Abstract
Purpose:
To quantify in vivo changes in miniscrew implant (MSI) stability over time using resonance frequency analysis, and to determine if pilot holes and placement sites affect changes in MSI stability.
Materials and Methods:
Twenty-two self-tapping MSIs (1.6 mm wide and 9 mm long) were placed in the maxillae of 2 adult beagle dogs (20 months old). The Osstell Mentor was used to measure the implant stability quotient (ISQ) weekly for 8 weeks. A split-mouth design was used to evaluate the effects of 1.1-mm wide, 3-mm deep pilot holes.
Results:
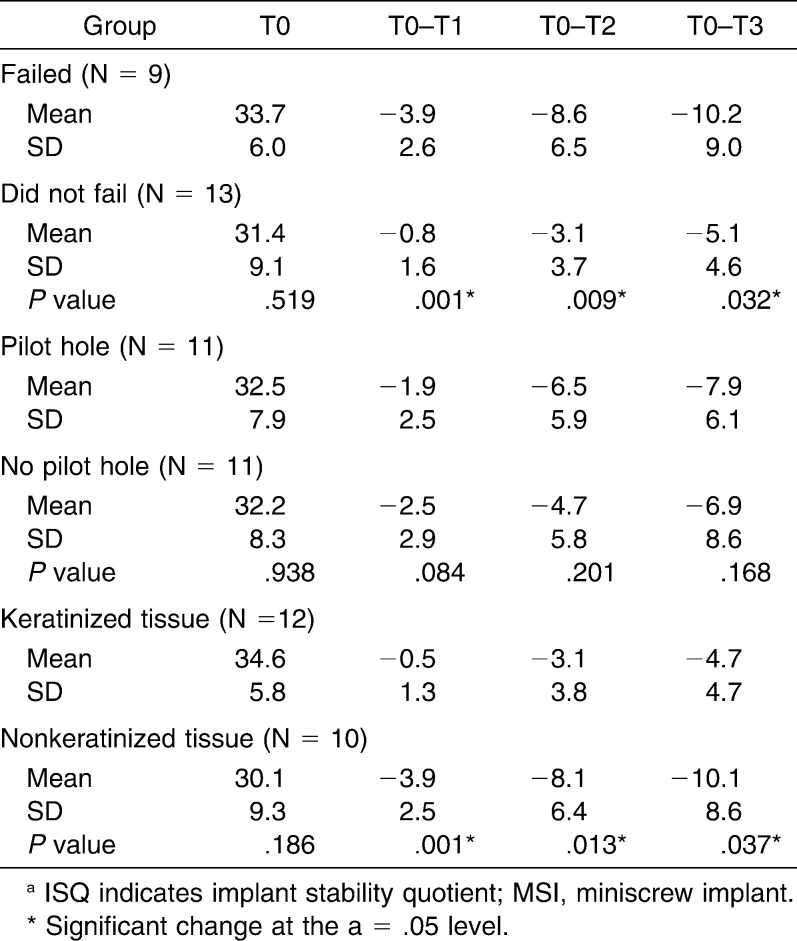
The MSIs that failed showed significantly (P < .05) greater decreases in ISQ values during the first 3 weeks than the MSIs that remained stable. All of the MSIs that failed (41%) had been placed in nonkeratinized tissue. MSIs that remained stable throughout the study also showed significant decreases in ISQ values during the first 3 weeks, followed by increases during the fourth and fifth weeks. Changes in ISQ values of MSIs inserted into bone with and without pilot holes were comparable (P > .05).
Conclusion:
Stability of unloaded MSIs decreased during the first 3 weeks and increased thereafter. Although the effects of pilot holes on stability could not be confirmed, placement of MSIs into nonkeratinized tissue negatively affected their stability and increased the likelihood of failures.
Keywords: Miniscrew implants, Stability, Resonance frequency analysis, Pilot holes, Longitudinal, keratinized tissue
INTRODUCTION
Miniscrew implants (MSIs) have become a popular means of providing skeletal anchorage in orthodontics. However, reported success rates of these devices vary from less than 50% to more than 95%.1–5 MSI failures have been related to various host factors,1,2,6 miniscrew factors,1,2,7–9 and treatment protocol factors.10–14 The ultimate cause of MSI failures is the loss of bone-to-implant contact, which changes throughout the primary and secondary phases of stability. To understand how bone-to-implant contact changes, and its relationship to healing, longitudinal assessments of MSI stability are necessary.
The stability of MSIs left in situ for some period of time has traditionally been measured using removal torque,15,16 histology,17,18 and pullout tests.19 Because these measures require the destruction of the bone-to-implant interface, they are not useful for longitudinal studies. The most promising noninvasive method to measure implant stability is resonance frequency analysis (RFA), which has been successfully used to study the stability of dental implants over time.20–22 RFA measures vibrations of the implant within bone.23,24 The stiffer the bone surrounding the implant, the higher the frequency of the measured vibration. Peri-implant bone density, cortical bone thickness, and percussion test values are all closely related to resonance frequencies of MSIs.25
Temporal changes in the stability of dental implants have been quantified using resonance frequencies. RFA measurements of endosseous implants placed in humans have shown significant decreases during the first 3–4 weeks and increases thereafter.20,22,26 Longitudinal changes in the stability of MSIs have not been experimentally investigated.
The primary aim of this pilot study was to determine how MSI stability changes over time using the longitudinal RFA to quantify MSI stability. The secondary aim was to determine whether pilot holes and tissue type affect MSI stability.
MATERIALS AND METHODS
The sample included 2 healthy, male, 20-month-old beagle dogs weighing approximately 15 kg. All procedures were approved by the Saint Louis University Animal Care Committee. Of animal models commonly used, human bone characteristic are best approximated by the properties of dog bone.27
As a prophylactic, 25 mg/kg of enrofloxacin (Bayer Health Care, Shawnee Mission, Kans) was administered intravenously during MSI placement and intramuscularly for 2 days after surgery. Carprofen (Pfizer Animal Health, Exton, Pa) was administered (4 mg/kg) subcutaneously as an analgesic. Induction of general anesthesia was accomplished by the intravenous (IV) administration of 7 mg/mL propofol (Abbot Animal Health, Chicago, Ill). Maintenance was achieved by 2–3% isoflurane (Baxter Healthcare Corporation, Deerfield, Ill) Postoperatively, the animals were given 0.2 mL IV acepromazine maleate (VEDCO, St Joseph, Mo) to calm postanesthetic excitement.
Lateral head films were used to determine MSI placement sites, which were chosen based on the availability of adequate space. MSI length prevented their insertion into the mandible without risk of fracture. Ten MSI insertion sites were identified for the first dog, and 12 insertion sites were identified for the second dog. One screw of each right- and left-side pair was randomly assigned to receive a pilot hole. All MSIs were placed in the maxillary premolar region: 12 were placed apical to the furcation in keratinized tissue (Figure 1), and 10 were placed apical to the root tips in nonkeratinized gingiva (Figure 2). The animals served as their own controls for evaluating the effects of pilot holes and placement sites.
Figure 1.
Placement of miniscrew implants in keratinized tissue of a dog.
Figure 2.
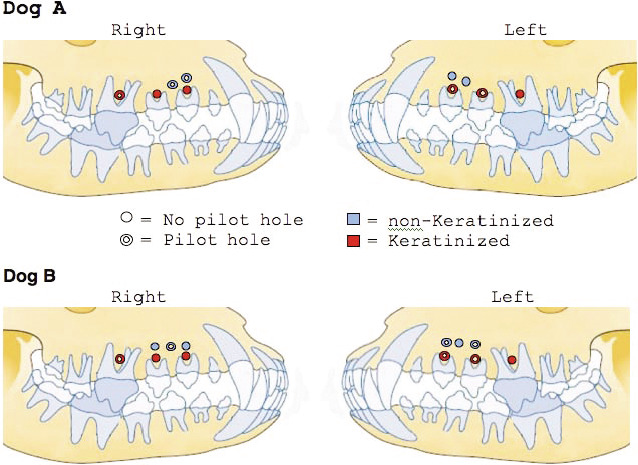
Miniscrew implants in dog A and dog B by pilot hole and soft-tissue location.
A 3.0-mm tissue punch (Premier Medical Products, Plymouth Meeting, Pa) was used to visualize the placement sites and prevent soft-tissue complications. Pilot holes were drilled using a 1.1-mm diameter drill (Sendax Spiral Drill, Imtec Corporation, Ardmore, Okla) at 1000 rpm and under constant irrigation with sterile saline solution. To control the depth of the hole, an endo stop was placed 3 mm from the drill tip.
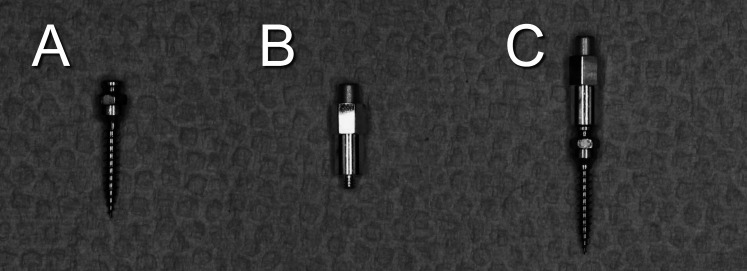
The MSIs were Ancoragem Ortodontica screws manufactured by Neodent (Curitiba, Brazil); they were self-drilling; were 9-mm long; and had a 1.6-mm external diameter, a 1.1-mm internal diameter, and a 0.7-mm pitch (Figure 3). The head of the screw was modified to include a 1.1-mm internal thread that accepts the Osstell Mentor Smartpeg type A3 (Osstell, Göteborg, Sweden). All MSIs were inserted by the primary author (DSU) using a hand driver.
Figure 3.
(A) Miniscrew implants (MSIs). (B) Smartpeg. (C) MSI attached to Smartpeg.
Resonance Frequency Analyses
RFA were conducted using an Osstell Smartpeg type A3, which was screwed into the head of the MSI and tightened with finger pressure according to the manufacturer's instructions. With the Osstell Mentor transducer held perpendicular to the MSI's long axis, resonance frequencies were taken parallel and perpendicular to the maxillary occlusal plane. All resonance frequencies were measured weekly by the primary author for 8 weeks and reported as implant stability quotients (ISQs).
Statistical Analysis
RFA was performed on MSIs that were immobile, partially mobile, or had been displaced. Because of the number of MSIs that failed, two data sets were evaluated, pertaining to (1) all the MSIs during the first 3 weeks, and (2) only those MSIs that remained intact throughout the study.
Preliminary analyses of all the MSIs that provided ISQ values over the first 3 weeks showed no statistically significant interaction between pilot holes and placement sites. Because of the small sample size, nonparametric tests were used to evaluate differences in ISQ values between screws placed (1) with and without pilot holes and (2) in keratinized gingiva and nonkeratinized gingival tissues. Wilcoxon signed-rank tests were used to evaluate changes in average ISQ between each successive time point. All statistical tests were performed using SPSS software Version 17.0 (SPSS, Chicago, Ill).
RESULTS
Failures
Of the 22 MSIs that were placed, nine (41%) failed during the course of the study (Figure 4). MSIs failed during the third (n = 4), fifth (n = 2), sixth (n = 2), and eighth (n = 1) weeks. All but one of the MSIs placed into nonkeratinized tissue failed. All failures occurred while attempting to unscrew the Smartpeg from the MSI.
Figure 4.
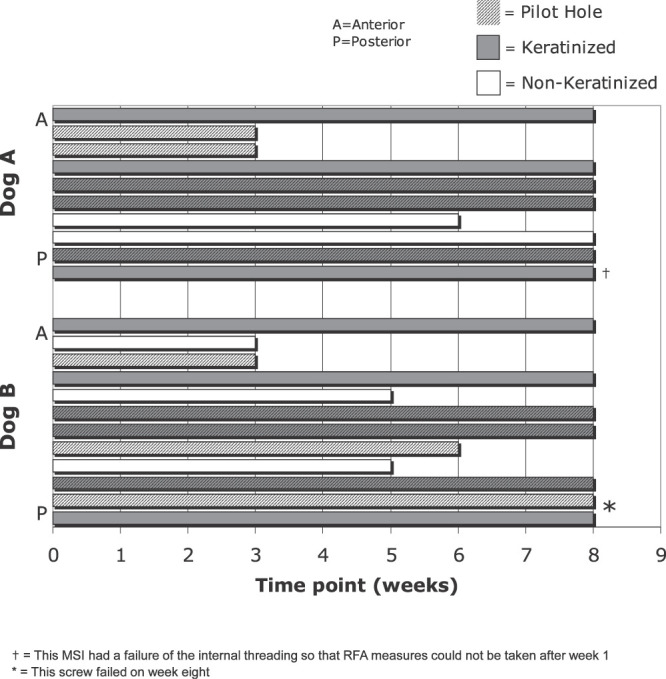
Timing of miniscrew implant failures by dog, anteroposterior location, tissue type, and pilot hole.
Changes Over the First 3 Weeks
Decreases in ISQ values over the first 3 weeks were significantly greater for the MSIs that failed than for those that did not (Table 1). The differences between the MSIs that failed and those that did not increased significantly, from 3.1 at the end of the first week to 5.1 at the end of the third week. MSIs placed in nonkeratinized tissue also showed significantly greater decreases in ISQ values over the first 3 weeks than MSIs placed in keratinized tissue (Figure 5). Although decreases in ISQ values tended to be greater for MSIs with pilot holes than for those without pilot holes, the differences were not statistically significant (Figure 6).
Table 1.
Means and SDs of Cumulative Changes of ISQ Values From Date of Insertion (T0) to 3 Weeks (T3), Along with Statistical Comparisons of MSIs That Failed Versus Those That Did Not Fail, Pilot Holes Versus No Pilot Holes, and Keratinized Versus Nonkeratinized Tissue.a
Figure 5.
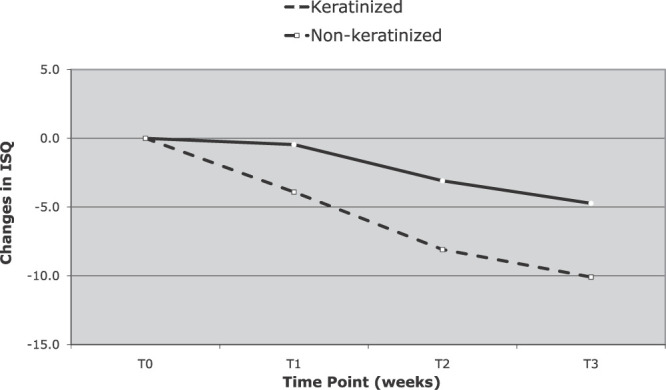
Change in implant stability quotient over time for miniscrew implants placed in keratinized and nonkeratinized tissue over the first 3 weeks.
Figure 6.
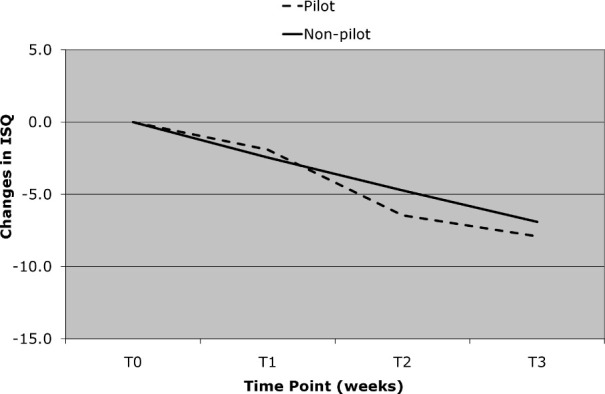
Change in implant stability quotient over time for miniscrew implants with pilot hole and no pilot hole over the first 3 weeks. No significant changes were found.
Changes Over 8 weeks
Based only on the MSIs that remained intact during the entire 8 weeks of the study (Figure 7), ISQ values decreased significantly during the first 3 weeks and increased significantly during the fourth and fifth weeks. The decrease that occurred during the sixth week, as well as the increases that occurred during the seventh, eighth and ninth weeks, were not statistically significant.
Figure 7.
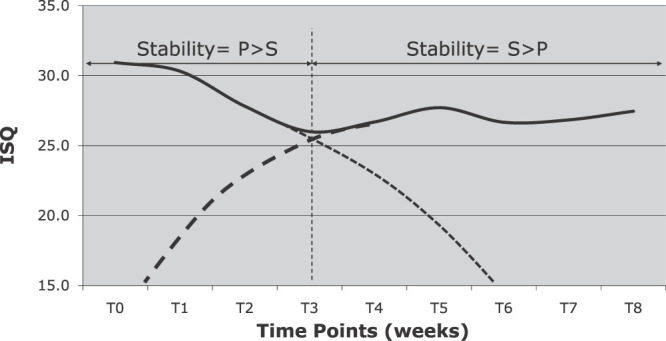
Changes in implant stability quotient over time for miniscrew implants that remained stable throughout the 8-week experimental period, with primary and secondary stability curves (dashed lined) superimposed.
DISCUSSION
The failures that occurred pertained exclusively to MSIs placed in nonkeratinized tissue. None of MSIs placed in keratinized tissue failed. The high success rate for MSIs placed in keratinized tissues compares well with rates reported for MSIs placed in keratinized tissue, which range from 91.7% to 100%.20,28,29 The consistency of these finding validates the use of RFA.
Placing MSIs in nonkeratinized mucosa is thought to be a clinical risk factor,1,2 and placement in nonkeratinized tissues has been shown experimentally to be problematic in dogs.30 The greater decreases in ISQs during the first 3 weeks indicated more bone loss around MSIs placed in nonkeratinized than in keratinized tissues. The failures were more likely due to peri-implant inflammation, which has been associated with the lack of keratinized tissue.2 The 3-mm tissue punch could have increased the risk of infection in nonkeratinized tissues, although it appeared to have had no effect in keratinized tissues.
Screwing the Smartpeg into the head of the MSI and unscrewing it after the recordings may have contributed to the high failure rate, because all of the failures occurred while attempting to unscrew the Smartpeg. This suggests that RFA may be limited, or perhaps even not appropriate, for measuring the stability of MSIs placed in regions of nonkeratinized tissues or in other regions with thin, less dense, cortical bone.
Upon placement, all of the MSIs in the present study exhibited adequate primary stability. It was the loss of stability during the first 3 weeks that determined whether or not MSIs remained stable or failed. Changes in stability have been previously used to identify dental implants at risk of failure.24,31–33 Based on the present findings, changes in stability might someday be used clinically to identify MSIs that are at the greatest risk of failing.
The overall stability of any MSI is due to the combined effects of primary and secondary stability.34 Based on the results in the present study, the point of transition from primary to secondary stability appears to occur at approximately 3 weeks (Figure 7), which compares closely to the point of transition identified for dental implants.20,21,33
Primary stability decreased immediately and significantly over the first 3 weeks following MSI placement. Decreases in ISQ values during the first 3 to 4 weeks have been previously reported after dental implant placement.20,26 Stability might be expected to start decreasing within the first week, when osteoclasts and mesenchymal cells, which appear by day four, begin removing bone damaged during MSI placement.17 Strategies to reduce trauma to bone during insertion or ways to accelerate healing should produce greater MSI stability.
Secondary stability, which is associated with healing and increases in total MSI stability, first became evident 4 weeks after miniscrew placement. For dental implants, increases in stability have been reported to begin around the fourth week after placement.21,26,35 Increases in overall stability can be explained by the new bone formation, which has been reported to begin around dental implants approximately 3 weeks after placement in dogs.36
Although secondary stability might be expected to increase until complete healing around the MSI has taken place, it leveled off after the fifth week in the present study. The dental implant literature indicates increases in stability well beyond 5 weeks.21,26,33 The lack of increase between weeks five and eight might be explained by the fact that Rimadyl (Pfizer, NY, NY), a nonsteroidal anti-inflammatory drug (NSAID), was administered by the veterinarian to both dogs during this time period to control pain. NSAIDs have been previously shown to inhibit bone formation because they inhibit prostaglandin formation.37
Whether or not the MSIs were placed with or without pilot holes appeared to have no appreciable effect on changes of ISQ values in the present study. Previous studies have shown that pilot holes decrease insertion torque and pullout strength.38–40 It is possible that small differences actually exist that RFA may not be sensitive enough to detect. A more plausible explanation relates to the stiffness of the bone, which is one of the factors that determine the resonance frequency of an implant. Because the stiffness of the bone is a function of its physical composition, it might be expected to remain the same whether or not a pilot hole is placed. It is also possible that the placement of a pilot hole causes as much trauma to the bone as does the placement of a MSI without a pilot hole.
CONCLUSIONS
Because of the small sample size, the results of this study should be considered preliminary. Within the limits of the study, it can be concluded that:
RFAs show that MSI stability changes over time; it decreases during the first 3 weeks after placement and increases between weeks three and five.
MSIs that fail show significantly greater decreases in stability during the first 3 weeks after placement than MSIs that remain stable.
MSIs placed in nonkeratinized tissue show significantly greater decreases in stability during the first 3 weeks after placement than MSIs placed in keratinized tissue.
Changes in stability of MSIs placed with or without pilot holes are comparable.
Acknowledgments
The miniscrew implants were provided by Neodent.
REFERENCES
- 1.Park H, Jeong S, Kwon O. Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofac Orthop. 2006;130:18–25. doi: 10.1016/j.ajodo.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Cheng S, Tseng I, Lee J, Kok S. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004;19:100–106. [PubMed] [Google Scholar]
- 3.Motoyoshi M, Hirabayashi M, Uemura M, Shimizu N. Recommended placement torque when tightening an orthodontic mini-implant. Clin Oral Implants Res. 2006;17:109–114. doi: 10.1111/j.1600-0501.2005.01211.x. [DOI] [PubMed] [Google Scholar]
- 4.Buschang P. H, Carrillo R, Ozenbaugh B, Rossouw P. E. 2008 survey of AAO members on miniscrew usage. J Clin Orthod. 2008;42:513–518. [PubMed] [Google Scholar]
- 5.Reynders R, Ronchi L, Bipat S. Mini-implants in orthodontics: a systematic review of the literature. Am J Orthod Dentofac Orthop. 2009;135:564.e1–e19. doi: 10.1016/j.ajodo.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Ashley E. T, Covington L. L, Bishop B. G, Breault L. G. Ailing and failing endosseous dental implants: a literature review. J Contemp Dent Pract. 2003;4:35–50. [PubMed] [Google Scholar]
- 7.Viwattanatipa N, Thanakitcharu S, Uttraravichien A, Pitiphat W. Survival analyses of surgical miniscrews as orthodontic anchorage. Am J Orthod Dentofac Orthop. 2009;136:29–36. doi: 10.1016/j.ajodo.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Miyawaki S, Koyama I, Inoue M, Mishima K, Sugahara T, Takano-Yamamoto T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofac Orthop. 2003;124:373–378. doi: 10.1016/s0889-5406(03)00565-1. [DOI] [PubMed] [Google Scholar]
- 9.Brinley C. L, Behrents R. G, Kim K. B, Condoor S, Kyung H. M, Buschang P. H. Effects of pitch and fluting on the primary stability of miniscrew implants. Angle Orthod. 2009;79:1156–61. doi: 10.2319/103108-554R.1. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson A. R, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101–107. doi: 10.1016/0022-3913(83)90174-9. [DOI] [PubMed] [Google Scholar]
- 11.Heidemann W, Gerlach K. L, Gröbel K. H, Köllner H. G. Drill free screws: a new form of osteosynthesis screw. J Craniomaxillofac Surg. 1998;26:163–168. doi: 10.1016/s1010-5182(98)80007-3. [DOI] [PubMed] [Google Scholar]
- 12.Matthews L. S, Hirsch C. Temperatures measured in human cortical bone when drilling. J Bone Joint Surg. 1972;54:297–308. [PubMed] [Google Scholar]
- 13.Büchter A, Wiechmann D, Koerdt S, Wiesmann H. P, Piffko J, Meyer U. Load-related implant reaction of mini-implants used for orthodontic anchorage. Clin Oral Implants Res. 2005;16:473–479. doi: 10.1111/j.1600-0501.2005.01149.x. [DOI] [PubMed] [Google Scholar]
- 14.Dalstra M, Cattaneo P, Melsen B. Load transfer of miniscrews for orthodontic anchorage. Orthodontics. 2004;1:53–62. [Google Scholar]
- 15.Johansson C, Albrektsson T. Integration of screw implants in the rabbit: a 1-year follow-up of removal torque of titanium implants. Int J Oral Maxillofac Implants. 1987;2:69–75. [PubMed] [Google Scholar]
- 16.Kim S, Cho J, Chung K, Kook Y, Nelson G. Removal torque values of surface-treated mini-implants after loading. Am J Orthod Dentofac Orthop. 2008;134:36–43. doi: 10.1016/j.ajodo.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 17.Berglundh T, Abrahamsson I, Lang N. P, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14:251–262. doi: 10.1034/j.1600-0501.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 18.Melsen B, Lang N. P. Biological reactions of alveolar bone to orthodontic loading of oral implants. Clin Oral Implants Res. 2001;12:144–152. doi: 10.1034/j.1600-0501.2001.012002144.x. [DOI] [PubMed] [Google Scholar]
- 19.Huja S. S, Litsky A. S, Beck F. M, Johnson K. A, Larsen P. E. Pull-out strength of monocortical screws placed in the maxillae and mandibles of dogs. Am J Orthod Dentofac Orthop. 2005;127:307–313. doi: 10.1016/j.ajodo.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Balshi S. F, Allen F. D, Wolfinger G. J, Balshi T. J. A resonance frequency analysis assessment of maxillary and mandibular immediately loaded implants. Int J Oral Maxillofac Implants. 2005;20:584–594. [PubMed] [Google Scholar]
- 21.Boronat López A, Balaguer Martínez J, Lamas Pelayo J, Carrillo García C, Peñarrocha Diago M. Resonance frequency analysis of dental implant stability during the healing period. Med Oral Patol Oral Cir Bucal. 2008;13:E244–E247. [PubMed] [Google Scholar]
- 22.Ersanli S, Karabuda C, Beck F, Leblebicioglu B. Resonance frequency analysis of one-stage dental implant stability during the osseointegration period. J Periodontol. 2005;76:1066–1071. doi: 10.1902/jop.2005.76.7.1066. [DOI] [PubMed] [Google Scholar]
- 23.Meredith N, Alleyne D, Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261–267. doi: 10.1034/j.1600-0501.1996.070308.x. [DOI] [PubMed] [Google Scholar]
- 24.Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 2000. 2008;47:51–66. doi: 10.1111/j.1600-0757.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- 25.Su Y. Y, Wilmes B, Hönscheid R, Drescher D. Application of a wireless resonance frequency transducer to assess primary stability of orthodontic mini-implants: an in vitro study in pig ilia. Int J Oral Maxillofac Implants. 2009;24:647–654. [PubMed] [Google Scholar]
- 26.Barewal R. M, Oates T. W, Meredith N, Cochran D. L. Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants. 2003;18:641–651. [PubMed] [Google Scholar]
- 27.Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663–670. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 28.Chaddad K, Ferreira A. F. H, Geurs N, Reddy M. S. Influence of surface characteristics on survival rates of mini-implants. Angle Orthod. 2008;78:107–113. doi: 10.2319/100206-401.1. [DOI] [PubMed] [Google Scholar]
- 29.Lim H, Eun C, Cho J, Lee K, Hwang H. Factors associated with initial stability of miniscrews for orthodontic treatment. Am J Orthod Dentofac Orthop. 2009;136:236–242. doi: 10.1016/j.ajodo.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Kim S. H, Lee S. J, Cho I. S, Kim S. K, Kim T. W. Rotational resistance of surface-treated mini-implants. Angle Orthod. 2009;79:899–907. doi: 10.2319/090608-466.1. [DOI] [PubMed] [Google Scholar]
- 31.Glauser R, Sennerby L, Meredith N, Rée A, Lundgren A, Gottlow J, Hämmerle C. H. F. Resonance frequency analysis of implants subjected to immediate or early functional occlusal loading. Successful vs. failing implants. Clin Oral Implants Res. 2004;15:428–434. doi: 10.1111/j.1600-0501.2004.01036.x. [DOI] [PubMed] [Google Scholar]
- 32.Friberg B, Sennerby L, Linden B, Gröndahl K, Lekholm U. Stability measurements of one-stage Brånemark implants during healing in mandibles. A clinical resonance frequency analysis study. Int J Oral Maxillofac Surg. 1999;28:266–272. [PubMed] [Google Scholar]
- 33.Huwiler M. A, Pjetursson B. E, Bosshardt D. D, Salvi G. E, Lang N. P. Resonance frequency analysis in relation to jawbone characteristics and during early healing of implant installation. Clin Oral Implants Res. 2007;18:275–280. doi: 10.1111/j.1600-0501.2007.01336.x. [DOI] [PubMed] [Google Scholar]
- 34.Raghavendra S, Wood M. C, Taylor T. D. Early wound healing around endosseous implants: a review of the literature. Int J Oral Maxillofac Implants. 2005;20:425–431. [PubMed] [Google Scholar]
- 35.Stadlinger B, Bierbaum S, Grimmer S, Schulz M. C, Kuhlisch E, Scharnweber D, Eckelt U, Mai R. Increased bone formation around coated implants. J Clin Periodontol. 2009;36:689–704. doi: 10.1111/j.1600-051X.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- 36.Roberts W. E. Bone tissue interface. J Dent Educ. 1988;52:804–809. [PubMed] [Google Scholar]
- 37.Fracon R. N, Teófilo J. M, Satin R. B, Lamano T. Prostaglandins and bone: potential risks and benefits related to the use of nonsteroidal anti-inflammatory drugs in clinical dentistry. J Oral Sci. 2008;50:247–252. doi: 10.2334/josnusd.50.247. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Shin H, Kyung H. Biomechanical and histological comparison of self-drilling and self-tapping orthodontic microimplants in dogs. Am J Orthod Dentofac Orthop. 2008;133:44–50. doi: 10.1016/j.ajodo.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Oktenoğlu B. T, Ferrara L. A, Andalkar N, Ozer A. F, Sarioğlu A. C, Benzel E. C. Effects of hole preparation on screw pullout resistance and insertional torque: a biomechanical study. J Neurosurg. 2001;94:91–96. doi: 10.3171/spi.2001.94.1.0091. [DOI] [PubMed] [Google Scholar]
- 40.Hung E, Oliver D, Kim K. B, Kyung H. M, Buschang P. H. Effects of pilot hole size and bone density on miniscrew implants' stability. Clin Implant Dent Relat Res. doi: 10.1111/j.1708-8208.2010.00269.x. March 12, 2010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]