Abstract
Background
Although long non-coding RNA differentiation antagonizing non-protein coding RNA (DANCR) has been reported to be involved in atherosclerosis (AS) development, its specific mechanism remains unclear.
Methods
DANCR expression levels in blood samples of AS patients and oxidized low-density lipoprotein (ox-LDL) treated vascular smooth muscle cells (VSMCs) and human umbilical vein endothelial cells (HUVECs) were detected by quantitative real-time polymerase chain reaction (qRT-PCR). The small interfering RNA targeting DANCR (si-DANCR) was used to silence DANCR expression. Cell viability was assessed by CCK-8 assay. Cell apoptosis was evaluated by flow cytometry. Levels of inflammatory cytokines, anti-oxidative enzyme superoxide dismutase (SOD) activity, and malonaldehyde (MDA) were detected by specific commercial kits. An animal AS model was established to confirm the role of DANCR/microR-214-5p/COX20 (the chaperone of cytochrome c oxidase subunit II COX2) in AS development.
Results
DANCR was significantly increased in the blood samples of AS patients and ox-LDL treated VSMCs and HUVECs. DANCR downregulation obviously increased viability and reduced apoptosis of ox-LDL-treated VSMCs and HUVECs. Meanwhile, DANCR downregulation reduced the levels of inflammatory cytokines, including interleukin (IL)-6 (IL-6), IL-1beta (IL-1β), IL-6 and tumor necrosis factor (TNF)-alpha (TNF-α) and MDA while increasing the SOD level in ox-LDL-treated VSMCs and HUVECs. DANCR regulated COX20 expression by acting as a competing endogenous RNA (ceRNA) of miR-214-5p. Rescue experiments demonstrated that miR-214-5p downregulation obviously attenuated si-DANCR-induced protective effects on ox-LDL-caused endothelial injury.
Conclusions
Our results revealed that DANCR promoted AS progression by targeting the miR-214-5p/COX20 axis, suggesting that DANCR might be a potential therapeutic target for AS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11658-022-00310-2.
Keywords: Atherosclerosis, DANCR, miR-214-5p, COX20
Introduction
Atherosclerosis (AS) is a chronic inflammatory disorder and the most common leading cause of cerebrovascular diseases (CVD) [1, 2]. Its pathogenesis is not well understood, which is one of the reasons for its high mortality [3]. It has been reported that endothelial cell dysfunction is an essential process for AS progression [4]. Oxidative low-density lipoprotein (ox-LDL) is an accepted substance for mimicking AS conditions in vitro via stimulating endothelial cell dysfunction [5]. Therefore, understanding the specific molecular mechanisms involved in ox-LDL-induced endothelial cell dysfunction may contribute to developing efficient diagnostic and therapeutic targets for AS.
Long non-coding RNAs (lncRNAs), a type of RNA transcripts more than 200 nucleotides in length, have been reported to participate in various biological and pathological processes [6]. Many lncRNAs have been shown to regulate AS progression by acting as competing endogenous RNAs (ceRNAs) of microRNAs (miRNAs) [7]. For example, OIP5-AS1 reduces ox-LDL-treated HUVEC injury via miR-30c-5p-mediated NF-kappaB signaling [8]. NEAT1 regulates endothelial functions in subclinical hypothyroidism by targeting the miR-126/TRAF7 axis [9]. PCA3 reduces lipid accumulation and AS progression by regulating the miR-140-5p/RFX7/ABCA1 signaling pathway [10]. Differentiation antagonizing non-protein coding RNA (DANCR), a widely studied lncRNA, has been reported to participate in the development of various human cancers such as non-small cell lung cancer [11] and colorectal cancer [12]. Recently, Zhang et al. found that DANCR participated in AS development by regulating ABCA1 [13], indicating that DANCR plays an important role in AS progression.
MiRNAs are another type of non-coding RNAs with regulatory functions [14]. MiR-214-5p, a newly identified miRNA, has been reported to regulate the progression of human diseases such as acute kidney injury [15], bone defects [16], prostate cancer [17], and breast cancer [18]. Interestingly, previous studies reported that DANCR could act as a ceRNA of miR-214-5p to impact the development of human diseases, such as non-small lung cancer [19] and pancreatic cancer [20]. Based on this evidence, we speculated that DANCR might play a role in AS progression through modulating miR-214-5p.
COX20 is a subunit of cytochrome c oxidase and is also known as the chaperone of cytochrome c oxidase subunit II (COX2) [21]. Although the function of COX20 in AS development remains unclear, COX2 could stimulate inflammation, thereby aggravating AS progression [22]. COX2 inhibition might aid in preventing AS progression [23]. As one of the essential chaperones of COX2 [21, 24], COX20 might also play an important role in AS. Our study aimed to investigate the potential regulatory relationship between DANCR and miR-214-5p and the role of COX20 in AS.
In this study, we confirmed that DANCR was significantly upregulated in the blood samples of AS patients and ox-LDL treated VSMCs and HUVECs. DANCR negatively regulated miR-214-5p by acting as its ceRNA. Furthermore, COX20 was identified as a direct target of miR-214-5p. Both in vitro and in vivo experiments demonstrated that DANCR/miR-214-5p/COX20 closely participated in AS progression and could be a potential therapeutic target for AS treatment.
Materials and methods
Clinical samples
A total of 60 volunteers, including 30 AS patients and 30 healthy controls, were recruited at Harrison International Peace Hospital between 2017 and 2020. Atherosclerosis was diagnosed with transthoracic echocardiography and carotid ultrasonography. The diagnostic criteria for AS required that the main blood vessels of the coronary artery have more than 50% stenosis. Meanwhile, patients with cancers, congestive heart failure, valvular heart diseases, hematological system diseases, autoimmune diseases, and/or infection were excluded. The blood samples from 60 volunteers were collected. All patients were diagnosed for the first time without any drug treatment, and blood samples were collected at the initial diagnosis stage. Table 1 shows the clinical characteristics of AS patients.
Table 1.
Baseline characteristics of the subjects
| Variables | Control group | AS group | p value |
|---|---|---|---|
| Sample size | 30 | 30 | – |
| Gender, number (%) | |||
| Female | 14 (46.7) | 13 (43.3) | 0.605 |
| Male | 16 (53.3) | 17 (56.7) | |
| Age (years), mean ± SD | 60.54 ± 9.21 | 64.60 ± 8.61 | 0.251 |
| BMI (kg/m2), media (IQR) | 22.89 (1.52) | 23.15 (2.53) | 0.49 |
| Diabetes, number (%) | |||
| Yes | 13 (43.3) | 15 (50) | 0.629 |
| No | 17 (56.7) | 15 (50) | |
| Hypertension, number (%) | |||
| Yes | 12 (40) | 18 (40) | 0.602 |
| No | 18 (60) | 12 (60) | |
| Smoke, number (%) | |||
| Yes | 14 (46.7) | 15 (50) | 0.667 |
| No | 16 (53.3) | 15 (50) | |
| Alcohol, number (%) | |||
| Yes | 16 (53.3) | 15 (50) | 0.7 |
| No | 14 (46.7) | 15 (50) | |
| TC (mmol/L) | 1.21 ± 0.55 | 1.45 ± 0.65 | 0.209 |
| TG (mmol/L) | 4.01 ± 0.98 | 4.35 ± 0.73 | 0.299 |
| LDL (mmol/L) | 2.02 ± 0.81 | 2.56 ± 0.64 | 0.384 |
| HDL (mmol/L) | 1.19 ± 0.47 | 1.17 ± 0.34 | 0.977 |
Data are presented as mean ± SD. Pearson χ2 test. *P < 0.05 was considered statistically significant
BMI Body Mass Index, TC total cholesterol, TG triglycerides, LDL low-density lipoprotein, HDL high-density lipoprotein
Cell culture and treatment
HEK-293A, VSMCs, and HUVECs were purchased from ATCC and cultured in RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, USA) in a 37 °C incubator with 5% CO2. To mimic the atherosclerosis condition, 1 × 106 cells were seeded into six-well plates. After being cultured overnight, cells were treated with 25, 50, and 100 μg/ml oxidized low-density lipoprotein (ox-LDL) (UnionBiol, Beijing, China) for 48 h, or treated with 50 μg/ml ox-LDL for 6, 12, 24, and 48 h. To explore the function of DANCR in AS, cells were treated with 50 μg/ml ox-LDL for 48 h.
Cell transfection
MiR-214-5p mimics, miR-214-5p inhibitor and their negative controls (miR-NC and inhibitor NC), as well as the small interfering RNA targeting DANCR (si-DANCR) and the negative control si-NC, were designed by and purchased from GenePharma (Shanghai, China). The sequence of si-DANCR is 5′-UCGGAGGUGGAUUCUGU UA-3′, and the sequence of si-NC is 5′-AGCCAACTATCCCTTCAGT-3′. 1 × 106 cells were seeded into six-well plates, and cell transfection was performed with 50 nM mimics/inhibitor/si-RNAs using Lipofectamine 2000 (Invitrogen), and subjected to 50 μg/ml ox-LDL for 48 h, then cells were used for the following analysis.
CCK-8 assay
In brief, 1 × 104 transfected VSMCs and HUVECs were seeded into 96-well plates and treated with 50 μg/ml ox-LDL for 48 h. Then 10 μl of CCK-8 reagent (CCK-8, Dojindo Molecular Technologies, Japan) was added, and cells were incubated for another 4 h. Finally, the absorbance at 450 nm was measured using a microplate reader.
Apoptosis analysis
Cell apoptosis was assessed using Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Beyotime, China). Briefly, 1 × 106 transfected VSMCs and HUVECs were seeded into 6-well plates and treated with 50 μg/ml ox-LDL for 48 h. Cells were then incubated with 5 μl of FITC and 5 μl of PI for 20 min. The percentage of apoptotic cells was calculated by FACSCalibur (BD Biosciences, Australia).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were extracted from blood samples and cells using TRIzol reagent (Invitrogen). cDNAs were synthesized via reverse transcription of approximately 1.2 μg of total RNAs using PrimeScript RT Master Mix (TaKaRa, Dalian, China). The real-time PCR reactions were performed using the Thermal Cycler Dice Real-Time PCR System (TaKaRa) at 95 ℃ for 10 s followed by 45 cycles of 95 ℃ for 5 s, 60 ℃ for 10 s, and 72 ℃ for 10 s and extension at 72 ℃ for 5 min. The RNA expression was analyzed using the 2−ΔΔCt method. GAPDH and U6 were used as the endogenous references for lncRNA/mRNA and miRNA, respectively. The primers are shown in Table 2.
Table 2.
The sequences of specific primers
| Gene name | Primer sequence (5ʹ to 3ʹ) |
|---|---|
| DANCR |
Forward: 5ʹ-GCCACTATGTAGCGGGTTTC-3ʹ Reverse: 5ʹ-ACCTGCGCTAAGAACTGAGG-3ʹ |
| miR-214-5p | Forward: 5ʹ-GCCGAGTGCCTGTCTACACT-3ʹ |
| Reverse: 5ʹ-CTCAACTGGTGTCGTGGA-3ʹ | |
| COX20 |
Forward: 5ʹ-TCTGTTGTGGCTGGCTTTGGAC-3ʹ Reverse: 5ʹ-CTTCTCTGGCAATTCTTTCCTGG-3ʹ |
| GAPDH |
Forward: 5ʹ-ATCCACGGGAGAGCGACAT-3ʹ Reverse: 5ʹ-CAGCTGCTTGTAAAGTGGAC-3ʹ |
| U6 |
Forward: 5ʹ-ACAGATCTGTCGGTGTGGCAC-3ʹ Reverse: 5ʹ-GGCCCCGGATTATCCGACATTC-3ʹ |
Western blot
Total proteins were extracted using RIPA lysis buffer. Equal amounts of proteins were separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. After being blocked, the membranes were incubated with primary antibodies against COX20 (1:1000, ab18197, Abcam), caspase 3 (1:1000, ab32147, Abcam), cleaved caspase 3 (1:1000, ab53699, Abcam), Bax (1:500, ab32503, Abcam), Bcl-2 (1:500, ab32124, Abcam) and GAPDH (1:1000, ab181602, Abcam) overnight at 4 °C. Protein signals were observed by a chemiluminescence detection system, and relative expression levels of target proteins were analyzed using Image-Pro Plus 6.0.
Oxidative stress assay
To evaluate oxidative stress in vitro, 1 × 104 transfected VSMCs and HUVECs were seeded into 96-well plates and treated with 50 μg/ml ox-LDL for 48 h. The anti-oxidative enzyme superoxide dismutase (SOD) activity and malonaldehyde (MDA) content were detected using the appropriate specific detection kits.
ELISA assay
About 1 × 104 transfected VSMCs and HUVECs were seeded into 96-well plates and treated with 50 μg/ml ox-LDL for 48 h. The levels of inflammatory cytokines including IL-1β, IL-6, and TNF-α were measured using appropriate specific commercial detection kits. The concentrations of high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and total cholesterol (TC) in mouse serum were detected using appropriate specific commercial detection kits.
Luciferase reporter assay
The binding sites among DANCR, miR-214-5p, and COX20 were predicted by TargetScan. To confirm their binding sites, wild type (WT) and mutant (MUT) DANCR and COX20 containing miR-214-5p binding sites were synthesized by GenePharma (Shanghai, China) and cloned into the luciferase reporter vector pmirGLO (Promega, Madison, Wisconsin). The recombinant luciferase reporter vectors were co-transfected with miR-214-5p mimics or miR-NC into VSMCs and HUVECs using Lipofectamine 2000. After transfection for 48 h, the relative luciferase activities were measured using the dual luciferase reporter system.
Adenovirus construction
The full-length DANCR and miR-214-5p were subcloned in an adenoviral shuttle vector, pAdtrace-Tox, to construct pAdtrace-DANCR or pAdtrace-miR-214-5p, which were further verified by DNA sequencing. The pAdtrace-DANCR or pAdtrace-miR-214-5p were transfected into AdEasier cells (BJ5183 cell) to generate recombinant adenovirus AAV-DANCR or AAV-miR-214-5p. The recombinant adenoviruses were amplified in HEK-293A cells, purified using CsCl2 banding, and dialyzed against 10 mM Tris-buffered saline with 10% glycerol. These viruses were titrated using the Adeno-X Rapid Titer kit (BD Biosciences Clontech, Palo Alto, CA, USA) according to the manufacturer’s instructions.
Animal model
All C57BL/6J mice, including 18 ApoE−/− mice and 6 normal mice (6–8 weeks, weighing 16–21 g), were purchased from Vital River Laboratory Animal Technology Co., Ltd., (Beijing, China). The animal model with AS was established by feeding with an atherogenic diet (10% cholesterol, 10% lard, 78% basal feed, and 2% cholate) for 8 weeks as previously described [25]. Normal mice in the sham group were fed with a normal diet (ND), and ApoE−/− mice in the AS model group received a high-fat diet (HFD) for 12 weeks. After that, 18 model mice were injected with 200 μl of adenovirus (1 × 1010 pfu/ml) overexpressing miR-214-5p and COX20 (AAV-miR-214-5p and AAV-COX20) through tail vein injection twice a week for the last 4 weeks. The empty adenoviruses without any exogenous gene were used as the negative control (AAV-mock). Finally, mice were anesthetized by intraperitoneally injecting 3% pentobarbital sodium (Sigma-Aldrich). After collecting blood samples, mice were sacrificed for the subsequent pathological morphologic analysis. Each group included six mice.
Hematoxylin and eosin (H&E) staining assay
After sacrifice, the aortas of mice were removed and sectioned into 5 μm thick aorta sections. The morphological changes were observed after H&E staining as described previously [26]. The images were captured using an optical microscope (Olympus) at 400× magnification.
Statistical analysis
Data were presented as mean ± SD, and statistical analysis was performed using SPSS 18.0 software. Each experiment was repeated three independent times. The difference between two groups was determined by Student’s t test or one-way ANOVA. p < 0.05 was considered as the significance threshold.
Results
DANCR was significantly upregulated in AS
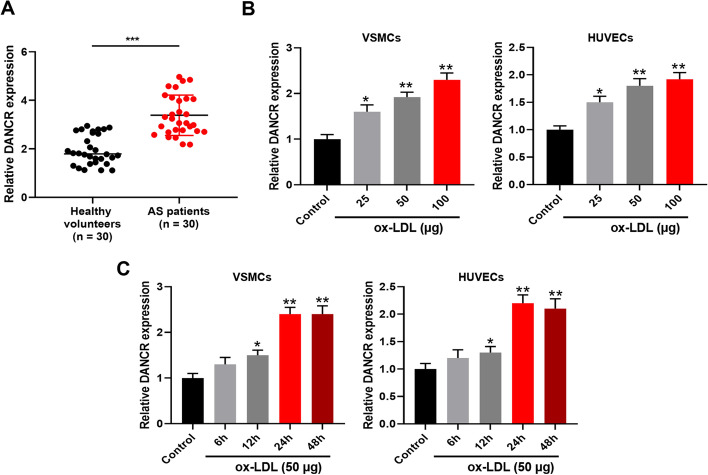
The clinical characteristics were not significantly different between AS patients and healthy controls (Table 1). However, DANCR was significantly upregulated in the blood samples of AS patients (Fig. 1A). To confirm this finding, we stimulated VSMCs and HUVECs with ox-LDL (25, 50, and 100 μg/ml) for 48 h and found that DANCR was significantly upregulated in ox-LDL treated cells (Fig. 1B). Later, 50 μg/ml ox-LDL was selected for the subsequent experiments. Figure 1C showed that DANCR was obviously upregulated in 50 μg/ml treated VSMCs and HUVECs. These results suggested that DANCR might play a potential role in AS.
Fig. 1.
DANCR was significantly upregulated in AS. A DANCR expression in blood samples of AS patients was evaluated by qRT-PCR. B VSMCs and HUVECs were subjected to ox-LDL (25, 50, and 100 μg/ml) treatment for 48 h. DANCR expression was evaluated by qRT-PCR. C VSMCs and HUVECs were subjected to 50 μg/ml ox-LDL treatment for different times. DANCR expression was evaluated by qRT-PCR. Each experiment was repeated three independent times. *p < 0.05, **p < 0.01, and ***p < 0.001
DANCR downregulation ameliorated ox-LDL-induced endothelial injury in vitro
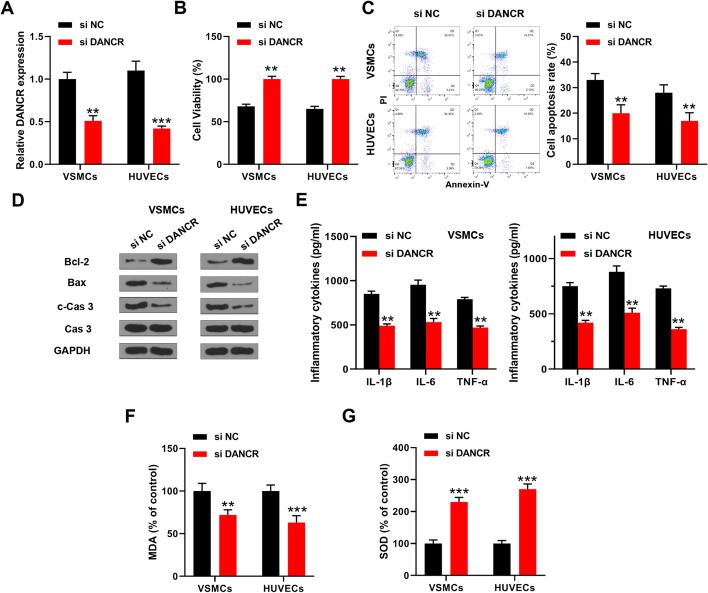
VSMCs and HUVECs were transfected with small interfering RNA targeting DANCR (si-DANCR) and then treated with 50 μg/ml ox-LDL for 48 h. The transfection efficiency was confirmed by qRT-PCR (Fig. 2A). Si-DANCR significantly increased viability and reduced apoptosis of ox-LDL-treated VSMCs and HUVECs compared with si-NC (Fig. 2B, C). The levels of apoptosis-related proteins were detected by Western blot, and the results showed that si-DANCR obviously increased the Bcl-2 level, while it reduced Bax and cleaved caspase 3 levels in ox-LDL-treated VSMCs and HUVECs (Fig. 2D). In addition, si-DANCR significantly reduced the levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) and MDA, while increasing the SOD level in ox-LDL-treated VSMCs and HUVECs (Fig. 2E–G). These results indicated that DANCR downregulation ameliorated ox-LDL-induced endothelial injury in vitro.
Fig. 2.
DANCR downregulation ameliorated ox-LDL-induced endothelial injury in vitro. VSMCs and HUVECs were transfected with si-DANCR or si-NC and then treated with 50 μg/ml ox-LDL for 48 h. A DANCR expression was detected by qRT-PCR. B CCK-8 assay. C Flow cytometry assay. D Expression of apoptosis-related proteins was detected by Western blot. The original images are shown in Additional file 1. E Expression of inflammatory cytokines was detected by ELISA assay. F, G Levels of MDA (F) and SOD (G) were evaluated by specific detection kits. Each experiment was repeated three independent times. **p < 0.01 and ***p < 0.001
DANCR served as a ceRNA of miR-214-5p
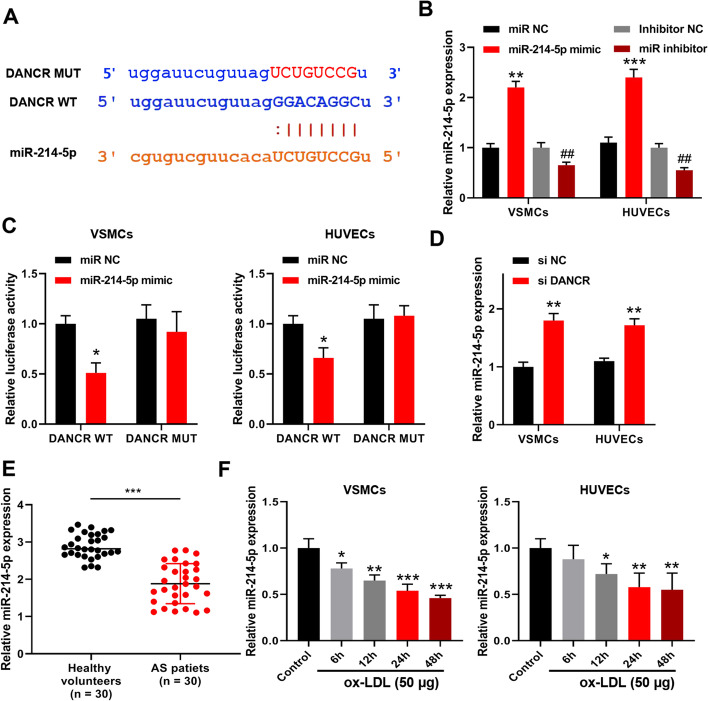
TargetScan was used to predict the binding site between DANCR and miR-214-5p, and the results are shown in Fig. 3A. To determine their binding relationship, miR-214-5p mimics and inhibitor were transfected into VSMCs and HUVECs, and the transfection efficiencies were confirmed by qRT-PCR (Fig. 3B). Luciferase reporter assay was performed, and the results indicated that miR-214-5p mimics significantly reduced the relative luciferase activity of DANCR WT compared with miR-NC in both VSMCs and HUVECs but had no effect on DANCR MUT (Fig. 3C). Meanwhile, si-DANCR significantly increased miR-214-5p expression in VSMCs and HUVECs compared with si-NC (Fig. 3D). Moreover, miR-214-5p was markedly downregulated in blood samples of AS patients compared with that in healthy volunteers (Fig. 3E). In addition, Fig. 3F showed a time-dependent reduction of miR-214-5p in 50 μg/ml ox-LDL-treated VSMCs and HUVECs. These results suggested that DANCR might serve as a ceRNA of miR-214-5p.
Fig. 3.
DANCR served as a ceRNA of miR-214-5p. A The binding site between DANCR and miR-214-5p was predicted by TargetScan. B MiR-214-5p mimics and inhibitor were transfected into VSMCs and HUVECs, and the transfection efficiencies were confirmed by qRT-PCR. C Luciferase reporter assay. D VSMCs and HUVECs were transfected with si-DANCR or si-NC, and miR-214-5p expression was detected by qRT-PCR. E MiR-214-5p expression in blood samples of AS patients was evaluated by qRT-PCR. F VSMCs and HUVECs were subjected to 50 μg/ml ox-LDL for different times. MiR-214-5p expression was evaluated by qRT-PCR. Each experiment was repeated three independent times. *p < 0.05, and **p < 0.01 vs. miR-NC or control group; ##p < 0.01 vs. inhibitor NC group
COX20 was a target of miR-214-5p
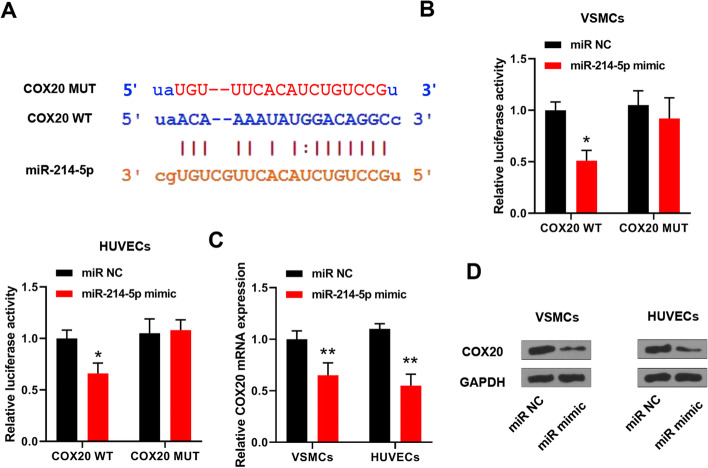
TargetScan was applied to predict the targets of miR-214-5p, and the results suggested that COX20 might be a direct target of miR-214-5p (Fig. 4A). The results of the luciferase reporter assay showed that miR-214-5p mimics significantly reduced the relative luciferase activity of COX20 WT compared with miR-NC in both VSMCs and HUVECs but did not affect that of COX20 MUT (Fig. 4B). Meanwhile, miR-214-5p mimics significantly reduced the COX20 level at both mRNA and protein levels in VSMCs and HUVECs compared with miR-NC (Fig. 4C, D). These results suggested that COX20 might be a direct target of miR-214-5p.
Fig. 4.
COX20 was a target of miR-214-5p. A The binding site between miR-214-5p and COX20 was predicted by TargetScan. B Luciferase reporter assay. C, D VSMCs and HUVECs were transfected with miR-214-5p mimics or miR-NC, and COX20 expression was evaluated by qRT-PCR (C) and Western blot (D). The original images are shown in Additional file 1. Each experiment was repeated three independent times. *p < 0.05, and **p < 0.01
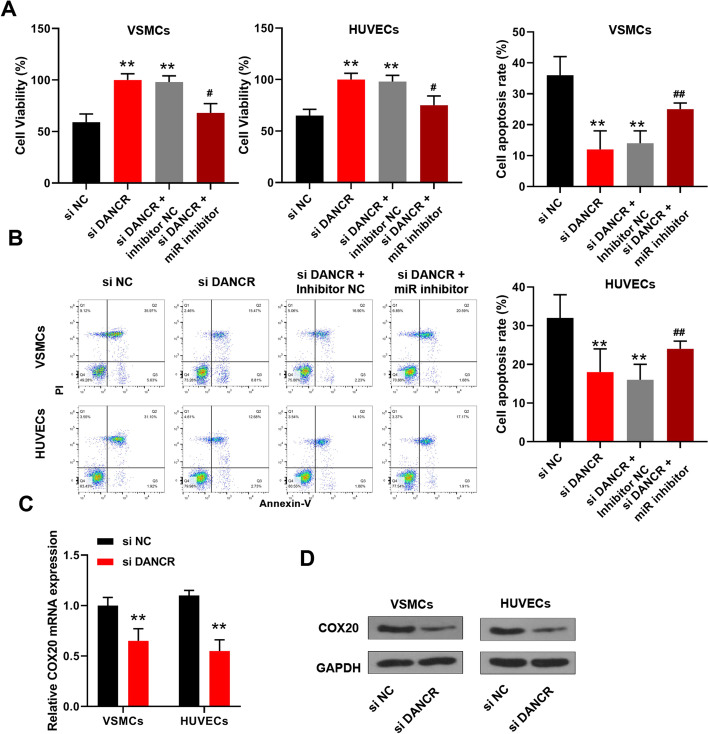
MiR-214-5p downregulation obviously attenuated the protection of si-DANCR against ox-LDL-induced endothelial injury
Next, rescue experiments were performed by co-transfecting si-DANCR and miR-214-5p inhibitor into VSMCs and HUVECs. CCK-8 and flow cytometry assays of these cells treated with 50 μg/ml ox-LDL for 48 h revealed that co-transfection of si-DANCR and miR-214-5p inhibitor significantly reduced cell viability and promoted apoptosis of ox-LDL-treated cells compared with the si-DANCR plus inhibitor NC group (Fig. 5A, B). In addition, si-DANCR obviously reduced COX20 expression at both mRNA and protein levels in ox-LDL-treated VSMCs and HUVECs compared with si-NC (Fig. 5C, D). These results indicated that miR-214-5p downregulation attenuated the protective role of si-DANCR in ox-LDL-induced endothelial injury via upregulating COX20.
Fig. 5.
MiR-214-5p downregulation obviously attenuated si-DANCR-induced protection against ox-LDL-induced endothelial injury. A, B VSMCs and HUVECs were transfected with si-DANCR, si-NC, or co-transfected with si-DANCR and inhibitor NC, or co-transfected with si-DANCR and miR-214-5p inhibitor, and then cells were treated with 50 μg/ml ox-LDL for 48 h. A CCK-8 assay. B Flow cytometry assay. C, D VSMCs and HUVECs were transfected with si-DANCR or si-NC, and then treated with 50 μg/ml ox-LDL for 48 h. COX20 expression was evaluated by qRT-PCR (C) and Western blot (D). The original images are shown in Additional file 1. Each experiment was repeated three independent times. **p < 0.01 vs. si-NC; ##p < 0.01 vs. si-DANCR plus inhibitor NC group
DANCR/miR-214-5p/COX20 participated in the development of AS in vivo
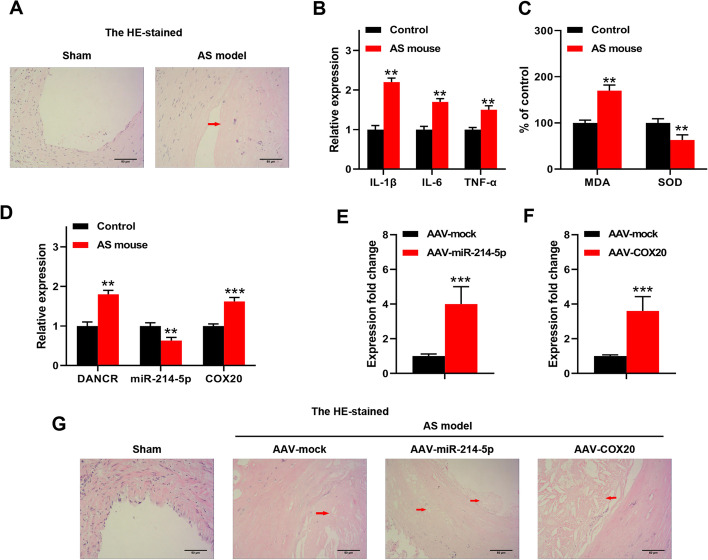
To investigate the role of the DANCR/miR-214-5p/COX20 axis in vivo, an animal model with AS was established. The results of H&E staining showed that the aortic sinus plaque area was notably reduced in the AS model group compared with the control group (Fig. 6A), suggesting that the AS model was successfully established. The levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) and MDA were significantly increased, while the level of SOD was decreased in the blood samples of the AS animal model compared with the control group (Fig. 6B, C). Meanwhile, both DANCR and COX20 were significantly upregulated, while miR-214-5p was downregulated in the blood samples of the AS animal model compared with the control group (Fig. 6D). In addition, the adenovirus overexpressing miR-214-5p and COX20 were injected into mice to overexpress them, and qRT-PCR results showed that AAV-miR-214-5p significantly increased the miR-214-5p level, and AAV-COX20 significantly increased the COX20 level in the blood samples of the AS animal model compared with the control group (Fig. 6E, F) Moreover, miR-214-5p overexpression obviously reduced the lipid deposition area of the AS animal model, and COX20 overexpression further increased the lipid deposition area (Fig. 6G). In addition, lipid profiles in mice serum were examined. The results showed that the levels of HDL-c, LDL-c, and TC in the AAV-mock group were significantly higher than those in the sham group, AAV-miR-214-5p reduced the levels of HDL-c and TC compared with the AAV-mock group, while AAV-COX20 increased the levels of LDL-c and TC compared with the AAV-mock group (Additional file 1: Fig. S1). These results suggested that DANCR/miR-214-5p/COX20 closely participated in the development of AS in vivo.
Fig. 6.
DANCR/miR-214-5p/COX20 participated in development of AS in vivo. A Representative micrographs of the lesion areas in the aortic sinus were observed by H&E staining at ×400 magnification, scale bar = 50 μm. B, C Levels of inflammatory cytokines (B), MDA, and SOD (C) in blood samples of AS animal model were detected by specific detection kits. D Expression of DANCR, miR-214-5p, and COX20 in blood samples of AS animal model was evaluated by qRT-PCR. E, F Adenovirus overexpressing miR-214-5p and COX20 were injected into mice, and expression of miR-214-5p (E) and COX20 (F) in blood samples of AS animal model was evaluated by qRT-PCR. G Representative micrographs of lesion areas in the aortic sinus were observed by H&E staining. ×400 magnification, scale bar = 50 μm. There were six mice in each group. Each experiment was repeated three independent times. **p < 0.01 and ***p < 0.001
Discussion
Increasing evidence has shown an important role of lncRNA-mediated pathogenic mechanisms involved in AS progression and also highlighted the regulatory axis of the lncRNAs–miRNA–mRNA network [27, 28]. The present study revealed a regulatory axis of DANCR/miR-214-5p/COX20 in ox-LDL induced endothelial injury and in an animal model in vivo, providing a potential targeted therapeutic means for AS.
Further, we identified that DANCR acted as a ceRNA of miR-214-5p, and the luciferase reporter assay confirmed their binding relationships. Rescue experiments showed that miR-214-5p downregulation obviously eliminated the protection of si-DANCR in ox-LDL-induced endothelial injury, and also that miR-214-5p was a direct downstream miRNA of DANCR [19, 20] and mediated the function of DANCR in AS progression. It has been reported that lncRNA-mediated cell proliferation and apoptosis of smooth muscle and endothelial cells, inflammation, and oxidative stress were closely associated with the pathogenic processes during AS progression [29–31]. Hence, we explored the effects of DANCR on these important factors and found that DANCR downregulation reduced the levels of inflammatory cytokines (IL-1β, IL-6, and TNF-α) and MDA while it increased the SOD level in ox-LDL-treated VSMCs and HUVECs. These data revealed that DANCR downregulation could efficiently reduce the inflammatory response and oxidative stress during AS progression, contributing to our understanding of the pathogenic mechanism during AS development. In addition, DANCR has been identified as the ceRNA of several target miRNAs other than miR-214-5p, such as miR-185-5p [32], miR-874-3p [33], miR-1301-3p [34], miR-185-5p [35], miR-345-5p [36], miR-125b-5p [37], and miR-144-3p [38]. Since one lncRNA might target one or more downstream miRNAs [39], we hypothesized that DANCR might also have other target miRNAs that participated in the function of this lncRNA in AS progression. We will investigate this hypothesis in subsequent experiments in the future.
To further explore the specific mechanism of miR-214-5p in AS, TargetScan was applied and the prediction showed that there was a putative binding site between miR-214-5p and the 3ʹ UTR of COX20. Luciferase reporter assay determined that miR-214-5p could directly bind to the 3ʹ UTR of COX20. DANCR could positively regulate the expression of COX20. COX20 is a mitochondrial inner membrane protein and has been identified as a chaperone of COX2, a subunit of cytochrome c oxidase [40]. Previous studies indicated that COX20 was important for the tolerance to oxidative stress [41]. However, the role of COX20 in AS progression has not been studied. Our results showed that COX20 was significantly upregulated in the blood samples of an AS animal model, and COX20 overexpression obviously promoted the progression of AS in vivo, indicating that the role of COX20 in AS might be due to its role in oxidative stress. This study investigated the roles of DANCR, miR-214-5p, and COX20 in the proliferation, apoptosis, inflammation, and oxidative stress of ox-LDL-treated VSMCs and HUVECs, reinforcing that this axis might be a novel therapeutic target for AS.
In this study, we also observed the significant changes in the lipid profile of the experimental mice after the injections of adenovirus genetic constructs (Additional file 1: Fig. S1), and these changes might be real reasons for acceleration or deceleration of atherosclerotic lesions. In addition, considering that the main target of adenoviral gene delivery is the liver [42], the possible role of DANCR/miR-214-5p/COX20 signaling in the regulation of lipid metabolism in hepatocytes was also suggested. This conjecture should be investigated in the future.
In summary, our study demonstrated that DANCR downregulation attenuated AS progression via regulating the miR-214-5p/COX20 axis, contributing to our understanding of the pathogenesis in AS.
Supplementary Information
Additional file 1: Fig. S1. Lipid profiles in mice. Serum lipid profile of C57BL/6J mice was measured. Six mice in each group. Each experiment was repeated three independent times. *p < 0.05, **p < 0.01, ***p < 0.001 vs. sham group; #p < 0.05 vs. AAV-mock group.
Acknowledgements
Not applicable.
Abbreviations
- DANCR
Differentiation antagonizing nonprotein coding RNA
- COX20
A subunit of cytochrome c oxidase and the chaperone of cytochrome c oxidase subunit II (COX2)
- CCK-8
Cell Counting Kit-8
- VSMCs
Vascular smooth muscle cells
- HUVECs
Human umbilical vein endothelial cells
- Ox-LDL
Oxidized low density lipoprotein
- SOD
Superoxide dismutase
- MDA
Malonaldehyde
- H&E
Hematoxylin and eosin
Authors’ contributions
RZ: study concepts, literature research, clinical studies, data analysis, experimental studies, manuscript writing and review; RZ, YH: study design, literature research, experimental studies and manuscript editing; JZ: definition of intellectual content, clinical studies, data acquisition and statistical analysis. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but are available on request from the corresponding author.
Declarations
Ethics approval and consent to participate
All patients signed written informed consent. This study was approved by the Human Ethics Committee of Harrison International Peace Hospital (No. 5189, October 2016), and the animal Ethics Committee of Harrison International Peace Hospital (No. 6323, May 2020). All procedures were performed in keeping with the standards set out in the Declaration of Helsinki and Laboratory Guidelines of Research in China and the National Institutes of Health Laboratory Animal Care and Use Guidelines.
Consent for publication
Not applicable.
Competing interests
All authors have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond, Engl: 1979) 2018;132(12):1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet (Lond, Engl) 2014;383(9921):984–998. doi: 10.1016/S0140-6736(13)61088-0. [DOI] [PubMed] [Google Scholar]
- 3.Milutinović A, Šuput D, Zorc-Pleskovič R. Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: an updated review. Bosn J Basic Med Sci. 2020;20(1):21–30. doi: 10.17305/bjbms.2019.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediat Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Williams D, Sur S, Wang JY, Jo H. Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vascul Pharmacol. 2019;114:76–92. doi: 10.1016/j.vph.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Li Q, Chen Y, Zhu Q. LncRNA OIP5-AS1 accelerates ox-LDL-treated HUVECs injury by NF-κB pathway via miR-30c-5p. Clin Hemorheol Microcirc. 2021;78(4):449–460. doi: 10.3233/CH-211130. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Liu J, Lu K, Qiu Y, Li X, Yue F, et al. Long non-coding RNA NEAT1 regulates endothelial functions in subclinical hypothyroidism through miR-126/TRAF7 pathway. Hum Cell. 2021;34(3):825–835. doi: 10.1007/s13577-021-00508-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhao ZW, Zhang M, Liao LX, Zou J, Wang G, Wan XJ, et al. Long non-coding RNA PCA3 inhibits lipid accumulation and atherosclerosis through the miR-140–5p/RFX7/ABCA1 axis. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(5):158904. doi: 10.1016/j.bbalip.2021.158904. [DOI] [PubMed] [Google Scholar]
- 11.Huang YF, Zhang Y, Fu X. Long non-coding RNA DANCR promoted non-small cell lung cancer cells metastasis via modulating of miR-1225-3p/ErbB2 signal. Eur Rev Med Pharmacol Sci. 2021;25(2):758–769. doi: 10.26355/eurrev_202101_24637. [DOI] [PubMed] [Google Scholar]
- 12.Xiong M, Wu M, Dan P, Huang W, Chen Z, Ke H, et al. LncRNA DANCR represses doxorubicin-induced apoptosis through stabilizing MALAT1 expression in colorectal cancer cells. Cell Death Dis. 2021;12(1):24. doi: 10.1038/s41419-020-03318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Li L, Wang J, Zhang T, Ye T, Wang S, et al. Recent advances in the regulation of ABCA1 and ABCG1 by lncRNAs. Clin Chim Acta. 2021;516:100–110. doi: 10.1016/j.cca.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 15.Guo C, Ye FX, Jian YH, Liu CH, Tu ZH, Yang DP. MicroRNA-214-5p aggravates sepsis-related acute kidney injury in mice. Drug Dev Res. 2021;65(9):105–116. doi: 10.1002/ddr.21863. [DOI] [PubMed] [Google Scholar]
- 16.He Q, Li R, Hu B, Li X, Wu Y, Sun P, et al. Stromal cell-derived factor-1 promotes osteoblastic differentiation of human bone marrow mesenchymal stem cells via the lncRNA-H19/miR-214–5p/BMP2 axis. J Gene Med. 2021;23(9):e3366. doi: 10.1002/jgm.3366. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Zhu B, Dong Z, Jiang H, Zhao X, Wu S. miR-214-5p targeted by LncRNA DANCR mediates TGF-β signaling pathway to accelerate proliferation, migration and inhibit apoptosis of prostate cancer cells. Am J Transl Res. 2021;13(4):2224–2240. [PMC free article] [PubMed] [Google Scholar]
- 18.Lv Y, Dong K, Gao H. Long non-coding RNA TDRG1 facilitates cell proliferation, migration and invasion in breast cancer via targeting miR-214-5p/CLIC4 axis. Cancer Biol Ther. 2021;22(3):248–256. doi: 10.1080/15384047.2020.1863120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YR, Wu YS, Wang WS, Zhang JS, Wu QG. Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214-5p/CIZ1 axis. Eur Rev Med Pharmacol Sci. 2020;24(5):2539–2547. doi: 10.26355/eurrev_202003_20521. [DOI] [PubMed] [Google Scholar]
- 20.Yao Z, Chen Q, Ni Z, Zhou L, Wang Y, Yang Y, et al. Long non-coding RNA differentiation antagonizing nonprotein coding RNA (DANCR) promotes proliferation and invasion of pancreatic cancer by sponging miR-214-5p to regulate E2F2 expression. Med Sci Monit. 2019;25:4544–4552. doi: 10.12659/MSM.916960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourens M, Boulet A, Leary SC, Barrientos A. Human COX20 cooperates with SCO1 and SCO2 to mature COX2 and promote the assembly of cytochrome c oxidase. Hum Mol Genet. 2014;23(11):2901–2913. doi: 10.1093/hmg/ddu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papageorgiou N, Zacharia E, Briasoulis A, Charakida M, Tousoulis D. Celecoxib for the treatment of atherosclerosis. Expert Opin Investig Drugs. 2016;25(5):619–633. doi: 10.1517/13543784.2016.1161756. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Hao R, Lan B, Mu Y, Dang F, Wang R. The protective effects of naproxen against interleukin-1β (IL-1β)- induced damage in human umbilical vein endothelial cells (HUVECs) Bioengineered. 2021;12(1):5361–5372. doi: 10.1080/21655979.2021.1955560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzi I, Oeljeklaus S, Aich A, Ronsör C, Callegari S, Dudek J, et al. The mitochondrial TMEM177 associates with COX20 during COX2 biogenesis. Biochim Biophys Acta. 2018;1865(2):323–333. doi: 10.1016/j.bbamcr.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siemeni T, Knöfel AK, Madrahimov N, Sommer W, Avsar M, Salman J, et al. In vivo development of transplant arteriosclerosis in humanized mice reflects alloantigen recognition and peripheral treg phenotype of lung transplant recipients. Am J Transplant. 2016;16(11):3150–3162. doi: 10.1111/ajt.13905. [DOI] [PubMed] [Google Scholar]
- 26.Fan M, Bai J. Adipose-derived stem cell transplantation inhibits vascular inflammatory responses and endothelial dysfunction in rats with atherosclerosis. Yonsei Med J. 2019;60(11):1036–1044. doi: 10.3349/ymj.2019.60.11.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Zhang F, Lu R, Feng Y, Li X, Zhang S, et al. Functional lncRNA-miRNA-mRNA networks in rabbit carotid atherosclerosis. Aging. 2020;12(3):2798–2813. doi: 10.18632/aging.102778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Zheng Z, Feng X, Zang X, Ding W, Wu F, et al. circRNA/lncRNA-miRNA-mRNA network in oxidized, low-density, lipoprotein-induced foam cells. DNA Cell Biol. 2019;38(12):1499–1511. doi: 10.1089/dna.2019.4865. [DOI] [PubMed] [Google Scholar]
- 29.Zhou T, Ding JW, Wang XA, Zheng XX. Long noncoding RNAs and atherosclerosis. Atherosclerosis. 2016;248:51–61. doi: 10.1016/j.atherosclerosis.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Libby P. Targeting inflammatory pathways in cardiovascular disease: the inflammasome, interleukin-1, interleukin-6 and beyond. Cells. 2021;10(4):52–60. doi: 10.3390/cells10040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Kitada M, Ogura Y, Koya D. Relationship between autophagy and metabolic syndrome characteristics in the pathogenesis of atherosclerosis. Front Cell Dev Biol. 2021;9:641852. doi: 10.3389/fcell.2021.641852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun W, Zu S, Shao G, Wang W, Gong F. Long non-coding DANCR targets miR-185–5p to upregulate LIM and SH3 protein 1 promoting prostate cancer via the FAK/PI3K/AKT/GSK3β/snail pathway. J Gene Med. 2021;23(7):e3344. doi: 10.1002/jgm.3344. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Liu L, Chen L, Liu H, Ren S, Tao Y. Long noncoding RNA DANCR confers cytarabine resistance in acute myeloid leukemia by activating autophagy via the miR-874-3P/ATG16L1 axis. Mol Oncol. 2021;15(4):1203–1216. doi: 10.1002/1878-0261.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng W, Di S, Xing S, Sun Z, Shen Z, Dou X, et al. Long non-coding RNA DANCR modulates osteogenic differentiation by regulating the miR-1301-3p/PROX1 axis. Mol Cell Biochem. 2021;476(6):2503–2512. doi: 10.1007/s11010-021-04074-9. [DOI] [PubMed] [Google Scholar]
- 35.Lu W, Huang Z, Wang J, Liu H. Long non-coding RNA DANCR accelerates colorectal cancer progression via regulating the miR-185-5p/HMGA2 axis. J Biochem. 2021;142(16):84–98. doi: 10.1093/jb/mvab011. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Li T, Liu G, Wang J, Yin Z, Kang J. LncRNA RHPN1-AS1 modulates cholangiocarcinoma progression and is related with poor clinical outcomes through miR-345-5p/YAP1 axis. J Gastroenterol Hepatol. 2020;12(16):141–158. doi: 10.1111/jgh.15374. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Jiang MN, Liu Y, Wu CQ, Liu H. Crosstalk between lncRNA DANCR and miR-125b-5p in HCC cell progression. Tumori. 2020;54(13):57–62. doi: 10.1177/0300891620977010. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Wang M, Zhou H, Lu S, Zhang B. Long noncoding RNA EBLN3P promotes the progression of liver cancer via alteration of microRNA-144-3p/DOCK4 signal. Cancer Manag Res. 2020;12:9339–9349. doi: 10.2147/CMAR.S261976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol (Clifton, NJ) 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 40.Hell K, Tzagoloff A, Neupert W, Stuart RA. Identification of Cox20p, a novel protein involved in the maturation and assembly of cytochrome oxidase subunit 2. J Biol Chem. 2000;275(7):4571–4578. doi: 10.1074/jbc.275.7.4571. [DOI] [PubMed] [Google Scholar]
- 41.Keerthiraju E, Du C, Tucker G, Greetham D. A role for COX20 in tolerance to oxidative stress and programmed cell death in Saccharomyces cerevisiae. Microorganisms. 2019;7(11):575–582. doi: 10.3390/microorganisms7110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs S, Bayer M, Taubert R. Effects of adenovirus-induced hepatocyte damage on chronic bile duct inflammation in a sclerosing cholangitis mouse model. Liver Int. 2019;39(12):2330–2340. doi: 10.1111/liv.14183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Lipid profiles in mice. Serum lipid profile of C57BL/6J mice was measured. Six mice in each group. Each experiment was repeated three independent times. *p < 0.05, **p < 0.01, ***p < 0.001 vs. sham group; #p < 0.05 vs. AAV-mock group.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants, but are available on request from the corresponding author.