Abstract
Background
There are currently no pharmacological therapies to address the intellectual disability associated with Down syndrome. Excitatory/inhibitory imbalance has been hypothesized to contribute to impairments in cognitive functioning in Down syndrome. Negative modulation of the GABAA-α5 receptor is proposed as a mechanism to attenuate GABAergic function and restore the excitatory/inhibitory balance.
Methods
Basmisanil, a selective GABAA-α5 negative allosteric modulator, was evaluated at 120 mg or 240 mg BID (80 or 160 mg for 12–13 years) in a 6-month, randomized, double-blind, placebo-controlled phase II trial (Clematis) for efficacy and safety in adolescents and young adults with Down syndrome. The primary endpoint was based on a composite analysis of working memory (Repeatable Battery for the Assessment of Neuropsychological Scale [RBANS]) and independent functioning and adaptive behavior (Vineland Adaptive Behavior Scales [VABS-II] or the Clinical Global Impression-Improvement [CGI-I]). Secondary measures included the Behavior Rating Inventory of Executive Functioning-Preschool (BRIEF-P), Clinical Evaluation of Language Fundamentals (CELF-4), and Pediatric Quality of Life Inventory (Peds-QL). EEG was conducted for safety monitoring and quantitatively analyzed in adolescents.
Results
Basmisanil was safe and well-tolerated; the frequency and nature of adverse events were similar in basmisanil and placebo arms. EEG revealed treatment-related changes in spectral power (increase in low ~ 4-Hz and decrease in high ~ 20-Hz frequencies) providing evidence of functional target engagement. All treatment arms had a similar proportion of participants showing above-threshold improvement on the primary composite endpoint, evaluating concomitant responses in cognition and independent functioning (29% in placebo, 20% in low dose, and 25% in high dose). Further analysis of the individual measures contributing to the primary endpoint revealed no difference between placebo and basmisanil-treated groups in either adolescents or adults. There were also no differences across the secondary endpoints assessing changes in executive function, language, or quality of life.
Conclusions
Basmisanil did not meet the primary efficacy objective of concomitant improvement on cognition and adaptive functioning after 6 months of treatment, despite evidence for target engagement. This study provides key learnings for future clinical trials in Down syndrome.
Trial registration
The study was registered on December 31, 2013, at clinicaltrials.gov as NCT02024789.
Supplementary Information
The online version contains supplementary material available at 10.1186/s11689-022-09418-0.
Keywords: Down syndrome, GABAA-α5, Cognition, Adaptive behavior, EEG
Background
Down syndrome (DS), the triplication of whole or part of chromosome 21, is the most common identifiable cause of intellectual disability with an incidence of 1 in 650 to 1 in 1000 live births per year worldwide [1, 2]. Among several co-occurring conditions, DS is associated with a unique cognitive and adaptive behavior profile [3, 4], which is of primary concern to many caregivers. Since more individuals with DS are active members of the community due to increased life expectancy, improving functional potential through development of pharmacotherapies may address these unmet needs. There is currently no therapeutic option available to treat the associated intellectual disability.
Although the etiology of the cognitive disability in people with DS remains unclear, cellular and anatomical abnormalities in the prenatal and perinatal forebrain and cerebellum suggest that early brain development is altered in individuals with DS [5–7]. Similar brain abnormalities have been described in mouse models of DS, such as the Ts65Dn which is the best characterized model [8–10]. Studies have suggested that the major functional defect in the postnatal Ts65Dn brain may be an imbalance between excitatory and inhibitory circuits [11–13]. Chronic treatment with selective GABAA-α5 negative allosteric modulators (NAMs)—such as α5IA [14], RO4938581 [15], and basmisanil [16]—improved synaptic plasticity and rescued cognitive and behavioral deficits in Ts65Dn mice, without inducing anxiety or convulsions, side effects observed with non-selective GABAA NAMs [17, 18]. Inhibition of GABAA-α5 receptors may represent an attractive mechanism to enhance cognition in individuals with DS.
Basmisanil (RO5186582, RG1662) is a potent NAM, which combines both binding and functional selectivity at GABAA-α5 subunit-containing receptors and has been shown to improve cognition in rats and monkeys [19]. GABAA-α5 hippocampal receptor occupancy between 30–65% was required for efficacy in preclinical studies [16, 19]. Basmisanil has shown a favorable safety and tolerability profile over a broad range of doses in healthy volunteer studies (BP25611 [ClinicalTrials.gov: NCT01667367], WP28214 [NCT01684891]; BP25129 [EudraCT: 2009-016097-33], WP25366 [2010-021554-19]), and in adults with DS (BP25543 [NCT01436955], BP25611 [NCT01667367]).
Given the absence of any effective therapy for the intellectual disability associated with DS, the supportive 5-week safety and tolerability profile established in individuals aged 18–30 years with DS (BP25543; Additional file 1) and the potential added benefit of earlier intervention, we aimed to assess the efficacy of extended basmisanil dosing on cognition and adaptive behavior in both adolescents and young adults with DS.
Methods
Participants
Male and female participants (12–30 years) with DS (standard trisomy 21, Robertsonian translocation, isochromosome 21, with reciprocal translocation, or mosaicism) were included. Minimum verbal abilities were required to participate in the study, as defined by a minimum raw score of 7 for adults, or 4 for adolescents, on the Clinical Evaluation of Language Fundamentals Preschool-2 (CELF-P) Word Classes subtests [20]. The IQ of participants was assessed at baseline only using the non-verbal Leiter 3 test [21].
Individuals with a diagnosis of autism spectrum disorder, major depressive disorder, a history of infantile spasms or epileptic encephalopathy, or a history of seizures within 2 years prior to the screening visit were not included in the trial. Participants consented or assented to participate, and written informed consent was obtained from their caregiver.
Study design
BP27832 (Clematis) was a randomized, double-blind, placebo-controlled, multi-country phase II study to investigate the efficacy and safety of basmisanil in adults (18–30 years) and adolescents (12–17 years) with DS (Additional file 2). The study was registered on December 31, 2013, at clinicaltrials.gov as NCT02024789, approved by local ethics committees, and conducted in accordance with the principles of the “Declaration of Helsinki” and Good Clinical Practice. A Roche-independent safety committee was responsible for the monitoring of safety data on a regular basis.
Eligible participants were randomized in a 1:1:1 ratio to receive either tablets of placebo, low or high dose of basmisanil, twice daily (BID) over 6 months (26 weeks). The low dose of basmisanil was 120 mg and the high dose was 240 mg, except for participants below 14 years where the low dose was age-adjusted to 80 mg and the high dose to 160 mg. Dose selection was based on an integrated evaluation of pharmacokinetics (PK), pharmacodynamics, PET (BP25611; Additional file 1), and safety data from prior clinical studies with basmisanil in healthy volunteers and adults with DS, coupled with preclinical safety and efficacy data. The aim was to have two effective dosing regimens: the low dose targeted exposures that would result in receptor occupancy in all individuals above a minimum threshold of 60% expected to be required for efficacy based on preclinical models of DS [19]; the high dose was selected to reach exposures predicted to maintain receptor occupancy above a near-maximal threshold (> 90%).
Primary and secondary efficacy
Efficacy assessments were performed at baseline and after 3 and 6 months of treatment. The primary efficacy analysis assessed the proportion of participants who showed improvement above pre-defined thresholds (i.e., above-threshold improvement) on a composite endpoint, concomitantly evaluating cognition and adaptive functioning, after 6 months of treatment. Above-threshold improvement on the composite endpoint was defined as (1) a relevant increase in raw scores from baseline in at least two out of three tasks from the Repeatable Battery for the Assessment of Neuropsychological Status ([RBANS]; at least 2 points for list learning and 1 point for list recognition and list recall); and (2) either an increase from baseline in the Vineland Adaptive Behavior Scales-II (VABS-II) composite standard score of ≥ 7 or a Down syndrome-specific Clinical Global Impression-Improvement (DS-CGI-I) score of ≤ 3 (minimally improved). The DS-CGI-I evaluation was based on scoring DS-specific anchors: communication/speech, activities of daily living, social functioning, and stubbornness/non-compliance (Additional file 3). The RBANS thresholds were identified based on the variability of each endpoint observed at baseline in the observational study, conducted in a comparable population in terms of average age and IQ [22]. They correspond to an effect size of approximately 0.3, i.e., 30% of the standard deviation observed in baseline raw scores for each task. These RBANS thresholds were then discussed in an advisory board meeting, with clinicians and clinical research experts in neurodevelopmental disorders and DS, to qualitatively assess the clinical meaningfulness of these changes. The selected thresholds were considered adequate across the age range if concomitant improvements could be observed on global functioning measures of established clinical relevance such as the CGI or the VABS. The secondary efficacy analyses evaluated change from baseline scores on each of the individual measures contributing to the composite endpoint, (RBANS learning, recognition and recall tasks raw scores; VABS-II composite standard score; DS-CGI-I score). Treatment effects on VABS-II domain standard scores (communication, daily living skills, and socialization), language (word classes tasks of Clinical Evaluation of Language Fundamentals-version 4 [CELF-4] raw scores), executive function (Behavior Rating Inventory of Executive Function Preschool [BRIEF-P] raw scores), and global quality of life (Pediatric Quality of Life Inventory [PedsQL] raw scores) were also evaluated.
Statistical analysis of efficacy endpoints
Fifty subjects per treatment group provide a power of 80% to detect a difference between each active dose and placebo when the frequency of participants with above-threshold improvement is 30% on active dose and 5% on placebo. This calculation was based on the two-sided χ2 test with continuity correction and significance declared at the two-sided 2.5% level to maintain the overall 5% level study-wise (as per Bonferroni adjustment for multiple comparisons).
The proportion of participants with above-threshold improvement was analyzed by means of a logistic regression model. This included treatment and visit and treatment by visit interaction, age, sex, and IQ at baseline as covariates, participant as repeated effect. The selected covariates were defined a priori in a statistical analysis plan, as sex and age may have an impact on drug pharmacokinetic properties, and age and IQ are expected to influence cognition, language, and adaptive behavior in individuals with DS. For all endpoints normally distributed a mixed model analysis of variance was applied to change from baseline scores, where applicable, with baseline, age, sex, and IQ at baseline as covariates, treatment and treatment by visit interaction, with visit as repeated measurements and participant as random. Inferential findings are provided for descriptive purposes only and without any confirmatory meaning. Multiple endpoints and multiple treatment comparisons were analyzed; however, due to the exploratory nature of the study, multiplicity was not statistically adjusted for, and the risk of false positive results should be taken into consideration in the interpretation of the results.
Pharmacokinetic assessments
Blood samples were collected for determination of plasma concentrations of basmisanil. Concentrations were measured by a specific liquid chromatography-mass spectrometry/mass spectrometry method. The following time points were included prior to dosing to assess trough concentrations of basmisanil at weeks 2, 6, and 12.
Safety assessments
Safety surveillance of participants included adverse event (AE) reporting, physical examinations, vital signs including 12-lead ECG recordings, clinical chemistry, hematology, and urinalyses. Comorbidities were monitored, such as ADHD (Conner’s questionnaire); sleep problems (Children’s sleep habits questionnaire); anxiety and depression (ADAMS questionnaire). As per regulatory guidance, suicidality monitoring was implemented using the pediatric and adult C-SSRS version.
EEG assessments
EEG recordings were primarily included to monitor the emergence of epileptiform abnormalities in adolescents and participants with a medical history of epilepsy, to confirm the favorable safety profile of basmisanil previously established in adults with DS (without a medical history of epilepsy, study BP25543). A 30-min EEG recording was performed at baseline (pre-dose), week 2, and week 20. Recordings from adolescents were used for the exploratory quantitative EEG analyses reported here. The exploratory quantitative analyses of the EEG data were restricted to spectral power, which provides a macroscopic measure of synchronized neuronal activity. No assumptions were made about spectral or spatial properties of possible treatment effects. The statistical analysis accounted for multiple comparisons across frequencies and electrodes using a cluster randomization approach.
To test for a PK-PD relationship we performed non-parametric correlations (Spearman rank correlation; one-tailed test, i.e., testing for a positive correlation for theta-band power and negative correlation for beta-band power) between individual measured trough exposure levels and theta and beta-band EEG power from the identified clusters both measured at week 2. Although the EEG was recorded 4–5 h post administration, and the PK sample before administration, the measured trough concentrations at steady state are considered as a reasonable proxy for the individual basmisanil concentration at the time of the EEG recording. For this analysis we used all dosed participants but only included participants with a PK sample and an EEG recording at week 2 (n = 37, low dose: n = 14, high dose: n = 23). Full details on the EEG acquisition and analysis can be found in Additional file 4.
Results
Enrollment
Between May 5, 2014, and October 1, 2015, 170 participants were randomized across 30 sites. For adults, the majority were recruited at US (60%), French (20%), and Spanish (13%) sites. For adolescents, the majority were recruited at Spanish (42%) and US (31%) sites.
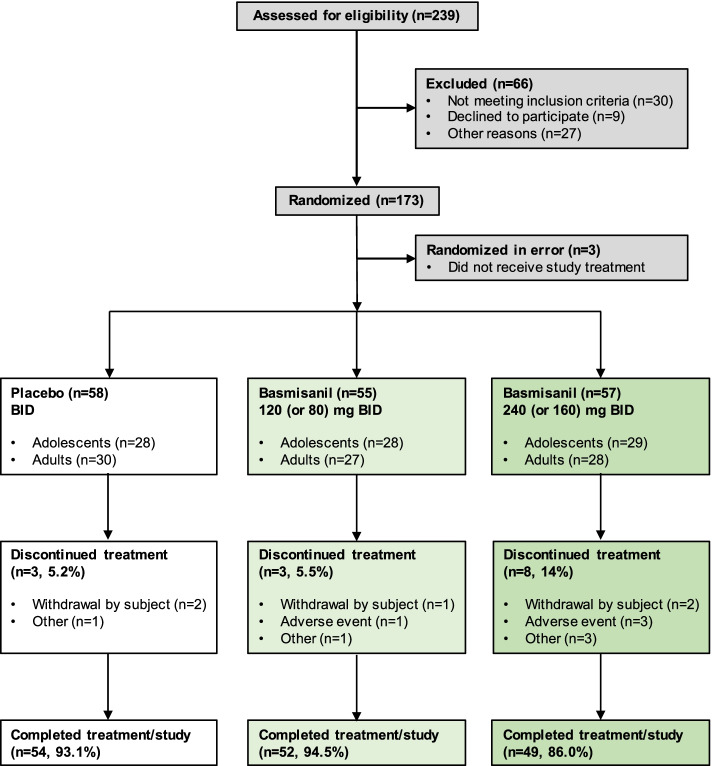
A total of 155 participants (91%) completed the study and were included in the analysis (Fig. 1). The proportion of participants who discontinued study medication prematurely was higher in the high-dose arm (8/57, 14%) than in placebo (3/58, 5.2%) and low-dose (3/55, 5.5%) arms. Withdrawals were mostly driven by non-safety-related reasons (placebo: 3/3; low dose: 2/3; high dose: 5/8). The majority of deviations to the protocol were assessments being performed outside the defined visit window due to scheduling issues. Seven participants were excluded from the efficacy analysis population (six did not meet CELF-P inclusion criterion, one had < 80% compliance rate to study medication).
Fig. 1.
Participant disposition (CONSORT diagram)
Participants’ demographics and baseline characteristics were similar across arms (Table 1). Approximately two-thirds of the study population were taking concomitant therapies; the most prescribed treatments across all groups were analgesics/non-steroidal anti-inflammatory drugs (17–31%), corticosteroids (3–19%), and penicillin drugs (9–18%).
Table 1.
Baseline characteristics
| Placebo | Basmisanil | ||
|---|---|---|---|
| 120 mg (80 mg) | 240 mg (160 mg) | ||
| n = 58 | n = 55 | n = 57 | |
| Age (years) | |||
| Mean ± SD | 18.7 ± 5.2 | 18.3 ± 4.9 | 18.7 ± 5.4 |
| Median | 18.0 | 17.0 | 17.0 |
| Minimum to maximum | 12–30 | 12–28 | 12–29 |
| 12–17 years: n (%) | 28 (48%) | 28 (51%) | 29 (51%) |
| 18–30 years: n (%) | 30 (52%) | 27 (49%) | 28 (49%) |
| Sex | |||
| Males: n (%) | 33 (57%) | 32 (58%) | 38 (67%) |
| Females: n (%) | 25 (43%) | 23 (42%) | 19 (33%) |
| Ethnicity | |||
| Hispanic or Latino: n (%) | 11 (19.0%) | 13 (23.6%) | 10 (17.5%) |
| Not Hispanic or Latino: n (%) | 38 (65.5%) | 35 (63.6%) | 39 (68.4%) |
| Unknown: n (%) | 9 (15.5%) | 7 (12.7%) | 8 (14.0%) |
| Race: n (%) | |||
| American Indian or Alaska Native | 1 (1.7%) | 0 | 0 |
| Asian | 1 (1.7%) | 1 (1.8%) | 0 |
| Black or African American | 0 | 2 (3.6%) | 0 |
| Multiple: White/Asian | 2 (3.4%) | 0 | 0 |
| White | 45 (77.6%) | 44 (80.0%) | 49 (86.0%) |
| Unknown | 9 (15.5%) | 8 (14.5%) | 8 (14.0%) |
| Formulationa | |||
| Granules: n (%) | 10 (17%) | 10 (18%) | 12 (21%) |
| Tablets: n (%) | 48 (83%) | 45 (82%) | 45 (79%) |
| CGI-severity | |||
| Mean ± SD | 3.7 ± 1.0 | 3.8 ± 0.9 | 3.9 ± 0.9 |
| Median | 4.0 | 4.0 | 4.0 |
| Minimum to maximum | 1–5 | 1–5 | 1–6 |
| ≤ 3: n (%) | 18 (31%) | 16 (31%) | 12 (22%) |
| > 3: n (%) | 40 (69%) | 36 (69%) | 43 (78%) |
| CELF-4 (word classes 1): mean ± SD | |||
| Receptive | 15.2 ± 4.3 | 15.2 ± 3.9 | 14.7 ± 4.9 |
| Expressive | 9.7 ± 5.4 | 9.6 ± 4.2 | 9.8 ± 5.1 |
| CELF-4 (word classes 2): mean ± SD | |||
| Receptive | 3.5 ± 4.1 | 2.98 ± 3.4 | 2.8 ± 3.2 |
| Expressive | 1.8 ± 2.3 | 1.4 ± 1.7 | 1.5 ± 1.9 |
| Anxiety/mood (ADAMS): mean ± SD | |||
| Depressed mood | 2.5 ± 2.9 | 2.1 ± 2.0 | 2.5 ± 3.1 |
| Anxiety | 2.6 ± 2.3 | 2.5 ± 2.5 | 2.6 ± 2.9 |
| Manic/hyperactive | 3.2 ± 2.4 | 4.0 ± 3.1 | 3.1 ± 2.8 |
| Obsessive/compulsive | 1.9 ± 2.0 | 1.9 ± 1.8 | 1.7 ± 1.8 |
| Social avoidance | 3.8 ± 3.9 | 4.3 ± 3.7 | 3.8 ± 3.9 |
| IQb | |||
| Mean ± SD | 52.8 ± 13.6 | 55.2 ± 14.6 | 55.6 ± 13.9 |
| Median | 49 | 53 | 57 |
| Minimum to maximum | 32–93 | 32–93 | 32–80 |
Abbreviations: ADAMS Anxiety, Depression and Mood Abnormalities, CELF Clinical Evaluation of Language Fundamentals, CGI Clinical Global Impression, SD standard deviation
aA granule formulation was available for individuals with difficulties swallowing tablets (assessed in comparative bioavailability study WP28978 [NCT02194244])
bIQ assessed by Leiter International Performance Scale-revised: a non-verbal intelligence test
Primary efficacy: composite endpoint analysis at 6 months
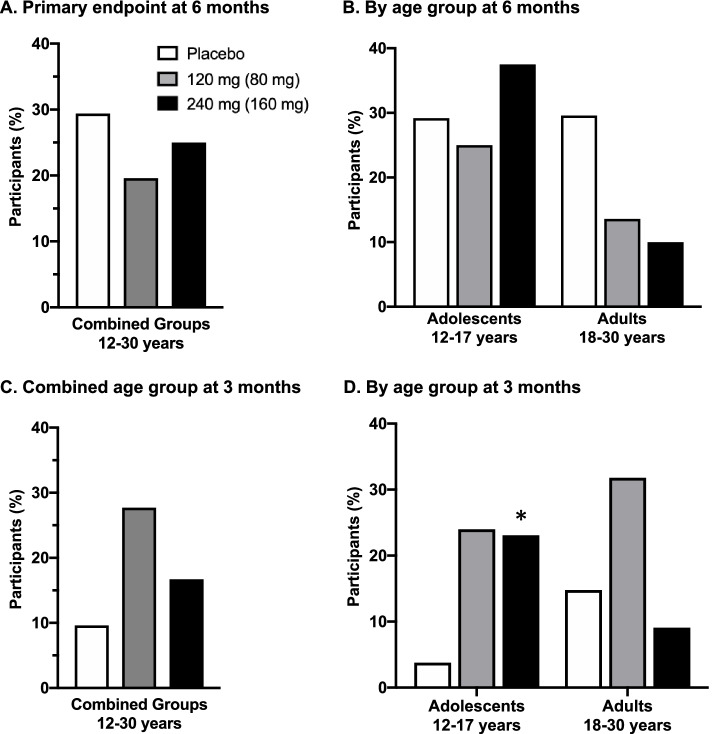
The findings of the study indicate lack of treatment effects on the primary endpoint. The proportion of participants with above-threshold improvement on the composite endpoint at 6 months was not different between basmisanil-treated groups and placebo (p = 0.262; Fig. 2A). Subgroup analyses by age (Fig. 2B), or by sex, language (English-speaking countries, Rest of the World), functioning level (IQ < 50, ≥ 50), and expressive abilities based on CELF−P score at screening (adolescents < 7 or ≥ 7; adults < 10 or ≥ 10) also showed lack of a treatment effect (data not shown).
Fig. 2.
Percent of participants with above-threshold improvement on the composite endpoint. A Primary efficacy endpoint after 6 months of treatment. Percent of participants with above-threshold improvement: B by age group (adolescents, adults) after 6 months of treatment; C combined age group after 3 months of treatment; D by age group (adolescents, adults) after 3 months of treatment. Above-threshold improvement on the composite endpoint was defined as having (1) a relevant increase in raw scores from baseline in at least two out of three tasks from the Repeatable Battery for the Assessment of Neuropsychological Status ([RBANS]; ≥ 2 points for list learning, ≥ 1 point for list recognition, ≥ 1 point for list recall); and (2) either an increase from baseline in the Vineland Adaptive Behavior Scales-II (VABS II) composite score of ≥ 7 or a Down syndrome-specific Clinical Global Impression-Improvement (DS-CGI-I) ≤ 3 (minimally improved). Efficacy assessments were performed at baseline and after 3 and 6 months of treatment. Statistics: *p < 0.05 vs. placebo-treated group
At 3 months there was no statistically significant difference in improvement overall (Fig. 2C), however, in the adolescents (Fig. 2D) a higher proportion of participants with above-threshold improvement was observed in both basmisanil-treated groups compared to the placebo group, with a nominal p-value of p = 0.043 at the high dose (low dose, nominal p-value: p = 0.063).
Additionally, no differences in the proportion of participants with above-threshold improvements were detected between placebo and basmisanil-treated groups on any of the individual components of the composite endpoint (RBANS, VABS-II and DS-CGI-I; Additional file 5).
Secondary efficacy outcome measures
There were no statistically significant differences between placebo and basmisanil-treated groups in secondary outcome measures evaluating changes from baseline (Table 2) in cognition (RBANS), adaptive behavior (VABS-II composite), language (CELF-4), executive function (BRIEF-P), or global quality of life (PedsQL). In both basmisanil and placebo groups, small improvements were observed in RBANS list learning, BRIEF-P and PedsQL (Table 2), as well as in the VABS-II domain scores of socialization, communication, and daily living skills (Additional file 6). Nearly all participants were able to reach CELF-4 Word Class 2 level and no improvements in receptive or expressive language abilities were observed over 6 months across treatment arms (Table 2).
Table 2.
Change from baseline scores at 3 and 6 months
| Assessment | Time point (month) | Placebo | 120 (80) mg | 240 (160) mg | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | p | Mean ± SD | n | p | ||
| RBANS | |||||||||
| List learning | 3 | 1.5 ± 5.2 | 54 | 2.3 ± 5.4 | 47 | 0.69 | 1.0 ± 5.12 | 48 | 0.86 |
| 6 | 3.1 ± 6.1 | 51 | 2.7 ± 6.2 | 47 | 0.56 | 2.4 ± 4.8 | 44 | 0.76 | |
| List recall | 3 | 0.5 ± 2.8 | 53 | 0.2 ± 2.2 | 47 | 0.49 | 0.4 ± 2.5 | 48 | 0.98 |
| 6 | 0.3 ± 2.4 | 51 | 0.2 ± 3.1 | 47 | 0.98 | -0.1 ± 2.4 | 44 | 0.83 | |
| List recognition | 3 | 0.0 ± 2.9 | 53 | 1.1 ± 2.3 | 47 | 0.09 | 0.8 ± 4.2 | 48 | 0.26 |
| 6 | 1.2 ± 3.2 | 51 | 1.4 ± 3.1 | 47 | 0.75 | 1.8 ± 3.9 | 44 | 0.29 | |
| VABS-II | |||||||||
| Composite score | 3 | 1.6 ± 5.0 | 53 | 0.98 ± 4.6 | 46 | 0.60 | 1.02 ± 3.7 | 47 | 0.62 |
| 6 | 2.4 ± 10.2 | 50 | 2.0 ± 4.02 | 46 | 0.79 | 2.02 ± 4.6 | 43 | 0.72 | |
| CELF-4 (word classes 1) | |||||||||
| Receptive | 3 | − 0.2 ± 3.7 | 54 | − 0.5 ± 4.1 | 47 | 0.74 | 1.1 ± 2.8 | 48 | 0.09 |
| 6 | 0.8 ± 3.6 | 51 | − 0.07 ± 3.4 | 46 | 0.28 | 1.5 ± 3.6 | 44 | 0.31 | |
| Expressive | 3 | 0.5 ± 3.8 | 54 | 0.6 ± 3.9 | 47 | 0.79 | 1.2 ± 3.2 | 48 | 0.22 |
| 6 | 0.9 ± 3.1 | 51 | 0.3 ± 3.8 | 46 | 0.34 | 1.3 ± 3.1 | 44 | 0.54 | |
| CELF-4 (word classes 2) | |||||||||
| Receptive | 3 | 0.1 ± 2.1 | 51 | − 0.2 ± 2.0 | 43 | 0.23 | 0.0 ± 2.1 | 43 | 0.60 |
| 6 | − 0.3 ± 3.6 | 47 | 0.0 ± 3.1 | 41 | 0.99 | 0.2 ± 2.3 | 40 | 0.52 | |
| Expressive | 3 | 0.2 ± 1.5 | 51 | − 0.07 ± 1.1 | 43 | 0.14 | 0.2 ± 1.5 | 43 | 0.99 |
| 6 | 0.1 ± 2.4 | 47 | − 0.07 ± 1.4 | 41 | 0.41 | 0.2 ± 1.6 | 40 | 0.78 | |
| BRIEF-Pa | |||||||||
| Global executive composite | 3 | − 4.1 ± 12.3 | 53 | − 6.6 ± 12.7 | 47 | 0.48 | − 5.2 ± 11.6 | 48 | 0.75 |
| 6 | − 4.1 ± 12.2 | 51 | − 7.8 ± 12.6 | 46 | 0.16 | − 7.9 ± 12.7 | 42 | 0.10 | |
| PedsQL | |||||||||
| Total scale score | 3 | 0.9 ± 14.0 | 54 | 2.7 ± 15.3 | 48 | 0.75 | 3.9 ± 12.1 | 45 | 0.19 |
| 6 | 1.7 ± 12.7 | 47 | 5.6 ± 12.5 | 46 | 0.31 | 3.5 ± 9.7 | 41 | 0.46 | |
See Additional file 12 for “change from baseline” scores by age group and time point
Abbreviations: BRIEF-P Behavior Rating Inventory of Executive Function-Preschool, CELF Clinical Evaluation of Language Fundamentals, PedsQL Pediatric Quality of Life Inventory, RBANS Repeatable Battery for the Assessment of Neuropsychological Status, SD standard deviation, VABS-II Vineland Adaptive Behavior Scales-II
aNegative change = improvement
Exploratory qEEG in adolescents
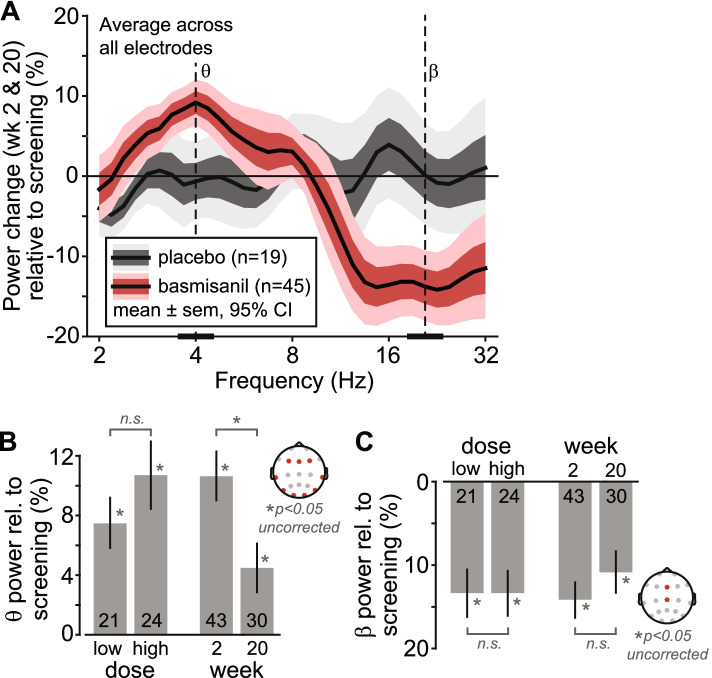
The baseline EEG power spectrum was characterized by a marked absence of an alpha peak, which is the most prominent feature of typical developing individuals, and exhibited a prominent peak in the theta frequency range around 4 Hz (Additional file 7: panel B). In response to basmisanil, relative spectral power at lower frequencies (~ 4-Hz, theta-frequency range) increased while relative power at higher frequencies (~ 20-Hz, beta-frequency range) decreased compared to baseline, but spectral power remained unchanged for placebo (Fig. 3A). Absolute power also revealed an increase in the theta- and decrease in the beta-frequency range in response to basmisanil (Additional file 7: panel G). These qualitative observations were confirmed by statistical analysis using cluster-randomization that accounted for multiple testing across all electrodes (n = 19) and frequencies (2–32 Hz). The analysis identified two clusters, i.e., differences between the combined dose groups and placebo that extended across frequencies and electrodes. A “positive cluster” in the theta-frequency range (power increase for dose groups relative to placebo, p = 0.022) and a “negative cluster” in the beta-frequency range (power decrease for dose groups relative to placebo, p = 0.0007; Additional file 7: panel B-E).
Fig. 3.
Quantitative EEG. A Change in EEG spectral power (average across week 2 and week 20 visits relative to baseline) for dosed (red) and the placebo (gray) groups. B, C Effects of assessment time-point (week 2 vs. week 20) and dose (low dose vs. high dose) for signal power extracted from the centers of the clusters identified in 1.2.3 (theta cluster, frequency range [3 bins]: ~ 3.5–4.5 Hz, electrodes: F3, Fz, F4, T7, T8, P7, P8, O1, O2; beta cluster, frequency range [3 bins]: ~ 19–22.5 Hz, electrodes: Fz, Cz). The top plots indicate the electrodes used for each extraction of signal power. The numbers at the base of the bars indicate the number of participants entering the analyses
For further characterization of the theta- and beta-band effects we extracted signal power from the “centers” of these clusters as pharmacodynamics parameter (Fig. 3B, C): power change from baseline (mean ± sem) and effect size for theta: 9.2±1.46%, d’ = 0.94; and beta: − 13.4±1.92%, d’ = − 1.04. These values are subject to a positive selection bias and should be considered as upper bounds.
There was no difference between the low or high dose (theta: p = 0.27, beta: p = 0.99; Fig. 3B, C). The EEG effects appeared weaker for week 20 compared to week 2. The decline was significant for the theta-band (p = 0.041, uncorrected for multiple testing) but not for the beta band (p = 0.27).
Neither the theta-band nor the beta-band EEG pharmacodynamic effects correlated with exposure (theta: rho = 0.217, p = 0.1; beta: rho = − 0.168, p = 0.16; n = 37). Numerically, the correlations were in the expected direction (positive for theta power, negative for beta) but lacked significance.
Pharmacokinetics
Comparable trough exposures were observed for the high dose between adults and adolescents aged 14–17 years (Additional file 8, Table 1). The low dose in adolescents aged 12–17 and the high dose in 12–13-year-olds resulted in lower exposures than adults. Overall, comparable average trough exposures were observed between adults and all adolescents (12–17 years) for the age-adjusted high doses, while differences were noted for the age-adjusted low doses, which resulted in slightly lower exposures in adolescents. Overall, the measured trough concentrations remained stable (Additional file 8, Table 3) and adherence to study medication was high throughout the study.
Predicted receptor occupancy
The low and high doses provided high predicted receptor occupancies of 83% and 92%, respectively, in the overall population (Additional file 8, Table 2), indicating a lack of separation of the two selected doses. At the high dose, the average predicted receptor occupancy at trough was comparable between adolescents (92%) and adults (93%). At the low dose, lower receptor occupancy was noted in adolescents (77%) compared to adults (87%) (Additional file 8, Table 1). There were no relevant differences in exposure or receptor occupancy between participants with and without above-threshold improvement (data not shown).
Safety
The frequency and nature of AEs were similarly distributed among placebo and basmisanil-treated participants (Table 3). There were no treatment-emergent epileptiform abnormalities noted during EEG monitoring in any participant.
Table 3.
Adverse events by treatment group
| Adverse events (in more than 5% of participants) | Placebo | 120 mg (80 mg) | 240 mg (160 mg) | |||
| n | % | n | % | n | % | |
| Infections and infestations | 32 | 55.2 | 24 | 43.6 | 24 | 42.1 |
| Gastrointestinal disorders | 18 | 31.0 | 12 | 21.8 | 15 | 26.3 |
| Nervous system disorders | 14 | 24.1 | 13 | 23.6 | 13 | 22.8 |
| Investigations | 12 | 20.7 | 5 | 9.1 | 6 | 10.5 |
| Skin and subcutaneous tissue disorders | 8 | 13.8 | 6 | 10.9 | 6 | 10.5 |
| Psychiatric disorders | 5 | 8.6 | 6 | 10.9 | 8 | 14.0 |
| General disorders and administration site conditions | 5 | 8.6 | 7 | 12.7 | 6 | 10.5 |
| Respiratory, thoracic, and mediastinal disorders | 4 | 6.9 | 7 | 12.7 | 2 | 3.5 |
| Musculoskeletal and connective tissue disorders | 2 | 3.4 | 5 | 9.1 | 5 | 8.8 |
| Eye disorders | 4 | 6.9 | 3 | 5.5 | 1 | 1.8 |
| Injury, poisoning and procedural complications | 0 | 0 | 4 | 7.3 | 3 | 5.3 |
| Serious adverse events | n | n | n | |||
| Suicidal ideation | 1 | 1 | ||||
| Altered state of consciousness | 1 | |||||
| Skin laceration | 1 | |||||
| Salmonellosis | 1 | |||||
Five serious AEs, reported in five participants, were considered not related to treatment (Table 3), and one event (altered state of consciousness) led to study withdrawal. In addition, non-serious AEs in three participants resulted in study withdrawal. Overall, the number of participants withdrawn from treatment due to AEs was low and did not point to a particular AE pattern (high-dose group [n = 3]: combination of “headache, nausea, vomiting” with treatment stop on study day 113; “sleep apnea syndrome” with treatment stop on day 45; and “nightmares” with treatment stop on day 98; low dose group [n = 1]: “altered state of consciousness” with treatment stop on day 60; placebo group: no subject withdrawn due to AE).
Vital sign monitoring did not reveal changes in heart rate and blood pressure (Additional file 9). QTcF analyses in ECG monitoring did not reveal an alert of relevant QTc prolongation (Additional file 10). Monitoring of co-occurring symptoms did not reveal notable changes as summarized in Additional file 11 and there was no signal on suicidality risk associated with basmisanil treatment.
Discussion
Clematis was the first phase II trial performed in the DS population with a compound specifically designed to address excessive inhibition in limbic brain areas, hypothesized to contribute to the intellectual disability associated with DS [14, 15]. Overall, the findings of this study indicate that 6 months of treatment with the GABAA-α5 receptor NAM basmisanil was safe and well-tolerated, but did not reveal any effects of treatment on primary and secondary measures of efficacy, suggesting it did not improve cognition or functioning in adults and adolescents with DS. The observed basmisanil exposures were stable and marginally lower in the adolescents. Although the exposures remained within the predicted range from the population PK model, both doses resulted in high average predicted receptor occupancy which did not clearly separate (low dose: 83% and high dose: 92%) and could thus be expected to be efficacious. The lack of differentiation between doses limits meaningful interpretations of dose-dependent treatment effects from both safety and efficacy perspectives in the overall population. In adolescents, there was a higher proportion of participants showing improvement on the primary endpoint after 3 months of treatment (nominal p-value < 0.05 at the high dose). This effect was not maintained after 6 months of treatment despite stable exposures and was not reflected in any of the secondary measures. The absence of differences in exposure-response relationships between participants with and without above-threshold improvements, across ages and doses (data not shown), corroborate a true lack of effect of basmisanil.
The primary endpoint was designed to capture potential improvements in intellectual functioning from multiple perspectives by combining direct measures of cognition (RBANS memory tasks), clinician ratings (DS-CGI-I), and caregiver-reported measure (VABS-II). These measures were selected based on their suitability for the population, reliability, stability over time, and feasibility of implementation, as previously determined in a 6-month observational study with a comparable study design and population [22, 23]. In the current study, the stability over time of most measures was not replicated; improvements were observed across placebo and treatment arms over 6 months on multiple variables including the VABS-II composite scores, DS-CGI-I, BRIEF-P, and PedsQL. The changes observed in this study, as compared to low natural improvement seen on the same measures in our previous non-interventional trial, may in part be attributed to the great anticipation of a potential therapeutic option among the DS community involved in this first large international clinical trial. The impact of treatment expectancy in clinical trials in pediatric neurodevelopmental disorders has been widely described, especially for caregiver-reported scales, and remains a key challenge for drug development [24, 25].
These changes were more pronounced in the adolescent population and are in line with published placebo response rates of 10–30% described in DS [26] and other neurodevelopmental conditions with intellectual disability, such as Fragile X syndrome or autism spectrum disorder [27]. In order to better control such effects, other researchers included regular cognitive training in both treated and placebo cohorts, with a run-in period, during a 6-month clinical trial in adults with DS [28].
The threshold for improvement on the primary composite endpoint combined improvements on RBANS memory tasks and global functioning on either the VABS-II or the DS-CGI-I. Because the DS-CGI-I anchors were mainly derived from the VABS-II domains, DS-CGI-I scores may not be independent of the caregiver perception captured by the VABS-II. The increases over time in VABS-II scores observed across groups may reflect treatment expectancy effects and directly (or indirectly via the DS-CGI-I) drive improvements on the primary endpoint. The composite endpoint is a multidimensional measure which increases the complexity of the analysis and interpretation and requires consistent effects to reach statistical significance. The choice of a composite endpoint, although a high bar objective, is unlikely to have masked effects as no beneficial treatment effects were detected on any of the individual components of the primary endpoint. Consistent with these findings, the analysis of secondary outcome measures did not show any beneficial effects of basmisanil over placebo after 6 months of treatment. Importantly, scores from the direct performance-based evaluations of cognition assessing memory (RBANS) and language (CELF), thought to be less sensitive to treatment expectancy bias, remained generally stable across age and treatment groups over the 6-month study duration, with the exception of the RBANS learning task. The small improvements observed in RBANS learning are in line with previous data from our observational study [22] and possibly reflect procedural learning due to repeated administration. Overall, this suggests that improvements in the placebo group are unlikely to have generally obscured treatment effects in the study. Of note, almost all participants were able to reach the second level of the CELF-4 and no floor effect was observed, suggesting that the CELF-4 word classes task can be used in future clinical trials with adults and adolescents with DS.
Exploratory quantitative analysis of EEGs recorded in adolescents was performed to test for effects on brain function. The absence of an alpha peak in the baseline EEG power spectrum is in line with previous findings in adults with DS reporting a shift to lower frequencies [29–31]. The basmisanil-induced pharmacodynamic effects, i.e., an increase in theta power (~4 Hz), and a decrease in beta power (~20 Hz) confirm the spectral signature of basmisanil that we have found previously in healthy volunteers [19] and demonstrate brain circuit engagement. In particular, EEG power in the beta frequency range has been linked to GABAA function through pharmacology [32, 33], in rare genetic conditions involving CNVs [34, 35] and SNPs in GABAA receptor genes [36, 37], and in modeling studies [38, 39]. Correlation analyses with individual basmisanil concentration did not reveal a significant dose dependence but were in the expected direction. The lack of a significance PK-PD relationship may relate to the overall high receptor occupancy (> 77% for all dose x age groups) where little dynamic range of the EEG PD effect may be expected, and to a limited sample size (Additional file 8: Table 1). In sum, the observed changes in the EEG in response to basmisanil can be considered evidence of functional target engagement.
While basmisanil exposure remained stable, the EEG effect in lower frequencies was weaker at week 20 compared to week 2, while remaining significantly higher than at baseline. The decrease in EEG power at lower frequencies may indicate compensatory or adaptive neuronal mechanisms that could result in tolerance. Tolerance is a well-described phenomenon for non-selective GABAA receptor positive allosteric modulators after long-term use [40]. However, it is important to point out that the beta-band EEG effect, with an established link to GABAA function did not significantly decline over time and no withdrawal effects were observed when the administration of basmisanil was stopped. Finally, there is no preclinical evidence suggesting that α5 subtype-selective compounds, such as basmisanil, lead to tolerance [41]. Tolerance to the effects of basmisanil is unlikely to underlie the lack of efficacy in this study.
Some study limitations should be noted. The detection of significant treatment effects of basmisanil may have been limited by the small sample size. Indeed a potential selection bias cannot be controlled for, albeit random treatment group assignment. Cognitive and behavioral measurements were not assessed during the first month of treatment; we are therefore unable to interpret potential improvements in relation to the early pharmacodynamic EEG changes observed. This would have also been helpful to interpret the trend observed after 3 months in adolescents, as well as the trends observed after 5 weeks of treatment on the RBANS tasks in a small exploratory phase IB trial in young adults with DS (BP25543; Additional file 1).
The detection of treatment effects of basmisanil may also have been hampered by the timing of the pharmacological intervention. Key brain development processes such as synaptogenesis and pruning [42] occur in early development before the age of 12 years. Modulation of GABAA-α5 receptors may therefore be more impactful during earlier stages of neural development, before long-term consequences of and adaptations to altered GABAergic inhibition have shaped brain function. Although our study did not demonstrate any evidence of age-dependent effects, a potential beneficial effect of basmisanil prior to the adolescent period cannot be fully excluded.
It is also conceivable that selective modulation of the GABAA-α5 receptor subtype or the maximal inhibitory effect of basmisanil on chloride channel current (~ 40%) [19] may not be sufficient to restore the excitatory/inhibitory imbalance hypothesized to underlie the cognitive profile of DS [15]. Alternatively, the “excitation/inhibition imbalance” working hypothesis may be invalid. Indeed, it relies solely on findings from the Ts65Dn mouse model of DS which has limitations with regards to predictive and translational relevance [43], and there is currently no clinical evidence of enhanced inhibition in individuals with DS. Since human chromosome 21 has approximately 200–300 genes, other pathways including metabolic pathways are likely involved [44]. Future trials may consider targeting more than one pathway at a time to maximize therapeutic potential.
Conclusions
Here we have described some of the challenges, and potential strategies to address them, from the perspective of investigators experienced with research in this population [45]. The low drop-out rate of around 9% illustrates the high dedication and motivation from the study participants and their caregivers. Standardization of scale administrations combined with high-quality and consistent training among the different sites and countries allowed us to achieve overall good quality of the data collected with moderate-to-low variability, consistent with what has been previously reported for DS or other conditions with intellectual disability. Independent of the negative outcome of the Clematis study, the learnings on outcome measures and feasibility of conducting international trials in DS, advocacy group relationships, and health authorities’ interactions, provide key information to support future clinical trials in DS and other populations with intellectual disabilities.
Supplementary Information
Additional file 1. Previous clinical information. Summary of PET and MAD data from Study BP25611 and Study BP25543.
Additional file 3. Primary and secondary assessment scales. Provides more detailed information on the scales, including the DS-CGI-I.
Additional file 4. EEG supplementary methods. Provides detailed methodology.
Additional file 5. Percent of Participants with Relevant Improvements for each Assessment of the Composite Endpoint by Age Group at 3 and 6 months. A table showing percent of participants with above-threshold improvements for each assessment by age group and time point.
Additional file 6. VABS-II: Change from baseline at 6 months. Figure showing VABS-II data: composite and individual scores for socialization, communication, and daily living skills.
Additional file 7. Quantitative EEG. Figures showing further analysis of EEG to support the EEG data in the main manuscript.
Additional file 8 Estimated Receptor Occupancy and Pharmacokinetics: Table 1: Estimated Receptor Occupancy from Geomean Trough Basmisanil Plasma Concentrations (ng/mL) by Age Group and Dose. Table showing trough concentrations and estimated receptor occupancy by age group and dose. Table 2: Geomean Trough Basmisanil Plasma Concentration (ng/mL) and Receptor Occupancy by dose. Table showing trough concentrations and estimated receptor occupancy by dose. Table 3: Geomean Trough Basmisanil Plasma Concentrations (ng/mL) in Adolescents and Adults by visit and dose. Table showing trough concentrations by age group, dose, and timepoint.
Additional file 9. Diastolic and systolic blood pressure. Table summarizing the change from baseline data at 2 weeks, 3 months and 6 months.
Additional file 10. ECG QTcF changes from baseline. Table summarizing the change from baseline at 2 weeks, 3 months and 6 months.
Additional file 11. Co-morbid Symptoms: Change from Baseline at 6months. Table summarizing Conner’s, ADAMS and CSHQ data.
Additional file 12. Change from baseline score for each assessment by age group and timepoint. Table summarizing change from baseline score by age group and timepoint.
Acknowledgements
We would like to thank all participants, families, caregivers, and principal investigators and their teams who contributed to the Clematis study. The EEG recording and review were performed using Biotrial EEG core lab platform. The authors thank Theresa M. Ballard PhD for providing medical writing support.
Clematis Study Group: Rafael De La Torre Fornell, IMIM, Human Pharmacology and Clinical Neurosciences, Spain; Paul Glue, University of Otago, New Zealand; Julie Hoover-Fong, John Hopkins University, USA; Sonja Uhlmann, Fundación Síndrome de Down, Spain; Jorge Malagón Valdez, Clínica Para La Atención Del Neurodesarrollo Aguascalientes, Mexico; Andrew Marshall, Wellington Hospital Research Office, New Zealand; Federico Martinón-Torres, Lorenzo Redondo-Collazo, Carmen Rodriguez-Tenreiro, Complejo Hospitalario Universitario de Santiago, Spain; Valeria Marquez Chin, Hospital Médica TEC 100, Mexico; Adriana G Michel Reynoso, Hospital Dr. Angel Leaño, Mexico; Ed A Mitchell, Rebecca F Slykerman, Trecia Wouldes, Sarah Loveday, Auckland Clinical Studies, New Zealand; Fernando Moldenhauer, Hospital Universitario de la Princesa, Spain; Ramon Novell, Biomedical Research Institute (IdIBGi), Spain; Cesar Ochoa, Rush University Medical Center, USA; Michael S Rafii, Alzheimer’s Therapeutic Research Institute, Keck School of Medicine of University of Southern California, USA; Anne-Sophie Rebillat, Institut Jérôme Lejeune, France; Damien Sanlaville, Groupement Hospitalier Est-Hopital Femme Mere Enfant, France; Pierre Sarda, CHU de Montpellier Hopital Arnaud de Villeneuve, France; Rohit Shankar, Cornwall Partnership NHS Foundation Trust, United Kingdom; Margaret Pulsifer, Casey L Evans, Alexandra M Silva, Mary Ellen McDonough, Massachusetts General Hospital and Harvard Medical School, USA; Maria Stanley, Lindsay M McCary, University of Wisconsin Madison, USA; Stefano Vicari, Ospedale Pediatrico Bambin Gesù Roma, Italy; William Wilcox, Emory University School of Medicine, USA; Giuseppe Zampino, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy; Alessandro Zuddas, University of Cagliari, Cagliari, Italy.
Abbreviations
- ADAMS
Anxiety, Depression and Mood Abnormalities
- ADHD
Attention-deficit/hyperactivity disorder
- AE
Adverse event
- BID
Twice daily
- BRIEF-P
Behavior Rating Inventory of Executive Function Preschool
- CELF-4
Clinical Evaluation of Language Fundamentals-version 4
- CELF-P
Clinical Evaluation of Language Fundamentals Preschool-2
- C-SSRS
Columbia-Suicide Severity Rating Scale
- DS
Down syndrome
- DS-CGI-I
Down syndrome-specific Clinical Global Impression-Improvement
- ECG
Electrocardiogram
- EEG
Electroencephalogram
- GABA
Gamma-aminobutyric acid
- NAM
Negative allosteric modulator
- PedsQL
Pediatric Quality of Life Inventory
- PET
Positron emission tomography
- PK
Pharmacokinetics
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- VABS-II
Vineland Adaptive Behavior Scales-II
Authors’ contributions
CG, JFH, MD, MCH, OK, SLC, JN, SP, LS, JV, CW, PF, PSK, XLD contributed to the conception and design of the Clematis trial. CG, OK, JN, LS, JV, PF, XLD were responsible for the primary and secondary endpoint analyses and interpretation. JFH was responsible for the EEG analysis and interpretation. CW was responsible for the safety analysis and interpretation. MD and SLC were responsible for the PK analysis and interpretation. PSK, BGS, JLC contributed to the acquisition and interpretation of primary and secondary outcome data. All authors were involved in discussions of the trial outcome and interpretation of the Clematis trial data. CG, MCH, JFH, XLD wrote the main manuscript. All authors contributed to revisions of the manuscript and have approved the final version.
Funding
This study was funded by F.Hoffmann-La Roche AG. F.Hoffmann-La Roche AG was involved in the design and conduct of the study and provided logistical support during the trial.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author [CG]. The data are not publicly available due to them containing information that could compromise research participant privacy/consent.
Declarations
Ethics approval and consent to participate
This study was approved by local ethics committees and was conducted in accordance with the principles of the “Declaration of Helsinki” and Good Clinical Practice.
Consent for publication
Not applicable
Competing interests
At the time of the study, P Fontoura, C Goeldner, MC Hernandez, JF Hipp, O Khwaja, X Liogier d’Ardhuy, J Noeldeke, S Pellicer, L Squassante, C Wandel were employees of F.Hoffmann-La Roche AG Switzerland; M Derks and S Lennon-Chrimes were employees of Roche Products Ltd. UK; J Visootsak was an employee of Roche New York. All employees (former and current) may be eligible for stock and stock options. P S Kishnani has no disclosures for Down syndrome-related research. J Lirio Casero has no disclosures. B G Skotko occasionally consults on the topic of Down syndrome through the Gerson Lehrman Group. He receives remuneration from Down syndrome non-profit organizations for speaking engagements and associated travel expenses. Dr. Skotko receives annual royalties from Woodbine House, Inc., for the publication of his book, Fasten Your Seatbelt: A Crash Course on Down Syndrome for Brothers and Sisters. Within the past 2 years, he has also received research funding from AC Immune and LuMind Research Down Syndrome Foundation to conduct clinical trials for people with Down syndrome. Dr. Skotko is occasionally asked to serve as an expert witness for legal cases where Down syndrome is discussed. Dr. Skotko serves in a non-paid capacity on the Honorary Board of Directors for the Massachusetts Down Syndrome Congress and the Professional Advisory Committee for the National Center for Prenatal and Postnatal Down Syndrome Resources. Dr. Skotko has a sister with Down syndrome.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Celia Goeldner, Email: celia.goeldner@roche.com.
Clematis Study Group:
Rafael De La Torre Fornell, Paul Glue, Julie Hoover-Fong, Sonja Uhlmann, Jorge Malagón Valdez, Andrew Marshall, Federico Martinón-Torres, Lorenzo Redondo-Collazo, Carmen Rodriguez-Tenreiro, Valeria Marquez Chin, Adriana G. Michel Reynoso, Ed A. Mitchell, Rebecca F. Slykerman, Trecia Wouldes, Sarah Loveday, Fernando Moldenhauer, Ramon Novell, Cesar Ochoa, Michael S. Rafii, Anne-Sophie Rebillat, Damien Sanlaville, Pierre Sarda, Rohit Shankar, Margaret Pulsifer, Casey L. Evans, Alexandra M. Silva, Mary Ellen McDonough, Maria Stanley, Lindsay M. McCary, Stefano Vicari, William Wilcox, Giuseppe Zampino, and Alessandro Zuddas
References
- 1.de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017;19(4):439–447. doi: 10.1038/gim.2016.127. [DOI] [PubMed] [Google Scholar]
- 2.de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in Europe. Eur J Hum Genet. 2021;29(3):402–10. [DOI] [PMC free article] [PubMed]
- 3.Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74(1):75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- 4.Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet. 2015;169(2):135–149. doi: 10.1002/ajmg.c.31439. [DOI] [PubMed] [Google Scholar]
- 5.Golden JA, Hyman BT. Development of the superior temporal neocortex is anomalous in trisomy 21. J Neuropathol Exp Neurol. 1994;53(5):513–520. doi: 10.1097/00005072-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Sidor B, Wisniewski KE, Shepard TH, Sersen EA. Brain growth in Down syndrome subjects 15 to 22 weeks of gestational age and birth to 60 months. Clin Neuropathol. 1990;9(4):181–190. [PubMed] [Google Scholar]
- 7.Weitzdoerfer R, Dierssen M, Fountoulakis M, Lubec G. Fetal life in Down syndrome starts with normal neuronal density but impaired dendritic spines and synaptosomal structure. J Neural Transm Suppl. 2001;61:59–70. doi: 10.1007/978-3-7091-6262-0_5. [DOI] [PubMed] [Google Scholar]
- 8.Baxter LL, Moran TH, Richtsmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9(2):195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti L, Galdzicki Z, Haydar TF. Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J Neurosci. 2007;27(43):11483–11495. doi: 10.1523/JNEUROSCI.3406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzi HA, Reeves RH. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 2006;1104(1):153–159. doi: 10.1016/j.brainres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Belichenko PV, Kleschevnikov AM, Masliah E, Wu C, Takimoto-Kimura R, Salehi A, et al. Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2009;512(4):453–466. doi: 10.1002/cne.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Cremades D, Hernandez S, Blasco-Ibanez JM, Crespo C, Nacher J, Varea E. Alteration of inhibitory circuits in the somatosensory cortex of Ts65Dn mice, a model for Down's syndrome. J Neural Transm (Vienna). 2010;117(4):445–455. doi: 10.1007/s00702-010-0376-9. [DOI] [PubMed] [Google Scholar]
- 13.Kurt MA, Davies DC, Kidd M, Dierssen M, Florez J. Synaptic deficit in the temporal cortex of partial trisomy 16 (Ts65Dn) mice. Brain Res. 2000;858(1):191–197. doi: 10.1016/s0006-8993(00)01984-3. [DOI] [PubMed] [Google Scholar]
- 14.Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, et al. Specific targeting of the GABA-A receptor alpha5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. J Psychopharmacol. 2011;25(8):1030–1042. doi: 10.1177/0269881111405366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Cue C, Martinez P, Rueda N, Vidal R, Garcia S, Vidal V, et al. Reducing GABAA alpha5 receptor-mediated inhibition rescues functional and neuromorphological deficits in a mouse model of down syndrome. J Neurosci. 2013;33(9):3953–3966. doi: 10.1523/JNEUROSCI.1203-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasser R, Hernandez MC, Thomas AW. Use of selective GABA A alpha 5 negative allosteric modulators for the treatment of central nervous system conditions. US2012115839(A1) 2012. p. 2012. [Google Scholar]
- 17.Little HJ, Nutt DJ, Taylor SC. Acute and chronic effects of the benzodiazepine receptor ligand FG 7142: proconvulsant properties and kindling. Br J Pharmacol. 1984;83(4):951–958. doi: 10.1111/j.1476-5381.1984.tb16536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2(8341):98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- 19.Hipp JF, Knoflach F, Comley R, Ballard TM, Honer M, Trube G, et al. Basmisanil, a highly selective GABAA-alpha5 negative allosteric modulator: preclinical pharmacology and demonstration of functional target engagement in man. Sci Rep. 2021;11(1):7700. doi: 10.1038/s41598-021-87307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson . Clinical Evaluation of Language Fundamentals - Preschool-2 (CELF-Preschool-2) 2004. [Google Scholar]
- 21.Glenn S, Cunningham C. Performance of young people with Down syndrome on the Leiter-R and British picture vocabulary scales. J Intellect Disabil Res. 2005;49(Pt 4):239–244. doi: 10.1111/j.1365-2788.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- 22.Liogier d’Ardhuy X, Edgin JO, Bouis C, de Sola S, Goeldner C, Kishnani P, et al. Assessment of cognitive scales to examine memory, executive function and language in individuals with Down syndrome: implications of a 6-month observational study. Front. Behav Neurosci. 2015;9:300. doi: 10.3389/fnbeh.2015.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiridigliozzi GA, Goeldner C, Edgin J, Hart SJ, Noeldeke J, Squassante L, et al. Adaptive behavior in adolescents and adults with Down syndrome: results from a 6-month longitudinal study. Am J Med Genet A. 2019;179(1):85–93. doi: 10.1002/ajmg.a.60685. [DOI] [PubMed] [Google Scholar]
- 24.Masi A, Lampit A, Glozier N, Hickie IB, Guastella AJ. Predictors of placebo response in pharmacological and dietary supplement treatment trials in pediatric autism spectrum disorder: a meta-analysis. Transl Psychiatry. 2015;5:e640. doi: 10.1038/tp.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeste SS, Geschwind DH. Clinical trials for neurodevelopmental disorders: at a therapeutic frontier. Sci Transl Med. 2016;8(321):321fs1. doi: 10.1126/scitranslmed.aad9874. [DOI] [PubMed] [Google Scholar]
- 26.Kishnani PS, Sommer BR, Handen BL, Seltzer B, Capone GT, Spiridigliozzi GA, et al. The efficacy, safety, and tolerability of donepezil for the treatment of young adults with Down syndrome. Am J Med Genet A. 2009;149A(8):1641–1654. doi: 10.1002/ajmg.a.32953. [DOI] [PubMed] [Google Scholar]
- 27.Erickson CA, Davenport MH, Schaefer TL, Wink LK, Pedapati EV, Sweeney JA, et al. Fragile X targeted pharmacotherapy: lessons learned and future directions. J Neurodev Disord. 2017;9:7. doi: 10.1186/s11689-017-9186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Torre R, de Sola S, Hernandez G, Farre M, Pujol J, Rodriguez J, et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Downʼs syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(8):801–810. doi: 10.1016/S1474-4422(16)30034-5. [DOI] [PubMed] [Google Scholar]
- 29.Babiloni C, Albertini G, Onorati P, Vecchio F, Buffo P, Sarà M, et al. Inter-hemispheric functional coupling of eyes-closed resting EEG rhythms in adolescents with Down syndrome. Clin Neurophysiol. 2009;120(9):1619–1627. doi: 10.1016/j.clinph.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Babiloni C, Albertini G, Onorati P, Muratori C, Buffo P, Condoluci C, et al. Cortical sources of EEG rhythms are abnormal in down syndrome. Clin Neurophysiol. 2010;121(8):1205–1212. doi: 10.1016/j.clinph.2010.02.155. [DOI] [PubMed] [Google Scholar]
- 31.Velikova S, Magnani G, Arcari C, Falautano M, Franceschi M, Comi G, et al. Cognitive impairment and EEG background activity in adults with Downʼs syndrome: a topographic study. Hum Brain Mapp. 2011;32(5):716–729. doi: 10.1002/hbm.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman H, Greenblatt DJ, Peters GR, Metzler CM, Charlton MD, Harmatz JS, et al. Pharmacokinetics and pharmacodynamics of oral diazepam: effect of dose, plasma concentration, and time. Clin Pharmacol Ther. 1992;52(2):139–150. doi: 10.1038/clpt.1992.123. [DOI] [PubMed] [Google Scholar]
- 33.Malizia AL, Gunn RN, Wilson SJ, Waters SH, Bloomfield PM, Cunningham VJ, et al. Benzodiazepine site pharmacokinetic/pharmacodynamic quantification in man: direct measurement of drug occupancy and effects on the human brain in vivo. Neuropharmacology. 1996;35(9-10):1483–1491. doi: 10.1016/s0028-3908(96)00072-x. [DOI] [PubMed] [Google Scholar]
- 34.Frohlich J, Miller MT, Bird LM, Garces P, Purtell H, Hoener MC, et al. Electrophysiological phenotype in angelman syndrome differs between genotypes. Biol Psychiatry. 2019;85(9):752–759. doi: 10.1016/j.biopsych.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frohlich J, Reiter LT, Saravanapandian V, DiStefano C, Huberty S, Hyde C, et al. Mechanisms underlying the EEG biomarker in Dup15q syndrome. Mol Autism. 2019;10:29. doi: 10.1186/s13229-019-0280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99(6):3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smit DJA, Wright MJ, Meyers JL, Martin NG, Ho YYW, Malone SM, et al. Genome-wide association analysis links multiple psychiatric liability genes to oscillatory brain activity. Hum Brain Mapp. 2018;39(11):4183–4195. doi: 10.1002/hbm.24238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38(3):315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 39.Traub RDWM, Jefferys JGR. Fast oscillations in cortical circuits. Cambridge: The MIT Press; 1999.
- 40.Gravielle MC. Activation-induced regulation of GABAA receptors: Is there a link with the molecular basis of benzodiazepine tolerance? Pharmacol Res. 2016;109:92–100. doi: 10.1016/j.phrs.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Vinkers CH, Olivier B. Mechanisms underlying tolerance after long-term benzodiazepine use: a future for subtype-selective GABA(A) receptor modulators? Adv Pharmacol Sci. 2012;2012:416864. doi: 10.1155/2012/416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med. 2016;22(11):1229–1238. doi: 10.1038/nm.4225. [DOI] [PubMed] [Google Scholar]
- 43.Sturgeon X, Gardiner KJ. Transcript catalogs of human chromosome 21 and orthologous chimpanzee and mouse regions. Mamm Genome. 2011;22(5-6):261–271. doi: 10.1007/s00335-011-9321-y. [DOI] [PubMed] [Google Scholar]
- 44.Dierssen M, Fructuoso M, Martinez de Lagran M, Perluigi M, Barone E. Down syndrome is a metabolic disease: altered insulin signaling mediates peripheral and brain dysfunctions. Front Neurosci. 2020;14:670. doi: 10.3389/fnins.2020.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hart SJ, Visootsak J, Tamburri P, Phuong P, Baumer N, Hernandez MC, et al. Pharmacological interventions to improve cognition and adaptive functioning in Down syndrome: Strides to date. Am J Med Genet A. 2017;173(11):3029–3041. doi: 10.1002/ajmg.a.38465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Previous clinical information. Summary of PET and MAD data from Study BP25611 and Study BP25543.
Additional file 3. Primary and secondary assessment scales. Provides more detailed information on the scales, including the DS-CGI-I.
Additional file 4. EEG supplementary methods. Provides detailed methodology.
Additional file 5. Percent of Participants with Relevant Improvements for each Assessment of the Composite Endpoint by Age Group at 3 and 6 months. A table showing percent of participants with above-threshold improvements for each assessment by age group and time point.
Additional file 6. VABS-II: Change from baseline at 6 months. Figure showing VABS-II data: composite and individual scores for socialization, communication, and daily living skills.
Additional file 7. Quantitative EEG. Figures showing further analysis of EEG to support the EEG data in the main manuscript.
Additional file 8 Estimated Receptor Occupancy and Pharmacokinetics: Table 1: Estimated Receptor Occupancy from Geomean Trough Basmisanil Plasma Concentrations (ng/mL) by Age Group and Dose. Table showing trough concentrations and estimated receptor occupancy by age group and dose. Table 2: Geomean Trough Basmisanil Plasma Concentration (ng/mL) and Receptor Occupancy by dose. Table showing trough concentrations and estimated receptor occupancy by dose. Table 3: Geomean Trough Basmisanil Plasma Concentrations (ng/mL) in Adolescents and Adults by visit and dose. Table showing trough concentrations by age group, dose, and timepoint.
Additional file 9. Diastolic and systolic blood pressure. Table summarizing the change from baseline data at 2 weeks, 3 months and 6 months.
Additional file 10. ECG QTcF changes from baseline. Table summarizing the change from baseline at 2 weeks, 3 months and 6 months.
Additional file 11. Co-morbid Symptoms: Change from Baseline at 6months. Table summarizing Conner’s, ADAMS and CSHQ data.
Additional file 12. Change from baseline score for each assessment by age group and timepoint. Table summarizing change from baseline score by age group and timepoint.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author [CG]. The data are not publicly available due to them containing information that could compromise research participant privacy/consent.