Abstract
Objective:
To assess the antiadherent and antibacterial properties of surface modified stainless steel orthodontic brackets with photocatalytic titanium oxide (TiO2) against Lactobacillus acidophilus.
Materials and Methods:
This study was done on 120 specimens of stainless steel preadjusted edgewise appliance (PEA) orthodontic brackets. The specimens were divided into four test groups. Each group consisted of 30 specimens. Groups containing uncoated brackets acted as a control group for their respective experimental group containing coated brackets. Surface modification of brackets was carried out by the radiofrequency (RF) magnetron sputtering method with photocatalytic TiO2. Brackets then were subjected to microbiological tests for assessment of the antiadherent and antibacterial properties of photocatalytic TiO2 coating against L acidophilus.
Results:
Orthodontic brackets coated with photocatalytic TiO2 showed an antiadherent effect against L acidophilus compared with uncoated brackets. The bacterial mass that was bound to the TiO2-coated brackets was less when compared with the uncoated brackets. Furthermore, TiO2-coated brackets had a bactericidal effect on L acidophilus, which causes dental caries.
Conclusion:
Surface modification of orthodontic brackets with photocatalytic TiO2 can be used to prevent the accumulation of dental plaque and the development of dental caries during orthodontic treatment.
Keywords: Photocatalytic, TiO2, Brackets, Antiadherent, Antibacterial, Lactobacillus acidophilus
INTRODUCTION
Enamel demineralization, or white spot lesions around orthodontic brackets, is a common unwanted side effect of orthodontic treatment. The oral environment with fixed appliance provides ideal conditions for colonization of microorganisms as a result of their inherent morphologic irregularities.1 Patients have difficulty in maintaining adequate oral hygiene, and the appliance provides additional sites for microorganisms to bind and colonize.1 The resultant increase in plaque accumulation and retention areas places the patient at higher risk for enamel demineralization adjacent to the appliance and exacerbates the effects of preexisting incipient carious lesions.
The incidence of enamel demineralization after fixed orthodontic treatment can involve up to 50% of patients. The incidence of such white spot lesions around orthodontic brackets has been demonstrated within 1 month of treatment.2,3
Among different fixed orthodontic appliances, brackets could play a significant role in enamel demineralization because they are attached to the teeth throughout the entire period of orthodontic treatment. Their complex design provides a unique environment that impedes proper access to tooth surfaces for cleaning. In a study done by Eliades et al.,4 it was seen that stainless steel represented the highest critical surface tension and energy and can be expected to have higher plaque retaining capacity.
In addition, stainless steel brackets were found to induce specific changes in the oral environment such as decreased pH, increased plaque accumulation,1,5,6 and elevated Streptococcus mutans and Lactobacillus acidophilus colonization.7–10 Among several pathogenic organisms that accumulate and colonize in the form of plaque, lactobacilli do not play a major part in initiation, but are important in progression, of the lesion. With established low pH, the number of lactobacilli increases and the number of S mutans decreases; this contributes to demineralization of the teeth once lesions are established. Preventing these lesions is an important concern for the orthodontist, because the lesions are unesthetic, unhealthy, and potentially irreversible.
Photocatalytic degradation of organic compounds in water-treatment processes and the decomposition of air pollutants have been studied for several decades.11 Semiconductor particles such as titanium oxide (TiO2), zinc oxide, tungsten oxide, cadmium sulfide, zinc sulfide, and iron oxide are considered ideal photocatalysts for these reactions. Among these, TiO2 has attracted considerable attention and has been reported to be the most useful substance in organic degradation processes on account of its chemically stable properties and absence of harmful effects on humans.
TiO2 photocatalysts have attracted great attention as alternative materials to aid in purification of water and air. TiO2 photocatalysts generate strong oxidizing power when illuminated with ultraviolet (UV) light with wavelength of less than 385 nm. They generate holes (h+) and hydroxyl radicals (OH) in the valence band, and electrons and superoxide ions (O2−) in the conduction band. With all this illuminated TiO2, photocatalysts can decompose organic compounds by participating in a series of oxidation reactions leading to the formation of carbon dioxide.12
Zheng et al.13 showed that photocatalytic action progressively increased cell permeability and subsequently allowed the free efflux of intracellular contents that eventually led to cell death. Free TiO2 particles also gained access to membrane-damaged cells, and a subsequent direct attack on intracellular components, such as alteration of the protein structure, could accelerate cell death. Investigators showed that TiO2-treated cells continued to lose their viability even after UV illumination stopped. Once lethal oxidation reactions are initiated by the TiO2 photocatalytic reaction in complex cellular systems, damage may continue in the dark via the Fenton reaction or the free radical chain reactions of lipid peroxidation.
Therefore, using this photocatalytic activity of TiO2 may provide effective results in preventing bacterial adhesion and growth around fixed orthodontic appliances. Chun et al.14 reported that TiO2 coating on the surface of orthodontic wires showed effective antiadherent properties against S mutans with the illumination of UV-A light. Similar results were observed in Porphyromonas gingivalis, which is a major pathogenic bacterium in periodontitis.
However, we need to study the photocatalytic effect of TiO2-coated orthodontic brackets (as they have greater potential to harbor microorganisms) against L acidophilus, as they actually cause progression of cariogenic lesions and are more acidogenic than other bacterial species because of their ability to produce lactic acid by fermenting carbohydrates.
Thus, this study was designed to evaluate the antiadherent and antibacterial properties of surface stainless steel orthodontic brackets modified with photocatalytic TiO2 against L acidophilus.
MATERIALS AND METHODS
This study was done on 120 specimens of stainless steel preadjusted edgewise appliance (PEA) maxillary central incisor brackets. The specimens were divided into four test groups for assessment of the antiadherent and antibacterial properties of photocatalytic TiO2 thin film–coated brackets. Each group consisted of 30 specimens. Groups containing uncoated brackets acted as control group for their respective experimental group containing coated brackets (Tables 1 and 2).
Table 1.
Groups of Brackets Used to Assess the Antiadherent Property of Surface Modified Orthodontic Brackets
Table 2.
Groups of Brackets Used to Assess the Antibacterial Property of Surface Modified Orthodontic Brackets
Bacterial Strains
L acidophilus strains were used for adhesion and viability tests. Lactobacilli were inoculated into 5 mL of MRS broth and were incubated for 24 hours at 37°C. For the adhesion test, 10% of an overnight-cultured broth was transferred to 10 mL of the MRS broth containing 10% sucrose and was incubated for 24 hours.
Preparation of Photocatalytic TiO2-Coated Orthodontic Brackets
Surface modification of stainless steel orthodontic brackets with photocatalytic titanium oxide was carried out by the radiofrequency (RF) magnetron sputtering method.
Sputtering processes remove surface atoms or molecular fragments from a solid cathode (target) by bombarding it with positive ions from an inert gas (argon) discharge, and deposit them on the nearby substrate to form a thin film. Substrates are placed in a vacuum chamber and are pumped down to a prescribed process pressure. Sputtering starts when a negative charge is applied to the target material, causing a plasma or glow discharge. Positively charged gas ions generated in the plasma region are attracted to the negatively biased target plate at a very high rate of speed. This collision creates a momentum transfer and ejects atomically sized particles from the target. These particles are deposited as a thin film onto the surface of the substrates (Figure 1a,b).
Figure 1.

(a) Radiofrequency (RF) magnetron sputtering unit. (b) Cathode (target) inside sputtering unit.
In the present study, sputtering was carried out on stainless steel orthodontic brackets (substrate) using titanium (Ti) as the target. A plasma generated inside the vacuumized chamber ejected surface atoms from the titanium target, which were sputtered onto the stainless steel brackets (substrate). The distance between the substrate and the target was kept constant at 7 cm, and sputtering was conducted for a period of 20 minutes. All brackets were sputtered at the same time to achieve a thin and uniform coating of titanium.
The sputtered titanium was further oxidized in an ambient environment inside an open air furnace at 500°C for 5 hours to provide a uniform coating of TiO2 on the stainless steel orthodontic brackets (Figure 2).
Figure 2.

Titanium oxide (TiO2) coated stainless steel brackets.
Evaluation of Bacterial Adhesion to Orthodontic Brackets
Before the adhesion test, the brackets were ultrasonicated for 5 minutes in 2-propanol to remove adventitious macroscopic contaminations and were dried in a desiccator. After cleaning and sterilizing in an autoclave, the brackets were preweighted using an analytical balance and were stored in an airtight container. In a sterile beaker containing 10 mL of an MRS broth, an overnight-cultured Lactobacillus culture broth was inoculated to a final concentration of 10%. After this, 1 mL of this suspension was pipetted into each of the Eppendorf tubes, and the brackets were immersed in it and were incubated for 24 hours at 37°C under illumination of UV-A black light (Philips Electronics TLD15W/ 08, F15T8BLB, Philips Electronics, Blue Bell, Pa) inside the laminar air flow chamber or inoculator (Figure 3). Brackets to which bacteria adhered were carefully removed and immersed in a 10% formaldehyde solution for 30 minutes to immobilize the cells.
Figure 3.
Brackets kept under ultraviolet (UV) exposure for antiadherent assay.
After a careful rinse with distilled water, the brackets were dried in a desiccator for 24 hours. The weight change of the brackets during the bacterial adhesion test was recorded with an analytical balance.
Antibacterial Activity Assay of Orthodontic Brackets
Antibacterial activities of the surface-modified orthodontic brackets were demonstrated against lactobacilli. First, the Lactobacillus culture broth was diluted with MRS broth to achieve an optical density of 1.0 at 660 nm. Ten milliliters of the diluted bacterial suspension was transferred onto petri dishes containing uncoated stainless steel brackets and TiO2-coated brackets. These dishes were illuminated with a UV-A black light inside the laminar air flow chamber with an intensity of 1.0 mW/cm2 for 60 minutes (Figure 4). After illumination, 100 µL of the bacterial suspension was serially diluted and plated onto MRS agar plates. Antibacterial activity was described as the survival rate by colony-forming units (CFUs) for lactobacilli (Figures 5, 6, and 7).
Figure 4.

Brackets kept under ultraviolet (UV) exposure for antibacterial assay.
Figure 5.
Colonies of Lactobacillus acidophilus on agar plate of control group.
Figure 6.
Colonies of Lactobacillus acidophilus on agar plate of test group.
Figure 7.

Colonies seen under digital colony counter.
Statistical Software
Statistical software, namely, SAS 9.0 (SAS Institute Inc, Cary, NC), the Statistical Package for the Social Sciences (SPSS), version 15.0 (SPSS Inc, Chicago, Ill), Stata 8.0 (StataCorp, College Station, Tex), MedCalc 9.0.1 (MedCalc Software bvba, Mariakerke, Belgium), and Systat 11.0 (Systat Software Inc, Chicago, Ill), was used for analysis of data; Microsoft Word and Excel were used to generate graphs, tables, and so forth. Descriptive statistical analysis was carried out in the present study. Results on continuous measurements are presented as means ± standard deviation (SD) (min-max), and results on categorical measurements are presented as number (%). Significance is assessed at the 5% level of significance. Student's t-test (two-tailed, independent) was used to find the significance of study parameters on a continuous scale between the two groups (intergroup analysis); Mann-Whitney U-test was used to analyze the CFUs in control and test groups; and Student's t-test (two-tailed, dependent) was used to find the significance of study parameters on a continuous scale within each group.
RESULTS
Adhesion of Lactobacilli to the Surface of Orthodontic Brackets
To compare photocatalytic activity, preweighted, intact, and TiO2-coated stainless steel brackets were immersed in an L acidophilus culture broth. After 24 hours' incubation with UV-A light illumination, the brackets were weighted again.
From the data shown in Table 3, it can be seen that Group 1 had an initial average weight of 0.0709 ± 0.0040 and a final average weight of 0.0738 ± 0.0044, with an increase in weight of 0.0029 (4.1%), which was statistically significant (P < .001).
Table 3.
Comparison of Weight: Initial, Final, and Change in Weight of Brackets in the Two Groups Studied
Group 2 had an initial average weight of 0.0722 ± 0.0009 and a final average weight of 0.0741 ± 0.0018, with an increase in weight of 0.0019 (2.6%), which was statistically significant (P < .001) (Figure 8). A 4.1% weight change in uncoated brackets was noted compared with a 2.6% change in surface modified brackets (0.0029 vs 0.0019 weight change). This is statistically significant, with a low change noted in the surface modified brackets group at P = .006. Thus, uncoated orthodontic brackets showed a greater increase in weight when compared with surface modified orthodontic brackets.
Figure 8.
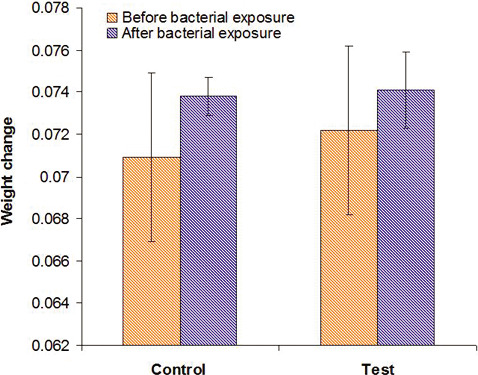
Graph showing comparison of weight change between coated and uncoated stainless steel brackets.
Antibacterial Activity of Surface Modified Orthodontic Brackets on Lactobacilli
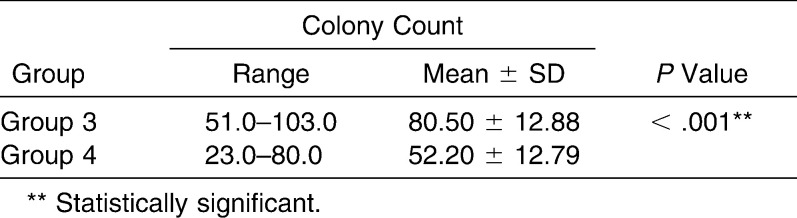
The survival rate of bacterial cells was calculated in terms of CFUs. In Table 4, it can be seen that in the dilution agar plate method, the survival rate of lactobacilli was 80.50 ± 12.88 CFUs in the case of group 3, whereas it was 52.20 ± 12.79 CFUs in the case of group 4. Antibacterial activity in CFUs is statistically significantly less in group 4 than in group 3 (80.50 vs 52.20 CFUs) with P < .001 (Figure 9).
Table 4.
Comparison of Colony Count
Figure 9.
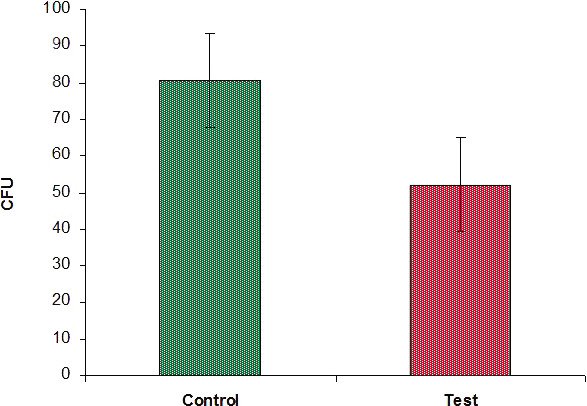
Graph showing comparison of colony counts between the two groups.
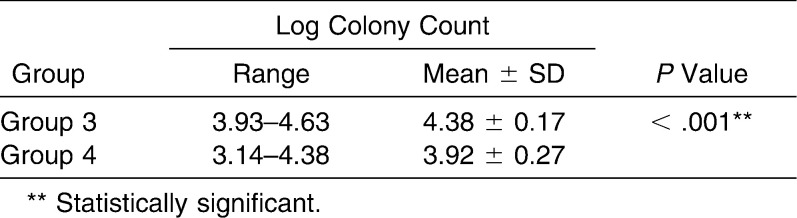
When the logarithm of colony count was compared, between groups 3 and 4 as given in Table 5, it was seen that the log colony count for group 3 was 3.93 to 4.63 (4.38 ± 0.17), whereas it was 3.14 to 4.38 (3.92 ± 0.27) for group XX. Antibacterial activity in log CFU is statistically significantly less in group 4 than in group 3 (3.92 vs 4.38 log CFUs) with P < .001 (Figure 10).
Table 5.
Comparison of Logarithm of Colony Count
Figure 10.
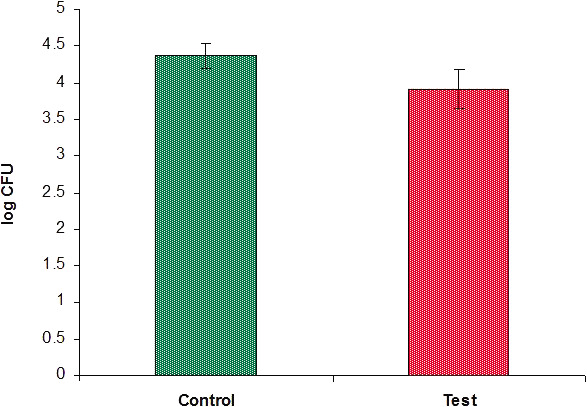
Graph showing comparison of logarithm of colony counts between the two groups.
Thus, the groups containing surface modified brackets showed a statistically significant decrease in the survival rate of lactobacilli expressed as CFUs and as the log of colony count when compared with groups containing uncoated brackets.
DISCUSSION
The photocatalytic effects of TiO2 as a surface coating can be used for the benefit of orthodontic patients by reducing microbial growth responsible for periodontal disease and enamel decalcification. In recent studies, surface modification of stainless steel orthodontic wires with photocatalytic TiO2 and TiAg (titanium silver) has led to positive results.14,15 The orthodontic brackets form an integral part of the fixed orthodontic appliances and supply a good shelter and habitat for the oral microorganism.
The present study was undertaken to modify the surfaces of stainless steel orthodontic brackets with photocatalytic TiO2 and to assess their antiadherent and antibacterial properties. It is possible that a sufficient amount of reactive oxygen species such as OH was released from the TiO2-coated wires as a result of UV-A light. Reduced adhesion of S mutans to the TiO2-coated wires might be a result of decomposition of surface organic molecules of S mutans such as the M protein. This phenomenon might further cause the cell walls of bacteria to become more fragile.
In this report, TiO2 coated on the surface of orthodontic brackets showed effective antiadherent properties against L acidophilus with illumination of UV-A light. The data presented in Table 3 show that it is possible that a sufficient amount of reactive oxygen species such as OH− was released from the TiO2-coated brackets as a result of UV-A light. Reduced adhesion of L acidophilus to the TiO2-coated brackets might be a result of decomposition of surface organic molecules of L acidophilus such as the M protein. This phenomenon might further cause the cell walls of bacteria to become more fragile. As is shown in these results, it is obvious that OH• radicals released from the photocatalytic TiO2 could directly influence the growth of this oral bacterium. Photocatalytic TiO2-coated orthodontic wires effectively reduced the survival rate of L acidophilus.
Titanium has historically maintained the reputation of being an inert, and relatively biocompatible, metal, but more recent scientific evidence suggests that titanium, or its corrosion by-products, may cause harmful reactions after traveling through the circulatory, or lymphatic, system. These corrosion by-products can cause reactions in the blood, in fibrotic tissue, and in osteogenic cells.
However, the amount of TiO2 essential to successfully carry out photocatalysis and the maximum lethal dose of TiO2 will have to be determined if its toxicity is to be avoided before it can be applied to orthodontic materials. The TiO2 coating on brackets is purely surface based and may be prone to wear during archwire sliding. Thus it is critical to assess the durability and sustainability of TiO2 coatings under clinical situations in the oral environment. Also it would be prudent to find out whether the thin coating of TiO2 in the slot would alter its frictional properties.
Titanium is one of the most inert and corrosion-resistant metals. It is often added to stainless steel to provide a stabilization effect by forming carbides in preference to chromium and by helping to maintain the passivating effect of stainless steel. The TiO2 coating may provide an additional layer of protection and may help in augmenting the passivating effect of stainless steel. However, studies have to be undertaken to analyze and assess the effects of TiO2 coating on the corrosion resistance of stainless steel brackets.
TiO2 may be found as a rutile or anatase crystalline structure. A rutile structure is more thermodynamically stable than an anatase structure, but an anatase structure is more photoactive. There is a strong possibility of wearing and aging of the TiO2 coating in the intraoral environment as a result of the unstable anatase structure. However, at more than 900°C, an anatase crystalline structure can be converted to rutile. This can be achieved by increasing the temperature and pressure of the RF sputtering unit during coating. Therefore, the crystalline structure of TiO2 is considered an important factor in photocatalytic activity.
CONCLUSIONS
The photocatalytic TiO2 coating prevented the adhesion of Lactobacillus acidophilus to the orthodontic brackets, hence demonstrating its antiadherent properties.
The photocatalytic TiO2 also demonstrated antibacterial effects against Lactobacillus acidophilus.
Surface modification of orthodontic brackets with photocatalytic TiO2 can be used to prevent the development of dental plaque and dental caries during orthodontic treatment.
REFERENCES
- 1.Balenseifen J. W, Madonia J. V. Study of dental plaque in orthodontic patients. J Dent Res. 1970;49:320–324. doi: 10.1177/00220345700490022101. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick L, Geiger A. M, Gwinnett A. J. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–98. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 3.Årtun J, Brobakken B. Prevalence of caries and white spot lesions after orthodontic treatment with multibanded appliance. Eur J Orthod. 1986;8:229–234. doi: 10.1093/ejo/8.4.229. [DOI] [PubMed] [Google Scholar]
- 4.Eliades T, Eliades G, Brantley W. A. Microbial attachment on orthodontic appliances: I. Wettability and early pellicle formation on bracket materials. Am J Orthod Dentofacial Orthop. 1995;108:351–360. doi: 10.1016/s0889-5406(95)70032-3. [DOI] [PubMed] [Google Scholar]
- 5.Menzaghi N, Saletta M, Garattini G, Brambilla E, Strohmenger L. Changes in the yeast oral flora in patients in orthodontic treatment. Prev Assist Dent. 1991;17:26–30. [PubMed] [Google Scholar]
- 6.Chatterjee R, Kleinberg I. Effect of orthodontic band placement on the chemical composition of human incisor tooth plaque. Arch Oral Biol. 1979;24:97–100. doi: 10.1016/0003-9969(79)90056-6. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbloom R. G, Tinanoff N. Salivary S. mutans levels in patients before, during, and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;100:35–37. doi: 10.1016/0889-5406(91)70046-Y. [DOI] [PubMed] [Google Scholar]
- 8.Scheie A. A, Arnesberg P, Krogstad O. Effects of orthodontic treatment on prevalence of Streptococcus mutans in plaque and saliva. Scand J Dent Res. 1984;92:211–217. doi: 10.1111/j.1600-0722.1984.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 9.Mattingly J. A, Sauer G. J, Yancey J. M, Arnold R. R. Enhancement of S mutans colonization by direct bonded orthodontic appliances. J Dent Res. 1983;62:1209–1211. doi: 10.1177/00220345830620120601. [DOI] [PubMed] [Google Scholar]
- 10.Sug-Joon A, Bum-Soon L, Shin-Jae L. Prevalence of cariogenic streptococci on incisor brackets detected by polymerase chain reaction. Am J Orthod Dentofacial Orthop. 2007;131:736–741. doi: 10.1016/j.ajodo.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 11.Lakshmi S, Renganathan R, Fujita S. Study on TiO2-mediated photocatalytic degradation of methylene blue. J Photochem Photobiol A. 1995;88:163–167. [Google Scholar]
- 12.Min C, Hyenmi C, Wonyong C, Jeyong Y. Linear correlation between inactivation of E. coli and OH− radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004;38:1069–1077. doi: 10.1016/j.watres.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Zheng H, Pin-Ching M, Blake D. M, Wolfrum E. J, Smolinski S. L. Bactericidal mode of titanium dioxide photocatalysis. J Photochem Photobiol A Chem. In press. [Google Scholar]
- 14.Chun M. J, Shim E, Kho E. H, et al. Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod. 2007;77:483–488. doi: 10.2319/0003-3219(2007)077[0483:SMOOWW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Choi J. Y, Chung C. J, Oh K. T, Choi Y. J, Kim K. H. Photocatalytic antibacterial effect of TiO2 film of TiAg on Streptococcus mutans. Angle Orthod. 2009;79:528–532. doi: 10.2319/012108-169.1. [DOI] [PubMed] [Google Scholar]