Abstract
Background
Exercise therapy is considered an important component of the treatment of arthritis. The efficacy of exercise therapy has been reviewed in adults with rheumatoid arthritis but not in children with juvenile idiopathic arthritis (JIA).
Objectives
To assess the effects of exercise therapy on functional ability, quality of life and aerobic capacity in children with JIA.
Search methods
The Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (The Cochrane Library), MEDLINE (January 1966 to April 2007), CINAHL (January 1982 to April 2007), EMBASE (January 1966 to October 2007), PEDro (January 1966 to October 2007), SportDiscus (January 1966 to October 2007), Google Scholar (to October 2007), AMED (Allied and Alternative Medicine) (January 1985 to October 2007), Health Technologies Assessment database (January 1988 to October 2007), ISI Web Science Index to Scientific and Technical Proceedings (January 1966 to October 2007) and the Chartered Society of Physiotherapy website (http://www.cps.uk.org) were searched and references tracked.
Selection criteria
Randomised controlled trials (RCTs) of exercise treatment in JIA.
Data collection and analysis
Potentially relevant references were evaluated and all data were extracted by two review authors working independently.
Main results
Three out of 16 identified studies met the inclusion criteria, with a total of 212 participants. All the included studies fulfilled at least seven of 10 methodological criteria. The outcome data of the following measures were homogenous and were pooled in a meta‐analysis: functional ability (n = 198; WMD ‐0.07, 95% CI ‐0.22 to 0.08), quality of life (CHQ‐PhS: n = 115; WMD ‐3.96, 95% CI ‐8.91 to 1.00) and aerobic capacity (n = 124; WMD 0.04, 95% CI ‐0.11 to 0.19). The results suggest that the outcome measures all favoured the exercise therapy but none were statistically significant. None of the studies reported negative effects of the exercise therapy.
Authors' conclusions
Overall, based on 'silver‐level' evidence (www.cochranemsk.org) there was no clinically important or statistically significant evidence that exercise therapy can improve functional ability, quality of life, aerobic capacity or pain. The low number of available RCTs limits the generalisability. The included and excluded studies were all consistent about the adverse effects of exercise therapy; no short‐term detrimental effects of exercise therapy were found in any study. Both included and excluded studies showed that exercise does not exacerbate arthritis. The large heterogeneity in outcome measures, as seen in this review, emphasises the need for a standardised assessment or a core set of functional and physical outcome measurements suited for health research to generate evidence about the possible benefits of exercise therapy for patients with JIA. Although the short‐term effects look promising, the long‐term effect of exercise therapy remains unclear.
Plain language summary
Exercise therapy in juvenile idiopathic arthritis (JIA)
This summary presents what we know from research about the effect of exercise therapy in JIA. The review shows that in children with JIA, exercise may not lead to any difference in a child's ability to function or move their joints fully, the number of joints with swelling, quality of life, overall wellbeing, pain or aerobic capacity. Aerobic capacity is the amount of oxygen the body consumes during exercise. If a person has low aerobic capacity, it generally means he or she is able to do less physical activity and may tire easily. The number of joints with pain was not measured in these studies. We often do not have precise information about side effects and complications. This is particularly true for rare but serious side effects. No short‐term adverse effects of exercise therapy were found in the studies that make up this review.
What is exercise therapy and what is JIA?
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease in children and is an important cause of short‐term and long‐term disability. In JIA the cause of the arthritis is unknown. It generally begins in children younger than age 16 years. It always lasts for at least six weeks. A physician will rule out other conditions that may be causing the symptoms before diagnosing JIA.
Several types of exercise therapy are described in this review, for example, physical training programs such as strength training for improving muscle strength and endurance exercise for improving overall fitness (either land based or in a pool).
Best estimate of what happens to children with JIA and exercise
Ability to function: a child's ability to function changed less than 1 more point on a scale of 0 to 3. Other studies state that a change of 0.13 on the score of the Childhood Health Assessment Questionnaire (CHAQ) is a clinically important improvement from the perspective of children and their parents. This level of change has not been found in this review
Quality of life: a child's quality of life changed between 2.5 and 4 more points on a scale of 1 to 50.
There may be little or no difference with exercise. It is possible that these differences are the result of chance.
Adverse effects: no short‐term effects have been reported after exercise therapy for children with JIA.
Background
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease in children and is an important cause of short‐term and long‐term disability. JIA is a disease of unknown aetiology that begins before the 16th birthday and persists for at least six weeks. A diagnosis is made when other known conditions are excluded (Petty 2004). Studies in developed countries have reported a prevalence that varies between 16 and 150 per 100,000 (Ravelli 2007). Data from two cross‐sectional studies indicate that children with arthritis are physically less active compared to healthy children (Henderson 1995; Takken 2003b). Moreover, it was found that physical activity was related to physical fitness (Takken 2003b) indicating that lower physical activity level leads to deconditioning and functional deterioration, which reinforces an inactive lifestyle (Bar‐Or 2004). Exercise therapy (for example, a training program) might prevent the deconditioning due to hypoactivity and break the vicious circle. Exercise therapy is considered as an integral part of the treatment of children with JIA (Ravelli 2007). Several types of exercise therapy can be distinguished, for example, physical training programs such as strength training for improving muscle strength and endurance exercise for improving cardiorespiratory fitness. Studies in adult rheumatoid arthritis (RA) patients have shown that these exercise modalities, or a combination of both, can improve physical fitness (muscle strength or maximal oxygen uptake) and function (De Jong 2003; Hakkinen 2003; Hakkinen 2004; Hakkinen 2005).
In both adult RA and JIA, the focus has shifted from inflammation parameters to more patient‐centred outcomes. For RA this resulted in the development of the OMERACT (Outcome Measures in Rheumatology) core set for RA (Omeract 1997) and in JIA the PRINTO (Pediatric Rheumatology International Trial Organization) core set (Giannini 1997). The OMERACT core set consists of patient and physician global assessment and measures of pain, disability and an acute‐phase reactant. The PRINTO core set consists of physician global assessment of disease activity, parent or patient (as appropriate for age) global assessment of overall wellbeing, functional ability, number of joints with active arthritis, number of joints with limited range of motion, erythrocyte sedimentation rate and health‐related quality of life (HRQoL) measurements.
A systematic review on the effects of dynamic exercise therapy for treating adult RA has shown that adults can benefit from exercise in terms of improved exercise capacity, muscle strength and range of motion (Van den Ende 1998). There is some evidence that children with JIA can benefit from exercise as well (Bacon 1991; Baldwin 1972). Other evidence showed that exercise does not exacerbate arthritis (Kirchheimer 1993; Klepper 1991). However, not all of these studies are controlled studies. A systematic review of randomised controlled studies can determine whether exercise therapy is effective for children with JIA. Therefore, we performed a systematic review on the effects of physical exercise therapy for children with JIA.
Objectives
The primary objective was to perform a systematic review on the effects of exercise therapy for children with JIA in terms of functional ability, range of motion, number of joints with swelling (active joint count), number of joints with pain, health‐related quality of life, parent or patient global assessment of overall wellbeing, pain, aerobic capacity and muscle strength.
Methods
Criteria for considering studies for this review
Types of studies
All full‐length randomised controlled trials were eligible for inclusion.
Types of participants
This review concerned children with juvenile idiopathic arthritis (juvenile rheumatoid arthritis (JRA), juvenile chronic arthritis (JCA), juvenile idiopathic arthritis (JIA)) under 18 years of age, including all subgroups ((extended) oligo (pauci) articular JIA, rheumatoid factor (RF) negative and RF positive polyarthritis, systemic onset JIA, psoriatic arthritis, enthesitis related arthritis and other arthritis) as diagnosed by a rheumatologist based on established criteria from national and international organizations (ILAR, ACR, EULAR). Studies of osteoarthritis were excluded as this is not relevant for children with JIA.
Types of interventions
Physical exercise therapy existing of:
Endurance training;
Strength training;
A combination of strength and endurance training;
Physical exercise during summer camps.
Comparators to these interventions were: A) placebo; B) therapy Y, where therapy Y is any therapy that can be considered as a placebo exercise therapy as attention is given that is not expected to improve physical function because of a very low exercise intensity, but which may also be beneficial to the participants; C) standard medical care; because it is very difficult to develop a real placebo for exercise therapy, children receiving assessment only will be considered as receiving placebo. In order to meet the inclusion criteria for this review all interventions must include an adequate description of the intervention including intensity, frequency, duration of training and mode of administration. Trial duration must be a minimum of two weeks.
Types of outcome measures
We included all the outcome measures recommended for use in clinical trials in the PRINTO‐core set (Giannini 1997) as well as training effects on exercise capacity and muscle strength. When reported, side effects, total number of dropouts and compliance with exercise were also included in the review.
Primary outcomes Functional outcome measures
Functional ability (as measured on functional tests and questionnaires (i.e. Juvenile Arthritis Functional Assessment Scale (JAFAS) (Lovell 1989), Childhood Health Assessment Questionnaire (CHAQ) (Sing 1994) and Juvenile Arthritis Functional Status Index (JASI) (Wright 1996))
Joint range of motion measures
Number of joints with swelling (active joint count)
Number of joints with pain
Health‐related quality of life (i.e. HR‐QoL, Child Health Questionnaire (CHQ) (Landgraf 1996))
Parent or patient global assessment of overall wellbeing
Pain
Adverse outcomes (safety of exercise therapy) and other outcomes
Any reported side effects (e.g. disease flares)
Total number of dropouts
Withdrawals due to inefficacy or negative effects
Secondary outcomes
Measures to evaluate the effects of exercise training on exercise capacity and muscle strength
Aerobic capacity (VO2peak) determined on maximal ergometer test
Aerobic capacity (VO2peak) estimated from submaximal ergometer test
Aerobic capacity estimated from field test measuring aerobic fitness
Muscle strength
Compliance with exercise
Search methods for identification of studies
Electronic searches
MEDLINE (January 1966 to April 2007)
CINAHL (January 1982 to April 2007)
EMBASE (to April 2007)
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, to April 2007)
PEDRO (http://ptwww.cchs.usyd.edu.au/pedro/‐) (to April 2007)
SportDiscus (to April 2007)
Google Scholar (to April 2007)
AMED (Allied and Alternative Medicine) (to April 2007)
Health Technologies Assessment database (to April 2007)
ISI Web Science Index to scientific and technical proceedings (to April 2007)
The Chartered Society of Physiotherapy website (http://www.csp.uk.org)
Other sources
Reference tracking ‐ reference lists of relevant studies and conference presentations were searched for further identification of published work
Personal communication
Search strategy Identifying trials: 1.randomized controlled trial.pt. 2.controlled clinical trial.pt. 3.randomized controlled trials.sh. 4.random allocation.sh. 5.double blind method.sh. 6.single blind method.sh. 7.1 or 2 or 3 or 4 or 5 or 6 8.(animal not (human and animal)).sh. 9.7 not 8 10.clinical trial.pt. 11.exp clinical trials/ 12.(clin$ adj25 trial$).ti,ab. 13.((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14.placebos.sh. 15.placebo$.ti,ab. 16.random$.ti,ab. 17.research design.sh. 18.volunteer$.ti,ab. 19.10 or 11 or 12 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20.19 not 8 21.20 not 9 22.9 or 21
Identifying juvenile arthritis patients: 23.Arthritis, Juvenile Rheumatoid.sh 24.Arthritis, Juvenile Chronic.tw 25.Arthrit$, Juvenile.tw 26.Arthrit$.tw 27.Child$.tw 28.Adolescence.tw 29.Child, Preschool.tw 30.Adult 31.or/23‐26 32.31 not 30 33.or/27‐29 34.32 and 33
Identifying therapy: 35.physical therapy.sh. 36.physical$.tw. 37.physio$.tw. 38.exercise.sh. 39.exercis$.tw. 40.rehabilitation.sh. 41.rehabilitation$.tw. 42.strengthening.tw. 43.hydro therapy.tw. 44.balneo therapy.tw. 45.spa therapy.tw. 46.water therapy.tw. 47.thalasso therapy.tw. 48.or/35‐51
Data collection and analysis
Selection of studies The search strategy identified a set of potentially relevant references. Two authors (TT, MvB) screened search results for potentially eligible studies. When titles and abstracts suggested a study was potentially eligible for inclusion, a full paper copy of the report was obtained. Disagreements between the two authors regarding a study's eligibility were resolved by discussion until consensus was reached or, where necessary, a third person (RHHE) acted as adjudicator.
In addition to extracting data, the review authors independently allocated each included trial to one of three methodology quality categories, based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Category A: low risk of bias ‐ plausible that bias is unlikely to seriously alter the results, all of the criteria met. Category B: moderate risk of bias ‐ plausible that bias raises some doubt about the results, one or more criteria partly met. Category C: high risk of bias ‐ plausible that bias seriously weakens confidence in the results, one or more criteria not met.
Data extraction and management Two independent observers (TT, MvB) independently extracted data using a standard extraction form. Agreements between observers were assessed using a weighted kappa statistic. Disagreements were discussed by the two review authors until a consensus was reached. If no consensus was reached, a third review author (RHHE) acted as adjudicator. Data were extracted at baseline and the end of the intervention period. If data were missing or further information was required, serious attempts were made to contact the first two study authors to request the required information.
Assessment of methodological quality of included studies Methodological quality was assessed independently by two review authors (TT, MvB) using the PEDro scale. The PEDro scale is based on the Delphi list developed by Verhagen et al (Verhagen 1998) which is based on 'expert consensus' not, for the most part, on empirical data. Two additional items not on the Delphi list (PEDro scale items 8 and 10) have been included in the PEDro scale. As more empirical data comes to hand it may become possible to weight scale items so that the PEDro score reflects the importance of individual scale items. The purpose of the PEDro scale is to help the users of the PEDro database to rapidly identify which RCT is likely to be internally valid (criteria 2 to 9) and could have sufficient statistical information to make the results interpretable (criteria 10 and 11). An additional criterion (criterion 1) that relates to the external validity (generalisability or applicability of the trial) has been retained so that the Delphi list is complete. However, this criterion is not used to calculate the PEDro score reported on the PEDro web site. The 11 criteria are: specification of eligibility criteria, random allocation, concealment of allocation, similarity between groups at baseline regarding the most important prognostic indicators, subject blinding, blinding of therapist, blinding of assessor, subject follow up, intention‐to‐treat analysis, between‐group statistical comparisons are reported for at least one key outcome, point measure and measure of variability for at least one key outcome. All selected methodological criteria were scored as yes or no, resulting in a range from 0 to 10. The PEDro Scale has shown moderate levels of interrater reliability (intraclass correlation coefficient 0.54, 95% confidence interval (CI) 0.39 to 0.71) (Sherrington 2000). To improve the reliability of this scale, any disagreement between the review authors was resolved by discussion with an independent review authors (RHHE) until a consensus was reached.
The scale assessed the following criteria:
Specification of eligibility criteria.
Random allocation.
Concealment of allocation.
Similarity between groups at baseline regarding the most important prognostic indicators.
Participant blinding.
Blinding of therapist.
Blinding of assessor.
Participant follow up.
Intention‐to‐treat analysis.
between‐group statistical comparisons reported for at least one key outcome.
Point measure and measure of variability for at least one key outcome.
Grading of evidence The grading system as described in the 2004 book 'Evidence‐based Rheumatology' (Tugwell 2004) and recommended by the Musculoskeletal Group was used.
Platinum: a published systematic review that has at least two individual controlled trials each satisfying the following. • Sample sizes of at least 50 per group ‐ if these do not find a statistically significant difference, they are adequately powered for a 20% relative difference in the relevant outcome. • Blinding of patients and assessors for outcomes. • Handling of withdrawals > 80% follow up (imputations based on methods such as last observation carried forward (LOCF) are acceptable). • Concealment of treatment allocation. Gold: at least one randomised clinical trial meeting all of the following criteria for the major outcome(s) as reported. • Sample sizes of at least 50 per group ‐ if these do not find a statistically significant difference, they are adequately powered for a 20% relative difference in the relevant outcome. • Blinding of patients and assessors for outcomes. • Handling of withdrawals > 80% follow up (imputations based on methods such as LOCF are acceptable). • Concealment of treatment allocation.
Silver: a randomised trial that does not meet the above criteria. Silver ranking would also include evidence from at least one study of non‐randomised cohorts that did and did not receive the therapy, or evidence from at least one high quality case‐control study. A randomised trial with a 'head‐to‐head' comparison of agents would be considered silver level ranking unless a reference were provided to a comparison of one of the agents to placebo showing at least a 20% relative difference.
Bronze: The bronze ranking is given to evidence if at least one high quality case series without controls (including simple before and after studies in which patients act as their own control) or if the conclusion is derived from expert opinion based on clinical experience without reference to any of the foregoing (for example, argument from physiology, bench research or first principles).
Measures of treatment effect All the trials to be included in the systematic review were entered into Review Manager 4.2. For continuous outcomes (functional ability, range of motion, number of joints with swelling (active joint count), number of joints with pain, quality of life, parent or patient global assessment of overall wellbeing, pain, aerobic capacity and muscle strength), a weighted mean difference between treatment and control groups was calculated, if possible. Dichotomous outcomes (number of side effects, total number of dropouts from study, compliance with therapy, physician and parent global assessment) were described. The results from the various studies were tested for heterogeneity using the chi‐square statistic, with a significance level of P = 0.05, and the I2 statistic where over 50% indicates substantial heterogeneity (Higgins 2005). Overall effects were only be estimated for groups of trials using the same intervention. As such, several individual meta‐analyses were performed. Meta‐analyses were conducted according to a fixed‐effect model. Where heterogeneity was significant, a random‐effects model was used. Potential publication bias was evaluated with the inverted funnel plot technique. A sensitivity analysis was conducted to evaluate the robustness of the meta‐analyses. This analysis examined the effects of methodological quality and potential differences in exercise frequency, intensity and duration.
Clinical relevance tables were compiled for pooled outcome measures as additional tables to improve the readability of the review. Weighted absolute change was calculated from the weighted mean difference (WMD) statistic in RevMan when trials using the same scale were pooled. Relative per cent change from baseline was calculated as the absolute benefit divided by the baseline mean of the control group. Since there were no statistically significant outcome measures, the number needed to treat (NNT) was not calculated.
Results
Description of studies
Review authors TT and MvB selected a total of 16 citations of full‐length reports and abstracts describing seven controlled exercise therapy trials (Epps 2005; Keller‐Marchand 2006; Moncur 1990; Öberg 1994; Singh‐Grewal 2007; Takken 2003; Vostrejs 1985). In one case, an article in the German language was obtained; this study was considered for inclusion because the review authors were able to read this language as well. Authors of abstracts were asked for a full‐length manuscript. Two authors of abstracts responded to our call (Fisher 1999; Keller‐Marchand 2006). However, they were not able to provide a full‐length version. Nor could they provide any details because the full‐length article was not submitted yet. The author of the third abstract did not reply (Moncur 1990). Four studies reported in seven controlled trials were identified by the review authors as randomised controlled trials (Epps 2005; Keller‐Marchand 2006; Singh‐Grewal 2007; Takken 2003). Three out of these four RCTs were included in this review; one was excluded because it was not a full‐length article. Following is a brief description of the three remaining studies. Epps 2005 carried out a RCT in which 78 children (43 girls, 35 boys; aged 4 to 19 years) with JIA were randomly allocated to receive a combined (hydrotherapy and land‐based physiotherapy) or a land‐based (only land‐based physiotherapy) training program. The children in both groups received 16 one‐hour treatment sessions over two weeks followed by local physiotherapy attendances for two months. Thirty‐nine children were allocated to the combined group. The primary outcome measures included improvement in disease status which was calculated from six core outcome measures: childhood health assessment questionnaire (CHAQ), physician's global assessment of disease activity, parent's global assessment of overall wellbeing, number of joints with limited range of motion (ROM), number of active joints and erythrocyte sedimentation rate; the secondary outcome measures included health‐related quality of life (CHQ‐PF50), muscle strength (peak power), cardiovascular fitness (time and maximal heart rate), pain (VAS scale) and patient satisfaction. These parameters were measured at baseline, two‐months follow up and six‐months follow up. The authors found that: "Two months after intervention 47% patients in the combined group and 61% patients in the land group had improved disease activity with 11 and 5% worsened, respectively". All secondary outcome measures demonstrated a mean improvement in both groups, with the combined group showing greater improvements compared with the land‐based group in physical aspects of HRQoL (improvement of 33 versus 28 in the land‐based group) and physical fitness.
Singh‐Grewal 2007 carried out an RCT in which 80 children (43 girls, 35 boys; aged 4 to 19 years) with JIA were randomly allocated to a high‐intensity aerobic training program (experimental group) or low‐intensity training program (control group). Both groups participated in a 12‐week, three times weekly training program consisting of high‐intensity aerobics in the experimental group and Qigong in the control group. Forty‐one children were allocated to the experimental group. The outcome measures included submaximal oxygen uptake at 3 km/hour (VO2submax), maximal oxygen uptake (VO2peak), peak power and functional ability (CHAQ). These parameters were measured at baseline and after completing the training program. The authors found that the exercise program was well tolerated in both groups. There was no difference in VO2submax (P = 0.43) or in any other exercise‐testing measure between the groups throughout the study period and no indication of improvement. The functional ability (CHAQ) was similar between groups (P = 0.80) although the within‐group change was statistically significant (mean difference ‐0.12; P < 0.0001) and clinically meaningful in magnitude.
Takken 2003 carried out an RCT in which 54 children (38 girls, 16 boys; aged 5 to 13 years) with JIA were randomly allocated to receive a training program consisting of a one hour per week supervised training program for approximately 20 sessions in a local pool or to a control group that received standard medical care assessment only. Twenty‐seven children were allocated to the experimental group. The outcome measures included functional ability (CHAQ and JAFAS), health‐related quality of life (CHQ), range of motion, joint status and physical fitness. These parameters were measured at baseline, three months after the start and immediately after the end of the training program. The authors found no significant effects on functional ability (P = 0.35, P = 0.55 for CHAQ and JAFAS, respectively), range of motion (P = 0.06), health‐related quality of life (P = 0.19, P = 0.09, P = 0.13 for JAQQ, CHQ‐PhS (physical summary score) and CHQ‐PsS (psychosocial summary score), respectively) and physical fitness (P = 0.46).
In summary, of the three studies none compared exercise therapy to a placebo, two studies compared exercise therapy to another therapy and one study compared exercise therapy to receiving standard medical care. The excluded studies are described in the 'Characteristics of excluded studies' table, which provides a summary of why studies were excluded from this review. The number of participating children varied from 54 to 80, with a median number of 78. The age range was between 4 years to 19 years of age. Functional ability, health‐related quality of life and aerobic capacity could be pooled for meta‐analysis as the same outcome measures were used in the included studies. Heterogeneity in test protocol, test equipment, outcome or failure to report the measures for range of motion, number of joints with swelling, number of joints with pain, parent or patient global assessment of overall wellbeing and pain made pooling of these outcome measurements inappropriate. The exercise therapy programs of the included studies showed a great range in duration and exercise frequency (see table 'Characteristics of included studies'). Moreover, both land‐based and pool‐based modalities were used.
Risk of bias in included studies
The following PEDro scores were obtained (maximal score = 10): Epps 2005: 8, Singh‐Grewal 2007: 8, Takken 2003: 7. None of the studies described blinding of the participants or therapists who administered the therapy. Based on the characteristics of the therapy, the included studies were categorised into one of three quality categories as described in the Cochrane Handbook for Systematic Reviews of Interventions version 4.2.5 (Higgins 2005). The categories were as follows. Epps 2005: moderate risk of bias due to no blinding of participants or therapists; all other criteria were met. Singh‐Grewal 2007: moderate risk of bias due to no blinding of participants or therapists; all other criteria were met. Takken 2003: moderate risk of bias due to no blinding of participants, therapists and assessors; all other criteria were met. The evidence on the outcome measures from the studies of Epps 2005, Singh‐Grewal 2007 and Takken 2003 were all graded as silver.
Effects of interventions
Primary outcomes Functional ability (pooled) There was no statistically significant change in functional ability (CHAQ) between exercise and the control (n = 198; WMD ‐0.07, 95% CI ‐0.22 to 0.08). Moreover, no significant differences were observed for studies which used exercise versus standard medical care assessment (Takken 2003) (P = 0.35) or when comparing two different exercise modes (Epps 2005; Singh‐Grewal 2007). This is expressed by I2 = 0 % in the test for heterogeneity between studies. Takken 2003 measured functional ability using the JAFAS as well. The scores were very low (a mean score of 0.15 at baseline for the experimental group). The JAFAS scores range from 0 to 2 and a score of 0.15 is very close to the lowest possible score on this instrument. This so called 'floor effect' makes improvement on this instrument almost impossible. The JAFAS showed no significant differences between the groups (P = 0.55).
Joint range of motion (descriptive) There was no statistically significant improvement in joint range of motion between the exercise and control groups. Epps 2005 reported a decrease in the number of joints with loss of range of motion after two months follow up in the combined group and in the land group (decrease of five and four joints, respectively); however, this decrease was not statistically significant. Takken 2003 also reported no significant changes in range of motion from baseline measurement to immediately after the intervention (P = 0.06). Both groups showed a very small decrease over time (a decrease of 0.02 and 0.07 on the EPROM score in the intervention group and control group, respectively). Singh‐Grewal 2007 reported that there was no worsening of range of motion (EPMROM) and also no differences between the groups (P = 0.35).
Number of joints with swelling (descriptive) None of the included studies reported a statistically significant difference in the number of joints with swelling. Epps 2005 reported a decrease in the number of active joints after two‐months follow up, in the combined group as well as the land group (a decrease of four and three active joints, respectively). Takken 2003 reported that the number of swollen and tender joints decreased in the intervention group (‐55%) while the number of swollen and tender joints increased in the control group (+21%). These differences were almost statistically significant (P = 0.07). Singh‐Grewal 2007 reported no significant changes in active joint count between the groups (P = 0.41). Number of joints with pain (descriptive) None of the included studies (Epps 2005; Singh‐Grewal 2007; Takken 2003) measured the number of joints with pain.
Health‐related quality of life (pooled) All outcome measures of health‐related quality of life indicated that there was no clinically important or statistically significant change in health‐related quality of life between exercise and control groups (CHQ‐PhS: n = 115; WMD ‐3.96, 95% CI ‐8.91 to 1.00; CHQ‐PsS: n = 112; WMD 2.57, 95% CI ‐0.69 to 5.82; JAQQ: n = 54; SMD 0.33, 95% CI ‐0.20 to 0.87; QoL: n = 70; SMD 0.17, 95% CI ‐0.30 to 0.64). Moreover, no significant differences for both CHQ outcome measures (P = 0.09, P = 0.13 for CHQ‐PhS and CHQ‐PsS, respectively) were observed between studies which used exercise versus standard medical assessment as control (Takken 2003) or when comparing two different exercise modes (Epps 2005). This is expressed with I2 = 0 % in the test for heterogeneity between the studies. Both JAQQ (Takken 2003) and QoL (Singh‐Grewal 2007) did not show any significant effects of the intervention over control or a different exercise mode (P = 0.19 and P = 0.47, respectively).
Parent or patient global assessment of overall wellbeing (descriptive) None of the included studies measuring parent or patient global assessment of overall wellbeing reported a statistical significant difference. Epps 2005 reported a decrease in scores on the VAS‐scale for parent global assessment of overall wellbeing after two months follow up in the combined group as well as in the land group (decrease of 6 mm and 7 mm, respectively). Singh‐Grewal 2007 and Takken 2003 did not measure parent or patient global assessment in their studies.
Pain (descriptive) None of the included studies measuring pain reported a significant decrease. Epps 2005 reported no significant decrease in pain, the change in pain was negligible. The pain score was decreased by 0.6 mm (on a 10 cm VAS scale) in the land group and increased by 7.3 mm in the combined exercise group. Singh‐Grewal 2007 reported low levels of pain on a 10 cm VAS scale during training sessions. The differences were not different between the two groups (median 0, range 0 to 10 in both groups; P = 0.09). Takken 2003 did not measure pain in their study.
Adverse outcomes All included studies assessed possible adverse outcomes; however, none of these studies reported negative effects of the exercise therapy. Dropouts Epps 2005 reported a total of six dropouts after randomisation. Four patients did not complete a two‐month assessment, two withdrew and two were lost to follow up. Two children could not be entered into the primary analysis because the preliminary definition of disease improvement was inconclusive. Therefore, 72 of 78 potential data sets were available for primary analysis. Singh‐Grewal 2007 reported a total of 10 dropouts after randomisation: six children from the experimental group and four from the control group. In the experimental group, four dropped out before and two after baseline testing. In the control group, one dropped out before and three after baseline testing. All reported a lack of time as the reason for dropping out. Takken 2003 reported one dropout during the training program; one boy stopped the training program after 15 sessions. Since he met the 75% criteria of 20 sessions, his data were not excluded from analysis.
Secondary outcomes
Aerobic capacity (pooled) There was no statistically significant change in aerobic capacity (VO2peak) between the exercise and control groups (n= 124; WMD 0.04, 95% CI ‐0.11 to 0.19). Moreover, no significant differences were observed for studies which used exercise versus standard medical care assessment (P = 0.46) (Takken 2003) or when comparing two different exercise modes (P = 0.35) (Singh‐Grewal 2007). This is expressed with I2 = 0 % in the test for heterogeneity between the studies. Epps 2005 did not measure VO2peak in her study.
Muscle strength (descriptive) Epps 2005 reported a small increase in the three muscle groups, in both groups, but none of the increases were statistically significant (P values were not provided). Singh‐Grewal 2007 and Takken 2003 did not measure muscle strength in their studies.
Compliance with exercise (descriptive) Epps 2005 reported that four patients did not complete a two‐month assessment, two withdrew and two were lost to follow up. Singh‐Grewal 2007 reported that completion of training sessions was 78% in the control group and 56% in the experimental group. An average of two sessions per week was completed by the experimental participants and 1.7 sessions by the control participants. The difference was most apparent in the number of home‐based sessions. Takken 2003 reported that the children attended a mean number of 19.6 ± 3.9 out of 20 training sessions.
Pooled outcome measures were also given in clinical relevance tables, where possible (see Additional Table 1; Table 2; Table 3; Table 4).
1. Table 1 Clinical relevance table for functional ability.
| Outcome (scale) | # patients (# trials) | Control baseline mean * | Wt absolute change | Relative % change* | NNT (B) or NNT (H) | Statistical significance | Quality of evidence |
| CHAQ (0 ‐ 3) | 200 (3) | 0.59 | ‐ 2,3% | ‐12% (I) | n/a | Not statistically significant | Silver |
| 95% confidence interval | (‐7.3%, 26%) | (‐37%; 14%) | |||||
| Legend CHAQ=Childhood Health Assessment Questionnaire | * using generic inverse variance method | I = Improvement | NNT/ B or H = Number Needed to Treat to Benefit or Harm n/ a = not applicable |
2. Table 2 Clinical relevance table for quality of life (CHQ‐PhS).
| Outcome (scale) | # patients (# trials) | Control baseline mean* | Wt absolute change | Relative % change* | NNT (B) or NNT (H) | Statistical significance | Quality of evidence |
| CHQ‐PhS (0‐50) | 115 (2) | 36.57 | ‐8% | ‐11% (I) | n/ a | Not statistically significant | Silver |
| 95% convidence interval | (‐17%; 2%) | (‐24%; 3%) | |||||
| Legend CHQ‐PhS=Child Health Questionnaire Physical Summary Score | * using generic inverse variance method | I = Improvement | NNT/ B or H= Number Needed to Treat to Benefit or Harm n/a= not applicable |
3. Table 3 Clinical relevance table for quality of life (CHQ‐PsS).
| Outcome (scale) | # patients (# trials) | Control baseline mean* | Wt Absolute change | Relative % change* | NNT (B) or NNT (H) | Statistical significance | Quality of evidence |
| CHQ‐PsS (0‐50) | 112(2) | 47.70 | 5% | 5.4%(I) | n/ a | Not statistically significant | Silver |
| 95% confidence interval | (‐1%; 12%) | (‐1%; 12%) | |||||
| Legend CHQ‐PsS=Child Health Questionnaire Psychosocial Summary Score | * using generic inverse variance method | I = Improvement | NNT/ B or H = Number Needed to Treat to Benefit or Harm n/a = not applicable |
4. Table 4 Clinical relevance table for aerobic capacity.
| Outcome (scale) | # patients (# trials) | Control baseline mean* | Wt absolute change | Relative % change * | NNT (B) or NNT (H) | Statistical significance | Quality of evidence |
| VO2peak (n/ a) | 124(2) | 1.0 | n/ a | 4%(d) | n/ a | Not statistically significant | Silver |
| 95% confidence interval | n/ a | (‐11%; 19%) | |||||
| Legend | * using generic inverse method | d=decrease | NNT/ B or H = Number Needed to Treat to Benefit of Harm n/a = not applicable |
Discussion
This review analysed the results of three randomised controlled trials (RCTs) for the effectiveness of exercise therapy in children with JIA. By applying strict selection criteria for inclusion, only full‐length reports of randomised controlled trials were included. All three trials met at least seven criteria on the PEDro scale. Due to the nature of the interventions, the criteria 'patient blinded' and 'care provider blinded' could not be scored. Therefore, a score of eight might be considered as the best score possible in this kind of intervention study. Evidence on the outcome measures in the included studies were all graded as 'silver' according to the grading system described by Tugwell 2004.
The trends for the outcome measures functional ability, joint range of motion, number of joints with swelling, health‐related quality of life and aerobic capacity were similar. These outcome measures were all in favour of treatment changes but were not statistically significant. Functional ability, health‐related quality of life and aerobic capacity were the only outcome measures which could be pooled into a meta‐analysis and, therefore, could be used to provide stronger evidence compared to the other outcome measures. Importantly, all outcome measures reported no worsening with exercise therapy in the short term. The study of Takken 2003 showed a decrease in the number of joints with swelling after aquatic fitness training; this was the only study where improvements almost reached statistical significance. The randomised design also allowed controlling for maturation and developmental effect on the outcome measures such as functional ability, health‐related quality of life, aerobic capacity and muscle strength. The size of the improvement on functional ability (CHAQ) in the pooled data (n = 231) is still clinically irrelevant when compared with the results of the study of Dempster 2001, who state that the minimal clinical important improvement on the CHAQ is a reduction in score of 0.13. Our meta‐analysis showed an average reduction of 0.07 and can not be considered clinically relevant. The CHAQ has been demonstrated to suffer from a floor effect whereby scores are clustered at the normal end of the scale, or near 0 (Huber 2001; Ruperto 1999; Bode 2003). This floor effect is also observed in this review and, therefore, might explain the missing significant improvement with exercise therapy.
Results for pain were contradictory. In the study of Epps 2005, the pain score was marginally decreased in the land‐based group but increased in the combined group. Because these differences were small (7.9 mm on a VAS scale from 0 to 100 mm) it is hard to determine if these changes could be explained by the different types of training or by measurement errors of the outcome measure. The study of Singh‐Grewal 2007 showed low levels of pain in both therapies. It is important to note that none of the participants of the included studies withdrew because of pain during exercise therapy. Parent or patient global assessment of overall wellbeing and muscle strength was only described in the study of Epps 2005. The evidence on this outcome measure is, therefore, inconclusive. The evidence in support of the effectiveness of exercise therapy on the number of joints with pain is also inconclusive as none of the included studies described this outcome measure in detail.
The excluded studies found comparable results for functional ability (Singh‐Grewal 2006; Takken 2001), health‐related quality of life (Singh‐Grewal 2006; Takken 2001) and aerobic capacity (Fisher 1999; Singh‐Grewal 2006). However, none of the studies reported improvements. Pain scores reported in the excluded studies (Klepper 1999; Fisher 1999; Singh‐Grewal 2006) did not show increases in pain. Furthermore, none of the excluded studies described the parent/ or atient global assessment of overall wellbeing or the number of joints with pain.
The following findings in the excluded non‐RCT studies were in contrast with the findings in the included RCT studies: the number of joints with swelling (Baldwin 1972; Klepper 1999; Moncur 1990), muscle strength (Fisher 1999; Öberg 1994) and joint ROM (Bacon 1991; Latzka 1982). The study of Baldwin 1972 reported, despite the lack of data, that the changes in the number of joints with swelling were not statistically significant. The studies of Moncur 1990 and Klepper 1999 both reported a significant decrease in the joint count after intervention. The studies of Fisher 1999 and Öberg 1994 reported muscle strength before and after intervention. Both studies reported significant increases in quadriceps strength; Fisher 1999 also reported a significant increase in hamstring strength. Both studies only studied two muscle groups. The studies of Bacon 1991 and Latzka 1982 both reported significant improvements in joint ROM but only described a few joints. The strength of this review lies in its rigorous methods, which include thorough grading of evidence, systematic appraisal of study quality and, where possible, the use of meta‐analysis. However, its main limitation is the low number of available RCTs. There were only seven controlled trials of exercise therapy for JIA found, of which three with a total number of 212 participants could be included. Because there are few RCTs, indirect methods of identifying publication bias such as funnel plots are of limited value and were not conducted. The limited number of studies, participants, and the heterogeneity of interventions and outcome measures limits the precision of the results of this review. Consequently, it also means that a single unidentified trial, or further trials, could have a substantial effect on the results and conclusions.
Overall, exercise therapy did not result in significant effects on functional ability, health‐related quality of life, aerobic capacity or pain. However, the low number of available RCTs limits the generalisability. The included and excluded studies are all consistent about adverse effects of exercise therapy; no short‐term detrimental effects of exercise therapy were found in any of the studies. Both the included and excluded studies showed that exercise does not exacerbate the arthritis. The large heterogeneity in outcome measures, as seen in this review, emphasises the need for a standardised assessment or a core set of functional and physical outcome measurements suited for health research to generate evidence about the possible effects of physical exercise for children with JIA. Despite the short‐term results of the intervention studies, the long‐term effect of exercise therapy remains unclear and warrants further research.
Authors' conclusions
Implications for practice.
Nowadays, exercise therapy is increasingly studied as treatment in childhood and juvenile arthritic conditions. This current review shows that the lack of statistically significant differences between intervention and control groups makes it difficult to conclude whether exercise therapy can be recommended as an effective treatment for JIA. Important to know is that both the included and the excluded studies were consistent about adverse effects of exercise therapy. None of the studies found short‐term detrimental effects of exercise therapy.
Implications for research.
The evidence regarding the efficacy of exercise therapy is drawn from a small number of randomised controlled trials. There was limited uniformity of outcome measures which limited the ability to pool data for a reliable meta‐analysis. The large heterogeneity in outcome measures, as is seen in this review, emphasises the need for a standardised assessment or a core set of functional and physical outcome measurements suited for health research to generate evidence about the possible benefits of exercise therapy for children with JIA, and only then can the true importance of exercise therapy be stated.
What's new
| Date | Event | Description |
|---|---|---|
| 14 June 2008 | Amended | Converted to new review format. CMSG ID C037‐R |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 11 December 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Supported in part by a grant from the Dutch Arthritis Association (IMP‐04‐01).
Data and analyses
Comparison 1. Functional ability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CHAQ | 3 | 198 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.22, 0.08] |
1.1. Analysis.
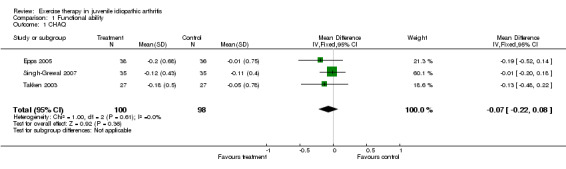
Comparison 1 Functional ability, Outcome 1 CHAQ.
Comparison 2. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CHQ‐PhS | 2 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐3.96 [‐8.91, 1.00] |
2.1. Analysis.
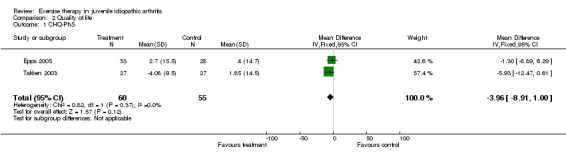
Comparison 2 Quality of life, Outcome 1 CHQ‐PhS.
Comparison 3. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CHQ‐PsS | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | 2.57 [‐0.69, 5.82] |
3.1. Analysis.
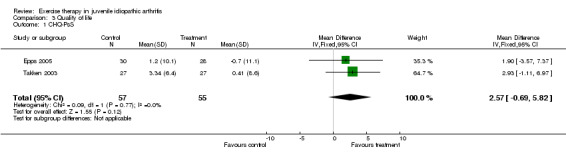
Comparison 3 Quality of life, Outcome 1 CHQ‐PsS.
Comparison 4. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 JAQQ and QoL (vAS) | 2 | 124 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.11, 0.60] |
| 1.1 JAQQ | 1 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.20, 0.87] |
| 1.2 QoL | 1 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.30, 0.64] |
4.1. Analysis.
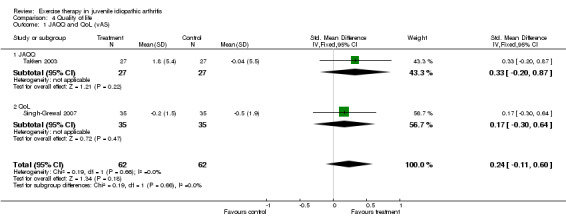
Comparison 4 Quality of life, Outcome 1 JAQQ and QoL (vAS).
Comparison 5. Aerobic capacity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VO2peak | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.11, 0.19] |
5.1. Analysis.
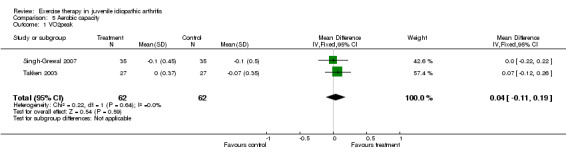
Comparison 5 Aerobic capacity, Outcome 1 VO2peak.
Comparison 6. Mean baseline functional ability calculated using generic inverse method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean baseline functional ability | 3 | generic inverse (Fixed, 95% CI) | 0.59 [0.47, 0.71] |
6.1. Analysis.
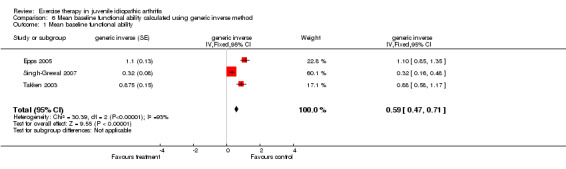
Comparison 6 Mean baseline functional ability calculated using generic inverse method, Outcome 1 Mean baseline functional ability.
Comparison 7. Mean baseline quality of life (CHQ‐PhS) calcualted using generic inverse method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CHQ‐PhS | 2 | quality of life (Fixed, 95% CI) | 36.57 [32.97, 40.18] |
7.1. Analysis.
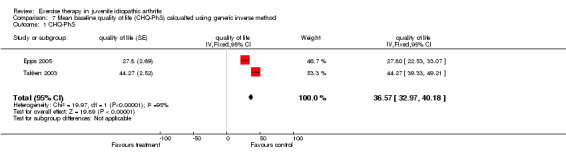
Comparison 7 Mean baseline quality of life (CHQ‐PhS) calcualted using generic inverse method, Outcome 1 CHQ‐PhS.
Comparison 8. Mean baseline quality of life (CHQ‐PsS) calculated using generic inverse method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 quality of life (CHQ‐PsS) | 2 | quality of life (Fixed, 95% CI) | 47.70 [44.91, 50.50] |
8.1. Analysis.
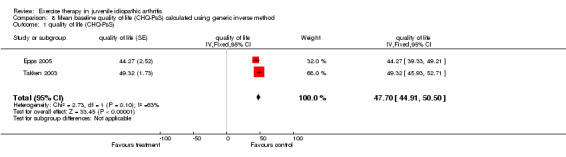
Comparison 8 Mean baseline quality of life (CHQ‐PsS) calculated using generic inverse method, Outcome 1 quality of life (CHQ‐PsS).
Comparison 9. mean baseline aerobic capacity calculated using generic inverse method.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 aerobic capacity | 2 | VO2peak (Fixed, 95% CI) | 1.00 [0.87, 1.14] |
9.1. Analysis.
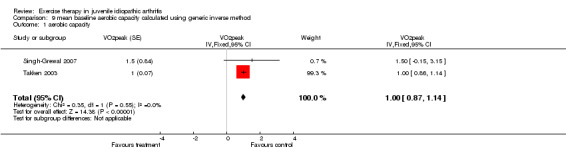
Comparison 9 mean baseline aerobic capacity calculated using generic inverse method, Outcome 1 aerobic capacity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Epps 2005.
| Methods | RCT comparing the effects of combined hydrotherapy programmes vs physiotherapy land techniques. Blinding: subjects and therapists were not blinded; assessors were blinded. Baseline: no significant differences, only difference the distribution of gender between the two groups. Dropouts: 4 dropouts after randomisation. | |
| Participants | 78 children with JIA (43 girls, 35 boys), aged 4‐19 years. 7 children had oJIA, 15 children had extended oJIA, 33 children pJIA, 10 children sJIA, 12 children had enthesitis‐related arthritis, and 1 child had psoriatic arhtriis with psoriasis. Exclusion: severe systemic disease or any other condition that is unstable, suffering from quotidian fevers, inablity to give informed consent or complete questionnaires owing to language barriers, musculoskeletal surgery within previous 6 months, neuromusclular condition which increases muscle tone, received intensive physiotherapy , no access to outpatient physiotherapy or hydrotherapy, and met general hydrotherapy exclusion criteria, such as chlorine allergy. | |
| Interventions | Patients in the combined and land groups received 16 1‐hour sessions of treatment over two weeks followed by physiotherapy attendances for 2 months. | |
| Outcomes | Improvement in disease status (CHAQ, physicians Measures: at baseline, after 2‐month follow up, and after 6‐month follow up. | |
| Notes | PEDro‐score: 8/10 Rank outcome measures: silver | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Singh‐Grewal 2007.
| Methods | RCT comparing the effectiveness of high intensity aerobic training vs low intensity training. Blinding: subjects and therapists were not blinded; assessors were blinded. Baseline: no significant differences, only difference were evident in the distribution of JIA subgroups between the two groups. Dropouts: 10 dropouts after randomisation; 6 from the experimental and 4 from the control group. | |
| Participants | 80 children with JIA (29 girls, 35 boys), aged 8 ‐16 years. 7 children had oJIA, 11 children had extended oJIA, 34 children pJIA, 7 children sJIA, 11 children enthesitis related JIA, 8 children psoriatic JIA, and 2 children with an "other" form of JIA. Exclusion: significant cardiac, pulmonary or metabolic comorbidity; moderate or severe hip pain while walking, and were unable to cooperate with training or testing. | |
| Interventions | Both groups participated in a 12‐week, three times weekly training program; high intensity in the experimental group and Qigong in the control group. | |
| Outcomes | Aerobic capacity (submaximal oxygen uptake (VO2submax), maximal oxygen uptake VO2max), and peak power (PP)), quality of life (QoL and HRQoL), and physical function (CHAQ). Measures: at enrollment, at baseline and within 2 weeks of completion of the training program. | |
| Notes | PEDro‐score: 8/10 Rank outcome measures: silver | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Takken 2003.
| Methods | RCT comparing an aquatic training program vs controls. Blinding: subject, therapist and assessors were not blinded. Baseline: no significant differences. Dropouts: one dropout ; data not excluded from analysis. | |
| Participants | 54 children with JIA (38 girls, 16 boys), aged 5 ‐13 years. 23 children had oJIA, 29 children pJIA and 2 children sJIA. Exclusion: a systemic disease with fever, low haemoglobin level and a general feeling of malaise; exercise contraindication by a medical specialist; a recipient of a bone marrow transplant; and not feeling confident in water. | |
| Interventions | All patients received their usual care and medical treatment during the study. The patients in the experimental group received an aquatic group (2‐4 children/ groep) exercise program, 1 h a week , supervised by an instructed community physical therapist, for 6 months. | |
| Outcomes | Included outcomes: function ability (CHAQ and JAFAS), Health‐related quality of life (JAQQ and CHQ), joint status (pEPMROM), and aerobic capacity (VO2peak, 6‐min walking test). Measures: at baseline, 3 months, and 6 months. | |
| Notes | PEDro‐score: 7/10 Rank outcome measures: silver | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bacon 1991 | Not a randomised trial pilot study |
| Baldwin 1972 | Not a randomised trial |
| Fisher 1999 | Inadequate data Only abstract Author was contacted but full manuscript was not published |
| Keller‐Marchand 2006 | Absence of data Only abstract Author was contacted but full manuscript was not published |
| Klepper 1999 | Not a randomised trial |
| Latzka 1982 | Not a randomised trial Inadequate data |
| Milliet 1996 | Not a randomised trial No heterogeneous population |
| Moncur 1990 | Inadequate data Only abstract Author was contacted but full manuscript was not published |
| Singh‐Grewal 2006 | Not a randomised trial |
| Takken 2001 | Not a randomised trial |
| Tork 1989 | Not a randomised trial Survey only Only one child in population |
| Vostrejs 1985 | Inadequate data Only abstract, author was contacted but did not reply |
| Öberg 1994 | Inadequate data |
Contributions of authors
TT, JVDN, and PJMH provided the idea. TT and MVB drafted the protocol. JVDN, RHHE, WK, and PJMH provided comments on the protocol and manuscript. TT entered the protocol and manuscript into RevMan and responded to peer review comments. TT and MVB conducted the searches and selected the trials. TT and MVB extracted, analysed and interpreted the data. TT, MVB, RHHE, JVDN, WK, and PJMH contributed to the methodological expertise and to the writing of the review.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Epps 2005 {published data only}
- Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, Woo P. Is hydrotherapy cost effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis. Health Technology Assessment 2005;9:1‐76. [DOI] [PubMed] [Google Scholar]
Singh‐Grewal 2007 {published data only}
- Singh‐Grewal D, Schneiderman‐Walker J, Wright V, Bar‐Or O, Selvadurai H, Cameron B, et al. The effects of vigorous exercise training on physical function in children with arthritis: a randomized, controlled, single‐blinded trial. Arthritis and Rheumatism 2007;57:1202‐10. [DOI] [PubMed] [Google Scholar]
Takken 2003 {published data only}
- Takken T, Net J, Kuis W, Helders PJM. Aquatic fitness training for children with juvenile idiopathic arthritis. Rheumatology 2003;42:1408‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bacon 1991 {published data only}
- Bacon MC, Nicholson C, Binder H, White PH. Juvenile rheumatoid arthritis. Aquatic exercise and lower‐extremity function. Arthritis and Rheumatism 1991;4:102‐5. [DOI] [PubMed] [Google Scholar]
Baldwin 1972 {published data only}
- Baldwin J. Pool therapy compared with individual home exercise therapy for juvenile rheumatoid arthritic patients. Physiotherapy 1972;58:230‐1. [PubMed] [Google Scholar]
Fisher 1999 {published data only}
- Fisher NM, Venkatraman JT, O'Neil KM. Effects of resistance exercise on children with juvenile arthritis. Arthritis and Rheumatism 1999;42(Suppl):396. [Google Scholar]
Keller‐Marchand 2006 {published data only}
- Keller‐ Marchand L, Farpour‐Lambert NJ, Hans D, Rizzoli R, Hofer MF. Effects of a weight‐bearing exercise program in children with juvenile idiopathic arthritis. Medicine and Science in Sports and Exercise 2006;Suppl(5):93‐4. [Google Scholar]
Klepper 1999 {published data only}
- Klepper SE. Effects of an eight‐week physical conditioning program on disease signs and symptoms in children with chronic arthritis. Arthritis Care and Research 1999;12:52‐60. [DOI] [PubMed] [Google Scholar]
Latzka 1982 {published data only}
- Latzka U, Böhler N, Lukeschitsch G, Pilz I. Österreichische therapieferien für kinder mit juveniler chronischer arthritis. Österreichische Ärztezeitung: Organ der Östereichischen Ärztekamme 1982;37:1561‐5. [Google Scholar]
Milliet 1996 {published data only}
- Milliet J, Carman D, Browne R. Summer camp: effects on function of children with autoimmune diseases. Arthritis Care and Reseach 1996;9:309‐14. [DOI] [PubMed] [Google Scholar]
Moncur 1990 {published data only}
- Moncur C, Marcus R, Johnson S. Pilot project of aerobic conditioning of subjects with juvenile arthritis. Arthritis Care and Research 1990;3(Suppl):149. [Google Scholar]
Singh‐Grewal 2006 {published data only}
- Singh‐ Grewal D, Wright V, Bar‐Or O, Feldman BM. Pilot study of fitness training and exercise testing in polyarticular childhood arthritis. Arthritis and Rheumatism 2006;55:364‐72. [DOI] [PubMed] [Google Scholar]
Takken 2001 {published data only}
- Takken T, Net J, Helders PJM. Do juvenile idiopathic arthritis patients benefit from an exercise program? A pilot study. Arthritis and Rheumatism 2001;45:81‐5. [DOI] [PubMed] [Google Scholar]
Tork 1989 {published data only}
- Tork SC, Douglas V. Arthritis water exercise program evaluation. Arthritis Care and Research 1989;2:28‐36. [DOI] [PubMed] [Google Scholar]
Vostrejs 1985 {published data only}
- Vostrejs M, Hollister JR. The effectiveness of an aquanastics program: a controlled study. Arthritis and Rheumatism 1985;28(Suppl):150. [Google Scholar]
Öberg 1994 {published data only}
- Öberg T, Karsnia A, Andersson Gäre B, Lagerstrand A. Physical training of children with juvenile chronic arthritis. Scandanavian Journal of Rheumatology 1994;23:92‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Bar‐Or 2004
- Bar‐Or O, Rowland TW. Pediatric Exercise Medicine. Human Kinetics, 2004. [Google Scholar]
Bode 2003
- Bode RK, Klein‐Gitelman S, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis and Rheumatism 2003;49:7‐15. [DOI] [PubMed] [Google Scholar]
De Jong 2003
- Jong Z, Munneke M, Zwinderman AH, Kroon HM, Jansen A, Ronday KH. Is a long‐term high‐intensity exercise program effective and safe in patients with rheumatoid arthritis? Results of a randomized controlled trail. Arthritis and Rheumatism 2003;48:2415‐24. [DOI] [PubMed] [Google Scholar]
Dempster 2001
- Dempster H, Porepa M, Young N, Feldman BM. The Clinical Meaning of Functional Outcome Scores in Children With Juvenile Arthritis. Arthritis and Rheumatism 2001;44:1768‐74. [DOI] [PubMed] [Google Scholar]
Giannini 1997
- Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis and Rheumatism 1997;40:1202‐9. [DOI] [PubMed] [Google Scholar]
Hakkinen 2003
- Hakkinen A, Hannonen P, Nyman K, Lyyski T, Hakkinen K. Effects of concurrent strength and endurance training in women with early or longstanding rheumatoid arthritis: comparison with healthy subjects. Arthritis and Rheumatism 2003;49:789‐97. [DOI] [PubMed] [Google Scholar]
Hakkinen 2004
- Hakkinen A. Effectiveness and safety of strength training in rheumatoid arthritis. Current Opinion in Rheumatology 2004;16:132‐7. [DOI] [PubMed] [Google Scholar]
Hakkinen 2005
- Hakkinen A, Pakarinen A, Hannonen P Kautiainen H, Nyman K, Kraemer WJ, Hakkinen K. Effects of prolonged combined strength and endurance training on physical fitness, body composition and serum hormones in women with rheumatoid arthritis and in healthy controls. Clinical and Experimental Rheumatology 2005;23:505‐12. [PubMed] [Google Scholar]
Henderson 1995
- Henderson CJ, Lovell DJ, Specker BL, Campaigne BN. Physical activity in children with juvenile rheumatoid arthritis. Arthritis Care and Research 1995;8(2):114‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Assessing methodological qualtiy. Section 6.7.1.. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. Chichester, UK: John Wiley & Sons, Ltd..
Huber 2001
- Huber AM, Hicks JE, Lachenbruch PA, Perez MD, Zemel LS, Rennebohm RM, et al. Juvenile Dermatomyositis Disease Activity Collaborative Study Group. Validation of the Childhood Health Assessment Questionnaire in the juvenile idiopathic myopathies. The Journal of Rheumatology 2001;28:1106‐11. [PubMed] [Google Scholar]
Kirchheimer 1993
- Kirchheimer JC, Wanivenhaus A, Engel A. Does sport negatively influence joint scores in patients with juvenile rheumatoid arthritis. An 8‐year prospective study. Rheumatology International 1993;12:239‐42. [DOI] [PubMed] [Google Scholar]
Klepper 1991
- Klepper SE. Effects of an eight‐week physical conditioning program on disease signs and symptoms in children with chronic arthritis. Arthritis Care and Research 1999;12:52‐60. [DOI] [PubMed] [Google Scholar]
Landgraf 1996
- Landgraf MA, Abetz L, Ware JE. The CHQ user's manual. First edition, editor Boston MA. New England Medical Center, 1996. [Google Scholar]
Lovell 1989
- Lovell DJ, Howe S, Shear E, Hartner S, McGirr G, Schulte M. Development of a disability measurement tool for juvenile rheumatoid arthritis. The Juvenile Arthritis Functional Assessment Scale. Arthritis and Rheumatism 1989;32:1390‐5. [DOI] [PubMed] [Google Scholar]
Omeract 1997
- OMERACT. OMERACT III. Outcome measures in arthritis clinical trials. Cairns, Australia, April 16‐19, 1996. Proceedings. The Journal of Rheumatology 1997;24:763‐802. [PubMed] [Google Scholar]
Petty 2004
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology Classification of Juvenile Idiopathic Arthritis: Second revision, Edmonton, 2001. The Journal of Rheumatology 2004;31:390‐2. [PubMed] [Google Scholar]
Ravelli 2007
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767‐78. [DOI] [PubMed] [Google Scholar]
Ruperto 1999
- Ruperto N, Ravelli A, Migliavacca D, Viola S, Pistorio A, Duarte C, et al. Responsiveness of clinical measures in children with oligoarticular juvenile chronic arthritis. The Journal of Rheumatology 1999;26:1827‐30. [PubMed] [Google Scholar]
Sherrington 2000
- Sherrington C, Herbert RD, Maher CG, Moseley AM. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Manual Theory 2000;5:223‐6. [DOI] [PubMed] [Google Scholar]
Sing 1994
- Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis and Rheumatism 1994;37:1761‐9. [DOI] [PubMed] [Google Scholar]
Takken 2003b
- Takken T, Net J, Kuis W, Helders PJ. Physical activity and health related physical fitness in children with juvenile idiopathic arthritis. Annals of the Rheumatic Diseases 2003;62:885‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tugwell 2004
- Tugwell P, Shea B, Boers M, Brooks P, Simon L, Strand V, Wells G (editors). Evidence‐based Rheumatology. London: BMJ Books, 2004. [Google Scholar]
Van den Ende 1998
- Ende CH, Vliet Vlieland TP, Munneke M, Hazes JM. Dynamic exercise therapy in rheumatoid arthritis: a systematic review. British Journal of Rheumatology 1998;37:677‐87. [DOI] [PubMed] [Google Scholar]
Verhagen 1998
- Verhagen AP, Vet HC, Bie RA, Kessels AG, Boers M, Bouter LM. The Delhi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. Journal of Clinical Epidemiology 1998;51:1235‐41. [DOI] [PubMed] [Google Scholar]
Wright 1996
- Wright FV, Kimber JL, Law M, Goldsmith CH, Crombie V, Dent P. The Juvenile Arthritis Functional Status Index (JASI): a validation study. Journal of Rheumatology 1996;23:1066‐79. [PubMed] [Google Scholar]


