Abstract
Objective:
To measure ion release from four sol-gel bioactive glass–containing orthodontic resin bonding agents (BAG-Bonds) following immersion into simulated body fluid (SBF) at pH values of 4 and 7.
Materials and Methods:
Four BAG-Bonds, two containing fluoride, were developed. Prepared in our laboratory, the BAG-Bonds were composed of a mixture of resin monomers and bioactive glasses (BAGs). Workability of the final BAG-Bonds determined the amount of filler added to each, and this varied according to BAG composition. Commercially available Transbond-XT was used as the control. Three disks (10 mm × 2 mm) of each material were individually suspended in 3.5 mL of SBF at pH 4 and pH 7. SBF was analyzed to measure pH and ions released at 1 hour, 10 hours, and 100 hours. Calcium was measured by atomic absorption analysis, phosphate by ultraviolet visible spectrometry, and fluoride by an ion-specific electrode. The data were compared using a three-way analysis of variance, with P ≤ .05.
Results:
Significant differences in calcium and phosphate ion release were found between the four BAG-Bonds and the control at multiple time points. Significant changes in pH were also found. There was no measureable release of fluoride from any of the materials.
Conclusions:
The BAG-Bonds showed the capacity for buffering acidic oral environments and significant release of calcium ions into their surrounding environment, and they hold the potential to be biomimetic bonding agents that may reduce white spot lesion formation.
Keywords: Bioactive glass, Orthodontic adhesive, Remineralization, White spot lesions, Ion release
INTRODUCTION
White spot lesions (WSLs) are clinically detectable manifestations of subsurface enamel demineralization, representing the first stage of caries formation.1 Nearly half of patients who receive fixed orthodontic appliance therapy develop WSLs as a result of poor oral hygiene and plaque retention around orthodontic appliances.2,3 With rapid accumulation of plaque, the incipient caries form as early as 4 weeks following the placement of fixed orthodontic appliances.1,2,4,5 WSLs have been found4–6 to result in a 10–50% reduction in enamel mineral content. Although WSLs may regress to a variable extent following bracket removal, they often persist, causing esthetic concerns.7,8 Given this ongoing side effect of orthodontic treatment, advances aimed at preventing WSLs continue to be an area of strong interest in orthodontics.
Previous investigation5 has shown that regular use of fluoridated toothpaste during fixed orthodontic treatment is often insufficient for prevention of WSLs. A number of supplemental regimens have been recommended,5,8 including fluoride-containing mouth rinses, gels, and varnishes. The effectiveness of fluoride, as applied through such applications, has been limited by several challenges, including the following: localizing the fluoride to those areas where it is needed; the substantivity of fluoride-containing products; and the lack of predictability related to patient compliance.8
Fluoride-releasing orthodontic bonding agents, such as glass-ionomer cements (GICs) and resin-modified glass ionomer cements (RMGICs), were developed in part for prevention of WSLs. Although these bonding agents have been shown to release fluoride in vivo, their bond strengths have been substantially lower than those of conventional resins,9–16 and reports have been mixed with regard to their anticariogenic effects.13,17 Although GICs and RMGICs are commercially available, no bonding agent is currently on the market that provides a biomimetic approach whereby calcium (Ca) and phosphate (PO4) ion release adjacent to brackets would potentially prevent demineralization.
Incorporating bioactive glass (BAG) into a composite resin may result in a biomimetic adhesive. Sol-gel BAG is a three-dimensional, cross-linked matrix of hydrolyzed alkoxides of SiO2, CaO, and P2O5.18 BAG has biomimetic properties when immersed in body fluids, leading to the formation of tooth-like hydroxylapatite.19,20 Under in vitro and in vivo conditions, BAGs release ions that interact with each other as well as with ions in the surrounding solution, forming a supersaturated solution and a Ca-phosphorus (P) precipitate. This process has the potential for subsequent remineralization of demineralized enamel.19,20
Additionally, in vitro investigations of an experimental GIC containing 30 wt% BAG have found that the bonding agent inhibited growth of the cariogenic bacteria Streptococcus mutans.21 Thus, this anticariogenic effect may also contribute to BAG's potential for preventing WSLs.
The purposes of this study were (1) to develop novel BAG-containing composite resins to be used for bonding of orthodontic brackets and (2) to test the hypotheses that (a) these novel resins would release ions into simulated body fluid (SBF) at pH 4 and pH 7 and (b) that the release of these ions would result in a change in the pH of the surrounding environment.
MATERIALS AND METHODS
Preparation of BAG-Bond
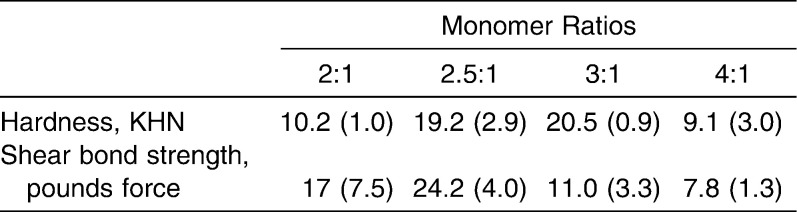
Four BAG–containing orthodontic resin bonding agents (62BAG-Bond, 65BAG-Bond, 81BAG-Bond, and 85BAG-Bond) were developed in our laboratories (Table 1). These were prepared by mixing (SpeedMixer DAC, Flack Tek, Landrum, SC) two resin monomers with one of four compositions of bioactive-glass (62BAG, 65BAG, 81BAG, or 85BAG), 0.4 wt% camphoroquinone (Polysciences, Warrington, Pa), and 0.8 wt% ethyl 4-dimethylaminobenzoate (Avocado Research Chemicals Ltd, Heysham, Lancaster, UK). The resin monomers were ethoxylated bisphenol A dimethacrylate (EOBPADMA) and BisGMA (both from Esstech Corp, Essington, Pa). The ratio of EOBPADMA to BisGMA used was 2.5∶1, selected after a pilot study comparing ratios of 3∶1, 2.5∶1, and 2∶1 showed that 2.5∶1 had the highest Knoop hardness and shear bond strength values (Table 2).
Table 1.
Bioactive Glass (BAG) and BAG–Containing Orthodontic Resin Bonding Agents (BAG-Bond) Compositions Prepared in Our Laboratory
Table 2.
Pilot Test for the Hardness and Shear Bond Strength Values for the Unfilled Resin Monomer Mixtures. Standard Deviations Are Shown in Parentheses. Knoop Hardness Values Were Obtained With a Duramin 5 Micro-Hardness Tester (Struers Corp, Westlake, Ohio) Using a Load of 200 p and a Time of 25 Seconds. Shear Bond Strength Was Evaluated Using Instron Universal Testing Machine (Instron Corp, Canton, Mass) at a Crosshead Speed of 0.5 mm/min
BAGs were synthesized in our laboratory by sol-gel methods,19 ball milled, sieved, and micronized (Sturtevant, Hanover, Mass). Average particle size ranged from 0.04 to 3.0 µm, as determined by laser particle size measurements (Beckman Coulter LS13 320, Brea, Calif). The BET method22 was used to measure the surface areas of each BAG. BAGs were added to the monomer mixture until the workability and viscosity of each product was similar to that of the control material, Transbond XT (3M Unitek, Monrovia, Calif). Viscosity was measured using a DV-III Ultra rheometer (Brookfield Engineering, Middleboro, Mass), while the workability of each formulation was evaluated by the same experienced dentist. This resulted in variable BAG-to-monomer ratios for each BAG-Bond and likely reflected differences in the surface areas of each BAG.
Sample Preparation
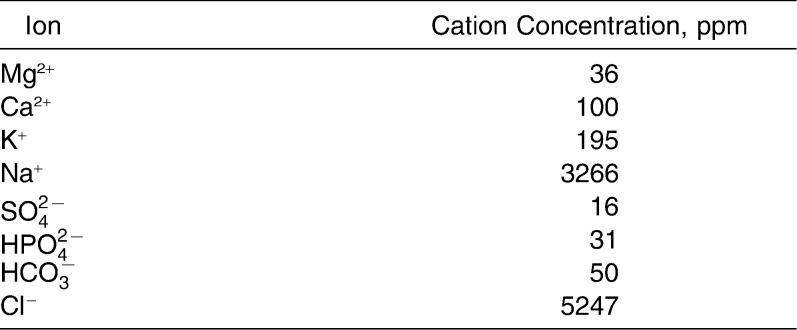
SBF (Table 3) was freshly prepared23 and titrated to desired pH levels of 4 (SBF4) and 7 (SBF7) using nitric acid. Cylindrical molds (10 mm × 2 mm) were made with polyvinylsiloxane impression material (Aquasil-Ultra, Dentsply, Milford, Del). Each adhesive was packed into a mold, and an end of a piece of nonwaxed dental floss was embedded in the material to allow for suspension of the disk in SBF. The filled molds were compressed between glass slides and light-cured (Demi, Kerr, Middleton, Wisc) for 40 seconds from each side. The curing light was measured with a radiometer (EFOS Inc, Williamsville, NY) before, during, and after sample preparation to ensure a constant output of >1000 mW/cm2. Disks of Transbond-XT prepared in the same manner served as controls.
Table 3.
Ionic Concentration of Starting Simulated Body Fluid
Each sample (n = 3 replicates/time point/SBF solution; total = 18 samples of each composition) was immersed into 3.5 mL of SBF (Figure 1) and continuously agitated on a biaxial orbital shaker (Thermo Fisher, Pittsburgh, Pa) at 37°C. At predetermined time points (1 hour, 10 hours, and 100 hours), samples were removed and the fluid was analyzed. A pilot experiment was performed on a group of samples at the 10-hour time point, and a power analysis was conducted on the resulting data to determine the sample size needed (n = 3) for statistical significance at the .05% level.
Figure 1.
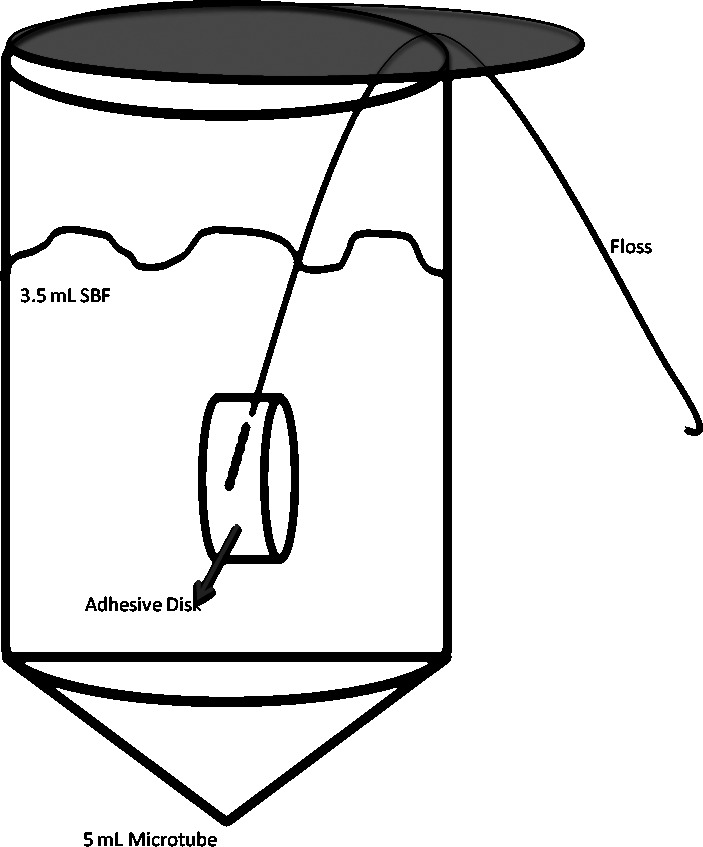
Sample configuration. Embedded floss suspended the disk to ensure that all surfaces were constantly exposed to the solution.
Sample Analysis
pH
The pH of the SBF samples at each time point was measured and recorded. Afterward, SBF7 samples were acidified to pH 4 using nitric acid to ensure that the released ions remained in the solution.
Ion analysis
Ca ion concentrations were measured using atomic absorption (AA) spectrometry (Analyst 300, Perkin Elmer, Waltham, Mass). Fluoride ion concentrations were determined by measurements using a fluoride ion–specific electrode (Orion, Thermo Fisher, Beverly, Mass) and comparison to a calibration curve. PO4 ion concentrations were determined by the vanadomolybdophosphoric acid colorimetric method,24 in which a spectrophotometer (Ultrospec II, LKB, Cambridge, UK) measured the color intensity of each sample at a wavelength of 400 nm, and the result was compared to a calibration curve.
Statistical Analysis
For comparison of pH levels and ion release, a three-way analysis of variance was run (SAS 9.1, SAS Inc, Cary, NC), with α = .05.
RESULTS
pH
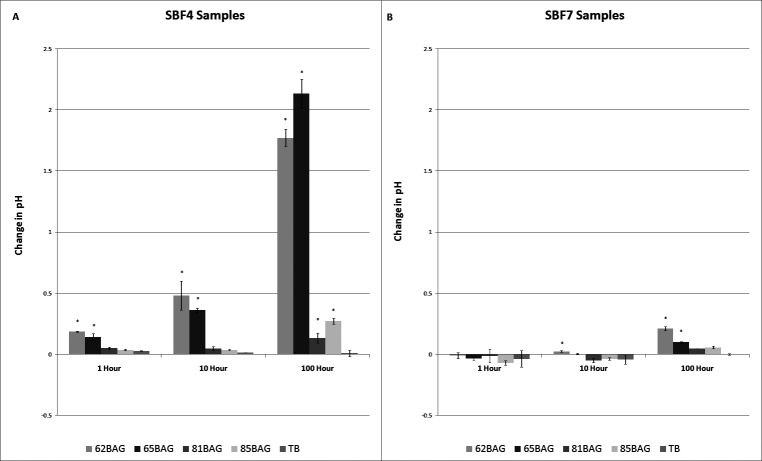
In SBF4, significant increases (P < .05) in pH were found with 62BAG-Bond and 65BAG-Bond at all time points and with 81BAG-Bond and 85BAG-Bond at 100 hours. In SBF7, significant increases were found with 62BAG-Bond at 10 hours and 100 hours and with 65BAG-Bond at 100 hours (Figure 2).
Figure 2.
Mean change in pH over time compared to Transbond XT. (A) Simulated body fluid (SBF) at pH 4; (B) SBF at pH 7. Note the difference in Y-axis scale. Significantly different from Transbond-XT (P < .05) is noted by an asterisk (*) above the columns.
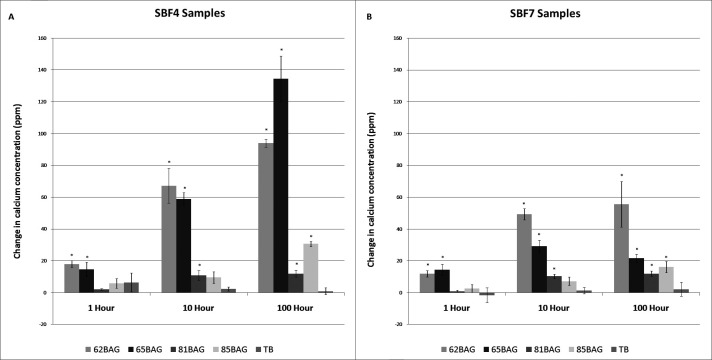
Calcium
In both SBF4 and SBF7, significant increases (P < .05) in Ca concentration were found with 62BAG-Bond and 65BAG-Bond at all time points, with 81BAG-Bond at 10 hours and 100 hours, and with 85BAG-Bond at 100 hours (Figure 3).
Figure 3.
Mean change in calcium concentration over time compared to Transbond XT. (A) Simulated body fluid (SBF) at pH 4; (B) SBF at pH 7. Significantly different from Transbond-XT (P < .05) is noted by an asterisk (*) above the columns.
Phosphate
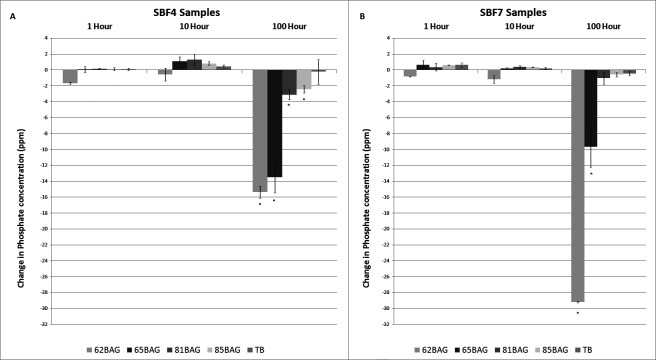
In SBF4, significant decreases (P < .05) in PO4 concentration were found with 62BAG-Bond, 65BAG-Bond, 81BAG-Bond, and 85BAG-Bond at 100 hours only. In SBF7, significant decreases (P < .0001) were found with 62BAG-Bond and 65BAG-Bond at 100 hours (Figure 4).
Figure 4.
Mean change in phosphate concentration over time compared to Transbond XT. (A) Simulated body fluid (SBF) at pH 4; (B) SBF at pH 7. Significantly different from Transbond-XT (P < .05) is noted by an asterisk (*) above the columns.
Fluoride
With both SBF solutions, no significant change in fluoride concentration was found with any of the BAG-Bonds at any time point.
DISCUSSION
This study evaluated the potential use of novel BAG-containing orthodontic resin bonding agents for the prevention of WSLs. In order to measure these potentials in vitro, samples of the light-cured adhesives were soaked in SBF at either pH 4 or pH 7. The resulting SBF solutions were analyzed over time for changes in pH and ion concentrations. Comparisons were made to a popular, commercially available adhesive, Transbond-XT.
The BAG-Bonds tested demonstrated differing buffering capacities (Figure 2). In SBF4, an environment below the critical pH25,26 for enamel demineralization, the buffering capacity of 62BAG-Bond and 65BAG-Bond was significant within 1 hour and increased profoundly by 100 hours. In comparison, 81BAG-Bond and 85BAG-Bond showed significant buffering capacity only at 100 hours. Differences in the buffering capacity among the BAG-Bonds can be attributed to their respective chemical compositions, whereby 62BAG-Bond and 65BAG-Bond contain more Ca than do 81BAG-Bond and 85BAG-Bond. Consequently, 62BAG-Bond and 65BAG-Bond had the potential to absorb more H+ ions as calcium was released.
In the noncariogenic SBF7 environment, a modest increase in pH was seen with the 62BAG and 65BAG resins at 100 hours, but this increase did not result in an overly basic environment, rising only to a maximum pH of 7.23, likely as a result of the low thermodynamic driving force27,28 for ion exchange at this pH.
The implications of BAG-Bond's ability to buffer cariogenic environments are significant because the rate of enamel demineralization is inversely proportional to the pH of its environment.29 When plaque pH drops below a critical pH, a change in the ion concentration gradients between the tooth surface and the plaque results in dissolution of enamel.25,26 The results of this study indicate that BAG-Bonds have the potential for decreasing the rate of enamel demineralization by increasing the pH adjacent to the bracket/tooth interface (Figure 2), or for entirely preventing enamel demineralization by maintaining the environment above the critical pH. This may be particularly applicable in adolescent patients because they have a greater propensity toward WSLs, with their tendency to have a higher critical pH than do adults.26
Significant increases in the Ca levels measured in SBF (Figure 3) were likely due to a Ca concentration gradient established between the SBF and each BAG-Bond. Ions from the SBF are known to displace Ca from the BAG-Bond in both the acidic and neutral environments.28 The greater amount of Ca released in SBF4 compared to SBF7 environments can be attributed to the higher concentration of H+ in SBF4. At the lower pH, an undersaturation of Ca and PO4 in the SBF would create a thermodynamic driving force27–29 that forces more Ca out of the BAG-Bond relative to the situation occurring in the higher pH. This is consistent with the current findings that with increased pH, less Ca had been released into solution.
Enamel demineralization and subsequent WSL formation are a result of the loss of Ca and PO4 from the enamel matrix during local ion concentration imbalances at the enamel surface. Dawes25 found that “the critical pH below which enamel dissolves is not constant but rather is inversely proportional to the amount of calcium and PO4 in saliva and plaque fluid.” With significant release of Ca, BAG-Bonds would have the ability to decrease the critical pH of the surrounding enamel environment. A decrease in critical pH would necessitate a greater reduction in pH from plaque activity before enamel dissolution could occur,25 resulting in a net decrease in risk for WSLs.
The significant decreases in PO4 ion levels measured in SBF (Figure 4) can be explained by supersaturation of the solution immediately surrounding the BAG-Bond and incorporation of the ions onto the resin surface by precipitation of Ca-P. The Ca-P precipitate is the precursor for hydroxylapatite formation.27 There is clinical evidence30 that WSLs can be remineralized if the enamel surface overlying the lesion remains intact. As long as the environment adjacent to the WSL is supersaturated with Ca and PO4, the Ca and PO4 can pass through the intact enamel layer and remineralize the subsurface lesion.25 In addition, precipitated Ca-P could act as a template for remineralization in areas where there has been superficial enamel erosion. Although it has been suggested25,30 that enamel erosions do not recalcify because they lack a matrix that supports crystal growth, the precipitated Ca-P may provide a matrix that is conducive for this remineralization.
The loss of PO4 from the surrounding environment may also deny an essential nutritional source for bacteria, thereby reducing plaque formation.31 During fixed orthodontic appliance therapy, increases in Streptococcus mutans and lactobacilli have been found around bracket margins and on the gingival third of teeth32–35; thus, having an antibacterial component associated with the adhesive would be advantageous for the reduction of WSLs.
Although 62BAG-Bond and 81BAG-Bond contain fluoride, no detectable amount of fluoride was released into solution. This may be attributable to the process of polymerization of the BAG-Bonds, whereby the fluoride becomes incorporated into the polymer matrix, unable to leach out into solution, or it may be that it becomes incorporated in the Ca-P precipitate as a precursor to highly insoluble fluoroapatite. Even though fluoride was not detectable within the SBF solution, it would likely be found within the ionic milieu at the surface of the adhesive/tooth interface, and, hence, it would be available for integration at the adhesive/enamel interface6 or for bactericidal activity.35
A limitation of this in vitro study is that it may not mimic the complex oral environment. The salivary flow rate, the amount of time required to replenish saliva, and its composition are important factors that affect the Ca and PO4 ions that are available surrounding the brackets. However, the immersion test used in the current study is an acceptable approach for initial evaluation of ion release into the storage medium. It should be kept in mind that the purpose of using BAG-containing orthodontic bonding agents is not to provide Ca and PO4 ions into the entire oral cavity but rather to release them when the pH reaches critical level. In this manner, there is the potential that the area adjacent to brackets would be saturated with these ions, and therefore demineralization could be either reduced or completely eliminated.
CONCLUSIONS
The results of this study indicate that combining BAG into resin adhesives may result in a “smart material” that provides a reservoir of crucial ions for remineralization or for the protection of enamel from demineralization in the following ways:
BAG-Bonds raise the pH of cariogenic environments and may also decrease critical pH, resulting in decreased mineral loss from teeth, ultimately preventing or reducing the severity of WSLs.
The reservoir of Ca and PO4 within BAG-Bond provides an ion source for precipitation of Ca-P onto the tooth surface, holding the potential for remineralization of demineralized enamel.
The removal of PO4 from the environment decreases the resources for bacterial metabolism and may have antimicrobial effects resulting in a more favorable environment for the reduction of WSLs.
REFERENCES
- 1.Chang H. S, Walsh L. J, Freer T. J. Enamel demineralization during orthodontic treatment. Aetiology and prevention. Aust Dent J. 1997;42:322–327. doi: 10.1111/j.1834-7819.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 2.Tufekci E, Dixon J. S, Gunsolley J. C, Lindauer S. J. Prevalence of white spot lesions during orthodontic treatment with fixed appliances. Angle Orthod. 2011;81:206–210. doi: 10.2319/051710-262.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovrov S, Hertrich K, Hirschfelder U. Enamel demineralization during fixed orthodontic treatment—incidence and correlation to various oral-hygiene parameters. J Orofac Orthop. 2007;68:353–363. doi: 10.1007/s00056-007-0714-1. [DOI] [PubMed] [Google Scholar]
- 4.Øgaard B, Rolla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94:68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 5.O'Reilly M. M, Featherstone J. D. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 6.Hallsworth A. S, Robinson C, Weatherbell J. A. Mineral and magnesium distribution within the approximal carious lesion of dental enamel. Caries Res. 1972;6:156–168. doi: 10.1159/000259787. [DOI] [PubMed] [Google Scholar]
- 7.Årtun J, Thylstrup A. A 3-year clinical and SEM study of surface changes of carious enamel lesions after inactivation. Am J Orthod Dentofacial Orthop. 1989;95:327–333. doi: 10.1016/0889-5406(89)90166-2. [DOI] [PubMed] [Google Scholar]
- 8.Benson P. E, Shah A. A, Millett D. T, Dyer F, Parkin N, Vine R. S. Fluorides, orthodontics and demineralization: a systematic review. J Orthod. 2005;32:102–114. doi: 10.1179/146531205225021033. [DOI] [PubMed] [Google Scholar]
- 9.Shamma I, Ngan P, Kim H, Kao E, Gladwin M, Gunel E, Brown C. Comparison of bracket debonding force between two conventional resin adhesives and a resin-reinforced glass ionomer cement: an in vitro and in vivo study. Angle Orthod. 1999;69:463–469. doi: 10.1043/0003-3219(1999)069<0463:COBDFB>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Coups-Smith K. S, Rossouw P. E, Titley K. C. Glass ionomer cements as luting agents for orthodontic brackets. Angle Orthod. 2003;73:436–644. doi: 10.1043/0003-3219(2003)073<0436:GICALA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Millett D. T, McCabe J. F. Orthodontic bonding with glass ionomer cement. Eur J Orthod. 1996;18:385–399. doi: 10.1093/ejo/18.4.385. [DOI] [PubMed] [Google Scholar]
- 12.Foster J. A, Berzins D. W, Bradley T. G. Bond strength of an amorphous calcium phosphate-containing orthodontic adhesive. Angle Orthod. 2008;78:339–344. doi: 10.2319/020807-60. [DOI] [PubMed] [Google Scholar]
- 13.Gaworski M, Weinstein M, Borislow A. J, Braitman L. E. Decalcification and bond failure: a comparison of a glass ionomer and a composite resin bonding system in vivo. Am J Orthod Dentofacial Orthop. 1999;116:518–521. doi: 10.1016/s0889-5406(99)70182-4. [DOI] [PubMed] [Google Scholar]
- 14.Bishara S. E, VonWald L, Olsen M. E, Laffoon J. F, Jakobsen J. R. Effect of light-cure time on the initial shear bond strength of a glass-ionomer adhesive. Am J Orthod Dentofacial Orthop. 2000;117:164–168. doi: 10.1016/s0889-5406(00)70227-7. [DOI] [PubMed] [Google Scholar]
- 15.Bishara S. E, Gordan V. V, VonWald L, Jakobsen J. R. Shear bond strength of composite, glass ionomer, and acidic primer adhesive systems. Am J Orthod Dentofacial Orthop. 1999;115:24–28. doi: 10.1016/s0889-5406(99)70312-4. [DOI] [PubMed] [Google Scholar]
- 16.Komori A, Ishikawa H. Evaluation of a resin-reinforced glass ionomer cement for use as an orthodontic bonding agent. Angle Orthod. 1997;67:189–196. doi: 10.1043/0003-3219(1997)067<0189:EOARRG>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Papagiannoulis L, Kakaboura A, Eliades G. In-vivo vs. in-vitro anticariogenic behavior of glass-ionomer and resin composite restorative materials. Dent Mater. 2002;18:561–569. doi: 10.1016/s0109-5641(01)00090-2. [DOI] [PubMed] [Google Scholar]
- 18.Hench L. L. The story of bioglass. J Mater Sci Mater Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell J. C. In-vivo aging of bioactive glasses and other graft materials. In: Eliades G, Eliades T, Brantley W, Watts D, editors. InVivo Aging of Dental Biomaterials Aging and Related Phenomena. Chicago, Ill: Quintessence; 2003. pp. 263–276. [Google Scholar]
- 20.Forsback A. P, Areva S, Salonen J. I. Mineralization of dentin induced by treatment with bioactive glass S534P4 in vitro. Acta Odontol Scand. 2004;62:14–20. doi: 10.1080/00016350310008012. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell J. C, Astashkina A, Park S, Baumgartner C. Antimicrobial effect of Sol-gel Bioactive glasses. J Dent Res. 2006;85(Spec Iss A):0541. [Google Scholar]
- 22.Brunauer S, Emmett P. H, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319. [Google Scholar]
- 23.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Kitson R. E, Mellon M. G. Colourimetric determination of phosphorus as molybdovanadophosphoric acid. Ind Eng Chem Anal Ed. 1944;16:379. [Google Scholar]
- 25.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–724. [PubMed] [Google Scholar]
- 26.Anderson P, Hector M. P, Rampersad M. A. Critical pH in resting and stimulated whole saliva in groups of children and adults. Int J Paediatr Dent. 2001;11:266–273. doi: 10.1046/j.1365-263x.2001.00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Margolis H. C, Zhang Y. P, Lee C. Y, Kent R. L, Jr, Moreno E. C. Kinetics of enamel demineralization in vitro. J Dent Res. 1999;78:1326–1335. doi: 10.1177/00220345990780070701. [DOI] [PubMed] [Google Scholar]
- 28.Meyer J. L, Eanes E. D. A thermodynamic analysis of amorphous to crystalline calcium phosphate transformation. Calcif Tiss Res. 1978;25:59–68. doi: 10.1007/BF02010752. [DOI] [PubMed] [Google Scholar]
- 29.Fosdick L. S, Stark A. C. Solubility of tooth enamel in saliva at various pH levels. J Dent Res. 1939;18:417–430. [Google Scholar]
- 30.ten Cate J. M, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;11:277–286. doi: 10.1159/000260279. [DOI] [PubMed] [Google Scholar]
- 31.Lemke M. J, Churchill P. F, Wetzel R. G. Effect of substrate and cell surface hydrophobicity on phosphate utilization in bacteria. Appl Environ Microbiol. 1995;61:913–919. doi: 10.1128/aem.61.3.913-919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams R. J. The effects of fixed orthodontic appliances on the carogenicity, quantity and microscopic morphology of oral lactobacilli. J Oral Med. 1967;22:88–99. [PubMed] [Google Scholar]
- 33.Bloom R. H, Brown L. R., Jr A study of the effects of orthodontic appliances on the oral microbial flora. Oral Surg Oral Med Oral Pathol. 1964;17:658–667. doi: 10.1016/0030-4220(64)90373-1. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbloom R. G, Tinanoff N. Salivary Streptococcus mutans levels in patients before, during and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;100:35–37. doi: 10.1016/0889-5406(91)70046-Y. [DOI] [PubMed] [Google Scholar]
- 35.Li Y. H, Bowden G. H. The effect of environmental pH and fluoride from the substratum on the development of biofilms of selected oral bacteria. J Dent Res. 1994;73:1615–1626. doi: 10.1177/00220345940730100601. [DOI] [PubMed] [Google Scholar]