ≥2, % | 20 | 18.42 | .249 |
| Crackly, % | 8.43 | 6.49 | .642 |
| Average time,b median (IQR), d | 300 (245–352) | 306 (254–363) | .352 |
| Radiological Features | Severe Disease (n = 117) | Moderate Disease (n = 147) | |
| Normal radiological pattern, % | 70.94 | 91.15 | .001 |
| RALE score, mean (SD) | 0.09 (0.05) | 0.00 (0.00) | .085 |
| RALE score 0, % | 97.06 | 99.35 | .176 |
| Pulmonary opacities, % | 10.34 | 2.19 | .006 |
| Parenchymal bands or reticulations, % | 21.05 | 7.25 | .001 |
| Hypoattenuation, % | 6.20 | 2.88 | .154 |
| Average time,b median (IQR), d | 260 (228–320) | 274 (225–353) | .262 |
| Pulmonary Functional Data | Severe Disease (n = 75) | Moderate Disease (n = 78) | |
|---|---|---|---|
| Functional data normalization, % | 50.66 | 64.10 | <.001 |
| FEV1, mean (SD), % predicted | 101.84 (21.38) | 103.70 (19.62) | .620 |
| FVC, mean (SD), % predicted | 103.44 (22.59) | 110.55 (20.55) | .074 |
| FEV/FVC, mean (SD), % | 78.46 (8.66) | 75.51 (7.53) | .033 |
| SpO2, mean (SD), % | 95.83 (4.05) | 97.18 (1.15) | .008 |
| DLCO, mean (SD), % predicted | 75.73 (24.68) | 83.79 (21.18) | .032 |
| Average time,b median (IQR), d | 277 (224–350) | 268 (218–344) | .609 |
| Serum Biomarkers | Severe Disease (n = 58) | Moderate Disease (n = 58) | |
|---|---|---|---|
| Lactate dehydrogenase, median (IQR), U/L | 366 (326–427) | 363 (316–410) | .445 |
| Ferritin, median (IQR), ng/mL | 114 (55.5–304.5) | 72.75 (45–143) | .047 |
| D-dimer, median (IQR), ng/mL | 415 (265–831) | 390 (242–502) | .374 |
| D-dimer >500 ng/mL, % | 36.36 | 25 | .131 |
| Average time,b median (IQR), d | 289 (273–306) | 275 (255–294) | .256 |
Abbreviations: DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; mMRC, modified Medical Research Council; RALE, Radiographic Assessment of Lung Edema; SpO2, oxygen saturation.
P values from 2-sample t tests, Wilcoxon rank-sum test, chi-square test, or Fisher exact test. Average time from initial symptoms to evaluation.
Time from initial symptoms to the follow-up data.
Prediction of Abnormalities in Long-term Chest Radiology Imaging
Ten months after discharge, abnormalities in chest radiology imaging were detected in 47 patients (17.8%). These patients with abnormalities on chest x-ray vs normal chest radiology were older (mean [SD] age, 68.8 [14.0] vs 60.4 [14.1] years; P < .001) and had a significantly longer hospital stay (median [IQR], 26 [22–38] vs 11 [3–22]; P < .001) and greater infection severity than patients with normal chest radiology (72.3% vs 38.2%; P < .001). Long-term FVC% predicted outcomes and DLCO% predicted outcomes at follow-up were significantly lower in patients with abnormalities on radiology imaging. Results of the multivariable analysis found that the number of days hospitalized and FVC% predicted at follow-up were associated with long-term abnormalities on chest radiology imaging (Table 4).
Table 4.
Relationship Between Radiological Features and Clinical Characteristics, COVID-19 Disease Severity and Features at Long-term Follow-up
| Total (n = 264) | Abnormal Radiological Pattern (n = 47) | Normal Radiological Pattern (n = 217) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 68.89 (14.02) | 60.40 (14.16) | <.001 |
| Never-smoker, % | 50 | 58.79 | .187 |
| Pulmonary disease, % | 24.44 | 32.16 | .203 |
| Body mass index, mean (SD), kg/m2 | 30.43 (4.46) | 30.17 (5.80) | .475 |
| Length of hospital stay, median (IQR), d | 26 (22–38) | 11 (3–22) | <.001 |
| Peak RALE score, mean (SD) | 4.73 (2.29) | 3.69 (2.47) | .014 |
| Severe disease, % | 72.34 | 38.25 | <.001 |
| Features at long-term follow-up (>180 d) | |||
|---|---|---|---|
| mMRC dyspnea, mean (SD) | 0.91 (0.78) | 0.82 (0.70) | .492 |
| FEV/FVC, mean (SD), % | 78.91 (7.80) | 76.60 (8.48) | .355 |
| FEV1, mean (SD), % predicted | 97.34 (18.15) | 104.28 (21.09) | .105 |
| FVC, mean (SD), % predicted | 96.89 (18.72) | 110.11 (21.84) | .007 |
| SpO2, % | 96.38 | 96.81 | .122 |
| DLCO, mean (SD), % predicted | 69.06 (21.04) | 83.56 (22.65) | <.001 |
| D-dimer, median (IQR), ng/mL | 302.5 (215–867.5) | 400 (242–551) | .812 |
| D-dimer >500 ng/mL, % | 35 | 27.06 | .326 |
| Predictors of long-term abnormalities on chest radiology imaging | |||
|---|---|---|---|
| OR (95% CI) | P Value | ||
| Age | 0.964 (0.928–1.003) | .072 | |
| Length of hospital stay | 0.966 (0.939–0.994) | .019 | |
| FVC at long-term follow-up | 1.026 (1.002–1.051) | .032 | |
Abbreviations: DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; mMRC, modified Medical Research Council; RALE, Radiographic Assessment of Lung Edema; SpO2, oxygen saturation.
Prediction of Long-term Lung Function Deficits
Ten months after discharge, impaired pulmonary diffusion was detected in 65 patients (42.4%). These patients with abnormal diffusion vs normal were older (mean [SD] age, 67.7 [14.7] vs 58.8 [11.9] years; P < .001), had different smoking histories (never-smoker: 43.6% vs 65.3%; P = .011), and history of lung disease was more prevalent (42.3% vs 27.1%; P = .045). Hospital stay was significantly longer in patients with abnormal diffusion vs normal (21.5 [10.5–32.5] vs 12.5 [2–26] days; P = .033). Results of the multivariable analysis found that age, FEV1% predicted, and dyspnea score at follow-up were independent risk factors associated with the presence of impaired DLCO% (Table 5). There was a significant negative correlation between DLCO% predicted and dyspnea score (R = –0.225; P = .006) and length of hospital stay (R = –0.1594; P = .0546), whereas RALE score and D-dimer levels showed no correlation with DLCO% predicted.
Table 5.
Relationship Between Lung Diffusion and Clinical Characteristics, COVID-19 Disease Severity and Features at long-term Follow-Up
| Total (n = 153) | DLCO <80% (n = 65, 42.48%) |
DLCO ≥80% (n = 88, 57.52%) |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 67.75 (14.74) | 58.80 (11.94) | <.001 |
| Never-smoker, % | 43.64 | 65.33 | .011 |
| Pulmonary disease, % | 42.37 | 27.16 | .045 |
| Body mass index, mean (SD), kg/m2 | 29.94 (4.81) | 30.43 (5.62) | .603 |
| Severe disease, No. (%) | 37 (56.92) | 38 (43.18) | .064 |
| Length of hospital stay, median (IQR), d | 21.5 (10.5–32.5) | 12.5 (2–26) | .033 |
| Peak RALE score, mean (SD) | 4.31 (2.28) | 3.73 (2.43) | .214 |
| Peak D-dimer, median (IQR), ng/mL | 1858 (971.5–6974) | 2358 (1157–6675) | .387 |
| Features at long-term follow-up (>180 d) | |||
|---|---|---|---|
| mMRC dyspnea, mean (SD) | 1.08 (0.66) | 0.70 (0.65) | <.001 |
| FEV/FVC, mean (SD), % | 75.43 (10.68) | 78.35 (5.53) | .078 |
| FEV1, mean (SD), % predicted | 93.29 (20.15) | 109.69 (17.90) | <.001 |
| FVC, mean (SD), % predicted | 98.98 (21.20) | 112.64 (20.60) | <.001 |
| DLCO, mean (SD), % predicted | 58.95 (17.69) | 95.27 (12.11) | <.001 |
| SpO2, mean (SD), % | 96.22 (1.19) | 97.05 (1.35) | <.001 |
| RALE score, mean (SD) | 0.12 (0.70) | 0.05 (0.44) | .497 |
| D-dimer, median (IQR), ng/mL | 701.55 (467.86– 935.24) | 531.85 (299.56–764.14) | .299 |
| Predictors of long-term DLCO impairment | |||
|---|---|---|---|
| OR (95% CI) | P Value | ||
| Age | 1.059 (1.014–1.105) | .008 | |
| Pulmonary disease | 1.711 (0.542–5.393) | .359 | |
| Never-smoker | 0.359 (0.125–1.029) | .057 | |
| Length of hospital stay | 0.989 (0.954–1.026) | .571 | |
| FEV1 at long-term follow-up | 0.962 (0.932–0.993) | .017 | |
| Dyspnea at long-term follow-up | 3.169 (1.439–6.977) | .004 | |
| Severe disease | 1.953 (0.440–8.653) | .378 | |
Abbreviations: DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; mMRC, modified Medical Research Council; RALE, Radiographic Assessment of Lung Edema; SpO2, oxygen saturation.
D-dimer Relationship at Long-term Follow-up
Ten months after discharge, abnormal D-dimer >500 ng/mL was detected in 31% of patients. These patients with abnormal D-dimer were older (mean [SD] age, 72.3 [14.1] vs 57.2 [12.5] years; P < .001) and had a higher peak D-dimer during hospitalization (median [IQR], 5062 [1800–22 840] vs 1748 [800–4568]; P < .020). At follow-up, impaired FEV1% predicted, SpO2, and DLCO% predicted functional data were more common in patients with abnormal D-dimer (Table 6).
Table 6.
Relationship Between D-dimer Features and Clinical Characteristics, COVID-19 Disease Severity and Features at Long-term Follow-up
| Abnormal D-dimer >500 ng/mL (n = 36) |
Normal D-dimer ≤500 ng/mL (n = 80) |
P Value | |
|---|---|---|---|
| Age, mean (SD), y | 72.34 (14.16) | 57.2 (12.53) | <.001 |
| Never-smoker, % | 62.50 | 61.54 | .554 |
| Pulmonary disease, % | 32.35 | 34.72 | .495 |
| Body mass index, mean (SD), kg/m2 | 30.46 (4.23) | 30.69 (6.12) | .950 |
| Length of hospital stay, median (IQR), d | 22 (10–32) | 14 (3–25) | .070 |
| Peak RALE score, mean (SD) | 4.62 (1.98) | 3.94 (2.47) | .186 |
| Peak D-dimer, median (IQR) | 5062 (1800–22 840) | 1748 (800–4568) | .020 |
| Severe disease, % | 57.14 | 43.75 | .131 |
| Features at long-term follow-up (>180 d) | |||
| mMRC dyspnea, mean (SD) | 1 (0.87) | 0.67 (0.59) | .066 |
| FEV/FVC, mean (SD), % | 74.85 (2.81) | 78.98 (1.00) | .349 |
| FEV1, mean (SD), % predicted | 96 (19.51) | 106.97 (18.80) | .028 |
| FVC, mean (SD), % predicted | 101.22 (25.86) | 110.65 (22.44) | .208 |
| SpO2, % | 96.09 | 97.04 | .018 |
| DLCO, mean (SD), % predicted | 71.73 (22.92) | 86.36 (20.83) | .006 |
| RALE score, mean (SD) | 0 (0) | 0.01 (0.11) | .505 |
Abbreviations: DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; mMRC, modified Medical Research Council; RALE, Radiographic Assessment of Lung Edema; SpO2, oxygen saturation.
DISCUSSION
To our knowledge, this is the first study reporting on long-term outcomes in respiratory follow-up after SARS-CoV-2 infection. Few reports have described sequelae in COVID-19 survivors, and this analysis is the first to investigate the long-term effects on changes in symptoms, pulmonary function, and chest radiology in patients with severe infection and moderate infection 1 year after infection.
This analysis demonstrates that nearly two-thirds of the cohort suffered symptoms almost a year after SARS-CoV-2 infection onset, particularly fatigue or muscle weakness and dyspnea. This is consistent with data from previous long-term SARS follow-up studies [19]. A follow-up study of SARS survivors showed that 40% of patients still had a chronic fatigue problem for a mean period of 41.3 months after SARS infection [20]. The underlying mechanism of fatigue or muscle weakness post-COVID-19 is likely multifactorial and might include the direct effects of viral infection, the immunological response, corticosteroid therapy, ICU stay, or isolation. In our analysis, fatigue or muscle weakness, chest pain and cough were more common in patients with moderate COVID-19 courses.
Our results showed significant improvement in symptoms between 5 and 10 months after infection onset. In patients with a severe course of COVID infection, dyspnea ≥2 mMRC was present in 55% of patients at midterm follow-up visits but only in 20% in the long term. This recovery has also been described in SARS. Many patients who recovered from SARS have complained of limitation in general physical function and/or shortness of breath in the early rehabilitation phase [20].
The results of radiological features showed that only 17.8% of patients had chest x-ray abnormalities in the long term, which was more frequent in patients with severe COVID-19 courses (29%) and associated with the number of days hospitalized. The rate of radiological anomalies was 40.2% in our series at an average follow-up of 5 months and was higher (58.2%) in patients with severe COVID-19 infection. These results indicate that radiological abnormalities caused by SARS-CoV-2 might get better over time. In relation to the abnormalities on chest x-ray after COVID infection, the first reports from discharged patients with SARS-CoV-2 pneumonia showed that 83% had chest x-ray alterations a few days after discharge [21, 22]. Data at 3 months showed that 74.5% had radiological abnormalities [23]. In our study, CT scans showed evidence of fibrosis as a combination of findings including parenchymal bands, coarse reticular patterns, irregular interfaces, and traction bronchiectasis in almost half of patients with severe COVID-19 courses and in a quarter of patients with moderate COVID infection, who underwent CT according to protocol. Data from a cohort of patients examined 153 days after hospital discharge showed abnormal CT findings in 44% [8].
In our cohort, the results of lung function assessment showed that 42.4% of patients had decreased pulmonary diffusion 10 months after infection onset. However, it is important to note that not all patients underwent pulmonary function testing, as per protocol. Pulmonary function was tested at the long-term follow-up in only 153 (55.6%) patients due to the chest x-ray not having cleared satisfactorily and/or the patient having ongoing respiratory symptoms. Of these, functional normalization dates were more present in patients with moderate COVID-19 infection vs the group of severe patients (64.1% vs 50.6%; P < .001) at 10 months from infection onset. If we consider the total number of patients in our cohort, assuming functional normality for those patients whose lung function was not tested due to resolution of symptoms and radiological findings, we could state that we detected functional alterations in 31.4% of patients with severe COVID-19 courses and in 27.7% of moderate patients at 10 months of mean follow-up. Previous reports from SARS outbreak survivors showed that 20% had long-term altered diffusion [3, 6].
In relation to lung function involvement after COVID-19 infection, the first reports from discharged patients with SARS-CoV-2 pneumonia showed that 47% had diffusion impairment after 1 month [22]. Data at 3 months showed that 24% had reduced DLCO, and opacities on the CT scan were present in 25% of patients, without finding differences between patients admitted to the ICU compared with those who were not severe [10]. A 6-month follow-up study demonstrated reduced pulmonary function in 56% of patients who required high-flow nasal cannula or invasive mechanical ventilation, compared with 29% of those who required only supplemental oxygen [8]. In our study, reduced DLCO was associated with age, FEV1% predicted, and dyspnea score during long-term follow-up. Smoking history, underlying pulmonary diseases, length of hospital stay, and severe disease were not associated with long-term diffusion impairment, despite 75.5% of patients with severe infection requiring ICU admission in our analyzed population. Previous studies demonstrated a negative correlation between the duration of mechanical ventilation during acute disease and pulmonary function at 4 months of follow-up [24].
Our study has several limitations. First, LUNG INJURY COVID-19 is a registry to assess pulmonary sequelae following COVID-19 infection in patients referred to the follow-up clinic in the Pneumology Department, and this may carry some bias. During the first wave of the pandemic, patients with more severe COVID-19 were controlled mainly by pulmonologists. This scenario gives us insight into the cases that will have the highest risk of developing complications. A large proportion of patients in our cohort required support therapies and care in intensive care units, which may result in overestimates of the long-term consequences of COVID-19. However, the sample size was large and stratified by COVID infection severity. Second, not all patients underwent lung function testing and CT as per protocol. This approach, in line with the guidance of the British Thoracic Society [12], was adopted to mitigate overload of the health system. Third, the presence of previous chronic lung abnormalities may be underestimated, as although the clinical history was actively searched, underlying asymptomatic lung disease, though unlikely, cannot be excluded. Fourth, we could not study serum biomarkers in the whole group, mainly for logistical reasons. Fifth, multivariable models did not adjust for coexisting medical comorbidities or differences in therapies that can impact association estimates.
In sum, a comprehensive, longitudinal approach to post-COVID-19 care will require strategies and resources to meet the common and divergent needs of these populations, addressing both pulmonary and extrapulmonary concerns and complications. Multiple centers are creating multidisciplinary ambulatory programs for the post-COVID-19 population to provide a comprehensive evaluation of post-COVID-19 complications, characterize and mitigate pulmonary sequelae of COVID-19, and address persistent symptoms experienced by COVID-19 survivors [12, 25].
CONCLUSIONS
This is the first study to show long-term respiratory outcomes after almost a year. Our cohort confirms that although a substantial proportion of patients have abnormalities in lung function and chest imaging at discharge, progressive improvement occurs in the following months. Only a small percentage of patients who have had a severe infection will continue to have chronic abnormalities. These results emphasize the importance of systematic follow-up after severe COVID-19, with appropriate management of pulmonary sequelae [25]. Comprehensive evaluation of survivors will refine our understanding of the clinical course of COVID-19 and facilitate the development of care plans to mitigate symptoms and complications for survivors.
Acknowledgments
The authors thank the investigators Elizabeth Gonzalez Revilla, Roberto Larrosa Barreiro, Celia Pinedo Sierra, Maria Jose Bernabe Barrios, and Elena Forcen Vicente De Vera, Pulmonology Department, Hospital Clínico San Carlos Madrid, Spain, who participated in the LUNG INJURY COVID-19 Study.
Financial support. This study has not been promoted or sponsored.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. G.V.C., M.C.R., J.L.A.S., F.M.S., A.A.H., R.H.H., and J.R.H. have intellectually contributed to this work and contributed to data analysis and interpretation of results. J.L.R.H. and M.C.R. wrote the manuscript. R.H.H. carried out the statistical analysis. All authors participated in drafting and revising the paper and assume accountability for all aspects of the work.
Patient consent. This study was approved by the Research Ethics Committee at Hospital Clínico San Carlos, Madrid, Spain (internal code 21/399-E). All patients provided informed consent before inclusion.
References
- 1. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis. J Rehabil Med 2020; 52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 4. Cheung OY, Chan JW, Ng CK, Koo CK.. The spectrum of pathological changes in severe acute respiratory syndrome (SARS). Histopathology 2004; 45:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ketai L, Paul NS, Wong KT.. Radiology of severe acute respiratory syndrome (SARS): the emerging pathologic-radiologic correlates of an emerging disease. J Thorac Imaging 2006; 21:276–83. [DOI] [PubMed] [Google Scholar]
- 6. Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax 2005; 60:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hui DS, Wong KT, Ko FW, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest 2005; 128:2247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur Respir J 2020; 57:2003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur Respir J 2020; 57:2003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 12. George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020; 75:1009–16. [DOI] [PubMed] [Google Scholar]
- 13. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW WJ.. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong HYF, Lam HYS, Fong AH, et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 2020; 296:E72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019; 200:e70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J 2017; 49:1600016. [DOI] [PubMed]
- 17. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J.. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722. [DOI] [PubMed] [Google Scholar]
- 18. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lam MH, Wing YK, Yu MW, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 2009; 169:2142–7. [DOI] [PubMed] [Google Scholar]
- 20. Chan KS, Zheng JP, Mok YW, et al. SARS: prognosis, outcome and sequelae. Respirology 2003; 8:S36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 2020; 21:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 2020; 55:2001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the National Prospective Observational Swiss COVID-19 Lung Study. Eur Respir J 2021; 57:2003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lutchmansingh DD, Knauert MP, Antin-Ozerkis DE, et al. A clinic blueprint for post-coronavirus disease 2019 RECOVERY. Learning from the past, looking to the future. Chest 2021; 159:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]