Abstract
The pharmacokinetics in serum and leukocyte (WBC) exposures of 1,500 mg of oral azithromycin administered as 3-day (500 mg/day, days 1 to 3) and 5-day (500 mg on day 1 and 250 mg/day on days 2 to 5) regimens were compared in 12 healthy volunteers. Serum, polymorphonuclear leukocytes, and mononuclear leukocytes were collected over a 12-day period from the start of each regimen. Results of the study indicate that the exposures of serum and both types of WBCs were similar with both regimens. Drug concentrations in day 12 WBCs were well above the MICs for all relevant community-acquired respiratory tract pathogens. Terminal half-lives in serum obtained by both regimens were essentially equal at 66 h and consistent with past reports. These results indicate that the standard 1,500-mg dose of oral azithromycin can be administered over either 5 or 3 days.
Azithromycin remains the sole agent developed and marketed within the azalide macrolide subclass. Due to its dibasic structure, azithromycin has demonstrated unique pharmacokinetic properties that differ significantly from those of classic macrolide agents (1, 4). Azithromycin’s pharmacokinetics are characterized by low concentrations in serum, secondary to rapid and significant uptake by fibroblasts and acute reactant cells like polymorphonuclear leukocytes (PMNs), monocytes, and lymphocytes (4, 11). Tissue infection site concentrations at least 1 log higher than corresponding concentrations in serum result from a combination of serum equilibrium, fibroblast drug release, and phagocyte drug delivery and release (2, 3, 9, 10, 15). The concentrations within leukocytes (WBCs) are even higher, reaching levels upwards of 3 log-higher concentrations than those in the surrounding serum (5, 10, 13, 18). This intracellular drug is still active and is delivered to the bacteria, when it is phagocytized, by the storage lysosomes merging with the phagosomes housing the trapped organism (4).
The result of this extensive tissue and cellular distribution and retention is an extended terminal half-life (t1/2) in serum of approximately 68 h (14). Due to this half-life and prolonged elevated infection site concentrations, short-course dosage regimens of 3 and 5 days have been investigated clinically in Europe and the United States, respectively (8). Both types of regimens have utilized the same total oral dose of azithromycin (1.5 g) and been shown to be at least as effective as their comparators.
Currently, the 5-day regimen is approved in the United States and the 3-day regimen is approved in several European nations. Recent comparison of the pharmacokinetics in plasma and urine obtained by both regimens has demonstrated that patient drug exposure is similar with both (17). The objective of this phase I SNDA study was to determine if the same total dose of 1.5 g administered over 3 and over 5 days provided similar extents of drug exposure (area under the curve [AUC]) not only in serum but also in granulocytes and monocytes/lymphocytes (M/L).
This protocol was approved by the Institutional Review Board of Bassett Healthcare. Twelve subjects were enrolled. All subjects provided written informed consent. All subjects were healthy as determined by medical history, physical exam, electrocardiogram, and laboratory screening (a complete blood count, serum chemistries, urinalysis, urine toxicology screen, and serum pregnancy tests in women of childbearing potential). Due to the extended washout period between study phases, all screening procedures and tests were repeated prior to the second study period. Subjects were between 18 and 50 years of age and within 10% of ideal body weight for their height and frame size. Women of childbearing potential utilized a hormonal or barrier method of birth control for 3 months prior to the study and agreed to continue contraceptive use throughout the study and for the 3 months following study completion. Subjects were required to be free of any drug exposure for 14 days prior to the start of the study. Exclusion criteria included a sensitivity to macrolides, recent history of drug or alcohol abuse, a negative blood alcohol test prior to the start of dosing for each period, an intention to donate blood during or immediately before or after the study, and the use of nicotine or nicotine delivery devices (tested via urine cotinine) within the past year.
This was an open-label, randomized, crossover study. A computer-generated randomization scheme was used to assign subjects to the following dosing regimens in random order: (i) oral azithromycin, 500 mg (two 250-mg tablets) daily for 3 days; (ii) oral azithromycin, 500 mg (two 250-mg tablets) on day 1 followed by 250 mg daily on days 2 through 5. Subjects fasted for at least 8 h prior to each azithromycin dose and continued fasting for 4 h after each dose. Subjects were not allowed to recline (thereby better assuring normal peristalsis) or drink caffeinated beverages for 4 h after each dose and were required to remain at the study site for 24 h after the first and last doses of each regimen to standardize conditions. All meals during the dosing and sampling periods were required to be low in fat content. Dosing periods were separated by an 8-week washout period.
Blood was sampled just prior to the start of dosing and at 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after administration of the first and last doses of both regimens. On day 2 of the 3-day regimen and days 2 to 4 of the 5-day regimen, blood was collected 24 h after the previous day’s azithromycin dose and 2 h after that day’s dose. After the final dose of each regimen, samples were collected every 24 h up until 288 h after the first dose. After centrifugation, serum was harvested and stored at −80°C until assayed.
Additionally, on the first day of each dosing period, 30-ml aliquots of blood were collected in tubes containing EDTA predose and at 4, 12, and 24 h postdose for WBC harvesting. Further samples were then collected at 48, 72, 96, 120, 144, 192, 240, and 288 h after the first dose of each regimen. The blood was layered in 3.5-ml amounts on top of 3.5 ml of PMN isolation medium (Robbins Scientific Corporation, Sunnyvale, Calif.) in borosilicate culture tubes and centrifuged at 1,280 rpm for 30 min at 20°C. When centrifugation was completed, the samples were layered from top to bottom in the following order: plasma layer, M/L layer, medium layer; PMN layer, medium layer, and packed erythrocytes. The M/L and PMN layers were then drawn off and pooled by cell type, subject, and draw time. The collected cells were diluted with an equal volume of 0.45% sodium chloride solution to promote erythrocyte lysis and then recentrifuged at 1,280 rpm for 10 min at 20°C. The supernatant was then decanted, and the PMN and M/L pellets were resuspended in 3.0 ml of Hanks buffered salt solution. A trypan blue exclusion test utilizing a 100-cell count was conducted to ensure sample viability, with ≥95% viability being acceptable. Cells were then diluted 1:10 into a gentian violet stain containing 3% acetic acid and were counted with a hemocytometer. Wright’s stain smears were also created to assess WBC differentials. To minimize error, all Wright’s stain smears were interpreted by the institution’s hematology department.
All serum and cell samples were assayed using a validated high-pressure liquid chromatography assay with electrochemical detection at BAS Analytics (West Lafayette, Ind.). As a brief summary, azithromycin and its N-propargyl derivative were extracted from the samples by a liquid-liquid extraction at an alkaline pH. The derivative served as the internal standard. After the addition of carbonate solution and internal standard to the plasma, the macrolides were extracted into methyl t-butyl ether. The ether layer was transferred to a clean tube and evaporated under nitrogen, and the reconstituted extract was washed with hexane to eliminate late-eluting peaks in the chromatogram. The extract was injected into an LCEC system with a hydrocarbon-coated aluminum oxide stationary phase and an alkaline phosphate buffer-acetonitrile mobile phase. At high potential, the tertiary amine on the macrolide’s desosamine ring was responsible for detectability. The detection curve was linear from 0.0104 to 1.00 μg/ml. Accuracies of the mean back-calculated concentrations ranged from 98.9 to 102.9%. Precision was ±11.8% or better. The interday accuracy of the means were 97.5% for the 0.0500-μg/ml, 97.7% for the 0.20-μg/ml, and 98.2% for the 0.50-μg/ml quality control samples.
All serum data were analyzed by noncompartmental methods with the TopFit version 2.0 computer program and a weighting scheme of 1/y2 (16). Serum exposure curves were extrapolated from the last data point to the estimated time of reaching 0 mg/l (AUC0–∞) and calculated by the trapezoidal method. Other fit or derived pharmacokinetic parameters included t1/2, total oral clearance (CLT/F [F denotes bioavailability]), and volume of distribution at steady state(Vss/F).
The concentration of azithromycin in PMNs and M/Ls was calculated by dividing the cell assay concentrations by the actual cell counts for the specific sample. This value was then divided by a composite cellular volume based on the actual percentages of PMNs, monocytes, and lymphocytes and the cells’ previously defined volumes (12). PMN and M/L exposure curves were then calculated by the trapezodial method through the final sampling time point (AUC0–288).
Comparison of the serum pharmacokinetic parameters and serum, PMN, and M/L exposure curves for the two dosing regimens was accomplished by using Wilcoxon’s signed rank test and the SYSTAT statistics computer software. During sample size calculations, the number of subjects in each treatment arm needed to provide approximately 74% power to detect a 25% difference in the mean azithromycin AUC was found to be 12. Significance was defined as achieving a P value of ≤0.05. Descriptive sample set data were also created with this software.
Twelve healthy volunteers (six males and six premenopausal females; age, 37.1 ± 7.1 years; weight, 67.2 ± 12.9 kg; estimated creatinine clearance [6], 77.9 ± 11.2 ml/min/1.73 m2) entered and completed the study. Adverse effects thought to be related to study drug were all associated with the gastrointestinal tract. They consisted of mild abdominal cramping or dyspepsia occurring approximately 1 h after dose administration and persisted for up to an hour. This occurred with both dosing regimens (3-day regimen, 3 of 12 subjects [25%]; 5-day regimen, 2 of 12 subjects [16.7%]), and all events resolved with no treatment.
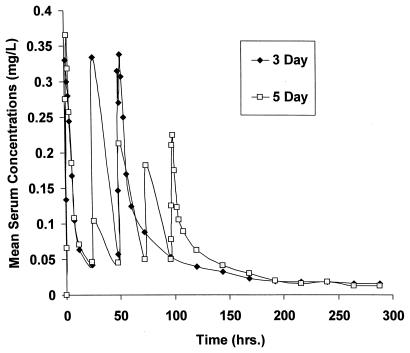
Although serum exposures for the two regimens (Table 1 and Fig. 1) did not differ significantly, subjects did have a higher mean azithromycin exposure (19.4 versus 15.9 mg · h/liter) with the 3-day regimen. The t1/2 values of the two dosing regimens (Table 1) also did not differ significantly and were consistent with the current product labeling of 68 h (14). CLT/F and Vss/F values were consistent with results from past studies (2, 5). Although there was a statistically significant difference between the distributional volumes of the regimens, the difference is most likely not clinically significant. When WBC exposures were compared it was noted that there were no significant differences for either cell type studied (Table 2) with the two dosing regimens. However, WBC exposures were associated with a high degree of variability, as was demonstrated by the high coefficients of variation for both regimens and cell types.
TABLE 1.
Serum exposures and pharmacokinetics for the 3- and 5-day regimens
| Parameter (units) | Mean ± SD for regimen
|
P value | |
|---|---|---|---|
| 3 Day | 5 Day | ||
| AUC0–∞ (mg · h/liter) | 19.4 ± 7.9 | 15.9 ± 4.8 | 0.06 |
| t1/2 (h) | 65.9 ± 14.7 | 66.1 ± 10.5 | 0.75 |
| CLt/F (liters/h) | 90.6 ± 38.7 | 102.5 ± 29.8 | 0.18 |
| Vss/F (liters/kg) | 121.2 ± 32.8 | 143.9 ± 33.1 | 0.03 |
FIG. 1.
Mean azithromycin concentrations in serum versus time for the 3- and 5-day regimens.
TABLE 2.
PMN and M/L exposures for the 3- and 5-day regimens
| Cell type | Median AUC0–288 (CV)a for regimen
|
P value | |
|---|---|---|---|
| 3 Day | 5 Day | ||
| PMN | 12,734.6 (89) | 11,797.7 (45) | 0.5 |
| M/L | 15,498.2 (133) | 15,625.8 (113) | 1.0 |
AUCs are in milligram · hours per liter; coefficients of variation (CV) are percents.
Both WBC types demonstrated significant retention of the azithromycin during each regimen. Median concentrations in cells at 12 days after the start of the 3-day and 5-day regimens were, respectively, 24 and 22 mg/liter for PMNs and 10 and 9 mg/liter for M/Ls. These are in sharp contrast to the corresponding concentrations in serum of 0.005 and 0.002 mg/liter, respectively.
Azithromycin is an azalide antibiotic with unique pharmacokinetic properties characterized by substantial tissue and cellular concentrations and retention. This allows it to be administered for short periods while maintaining high infection site concentrations for much longer periods (8). Currently, azithromycin is given at 1.5 g over 5 days in the United States and over 3 days in other countries. In a previous study, the 3-day regimen was demonstrated to result in higher serum exposure than the 5-day azithromycin regimen (17). This study was conducted to verify the serum exposures and pharmacokinetics demonstrated by both regimens as well as to investigate whether the WBC exposure curves were also similar.
As was demonstrated by the data, the serum exposure curves for the two regimens are indeed similar and support the above-mentioned study in that the 3-day regimen did provide higher exposure on average than the 5-day regimen. The presence of additional study subjects may have resulted in a statistically significant difference. As has been repeatedly demonstrated in the past, azithromycin is extensively distributed in the body (Vss/F > 100 liters/kg), and as demonstrated in Table 2, large amounts of the drug are found within the WBCs (2, 5). CLT/F of 90 to 100 liters/hr resulted in t1/2 values that are consistent with current product labeling for azithromycin but much longer than for other macrolides (2, 14).
The two dosing regimens also resulted in similar PMN and M/L exposures with trough (defined as last time point) concentrations detectable and well above the MIC for any relevant community-acquired respiratory pathogen. As has been reported previously, concentrations in WBC are at least 2 log units higher than corresponding concentrations in serum (13). This is consistent with azithromycin’s extended antibacterial activity, especially intracellularly, due to the correlation of AUC above MIC with efficacy (7). The relative high degree of variability of the WBC exposures can be explained by a couple of issues that are inherent to WBC research currently. First, WBC counts and the mix of type of WBCs can vary from day to day within any given subject. Secondly, and most importantly, despite our utilizing state-of-the art WBC separation technology, the technology available for WBC separation is imperfect. Although we utilized a separation medium that was felt to be superior to media used previously, there is not yet a medium that gives consistent separation results.
The results of this study indicate that azithromycin, at a total dose of 1.5 g, provides similar serum, granulocyte, and M/L exposures when it is administered over 3 and over 5 days.
Acknowledgments
We thank Ruth Blackman, Linda Stragand, Roberta Steere, Jennifer Amsden, Laura Cabelus, Anne Menhinick, and Lucia LaBoy-Goral for their key roles and assistance during this study. We also recognize Rahlene Welch for her invaluable assistance in preparing the manuscript and Joseph S. Bertino for his manuscript reviews.
This study was supported by a grant (066-087) from Pfizer Inc.
REFERENCES
- 1.Amsden G W. Erythromycin, clarithromycin, and azithromycin: are the differences real? Clin Ther. 1996;18:56–72. doi: 10.1016/s0149-2918(96)80179-2. [DOI] [PubMed] [Google Scholar]
- 2.Amsden G W, Ballow C H, Forrest A. Comparison of the plasma, urine and blister fluid pharmacokinetics of clarithromycin and azithromycin in normal subjects. Clin Drug Invest. 1997;13:152–161. [Google Scholar]
- 3.Baldwin D R, Wise R, Andrews J M, Ashby J P, Honeybourne D. Azithromycin concentrations at the site of pulmonary infections. Eur Respir J. 1990;3:886–890. [PubMed] [Google Scholar]
- 4.Ballow C H, Amsden G W. Azithromycin: the first azalide antibiotic. Ann Pharmacother. 1992;26:1253–1261. doi: 10.1177/106002809202601014. [DOI] [PubMed] [Google Scholar]
- 5.Ballow C H, Amsden G W, Highet V S, Forrest A. Pharmacokinetics of oral azithromycin in serum, urine, polymorphonuclear leukocytes and inflammatory vs. non-inflammatory skin blisters. Clin Drug Invest. 1998;15:159–167. doi: 10.2165/00044011-199815020-00009. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 7.Craig W A. Postantibiotic effects and the dosing of macrolides, azalides, and streptogramins. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker; 1997. pp. 27–38. [Google Scholar]
- 8.Foulds, G., and R. B. Johnson. 1993. Selection of dose regimens of azithromycin. J. Antimicrob. Chemother. 31(Suppl. E):39–50. [DOI] [PubMed]
- 9.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73–82. [DOI] [PubMed]
- 10.Gladue R P, Bright L M, Isaacson R E, Newborg M F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989;3:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladue R P, Snider M E. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob Agents Chemother. 1990;34:1056–1060. doi: 10.1128/aac.34.6.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nibbering P H, Zomerdijk T P L, Corsèl-Van Tilburg A J, Van Furth R. Mean cell volume of human blood leukocytes and resident and activated murine macrophages. J Immunol Methods. 1990;129:143–145. doi: 10.1016/0022-1759(90)90432-u. [DOI] [PubMed] [Google Scholar]
- 13.Olsen K M, San Pedro G S, Gann L P, Gubbins P O, Halinski D M, Campbell G D., Jr Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob Agents Chemother. 1996;40:2852–2855. doi: 10.1128/aac.40.11.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfizer Inc. Product information, Zithromax. Groton, Conn: Pfizer Inc.; 1997. [Google Scholar]
- 15.Shepard, R. M., and F. C. Falkner. 1990. Pharmacokinetics of azithromycin in rats and dogs. J. Antimicrob. Chemother. 25(Suppl. A):49–60. [DOI] [PubMed]
- 16.Tanswell P, Koup J. TopFit: a PC based pharmacokinetic/pharmacodynamic data analysis program. Int J Clin Pharmacol Ther Toxicol. 1993;31:514–520. [PubMed] [Google Scholar]
- 17.Wildfeuer, A., H. Laufen, M. Leitold, and T. Zimmermann. 1993. Comparison of the pharmacokinetics of three-day and five-day regimens of azithromycin in plasma and urine. J. Antimicrob. Chemother. 31(Suppl. E):51–56. [DOI] [PubMed]
- 18.Wildfeuer A, Laufen H, Müller-Wening D, Haferkamp O. Interaction of azithromycin and human phagocyte cells. Arzneim-Forsch. 1990;39:755–758. [PubMed] [Google Scholar]