ABSTRACT
A 72-year-old man with type II diabetes mellitus presented with sudden painless vision loss and an inferior visual field defect in his right eye. He had previously tested positive for COVID-19 disease with the symptoms starting 13 days before the onset of vision loss. His decimal visual acuity, 55 days after the onset of visual symptoms, was 0.3 and this decreased over the following week to counting fingers. 24–2 visual field analysis revealed an inferior altitudinal defect. Dilated fundus examination revealed mild optic disc swelling in the right eye. The left eye was normal. He was diagnosed with non-artertic anterior ischaemic optic neuropathy (NAION). On spectral domain optical coherence tomography there was retinal thinning in the supero-temporal foveal area. Macular ganglion cell layer – inner plexiform retinal layer complex analysis showed progressive atrophy that developed from the supero-temporal to the infero-nasal fovea. COVID-19 infection may lead to NAION.
KEYWORDS: COVID-19, non-arteritic anterior ischaemic optic neuropathy, OCT, progressive macular ganglion cell loss
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has led to a global pandemic with more than 100 million confirmed cases and 2 million deaths to date.1,2 While the main manifestations are in the respiratory tract, multi-system organ involvement has also been reported.2 SARS-CoV-2 causes a pro-thrombotic state due to a mechanism that has not yet been fully elucidated.3,4 There are a growing number of reports of ophthalmological complications of COVID-19 in the literature. Reported posterior segment manifestations include optic neuritis,5,6 cotton-wool spots and microhaemorrhages,4 central retinal artery occlusion,7 reduction in foveal vascular density,3 acute macular neuroretinopathy,8 retinal nerve fibre layer infarcts,9 and ocular neuropathy with panuveitis.10
Rho et al. reported the first case of non-arteritic anterior ischaemic optic neuropathy (NAION) following COVID-19 infection in a patient with diabetic retinopathy.11 NAION is caused by occlusion of the short posterior ciliary arteries resulting in partial or total infarction of the optic nerve head.6 Major risk factors include crowded optic discs, hypertension, diabetes mellitus, and hyperlipidaemia.11 The usual presentation is in the 6th-7th decades with sudden, painless vision loss and mostly inferior altitudinal visual field defects. Diffuse or sectorial hyperaemic optic disc swelling with a few peri-papillary splinter haemorrhages may be seen. The swelling gradually resolves and pallor develops 3–6 weeks after onset.12 The optic nerve damage is usually severe and irreversible, depending on the extent of the occluded arteries.6 The usual duration of mild COVID-19 symptoms is reported as two weeks.13 Here we report a diabetic patient who developed NAION on the 13th day after contracting COVID-19.
Case report
A 72-year-old male dentist presented with sudden, painless inferior visual field loss and blurred vision in his right eye (OD). He had had type II diabetes mellitus for 10 years that was well controlled with oral anti-diabetic medication (Metformin). His fasting blood glucose was 127 mg/dL and his HBA1c was 5.4%. He also had hypertension controlled with medication, was on aspirin and also sulfasalazine for ulcerative colitis. He smoked 10 cigarettes per day.
He had had a positive polymerase chain reaction test for COVID 19. He developed flu-like symptoms 13 days before the onset of visual complaints. Although he did not have a cough, a computed tomography (CT) scan of the chest revealed hazy opacification. He commenced 200 mg favipiravir tablets, eight twice per day on the first day and continued with one tablet twice per day. Five days after onset his COVID-19 symptoms had resolved.
On presentation with his loss of vision the inferior visual field defect OD was confirmed by confrontation testing. A Neurology consultation, blood tests and imaging were performed on the same day. His erythrocyte sedimentation rate (ESR) was 10 mm/hr and his C-reactive protein (CRP) was 0.5 mg/L. Bilateral carotid Doppler ultrasonography revealed minimal intimal thickening. Cranial magnetic resonance imaging with contrast revealed a few subcortical ischaemic foci. A CT scan of the chest revealed atherosclerotic calcification of the aortic arch and bilateral subpleural thickening suggesting interstitial lung disease.
He was referred to our hospital and was seen 55 days after the onset of visual symptoms. His best corrected decimal visual acuity was 0.3 OD and 1.0 in the left eye (OS). A mild colour vision defect OD was detected using the Ishihara pseudoisochromatic plates. His intraocular pressure by Goldmann applanation tonometry was 19 mmHg OD and 20 mmHg OS. There was no apparent relative afferent pupillary defect on the right. A dilated fundus examination revealed mild optic disc swelling OD (Figure 1a). The left eye was normal. Spectral domain optical coherence tomography (SD-OCT: Heidelberg Engineering GmbH, Heidelberg, Germany) macular thickness analysis revealed retinal thinning of the supero-temporal fovea with a central thickness of 245 µm. SD-OCT (Cirrus HD-OCT 5000, Carl Zeiss Meditec Inc, Dublin, Ca, USA) retinal nerve fibre layer (RNFL) analysis revealed superior thickening due to oedema OD (Figure 2a). Macular ganglion cell – inner plexiform layer (GCIPL) complex analysis (by Cirrus HD-OCT) showed GCIPL loss of the superior fovea OD. The minimum GCIPL thickness was 63 µm in that area (Figure 3a). The supero-temporal GCIPL thickness was 84 µm OS. Since the ESR and CRP were normal and the patient had no scalp tenderness or thickened temporal arteries, giant cell arteritis was excluded. A diagnosis of NAION was made. Three days later, his visual acuity dropped to 0.2 OD. The optic disc oedema had decreased. Fluorescein angiography revealed mild optic disc hyperfluorescence OD (Figure 1b).
Figure 1.
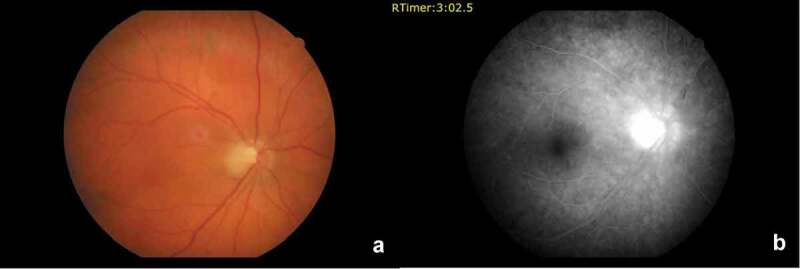
(a) Colour fundus photograph shows mild temporal optic disc oedema in the right eye. (b) Fluorescein angiogram revealing late optic disc hyper-fluorescence in the right eye.
Figure 2.
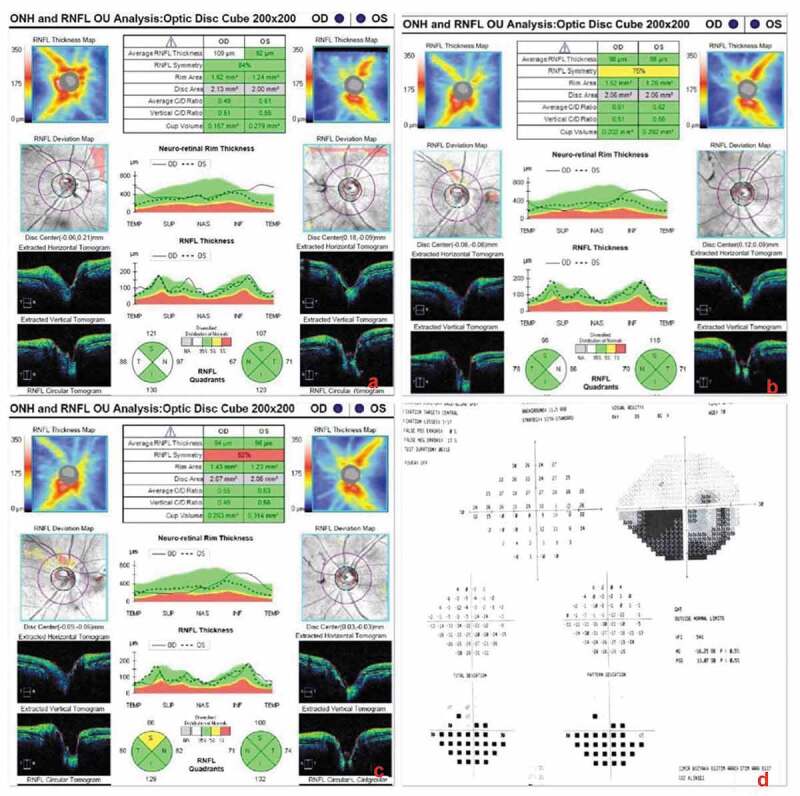
Retinal nerve fibre layer (RNFL) analysis by SD-OCT (Cirrus). (a) Initial RNFL thickening in the right eye due to optic disc oedema. (b,c) Second and third analyses one week apart revealing a superior RNFL defect in the right eye indicating sectorial optic atrophy. The RNFL in the left eye was normal. (d) 24–2 Humphrey visual field analysis of the right eye that is quite reliable with 1/17 fixation losses, 0% false positive errors and 13% false negative errors, showing an inferior altitudinal defect.
Figure 3.
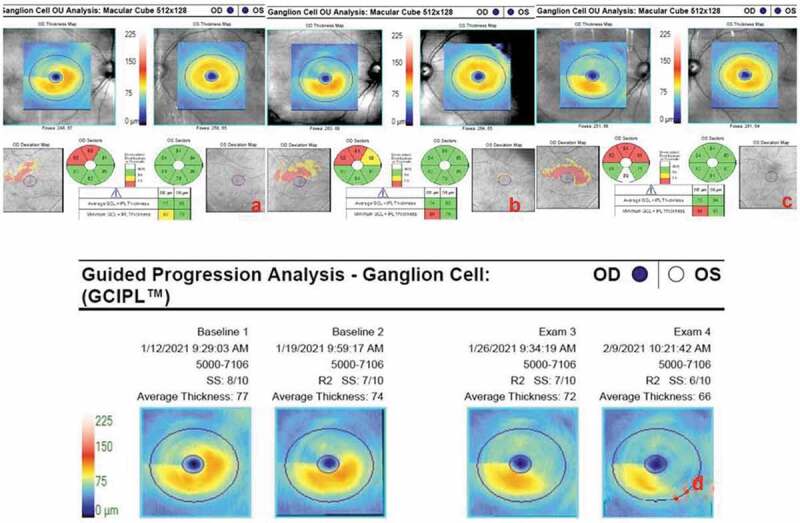
Serial macular ganglion cell – inner plexiform layer (GCIPL) complex analyses of both eyes: (a) On day 55 ischaemic GCIPL loss of the supero-temporal fovea with a minimum thickness of 65 µm. The left GCIPL thickness is normal at 84 µm. (b) The same analysis repeated on day 62 reveals further atrophy enlarging supero-nasally. (c) Eventual atrophy involving the infero-nasal part of the macula can be seen on day 69. (d) Automated progression analysis showing ongoing GCIPL atrophy through 3 weeks until day 76.
Four days after this, 62 days after the onset of visual symptoms, his vision decreased to counting fingers at 3.5 m OD. Central 24–2 visual field testing (Humphrey Field Analyser 745i, Carl Zeiss Meditec Inc, Dublin, Ca, USA) revealed an inferior altitudinal defect OD (Figure 2d). Temporal pallor of the optic disc was visible OD. The central macular thickness on SD-OCT was 216 µm OD reflecting 29 µm thinning since the previous week. The minimum GCIPL thickness was 54 µm OD indicating progressive loss (Figure 3b). Atrophy involving the infero-nasal fovea OD was detected on day 69 (Figure 3c). Automated progression analysis revealed progressive GCIPL atrophy OD through three weekly visits until day 76 (Figure 3d).
Discussion
Infection with SARS-CoV-2 leads to a severe inflammatory response with hypercoagulability and hypoxaemia.4,11 Disturbances in endothelial cells that are followed by intense thrombin generation and reduced fibrinolysis leads to the hypercoagulability. Thus, thrombosis within the arterioles supplying the optic nerve may be triggered.6 Rho et al. reported the first NAION case occurring two weeks after the onset of COVID-19 symptoms.11 Likewise, our patient had NAION on the 13th day of the disease course. As far as we know, our patient is the second reported NAION case associated with COVID-19. Our patient also had diabetes mellitus but no retinopathy. He had no plaques in the internal carotid and he was using aspirin. These findings suggest that COVID-19 may have had a causal relationship with our patient’s NAION. However, it could have been a coincidence, since our patient is a diabetic, hypertensive male aged 72 years, which are all well known risk factors for NAION.
Progressive NAION has been reported in a quarter of the cases. Possibly, swollen axons in one ischaemic part may lead to secondary infarction in another part of the optic disc.14 Likewise, our patient had progressive NAION with progressive GCIPL atrophy and corresponding vision loss. It is important to clarify the mechanisms by which neurovascular disorders occur in COVID-19 infection.6,15 SARS-CoV-2 adheres to angiotensin-converting enzyme (ACE-2) receptors on cell membranes to gain entry to the cells. ACE-2 receptors are found in multiple organs including the retina.4 It means that the virus can attack extra-pulmonary organs.6 Viral endothelitis, blood viscosity, complement mediated microangiopathy and silent hypoxia may play role in micro-circular damage.4,6,11,15 Overall, COVID-19 leads to rare and atypical occlusions in both veins and arteries such as renal, mesenteric and myocardial vessels.2 OCT angiography studies in patients recovered from COVID-19 have revealed defects in radial peripapillary2 and superficial to deep retinal capillary plexus.16 Occlusion of the short posterior ciliary arteries causes NAION. Macular GCIPL thinning is reported as an early indicator of axonal damage9 and shows better correlation with visual field defects than RNFL analysis.17 Macular thickness is also reduced in NAION patients.9 Our patient also had superior foveal thinning. SD-OCT studies have also demonstrated hyper-reflective lesions at the inner retinal layers and disruption of the ellipsoid zone in COVID-19 infected patients.18
It is not certain whether COVID-19 infection was causal or coincidental in the development of NAION in our patient. COVID-19 infection could be an additional risk factor for NAION in older patients with diabetes mellitus and hypertension. These patients should be observed closely for the development of neurovascular complications should they contract COVID-19. More work is required to ascertain whether prophylactic treatment with anti-platelet or anticoagulants may be beneficial in patients with active or recently resolved COVID-19 to prevent such complications.
References
- 1.World Health Organisation . WHO coronavirus disease COVID-19 dashboard. https://covid19.who.int. Accessed March 28, 2021.
- 2.Savastano A, Crincoli E, Savastano M, et al. Peripapillary retinal vascular involvement in early post-COVID-19 patients. J Clin Med. 2020;9(9):2895. doi: 10.3390/jcm9092895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Má Z, Banderas García S, Sánchez A. et al. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol. 2020;1–5. doi: 10.1136/bjophthalmol-2020-317953. [DOI] [PubMed] [Google Scholar]
- 4.Landecho MF, Yuste JR, Gándara E, et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med. 2021;289(1):116–120. doi: 10.1111/joim.13156. [DOI] [PubMed] [Google Scholar]
- 5.Sawalha K, Adeodokun S, Kamoga GR.. COVID-19-induced acute bilateral optic neuritis. J Invest Med High Impact Case Rep 2020;8. doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos A, Catharino S, Antônio M, et al. COVID-19 related optic neuritis: case report. J Clin Neurol Neurosci. 2017;8. doi: 10.21767/2171-6625.1000176. [DOI] [Google Scholar]
- 7.Montesel A, Bucolo C, Mouvet V, Moret E, Eandi CM. Case report: central retinal artery occlusion in a COVID-19 patient. Front Pharmacol. 2020;11:11. doi: 10.3389/fphar.2020.588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gascon P, Briantais A, Bertrand E, et al. Covid-19-associatedretinopathy: a case report. Ocul Immunol Inflamm. 2020;28(8):1293–1297.doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 9.Rebolleda G, Diez-Alvarez L, Casado A, et al. OCT: new perspectives in neuro-ophthalmology. Saudi J Ophthalmol. 2015;29(1):9–25.doi: 10.1016/j.sjopt.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.François J, Collery AS, Hayek G, et al. Coronavirus disease 2019-associated ocular neuropathy with panuveitis: a case report. JAMA Ophthalmol. 2020. doi: 10.1001/jamaophthalmol.2020.5695. [DOI] [PubMed] [Google Scholar]
- 11.Rho J, Dryden SC, McGuffey CD, Fowler BT, Fleming JA. Case of non-arteritic anterior ischemic optic neuropathy with COVID-19. Cureus. 2020;12(12). doi: 10.7759/cureus.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanski J, Bowling B. Kanski’s Clinical Ophthalmology. In:7th ed. London: Elsevier Saunder Limited; 2011: p:796. [Google Scholar]
- 13.Cevik M, Bamford CGG, Ho A. COVID-19 pandemic—a focused review for clinicians. Clin Microbiol Infect. 2020;26(7):842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto H, Hata M, Kashii S, et al. Analysis of retinal nerve fibre thickening in progressive and non-progressive non-arteritic anterior ischaemic optic neuropathy using optical coherence tomography. Neuro-Ophthalmology.2020;44(5):307–314.doi: 10.1080/01658107.2020.1755991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoli F, Veritti D, Danese C, et al. Ocular findings in COVID-19 patients: a review of direct manifestations and indirect effects on the eye. J Ophthalmol. 2020;2020. doi: 10.1155/2020/4827304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrishami M, Emamverdian Z, Shoeibi N. et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol. 2020;56:24–30. doi: 10.1016/j.jcjo.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee TH, Heo H, Park SW. Clinical usefulness of spectral-domain optical coherence tomography in glaucoma and NAION. Chonnam Med J. 2016;52(3):194. doi: 10.4068/cmj.2016.52.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zago Filho LA, Lima LH, Melo GB, Zett C, Farah ME. Vitritis and outer retinal abnormalities in a patient with COVID-19. Ocul Immunol Inflamm. 2020;28(8):1298–1300. doi: 10.1080/09273948.2020.1821898. [DOI] [PubMed] [Google Scholar]


