Abstract
Background
Face mask wearing has been an important part of the response to the COVID-19 pandemic. As vaccination coverage progresses in countries, relaxation of such practices is increasing. Subsequent COVID-19 surges have raised the questions of whether face masks should be encouraged or required and for how long. Here, we aim to assess the value of maintaining face masks use indoors according to different COVID-19 vaccination coverage levels in the USA.
Methods
In this computational simulation-model study, we developed and used a Monte Carlo simulation model representing the US population and SARS-CoV-2 spread. Simulation experiments compared what would happen if face masks were used versus not used until given final vaccination coverages were achieved. Different scenarios varied the target vaccination coverage (70–90%), the date these coverages were achieved (Jan 1, 2022, to July 1, 2022), and the date the population discontinued wearing face masks.
Findings
Simulation experiments revealed that maintaining face mask use (at the coverage seen in the USA from March, 2020, to July, 2020) until target vaccination coverages were achieved was cost-effective and in many cases cost saving from both the societal and third-party payer perspectives across nearly all scenarios explored. Face mask use was estimated to be cost-effective and usually cost saving when the cost of face masks per person per day was ≤US$1·25. In all scenarios, it was estimated to be cost-effective to maintain face mask use for about 2–10 weeks beyond the date that target vaccination coverage (70–90%) was achieved, with this added duration being longer when the target coverage was achieved during winter versus summer. Factors that might increase the transmissibility of the virus (eg, emergence of the delta [B.1.617.2] and omicron [B.1.1.529] variants), or decrease vaccine effectiveness (eg, waning immunity or escape variants), or increase social interactions among certain segments of the population, only increased the cost savings or cost-effectiveness provided by maintaining face mask use.
Interpretation
Our study provides strong support for maintaining face mask use until and a short time after achieving various final vaccination coverage levels, given that maintaining face mask use can be not just cost-effective, but even cost saving. The emergence of the omicron variant and the prospect of future variants that might be more transmissible and reduce vaccine effectiveness only increases the value of face masks.
Funding
The Agency for Healthcare Research and Quality, the National Institute of General Medical Sciences, the National Science Foundation, the National Center for Advancing Translational Sciences, and the City University of New York.
Introduction
Although many people in the USA adopted face mask wearing during the COVID-19 pandemic beginning in April, 2020, the spring of 2021 saw the relaxation of such practices, despite vaccination rates being well below potential herd-immunity thresholds.1, 2, 3, 4 For example, in mid-May, 2021, the Centers for Disease Control and Prevention indicated that people who were vaccinated no longer needed to wear face masks while indoors in public, which prompted many individuals who were vaccinated or unvaccinated to stop wearing face masks.5, 6 Subsequent COVID-19 surges, such as the ones fuelled by the delta (B.1.617.2) and omicron (B.1.1.529) variants, did prompt the reinstitution of face mask requirements to some degree in certain jurisdictions, such as Los Angeles County, CA, and Washington, DC.7, 8, 9 However, face mask use in 2021 remained lower than it was in 2020, even though evidence has shown how face masks can potentially decrease the amount of SARS-CoV-2 particles that an infectious person can emit into the surroundings.10 Face masks might also reduce the amount of virus that the wearer of a face mask inhales.10 This decrease in viral particles, in turn, could decrease the transmission of SARS-CoV-2 and the resulting burden of COVID-19.10, 11 Therefore, the question is whether face mask use should be encouraged or even required in public indoor locations such as grocery stores and public transportation, and how long this encouragement or these requirements should be maintained. To address this question and simulate different scenarios of face mask wearing, we developed and used a computational Monte Carlo simulation model representing the USA and the impact of SARS-CoV-2.
Research in context.
Evidence before this study
We searched MEDLINE via PubMed on Sept 28, 2021, with key terms such as “face masks”, “COVID-19”, “SARS-CoV-2”, and “non-pharmaceutical interventions”, for studies (restricted to the English language) published between Dec 1, 2019, and Sept 28, 2021, assessing the impact and value of wearing face masks on SARS-CoV-2 transmission and subsequent clinical and economic outcomes. Previous studies have shown that face masks can decrease transmission of SARS-CoV-2, including laboratory studies and retrospective observational studies. However, it has not been clear what the value of maintaining more widespread face mask use might be across a wide range of different circumstances, especially when vaccination coverage has reached different levels. There has also been a scarcity of economic studies on face mask use that in turn could help better guide policy making, such as decisions on whether to institute mask requirements.
Added value of this study
Our study estimated that maintaining face mask use (at the levels seen in the USA from March, 2020, to July, 2020) until target vaccination coverages were achieved was cost-effective and in many cases cost saving from both the societal and third-party payer perspectives across nearly all scenarios explored. For example, it was always cost-effective and usually cost saving when the cost of face masks per person per day was $1·25 or less. In fact, in all scenarios, it was cost-effective to maintain face mask use for somewhere from 2 weeks to 10 weeks beyond the date that target vaccination coverage was achieved, with this added duration being longer when the target coverage was achieved during winter versus summer. Our results also showed that anything that could increase the transmissibility of the virus (eg, emergence of the delta [B.1.617.2] and omicron [B.1.1.529] variants), or decrease vaccine effectiveness (eg, waning immunity and escape variants), or increase social interactions among certain segments of the population, only increased the cost savings or cost-effectiveness provided by maintaining face mask use. Even if 100% of people who are symptomatic and infectious isolated, maintaining face masks remained cost saving or cost-effective.
Implications of all the available evidence
Available evidence supports maintaining face mask use to at least the levels seen in the USA from March, 2020, to July, 2020, until and beyond various target vaccination coverages. Recent developments in the pandemic, such as the emergence of the delta and omicron variants and the finding that vaccine protection wanes over time only further increases the value of face masks. Therefore, there could be substantial value in providing incentives for or requiring the use of face masks indoors. Since such use would be cost-effective and even cost saving across many different circumstances, governments, businesses, and third-party payers might consider subsidising the purchases of such face masks.
Methods
Model structure
For this computational simulation-model study, we adapted our previously described stochastic computational model12, 13, 14, 15 (developed in Microsoft Excel with the Crystal Ball add-in) that represented the spread and impact of SARS-CoV-2 in the USA with a population of 327 167 434 people. The initial model structure and how people mix with each other is presented (appendix p 1). The figure also shows the different mutually exclusive compartments that each person can be in on a given simulated day and the equations that govern how and when an individual will move among them. For example, an individual can move from being susceptible to exposed when they interact with an individual who is infectious on the basis of the following equation:
where β is the transmission coefficient and equals Rt divided by the infectious period duration in turn divided by population size. Rt is the reproduction number of the virus on a given day t and is the basic reproduction number (R0; average number of secondary cases generated by one infectious case in a completely susceptible population), adjusted by observed seasonal variation. S is the number of individuals who are susceptible, and I is the number of individuals who are infectious. When an individual eventually becomes infectious, they have a probability of being asymptomatic (Ia) or symptomatic (Is).
Each person who is symptomatic in turn travels through a probability tree of different possible sequential age-specific COVID-19 clinical outcomes (appendix p 1).12, 13, 14, 15, 16, 17 The probabilities along with their distributions for each of the branches in the tree are provided here (appendix pp 4–6). Although the initial model structure attempted to account for the less heterogenous mixing that has been occurring during the pandemic because of COVID-19 precautions, additional iterations of the model explored the effects of further stratifying the population by age (an example of a model stratified by children and adults is shown on appendix p 2) and giving different age groups different mixing patterns with each other following previous studies.18, 19
Vaccination
As described in previous publications,12, 13, 14, 15 getting vaccinated decreases the risk of an individual getting infected when interacting with someone who is infectious by 1 minus the vaccine efficacy at preventing infection. Once infected, an individual who is vaccinated has a lower probability (1 – vaccine efficacy at preventing severe disease; appendix p 5) of more severe outcomes. Such protection begins 2 weeks after vaccination.20, 21, 22 Vaccination has various probabilities of causing minor (eg, fever, soreness, or headache) and major (eg, allergic reaction or anaphylaxis, pericarditis, or myocarditis resulting in hospital admission and treatment) side-effects.
Face masks
Each day, any individual, whether vaccinated or unvaccinated, can wear face masks, which in turn decreases the probability of transmission between an individual who is infectious and one who is susceptible to infection proportionally by face mask effectiveness (appendix p 5).23 Wearing face masks in turn attenuates Rt (ie, the average number of cases generated by one infectious case at a time t) by 1 minus effectiveness of face masks, with effectiveness being face mask efficacy multiplied by compliance with their use. For our baseline scenario, we assumed an estimated effectiveness of 18% (95% CI 16–20%)23 reported from March to July, 2020. This estimated effectiveness translates to a compliance of 28·4% (95% CI 25·3–31·6%), when considering the type of masks used (ie, N95, surgical, or cloth), their reported use (appendix p 5),24 and their reported efficacy (99% for N95 masks, 59% for surgical masks, and 51% for cloth masks)25 during the same time period.
Costs
Each time any event or outcome (eg, vaccination, hospital admission and treatment, and death) occurred in a model run, it accrued corresponding costs and health effects. These costs and health effects are presented here (appendix pp 4–6). The perspective of the third-party payer includes direct medical costs (eg, hospital admission and treatment), whereas the societal perspective includes direct and indirect costs (ie, productivity losses caused by absenteeism and presenteeism). For each scenario, we calculated the incremental cost-effectiveness ratio (ICER) of scenario A versus scenario B as:
where health effects are measured in quality-adjusted life years (QALYs) lost. Death results in the loss of the net present value of QALYs for the remainder of an individual's lifetime.26 We considered face mask use to be cost-effective if the ICER was up to US$50 000 per QALY. All costs are reported in 2021 values, inflating all past costs and discounting all future costs using a 3% annual rate.
Experimental scenarios
Each scenario simulated the entire course of the pandemic to date in the USA (model validation on appendix p 2), using reported case data and adjusting for potential underreporting on the basis of data used in our work with the New York Times.27, 28, 29 Each simulation experiment consisted of running the model 1000 times (Monte Carlo simulations) with each parameter drawing values from the distributions (appendix pp 4–6), and comparing what would happen if face masks were used as they were in March, 2020, to July, 2020, in the USA with what would happen with no mask use until various final vaccination coverages were achieved. Different scenarios varied the target vaccination coverage of the entire population (70–90%), the date this coverage was achieved (defined as 2 weeks after vaccination coverage occurred to account for the lag time required to achieve immune protection, varied from Jan 1, 2022, to July 1, 2022), and the date the population discontinued wearing face masks. In sensitivity analyses, we varied R0 (2·5–10·0)30, 31, 32, 33, 34, 35, 36 to account for different possible variants, vaccine efficacy to prevent infection (30–90%) to account for waning immunity and different variants, natural immunity after infection (64–95%),37, 38, 39, 40, 41, 42 and different face mask characteristics, such as face mask effectiveness, cost, and replacement frequency (baseline $0·32 per person per day). Additional scenarios explored the percentage of people who self-isolated when infected.
Role of the funding source
The funders of the study did not have any role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Maintaining face mask use (at the amount of use seen in the USA from March, 2020, to July, 2020) until target vaccination coverages were achieved was cost-effective and in many cases cost saving across nearly all scenarios explored. For example, it was always cost-effective and usually cost saving when the cost of face masks per person per day was up to $1·25. In fact, in all scenarios, it was cost-effective to maintain face mask use for about 2–10 weeks beyond the date that target vaccination coverage was achieved, with this added duration being longer when target coverage was achieved during winter versus summer. What follows are the major drivers that affected the findings.
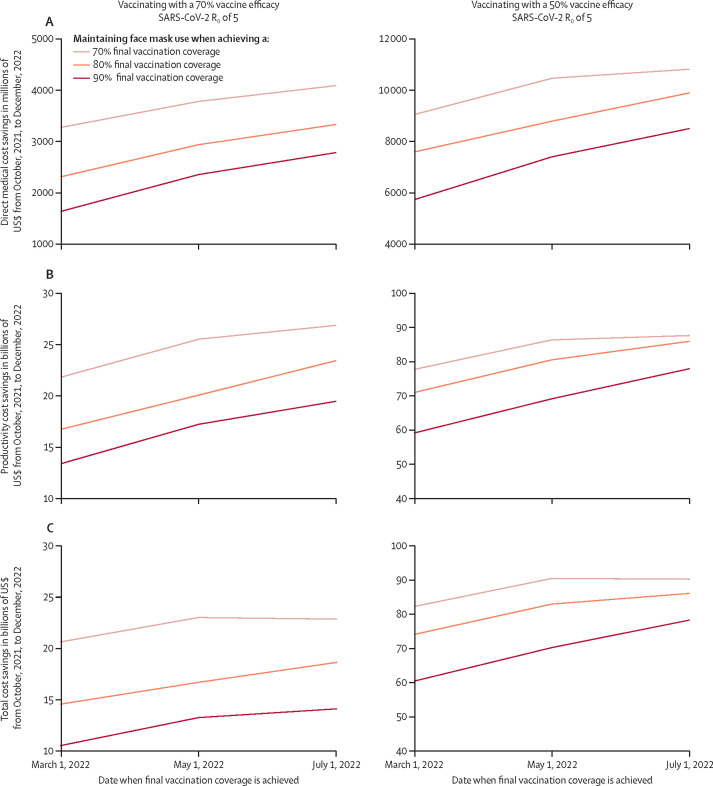
The value of face masks increased in more than a linear manner as final vaccination coverage decreased (Figure 1, Figure 2 ). If the USA were to achieve an 80% vaccine coverage by March 1, 2022, simulations show that maintaining face mask use until then would avert US$14·6 billion (95% CI 13·8–15·3) in societal costs and $2·3 billion (95% CI 2·2–2·4) in third-party payer costs as well as 6·29 million (95% CI 6·28–6·3) cases, 138 600 (95% CI 137 600–139 700) hospital admissions and treatment, and 16 100 (95% CI 15 900–16 300) deaths, saving 180 000 (95% CI 172 500–187 600) QALYs (70% vaccine efficacy to prevent infection; these outcomes per 100 000 people are shown in the table ; Figure 1, Figure 2). However, achieving only a 70% coverage would increase these savings to $20·6 billion (19·8–21·5) in societal costs, $3·27 billion (3·20–3·34) in third-party payer costs, 8·3 million (8·29–8·34) cases, 193 500 (192 100–194 800) hospital admissions and treatment, 22 700 (22 500–22 900) deaths, and 252 900 (243 700–262 000) QALYs.
Figure 1.
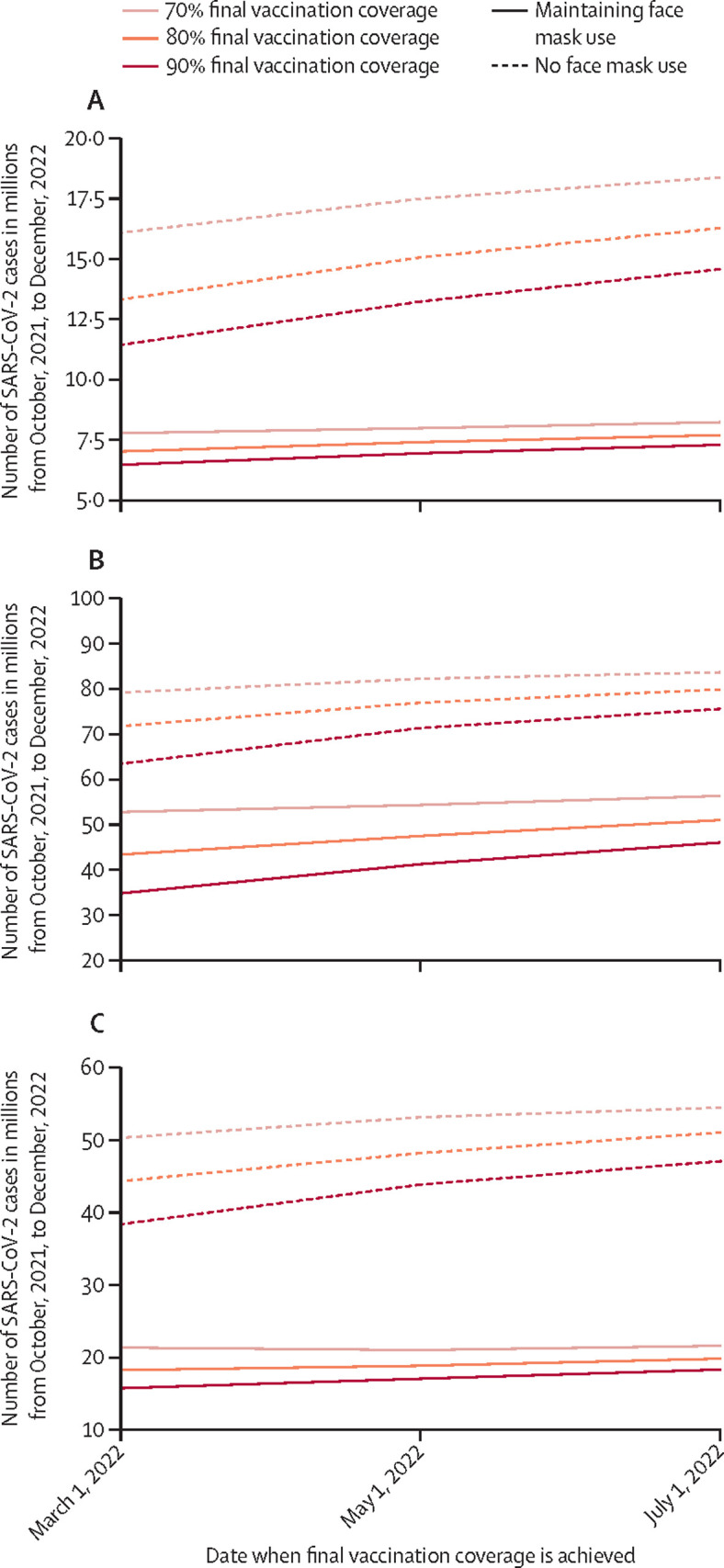
Simulated number of SARS-CoV-2 cases with and without face masks at different vaccination coverages
(A) Vaccination with 70% efficacy against infections, with SARS-CoV-2 R0=5. (B) Vaccination with 70% efficacy against infections, with SARS-CoV-2 R0=8. (C) Vaccination with 50% efficacy against infections, with SARS-CoV-2 R0=5. Vaccination coverage is defined as the time at which immune protection was achieved, 2 weeks after vaccination.
Figure 2.
Estimated cost savings associated with maintaining face mask use
(A) Direct medical cost savings when maintaining face mask use. (B) Productivity cost savings when maintaining face mask use. (C) Total societal cost savings when maintaining face mask use. Final coverage defined as when immune protection is achieved, 2 weeks after vaccination.
Table.
Difference between not wearing face masks and maintaining face mask use when achieving target vaccination coverages at different dates with different vaccine efficacies
| Total SARS-CoV-2 infections (per 100 000 people) | Hospital admissions and treatment (per 100 000 people) | Deaths (per 100 000 people) | QALYs lost (per 100 000 people) | Direct medical costs (in millions of US$ per 100 000 people) | Productivity losses (in millions of US$ per 100 000 people) | |
|---|---|---|---|---|---|---|
| 70% vaccination coverage achieved by Jan 1, 2022, with 70% vaccine efficacy to prevent infection | ||||||
| No face mask use | 4365·4 (4317·5–4391·4) | 103·8 (100·7–106·8) | 12·2 (11·5–12·7) | 134·1 (105·6–166·0) | 3·8 (3·6–4·0) | 12·4 (10·0–14·5) |
| Using face masks until reaching the target vaccination coverage | 2473·4 (2342·3–2575·1) | 60·4 (57·7–63·0) | 7·1 (6·7–7·5) | 76·1 (59·0–93·3) | 3·1 (2·9–3·3) | 7·2 (6·0–8·6) |
| Using face masks for 1 month after reaching target vaccination coverage | 2300·4 (2209·3–2371·2) | 56·8 (54·3–59·0) | 6·6 (6·2–7·0) | 72·2 (56·0–89·1) | 3·1 (2·9–3·3) | 6·9 (5·6–8·0) |
| 70% vaccination coverage achieved by March 1, 2022, with 50% vaccine efficacy to prevent infection | ||||||
| No face mask use | 15 526·6 (14 409·9–16 540·3) | 294·0 (273·0–317·1) | 34·3 (31·8–37·0) | 388·7 (311·1–476·5) | 7·1 (6·7–7·5) | 40·6 (33·9–49·6) |
| Using face masks until reaching the target vaccination coverage | 6486·4 (6275·3–6729·1) | 130·7 (125·1–136·6) | 15·2 (14·4–16·1) | 167·2 (131·4–210·4) | 4·3 (4·1–4·5) | 18·0 (14·9–21·4) |
| Using face masks for 1 month after reaching target vaccination coverage | 6162·2 (5962·5–6385·0) | 124·3 (118·8–130·2) | 14·6 (13·7–15·4) | 160·3 (128·1–200·4) | 4·2 (4·0–4·4) | 16·8 (14·0–20·2) |
| 80% vaccination coverage achieved by March 1, 2022, with 90% vaccine efficacy to prevent infection | ||||||
| No face mask use | 1689·8 (1674·5–1721·9) | 51·9 (50·4–53·5) | 6·0 (5·8–6·4) | 64·1 (49·4–81·0) | 4·5 (4·1–4·9) | 5·9 (5·0–6·9) |
| Using face masks until reaching the target vaccination coverage | 1210·1 (1191·0–1239·0) | 37·1 (36·0–38·4) | 4·3 (4·1–4·5) | 46·1 (35·8–58·5) | 4·3 (3·9–4·6) | 4·6 (3·9–5·4) |
| Using face masks for 1 month after reaching target vaccination coverage | 1208·0 (1189·1–1234·0) | 37·1 (35·8–38·4) | 4·3 (4·1–4·5) | 45·8 (36·4–58·0) | 4·2 (3·9–4·6) | 4·6 (3·9–5·5) |
| 80% vaccination coverage achieved by March 1, 2022, with 70% vaccine efficacy to prevent infection | ||||||
| No face mask use | 4091·2 (4038·8–4119·0) | 95·4 (92·3–98·5) | 11·1 (10·6–11·7) | 121·3 (95·5–151·1) | 5·2 (4·9–5·6) | 12·1 (9·9–14·4) |
| Using face masks until reaching the target vaccination coverage | 2150·3 (2104·1–2195·5) | 52·9 (51·1–54·8) | 6·2 (5·9–6·5) | 68·0 (52·2–84·4) | 4·5 (4·1–4·9) | 7·0 (5·9–8·4) |
| Using face masks for 1 month after reaching target vaccination coverage | 2138·3 (2089·1–2184·8) | 52·9 (51·0–54·5) | 6·1 (5·9–6·5) | 67·3 (54·1–84·7) | 4·5 (4·1–4·9) | 7·0 (5·9–8·3) |
| 80% vaccination coverage achieved by July 1, 2022, with 50% vaccine efficacy to prevent infection | ||||||
| No face mask use | 15 646·6 (14 536·2–16 743·6) | 299·6 (275·5–319·3) | 34·7 (31·9–37·5) | 388·6 (306·6–481·5) | 8·7 (8·2–9·2) | 41·9 (34·3–50·5) |
| Using face masks until reaching the target vaccination coverage | 6004·9 (5786·5–6258·1) | 122·5 (116·8–128·8) | 14·3 (13·5–15·2) | 156·4 (125·6–197·0) | 5·7 (5·3–6·0) | 17·1 (14·1–20·4) |
| Using face masks for 1 month after reaching target vaccination coverage | 5984·0 (5780·2–6234·5) | 122·4 (116·9–128·4) | 14·3 (13·4–15·1) | 160·7 (128·5–198·4) | 5·7 (5·3–6·1) | 16·9 (13·9–20·3) |
Data are presented as median (IQR). The date at which vaccination coverage is achieved occurs 2 weeks after vaccination to account for the 2 weeks that it might take for the full onset of immune protection. Results are the number of cases, clinical, and economic outcomes occurring from October, 2021, to December, 2022.
If the USA were to achieve a 90% coverage by May 1, 2022, simulations show that face mask use would avert $13·3 billion (12·5–14·1) in societal costs and $2·4 billion (2·2–2·5) in third-party payer costs, as well as 6·29 million (6·27–6·30) cases, 136 700 (135 700–137 800) hospital admissions and treatment, and 16 000 (15 700–16 100) deaths, saving 181 500 (173 900–198 200) QALYs (Figure 1, Figure 2). These savings would increase to $16·7 billion (15·9–17·5) in societal costs, $2·9 billion (2·8–3·1) in third-party payer costs, 7·66 million (7·63–7·69) cases, 174 900 (173 700–176 100) hospital admissions and treatment, 20 500 (20 300–20 700) deaths, and 223 700 (215 100–232 400) QALYs if only achieving 80% coverage (Figure 1, Figure 2).
For a given final vaccination coverage level, the longer it takes to reach that level, the greater the value of face masks (Figure 1, Figure 2; table). For example, as shown, if an 80% vaccination coverage is achieved at May 1, 2022 (70% vaccine efficacy), maintaining face masks until then would avert 7·66 million cases. However, if this same coverage was reached 2 months later on July 1, then these results change to $18·7 billion (17·8–19·5) in societal costs, $3·3 billion (3·2–3·5) in third-party payer costs, 8·57 million (8·55–8·60) SARS-CoV-2 cases, 200 000 (198 000–201 000) hospital admissions and treatment, and 23 200 (23 000–23 500) deaths averted, saving 264 000 (255 000–274 000) QALYs (Figure 1, Figure 2).
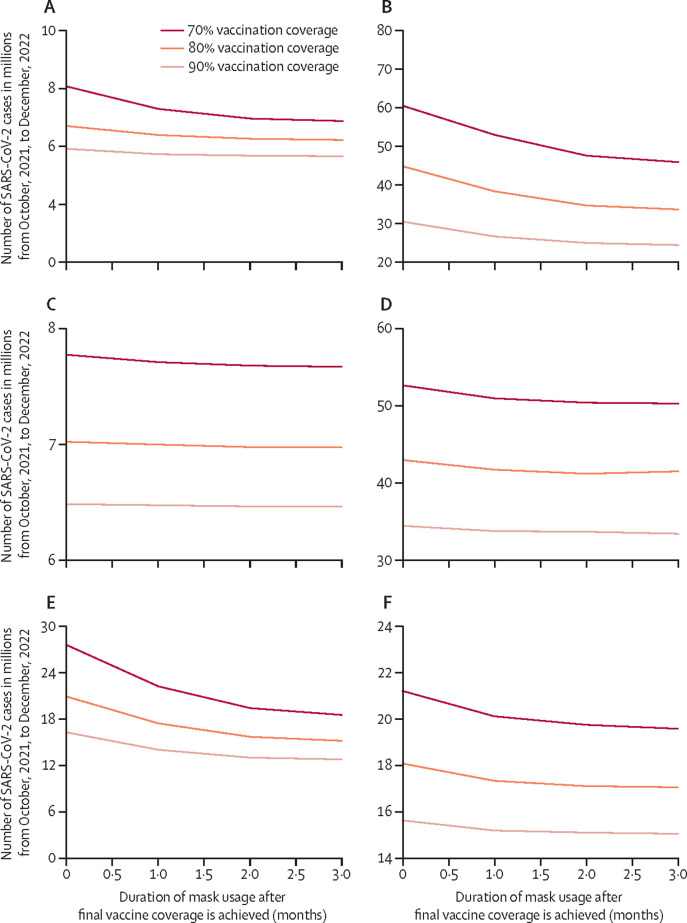
Varying R0 showed that the emergence of more transmissible variants such as the delta and now omicron variants has further boosted the value of face masks (figure 1). For example, as aforementioned, when the R0 is 5, corresponding to the delta variant, maintaining face masks would avert $20·6 billion (19·8 to 21·5) in societal costs (70% coverage by March 1, 2022). A higher R0 of 10, corresponding to the omicron variant, pushes these numbers averted up to $49·5 billion (43·6 to 55·4) in societal costs, $5·2 billion (5·0 to 5·4) in third-party payer costs, and 17·9 million (17·8 to 18·1) cases versus no mask use and maintaining 1 more month (until April) provided value, averting an additional $1·5 billion (−13·7 to 6·8) in societal costs, $148·6 million (−10·4 to 307·6) in third-party payer costs, and 856 000 (710 200 to 1 000 000) cases. Lowering the R0 to 2·5, corresponding to the original virus, would lower these values to 581 350 (578 950 to 583 800) cases and face mask use would not be cost-effective.
The emergence of variants and waning immunity might also reduce vaccine effectiveness, which in turn will also increase the value of face masks (Figure 1, Figure 2; table). For example, with a 50% vaccine efficacy, maintaining face mask use until reaching a 70% coverage by March 1, 2022 would avert $82·3 billion (79·7–84·9) in societal costs and $9·1 billion (8·9–9·2) in third-party payer costs as well as 29·1 million (28·8–29·5) SARS-CoV-2 cases, 533 500 (527 700–539 300) hospital admissions and treatment, and 62 600 (61 800–63 300) deaths, saving 737 900 (714 700–762 200) QALYs. As another example, with a 30% efficacy, face mask use until reaching a 70% coverage by March 1, 2022 would avert $95·1 billion (90·2–100·0) in societal costs, $10·7 billion (10·5–10·9) in third-party payer costs, and 34·0 million (33·7–34·3) cases. Maintaining masks for 2 more months until May would be cost-effective (ICER $48 421 per QALY) averting an additional 1·2 million (0·9–1·6) cases compared to maintaining masks for 1 additional month (figure 3 ). Even with a 90% vaccine efficacy, face mask use provided value, averting $1·7 billion (1·4–2·1) in societal costs, $1·0 billion (0·9–1·1) in third-party payer costs, and 1·93 million (1·92–1·93) cases compared to no mask use when achieving coverage by March 1, 2022 (Figure 1, Figure 2, Figure 3).
Figure 3.
Simulated number of SARS-CoV-2 cases when face masks are used for different durations after the final vaccination coverage is achieved
(A) 70% vaccine efficacy with SARS-CoV-2 R0=5, and vaccination coverage achieved by Jan 1, 2022. (B) 70% vaccine efficacy with SARS-CoV-2 R0=8, and vaccination coverage achieved by Jan 1, 2022. (C) 70% vaccine efficacy with SARS-CoV-2 R0=5, and vaccination coverage achieved by March 1, 2022. (D) 70% vaccine efficacy with SARS-CoV-2 R0=8, and vaccination coverage achieved by March 1, 2022. (E) 50% vaccine efficacy with SARS-CoV-2 R0=5, and vaccination coverage achieved by Jan 1, 2022. (F) 50% vaccine efficacy with SARS-CoV-2 R0=5, and vaccination coverage achieved by March 1, 2022. Maintaining face mask use for longer provided more benefits with lower vaccine efficacies and with increases in R0. Note the differences in scales across the panels, which was done to more readily see when outcomes levelled off with increases in the time face masks were used.
Increasing the effectiveness of face masks (eg, average compliance of 44·2%), decreased the total number of infections when using masks and thus increased the value of face mask wearing. For example, when an 80% vaccination coverage was achieved by March 1, 2022 (vaccine efficacy 70%; R0=5) maintaining face mask use averted $18·0 billion (17·3–18·7) in societal costs and $3·0 billion (2·9–3·1) in third-party payer costs, as well as 8·15 million (8·14–8·17) cases, 179 400 (178 400–180 400) hospital admissions and treatment, and 20 900 (20 700–21 100) deaths, saving 235 900 (228 800–242 900) QALYs.
Mask use in March, 2020, to July, 2020, with that particular combination of N95, surgical, and cloth masks, would have resulted in a cost per person per day of $0·32. Adjusting different usage and characteristics (eg, frequency of replacement and cost per mask; appendix p 8) so that the cost per person per day increased to $0·94 resulted in decreases in cost savings, but face mask use would remain cost-effective when achieving 90% coverage by July 1, 2022 (ICER $8293 per QALY) and would remain cost-effective, increasing the ICER to $36 092 per QALY, when further increasing this cost to $1·17. As another example, when increasing this cost to $1·25 per person per day, face masks would remain cost saving for most scenarios and would be cost-effective (ICER $36 092 per QALY) when achieving a 90% coverage by July 1, 2022 (70% vaccine efficacy; R0=5). When increasing the cost to $1·39 per person per day, maintaining face masks remained cost saving (appendix p 8) and would be cost-effective if achieving 80% coverage by July 1, 2022 (ICERs ≤$32 319 per QALY) and when achieving a 90% coverage by May 1, 2022 (ICERs ≤$43 161 per QALY). However, when face masks cost $1·39 per person per day, maintaining use would not be cost-effective (ICER $63 891 per QALY) if achieving 90% coverage by July 1, 2022 (appendix).
Further stratifying the population by age groups increased the number of infections and thus the impact and value of face mask wearing. For example, when achieving 70% vaccination coverage by May 1, 2022 (70% vaccine efficacy; R0=5), maintaining face mask use (18% effectiveness) until then would save $81·6 billion (78·7–84·5) in societal costs and $11·2 billion (11·1–11·3) in third-party payer costs, averting 29·8 million (29·6–30·2) cases and 668 400 (661 000–675 900) hospital admissions and treatment, saving 871 600 (838 700–904 600) QALYs.
Increasing the percentage of symptomatic individuals who remain isolated throughout their infectious period did reduce the value of face masks to some degree. However, even when assuming that 100% of people who were infectious and symptomatic stayed isolated, face mask use would still be cost saving, saving $359·7 million (−12·8 to 732·2) in societal costs and $575·9 million (503 to 648) in third-party payer costs, averting 1·62 million cases (1·56 to 1·67) and 3950 deaths (3800 to 4100; mask cost $0·32 per person per day; 50% vaccine efficacy; 70% vaccination coverage by March 1, 2022; R0=5). The only time it would not be cost-effective would be when achieving 90% coverage by Jan 1, 2022, and when vaccine efficacy is at least 70% or face mask costs more than $0·50 per person per day·
Discussion
The results of this study re-emphasise that vaccination alone is not enough to control the pandemic and prevent deaths and suffering, as well as the importance of multilayered interventions. As has been described previously, each available intervention has different limitations.43, 44, 45 Combining several layers of interventions can not only cover up these gaps but also further enhance each layer.46, 47, 48 Our study shows that face mask use can be cost-effective and, in many cases, cost saving, meaning that face mask use would pay for itself. This finding provides strong support for governments, third-party payers, and other organisations to provide face masks to the general public. Moreover, our study showed face mask use should not end as soon as certain amounts of vaccination coverage are achieved, even if these coverages exceed herd-immunity thresholds (eg, ranging from 60% for an R0 of 2·5 and 90% for an R0 of 10). That is because virus transmission does not immediately stop once such coverage levels are reached. Instead, face mask use could prevent additional COVID-19 cases until transmission eventually subsides after 2–10 weeks. Our study suggests that there are clear, finite times during which people should continue masking.
The continuing uncertainty of the pandemic further increases face masks' value. Decreasing vaccine effectiveness, as has been the case with waning immunity and the emergence of new variants, only increases the value of face mask wearing. This is the case with increasing transmissibility of the virus, which has been seen with the omicron and delta variants and the current winter surge. Such may be the case in outbreak situations too if vaccine efficacy is lower and transmissibility is higher.
Our experiments also show the value of face mask wearing even as other interventions might change. For example, even if every person who is symptomatic from COVID-19 were to isolate themselves for the full duration of their infectious stage, face mask wearing would still be cost-effective and close to cost saving (eg, when face masks cost ≤US$0·50 and last 2 days). Such a scenario would not be very realistic given that many people do not get tested for COVID-19 or might not remain isolated throughout their infectious period.49, 50, 51, 52, 53 This finding demonstrates that although increasing testing might be helpful, it alone will not be enough to control the pandemic and will not remove the need for face mask wearing.
Additionally, our study supports face mask use across the population and not just among specific age groups or in people who have particular mixing patterns. In fact, the more we substratified the population and made mixing patterns heterogeneous, the more the value of face masks increased. This increase in value is caused by the fact that more intense mixing occurs among certain population strata, increasing transmission of the virus and the number of COVID-19 cases.
Our study also estimates the value of increasing face mask effectiveness and adherence. When increasing face mask effectiveness by 10% (implying mask compliance is 44·2%), the relative reduction in cases is greater, with a 17–20% reduction in cases, hospital admissions and treatment, and deaths. Nevertheless, even if there are shortages in more effective face masks (eg, N95 masks), wearing any face mask (eg, cloth masks) is better than not wearing one. This is because people who are infected with SARS-CoV-2 are less likely to transmit the virus to others when wearing a mask, even if it is made of cloth.25
Although our model represented the USA, our results could be applicable to other country settings. The value of face mask wearing was robust to changes in mixing patterns, vaccination coverage, vaccine efficacies, and transmission parameters, which covers a lot of the diversity seen across the world including in low-income and middle-income countries. For example, the 50% vaccine efficacy scenarios are similar to countries primarily using inactivated-virus vaccines,54 such as Bahrain, Chile, and Hungary55 and the 80% vaccination coverage with 70% vaccine efficacy scenario is similar to current situations in Spain and Australia.56 These results can help provide a general estimate for how long after reaching different coverage levels masks can still provide value.
All models, by definition, are simplifications of real life and cannot account for every possible outcome.57 Model inputs drew from various sources and time points during the pandemic, and new data on SARS-CoV-2 continues to emerge. We did not vary the effectiveness of face masks on the transmissibility of the virus over the duration of the simulation, however this effectiveness might vary from day to day and over time and with state-level and local-level policies.58, 59, 60 Our scenarios assume coverage of the entire population; however, some populations are not yet eligible for vaccination (eg, children aged <5 years). We attempted to be conservative in estimating the value of face masks. For example, we did not include all costs face masks can avert, such as caregiver-productivity losses or declines in economic activity (eg, job loss) nor did we consider QALY losses that could occur during quarantine or isolation (eg, mental health declines or worry of hospital admission and treatment). Incorporating these would further increase the value of wearing face masks.
This study helps quantify the value of maintaining face mask use until certain vaccination coverages are achieved and how doing so can be not just cost-effective, but even cost saving under a wide variety of circumstances. We found substantial value in continuing face mask wearing 2–10 weeks beyond the achievement of target vaccination coverage thresholds to reduce residual SAR-CoV-2 transmission. The emergence of the omicron variant and the prospect of future variants that might be more transmissible and reduce vaccine effectiveness only increases the value of face masks.
Data sharing
All relevant data are contained within the manuscript text and appendix.
Declaration of interests
PJH and MEB codirect the Texas Children's Center for Vaccine Development and with US are codevelopers of vaccines against emerging and neglected diseases including coronaviruses such as COVID-19. Baylor College of Medicine non-exclusively licensed a COVID-19 vaccine construct to Biological E, an India-based manufacturing company. These authors have no financial stakes in any COVID-19 vaccine candidates under development. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the Agency for Healthcare Research and Quality (AHRQ) via grant 1R01HS028165-01, the National Institute of General Medical Sciences as part of the Models of Infectious Disease Agent Study network under grants R01GM127512 and 3R01GM127512-01A1S1, and by the National Science Foundation proposal number 2054858, the National Center for Advancing Translational Sciences of the National Institutes of Health via award number U54TR004279, and by the City University of New York (CUNY) in support of the Pandemic Response Institute (PRI). Statements in the manuscript do not necessarily represent the official views of, or imply endorsement by, the National Institute of Health, AHRQ, the US Department of Health and Human Services, CUNY, or the PRI.
Contributors
All authors conceived and designed the question, model, and experiments. All authors collected, accessed, and verified the data. SMB, KJO, KLC, PTW, MCF, SSS, and SNC developed the model. SMB, KJO, MCF, MEB, US, KLC, SNC, PTW, PJH, and BYL parameterised the model. All authors analysed the data. SMB, KJO, MCF, MEB, US, KLC, PTW, SNC, PJH, and BYL helped draft the paper. All authors edited the paper and were included in the decision to submit for publication.
Supplementary Material
References
- 1.Raifman J, Nocka K, Jones D, Bor J, Lipson S, Jay J. CUSP COVID-19 US state policies. Aug 27, 2021. https://statepolicies.com/data/library/
- 2.Simmons-Duffin S. Confused by CDC's latest mask guidance? Here's what we've learned. May 15, 2021. https://www.npr.org/sections/health-shots/2021/05/14/996879305/confused-by-cdcs-latest-mask-guidance-heres-what-weve-learned
- 3.Miller Z, Balsamo M. ‘Great day for America': vaccinated can largely ditch masks. May 13, 2021. https://apnews.com/article/coronavirus-masks-cdc-guidelines-9d10c8b5f80a4ac720fa1df2a4fb93e5
- 4.Lee BY. Is it too early to say fully vaccinated don't need face masks, social distancing? Here are 5 COVID-19 concerns. May 14, 2021. https://www.forbes.com/sites/brucelee/2021/05/14/cdc-says-vaccinated-dont-have-to-wear-face-masks-social-distance-here-are-5-continuing-covid-19-concerns/?sh=3d7046854f2f
- 5.Lovelace B. CDC says fully vaccinated people don't need to wear face masks indoors or outdoors in most settings. May 13, 2021. https://www.cnbc.com/2021/05/13/cdc-says-fully-vaccinated-people-dont-need-to-wear-face-masks-indoors-or-outdoors-in-most-settings.html
- 6.Edwards E, Bennett G, Alba M. Fully vaccinated? You can ditch the mask, CDC says. May 13, 2021. https://www.nbcnews.com/health/health-news/cdc-plans-drop-mask-requirements-fully-vaccinated-people-n1267249
- 7.Danner C. Mask mandates are back—even for the vaccinated. Here's what to know. Aug 7, 2021. https://nymag.com/intelligencer/article/what-to-know-about-mask-mandates-including-for-vaccinated.html
- 8.Horng E, Gallardo M, ABC7 Chicago Digital Team Chicago reinstates indoor mask mandate, regardless of vaccination status, health officials announce. Aug 18, 2021. https://abc7chicago.com/chicago-face-masks-mask-mandate-chicaog/10957531/
- 9.Money L, Lin R-G, II, Hernadez M. L.A. County will require masks indoors amid alarming rise in coronavirus cases. July 15, 2021. https://www.latimes.com/california/story/2021-07-15/l-a-county-will-require-masks-indoors-amid-covid-19-surge
- 10.Howard J, Huang A, Li Z, et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyu W, Wehby GL. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff. 2020;39:1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch SM, O'Shea KJ, Ferguson MC, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59:493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartsch SM, O'Shea KJ, Wedlock PT, et al. The benefits of vaccinating with the first available COVID-19 coronavirus vaccine. Am J Prev Med. 2021;60:605–613. doi: 10.1016/j.amepre.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartsch SM, Wedlock PT, O'Shea KJ, et al. Lives and costs saved by expanding and expediting coronavirus disease 2019 vaccination. J Infect Dis. 2021;224:938–948. doi: 10.1093/infdis/jiab233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BY, Bartsch SM, Ferguson MC, et al. The value of decreasing the duration of the infectious period of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. PLoS Comp Biol. 2021;17 doi: 10.1371/journal.pcbi.1008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020;39:927–935. doi: 10.1377/hlthaff.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wedlock PT, O'Shea KJ, Conte M, et al. Estimated number of N95 respirators needed for healthcare workers in acute care hospitals during the COVID-19 coronavirus pandemic. Infect Control Hosp Epidemiol. 2021;42:1318–1326. doi: 10.1017/ice.2020.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mossong J, Hens N, Jit M, et al. POLYMOD social contact data (version 1.1) 2017. [DOI]
- 20.Centers for Disease Control and Prevention Key things to know about COVID-19 vaccines. Oct 7, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/keythingstoknow.html#:~:text=It%20typically%20takes%202%20weeks,enough%20time%20to%20build%20protection
- 21.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B, Huang AT, Garcia-Carreras B, et al. Effect of specific non-pharmaceutical intervention policies on SARS-CoV-2 transmission in the counties of the United States. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan P, Hellstorm S, Hughes J, Rothberg J. Survey: in the US, people say their use of masks may endure. July 1, 2020. https://www.mckinsey.com/featured-insights/americas/survey-in-the-us-people-say-their-use-of-masks-may-endure
- 25.Lindsley WG, Blachere FM, Law BF, Beezhold DH. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aero Sci Tech. 2021;55:449–457. doi: 10.1080/02786826.2020.1862409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman S, Maculloch B, Batz MB. US Department of Agriculture Economic Research Service; Atlanta, GA: 2015. Economic bruden of major foodborne illnesses aquired in the United States. [Google Scholar]
- 27.Centers for Disease Control and Prevention United States COVID-19 cases and deaths by state over time. Oct 2, 2021. https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36
- 28.National Center for Immunization and Respiratory Diseases. Division of Viral Diseases Estimated disease burden of COVID-19. July 27, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html
- 29.Conlen M, Smart C. When could the United States reach herd immunity? It's complicated. Feb 20, 2021. https://www.nytimes.com/interactive/2021/02/20/us/us-herd-immunity-covid.html
- 30.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou T, Liu Q, Yang Z, et al. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J Evid Based Med. 2020;13:3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention COVID-19 pandemic planning scenarios. May 20, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
- 34.Liu Y, Rocklov J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28 doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scientific Pandemic Influenza Group on Modelling Os-gS-M-O SPI-M-O: summary of further modelling of easing restrictions: roadmap step 4 on 19th July 2021. July 7, 2021. https://www.gov.uk/government/publications/spi-m-o-summary-of-further-modelling-of-easing-restrictions-roadmap-step-4-on-19-july-2021-7-july-2021
- 36.Nishiura H, Ito K, Anzai A, Kobayashi T, Piantham C, Rodriguez-Morales AJ. Relative reproduction number of SARS-CoV-2 Omicron (B.1.1.529) compared with Delta variant in South Africa. J Clin Med. 2021;11:30. doi: 10.3390/jcm11010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Russell RM, Bibollet-Ruche F, et al. Predictors of nonseroconversion after SARS-CoV-2 Infection. Emerg Infect Dis. 2021;27:2454–2458. doi: 10.3201/eid2709.211042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masia M, Telenti G, Fernandez M, et al. SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oved K, Olmer L, Shemer-Avni Y, et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wellinghausen N, Plonne D, Voss M, Ivanova R, Frodl R, Deininger S. SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons. J Clin Virol. 2020;130 doi: 10.1016/j.jcv.2020.104542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haug N, Geyrhofer L, Londei A, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Human Behav. 2020;4:1303–1312. doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 44.Iezadi S, Gholipour K, Azami-Aghdash S, et al. Effectiveness of non-pharmaceutical public health interventions against COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendez-Brito A, El Bcheraoui C, Pozo-Martin F. Systematic review of empirical studies comparing the effectiveness of non-pharmaceutical interventions against COVID-19. J Infect. 2021;83:281–293. doi: 10.1016/j.jinf.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deziel NC, Allen JG, Scheepers PTJ, Levy JI. The COVID-19 pandemic: a moment for exposure science. J Expo Sci Environ Epidemiol. 2020;30:591–593. doi: 10.1038/s41370-020-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren X, Weisel CP, Georgopoulos PG. Modeling effects of spatial heterogeneities and layered exposure interventions on the spread of COVID-19 across New Jersey. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph182211950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escandon K, Rasmussen AL, Bogoch, et al. COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection. BMC Infect Dis. 2021;21:710. doi: 10.1186/s12879-021-06357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bodas M, Peleg K. Self-isolation compliance in the COVID-19 era influenced by compensation: findings from a recent survey in Israel. Health Affairs. 2020;39:936–941. doi: 10.1377/hlthaff.2020.00382. [DOI] [PubMed] [Google Scholar]
- 50.Eraso Y, Hills S. Self-Isolation and quarantine during the UK's first wave of COVID-19. A mixed-methods study of non-adherence. Int J Environ Res Pub Health. 2021;18 doi: 10.3390/ijerph18137015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith LE, Amlôt R, Lambert H, et al. Factors associated with adherence to self-isolation and lockdown measures in the UK: a cross-sectional survey. Pub Health. 2020;187:41–52. doi: 10.1016/j.puhe.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith LE, Potts HWW, Amlôt R, Fear NT, Michie S, Rubin GJ. Adherence to the test, trace, and isolate system in the UK: results from 37 nationally representative surveys. BMJ. 2021;372:n608. doi: 10.1136/bmj.n608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steens A, Freiesleben de Blasio B, Veneti L, et al. Poor self-reported adherence to COVID-19-related quarantine/isolation requests, Norway, April to July 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.37.2001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayme J. Round table: institutional psychotherapy and compartmentalized practice. Ann Med Psychol. 1987;145:714–716. [PubMed] [Google Scholar]
- 55.Alhinai ZA, Elsidig N. Countries with similar COVID-19 vaccination rates yet divergent outcomes: are all vaccines created equal? Int J Infect Dis. 2021;110:258–260. doi: 10.1016/j.ijid.2021.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holder J. Tracking coronavirus vaccinations around the world. Jan 14, 2022. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html
- 57.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008;46:1139–1141. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 58.Institute for Health Metrics and Evaluation COVID-19 results briefing. March 6, 2021. http://www.healthdata.org/sites/default/files/files/Projects/COVID/2021/102_briefing_United_States_of_America_4.pdf
- 59.Crane MA, Shermock KM, Omer SB, Romley JA. Change in reported adherence to nonpharmaceutical interventions during the COVID-19 pandemic, April–November 2020. JAMA. 2021;325:883–885. doi: 10.1001/jama.2021.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guy GP, Jr, Lee FC, Sunshine G, et al. Association of state-issued mask mandates and allowing on-premises restaurant dining with county-level COVID-19 case and death growth rates: United States, March 1–December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:350–354. doi: 10.15585/mmwr.mm7010e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are contained within the manuscript text and appendix.