Abstract
Objective:
To evaluate and compare the effects of circumferential supracrestal fiberotomy in vivo (using diode, CO2, and Er∶YAG lasers) on the morphology and chemical composition of the root surface.
Materials and Methods:
Forty healthy premolar teeth, intended for extraction for orthodontic reasons, were used in this study. Root surfaces were treated using different laser methods, as follows: (1) control; (2) Er∶YAG laser (2.94 µm, 100 mJ, 10 Hz); (3) diode laser (808 nm, 1.2 W, continuous wave); and (4) CO2 laser (10.6 µm, 3 W, continuous wave). Subsequently, the teeth were removed and subjected to scanning electron microscopic (SEM) examination and energy dispersive x-ray (EDX) spectrometric analysis.
Results:
SEM analysis indicated that no thermal changes, including melting or carbonization, were observed following the lasing procedures. EDX analysis showed that the laser procedures resulted in similar mineral contents (weight % of calcium and phosphate) as compared to those in the control group.
Conclusion:
Based on these findings, we concluded that laser-aided procedures, when used at appropriate laser settings, preserve the original morphology and chemical composition of cementum.
Keywords: Circumferential supracrestal fiberotomy, Lasers, Energy dispersive x-ray (EDX) spectrometric analysis, Root surface, Scanning electron microscopic (SEM) examination
INTRODUCTION
Long-term stability is a concern in orthodontic treatment. One of the major causes of rebound after orthodontic tooth movement is the network of elastic supracrestal gingival fibers. As teeth are moved to new positions, these fibers tend to stretch, and they remodel very slowly.1–3 Within 4 to 6 months, the collagenous fiber networks within the gingiva have normally completed their reorganization, but the elastic supracrestal fibers remodel slowly and can still exert forces capable of displacing a tooth 1 year after removal of an orthodontic appliance.
To relieve the orthodontically moved tooth of the forces exerted by the stretched fibers, circumferential supracrestal fiberotomy (CSF) was introduced.4,5 With conventional CSF, performed with a scalpel blade, intergingival, transgingival, transseptal, and semicircular fibers are transected. CSF appears to help the tissue remodel and decreases relapse of orthodontically moved teeth. The literature indicates6 that periodontal problems, such as pocket formation, loss of attached gingiva, and gingival recession, do not occur after CSF.
In recent years, the use of laser irradiation has been tested as an alternative treatment to conventional CSF.7 Laser-aided CSF (L-CSF) proved to be effective in decreasing rates of relapse following tooth rotation. In our previous study,7 the diode laser CSF procedure did not cause any injury to teeth or bone. Histological studies failed to show any evidence of bone necrosis, sequestration, or destruction. Rearrangement of the fibrous structures was observed; the organizational pattern of experimental teeth resembled that of non-rotated teeth, in which large fiber bundles were seen interconnected with thin fibers.
L-CSF offers numerous advantages compared with conventional surgery by blade. One of the advantages is accelerated wound healing due to the biostimulatory effects of laser.8 Coagulation of blood vessels and sealing of lymphatics enable practitioners to cause less bleeding during the procedure and to maintain a clear and clean surgical field.8 The last advantage of L-CSF involves the sterilization of the wound during ablation, and this makes it possible for the patient to experience minimal swelling and lower rates of postoperative infection.9
The lasers most commonly used in periodontics are diode lasers, the Er∶YAG laser, and the CO2 laser. Several researchers10,11 reported thermal side effects, such as melting, cracking, or carbonization, when CO2 or Nd∶YAG lasers were used directly on root surfaces. In addition, limited information about the effects of diode laser radiation on root surfaces currently exists. Recent studies12 have shown that this type of laser may cause damage to periodontal hard tissues if irradiation parameters are not suitable. The aim of this study was to investigate the effects of CSF performed by three types of lasers on the physical and chemical characteristics of the human root.
MATERIALS AND METHODS
This study included 40 premolar teeth scheduled for extraction as part of orthodontic treatment. All patients provided informed consent. Each tooth satisfied the following criteria: (1) absence of caries and filling materials, (2) absence of root fractures or anatomical abnormalities, and (3) absence of calculus deposits. Patients had not received any periodontal treatment in the previous 6 months. The teeth were divided into four groups according to the laser used, as follows: (1) control (conservative CSF), (2) Er∶YAG laser, (3) diode laser, and (4) CO2 laser groups. The research protocol was reviewed and approved by the institutional review board of Kyung Hee University Medical Center (IRB No. KHD IRB 2009-12).
Laser-Aided CSF
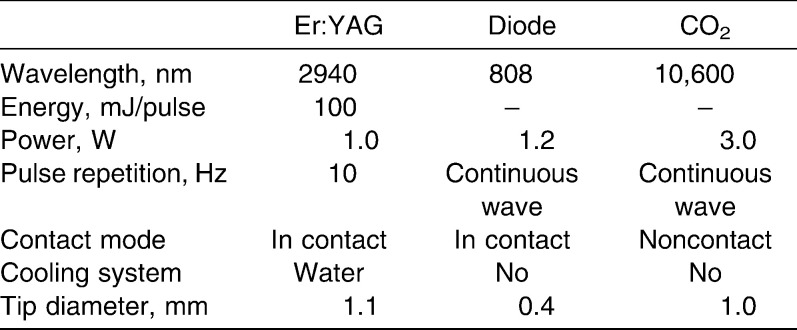
Three dental lasers were used in this study (Table 1; Figure 1). An Er∶YAG laser (KEY laser, Kavo, Germany) with a wavelength of 2.94 µm was used at an energy setting of 100 mJ/pulse and a pulse repetition of 10 Hz, with a tip diameter of 1.1 mm, focused and in contact, with water cooling. A gallium-aluminum-arsenide diode laser (SoftLase Pro; Zap Lasers, Pleasant Hill, CA) with a wavelength of 808 nm was used in continuous wave mode at a power output of 1.2 W (soft tissue cutting mode), with a 0.4-mm–diameter fiber tip. A CO2 laser (Panalas C05∑, Panasonic, Japan) with a wavelength of 10.6 µm was used in continuous wave, noncontact mode at a power output of 3 W. A metal tip of 1.0 mm in diameter was used for the sharp incision of tissues.
Table 1.
List of Parameters Used for Each Laser Type
Figure 1.
Three dental lasers used in this study. (a) Er∶YAG laser (KEY Laser, Kavo, Germany); (b) diode laser (SoftLase Pro; Zap Lasers, Pleasant Hill, CA); and (c) CO2 laser (Panalas C05∑, Panasonic, Osaka, Japan).
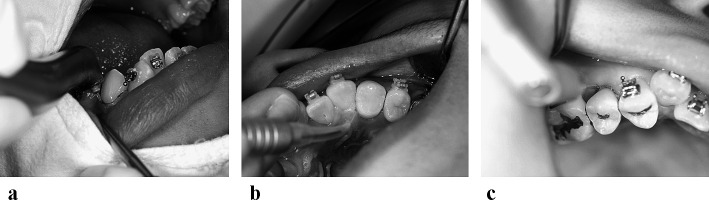
Immediately before L-CSF was performed, the sulcus depth around the tooth was measured by probing. The maximum depth of insertion of the tip was determined as the sum of the sulcus depth and the biologic width (approximately 2 mm).13 The laser tip was inserted through the connective tissue attachment just above the alveolar crest. Intrasulcular ablation was performed around the tooth circumference with the system configured in soft tissue cutting mode. The laser tip was directed at an angle of 10–15° to the radicular surface and was applied continuously in an apico-coronal direction (Figure 2).
Figure 2.
Laser-aided circumferential supracrestal fiberotomy. (a) Er∶YAG laser (KEY Laser, Kavo, Germany); (b) diode laser (SoftLase Pro; Zap Lasers, Pleasant Hill, CA); and (c) CO2 laser (Panalas C05∑, Panasonic, Osaka, Japan).
The working time was 5 minutes for each tooth. Immediately after laser ablation, the teeth were extracted. All root surfaces appeared unaltered by the extraction procedure. After extraction, any remaining tags of the periodontal ligament were carefully removed using a blade (group 1) or a laser (groups 2–4). Teeth were fixed in a 2% glutaraldehyde solution in phosphate-buffered saline.
Scanning Electron Microscopic Analysis
Fixed specimens were dehydrated through increasing concentrations of ethanol for 30 minutes. After dehydration in hexamethyldisiloxane for 30 minutes, the specimens were sputter-coated with gold before scanning electron microscopic (SEM) examination. All specimens were then examined and photographed by SEM on the mesial side of the root, 2 mm apical to the cementoenamel junction, in an area with dimensions of 9 mm2. This analysis was conducted by a Stereoscan 440 microscope (Leica, Cambridge, UK). Photomicrographs on the root surfaces were taken at magnifications of 200× and 2000× to obtain representative images of each group.
Energy Dispersive X-Ray Analysis
We used energy dispersive x-ray (EDX) analysis to evaluate the change of root surfaces treated using different lasers. EDX is an analytical technique used for the elemental analysis or chemical characterization of a sample. It is one of the variants of x-ray fluorescence spectroscopy that relies on the investigation of a sample through interactions between electromagnetic radiation and matter, analyzing x-rays emitted by the matter in response to being bombarded with charged particles. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing x-rays that are characteristic of an element's atomic structure to be uniquely identified. In this study, calcium (Ca) and phosphate (P) minerals were measured in the root surface and Ca/P ratios were calculated.
SEM and EDX analysis were performed at the Center for Research Facilities at Kyung-Hee University in Korea.
Statistical Analysis
A software package (SPSS 11.0, SPSS Inc, Chicago, Ill) was used for statistical analysis. The Ca content (Ca weight %), phosphorus content (P weight %), and Ca/P weight ratio from the lased and control areas were recorded and analyzed using the Kruskal-Wallis test. Results are expressed as the mean ± standard deviation (SD), and a value of P < .05 was considered to be significant.
RESULTS
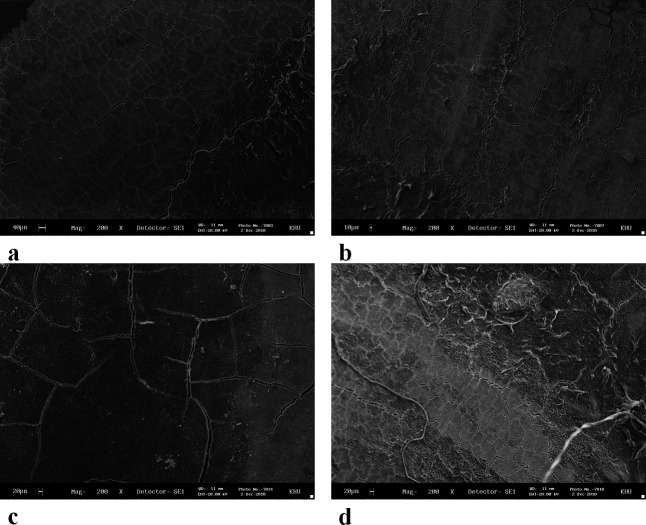
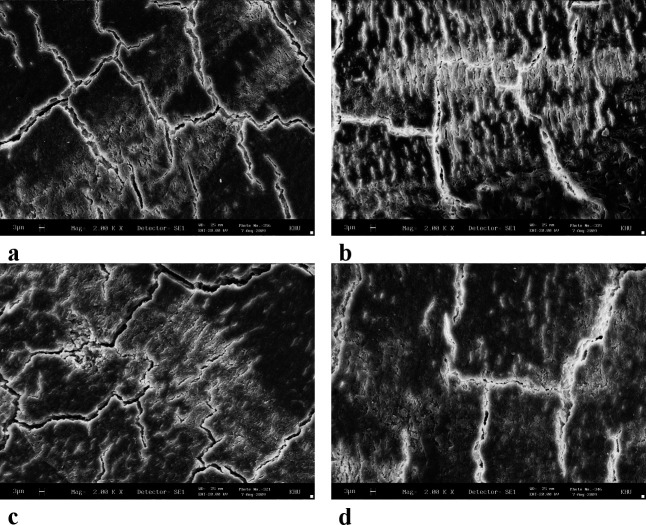
The SEM images showed that the surface texture of the lased root surfaces (Er∶YAG, diode, and CO2 lasers) were not changed in comparison with those of the control group. Loss of cementum was absent or minimal, with no signs of thermal side effects such as melting or carbonization (Figures 3 and 4).
Figure 3.
Scanning electron microscopic (SEM) photograph of a root surface. (a) Control; (b) Er∶YAG laser; (c) diode laser; and (d) CO2 laser (original magnifications: a–d: 200×).
Figure 4.
Scanning electron microscopic (SEM) photograph of a root surface. (a) Control; (b) Er∶YAG laser; (c) diode laser; and (d) CO2 laser (original magnifications: a–d: 2000×).
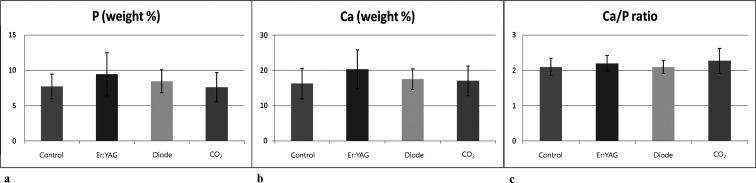
The results of EDX analysis are shown in Table 2 and Figure 5. The results showed that the contents of Ca (Ca weight %) in the samples of lased roots did not show significant change in the irradiated areas compared with the control areas (P = .301). The content of P (P weight %) did not increase (P = .441). No significant changes in the Ca/P weight ratio were shown between the lased and unlased areas (P = .546).
Table 2.
Percentages of Elemental Ion Concentrations Found on Energy Dispersive X-Ray (EDX) Analysis After Treatment of Root Surfaces (mean ± standard deviation [SD]; n = 10)a
Figure 5.
Percentages of elemental ion concentrations found on energy dispersive x-ray (EDX) analysis after treatment of root surfaces. (a) Phosphorus (P) mineral values; (b) calcium (Ca) mineral values; and (c) Ca/P ratios.
DISCUSSION
The results of this study indicated that CSF by Er∶YAG, diode, or CO2 lasers at a power output set to soft tissue ablation mode all preserve the original morphology and chemical composition of the root surface in vivo.
It is well known that the effects of laser irradiation on tissue depend on the physical properties of the laser energy, such as wavelength, power output, pulse duration, spot size, and irradiation time. The resulting effects are also dependent on the optical properties of the irradiated tissue, such as optical density, structure, and maximal absorption.14 Dental hard tissues are composed of hydroxyapatite crystals, organic components, and water.
The wavelength of an Er∶YAG laser (2.94 µm) is well absorbed by water because the peak is close to the absorption coefficient of water.15 The Er∶YAG laser has an excellent capacity for ablating both soft and hard tissues with minimal heat-related side effects. The resulting root surface alterations have previously been examined16,17 in vitro and were described as microstructured, with a partial loss of cementum and sometimes dentine but with no cracks or thermal side effects, such as the melting observed after CO2 or Nd∶YAG laser irradiation. A direct relationship between ablation depth and the energy settings used was observed.17 The mechanism of tissue damage caused by the Er∶YAG laser is probably not related to thermal side effects but rather to the microexplosions associated with water evaporation within the cementum.18 In our study, use of an energy setting of 100 mJ resulted in smooth root surface morphology and no significant changes in the chemical composition of the root surface. These findings are consistent with the results of previous studies of the root surface.19
Diode lasers operate in continuous and/or pulsed modes and are very effective for soft tissue applications, offering excellent incision, hemostasis, and coagulation.19 Root surface alterations after diode laser irradiation have been examined both in vivo12 and in vitro.19,20 Irradiation at a power output of 1 W or less had virtually no negative effects on the root surface, whereas lasing at 1.5 W, 2.0 W, and 2.5 W resulted in partial or total carbonization of root samples. In contrast, lasing dry specimens and specimens moistened with saline resulted in no detectable alterations, regardless of irradiation time and the power output applied.20 This phenomenon might be explained by the high absorption by hemoglobin and other pigments of the laser light at 810 nm.12 However, in this study, CSF using diode laser irradiation at a power output of 1.2 W did not cause any negative thermal side effects on the root surface. Because a laser beam is irradiated from the end of a 0.4-mm–diameter laser tip, the sides of the tip cannot be used for transection; therefore, an up-and-down stroking movement is initially required. As the diode laser tip is made of relatively low-strength fiber, the tip could occasionally break.7
The CO2 laser has a wavelength of 10.6 µm and can be used in both pulsed and continuous wave modes. This laser is efficient in soft tissue vaporization, as most soft tissues have high water content and because the CO2 laser radiation is absorbed easily by water.21–23 The scattering of laser energy toward the surrounding tissues is low and the layer of heat-altered tissue that remains after vaporization is relatively thin; however, the vaporization temperature is high and the irradiated surface is carbonized easily. Heat accumulates rapidly in the inorganic components of hard tissues because apatite crystals, a major component of hard tissues, absorb even more of the energy of the CO2 laser than does water.24 At the level of 5–11 W, CO2 laser–induced surface changes included cavitation, globules of melted and resolidified mineral, surface crazing, and production of a superficial char layer.25 At the level of 3 W, a CO2 laser completely removed the smear layer, with minimal changes in the diameter of the dentinal tubules.26 In our study, using an energy setting of 3 W resulted in a smooth root surface morphology and no significant change in chemical composition of the root surface. However, because of the diameter of the hollow delivery tip (>1 mm) that is required to transmit the energy beam, and given the use of the noncontact mode, CO2 lasers are difficult to use for the CSF procedure.
When applying lasers in vivo, thermal side effects have been a major concern. Heat generation during laser irradiation could cause inflammation and necrosis of the pulp and alveolar bone. A temperature increase of up to 5°C in the pulp has been reported27 as safe for pulpal survival. Er∶YAG (100 mJ/pulse, 10 Hz, 30 seconds) and diode (1.4 W, 30 seconds) laser irradiation of the root surface induced a temperature elevation of less than 5°C in the pulp chambers.19 Exposure of bone to heating above 47°C is reported to induce cellular damage leading to osseous resorption, while temperature levels of >60°C result in tissue necrosis. A diode laser (0.8–1.2 W)28 induced an increase in bone surface temperature of 10–11°C, and a CO2 (4–8 W)29 laser induced an increase of 1.4–2.1°C when the overlying soft tissues were irradiated. Thus, laser irradiation at the level of soft tissue incision is unlikely to cause thermal side effects on the pulp or alveolar bone. However, for confirmation, further histological or cytological studies are needed.
EDX is an analytical technique used for the elemental analysis or chemical characterization of a sample. In recent years, several studies30,31 have reported chemical changes of the root surface after laser irradiation. The quantities of Ca and P increased significantly after Er∶YAG laser (120 mJ/pulse, 10 Hz) irradiation and decreased after Nd∶YAG laser (1.5 W, 10 Hz) irradiation.30 After Er,Cr∶YSGG laser (1.5 W, 10 Hz) irradiation, decreased P levels and increased Ca levels were observed.31 In our study, after Er∶YAG laser (100 mJ/pulse, 10 Hz), diode laser (1.2 W, continuous wave), and CO2 laser (3.0 W, continuous wave) irradiation there were no significant changes in the quantities of Ca and P. Analysis by EDX is an appropriate method, as a result of our study design, with which to measure levels of the minerals, which are important for root structure and to providing a proper surface for repair of the periodontal attachment.
CONCLUSION
Within the limits of this study, the in vivo results indicate that laser-aided CSF procedures, when used at appropriate settings, preserve the original morphology and chemical composition of cementum.
Acknowledgments
This study was supported by the Kyung Hee University program for the young researcher of medical science (2010).
REFERENCES
- 1.Thompson H. E. Orthodontic relapses analyzed in a study of connective tissue fibers. Am J Orthod. 1959;45:93–109. [Google Scholar]
- 2.Edwards J. G. A study of the periodontium during orthodontic rotation of teeth. Am J Orthod. 1968;54:441–461. doi: 10.1016/0002-9416(68)90199-1. [DOI] [PubMed] [Google Scholar]
- 3.Wiser G. M. Resection of the supra-alveolar fibers and the retention of orthodontically rotated teeth. Am J Orthod. 1996;52:855. [Google Scholar]
- 4.Edwards J. G. A surgical procedure to eliminate rotational relapse. Am J Orthod. 1970;57:35–46. doi: 10.1016/0002-9416(70)90203-4. [DOI] [PubMed] [Google Scholar]
- 5.Crum R. E, Andreason G. F. The effect of gingival fiber surgery on the retention of rotated teeth. Am J Orthod. 1974;65:626–638. doi: 10.1016/0002-9416(74)90257-7. [DOI] [PubMed] [Google Scholar]
- 6.Block R. W, Kerns D. G, Regennitter F. J, Kerns L. L. The circumferential supracrestal fiberotomy. Gen Dent. 1998;55:48–54. [PubMed] [Google Scholar]
- 7.Kim S. J, Paek J. H, Park K. H, Kang S. G, Park Y. G. Laser-aided circumferential supracrestal fiberotomy and low-level laser therapy effects on relapse of rotated teeth in beagles. Angle Orthod. 2010;80:385–390. doi: 10.2319/051609-268.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarver D. M, Yanosky M. Principles of cosmetic dentistry in orthodontics: part 2. Soft tissue laser technology and cosmetic gingival contouring. Am J Orthod Dentofacial Orthop. 2005;127:85–90. doi: 10.1016/j.ajodo.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Moritz A. Oral Laser Application. Chicago, Ill: Quintessence; 2006. [Google Scholar]
- 10.Tucker D, Cobb C. M, Rapley J. W, Killoy W. J. Morphologic changes following in vitro CO2 laser treatment of calculus-ladened root surfaces. Lasers Surg Med. 1996;18:150–156. doi: 10.1002/(SICI)1096-9101(1996)18:2<150::AID-LSM4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Tewfik H. M, Garnick J. J, Schuster G. S, Sharawy M. M. Structural and functional changes of cementum surface following exposure to a modified Nd∶YAG laser. J Periodontol. 1994;65:297–302. doi: 10.1902/jop.1994.65.4.297. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz F, Sculean A, Berakdar M, Szathmari L, Georg T, Becker J. In vivo and in vitro effects of an Er∶YAG laser, a GaAlAs diode laser, and scaling and root planing on periodontally diseased root surfaces: a comparative histologic study. Lasers Surg Med. 2003;32:359–366. doi: 10.1002/lsm.10179. [DOI] [PubMed] [Google Scholar]
- 13.Berglundh T, Marinello C. P, Lindhe J, Thilander B, Liljenberg B. Periodontal tissue reactions to orthodontic extrusion. J Clin Periodontol. 1991;18:330–336. doi: 10.1111/j.1600-051x.1991.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 14.Keller U, Hisbst R. Experimental studies of the application of the Er∶YAG laser on dental hard substances (II). Light microscopic and SEM investigations. Lasers Surg Med. 1989;9:345–351. doi: 10.1002/lsm.1900090406. [DOI] [PubMed] [Google Scholar]
- 15.Walsh J. T, Flotte T. J, Deutsch T. F. Er∶YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med. 1989;9:314–326. doi: 10.1002/lsm.1900090403. [DOI] [PubMed] [Google Scholar]
- 16.Aoki A, Ando A, Watanabe H, Ishikawa I. In vitro studies on laser scaling of subgingival calculus with an Er∶YAG laser. J Periodontol. 1996;65:1097–1106. doi: 10.1902/jop.1994.65.12.1097. [DOI] [PubMed] [Google Scholar]
- 17.Folwaczny M, Mehl A, Haffner C, Benz C, Hickel R. Root substance removal with Er∶YAG laser radiation at different parameters using a new delivery system. J Periodontol. 2000;71:147–155. doi: 10.1902/jop.2000.71.2.147. [DOI] [PubMed] [Google Scholar]
- 18.Fujii T, Baehni P. C, Kawai O, Kawakami T, Matsuda K, Kowashi Y. Scanning electron microscopic study of the effects of Er∶YAG laser on root cementum. J Periodontol. 1998;69:1283–1290. doi: 10.1902/jop.1998.69.11.1283. [DOI] [PubMed] [Google Scholar]
- 19.Theodoro L. H, Haypek P, Bachmann L, Garcia V. G, Sampaio J. E, Zezell D. M, Eduardo Cde P. Effect of Er∶YAG and diode laser irradiation on the root surface: morphological and thermal analysis. J Periodontol. 2003;74:838–843. doi: 10.1902/jop.2003.74.6.838. [DOI] [PubMed] [Google Scholar]
- 20.Kreisler M, Al Haj H, Daublander M, Gotz H, Duschner H, Willershausen B, D'Hoedt B. Effect of diode laser irradiation on root surfaces in vitro. J Clin Laser Med Surg. 2002;20:63–69. doi: 10.1089/104454702753768034. [DOI] [PubMed] [Google Scholar]
- 21.Hale G. M, Querry M. R. Optical constants of water in the 200 nm to 200 µm wavelength region. Appl Optics. 1973;12:555–563. doi: 10.1364/AO.12.000555. [DOI] [PubMed] [Google Scholar]
- 22.Pick R. M, Colvard M. D. Current status of lasers in soft tissue dental surgery. J Periodontol. 1993;64:589–602. doi: 10.1902/jop.1993.64.7.589. [DOI] [PubMed] [Google Scholar]
- 23.White J. M, Chaudhry S. I, Kudler J. J, Sekandari N, Schoelch M. L, Silverman S., Jr Nd∶YAG and CO2 laser therapy of oral mucosal lesions. J Clin Laser Med Surg. 1998;16:299–304. doi: 10.1089/clm.1998.16.299. [DOI] [PubMed] [Google Scholar]
- 24.Featherstone J. D. Caries detection and prevention with laser energy. Dent Clin North Am. 2000;44:955–969. [PubMed] [Google Scholar]
- 25.Israel M, Cobb C. M, Rossmann J. A, Spencer P. The effects of CO2, Nd∶YAG and Er∶YAG lasers with and without surface coolant on tooth root surfaces. An in vitro study. J Clin Periodontol. 1997;24:595–602. doi: 10.1111/j.1600-051x.1997.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 26.Misra V, Mehrotra K. K, Dixit J, Maitra S. C. Effect of a carbon dioxide laser on periodontally involved root surfaces. J Periodontol. 1999;70:1046–1052. doi: 10.1902/jop.1999.70.9.1046. [DOI] [PubMed] [Google Scholar]
- 27.Zach L, Cohen G. Pulp response to externally applied heat. Oral Surg Oral Med Oral Pathol. 1965;19:515–530. doi: 10.1016/0030-4220(65)90015-0. [DOI] [PubMed] [Google Scholar]
- 28.Fontana C. R, Kurachi C, Mendonça C. R, Bagnato V. S. Temperature variation at soft periodontal and rat bone tissues during a medium-power diode laser exposure. Photomed Laser Surg. 2004;22:519–522. doi: 10.1089/pho.2004.22.519. [DOI] [PubMed] [Google Scholar]
- 29.Spencer P, Cobb C. M, Wieliczka D. M, Glaros A. G, Morris P. J. Change in temperature of subjacent bone during soft tissue laser ablation. J Periodontol. 1998;69:1278–1282. doi: 10.1902/jop.1998.69.11.1278. [DOI] [PubMed] [Google Scholar]
- 30.Gómez C, Bisheimer M, Costela A, García-Moreno I, García A, García J. A. Evaluation of the effects of Er∶YAG and Nd∶YAG lasers and ultrasonic instrumentation on root surfaces. Photomed Laser Surg. 2009;27:43–48. doi: 10.1089/pho.2008.2236. [DOI] [PubMed] [Google Scholar]
- 31.Hakki S. S, Berk G, Dundar N, Saglam M, Berk N. Effects of root planing procedures with hand instrument or erbium, chromium:yttrium-scandium-gallium-garnet laser irradiation on the root surfaces: a comparative scanning electron microscopy study. Lasers Med Sci. 2010;25:345–353. doi: 10.1007/s10103-009-0643-x. [DOI] [PubMed] [Google Scholar]