ABSTRACT
Upon discovery that the Boquila trifoliolata is capable of flexible leaf mimicry, the question of the mechanism behind this ability has been unanswered. Here, we demonstrate that plant vision possibly via plant-specific ocelli is a plausible hypothesis. A simple experiment by placing an artificial vine model above the living plants has shown that these will attempt to mimic the artificial leaves. The experiment has been carried out with multiple plants, and each plant has shown attempts at mimicry. It was observed that mimic leaves showed altered leaf areas, perimeters, lengths, and widths compared to non-mimic leaves. We have calculated four morphometrical features and observed that mimic leaves showed higher aspect ratio and lower rectangularity and form factor compared to non-mimic leaves. In addition, we have observed differences in the leaf venation patterns, with the mimic leaves having less dense vascular networks, thinner vascular strands, and lower numbers of free-ending veinlets.
KEYWORDS: Boquila trifoliolata, chameleon-like leaves, leaf mimicry, plant ocelli, plant vision, vascular network
Introduction
Seven years ago, Gianoli and Carrasco-Urra reported on their discovery of Boquila trifoliolata (Lardizabalaceae), a woody vine from temperate rainforests of southern Chile, capable of complex leaf mimicry, when leaves of up to three different host plants were mimicked by leaves of one B. trifoliolata plant.1 However, according to a side-by-side published commentary, the absence of any plausible hypothesis for such a phenomenon makes this report unexplainable and mysterious.2
Gianoli and Carrasco-Urra preferred some chemical volatile signals released from the host plants, which would allow the B. trifoliolata to mimic leaves of host plants.1,3 As an alternative proposal, they also speculated that horizontal gene transfer between host plant and Boquila vine, mediated perhaps via airborne microbes, might allow this leaf mimicry. They proposed this scenario because B. trifoliolata leaves mimic the nearest foliage, irrespective if these leaves are from the host plants or some other neighboring plants.1,3 The complexity of this mimicry, when B. trifoliolata leaves were shown to mimic shapes, colors, leaf orientations, petiole lengths, and vein conspicuousness and patterns may have a third hypothesis, totally different from the volatile signals from host plants or gene transfer via airborne microbes. This third hypothesis would support the possibility that plant vision based on plant ocelli4,5 is behind this unique form of plant behavior.6,7
The plant ocelli concept was elaborated by Gottlieb Haberlandt in 1905 and two years later supported by Francis Darwin8 which consists of the upper epidermis cells have a planoconvex or convex shape acting as lenses, allowing the convergence of light radiation into light-sensitive subepidermal cells.5 With the discovery that the B. trifoliolata is able to mimic the leaves of the nearest plant,1,3 we have been given a rare opportunity to test plant vision in more detail. The simplest way to test the vision hypothesis with the B. trifoliolata would be to see if it would mimic a non-living leaf shape from an artificial plant. In this study, B. trifoliolata was exposed to the artificial plastic plant with a characteristic leaf shapes. The results of this study show that this is indeed the case as leaves of B. trifoliolata mimicked leaves of the artificial plant. Hopefully, this report will stimulate more experiments in future to improve our understanding of the plant sensory abilities.
Results and discussion
Boquila trifoliolata grows in very wet conditions in the Valdivian temperate rainforest. The standard leaves of the B. trifoliolata plants show a variation of leaf shapes and the number of lobes. The majority of leaves have three lobes with blunted tips (Figure 1a). Variation of the number of lobes can be seen with some leaves having multiple lobes and others having less than three. Some leaves showed similar pattern to the fake leaves with respect to lobe variation (Figure 1b). In this research, lower leaves were used as control (non-mimic) leaves due to being below the line of the opaque shelf 1, therefore without direct visual contact with the false leaves (Figure 2).
Figure 1.
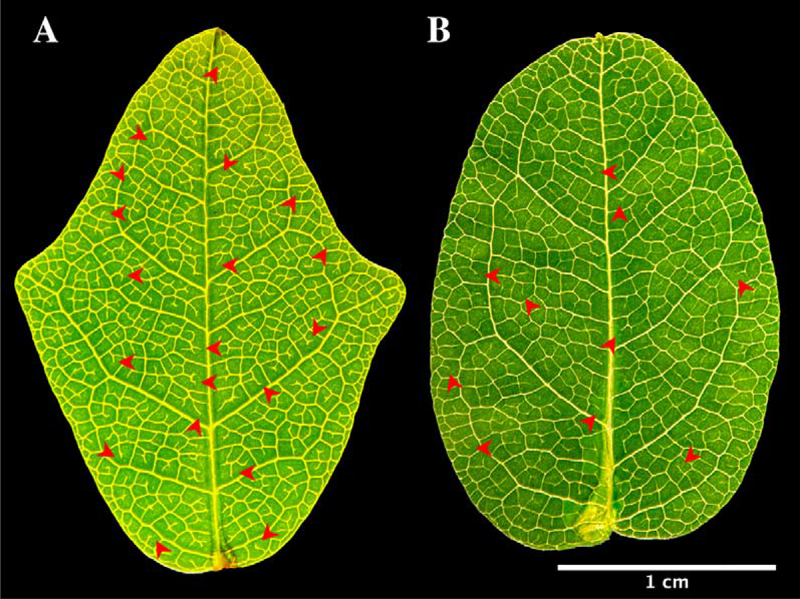
Leaf shapes in Boquila trifoliolata. (a) Non-mimic leaf, with three lobes, dense vascular network. (b) Mimic leaf, with a single lobe in the apex, less dense vascular network. Red asterisks shows examples of free-ending veinlets.
Figure 2.
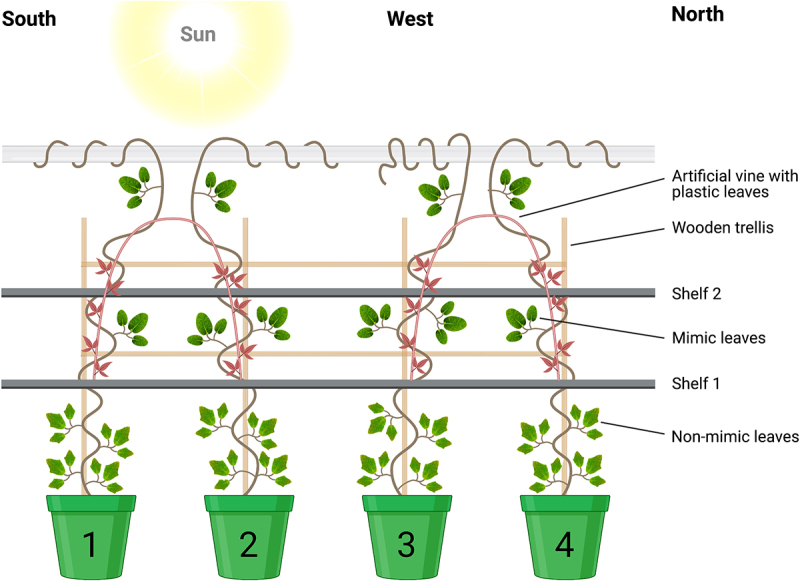
Experimental design. Four Boquila trifoliolata plants lined up side-by-side in front of a window and the artificial model vine plant with plastic leaves (red). Leaves below shelf 1 is the non-mimic (control) leaves. Leaves above shelf 1 is the mimick leaves. Created with BioRender.com.
As the vine grows toward the artificial plant, the leaves of B. trifoliolata take a much different shape. The plants show obvious mimicry attempts to the closest false leaves of model plants, though some leaves still maintain a single lobe (Figure 3). The artificial plant, due to the imperfections in manufacture, has differently shaped leaves. However, all leaves showed more longitudinal shapes (Figure 4). To evaluate the mimicry attempts of Boquila leaves, we have classified them, regarding their age, into three basic groups of young, middle-age and old age leaves.
Figure 3.
Single lobe mimic leaf. Mimicry attempt to the plastic leaves of artificial host plant.
Figure 4.

Plastic leaf of a model artificial plant with longitudinal shape.
Moreover, we used a leaf recognition algorithm for quantification of leaf forms (for details, see the Materials and Methods section).9 We have observed that the middle-age and old non-mimic leaves had significantly greater leaf area, perimeter, length, and width than the mimic leaves (Figure 5). Regarding young leaves, we observed significant differences in leaf widths. By establishing a relationship between these four parameters, we calculated different variables. We observed that the non-mimic leaves had greater rectangularity.9 Regardless of their age, leaf shapes took on a more uniform rectangle-like forms (Figure 6). Furthermore, when we compared the ratio between leaf diameter and leaf length (form factor), we observed that the young middle-aged and old leaves of the non-mimic plants had this ratio higher (Figure 6). However if we compared the ratio between length and diameter (aspect ratio), we found that mimic leaves had higher values, which means a more slender shapes (forms).
Figure 5.
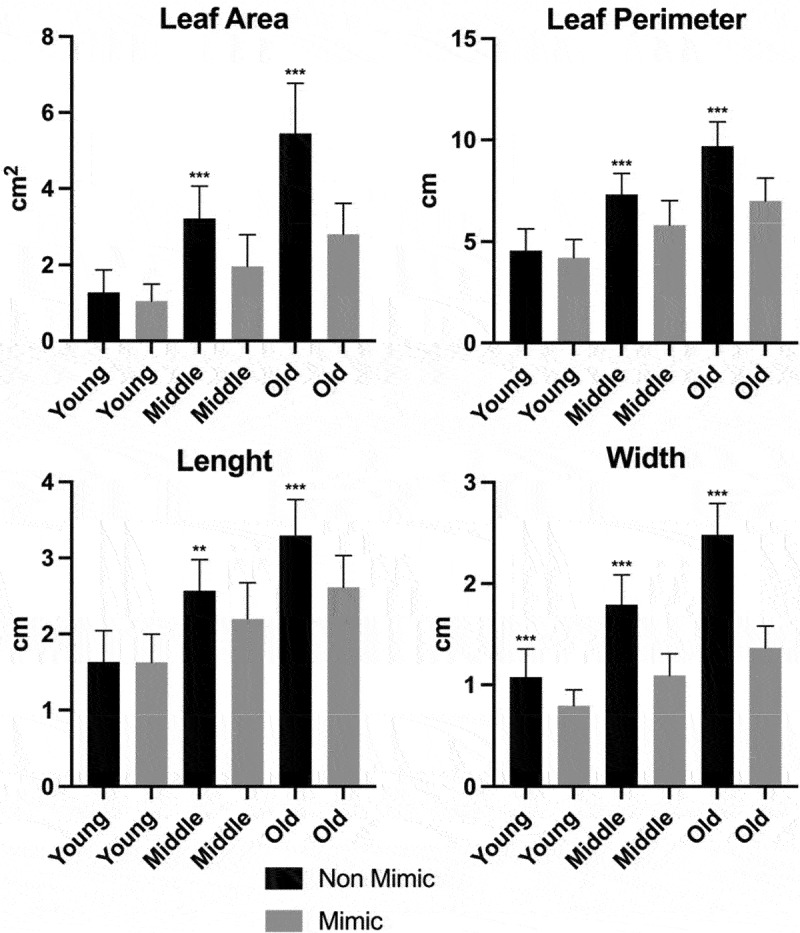
Morphometric analysis of Boquila trifoliolata leaves. Black bars correspond to non-mimic leaves (control), without contact with plastic leave. Gray bars correspond to mimic leaves, with close contact with plastic leaves. Leaves were classify into young, middle and old regarding their age. Measurements performed in 16 biological repetitions and two-tailed Student’s t-test was used to identify significant differences between mimick and non-mimic leaves. P-values<0.05 were considered significant (***P < .001; **P < .01; *P < .05). The error bars reported in all graphs represent standard deviation.
Figure 6.
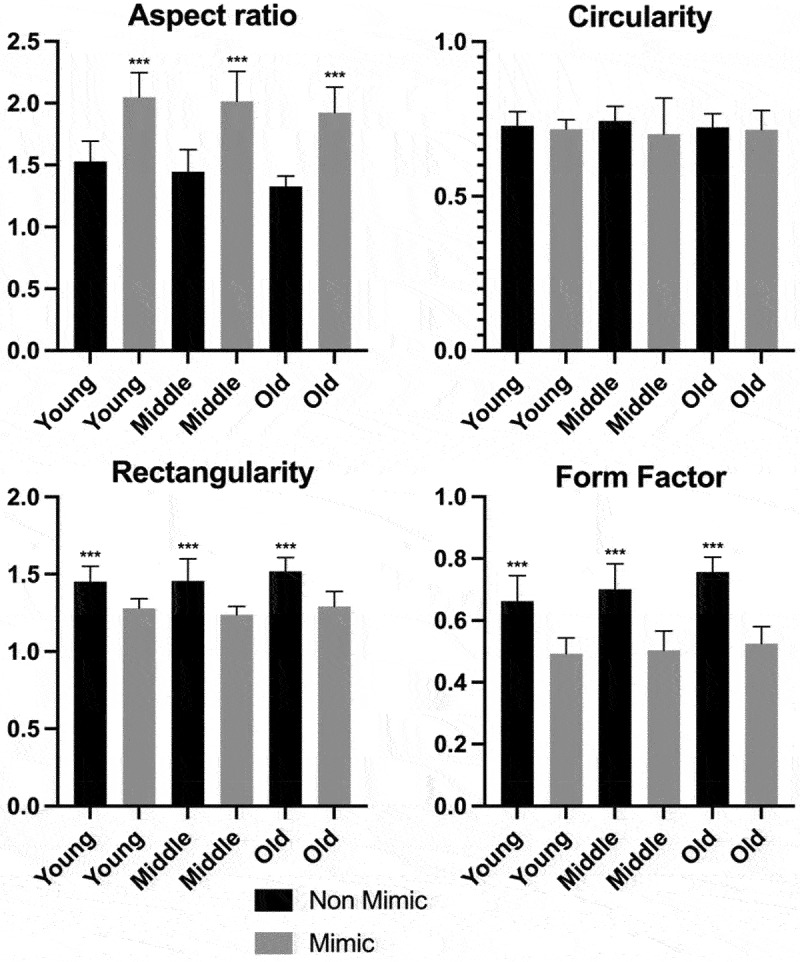
Morphometric analysis of Boquila trifoliolata leaves. Aspect ratio is the ratio of leaf length and width. Circularity describes the difference between a leaf and a circle. Rectangularity describes the similarity between a leaf and a rectangle. Form factor is the ratio between leaf width and length. Measurements performed in 16 biological repetitions and two-tailed Student’s t-test was used to identify significant differences between mimick and non-mimic leaves. P-values<0.05 were considered significant (***P < .001; **P < .01; *P < .05). The error bars reported in all graphs represent standard deviation.
These last two parameters (aspect ratio and form factor) show us that mimic leaves are generally longer rather than wider, indicating that they are more similar to the elongated plastic leaves that were placed next to the Boquila plants, as a model of host plant. The non-mimic leaves showed similar values for lengths, having their form factor values close to 1 (similar width and length values result in the form factor values close to 1), the more similar the leaves are in length and width. Corroborating these data, we obtained rectangularity, showing us that the non-mimics are more roundish in shape, in comparison to the slender mimic leaves.
The mimicry began just below the artificial vine (between shelfs 1 and 2) and when more leaves were facing the model leaves, it seemed to affect the detail of mimicry. This suggests that the lower leaves sample details of the leaves next to them and pass the obtained information to the next set of growing leaves. New leaves are formed in the mimic shape and young leaves grow larger in that shape. This suggests that lower leaves play some roles in the leaf mimicry.
An interesting aspect was observed about the venation pattern when we analyzed the leaves under binocular microscopy. It was observed that non-mimic leaves had more free-ending veinlets, represented by tiny veinlets having their extremities ending freely in the leaf mesophyll10 (red arrow heads in Figure 1). Greater amounts of the free-ending veinlets were observed in non-mimic leaves in young leaves as well as middle-aged and old leaves (Figure 7). It is well known that the development and patterning of the veins progresses in a basipetal direction (from the leaf apex toward the base); therefore, the more advanced stage of the venation networks can be found at the leaf apex than at its base.10,11 In comparison to the non-mimic standard leaves, mimic leaves show lower numbers of free-ending veinlets and less dense vascular networks (Figure 1). This feature is an indication of high auxin concentrations at the leaf margins, suggesting that perhaps these leaves have altered patterns of auxin biosynthesis and polar auxin transport.10,11 This can be interpreted as an attempt to modify their leaf shape, trying to mimic the plastic leafs.
Figure 7.
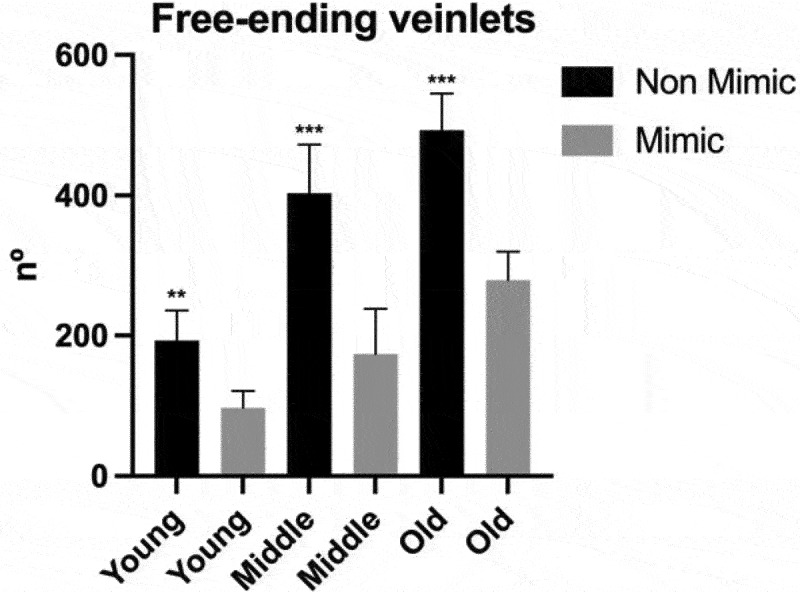
Quantification of the free-ending veinlets. Number of free-ending veinlets per leaf. Black bars correspond to non-mimic leaves (control), without contact with plastic leave. Gray bars correspond to mimic leaves, with close contact with plastic leaves. Leaves were classify into young, middle and old regarding their age. Measurements performed in 4 biological repetitions and two-tailed Student’s t-test was used to identify significant differences between mimick and non-mimic leaves. P-values<0.05 were considered significant (***P < .001; **P < .01; *P < .05). The error bars reported in all graphs represent standard deviation.
It appears that over the months, B.trifoliolata plants improved their mimicking of the plastic host plant significantly (Figure 8). The mimic leaves doubled in size from one analysis to the next (first analysis December 2020, second analysis June 2021) and the form factor has reduced significantly, approaching the form factor of the plastic leaves having slender shapes (form factors close to the value 1). This improved ability of B.trifoliolata plants to mimic shapes and sizes of plastic leaves implicates learning and memory processes in plant mimicry.
Figure 8.
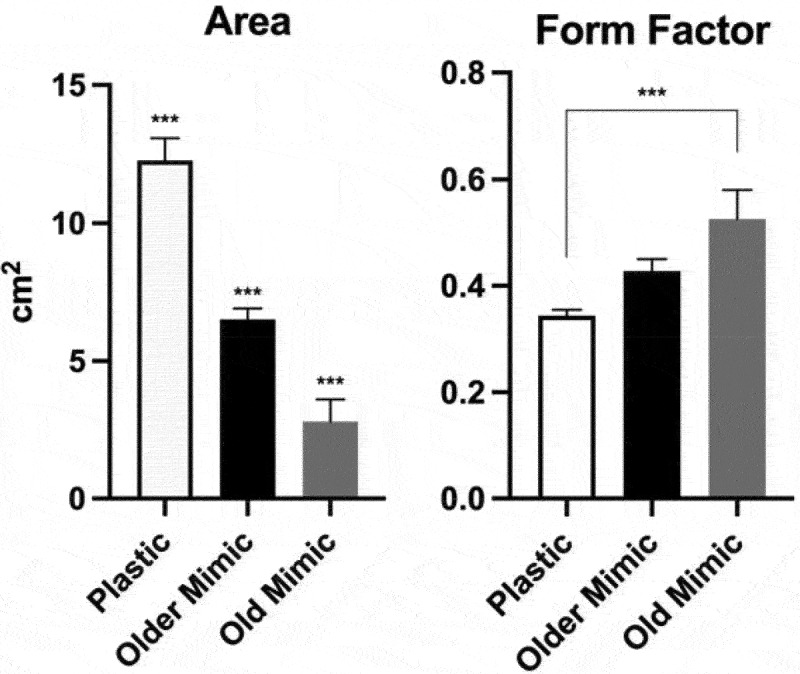
Leaf area and form factor (ratio between leaf width and length) of plastic leaves, old mimic leaves and older mimic leaves (leaves one year older). All three groups showed significant differences in leaf area. Only the plastic and old mimic groups showed a difference between each other, the other interactions showed no significant differences. The data were submitted to one-way analysis of variance (ANOVA) and the mean values were compared by Tukey test (***P < .001; **P < .01; *P < .05). The error bars reported in all graphs represent standard deviation.
Leaf mimicry attempts have been observed on all shoots growing near the artificial model (host) plant. Some mimicking leaves are not perfect in their mimicry, similarly to their attempts at serrated leaves in nature.1 Perhaps due to the uneven edges on the artificial plant, all leaves in contact with the artificial vine have a markedly different shape than the non-mimic leaves below the shelf. Our results showed that leaves of B. trifoliolata mimic artificial leaves, changing their shape to a more longitudinal shape devoid of lobes. This goes in the opposite direction of the two hypotheses proposed by Gianoli & Carrasco-Urra 2014, which speculated that the leaves of Boquila could pick up airborne chemicals released by other trees or use genes from its host via parasite or microbe. Our present analysis favors plant vision based on plant-specific leaf ocelli.4,5
Outlook and perspectives
Up to this point, the leading explanation for leaf mimicry in the B. trifoliolata has been volatile signaling and horizontal gene transfer.1,3 Volatile signaling and horizontal gene transfer in plants have been proposed.12,13 However, since the B. trifoliolata can mimic leaves when not in contact with the host plant makes this unlikely and hard to test. Volatile signaling does show promise and can be easily tested, as in a recent study has shown that Cuscuta racemose can choose between different hosts plants at a certain distance.14
Recent research into plant perception and communication has provided new surprising details into the life of plants enjoying not only ability of communication through chemical volatiles but also perception of acoustic signals.13,15,16 Moreover, research done on the visual capabilities of algae and protists clearly suggest vision already in unicellular organisms.17–23 Experimental testing of the ocelli-based plant vision, as it was done by Harold Wager,4 would be the logical next step in our quest for understanding the plant sensory complexity.
Currently, in a cooperation with the group of Prof. Maximilian Weigend, we are growing several Boquila plants in the Botanical Garden of the University of Bonn. These plants will allow us to perform these critical experiments in our future studies.
Materials and methods
Plant material and growth conditions
Boquila trifoliolata plants were purchased from a local store placed in Port Townsend Washington and arrived in 15.24 cm pots. Shortly after arrival plants were reported in 25.4 cm pots filled with high nutrient potting soil with a pH of 6.3, 0.30% nitrogen, 0.45% phosphate, 0.05% potassium, and 1.00% calcium. The plants were watered with distilled water (approximately 236 ml) until they reached field capacity every other day to keep the soil moist. A stone humidifier was placed near the plants to maintain a higher humidity. The experiment was conducted in Magna, Ut, USA (40°42ʹN, 112°06ʹW) during the period from September 2019 to October 2020. The plants were placed in front of a large west facing window. The first leaves sample for analysis was collected in December 2020 and the second sample was collected in June 2021.
Each plant was assigned a number and placed on a growing rack. Two artificial vines were placed above the plants on a wooden trellis. During the winter, the plants grew quickly through the leaves showed poor mimicry of the artificial plants leaves. The original plant that we had did not show good evidence of mimicry until the spring and summer. We decided to continue the experiment and see if there were better results in the warmer months.
Experimental design
The plants were lined up side by side in front of the window through which they received sunlight coming from the west direction. Above the plant pots, two opaque shelves (shelf 1 and shelf 2) were placed to keep the lower parts away from the artificial vine and plastic leaves. Two White Wisteria Garland artificial vines were purchased from a local store, and the flowers were removed so only the silk leaves would remain. The artificial vines were covered with fake leaves (Figure 4) and placed 28 cm above the top of the pots containing B. trifoliolata, so the artificial vine with fake leaves were not visible below the shelf 1, Figure 2 (The plastic plants are in red in Figure 2 for easy discrimination between the mimic leaves. However, in reality, the plastic leaves are green, as shown in Figure 3). As the plants grew, wires were placed adjacent to growing shoots to guide then toward the artificial vines if they did not attach to the trellis. Plants were observed daily with notes taken of new shoot growth and wires were added as needed to bring the new shoots closer to the artificial leaves.
Leaf morphology analysis
The plants were classified into three groups in relation to the leaves age;
Young: juvenile, newly formed leaves;
Middle: middle-aged leaves;
Old: fully formed leaves.
In addition, we classified the leaves into two additional groups that were compared to each other: mimic group and non-mimic group.
The leaves were analyzed based on four basic geometrical features:9
Leaf area (A): total leaf area, calculated in pixels of the entire leaf with the help of the Plugin LeafJ;24
Perimeter (P): the leaf perimeter was calculated by counting the pixels consistent with the leaf margin;
Length (L): distance between the two terminals of the main vein;
Width (W): the longest distance between two points that intersect the straight line of length at a 90 degree angle.
Using the four basic geometrical features, we define four digital morphological features, used for leaf analysis:
Aspect ratio: is defined as the ratio of length to width;
Circularity: describes the difference between a leaf and a circle, according to the following equation:
Rectangularity: describes the similarity between a leaf and a rectangle, according to the following equation:
Form Factor: ratio between width and length.
The free-ending veinlets were analyzed using photos taken with a binocular microscope at 0.8x magnification. The photos were analyzed and the free-ending veins were counted using ImageJ software (Cell counter analyzer plugin). A minimum of 6 leaves were used for each group.
The largest leaves of the branch containing three leaves were removed, then these leaves were photographed with a camera (Canon EOS 1000D, Canon Inc., Tokyo, Japan) and binocular microscope (Leica MZ FL III with Leica DFC 290, Leica Microsystems, Wetzler Deutschland). The images were analyzed with Adobe Photoshop 2021 (22.3.0, Adobe Inc., San José, CA, USA) and Fiji ImageJ (LeafJ Plugin24).
Data analysis
Data were analyzed using the statistical software GraphPad Prisma (9.1.0, GraphPad Software, San Diego, CA, USA). All data were obtained from 16 biological repetitions and two-tailed Student’s t-test was used to identify significant differences between mimic and non-mimic leaves. One-way analysis of variance (ANOVA) was used to identify significant differences of plastic leaves from older and old mimic leaves and the mean values were compared by Tukey test (***P < .001; **P < .01; *P < .05). The error bars reported in all graphs represent standard deviation.
Supplementary Material
Acknowledgments
The authors acknowledge Ursula Mettbach (University of Bonn, Germany) for skillful technical assistance and the Stiftung Zukunft Jetzt! (Munich, Germany) for scholarship to F.Y.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Gianoli E, Carrasco-Urra F.. Leaf mimicry in a climbing plant protects against herbivory. Curr Biol. 2014;24:984–8. doi: 10.1016/j.cub.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Pannell JR. Leaf mimicry: chameleon-like leaves in a patagonian vine. Curr Biol. 2014;24:R357–R359. doi: 10.1016/j.cub.2014.03.066. [DOI] [PubMed] [Google Scholar]
- 3.Gianoli E. Eyes in the chameleon vine? Trends Plant Sci. 2017;22:4–5. doi: 10.1016/j.tplants.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Wagner H. The perception of light in plants. Ann Bot. 1909;23:459–489. doi: 10.1093/oxfordjournals.aob.a089231. [DOI] [Google Scholar]
- 5.Haberlandt G. Die Lichtsinnesorgane der Laubblätter.(Whilhelm Engelmann),1905. [Google Scholar]
- 6.Mancuso S, Baluška F. Plant ocelli for visually guided plant behavior. Trends Plant Sci. 2017;22:5–6. doi: 10.1016/j.tplants.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Baluška F, Mancuso S. Vision in plants via plant-specific ocelli? Trends Plant Sci. 2016;21:727–730. doi: 10.1016/j.tplants.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Darwin F. Lectures on the physiology of movement in plants. New Phytol. 1907;6:69–76. doi: 10.1111/j.1469-8137.1907.tb06045.x. [DOI] [Google Scholar]
- 9.S. G. Wu, F. S. Bao, E. Y. Xu, Y. Wang, Y. Chang and Q. Xiang, “A Leaf Recognition Algorithm for Plant Classification Using Probabilistic Neural Network,„ 2007 IEEE International Symposium on Signal Processing and Information Technology, 2007, pp. 11-16, Giza, Egypt. doi: 10.1109/ISSPIT.2007.4458016. [DOI] [Google Scholar]
- 10.Aloni R, Schwalm K, Langhans M, Ullrich CI. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta. 2003;216:841–853. doi: 10.1007/s00425-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 11.Aloni R. Vascular Differentiation and Plant Hormones.(Springer International Publishing), 2021. doi: 10.1007/978-3-030-53202-4. [DOI] [Google Scholar]
- 12.Das A, Lee S-H, Hyun TK, Kim S-W, Kim J-Y. Plant volatiles as method of communication. Plant Biotechnol Rep. 2013;7:9–26. doi: 10.1007/s11816-012-0236-1. [DOI] [Google Scholar]
- 13.Bock R. The give-and-take of DNA: horizontal gene transfer in plants. Trends Plant Sci. 2010;15:11–22. doi: 10.1016/j.tplants.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Parise AG, Reissig GN, Basso LF, Senko LGS, Oliveira TFDC, De Toledo GRA, Ferreira AS, Souza GM. Detection of different hosts from a distance alters the behaviour and bioelectrical activity of Cuscuta racemosa. Front. Plant Sci. 2021;12:1–21. doi: 10.3389/fpls.2021.594195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagliano M, Mancuso S, Robert D. Towards understanding plant bioacoustics. Trends Plant Sci. 2012;17:323–325. doi: 10.1016/j.tplants.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigo-Moreno A, Bazihizina N, Azzarello E, Masi E, Tran D, Bouteau F, Baluska F, Mancuso S. Root phonotropism: early signalling events following sound perception in Arabidopsis roots. Plant Sci. 2017;264:9–15. doi: 10.1016/j.plantsci.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kessler JO, Nedelcu AM, Solari CA, Shelton DE. Cells acting asz lenses: a possible role for light in the evolution of morphological asymmetry in multicellular volvocine algae. In: Evolutionary transitions to multicellular life. Springer, Dordrecht; 2015. p. 225–243. doi: 10.1007/978-94-017-9642-2_12. [DOI] [Google Scholar]
- 18.Gavelis GS, Hayakawa S, White III RA, Gojobori T, Suttle CA, Keeling PJ, Leander BS. Eye-like ocelloids are built from different endosymbiotically acquired components. Nature. 2015;523:204–207. doi: 10.1038/nature14593. [DOI] [PubMed] [Google Scholar]
- 19.Richards TA, Gomes SL. How to build a microbial eye. Nature. 2015;523:166–167. doi: 10.1038/nature14630. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa S, Takaku Y, Hwang JS, Horiguchi T, Suga H, Gehring W, Ikeo K, Gojobori T. Function and evolutionary origin of unicellular camera-type eye structure. PLoS One. 2015;10:e0118415. doi: 10.1371/journal.pone.0118415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson D-E, Colley NJ. Comparative vision: can bacteria really see? Curr Biol. 2016;26:R369–R371. doi: 10.1016/j.cub.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 22.Schuergers N, Lenn T, Kampmann R, Meer MV, Esteves T, Temerinac-Ott M, Korvink JG, Lowe AR, Mullineaux CW, Wilde A. Cyanobacteria use micro-optics to sense light direction. Elife. 2016;5:1–16. doi: 10.7554/eLife.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson D-E, Marshall J. Lens eyes in protists. Curr Biol. 2020;30:R458–R459. doi: 10.1016/j.cub.2020.01.077. [DOI] [PubMed] [Google Scholar]
- 24.Maloof JN, Nozue K, Mumbach MR, Palmer CM. LeafJ: an ImageJ plugin for semi-automated leaf shape measurement. J Vis Exp. 2013:2–7. doi: 10.3791/50028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


