Abstract
Quinupristin-dalfopristin may be useful for treatment of organisms causing peritoneal dialysis-related peritonitis, including methicillin-resistant coagulase-negative staphylococci, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci. The pharmacokinetic profiles of single intravenous doses of this combination streptogramin antibiotic of 7.5 mg/kg of body weight were characterized for eight noninfected patients receiving continuous ambulatory peritoneal dialysis. Comparison was made to pharmacokinetic profiles determined for eight healthy volunteers matched by age, sex, and race. Drug was measured in dialysate up to 6 h following the dose. Plasma and dialysate were assayed for parent compounds and metabolites. Mean pharmacokinetic parameters were compared between groups. No statistically significant differences were observed between groups for maximal concentrations in plasma, times to maximal concentration, areas under the curve, distribution volumes, rates of total body clearance, or half-lives in plasma for quinupristin and dalfopristin. No statistically significant differences were observed in maximal concentrations in plasma, times to maximal concentration, areas under the curve, or half-lives for cysteine, the glutathione conjugates of quinupristin, or the pristinamycin IIA metabolite of dalfopristin. The measurements in dialysate of the parent and most metabolites were below the expected MICs. Dialysis clearance was insignificant. Quinupristin-dalfopristin was well tolerated in both groups, causing only mild adverse events that resolved prior to discharge from the study. The disposition of quinupristin, dalfopristin, or their primary metabolites following a single dose was unaltered in patients receiving peritoneal dialysis. Intravenous dosing of this antibiotic combination is unlikely to be adequate for the treatment of peritonitis associated with peritoneal dialysis.
Antibiotic use continues to be common for patients receiving peritoneal dialysis. Peritonitis and catheter-related infections are frequent complications of this dialysis modality. Concerns over the emergence of methicillin- and vancomycin-resistant microorganisms have led to recent changes in treatment recommendations for these infections (6). Methicillin-resistant strains of Staphylococcus aureus and coagulase-negative staphylococci have been identified for many years in peritoneal dialysis patients. More recently, vancomycin-resistant Enterococcus faecium and a strain of S. aureus with intermediate susceptibility to vancomycin have been observed in peritoneal dialysis patients (2, 7, 9). In addition, antibiotic-resistant strains are a growing cause of nosocomial infections (12). Quinupristin-dalfopristin, a new semisynthetic combination streptogramin, has shown in vitro and in vivo activities against many bacterial strains, including those with methicillin and vancomycin resistance (4, 10, 11, 13).
Quinupristin-dalfopristin (Synercid; Rhône-Poulenc Rorer) is the first injectable streptogramin. It is a combination of two semisynthetic derivatives of pristinamycin I and pristinamycin II in a fixed 30/70 ratio. The two components act synergistically against gram-positive organisms by inhibiting bacterial protein synthesis (1). The combination is usually bactericidal in vitro. The drug is not active against gram-negative bacilli.
Previous studies of healthy volunteers have been conducted to establish the pharmacokinetic parameters for quinupristin-dalfopristin. Following intravenous administration of 14C-quinupristin-dalfopristin, approximately 75% of the dose of drug-related components was excreted unchanged in the feces while less than 20% was recovered in the urine (5). Unchanged quinupristin accounted for 35% of total radioactivity of the pristinamycin I components excreted in the urine, and its main metabolite, a cysteine conjugate (RPR 100391), accounted for 38%. No unchanged dalfopristin was recovered in urine, but its metabolite pristinamycin IIA (RP 12536) represented 70% of total radioactivity of the pristinamycin II components excreted in the urine. RPR 100391 and RP 12536 possess in vitro antibacterial activities comparable to those of quinupristin and dalfopristin, respectively. The apparent elimination half-life (t1/2β) of each active compound was approximately 1.5 h for healthy volunteer subjects (3). Levels of protein binding of quinupristin and dalfopristin in healthy subjects were 23 to 32% and 50 to 56%, respectively (11a). The objectives of the present study were to characterize the pharmacokinetic profiles of quinupristin-dalfopristin in patients receiving continuous ambulatory peritoneal dialysis (CAPD), to determine the rate of antibiotic excretion into the peritoneal effluent, and to compare the pharmacokinetic parameters of quinupristin-dalfopristin in CAPD patients to those observed for healthy volunteers with normal renal function.
MATERIALS AND METHODS
Eight noninfected CAPD patients and eight healthy volunteers matched for sex, race, and age (±5 years) participated in this open-label, matched-control study. All participants were between the ages of 18 and 75 years, were not pregnant, and were within 25% of their ideal body weights. All CAPD patients had been receiving CAPD and had been peritonitis-free for at least 1 month. Patients were excluded if they smoked or if they had a positive serology result for hepatitis B, hepatitis C, or human immunodeficiency virus. Concomitant medications that might have affected hepatic microsomal enzymes were not allowed for 1 month prior to or during the study. All other medications routinely prescribed for dialysis patients were continued. All participants gave written informed consent. We obtained a baseline medical history and electrocardiogram and performed a physical examination and routine clinical laboratory testing prior to administration of the study drug. A clinical evaluation of each participant was repeated on the day of the study prior to drug administration and 10 h later upon completion of the study. All CAPD patients were studied in the General Clinical Research Center at the University of Wisconsin—Madison. Healthy volunteers were studied at the Corning Besselaar Clinical Research Unit, Inc. (Covance Clinical Research Unit, Inc.) in Madison, Wis.
Quinupristin-dalfopristin was given intravenously as a single dose of 7.5 mg/kg of actual body weight. The drug was administered with an infusion pump over 60 min in 250 ml of dextrose–5% water. All CAPD patients were subjected to a 1.5% dextrose dialysis exchange just prior to the start of drug dosing. The subsequent exchange was performed 6 h later.
Blood sampling.
Two blood samples (4 ml each) were withdrawn from the arm opposite that receiving the drug infusion at the following times: time zero (prior to study drug administration); 15, 30, 60, 70, 80, 90, and 105 min after the start of infusion; and 2, 2.5, 3, 4, 5, 6, 8, and 10 h after the start of the infusion. Samples were withdrawn into two vacuum tubes, each of which contained 0.5 ml of 3.8% citrate. Immediately after collection, 9 ml of citrated blood was transferred into a tube containing 2 ml of 0.25 N hydrochloric acid kept on ice. The mixture was stirred gently by hand, and the tube was centrifuged immediately at 2,000 × g and 4°C for 15 min. The resulting plasma supernatants were stored at −20°C.
Dialysate sampling.
Dialysate effluent (50 ml) was withdrawn through the peritoneal dialysis catheter at 1.25, 2, 3, and 6 h following the start of the quinupristin-dalfopristin infusion. Buffer solution (5 ml, pH 3) was added immediately. Samples were stored at −20°C.
Sample analysis.
Concentrations of quinupristin and its metabolites RPR 100391 (cysteine conjugate) and RP 69012 (glutathione conjugate) and of dalfopristin and its metabolite RP 12536 (pristinamycin IIA) in plasma and dialysate were determined by a specific and sensitive high-performance liquid chromatography method with UV and fluorescence detection after liquid-solid extraction (8). With plasma, the assay was linear from a limit of quantitation of 0.025 to 5 μg/ml for quinupristin, dalfopristin, and RP 12536 and a limit of quantitation of 0.01 to 1 μg/ml for RP 69012 and RPR 100391, with a 1.35-ml sample volume. With dialysate, the assay was linear from a limit of quantitation of 0.2 to 2 μg/ml for quinupristin, dalfopristin, and RP 12536 and a limit of quantitation of 0.01 to 1 μg/ml for RP 69012 and RPR 100391, with a 1.10-ml acidified-dialysate sample volume. Precision and accuracy of the assay were evaluated by the determination of quality controls at three different levels (0.2, 1, and 2 μg/ml for quinupristin, dalfopristin, and RP 12536 and 0.1, 0.5, and 1 μg/ml for RP 69012 and RPR 100391). With plasma, the coefficients of variation were lower than 11.7% and accuracy was between 96 and 104.8%. With dialysate, the coefficients of variation were lower than 17.7% and accuracy was between 90.7 and 114.3%.
Pharmacokinetic analysis.
Noncompartmental pharmacokinetic analysis was performed with WinNonlin, version 1.1 (Pharsight of North Carolina). The maximum concentration of drug in plasma (Cmax) and the time at which Cmax occurred (Tmax) were determined from the experimental concentration-time curve in plasma. The area under the concentration-time curve (AUC) in plasma from time zero to the time of the last detectable sample (AUC0–t) was determined by the linear trapezoidal rule. The AUC in plasma from time zero to infinity (AUC0–∞) was calculated by the trapezoidal rule with extrapolation to infinity. The rate constant for elimination (kel) was calculated from the log-linear regression of the terminal concentration-time data. The apparent t1/2β was calculated with the equation ln 2/kel. For the metabolites, this apparent elimination half-life is an aggregate of metabolite formation and elimination. The clearance (CL) of the parent drugs from plasma was calculated by dividing the dose by the AUC0–∞. The volume of distribution (V) was calculated by dividing the CL by the kel. Dialysis CL was determined from the equation VdCd/AUC0–t, where Vd is the volume of dialysate, Cd is the dialysate drug concentration, and t is the dwell time. CL from plasma and V were not calculated for the metabolites RP 69012, RPR 100391, and RP 12536.
Statistical analysis.
Mean pharmacokinetic parameters were compared between groups by t test (parametric) or exact two-way Wilcoxon rank-sign test (nonparametric) analysis. A P of <0.05 was considered significant. Pharmacokinetic data are expressed as means ± standard deviations (SD). Descriptive statistics were used to summarize the safety evaluation.
RESULTS
Patient demographics.
The mean age for all participants was 43.6 years (range, 19 to 67 years). The mean weight for the CAPD patients was 72.2 kg (range, 60 to 104 kg); the mean height for all participants was 172.2 cm (range, 159 to 182 cm). There were 10 males (5 in each group) and 6 females (3 in each group). Potential participants were excluded if they exhibited any evidence of liver disease. Baseline blood pressures and heart rates were slightly higher in the CAPD patients and remained so throughout the study.
Pharmacokinetics.
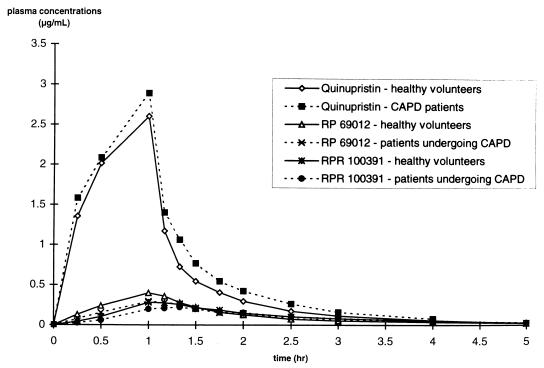
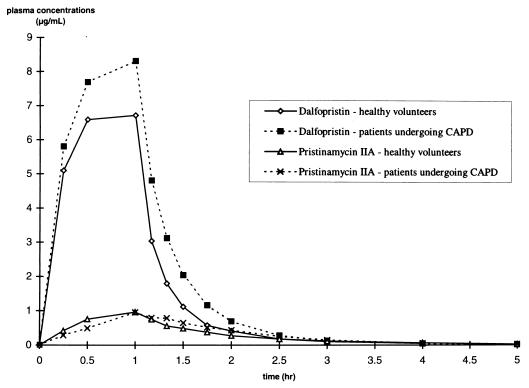
The mean concentration-time profiles in plasma for quinupristin, RPR 100391, and RP 69012 are presented in Fig. 1. Quinupristin concentrations could not be determined accurately for CAPD patients 2 and 3 due to suspected interference with furosemide in the plasma. The mean concentration-time profiles in plasma for dalfopristin and pristinamycin IIA are presented in Fig. 2. The pharmacokinetic parameters are presented in Tables 1 and 2. The peak concentrations of all metabolites in plasma were achieved at nearly the same time as the peak concentrations of the parent compounds in plasma for both healthy volunteers and CAPD patients. Mean total CL from plasma for both parent compounds was high for each group of study participants. There was no statistically significant decrease in CL from plasma or V values for the CAPD patients compared to values for the healthy subjects for either quinupristin or dalfopristin. Moderate increases in the AUC0–t values for quinupristin (+18%) and dalfopristin (+29%) were observed for the CAPD patients compared to those for the healthy volunteers; however, these differences were not statistically significant. The AUC0–t values of the metabolites were comparable in both groups.
FIG. 1.
Mean concentrations of quinupristin, RP 69012, and RPR 100391 in plasma versus time.
FIG. 2.
Mean concentrations of dalfopristin and RP 12536 in plasma versus time.
TABLE 1.
Pharmacokinetic parameters of quinupristin and its metabolites RP 69012 and RPR 100391a
| Group | Quinupristin (RP 57669)
|
RP 69012 (glutathione conjugate)
|
RPR 100391 (cysteine conjugate)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC0–∞ (μg · h/ml) | t1/2β (h) | CL (liter/h · kg) | V (liters/kg) | Cmax (μg/ml) | Tmax (h) | AUC0–∞ (μg · h/ml) | t1/2β (h) | Cmax (μg/ml) | Tmax (h) | AUC0–∞ (μg · h/ml) | t1/2β (h) | |
| Patients | 2.89 ± 0.85 | 1 | 3.39 ± 0.96 | 0.83 ± 0.13 | 0.71 ± 0.20 | 0.85 ± 0.29 | 0.320 ± 0.06 | 1.17 | 0.653 ± 0.20 | 2.39 ± 1.34 | 0.232 ± 0.08 | 1.25 | 0.690 ± 0.22 | 2.38 ± 1.06 |
| Volunteers | 2.60 ± 0.43 | 1 | 2.90 ± 0.41 | 0.93 ± 0.15 | 0.79 ± 0.12 | 1.07 ± 0.27 | 0.405 ± 0.13 | 1 | 0.722 ± 0.25 | 1.75 ± 0.61 | 0.289 ± 0.09 | 1.17 | 0.656 ± 0.25 | 1.44 ± 0.39 |
Values are means ± SD. Differences between values were not statistically significant (P > 0.05).
TABLE 2.
Pharmacokinetic parameters of dalfopristin and its metabolite RP 12536a
| Group | Dalfopristin (RP 54476)
|
RP 12536 (metabolite)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmax (h) | AUC0–∞ (μg · h/ml) | t1/2β (h) | CL (liter/h · kg) | V (liter/kg) | Cmax (μg/ml) | Tmax (h) | AUC0–∞ (μg · h/ml) | t1/2β (h) | |
| Patients | 8.52 ± 3.52 | 1 | 9.72 ± 4.53 | 0.76 ± 0.29 | 0.67 ± 0.36 | 0.68 ± 0.30 | 0.974 ± 0.32 | 1 | 1.62 ± 0.48 | 0.84 ± 0.39 |
| Volunteers | 7.09 ± 2.70 | 1 | 7.60 ± 2.81 | 0.71 ± 0.18 | 0.77 ± 0.30 | 0.77 ± 0.34 | 1.05 ± 0.29 | 1 | 1.54 ± 0.28 | 1.15 ± 0.23 |
Values are means ± SD. Differences between values were not statistically significant (P > 0.05).
Dalfopristin and a glutathione conjugate of quinupristin (RP 69012) were not detected in dialysis effluent. Quinupristin was detected at very low concentrations (0.24 μg/ml) in the dialysate effluents of only two patients at 1.25 h after the start of the infusion. Pristinamycin IIA was present in dialysate at 1.25, 2, and 3 h after the start of infusion at low concentrations compared to those observed in plasma. A mean Cmax of 0.207 μg/ml in dialysate was reached at 2 h compared to that of 0.974 μg/ml in plasma at the end of infusion. Dialysate pristinamycin IIA concentrations at 6 h were nondetectable. The cysteine conjugate of quinupristin (RPR 100391) appeared slowly in dialysate, reaching a mean Cmax at 6 h after the start of infusion. The dialysate RPR 100391 concentrations were low compared to those observed in plasma. RPR 100391 was the only compound for which peritoneal CL could be calculated (0.56 ± 0.28 liter/h). Peritoneal CL values for quinupristin, dalfopristin, and their respective metabolites were negligible. Due to the very low rate of dialysate CL for all the compounds, no rate of appearance of the intravenously administered product into the peritoneal fluid was estimated.
Safety results.
There were no clinically significant changes in any of the laboratory parameters from baseline through study termination 10 h after the start of infusion. There were 34 adverse events, 28 in the CAPD group and 6 in the healthy volunteer group, as noted in Table 3. All adverse events were mild, and they resolved prior to discharge from the study. Six patients and one healthy volunteer experienced pain at the infusion site. No patients had inflammation at the infusion site, but three of the healthy volunteers did. Five of the CAPD patients, but none of the healthy volunteers, experienced nausea.
TABLE 3.
Adverse events observed during the study
| Adverse event | No. of patients | No. of volunteers |
|---|---|---|
| Stiff neck | 1 | 0 |
| Dry mouth | 2 | 0 |
| Sedation | 1 | 0 |
| Sneezing | 1 | 0 |
| Coughing | 2 | 0 |
| Arm, hand, and/or shoulder stiffness | 3 | 0 |
| Metallic taste | 1 | 0 |
| Nausea | 5 | 0 |
| Numbness in nose | 1 | 0 |
| Headache | 1 | 1 |
| Emesis | 1 | 0 |
| Numbness in lips | 1 | 0 |
| Tingling in shoulders | 1 | 0 |
| Light-headedness | 0 | 1 |
| Burning or discomfort at i.v. sitea | 4 | 1 |
| Aching arm above i.v. site | 2 | 0 |
| Erythema in arm at i.v. site | 0 | 2 |
| Dizziness | 1 | 1 |
i.v. site, site of intravenous injection.
DISCUSSION
Previous studies of healthy volunteers have demonstrated that following a 1-h infusion, quinupristin and dalfopristin undergo rapid elimination, with t1/2β values of about 1 h. Approximately 75% of the dose of each compound is fecally excreted. Less than 20% of the dose is excreted in the urine as either parent drug or metabolites (5). In a previous study of intravenous quinupristin-dalfopristin given to patients with severe chronic renal failure, the disposition profiles of quinupristin were comparable in patients with severe renal failure and in healthy volunteers. However, the elimination of quinupristin metabolites may have been somewhat impaired, as indicated by selective bioassay. The elimination of dalfopristin was slightly modified in the renal-failure patients. The mean Cmax and AUC0–∞ values for dalfopristin were about 1.3 times higher than those estimated for healthy volunteers (11a).
For quinupristin and dalfopristin, the results of the present study are consistent with the results of the earlier study of patients with chronic renal failure. While not statistically significant, the slight-to-moderate increases in AUC0–t values in plasma suggest that nonrenal CL of these compounds may be slightly reduced in patients undergoing CAPD. The pharmacokinetic parameters for the metabolites in our study showed some differences compared to those of the earlier study of chronic renal failure. In the earlier study, statistically significant increases were observed in the AUC0–t values of quinupristin and active metabolites, as determined by bioassay, suggesting an increase of quinupristin metabolite concentrations. In the present study, no differences in AUC0–t values for quinupristin metabolites were observed between CAPD patients and healthy volunteers. This difference between the two studies may be explained by differences in analytical techniques used (bioassay versus high-performance liquid chromatography) or by the contribution of CL via peritoneal dialysis. This latter hypothesis seems unlikely given the molecular weights of quinupristin and its metabolites. The pharmacokinetic parameters observed in the present study for the healthy volunteers were similar to those seen in previous studies with volunteer groups.
Patients receiving peritoneal dialysis may develop community-acquired or nosocomial infections for which quinupristin-dalfopristin may be indicated. The results of this study indicate that peritoneal CL of both drugs and their metabolites is a relatively insignificant contributor to total body CL. Because insignificant amounts of both parent drugs and their metabolites are excreted into dialysis effluent, intravenous dosing of quinupristin and dalfopristin is unlikely to be adequate for the treatment of peritoneal dialysis-related peritonitis. While the data from this study suggest that dose adjustments will not be required for intravenous administration to patients receiving CAPD, future multiple-dose studies will need to confirm dosing recommendations for therapeutic use. Currently, there are no data regarding the intraperitoneal dosing of this antibiotic combination.
ACKNOWLEDGMENTS
This work was supported by a grant from Rhône-Poulenc Rorer and in part by NIH grant M01 RR03186 from the National Center for Research Resources to the University of Wisconsin Medical School.
We thank the following individuals for their invaluable assistance: Maureen Wakeen, Jim Mroczynski, Linda Lorentzen, Pierre Delplanque, Abhik Bhattacharya, and the members of the staffs of the University of Wisconsin General Clinical Research Center and the Corning Besselaar Clinical Research Unit, Inc. (Covance Clinical Research Unit, Inc.).
REFERENCES
- 1.Bouanchaud, D. H. 1992. In-vitro and in-vivo synergic activity and fractional inhibitory concentration (FIC) of the components of a semisynthetic streptogramin, RP 59500. J. Antimicrob. Chemother. 30(Suppl. A):95–99. [DOI] [PubMed]
- 2.Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:765–766. [PubMed] [Google Scholar]
- 3.Etienne, S. D., G. Montay, A. Le Liboux, A. Frydman, and J. J. Garaud. 1992. A phase I, double-blind, placebo-controlled study of the tolerance and pharmacokinetic behavior of RP 59500. J. Antimicrob. Chemother. 30(Suppl. A):123–131. [DOI] [PubMed]
- 4.Finch, R. G. 1996. Antibacterial activity of quinupristin/dalfopristin. Rationale for clinical use. Drugs 1(Suppl. 1):31–37. [DOI] [PubMed]
- 5.Gaillard C, Van Cantfort J, Montay G, Piffard D, Le Liboux A, Etienne S, Scheen A, Frydman A. Program and abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. Disposition of the radiolabelled streptogramin RP 59500 in healthy male volunteers, abstr. 1317; p. 330. [Google Scholar]
- 6.Keene W F, Alexander S R, Bailie G R, Boeschoten E, Gokal R, Golper T A, Holmes C J, Huang C-C, Kawaguchi Y, Piraino B, Riella M, Schaefer F, Vas S. Peritoneal dialysis-related peritonitis treatment recommendations: 1996 update. Perit Dial Int. 1996;16:557–573. [PubMed] [Google Scholar]
- 7.Lai K K. Treatment of vancomycin-resistant Enterococcus faecium infections. Arch Intern Med. 1996;156:2579–2584. [PubMed] [Google Scholar]
- 8.Le Liboux A, Pasquier O, Montay G. Simultaneous high-performance liquid chromatographic determination of quinupristin, dalfopristin and their main metabolites in human plasma. J Chromatogr B. 1998;708:161–168. doi: 10.1016/s0378-4347(98)00005-x. [DOI] [PubMed] [Google Scholar]
- 9.Lynn W A, Clutterbuck E, Want S, Markides V, Lacey S, Rogers T R, Cohen J. Treatment of CAPD-peritonitis due to glycopeptide-resistant Enterococcus faecium with quinupristin/dalfopristin. Lancet. 1994;344:1025–1026. doi: 10.1016/s0140-6736(94)91687-x. [DOI] [PubMed] [Google Scholar]
- 10.Mulazimoglu L, Drenning S D, Yu V L. In vitro activities of two novel oxazolidinones (U100592 and U100766), a new fluoroquinolone (trovafloxacin), and dalfopristin-quinupristin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1996;40:2428–2430. doi: 10.1128/aac.40.10.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qadri S M, Ueno Y, Abu Mostafa F M, Halim M. In vitro activity of quinupristin/dalfopristin, RP59500, against gram-positive clinical isolates. Chemotherapy. 1997;43:94–99. doi: 10.1159/000239542. [DOI] [PubMed] [Google Scholar]
- 11a.Rhône-Poulenc Rorer. Data on file. Rhône-Poulenc Rorer, Collegeville, Pa.
- 12.Schaberg, D. R., D. H. Culver, and R. P. Gaynes. 1991. Major trends in the microbial etiology of nosocomial infections. Am. J. Med. 91(Suppl. 3B):72S–75S. [DOI] [PubMed]
- 13.Shonekan D, Handwerger S, Mildvan D. Comparative in-vitro activities of RP59500 (quinupristin/dalfopristin), CL 329,998, CL 331,002, trovafloxacin, clinafloxacin, teicoplanin and vancomycin against Gram-positive bacteria. J Antimicrob Chemother. 1997;39:405–409. doi: 10.1093/jac/39.3.405. [DOI] [PubMed] [Google Scholar]